Abstract
Quorum sensing is a process in which bacteria secrete and sense a diffusible molecule, thereby enabling bacterial groups to coordinate their behavior in a density-dependent manner. Quorum sensing has evolved multiple times independently, utilizing different molecular pathways and signaling molecules. A common theme among many quorum-sensing families is their wide range of signaling diversity—different variants within a family code for different signal molecules with a cognate receptor specific to each variant. This pattern of vast allelic polymorphism raises several questions—How do different signaling variants interact with one another? How is this diversity maintained? And how did it come to exist in the first place? Here we argue that social interactions between signaling variants can explain the emergence and persistence of signaling diversity throughout evolution. Finally, we extend the discussion to include cases where multiple diverse systems work in concert in a single bacterium.
Keywords: quorum sensing, evolution, social evolution, diversification, pherotypes, cell-cell communication
Introduction
Quorum sensing (QS) is a process where bacteria secrete a diffusible signal molecule that they can also sense (71, 75, 112, 116). QS is widespread in the bacterial world and has evolved independently multiple times. It is not surprising, therefore, that QS systems vary in the type of signal molecule used, its mode of sensing, the conditions under which the signal is produced and sensed, and the type of response mediated. Yet, by its definition, QS will lead to a density-dependent response. Other parameters, such as the presence of flow (27, 51), stability of the signaling molecule (46), and spatial confinement (43, 85) may also influence the response through their effect on the concentration of the signal in the intercellular milieu. Several excellent recent reviews have discussed the dynamics of QS signaling, and the reader is referred to them for further discussion of these subjects (44, 70).
While QS controls a variety of traits in different bacteria, some general trends can be identified. Most notably, QS often controls social behaviors—behaviors with a positive fitness effect on other bacteria, or whose benefit to the responding cell depends on the presence of other bacteria in the environment. This includes secretion of enzymes and other substances that modify the environment, secretion of toxins that affect other strains or species, and horizontal gene transfer processes. To understand the evolutionary and ecological aspects of QS, it is therefore crucial to take into account bacterial social interactions.
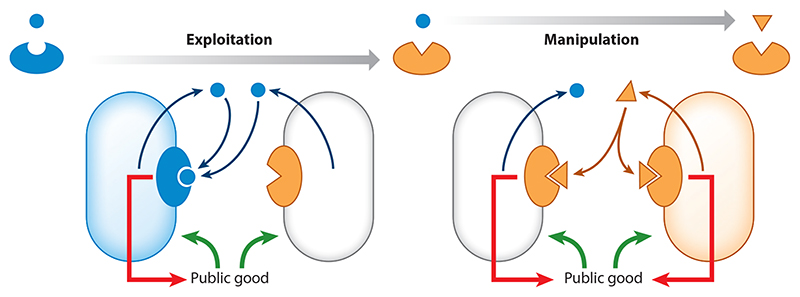
In addition to communication among bacteria, intercellular signaling allows for complex interactions between bacteria and the mobile elements they contain, as well as the hosts they inhabit. Eavesdropping on bacterial communication, for instance, has been shown to be a strategy employed by both plant and animal hosts (62, 69) and by selfish genetic parasites of bacteria (25, 97). Manipulation of QS has also been shown to occur, both by signal disruption (24) and by production of molecules that activate or inhibit QS receptors (60, 80, 106). This is sometimes also referred to as quorum quenching. From a technological standpoint, eavesdropping and manipulation present an opportunity for developing new methods for detection and control of bacterial populations. This is currently a topic of extensive research (116).
Another striking and prevalent feature of QS systems, the focus of this review, is the large repertoire of signaling molecules observed within a given QS family (40). This is especially true for gram-positive bacteria, where hundreds, if not thousands, of distinct peptides act as QS signals. Furthermore, signals are paired with cognate receptors that are often tuned to sense them specifically. We will use the term signaling variants to refer to such divergent signal-receptor pairs within a family.
In the following, we first overview the observed magnitude and characteristics of diversification in different QS families. We then discuss the ecological forces that may shape this diversity, focusing on the role of social interactions. Continuing with ecology, we examine some of the interactions that may occur between different variants, such as manipulation and eavesdropping, and review the limited empirical evidence in this regard. Moving from ecology to evolution, we discuss complications arising from the required coevolution of receptor and signal for the formation of a novel signaling variant. Finally, we examine the evolutionary forces underlying acquisition of multiple QS systems by a single bacterium and its ecological implications.
Patterns of Diversity In Quorum-Sensing Families
Though different families of QS systems vary widely in all attributes, a shared feature among many of them is a considerable level of signaling diversity. Table 1 summarizes the molecular architecture and patterns of signaling diversity found in many of the well-studied QS families. These include both gram-negative- and gram-positive-associated families. Gram-negative families utilize small molecules as signals and either membranal or cytoplasmic receptors for their sensing (65). Gram-positive QS systems are distinguished into two superfamilies, both utilizing genetically encoded peptides as signal molecules. The first is based on long or post-translationally modified peptide signals and a membranal histidine kinase receptor. The second superfamily, termed RNPP, is based on small unmodified peptide signals (5–10 amino acids) that are imported through an oligopeptide permease to interact with a cytoplasmic receptor (54, 67).
Table 1. Major quorum-sensing families and their diversity patterns.
| Family name | Molecular and population-level characteristics | References |
|---|---|---|
|
Gram-negative-associated families
Signal: small molecule produced by a specific enzyme | ||
| LuxRI | Receptor: intracellular, TF Signal: acyl homoserine lactone Number of variants: ~20 Variation types: acyl chain length (4–16 carbons), acyl C3 modification, noncanonical Distribution: gram-negative, mostly interspecific |
32, 75 |
| LuxMN | Receptor: membranal, histidine kinase Signal: acyl homoserine lactone Number of variants: ~20 (including cross talk with LuxRI variants) Variation types: acyl chain length (4–16 carbons), acyl C3 modification, noncanonical Distribution: vibrios, interspecific |
11, 49 |
| LuxPQS | Receptor: membranal (periplasmic), histidine kinase Signal: AI-2 Number of variants: 1 Variation types: no variation Distribution: signal produced by many bacteria; sensed by few |
11, 16, 86 |
| DSF | Receptor: membranal histidine kinase Signal: cis-2-unsaturated fatty acids Number of variants: ≳10 Variation types: chain length and branching Distribution: various gram-negative species |
21, 121 |
|
Gram-positive membranal receptor superfamily
Receptor: membranal; acts as a histidine kinase on a transcriptional response regulator Signal: modified or long peptide | ||
| Agr | Signal: cyclic peptide (6–10 amino acids) Number of variants: ≫10 Variation types: sequence, cyclization moiety, noncyclic peptide tail Distribution: gram-positive bacteria, intraspecific (e.g., 4 variants in Staphylococcus aureus) |
48, 56, 73, 104, 118 |
| ComQXP | Signal: tryptophan-prenylated peptide (6–10 amino acids) Number of variants: ~10 Variation types: peptide sequence and length, prenylation moiety Distribution: Mostly Bacillus subtilis group, intraspecific |
23, 107, 108 |
| ComABCD | Signal: long unmodified peptide (~20 amino acids) Number of variants: ~10 Variation types: peptide sequence and length Distribution: streptococci, intraspecific (e.g., 2 variants in Streptococcus pneumoniae) |
2, 39, 83, 115 |
|
Gram-positive RNPP superfamily
Receptor: cytoplasmic (mostly transcription factor) Signal: small, unmodified peptide Variation types: peptide sequence and length Distribution: intraspecific | ||
| Rap-Phr | Distribution: bacilli, multiple pherotypes per strain, including mobile elements Number of variants: ~100 (> 50 variants in B. subtilis group) |
29, 55, 79 |
| NprR-NprX | Distribution: Bacillus cereus group Number of variants: ~10 |
77 |
| PlcR-PapR | Distribution: B. cereus group Number of variants: ~10 |
98, 99 |
| PrgX-iCF10 | Distribution: enterococcus plasmids Number of variants: ~10 |
24 |
| ComRS/Rgg | Distribution: streptococci, multiple pherotypes per strain Number of variants: ≫10 |
30, 95 |
| AimR-AimP | Distribution: bacillus mobile elements, multiple pherotypes per bacterial strain Number of variants: > 150 |
28, 101 |
| AloR-AloP | Distribution: Paenibacillus (P. polymyxa), multiple pherotypes per strain Number of variants: ≫10 |
109 |
| QsrR-QsrP | Distribution: clostridia (Clostridium acetobutylicum), multiple pherotypes per strain Number of variants: ≫10 | 53 |
| TprA | Distribution: streptococci, mobile elements Number of variants: ~3 |
45 |
Generally, the number of different signaling molecules in the gram-negative small-moleculebased QS systems is rather small. The acyl homoserine lactone (AHL)-based luxRI family, for example, has ~20 signal variants, while others have even fewer. Some systems, and specifically the AI-2 systems, are based on a metabolic by-product produced by many species. These molecules may therefore be regarded as a cue rather than a signal (86). Maybe due to the small repertoire of distinct signals overall, gram-negative species typically do not show intraspecific diversity in the signaling molecule of a given QS system (but cf. 10, 68). Diversification of the signal is thus interspecific and is generally accompanied by diversification of the QS response regulon along with other traits.
The level of diversity of gram-positive-associated systems is far higher than that of their gram-negative counterparts—each of the superfamilies contains multiple families, coding for many different signaling variants. In these systems, diversity is often intraspecific, with few to dozens of signaling variants in a given species. These intraspecific variants are referred to as pherotypes. The phylogenetic tree of pherotypes often diverges significantly from the housekeeping phylogeny of the species and from the phylogeny of the QS regulon (4, 8, 30, 63, 64, 87, 99). This suggests that pherotypes are horizontally transferred and decouple signaling diversity from the regulon controlled by the QS systems. For example, in the ComQXP system of Bacillus subtilis, there are ~10 pherotypes. In this system, only the extracellular part of the comP receptor gene and the signaling genes, comQ and comX, show high diversity and are horizontally transferred. On the other hand, phylogeny of the intracellular part of the ComP receptor that mediates kinase activity on the ComA response regulator conforms to the housekeeping phylogeny, and the same is true for ComA and many of its target genes (3, 4, 59, 107).
The Social Nature of Bacterial Quorum Sensing
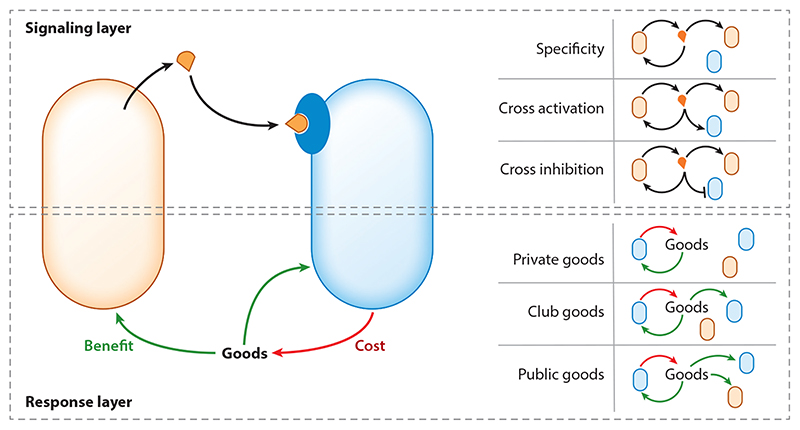
Throughout this review we argue that social interactions underlie the existing signaling diversity found in various QS systems. Bacterial QS can be thought of as a two-layered social interaction system, consisting of the signaling and response layers (Figure 1). These two layers combine to produce the overall ecological interactions between different signaling variants. Understanding the patterns of social interactions at these two layers is therefore crucial for explaining the emergence of novel signaling variants, their maintenance in the population, and their impact on other bacterial traits.
Figure 1. Interactions between signaling variants are composed of two layers: a signaling layer (top) and a response layer (bottom).
The image illustrates the impact of each layer—a signaling bacterium [light orange, also coding for its own receptor (not shown)] can impact the receptor of a different signaling variant [light blue, also coding for its own signal (not shown)]. The responding cell produces a good (green arrows) that can benefit itself or others. Production of goods is costly (red arrows). The right-hand panels illustrate different possible scenarios for interactions at either layer.
The Signaling Layer
The signaling layer consists of interactions between the signals produced by different variants, and the receptors that bind them (Figure 1). Signals can activate a receptor, inhibit its activation, or not interact with it at all. Crucially, in a given signaling variant the signal can always, by definition, activate its cognate receptor. When considering the interactions between receptors and signals within a given QS family, one can identify several patterns:
Signaling optimization: Generally, the receptor of a signaling variant is optimized to sense its cognate signal compared to signals of other variants. Signaling optimization was observed in many specific studies where a multitude of signals from a given family have been experimentally assayed for their effect on a given receptor (e.g., 4, 48, 72, 92, 96). This basic observation is in agreement with the general notion of QS as a means of communication between related bacteria [or for sensing oneself (9, 85)]. Another implication of signaling optimization is that signal and receptor coevolve to maintain their optimal affinity (see more below). In gram-positive systems, where the primary sequence of the signal is genetically encoded, this can be readily observed by comparing the phylogenetic relations between the receptor and signal genes. Such studies have been done for multiple systems and indeed show tight coevolution between cognate receptors and signals (e.g., 4, 29, 115, but cf. 64).
Cross activation: A receptor may also respond to other signaling molecules in the family, but typically with reduced sensitivity (e.g., 4, 31, 64, 96). In some of the observed cases, cross activation seems to reflect the evolutionary distance between the variants, as receptors with higher similarity tend to cross interact more in their signals (107). Cross activation between two signal-receptor pairs is sometimes symmetric—each receptor is similarly cross activated by the noncognate signal but is often asymmetric; one receptor is cross activated by the noncognate signal more than the reverse.
Full orthogonality: In many cases QS systems can be regarded as orthogonal—a signal of one system will not activate the receptor of another variant. In gram-negative bacteria, this is typically the case between highly diversified signaling molecules, such as between receptors of short-chain AHL (4–6 carbons in the acyl tail) and long-chain ones (12–14 carbons), while more similar molecules sometime cross activate (e.g., 105). Gram-positive pherotypes are often fully orthogonal. For example, a recent study of the Rap-Phr system of Bacillus subtilis found almost no cross talk between 10 divergent Rap-Phr pherotypes (29). Similarly, a study of cross interactions between ComRS variants from several Streptococcus species showed a combination of fully orthogonal interactions and partial cross activation (96). Multiple works have analyzed the molecular determinants of specificity between QS variants (e.g., 17, 34, 49, 76, 92, 96). We do not elaborate on these molecular characteristics, as they are very different between families. The reader is referred to an excellent recent review on these aspects (40).
Cross inhibition: In some cases, a noncognate signal can interfere with the activation of a receptor by its cognate signal. Such cross inhibitory interaction was initially described between different Staphylococcus aureus Agr pherotypes (48). Since then, it has been identified in additional systems, including the ComQXP pherotypes family of B. subtilis (107, 108), the luxMN variants in Vibrio (49), and the Chromobacterium violaceum pherotypes (68). From a molecular perspective, the simplest mechanism for obtaining cross inhibition is by competitive inhibition on the binding site (92). However, there is evidence suggesting that for certain cases, binding of a noncognate signal may have a direct effect on receptor conformation (35, 49).
The Response Layer
From a social perspective, we can distinguish between three levels of sociality in bacterial QS-dependent responses. Binding of a signal may illicit a response that can provide a fitness benefit exclusively to the responding bacterium (private good), exclusively to related bacteria (club good), or nonexclusively to neighboring bacteria (public good). Most of the activities shown to be regulated by QS fall into the rubric of public goods, and we view this as critical for understanding QS diversity, as discussed below. For example, Pseudomonas aeruginosa utilizes QS to regulate the secretion of degradative enzymes, surfactants, and host-directed toxins (94). Public goods production is cooperative (Figure 1), and as such, it is susceptible to exploitation by cheaters, which enjoy the benefits of cooperation without bearing the cost of public goods production (37, 65, 84, 103, 113). In QS-regulated public goods a receptor null mutant acts as a cheater, as has been described in multiple systems (5, 22, 81, 82, 89, 93, 117). For cooperative behaviors to be maintained through-out evolution, exploitation must be suppressed. One way by which exploitation can be avoided is if the population is sufficiently structured, i.e., cooperators tend to interact mostly with other cooperators and cooperation is maintained by kin selection (22, 38, 114).
We consider a response of one variant as a club good if it does not provide a benefit to another signaling variant in the population. Club goods require that signaling diversity be correlated with genetic factors that govern the ability to enjoy the goods. For interactions between pherotypes, club goods are expected to be rare, as pherotype variability is typically uncorrelated with other genes. Club goods are observed, for example, in the Blp QS system of streptococci, where the QS variants are the regulatory part of a locus encoding for a variable toxin system (64) and often covary with them.
Finally, QS may regulate the production of private goods, which benefit only the responding cells. These responses are not exploitable, and their expression can select against the rise of cheater QS mutants (20, 111). However, the ecological relevance of QS regulation of private goods is yet unclear (95, 100).
The signaling and response layers together form the overall relevant ecological and evolutionary interactions between signaling variants. From this perspective, QS can be thought of as a kin-discrimination process, as it allows cells to differentially respond to the presence of kin and nonkin (102, 110). The ecological process leading to diversification of QS variants can therefore be analyzed using concepts and methods described in this field (7, 36, 88), or in the related field of the evolution of green-beard genes (33, 47).
The Ecological Implications of Quorum-Sensing Diversity
The apparent diversity of QS systems, and intraspecific pherotype diversity in particular, suggests that there are selection forces that act to create and maintain signaling diversity. The social consequences of QS imply that selection in favor of diversity may emerge from interactions between signaling variants. Before delving into this possibility, we wish to mention an alternative hypothesis according to which diversity is driven by the different chemical nature of signals within a family, which may affect their properties in a given environment (46). For example, it was shown that homoserine lactones of different acyl side chain length have different levels of hydrophobicity (42). This can affect their stability (19) and distribution pattern in oil-water interfaces (42). However, for many of the abovementioned families, the chemical characteristics of the different signals are similar (molecule size, general shape, charge, hydrophobicity), and they likely interact similarly with the abiotic environment.
To ask whether signaling diversity can be maintained by social interactions, one must take into account the combination of interactions between variants at both layers and the resulting selection patterns that emerge (Figure 2). When considering interactions between two strains, one can identify multiple scenarios. Here we focus mostly on interactions between strains carrying different pherotypes, and so interactions at the layer of response are assumed to be symmetric—either both strains produce private goods or both produce public goods. We note that for our considerations, club goods lead to similar selection patterns as private goods, and we do not distinguish between them.
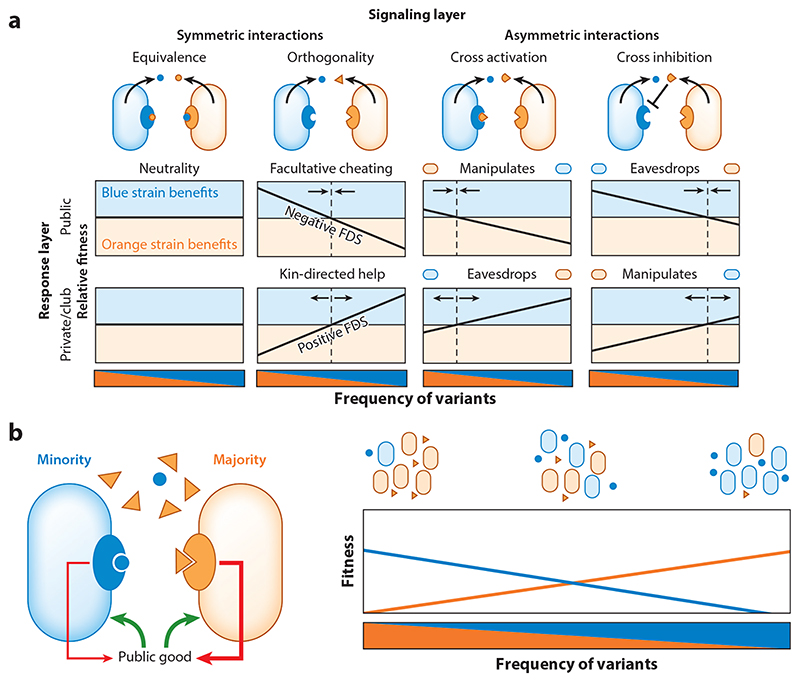
Figure 2. Social interactions between QS variants.
(a) The different interactions between signaling variants and the resulting frequency-dependent selection scheme between them depending on whether QS response is cooperative (public goods) or competitive (private or club goods). Production of public goods leads to negative frequency-dependent selection, while private goods lead to positive frequency-dependent selection. Asymmetric interactions lead to manipulation/eavesdropping by modifying the frequency point of equal fitness. Dashed lines indicate the frequency of equal fitness, and arrows represent the direction in which frequency would change due to selection. Blue and orange sections represent a fitness advantage in favor of the blue and orange strains, respectively. (b) A more detailed examination of the case of orthogonal variants with production of public goods. The left-hand illustration demonstrates the cost (red arrows) and benefit (green arrows) when the blue strain is in the minority. The extent of cost payed or benefit gained is represented by line width. The blue minority pays less of a cost because it senses less of its signal. The right-hand graph illustrates the fitness of both strains in relation to their frequency in the population. Fitness is equal at equal frequencies. Abbreviations: FDS, frequency-dependent selection; QS, quorum sensing.
First, we consider the simple case where the two signaling variants are equivalent—each signal activates its own receptor and the other’s to the same extent. Under these conditions, the variants behave like neutral alleles and their fate is determined by genetic drift irrespective of the type of response regulated by QS (Figure 2a). Specificity is therefore crucial for understanding the maintenance of multiple pherotypes. In all other cases, where signals are not equivalent, we argue that the fate of the variants is strongly dependent on the type of response mediated. If cells respond to the signal by producing public goods, then the two strains would stably coexist by minority selection [also known as negative frequency-dependent selection (Figure 2a)]. The minority strain would sense less of its signal than the majority strain and would therefore produce less of the public goods, effectively exploiting the majority. As the minority frequency increases, the difference in public good production between the strains would decrease, reducing the strength of selection. In the simple case of orthogonal signaling variants, the strains would stably coexist at an equal frequency of 50%. This mechanism of selection is referred to as facultative cheating (90) (Figure 2a, b). If, on the other hand, cells respond by producing private goods, selection favors the majority (also known as positive frequency-dependent selection) (Figure 2a). The majority strain would sense a higher level of the QS signal and would therefore produce more of the private goods, increasing its relative benefit compared to the minority. This would allow it to take over the population and eliminate diversity.
Two studies have examined the consequences of selection between signaling variants with a public goods response (Figure 2b). Pollak et al. (81) studied the patterns of selection between pherotypes of the ComQXP system in B. subtilis. A major activity regulated by this system is the production and secretion of surfactin—a potent surfactant whose level can reach 10% of the bacterial biomass (18). Surfactin production is necessary for swarming motility over semisolid surfaces (50). The work established that surfactin is a public good, allowing a QS mutant to exploit the wild type over its production. Subsequently, minority selection was shown for the interaction between four pherotypes introduced into an isogenic background. The coexistence ratio of a closely examined pair diverted from 1:1, as one of the pherotypes had a higher effective activity than the other. In this case, it was shown that coexistence occurred at a frequency where the cost of public goods production by the two strains is equal. Similar results were found when orthogonal pherotypes of the PlcR-PapR system in Bacillus thuringiensis were studied in well-mixed insect larva homogenate (119). An oral larva infection model gave more complicated results, due to the highly structured nature of the population, as well as other effects (120).
When QS controls the production of public goods, selection between nonorthogonal signaling variants is still negative-frequency dependent due to facultative cheating by the minority, but with a shifted equilibrium frequency. Cross activation in this case amounts to manipulation, as signaling by the manipulator strain causes the other strain to produce additional public goods, which the manipulator enjoys without suffering the cost of production. Manipulation under these conditions offsets the coexistence balance in favor of the manipulator strain. Conversely, if the interaction between pherotypes is cross inhibitory, then the inhibited strain is effectively eavesdropping on the inhibitor strain. Cross inhibition leads to reduction in the production of public goods by the inhibited strain, allowing it to exploit the inhibitor. Similar to the effect of manipulation, cross inhibition would offset the coexistence balance in favor of the inhibited eavesdropper strain.
If the response to QS is private, then the adaptive implications of the interactions between variants are reversed; cross activation is an eavesdropping mechanism by the activated strain, while cross inhibition is a manipulation by the inhibiting strain. These asymmetric pherotype interactions still lead to positive-frequency-dependent selection but shift the tipping frequency, above which one strain takes over the other to benefit the manipulator or eavesdropper strains (Figure 2a).
The ecological implications of cross inhibition and cross activation have not been widely explored. One work where eavesdropping by asymmetric cross activation was studied concerns interactions between bacteria with QS-dependent production of club goods. This research explored the consequences of cross activation between two species coding for LuxIR-type systems (15) (Table 1). Burkholderia thailandensis codes for the BtaIR2 3OH-C8-HSL QS system, which controls the production of antibiotic bactobolin A. Chromobacterium violaceum codes for the CvlIR C6-HSL QS system, which regulates the production of the antimicrobial violacein. Each species is sensitive to the toxin of the other. Based on this, one would expect to find a pattern of positive-frequency-dependent selection. The authors find that in addition to this interaction, the promiscuous receptor of C. violaceum is activated by the B. thailandensis signal. This results in eavesdropping of B. thailandensis by C. violaceum (Figure 2a). It is claimed that this interaction may explain the evolution and maintenance of promiscuous receptors sensitive to nonendogenous signals. On the other hand, such eavesdropping may lead to selection in favor of mutations that allow escape from eavesdropping. In the context of the LuxIR family, with its limited signal diversity (Table 1), this may not be possible. Similar arguments were also made for the evolution of the Blp system described above (64).
Gram-positive RNPP systems are also utilized by mobile elements to control their horizontal transfer. In these systems, the receptor activates horizontal transfer (by either conjugation or virion formation) in the absence of signal (the signal represses receptor activity). This logic ensures that horizontal transfer would not occur if a majority of the population contains the mobile element. Mobile-element-associated systems also exhibit high signaling diversity (Table 1). To understand the ecological implications of this diversity, we separately consider cases where the two elements are able to productively infect a cell inhabited by the other (hetero-immune) or not (homo-immune). If the elements are homo-immune, their transfer rate should depend on the frequency of both elements within the population, implying that diversity would not be selected for. In contrast, if they are hetero-immune, the transfer rate of an element should depend only on its own frequency in the population, and therefore selection in favor of diversity is expected.
To conclude, the vast diversity of signaling variants can be explained by considering the social context of bacterial interactions. Viewing this through the framework of social evolution, greater signaling diversity corresponds to fine-tuning of QS as a mechanism of kin discrimination. The higher the specificity of the signal, the more its reliability as an indicator of kin frequency increases, allowing bacteria to invest in cooperative behaviors only when the population is genetically related to them. A prerequisite for this argument is that QS controls the production of public goods, and observations indicate that this is indeed often the case. We note that from a theoretical standpoint public goods production can be selected for in structured populations. A problem arising from this is that our arguments regarding the maintenance of signaling diversity require a mixed population. Reconciling this seeming inconsistency, a recent theoretical work showed that structured populations that eliminate cheaters could still maintain signaling diversity (12). Finally, selection for signaling diversity may also be shaped by interactions with other organisms that either eavesdrop or manipulate bacterial QS to their benefit. To the best of our knowledge, there is no study exploring this possibility.
Molecular Coevolution of Signaling Diversity
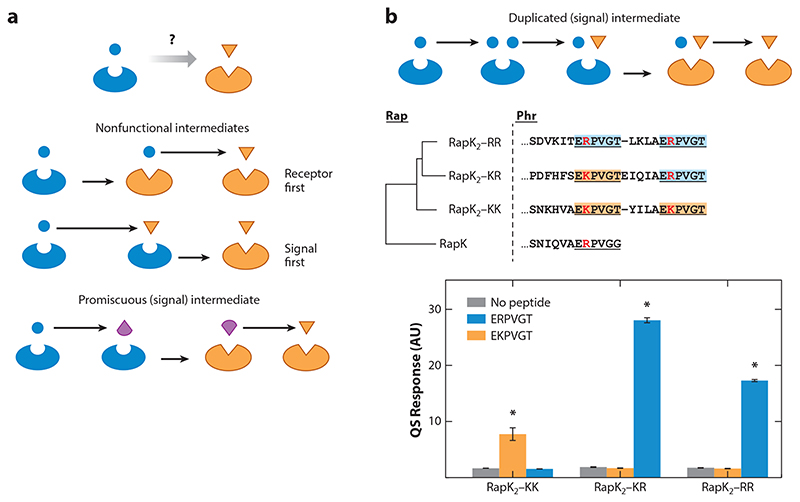
So far we have provided an explanation for how signaling diversity can be maintained once it has been established. But how and why did this diversity come to exist in the first place? The evolution of a novel QS signaling variant is, by definition, a coevolutionary process at the molecular level—to obtain a novel variant, mutations must occur in both the signaling and reception genes. Molecular coevolution of interacting pairs and the formation of divergent specific variants are general problems in the field of molecular evolution and population genetics. The multistep nature of this process requires one to take into account the order of intermediate evolutionary states and their functional consequences (Figure 3a). The shortest path to diversification is one in which consecutive mutations in the genes for the two interacting partners lead to the evolution of a novel interacting pair. A clear problem with this direct path is that in the intermediate mutant that has mutated only one of the partners, the interaction would be suboptimal compared to either the ancestral or the novel pairs. As interaction is assumed to be beneficial, it is unclear how this less-fit intermediate could evolve in the context of its ancestral strain, what is also known as a valley-crossing problem in evolution (52). This obvious problem is at the heart of much of the studies regarding the evolution of interacting pairs.
Figure 3. Coevolution of signal and receptor.
(a) Pathways of coevolution—direct through a nonfunctional intermediate and indirect through a promiscuous intermediate. The indirect route may also work through a receptor intermediate (not shown). Arrows represent mutations, and changes in shape and color signify changes in the receptor or signal. (b) Evolution through a duplicated intermediate. A scheme of the evolutionary path (top) and evidence for it in the Rap-Phr system. Three related Rap-Phr systems have a duplicated Phr mature signal with either an arginine or a lysine at the second residue. The phylogenetic tree shows the evolutionary relation between the three receptors and the closely related RapK receptor. The terminal part of the Phr genes is shown for the three divergent systems, and the mature signals are indicated with their variant residues highlighted in red. The bottom panel shows the response of the receptors to the two signal variants. Panel b adapted with permission from Reference 29.
One way this can be solved is by an additional, promiscuous, intermediate variant of one of the partners (Figure 3a). This promiscuous variant can interact with both the ancestral and novel forms of its counterpart. In this scenario the promiscuous allele evolves first, followed by mutations in its counterpart. A second mutation in the promiscuous variant then results in the novel, divergent, and specific pair. In contrast with the direct valley-crossing path, this longer, indirect path is entirely composed of neutral steps. A promiscuous-intermediate path for diversification was suggested to take place in many interacting pairs, both in eukaryotic signaling systems (e.g., 91) and in prokaryotic ones (1). In the latter work, an exhausting mutagenesis of specificity-determining residues in an antitoxin and analysis of mutant interactions with two related toxins identified multiple molecular paths that allow a transition between toxin-antitoxin pairs through promiscuous intermediates. To the best of our knowledge no similar analysis has been performed on QS variants.
A similar solution involves the duplication of a full pair or one partner. In this case the duplicated partner serves as a replacement for the promiscuous variant—one of the duplicates maintains interaction with the ancestral counterpart, allowing the second duplicate to mutate into its novel form with no selective disadvantage (Figure 3b). This process is similar to the general evolutionary mechanism of neofunctionalization of duplicated genes (74), but it has not been much explored in the context of molecular coevolution of interacting pairs. Interestingly, there is direct evidence for this path in the evolution of the Rap-Phr and NprR-NprX QS systems (29) (Figure 3b; Table 1). A detailed analysis of Rap-Phr homologs in hundreds of B. subtilis and Bacillus cereus group genomes identified close to 100 variants. As with other RNPP superfamily signals, the Phr gene product is typically ~50 amino acids long and is composed of a signal sequence and, typically, a single propeptide that is cleaved into the mature peptide extracellularly (54, 67). It was found that >5% of the variants had intragenic duplications (or multiplications) of the propeptide region and in some cases full gene duplications. While some multiplications had the same putative mature signal, others showed variation in this sequence. A specific triplet of closely related receptors (homologs of RapK named RapK2-KK, RapK2-KR, and RapK2-RR) were further analyzed experimentally (Figure 3b). The three receptors had a cognate signaling gene coding for duplications of the mature signal E[K/R]PVGT. The signaling peptide duplications both code for the lysine allele (KK variant) or both code for the arginine allele (RR variant), or they code for one of each (KR variant). Despite the similar chemical nature of arginine and lysine, it was found that the KK receptor responds only to the lysine-containing mature signal allele, while the KR and RR respond only to the arginine-containing mature peptide (Figure 3b). This indicates that these variants had undergone diversification and suggests that this had been assisted by the duplication event. The vast number of duplicates suggests that duplication may be selected for in this family (perhaps for increasing signaling level under some conditions) and that it may serve as a general mediator of divergence. Similar duplications were also observed in the NprR-NprX QS system of the B. cereus group, which is phylogenetically and mechanistically related to the Rap-Phr system (78). Intriguingly, similar mechanisms may be at place in the evolution of yeast mating pheromone (61).
We have described several processes that overcome valley-crossing by moving through an indirect path. Are there mechanisms that enable a transition through a nonfunctional (or less functional) intermediate? A general answer is that this can occur if divergence proceeds in multiple small steps in small populations. Each step begins with a mutation in one of the partners, which only slightly reduces the affinity of interaction, followed by a compensatory mutation in the other partner. If population size is sufficiently small, this process is governed by neutral drift (57). It is unclear, however, whether this requirement is met in bacterial populations. Additionally, even a single mutation may lead to a sharp reduction in affinity, as in the example mentioned above (Figure 3b).
An alternative that may allow the direct route can occur if there are contexts where the non-functional pair is adaptive. The social nature of QS immediately provides such a context, as non-interacting receptor mutants can function as cheaters, which do not produce public goods (81, 93, 113). A diversification process of this sort has been analyzed theoretically (26) (Figure 4). At the first step, the receptor of an ancestral pherotype mutates into its novel form, making the QS system nonfunctional and the strain a cheater of its ancestor. This is followed by a compensatory mutation in the signal, which forms a novel functional pherotype. The novel pherotype is immune to cheating by the intermediate; its signal activates the receptor of the intermediate, thereby manipulating it to produce public goods. Immunity is sufficient for selection in favor of the novel pherotype over the intermediate in structured populations. Once the novel pherotype forms, it coexists with the ancestral pherotype by the negative-frequency-selection mechanisms described above. Note that this scenario breaks the symmetry between counterparts—it specifically predicts that the receptor would mutate first, followed by signal changes. The asymmetry is due to the different social roles of reception (response layer) and signaling (signaling layer), as discussed above. It is worth noting that similar mechanisms may be at action in other social systems (52). For example, a similar model was proposed for the evolution of hetero-immunity in latent phages (13).
Figure 4. Social evolution of a novel pherotype.
Under the assumption that quorum sensing controls public goods, a new variant can evolve through a nonfunctional intermediate with a mutated receptor. The intermediate exploits its functional ancestral strain but is manipulated by the novel functional strain. Gray arrows indicate mutations. The illustrations below each of these arrows indicate the social interaction between variants, with costs and benefits indicated by red and green arrows, respectively.
In summary, several mechanisms may contribute nonexclusively to the evolutionary divergence of pherotypes in bacterial QS systems. One mechanism may be neutral divergence through a promiscuous or duplicated intermediate, while another adaptive mechanism may take advantage of the social component of QS evolution.
Refining Kin Discrimination by Accumulation of Multiple Quorum-Sensing Systems
We have ascribed the diversification of QS systems to the evolutionary pressure for increased kin recognition. Another general way by which kin recognition can increase in resolution is by responding synergistically to multiple recognition cues originating from different loci (58, 102, 110). Such a design has been demonstrated in two well-studied genera: Vibrio and Bacillus. Vibrio harveyi codes for three membranal receptors that respond to different signal families (Table 1): AI-2, a nonspecific signal; AHL, a prevalent gram-negative signal; and CAI-1, a vibrio-specific signal. Each of the receptors acts as a kinase in the absence of signal and acts as a phosphatase in its presence. This arrangement leads to an effective AND gate (or coincidence detection) between the signals in activating a QS response, as is required to increase kin-recognition specificity (14, 41, 66). A similar picture is seen in B. subtilis Rap-Phr systems, where multiple Rap-Phr pairs regulate the response regulators Spo0F and ComA (6, 30, 54). An unbound Rap receptor represses the activity of a cognate response regulator, and Phr binding to Rap represses its activity, thus derepressing the regulator (55). The general design of signal transduction in both systems can thus be roughly described as double negative.
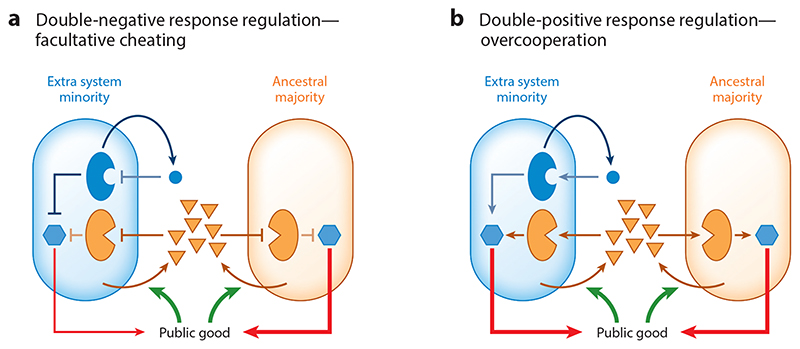
As was illustrated in a recent study (30), a strain that evolved from its ancestor by acquiring an additional double-negative QS system will add it as an additional input to an AND gate formed by the existing systems. Such a strain would not respond in a population where it is a minority. If QS controls public goods, then this would lead to facultative cheating—the evolved strain exploits its ancestor as a minority and returns to cooperate as its frequency increases (Figure 5a). On the other hand, if the acquired QS acts through double-positive regulation—the receptor activates public goods production in the presence of the signal—acquisition would not occur, as this design will form an OR gate between the signals and will be neutral, or slightly deleterious as a minority (Figure 5b). The above work demonstrated the distinction between double-negative and doublepositive regulation both mechanistically and through competition between strains coding for a different number of systems of each type. This was established both in B. subtilis and in V. harveyi. Interestingly, two additional RNPP families with multiple parallel systems in every strain have recently been identified (53, 109). The above model predicts that they will act through a doublenegative type of regulation on the same regulator.
Figure 5. Accumulation of multiple parallel quorum-sensing (QS) systems.
Two architectures of QS regulation of public goods yield different selection on accumulation of parallel systems. (a) In a double-negative architecture, the signal prevents the receptor from inhibiting public goods production. Here, a strain with an additional system would not produce public goods as a minority due to repression by the novel, signal-free receptor. It will therefore cheat its ancestor. Hexagons represent response regulators that control production of public goods and are affected by the receptors. (b) In a double-positive architecture, the signal induces the receptor to activate public goods production. Here, a strain with an additional system will be counterselected as a minority by overcooperation—as a minority, this strain would produce equal or higher amounts of public goods compared to the ancestral majority.
Concluding Remarks
The tower of Babel narrative is an origin story meant to explain the puzzling diversity of human languages. This review presents our own (evidence-based) narrative meant to explain the puzzling diversity of bacterial communication systems. One story relies on intervention by a higher power, while the other is propelled by the forces of selection. However, at the heart of both lies the critical link between communication and cooperation. Signals and their diversity play a crucial role in interactions occurring in all domains of life. In that, signaling diversity provides an important basis for our understanding of the evolution of cooperation, mating behaviors, virulence, and speciation. QS is a form of signaling where these evolutionary processes can be understood in fine detail. Yet, empirical research focusing on the consequences of signaling diversity on bacterial ecology and evolution is conspicuously sparse.
As with many other aspects of bacterial life, a key issue is our ability to understand QS in its natural context, and this is still lacking as well. Other avenues of research that remain to be further explored include the design principles of different QS systems, as well as the diversity of QS systems utilized by genetic elements. Finally, in addition to being an intriguing natural phenomenon, external manipulation of QS has also been suggested as a promising approach for controlling bacterial populations. It is worth noting that the signaling diversity of QS systems, in particular that of pherotypes observed in gram-positive bacteria, may present some obstacles for this approach.
Signal: a secreted molecule that elicits a response in recipients that benefits both senders and receivers
Eavesdropping: occurs when an actor intercepts a signal and acts in its own benefit at the expense of the sender
Manipulation: occurs when a secreted molecule elicits a response that benefits the senders but not receivers
Signaling variants: alleles of functioning quorum-sensing signal-receptor pairs
Cue: a secreted molecule that elicits a response in recipients that benefits the receivers but not senders
Pherotypes: intraspecific signaling variants
Private goods: goods that benefit the producer exclusively
Club goods: goods that exclusively benefit a related group of bacteria
Public goods: goods that benefit different bacteria, independent of relatedness
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Nitzan Aframian, Email: aframian@mail.tau.ac.il.
Avigdor Eldar, Email: avigdor@gmail.com.
Literature Cited
- 1.Aakre CD, Herrou J, Phung TN, Perchuk BS, Crosson S, Laub MT. Evolving new protein-protein interaction specificity through promiscuous intermediates. Cell. 2015;163(3):594–606. doi: 10.1016/j.cell.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan E, Hussain HA, Crawford KR, Miah S, Ascott ZK, et al. Genetic variation in comC the gene encoding competence-stimulating peptide (CSP) in Streptococcus mutans . FEMS Microbiol Lett. 2007;268(1):47–51. doi: 10.1111/j.1574-6968.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 3.Ansaldi M, Dubnau D. Diversifying selection at the Bacillus quorum-sensing locus and determinants of modification specificity during synthesis of the ComX pheromone. J Bacteriol. 2004;186(1):15–21. doi: 10.1128/JB.186.1.15-21.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol Microbiol. 2002;44:1561–73. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 5.Asfahl KL, Schuster M. Social interactions in bacterial cell-cell signaling. FEMS Microbiol Rev. 2017;41(1):92–107. doi: 10.1093/femsre/fuw038. [DOI] [PubMed] [Google Scholar]
- 6.Auchtung JM, Lee CA, Monson RE, Lehman AP, Grossman AD. Regulation of a Bacillus sub-tilis mobile genetic element by intercellular signaling and the global DNA damage response. PNAS. 2005;102(35):12554–59. doi: 10.1073/pnas.0505835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axelrod R, Hammond RA, Grafen A. Altruism via kin-selection strategies that rely on arbitrary tags with which they coevolve. Evolution. 2004;58(8):1833–38. doi: 10.1111/j.0014-3820.2004.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 8.Bannoehr J, Ben Zakour NL, Waller AS, Guardabassi L, Thoday KL, et al. Population genetic structure of the Staphylococcus intermedius group: insights into agr diversification and the emergence of methicillin-resistant strains. J Bacteriol. 2007;189(23):8685–92. doi: 10.1128/JB.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bareia T, Pollak S, Eldar A. Self-sensing in Bacillus subtilis quorum-sensing systems. Nat Microbiol. 2018;3(1):83–89. doi: 10.1038/s41564-017-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnard AML, Salmond GPC. Quorum sensing in Erwinia species. Anal Bioanal Chem. 2007;387(2):415–23. doi: 10.1007/s00216-006-0701-1. [DOI] [PubMed] [Google Scholar]
- 11.Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13(2):273–86. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Zion I, Pollak S, Eldar A. Clonality and non-linearity drive facultative-cooperation allele diversity. ISME J. 2019;13(3):824–35. doi: 10.1038/s41396-018-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berngruber TW, Weissing FJ, Gandon S. Inhibition of superinfection and the evolution of viral latency. J Virol. 2010;84(19):10200–8. doi: 10.1128/JVI.00865-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridges AA, Bassler BL. The intragenus and interspecies quorum-sensing autoinducers exert distinct control over Vibrio cholerae biofilm formation and dispersal. PLOS Biol. 2019;17(11):e3000429. doi: 10.1371/journal.pbio.3000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler JR, Heilmann S, Mittler JE, Greenberg EP. Acyl-homoserine lactone-dependent eaves-dropping promotes competition in a laboratory co-culture model. ISME J. 2012;6(12):2219–28. doi: 10.1038/ismej.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415(6871):545–49. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 17.Collins CH, Leadbetter JR, Arnold FH. Dual selection enhances the signaling specificity of a variant of the quorum-sensing transcriptional activator LuxR. Nat Biotechnol. 2006;24(6):708–12. doi: 10.1038/nbt1209. [DOI] [PubMed] [Google Scholar]
- 18.Cooper DG, Macdonald CR, Duff SJB, Kosaric N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol. 1981;42(3):408–12. doi: 10.1128/aem.42.3.408-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornforth DM, Popat R, McNally L, Gurney J, Scott-Phillips TC, et al. Combinatorial quorum sensing allows bacteria to resolve their social and physical environment. PNAS. 2014;111(11):4280–84. doi: 10.1073/pnas.1319175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandekar AA, Chugani S, Greenberg EP. Bacterial quorum sensing and metabolic incentives to cooperate. Science. 2012;338(6104):264–66. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, Wu J, Tao F, Zhang L-H. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev. 2011;111(1):160–73. doi: 10.1021/cr100354f. [DOI] [PubMed] [Google Scholar]
- 22.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450(7168):411–14. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 23.Dogsa I, Choudhary KS, Marsetic Z, Hudaiberdiev S, Vera R, et al. ComQXPA quorum sensing systems may not be unique to Bacillus subtilis: a census in prokaryotic genomes. PLOS ONE. 2014;9(5):e96122. doi: 10.1371/journal.pone.0096122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411(6839):813–17. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 25.Dunny GM. Enterococcal sex pheromones: signaling, social behavior, and evolution. Annu Rev Genet. 2013;47:457–82. doi: 10.1146/annurev-genet-111212-133449. [DOI] [PubMed] [Google Scholar]
- 26.Eldar A. Social conflict drives the evolutionary divergence of quorum sensing. PNAS. 2011;108(33):13635–40. doi: 10.1073/pnas.1102923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emge P, Moeller J, Jang H, Rusconi R, Yawata Y, et al. Resilience of bacterial quorum sensing against fluid flow. Sci Rep. 2016;6:33115. doi: 10.1038/srep33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erez Z, Steinberger-Levy I, Shamir M, Doron S, Stokar-Avihail A, et al. Communication between viruses guides lysis-lysogeny decisions. Nature. 2017;541(7638):488–93. doi: 10.1038/nature21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Even-Tov E, Omer Bendori S, Pollak S, Eldar A. Transient duplication-dependent divergence and horizontal transfer underlie the evolutionary dynamics of bacterial cell-cell signaling. PLOS Biol. 2016;14(12):e2000330. doi: 10.1371/journal.pbio.2000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Even-Tov E, Omer Bendori S, Valastyan J, Ke X, Pollak S, et al. Social evolution selects for redundancy in bacterial quorum sensing. PLOS Biol. 2016;14(2):e1002386. doi: 10.1371/journal.pbio.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleuchot B, Guillot A, Mézange C, Besset C, Chambellon E, et al. Rgg-associated SHP signaling peptides mediate cross-talk in streptococci. PLOS ONE. 2013;8(6):e66042. doi: 10.1371/journal.pone.0066042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–51. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 33.Gardner A, West SA. Greenbeards. Evolution. 2010;64(1):25–38. doi: 10.1111/j.1558-5646.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 34.Geisinger E, George EA, Muir TW, Novick RP. Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J Biol Chem. 2008;283(14):8930–38. doi: 10.1074/jbc.M710227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisinger E, Muir TW, Novick RP. agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. PNAS. 2009;106(4):1216–21. doi: 10.1073/pnas.0807760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grafen A. Do animals really recognize kin? Anim Behav. 1990;39(1):42–54. [Google Scholar]
- 37.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–27. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton WD. The genetical evolution of social behaviour: I. J Theor Biol. 1964;7(1):1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 39.Håvarstein LS, Gaustad P, Nes IF, Morrison DA. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21(4):863–69. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 40.Hawver LA, Jung SA, Ng W-L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol Rev. 2016;40(5):738–52. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi . J Bacteriol. 2004;186(20):6902–14. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henkel M, Schmidberger A, Kühnert C, Beuker J, Bernard T, et al. Kinetic modeling of the time course of N-butyryl-homoserine lactone concentration during batch cultivations of Pseudomonas aeruginosa PAO1. Appl Microbiol Biotechnol. 2013;97(17):7607–16. doi: 10.1007/s00253-013-5024-5. [DOI] [PubMed] [Google Scholar]
- 43.Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J-U. Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol. 2007;5(3):230–39. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 44.Hense BA, Schuster M. Core principles of bacterial autoinducer systems. Microbiol Mol Biol Rev. 2015;79(1):153–69. doi: 10.1128/MMBR.00024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoover SE, Perez AJ, Tsui H-CT, Sinha D, Smiley DL, et al. A new quorum-sensing system (TprA/PhrA) for Streptococcus pneumoniae D39 that regulates a lantibiotic biosynthesis gene cluster. Mol Microbiol. 2015;97(2):229–43. doi: 10.1111/mmi.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horswill AR, Stoodley P, Stewart PS, Parsek MR. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal Bioanal Chem. 2007;387(2):371–80. doi: 10.1007/s00216-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansen VAA, van Baalen M. Altruism through beard chromodynamics. Nature. 2006;440(7084):663–66. doi: 10.1038/nature04387. [DOI] [PubMed] [Google Scholar]
- 48.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276(5321):2027–30. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 49.Ke X, Miller LC, Bassler BL. Determinants governing ligand specificity of the Vibrio harveyi LuxN quorum-sensing receptor. Mol Microbiol. 2015;95(1):127–42. doi: 10.1111/mmi.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis . Mol Microbiol. 2004;49(3):581–90. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim MK, Ingremeau F, Zhao A, Bassler BL, Stone HA. Local and global consequences of flow on bacterial quorum sensing. Nat Microbiol. 2016;1:15005. doi: 10.1038/nmicrobiol.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komarova NL. Spatial interactions and cooperation can change the speed of evolution of complex phenotypes. PNAS. 2014;111(Suppl. 3):10789–95. doi: 10.1073/pnas.1400828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotte A-K, Severn O, Bean Z, Schwarz K, Minton NP, Winzer K. RRNPP-type quorum sensing affects solvent formation and sporulation in Clostridium acetobutylicum . Microbiology. 2020;166(6):579–92. doi: 10.1099/mic.0.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazazzera BA, Grossman AD. The ins and outs of peptide signaling. Trends Microbiol. 1998;6(7):288–94. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 55.Lazazzera BA, Solomon JM, Grossman AD. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis Cell. 1997;89(6):917–25. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 56.Le KY, Otto M. Quorum-sensing regulation in staphylococci—an overview. Front Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch M, Hagner K. Evolutionary meandering of intermolecular interactions along the drift barrier. PNAS. 2015;112(1):E30–38. doi: 10.1073/pnas.1421641112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyons NA, Kraigher B, Stefanic P, Mandic-Mulec I, Kolter R. A combinatorial kin discrimination system in Bacillus subtilis . Curr Biol. 2016;26(6):733–42. doi: 10.1016/j.cub.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandic-Mulec I, Kraigher B, Cepon U, Mahne I. Variability of the quorum sensing system in natural isolates of Bacillus sp. Food Technol Biotechnol. 2003;41(1):23–28. [Google Scholar]
- 60.Manefield M, DeNys R, Kumar N, Read R, Givskov M, et al. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145(2):283–91. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 61.Martin SH, Wingfield BD, Wingfield MJ, Steenkamp ET. Causes and consequences of variability in peptide mating pheromones of ascomycete fungi. Mol Biol Evol. 2011;28(7):1987–2003. doi: 10.1093/molbev/msr022. [DOI] [PubMed] [Google Scholar]
- 62.Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anollés G, et al. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. PNAS. 2003;100(3):1444–49. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller EL, Evans BA, Cornejo OE, Roberts IS, Rozen DE. Pherotype polymorphism in Streptococcus pneumoniae has no obvious effects on population structure and recombination. Genome Biol Evol. 2017;9(10):2546–59. doi: 10.1093/gbe/evx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller EL, Kjos M, Abrudan MI, Roberts IS, Veening JW, Rozen DE. Eavesdropping and crosstalk between secreted quorum sensing peptide signals that regulate bacteriocin production in Streptococcus pneumoniae . ISME J. 2018;12(10):2363–75. doi: 10.1038/s41396-018-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitri S, Richard Foster K. The genotypic view of social interactions in microbial communities. Annu Rev Genet. 2013;47:247–73. doi: 10.1146/annurev-genet-111212-133307. [DOI] [PubMed] [Google Scholar]
- 66.Mok KC. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 2003;22(4):870–81. doi: 10.1093/emboj/cdg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monnet V, Gardan R. Quorum-sensing regulators in Gram-positive bacteria: ‘cherchezle peptide.’. Mol Microbiol. 2015;97(2):181–84. doi: 10.1111/mmi.13060. [DOI] [PubMed] [Google Scholar]
- 68.Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T. N-Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC12472. FEMS Microbiol Lett. 2008;12472:124–30. doi: 10.1111/j.1574-6968.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 69.Moura-Alves P, Puyskens A, Stinn A, Klemm M, Guhlich-Bornhof U, et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366(6472):eaaw1629. doi: 10.1126/science.aaw1629. [DOI] [PubMed] [Google Scholar]
- 70.Mukherjee S, Bassler BL. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol. 2019;17(6):371–82. doi: 10.1038/s41579-019-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neiditch MB, Capodagli GC, Prehna G, Federle MJ. Genetic and structural analyses of RRNPP intercellular peptide signaling of gram-positive bacteria. Annu Rev Genet. 2017;51:311–33. doi: 10.1146/annurev-genet-120116-023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng W-L, Perez LJ, Wei Y, Kraml C, Semmelhack MF, Bassler BL. Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems. Mol Microbiol. 2011;79(6):1407–17. doi: 10.1111/j.1365-2958.2011.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–64. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 74.Ohno S. Evolution by Gene Duplication. Springer Sci. Bus. Media; New York: 2013. [Google Scholar]
- 75.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14(9):576–88. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parashar V, Jeffrey PD, Neiditch MB. Conformational change-induced repeat domain expansion regulates Rap phosphatase quorum-sensing signal receptors. PLOS Biol. 2013;11(3):e1001512. doi: 10.1371/journal.pbio.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perchat S, Dubois T, Zouhir S, Gominet M, Poncet S, et al. A cell-cell communication system regulates protease production during sporulation in bacteria of the Bacillus cereus group. Mol Microbiol. 2011;82(3):619–33. doi: 10.1111/j.1365-2958.2011.07839.x. [DOI] [PubMed] [Google Scholar]
- 78.Perchat S, Talagas A, Zouhir S, Poncet S, Bouillaut L, et al. NprR, a moonlighting quorum sensor shifting from a phosphatase activity to a transcriptional activator. Microb Cell. 2016;3(11):573–75. doi: 10.15698/mic2016.11.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perego M, Hoch JA. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis . PNAS. 1996;93(4):1549–53. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562(7728):532–37. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pollak S, Omer-Bendori S, Even-Tov E, Lipsman V, Bareia T, et al. Facultative cheating supports the coexistence of diverse quorum-sensing alleles. PNAS. 2016;113(8):2152–57. doi: 10.1073/pnas.1520615113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pollitt EJG, West SA, Crusz SA, Burton-Chellew MN, Diggle SP. Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus . Infect Immun. 2014;82(3):1045–51. doi: 10.1128/IAI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pozzi G, Masala L, Iannelli F, Manganelli R, Håvarstein LS, et al. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J Bacteriol. 1996;178(20):6087–90. doi: 10.1128/jb.178.20.6087-6090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 85.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10(8):365–70. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 86.Rezzonico F, Duffy B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol. 2008;8(1):154. doi: 10.1186/1471-2180-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson DA, Monk AB, Cooper JE, Feil EJ, Enright MC. Evolutionary genetics of the accessory gene regulator (agr) locus in Staphylococcus aureus . J Bacteriol. 2005;187(24):8312–21. doi: 10.1128/JB.187.24.8312-8321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rousset F, Roze D. Constraints on the origin and maintenance of genetic kin recognition. Evolution. 2007;61(10):2320–30. doi: 10.1111/j.1558-5646.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 89.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. PNAS. 2007;104(40):15876–81. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santorelli LA, Thompson CRL, Villegas E, Svetz J, Dinh C, et al. Facultative cheater mutants reveal the genetic complexity of cooperation in social amoebae. Nature. 2008;451(7182):1107–10. doi: 10.1038/nature06558. [DOI] [PubMed] [Google Scholar]
- 91.Sayou C, Monniaux M, Nanao MH, Moyroud E, Brockington SF, et al. A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science. 2014;343(6171):645–48. doi: 10.1126/science.1248229. [DOI] [PubMed] [Google Scholar]
- 92.Schaefer AL, Hanzelka BL, Eberhard A, Greenberg EP. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol. 1996;178(10):2897–901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schuster M, Sexton JD, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 94.Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185(7):2066–79. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schuster M, Sexton DJ, Hense BA. Why quorum sensing controls private goods. Front Microbiol. 2017;8:885. doi: 10.3389/fmicb.2017.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shanker E, Morrison DA, Talagas A, Nessler S, Federle MJ, Prehna G. Pheromone recognition and selectivity by ComR proteins among Streptococcus species. PLOS Pathog. 2016;12(12):e1005979. doi: 10.1371/journal.ppat.1005979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysislysogeny decision. Cell. 2019;176(1-2):268–80.:e13. doi: 10.1016/j.cell.2018.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slamti L, Lereclus D. A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 2002;21(17):4550–59. doi: 10.1093/emboj/cdf450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slamti L, Lereclus D. Specificity and polymorphism of the PlcR-PapR quorum-sensing system in the Bacillus cereus group. Society. 2005;187(3):1182–87. doi: 10.1128/JB.187.3.1182-1187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith P, Cozart J, Lynn BK, Alberts E, Frangipani E, Schuster M. Bacterial cheaters evade punishment by cyanide. iScience. 2019;19:101–9. doi: 10.1016/j.isci.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stokar-Avihail A, Tal N, Erez Z, Lopatina A, Sorek R. Widespread utilization of peptide communication in phages infecting soil and pathogenic bacteria. Cell Host Microbe. 2019;25(5):746–55.:e5. doi: 10.1016/j.chom.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strassmann JE, Gilbert OM, Queller DC. Kin discrimination and cooperation in microbes. Annu Rev Microbiol. 2011;65:349–67. doi: 10.1146/annurev.micro.112408.134109. [DOI] [PubMed] [Google Scholar]
- 103.Strassmann JE, Zhu Y, Queller DC. Altruism and social cheating in the social amoeba Dictyostelium discoideum . Nature. 2000;408(6815):965–67. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- 104.Sung JML, Chantler PD, Lloyd DH. Accessory gene regulator locus of Staphylococcus intermedius . Infect Immun. 2006;74(5):2947–56. doi: 10.1128/IAI.74.5.2947-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Swem LR, Swem DL, O’Loughlin CT, Gatmaitan R, Zhao B, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009;35(2):143–53. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant-Microbe Interact. 2000;13(6):637–48. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- 107.Tortosa P, Logsdon L, Kraigher B, Itoh Y. Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system. Society. 2001;183(2):451–60. doi: 10.1128/JB.183.2.451-460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tran L-SP, Nagai T, Itoh Y. Divergent structure of the ComQXPA quorum-sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis . Mol Microbiol. 2000;37(5):1159–71. doi: 10.1046/j.1365-2958.2000.02069.x. [DOI] [PubMed] [Google Scholar]
- 109.Voichek M, Maaß S, Kroniger T, Becher D, Sorek R. Peptide-based quorum sensing systems in Paenibacillus polymyxa . bioRxiv. 2019:767517. doi: 10.26508/lsa.202000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wall D. Kin recognition in bacteria. Annu Rev Microbiol. 2016;70:143–60. doi: 10.1146/annurev-micro-102215-095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. PNAS. 2015;112(7):2187–91. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 113.West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- 114.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4(8):597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 115.Whatmore AM, Barcus VA, Dowson CG. Genetic diversity of the streptococcal competence com gene locus. J Bacteriol. 1999;181(10):3144–54. doi: 10.1128/jb.181.10.3144-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551(7680):313–20. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilder CN, Diggle SP, Schuster M. Cooperation and cheating in Pseudomonas aeruginosa: the roles of the las, rhl and pqs quorum-sensing systems. ISME J. 2011;5(8):1332–43. doi: 10.1038/ismej.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wuster A, Babu MM. Conservation and evolutionary dynamics of the agr cell-to-cell communication system across firmicutes. J Bacteriol. 2008;190(2):743–46. doi: 10.1128/JB.01135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou L, Slamti L, Lereclus D, Raymond B. Optimal response to quorum-sensing signals varies in different host environments with different pathogen group size. mBio. 2020;11(3):e00535-20. doi: 10.1128/mBio.00535-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L, Slamti L, Nielsen-LeRoux C, Lereclus D, Raymond B. The social biology of quorum sensing in a naturalistic host pathogen system. Curr Biol. 2014;24(20):2417–22. doi: 10.1016/j.cub.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 121.Zhou L, Zhang L-H, Cámara M, He Y-W. The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 2017;25(4):293–303. doi: 10.1016/j.tim.2016.11.013. [DOI] [PubMed] [Google Scholar]