Abstract
Background
Congenital hyperinsulinism (CHI) is a cause of persistent hypoglycemia due to unregulated insulin secretion from pancreatic β-cells. Histologically, there are two major subgroups, focal and diffuse. Focal CHI is typically unresponsive to diazoxide and can be cured with surgical removal of the focal lesion.
Aims
We report on three patients with focal CHI to illustrate the marked clinical, genetic, radiological, and histological heterogeneity.
Methods and Results
The first two patients had focal CHI due to a paternal (c.3992–9G→A) ABCC8 mutation. One of these patients was fully responsive to a small dose (5 mg/kg · d) of diazoxide, whereas the other patient was medically unresponsive. In both patients, the focal lesions were accurately localized preoperatively by [18F]dihydroxyphenylalanine (DOPA) positron emission tomography (PET) and surgically resected. The third patient had a paternally inherited ABCC8 (A1493T) mutation, and the initial [18F]DOPA PET scan indicated extensive uptake of DOPA in the body and tail of the pancreas. However, despite surgical resection of the body and tail, this patient continued to have severe CHI. A subsequent [18F]DOPA PET scan now showed markedly increased DOPA uptake in the remaining body and head of the pancreas. This focal lesion occupied virtually the whole of the pancreas.
Conclusions
These three cases illustrate that focal lesions even with the same genotype (c.3992–9G→A) may have a different clinical presentation and that [18F]DOPA PET scans in very large focal lesions may be difficult to interpret. (J Clin Endocrinol Metab 97: E94–E99, 2012)
Congenital hyperinsulinism (CHI) is a cause of persistent hypoglycemia due to unregulated insulin secretion. Histologically, there are two major subgroups, diffuse and focal (1). Diffuse CHI involves the whole pancreas, whereas in focal CHI (focal adenomatous hyperplasia), the abnormality is confined to a single region. Histologically, the focal region is characterized by nodular hyperplasia of islet-like cell clusters with ductuloinsular complexes and scattered giant ß-cell nuclei (1–3). Focal CHI occurs due to the inheritance of a paternal ABCC8/KCNJ11 mutation followed by the somatic loss of the maternal chromosome 11p15 region within the focal lesion only (4–7). This somatic loss of the maternal 11p15 region leads to hemizygosity of the paternally derived ABCC8/KCNJ11 allele and also causes an imbalance in the imprinted genes within this chromosomal region.
Focal CHI is generally medically unresponsive and requires surgery to remove the focal lesion. Accurate pre-operative localization and complete resection of the focal lesion can cure the patient. [18F]Dihydroxyphenylalanine (DOPA) positron emission tomography (PET) scanning allows accurate preoperative localization of focal lesions before any planned surgery (8), although in some population, it may not be so accurate (9). We report on three patients with focal CHI to illustrate the marked heterogeneity of this disease. We show that patients who have focal CHI due to the same genetic mutation can have markedly different clinical presentations and that [18F]DOPA PET scanning may be difficult to interpret in very large focal lesions which involve virtually the entire pancreas.
Materials and Methods
Ethics
This study was approved by the Ethics Committee of Great Ormond Street Children’s Hospital and the Institute of Child Health.
[18F]DOPA PET scan
The PET/computed tomography (CT) scans (Siemens Biograph, Berlin, Germany) were performed as described previously (10).
Histology
Routine histological procedures were performed as previously reported (11).
Genetics
DNA was extracted from peripheral leukocytes and the KCNJ11 and ABCC8 genes amplified and sequenced as previously described (11). Sequences were compared with published sequences (NM_000525 and NM_000352.2) using Mutation Surveyor software (SoftGenetics, State College, PA). Parental samples were tested for mutations identified in the probands, and microsatellite analysis confirmed de novo mutations. To determine the chromosome of origin of a de novo mutation, primers were designed to amplify a single copy of the ABCC8 gene using a primer complementary to an informative single-nucleotide polymorphism, S1370A;c.4108T→G, which was within close proximity to the mutation (primers available on request).
Loss of heterozygosity (LOH) within the focal lesion was investigated by microsatellite analysis. Ten markers spanning chromosome 11p15 were amplified using DNA extracted from the focal lesion, from surrounding normal pancreatic tissue when available, and from the patient and parents’ peripheral leukocytes. The allele peak heights were compared using GeneMarker software (SoftGenetics).
Clinical cases
Clinical and biochemical details are summarized in Table 1.
Table 1. Summary of the clinical, genetic, and biochemical aspects of the patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Gestational age (wk) | 39 | 39 | 40 |
| Birth weight (kg) | 4.3 | 5.09 | 4.8 |
| Age at presentation (h) | 14 | 1 | 5 |
| Symptoms of hypoglycemia | Lethargy and floppiness | Seizures and cyanosis | Seizures |
| Maximum glucose infusion rate (mg/kg · min) | 11 | 20 | 21 |
| Blood glucose (mmol/liter) | 2.6 | 1.6 | 1.1 |
| Insulin (mU/liter) | 3.4 | 10 | 126 |
| Nonesterified fatty acids (mmol/liter) | <0.05 | <0.05 | <0.05 |
| Ketone bodies (mmol/liter) | <0.05 | <0.05 | <0.05 |
| Diazoxide response | Yes (5 mg/ kg · d) | No | No |
| ABCC8 gene mutation | c.3992-9G→A | c.3992-9G→A | c.4477G→A |
Results
Patient 1
Genetics
Sequencing analysis identified a previously reported heterozygous ABCC8 de novo mutation, c.3992-9G→A (12). Microsatellite analysis confirmed family relationships, and allele-specific PCR demonstrated that the mutation had arisen on the paternal chromosome.
[18F]DOPA PET scanning
This patient underwent an [18F]DOPA PET/CT scan of the pancreas that confirmed the presence of a focal lesion located in the cranial part of the body positioned more to the backside (dorsal) measuring 4 × 8 × 7mm. The patient underwent surgery, and the focal lesion was removed, curing the patient.
Histology
The histology of the focal lesion showed nodules of endocrine tissue with large nuclei. The lesion measured 0.8 cm in diameter, and the remaining pancreas showed normal resting islets.
LOH studies
Microsatellite analysis of 10 markers spanning the differentially methylated region and the ABCC8 gene demonstrated a loss the maternal allele in the pancreatic lesion when compared with the leukocyte DNA.
Patient 2
Genetics
A previously reported heterozygous ABCC8 gene splicing mutation c.3992-G→A was identified (12), and family member testing identified the mutation in the unaffected father.
[18F]DOPA PET scanning
The [18F]DOPA PET showed a large focal lesion in the head of the pancreas (standard uptake value ratio of 2.3) with nearly no tracer uptake in the body and tail of the pancreas. The operation involved resection of the head of the pancreas and pancreato-jejunostomy. Postoperatively, the patient was cured with no postoperative complications.
Histology
A focal lesion consisting of nodules of endocrine tissue with large endocrine nuclei was identified and measuring 0.7 cm in diameter. Histology of the pancreas outside the focal lesion showed normal resting islets.
LOH studies
Microsatellite analysis of 10 markers spanning the differentially methylated region and the ABCC8 gene demonstrated a reduced peak height for the maternal allele in the pancreatic lesion when compared with the leukocyte DNA.
Patient 3
Genetics
A previously reported heterozygous ABCC8 mutation, A1493T; c.4477G→A (13) was found. Mutation testing in the unaffected parents showed the proband had inherited the mutation from her father.
[18F]DOPA PET scanning
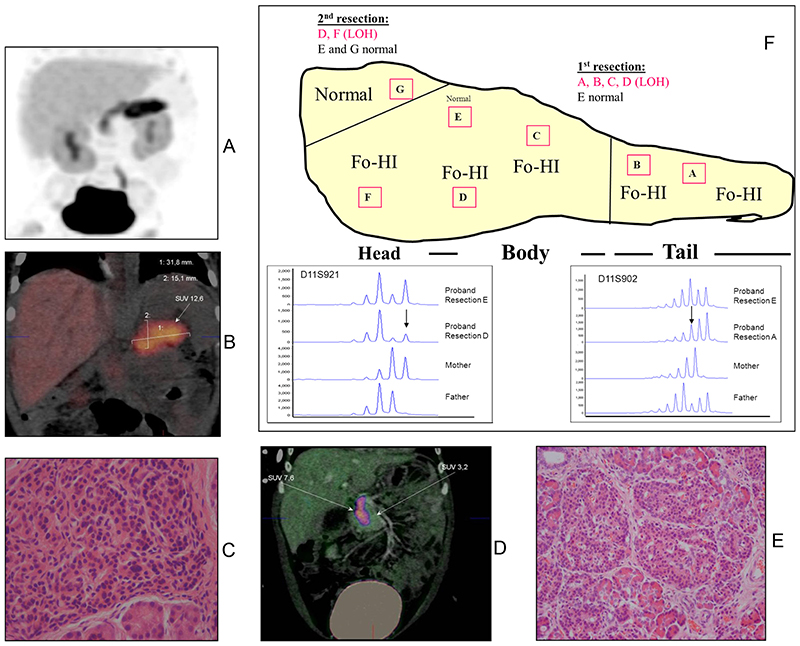
The results of the initial [18F]DOPA PET/CT scan showed intense tracer uptake in the tail and parts of the body (Fig. 1, A and B). Because the affected region was unusually large (32 × 25 × 15 mm), this was interpreted either as a very large focus or as an atypical form of CHI. Based on the [18F]DOPA PET scan findings, the child underwent a laparoscopic pancreatectomy involving the removal of the tail and the body of the pancreas.
Fig. 1.
Panels A and B, First [18F]DOPA PET/CT scan showing uptake of [18F]DOPA in tail and body of pancreas in patient 3. Panel C, Hematoxylin and eosin staining of the resected pancreas showing histological changes of focal disease. Panel D, Second [18F]DOPA PET/CT scan now showing uptake of [18F]DOPA in remaining head of pancreas in patient 3. Panel E, Hematoxylin and eosin staining of the resected pancreatic head region with histological changes of focal CHI. Panel F, Reconstruction of the extent of the focal lesion based on regions of abnormal histology and loss of maternal heterozygosity for 11p15 in patient 3. Electropherograms demonstrating the result of microsatellite analysis for two of the informative markers on chromosome 11, D11S902 and D11S921, are provided for the proband and her parents. Alleles of the same size (base pairs) occur at the same position on the x-axis. The y-axis indicates the product quantity (arbitrary units). The results for the markers amplified from unaffected tissue (resection E) show normal biparental inheritance as demonstrated by the preferential amplification of the smaller product. In contrast, the results for the markers amplified from affected tissue (resections D and A) illustrate LOH for the maternal allele (indicated by arrow). Fo-HI, Focal hyperinsulinism.
However, despite the removal of the tail and body of the pancreas, the child still had hypoglycemia. She was restarted on octreotide, but once again could not be weaned off iv dextrose. Six weeks after the first [18F]DOPA PET scan, the patient underwent a repeat scan. On this occasion, the remaining head of the pancreas showed markedly increased tracer uptake involving virtually the whole of the head (Fig. 1D). Based on these findings, the patient thus underwent a second pancreatectomy to remove the remaining head of the pancreas. However, despite this second pancreatectomy, the patient’s blood glucose levels could only be stabilized on continuous gastrostomy feeds.
Histology
First resection
(see Fig. 1, C, E, and F). Macroscopically, there was no focal lesion. Microscopically frozen sections from all specimens labeled A, B, C, and D showed nodules of endocrine tissue with large endocrine nuclei. In specimen E, however, in addition to lesion tissue, occasional islets of Langerhans with small resting endocrine nuclei (suggesting normal tissue) were also seen. The lesion was widespread, being present in virtually every segment and reminiscent of a giant focal lesion previously reported (14, 15).
Second resection
The second resection involved removing the major portion of the head of the pancreas. Once again, macroscopically, the pancreas head appeared normal. However, microscopically, the portion F had nodules of irregular islet tissue with occasional large endocrine nuclei, whereas the remaining portion G showed normal endocrine histology.
LOH studies
Microsatellite analysis demonstrated that four markers (D11S2071, D11S1397, D11S1888, and D11S902) had a reduced peak height for the maternal allele in the pancreatic lesion when compared with the normal tissue and leukocyte DNA. These results confirmed LOH from 11p15.5 to 11p15.1, which encompasses the differentially methylated region and the ABCC8 gene, within the focal lesion of the pancreas. Sections A, B, C, D, and F all showed evidence of LOH for the maternal 11p15 allele (Fig. 1F) Using the information from all the histological samples and the data from the LOH studies for the maternal 11p15 allele, we were able to construct a model of the focal lesion as depicted in Fig. 1F. The whole pancreas was virtually composed of the focal lesion except a small segment of normal pancreatic tissue in the superior aspect of the head of the pancreas.
Discussion
These three cases illustrate the complex heterogeneity of focal CHI in terms of clinical presentation, histology, and imaging with [18F]DOPA PET/CT scan. Typically focal CHI does not respond to treatment with diazoxide and requires surgery. The first two patients were heterozygous for the previously reported paternally inherited ABCC8 splicing mutation, c.3992-9G→A. In both patients, a focal lesion was radiologically confirmed preoperatively by the [18F]DOPA PET scan. Histologically, both focal lesions were approximately the same size (0.7 and 0.8 cm), but their location was different. However, their clinical response to treatment with diazoxide was very different with the first patient responding to a small dose of diazoxide, whereas the second patient had no response at all. These observations suggest that unknown genetic or environmental factors may be influencing the expression of the phenotype, and not all focal lesions present with severe medically unresponsive CHI.
Two frequent mutations in the ABCC8 gene, c.3992–9G→A and p.F1388del, are associated with 88% of CHI in the Ashkenazi Jewish population (12). In this patient group with these two mutations, there is marked intra- and interfamilial phenotypic heterogeneity. This clinical heterogeneity may be due to modifying genes or other unknown factors that influence the expression of the CHI phenotype. Previous studies have documented that most Ashkenazi Jewish patients with CHI can be managed medically without the need for a pancreatectomy (16). Our study suggests that some patients of Ashkenazi Jewish origin with paternally inherited c.3992–9G→A mutation in the ABCC8 gene might have mild focal CHI, thus explaining the response to medical therapy with diazoxide and or octreotide.
Typical focal forms of CHI are small (2–10 mm in diameter) and well circumscribed, and if completely resected surgically, the patient is cured. However, some focal forms are not so well defined and have branches (or tentacles); yet others can be large in size (14, 15). The size of the focal lesion is determined by the timing of the paternal uniparental isodisomy event (15). In the first two patients, it is likely that the loss of maternal heterozygosity occurred late in gestation, whereas in the third patient, the loss of the maternal heterozygosity must have occurred earlier.
Patient 3 had a focal lesion occupying virtually the whole pancreas. The first [18F]DOPA PET scan showed tracer uptake in the tail and body, but after surgical removal of the tail and body, there was marked uptake of [18F]DOPA tracer in the remaining head region. It is unclear why the [18F]DOPA tracer uptake in the head of the pancreas was suppressed during the first PET scan. The two different [18F]DOPA PET scan findings point toward mechanisms that might result in incomplete tracer uptake in different regions of the pancreas in very large focal lesions.
The principle of the [18F]DOPA PET scan depends on DOPA being taken up by the β-cells and being converted to dopamine by aromatic L-amino acid decarboxylase (AADC). Pancreatic β-cells have high AADC activity, but the role of this enzyme in insulin secretion is still unknown. Some studies have shown that l-DOPA accumulates within the β-cell secretory granules and inhibits insulin secretion (17, 18), yet other studies in patients with CHI have shown no effect on insulin secretion when administered an AADC inhibitor (19). However, there are no data to suggest that different regions of the pancreas take up DOPA at different rates.
In summary, focal lesions with the same genotype can have very different clinical presentations, and [18F]DOPA PET scans may be difficult to interpret in patients with large focal lesions that occupy virtually the entire pancreas. More case studies of patients with large focal lesions are required to better understand the role of [18F]DOPA PET in this group of patients.
Acknowledgments
Address all correspondence and requests for reprints to: Dr. K. Hussain, Developmental Endocrinology Research Group, Clinical and Molecular Genetics Unit, Institute of Child Health, University College London, 30 Guilford Street, London WC1N 1EH, United Kingdom. E-mail: K.Hussain@ich.ucl.ac.uk.
This work was supported by the Wellcome Trust (Project Grant WT081188AIA).
Abbreviations
- AADC
l-Amino acid decarboxylase
- CHI
congenital hyperinsulinism
- CT
computed tomography
- DOPA
dihydroxyphenylalanine
- LOH
lossof heterozygosity
- PET
positron emission tomography
Footnotes
Disclosure Summary: There are no conflicts of interest for any authors.
Contributor Information
Virpi V. Smith, Surgery, Great Ormond Street, Hospital for Children National Health Service Trust, London, and The Institute of Child Health, University College London, London WC1N 1EH, United Kingdom
Michael Ashworth, Histology, Great Ormond Street, Hospital for Children National Health Service Trust, London, and The Institute of Child Health, University College London, London WC1N 1EH, United Kingdom.
Oliver Blankenstein, Department of Endocrinology, Charité-University Medicine, 10117 Berlin, Germany.
Agostino Pierro, Surgery, Great Ormond Street, Hospital for Children National Health Service Trust, London, and The Institute of Child Health, University College London, London WC1N 1EH, United Kingdom.
Khalid Hussain, Departments of Endocrinology, Great Ormond Street, Hospital for Children National Health Service Trust, London, and The Institute of Child Health, University College London, London WC1N 1EH, United Kingdom.
References
- 1.Rahier J, Guiot Y, Sempoux C. Persistent hyperinsulinaemic hypoglycaemia of infancy: a heterogeneous syndrome unrelated to nesidioblastosis. Arch Dis Child Fetal Neonatal Ed. 2000;82:F108–F112. doi: 10.1136/fn.82.2.F108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goossens A, Gepts W, Saudubray JM, Bonnefont JP, Nihoul-FeketeHeitz PU, Klöppel G. Diffuse and focal nesidioblastosis. A clinicopathological study of 24 patients with persistent neonatal hyperinsulinemic hypoglycemia. Am J Surg Pathol. 1989;13:766–775. [PubMed] [Google Scholar]
- 3.Klöppel G, Reinecke-Lüthge A, Koschoreck F. Focal and diffuse β-cell changes in persistent hyperinsulinemic hypoglycemia of infancy. Endocr Pathol. 1999;10:299–304. doi: 10.1007/BF02739772. [DOI] [PubMed] [Google Scholar]
- 4.Verkarre V, Fournet JC, de Lonlay P, Gross-Morand MS, Devillers M, Rahier J, Brunelle F, Robert JJ, Nihoul-Fékété C, Saudubray JM, Junien C. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998;102:1286–1291. doi: 10.1172/JCI4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lonlay P, Fournet JC, Rahier J, Gross-Morand MS, Poggi-Tra-vert F, Foussier V, Bonnefont JP, Brusset MC, Brunelle F, Robert JJ, Nihoul-Fékété C, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest. 1997;100:802–807. doi: 10.1172/JCI119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damaj L, le Lorch M, Verkarre V, Werl C, Hubert L, Nihoul-Fékété C, Aigrain Y, de Keyzer Y, Romana SP, Bellanne-Chantelot C, de Lonlay P, et al. Chromosome 11p15 paternal isodisomy in focal forms of neonatal hyperinsulinism. J Clin Endocrinol Metab. 2008;93:4941–4947. doi: 10.1210/jc.2008-0673. [DOI] [PubMed] [Google Scholar]
- 7.Bellanné-Chantelot C, Saint-Martin C, Ribeiro MJ, Vaury C, Verkarre V, Arnoux JB, Valayannopoulos V, Gobrecht S, Sempoux C, Rahier J, Fournet JC, et al. ABCC8 and KCNJ11 molecular spectrum of 109 patients with diazoxide-unresponsive congenital hyperinsulinism. J Med Genet. 2010;47:752–759. doi: 10.1136/jmg.2009.075416. [DOI] [PubMed] [Google Scholar]
- 8.Otonkoski T, Nänto-Salonen K, Seppanen M, Veijola R, Huopio H, Hussain K, Tapanainen P, Eskola O, Parkkola R, Ekström K, Guiot Y, et al. Non-invasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55:13–18. [PubMed] [Google Scholar]
- 9.Masue M, Nishibori H, Fukuyama S, Yoshizawa A, Okamoto S, Doi R, Uemoto S, Tokumi T, Kasai T, Yorifuji T. Diagnostic accuracy of [18F]fluoro-L-DOPA PET scan for persistent congenital hyperinsulinisminJapan. ClinEndocrinol (Oxf) 2011;75:342–346. doi: 10.1111/j.1365-2265.2011.04072.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohnike K, Blankenstein O, Christesen HT, De Lonlay J, Hussain K, Koopmans KP, Minn H, Mohnike W, Mutair A, Otonkoski T, Rahier J, et al. Proposal for a standardized protocol for 18F-DOPA-PET (PET/CT) in congenital hyperinsulinism. Horm Res. 2006;66:40–42. doi: 10.1159/000093471. [DOI] [PubMed] [Google Scholar]
- 11.Ismail D, Smith VV, de Lonlay P, Ribeiro MJ, Rahier J, Blankenstein O, Flanagan SE, Bellanné-Chantelot C, Verkarre V, Aigrain Y, Pierro A, et al. Familial focal congenital hyper-insulinism. J Clin Endocrinol Metab. 2011;96:24–28. doi: 10.1210/jc.2010-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, Landau H, Stanley CA, Thornton PS, Clement JP, 4th, Bryan J, Aguilar-Bryan L, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822. doi: 10.1093/hmg/5.11.1813. [DOI] [PubMed] [Google Scholar]
- 13.Kassem SA, Ariel l, Thornton PS, Hussain K, Smith V, Lindley KJ, Aynsley-Green A, Glaser B. p57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes. 2001;50:2763–2769. doi: 10.2337/diabetes.50.12.2763. [DOI] [PubMed] [Google Scholar]
- 14.Suchi M, MacMullen CM, Thornton PS, Adzick NS, Ganguly A, Ruchelli ED, Stanley CA. Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod Pathol. 2006;19:122–129. doi: 10.1038/modpathol.3800497. [DOI] [PubMed] [Google Scholar]
- 15.Giurgea I, Sempoux C, Bellanné-Chantelot C, Ribeiro M, Hubert L, Boddaert N, Saudubray JM, Robert JJ, Brunelle F, Rahier J, Jaubert F, et al. The Knudson’s two-hit model and timing of somatic mutation may account for the phenotypic diversity of focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2006;91:4118–4123. doi: 10.1210/jc.2006-0397. [DOI] [PubMed] [Google Scholar]
- 16.Glaser B, Hirsch HJ, Landau H. Persistent hyperinsulinemic hypoglycemia of infancy: long-term octreotide treatment without pancreatectomy. J Pediatr. 1993;123:644–650. doi: 10.1016/s0022-3476(05)80970-9. [DOI] [PubMed] [Google Scholar]
- 17.Raffo A, Hancock K, Polito T, Xie Y, Andan G, Witkowski P, Hardy M, Barba P, Ferrara C, Maffei A, Freeby M, et al. Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrinol. 2008;198:41–49. doi: 10.1677/JOE-07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ericson LE, Håkanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic B-cells following injection of l-DOPA Localization to secretory granules and inhibition of insulin secretion. Diabetologia. 1977;13:117–124. doi: 10.1007/BF00745138. [DOI] [PubMed] [Google Scholar]
- 19.de Lonlay P, Simon-Carre A, Ribeiro MJ, Boddaert N, Giurgea I, Laborde K, Bellanné-Chantelot C, Verkarre V, Polak M, Rahier J, Syrota A, et al. Congenital hyperinsulinism: pancreatic [18F] fluoro-L-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of DOPA decarboxylase and insulin secretion. J Clin Endocrinol Metab. 2006;91:933–940. doi: 10.1210/jc.2005-1713. [DOI] [PubMed] [Google Scholar]