Abstract
Context
Activating mutations in genes encoding the Kir6.2 (KCNJ11) and SUR1 (ABCC8) subunits of the pancreatic ATP-sensitive K+ channel are a common cause of permanent neonatal diabetes (PNDM). All Kir6.2 mutations identified to date are missense mutations. We describe here a novel in-frame deletion (residues 28-32) in Kir6.2 in a heterozygous patient with PNDM without neurological problems that are detectable by standard evaluation.
Objective
The aim of the study was to identify the mutation responsible for neonatal diabetes in this patient and characterize its functional effects.
Design
Wild-type and mutant Kir6.2/SUR1 channels were examined by heterologous expression in Xenopus oocytes.
Results
The Kir6.2-28Δ32 mutation produced a significant decrease in ATP inhibition and an increase in whole-cell KATP currents, explaining the diabetes of the patient. Tolbutamide block was only slightly reduced in the simulated heterozygous state, suggesting that the patient should respond to sulfonylurea therapy. The mutation decreased ATP inhibition indirectly, by increasing the intrinsic (unliganded) channel open probability. Neither effect was observed when Kir6.2 was expressed in the absence of SUR1, suggesting that the mutation impairs coupling between SUR1 and Kir6.2. Coimmunoprecipitation studies further revealed that the mutation disrupted a physical interaction between Kir6.2 and residues 1-288 (but not residues 1-196) of SUR1.
Conclusions
We report a novel KCNJ11 mutation causing PNDM. Our results show that residues 28-32 in the N terminus of Kir6.2 interact both physically and functionally with SUR1 and suggest that residues 196-288 of SUR1 are important in this interaction. (J Clin Endocrinol Metab 94: 2551–2557, 2009)
Gain-of-function mutations in KCNJ11 (Kir6.2) and ABCC8 (SUR1) are a common cause of neonatal diabetes (1–4). Kir6.2 and SUR1 coassemble in a 4:4 stoichiometry to form the ATP-sensitive potassium (KATP) channel (5, 6), which plays a crucial role in insulin secretion from pancreatic β-cells (1, 7). When metabolism is low, this channel is open, and the ensuing K+ efflux keeps the β-cell hyperpolarized, thereby preventing calcium influx and insulin secretion. In response to enhanced glucose metabolism, the KATP channel closes, which leads to membrane depolarization, activation of voltage-gated calcium channels, calcium influx, and insulin secretion. Sulfonylurea drugs, widely used to treat type 2 diabetes, bypass metabolism and close the KATP channel directly, thus stimulating insulin release (8). These drugs are also very effective at treating neonatal diabetes (9).
Metabolic regulation of the KATP channel involves both Kir6.2 and SUR1 subunits. The ion-conducting pore is composed of a tetramer of Kir6.2 subunits, and ATP binding to the cytosolic domain of one or more Kir6.2 subunits closes the channel (7, 10). Conversely, Mg nucleotides (MgATP, MgADP) open the channel via interaction with SUR1 (10–12). However, SUR1 also confers other properties upon Kir6.2, including sensitivity to inhibition by sulfonylurea drugs, an increased intrinsic open probability (Po), and enhanced ATP sensitivity (10, 13).
To date, more than 30 separate activating mutations in KCNJ11 have been found to cause neonatal diabetes (1–3, 14). All of these act by reducing channel inhibition by ATP (1), and the extent to which ATP block is compromised appears to determine the severity of the clinical phenotype. Those mutations that cause the greatest reduction in ATP inhibition are associated with a more severe phenotype in which developmental delay, muscle weakness, and epilepsy accompany the neonatal diabetes, a condition known as DEND syndrome (4, 15). Mutations that cause a lesser reduction in ATP block give rise to neonatal diabetes without neurological complications. This suggests that a smaller decrease in ATP sensitivity leads to an increase in KATP current that hyperpolarizes only pancreatic β-cells, whereas larger increases in KATP current can also influence the function of extrapancreatic tissues (4).
KCNJ11 mutations can decrease ATP sensitivity in several ways. For example, they may reduce ATP binding to Kir6.2, or they may influence ATP sensitivity indirectly by enhancing the amount of time the channel spends in the open state (1, 14, 15). Reflecting this, most mutations lie either in the predicted ATP-binding site or in regions of the protein associated with channel opening and closing (gating) (1).
All KCNJ11 neonatal diabetes mutations identified to date are missense mutations that result in a single amino acid substitution. In this study, we report a novel type of KCNJ11 mutation: an in-frame deletion of five amino acids (A28-R32) from the N terminus of Kir6.2. This mutation causes neonatal diabetes with-out neurological effects that are detectable by standard evaluation. Functional analysis reveals that the mutation reduces ATP inhibition of the KATP channel indirectly, by increasing the intrinsic (unliganded) Po of the channel. However, neither of these effects is observed when Kir6.2 is expressed in the absence of SUR1. The results further show that residues 28-32 in the N terminus of Kir6.2 interact both physically and functionally with SUR1. They also illustrate how naturally occurring mutations can provide novel insights into KATP channel function.
Materials and Methods
Molecular genetic analysis
Genomic DNA was extracted from peripheral lymphocytes using standard procedures. The KCNJ11 gene was amplified and sequenced as previously described by Flanagan and colleagues (34). Parental samples were tested for the mutation, and family relationships were confirmed using a panel of six microsatellite markers on chromosome 20: D20S851, D20S171, D20S103, D20S107, D20S481, and D20S477. All samples were taken with the consent of the subjects.
Molecular biology
Quick-change mutagenesis was used to conservatively introduce two NotI restriction sites on either side of the region to be deleted in human or mouse Kir6.2. Digestion with NotI followed by ligation resulted in an in-frame deletion of residues 28-32 (ARQRR) inclusive (Kir6.2-28Δ32). All DNAconstructs were cloned into the pBF vector. cRNA was prepared using the mMessage mMachine SP6 kit (Ambion, Austin, TX). Electrophysiological experiments were performed using human Kir6.2 with glutamate at residue 23 (GenBank D50582). Surface expression assays and coimmunoprecipitation studies were performed using mouse Kir6.2 (GenBank D50581), containing an HA-tag in the extracellular domain of the protein (16). RatSUR1 (GenBank L40624) was used for all studies. We also used constructs consisting of residues 1-196 of SUR1 [trans-membrane domain 0 (TMD0) or SUR1(1-196)] or residues 1-288 of SUR1 [SUR1(1-288)]; both constructs carried an N-terminal FLAG tag.
Oocyte preparation
Xenopus oocytes were prepared as previously reported (17). Oocytes were coinjected with approximately 2 ng of SUR1 mRNA and approximately 0.1 ng wild-type or mutant Kir6.2 mRNA. For each batch of oocytes, all mutations were injected to enable direct comparison of their effects. Oocytes were maintained in Barth’s solution and studied 1–4 d after injection (18).
Coimmunoprecipitations
Briefly, oocytes were lysed by passage through hypodermic needles, membranes were isolated and solubilized, and immunoprecipitation of Kir6.2ΔC was performed using anti-HA antibodies. Bound SUR1(1-196) was detected by Western blotting with an anti-FLAG antibody and normalized to total SUR1(1-196) expression levels, because this was variable. Levels of mutant and wild-type Kir6.2ΔC precipitated by the HA antibody were found to be the same in two samples tested, so normalization to Kir6.2ΔC levels was unnecessary. For coimmunoprecipitations using SUR1(1-288), the experiment was performed in the reverse manner, i.e. FLAG-tagged SUR1(1-288) was immunoprecipitated with an anti-FLAG antibody, and bound Kir6.2ΔC was detected using anti-HA antibodies. Some variation was found in the level of SUR1(1-288) precipitated by the FLAG antibody; therefore, these assays were normalized for both Kir6.2ΔC expression and SUR1(1-288) precipitated. Data were quantified using densitometry.
Surface expression
Surface expression assays were performed using the method of Zerangue et al. (16). Briefly, oocytes were incubated with rat monoclonal anti-HA antibody, followed by a secondary anti-rat antibody conjugated to horseradish peroxidase. The horseradish peroxidase was used to drive a chemiluminescent reaction using Femto Maximum Sensitivity Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL), and surface expression levels were detected as light using a luminometer.
Electrophysiology
Whole-cell currents were recorded using a two-electrode voltage-clamp (18) in response to voltage steps of ± 20 mV from a holding potential of −10 mV in a solution containing (in millimoles/liter) 90 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES (pH 7.4 with KOH). Metabolic inhibition was induced by 3 mmol/liter Na-azide, and 0.5 mmol/liter tolbutamide was used to block KATP channels, as indicated. Macroscopic currents were recorded from giant inside-out patches (18). The pipette solution contained (in millimoles/liter) 140 KCl, 1.2 MgCl2, 2.6 CaCl2, and 10 HEPES (pH 7.4 with KOH). The Mg-free internal (bath) solution contained (in millimoles/liter) 107 KCl, 1 K2SO4, 10 EGTA, 10 HEPES (pH 7.2 with KOH), and K2ATP, as indicated. Mg-containing solution was made by adding 2 mmol/liter MgCl2 to the Mg-free solution and using MgATP rather than ATP.
The macroscopic slope conductance was measured between −20 and −100 mV, in response to 3-sec voltage ramps from −100 mV to +110 mV (holding potential, −10 mV). To control for possible rundown, Gc was taken as the mean of the conductance in control solution before and after ATP application. ATP concentration-response curves were fit with the Hill equation: G/Gc = 1/ (1 + ([ATP]/IC50)h), where [ATP] is the ATP concentration, IC50 is the [ATP] at which inhibition is half maximal, and h is the slope factor (Hill coefficient). The mean ± SEM values of IC50 and h given in the text were calculated by fitting each individual dose-response curve and calculating the mean of the values obtained. The IC50 and h values given in the figure legends are the best fit of equation 1 to the mean data.
Single-channel currents were recorded at −60 mV from inside-out patches, as described (15). Open probability was determined from current records of approximately 1-min duration as I/iN, where I is the macroscopic current, i is the single-channel current amplitude, and N is the number of channels. Data are given as mean ± SEM.
Results
Patient characteristics
The male patient was born at term after an uneventful pregnancy with a birth weight of 3070 g. He was diagnosed with diabetes at the age of 3 months. The diagnosis was made during an episode of acute gastroenteritis when he presented with a blood glucose level of 440 mg/dl and a markedly elevated glycosylated hemoglobin of 13.3%. No β-cell antibodies were present (ICA, GAD, IA2, IAA). Insulin therapy was started, and stable metabolic control was achieved with 2 to 3 daily injections of 0.75 U of NPH insulin. His insulin requirement varied between 0.15 and 0.4 U/kg · d. There were no episodes of diabetic ketoacidosis despite several infections and a temporary interruption of insulin therapy for several days.
At 3 yr 9 months of age, after identification of a KCNJ11 mutation, the patient was transferred to glibenclamide and was treated with 3 daily doses totaling 3.5 mg/d. This resulted in stable metabolic control with an glycosylated hemoglobin between 6.5 and 6.9%. Because no severe hypoglycemic episodes were encountered, the frequency of self-monitoring blood glucose was reduced to three measurements per day. The patient has normal psychomental development, no seizures, and a normal electroencephalogram; a formal developmental test was normal. To date, no increase in glibenclamide dose has been required, and he is currently treated with a dose of 0.2 mg/kg · d. His current height (between the 3rd and 10th centiles) and weight (between the 25th and 50th centiles) are in the normal range and consistent with parental height. Examination for diabetic retinopathy and nephropathy were both normal.
Sequencing of the patient’s KCNJ11 gene identified a novel 15-bp deletion (c.81_95del), which resulted in loss of five amino acids (alanine 28 to arginine 32, inclusive) in the N terminus of Kir6.2. This mutation is presumably caused by a rare recombination event because the sequences flanking the deleted region are identical (GCCCGC). Mutation testing in both parents demonstrated that the mutation had arisen de novo in the proband. Microsatellite analysis confirmed family relationships, and a younger child born to the family is healthy. This is the first permanent neonatal diabetes mellitus case described to date that is caused by an in-frame deletion in Kir6.2.
Whole-cell KATP currents
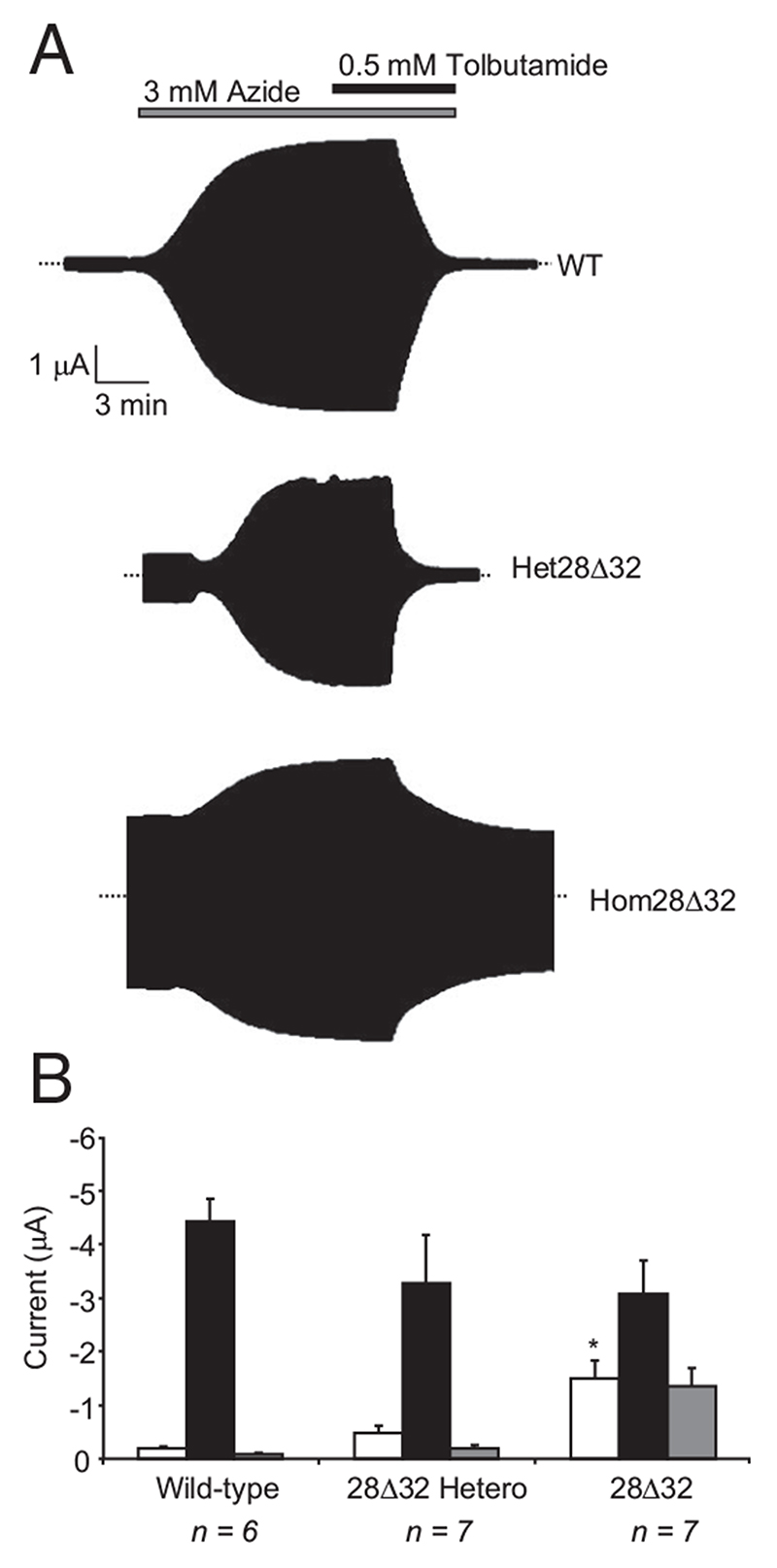
To assess the functional effects of the 28Δ32 mutation, we first measured whole-cell currents in Xenopus oocytes. Wild-type KATP channels exhibited characteristically low resting currents (Fig. 1), due to the high intracellular ATP concentration, but addition of the metabolic inhibitor azide increased the current approximately 20-fold. The sulfonylurea tolbutamide (0.5 mmol/liter), a specific KATP channel blocker, reduced this current to resting levels.
Fig. 1.
A, Representative whole-cell currents recorded from Xenopus oocytes coexpressing SUR1 and either Kir6.2, Kir6.2-28Δ32 (hom28Δ32), or both Kir6.2 and Kir6.2-28Δ32 (het28Δ32) in response to voltage steps of ± 20 mV from a holding potential of −10 mV. B, Mean steady-state whole-cell KATP currents recorded from oocytes coexpressing SUR1 and either wild-type or mutant Kir6.2, as indicated. Currents were evoked by a voltage step from −10 to −30 mV before (control, white bars) and after (black bars) application of 3 mM azide, and in the presence of 3 mM azide plus 0.5 mM tolbutamide (gray bars). The number of oocytes is indicated below the bars. *, P ≤ 0.05 against control (t test).
Homozygous Kir6.2-28Δ32/SUR1 (hom28Δ32) channels had much larger resting currents than wild-type channels (Fig. 1). These currents were further increased (~2-fold) by azide but showed a reduced tolbutamide block: 60 ± 4.4% (n = 7) block compared with 98 ± 0.6% (n = 6) block for wild-type channels. The heterozygous state (seen in the patient) was simulated by coinjection of equal amounts of wild-type and mutant Kir6.2 mRNAs (with SUR1 mRNA). Resting heterozygous (het28Δ32) currents were significantly larger than wild-type: 13 ± 2% (n = 7) vs. 4 ± 0.8% (n = 6) for het28Δ32 and wild-type currents, respectively (P < 0.01), compared with 41 ± 4% (n = 7) for hom28Δ32 currents. Heterozygous KATP currents were inhibited 95 ± 5% (n = 7) by tolbutamide, which explains why sulfonylurea therapy is effective for this patient (9).
ATP sensitivity
The increase in the resting whole-cell current suggests that the 28Δ32 mutation reduces the ATP sensitivity of the KATP channel. We therefore compared the ability of ATP to inhibit wild-type and 28Δ32 KATP channels in inside-out patches.
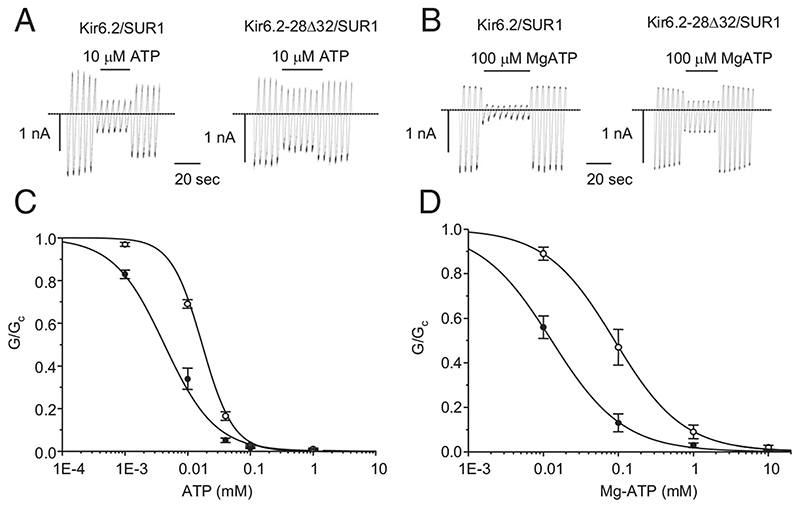
Experiments were first carried out in the absence of Mg2+. This enables the effects of ATP on Kir6.2 to be distinguished from those on SUR1 because ATP interaction with Kir6.2 (but not SUR1) is independent of Mg2+ (10, 19). The 28Δ32 mutation caused a significant reduction in ATP inhibition (Fig. 2A); half-maximal block (IC50) was produced by 15.0 μM ATP and 6.0 μM ATP for hom28Δ32 and wild-type channels, respectively. Interestingly, the Hill coefficient also increased significantly (from 1.3 for wild-type to 2.0 for hom28Δ32; P < 0.02), which implies that the mutation affects cooperative interactions between KATP channel subunits.
Fig. 2.
A and B, Representative KATP currents recorded in response to successive voltage ramps from −110 to +100 mV in an inside-out patch excised from a Xenopus oocyte coexpressing the indicated homozygous KATP channels in the absence (A) and presence (B) of Mg2+. The dashed line indicates the zero current level. C and D, Mean relationship between [ATP] (C) or [MgATP] (D) and KATP conductance (G), expressed relative to the conductance in the absence of nucleotide (Gc), for Kir6.2/SUR1 (WT, black filled circles) and homKir6.2-28Δ32/SUR1 (hom28Δ32, unfilled circles) channels. The lines are the best fit of the Hill equation to the mean data: panel C, WT, IC50 = 5 μM, n = 9; hom28Δ32, IC50 = 16 μM, n = 10. Panel D, WT, IC50 = 13 μM, n = 6; hom28Δ32, IC50 = 89 μM, n = 5. Figures are derived from the fit of the mean data.
In the presence of Mg2+ (Fig. 2B), the sensitivity to ATP inhibition was further reduced by the 28Δ32 mutation, with the IC50 being 111 μM for hom28Δ32 channels vs. 18 μM for wild-type channels ((Table 1). The Hill coefficient was unaffected. Thus, the Kir6.2-28Δ32 mutation causes decreased inhibition by ATP in both the presence and absence of Mg2+. Importantly, the current amplitude at physiological levels of MgATP (3 mM) was increased (Fig. 2B).
Table 1. Biophysical properties of KATP channels.
| Kir6.2ΔC | Kir6.2ΔC + SUR1(1-196) | Kir6.2ΔC + SUR1(1-288) | Kir6.2 + SUR1 | |
|---|---|---|---|---|
| Po | ||||
| WT | 0.03 ± 0.01 (n = 5) | 0.67 ± 0.03 (n = 5) | 0.58 ± 0.06 (n = 8) | 0.24 ± 0.05 (n = 7) |
| 28Δ32 | 0.04 ± 0.01 (n = 5) | 0.68 ± 0.03 (n = 5) | 0.77 ± 0.04 a (n = 8) | 0.80 ± 0.03 b (n = 5) |
| ATP IC50 (μM) | ||||
| WT | 97 ± 2 (n = 7) | 330 ± 20 (n = 6) | 145 ± 23 (n = 5) | 18 ± 2 (n = 6) |
| 28Δ32 | 162 ± 16 a (n = 7) | 365 ± 88 (n = 5) | 209 ± 25 (n = 5) | 111 ± 37 b (n = 5) |
n, Number of oocytes tested. IC50 for Kir6.2ΔC + SUR1(1-196) and Kir6.2ΔC + SUR1(1-288) were statistically significantly different from Kir6.2ΔC alone and from each other. There was no significant difference between the Po of Kir6.2ΔC + SUR1(1-196) and Kir6.2ΔC + SUR1(1-288), or between the Po of Kir6.2ΔC + SUR1(1-196) and Kir6.2ΔC 28Δ32 + SUR1(1-288).
P < 0.01 compared to wild-type.
P < 0.001 compared to wild-type.
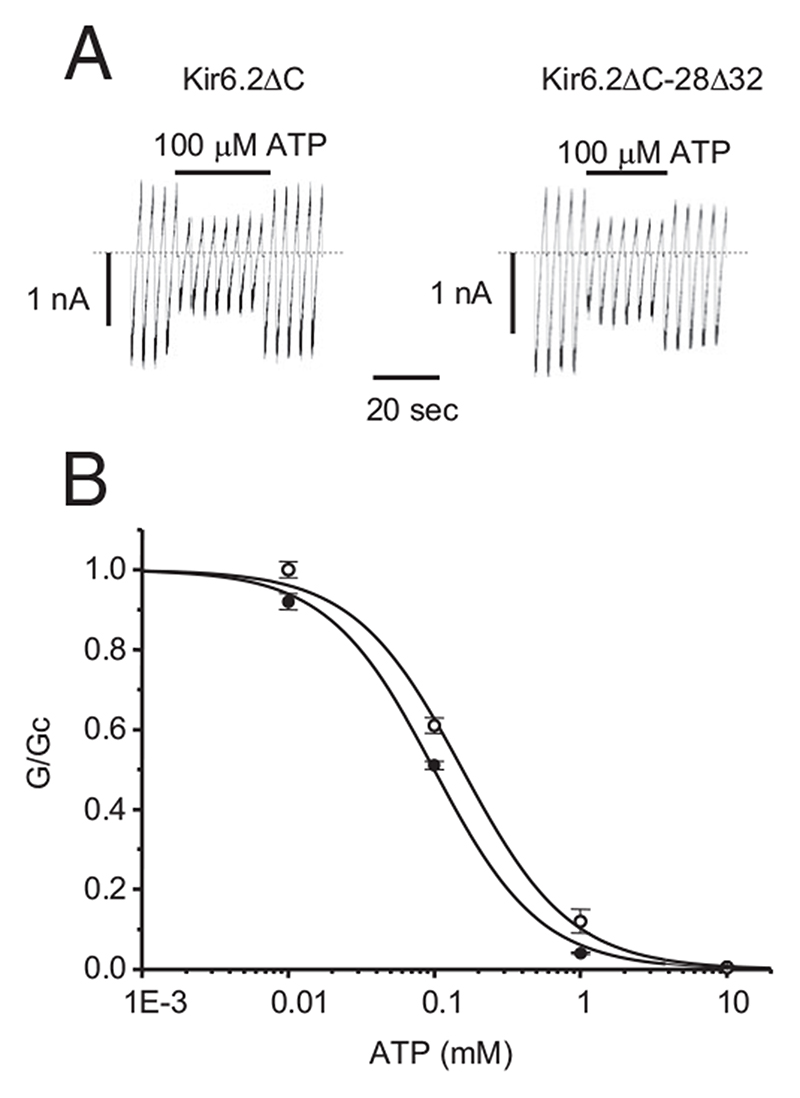
To determine whether the reduced ATP sensitivity of hom28Δ32 channels is intrinsic to Kir6.2 or is due to an altered interaction with SUR1, we used Kir6.2ΔC, a truncated form of Kir6.2 that expresses in the absence of SUR1 (10). As Fig. 3 shows, the ATP sensitivity of Kir6.2ΔC channels was also reduced slightly but significantly by the 28Δ32 mutation, being 97 μM for homKir6.2ΔC-28Δ32 channels compared with 162 μM for Kir6.2ΔC (Table 1; P < 0.01). This suggests that at least part of the reduction in ATP sensitivity may be intrinsic to Kir6.2, but that the major part is due an impaired interaction with SUR1.
Fig. 3.
A, Representative KATP currents recorded in response to successive voltage ramps from −110 to +100 mV in an inside-out patch excised from a Xenopus oocyte expressing the indicated homozygous Kir6.2ΔC construct. The dashed line indicates the zero current level. B, Mean relationship between [ATP] and Kir6.2ΔC conductance (G), expressed relative to the conductance in the absence of nucleotide (Gc), for Kir6.2ΔC (black filled circles) and homKir6.2ΔC- 28Δ32 (unfilled circles) channels. The lines are the best fit of the Hill equation to the mean data: WT, IC50 = 99 μM, n = 7; hom28Δ32, IC50 = 156 μM, n = 7. Figures are derived from the fit of the mean data.
Mechanism of reduced ATP sensitivity
The reduction in ATP sensitivity of Kir6.2 could be caused by reduced ATP binding, impairment of the mechanism by which ATP binding is transduced into channel closure, or (indirectly) by altered channel gating (15). To address the latter possibility, we performed single-channel recordings in the absence of ATP, where intrinsic gating can be assessed. The mean Po was markedly enhanced by the 28Δ32 mutation, being 0.24 for Kir6.2/SUR1 compared with 0.8 for homKir6.2-28Δ32/SUR1 channels ((Table 1). Interestingly, there was no significant effect of the mutation on the intrinsic Po of Kir6.2ΔC channels ((Table 1). This suggests that the 28Δ32 mutation does not stabilize the open state of Kir6.2 per se, but rather that it affects the regulation of channel gating by SUR1. The enhanced Po produced by the mutation when SUR1 is present leads to the lower ATP sensitivity.
It is well established that SUR1 has a marked influence on the KATP channel kinetics, as exemplified by the increase in Po of Kir6.2 when coexpressed with SUR1 (10, 13). Previous studies have shown that this increase in Po is largely conferred by the first five transmembrane domains of SUR1 (TMD0: residues 1-196) (20, 21) and the cytosolic loop that links TMD0 to TMD1 (the CL3 linker) (22). SUR1 also enhances the ATP sensitivity of Kir6.2 (10); the mechanism is unknown but it does not involve either TMD0 (20) or the CL3 linker (22). Thus, any reduction in ATP inhibition produced by TMD0/CL3 is likely to be mediated via changes in Po.
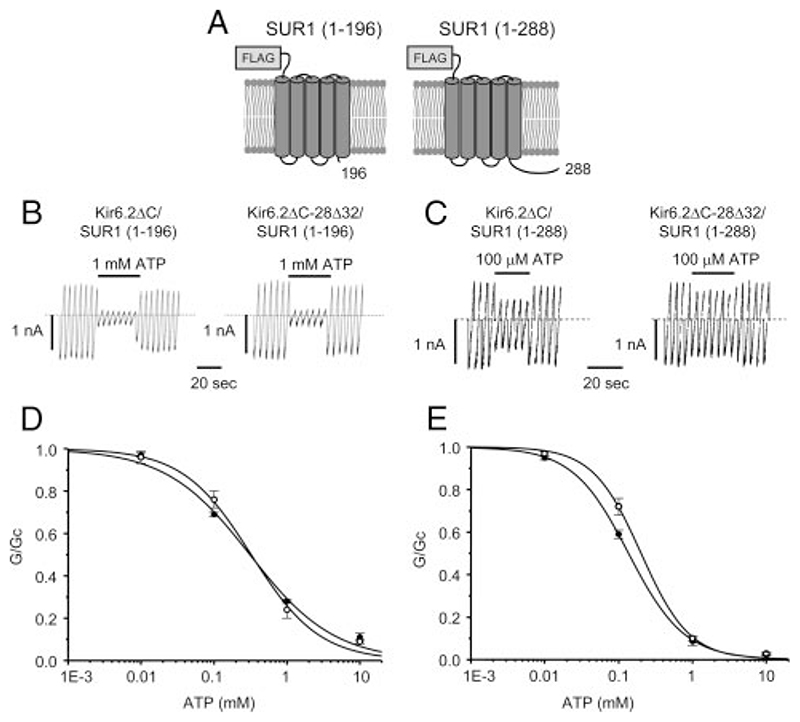
As previously reported, the Po of Kir6.2ΔC was much lower than that of Kir6.2/SUR1 ((Table 1) (13). Coexpression of Kir6.2ΔC with residues 1-196 of SUR1 [SUR1(1-196)] resulted in an increase in Po to a value significantly higher than that found for coexpression with full-length SUR1 ((Table 1), in agreement with previous studies (21, 22). Similar results were found when Kir6.2ΔC was coexpressed with the first 288 amino acids of SUR1 [SUR1 (1-288)], which includes both TMD0 and most of the CL3 linker.
As Fig. 4, B and D, shows, no significant difference in ATP inhibition was observed between wild-type and mutant Kir6.2ΔC, when coexpressed with SUR1(1-196), suggesting that this region of SUR1 is not sufficient to confer sensitivity to the 28Δ32 mutation. In agreement with this idea, the mutation did not change the Po of Kir6.2ΔC/SUR1(1-196) channels ((Table 1).
Fig. 4.
A, Schematic representation of the SUR1(1-196) and SUR1(1-288) constructs, showing transmembrane helices and an N-terminal FLAG tag. B and C, Representative KATP currents recorded in response to successive voltage ramps from −110 to +100 mV in an inside-out patch excised from a Xenopus oocyte coexpressing the indicated homozygous Kir6.2ΔC construct in the presence of SUR1(1–196) (B) and/or SUR1(1-288) (C). The dashed line indicates the zero current level. D and E, Mean relationship between [ATP] and Kir6.2ΔC conductance (G), expressed relative to the conductance in the absence of nucleotide (Gc), for Kir6.2ΔC (black filled circles) and homKir6.2ΔC-28Δ32 (unfilled circles) channels in the presence of either SUR1(1-196) (D) or SUR1(1-288) (E). The lines are the best fit of the Hill equation to the mean data: D, WT, IC50 = 311 μM, n = 6; hom28Δ32, IC50 = 325 μM, n = 7. E, WT, IC50 = 136 μM, n = 5; hom28Δ32, IC50 = 202 μM, n = 5. Figures are derived from the fit of the mean data.
Figure 4, C and E, shows that the ATP sensitivity of Kir6.2/ SUR1(1-288) channels was also not significantly affected by the mutation, but that there was a small but significant increase in Po ((Table 1). This suggests that the 28Δ32 mutation alters an interaction between the N terminus of Kir6.2 and a region of SUR1 containing the CL3 linker (residues 196-288), which results in stabilization of the open state of Kir6.2 by SUR1.
The dramatic difference in ATP inhibition when wild-type Kir6.2 is coexpressed with full-length SUR1, rather than SUR1(1-196) or SUR1(1-288), reflects an ability of SUR1 to enhance ATP block that does not involve residues 1-288, as previously described (22). The lower ATP sensitivity of Kir6.2/SUR1 channels produced by the mutation may reflect disruption of this effect, or be a secondary consequence of the increase in Po, or both.
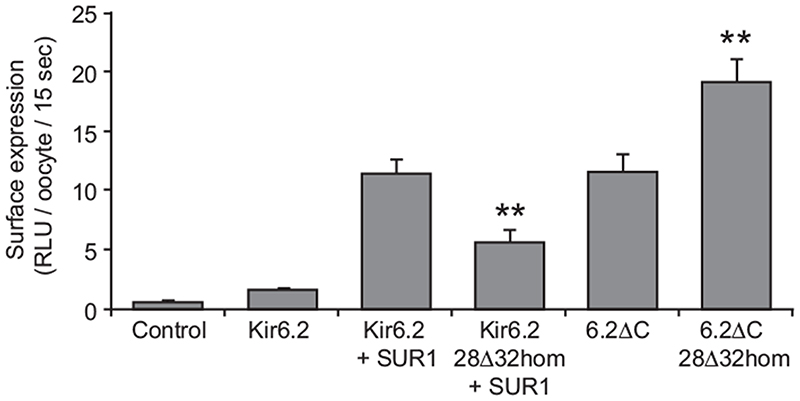
Surface expression
Because azide-activated whole-cell currents were somewhat smaller for hom28Δ32 channels, we next examined whether channel density in the plasma membrane was decreased by the mutation (16). As Fig. 5A shows, surface expression of Kir6.2/ SUR1 was significantly reduced by the 28Δ32 mutation (P < 0.01), suggesting that the mutation disrupts surface trafficking or assembly of the complex. However, Kir6.2ΔC surface expression was significantly increased by the 28Δ32 mutation (Fig. 5B; P < 0.01). Thus, the reduction in channel density requires the presence of SUR1, suggesting that the mutation alters an interaction between Kir6.2 and SUR1.
Fig. 5.
Relative levels of surface expression in Xenopus oocytes of HA-tagged Kir6.2 (wild-type or mutant) coexpressed with or without SUR1, and HA-tagged Kir6.2ΔC (wild-type or mutant), as indicated. Data are the mean ± sem of 18–52 individually assayed oocytes from three different preparations. Control oocytes were uninjected. Surface expression is expressed as the relative light units (RLU) recorded in 15 sec from individual oocytes. **, P < 0.01 compared with wild-type for both Kir6.2-28Δ32 and Kir6.2ΔC-28Δ32.
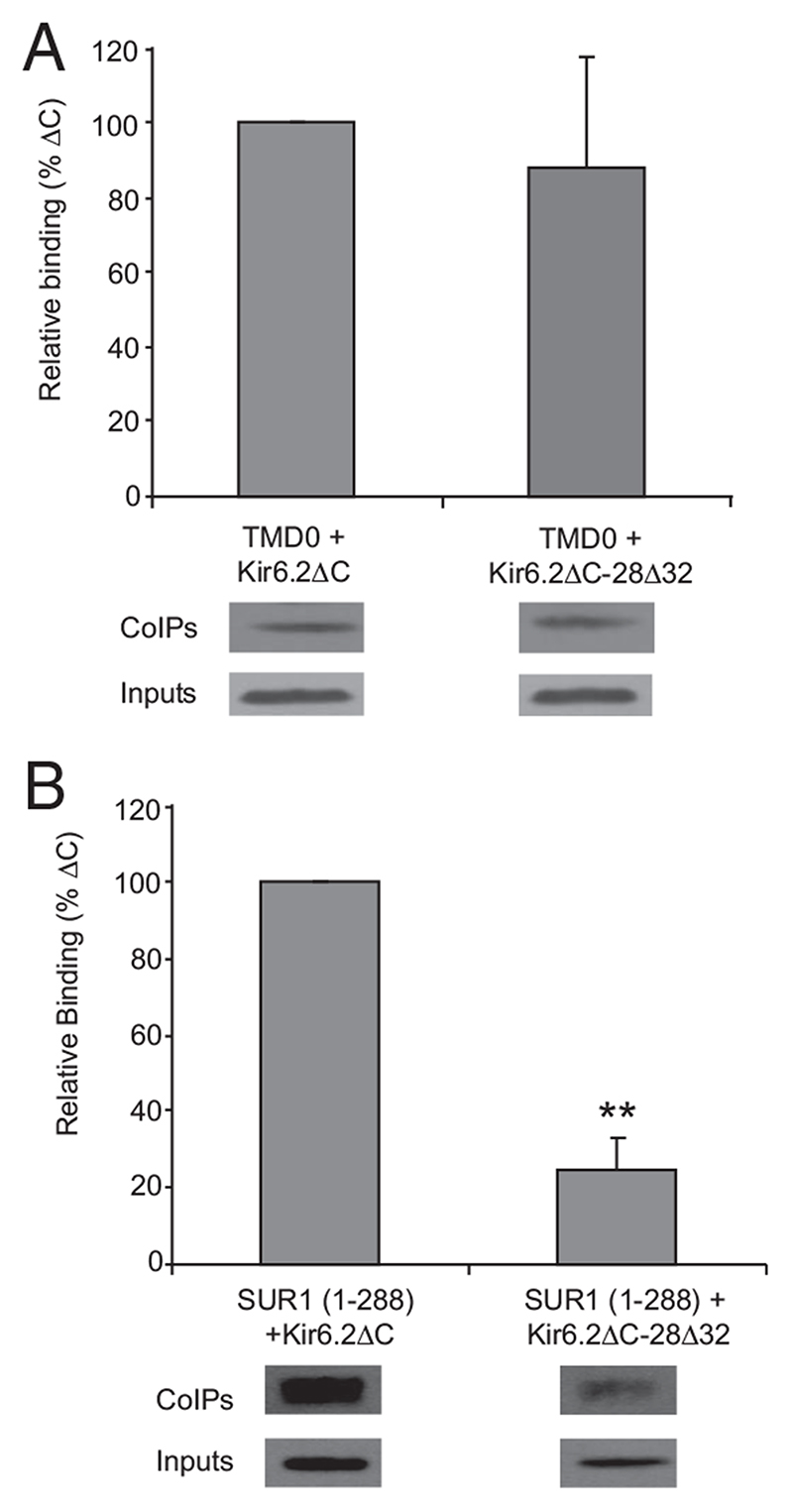
Physical interaction between Kir6.2 and SUR1
Our results suggest that the 28Δ32 mutation affects a functional and a physical interaction between Kir6.2 and residues 196-288 in SUR1. To confirm this idea, we performed coimmunoprecipitation studies in Xenopus oocytes expressing tagged versions of Kir6.2ΔC (wild-type and mutant) and either residues 1-196 or 1-288 of SUR1. Figure 6A shows that the interaction between SUR1(1-196) and Kir6.2ΔC is not significantly affected by the 28Δ32 mutation. In contrast, the interaction between SUR1(1-288) and Kir6.2ΔC is significantly reduced (P < 0.01) (Fig. 6B). This supports the idea that residues 196-288 of SUR1 form an essential part of the binding site between SUR1 and the N terminus of Kir6.2 and that this interaction is disrupted by deletion of residues 28-32 in Kir6.2.
Fig. 6.
Coimmunoprecipitation assays performed on oocytes expressing FLAG-tagged SUR1(1-196) and either HA-tagged Kir6.2ΔC or HA-tagged Kir6.2ΔC- 28Δ32. A, Top, Binding of Kir6.2ΔC and Kir6.2ΔC-28Δ32 to TMD0 [SUR1(1-196), n = 6], expressed as a percentage of binding to Kir6.2ΔC. Bound protein levels were normalized to the expression levels of SUR1(1-196). Bottom, upper panel, Representative anti-FLAG Western blots from individual experiments, showing SUR1(1-196) binding to Kir6.2ΔC and Kir6.2ΔC-28Δ32 (corresponding to the graph above). Bottom, lower panel, Representative Western blots of the oocyte lysate inputs into the binding assay, showing expression levels of TMD0. B, Top, Binding of Kir6.2ΔC and Kir6.2ΔC-28Δ32 to SUR1(1-288) (n = 4), expressed as a percentage of the binding of Kir6.2ΔC. **, P < 0.01 compared with Kir6.2ΔC. Bound protein levels were normalized to the precipitated levels of SUR1(1-288) and expression levels of Kir6.2ΔC. Bottom, upper panel, Representative anti-HA Western blots from individual experiments, showing Kir6.2ΔC or Kir6.2ΔC- 28Δ32 binding to SUR1(1-288), corresponding to the graph above. Bottom, lower panel, Representative Western blots of the oocyte lysate inputs into the binding assay, showing expression levels of Kir6.2ΔC or Kir6.2ΔC-28Δ32.
Discussion
Our results reveal that het28Δ32 channels have significantly larger resting whole-cell currents than wild-type channels. In pancreatic β-cells, a similar increase in current would be expected to result in β-cell hyperpolarization and could therefore account for the diabetic phenotype of the patient. The fact that tolbutamide blocked het28Δ32 channels as effectively as wild-type channels explains why the patient was able to respond to sulfonylurea therapy; when tolbutamide blocks mutant KATP channels to this extent, patients carrying the same mutation are often able to transfer from insulin to sulfonylureas (9).
As is the case for other neonatal diabetes mutations (1, 14), the increase in whole-cell current is caused by a reduced ability of ATP to block KATP channels containing the het28Δ32 mutation. This results in a small increase in current at physiological levels of ATP: about 2.5% of unblocked hom28Δ32 current remains at 3 mM, and the het28Δ32 current will be even smaller. Most mutations that give rise to neonatal diabetes alone result in 4–16% of remaining current at 3 mM MgATP (14, 23). DEND syndrome is associated with much larger currents. Thus, the fact that the 28Δ32 mutation caused only a small increase in current explains why our patient had no neurological symptoms that were detectable by the standard evaluations performed in this study.
Molecular mechanism of ATP insensitivity
Single-channel analysis showed that the 28Δ32 mutation greatly increased the intrinsic Po of the KATP channel. It is well established that stabilization of the channel open state leads to a decrease in ATP sensitivity (15), and many neonatal diabetes mutations act in this way (24–27). Because the 28Δ32 mutation has no effect on the Po when Kir6.2 is expressed in the absence of SUR1, it appears that the mutation does not directly stabilize the open state of the channel. Rather, it affects the regulation of the open state of the pore by SUR1.
Previous studies also support a role for the N terminus of Kir6.2 in the modulation of channel gating by SUR1. For example, serial truncations from the N terminus of Kir6.2 result in a progressive increase in Po (and a reduction in ATP sensitivity) that is manifest only in the presence of SUR1 (28–30). Furthermore, mutations at two residues within the Kir6.2 N terminus (F35, Q52) increase Po in the presence but not absence of SUR1, and thereby lead to neonatal diabetes (24, 26). Mutations near the C terminus of Kir6.2 (Y330C, F333I) also affect its functional interaction with SUR1 (31), indicating that there are multiple sites of interaction between these two proteins. Interestingly, a peptide containing the first 53 amino acids of Kir6.2 also increased Po, suggesting that it competes with Kir6.2 for binding to SUR1, and thereby disrupts the interaction between these two subunits (32).
Which region of SUR1 is involved in the interaction 28-32 of Kir6.2?
It is well established that TMD0 of SUR1 influences the gating of Kir6.2 and that this region of SUR is responsible for the differences in gating of Kir6.2/SUR1 and Kir6.2/SUR2 channels (20, 21, 33). The CL3 linker of SUR1 has also been implicated in regulation of gating and appears to contain domains that both enhance and inhibit channel activity (22).
Our studies show that coexpression of Kir6.2ΔC with SUR1(1-196) of SUR1 enhances Po, as previously reported (21). However, the 28Δ32 mutation has no effect on the gating of these channels, indicating that any interaction of Kir6.2 with residues 1-196 of SUR1 is not sufficient to modulate gating. In contrast, when Kir6.2ΔC-28Δ32 was coexpressed with residues 1-288 of SUR1, the Po was very similar to that found with full-length SUR1, suggesting that the mutation disrupts an interaction that involves residues 196-288 of SUR1 (the CL3 linker).
The data presented here confirm that residues 1-196 of SUR1 interact with Kir6.2 to enhance the intrinsic Po and thus reduce KATP channel ATP sensitivity (21, 22). They further show that residues 28-32 of Kir6.2 are not involved in this interaction because their deletion does not influence Po. In contrast, our results suggest that residues 196-288 form part of a region of SUR1 that interacts with the N terminus of Kir6.2 to decrease Po and that deletion of residues 28-32 in Kir6.2 disrupts this inhibitory interaction, while leaving activating interactions (e.g. the interaction between Kir6.2 and TMD0) intact. The net result is stabilization of the open state of Kir6.2, which leads to a secondary decrease in ATP sensitivity. Importantly, this functional interaction is mirrored by a physical interaction, identified in coimmunoprecipitation studies.
Summary.
Several studies have demonstrated that SUR1 has both a stimulatory and an inhibitory effect on the gating of Kir6.2 (21, 22). In this study, we have, for the first time, defined the regions of Kir6.2 and SUR1 that contribute to one specific inhibitory interaction between these two proteins: namely, residues 28-32 of Kir6.2 and 196-288 of SUR1. These domains interact both functionally and physically, suggesting that this is a fairly strong structural interaction. Disruption of this interaction is of clinical importance because it leads to neonatal diabetes.
Acknowledgments
We thank Prof. Andrew Hattersley (A.T.H.) of Exeter University for constant support and advice.
Abbreviations
- KATP
ATP-sensitive potassium
- Po
open probability
- TMD
transmembrane domain
Footnotes
Disclosure Summary: T.J.C., K.S., R.W.H., S.E.F., S.E., and F.M.A. have nothing to declare.
Contributor Information
Tim J. Craig, Henry Wellcome Centre for Gene Function, Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford OX1 3PT, United Kingdom
Kenju Shimomura, Henry Wellcome Centre for Gene Function, Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford OX1 3PT, United Kingdom.
Reinhard W. Holl, Institute of Epidemiology, University of Ulm, D-89081 Ulm, Germany
Sarah E. Flanagan, Institute of Biomedical and Clinical Research, Peninsula Medical School, Exeter EX2 5DW, United Kingdom
Sian Ellard, Institute of Biomedical and Clinical Research, Peninsula Medical School, Exeter EX2 5DW, United Kingdom.
Frances M. Ashcroft, Henry Wellcome Centre for Gene Function, Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford OX1 3PT, United Kingdom
References
- 1.Ashcroft FM. The Walter B. Cannon Physiology in Perspective Lecture, 2007.ATP-sensitive K+ channels and disease: from molecule to malady. AmJ Physiol Endocrinol Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 2.Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- 3.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 4.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 5.Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 8.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 9.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 10.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphony-lurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 11.Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP.Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, 4th, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 13.Proks P, Ashcroft FM. Phentolamine block of KATP channels is mediated by Kir6.2. Proc Natl Acad Sci USA. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 17.Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 18.Gribble FM, Ashfield R, Ammälä C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed inXenopusoocytes. JPhysiol. 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proks P, Shimomura K, Craig TJ, Girard CA, Ashcroft FM. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum Mol Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. [DOI] [PubMed] [Google Scholar]
- 20.Gribble FM, Proks P, Corkey BE, Ashcroft FM. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem. 1998;273:26383–26387. doi: 10.1074/jbc.273.41.26383. [DOI] [PubMed] [Google Scholar]
- 21.Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. doi: 10.1093/emboj/cdg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babenko AP, Bryan J. SUR domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–41580. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- 23.Ashcroft FM. K(ATP) channels and insulin secretion: a key role in health and disease. Biochem Soc Trans. 2006;34:243–246. doi: 10.1042/BST20060243. [DOI] [PubMed] [Google Scholar]
- 24.Tammaro P, Proks P, Ashcroft FM. Functional effects of naturally occurring KCNJ11 mutations causing neonatal diabetes on cloned cardiac KATP channels. J Physiol. 2006;571:3–14. doi: 10.1113/jphysiol.2005.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard CA, Shimomura K, Proks P, Absalom N, Castano L, Perez de Nanclares G, Ashcroft FM. Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch. 2006;453:323–332. doi: 10.1007/s00424-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 26.Proks P, Girard C, Baevre H, Njølstad PR, Ashcroft FM. Functional effects of mutations at F35 in the NH2-terminus of Kir6.2 (KCNJ11), causing neonatal diabetes, and response to sulfonylurea therapy. Diabetes. 2006;55:1731–1737. doi: 10.2337/db05-1420. [DOI] [PubMed] [Google Scholar]
- 27.Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, Ashcroft FM. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babenko AP, Gonzalez G, Bryan J. The N-terminus of KIR6.2 limits spontaneous bursting and modulates the ATP-inhibition of KATP channels. Biochem Biophys Res Commun. 1999;255:231–238. doi: 10.1006/bbrc.1999.0172. [DOI] [PubMed] [Google Scholar]
- 29.Reimann F, Tucker SJ, Proks P, Ashcroft FM. Involvement of the N-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J Physiol. 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of β cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 31.Tammaro P, Girard C, Molnes J, Njølstad PR, Ashcroft FM. Kir6.2 mutations causing neonatal diabetes provide new insights into Kir6.2-SUR1 interactions. EMBO J. 2005;24:2318–2330. doi: 10.1038/sj.emboj.7600715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babenko AP, Bryan J. SUR-dependent modulation of KATP channels by an N-terminal KIR6.2 peptide. Defining intersubunit gating interactions. J Biol Chem. 2002;277:43997–44004. doi: 10.1074/jbc.M208085200. [DOI] [PubMed] [Google Scholar]
- 33.Babenko AP, Gonzalez G, Bryan J. Two regions of sulfonylurea receptor specify the spontaneous bursting and ATP inhibition of KATP channel isoforms. J Biol Chem. 1999;274:11587–11592. doi: 10.1074/jbc.274.17.11587. [DOI] [PubMed] [Google Scholar]
- 34.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]