Abstract
CriPec® technology enables the generation of drug-entrapped biodegradable core-crosslinked polymeric micelles (CCPM) with high drug loading capacity, tailorable size and drug release kinetics. Docetaxel-entrapped CCPM, also referred to as CPC634, have demonstrated favorable pharmacokinetics, tolerability and enhanced tumor uptake in patients, and clinical efficacy evaluation is ongoing. CPC634 is currently stored (shelf life >5 years) and shipped as a frozen aqueous dispersion at temperatures below -60 °C to prevent premature release of docetaxel and hydrolysis of the core-crosslinks. Consequently, like many other aqueous nanomedicine formulations, CPC634 relies on cold chain supply, which is unfavorable for commercialization. Lyophilization can help to bypass this issue. We therefore set out to develop a freeze-drying methodology for CCPM, employing CPC634 as a model formulation, and using a shelf freeze-dryer and sucrose and trehalose as cryoprotectants. We studied the residual moisture content and reconstitution behavior of the CPC634 freeze-dried cake, as well as the size, polydispersity index, morphology, drug retention and release kinetics of reconstituted CPC634. Subsequently, the freeze-drying methodology was validated in an industrial setting, yielding a CPC634 freeze-dried cake with a moisture content of less than 0.1 wt%. It was found that trehalose-cryoprotected CPC634 could be rapidly reconstituted in less than 5 min at room temperature. Critical quality attributes such as size, morphology, drug retention and release kinetics of trehalose-cryoprotected freeze-dried CPC634 upon reconstitution were identical to those of non-freeze-dried CPC634. These findings provide proof-of-concept for the lyophilization of drug-containing CCPM and they are readily translatable to large-scale manufacturing for future commercialization.
Keywords: Nanomedicine, Tumor targeting, Polymeric micelles, Drug delivery, Lyophilization
Abbreviations
- CCPM
core-crosslinked polymeric micelles
- DLS
dynamic light scattering
- DSC
differential scanning calorimetry
- DTX
docetaxel
- PDI
polydispersity index
- PM
polymeric micelles
- TEM
transmission electron microscopy
- Tg
glass transition temperature
- UPLC
ultra-performance liquid chromatography.
1. Introduction
Polymeric micelles (PM) can entrap drug molecules either via physical interaction (e.g. hydrophobic interactions and π-π stacking) or via chemical interaction (e.g. covalent core-crosslinking), and they are attractive vehicles for targeted drug delivery to pathological sites exploiting the so-called EPR effect [1–5]. PM based on CriPec® technology platform are size-tunable (30-100 nm) core-crosslinked polymeric micelles (CCPM) composed of thermosensitive methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide lactate] (mPEG-b-pHPMAmLacn) block copolymers. During the manufacturing of CCPM, the block copolymers self-assemble into micellar structures in aqueous solutions and then crosslinked in the micellar core by means of free radical polymerization. Meanwhile, drug molecules are transiently covalently attached to the crosslinked micellar core via hydrolysable linkages that enable release of native drug molecules at the target site with predetermined kinetics [6, 7]. Covalent core-crosslinking of PM prevents premature micelle disintegration and minimizes drug release in the bloodstream, together contributing to prolonged circulation and enhanced drug delivery to pathological sites [7–9]. So far, a variety of therapeutics agents such as chemotherapeutic drugs, corticosteroids and peptides have been successfully entrapped in CCPM, resulting in superior therapeutic performance [7, 10, 11].
Docetaxel (DTX)-entrapped CCPM, also known as CPC634, is the clinically most advanced CriPec® nanomedicine where docetaxel is conjugated via a hydrolytically sensitive ester bond to the core of the micelles. CPC634 is currently under evaluation in a phase 2 clinical trial in patients with ovarian cancer [12]. In the clinically used formulation, the DTX-entrapped CCPM are dispersed in an aqueous buffer. However, the formulation cannot be stored in the fridge (4 °C) or at room temperature (20-25 °C) for prolonged periods of time because of inherent hydrolysis of the ester bond between DTX and the CCPM and thereby premature release of the drug [10, 11]. Consequently, the clinical batches of CPC634 are stored at < -60 °C as a frozen dispersion. So far, CPC634 aqueous formulation has demonstrated good stability for at least 5 years upon storage at < -60 °C.
Currently, the supply of CPC634 to clinics relies on a cold chain comprising of shipment on dry ice, continuous temperature logging and < -60 °C freezers at each clinical site, all contributing to a very expensive mode of transportation. To circumvent this, we developed a freeze-drying process for lyophilization of CPC634 which likely can also be exploited for other (CriPec®) nanoformulations. Freeze-drying is a water removal process by sublimation of ice under high vacuum, which offers long shelf-life and ease of transportation of pharmaceutical and food products [13–16]. Freeze-drying has already been employed for CCPM and several other polymeric micelles nanoformulations (e.g. Genexol-PM®, NK105 and NK-6004 Nanoplatin™ [17, 18, 19]), as well as used for stabilization of other nanomedicine formulations such as liposomes and lipo/polyplexes [20–23]. A freeze-drying process typically involves three steps: (1) freezing; (2) primary drying, i.e. removal of bulk water molecules; and (3) secondary drying, i.e. removal of residual/bound water molecules. For therapeutic proteins and nano-formulations, harsh treatments such as freezing and drying can deform, damage and even disintegrate them, thus compromising their pharmaceutical properties. Cryoprotectants, which comprise sugars, polymers and surfactants, are commonly used to stabilize the formulation, by means of vitrification [14, 24]. Additionally, sugars provide cryoprotection via water replacement mechanism i.e. hydrogen bonding between hydroxyl groups of sugar and hydrogen accepting groups in proteins and PEGylated nanoparticles [25, 26]. For each formulation, an individually optimized protocol is needed to identify the best cryoprotectant, concentration and freeze-drying settings (including vacuum pressure, temperature and duration) to eventually obtain a freeze-dried product with unaltered physicochemical and pharmaceutical properties after reconstitution [14, 24, 27–29].
In the present study, efforts to establish cryoprotectant selection and freeze-drying settings for obtaining lyophilized DTX-entrapped CCPM are described (Fig. 1). An aqueous dispersion of CPC634 was employed as the starting formulation. Standard cryoprotectants based on sugars were assessed and two different shelf freeze-dryers were used. Further, differential scanning calorimetry (DSC) was employed to determine glass transition temperature (Tg) of CPC634 aqueous dispersion containing different cryoprotectants. Extensive post freeze-drying characterizations, evaluating cake appearance, residual moisture content, reconstitution behavior, size, polydispersity index (PDI), drug retention and drug release kinetics were performed.
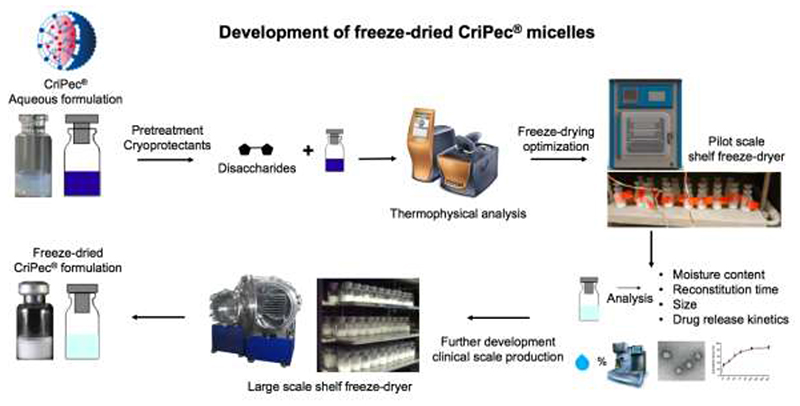
Figure 1.
Study design. The feasibility of freeze-drying CriPec®-based core-crosslinked polymeric micelles (CCPM) was investigated using disaccharides (sucrose and trehalose) as cryoprotectants. The glass transition temperature (Tg) of aqueous solutions containing CPC634 (i.e. clinical-stage docetaxel-CCPM) plus cryoprotectant was determined using differential scanning colorimetry (DSC) to optimize temperature setting and avoid cake collapse during freeze-drying. Pilot scale shelf freeze-dryers equipped with temperature sensor and pirani gauge were employed to perform freeze-drying and optimal settings were identified. Next, a systematic analysis of the freeze-dried cakes and reconstituted formulations was performed, evaluating key quality attributes such as moisture content, reconstitution time, size, PDI, transmission electron microscopy (TEM), drug retention and release kinetics. These results confirm the feasibility to generate lyophilized CCPM formulations for clinical evaluation and commercial application.
2. Materials and Methods
2.1. Materials
Docetaxel (DTX) was obtained from Phyton Biotech GmbH (Ahrensburg, Germany). N,N’-dicyclohexyl-carbodiimide (DCC), 4-dimethylaminopyridine (DMAP), 4-methoxyphenol, methacrylic anhydride, ammonium acetate, formic acid, Mukaiyama’s reagent (2-chloro-1-methylpyridinium iodide), potassium peroxymonosulfate (oxone), potassium persulfate (KPS), lactic acid, N, N, N’, N’ tetramethyl-ethylenediamine (TEMED) and trifluoroacetic acid (TFA) were obtained from Sigma Aldrich (Zwijndrecht, the Netherlands). Dichloromethane (DCM), N,N-dimethylformamide (DMF) and acetonitrile (ACN) were purchased from Biosolve (Valkenswaard, the Netherlands). Absolute ethanol and triethylamine were purchased from Merck (Darmstadt, Germany). The macro-initiator (mPEG5000)2-ABCPA and 2-(2-(methacryloyloxy)ethylthio) acetic acid (linker) were synthesized as described previously [10, 30]. Sucrose and trehalose were purchased from Merck (Darmstadt, Germany). Three-ml sample glass vials (soda-lime glass) with silicon caps were purchased from VWR (Langenfeld, Germany). All materials were analytical grade and used without purification.
2.2. Preparation of CPC634 dispersion
The detailed synthesis of derivatized DTX and CPC634 is described in [6, 7]. Briefly, an ice-cold aqueous solution of methacrylated mPEG5000-b-pHPMAmLacn (n=1 or 2, 22.6 kDa) block copolymer (83 v%, 24 mg/mL) was mixed with TEMED (2.5 v%, 120 mg/mL) dissolved in ammonium acetate buffer (150 mM, pH 5). Subsequently, derivatized DTX (10 v%, 20 mg/mL DTX equivalents, dissolved in ethanol) was added, followed by rapid heating to 60 °C under vigorous stirring for 1 min to form micelles. The micellar aqueous dispersion was then transferred into a vial containing KPS (4.5 v%, 30 mg/mL) dissolved in ammonium acetate buffer (150 mM, pH 5). The polymeric micelles were covalently stabilized by free radical polymerization of the methacrylate moieties of derivatized DTX and methacrylated mPEG-b-pHPMAmLacn under a N2 atmosphere for 1 h at room temperature, to obtain CPC634 micelles. The final feed concentrations of block copolymer and DTX equivalents were 20 and 2.0 mg/mL, respectively. Next, the obtained CPC634 dispersion was filtered through a 0.2 μm cellulose membrane filter to remove possible aggregates, and then purified in ammonium acetate buffer (20 mM, pH 5, supplemented with 130 mM NaCl) using a KrosFlo Research IIi Tangential Flow Filtration (TFF) System equipped with modified polyethersulfone (mPES) MicroKros® filter modules (MWCO 100 kDa). The chemical structures and schematics of methacrylated mPEG5000-b-pHPMAmLacn block copolymer, derivatized docetaxel and CPC634 are illustrated in Fig. S1. To prepare materials for freeze-drying experiments, a clinical batch of CPC634 (2 mg/mL docetaxel equiv.) was buffer swapped into predetermined aqueous solutions, followed by concentration to 15 mg/mL docetaxel equiv. (135 mg/mL polymer equiv.) by TFF and subsequent storage at < -60 °C.
2.3. Differential scanning calorimetry (DSC) analysis
Determination of glass transition temperature (Tg) of CPC634 aqueous dispersions (block copolymer and derivatized DTX (DTX equivalents) concentration were (109 and 12 mg/ml, respectively) in sodium acetate buffer (20 mM, pH 5) with two cryoprotectants (sucrose and trehalose 10 wt%) using a TA Instruments Discovery DSC device was done as follows. Twenty μl of CPC634 cryoprotectant dispersion was added in hermetic aluminum pan which was sealed with aluminum lid. The pan containing the sample was frozen to -70 °C and then gradually heated to 50 °C at a rate of 10 °C/minute, followed by cooling down again to -70 °C and re-heating to 50 °C, always at a rate of 10 °C/minute. A pan containing 20 μl water was used as reference. The Tg was determined with the help of TRIOS software. The reported Tg values were obtained from the last heat treatment.
2.4. Freeze-drying and reconstitution
Freeze-drying of CPC634 was carried out using Lyoph-Pride 03, a pilot-scale shelf freeze-dryer (ilshinBioBase, Dongduchon, South Korea) equipped with a temperature sensor for recording the product temperature. Three-ml glass vials were filled with 1 ml of CPC634 aqueous dispersion in sodium acetate buffer (20 mM, pH 5) containing 12 mg of derivatized DTX (DTX equivalents), 109 mg block copolymer and different cryoprotectants (sucrose and trehalose) at different weight ratios (10, 5 and 2.5 wt%). the glass vials (partially capped with silicon caps) were loaded in the freeze-dryer onto the stainless-steel shelf set at 4 °C. Freezing of the dispersions was initiated by setting the shelf-temperature at -45 °C for 3 hours at atmospheric pressure. This was followed by primary freeze-drying at -25 °C shelf temperature and -70 °C condenser temperature under 0.3 mbar chamber pressure for 17 hours. Subsequently, secondary drying was conducted at shelf temperature 20 °C and a condenser temperature of -70 °C at 0.3 mbar for 4 hours. Lastly, the chamber pressure was adjusted back to atmospheric pressure and the vials were sealed in the freeze-dryer with silicon caps prior to unloading. The above freeze-drying cycle parameters (shelf temperature and vacuum pressure) were selected based on trial freeze-drying experiments, conducted at different vacuum pressure (0.1, 0.2 and 0.3 mbar) and at different shelf temperatures (-35, -30 and -25 °C) and by hourly recording product temperature using integrated temperature sensor needles inserted in the vials.
Further development of the CCPM freeze-drying process was conducted in an industrial setting. Using a GT3 shelf freeze-dryer (Hof Sonderanlagenbau GmbH, Lohra, Germany) equipped with a pirani gauge sensor, a Baratron® capacitance manometer for vapor pressure measurement and a product temperature sensor. Glass vials filled with 1 ml samples (each vial containing: 12 mg of DTX, 70 mg block copolymer and trehalose 5 wt%) were sealed with steam permeable LyoProtect® bags to avoid contamination from the freeze-dryer and were further loaded on the freeze-dryer shelf with a temperature of 20 °C. Freezing was carried by adjusting shelf temperature to -45 °C for 4 h. Subsequently, primary drying was conducted at -10 °C shelf temperature and -70 °C condenser temperature at 0.05 mbar for 33 h. Next, secondary drying was conducted by ramping the shelf temperature to 30 °C and keeping the condenser temperature and vacuum pressure unaltered for 18 h. Vials were sealed with caps under nitrogen at 750 mbar chamber pressure before unloading the vials from the freeze-dryer.
The freeze-dried CPC634 samples were reconstituted with 2 ml of 0.9% NaCl solution. After addition of the NaCl solution, the vials were left undisturbed on the shelf while observing their reconstitution behavior. Complete dissolution of the freeze-dried CCPM cake was examined visually and the time for complete dissolution was established. Following this, pH of reconstituted samples was measured with Five Easy™ pH meter (Mettler Toledo BV, Tiel, the Netherlands).
2.5. Moisture content analysis
The moisture content of the CPC634 freeze-dried cakes was analyzed using a TA Instruments - TGA Q50 device. Around 10 mg of the freeze-dried sample (accurately weighed) was loaded into platinum pan. The pan was placed on the sample holder followed by heating to 110 °C for 8 min in the instrument’s furnace. The change in wt% after heat treatment was used to calculate the moisture content of the freeze-dried cakes. The measurements were conducted in triplicates. For the industrial setting experiments, the residual moisture content of the samples was determined via Karl Fischer titration using a 756 Karl Fischer coulometer (Deutsch Metrohm GmbH, Filderstadt, Germany). For each measurement, around 100 mg of each lyophilizate was accurately weighed and transferred into a Karl-Fischer glass vial. The vial was sealed with a crimp cap and transferred into the oven of the Karl Fischer coulometer which was heated to 100 °C for 5 min. The septum of the cap was penetrated by an injection needle, and the generated water vapor was transferred into the titration chamber of the Karl Fischer coulometer by a dry nitrogen flow. Empty glass vials were used for blank correction. The measurements were conducted in duplicate.
2.6. Size measurements
The samples for size measurements were prepared by diluting reconstituted CPC634 samples by 100 folds with Millipore water. The size of the micelles was measured by dynamic light scattering (DLS) using a Malvern Zetasizer nano series ZS90 with a measurement angle of 90° and a temperature of 25 °C. DLS results are presented as z-average particle size diameter and polydispersity index (PDI). For each sample, the reported values are the average of 3 independent measurements. Morphological analysis of CPC634 was performed using transmission electron microscope (TEM). For sample preparation, CPC634 particles were allowed to adsorb on glow-discharged formvar-carbon-coated nickel grids (Maxtaform, 200 mesh, Plano, Wetzlar, Germany) for 10 min. Negative staining was performed with 0.5% uranyl acetate (Science Services GmbH, Munich, Germany). Samples were examined using a TEM LEO 906 (Carl Zeiss, Oberkochen, Germany), operating at an acceleration voltage of 60 kV. Particle size was determined from TEM image using ImageSP software (SYSPROG, Belarus).
2.7. Drug retention and drug release kinetics analysis
The amounts of docetaxel (DTX) and 7-epi-DTX (epimer of docetaxel) in aqueous dispersions of CPC634 was determined by ultra-performance liquid chromatography (UPLC). Drug release kinetics analysis was performed according to a previously established protocol [7]. Release of DTX from CPC634 was studied in phosphate buffer (100 mM, pH 7.4), containing 33 mM NaCl and 1% polysorbate 80 (v/v) (to solubilize released DTX and 7-epi DTX) at 37 °C. CPC634 dispersion was incubated at 37 °C and the samples were collected at different time points and analyzed for released DTX using UPLC. The concentration of released DTX and 7-epi DTX was determined by injecting 7 μL of the mixture into a UPLC system (Waters, USA) equipped with an ultraviolet/visible light detector (DAD, Waters). An Acquity HSS T3 1.8 μm, 2.1 x 50 mm column (Waters) was used with a gradient from 100% eluent A (70% H2O/30% ACN/0.1% formic acid %v/v/v) to 100% B (10% H2O/90% ACN/0.1% formic acid % v/v/v) in 14 min with a flow rate of 0.7 mL/min and UV detection at 227 nm. DTX standards dissolved in 70% v/v ACN at a concentration of 50 μg/mL were used for quantification. Released DTX was calculated based on total DTX levels present in the formulation. The latter was determined by a separate assay which is based on full conversion of DTX present in CPC634 into a stable final degradation product benzoic acid under strongly basic conditions followed by chromatographic separation and UV detection.
2.8. statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). The two-tailed student’s t test and the Mann-Whitney U test were used for the analysis. A p-values of <0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Glass transition temperatures (Tg) and freeze-drying of CPC634
CPC634 micelles were freeze-dried using sucrose and trehalose as cryoprotectant, both at 10 wt%. DSC analysis of the resulting dispersions was done to determine the Tg of the systems since this is an important parameter for the development of the desired freeze-dried product. Especially for nanoformulations, carrying out the primary drying at temperatures above the Tg can result in structural deformation and/or aggregation as a result of defects in the freeze-dried cake [14, 24].
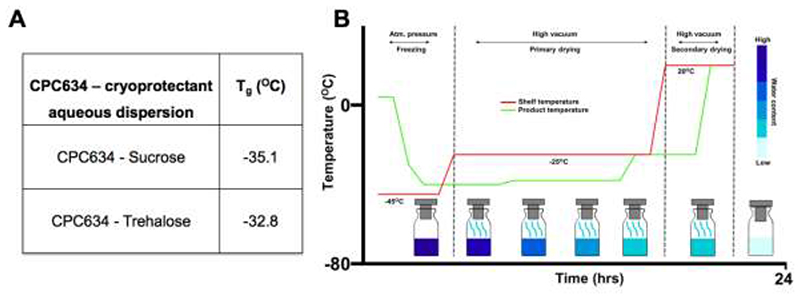
Fig. 2A shows Tg values of CPC634-cryoprotectant dispersions obtained by DSC heat/cool/heat analysis. The Tg of the CPC634-trehalose dispersion was slightly higher than that of the CPC634-sucrose dispersion (-32.8 and -35.1 °C, respectively). As illustrated in Fig. 2B, a conventional three-step freeze-drying procedure, i.e. freezing, primary drying and secondary drying, using a Lyoph-Pride 03 pilot scale shelf freeze-dryer was employed. Based on the obtained Tg values, specifically considering CPC634-trehalose Tg, the vacuum pressure and shelf temperature was adjusted to keep the product temperature below the Tg of the frozen dispersions during early hours of primary drying (Fig. 2B).
Figure 2.
Glass transition temperatures of the CPC634 - cryoprotectant dispersions and freeze-drying cycle. The table shows the Tg values of CPC634 with 10 wt% sucrose and trehalose as cryoprotectants (A). Schematic representation of the freeze-drying cycles indicating duration, vacuum pressure, shelf temperature, product temperature and water content in the obtained cakes, i.e. freezing, primary drying and secondary drying (B).
Based on the freeze-drying cycle optimization experiments, it was found that primary drying works best at a chamber pressure 0.3 mbar and at a shelf temperature -25 °C, applied for 17 hours. The endpoint of primary drying is ideally decided when decline in chamber vapor pressure reached the lowest possible level and when product temperature reaches that of the shelf [31]. Vapor pressure is usually measured by a built-in pirani gauge sensor, but since the freeze-dryer did not have such a sensor, the overlap of product and shelf temperature were considered as an endpoint of the primary drying. Secondary drying was carried out by ramping up the shelf temperature to 20 °C for 4 hours, to remove bound water molecules from the CCPM cake. The endpoint of the secondary drying was also determined based on time point where both product as well as shelf temperature were the same.
3.2. Effect of freeze-drying on CPC634 size, PDI and morphology
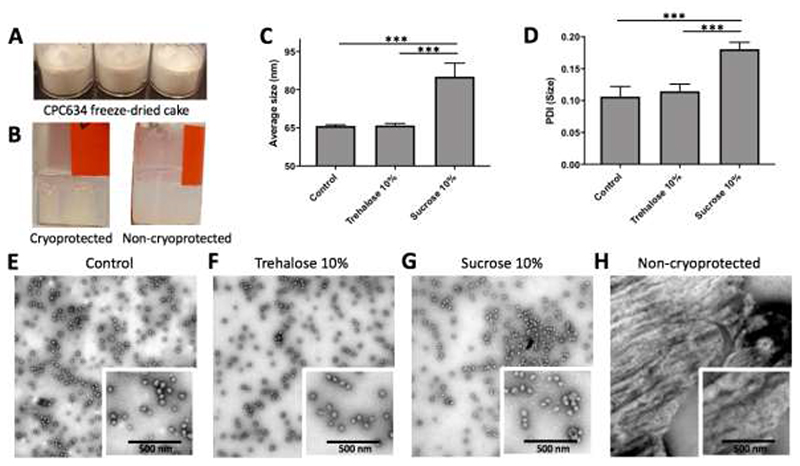
The freeze-dried CPC634-trehalose, CPC634-sucrose and non-cryoprotected CPC634 cakes were well-defined with no obvious defects (cracks, shrinking) (Fig. 3A). The samples were reconstituted with 2 ml of 0.9% NaCl solution without applying any external force, such as shaking or vortexing. The freeze-dried cakes cryoprotected with trehalose and sucrose contained 1.46 wt% and 1.53 wt% residual moisture, respectively. After reconstitution, the non-cryoprotected CPC634 sample was turbid with visible precipitates (Fig. 3B), demonstrating that the use of a cryoprotectant is required. In comparison, reconstitution of freeze-dried CPC634-trehalose and CPC634-sucrose samples was completed in less than 5 minutes and in 15 minutes, respectively. In both cases, the reconstituted dispersions were opalescent and homogenous with a pH of 5.1, alike the aqueous formulations before freeze-drying (Fig. 3B).
Figure 3.
Analysis of freeze-dried cake appearance, and size, PDI and CPC634 morphology before and after freeze-drying/reconstitution. Representative image showing optimal freeze-dried cake morphologies of CPC634 cryoprotected with trehalose and sucrose and non-cryoprotected CPC634, with no signs of cracks or shrinkage (A). Reconstituted CPC634 cryoprotected with trehalose and sucrose were opalescent. Reconstituted CPC634 without cryoprotectant was turbid even after 60 minutes (B). DLS measurements showing that CPC634 cryoprotected with trehalose was similar to the parental control formulation, whereas a significant increase in the mean size and PDI was observed for reconstituted CPC634 cryoprotected with sucrose (C, D). Values represent average ± SD of four different batches, all measured in triplicates. *** indicates p < 0.005. Representative TEM images (E-H) of non-freeze-dried CPC634 aqueous control formulation, and of reconstituted CPC634 cryoprotected with trehalose, sucrose and non-cryoprotectant.
DLS measurements showed that after reconstitution there was no change in the mean size, size distribution and PDI of CPC634 cryoprotected with 10 wt% trehalose when compared to the non-freeze-dried particles (Fig. 3C, Fig. S2A and Fig. 3D). Conversely, a statistically significant increase in the mean size (from 65 to 85 nm) and PDI (from 0.10 to 0.18) of CPC634 cryoprotected with sucrose 10 wt% was observed (Fig. 3C, D). Similarly, DLS size distribution analysis indicated increase in size of CPC634 cryoprotected with sucrose 10 wt% (Fig. S2A). Due to substantial aggregation, DLS measurements were not performed on the reconstituted non-cryoprotected micelles.
The TEM images of Fig. 3E-H illustrated the morphology of a non-freeze-dried CPC634 control formulation, reconstituted CPC634 cryoprotected with trehalose and sucrose, as well as reconstituted non-cryoprotected CPC634. In line with the DLS findings, the TEM images and size analysis clearly demonstrated that the mean size, size distribution and shape of trehalose protected CPC634 were identical to those of the parental non-freeze-dried control formulation (Fig. 3C, 3F and Fig. S2A, C). Analogously, the TEM images of CPC634 cryoprotected with sucrose showcased the increase in the mean size and size distribution which was also observed using DLS (Fig. 3G and Fig. S2C). The TEM images of non-cryoprotected CPC634 showed the formation of large aggregates of the CPC634 micelles.
Together, these results demonstrate that 10 wt% trehalose efficiently preserves the structural integrity of CPC634 during freeze-drying while 10 wt% sucrose was found to be less suitable for the freeze-drying of CPC634. This could be due to the fact that trehalose decreases water crystallization better than sucrose by having greater destructing effect on the tetrahedral structure of water than sucrose [32, 33]. Furthermore, the primary freeze-drying settings (i.e. vacuum pressure and shelf temperature) used in our experiments together with the lower Tg of sucrose may have resulted in less uniform vitrification during freeze-drying, as the product temperature was very close to the Tg of sucrose, which could have led to the aggregation of micelles [34].
3.3. Effect of CPC634 freeze-drying on drug retention and release kinetics
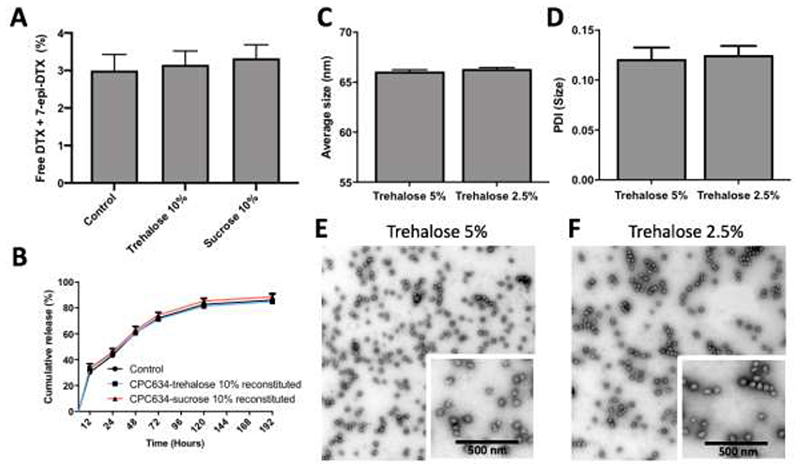
Freeze-drying can affect the drug retention of nanomedicines by causing physical and mechanical stress. Since premature drug release of nanomedicine formulations is unwanted, the so-called burst release was quantified. The amounts of free docetaxel (DTX) plus 7-epi-DTX (epimer of DTX, its main degradation product in aqueous solutions [35, 36]) in reconstituted CPC634 cryoprotected with trehalose and sucrose was determined using ultra-performance liquid chromatography (UPLC) [7]. Furthermore the in vitro drug release behavior of reconstituted CPC634 was evaluated under physiological conditions up to 192 h. Fig. 4A clearly demonstrates that the free DTX plus 7-epi-DTX content was identical in reconstituted CPC634 cryoprotected with either trehalose or sucrose compared to non-freeze-dried parental control CPC634. In line with this, investigation of the in vitro DTX release kinetics under physiological conditions (pH 7.4, 37 °C) revealed that freeze-drying with trehalose and sucrose had not affected the drug release kinetics of CPC634 compared to the parental control formulation (Fig. 4B).
Figure 4.
Analysis of drug retention and in vitro drug release kinetics, as well as size, PDI and morphology upon using low amounts of cryoprotectants. The amount of free DTX plus 7-epi-DTX in reconstituted CPC634 cryoprotected with 10 wt% trehalose and sucrose as compared to non-freeze-dried/parental CPC634 control (A). DTX release profile of parental CPC634 formulation (control) and reconstituted CPC634 cryoprotected with trehalose and sucrose under physiological conditions (B). Sizes and PDI of reconstituted CPC634 cryoprotected with trehalose 5 wt% and 2.5 wt%, determined by DLS analysis (C, D). Values represent average ± SD of three different batches, measured in triplicates. Representative TEM images (E, F) of reconstituted CPC634 cryoprotected with 5 wt% and 2.5 wt% trehalose.
Together, these findings demonstrate that CPC634 drug retention and drug release kinetics are not affected by freeze-drying when cryoprotected with either trehalose 10 wt% or sucrose 10 wt%. CPC634 cryoprotected with sucrose showed a slight increase in size and PDI (Fig. 3), but the freeze-drying process did not impact drug release kinetics given the latter is driven by chemical hydrolysis of the ester linkage between docetaxel and the CCPM. Conversely, freeze-drying of several nanoformulations incorporating physically bound drug showed not only increased size and PDI, but also noticeable drug leakage and altered (faster) drug release profile [37–39]. In contrast to these physical assemblies, CPC634 is a core crosslinked nanoparticle with covalent docetaxel entrapment with initial structural integrity and a well-defined biodegradable profile over time under physiological conditions. The findings of figure 4B indicate that core-crosslinking of CPC634 prevented drug leakage. More specifically, during the freeze-drying process, the low moisture content and temperature prevented premature hydrolysis of the ester bond linking DTX and CCPM core.
3.4. CPC634 freeze-drying using lower cryoprotectant amounts
The above results demonstrate better performance of trehalose over sucrose in protecting CPC634 during freeze-drying when applied at an amount of 10 wt%. Considering this outcome, in the next step the feasibility of CPC634 freeze-drying with lower amounts of trehalose was studied in order to reduce excipient amount. Therefore, CPC634 was freeze-dried using 5 and 2.5 wt% of trehalose. Readouts upon reconstitutions were CPC634 size, PDI and morphology, as assessed by DLS and TEM. As shown in Fig. 4C, Fig. S2A and Fig. 4D, the mean size (65 nm), size distribution and PDI (0.1) of CPC634 cryoprotected with 5 wt% and 2.5 wt% of trehalose were similar to that of non-freeze-dried CPC634. In line with this, the TEM images and particle size analysis in Fig. 4E, 4F and Fig. S2C show that 5 wt% and 2.5 wt% of trehalose efficiently cryoprotected CPC634, retaining a morphology and size identical to non-freeze-dried parental CPC634 (Fig. 3E and Fig. S2C). The CPC634 freeze-dried cake cryoprotected with 5 wt% and 2.5 wt% of trehalose had a residual moisture content of 1.8 ± 0.4 wt%, and could be successfully reconstituted within 5 minutes with a pH of 5.1, similar to the parental control formulation. These findings indicate that trehalose even at an amount as low as 2.5 wt% can provide proper protection of CCPM during freeze-drying. The drug content and in vitro drug release kinetics of these samples were not analyzed as no substantial changes in their mean size and PDI were observed compared to parental control formulation.
3.5. Towards industrial freeze-drying of CPC634
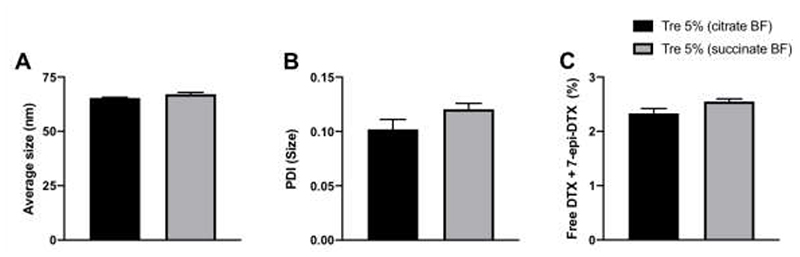
The freeze-drying process was optimized with the aim to further reduce the moisture content of the CPC634 freeze-dried cake to further slowdown possible hydrolysis of ester bond between DTX and core of CPC634 upon long term storage. This was carried out using an advanced freeze-dryer equipped with a pirani gauge sensor, a Baratron® capacitance manometer for vapor pressure analysis, and a product temperature sensor. To start with, cryoprotectant of the median concentration, i.e. 5 wt% trehalose, was selected from the abovementioned results. In this setting, the effect of commercially suitable buffer systems (20 mM succinate and citrate buffer systems, pH 5.0) were studied on CPC634 freeze-drying. In line with the above findings, Fig. 5A, Fig. S2B and Fig. 5B clearly show that the mean size, size distribution and PDI of CPC634 in the citrate and succinate buffer cryoprotected with 5 wt% trehalose were similar to those of non-freeze-dried control formulation.
Figure 5.
CCPM freeze-drying in an industrial setting. Size (A), PDI (B) and free drug content (C) of freeze-dried CPC634 cryoprotected with 5 wt% trehalose in citrate and succinate buffer systems. Values represent average ± SD of five different batches, all measured in triplicates.
In parallel, we investigated the effect of freeze-drying on DTX retention in CPC634 cryoprotected with 5 wt% trehalose in succinate and citrate buffer systems. As shown in Fig. 5C, the amount of free DTX plus 7-epi-DTX had not changed after freeze-drying when cryoprotected with trehalose in citrate as well as succinate buffer.
The residual water content was found to be 0.055 wt% and 0.060 wt% for CPC634 freeze-dried cakes cryoprotected with trehalose 5 wt% in combination with citrate and succinate buffer, respectively. Reconstitution analysis showed that reconstitution was 15 folds faster for the CPC634 freeze-dried cake cryoprotected with trehalose in citrate buffer (2 min) compared to succinate buffer (30 min). This could be due to elevated water penetration resistance in CPC634 freeze-dried cakes consisting trehalose and succinate buffer compared to CPC634 freeze-dried cakes consisting trehalose and citrate buffer. Decrease in the overall pore size and pore volume of freeze-dried cake can drastically delay reconstitution by impeding water penetration [40]. Crystallization of buffer salts during freeze-drying decreases the pore size and volume of the freeze-dried cake thus, hindering water penetration [41, 42]. In this regard, succinate buffer shows higher crystallization compared to citrate buffer after freeze-drying even in presence of trehalose at acidic pH [41, 43, 44]. The reconstituted CPC634 cryoprotected with 5 wt% trehalose in combination with citrate (pH 5.1) and succinate (pH 5.0) buffer systems also showed identical pH as their respective non-freeze-dried parental control formulations.
Overall, these findings indicate that trehalose efficiently provides protection to CPC634 during freeze-drying when used with citrate buffer (pH 5.0). Further, our findings suggest that buffers play a crucial role in freeze-drying and that they can influence the outcome such as size and reconstitution time. The use of a more advanced shelf freeze-dryer at the industrial setting convincingly provided a well-controlled freeze-drying protocol and freeze-dried products with lower moisture content and shorter reconstitution time. These outcomes provide high confidence in further large-scale manufacturing development and optimal storage stability of CPC634 and other CriPec® based freeze-dried products.
4. Conclusion
Our study exemplifies the feasibility of freeze-drying drug-entrapped CCPM upon the use of trehalose as a cryoprotectant. The lyophilized material was rapidly reconstitutable with unaltered pharmaceutical features such as size, PDI, drug retention and drug release kinetics. Via additional validation in an industrial setting, our findings support large-scale development and future commercialization of freeze-dried CriPec®-based CCPM drug products.
Supplementary Material
5. Acknowledgments
This research is funded by the European Union (H2020-MSCA-ITN-2014 642028: NABBA and ERANET-EuroNanoMed-III: NSC4DIPG), by the German Research Foundation (DFG: GRK2375 (#331065168), SFB1066 and LA2937/4-1), by the European Research Council (ERC Consolidator Grant Meta-Targeting (#864121)), and by the Phospholipid Research Center (Heidelberg, Germany; RBA-2019-076/1-1).
Footnotes
Conflict of Interest
Cristal Therapeutics supplied test materials, performed partial analytical characterization and covered external CDMO cost for the conducted study. Cristianne J. F. Rijcken, Qizhi Hu, Claudio Colombo and Michiel van Geijn were employees of Cristal Therapeutics during the conduct of the study. Jan Wit provided paid pharmaceutical consultancy to Cristal Therapeutics and contributed to the conducted study.
References
- 1.Shi Y, Lammers T, Storm G, Hennink WE. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromolecular Bioscience. 2017;01:1600160. doi: 10.1002/mabi.201600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chemical Reviews. 2018;06:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 3.Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, et al. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;02:93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng C, Jiang Y, Cheng R, Meng F, Zhong Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today. 2012;10:467–480. [Google Scholar]
- 5.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2011;03:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Hu Q, Rijcken CJ, van Gaal E, Brundel P, Kostkova H, et al. Tailoring the physicochemical properties of core-crosslinked polymeric micelles for pharmaceutical applications. Journal of Controlled Release. 2016;12:314–325. doi: 10.1016/j.jconrel.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Hu Q, Rijcken CJ, Bansal R, Hennink WE, Storm G, et al. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials. 2015;06:370–378. doi: 10.1016/j.biomaterials.2015.02.085. [DOI] [PubMed] [Google Scholar]
- 8.Rijcken CJ, Snel CJ, Schiffelers RM, van Nostrum CF, Hennink WE. Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterisation and in vivo studies. Biomaterials. 2007;12:5581–5593. doi: 10.1016/j.biomaterials.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Talelli M, Rijcken CJ, Hennink WE, Lammers T. Polymeric micelles for cancer therapy: 3 C’s to enhance efficacy. Current Opinion in Solid State and Materials Science. 2012;12:302–309. [Google Scholar]
- 10.Crielaard BJ, Rijcken CJ, Quan L, Van Der Wal S, Altintas I, et al. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angewandte Chemie International Edition. 2012;07:7254–7258. doi: 10.1002/anie.201202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Van Gaal EV, Brundel P, Ippel H, Hackeng T, et al. A novel approach for the intravenous delivery of leuprolide using core-cross-linked polymeric micelles. Journal of Controlled Release. 2015;04:98–108. doi: 10.1016/j.jconrel.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov. [accessed: April 2016]; clinicaltrials.gov/ct2/show/NCT02442531?term=NCT02442531&rank=1. [Google Scholar]
- 13.Ojha T, Pathak V, Drude N, Weiler M, Rommel D, et al. Shelf-Life Evaluation and Lyophilization of PBCA-Based Polymeric Microbubbles. Pharmaceutics. 2019;09:433. doi: 10.3390/pharmaceutics11090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Advanced Drug Delivery Reviews. 2006;12:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Talsma H, Cherng JY, Lehrmann H, Kursa M, Ogris M, et al. Stabilization of gene delivery systems by freeze-drying. International Journal of Pharmaceutics. 1997;11:233–238. doi: 10.1016/s0378-5173(97)00244-5. [DOI] [PubMed] [Google Scholar]
- 16.Gray SA, Coler RN, Carter D, Siddiqui AA. Progress in molecular biology and translational science. Elsevier; 2016. Translational Activities to Enable NTD Vaccines. [DOI] [PubMed] [Google Scholar]
- 17.Miyata K, Kakizawa Y, Nishiyama N, Yamasaki Y, Watanabe T, et al. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. Journal of Controlled Release. 2005;12:15–23. doi: 10.1016/j.jconrel.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Lee SW, Kim YM, Cho CH, Kim YT, Kim SM, et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3021) Cancer Research and Treatment: official Journal of Korean Cancer Association. 2018;01:195. doi: 10.4143/crt.2016.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Advanced Drug Delivery Reviews. 2008;04:899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. Journal of Controlled Release. 2010;03:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Van Winden ECA, Crommelin DJA. Long term stability of freeze-dried, lyoprotected doxorubicin liposomes. European Journal of Pharmaceutics and Biopharmaceutics. 1997;06:295–307. [Google Scholar]
- 22.Cherng JY, Van de Wetering P, Talsma H, Crommelin DJ, Hennink WE. Freezedrying of poly ((2-dimethylamino) ethyl methacrylate)-based gene delivery systems. Pharmaceutical Research. 1997;12:1837–1840. doi: 10.1023/a:1012164804441. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Anchordoquy TJ. Effects of moisture content on the storage stability of dried lipoplex formulations. Journal of Pharmaceutical Sciences. 2009;09:3278–3289. doi: 10.1002/jps.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang XC, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharmaceutical research. 2004;02:191–200. doi: 10.1023/b:pham.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 25.Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WL. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. European Journal of Pharmaceutics and Biopharmaceutics. 2017;04:288–295. doi: 10.1016/j.ejpb.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophysical Journal. 1996;10:2087–2093. doi: 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsinontides SC, Rajniak P, Pham D, Hunke WA, Placek J, et al. Freeze drying — principles and practice for successful scale-up to manufacturing. International Journal of Pharmaceutics. 2004;08:1–16. doi: 10.1016/j.ijpharm.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Fonte P, Reis S, Sarmento B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. Journal of Controlled Release. 2016;03:75–86. doi: 10.1016/j.jconrel.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Horn J, Friess W. Detection of collapse and crystallization of saccharide, protein, and mannitol formulations by optical fibers in lyophilization. Frontiers in Chemistry. 2018;01:4. doi: 10.3389/fchem.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talelli M, Rijcken CJ, Oliveira S, van der Meel R, en Henegouwen PMVB, et al. Reprint of” Nanobody—Shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting”. Journal of Controlled Release. 2011;07:93–102. doi: 10.1016/j.jconrel.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Patel SM, Doen T, Pikal MJ. Determination of end point of primary drying in freeze-drying process control. Aaps Pharmscitech. 2010;03:73–84. doi: 10.1208/s12249-009-9362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson C, Swenson J. Structural Comparison between Sucrose and Trehalose in Aqueous Solution. The Journal of Physical Chemistry B. 2020;03:3074–3082. doi: 10.1021/acs.jpcb.9b09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellavia G, Cottone G, Giuffrida S, Cupane A, Cordone L. Thermal Denaturation of Myoglobin in Water- Disaccharide Matrixes: Relation with the Glass Transition of the System. The Journal of Physical Chemistry B. 2009;08:11543–11549. doi: 10.1021/jp9041342. [DOI] [PubMed] [Google Scholar]
- 34.Jain NK, Roy I. Effect of trehalose on protein structure. Protein Science. 2009;01:24–36. doi: 10.1002/pro.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manjappa AS, Goel PN, Vekataraju MP, Rajesh KS, Makwana K, et al. Is an alternative drug delivery system needed for docetaxel? The role of controlling epimerization in formulations and beyond. Pharmaceutical Research. 2013;10:2675–2693. doi: 10.1007/s11095-013-1093-5. [DOI] [PubMed] [Google Scholar]
- 36.Kumar D, Tomar RS, Deolia SK, Mitra M, Mukherjee R, et al. Isolation and characterization of degradation impurities in docetaxel drug substance and its formulation. Journal of Pharmaceutical and Biomedical Analysis. 2007;03:1228–1235. doi: 10.1016/j.jpba.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 37.de Chasteigner S, Cavé G, Fessi H, Devissaguet JP, Puisieux F. Freeze-drying of itraconazole-loaded nanosphere suspensions: a feasibility study. Drug Development Research. 1996;06:116–124. [Google Scholar]
- 38.Fonte P, AraÚjo F, Seabra V, Reis S, van de Weert M, et al. Co-encapsulation of lyoprotectants improves the stability of protein-loaded PLGA nanoparticles upon lyophilization. International journal of Pharmaceutics. 2015;12:850–862. doi: 10.1016/j.ijpharm.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Fonte P, Soares S, Costa A, Andrade JC, Seabra V, et al. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter. 2012;10:329–339. doi: 10.4161/biom.23246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beech KE, Biddlecombe JG, Van Der Walle CF, Stevens LA, Rigby SP, et al. Insights into the influence of the cooling profile on the reconstitution times of amorphous lyophilized protein formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2015;10:247–254. doi: 10.1016/j.ejpb.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Pikal MJ. Freeze-drying of mannitol-trehalose-sodium chloride-based formulations: The impact of annealing on dry layer resistance to mass transfer and cake structure. Pharmaceutical Development and Technology. 2004;01:85–95. doi: 10.1081/pdt-120027421. [DOI] [PubMed] [Google Scholar]
- 42.Shalaev EY, Gatlin LA. Formulation and process development strategies for manufacturing biopharmaceuticals. John Wiley & Sons; 2010. The Impact of Buffer on Solid-State Properties and Stability of Freeze-Dried Dosage Forms. [Google Scholar]
- 43.Desu HR, Narishetty ST. Sterile Product Development. Springer; New York: 2013. Challenges in freeze-thaw processing of bulk protein solutions; pp. 167–203. [Google Scholar]
- 44.Sundaramurthi P, Suryanarayanan R. The effect of crystallizing and non-crystallizing cosolutes on succinate buffer crystallization and the consequent pH shift in frozen solutions. Pharmaceutical Research. 2011;02:374–385. doi: 10.1007/s11095-010-0282-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.