Abstract
Twenty-five years after the approval of the first anticancer nanodrug, we have to start re(de)fining tumor-targeted drug delivery alongside advances in immuno-oncology. Because cancer is characterized by an immunological imbalance that goes beyond the primary tumor, we should focus on targeting, engaging and modulating cancer-associated immune cells in the tumor microenvironment, in the circulation and in immune cell-enriched tissues. When designed and applied rationally, nanomedicines will assist in restoring the immunological equilibrium at the whole-body level, which holds potential for cancer therapy, but also for the treatment of a wide range of other disorders.
Keywords: Nanomedicine, Immunotherapy, Tumor microenvironment, Immune Cell Targeting, Drug Targeting, Cancer, Drug Delivery
Introduction
Cancer nanomedicine still focuses primarily on delivering chemotherapeutic drugs directly to cancer cells (Table 1). With this perspective, we aim to contribute to a paradigm shift in which nanoparticles are no longer packed with chemotherapeutics for the direct eradication of cancer cells, but instead loaded with immunomodulatory agents and targeted to cancer-associated immune cells. We describe various possibilities for in vivo immune cell targeting (in contrast to their ex vivo manipulation), and propose to target immune cell populations inside and outside the tumor microenvironment. Preclinical as well as clinically already translated nanoparticles can be re-purposed in such endeavor since they can prolong the circulation half-life of therapeutic agents and shift their biodistribution profile towards target tissues and/or cells [1–4].
Table 1. Nanomedicine publication trends.
| Scopus search on 2021.08.01 | Number of studies per year | ||||
|---|---|---|---|---|---|
| Terms in “title, abstract, keywords” | 2016 | 2017 | 2018 | 2019 | 2020 |
| “nanomedicine” AND “chemotherapy” | 276 | 295 | 370 | 416 | 470 |
| “nanomedicine” AND “immunotherapy” | 67 | 74 | 128 | 177 | 247 |
| “nanomedicine” AND “immunomodulation” | 27 | 37 | 27 | 38 | 42 |
Traditional tumor targeting in nanomedicine
As mentioned above, the cancer nanomedicine field has historically invested heavily in directly killing cancer cells. The hope behind this traditional approach is that nanomedicines improve the accumulation of chemotherapeutic agents in tumor tissue and limit their off-target localization, which ultimately increases the efficacy-to-toxicity ratio [5]. The common consideration in this regard is that nanomedicine formulations preferentially accumulate in cancerous lesions based on enhanced vascular leakiness and defective lymphatic drainage associated with solid malignancies [6,7]. In such cases, a high degree of intra-tumor accumulation is clearly the desired outcome. In many situations, however, the injected nanomedicine dose that reaches the tumor is low, averaging between 0.1 and 10% of the injected dose, both in animal models and in patients [8]. This notion implies that 90 to 99.9% of the injected nanodrug dose ends up in organs and tissues other than the tumor (e.g., liver and spleen), or is rapidly cleared. In addition, significant heterogeneity in tumor accumulation is observed, both within a tumor in an individual patient as well as between different tumors in different patients. This complicates clinical translation [9,10].
Targeting the immune system with nanomedicines
Even with targeting capabilities increased beyond 10%, nanomedicine treatment cannot guarantee improved therapeutic responses in cancer patients. This is particularly true when nanodrugs are used as monotherapies. Remission is often temporary and typically followed by relapse resulting from the re-establishment of pro-tumorigenic conditions, e.g., by progenitor immune cells [11] or by re-activation of cancer stem cells [12]. In such situations, we consequently often end up in a continuous vicious circle of ‘detection, treatment, response, and relapse’. This implies that truly curative anticancer therapy requires more holistic treatment concepts which not only include direct eradication of cancer cells by chemotherapeutic agents, but also modulation of cancer growth-promoting phenomena that occur inside and outside of tumor tissue, involving - most importantly - the immune system. In this context, it is crucial to understand that the long circulation properties that the various nanomedicines possess upon systemic administration will benefit their accumulation in anticipated target organs by avoiding rapid clearance by phagocytes in liver and spleen [13]. Nanomedicine formulations used for immune cell targeting must therefore be able to evade clearance from the blood stream, they must be easily functionalizable with targeting moieties and they should be loadable with different types of payloads. These features profit from the notion that nanomedicines are a very versatile and readily available toolbox in comparison to other (bio)technological tools; conventional antibodies, for instance, can only be directed against one therapeutic target, and microscale drug delivery systems do not possess long-circulation properties.
Targeting immune cells in the tumor microenvironment
The immune system strongly affects tumorigenesis and malignant disease progression. This notion has resulted in the development of “nano” concepts aimed at targeting and modulating the tumor immune micro-environment (TIME; [14]) to promote immune-cell mediated anticancer responses (Fig. 1 and Table 2) [15–17]. First results have been very encouraging. For example, polymeric nanoparticles loaded with a TLR7/8 agonist and TGF-β inhibitor, were functionalized with CD8a and PD-1 to target intratumoral PD-1+ or CD8+ cytotoxic T cells. Specific delivery of immunomodulatory cargo to these cells primed the cytotoxic activity of CD8+ T cells in the tumor, allowed for enhanced infiltration of these cells, and sensitized the TIME for better response to subsequent antibody-based anti-PD-1 immunotherapy [18]. Another interesting example is targeting FoxP3+ regulatory T cells with hybrid polymeric-lipid NPs surface-functionalized with the peptide tLyp1. These NPs were loaded with the kinase inhibitor imatinib, to inhibit regulatory T cells in the TIME. Combining them with anti-CTLA-4 antibodies reduced the numbers of intratumoral regulatory T cells and elevated the number of cytotoxic CD8+ T cells [19]. Similar conceptual approaches have been conceived for modulating myeloid cells inside the TIME. These have e.g., included the design of β-cyclodextrin NPs containing TLR7/8 agonists [20], as well as of liposomes functionalized with a CD206 targeting ligand loaded with CSF1R and SHP2 inhibitors [21], with the aim of exploiting the phenotypic plasticity of macrophages [22] and induce their polarization from a immuno-suppressive M2-like towards a tumor-suppressive M1-like phenotype. This phenotypic change enhances the phagocytic capability of macrophages, helps to control tumor growth, and protects against tumor re-challenge. Macrophage polarization and TIME modulation can also be achieved through NP-mediated genetic reprogramming. Loading polymeric NPs with mRNA encoding for the M1-inducing proteins IRF5 and IKKβ and targeting of these NPs to M2-like macrophages induced their re-polarization toward an anti-tumor phenotype in three different tumor models [23]. In animal models, this approach showed an efficacy not only against primary tumors but also against metastasis, and the NPs further proved to be active in reprogramming human macrophages.
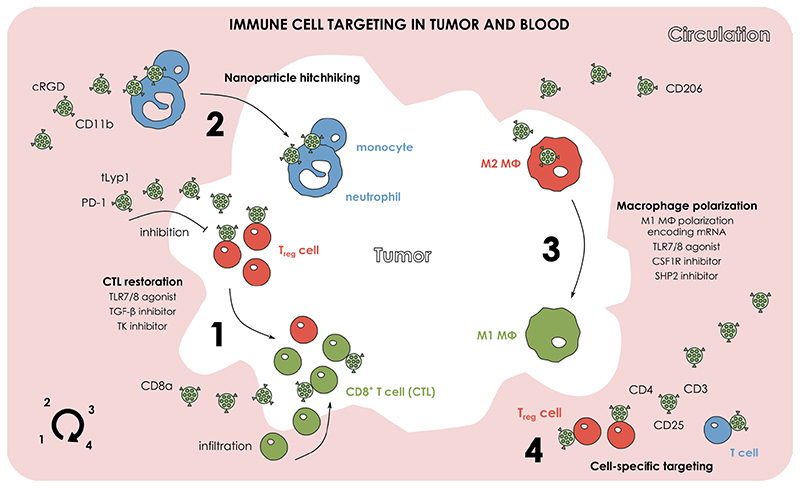
Figure 1. Nanoparticle targeting of the immune system in tumor and blood.
Nanotherapies can aim for the holistic manipulation of the immune system. (1) Nanoparticles (NP) can help to inhibit regulatory T cells, elicit a strong CD8+ T cell infiltration and consequently restore the cytotoxic T lymphocyte (CTL) populations in the tumor microenvironment (TME). (2) NPs can exploit the inherent tumor-homing capabilities of myeloid immune cells and be delivered to tumors via immune cell hitchhiking. (3) NPs can be used for directly inhibiting the activity of M2-like macrophages in the TME, and deliver immunomodulatory cargo that can polarize M2-like towards M1-like macrophages. (4) NPs can be specifically modified and target or inhibit specific immune cell sub-populations in circulation.
Table 2. Nanomedicines targeting immune cells in tumor, blood, and immune cell-enriched organs.
Selected representative publications demonstrate the use of nanoparticles for immunomodulatory, nanoparticle-mediated transportation, and immune cell tracking purposes in cancer and inflammatory diseases.
| Nanoparticle | Targeting decoration | Payload | Payload function | Tissue target | Cell target | Major outcome | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | PLGA-based polymeric micelle | CD8a PD-1 | R848 | TLR7/8 agonist | Tumor Blood Spleen LN | CD8+ T cell PD-1+ T cell | ↑ survival ↑ tumor-infiltrating CD8+ T cells sensitizing tumors to anti-PD-1 therapy | [18] |
| SD-208 | TGF-β inhibitor | |||||||
| 2 | PLGA-lipid hybrid nanoparticle | tLyp1 | Imatinib | Tyrosine kinase inhibitor | Tumor | FoxP3+ Treg cell | ↑ survival ↑ tumor inhibition ↓ FoxP3+ Treg cell ↑ CD8+ T cell potentiated anti-CTLA-4 therapy | [19] |
| 3 | β-cyclodextrin nanoparticle | - | R848 | TLR7/8 agonist | Tumor | M2 macrophage | polarized M2 → M1 macrophages controlled tumor growth protected against tumor re-challenge potentiated anti-PD-1 therapy | [20] |
| 4 | Liposome | CD206 | BLZ945 | CSF1R inhibitor | Tumor | M2 macrophage | polarized M2 → M1 macrophages ↑ phagocytic capabilities ↑ anti-tumor efficacy | [21] |
| SHP099 | SHP2 inhibitor | |||||||
| 5 | Polymeric nanoparticle | CD206 | IRF5 mRNA IKKβ mRNA | Encoding M1 macrophage polarization factors | Tumor | M2 macrophage | polarized M2 → M1 macrophages ↑ survival ↑ T cell infiltration control tumor metastases potential to reprogram human macrophages | [23] |
| 6 | Liposome Nanoemulsion | cRGD | (for imaging purposes) | Blood | Neutrophils Ly6C- monocytes | nanoparticle hitchhiking with phagocytes in breast cancer | [24] | |
| 7 | Liposome | cRGD | Edaravone | Neuroprotective | Blood | Neutrophils Monocytes | nanoparticle hitchhiking with phagocytes in cerebral ischemia ↓ infarcted volume | [26] |
| 8 | Polymeric nanoparticle Gold nanorod | CD11b | Pyropheophorbide-a (not loaded) | Photosensitizer | Blood | Neutrophils | ↑ neutrophil tumor infiltration upon photosensitization ↑ survival ↓ tumor growth | [27] |
| 9 | Liposome | CD3 CD4 CD25 Ly6C | CD45 siRNA | (for testing CD45 silencing) | Blood LN | CD3+ T cells CD4+ T cells CD25+ Treg cells Ly6C+ monocytes | cell-specific siRNA delivery ↓ colitis by targeting Ly6C+ pro-inflammatory monocytes in an IBD model | [28] |
| TNF siRNA | To inhibit the expression of the pro-inflammatory mediator TNFα | |||||||
| 10 | Liposome | Ly6C | IL10 modified mRNA | Expressing the antiinflammatory cytokine IL10 | Spleen | Ly6C+ monocytes | ↑ IL10 in liver, spleen, colon ↓ colitis by targeting Ly6C+ monocytes in an IBD model | [29] |
| 11 | HDL nanoformulation | - | (for imaging purposes) | Spleen BM Blood | Neutrophils Ly6C+ monocytes Ly6C- monocytes | ↑ infiltration of Ly6C+ monocytes in intermediate atherosclerosis ↑ infiltration of neutrophils in advanced stage atherosclerosis Recruitment of myeloid cells in myocardial infarction | [33] | |
| 12 | Lipid nanoparticle | SORT molecules | hEPO mRNA or IL-10 mRNA | To verify the expression of hEPO and IL-10 | Spleen Liver Lung | Macrophages B cells T cells others | Organ-specific targeting Effective mRNA expression in organ specific manner Effective gene editing in organ specific manner | [34] |
| Cre mRNA | To activate tdTom expression | |||||||
| Cas9 protein / sgTom1 | To improve the delivery of Cas9 RNPs | |||||||
| Cas9 mRNA / sgPTEN | To edit PTEN for anti-cancer purposes | |||||||
| Cas9 mRNA / sgPCSK9 | To edit PCSK9 for anti-atherosclerotic purposes | |||||||
| 13 | Lipid nanoparticle | - | gp100 mRNA TRP2 mRNA | Encoding tumor-associated antigens | LN | Dendritic cells Neutrophils Macrophages B cells | Successful anticancer vaccination ↑ cytotoxic CD8+ T cell response ↑ survival ↓ tumor growth | [43] |
| 14 | Polymeric micelle | - | Adpgk | peptide neoantigen | LN | Dendritic cells Macrophages | Successful anti-cancer vaccination ↑ cytotoxic CD8+ T cell response ↑ survival ↓ tumor growth Potentiating anti-PD-1 therapy | [45] |
| R848 | TLR7/8 agonist | |||||||
| CpG | TLR9 agonist | |||||||
| 15 | Liposome | - | gp70 RNA | Encoding endogenous antigen of Moloney murine leukaemia virus | LN BM Spleen Lung | Dendritic cells | Activation of NK, T, B cells ↑ survival ↓ tumor growth Induction of systemic INFα in patients Priming and amplification of T cells against the antigens in patients | [46] |
| OVA RNA | Encoding an ovalbumin epitope expressed in B16F10 cell line | |||||||
| Hemagglutinin RNA | Encoding influenza virus hemagglutinin | |||||||
| NY-ESO-1 RNA MAGE-A3 RNA tyrosinase RNA TPTE RNA | Encoding tumor antigens for clinical use | |||||||
| 16 | Polymer / lipid TransIT reagent | - | CD3 × CLDN6 mRNA CLDN18.2 × CD3 mRNA EpCAM × CD3 mRNA CD3 × (CLDN6)2 mRNA | In vitro-transcribed mRNA encoding bispecific antibodies against T cells, cancer cells, epithelial cells | Liver | T cells | ↑ plasma levels of bi-functional proteins Elimination of advanced xenograft tumors Activated T cells on cell-target specific manner ↑ cytotoxic T cells in tumors | [47] |
Targeting circulating immune cells
Circulating immune cells can recognize and interact with nanomedicine formulations in the bloodstream prior to homing to diseased areas (Fig. 1 and Table 2). For example, investigation of αvβ3-integrin-specific cRGD liposomes and nanoemulsions revealed that in addition to targeting integrins on the tumor endothelium, circulating phagocytes (predominantly neutrophils) take up these NPs and transport them into tumor tissue [24]. This observation rationalizes the use of immune cells as drug delivery vehicles [25]. The interaction of cRGD liposomes with circulating phagocytes also resulted in co-migration into ischemic brain tissue [26], a tissue that is difficult to reach using conventional drug targeting strategies. While the cRGD liposomes in this study were loaded with edaravone (i.e., a neuroprotective agent, aiming to lower infarct volumes), similar approaches can be envisaged for increasing anticancer and/or immunomodulatory drug deposition in the brain. Besides cRGD, circulating neutrophils have also been targeted by decorating polymeric NPs and gold nanorods with anti-CD11b. Subsequent TIME priming via photosensitization, with the photosensitizer Pyropheophorbide-a, resulted in enhanced infiltration of the NP-loaded neutrophils in tumor tissue upon illumination with laser light [27]. Analogously, surface decoration of lipid NPs with monoclonal antibodies resulted in a modular liposome platform suitable for immune cell-specific siRNA delivery [28]. This platform showed impressive versatility in specifically targeting different immune cell populations when functionalized with CD3, CD4, CD25, and Ly6C antibodies. Therapeutically, anti-Ly6C-decorated liposomes loaded with siRNA successfully inhibited the expression of the pro-inflammatory mediator TNFα in Ly6C+ monocytes in a colitis model. The same modular platform was also applied for mRNA delivery in an inflammatory bowel disease model, targeting Ly6C+ monocytes and inducing the expression of the anti-inflammatory interleukin-10 [29]. These examples support the exploration of cancer nanomedicine engineering towards targeting immunomodulatory cargo to circulating immune cells [30]. Furthermore, by determining the composition of tumor-infiltrating immune cells and by exploiting immune cell-NP interactions [24,31,32], immune cells can function as chariots for delivering therapeutic cargo to tumors and metastases.
Targeting myeloid and lymphoid immune cell-enriched tissues
Targeting immune cells entails delivery to tissues enriched in immune cells, such as bone marrow, liver, lymph nodes, and spleen (Fig. 2 and Table 2) [33–36]. Research on the concept to achieve organ-specific targeting has enabled the development of a lipid NP platform that allowed for the targeted delivery of mRNA or gene editing in a tissue-specific manner [34,37]. By capitalizing on the biophysical properties of different lipid components, three major categories of lipid NPs achieved a specific targeting to spleen, liver, or lungs and a direct association of the NPs with residual macrophages, B cells, and T cells. Loading these NPs with various therapeutic RNAs (i.e., hEPO mRNA and IL-10 mRNA) or Cas9 mRNA / sgRNA combinations (i.e., Cas9 mRNA plus sgPTEN, and Cas9 mRNA plus sgPCSK9) demonstrated organ-specific action for applications in inflammatory disease, cancer and atherosclerosis. Such a re-direction may benefit therapeutic approaches focusing on eliminating malignant cells responsible for blood disorders [38], or on reversing the aftermath of such conditions, i.e., bone marrow fibrosis [39], splenomegaly [40], and splenic lymphomas [37].
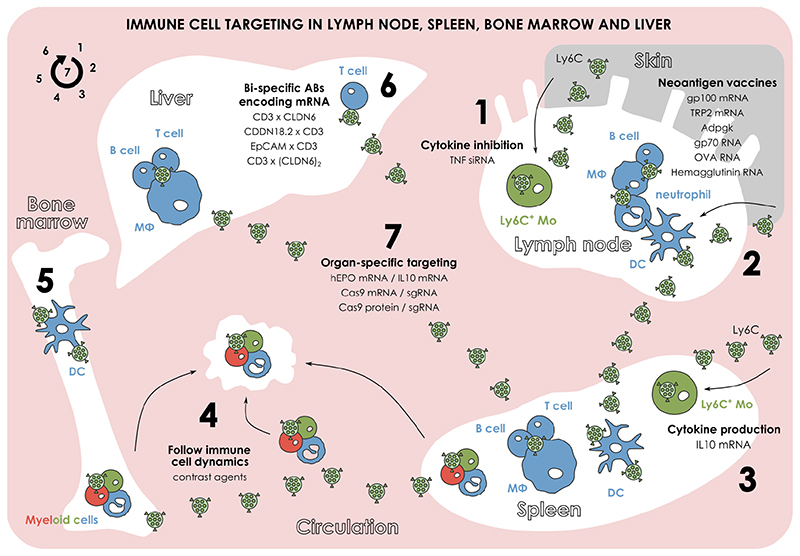
Figure 2. Nanoparticle targeting of the immune system in immune cell-enriched organs.
(1) Subcutaneous administration of nanoparticles (NP) can lead to lymph node targeting. Surface decoration of such NPs can allow for the specific targeting of lymph node resident monocytes and stimulate cytokine inhibition. (2) NPs can deliver neoantigen RNA cargo in specific cells in lymph node and spleen for eliciting a personalized anti-cancer response. (3) Surface decoration of NPs with various motifs can enable the targeting of spleen resident monocytes and the stimulation of cytokine production. (4) NPs functionalized with contrast agents can be used for targeting myeloid cells in various tissues (e.g., bone marrow, spleen) and visualize their transportation to a cancerous or inflammatory lesion. (5) Similarly to the delivery of neoantigen vaccines in lymph node and spleen, targeting of NPs in dendritic cells in the bone marrow can also be used for eliciting anti-cancer responses. (6) NPs can deliver specific antibody-encoding mRNAs in the liver for targeting T cells, cancer, and epithelial cells and eventually stimulating the anti-cancer T cell responses. (7) By synthesizing NPs with desired biophysical characteristics, organ-specific targeting can be achieved and used for delivering RNA therapeutics or applying gene editing.
NP developed for delivering mRNA vaccines also target immune cells [41,42]. A subcutaneously injected lipid NP mRNA vaccine loaded with gp100 mRNA or TRP2 mRNA led to improved mRNA translation in antigen presenting cells, i.e., dendritic cells, neutrophils, macrophages, and B cells. The resulting increased transfection rate in regional lymph nodes elicited a strong CD8+ T cell-mediated response against melanoma [43]. Along the same line of thinking, nanomedicine has also been strongly integrated in neoantigen vaccine approaches [44]. A subcutaneously administered micellar nanovaccine delivered a peptide neoantigen (Adpgk) together with a TLR 7/8 agonist (R848) and a TLR9 receptor agonist (CpG) to immature dendritic cells residing in the lymph nodes [45]. Combining this nanovaccine with anti-PD-1 treatment resulted in regression of Adpgk-positive colorectal cancer. Lymphoid tissues can also be targeted for neoantigen mRNA expression after systemically injecting lipoplexes [46]. Indeed, an intravenously injected lipoplex delivered cancer-specific antigen-encoding RNA (i.e., gp70 RNA, OVA RNA, and hemagglutinin RNA) to dendritic cells in spleen, bone marrow, lymph nodes and lungs, and elicited strong antigen-specific responses against melanoma and colon carcinoma via activation of NK cells, B cells and T cells. Of note, this lipid NP platform constitutes the first nanomedicine-based neoantigen vaccination in clinical evaluation, displaying promising targeting of lymphoid dendritic cells in the spleen, lymph nodes, and bone marrow with various RNAs encoding tumor-specific antigens. Additionally, mRNA-encoding bispecific antibodies can be directed to the liver by employing T cell-specific NPs [47]. These therapeutic NPs were based on a single mRNA strand that produced a combination of antibodies against T cells, cancer cells and epithelial cells (via CD3 x CLDN6 mRNA, CLDN18.2 × CD3 mRNA, EpCAM × CD3 mRNA, and CD3 × (CLDN6)2 mRNA) enabling stable and prolonged production of high plasma levels of bi-functional proteins targeting T cells and activating them in a cell-specific manner [47].
Finally, it’s worth mentioning that NPs have been used as tools for imaging-assisted immune cell tracking. HDL nanobiologics can be taken up by myeloid cells in the spleen and bone marrow, enabling the visualization of myeloid cell dynamics in atherosclerosis and myocardial infarction [33]. The functionalization of these NPs with different contrast agents allowed for the utilization of complementary imaging modalities, which together illustrated distinct immune cell migration patterns at different disease stages. It was for instance found that the infiltration of Ly6C+ monocytes was dominant in intermediate-stage atherosclerosis, while neutrophil infiltration was dominant in advanced-stage atherosclerosis [33].
Benefits of in vivo immune cell targeting
Targeting strategies involving immune cells typically rely on ex vivo methodology. For example, ex vivo decoration of monocytic myeloid cells with interferon-γ immunomodulatory “backpacks” polarized these cells towards an M1-like anti-tumor phenotype [48]. Their subsequent intratumoral injection not only preserved this phenotype, but remarkably also polarized neighboring tumor-associated macrophages toward an M1-like anti-tumor phenotype. Furthermore, ex vivo methodologies were developed for conjugating NPs to the cell surface of T cells. This led to the development of an interleukin-15-loaded nanogel backpack attached to the surface of T cells [49]. This technology rendered multiple therapeutic benefits, including an increased delivery of IL-15, an intratumoral increase of CD8+ T cells, and consequently, an improved CAR-T cell response [50]. As immune cell isolation, ex vivo manipulation and subsequent systemic re-infusion may lead to the rapid recognition of the injected cells by the mononuclear phagocyte system via e.g., efferocytosis [51,52], we speculate that such ex vivo approaches can be refined and improved by performing direct in vivo targeting of immune cells. In vivo immune cell targeting also provides the possibility of inhibiting (or even killing) immune cells in the circulation, thereby modulating the number and type of immune cells infiltrating tumors and metastases. The utilization of NPs for such concepts bypasses a key drawback of NP design and traditional tumor targeting, i.e., the sometimes very rapid recognition of nanoformulations by the immune system. Last but not least, at the patient level, direct in vivo targeting approaches circumvent labor-intensive immune cell isolation, ex vivo manipulation and re-injection into the patient.
Nanomedicine-assisted immune cell imaging
Immunomodulatory strategies require profound knowledge of immune cell count and composition in disease conditions. This knowledge can be obtained by developing nanoparticle-assisted methodologies that can accurately visualize through multiscale and multimodal imaging techniques the presence of a specific immune cell subset in the target tissue [53,54]. The target tissue can be the pathological site, e.g., a tumor, as well as immunoregulating organs, e.g., bone marrow and spleen. NP libraries can be constructed based on well-known manufacturing procedures, which allow for tuning of NP accumulation in different tissues and cell types by controlling parameters such as composition, size, surface decoration and ligand density [31,55]. This engineering versatility is important because each cancer case bears its own immunological signature [56,57]. Given the fact that this immunological signature may change over time, such alternations might be assessed via multiple rounds of NP-assisted imaging. In this regard, NPs will act as a supportive tool to biopsies that are typically not performed multiple times. A major application, in which this approach can be extended, is to decipher between hot and cold tumors, a process that typically is evaluated via biopsy and ex vivo histological analysis [58]. Such a strategy will therefore allow us for better monitoring patients in terms of disease progression, response to therapy, and assist in (re)allocating patients in treatment groups. In the long run, we foresee that the systematic visualization of the NP-immune cell engagement to not only benefit cancer applications but various pathologies strongly characterized by immune cell abnormalities.
Concluding remarks
We anticipate that immune system modulation by means of targeted nanomedicines will play a prominent role in future oncological interventions. Classification of patients and disease stage based on their immunological signature [59] is already established as an important prognostic marker for achieving good treatment outcomes. In this regard, expanding our toolbox with nanomedicines that selectively target certain immune cell populations can help (re)directing the immune system against tumor progression and recurrence. Shifting our experimental attention from traditional tumor targeting towards more extensive engagement of the immune system will open up a new era in nanomedical cancer therapy.
Acknowledgements
The authors gratefully acknowledge financial support by the European Research Council (ERC: Meta-Targeting (864121)), the European Union (European Fund for Regional Development: TAKTIRA (EFRE-0801767), the German Research Foundation (DFG: SFB/TRR57, SFB1066, GRK/RTG 2375 (Tumor-targeted Drug Delivery; Project number: 331065168), KO 2155/6-1 and KO 2155/7-1), and the German Federal Ministry of Education and Research (BMBF: PP-TNBC, Project number: 16GW0319K).
Footnotes
Conflicts of interest
SK reports research funding from Novartis, Janssen, AOP Orphan Pharmaceuticals AG, and Bristol-Myers Squibb as well as consultancy honoraria from Novartis, Incyte/Ariad, Bristol-Myers Squibb, AOP Orphan Pharmaceuticals AG, Pfizer, Celgene, Bayer, Roche, CTI, and Shire. TL reports research funding and consultancy honoraria from Cristal Therapeutics.
References
- 1.Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine. 2019;4(3) doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Advanced Drug Delivery Reviews. 2017;122:20–30. doi: 10.1016/J.ADDR.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Li S-D, Huang L. Pharmacokinetics and Biodistribution of Nanoparticles. Molecular Pharmaceutics. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 4.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. Journal of Controlled Release. 2012;161(2):175–187. doi: 10.1016/J.JCONREL.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced Drug Delivery Reviews. 2014;66:2–25. doi: 10.1016/J.ADDR.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced Drug Delivery Reviews. 2015;91:3–6. doi: 10.1016/J.ADDR.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1(5):16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 9.de Maar JS, Sofias AM, Porta Siegel T, Vreeken RJ, Moonen C, Bos C, Deckers R. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics. 2020;10(4):1884–1909. doi: 10.7150/thno.38625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nature Nanotechnology. 2019;14(11):1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildes TJ, Flores CT, Mitchell DA. Concise Review: Modulating Cancer Immunity with Hematopoietic Stem and Progenitor Cells. STEM CELLS. 2019;37(2):166–175. doi: 10.1002/stem.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H. The cancer stem cell: premises, promises and challenges. Nature Medicine. 2011;17(3):313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 13.Lammers T, Sofias AM, van der Meel R, Schiffelers R, Storm G, Tacke F, Koschmieder S, Brümmendorf TH, Kiessling F, Metselaar JM. Dexamethasone nanomedicines for COVID-19. Nature Nanotechnology. 2020;15(8):622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Lammers T. Combining Nanomedicine and Immunotherapy. Accounts of Chemical Research. 2019;52(6):1543–1554. doi: 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nature Reviews Immunology. 2020:1–14. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Chen Q, Feng L, Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55–73. doi: 10.1016/J.NANTOD.2018.06.008. [DOI] [Google Scholar]
- 18.Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nature Communications. 2017;8(1):1747. doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou W, Thapa RK, Jiang L, Soe ZC, Gautam M, Chang J-H, Jeong J-H, Ku SK, Choi H-G, Yong CS, Kim JO. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. Journal of Controlled Release. 2018;281:84–96. doi: 10.1016/J.JCONREL.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nature Biomedical Engineering. 2018;2(8):578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh A, Kumar S, Nandi D, Kulkarni A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Advanced Materials. 2019;31(51):1904364. doi: 10.1002/adma.201904364. [DOI] [PubMed] [Google Scholar]
- 22.Murray PJ. Macrophage Polarization. Annual Review of Physiology. 2017;79(1):541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nature Communications. 2019;10(1):3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sofias AM, Toner YC, Meerwaldt AE, van Leent MMT, Soultanidis G, Elschot M, Gonai H, Grendstad K, Flobak Å, Neckmann U, Wolowczyk C, et al. Tumor Targeting by αvβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano. 2020:acsnano.9b08693. doi: 10.1021/acsnano.9b08693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combes F, Meyer E, Sanders NN. Immune cells as tumor drug delivery vehicles. Journal of Controlled Release. 2020;327:70–87. doi: 10.1016/J.JCONREL.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Hou J, Yang X, Li S, Cheng Z, Wang Y, Zhao J, Zhang C, Li Y, Luo M, Ren H, Liang J, et al. Accessing neuroinflammation sites: Monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Science Advances. 2019;5(7):eaau8301. doi: 10.1126/sciadv.aau8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu D, Dong X, Zhao Q, Gu J, Wang Z. Photosensitization Priming of Tumor Microenvironments Improves Delivery of Nanotherapeutics via Neutrophil Infiltration. Advanced Materials. 2017;29(27):1701021. doi: 10.1002/adma.201701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedmi R, Veiga N, Ramishetti S, Goldsmith M, Rosenblum D, Dammes N, Hazan-Halevy I, Nahary L, Leviatan-Ben-Arye S, Harlev M, Behlke M, et al. A modular platform for targeted RNAi therapeutics. Nature Nanotechnology. 2018;13(3):214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 29.Veiga N, Goldsmith M, Granot Y, Rosenblum D, Dammes N, Kedmi R, Ramishetti S, Peer D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nature Communications. 2018;9(1):1–9. doi: 10.1038/s41467-018-06936-1. 2018 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sofias AM, Andreassen T, Hak S. Nanoparticle Ligand-Decoration Procedures Affect in Vivo Interactions with Immune Cells. Molecular Pharmaceutics. 2018;15(12):5754–5761. doi: 10.1021/acs.molpharmaceut.8b00908. [DOI] [PubMed] [Google Scholar]
- 31.Tang J, Baxter S, Menon A, Alaarg A, Sanchez-Gaytan BL, Fay F, Zhao Y, Ouimet M, Braza MS, Longo VA, Abdel-Atti D, et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proceedings of the National Academy of Sciences. 2016;113(44):E6731–E6740. doi: 10.1073/pnas.1609629113. https://www.pnas.org/content/113/44/E6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stankovic B, Bjørhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold ES, Woldbæk PR, Helland Å, et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Frontiers in Immunology. 2019;9:3101. doi: 10.3389/fimmu.2018.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senders ML, Meerwaldt AE, van Leent MMT, Sanchez-Gaytan BL, van de Voort JC, Toner YC, Maier A, Klein ED, Sullivan NAT, Sofias AM, Groenen H, et al. Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging. Nature Nanotechnology. 2020:1–8. doi: 10.1038/s41565-020-0642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nature Nanotechnology. 2020;15(4):313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nature Reviews Materials. 2019;4(6):415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W, Zhou Z, Lau J, Hu S, Chen X. Functional T cell activation by smart nanosystems for effective cancer immunotherapy. Nano Today. 2019;27:28–47. doi: 10.1016/J.NANTOD.2019.05.004. [DOI] [Google Scholar]
- 37.van der Meel R. Nanotechnology for organ-tunable gene editing. Nature Nanotechnology. 2020;15(4):253–255. doi: 10.1038/s41565-020-0666-9. [DOI] [PubMed] [Google Scholar]
- 38.Baumeister J, Chatain N, Hubrich A, Maié T, Costa IG, Denecke B, Han L, Küstermann C, Sontag S, Seré K, Strathmann K, et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34(4):1062–1074. doi: 10.1038/s41375-019-0629-z. [DOI] [PubMed] [Google Scholar]
- 39.Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R, Hoffman R, Mascarenhas J. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660–671. doi: 10.3324/haematol.2015.141283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song M-K, Park B-B, Uhm J-E. Understanding Splenomegaly in Myelofibrosis: Association with Molecular Pathogenesis. International Journal of Molecular Sciences. 2018;19(3):898. doi: 10.3390/ijms19030898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong Z, Mc Cafferty S, Combes F, Huysmans H, De Temmerman J, Gitsels A, Vanrompay D, Portela Catani J, Sanders NN. mRNA therapeutics deliver a hopeful message. Nano Today. 2018;23:16–39. doi: 10.1016/J.NANTOD.2018.10.005. [DOI] [Google Scholar]
- 42.Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi: 10.1016/J.NANTOD.2019.100766. [DOI] [Google Scholar]
- 43.Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Letters. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Molecular Cancer. 2019;18(1):128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, Niu G, Su T, Zhu G, Lu G, Zhang L, et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Science Advances. 2020;6(12):eaaw6071. doi: 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 47.Stadler CR, Bähr-Mahmud H, Celik L, Hebich B, Roth AS, Roth RP, Karikó K, Türeci Ö, Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nature Medicine. 2017;23(7):815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 48.Shields CW, Evans MA, Wang LL-W, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S. Cellular backpacks for macrophage immunotherapy. Science Advances. 2020;6(18):eaaz6579. doi: 10.1126/sciadv.aaz6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature Medicine. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang L, Zheng Y, Melo MB, Mabardi L, Castaño AP, Xie Y-Q, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nature Biotechnology. 2018;36(8):707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Combes F, Sofias AM, Mc Cafferty S, Huysmans H, De Temmerman J, Hak S, Meyer E, Sanders NN. Mononuclear but Not Polymorphonuclear Phagocyte Depletion Increases Circulation Times and Improves Mammary Tumor-Homing Efficiency of Donor Bone Marrow-Derived Monocytes. Cancers. 2019;11(11):1752. doi: 10.3390/cancers11111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravichandran KS. Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways. Immunity. 2011;35(4):445–455. doi: 10.1016/J.IMMUNI.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy CE, White JM, Viola NT, Gibson HM. In vivo Imaging Technologies to Monitor the Immune System. Frontiers in Immunology. 2020;11:1067. doi: 10.3389/fimmu.2020.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapelin F, Capitini CM, Ahrens ET. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. Journal for ImmunoTherapy of Cancer. 2018;6(1):105. doi: 10.1186/s40425-018-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramishetti S, Hazan-Halevy I, Palakuri R, Chatterjee S, Gonna SN, Dammes N, Freilich I, Shmuel LK, Danino D, Peer D. A Combinatorial Library of Lipid Nanoparticles for RNA Delivery to Leukocytes. Advanced Materials. 2020;32(12) doi: 10.1002/ADMA.201906128. [DOI] [PubMed] [Google Scholar]
- 56.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV, Kerwin CM, Choi S, et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity. 2019;50(5):1317–1334.:e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friebel E, Kapolou K, Unger S, Núñez NG, Utz S, Rushing EJ, Regli L, Weller M, Greter M, Tugues S, Neidert MC, et al. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell. 2020;0(0) doi: 10.1016/j.cell.2020.04.055. [DOI] [PubMed] [Google Scholar]
- 58.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nature Reviews Drug Discovery. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 59.Halama N. The next age of immunotherapy: optimisation, stratification and therapeutic synergies. British Journal of Cancer. 2019;120(1):1–2. doi: 10.1038/s41416-018-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]