Abstract
Background
Deficiency of the thyroid hormone transporter MCT8 causes severe intellectual and motor disability and high serum T3 concentrations. Chronic thyrotoxicosis leads to progressive deterioration in body weight, tachycardia and muscle wasting, predisposing to significant morbidity and mortality. Treatment that safely alleviates peripheral thyrotoxicosis and reverses cerebral hypothyroidism is unavailable. We investigated the effects of the T3-analogue Triac in patients with MCT8 deficiency.
Methods
In an investigator-initiated, open-label, single group, international, multi-centre phase 2 trial, male paediatric and adult patients with MCT8 deficiency received Triac orally, according to a predefined dose-escalation protocol. The prespecified primary endpoint was the change in serum T3 concentrations from baseline to month 12. Prespecified secondary endpoints were changes in body weight, heart rate, blood pressure and biochemical markers of thyroid hormone action from baseline to month 12. Safety measures and adverse events were documented. This trial (NCT02060474) is finished.
Findings
Between October 2014 and June 2017, 46 subjects were enrolled, of whom 40 completed 12 months of Triac treatment. Mean serum T3 concentrations decreased from 4·97±1·55 to 1·82±0·69 ng/dl (mean decrease 3·15, 95%CI: 2·68-3·62, p<0·0001), at a median dose of 37 (IQR, 29-47) μg/kg body weight/day Triac. From baseline to month 12, mean weight to age z score improved by 0·27 (0·03-0·50) SDs (p=0·025), resting heart rate decreased by 9 (2-16) bpm (p=0·010), 24h heart rate by 5 (1-9) bpm (p=0·012), systolic blood pressure decreased 18 (6-29) percentile (p=0·0037), sex hormone binding globulin concentrations decreased 35 (15-55) nmol/l (p=0·0013), creatine kinase concentrations increased 53 (27-78) U/l (p<0·0001), diastolic blood pressure and total cholesterol remained unchanged. 43 (93%) patients had at least one adverse event. Seven treatment-related adverse events (transiently increased perspiration or irritability) occurred in six (13%) patients. 26 serious adverse events considered unrelated to treatment (mostly hospitalizations due to infections) occurred in 18 (39%) patients.
Interpretation
Key features related to peripheral thyrotoxicosis in patients with MCT8 deficiency were alleviated under Triac treatment in paediatric and adult patients with MCT8 deficiency.
Funding
Dutch Scientific Organization, Sherman Foundation, NeMO Foundation, Wellcome Trust, NIHR Cambridge Biomedical Centre, Toulouse University Hospital, and “Una Vita Rara ONLUS”
Introduction
Intracellular thyroid hormone action requires membrane transporter proteins that facilitate cellular entry of thyroid hormones. Monocarboxylate transporter 8 (MCT8) is a specific thyroid hormone transporter and crucial for transport of triiodothyronine (T3) and thyroxine (T4) in different tissues, including the brain 1. Mutations in the SLC16A2 gene (located on the X-chromosome), encoding MCT8, cause MCT8 deficiency (or Allan-Herndon-Dudley syndrome), a rare disorder with an estimated prevalence of 1:70 000 males 2–4. As a consequence of impaired thyroid hormone entry into the brain, male subjects with MCT8 deficiency exhibit severe intellectual and motor disability and fail to achieve early developmental milestones. The endocrine hallmark of MCT8 deficiency is elevated serum T3 concentrations together with reduced free T4 and normal TSH concentrations. Peripheral tissues that rely on transporters other than MCT8 are exposed to markedly elevated serum T3 concentrations 5. Such chronic tissue thyrotoxicosis in MCT8 deficiency leads to tachycardia, muscle wasting, hypermetabolism, and progressive reduction in body weight for age, constituting significant morbidity and mortality 5. Thus, it is imperative to treat the permanent hyperthyroidism present in this disorder. However, standard anti-thyroid drug therapy with methimazole has been shown to be ineffective 4. The alternative anti-thyroid drug propylthiouracil (PTU) has the potential to reduce serum T3 concentrations, through its inhibitory effect on the type 1 deiodinase, but received a black box warning by the U.S. Food and Drug Administration due to risk of severe hepatotoxicity. Accordingly, PTU is not recommended as therapy for hyperthyroidism and its use, particularly in children, is strongly discouraged by current guidelines6,7. The unfavorable safety profile of PTU is particularly relevant in the context of the frequent necessity to use other drugs (e.g. anticonvulsants) with hepatotoxic side effects in MCT8 deficient patients. Optimal therapy should safely alleviate peripheral thyrotoxicosis and restore euthyroidism in the brain, but as yet such treatment is not available.
Triiodothyroacetic acid (Triac) is a thyroid hormone analogue whose cellular entry is not dependent on MCT8 8–10. Triac can inhibit TSH secretion in humans, thereby lowering endogenous thyroid hormone production, yet has relatively weak thyromimetic activity in peripheral tissues 11,12. Preclinical studies indicate that Triac restores abnormal neuronal development and myelination in animal models of MCT8 deficiency if administrated in early postnatal life 8,13. Here, we report the results of a clinical trial that evaluated the effectiveness and safety of Triac treatment for peripheral thyrotoxicosis in paediatric and adult patients with MCT8 deficiency. Given the wide spectrum of patient age, neurocognitive changes were only evaluated in an exploratory way.
Methods
Study design and participants
In this investigator-initiated, multi-centre, open-label, single group, phase 2 pragmatic trial, we investigated the effectiveness and safety of Triac in patients with MCT8 deficiency. Patients were enrolled at 11 sites in 8 countries in Europe and 1 site in South-Africa (Fig. S1) and received Triac for 12 months. All participants discontinued treatment with anti-thyroid drugs and/or levothyroxine (if applicable). After a wash-out period of at least 4 weeks, baseline measurements were undertaken.
Patients with MCT8 deficiency, confirmed by the presence of a mutation in the SLC16A2 gene, were eligible to participate, irrespective of their age, and comorbidities. Patients were known to the investigators through direct care; also, patients were included whose doctors or parents had become aware of this trial through ClinicalTrials.gov registration. Key exclusion criteria were major illness or recent major surgery, enrollment in other randomized controlled trials, allergy to components in Triac tablets, or the presence of any major contraindications to Triac treatment. Patients could be withdrawn from the trial at wish of the parents or guardians, in case continued participation was considered harmful by the investigators for the participants` health due to occurrence of dose-limiting toxicities, in case of non-adherence to the trial protocol, premature termination of the trial, or loss to follow-up (Supplementary Table S1). Parents or guardians provided written informed consent for their children to participate. The institutional review board at each participating site approved the study protocol and all of its amendments (see Supplementary documents). The trial was intended as a national study in The Netherlands, but was amended to allow additional enrollment of patients outside The Netherlands. To enable ascertainment of long-term effectiveness and safety, the first patients enrolled in The Netherlands, who had completed the 12 months of treatment, were offered the opportunity to enter an open-label treatment extension period, whose end was defined as the completion date of the last patient in other countries. An independent data safety monitoring board monitored patient safety.
Study procedures
All patients were treated with Triac (Téatrois tablets 350 microgram, Rare Thyroid Therapeutics) by individualized dose-escalation, following a pre-defined dose-escalation protocol. After the initial dose of Triac (350 microgram) was administered and no predefined dose-limiting toxicities were observed, the daily dose was increased progressively in 350 microgram steps, with a goal of attaining serum total T3 concentrations within the target range of 1·4-2·5 nmol per liter. The maintenance Triac dose was continued throughout the rest of the study period (Fig. S2A and B), but could be further adjusted according to the dose-escalation protocol if T3 concentrations were outside the target range during control visits.
Patients were assessed for study outcomes at baseline and 12 months after starting Triac administration. In the interval, patients were evaluated and screened for clinical and biochemical signs of hypothyroidism or hyperthyroidism, adverse events were recorded and adherence to therapy was assessed. An overview of the timing of all study measures is provided in Table S2. All study procedures were specified in standard operating procedures, and were performed by well-trained investigators. Neuropsychological tests were conducted according to their manual. All biochemical measurements were performed in a central laboratory (Erasmus Medical Centre). To account for any interference of Triac in the measurement of serum T3 concentrations, conventional methods were employed to correct for cross-reactivity (details are provided in the Supplementary Appendix).
Outcomes
The prespecified primary endpoint was the change in the serum T3 concentrations from baseline to month 12. The prespecified co-primary endpoints were the change in serum TSH, free and total T4 and total reverse T3 (rT3) concentrations from baseline to month 12.
Prespecified secondary endpoints were the change from baseline to month 12 in body weight (expressed as body weight for age z score, to account for the natural development in children), mean and resting heart rate (in beats per minute), measured by 24 h ambulatory cardiac monitoring and electrocardiography, respectively, blood pressure (in mmHg and percentiles, based on reference ranges in healthy subjects 14,15; mean of two measurements), and well-established biochemical parameters that reflect thyroid hormone activity in the liver (sex hormone binding globulin and total cholesterol) and muscle (creatine kinase). Body weight was selected over BMI, as accurate height measurements can be hampered by scoliosis and contractures.
An overview of prespecified exploratory measures, including neuropsychological tests, is provided in Supplementary Table S2 and fully defined in the Statistical Analysis Plan, for which the endpoint was the change from baseline to month 12.
Prespecified safety assessments included documentation of adverse events; echocardiography and 24 hour monitoring of heart rhythm; bone mineral density measurement; biochemical evaluations, including renal and liver function tests and bone turnover markers. Details are provided in the Statistical Analysis Plan.
Post-hoc endpoints were the change from baseline to the end of the treatment extension period (TEP) for endpoints measured in the extension participants (see Supplementary Table S2), as well as the change from baseline to month 12 in total fat mass and percentage, and lean body mass by DXA, the number of premature atrial contractions per 24 h, HDL/LDL cholesterol ratio, and the change in body weight in kilograms. In addition, changes in neuropsychological test results were examined after stratification by age.
Although the measurement of energy expenditure and hair cortisol concentrations were prespecified endpoints, their acquisition was compromised by technical difficulties and are, therefore, not reported.
Statistical analysis
A power calculation was performed based on a one sample t-test estimating the difference in serum T3 concentrations after 12 months of treatment, using a mean ± SD serum T3 concentrations of 4·3 ± 1·2 nmol/L derived from a historical group of 31 patients with MCT8 deficiency. With a total of 10 patients we would have 80% power (at α=0·05) to detect a mean decrease in serum T3 concentrations to 3·3 nmol per liter. In this way, we ensured sufficient power to detect a decrease in T3 levels to the upper limit of the intended target range (1·4 - 2·5 nmol per liter). Following approval by all relevant ethical committees, additional patients were recruited to ascertain centralized documentation of the effects of Triac in this rare disorder, and to provide more meaningful data on secondary outcomes and safety measures. Being the first trial in MCT8 deficiency, the exact drop-out rates and effect sizes were difficult to predict beforehand.
For statistical analyses, we used GraphPad Prism version 6 (La Jolla, CA). Two-sided P values of less than 0·05 denote statistical significance.
Analyses of the prespecified primary endpoints were based on the full analysis set, which included all patients receiving at least one dose of Triac, and had at least one control visit following the baseline evaluation. As such, drop-outs were fully considered in the analyses, and their last available measurement was considered.
The primary analyses of prespecified secondary endpoints were based on all patients that completed 12 months of treatment. Supporting this data, the analyses of prespecified secondary endpoints were also carried out on all trial participants including patients that did not complete the 12 months treatment. The analyses of prespecified exploratory endpoints and the post-hoc analyses were based on all patients that completed 12 months of treatment, unless otherwise stated. Analyses of safety endpoints was based on the safety population, which included all patients exposed to the study drug.
For all prespecified primary and secondary endpoints P values and 95% confidence intervals (CI) were calculated for the mean change from baseline to month 12 with the use of paired Student`s t-tests. Serum TSH and creatine kinase concentrations were first log-transformed to normalize the distribution. For all prespecified exploratory and safety measures, 95% CI were calculated for the mean change from baseline to 12 months of Triac treatment. Post-hoc analyses of neurocognitive endpoints after stratification by age were descriptive. Longitudinal analyses of the primary and secondary endpoints in the treatment extension period were based on all patients that received at least one dose of Triac after enrollment in the long-term TEP and were carried out using paired t-tests, comparing baseline versus TEP.
Missing data can mainly be attributed to the poor clinical condition of patients, their inability to follow instructions and common manifestations of MCT8 deficiency such as scoliosis and dystonic posturing that hamper investigations for which patients needed proper positioning. With the assumption that omission of data occurred randomly and given the broad age range of the participants and small group size, pair-wise deletion was used to adjust for missing data that were only captured at T0 and T12 and for missing data that were captured throughout the study the last available measurement was considered. For primary and secondary endpoints, post-hoc sensitivity analyses were performed, excluding drop-outs and cases for whom the last available measurement had been used in the primary analyses. Because of the rarity of MCT8 deficiency and the small number of patients in this trial, individual patient data beyond what is reported in this manuscript will not be shared to safeguard patient privacy.
The trial is registered with ClinicalTrials.gov, number NCT02060474.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
From October 2014 through June 2017, a total of 50 patients were screened and found eligible to participate, of whom 46 were enrolled in the trial to receive Triac. Forty patients completed the 12–month intervention period, of whom ten patients were included in the long-term (median [IQR], 40·4 [38·1-41·3]-month) treatment extension period (Fig. S2B).
The demographic and clinical characteristics of patients are shown in Table 1. At baseline, the median age was 7·1 (range 0·8-66·8) years. Mean serum T3 concentrations were 4·91 (±1·57) nmol per liter, exceeding 1·6 times the upper limit of the normal range for age. The mean z score for weight for age was -2·84 (±1·88) SD and 30 out of 46 enrolled patients were underweight (z score < -2). All patients had severe intellectual and motor disability with 41 out of 46 patients being wheelchair bound, and not reaching early developmental milestones such as independent sitting. Resting heart rate was markedly elevated (> 90th percentile) in 19 (43%) patients and systolic hypertension was present in 12 (34%) patients.
Table 1. Demographical and clinical characteristics at baseline.
| Characteristic | N =46 |
|---|---|
| Age - yr | |
| Median (range) | 7·1 (0·8-66·8) |
| Age composition – no. (%) | |
| <4 years | 11 (24) |
| 4-10 years | 19 (41) |
| 11-18 years | 11 (24) |
| adults | 5 (11) |
| Sex – no. (%) | |
| Male | 46 (100) |
| Ethnicity – no. (%) | |
| White | 44 (96) |
| European | 39 (85) |
| Middle-Eastern | 2 (4) |
| North-Africa | 3 (7) |
| Asian | 1 (2) |
| Other | 1 (2) |
| Patients per country – no (%) | |
| Netherlands | 14 (30) |
| South Africa | 1 (2) |
| Czech Republic | 1 (2) |
| Romania | 3 (7) |
| Belgium | 2 (4) |
| France | 7 (15) |
| Germany | 3 (7) |
| Italy | 5 (11) |
| United Kingdom | 10 (22) |
| Living situation – no. (%) | |
| At home | 34 (74) |
| Institution | 5 (11) |
| Both | 7 (15) |
| Level of development – no. (%) | |
| Wheelchair bound | 41 (89) |
| None or poor head control | 32 (70) |
| Able to sit independently | 5 (11) |
| T3 concentration – ng/dl | |
| Mean (±SD) | 4·91 (±1·58) |
| Body weight - weight for age z score | |
| Mean (±SD) | -2·84 (±1·88) |
| Underweight – no. (%)* | 30 (65) |
| Feeding tube – no. (%) | 20 (43) |
| Tachycardia at rest – no. (%)† | 19 (43) |
| Systolic hypertension – no. (%)‡ | 12 (34) |
Underweight was defined following WHO criteria (z score < -2).
Tachycardia was defined as a resting heart rate above the 90th percentile for the corresponding age, using cut-offs described in Fleming et al. 2011 15. Resting heart rate was available in 44 patients.
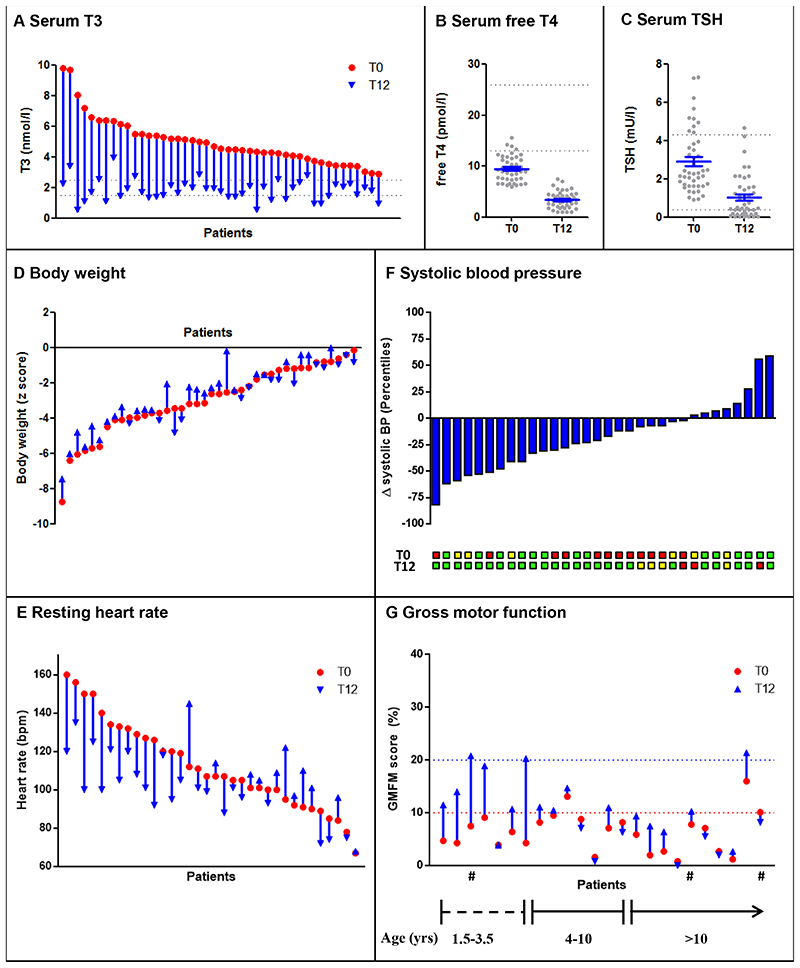
Prespecified analysis of effectiveness for the primary endpoints included all patients who received Triac and had at least one follow-up measurement of thyroid function (N=45). Serum T3 concentrations significantly decreased by month 12 (median [IQD], 13·0 [12·4-13·9]), with a mean change of 3·15 (95% CI, 2·68-3·62) nmol per liter (P<0·0001) (Figure 1A), representing a mean reduction of 61 (95% CI, 56-66) percent. Thirty-four out of 40 patients attained serum T3 levels within the target range by month 12 with the remainder just above the target range, but within target range during the preceding control visit. All drop-outs had T3 concentrations within target range at time of the last available measurement (median [IQD] follow-up: 7·0 [3·9-9·3] months). The median time to achieve serum T3 concentrations within the target range was 2·5 [1·5-3·7] months, requiring a mean daily Triac dose of 38·3±15·3 (range: 6·4-84·3) microgram per kilogram body weight, divided into a median of 3 [2-3] administrations per day (Fig. S4A and B). By month 4, 77% of patients attained T3 concentrations within target range (Fig. S4C). The median daily Triac dose remained stable after the dose escalation phase, although 7 (16%) patients required further dose adjustments to maintain T3 concentrations within target range (Fig. S4D and E). The median daily Triac dose at month 12 was 37·0 [28·9-47·2] microgram per kilogram body weight. Serum free T4 concentrations decreased during the dose-escalation phase and were maintained throughout the rest of the study (Fig. S3F). By month 12, serum free T4 concentrations were significantly reduced with a mean change of 6·1 (95% CI, 5·4-6·8) pmol per liter (P<0·0001) (Figure 1B), and serum TSH concentrations were reduced, with a mean change of 1·89 (95% CI, 1·39-2·39) mU per liter (P<0·0001) (Figure 1C). In addition, serum total T4 concentrations decreased by 31·6 (95% CI, 28·0-35·2) nmol/l (p<0·0001) and reverse T3 by 0·08 (95% CI, 0·05-0·10) nmol/l (p<0·0001) (Table 2).
Figure 1. Change from baseline to month 12 in prespecified primary and secondary outcome measures.
Panel A shows the significant decrease in serum T3 concentrations from baseline (red dot) to month 12 (blue arrow head) (P<0·0001). Panel B shows the significant decrease in mean serum free T4 concentrations from baseline (T0) to month 12 (T12) (P<0·0001). Grey dots represent individual patients and the mean and standard error of the mean are presented in blue. Similarly, panel C shows the significant decrease in mean serum TSH concentrations seen at month 12 (P<0·0001). Panel D represents the change in body weight (expressed as body weight for age z scores) between baseline (red dot) and month 12 (blue arrow head) of Triac treatment. The majority of the patients had an improvement in body weight (P=0·025). Panel E represents the resting heart rate, determined by electrocardiogram, per patient at baseline (red dot) and after 12 months of Triac treatment (blue arrowhead), indicating an improvement in the majority of the patients (P=0·010). Panel F shows the decrease in mean systolic blood pressure, expressed in percentiles after correction for age and body height, at month 12 (P=0·0037). The number of patients with a classification of normal systolic blood pressure (green box), elevated systolic blood pressure (yellow box) or systolic hypertension (red box) of each patient is shown at baseline (T0) and month 12 (T12), according to the American Academy of Pediatrics guidelines 14 and American College of Cardiology/American Heart Association (ACC/AHA)15. Panel G presents the gross motor function for each patient at baseline and at month 12. All patients had a low score at baseline, in the majority of cases not exceeding the 10 percent score (red dotted line), exemplified by poor or no head control, indicating a gross delay in motor development. No clinically relevant change is seen in the majority of patients. Some patients below 4 years of age exceeded the 20 percent score at month 12 (blue dotted line), exemplified by achievement of head control in different postural positions and independent sitting. Gross motor function was assessed with the Gross Motor Function Measure-88 (GMFM-88) (in which scores range from 0 to 100%, with higher scores indicating better motor function and where a 100% score is achieved by a normal developing child of 4 years of age)17. Patients with the same inactivating F230del mutation are indicated with an #.
Table 2. Effects of Triac on prespecified and post-hoc outcome measures.
| Primary efficacy measures (n=45) | ||||
| T3 (nmol/l) | 4·97 (±1·55) | 1·82 (±0·69) | -3·15 (-3·62--2·68) | <0.001 |
| TSH (mU/l)* | 2·91 (±1·68) | 1·02 (±1·14) | -1·89 (-2·39--1·39) | <0·001 |
| Free T4 (pmol/l) | 9·5 (±2·5) | 3·4 (±1·6) | -6·1 (-6·8 - -5·4) | <0·001 |
| Total T4 (nmol/l) | 56·0 (±13·0) | 24·4 (±9·4) | -31·6 (-35·2--28·0) | <0·001 |
| Reverse T3 (nmol/l) | 0·12 (±0·10) | 0·04 (±0·04) | -0·08 (-0·10--0·05) | <0·001 |
| Secondary efficacy measures (n=40) ‡ | ||||
| Weight for age (z score) | -2·98 (±1·93) | -2·71 (±1·79) | 0·27 (0·03 - 0·50) | 0·03 |
| Resting heart rate (bpm) (n=34) | 112 (±23) | 104 (±17) | -9 (-16 - -2) | 0·01 |
| Mean heart rate 24 h (bpm) (n=31) | 102 (±14) | 97 (±9) | -5 (-9 - -1) | 0·01 |
| Blood pressure (n=32) | ||||
| Systolic (mm Hg) | 108 (±8) | 102 (±10) | -5 (-9 - -1) | 0·009 |
| Systolic (Percentile) † | 78 (±24) | 61 (±29) | -18 (- 29- -6) | 0·004 |
| Diastolic (mm Hg) | 64 (±9) | 62 (±9) | -2 (-6 – 2) | 0·35 |
| Diastolic (Percentile) † | 74 (±22) | 67 (±22) | -6 (-17 – 4) | 0·24 |
| SHBG (nmol/l) (n=39) | 212 (±91) | 178 (±76) | -35 (-55 - -15) | 0·001 |
| Total cholesterol (mmol/l) | 3.2 (±0.7) | 3.4 (±0.7) | 0.2 (0.0-0.3) | 0·06 |
| CK (U/l) * | 108 (±90) | 161 (±117) | 53 (27-78) | <0·001 |
| Exploratory measures (n=40) ‡ | ||||
| Height (m) | 1·20 (±0·23) | 1·26 (±0·22) | 0·06 (0·04-0·07) | |
| Height for age (z score) | -1·96 (±1·5) | -1·98 (±1·5) | -0·02 (-0·19– 0·16) | |
| BMI (kg/m2) | 14·2 (±2·7) | 14·6 (±2·9) | 0·3 (-0·09 - 0·77) | |
| BMI for age (z score) | -2·56 (±2·56) | -2·24 (±2·60) | 0·32 (-0·14 – 0·77) | |
| TBG (mg/l) | 18·2 (±3·3) | 19·7 (±4·6) | 1·5 (0·3-2·8) | |
| Albumin (g/l) | 46 (±2·2) | 46·8 (±2·1) | 0·9 (-0·1-1·9) | |
| Creatinine (μmol/l) | 33 (±12) | 38 (±14) | 5 (3-7) | |
| LDL cholesterol (mmol/l) | 1·80 (±0·53) | 1·85 (±0·53) | 0·06 (-0·07-0·19) | |
| HDL cholesterol (mmol/l) | 1·20 (±0·30) | 1·37 (±0·31) | 0·18 (0·10-0·26) | |
| Triglycerides (mmol/l) | 0·69 (±0·34) | 0·72 (±0·35) | 0·02 (-0·14– 0·09) | |
| Ferritin (μg/l) | 45 (±40) | 29 (±19) | -16 (-27 – -4) | |
| Thyroglobulin (μg/l) § | 11·7 (7·1-28·9) | 4·1 (1·5-6·8) | -9·2 (-23·6- -2·7) | |
| Post-hoc measures (n=40) ‡ | ||||
| Weight (kg) | 21·8 (±12·2) | 24·5 (±12·6) | 2·7 (1·9-3·5) | |
| Body fat (kilogram) (n=15) | 5·1 (±3·9) | 6·2 (±4·2) | 1·1 (0·2-2·1) | |
| Body fat (%) (n=15) | 22·8 (±9·8) | 25·1 (±10·0) | 2·3 (-1·0 – 5·6) | |
| Lean body mass (kilograms) (n=15) | 15·7 (±6·9) | 16·9 (±6·8) | 1·2 (0·8-1·7) | |
| HDL / LDL cholesterol (ratio) | 0·70 (±0·19) | 0·78 (±0·22) | 0·08 (0·02-0·14) | |
| Premature atrial complexes§ (n=31) | 48 (1-1322) | 0 (0-12) | -22 (-1150- 0) | |
The prespecified analysis of primary efficacy measures included all patients who received Triac and had at least one follow-up measurement of thyroid function (N=45), and included five patients who dropped-out for whom the last available measurement was considered.
The primary analyses of prespecified secondary and exploratory outcomes, as well as post-hoc outcomes were based on the available data from patients who completed the 12–month intervention period (n=40, unless otherwise indicated between brackets of the individual parameter). In case month 12 measurements were not available, the last available observation in the same patient was used instead, which accounted for <3% of data points included in the analyses.
Post-hoc sensitivity analyses excluding those patients with T12 measurements (for whom the last available measurement was considered) showed results of similar size and significance.
TSH and CK concentrations were log-transformed to ensure a normal distribution before paired T-tests were conducted. Non-transformed means (±SD) and mean changes (95% CI) are presented to increase interpretability.
Percentile scores based on age and body height.(Flyn & Wellton)
Post-hoc exploratory analyses entail parameters that have been captured during pre-defined study procedures and provide relevant additional information on the treatment effect.
The median (change) with the interquartile range are provided due to violation of normality assumptions. Reported confidence intervals for exploratory measures have not been adjusted for multiplicity, and the intervals should not be used to infer definitive treatment effects.
Primary analyses of prespecified secondary were based on 40 patients who completed the 12–month intervention period. Body weight for age z scores improved significantly at month 12 by 0·27 (95% CI , 0·03-0·50, P = 0·0253) SDs (Figure 1D and Fig. S6A), which equates to a mean increase in body weight of 2·7 (95% CI, 1·9-3·5) kilograms (p<0·0001), whereas the natural course of body weight for age z score in untreated patients shows a progressive reduction over time (Fig. S6B).
Resting heart rate measured by electrocardiography decreased by 9 (95% CI, 2-16) beats per minute (P = 0·010) (Figure 1E). Mean heart rate measured by 24 h cardiac monitoring decreased by 5 (95% CI, 1-9) beats per minute at month 12 (P = 0·012) (Fig. S7A). Post-hoc analyses showed that premature atrial contractions largely subsided in the majority of patients in whom these were present at T0 (Fig. S7B). Mean systolic blood pressure decreased at month 12 from 78th percentile to 61th percentile (delta = 18, 95% CI, 6-29, P = 0·0037) (Figure 1F). The number of subjects with systolic hypertension decreased from 12 at baseline to 3 at month 12.
The concentrations of sex-hormone binding globulin decreased by 35 (95% CI, 15-55, P=0·0013) nmol per liter at month 12. The increase in mean serum total cholesterol concentration of 0·2 (95% CI, 0·0-0·3) mmol per liter was not significant (P=0·056) (Table 2). Serum creatine kinase concentrations increased by 53 (95% CI, 27-78, P<0·0001) U per liter at month 12 (Table 2). The effects of Triac treatment on secondary outcomes were maintained when the analyses was carried out using the whole trial population, including patients that did not complete the 12 months treatment (Table S3). Data on the exploratory outcome measures are shown in Table 2.
The greatest increase in gross motor function was observed in patients in whom Triac treatment was started before the age of 4 years (Figure 1G). At baseline, none of the patients with a completely inactivating MCT8 mutation reached a score above 20% on the Gross Motor Function Measure (GMFM)-G88 17, roughly reflecting the ability to sit independently and achieve full head control in different postural positions. Two out of seven of such patients who started treatment before the age of 4 years reached this developmental level at month 12. Full details of other neurological and neuropsychological parameters are shown in Table S4 and Table S5 of the Supplementary Appendix.
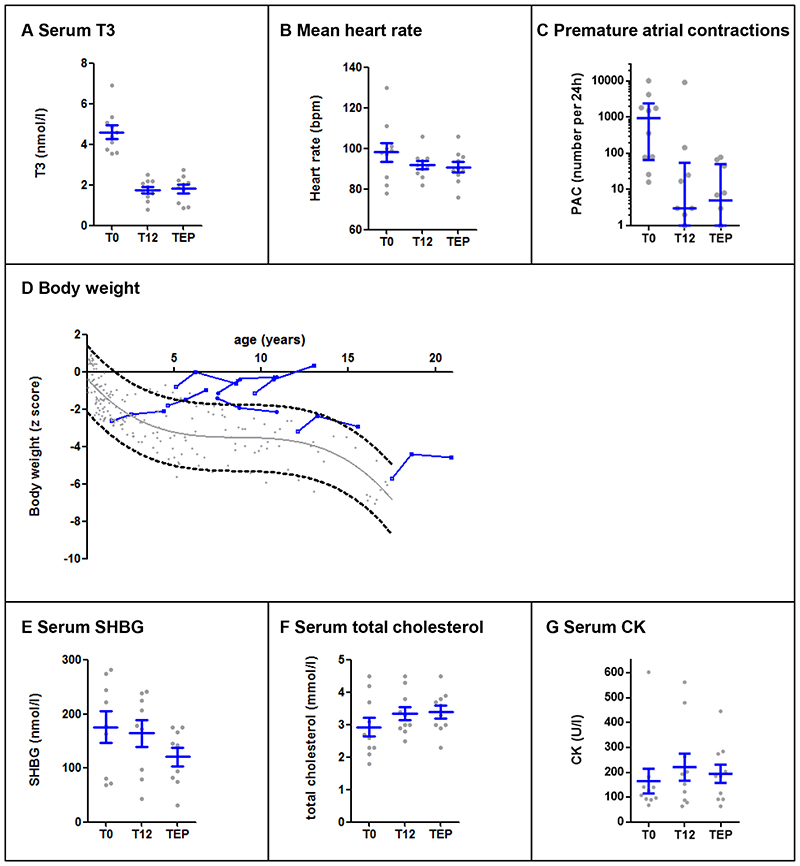
Post-hoc analyses of the treatment extension endpoints included ten patients. During the long-term treatment extension period, the reduction in T3 concentrations persisted in all patients (Figure 2A and Table S6) and TSH and free T4 concentrations were maintained (Fig. S9A and S9B). The reduction in mean heart rate was sustained (Figure 2B). The occurrence of premature atrial contractions was reduced to less than 100 per 24 h in all patients enrolled in the treatment extension period, and completely subsided in 3 out of 7 children (Figure 2C). Atrial fibrillation, present in one child at baseline, subsided at month 12 and 36. The improvement of body weight for age z score was maintained with a mean increase of 0·52 [95% CI, 0·02- 1·02] SDs at month 12 and 0·62 [95% CI, 0·12 – 1·12] at month 32. Body weight (Figure 2D), height (Fig. S10A) and BMI (Fig. S10B) trajectories deviated positively from their anticipated natural course in 7 out of the 8 paediatric patients. Effects on circulating tissue markers of thyroid hormone action were maintained in the long-term treatment extension period (Figure 2E-G).
Figure 2. Change from baseline to month 12 and end of the treatment-extension period (range 26-42 months) in primary and secondary outcome measures.
Panel A shows the significant decrease in serum T3 concentrations from baseline (T0) to the end of the treatment extension period (TEP) (P<0·0001). Grey dots represent measurements in the individual patients, the mean and SEM are displayed in blue. Panel B shows the mean heart rate, measured by 24 h cardiac monitoring, and panel C shows the post-hoc analysis of the number of premature atrial contractions. Grey dots represent measurements in the individual patients, the median and interquartile range are displayed in blue. Panel D presents the development of body weight of each of the paediatric patients enrolled in the treatment extension period (blue lines) during Triac treatment in comparison to the natural course of body weight development in MCT8 deficiency (represented by a trend line with 95% error band and grey dots of individual historical measurements). The natural course is based on historical measurements from growth charts and medical records of the patients enrolled in the study. All measurements were captured before Triac treatment was commenced. Although not undertaken under standardized conditions, these measurements have been carried out in the context of regular medical care in patients with different co-morbidities, co-medication, and socio-economic backgrounds and, as such, are likely to constitute a representative set of clinical observations. Seven out of 8 patients show a clear deviation from the expected natural course. Panel E shows the sex-hormone binding globulin concentrations, panel F the total cholesterol concentrations, and panel G the creatine kinase concentrations at baseline (T0), month 12 (T12) and end of the treatment extension period (TEP). Grey dots represent measurements in the individual patients, means and SE (for sex-hormone binding globulin and total cholesterol) and median and interquartile range (creatine kinase) are displayed in blue. Means (±SD) and mean changes with corresponding 95% CI values are provided in Table S4 in the Supplementary Appendix.
All patients who received at least 1 dose of Triac were included in analyses of drug safety (N=46). 43 (93%) patients had at least one adverse event. All seven adverse events that were suspected to be related to Triac treatment, occurring in six (13%) patients, were mild and involved a transient increase in perspiration (3 patients) and irritability (3 patients). The onset of these events coincided with the start of Triac treatment or modification in Triac dosage and spontaneously resolved after a few days. No patients required a dose reduction or discontinued participation due to drug-related toxicity. Adverse events occurring in more than 10 percent of patients and all serious adverse events are listed in Table 3, and a complete overview of adverse events is provided in Table S7. The majority of events were classified as mild requiring no or symptomatic treatment, and resolved while the patients continued to receive Triac. Of the safety measures prespecified in the protocol (Table S2), no clinically relevant changes in cardiac structure or function were observed. Serum β-carboxy-terminal collagen crosslinks increased by 0·14 (95% CI, 0·03-0·25) μg per liter and bone-specific alkaline phosphatase concentrations increased by 8·4 (95% CI, 2·7-14·1) μg per liter, without affecting bone mineral density (Table S8) consistent with the physiological increase of bone turn-over markers in paediatric patients during development. No notable changes in serum electrolytes, serum urea, or random plasma glucose concentrations were observed. There were no significant changes in hematopoietic parameters, except three patients in whom mild anemia, ascribed to nutritional deficiency, was detected at month 12 and which could not be linked to the intervention. Twenty-five out of 46 subjects showed mildly elevated serum concentrations of alanine aminotransferase, aspartate aminotransferase or gamma-glutamyl transferase at baseline, attributed to the concomitant use of hepatotoxic medications and these parameters did not deteriorate further. Two subjects showed an increase in aminotransferase levels, attributed to commencement or dose adjustment of anticonvulsant drugs with known hepatotoxicity (levetiracetam and lamotrigine).
Table 3. Summary of adverse events by system organ class and preferred term.
| Adverse events * † | 43 (93) | 150 |
| Adverse events occurring in >10% of patients | ||
| Gastrointestinal disorders | ||
| Diarrhoea | 5 (11) | 5 |
| Gastroenteritis | 11 (24) | 12 |
| Vomiting | 5 (11) | 5 |
| General disorders and administration site conditions | ||
| Influenza (like illness) | 9 (20) | 12 |
| Infections and infestations | ||
| Bronchitis | 6 (13) | 6 |
| Otitis media | 5 (11) | 5 |
| Respiratory, thoracic and mediastinal disorders | ||
| Nasopharyngitis | 11 (24) | 14 |
| Upper respiratory tract Infection | 9 (20) | 9 |
| Serious adverse events ‡ | 18 (39) | 26 |
| Gastrointestinal disorders | ||
| Gastroenteritis | 2 (3) | 3 |
| Enterocolitis | 1 (2) | 1 |
| General disorders and administration site conditions | ||
| Multiple organ dysfunction syndrome | 1 (2) | 1 |
| Hepatobiliary disorders | ||
| Hepatic failure | 1 (2) | 1 |
| Infections and infestations | ||
| Bronchitis | 2 (3) | 2 |
| Pneumonia | 2 (3) | 2 |
| Clostridium difficile infection | 1 (2) | 1 |
| Investigations | ||
| Gastroscopy | 1 (2) | 1 |
| Nervous system disorders | ||
| Seizures (increase) | 2 (3) | 2 |
| Product issues | ||
| Device malfunction § | 2 (3) | 2 |
| Renal and urinary tract disorders | ||
| Urinary tract infection | 1 (2) | 1 |
| Respiratory, thoracic and mediastinal disorders | ||
| Bronchiolitis | 3 (7) | 3 |
| Respiratory distress | 1 (2) | 2 |
| Upper respiratory tract infection | 1 (2) | 1 |
| Surgical and medical procedures | ||
| Hip surgery | 1 (2) | 1 |
| Drug therapy | 1 (2) | 2 |
| Fatal adverse events ¶ | 1 (2) | 1 |
| Adverse events leading to premature treatment discontinuation | 1 (2) | 1 |
| Immune system disorders | ||
| Autoimmune thyroid disorder | 1 (2) | 1 |
| Grade of events | ||
| Severe | 12 (26) | 17 |
| Moderate | 9 (20) | 10 |
| Mild | 39 (85) | 123 |
| Relationship to the product | ||
| Probable ** | 6 (13) | 7 |
| Unlikely | 43 (93) | 143 |
Adverse events were classified according system organ class and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA).
Adverse events were defined as those that occurred between the administration of the first dose and 30 days after administration of the final dose and deemed unrelated to the study treatment by the investigators. Listed adverse events include those cases designated as serious adverse events.
A patient with multiple events within a category was counted only once in that category.
A serious adverse event was defined as any untoward medical occurrence or effect that at any dose resulted in death, was life-threatening, resulted in hospitalization or prolongation of hospitalization, resulted in persistent or clinically significant disability or incapacity other than may be expected by the effects of the disease-specific mutation, or was deemed to be serious for any other reason.
Device malfunction denote hospitalizations for a dysfunctional ventriculoperitoneal drain, or percutaneous enteral feeding tube.
One patient died from pulmonary sepsis leading to multi-organ failure; postmortem examination confirmed the clinical diagnosis and other causes were excluded.
Adverse events with a probable relationship to the product as deemed by the investigators (adverse reactions) were defined as those adverse events that occurred between the administration of the first dose and 30 days after administration of the final dose and for which a causal relation with Triac could not be excluded.
The majority of serious adverse events concerned intermittent infections which were treated with antibiotics and supportive care. Other causes of hospitalization are displayed in Table 3. All serious adverse events were considered as secondary to MCT8 deficiency and, thus, unrelated to Triac (Table 3). In three subjects with pre-existing seizures an increase in seizure frequency was reported. In one subject this coincided with a gastro-intestinal infection and two subjects had a history of seizures that were difficult to control. Hospitalization was required in two of them in order to treat prolonged seizure or optimize anticonvulsant therapy. Triac treatment was continued during hospital admission in all but one cases. In this case, the occurrence of hepatic insufficiency resulted in hospitalization during which Triac treatment was temporarily withheld; hepatic insufficiency resolved with reduction in anticonvulsant drug dosage and supportive measures. One patient died from pulmonary sepsis leading to multi-organ failure; postmortem examination confirmed the clinical diagnosis and other causes were excluded.
Discussion
In this investigator-initiated international multicentre trial in patients with MCT8 deficiency, serum T3 concentrations were effectively reduced under Triac treatment, and improvements were observed in clinically relevant outcome measures including body weight, heart rate and rhythm, blood pressure and biochemical markers of thyroid hormone action in different tissues.
During treatment with Triac, serum T3 concentrations declined, with 77% of patients achieving serum T3 concentrations within the normal range by 4 months of treatment, requiring a median dose of 38.9 microgram per kilogram per day. This effect was maintained in those patients enrolled in the treatment extension period.
Many key clinical outcomes improved during Triac treatment for 12 months, of which 8 months was at maintenance dosage. An increase in body weight for age z score was noted, with subjects enrolled in the treatment extension period showing a reversal of the natural course of the disorder, with progressive deterioration of body weight, often necessitating enteral tube feeding. In this vulnerable population, being severely underweight is associated with adverse clinical outcomes, including an increased risk of infections which commonly occur in the study population 18. Heart rate decreased predominantly in subjects with elevated heart rate at baseline, for whom this reduction is clinically most relevant. Systolic blood pressure decreased and hypertension resolved in the majority of subjects on Triac treatment. Premature atrial complexes, which are more prevalent in patients with hyperthyroidism 19, subsided in the majority of cases. The high frequency of premature atrial complexes observed in a subset of untreated patients is uncommon in healthy individuals, particularly in children 20, and predisposes to other arrhythmias and cardiac death 21–23. Indeed, sudden death is a frequent cause of mortality in patients with MCT8 deficiency and in one subject an episode of atrial fibrillation was captured during baseline assessment. Thus, improving body weight and the cardiovascular status may ameliorate important risk factors for premature death in MCT8 deficiency.
Triac treatment was associated with reversal of the hypermetabolic state in different tissues (liver, kidney, muscle), reflected by a reduction in serum sex hormone binding globulin concentrations and increase in serum creatinine and creatine kinase concentrations. The positive clinical and biochemical outcomes were sustained in the subgroup followed during the long-term treatment extension period. Except for a decrease in serum T3 concentrations, such effects were not seen in an observational study with diiodothyropropionic acid (DITPA) in four patients 24. With DITPA treatment, (F)T4 and rT3 increased with TSH concentrations remaining unchanged, while with Triac treatment TSH concentrations decreased with a concomitant reduction in (F)T4, T3 and rT3 concentrations. These differential changes in thyroid function tests may point to a different mode of action of DITPA versus Triac.
The consistent reduction in serum T3 concentrations coincided with improvement in body weight, cardiovascular parameters and markers of thyroid hormone action in different tissues. Although the study design, including the lack of a control group and open-label character, does not allow proving causality based on this study alone, the observed unidirectional changes in serum T3 concentrations can likely be attributed to Triac treatment, given the substantial body of preclinical and clinical studies on the effects of Triac (reviewed in 10).
The Triac dose used in this trial was within the range used in previous clinical studies to adequately replace hypothyroid patients (23-48 μg/kg per day) (e.g. 25,26, summarized in 10). Therefore, the thyromimetic effects of Triac in peripheral organs likely compensate adequately for the observed reduction in serum T4 concentrations. This is supported by the absence of clinical and biochemical signs of hypothyroidism which was actively monitored for throughout the study.
It is unknown if the further reduction in circulating T4 concentrations under Triac treatment aggravates the hypothyroid state in the brain in human MCT8 deficiency 27,28. Although MCT8 is believed to be the primary transporter that facilitates both T3 and T4 transport across the human blood-brain barrier, it cannot be excluded that other factors can contribute 29. In various animal models that recapitulate the neuro-motor phenotype of human MCT8 deficiency, it has been reported that Triac resolves brain hypothyroidism and normalizes brain development 8,13. The current trial was not designed to detect whether Triac also modulates neurodevelopment in human MCT8 deficiency, since it lacked specific neurodevelopmental outcomes and enrolled patients of all ages. Therefore, the majority of patients would have passed the small window of opportunity to modulate brain development. A further phase 2 trial will investigate the effects of Triac on neurodevelopmental outcomes in very young children.
The most commonly reported adverse events were all deemed consequences of MCT8 deficiency and unrelated to the intervention. Triac treatment only transiently increased signs of mild hyperthyroidism (perspiration and irritability) after commencement or dose adjustment of treatment in a minority of patients. Although some previous studies have suggested that Triac could increase bone resorption 26,30, we found only marginal increases in bone turnover markers which followed the physiological changes observed during development in children and were not accompanied by noticeable changes in bone mineral density. Although the current observations suggest that Triac treatment is generally well-tolerated, the acquisition of additional safety data in consecutive trials is warranted to extend knowledge of drug safety in this population.
Since we did not select for patient characteristics, the study population comprises a heterogenic sample which constitutes a large proportion of the currently reported patients, and is, as such, representative for the clinical setting. Inherent to studies in such a heterogenic population, the effect size of outcomes varied within the study cohort, suggesting inter-individual variation in degree of benefit between patients. The small sample size did not allow identification or statistical control for factors other than the intervention that may modulate the treatment effects. Moreover, the limited physical and cognitive abilities of the participants, inherent to the disorder, precluded capturing some study parameters in all patients. Together with the presence of drop-outs, this may have, unavoidably, caused selection bias in the data used for statistical analyses.
Being severely underweight and cardiovascular dysfunction are important clinical sequelae of chronic peripheral thyrotoxicosis, causing significant morbidity and mortality in MCT8 deficiency. This study indicates that several key features related to the peripheral phenotype of MCT8 deficiency are alleviated under Triac treatment in paediatric and adult patients.
Supplementary Material
Acknowledgements
Supported by the Netherlands Organisation for Health Research and Development (project number 113303005) (to WEV), the Sherman Foundation (to WEV), the NeMO Foundation (IFMC), the Wellcome Trust (210755/Z/18/Z) (to KC) and NIHR Cambridge Biomedical Centre (to CM, KC), Toulouse University Hospital (to IOP), the Italian MCT8 family association “Una Vita Rara ONLUS” (to DT). The centres in Rotterdam, Berlin, Paris and Toulouse are part of the European Reference Network on rare endocrine conditions (Endo-ERN).
We thank Laboratoires Théranol-Deglaude, and Rare Thyroid Therapeutics for providing the study drug. We thank the patients and their families and care-givers who participated in this trial.
Footnotes
Author Contributions
SG, WEV, RPP, TJV, IFMC, MCYW, FKA, YBR: design of the study
WEV: Principle Investigator
SG: Study Coordinator
SD, JL, MC, LD, HK, DC, FZ, IOP, AS, MP, KC: Principal Investigators at trial sites
All authors: substantial contribution to the coordination and execution of study procedures, and/or data acquisition, and/or interpretation of data for the work
SG and WEV: analyzed the data and wrote the initial draft manuscript on behalf of the authors
All authors critically reviewed and revised the report and have read and approved the final version (except for TJV, LM, MM, who unexpectedly passed away during finalization of the study and manuscript).
Declaration of Interest
The Erasmus MC may receive royalties from Rare Thyroid Therapeutics in the future. None of the authors have personal disclosures relevant to this work.
Contributor Information
Stefan Groeneweg, Academic Center for Thyroid Diseases Erasmus Medical Center, all Rotterdam, The Netherlands.
Prof Robin P. Peeters, Academic Center for Thyroid Diseases Erasmus Medical Center, all Rotterdam, The Netherlands.
Carla Moran, Wellcome Trust-MRC Institute of Metabolic Science University of Cambridge, Cambridge, UK.
Athanasia Stoupa, Paediatric Endocrinology, Diabetology and Gynaecology department, Necker Children’s University Hospital, Imagine Institute affiliate, Paris, France.
Françoise Auriol, Department of Paediatric Endocrinology and Genetics, Children Hospital, University Toulouse Hospital, Toulouse, France.
Davide Tonduti, Child Neurology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Alice Dica, Paediatric Neurology Clinic, Alexandru Obregia Hospital, Bucharest.
Laura Paone, Division of Endocrinology, Bambino Gesu’ Children’s Research Hospital IRCCS, Rome, Italy.
Klara Rozenkova, Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic.
Jana Malikova, Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic.
Adri van der Walt, Panorama Medical Centre, Cape Town, South-Africa.
Irenaeus F.M. de Coo, Sophia Children`s Hospital, Department of Paediatric Neurology.
Anne McGowan, Wellcome Trust-MRC Institute of Metabolic Science University of Cambridge, Cambridge, UK.
Greta Lyons, Wellcome Trust-MRC Institute of Metabolic Science University of Cambridge, Cambridge, UK.
Femke K. Aarsen, Sophia Children’s Hospital, Department of Paediatric Neurology
Diana Barca, Paediatric Neurology Clinic, Alexandru Obregia Hospital, Bucharest; Carol Davila University of Medicine, Department of Neurosciences, Paediatric Neurology Discipline II, Bucharest.
Ingrid. M. van Beynum, Division of Paediatric Cardiology, all Rotterdam, The Netherlands.
Marieke M. van der Knoop, Sophia Children’s Hospital, Department of Paediatric Neurology.
Jurgen Jansen, Department of Paediatrics, Meander Medical Center, Amersfoort, the Netherlands.
Martien Manshande, Academic Center for Thyroid Diseases; The Netherlands Julianadorp.
Roelineke J. Lunsing, Department of Child Neurology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Stan Nowak, Department of Paediatrics, Refaja Hospital, Stadskanaal, The Netherlands.
Corstiaan A. den Uil, Department of Cardiology and Intensive Care Medicine.
Prof M. Carola Zillikens, From the Erasmus Medical Center, Department of Internal Medicine.
Frank E. Visser, The Netherlands Heeren Loo, Ermelo.
Paul Vrijmoeth, General Practitioner Baalderborg, Hardenberg, The Netherlands.
Marie Claire Y. de Wit, Sophia Children’s Hospital, Department of Paediatric Neurology.
Nicole I. Wolf, The Netherlands Department of Child Neurology, Emma Children’s Hospital, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam and Amsterdam Neuroscience, Amsterdam.
Angelique Zandstra, General Practitioner Latyrus, Dedemsvaart, The Netherlands.
Gautam Ambegaonkar, Department of Paediatric Neurology, Cambridge University Hospital NHS Foundation Trust, UK.
Yogen Singh, Department of Paediatric Cardiology, Addenbrooke’s Hospital.
Prof Yolanda B. de Rijke, Department of Clinical Chemistry
Prof Enrico S. Bertini, Unit of Neuromuscular and Neurodegenerative Disorders.
Prof Jan Lebl, Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic.
Marco Cappa, Division of Endocrinology, Bambino Gesu’ Children’s Research Hospital IRCCS, Rome, Italy.
Prof Linda De Meirleir, Paediatric Neurology Unit, Department of Paediatrics, UZ Brussel, Belgium.
Prof Heiko Krude, Department of Paediatric Endocrinology and Diabetology, Charité-Universitätsmedizin Berlin, Berlin, Germany.
Prof Dana Craiu, Paediatric Neurology Clinic, Alexandru Obregia Hospital, Bucharest; Carol Davila University of Medicine, Department of Neurosciences, Paediatric Neurology Discipline II, Bucharest.
Federica Zibordi, Child Neurology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Isabelle Oliver Petit, Department of Paediatric Endocrinology and Genetics, Children Hospital, University Toulouse Hospital, Toulouse, France.
Prof Michel Polak, Paediatric Endocrinology, Diabetology and Gynaecology department, Necker Children’s University Hospital, Imagine Institute affiliate, Paris, France.
Krishna Chatterjee, Wellcome Trust-MRC Institute of Metabolic Science University of Cambridge, Cambridge, UK.
Prof Theo J. Visser, Academic Center for Thyroid Diseases
W. Edward Visser, Academic Center for Thyroid Diseases.
References
- 1.Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278(41):40128–35. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 2.Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364(9443):1435–7. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 3.Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–75. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser WE, Vrijmoeth P, Visser FE, Arts WF, van Toor H, Visser TJ. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin Endocrinol (Oxf) 2013;78(2):310–5. doi: 10.1111/cen.12023. [DOI] [PubMed] [Google Scholar]
- 5.Groeneweg S, Visser WE, Visser TJ. Disorder of thyroid hormone transport into the tissues. Best Pract Res Clin Endocrinol Metab. 2017;31(2):241–53. doi: 10.1016/j.beem.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med. 2009;360(15):1574–5. doi: 10.1056/NEJMc0809750. [DOI] [PubMed] [Google Scholar]
- 7.Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26(10):1343–421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 8.Kersseboom S, Horn S, Visser WE, et al. In vitro and mouse studies supporting therapeutic utility of triiodothyroacetic acid in MCT8 deficiency. Mol Endocrinol. 2014;28(12):1961–70. doi: 10.1210/me.2014-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messier N, Langlois MF. Triac regulation of transcription is T(3) receptor isoform- and response element-specific. Mol Cell Endocrinol. 2000;165(1-2):57–66. doi: 10.1016/s0303-7207(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 10.Groeneweg S, Peeters RP, Visser TJ, Visser WE. Triiodothyroacetic acid in health and disease. J Endocrinol. 2017;234(2):R99–R121. doi: 10.1530/JOE-17-0113. [DOI] [PubMed] [Google Scholar]
- 11.Bracco D, Morin O, Schutz Y, Liang H, Jequier E, Burger AG. Comparison of the metabolic and endocrine effects of 3,5,3’-triiodothyroacetic acid and thyroxine. J Clin Endocrinol Metab. 1993;77(1):221–8. doi: 10.1210/jcem.77.1.8325946. [DOI] [PubMed] [Google Scholar]
- 12.Burger AG, Engler D, Sakoloff C, Staeheli V. The effects of tetraiodothyroacetic and triiodothyroacetic acids on thyroid function in euthyroid and hyperthyroid subjects. Acta Endocrinol (Copenh) 1979;92(3):455–67. doi: 10.1530/acta.0.0920455. [DOI] [PubMed] [Google Scholar]
- 13.Zada D, Tovin A, Lerer-Goldshtein T, Appelbaum L. Pharmacological treatment and BBB-targeted genetic therapy for MCT8-dependent hypomyelination in zebrafish. Dis Model Mech. 2016;9(11):1339–48. doi: 10.1242/dmm.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 16.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31(3):341–52. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 18.Falagas ME, Athanasoulia AP, Peppas G, Karageorgopoulos DE. Effect of body mass index on the outcome of infections: a systematic review. Obes Rev. 2009;10(3):280–9. doi: 10.1111/j.1467-789X.2008.00546.x. [DOI] [PubMed] [Google Scholar]
- 19.von Olshausen K, Bischoff S, Kahaly G, et al. Cardiac arrhythmias and heart rate in hyperthyroidism. Am J Cardiol. 1989;63(13):930–3. doi: 10.1016/0002-9149(89)90142-2. [DOI] [PubMed] [Google Scholar]
- 20.Scott O, Williams GJ, Fiddler GI. Results of 24 hour ambulatory monitoring of electrocardiogram in 131 healthy boys aged 10 to 13 years. Br Heart J. 1980;44(3):304–8. doi: 10.1136/hrt.44.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121(17):1904–11. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 22.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 23.Perez MV, Dewey FE, Marcus R, et al. Electrocardiographic predictors of atrial fibrillation. Am Heart J. 2009;158(4):622–8. doi: 10.1016/j.ahj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97(12):4515–23. doi: 10.1210/jc.2012-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechelany C, Schlumberger M, Challeton C, Comoy E, Parmentier C. TRIAC (3,5,3’-triiodothyroacetic acid) has parallel effects at the pituitary and peripheral tissue levels in thyroid cancer patients treated with L-thyroxine. Clin Endocrinol (Oxf) 1991;35(2):123–8. doi: 10.1111/j.1365-2265.1991.tb03509.x. [DOI] [PubMed] [Google Scholar]
- 26.Sherman SI, Ringel MD, Smith MJ, Kopelen HA, Zoghbi WA, Ladenson PW. Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J Clin Endocrinol Metab. 1997;82(7):2153–8. doi: 10.1210/jcem.82.7.4054. [DOI] [PubMed] [Google Scholar]
- 27.Barez-Lopez S, Obregon MJ, Martinez-de-Mena R, Bernal J, Guadano-Ferraz A, Morte B. Effect of Triiodothyroacetic Acid Treatment in Mct8 Deficiency: A Word of Caution. Thyroid. 2016;26(5):618–26. doi: 10.1089/thy.2015.0388. [DOI] [PubMed] [Google Scholar]
- 28.Visser WE, Heuer H, Visser TJ. Triiodothyroacetic Acid Treatment in MCT8 Deficiency: A Word of Nuance. Thyroid. 2016;26(5):615–7. doi: 10.1089/thy.2016.0191. [DOI] [PubMed] [Google Scholar]
- 29.Bernal J, Guadano-Ferraz A, Morte B. Thyroid hormone transporters--functions and clinical implications. Nat Rev Endocrinol. 2015;11(7):406–17. doi: 10.1038/nrendo.2015.66. [DOI] [PubMed] [Google Scholar]
- 30.Brenta G, Schnitman M, Fretes O, et al. Comparative efficacy and side effects of the treatment of euthyroid goiter with levo-thyroxine or triiodothyroacetic acid. J Clin Endocrinol Metab. 2003;88(11):5287–92. doi: 10.1210/jc.2003-030095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.