Abstract
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) disease (COVID-19) pandemic has caused millions of deaths worldwide. Genome-wide association studies (GWAS) identified the 3p21.31 region as conferring a two-fold increased risk of respiratory failure. Here, using a combined multiomics and machine-learning approach, we identify the gain-of-function risk A allele of a single-nucleotide polymorphism (SNP), rs17713054G>A, as a probable causative variant. We show with chromosome conformation capture and gene expression analysis that the rs17713054-affected enhancer upregulates the interacting gene, Leucine Zipper Transcription Factor Like 1 (LZTFL1). Selective spatial transcriptomic analysis of COVID-19 patient lung biopsies shows the presence of signals associated with epithelial-mesenchymal transition (EMT), a viral response pathway that is regulated by LZTFL1. We conclude that pulmonary epithelial cells undergoing EMT, rather than immune cells, are likely to be responsible for the 3p21.31 associated risk. As the 3p21.31 effect is conferred by a gain-of-function, LZTFL1 may provide a therapeutic target.
Keywords: COVID-19, Coronavirus, gene regulation, chromosome conformation capture, machine learning, respiratory epithelium
Introduction
The COVID-19 pandemic is estimated to have caused over 4.6 million deaths so far1,2. The predominant cause of mortality is pneumonia and severe acute respiratory distress syndrome3. However, COVID-19 can cause multiple organ failure through cytokine release, microvascular and macrovascular thrombosis, endothelial damage, acute kidney injury and myocarditis4–6. GWAS are important for identifying candidate genes and pathways that predispose to complex diseases7 and genetically validated drug targets are more likely to lead to approved drugs8. Two large GWAS have been carried out to determine whether common variants drive susceptibility to severe COVID-199,10. Both studies identified a region of chromosome 3p21.31 as having the strongest association, whilst a third study also identified this locus as conferring susceptibility to infection11. The 3p21.31 risk haplotype, which arises from Neanderthal DNA12 and is currently unexplained with regards to the causal variant(s), causal gene(s) and specific role in COVID-19, confers a two-fold increased risk of respiratory failure from COVID-199,10, and an over two-fold increased risk of mortality for under 60 year-olds13. Additionally, the risk variants at this locus are carried by >60% of individuals with South Asian ancestries (SAS), compared to 15% of white European ancestry (EUR) groups, partially explaining the ongoing higher death rate in this population in the United Kingdom14,15.
Identifying the causal gene(s) and mechanism(s) behind GWAS hits poses several challenges. First, a causative variant is usually in linkage disequilibrium (LD) with many other variants and these can take different forms (SNPs, insertions, deletions and structural polymorphisms). Secondly, the genetic signals are completely cell-type agnostic, which makes it challenging to identify appropriate experimental models for further investigation. Thirdly, there are multiple mechanisms by which variants can have an effect. Alteration of the protein coding sequence or RNA splicing, both of which are relatively straightforward to disentangle, account for fewer than 20% of associations in polygenic disease16. The remaining variants and their target gene(s) can be very difficult to decode. Many are thought to lie within cis-regulatory elements17, such as enhancers, which are short DNA sequences that often control tissue- and developmental-stage-specific gene expression. Deciphering the variants that affect enhancers is challenging because many enhancers are only active in specific cell types or at specific times, enhancers are often distant in the linear DNA sequence (often 104-106 bp) from the genes they control, and the effects of sequence changes are not straightforward to predict.
We have developed a comprehensive platform for decoding the effects of sequence variation identified by GWAS16 (Extended Data Fig. 1a). This combines computational and “wet lab” approaches to delineate the identity of causative variants, cell types involved and effector genes. Initially, GWAS-identified haplotypes are screened for potential protein coding sequence variants. Variants altering splice sites are then assessed using a combination of machine learning18 and RNA-seq analysis. Conventional genomic approaches are then combined with machine learning19 to define whether variants lie within, and affect, cis-regulatory sequences from a panel of disease relevant cell types; this allows for the identification of the key cell type(s) and to determine the likely causative variant. Subsequently, chromosome conformation capture (3C) analysis20–22 is used to identify the gene promoters, which physically contact the candidate enhancer sequence in the relevant cell type(s), and these data are integrated with gene expression analyses. Finally, genome editing is used to validate regulatory effects of prioritized variants.
Here, we have applied this approach to identify rs17713054 as a probable causative variant and LZTFL1 as a candidate effector gene in pulmonary epithelial cells as contributing to the strong COVID-19 association at the 3p21.31 locus; with EMT identified as a relevant infection response pathway.
Results
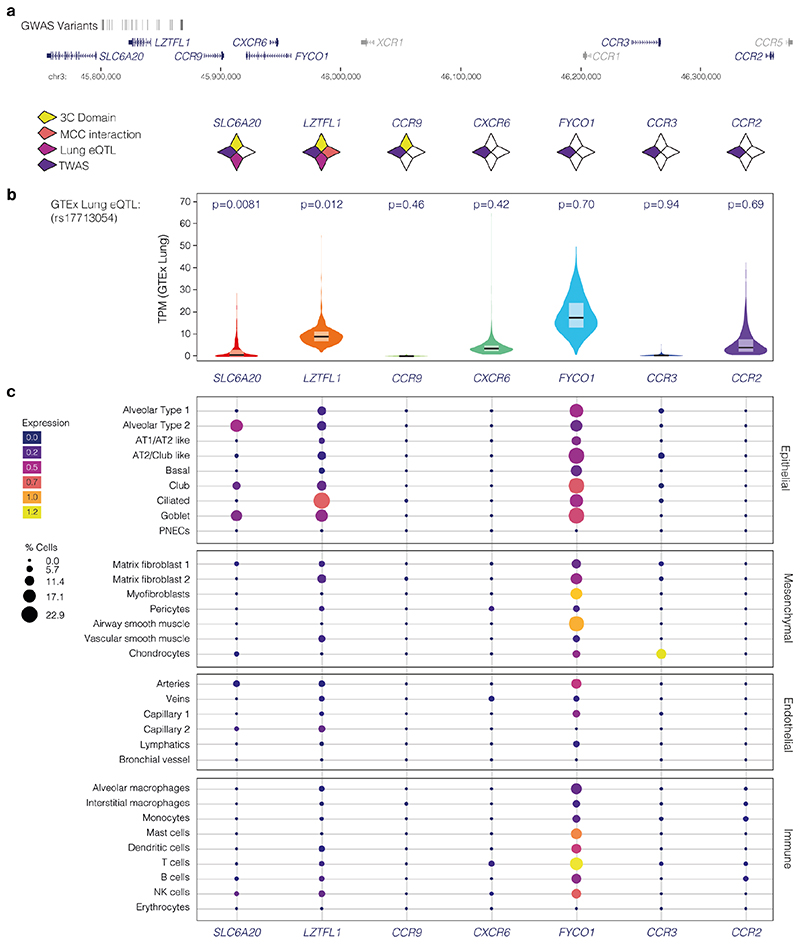
The rs17713054 risk allele generates a CEBPB motif
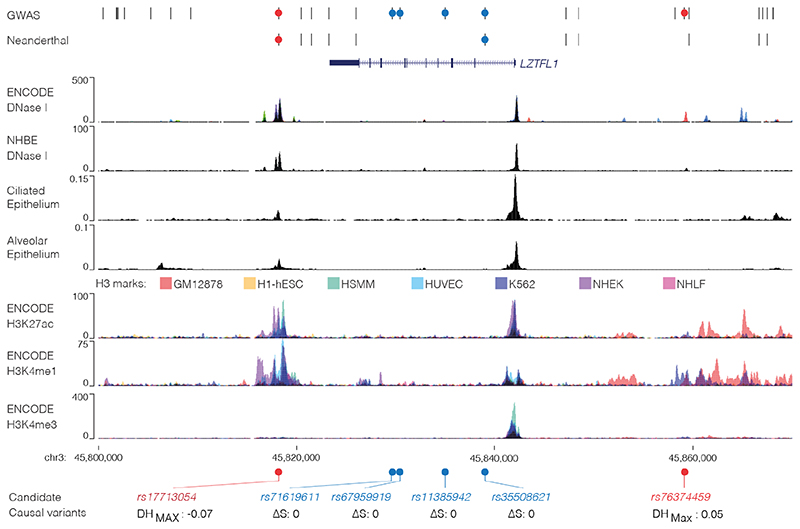
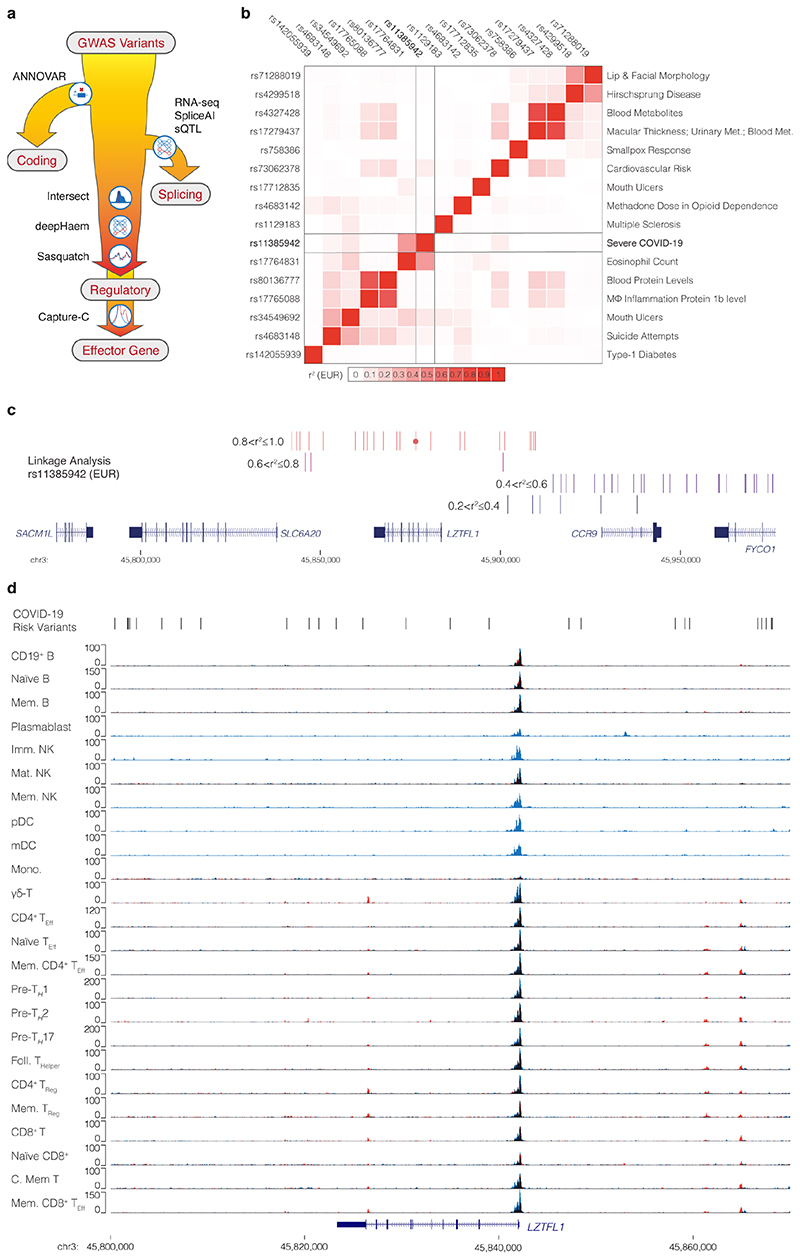
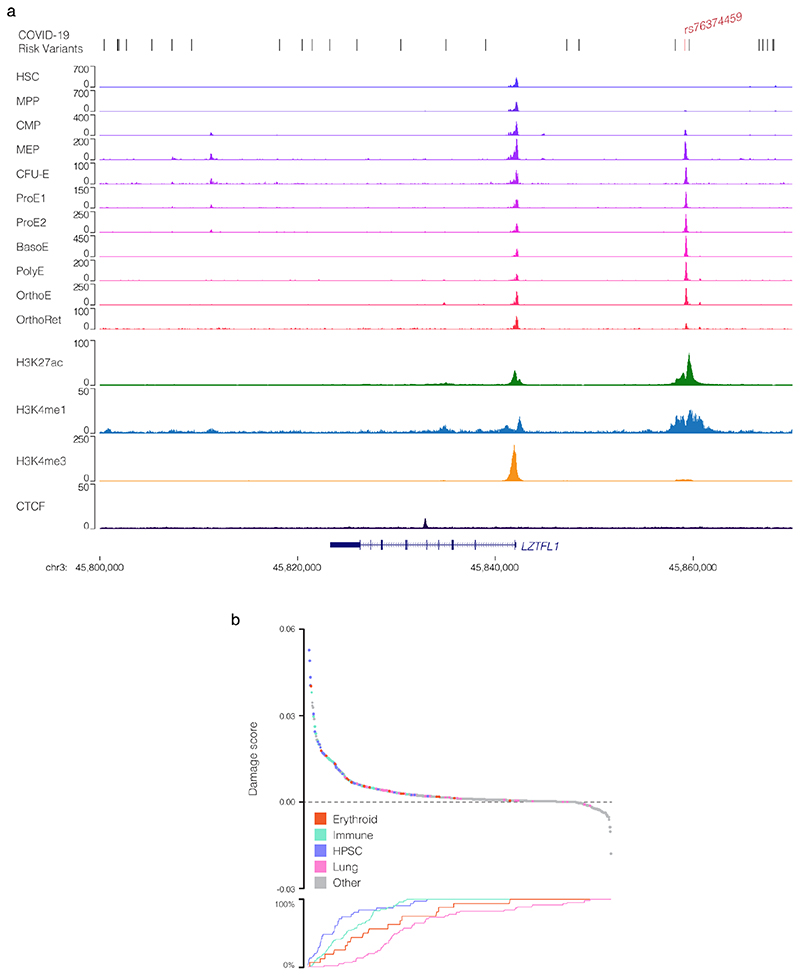
The 3p21.31 region contains variants associated with the autoimmune diseases type-1 diabetes23 and multiple sclerosis24, though the lead and tag variants identified in these studies are not in high LD with those associated with COVID-19 severity (Extended Data Fig. 1b). There are 28 candidate risk variants in LD with the original genome-wide significant SNPs9 at 3p21.31 (r2 > 0.8, EUR; Extended Data Fig. 1c). None of these variants affect coding sequences. One SNP, rs35624553 is in the 3’ UTR of the gene LZTFL1 (Fig. 1), but this is not a conserved miRNA binding site25 and neither miRdSNP26 nor MicroSNiPer27 predict the variant alters miRNA binding. Four other variants are within LZTFL1 introns, including the lead SNP rs113859429. None of these are predicted to alter mRNA splicing of LZTFL1, either by machine learning with SpliceAI18 or splicing quantitative trait loci (sQTL) based approaches28, and the nearest exon junction to these variants is ~500 bp (Fig. 1). Therefore, a cis-regulatory mechanism seems to be the most likely explanation for this haplotype.
Figure 1. Identification of a potentially causative COVID-19 risk variant.
COVID-19 risk variants from GWAS were assessed for multiple mechanisms. All genome-wide significant variants and linked variants are shown (GWAS) as are variants present in the Vindija Neanderthal12 risk haplotype. Circles indicate variants assessed for splicing changes (blue circles, SpliceAI18: ΔS score [0-1, where 1 is most damaging]), and presence in cis-regulatory elements using open chromatin in 95 ENCODE overlaid DNase I datasets (red circles), normal human bronchial epithelial cells (NHBE), and single-cell ATAC-seq from fetal ciliated epithelium and alveolar epithelium34. Histone H3 modification tracks show presence of marks associated with active transcription (H3K27ac) at enhancers (H3K4me1) and promoters (H3K4me3). Variants in open chromatin are given deepHaem damage scores (DH, 0-1) with sign indicating increased (-) or decreased (+) accessibility. Region shown is chr3:45,800,000-45,870,000, hg38.
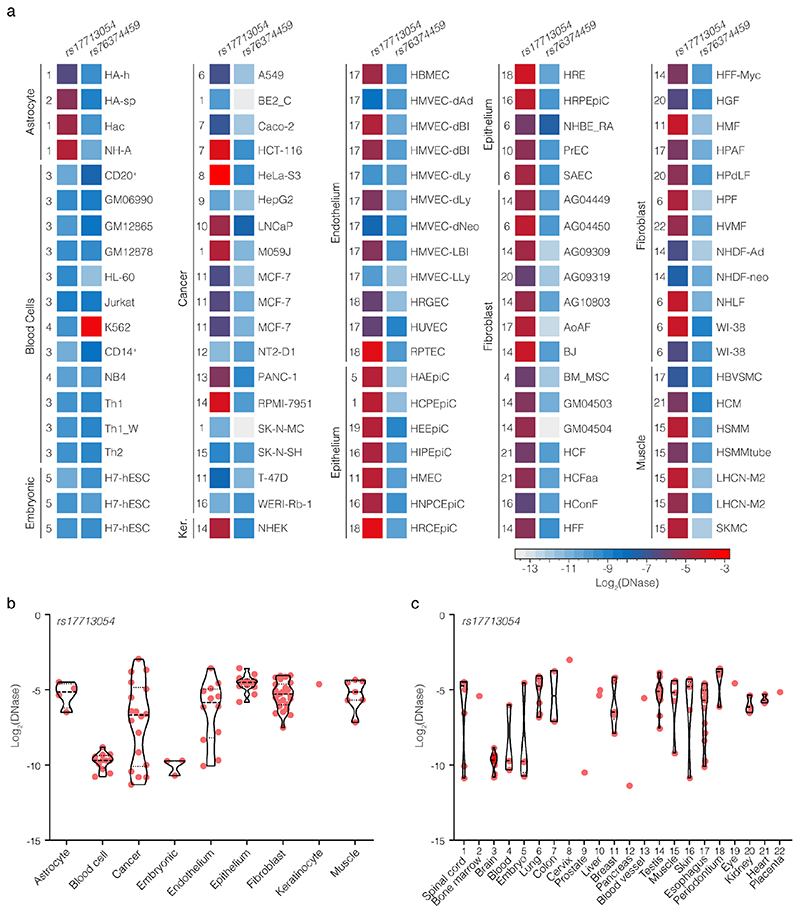
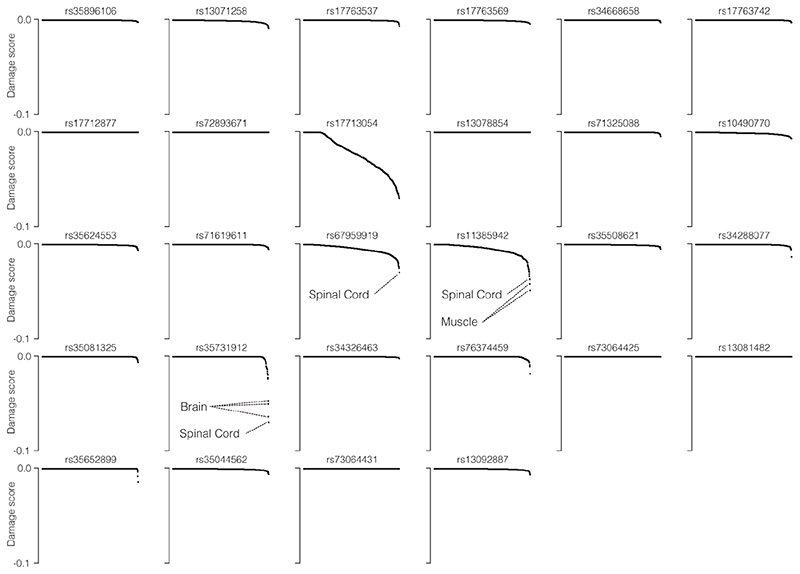
We first examined open chromatin from 24 diverse immune cell populations29 (including T, B, Natural Killer and dendritic cells) in resting and stimulated states, but did not identify any of the 28 severe COVID-19 associated variants at 3p21.31 in open chromatin (Extended Data Fig. 1d); making it unlikely that a cis-regulatory mechanism in these immune cell types is responsible. By considering open-chromatin data from 95 diverse cell types we identified two SNPs, rs17713054 and rs76374459, which lie in open chromatin30 (Fig. 1, Extended Data Fig. 2). Machine-learning approaches have proven accurate at predicting allele-specific changes in transcription factor binding and chromatin accessibility31,32, including for de novo gain-of-function changes33. We have previously developed a machine-learning model, deepHaem19, which uses 694 DNase I hypersensitivity and ATAC-seq datasets to predict changes to active regulatory elements. Importantly, deepHaem predicted that the 26 variants not in open chromatin have no strong gain-of-function effect in any cell type (Extended Data Fig. 3).
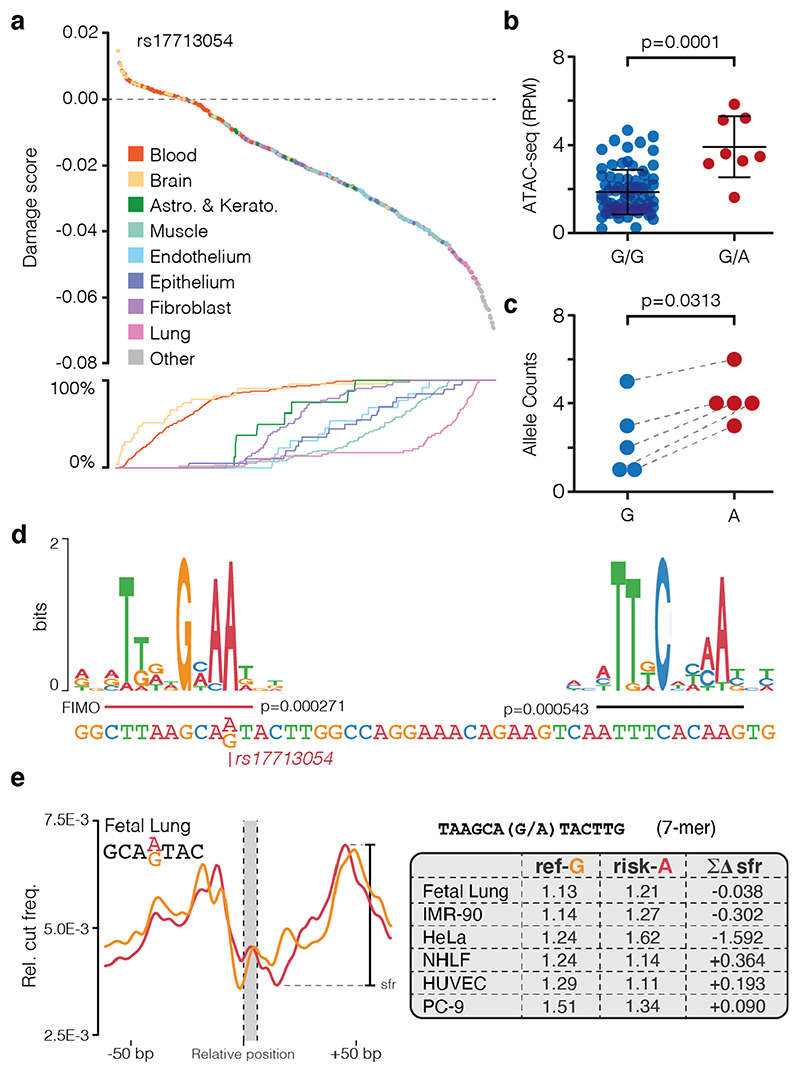
Of the two variants in open chromatin, rs76374459 is unlikely to be causative. It is not contained within the Vindija Neanderthal risk haplotype12 and is not in tight LD with the 3p21.31 lead SNPs from either of two GWAS9,10 (rs11385942 r2 = 0.737/0.058, EUR/SAS; rs73064425 r2 = 0.747/0.058, EUR/SAS). In addition, it is in an erythroid-specific enhancer, a cell-type not strongly implicated in SARS-CoV-2 infection, and it is not predicted by machine learning to cause damaging effects (Fig. 1, Extended Data Figs. 2,4). In contrast, rs17713054 is likely to be a causative SNP as it is in tight LD with both lead SNPs (rs11385942 r2 = 1.0/1.0, EUR/SAS; rs73064425 r2 = 0.986/0.995, EUR/SAS), is located in open chromatin in numerous COVID-19-relevant cell types including epithelial and endothelial cells (Fig. 1, Extended Data Fig. 2), where it is marked by epigenetic modifications associated with active enhancers (histone H3 lysine-4 monomethylation [H3K4me1] and histone H3 lysine-27 acetylation [H3K27ac]). Inspection of single-cell ATAC-seq from healthy lung34,35 shows this enhancer is present in several lung epithelial cell types, including the ciliated epithelia and club cells, which line the respiratory tract, and in Type I and Type II pneumocytes, which form the alveoli (Fig. 1, Extended Data Fig. 5). Interestingly, deepHaem predicts the rs17713054 risk allele, which is the minor allele A (MAF: 0.0817 EUR, 0.377 SAS36), acts as a gain-of-function by augmenting an existing enhancer; resulting in increased chromatin accessibility in both epithelial and endothelial cells, and particularly in primary lung tissue (Fig. 2a). Analysis of ATAC-seq for human aortic endothelial cells (HAECs) from 48 individuals37 showed that the rs17713054-containing enhancer was significantly more accessible in heterozygous A/G donors than homozygous G/G donors (Fig. 2b), and, in heterozygous samples, more reads originated the risk A allele than the non-risk G allele (Fig. 2c).
Figure 2. rs17713054 creates a CEBPB motif.
a, Ranked deepHaem chromatin accessibility damage scores for the risk A allele of rs17713054 in 694 cell-types including primary cells. Line plot shows cumulative percentage of samples for each tissue, indication that lung tissue is enriched in the highly ranked damaging variants. b, Quantification of ATAC-seq reads in the rs17713054 enhancer (chr3:45,817,661-45,818,660, hg38) from aortic endothelium. Bars show mean and one standard deviation. Two-tailed Mann-Whitney rank sum test, testing different accessibility of the two genotypes, G/G n = 78 and G/A n = 8 independent experiments. c, ATAC-seq reads over rs17713054 alleles in heterozygous individuals, grey lines denote paired counts from a single replicate. One-sided Wilcoxon matched-pairs signed rank test, testing higher accessibility of the A allele, n = 5. Three replicates were excluded due to low coverage. d, CEBPB DNA binding motif over sequence around the rs17713054 risk-A and non-risk-G alleles. P values for motifs were determined using FIMO with reference and variant sequence for the entire enhancer and Jaspar motif MA0466.1. The motif over rs17713054 was only identified in sequence with the A allele. e, Sasquatch DNase I hypersensitivity profile and shoulder-footprint ratio (sfr) scores for rs17713054 risk and non-risk (ref-G) alleles using DNase I datasets for a subset of cells with open chromatin at this site. Larger sfr scores indicate a deeper footprint associated with greater likelihood of being bound by a transcription factor. Δsfr scores are generated by subtracting risk-A sfr from ref-G sfr, negative values show an increased footprint depth in the risk allele.
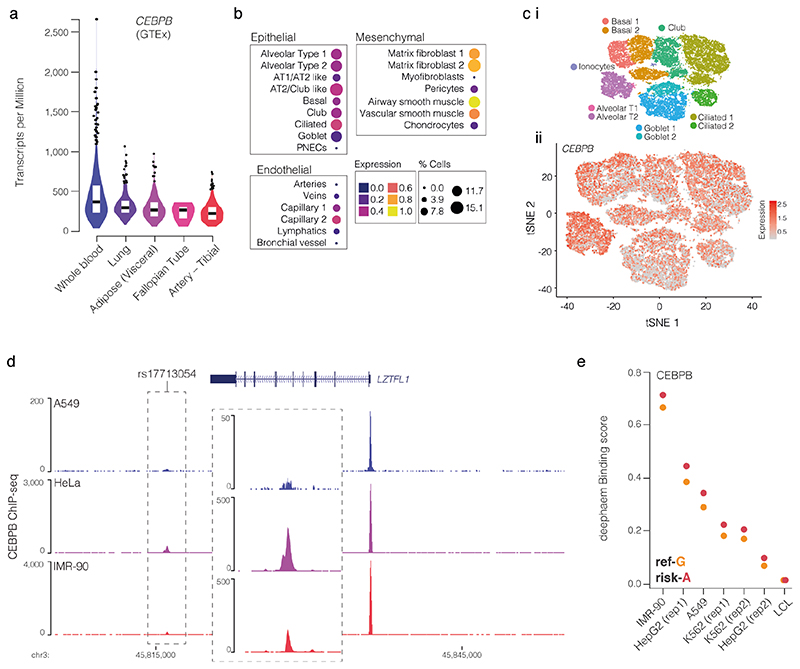
Sequence analysis shows that the risk allele generates a second CCAAT/enhancer binding protein beta (CEBPB) motif38 in the enhancer (Fig. 2d). The biological relevance of this new motif is supported by strong expression of CEBPB in lung tissue28, and ChIP-seq of CEBPB in HeLa, A549 alveolar basal epithelial adenocarcinoma, and IMR-90 lung fibroblast cells39 – which are homozygous G/G non-risk – shows weak binding at the enhancer (Extended Data Fig. 6a-d). Furthermore, deepHaem predicts rs17713054-A leads to increased CEBPB binding in IMR-90 and A549 cells (Extended Data Fig. 6e). An orthogonal DNase I hypersensitivity footprinting based approach, Sasquatch40, uses genome-wide, cell-type-specific motif footprints to predict how sequence-specific changes alter transcription factor binding. This found that motifs containing either allele have strong DNase I footprints. When comparing motifs with the risk-A allele with the non-risk G allele, risk-A motifs showed a weak gain in accessibility in fetal lung and IMR-90 lung fibroblast cells (Fig. 2e), corroborating a gain-of-function mechanism.
rs1773054 enhancer interacts with LZTFL1 promoter
The 3p21.31 locus is gene dense and contains several candidates that could potentially be involved in COVID-19 pathogenesis. These include three chemokine receptors: CCR9 (which encodes a lymphocyte-expressed C-C chemokine receptor41); CXCR6 (which is associated with sarcoidosis and is a co-receptor for HIV42,43) and XCR1 (which encodes a X-C chemokine receptor). Transcriptome-wide association study (TWAS) analysis has also identified CCR2, CCR3 and FYCO1, which lie up to 500 kb away, as candidate effector genes for the 3p21.31 COVID-19 association10. In addition, there are the two nearest genes that are less well studied: SLC6A20 (the SIT1 imino acid transporter associated with glycinuria44) and LZTFL1 (Leucine zipper transcription factor-like 145), homozygous loss of which causes the classical ciliopathy Bardet-Biedl Syndrome46,47.
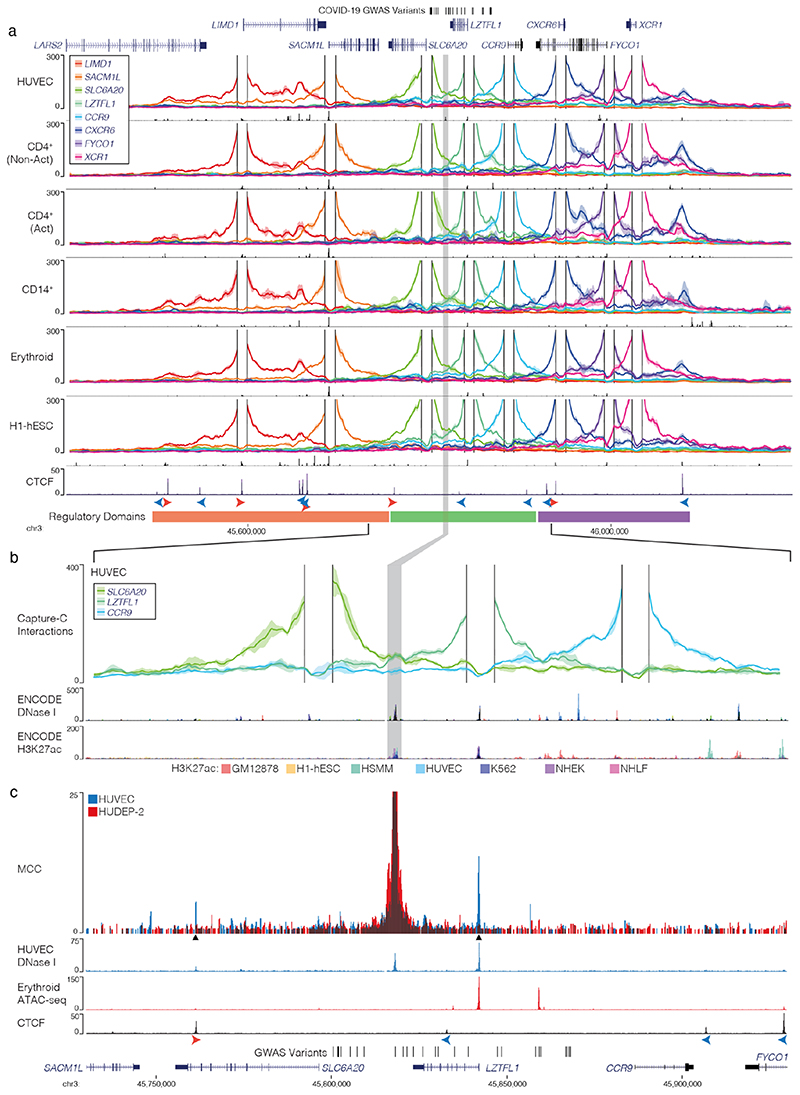
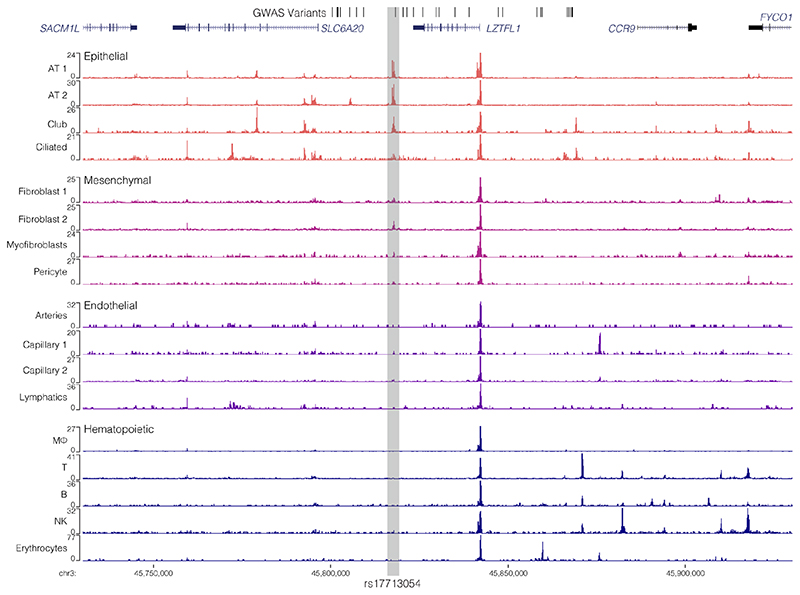
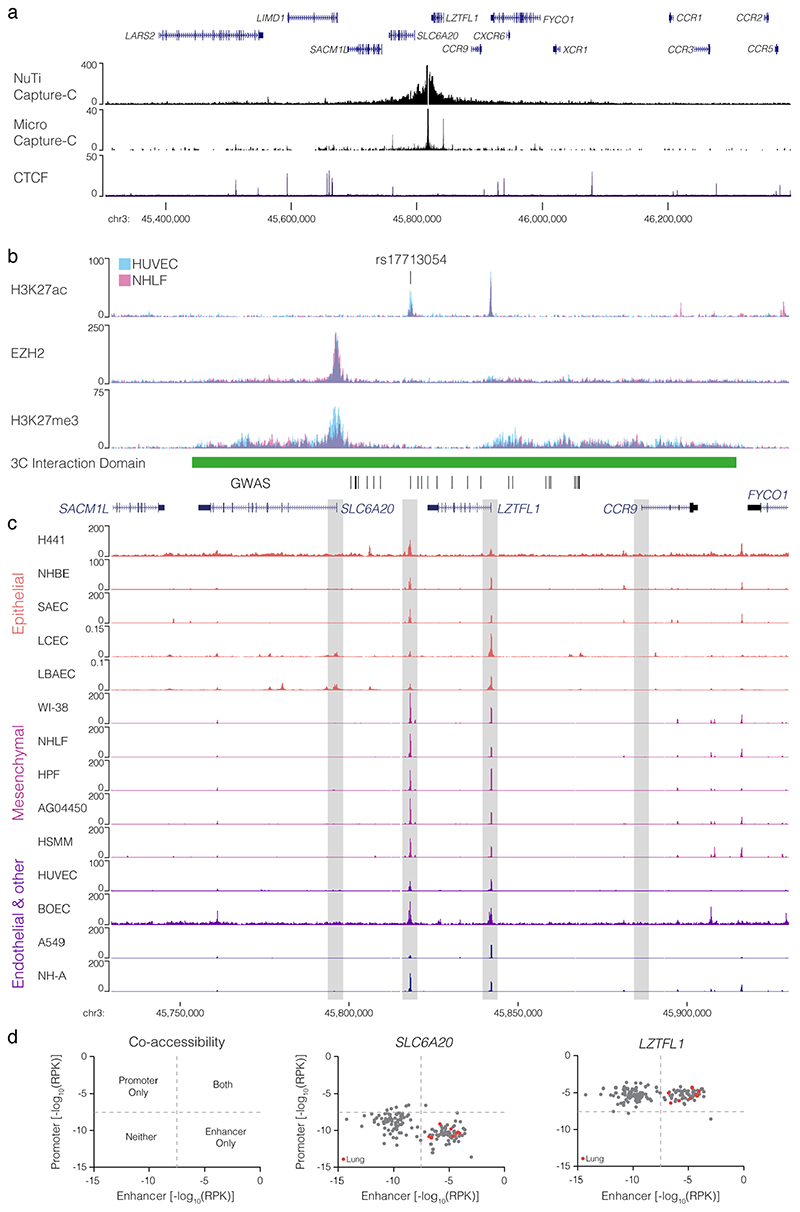
To identify candidate target genes of the rs17713054 enhancer we performed NuTi Capture-C20,21 from the promoters of genes in surrounding regulatory domains (Methods) in primary human umbilical vein endothelial cells (HUVEC) in which the rs17713054 enhancer is accessible, as well as resting and stimulated primary CD4+ T-cells, primary CD14+ monocytes, CD71+ CD235+ erythroid cells, and embryonic stem cells (H1-hESCs), where the enhancer is not accessible. In all cell types tested, all 28 COVID-19-associated variants fell within a domain of interaction that contained only the promoters of LZTFL1, SLC6A20, and CCR9, and is delimited by convergent CTCF boundary motifs (Fig. 3a). Within this domain, the promoters of both LZTFL1 and SLC6A20 interacted more strongly with the rs17713054 enhancer than CCR9 (Fig. 3b). Reciprocal Capture-C from the rs17713054 enhancer also showed its interactions were primarily constrained to the same domain (Extended Data Fig. 7a). Notably, inside this domain, several tissue-specific enhancers could be seen for immune, erythroid and endothelial cell types; altering the interaction profile of the ubiquitously accessible LZTFL1 promoter and indicating dynamic regulation (Supplementary Fig. 1).
Figure 3. The interaction landscape of the severe COVID-19 risk locus.
a, DpnII Capture-C derived mean interaction count (n = 3 for all except CD14+: n = 2) and one standard deviation (shading) for gene promoters in human vein endothelial cells (HUVEC), resting and activated T-Cells (CD4+ Non-Act/Act), monocytes (CD14+), CD235+ CD71+ erythroid cells and human embryonic stem cells (H1-hESCs). The enhancer containing rs17713054 is highlighted by a grey box. ATAC-seq/DNase I for each cell-type is shown underneath in black. CTCF track shows binding of the CCAAT-binding factor which acts as a boundary with forward and reverse motif orientation shown with arrowheads (red and blue respectively). Three broad regulatory domains were identified as regions with overlapping interactions. Region: chr3:45,400,000-46,200,000, hg38. Per fragment interactions were smoothed using 400-bp bins and an 8-kb window. b, The rs17713054 regulatory domain in endothelial cells (HUVEC). Overlaid DNase I shows accessible sites in 95 cell types and H3K27ac shows active elements. Region: chr3:45,730,000-45,930,000, hg38. Per fragment interactions were smoothed using 250-bp bins and a 5-kb window. Solid line shows mean interaction count (n = 3 independent samples) with one standard deviation (shading). c, Micro Capture-C (MCC) of the rs17713054 enhancer in endothelial (HUVEC, blue) and erythroid (HUDEP-2, red) cells with tissue specific open chromatin tracks (n = 3). Peak analysis of MCC using LanceOtron to compare HUVEC and HUDEP-2 profiles identified two significantly enriched peaks in HUVEC cells (black triangles, P ≤ 1 × 10-999) which correspond to the LZTFL1 promoter and the upstream CTCF site.
We went on to perform Micro Capture-C (MCC), a 3C method that provides higher resolution data than conventional approaches22, from the rs17713054 enhancer in endothelial cells. MCC in HUVECs delineated significant tissue-specific interaction with the LZTFL1 promoter and the nearest upstream boundary CTCF site but no other significant peaks of interactions with any of the other gene promoters in the region (Fig. 3c, Extended Data Fig. 7a). Importantly, we did not find a peak of interaction with SLC6A20, likely because ENCODE datasets show that SLC6A20 carries Polycomb repression marks in endothelial (HUVEC) and fibroblast (NHLF) cells (Extended Data Fig. 7b). Additionally, the LZTFL1 promoter is more consistently accessible in cells where rs17713054 is also accessible (Extended Data Fig. 7c,d). Therefore, LZTFL1 seems to be the most likely direct regulatory target of the rs17713054-containing epithelial-endothelial-fibroblast enhancer.
rs17713054-A associates with higher gene expression in lung
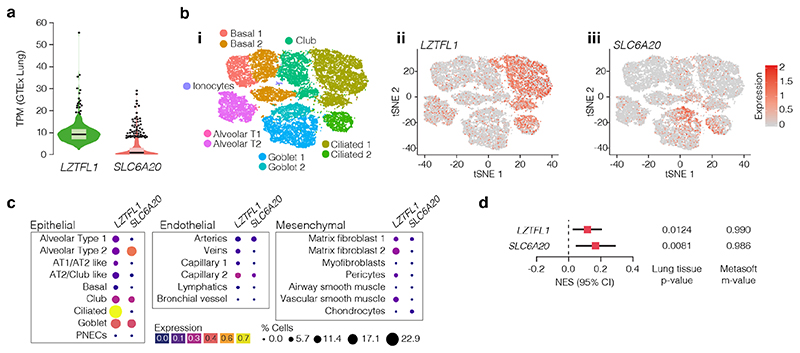
Disease biology, deepHaem, TWAS analysis10 and a Phenome-wide association study11 (PheWAS) identified lung tissue and function as key for the 3p21.31 COVID-19 association. Analysis of whole-lung RNA-seq28 showed that LZTFL1 is strongly expressed in the lung (Fig. 4a), and single-cell RNA-seq48 shows that LZTFL1 is present throughout the respiratory epithelium, but predominantly expressed in ciliated cells (Fig. 4b,c). Of the other candidate genes identified here and elsewhere10,49,50 (SLC6A20, CCR2, CCR3, CCR9, CXCR6, and FYCO1), only SLC6A20 and FYCO1 are consistently expressed in both lung bulk and single-cell RNA-seq datasets, though CCR2 and CXCR6 were found in bulk RNA-seq. FYCO1 was found in most cell types and SLC6A20 was restricted to Goblet cells and Alveolar Type II pneumocytes (Fig. 4, Extended Data Fig. 8). Analysis using the Genotype-Tissue Expression28 (GTEx) portal for expression quantitative trait loci (eQTLs) showed that the rs17713054-A risk-allele is associated with higher levels of expression in the lung of LZTFL1 and SLC6A20 but not the other genes (Fig. 4d, Extended Data 8). Colocalization analysis51 shows that these GWAS and eQTL associations are more likely as a result of a single variant (PP = 0.2657) than two distinct variants (PP = 0.0566).
Figure 4. Pulmonary expression analysis of LZTFL1 and SLC6A20.
a, GTEx whole-lung RNA-seq expression profiles for LZTFL1 and SLC6A20 as transcripts per million (TPM). For violin plots, minima and maxima are the top and bottom of the violin, black lines show means, ends of the pale regions denote first and third quartiles, and black dots denote outliers (n = 578 independent samples). b, 10x Genomics Chromium droplet single-cell RNA sequencing (scRNA-seq) from upper and lower airways and lung parenchyma48 from healthy volunteers or deceased transplant donors with ten epithelial populations (i). scRNA-seq expression profiles for LZTFL1 (ii) and SLC6A20 (iii). c, Chromium single-nucleus RNA-seq35 from non-diseased adult lung (n = 3) with 22 epithelial, endothelial and mesenchymal populations, including alveolar type 1 (AT) and type 2 (AT2) pneumocytes and pulmonary neuroendocrine cells (PNECs). d, GTEx eQTL analysis the rs17713054 risk-A allele in lung (n = 515 independent samples). Normalized effect size (NES) is the slope of the linear regression comparing the alternate (A) allele to the reference (G) allele. NES are calculated in a normalized space where magnitude has no direct biological interpretation. Lines show the 95% confidence interval, with significance values for single tissue (two-sided P value without multiple test correction) and multi-tissue (posterior probability/m-value) analyses.
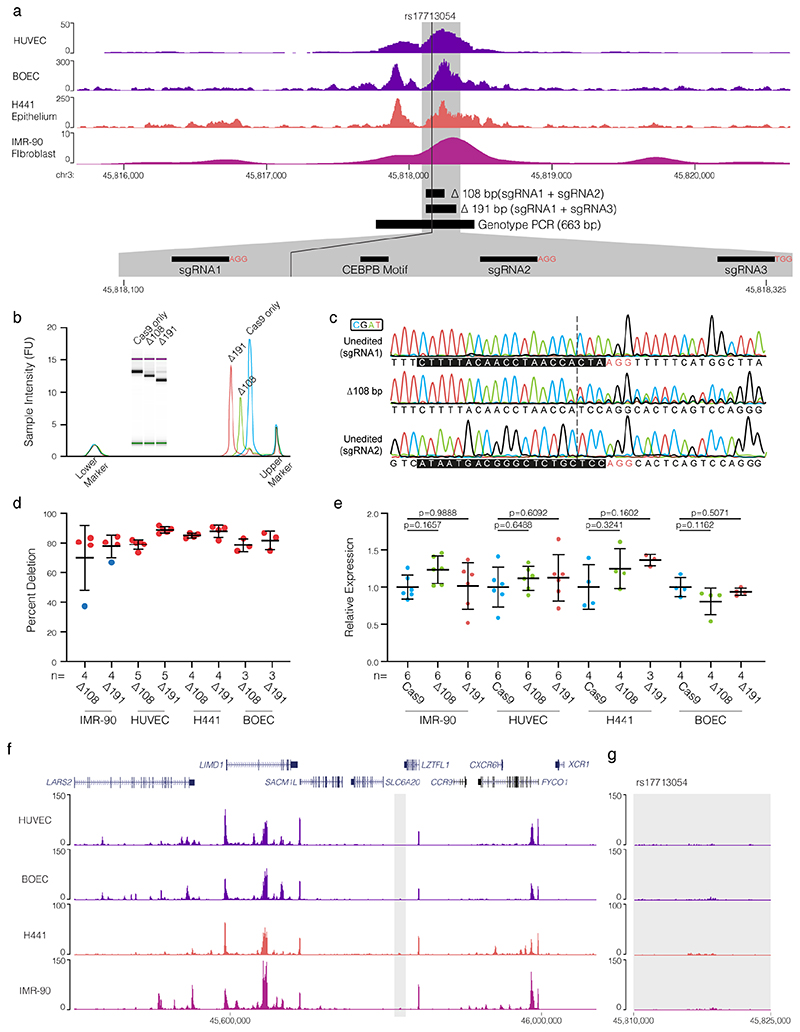
CRISPR-Cas9 genome editing52 allows the possibility to test the role of the rs17713054 enhancer in regulation of LZTFL1 and SLC6A20. As the enhancer shows accessibility in epithelial, endothelial, and mesenchymal cells (Extended Data Fig. 9a) we used CRISPR-Cas9 ribonucleoprotein editing to delete either a 108-bp or a 191-bp region at high efficiency (>70%) from H441 distal lung epithelial cells, adult blood outgrowth endothelial cells, HUVECs and IMR-90 lung fibroblast cells (Extended Data Fig. 9b-d, Supplementary Fig. 2). Using real-time qPCR we detected no-effect on LZTFL1 expression following enhancer deletion (Extended Data Fig. 9e) – consistent with a report that CRISPRi in the 16HBE14o- bronchial epithelial cell line had no effect on nearby gene expression50. As SLC6A20 is Polycomb repressed in fibroblasts and endothelial cells it was undetectable by RT-qPCR. To understand the unexpected result, we generated H3K27ac ChIP-seq in all four cell types (Extended Data Fig. 9f,g). The rs17713054 enhancer lacked strong H3K27ac and is likely inactive; explaining the lack of effect seen by deletion. Therefore, a suitable cell model for testing the effects of rs17713054, particularly in the lung epithelium, is not currently available.
Epithelial dysfunction in COVID-19 lung
Given that the rs17713054 enhancer is present and LZTFL1 is expressed in lung epithelial cells; the respiratory epithelium is of particular interest for understanding the association at 3p21.31. EMT, a developmental pathway that allows terminally differentiated epithelial cells to de-differentiate and acquire mesenchymal identity, plays a key role in the innate immune response and is a consequence of lung inflammation, involved in both the development and resolution of pneumonitis53–56. SARS-CoV-2 is known to induce EMT in both lung carcinoma cell lines and in the respiratory tract57,58 and LZTFL1 is known to regulate EMT through Wnt/β-catenin, hedgehog and TGF-β signalling59,60. In the context of malignancy, increased levels of LZTFL1 inhibits EMT, whereas decreased LZTFL1 promotes EMT45,59,60.
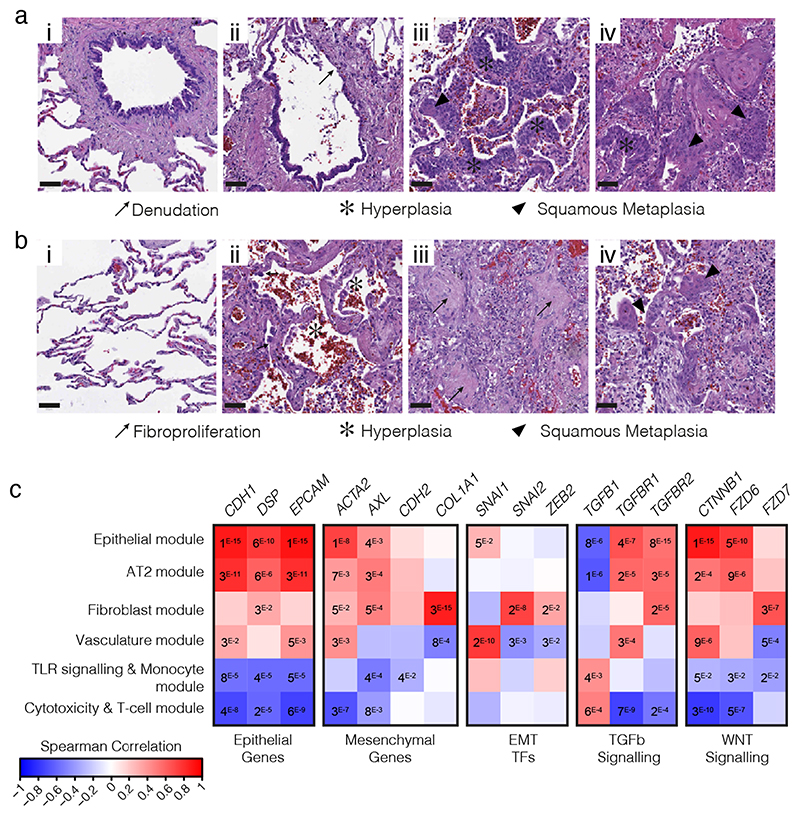
Defining EMT in complex tissues is challenging due to its diverse and dynamic nature, but can be achieved through a combined assessment of cellular reorganization, an abundance of fibroblasts (which are products of EMT), presence of EMT promoting signaling pathways and co-expression of epithelial and mesenchymal markers61. Consistent with work by others62,63, we saw widespread epithelial dysfunction and diffuse alveolar damage (DAD) with reorganization indicative of EMT evident in post-mortem biopsies of three COVID-19 patients. Dysfunction in ciliated airways included denudation, hyperplasia, and squamous metaplasia (Fig. 5a). Features of DAD included pneumocyte hyperplasia, hyaline membrane deposition, immune inflammation, fine- and focal-fibrosis, and squamous metaplasia (Fig. 5b). Between the areas of interstitial expansion and fibrotic foci, there was an accumulation of fibroblasts, generally absent from healthy lung tissue.
Figure 5. COVID-19 patient lungs show signals of EMT.
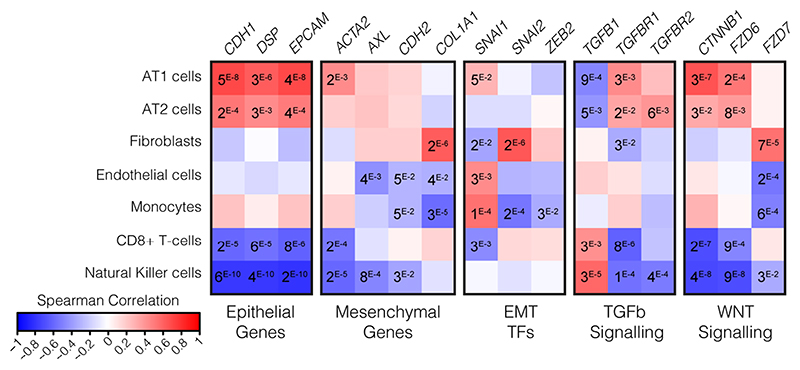
Hematoxylin and eosin (H&E) stained biopsies of the ciliated respiratory epithelium on bronchiole (a) and of alveolar space (b) in healthy lung (i) and COVID-19 patient lung (ii-iv). COVID-19 patient samples are representative images from staining of biopsies from 3 individuals and show loss of ciliated cell lined bronchioles (denudation) and loss of alveolar monolayers populated by alveolar type I pneumocytes with few type II pneumocytes, with alveolar wall expansion and fine interstitial fibrosis. Scale bars show 50 μM. c, Spearman correlation of gene expression profiles for EMT-related genes with the eigengenes of cell-type modules identified by WGCNA analysis from spatially resolved expression data from COVID-19 patient lung. P values were identified by two-sided Hmisc analysis (without multiple test correction), values for significant correlations (P < 0.05) are shown and all correlation and P values are in Source Data.
We previously generated selective spatial transcriptomics from 46 areas of post-mortem biopsies from critical COVID-19 patients covering a spectrum of alveolar injury64. To explore expression profiles of EMT relevant genes we used both a cell deconvolution approach65, to estimate cell abundance through gene transcripts, and a Weighted Gene Correlation Network Analysis66 (WGCNA), to identify modules of co-regulated gene expression patterns that were assigned to cell-types or biological processes. As expected, epithelial marker genes (CDH1, EPCAM) were naturally associated with Alveolar Type I (AT1) and Type II (AT2) pneumocytes, as well as both of the Epithelial and Type II pneumocyte WGCNA modules (Fig 5c, Extended Data Fig. 10). However, AT1, was also positively associated with the hallmark EMT gene ACTA2 (α-smooth muscle actin; Hmisc rcorr asymptomatic P = 0.0014), as were both the AT2 and Epithelial modules (P = 0.0069 and 9.59 × 10-9 respectively). These two modules were also positively associated with a second mesenchymal EMT marker gene, the receptor tyrosine kinase encoding AXL (P = 0.0002 and P = 0.0031). We next investigated EMT-associated transcription factors, finding SNAI1 (Snail Family Transcriptional Repressor 1) positively associated with the epithelial module (P = 0.0491) and AT1 cells (P = 0.0432), while fibroblasts associated with SNAI2 (P = 1.08 × 10-6) and the fibroblast module associated with both SNAI2 (P = 1.54 × 10-8) and ZEB2 (Zinc finger E-box-binding homeobox 2; P = 0.0144). Finally, we investigated the Wnt/β-catenin and TGF-β pathways, finding both pneumocyte subtypes (AT1, AT2) and both epithelial modules associated with TGF-β signaling receptor genes (TGFBR1 and TGFBR2) and WNT signaling genes which encode β-catenin and frizzled receptors (CTNNB1, FZD6 and FZD7). By contrast, neither CD8+ T-cells nor the cytotoxicity and T-cell module expressed epithelial or mesenchymal genes, but they did express TGFB1 (P = 0.0029 and P = 0.0005 respectively). The colocalized expression of mesenchymal genes with epithelial cells, along with expression of EMT transcription factors and associated signaling pathways is indicative of the EMT process, highlighting the relevance of this cellular reorganization pathway in COVID-19. The modulation of EMT by LZTFL1 may therefore be of relevance to the pathological outcome of COVID-19 infection.
Discussion
We have applied a machine learning and molecular biology platform for decoding GWAS hits, and identified a relatively unstudied gene, LZTFL1, as a candidate causal gene potentially responsible for the two-fold increased risk of respiratory failure from COVID-19 associated with 3p21.31. The risk allele of the SNP, rs17713054-A, leads to increased transcription, through augmentation of an epithelial-endothelial-fibroblast enhancer, facilitated by addition of a second CEBPB binding motif.
MCC identified LZTFL1 as the only gene to specifically interact with the rs17713054 enhancer. However, it is possible LZTFL1 may not be the sole causal gene at 3p21.31. Two TWASs identified 11 candidate genes at this locus10,49, including LZTFL1 and SLC6A20, but only these two genes have strong 3C contacts with the rs17713054 enhancer and lung eQTLs. TWASs are unable to differentiate between direct and indirect regulation67. The absence of a 3C interaction with COVID-19 severity associated variants suggests that there may be an indirect effect for the remainder of the genes; with the caveat that it is possible that a direct effect may occur in an untested cell type. Whilst the ultra-high resolution MCC approach only identified physical contacts between LZTFL1 and rs17713054; traditional 3C found both CCR9 and SLC6A20 to be in the same regulatory domain. CCR9 is not expressed in lung and rs17713054 is not in an active enhancer in immune cells, where CCR9 is expressed. Both LZTFL1 and SLC6A20 have higher expression in the presence of the rs17713054 risk-allele, and it is plausible that in cells where SLC6A20 is not Polycomb repressed (e.g. Goblet and Alveolar Type II pneumocytes) it also directly interacts with the rs17713054 enhancer and would thus be affected by the risk allele.
The biological relevance of SLC6A20 to COVID-19 is unclear. It is primarily expressed in the kidneys and gastrointestinal tract, and its associated Mendelian disease causes renal calculi due to failure of reuptake of glycine in the nephron44. Nevertheless, its function as an imino acid transporter is modulated by levels of angiotensin-converting enzyme 268 (ACE2); which is a cell receptor for SARS-CoV-269. Conversely, LZTFL1 is widely expressed in pulmonary epithelial cells, including ciliated epithelial cells, which have been identified as one of the main cellular targets for SARS-CoV-2 infection70. Furthermore, homozygous loss of LZTFL1 causes a classical ciliopathy: Bardet-Biedl syndrome46,47. The association of 3p21.31 variants with susceptibility to SARS-CoV-2 infection, as well as disease severity, highlights the importance of the respiratory epithelium for this locus11. LZTFL1 encodes a cytosolic leucine-zipper protein, which associates with the epithelial marker E-cadherin and is involved in the trafficking of numerous signaling molecules45,71–74. We note that upregulation of LZTFL1 in the context of malignancy inhibits EMT45,59,60, a pathway known to be part of both wound healing and immune responses53–56.
Examination of post-mortem COVID-19 lung biopsies demonstrates widespread epithelial dysfunction with EMT signatures62,63. Consistently, single-cell RNA-seq shows a reduction in total numbers of epithelial cells following infection75, with a lower epithelial composition correlating with a more rapid progression from symptom onset to death76. The samples analyzed here showed few areas of healthy tissue and it is possible that inflammation or Neutrophil Extracellular Traps, rather than direct viral infection, was driving this epithelial dysfunction58, and that LZTFL1 acts earlier in disease progression, contributing to poor structural resolution of inflammation. Expression profiling of nasal epithelia from COVID-19 patients has detected EMT signals in the upper respiratory tract57. Similarly, SARS-CoV-2 infection of both a reconstructed human bronchial epithelium model and Syrian hamster induced de-differentiation of airway ciliated cells77, highlighting the relevance of this pathway and cell-type. As such, an effect of the 3p21.31 locus in the early epithelial response may contribute to susceptibility to SARS-CoV-2 infection11. Although both influenza and SARS-CoV-2 have been shown to induce EMT57,78, its role in viral infection is not entirely clear. While chronic EMT leads to fibrosis and severe inflammation, acute EMT may be a beneficial response. In the context of viral infection, EMT leads to a reduction of two of the cell receptors of SARS-CoV-2: ACE2 and transmembrane serine protease 2 (TMPRSS2)57,79. A reduction in these cell surface markers as a result of EMT could reduce viral load by decreasing infection efficiency and preventing severe disease. Conversely, EMT allows for epithelial cells to proliferate, repair damaged tissue and replace lost cells – which may be required to overcome severe disease.
For the 3p21.31 COVID-19 risk locus, higher risk is associated with increased expression of LZTFL1, a known EMT inhibitor. Higher levels of LZTFL1 may delay the positive effects of an acute EMT response, blocking a reduction in ACE2 and TMPRSS2 levels and/or through slowing EMT-driven tissue repair. Further investigation of the potential role of LZTFL1 and EMT in pulmonary pathogenesis is needed. Our findings suggest that a gain-of-function variant in an inducible enhancer, causing increased expression of LZTFL1 may be associated with a worse outcome. This raises the possibility that LZTFL1 could be a potential therapeutic target for treatment or prevention of COVID-19.
Methods
Human research ethics compliance
All samples and information were collected with written and signed informed consent. For erythroid cells, peripheral blood was obtained with approval from North West Research Ethics Committee of NHS National Research Ethics Services, UK (03/08/097). Blood samples for CD4+ cells were obtained from donors recruited from the Cambridge BioResource. The study was approved by East of England – Cambridgeshire and Hertfordshire Research Ethics Committee (05/Q0106/20). CD14+ samples were isolated from healthy donors with approval from the Oxfordshire Research Ethics Committee COREC (06/Q1605/55). Patient samples were acquired and analyzed with approval from the ethics committee of the University of Navarra, Spain (15/05/2020) and the Medical Sciences Interdivisional Research Ethics Committee of the University of Oxford (Approval R76045/RE001). Patient samples and, hematopoietic stem and progenitor cells from healthy donors were stored in accordance with Human Tissue Authority (License 12433).
Cell isolation, culture and stimulation
Human ESC line H1 (H1-hESC [https://scicrunch.org/resolver/CVCL_97711; WA01 WiCell, RRID:CVCL_9771) was grown on Matrigel (Corning) coated plates in mTeSR1 medium (StemCell technologies). Cells were harvested as a single-cell suspension using Accutase (EDM Millipore); fixation were carried out in mTeSR1 medium. Primary neonatal Human Umbilical Vein Endothelial Cells (HUVEC; Lonza; CC-2517, Gibco; C0035C, PromoCell; C-12200) were expanded in endothelial cell growth medium (Sigma) up to five passages following the manufacturer’s protocol. For passaging, HUVECs were grown to 60% confluence, washed with HBSS at room temperature and sub-cultured following light trypsination using Trypsin-EDTA (Sigma) at room temperature with trypsin inhibitor (Sigma) added upon rounding of the cells to achieve gentle release from the flask. HUVECs were fixed in RPMI supplemented with 10% FBS. For erythroid cells, CD34+ hematopoietic stem and progenitor cells were isolated from peripheral blood of two healthy males and one healthy female and differentiated ex vivo for 13 days as previously described82. CD4+ T-cells were enriched from whole blood (93–99% pure, RosetteSep Human CD4+ T-cell Enrichment Cocktail, StemCell Technologies) and were plated at 250,000 cells per well in U-96 well plates (Greiner) and cultured in medium alone or stimulated with anti-CD3/CD28 T-activator beads (Dynabeads, Life Technologies) at a ratio of 0.3 beads per cell for 4 hours at 37°C in X-VIVO-15 (Lonza), 1% AB serum (Lonza) and penicillin/ streptomycin (Life Technologies). Non-activated or activated CD4+ T-cells were pooled after 4 hours of culture and fixed in growth media. For CD14+ cells, peripheral blood mononuclear cells were obtained by Ficoll-Paque (GE healthcare) density centrifugation of whole blood collected into EDTA tubes (BD vacutainer system) or leukocyte cones (NHS Blood and Transport). Monocyte isolation was carried out by positive selection using magnetic-activated sorting with CD14+ beads (MACS, Miltenyi) according to the manufacturer’s instructions. IMR-90 [https://scicrunch.org/resolver/CVCL_03471 lung fibroblasts (ATCC; CCL-186, RRID:CVCL_0347) were cultured in Eagle’s Minimal Essential Medium supplemented with 10% FBS, 1 mM sodium pyruvate (Gibco), 1× MEM Non-Essential Amino Acids (Gibco) and Penicillin-Streptomycin (100 U ml-1 each) Cell were sub-cultured every 3 days following light trypsination using 0.05% Trypsin-EDTA (Gibco). Blood outgrowth endothelial cells (BOECs) were isolated as previously described83. Briefly 20-40 ml of fresh blood was diluted 1:1 with PBS, layered over Histopaque-1077 (Sigma) and centrifuged for 15 minutes at 500 ×g, brake off. Peripheral blood mononuclear cells were washed with PBS then resuspended in EGM-2 Bullet kit growth media (Lonza) supplemented with 10% heat inactivated FBS. Cells were cultured for 21-28 days in collagen coated flasks until BOEC colonies formed. BOEC colonies were passaged by light trypsinisation. BOEC cells were passaged twice before any experimentation to ensure endothelial cell purity, which was also confirmed by FACS and immunofluorescence. BOEC cells were fixed in growth medium. NCI-H441 [https://scicrunch.org/resolver/CVCL_15611 cells (ATCC; HTB-174, RRID:CVCL_1561) were grown in RPMI 1640 medium (Gibco) supplemented with 10% non-heat inactivated FBS (Sigma) and 1% Penicillin-Streptomycin (Gibco), cells were given fresh media every 2 days and passaged by light trypsination twice weekly. Human Umbilical Derived Erythroid Progenitor line 2 cells84 (HUDEP-2 [https://scicrunch.org/resolver/CVCL_VI061, RRID:CVCL_VI06) were provided by RIKEN and were maintained at 0.7-1.5 × 106 cells ml-1 in HUDEP expansion medium (SFEM, 50 ng ml-1 SCF, 3 IU ml-1 EPO, 10 μM DEX, 1% L-Glu, 1% Penstrep) and changed into fresh medium containing 2× doxycycline every 2 days.
Variant effect sequence predictions
Linkage analysis was determined using the LDlink webtool (v5.1, LDproxy, LDpair; https://ldlink.nci.nih.gov/). Candidate variants either achieved genome wide significance in the first COVID-19 GWAS9, or were in tight linkage (r2 > 0.8) with lead variants from the first two large COVID-19 GWAS9,10. The deepHaem convolutional neural network19 was trained with 4,384 ENCODE peaks calls (694 open chromatin DNase I/ATAC-seq, 1,750 transcription factor ChIP-seq and 1,940 histone modification ChIP-seq) and is available via GitHub (Model 4; https://github.com/rschwess/deepHaem). Identification of CEBPB motifs was performed by FIMO85 analysis of reference and variant containing enhancer sequence (chr3:45,817,661-45,818,660, hg38) with the JASPAR86 motif MA0466.1. Sasquatch40 was run using default Workflow 3 settings (7-mer, propensity-based [Erythroid], exhaustive) on the web interface (https://sasquatch.molbiol.ox.ac.uk/cgi-bin/foot.cgi). Masked SpliceAI18 predictions for each variant were extracted from the coding genome scan for substitutions, 1 base insertions, and 1-4 base deletions (https://github.com/Illumina/SpliceAI). Conserved miRNA binding sites were identified using TargetScan25 (http://www.targetscan.org/vert_71/). SNP predictions were identified using the miRdSNP26 database (http://mirdsnp.ccr.buffalo.edu/browse-genes.php) and the MicroSNiPer27 webtool (http://vm24141.virt.gwdg.de/services/microsniper/index.php), using 6-mer, 7-mer, 8-mer and 9-mer settings.
Colocalization analysis
Harmonized summary statistics for severe COVID-199 were downloaded from the GWAS Catalog87 (GCST90000256). Summary statistics for all lung eQTL-variant pairs (V8) in European-Americans (EUR) were downloaded from the GTEx portal28. Coloc51 (v5.0.1) analysis of variants within 200 kb of the predicted causal variant (rs17713054) was implemented in R. Inputs of GWAS size (n = 3,795), GWAS case frequency (0.419), eQTL study size (n = 515), as well as association β, standard errors, minor allele frequencies, and z-scores were used in a sensitivity analysis88 which showed a prior probability of co-localization (p12) of 1 × 10-5 tested approximately equal prior probability of both H3 (two distinct causal variants for the GWAS and eQTL trait) and H4 (a single causal variant).
Chromosome conformation capture (3C)
Gene promoters were selected for Capture-C using 10-kb resolution Hi-C data on the 3D Genome Browser89 [url:http://3dgenome.fsm.northwestern.edu/index.html] from a range of cell types to identify putative regulatory domains and interactions with rs17713054. Capture-C was performed as previously described with either the NG or NuTi method20,21,90. Briefly 5-20 million cells were fixed with 2% formaldehyde, 3C libraries were generated using the high resolution DpnII enzyme. Targeted enrichment was performed using SeqCap reagents (Roche) and 100-mer biotinylated oligonucleotides (Supplementary Table 2) at the optimal titrated concentration21. Libraries were sequenced using 75 bp paired-end reads on an Illumina NextSeq Platform to generate over 250,000 reads per viewpoint per sample. For Micro Capture-C22 (MCC), aliquots of 1-2 × 107 cells were fixed for 10 minutes with 2% formaldehyde in 10 ml of growth medium. Formaldehyde was quenched with 125 mM glycine, and cells were pelleted (5 minutes, 500 ×g, 4°C) and washed with PBS. Cells were resuspended in 1 ml PBS and permeabilized with 0.005% Digitonin. Cells were pelleted and resuspended in 800 μl of reduced calcium content micrococcal nuclease buffer (10 mM Tris-HCl pH 7.5, 1 mM CaCl2). Chromatin was digested for 1 hour at 37°C inside intact, permeabilised cells in three separate reactions using 5-120 Kunitz U of micrococcal nuclease (NEB). Digestion was quenched by with 5 mM ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA; Sigma). Cells were pelleted and washed with PBS, prior to end-repair and phosphorylation; cells were resuspended in 400 μl DNA ligase buffer (Thermo Scientific) supplemented with 400 μM of each of dATP, dCTP, dGTP and dTTP and 5 mM EGTA, 200 U ml-1 T4 Polynucleotide U Kinase PNK (NEB) and 100 U ml-1 DNA Polymerase I Large Klenow Fragment (NEB) for 2 hours at 37°C. To ligate DNA fragments, T4 DNA ligase (Thermo Scientific) was added at 300 U ml-1 and the reaction was incubated at room temperature for 8 hours. Chromatin was de-crosslinked with Proteinase K at 65°C for over 4 hours and DNA extracted using either phenol chloroform with RNase treatment (Roche) and ethanol precipitation or using the DNeasy Blood and Tissue Kit (Qiagen). MCC libraries were sonicated to 200-bp fragments and indexed using NEBNext Ultra II indexing reagents (NEB) with the following modifications; 2 μg of DNA was indexed, 5 μl of adaptor was used, bead clean-ups were performed with 1.5 volumes of Ampure XP beads, and with Herculase II PCR reagents (Agilent) used for the indexing PCR. Target enrichment was performed using double capture with 120-bp biotinylated oligonucleotides (Supplementary Table 3) with SeqCap Reagents (Roche). Enriched libraries were sequenced on the NextSeq platform using 150-bp paired-end reads to generate ~1 M reads per viewpoint.
3C data analysis
NuTi Capture-C data were mapped to hg38 using CCseqBasicS91 (github.com/Hughes-Genome-Group/CCseqBasicS) using bowtie2. Briefly, CCseqBasic592 trims adaptor sequences, flashes read pairs, in silico digests fragments and uses maps reads before identifying sequences as either capture and reporter. Replicates were compared using the CaptureCompare93 (github.com/Hughes-Genome-Group/CaptureCompare), which normalizes cis reporter counts per 100,000 cis reporters, generates per fragment mean counts for each cell type, then bins reporter counts in equal sized regions for generating a windowed profile. For MCC, adapters were removed using TrimGalore94 (v0.3.1) then fragments reconstructed with FLASH95 (v1.2.11) into single sequences using the central area of overlapping reads. Fragments were mapped to the oligonucleotide DNA sequence ±350 bp using BLAT96 (v35) to identify ligation junctions, allowing splitting of reads into new paired fastq files using MCCsplitter.pl and subsequent mapping to hg38 with bowtie297 (v2.3.5). PCR duplicates were removed from alignment files with MCCanalyser.pl using both sonicated ends and ligation junction with a wobble of ±2 bp. MCCsplitter.pl and MCCanalyser.pl are available for academic use through the Oxford University Innovation software store https://process.innovation.ox.ac.uk/software/p/16529a/micro-capture-c-academic/1. MCC tissue-specific peaks for rs17713054 were called using LanceOtron98 on the webtool “Find and Score Peaks with Inputs” (https://lanceotron.molbiol.ox.ac.uk) using the HUDEP2 MCC profile as an input track.
Genome editing
For deletion of the rs17713054 enhancer, cells were transfected with 5 μg Alt-R SpCas9 nuclease V3 ribonucleoprotein (IDT) and 0.1 nmol each of two guide RNAs (Supplementary Table 4). All transfections were carried out with 1-2 × 105 cells in 20 μl reactions using a 4D-Nucleofector (Lonza); IMR-90 Fibroblast cells were electroporated using Amaxa Cell Line Nucleofector Kit V reagents (Lonza) with program CM-120, HUVECs and BOECs were electroporated using Amaxa P5 Primary Cell 4D-Nucleofector X Kit S reagents (Lonza) with program CA-167, and H441 epithelial cells were electroporated using P3 Primary Cell 4D-Nucleofector X Kit S reagents (Lonza) with program EL-10. Cells were cultured for 24 hours in 2 ml antibiotic-free growth media in a single well of a 6-well plate before expansion in fully supplemented media. Bulk DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen), and the edited region (chr3:45,817,769-45,818,459; hg38) amplified using Platinum PCR Supermix (Invitrogen) with 5’-GGAAAGAACACGCATAAACCATA-3’ (Forward primer) and 5’-CTCATCCCACAGTGAACTAAGAA-3’ (Reverse primer). Editing efficiency was determined using a D1000 Tapestation, and Sanger Sequencing with the forward primer and Synthego ICE analysis [https://ice.synthego.com/#/].
Quantitative reverse transcription PCR
For expression analysis, cells were grown to >80% confluence in a single well of a 6-well plate. Cells were lysed by addition of 1 ml TRI Reagent (Sigma), snap frozen and stored at -80°C for less than 6 months. RNA was separated by addition of 100 μl of 1-bromo-3-chloropropane, centrifugation in a Phase Lock Gel-Heavy tube (5Prime) for 5 minutes at 10,000 ×g, and precipitation in an equal volume of isopropanol (500 μl) with 1 μl of GlycoBlue (Thermo Fisher). DNA was removed using DNA-free DNA Removal Kit (Invitrogen) and cDNA generated using 1 μg of total RNA with SuperScript III First-Strand Synthesis SuperMix reagents (Thermo Fisher). Quantitative PCR was performed using a 1:10 dilution of cDNA, TaqMan Universal PCR Master Mix II without UNG (Thermo Fisher) and TaqMan Gene Expression Assays (Thermo Fisher) for LZTFL1 (Hs00947898_m1), SLC6A20 (Hs00610960_m1) and RPL18 (Hs00965812_g1) with FAM. LZTFL1 expression was normalized to RPL18 and relative expression calculated by normalizing to the mean expression of LZTFL1 in RNP treated cells from samples of the same cell type processed in the same batch.
Chromatin Immunoprecipitation (ChIP)
For ChIP, single-cell suspensions of 106 cells ml-1 in growth media were generated after light trypsin treatment. Cells were fixed by addition of 1% formaldehyde for 10 minutes at room temperature, which was quenched by addition of glycine at a final concentration of 125 mM. Fixed cells were washed with PBS and snap frozen. Cell lysis and immunoprecipitation was carried out using ChIP Assay Kit (Merk Millipore) on 5 × 106 cells in 2 ml of dilution buffer incubated overnight at 4°C with 1 μl polyclonal rabbit anti-H3K27ac (AbCam, ab4729, 0.3 μg, lot: GB3205523-I; 1:2,000). DNA was isolated by phenol- chloroform isoamylalcohol extraction and ethanol precipitation then indexed using NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB). Libraries were sequenced using 39-bp paired-end reads on a NextSeq Platform. Reads were mapped to hg38 using bowtie297, PCR duplicates filtered using samtools99, and bigwigs generated with deeptools100.
Fluorescence-activated cell sorting (FACS) analysis
For FACS, ~105 cells were resuspended in 100 μl staining buffer (PBS with 10% FBS) and incubated with 1 μl each APC conjugated mouse anti-CD14 (2 ng, Clone: M5E2, BioLegend Cat: 301807, Lot: B266608, 1:100), PE conjugated mouse anti-CD309/VEGFR2 (2 ng, Clone: 7D4-6, BioLegend Cat: 359903, Lot: B245460, 1:100), FITC conjugated mouse anti-CD31/PECAM (2 ng, Clone: WM59, BioLegend Cat: 303103, Lot: B287895, 1:100), and PE/Cy7 conjugated mouse anti-CD34 (0.5 ng, Clone: 561, BioLegend Cat: 343616, Lot: B257238, 1:100) for 20 minutes at 4°C. Cell were diluted with 90 μl staining buffer with 1:5,000 Hoechst 33258 (Thermo Fisher) and analyzed on an Attune NxT Flow Cytometer. Voltages and compensation were set using single stain samples with UltraComp eBeads (Thermo Fisher) for antibodies and cells for Hoechst. Negative and positive populations were established using Fluorescence Minus One Controls. Mononuclear cells were gated using forward scatter (FSC) and side scatter, single cells gated using FSC-area and FSC-height, and live cells selected using a Hoechst negative gate in FlowJo (v10.7).
Assay for Transposase-Accessible Chromatin
ATAC-seq was performed as published101,102 with 7.5 × 104 cells per technical replicate, and two to four technical replicates per samples. After spinning at 500 ×g for 15 minutes, cells were resuspended in lysis buffer (10 mM Trish-HCl pH 7.5, 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL CA-630), centrifuged and nuclei washed with PBS. Nuclei were pelleted, PBS discarded and resuspended in tagmentation buffer (25 μl 2× TD buffer, 2.5 μl Tn5 Transposase [Illumina], and 22.5 μl water) then incubated at 37°C for 30 minutes. After the transposition DNA was extracted using MinElute PCR purification kit (Qiagen), half the DNA was amplified for sequencing using NEBNext High-Fidelity 2× PCR Master Mix (NEB) and further purified with QIAquick PCR purification kit (Qiagen). Libraries were sequenced using 39-bp paired-end reads on a NextSeq Platform. Reads were mapped to hg38 using bowtie2 in NGseqBasic102.
Immunofluorescence staining and microscopy
Cells were grown for 24-48 hours on sterilized coverslips under standard growth conditions and fixed in 4% vol/vol para-formaldehyde (PFA) in 0.25 M HEPES for 15 minutes, followed by permeabilization in 0.2% vol/vol Triton X-100 in PBS for 10 minutes. Following blocking with 10% vol/vol fetal calf serum in PBS, von Willebrand’s factor (VWF) was detected using mouse anti-VWF 1:100 (Clone F8/86, MA5-14029, Invitrogen) and goat anti-mouse Alexa Fluor 488 1:500 (A32723, Thermo Fisher Scientific). DNA was stained with 1 μg ml-1 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) in PBS and after washing coverslips were mounted in Vectashield (Vector Laboratories). Widefield fluorescence imaging was performed on a DeltaVision Elite system (Applied Precision) using a UPLFLN 40× 1.30NA oil immersion objective (Olympus), a CoolSnap HQ2 CCD camera (Photometrics), DAPI (excitation 390/18; emission 435/40) and FITC (excitation 475/28; emission 525/45) filters. 12-bit image stacks were acquired with a z-step of 200 nm giving a voxel size of 161.3 nm × 161.3 nm × 200 nm. All images were acquired using the same exposure settings. Using Fiji103, 3D images were flattened by maximum intensity projection and displayed at the same minimum/maximum intensity settings. Images were cropped for publication in Adobe Photoshop (v22.4.1).
Patients tissue analyses
Healthy lung samples were sourced from chronic obstructive pulmonary disease (COPD) patients during lung tumor resection, with a sample of normal lung acquired away from the tumor. Medical records of COVID-19 patients were retrospectively reviewed104 and three were selected for in-depth analysis based on: their clinical manifestation of acute respiratory distress syndrome (ARDS), typical COVID-19 histology (with a 4-5 score on the Brescia-COVID Respiratory Severity Scale), and a lung-restricted (absence in heart, liver and kidney biopsies) presence of SARS-CoV-2. Post-mortem lung tissues were obtained through open biopsy shortly after death and processed as fully described previosly104. In brief, tissues were immediately fixed in neutral buffered formalin for <24 hours and then paraffin embedded. Sections (5 μm each) were cut from Wedge biopsies (mean size 1.78 cm2, standard deviation 0.55 cm2) for Hematoxylin and Eosin (H&E) analysis. Sections were analyzed by NanoString GeoMx Digital Spatial Profiling (DSP) with normalization and downstream analysis by weighted gene correlation network analysis66 (WGCNA) and cell deconvolution65 as previously described64. For deconvolution with SpatialDecon in R (v1.0.0), cell profiles were obtained from the Human Cell Atlas healthy lung single-cell RNA-seq appended with neutrophil data105 using the R package dataset “Lung_plus_neut” dataset. Seven relevant cell types were selected for expression analysis from a total of 26 cell types. WGCNA was performed using the WGCNA R package (v1.70-3) and generated 17 biologically assignable modules, of which six were selected for further analysis. Spearman correlation and non-adjusted P value generation was performed with the Hmisc R package (v4.5-0) and visualized with corrplot (v0.84).
Public Data Set analysis
Unless stated, ENCODE datasets were accessed using the UCSC genome browser106,107, which was also used to generate track figures. ENCODE DNase I bigwigs (hg38) were downloaded from ENCODE portal (https://www.encodeproject.org/) and analyzed with deeptools100 (multiBigwigSummary). Capture-C was analyzed using the CaptureCompendium suite91 mapping to hg38 with bowtie297 and using default settings. ATAC-seq and H3K27ac ChIP-seq data from erythroid progenitors, immune cells29,80,81 and aortic endothelium37 were downloaded from the Gene Expression Omnibus (GSE74912, GSE115684, GSE118189, GSE139377) and analyzed using NGseqBasic102 with default settings for bowtie297. Aortic endothelial samples were genotyped by counting two or more reads from either allele in combined ATAC-seq and ChIP-seq data. For allelic skew analysis, aortic endothelium ATAC-seq from heterozygous individuals was mapped with bowtie297 and processed using WASP108 to correct for reference genome mapping bias. Three replicates with fewer than four remaining reads were excluded from analysis. Mature erythroid chromatin modification and CTCF data (GSE125926) have been previously reported by our group16, CTCF motifs were identified using MEME-SUITE85 tools, (meme --dna --nmotifs 1 --w 19 --mod zoops --maxsize 1102788; fimo --thresh 1e-4 --motif 1). Single-cell RNA-seq data35,48 were sourced from online portal (https://asthma.cellgeni.sanger.ac.uk/, https://www.lungepigenome.org/gene-expression/) on 9th Oct. 2020 and 19th May 2021 respectively. Single-cell ATAC-seq data34,35 were sourced from online portals (https://descartes.brotmanbaty.org/bbi/human-chromatin-during-development/dataset/lung, https://www.lungepigenome.org/) on 19th May 2021. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The multi-tissue eQTL and expression level data were obtained from the GTEx Portal V8 on the 14th Oct. 2020 (https://gtexportal.org/home/snp/rs17713054).
Extended Data
Extended Data Figure 1. 3p21.31 severe COVID-19 locus SNPs are not in immune regulatory elements.
a, To decode GWAS variants either all genome wide significant variants and/or variants in linkage disequilibrium with sentinel variants are assessed for protein coding changes with ANNOVAR. Remaining variants are then assessed for changes in splicing of expressed genes using the SpliceAI machine learning approach18 or splicing quantitative trait loci (sQTL). Variants are then intersected with open chromatin with a panel of disease relevant cell types to asses cis-regulatory element altering potential. This potential is assessed for effects on open chromatin with deepHaem19 or transcription factor binding with both deepHaem and Sasquatch40. Finally, variants in enhancers are linked to target effector genes using high resolution chromosome conformation capture with NG/NuTi Capture-C20,21 or Micro Capture-C22. b, Heatmap of linkage disequilibrium (European; EUR) between a severe COVID-19 lead SNP (rs11385942) with lead SNPs for other GWAS traits identified in the region (chr3:45,710,500-45,954-500, hg38). c, Linkage analysis for a 3p21.31 severe COVID-19 lead SNP (rs11385942 - circle) showing variants within 100 kb and r2>0.2. No variants with r2>0.6 were seen beyond this range. d, Overlaid tracks of ATAC-seq from sorted populations of resting (blue) and stimulated (red) immune cells29. Overlapping signal appears black. Abbreviations: Memory (Mem.), Immature (Imm.), Mature (Mat.), Natural Killer cells (NK), Plasmacytoid Dendritic cells (pDC), Myeloid Dendritic cells (mDC), Monocytes (Mono.), Effector (Eff.), Helper (H.), Regulatory (Reg.), and Central (C.). Region: chr3:45,800,000-45,870,000, hg38.
Extended Data Figure 2. DNase I accessibility over COVID-19 SNPs.
a. DNase I signal in each of 95 ENCODE datasets for rs17713054 (chr3:45,817,661-45,818,660, hg38) and rs7634459 (chr3:45,859,001-45,859,500, hg38) which were found in open chromatin. Datasets are grouped according to cell-type, numbers indicate tissue of origin (see panel c). Violin plots of ENCODE DNase I accessibility over rs17713054 grouped by cell type (b) and tissue of origin (c). Each sample is shown as a red dot, dashed lines show mean, dotted lines show quartiles.
Extended Data Figure 3. deepHaem prediction of de novo open chromatin elements.
deepHaem19 negative damage score, which predict gain-of-accessibility, for the 28 candidate COVID-19 severity variants in 694 cell-types. Positive scores (loss-of-function) were adjusted to zero. In general, variants generating de novo regulatory elements33 have scores lower than – 0.1, which was not true for any variant in any cell type.
Extended Data Figure 4. rs76374459 is likely benign in an erythroid enhancer.
ATAC-seq from progenitor80 and differentiating erythroid cells81. Haematopoietic Stem Cells (HSC), Multi-Potent Progenitors (MPP), Common Myeloid Progenitors (CMP), Myeloid-Erythroid Progenitors (MEP) from bone marrow or peripheral blood and erythroid Colony Forming Units (CFU-E), Pro-erythroblasts (ProE1, ProE2), Basophilic Erythroblasts (BasoE), Polychromatic Erythroblasts (PolyE), Orthochromatic Erythroblasts (OrthoE) and Orthochromatic/Reticulocytes (OrthoRet). ChIP-seq tracks from CD71+ CD23+ mature erythroid cells16 show presence of marks associated with active transcription (H3K27ac), enhancers (H3K4me1), promoters (H3K4me3) and boundaries (CTCF). b, deepHaem damage score for the risk-C allele versus non-risk-G allele of rs76374459 associated with severe COVID-19 in 694 cell-types. rs763774458 is found in open chromatin through-out erythropoiesis. A positive score predicts loss of accessibility, a negative score predicts increased accessibility.
Extended Data Figure 5. Single nucleus ATAC-seq in adult lung.
Chromium single nucleus ATAC-seq from non-diseased adult lung35 (n=3) with 17 epithelial, endothelial, mesenchymal and hematopoietic populations, including Alveolar Type (AT) 1 and 2 Pneumocytes, Macrophage (MΦ) and Natural Killer (NK) cells. The rs17713054 containing element is highlighted in grey.
Extended Data Figure 6. Pulmonary expression and binding analysis of CEBPB.
a, GTEx top five expressed tissues for CEBPB. For violin plots, minima and maxima are the top and bottom of the violin, black lines show means, ends of the pale regions denote first and third quartiles, and black dots denote outliers. Data from independent samples for Whole blood (n=755), Lung (n=578) Adipose (n=541), Fallopian Tube (n=9), Artery (n=663). b, Chromium single nucleus RNA-seq from non-diseased adult lung35 (n=3 independent samples) with 22 epithelial, endothelial and mesenchymal populations, including Alveolar Type (AT) 1 and 2 Pneumocytes and Pulmonary Neuroendocrine cells (PNECs). c, 10x Genomics Chromium droplet single-cell RNA sequencing (scRNA-seq) from upper and lower airways and lung parenchyma34 from healthy volunteers or deceased transplant donors with ten epithelial populations (i) with expression profiles for CEBPB (ii). d, ENCODE ChIP-seq for CEBPB in A549 alveolar basal epithelial adenocarcinoma cells, HeLa cells, and IMR-90 lung fibroblast cells with inset region (chr3:45,805,000-45,855,000; hg38) showing the rs17713054 containing enhancer. e, DeepHeam ChIP-seq binding prediction score for CEBPB in lung fibroblast (IMR-90), alveolar basal epithelial adenocarcinoma (A549), the erythroleukaemia line (K562), human endothelial kidney cells (HEK293), and the GM12878 lymphoblastoid cell line (LCL) predicts increased binding to the risk-A allele.
Extended Data Figure 7. LZTFL1 is a most likely target of rs17713054.
a, NuTi Capture-C and Micro Capture-C from the rs17713054 enhancer in Endothelial cells (HUVEC) shows specific interaction with only the promoter of LZTFL1 and an upstream CTCF site (triangles). CTCF track shows binding of the CCAAT-binding factor which acts as a boundary. b, ENCODE ChIP-seq for the active chromatin mark (H3K27ac), the repressive chromatin mark (H3K27me3) and EZH2, a member of the Polycomb Repressive Complex 2, in endothelial (HUVEC) and normal human lung fibroblast (NHLF) cells. Green bar denotes the 3C regulatory domain as identified by 3C analysis. c, ENCODE DNase I seq tracks from a range of cell types and tissues, including airway epithelium and bronchial epithelium, where the rs17713054 enhancer is active. In these cell types the LZTFL1 promoter is DNase I accessible, but neither the CCR9 promoter nor the SLC6A20 promoter are. Region shown is chr3: 45,730,000-45,930,000 (hg38). d, Paired accessibility analysis of read counts per kilobase (RPK) over the LZTFL1 and SLC6A20 promoters and the rs17713054 enhancer in 156 ENCODE, immune and erythroid open chromatin datasets. Only the LZTFL1 promoter is widely accessible in the same cells as the affected enhancer.
Extended Data Figure 8. Expression and eQTL analysis of 3p21.31 candidate lung effector genes.
a, Genomic position of genes identified as 3p21.31 candidate causal genes with method of identification, including two TWASs10,49. b, GTEx whole lung RNA-seq expression profiles for candidate causal genes as transcripts per million (TPM) with rs17713054 eQTL two-sided p-value for lung. For violin plots, minima and maxima are the top and bottom of the violin, black lines show means, ends of the pale regions denote first and third quartiles, and black dots denote outliers. n=578 independent samples. c, Chromium single nucleus RNA-seq35 from non-diseased adult lung (n=3), including Alveolar Type 1 (AT) and Type 2 (AT2) Pneumocytes and Pulmonary Neuroendocrine cells (PNECs).
Extended Data Figure 9. CRISPR/Cas9 deletion of the rs17713054 enhancer.
a, ENCODE DNase I-seq in. HUVEC and IMR-90 cells and ATAC-seq in Blood Outgrowth Endothelial Cells (BOECs) and H441 epithelial cells showing the rs17713054 containing enhancer with schematic of generated deletions and short guide RNA (sgRNA) binding sites. b, Example D1000 trace of genotyping PCR product amplified from cells transfected with Cas9 protein only, Cas9 protein with sgRNA1+2 (Δ108), or Cas9 protein with sgRNA1+3 (Δ191). c, Example Sanger sequencing trace following ICE analysis over the sgRNA1 and sgRNA2 binding sites in unedited cells, and the double strand break repair site in cells containing the 108 bp deletions. sgRNA sequence shown by black boxes, PAM sites shown with red letters. d, Calculated deletion efficiency for each sgRNA pair and cell type. Transfections failing to achieve >70% deletion (blue circles) were excluded from expression analyses. n shown are for independent transfections e, Expression of LZTFL1 normalized to RPS18 and expressed as relative to the mean expression in Cas9 only treated cells for each cell type. Corrected p-values from an ordinary one-way ANOVA with Dunnett’s multiple comparisons test. n shown are for independent samples from at least 3 independent transfections. For d,e bars show mean and one standard deviation. f, ChIP-seq for the active transcription marker (H3K27ac) was performed in umbilical vein endothelial cells (HUVECs), blood outgrowth endothelial cells (BOECs), H441 lung epithelial cells, and IMR-90 lung fibroblast cells. The rs17713054 enhancer (grey box, g) lacks strong modification under standard growth conditions in these cells.
Extended Data Figure 10. COVID-19 patient lung shows signals of EMT.
Spearman correlation of gene expression profiles for EMT-related genes with the cell-types identified by deconvolution. AT1: Alveolar Type1 pneumocytes, AT2: Alveolar Type2 pneumocytes. P-values were identified by two-sided Hmisc analysis (without multiple test correction), values for significant correlations are shown and all correlation and p-values are in Source Data.
Supplementary Material
Acknowledgements
We would like to thank Priscila Hirschfeld, Matt Gosden, Steph Carpenter, Caz Harrold, Lea Nussbaum, Yavor Bozhilov, Andrew King, Mohsin Badat, Minnie Salmon, Lance Hentges, Andrew Brown, Guiseppe Scozzafava, Alicia Lledó Lara, Tim Rostron, Jason Torres, Chris Eijsbouts, Valentina Iotchkova, and Martin Sergeant for their help with cell culture, experiments, and data analysis, Rob Beagrie for critical reading of the manuscript, and Doug Higgs for nurturing this research.
This GWAS approach was developed as part of the WIGWAM Consortium (Wellcome Investigation of Genome Wide Association Mechanisms) funded by a Wellcome Strategic Award (106130/Z/14/Z, J.R.H.). This work was also supported by Medical Research Council (MRC) Core Funding (MC_UU_00016/14, J.R.H.). D.J.D. received funding from the Oxford University Medical Science Internal Fund: Pump Priming (0006152). R.S. was supported by a Wellcome Doctoral Programme (203728/Z/16/Z). S.N.S. received Kennedy Trust for Rheumatology Research Core support (KENN171803). F.I. received support from Wellcome (211122/Z/18/Z). J.O.J.D. is funded by an MRC Clinician Scientist Award (MR/R008108) and received Wellcome support (098931/Z/12/Z). J.C.K. is a Wellcome Investigator (WT204969/Z/16/Z) and supported by NIHR Oxford Biomedical Research Centre and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (2018-I2M-2-002). A.M.M., D.R.G., and S.C.H. received support from Wellcome (110579/Z/15/Z). J.A.T. is supported by a Strategic Award from Wellcome (107212/Z/15/Z), JDRF (5-SRA-2015-130-A-N and 4-SRA-2017-473-A-N) and the Wellcome Core Award to the Wellcome Centre for Human Genetics (203141/Z/16/Z). C.E.A. and I.M. were supported by Banco Bilbao Vizcaya (BBVA) Foundation, “Ayudas a Equipos de Investigación Científica SARS-CoV-2 y COVID-19”. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Author Contributions Statement
D.J.D., A.J.C., O.M., N.R., and A.M.M. isolated, cultured, and fixed cells and processed 3C material. D.J.D., P.H., A.R.C., J.B., C.E.d.A., I.M., F.I., and J.O.J.D. designed and performed experiments. D.J.D., P.H., A.R.C., R.S., J.B., and S.N.S. analyzed data. C.E.d.A., I.M., D.R.G., S.C.H., J.C.K., J.A.T., F.I., and J.R.H. acquired funding and oversaw the project. D.J.D., J.O.J.D. and J.R.H. conceived the work, generated figures, and wrote the manuscript.
Competing Interests Statement
J.R.H. and J.O.J.D. are founders and shareholders of, and J.R.H., J.O.J.D., D.J.D. and R.S. are paid consultants for Nucleome Therapeutics. J.R.H and J.O.J.D. hold patents for Capture-C (WO2017068379A1, EP3365464B1, US10934578B2) and have a patent application for MCC. J.A.T. is member of the GSK Human Genetics Advisory Board. These authors declare no other financial or non-financial interests. The remaining authors declare no competing interests.
Contributor Information
COvid-19 Multi-omics Blood ATlas (COMBAT) Consortium:
A full list of consortium members can be found in Supplementary Table 1. Authors who are COMBAT consortium members:, Damien J. Downes, Ron Schwessinger, Julian C. Knight, John A. Todd, Stephen N. Sansom, Fadi Issa, and Jim R. Hughes
Data availability
Capture-C, Micro Capture-C, ATAC-seq and ChIP-seq data generated for this study (Fig 3, Extended Data Figs. 7, 9 and Supplementary Figs. 1, 2) are available from the Gene Expression Omnibus (GSE159867, GSE175791). Processed Capture-C data can be visualized on UCSC (http://datashare.molbiol.ox.ac.uk//datashare/project/fgenomics/publications/Downes_2021_Covid_GWAS/hub.txt) or on the CaptureSee website (https://capturesee.molbiol.ox.ac.uk/projects/capture_compare/3718). Numerical values for Figs. 2a-c, and 5d, and Extended Data Figs. 2, 3, 4, 6, 7, 9, 10 are available in Source Data. Expression data (Fig. 3, Extended Data Figs. 6,8) was from publicly available sources: GTEx (https://gtexportal.org), the Lung Cell Atlas (https://asthma.cellgeni.sanger.ac.uk/) and the Lung Epigenome (https://www.lungepigenome.org/). Publicly available open chromatin data (ATAC-seq/DNase-seq), transcription factor binding data (ChIP-seq), and epigenetic modifications (ChIP-seq) data, (Figs. 1,2, Extended Data Figs. 1, 2, 4-7, 9, Supplementary Figs. 1,2) were sourced from the ENCODE portal (https://www.encodeproject.org/), the Gene Expression Omnibus (GSE74912, GSE115684, GSE118189, GSE125926), the UCSC genome browser (https://genome.ucsc.edu), descartes human developmental accessibility atlas https://descartes.brotmanbaty.org/bbi/human-chromatin-during-development/) and the Lung Epigenome (https://www.lungepigenome.org/). Masked splicing prediction effects were downloaded from the SpliceAI database (https://github.com/Illumina/SpliceAI). CEBPB motif (MA0466.1) was downloaded from the JASPAR database (http://jaspar.genereg.net). Conserved miRNA sites were identified on miRdSNP (http://mirdsnp.ccr.buffalo.edu/browse-genes.php).
Code availability
All custom analysis code and links to software are available on Github https://github.com/Hughes-Genome-Group/Downes_2021_LZTFL1_Covid.git. Note, MCCsplitter.pl and MCCanalyser.pl are only available for academic use through the Oxford University Innovation software store https://process.innovation.ox.ac.uk/software/p/16529a/micro-capture-c-academic/1.
References
- 1.Zhu N, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini JJ, Hotchkiss JR, Broccard AF. Bench-to-bedside review: Microvascular and airspace linkage in ventilator-induced lung injury. J Am Med Assoc. 2020;323:2330. doi: 10.1186/cc2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID- 19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visscher PM, et al. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King EA, Wade Davis J, Degner JF. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019;15:1–20. doi: 10.1371/journal.pgen.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellinghaus D, et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pairo-Castineira E, et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2020 doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 11.Initiative, T. C.-19 H. G. Mapping the human genetic architecture of COVID-19. Nature. 2021 doi: 10.1101/2021.03.10.21252820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020 doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi T, et al. Age-dependent impact of the major common genetic risk factor for COVID-19 on severity and mortality. medRxiv. 2021:2021.03.07.21252875. doi: 10.1172/JCI152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nafilyan V, et al. Ethnic differences in COVID-19 mortality during the first two waves of the Coronavirus Pandemic: a nationwide cohort study of 29 million adults in England. Eur J Epidemiol. 2021 doi: 10.1007/s10654-021-00765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICNARC. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland. 2021
- 16.Downes DJ, et al. An integrated platform to systematically identify causal variants and genes for polygenic human traits. bioRxiv. 2019:813618. doi: 10.1101/813618. [DOI] [Google Scholar]
- 17.Hindorff LA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaganathan K, et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell. 2019;176:535–548.:e24. doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Schwessinger R, et al. DeepC: predicting 3D genome folding using megabase-scale transfer learning. Nat Methods. 2020 doi: 10.1038/s41592-020-0960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies JOJ, et al. Multiplexed analysis of chromosome conformation at vastly improved sensitivity. Nat Methods. 2016;13:74–80. doi: 10.1038/nmeth.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downes DJ, et al. High-resolution targeted 3C interrogation of cis-regulatory element organisation at genome-wide scale. Nat Commun. 2020 doi: 10.1038/s41467-020-20809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua P, et al. Defining genome architecture at base-pair resolution. Nature. 2021 doi: 10.1038/s41586-021-03639-4. [DOI] [PubMed] [Google Scholar]
- 23.Robertson CC, et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells,genes and drug targets for type 1 diabetes. Nat Genet. 2021 doi: 10.1038/s41588-021-00880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsopoulos NA, et al. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science (80-) 2019;365 doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:1–38. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno AE, et al. miRdSNP: A database of disease-associated SNPs and microRNA target sites on 3’UTRs of human genes. BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barenboim M, Zoltick BJ, Guo Y, Weinberger DR. MicroSNiPer: A web tool for prediction of SNP effects on putative microRNA targets. Hum Mutat. 2010;31:1223–1232. doi: 10.1002/humu.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genomics H. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderon D, et al. Landscape of stimulation-responsive chromatin across diverse human immune cells. Nat Genet. 2019;51:1494–1505. doi: 10.1038/s41588-019-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurman RE, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley DR, Snoek J, Rinn JL. Basset: learning the regulatory code of the accessible genome with deep convolutional neural networks. Genome Res. 2016:990–999. doi: 10.1101/gr.200535.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Troyanskaya OG. Predicting effects of noncoding variants with deep learning-based sequence model. Nat Methods. 2015;12 doi: 10.1038/nmeth.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozhilov YK, et al. A gain-of-function single nucleotide variant creates a new promoter which acts as an orientation-dependent enhancer-blocker. Nat Commun. 2021 doi: 10.1038/s41467-021-23980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domcke S, et al. A human cell atlas of fetal chromatin accessibility. Science (80-) 2020;370 doi: 10.1126/science.aba7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang A, et al. Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of sars-cov2 host genes. Elife. 2020;9:1–28. doi: 10.7554/eLife.62522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan L, et al. National Center for Biotechnology Information. National Library of Medicine; U.S: 2020. ALFA: Allele Frequency Aggregator. Available at: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ [Google Scholar]
- 37.Stolze LK, et al. Systems Genetics in Human Endothelial Cells Identifies Non-coding Variants Modifying Enhancers, Expression, and Complex Disease Traits. Am J Hum Genet. 2020;106:748–763. doi: 10.1016/j.ajhg.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendricks-Taylor LR, et al. The CCAAT/enhancer binding protein (C/EBPα) gene (CEBPA) maps to human chromosome 19q13.1 and the related nuclear factor NF-IL6 (C/EBPβ) gene (CEBPB) maps to human chromosome 20q13.1. Genomics. 1992;14:12–17. doi: 10.1016/s0888-7543(05)80276-9. [DOI] [PubMed] [Google Scholar]
- 39.ENCODE. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwessinger R, et al. Sasquatch: predicting the impact of regulatory SNPs on transcription factor binding from cell- and tissue-specific DNase footprints. Genome Res. 2017;27:1730–1742. doi: 10.1101/gr.220202.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehara S, Grinberg A, Farber JM, Love PE. A Role for CCR9 in T Lymphocyte Development and Migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 42.Liao F, et al. STRL33, A Novel Chemokine Receptor-like Protein, Functions as a Fusion Cofactor for Both Macrophage-tropic and T Cell Line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agostini C, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–1298. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- 44.Broer S, et al. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J Clin Invest. 2008;118:3881–3892. doi: 10.1172/JCI36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, et al. Tumor-suppressive functions of leucine zipper transcription factor-like 1. Cancer Res. 2010;70:2942–2950. doi: 10.1158/0008-5472.CAN-09-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaghloul N, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marion V, et al. Exome sequencing identifies mutations in LZTFL1, a bbsome and smoothened trafficking regulator, in a family with bardetebiedl syndrome with situs inversus and insertional polydactyly. J Med Genet. 2012;49:317–321. doi: 10.1136/jmedgenet-2012-100737. [DOI] [PubMed] [Google Scholar]
- 48.Vieira Braga FA, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 49.Pathak GA, et al. Integrative genomic analyses identify susceptibility genes underlying COVID-19 hospitalization. Nat Commun. 2021:1–11. doi: 10.1038/s41467-021-24824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Y, et al. Genome and epigenome editing identify CCR9 and SLC6A20 as target genes at the 3p21.31 locus associated with severe COVID-19. Signal Transduct Target Ther. 2021;6:2020–2022. doi: 10.1038/s41392-021-00519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giambartolomei C, et al. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mali P, et al. RNA-Guided Human Genome Engineering via Cas9. Science (80-) 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 54.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Stewart CA, et al. Lung cancer models reveal SARS-CoV-2-induced EMT contributes to COVID-19 pathophysiology. J Thorac Oncol. 2021 doi: 10.1016/j.jtho.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandolfi L, et al. Neutrophil extracellular traps induce the epithelial-mesenchymal transition: implications in post-COVID-19 fibrosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.663303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Q, et al. LZTFL1 suppresses lung tumorigenesis by maintaining differentiation of lung epithelial cells. Oncogene. 2016;35:2655–2663. doi: 10.1038/onc.2015.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, et al. LZTFL1 suppresses gastric cancer cell migration and invasion through regulating nuclear translocation of P-catenin. J Cancer Res Clin Oncol. 2014;140:1997–2008. doi: 10.1007/s00432-014-1753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang J, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J, et al. Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell. 2020;11:680–687. doi: 10.1007/s13238-020-00752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borczuk AC, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cross AR, et al. Spatial transcriptomic characterization of COVID-19 pneumonitis identifies immune pathways related to tissue injury. bioRxiv. 2021:2021.06.21.449178. doi: 10.1101/2021.06.21.449178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danaher P, et al. Advances in mixed cell deconvolution enable quantification of cell types in spatially- resolved gene expression data. bioRxiv. 2020:2020.08.04.235168. doi: 10.1101/2020.08.04.235168. [DOI] [Google Scholar]
- 66.Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gusev A, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48:245–252. doi: 10.1038/ng.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singer D, et al. Defective intestinal amino acid absorption in Ace2 null mice. Am J Physiol - Gastrointest Liver Physiol. 2012;303:686–695. doi: 10.1152/ajpgi.00140.2012. [DOI] [PubMed] [Google Scholar]
- 69.Vuille-dit-Bille RN, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 70.Ravindra NG, et al. Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes. PLoS Biol. 2021;19:1–24. doi: 10.1371/journal.pbio.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Promchan K, Natarajan V. Leucine zipper transcription factor-like 1 binds adaptor protein complex-1 and 2 and participates in trafficking of transferrin receptor 1. PLoS One. 2020;15:1–25. doi: 10.1371/journal.pone.0226298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starks RD, et al. Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins. PLoS Genet. 2015;11:1–16. doi: 10.1371/journal.pgen.1005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Q, et al. Lztfl1/BBS17 controls energy homeostasis by regulating the leptin signaling in the hypothalamic neurons. J Mol Cell Biol. 2018;10:402–410. doi: 10.1093/jmcb/mjy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seo S, et al. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and smoothened. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melms JC, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delorey TM, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021 doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinot R, et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun. 2021:1–16. doi: 10.1038/s41467-021-24521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruan T, et al. H1N1 Influenza Virus Cross-Activates Gli1 to Disrupt the Intercellular Junctions of Alveolar Epithelial Cells. Cell Rep. 2020;31:107801. doi: 10.1016/j.celrep.2020.107801. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann M, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.:e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corces MR, et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat Genet. 2016;48:1193–1203. doi: 10.1038/ng.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ludwig LS, et al. Transcriptional States and Chromatin Accessibility Underlying Human Erythropoiesis. CellReports. 2019;27:3228–3240.:e7. doi: 10.1016/j.celrep.2019.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott C, et al. Recapitulation of erythropoiesis in congenital dyserythropoietic anaemia type I (CDA-I) identifies defects in differentiation and nucleolar abnormalities. Haematologica. 2020:1–27. doi: 10.1101/744367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin-Ramirez J, Hofman M, Van Den Biggelaar M, Hebbel RP, Voorberg J. Establishment of outgrowth endothelial cells from peripheral blood. Nat Protoc. 2012;7:1709–1715. doi: 10.1038/nprot.2012.093. [DOI] [PubMed] [Google Scholar]
- 84.Kurita R, et al. Establishment of Immortalized Human Erythroid Progenitor Cell Lines Able to Produce Enucleated Red Blood Cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bailey TL, et al. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37:202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fornes O, et al. JASPAR 2020: Update of the open-Access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buniello A, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallace C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020;16:1–20. doi: 10.1371/journal.pgen.1008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, et al. The 3D Genome Browser: A web-based browser for visualizing 3D genome organization and long-range chromatin interactions. Genome Biol. 2018:1–12. doi: 10.1101/112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Downes DJ, Hughes JR. Chromosome Conformation Capture with Nuclear Titrated Capture-C (NuTi Capture-C) Protoc Exch. 2020:1–20. [Google Scholar]
- 91.Telenius JM, et al. CaptureCompendium: a comprehensive toolkit for 3C analysis. bioRxiv. 2020:1–18. doi: 10.1101/2020.02.17.952572. [DOI] [Google Scholar]
- 92.Telenius JM, Davies JOJ, Hughes JR, CCseqBasic . GitHub. 2020. [DOI] [Google Scholar]
- 93.Downes DJ, et al. CaptureCompare. GitHub. 2020 doi: 10.5281/zenodo.4194345. [DOI] [Google Scholar]
- 94.Krueger F. Trim Galore. 2015 https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/
- 95.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kent WJ. BLAT---The BLAST-Like Alignment Tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hentges LD, Sergeant MJ, Downes DJ, Hughes JR, Taylor S. LanceOtron: a deep learning peak caller for ATAC-seq, ChIP-seq, and DNase-seq. bioRxiv. 2021:2021.01.25.428108. doi: 10.1101/2021.01.25.428108. [DOI] [Google Scholar]
- 99.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–8. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Telenius JM, Hughes JR. NGseqBasic - a single-command UNIX tool for ATAC-seq, DNaseI-seq, Cut-and-Run, and ChIP-seq data mapping, high-resolution visualisation, and quality control. bioRxiv. 2018:393413. doi: 10.1101/393413. [DOI] [Google Scholar]
- 103.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Recalde-Zamacona B, et al. Histopathological findings in fatal COVID-19 severe acute respiratory syndrome: Preliminary experience from a series of 10 Spanish patients. Thorax. 2020;75:1116–1118. doi: 10.1136/thoraxjnl-2020-215577. [DOI] [PubMed] [Google Scholar]
- 105.Desai N, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat Commun. 2020;11 doi: 10.1038/s41467-020-20139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kent WJ, et al. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenbloom KR, et al. ENCODE Data in the UCSC Genome Browser: year 5 update. 2013;41:56–63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van de Geijn B, Mcvicker G, Gilad Y, Pritchard JK. WASP: Allele-specific software for robust molecular quantitative trait locus discovery. Nat Methods. 2015;12:1061–1063. doi: 10.1038/nmeth.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Capture-C, Micro Capture-C, ATAC-seq and ChIP-seq data generated for this study (Fig 3, Extended Data Figs. 7, 9 and Supplementary Figs. 1, 2) are available from the Gene Expression Omnibus (GSE159867, GSE175791). Processed Capture-C data can be visualized on UCSC (http://datashare.molbiol.ox.ac.uk//datashare/project/fgenomics/publications/Downes_2021_Covid_GWAS/hub.txt) or on the CaptureSee website (https://capturesee.molbiol.ox.ac.uk/projects/capture_compare/3718). Numerical values for Figs. 2a-c, and 5d, and Extended Data Figs. 2, 3, 4, 6, 7, 9, 10 are available in Source Data. Expression data (Fig. 3, Extended Data Figs. 6,8) was from publicly available sources: GTEx (https://gtexportal.org), the Lung Cell Atlas (https://asthma.cellgeni.sanger.ac.uk/) and the Lung Epigenome (https://www.lungepigenome.org/). Publicly available open chromatin data (ATAC-seq/DNase-seq), transcription factor binding data (ChIP-seq), and epigenetic modifications (ChIP-seq) data, (Figs. 1,2, Extended Data Figs. 1, 2, 4-7, 9, Supplementary Figs. 1,2) were sourced from the ENCODE portal (https://www.encodeproject.org/), the Gene Expression Omnibus (GSE74912, GSE115684, GSE118189, GSE125926), the UCSC genome browser (https://genome.ucsc.edu), descartes human developmental accessibility atlas https://descartes.brotmanbaty.org/bbi/human-chromatin-during-development/) and the Lung Epigenome (https://www.lungepigenome.org/). Masked splicing prediction effects were downloaded from the SpliceAI database (https://github.com/Illumina/SpliceAI). CEBPB motif (MA0466.1) was downloaded from the JASPAR database (http://jaspar.genereg.net). Conserved miRNA sites were identified on miRdSNP (http://mirdsnp.ccr.buffalo.edu/browse-genes.php).
All custom analysis code and links to software are available on Github https://github.com/Hughes-Genome-Group/Downes_2021_LZTFL1_Covid.git. Note, MCCsplitter.pl and MCCanalyser.pl are only available for academic use through the Oxford University Innovation software store https://process.innovation.ox.ac.uk/software/p/16529a/micro-capture-c-academic/1.