Summary
There is evidence that a number of medical conditions and co-morbidities are associated with obesity in young children. This review explored whether there is evidence of associations with other conditions or co-morbidities. Observational studies of young children (mean age < 10 years) were identified using electronic searches of five databases (MEDLINE, Embase, CINAHL, AMED and SPORTDiscus). Of 27 028 studies screened, 41 (comprising 44 comparisons) met the inclusion criteria. These studies provided data on five distinct diseases/conditions: asthma (n = 16), vitamin D deficiency (n = 10), iron deficiency (n = 10), allergies (n = 4) and flat-footedness (n = 4). Thirty-two studies were appropriate for meta-analysis using random-effects models, and revealed obesity was significantly associated with having asthma (OR 1.5, 95% CI 1.3–1.7), vitamin D deficiency (OR 1.9, 95% CI 1.4–2.5) and iron deficiency (OR 2.1, 95% CI 1.4–3.2). Heterogeneity (I 2) ranged from 57% to 61%. Narrative synthesis was conducted for all studies. There was no evidence of a consistent association between obesity in young children and eczema, dermatitis or rhinitis due to the low number of studies. However, there was an association with flat-footedness. These results have implications for health policy and practice and families. Further research leading to a greater understanding of the associations identified in this review is suggested.
Keywords: associations, childhood obesity, co-morbidities, meta-analysis
1. Introduction
While having obesity in childhood is a known predictor of numerous health conditions in adulthood, 1,2 a growing evidence base has demonstrated the adverse health effects obesity has during childhood and adolescence. Specifically, an abundance of research has demonstrated the link between having childhood obesity and cardio-metabolic disease markers such as high cholesterol, hypertension and abnormal glucose tolerance, with children with obesity at a threefold increased risk of hypertension than children without obesity for example.3–5 A recent systematic review of observational studies and randomized trials demonstrated that 5- to 15-year-old children with obesity had 7.49 mmHg higher systolic blood pressure, 0.15 mmol L−1 higher total cholesterol, 0.26 mmol L−1 higher triglycerides and significantly higher fasting insulin and insulin resistance than children without obesity. 6 The latter finding further supports recent research demonstrating the association between having childhood obesity and the development of type 2 diabetes in youth.7
While the relationship between having childhood obesity and cardio-metabolic risk factors has been well established by published research, including several systematic reviews, the potential relationship between having obesity in childhood and other co-morbid conditions is not as clearly understood. Furthermore, a focus on these co-morbidities in younger children with obesity is limited to date. Epidemiological research has demonstrated associations between childhood obesity and increased risk of asthma, sleep apnoea, vitamin D deficiency, non-alcoholic fatty liver disease, dental caries, eye disorders, atopic disease and musculoskeletal complaints among other conditions.8–16 However, recent systematic reviews investigating the health impacts of having childhood obesity have not been definitive regarding these conditions. Specifically, asthma has increasingly been linked to having childhood obesity; however, a review by Pulgaron identified a number of studies in which no association between childhood obesity and asthma was reported.17 Another systematic review and meta-analysis of 48 epidemiological studies demonstrated a weak but significant link between asthma and overweight/obesity in children. 18 However, a systematic review of 10 longitudinal studies demonstrated that children who had obesity as a child were more likely to suffer from asthma either in childhood or in adolescence,19 a finding supported by an umbrella review of risk factors for childhood obesity.20
There are a number of plausible explanations for the equivocal findings observed for the relationship between having some co morbidities and childhood obesity. However, one issue that is rarely discussed sufficiently in the literature is the methodological issues that may arise from the practice of combining children with overweight and obesity during participant recruitment and subsequent analyses. Many observational studies grouped children with obesity and overweight as a combined exposure variable, instead of considering both conditions as two distinct groups. Participants with overweight and obesity would rarely be combined in adult studies of co-morbidities but are routinely combined in paediatric studies. Possible reasons for this may be that there is relatively low prevalence of children with obesity in some populations historically, in comparison with overweight; therefore, recruiting an adequately powered sample to measure the outcomes of interest may be more difficult if the inclusion criteria are limited to individuals with obesity. While overweight without obesity in childhood is associated with numerous health conditions, 21 the grouping of participants with overweight and obesity together without appropriate stratification or subgroup analysis may dilute the real relationship between true obesity and the outcome of interest, which in some cases is considerably more pronounced than the effects of having overweight alone.6,22 Such issues are evident from studies that have stratified by body mass index (BMI) percentile, where the risk of co-morbidity rises as BMI increases, or prevalence is higher in groups with obesity compared with groups with overweight as defined by standardized cut-offs.11,22
Another potential source of inconsistent findings in research on the co-morbidities of child and adolescent obesity may be the numerous different age ranges used to define ‘childhood’ among the published literature. While some studies will distinguish childhood from adolescence using either internationally recognized categories, or researcher-defined cut-offs, others will take an all-encompassing approach and group all participants together from early childhood up to late adolescence. An issue with this approach is that the physiological, behavioural and metabolic differences between a young child and an older adolescent may have a significant influence on the outcome of interest. 23
The aim of this current systematic review was therefore to update and synthesize the evidence base on the physical co-morbidities of childhood obesity, focusing specifically on the impact of obesity (rather than overweight) in children under 10 years old and excluding co-morbidities of childhood obesity that have been well-established by previous systematic reviews and meta-analyses.
2. Methods
This systematic review was prospectively registered with PROSPERO-registration number: CRD42018079387. The Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) checklist was used to inform the conduct and reporting of this review. The methods used were guided by two expert Cochrane reviewers (AM and CS).
2.1. Inclusion and exclusion criteria
Observational studies of a cross-sectional, longitudinal or case-control design were included if they reported one measure of adiposity (e.g., BMI) in childhood (WHO definition; ≤9.9 years) and measured at least one physical health outcome in either childhood or adolescence (age 0–19 years). Studies that included children older than 10 years of age were included if the mean age of the overall sample was ≤9.9 years. Therefore, the overall age range of children within the included studies was 2–19 years (a number of studies stratified by age groups). Included studies were required to contain both groups with obesity and groups without obesity as a comparison within the sample and be published in English. Studies were also required to include both children with and without the respective co-morbid conditions. Studies that only recruited children with co-morbidities were excluded.
A number of co-morbid conditions including cardiovascular disease markers, diabetes, dental carries, sleep apnoea and metabolic syndrome were not incorporated into the search strategy for this review as the relationship is either well established, or a recent good-quality systematic review has been published relating to these conditions.24–26 Therefore, any studies reporting exclusively on these outcomes concerning childhood obesity were excluded. However, all other potential co-morbidities of childhood obesity were deemed eligible for inclusion in the review providing they met the inclusion criteria. Studies were excluded if
The study population had a prior health condition that would limit generalizability of the study findings (such as children born preterm or who had a disability).
Studies that exclusively recruited children who had the outcome of interest (co-morbidity) without an unaffected comparison group (as this would not allow for the assessment of weight status on the incidence of the condition).
All participants in the study population had the exposure (obesity) without a comparison group that did not have obesity.
Reported exclusively non-physical conditions (i.e., mental health conditions).
Recognized definitions of obesity were not used or reported in the study (e.g., ≥95th percentile for age and gender using national or international growth charts).
Children with obesity and overweight were combined without stratification during analysis.
The mean age of the sample was over 9.9 years at the time of obesity exposure.
Anthropometry or presence of morbidities was obtained through parental or self-report (parental reporting of doctor diagnosis of condition was included).
2.2. Search strategy and study selection
A computerized search of five electronic bibliographic databases (MEDLINE, Embase, CINAHL, AMED within Ovid and SPORTDiscus within Ebsco database platforms) was undertaken from January 2001 to December 2016. A forward citation search was also conducted on all eligible studies up to December 2018. This allowed for the identification of relevant studies that had been published during the time that had elapsed between the original search and completion of full-text screening. A comprehensive systematic review was published in 2003 in this subject area5 limiting the need to search databases from their inception. The search strategy used was checked and approved by a specialist librarian in addition to an experienced systematic reviewer (AM) before being executed in two database platforms (Ovid and EBSCOhost). The search strategy consisted of three categories, namely, (i) population group, incorporating truncated terms such as ‘child*,’ ‘infan*’ and ‘adolesc*.’ (ii) Exposure, using adiposity-related terms (e.g., ‘body mass index,’ ‘obes*’ and ‘adipos*). (iii) Outcomes, where broad headings were initially used (e.g., ‘health,’ ‘comorbid* or co-morbid*’) before becoming more focused on specific health conditions that were identified as potential co-morbidities of obesity from the published literature (e.g., ‘pes planus,’ ‘asthma,’ ‘musculoskeletal diseases’). Where possible, MESH-headings were used in addition to free-text words to account for databases without the MESH function and specific conditions not covered under MESH-headings. The search was restricted to children, human subjects and primary research studies.
Identified studies were independently screened for eligibility by two reviewers (SM and JG), initially by title and abstract (a random 20% of identified papers were double-screened), before double full-text screening was conducted for all papers that were deemed eligible after title and abstract screening. In addition to the two reviewers discussing any inconsistencies in identification, where consensus could not be reached, a third reviewer (JRR) was included in the discussion to support resolution of the decision. Following full-text screening, eligible studies had their reference lists searched for potentially relevant studies, as did relevant published systematic reviews. Relevant titles were exported to an excel spreadsheet and screened using the same methods as initially applied to the articles identified through database searches.
2.3. Quality assessment
The quality of eligible studies was assessed using an adapted version of the Newcastle–Ottawa scale (NOS) previously used in a review by Herzog et al.27 The tool has been adapted to assess the quality of cross-sectional studies in addition to cohort and case–control studies. The scale assesses studies against the following criteria: (i) selection of the sample; (ii) comparability of the sample/participants; (iii) assessment of exposure and outcomes. Stars are awarded for high-quality aspects of each study against the three aforementioned criteria, with a total of nine stars available for case–control and cohort studies and eight stars available for cross-sectional designs. Studies awarded less than five stars were classed as having a high risk of bias, while an award of ≥5 stars indicated low risk of bias. The scale has been specifically designed for non-randomized studies and does not report summary scores, which have been shown to be unreliable.28 Two researchers independently appraised each study before discussing any disagreements. No formal overall assessment of the quality of evidence was undertaken, as this review only included observational studies, which are deemed by GRADE29 to be of either low to very low quality of evidence.
2.4. Data extraction
The following data were extracted from each study: authors, publication year, study design, study population characteristics (sample size, geographic location, age ranges, % female), method of recruitment, exposure (exposure assessment method, the definition of obesity), outcomes (outcome assessment method, the definition of outcome, number of outcomes), method of analysis, reported effect estimates (odds ratios, relative risk, proportions, prevalence and relevant confidence intervals), level of significance reported and confounders controlled for in the analysis. We also planned to collect any data on the socio-economic status (SES) of children under study, given the known association between some medical conditions and SES in adults, and this field was included in our data extraction form. Data were extracted by two researchers independently using the pre-designed form, which was piloted on a random sample of studies prior to full data extraction commencing.
2.5. Data synthesis and meta-analysis
Both meta-analysis and narrative synthesis were performed for this review. Due to the inconsistent nature of information reported in a number of included studies, coupled with considerable study heterogeneity, it was not possible to include all the studies in the meta-analyses. Where narrative synthesis was adopted, recommendations outlined in the Cochrane handbook of systematic reviews were followed,30 whereby the characteristics of each study were summarized in terms of the study design, risk of bias and study context for each outcome. This was then followed by an exploration of the similarities and differences between each studies’ findings.
Random-effects meta-analysis was conducted on three outcomes in this review: asthma, vitamin D deficiency and iron deficiency. Odds ratios and corresponding 95% confidence intervals were collated from studies reporting these results. For studies that did not report odds ratios, information was collected on the number of children with obesity versus children without obesity, in addition to the number who presented with the outcome of interest within these two exposure groups. Pooled odds ratios and 95% confidence intervals were generated using random-effects method on MetaXL meta-analysis software (Version 5.3; EpiGear International Pty Ltd). Forest plots were generated for each outcome, and funnel plots were used to visually assess publication bias. Heterogeneity and inconsistency were assessed using Cochran’s Q statistical test, with the inconsistency test (I 2 > 50%) used to indicate moderate heterogeneity.
Where a significant proportion of the studies were of high risk of bias, a sensitivity analysis was conducted to assess whether their removal from the model significantly affected the overall result. For all analyses, the level of significance was set at ≤.05.
3. Results
3.1. Description of included studies
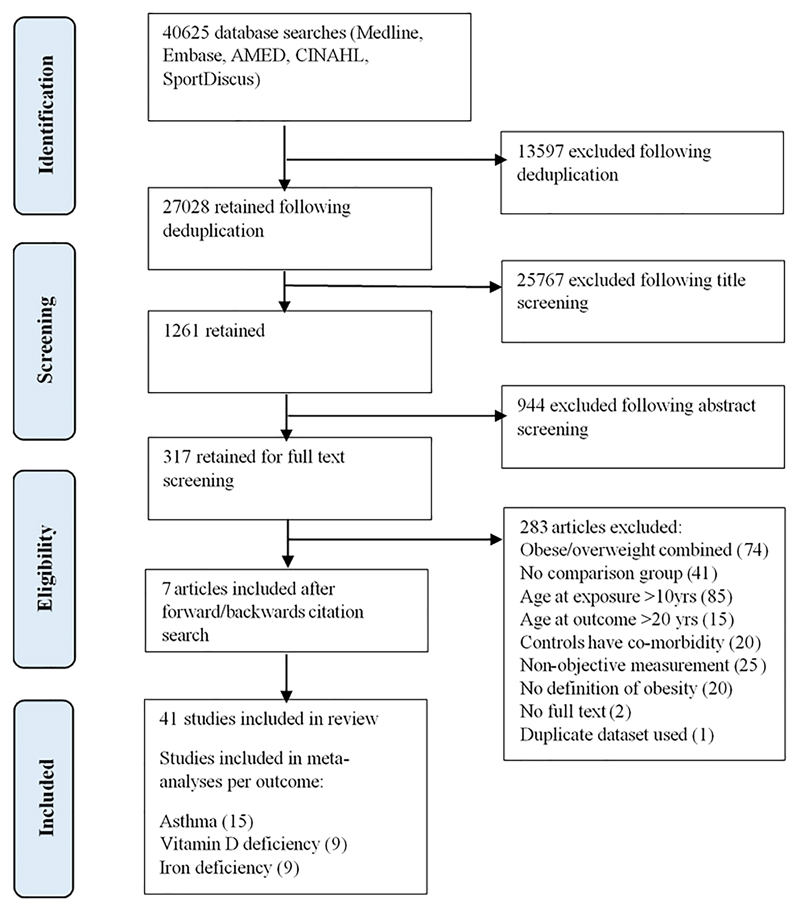
Of the 27 028 studies identified following database searches and deduplication, 41 met the inclusion criteria (Figure 1). These studies presented results investigating relationships between childhood obesity and five distinct health outcomes: asthma (n = 16),31–45 vitamin D deficiency (n = 10),46–55 iron deficiency (n = 10),34,56–64 flat-footedness/pes planus (n = 4)65–68 and allergies (n = 4).34,39,69,70 Two of the studies identified reported results for more than one of the outcomes.
Figure 1. PRISMA flow diagram for study identification and inclusion.
Although one of the original aims of the review was to investigate obesity in children under <10 years and co-morbidity in later childhood or adolescence, we did not identify any longitudinal studies that followed young children into adolescence. Therefore, all analyses investigated the associations between having obesity and co-morbid conditions during childhood defined as under 10 years of age.
3.2. Study characteristics
Study characteristics are summarized in Tables 1–5. The majority of included studies were cross-sectional studies (n = 29), followed by case–control studies (n = 8). Four studies were longitudinal, three being prospective cohort studies, and one a Mendelian randomization study. Studies varied considerably by sample size, from a case–control study with 100 participants to a repeat cross-sectional study totalling 36 152 participants. All studies involved the objective measurement of anthropometry by trained practitioners. All outcomes were objectively measured using established protocols, with the exception of asthma and allergies, for which the majority of studies employed valid diagnostic survey methods to obtain confirmation via parental report of diagnosis by a health professional.
Table 1. Stud ies reporting on the relationship between obesity and asthma (n = 16).
| Author and publication year | Study design | Country | Sample size (n) | Age range or mean age | Obesity definition | Outcome definition | Outcome identification | Covariates | Results | Study quality (based on NOS score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadiafshar et al. | Case control | Iran | 400 | 6–15 years | CDC growth charts | Report of doctor diagnosis | Asthma—history, clinical findings and pulmonary function test | - | Obesity increased odds of asthma: (OR 2.44; 95% CI 1.2–4.97) | High risk of bias (4/9) |
| Black et al. | Prospective cohort | United States | 14 987 | 6–10 years | CDC growth charts: moderately obese (>95th percentile OR BMI ≥ 30) and extremely obese (≥99th percentile OR BMI ≥ 35) | Asthma Index ICD-9 code 493 | Parental questionnaire | Sex, race/ethnicity, insurance payer | Obesity increased the risk of asthma: moderate obese: overall (HR 1.32 95% CI 1.26–1.39), girls HR 1.36 95% CI 1.27–1.46), boys (HR 1.28 95% CI 1.21–1.36) Extreme obese: overall (HR 1.49 95% CI 1.41–1.5), girls (HR 1.56 95% CI 1.43–1.71), boys (HR 1.43 95% CI 1.33–1.5) |
Low risk of bias (9/9) |
| Skinner et al. | Cross-sectional | United States | 2792 | 3–5 years | ≥95th percentile obese; ≥99th percentile very obese | Report of doctor diagnosis of asthma | Parental questionnaire/interview | Age, race/ethnicity, income, insurance status | Obese or very obese status increased odds of asthma in boys but not in girls: very obese: boys (OR 2.51 95% CI 1.36–4.64; girls OR 1.33 95% CI 0.46–3.84). Obese: boys (OR 2.42 95% CI 1.15–5.11; girls OR 1.15 95% CI 0.56–2.37) |
Low risk of bias (8/8) |
| Granell et al. | Mendillian randomization | United Kingdom | 4835 (2376 girls) | 7 and 9 years | ≥95th percentile | Report of doctor diagnosis of asthma | Parental questionnaire | - | Obesity increased the risk of asthma at 7 years old (RR 0.21; 95% CI 0.14–0.31) | Low risk of bias (9/9) |
| Guibas et al. | Cross-sectional | Greece | 1622 (789 girls) | 2–5 years | ≥95th percentile | Report of doctor diagnosis of asthma | Parental questionnaire | Prenatal smoking, gestational age, birthweight, gender, parity, breastfeeding, passive smoking at home, nationality, parental educational level | Obese status did not significantly increase odds of asthma (OR 1.54; 95% CI 0.85–280) | Low risk of bias (6/8) |
| Kwon et al. | Cross-sectional | United States | 853 (431 girls) | 7.5 years | U.K. 1990 growth charts | Report of doctor or nurse diagnosis of asthma and evidence of asthma-like symptoms or asthma-related emergency care use during the past year | Parental questionnaire | Age, race/ethnicity, nativity, household smoking exposure | Obese status increased odd of asthma in boys and girls: (boys OR 2.4; 95% CI 1.4–4.3; girls OR 2.1; 95% CI 1.2–3.8) | Low risk of bias (7/8) |
| Romeiu et al. | Cross-sectional | United States | 3337 | 2–5 years | IOTF cut-offs | Report of doctor diagnosis of asthma and report of current asthma symptoms | Parental questionnaire | Wheezing, atopy, physical activity, vitamin C consumption, dietary intake, race, poverty income ratio, passive smoking, parental asthma, hay fever ever | Obese status did not significantly increase the odds of asthma (OR 1.41; 95% CI.56–3.57) | Low risk of bias (7/8) |
| Suglia et al. | Cross-sectional | United States | 1815 | 3 years | CDC growth charts | Report of doctor diagnosis of asthma which had been active within the last year | Parental questionnaire | Sex, race/ethnicity, low birth weight, maternal education, parent marital status, maternal age, public assistance, daycare attendance, maternal depression, intimate partner violence, child neglect, housing quality, tobacco exposure | Obese status increased odds of asthma in boys and girls (whole sample OR 2.26; 95% CI 1.5–3.3. Boys OR 2.55; 95% CI 1.5–4.3. Girls OR 1.97; 95% CI 1.1–3.7) | Low risk of bias (7/8) |
| Tai et al. | Cross-sectional | Australia | 1509 (737 girls) | 4–5 years | CDC growth charts | Report of doctor diagnosis of asthma | Parental questionnaire | Sex | Obese status increased odds of asthma (OR 2.96; 95% CI 1.84–4.75) | Low risk of Bias (5/8) |
| Wake et al. | Cross-sectional | Australia | 13 879 | 2–7 years | CDC growth charts | Report of doctor diagnosis of asthma with medication use in last 12 months | Parental questionnaire | - | Asthma prevalence: 2–3 yrs = normal weight—10.1 (0.5); obese—13.5(2.4). 4–5 yrs = normal weight—14.5 (0.6); obese—19.1 (2.5). 6–7 yrs = normal weight—15.4 (0.6); obese—19.4 (2.6) | Low risk of bias (7/8) |
| Yao et al. | Cross-sectional | China | 12 092 (5761 girls) | 8.2 years | IOTF cut-offs | Report of doctor diagnosis of asthma | Parental questionnaire | Age, sex | Obese status increased the odds of asthma (OR 1.242; 95% CI .080–1.429) | Low risk of bias (5/8) |
| Amra et al. | Cross-sectional | Iran | 2413 | 7–12 years | IOTF cut-offs | Report of doctor diagnosis of asthma | Parental questionnaire | Sex, age, parental smoking, family history | Obese status was significantly associated with asthma | High risk of bias (4/8) |
| Akinbami et al. | Repeat cross-sectional | United States | 36 152 | 2–19 years | Cole et al. | CDC asthma surveillance definition | Parental questionnaire | Age, sex, income status | Obese status significantly increased odds of asthma in White, Black and Mexican American children (White OR 1.7; 95% CI 1.4–2.2. Black OR 1.8; 95% CI 1.6–2.1. Mexican American OR 1.4; 95% CI 1.1–1.8) | Low risk of bias (7/8) |
| den Dekker et al. | Cross-sectional | Netherlands | 6178 | 6.2 years | ≥95th percentile | Global Initiative for Asthma definition | Parental questionnaire | Maternal age, pre-pregnancy BMI, educational level, history of asthma and atopy, psychological distress during pregnancy, parity, smoking during pregnancy, child’s sex, gestational age at birth, birth weight, ethnicity, breastfeeding, pet keeping, physical activity, lower respiratory tract infections, current height | Obese status did not increase the odds of asthma (OR 0.90; 95% CI 0.36–2.22) | Low risk of bias (7/8) |
| Lei et al. | Cross-sectional | China | 3327 (1663 girls) | 2–14 years | CDC growth charts | Report of doctor diagnosis of asthma | Parental questionnaire | - | Obese status did not increase the odds of asthma (overall OR 1.09; 95% CI 0.68–1.72; boys OR 1.0; 95% CI 0.58–1.79; girls OR 1.15; 95% CI 0.54–2.44) | Low risk of bias (6/8) |
| Shachter et al. | Cross-sectional | Australia | 5993 (2976 girls) | 9.8 years | IOTF cut-offs | Report of doctor diagnosis of asthma, with present symptoms | Parental questionnaire | Family history of asthma, sex, atopy status, exposure to cigarette smoke | Obese status did not increase the odds of asthma (OR 0.87; 95% CI 0.65–1.17) | Low risk of bias (7/8) |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; IOTF, International Obesity Task Force; NOS, Newcastle–Ottawa Scale.
Table 5. Stud ¡es reporting on the relationship between obesity and allergies (n = 4).
| Author and publication year | Study design | Country | Sample size (n) | Age range or mean age | Obesity definition | Outcome definition | Outcome identification | Covariates | Results | Study quality (based on NOS score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lei et al. | Cross-sectional | China | 3327 (1,663 girls) | 2–14 years | Chinese growth charts | Allergic rhinitis and its impact on asthma criteria and atopic dermatitis using the Hanifin and Rajka criteria | Clinical examination by doctor | - | Rhinitis overall: 1.33 (1.04–1.72); girls: 1.48 (1.00–2.18); boys: 1.20 (0.86–1.67). Dermatitis overall: 1.33 (1.02–1.74); girls: 1.42 (0.93–2.16); boys: 1.24 (0.87–1.75) | Low risk of bias (6/8) |
| Silverberg et al. | Case control | United States | 1242 (592 girls) | 7 years | WHO growth reference charts for <2 year olds; CDC growth charts for > 2 year olds | International Classification of Diseases–ninth revision diagnostic code 691.8 for atopic dermatitis | Clinical examination by doctor | Sex; season of birth; comorbid asthma, allergic rhinoconjunctivitis and food allergy; race/ethnicity; and immunization up-to-date, age at the time of the study and at first diagnosis of atopic dermatitis, height, height for age, weight, weight for age, head circumference, head circumference for age | Obese status increased odds of atopic dermatitis (OR 2.00; 95% CI 1.22–3.26) | Low risk of bias (8/9) |
| Skinner et al. | Cross-sectional | United States | 2792 | 3–5 years | CDC growth charts | Clinical guidelines for eczema diagnosis | Parental report of doctor diagnosis of eczema | Age, race/ethnicity, income, insurance status | Obese or very obese status did not increase odds of eczema diagnosis in boys or girls | Low risk of bias (8/8) |
| Weinmayr et al. | Cross-sectional | Multicentre: Brazil, Estonia, Georgia, Germany, Ghana, Greece, India, Italy, Latvia, Netherlands, New Zealand, Norway, Palestine, Spain, Sweden, Turkey | 10 652 | 9.4 years | Cole et al. | Clinical guidelines for allergic presentations | Clinical examination by trained fieldworker, skin prick test | Sex | Skin prick test = OR 1.13 (0.91; 1.42), examined eczema without wheeze = 2.07 (1.03; 4.17) | Low risk of bias (7/8) |
Abbreviations: CDC, Centers for Disease Control and Prevention; NOS, Newcastle–Ottawa Scale.
3.3. Study quality
Overall, cohort studies were rated as having a lower risk of bias than the case–control studies, with mean NOS scores of 7.4/9 and 6.3/9, respectively (a higher score equates to a lower risk of bias). Crosssectional studies had a mean score of 6.3 out of a possible 8 stars. In general, studies of all designs did not adequately describe or justify sample sizes or demonstrate the representativeness of the sample to the general population. Because of the stringent inclusion criteria adopted for this review, the included studies all scored highly on the NOS items pertaining to ‘assessment of exposures and outcomes.’ Six studies received a rating of below 5 stars (four cross-sectional and two case–control) and were deemed to have a high risk of bias. The NOS score and corresponding risk of bias for each study are summarized in Tables 1–5.
3.4. Association between childhood obesity and asthma
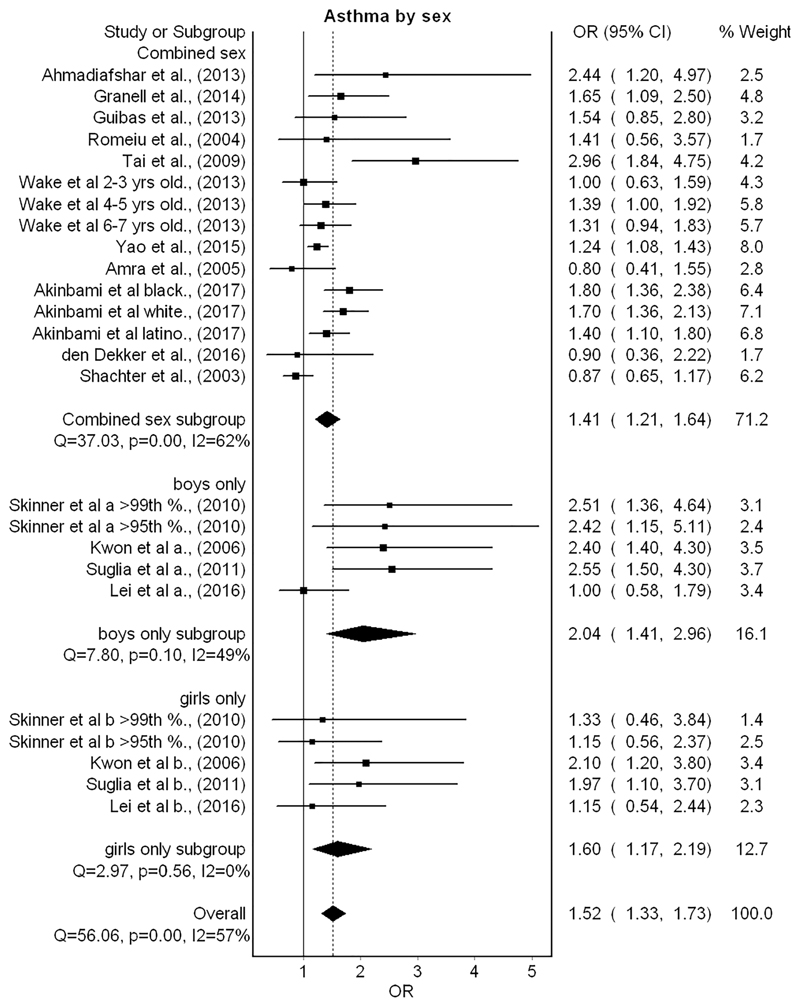
Fifteen studies were grouped comparing the odds of asthma diagnosis between children with and without obesity.31–45,71 Six studies presented results separately for different subgroups within the study sample (i.e., by age group and ethnicity),32,34,38,39,42,44 and these results are presented separately in the forest plot output (Figure 2). Additionally, subgroup analysis of four studies that presented results separately for boys and girls is also included in the forest plot. The metaanalysis demonstrated that having childhood obesity significantly increased the odds of asthma diagnosis by over 50% in comparison with children without obesity (OR 1.5; 95% CI 1.3–1.7). Inconsistency was moderate (I 2 = 57%). In subgroup analysis by sex, boys showed higher odds than girls for having asthma and obesity (OR 2.0; 95% CI 1.4–2.9 and OR 1.6; 95% CI 1.2–2.2, respectively). However, this finding was not statistically significant (p > .05). Two of the studies were assessed as having a high risk of bias.31,33 However, removal of these studies from the model in a sensitivity analysis did not lead to a statistically significant change in the pooled result (OR 1.5; 95% CI 1.3–1.7). No publication bias was indicated by the funnel plot (Figure 3).
Figure 2. Forest plot for random effects meta-analysis of studies investigating relationships between childhood obesity and asthma (OR = odds ratio; CI = confidence interval).
Figure 3.
Funnel plot for studies reporting on the relationship between obesity and asthma in Meta-analysis. (ES = effect size)
One additional study met the inclusion criteria for the review but was not appropriate for inclusion in the meta-analysis, as it was a large prospective cohort study,71 which presented time-to-event analysis that would be poorly interpreted by conversion to odds. The study was evaluated as having a low risk of bias and reported that having moderate obesity (≥95th centile) and extreme obesity (≥99th centile) both significantly increased the risk of asthma diagnosis.71
3.5. Association between childhood obesity and vitamin D deficiency
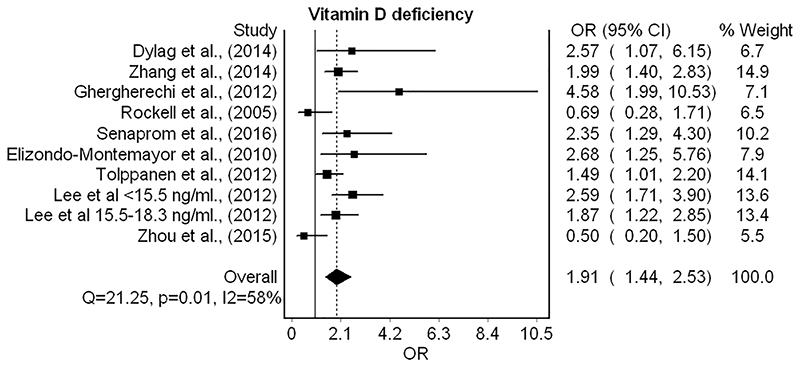
Nine separate studies were included in the meta-analysis of vitamin D deficiency and obesity,46–48,50–55 resulting in a pooled odds ratio of 1.9 (95% CI 1.4–2.5) (Figure 4). The studies showed moderate heterogeneity (I 2 = 58%). The majority of studies defined deficiency as <20 ng ml−1, and one study reported separate odds ratios for deficiency and severe deficiency, which have been added to the forest plot separately.50 Studies that reported serum levels in nmol L−1 were less uniform, with deficiency cut-offs ranging from <17.5 to <30 nmol L−151,55 (see Table 2). Two of the studies included in the meta-analysis had a high risk of bias.46,48 When these studies were removed during sensitivity analysis, the pooled effect size was reduced to OR 1.7 (95% CI 1.3–2.3).
Figure 4. Forest plot for random effects meta-analysis of studies investigating relationships between childhood obesity and vitamin D deficiency.
Table 2. Stud ies reporting on the relationship between obesity and vitamin D deficiency (n = 10).
| Author and publication year | Study design | Country | Sample size (n) | Age range or mean age | Obesity definition | Outcome definition | Outcome identification | Covariates | Results | Study quality (based on NOS score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dylag et al. | Cross-sectional | Poland | 100 (55 girls) | 1–5 years | WHO growth reference charts | Optimal vitamin D levels: >30 ≤ 50 ng ml−1; suboptimal vitamin D levels: ≤30 ≥ 20 ng ml−1; vitamin D Deficiency: <20 ng ml−1 | Blood test/assay | Age | Significantly lower mean difference in vitamin D concentrations: 23.6 ± 10.8 obese, 26.6 ± 9.8 non-obese. | High risk of bias (4/8) |
| Elizondo-Montemayor et al. | Cross-sectional | Mexico | 198 (98 girls) | 9 years | WHO growth reference charts | Optimal vitamin D levels: ≥30 ng ml−1; vitamin D insufficiency: 21–29 ng ml−1; vitamin D deficiency: <20 ng ml−1 | Overnight fasting blood sample assessed using competitive immunolumi nometric direct assay | Skin phototype, physical activity, screen time, vitamin use, diet | Obese status increased the odds of vitamin D deficiency (OR 2.679; 95% CI 1.245–5.765) | Low risk of bias (6/8) |
| Ghergherechi et al. | Case control | Iran | 109 | 8.9 years | >95% centile for age and gender | <20 ng dl−1 vitamin D deficiency; <10 ng dl−1 severe vitamin D deficiency | Blood test/assay | Age, sex, height | Vitamin D deficiency obese group = 76.9%; non-obese = 42.1%. Severe vitamin D deficiency obese group = 44.2%; non-obese = 17.5% | High risk of bias (4/9) |
| Jazar et al. | Cross-sectional | Jordan | 200 (100 girls) | 3.3 years | CDC growth charts | Vitamin D insufficiency, from 15 to 20 ng ml−1; vitamin D deficiency, ≤15 ng ml−1; severe vitamin D deficiency ≤ 5 ng ml−1 | Blood test/assay | Duration of breastfeeding, duration of formula feeding, duration of outdoor physical activity, calcium intake and dietary vitamin D intake | Significantly lower mean serum vitamin D levels in obese participants compared with controls (obese serum vit D levels = 13.0 ± 2.5 v normal weight 25.4 ±0.6) | High risk of bias (4/8) |
| Lee et al. | Cross-sectional | South Korea | 1660 (756 girls) | 9 years | BMI ≥ 95th percentile for age and sex | <20 ng ml−1 vitamin D deficient | Blood collected after overnight fasting and 25(OH)D concentrations measured by chemiluminescent immunoassay | - | Obese status increased odds of having lower mean serum vitamin D levels | Low risk of bias (5/8) |
| Rockell et al. | Cross-sectional | New Zealand | 1585 (784 girls) | 5–14 years | IOTF cut-offs | Vitamin D deficient <17.5 nmol L−1; vitamin D insufficient: <37.5 nmol L−1 | Blood sample/assay | Age, ethnicity, latitude (North vs. South Island), season (‘summer’ vs. ‘winter’ months) | Both vitamin D deficiency and insufficiency were significantly associated with obese status | Low risk of bias (7/8) |
| Senaprom et al. | Cross-sectional | Thailand | 477 (239 girls) | 7.8 years | BMI-for-age Z score (BAZ) >3 SD above the median for children aged 3–5.9 years (WHO, 2006) and as a BAZ > 2 SD above the median for children aged 6–13 years |
Vitamin D deficiency <50 nmol l−1 | Fasting blood sample analysed by chemiluminescence immunoassay | - | Obese status was significantly associated with vitamin D deficiency | Low risk of bias (5/8) |
| Tolppanen et al. | Prospective cohort | United Kingdom | 7555 (3744 girls) | 9.8 years | Cole et al. international BMI cut-off values | Vitamin D deficiency < 20 ng ml−1 | Non-fasting blood samples assayed using HPLC tandem mass spectrometry | - | Odds of vitamin D deficiency in obese participants was 1.49 (95% CI 1.01–2.20) | Low risk of bias (7/9) |
| Zhang et al. | Cross-sectional | China | 1488 (656 girls) | 8.8 years | Chinese obesity task force cut-off values | Vitamin D deficiency = <20 ng ml−1; vitamin D insufficiency = 20–30 ng ml−1: vitamin D sufficiency ≥ 30 ng ml−1 | Blood sample and liquid chromatography | Age, gender, dietary energy intake, energy expenditure | Significantly higher prevalence of vitamin D deficiency among obese participants compared with normal weight | Low risk of bias (7/8) |
| Zhou et al. | Cross-sectional | Australia | 221 (105 girls) | 1–5 years | WHO growth reference charts | Deficiency = vit D < 30 nmol L−1; insufficiency = Vit D ≥ 30 and <50 nmol L−1 | Non-fasting blood sample and assay | - | No significant difference between mean serum vitamin D levels in obese and normal weight individuals | Low risk of bias (6/8) |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; HPLC, high-performance liquid chromatography; IOTF, International Obesity Task Force; NOS, Newcastle–Ottawa Scale.
One additional study49 reported insufficient data to allow for pooling within the meta-analysis and was judged as having a high risk of bias.49 This study reported significantly lower vitamin D levels in children with obesity, due to its small sample size, this study would not have significantly influenced the overall effect size in pooled analysis had it been included.
3.6. Association between childhood obesity and iron deficiency
Ten studies investigated the relationship between childhood obesity and iron deficiency34,56–64 of which nine were appropriate for meta-analysis (Figure 5). Two of these studies conducted separate analyses by sex,34,59 with both subgroups included in the model individually. Meta-analysis revealed that having obesity doubled the odds of iron deficiency diagnosis (OR 2.1; 95% CI 1.4–3.2). However, the removal of one study56 with a large effect size during sensitivity analysis reduced the association (OR 1.8; 95% CI 1.3–2.6).
Figure 5. Forest plot for random effects meta-analysis of studies investigating relationships between childhood obesity and iron deficiency.
One case–control study60 assessed as having a low risk of bias was not appropriate for meta-analysis due to insufficient reporting of data necessary for calculation of odds and found children with obesity to have significantly different markers of iron deficiency than the control group. Specifically, children with obesity had significantly lower iron, transferrin saturation and total-iron binding capacity along with higher ferritin, soluble transferrin receptors and hepcidin-25 than children of normal weight.
3.7. Association between childhood obesity and pes planus (flat-footedness)
Four studies investigated the relationship between childhood obesity and having flat-footedness.65–68 Of these, one was a longitudinal study,65 two were of a cross-sectional design66,67 and one was a case control study.68 All four studies were of low risk of bias and reported a statistically significant association between having flat-footedness and obesity. One study66 investigated both bilateral and unilateral flat-footedness but only found having obesity to significantly increase the odds of the bilateral condition (OR 1.9; 95% CI 1.2–2.9), while the remaining three studies investigated bilateral flat-footedness only. All four studies were assessed as having a low risk of bias following quality assessment (Table 4).
Table 4. Stud ies reporting on the relationship between obesity and musculoskeletal disorders (n = 4).
| Author and publication year | Study design | Country | Sample size (n) | Age range or mean age | Obesity definition | Outcome definition | Outcome identification | Covariates | Results | Study quality (based on NOS score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen 1 et al. | Prospective cohort | Taiwan | 580 (283 girls) | 3–5 years | Taiwanese FDA definitions of obesity for children and adolescents | Flatfoot = AB distance by CSI > 62.70%. CSI is defined as the ratio of the minimum width of the midfoot arch region (B) to the maximum width of the metatarsus region (A) | Clinician measurement using digital footprint mat | Age | Prevalence of flatfoot was significantly higher in obese children | Low risk of bias (6/9) |
| Chen 2 et al. | Cross-sectional | Taiwan | 1598 (765 girls) | 3–6 years | Taiwanese FDA definitions of obesity for children and adolescents | Clinical presentations of malformation of the medial longitudinal arch in a weight bearing position | Clinician examination of foot | Age, sex, joint laxity, W sitting | Obese status increased the odds of bilateral flatfoot, but did not increase odds of unilateral flatfoot (Bilateral OR 1.90; 95% CI 1.22–2.95; unilateral OR 1.39; 95% CI 0.80–2.41) | Low risk of bias (6/8) |
| Ezema et al. | Cross-sectional | Nigeria | 474 (253 girls) | 6–10 years | CDC growth charts | Plantar arch index value >1.15 | Ink footprint test | - | Prevalence of flatfoot was significantly higher in obese children | Low risk of bias (7/8) |
| Riddiford-Harland et al. | Case control | Australia | 150 (98 girls) | 8.3 years | Cole et al. | Clinical presentation of reduced foot arch on ultrasound | Ultrasound | Age, sex | Prevalence of flatfoot was significantly higher in obese children | Low risk of bias (8/9) |
Abbreviations: CDC, Centers for Disease Control and Prevention; NOS, Newcastle–Ottawa Scale.
3.8. Association between childhood obesity and allergies
Four studies assessed the relationship between childhood obesity and allergic conditions.34,39,69,70 Two distinct conditions were investigated within the studies; all four studies assessed eczema/dermatitis, and one study also included rhinitis as an outcome.39 Additionally, one study reported results from the skin prick test.70 Three of these studies had a cross-sectional design, and one was a case–control study.69 All four studies had a low risk of bias (Table 5). Three of the four studies found having obesity to increase the odds of eczema/dermatitis diagnosis; however, these effects were small to moderate.39,69,70 One study reported no association between eczema and obesity/severe obesity.34 Having obesity was found to slightly increase the odds of rhinitis diagnosis in one study (OR 1.3; 95% CI 1.0–1.7) when the sample was analysed collectively. However, a differential effect by sex was reported, as the association was only evident in girls and not boys.39 Obesity was not found to significantly increase the odds of a positive skin prick test (OR 1.1; 95% CI 0.9–1.4).
4. Discussion
This systematic review and meta-analysis investigated associations between obesity in young children and multiple co-morbid conditions. Though this topic has been studied through both primary research and recent systematic reviews,17,18,72–78 we investigated obesity as a distinct condition from overweight. This is in contrast to similar systematic reviews in the subject area, which have included studies that combine individuals with overweight or obesity, or do not stratify by weight status in the analysis.17–19,73,78 We also used more stringent inclusion criteria to define childhood as children under 10 years, potentially offsetting the physiological, cultural and behavioural effects that later childhood/adolescence can have on obesity co-morbidities.79,80
The results of our review offer a number of important findings. Firstly, the meta-analysis of childhood obesity and asthma diagnosis appears to support previous results from systematic reviews in this area,18,77,78 while also further distinguishing the effects of having obesity considered explicitly from overweight. Chen et al.77 reported a significantly higher risk of incident asthma among children and adolescents with obesity compared with children without obesity (relative risk 2.02; 95% CI 1.16–3.50), while a narrative synthesis by Papoutsakis et al.18 concluded that there was a clear relationship between childhood obesity and asthma incidence. Our finding that having obesity at a young age increased the odds of asthma by over 50% indicates that there is a possible relationship between the two conditions. However, we did not identify any longitudinal studies that were appropriate for meta-analysis based on our inclusion criteria, meaning the cross-sectional data that our findings are based on cannot offer any indication of a causal link between obesity and asthma. One cohort study included in our narrative synthesis found that not only did having a higher BMI predispose children to subsequent asthma development, but children with both asthma and obesity or overweight were also more likely to develop a severe asthma phenotype than healthy weight children with asthma.71 A meta-analysis of six longitudinal studies also found that asthma risk increased by 35% among children with obesity/overweight.78 Conversely, Chen et al. reported that asthma in fact preceded the onset of obesity even after controlling for glucocorticosteroid usage, with children with asthma having 51% higher risk of developing obesity at follow-up than children without asthma.81
There may be legitimate physiological and behavioural explanations for both directions of the relationship. Firstly, physiological consequences of obesity such as reduced lung and tidal volume, low-grade systemic inflammation and changes in adipose-derived hormones likely promote the onset of asthma.82 Conversely, children with normal weight and asthma may be at a higher risk of developing overweight and obesity due to the observed tendency for children with asthma to avoid moderate-vigorous physical activity,83,84 an important protective factor against excess weight gain.85 Additionally, asthma medications such as glucocorticosteroids are theorized to promote weight gain through increased lipid metabolism and storage.81
Of the four identified studies relating to musculoskeletal disorders included in our review, all related to flat-footedness, and all found having obesity to significantly increase risk. Other musculoskeletal disorders have been studied in relation to childhood obesity; however, these did not meet our inclusion criteria. A recent review by Paulis and colleagues found musculoskeletal pain to be related to childhood overweight and obesity,73 supporting the findings of our review that obesity may have structural/biomechanical consequences. Potential physiological explanations for this are expressed in the literature, with excess fat deposits on feet or excess load-bearing due to excess weight causing arches to collapse in children with obesity.68
This review identified vitamin D deficiency as a condition that is associated with obesity in young children, a finding that has only previously been investigated in a small number of systematic reviews.17,74,75 Periera-Santos and colleagues found that obesity in children and adolescents increased the prevalence of vitamin D deficiency by 37% in a meta-analysis of eight studies.74 Another meta-analysis reported a pooled odds ratio of 3.43 (95% CI 2.33–5.06).75 Our finding from the present review that having obesity increases the odds of vitamin D deficiency further supports these findings. However, as with asthma, the studies that met the inclusion criteria for this review were all cross-sectional in nature, and therefore, a causal relationship could not be confirmed. Physiological mechanisms of vitamin D deficiency as a consequence of obesity have been discussed in the literature86; however, the nature of the relationship is still poorly understood. A longitudinal study of Colombian 5–12 year olds found vitamin D deficient participants had a 0.1/year greater change in BMI than vitamin D sufficient children,9 indicating that the physiological effects of obesity such as impaired hydroxylation may contribute to lower vitamin D levels in children with obesity.87 It is also important to consider that vitamin D deficiency has been shown to increase asthma severity, which may indicate each condition may mediate any relationship with obesity.88 Despite this, few studies included in this review controlled for this potential confounding (Tables 1 and 2). Furthermore, it is theorized that having obesity may impair the bioavailability of vitamin D for bloodstream absorption, as it is fat-soluble, and instead stored in adipose tissue reservoirs,89 further highlighting the complexity of the relationship between the two conditions.
This review also found that childhood obesity increased the odds of having iron deficiency, which to our knowledge is only the second such meta-analysis to demonstrate this relationship and the first to do so exclusively in children aged <10 years.76 An important observation that applies to both vitamin D and iron deficiency is that they are both nutritional deficiencies. It could therefore be that causation may be related to diet quality, as children with obesity have been shown to have poorer nutritional intake (lower nutrient density, consuming less iron-rich foods) than children with normal weight in epidemiological studies.90 A small number of studies included in our review controlled for diet in their analysis,47,49,58 still finding the conditions to be associated with obesity. However, the majority of studies concerning vitamin D and iron status did not include diet/nutrient intake as a covariate, which may have confounded the results obtained for these studies. In the case of vitamin D, this can be extended to include time spent outdoors (or physical activity as a proxy measure of ultraviolet light exposure), as it has been demonstrated that children with obesity spend less time in outdoor play and longer periods sedentary indoors.91 With vitamin D levels mediated by sunlight exposure,47 this could potentially explain the differences observed in children with obesity from a behavioural perspective. Therefore, the results of a number of included studies that did not control for these covariates should be interpreted with caution.46,48,50–53,55 In the case of iron deficiency, while mechanisms explaining effects of obesity on the condition are not fully understood, individuals with obesity have both increased iron requirements (secondary to increased blood volume) and reduced iron absorption (secondary to increased inflammation).92 Interventions to reduce weight status in children with vitamin D and iron deficiency would therefore enhance understanding of the causal effects of having obesity in these conditions.
While the inclusion criteria adopted for this review are a strength of this study, there are a number of limitations that should be considered when interpreting the findings. We decided against conducting an overall assessment of the quality of evidence for each outcome by using GRADE assessment criteria.29 Despite this, an alternative assessment of some key quality indicators adds further context to the strength of the evidence presented in this review. Specifically, the majority of studies were cross-sectional or case–control studies, which are more susceptible to bias than longitudinal studies of the same methodological rigour. Secondly, inconsistency was evident as the moderate–high heterogeneity observed within the meta-analyses in this review reflects the fact that a number of included studies did not adequately control for confounding factors in their analyses. It is therefore possible that the results of this review may have been affected by residual confounding and should be interpreted with caution. Definitive causal effects of childhood obesity on the co-morbid conditions identified in this review have still to be established, but plausible mechanisms have been identified as discussed above. It would therefore be beneficial for behavioural and environmental obesity treatment interventions to include measurement of morbidity as an outcome in evaluations, to determine if reductions in weight status are also accompanied by improvements in disease symptoms/presentation.
This systematic review and meta-analysis identified a number of co-morbidities of childhood obesity that were not well established previously. Evidence of an association between childhood obesity and diagnosis of asthma, vitamin D deficiency, flat-footedness and allergies is reported, in addition to the novel finding that iron deficiency is a potential co-morbidity of childhood obesity. Additionally, it appears that a better understanding of any important inequalities (by SES) in the relationship between obesity and health conditions in young children is needed to help support policy and practice with regard to obesity prevention in children.93 Healthcare professionals may find our results helpful when treating pediatric patients with obesity, in terms of additional assessment and consideration for the co-morbid conditions identified and investigated in this review. The potential for obesity to cause harm as early as childhood is apparent, and efforts to prevent obesity in the early years could, in turn, alleviate the health burden of conditions associated with having excess weight in childhood.
Table 3. Stud ies reporting on the relationship between obesity and iron deficiency (n = 10).
| Author and publication year | Study design | Country | Sample size (n) | Age range or mean age | Obesity definition | Outcome definition | Outcome identification | Covariates | Results | Study quality (based on NOS score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abd-El Wahed et al. | Case control | Egypt | 120 (62 girls) | 9.25 years | CDC growth charts | The presence of two or more of the following abnormal parameters: Mean corpuscular volume (MCV) is 76 fl or less; serum TS 15% or less; Serum ferritin less than 10 mg ml−1 | Blood sample/assay | Age, sex | Obese status increased odds of iron deficiency (OR 7.09; 95% CI 3.16–15.92) | Low risk of bias (8/8) |
| Brotanek et al. | Cross-sectional | United States | 960 (434 girls) | 1–3 years | Weight-for-length status of ≥95th percentile | Ages 1–2 years, iron deficiency <10% transferrin saturation < 10 g L−1 of serum ferritin and >1.42 mol L−1 of red blood cells erythrocyte protoporphyrin. For 3-year-old children, <12% < 10 g L−1, and >1.24 mol L−1 of red blood cells | Blood sample/assay | Race/ethnicity, interview language, preschool/day care attendance | Obese status increased odds of iron deficiency (OR 3.34; 95% CI 1.10–10.12) | Low risk of bias (8/8) |
| Cepeda-Lopez et al. | Cross-sectional | Mexico | 1174 (49% girls) | 8.17 years | WHO growth reference charts | Either (1) low serum iron (<60 ug dl−1) or (2) elevated TIBC (>360 ug dl−1)and low %TS (<20%) values | Blood sample/assay | Age, sex, region, area, caregiver education | Obese status increased the odds of iron deficiency (OR 3.96; 95% CI 1.34–11.67) | Low risk of bias (7/8) |
| Skinner et al. | Cross-sectional | United States | 2792 | 3–5 years | CDC growth charts | Taking medication for anaemia and laboratory values of hemoglobin <11 g dl−1.16 | Blood sample/assay | Age, ethnicity, income, insurance status | Obese status increased the odds of anaemia in boys but not in girls (boys OR 3.51; 95% CI 1.06–11.91; girls OR 1.02; 95% CI 0.35–2.98) | Low risk of bias (8/8) |
| Crivelli et al. | Cross-sectional | Tajikistan | 1320 (653 girls) | 2–5 years | WHO growth reference charts | WHO cut-off value for iron deficiency in children (Hb < 11 g dl−1) | Finger prick test using Drabkin’s reagent for Hb analysis | Age, sex, location, parental education, region | Obese status did not increase odds of iron deficiency in boys or girls (boys: OR 1.05 95% CI 0.55–2.0; girls: OR 0.85 95% CI 0.38–1.86) | Low risk of bias (7/8) |
| Hamza et al. | Case control | Egypt | 100 (42 girls) | 9.8 years | Cole et al. | Fe deficient when 2 or more Fe profile values were abnormal for age and gender: serum Fe < 20 μg dl−1, TICB >494 μg dl−1, ferritin <12 μg dl−1, TS < 16% (2), and sTfR > 8.3 mg L−1 | Blood sample/assay | Age | Fe, TS and TIBC were significantly lower, while ferritin, sTfR and hepcidin-25 were significantly higher in obese children versus controls | Low risk of bias (8/9) |
| Ibrahim et al. | Case control | Jordan | 150 (61 girls) | 2.1 years | WHO growth reference charts | Internationally accepted cut-off values for biochemical iron markers: Hb (g L−1 = 9.5–14.5; SF (ng ml−1) -29–160; and SI (μg dl−1) - 25–115. | Blood sample/assay | Age | Odds of iron deficiency in obese group compared with normal weight was 3.7 (95% CI 0.9–14.5) | Low risk of bias (6/8) |
| Konstantyner et al. | Cross-sectional | Brazil | 1325 | 1–2 years | WHO growth reference charts | Mild iron deficiency anemia: Hb < 11.0 g dl−1; moderate iron deficiency anemia: Hb < 9.5 g dl−1 | High-performance liquid chromatography (HPLC) of dried blood spot samples | - | Obese status did not increase the odds of mild or moderate anaemia: mild anaemia: OR 1.11 (0.46; 2.64); moderate anemia: 2.41 (0.80; 7.30) | Low risk of bias (7/8) |
| Nead et al. | Cross-sectional | United States | 9698 | 2–16 years | CDC growth charts | Iron-deficient if 2 of 3 values were abnormal for age and gender. Anemia = Hemoglobin cutoff points used to define anemia were based on the fifth percentiles for the reference groups | Blood sample/assay | Age, gender, race/ethnicity, poverty status, caretaker education | Obese status increased the odds of iron deficiency (OR 2.3; 95% CI 1.4–3.9) | Low risk of bias (6/8) |
| Sharif et al. | Case control | Iran | 100 children (49 girls) | 9.5 years | CDC growth charts | Serum iron levels less than 50 μg dl−1 and TIBC higher than 450 μg dl−1 were defined as iron deficiency | Blood sample biochemistry method and plasma ferritin by ELISA method | - | Prevalence of iron deficiency significantly higher in obese versus normal weight children (48% vs. 28%) | Low risk of bias (5/9) |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; ELISA, enzyme-linked immunosorbent assay; IOTF, International Obesity Task Force; NOS, Newcastle–Ottawa Scale.
Acknowledgements
This research was funded by the Cunningham Trust. AM was supported by the UK Medical Research Council (grant number MC_UU_12017/14) and the Scottish Government Chief Scientist Office (grant number SPHSU14). We are grateful to the reviewers who provided useful and insightful comments during peer review.
Funding information
The Cunningham Trust, Grant/Award Number: No specific award number; UK Medical Research Council (MC_UU_12017/14); Scottish Government Chief Scientist Office (SPHSU14)
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60(3):222–228. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35(7):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 3.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40(4):441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 4.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117(5):1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 5.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012 Sep 25;345(2):e4759. doi: 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metabol. 2003;88(4):1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert-Diamond D, Baylin A, Mora-Plazas M, et al. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92(6):1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddiford-Harland D, Steele J, Storlien L. Does obesity influence foot structure in prepubescent children? Int J Obes (Lond) 2000;24(5):541–544. doi: 10.1038/sj.ijo.0801192. [DOI] [PubMed] [Google Scholar]
- 11.Von Kries R, Hermann M, Grunert V, Von Mutius E. Is obesity a risk factor for childhood asthma? Allergy. 2001;56(4):318–322. doi: 10.1034/j.1398-9995.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonuck K, Chervin RD, Howe LD. Sleep-disordered breathing, sleep duration, and childhood overweight: a longitudinal cohort study. J Pediatr. 2015;166(3):632–639. doi: 10.1016/j.jpeds.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez-Nava F, Vázquez-Rodríguez EM, Saldívar-González AH, Lin-Ochoa D, Martínez-Perales GM, Joffre-Velázquez VM. Association between obesity and dental caries in a group of preschool children in Mexico. J Public Health Dent. 2010;70(2):124–130. doi: 10.1111/j.1752-7325.2009.00152.x. [DOI] [PubMed] [Google Scholar]
- 14.Akinci A, Cetinkaya E, Aycan Z, Oner O. Relationship between intraocular pressure and obesity in children. J Glaucoma. 2007;16(7):627–630. doi: 10.1097/IJG.0b013e318057528a. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadizar F, Vijverberg SJ, Arets HG, et al. Childhood obesity in relation to poor asthma control and exacerbation: a meta-analysis. Eur Respir J. 2016;48(4):1063–1073. doi: 10.1183/13993003.00766-2016. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa-Munoz J, Chinn S, Rona R. Association between obesity and asthma in 4-11 year old children in the UK. Thorax. 2001;56(2):133–137. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulgaron ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. 2013;35(1):A18–A32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papoutsakis C, Priftis KN, Drakouli M, et al. Childhood overweight/-obesity and asthma: is there a link? A systematic review of recent epidemiologic evidence. J Acad Nutr Diet. 2013;113(1):77–105. doi: 10.1016/j.jand.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Noal R, Menezes A, Macedo S, Dumith S. Childhood body mass index and risk of asthma in adolescence: a systematic review. Obes Rev. 2011;12(2):93–104. doi: 10.1111/j.1467-789X.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- 20.Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4(6):1111–1122. doi: 10.1016/j.jaip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell LM, Byrne S, Thompson A, et al. Increasing body mass index zscore is continuously associated with complications of overweight in children, even in the healthy weight range. J Clin Endocrinol Metabol. 2006;92:517–522. doi: 10.1210/jc.2006-1714. [DOI] [PubMed] [Google Scholar]
- 22.Chang J-H, Wang S-H, Kuo C-L, Shen HC, Hong Y-W, Lin L-C. Prevalence of flexible flatfoot in Taiwanese school-aged children in relation to obesity, gender, and age. Eur J Pediatr. 2010;169(4):447–452. doi: 10.1007/s00431-009-1050-9. [DOI] [PubMed] [Google Scholar]
- 23.Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34(2):350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Manohar N, Hayen A, Fahey P, Arora A. Obesity and dental caries in early childhood: A systematic review and meta-analyses. Obes Rev. 2020;21(3):e12960. doi: 10.1111/obr.12960. [DOI] [PubMed] [Google Scholar]
- 25.McCurley JL, Crawford MA, Gallo LC. Prevention of type 2 diabetes in US hispanic youth: a systematic review of lifestyle interventions. Am J Prev Med. 2017;53(4):519–532. doi: 10.1016/j.amepre.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen IG, Holm J-C, Homøe P. Obstructive sleep apnea in obese children and adolescents, treatment methods and outcome of treatment—a systematic review. Int J Pediatr Otorhinolaryngol. 2016;87:190–197. doi: 10.1016/j.ijporl.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13(1):154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss PH, Rehfuess EA. Quality appraisal in systematic reviews of public health interventions: an empirical study on the impact of choice of tool on meta-analysis. J Epidemiol Community Health. 2013;67(1):98–104. doi: 10.1136/jech-2011-200940. [DOI] [PubMed] [Google Scholar]
- 29.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers M, Sowden A, Petticrew M, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews: effectiveness of interventions to promote smoke alarm ownership and function. Evaluation. 2009;15(1):49–73. [Google Scholar]
- 31.Ahmadiafshar A, Tabbekhha S, Mousavinasab N, Khoshnevis P. Relation between asthma and body mass index in 6-15 years old children. Acta Med Iran. 2013;51(9):615–619. [PubMed] [Google Scholar]
- 32.Akinbami LJ, Rossen LM, Fakhouri TH, Simon AE, Kit BK. Contribution of weight status to asthma prevalence racial disparities, 2-19 year olds, 1988-2014. Ann Epidemiol. 2017;27:472–478.:e3. doi: 10.1016/j.annepidem.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amra B, Rahmani A, Salimi S, Mohammadzadeh Z, Golshan M. Association between asthma and body mass index in children. Iran J Allergy Asthma Immunol. 2005;4(1):33–38. [PubMed] [Google Scholar]
- 34.Cockrell Skinner A, Perrin EM, Steiner MJ. Healthy for now? A crosssectional study of the comorbidities in obese preschool children in the United States. Clin Pediatr. 2010;49(7):648–655. doi: 10.1177/0009922810362098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granell R, Henderson AJ, Evans DM, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11(7):e1001669. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guibas G, Manios Y, Xepapadaki P, et al. The obesity-asthma link in different ages and the role of Body Mass Index in its investigation: findings from the G enesis and H ealthy G rowth S tudies. Allergy. 2013;68(10):1298–1305. doi: 10.1111/all.12245. [DOI] [PubMed] [Google Scholar]
- 37.Herman T, Ros KP, de Jongste JC, Reiss IK, Jaddoe VW, Duijts L. Body fat mass distribution and interrupter resistance, fractional exhaled nitric oxide, and asthma at school-age. J Allergy Clin Immunol. 2017;139:810–818.:e6. doi: 10.1016/j.jaci.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Kwon HL, Ortiz B, Swaner R, et al. Childhood asthma and extreme values of body mass index: the Harlem Children’s Zone Asthma Initiative. J Urban Health. 2006;83(3):421–433. doi: 10.1007/s11524-006-9050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei Y, Yang H, Zhen L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China) Asia Pac Allergy. 2016;6(2):101–104. doi: 10.5415/apallergy.2016.6.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romieu I, Mannino DM, Redd SC, McGeehin MA. Dietary intake, physical activity, body mass index, and childhood asthma in the Third National Health And Nutrition Survey (NHANES III) Pediatr Pulmonol. 2004;38(1):31–42. doi: 10.1002/ppul.20042. [DOI] [PubMed] [Google Scholar]
- 41.Schachter L, Peat J, Salome C. Asthma and atopy in overweight children. Thorax. 2003;58(12):1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suglia SF, Chambers EC, Rosario A, Duarte CS. Asthma and obesity in three-year-old urban children: role of sex and home environment. J Pediatr. 2011;159:14–20.:e1. doi: 10.1016/j.jpeds.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai A, Volkmer R, Burton A. Association between asthma symptoms and obesity in preschool (4-5 year old) children. J Asthma. 2009;46(4):362–365. doi: 10.1080/02770900902759260. [DOI] [PubMed] [Google Scholar]
- 44.Wake M, Clifford S, Patton G, et al. Morbidity patterns among the underweight, overweight and obese between 2 and 18 years: population-based cross-sectional analyses. Int J Obes (Lond) 2013;37(1):86–93. doi: 10.1038/ijo.2012.86. [DOI] [PubMed] [Google Scholar]
- 45.Yao J, Zhou Y, Wang J, et al. Relationship between obesity and sex, and prevalence of asthma-like disease and current wheeze in Han children in Nanjing, China. J Int Med Res. 2015;43(1):139–146. doi: 10.1177/0300060514548289. [DOI] [PubMed] [Google Scholar]
- 46.Dylag H, Rowicka G, Strucinska M, Riahi A. Assessment of vitamin D status in children aged 1-5 with simple obesity. Rocz Panstw Zakl Hig. 2014;65(4):325–330. [PubMed] [Google Scholar]
- 47.Elizondo-Montemayor L, Ugalde-Casas PA, Serrano-González M, Cuello-García CA, Borbolla-Escoboza JR. Serum 25-hydroxyvitamin d concentration, life factors and obesity in Mexican children. Obesity. 2010;18(9):1805–1811. doi: 10.1038/oby.2009.448. [DOI] [PubMed] [Google Scholar]
- 48.Ghergherehchi R, Tabrizi A. Vitamin D deficiency and secondary hyperparathyroidism in pediatrics obesity. Caspian J Intern Med. 2010;1:119–127. [Google Scholar]
- 49.Jazar AS, Takruri HR, Bulos NAK. Vitamin D status in a sample of preschool children aged from 1 to 6 years visiting the pediatrics clinic at Jordan University hospital. Jordan Med J. 2011;171:1–18. [Google Scholar]
- 50.Lee S, Kim SM, Park H, et al. Serum 25-hydroxyvitamin D levels, obesity and the metabolic syndrome among Korean children. Nutr Metab Cardiovasc Dis. 2013;23(8):785–791. doi: 10.1016/j.numecd.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Rockell JE, Green TJ, Skeaff CM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5-14 y. J Nutr. 2005;135(11):2602–2608. doi: 10.1093/jn/135.11.2602. [DOI] [PubMed] [Google Scholar]
- 52.Senaprom S, Yamborisut U, Rojroongwasinkul N, et al. Factors associated with vitamin D status among Thai children aged 3-13 years. Southeast Asian J Trop Med Public Health. 2016;47:277–286. [PubMed] [Google Scholar]
- 53.Tolppanen A-M, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-hydroxyvitamin D3 and D2 concentrations and vitamin D deficiency in children. J Clin Endocrinol Metabol. 2012;97(4):1202–1210. doi: 10.1210/jc.2011-2516. [DOI] [PubMed] [Google Scholar]
- 54.Zhang HQ, Teng JH, Li Y, et al. Vitamin D status and its association with adiposity and oxidative stress in schoolchildren. Nutrition. 2014;30:1040–1044. doi: 10.1016/j.nut.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Zhou SJ, Skeaff M, Makrides M, Gibson R. Vitamin D status and its predictors among pre-school children in A delaide. J Paediatr Child Health. 2015;51(6):614–619. doi: 10.1111/jpc.12770. [DOI] [PubMed] [Google Scholar]
- 56.Abd-El Wahed MA, Mohamed MH, Ibrahim SS, El-Naggar WA. Iron profile and dietary pattern of primary school obese Egyptian children. J Egypt Public Health Assoc. 2014;89(2):53–59. doi: 10.1097/01.EPX.0000451827.84315.5c. [DOI] [PubMed] [Google Scholar]
- 57.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics. 2007;120(3):568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 58.Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93(5):975–983. doi: 10.3945/ajcn.110.005439. [DOI] [PubMed] [Google Scholar]
- 59.Crivelli M, Wyss K, Grize L, Matthys B, Aebi T, Zemp E. Are overweight and obesity in children risk factors for anemia in early childhood? Results from a national nutrition survey in Tajikistan. Int J Public Health. 2018;63(4):491–499. doi: 10.1007/s00038-018-1088-4. [DOI] [PubMed] [Google Scholar]
- 60.Hamza RT, Hamed AI, Kharshoum RR. Iron homeostasis and serum hepcidin-25 levels in obese children and adolescents: relation to body mass index. Horm Res Paediatr. 2013;80(1):11–17. doi: 10.1159/000351941. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim LS, Tayyem RF. Evaluation of iron deficiency and the intake of macro- and micronutrients among normal, overweight, and obese children under 5 years in Amman. Iran J Ped Hematol Oncol. 2018;8(1):21–36. [Google Scholar]
- 62.Konstantyner T, Roma Oliveira TC, de Aguiar Carrazedo Taddei JA. Risk factors for anemia among Brazilian infants from the 2006 National Demographic Health Survey. Anemia. 2012;2012:1–7. doi: 10.1155/2012/850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114(1):104–108. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]
- 64.Sharif M, Madani M, Tabatabaie F. Comparative evaluation of iron deficiency among obese and non-obese children. Iran J Ped Hematol Oncol. 2014;4:160–166. [PMC free article] [PubMed] [Google Scholar]
- 65.Chen K-C, Tung L-C, Yeh C-J, Yang J-F, Kuo J-F, Wang C-H. Change in flatfoot of preschool-aged children: a 1-year follow-up study. Eur J Pediatr. 2013;172(2):255–260. doi: 10.1007/s00431-012-1884-4. [DOI] [PubMed] [Google Scholar]
- 66.Chen K-C, Yeh C-J, Tung L-C, Yang J-F, Yang S-F, Wang C-H. Relevant factors influencing flatfoot in preschool-aged children. Eur J Pediatr. 2011;170(7):931–936. doi: 10.1007/s00431-010-1380-7. [DOI] [PubMed] [Google Scholar]
- 67.Ezema C, Abaraogu U, Okafor G. Flat foot and associated factors among primary school children: A cross-sectional study. Hong Kong Physiother J. 2014;32(1):13–20. [Google Scholar]
- 68.Riddiford-Harland D, Steele J, Baur L. Are the feet of obese children fat or flat? Revisiting the debate. Int J Obes (Lond) 2011;35(1):115–120. doi: 10.1038/ijo.2010.119. [DOI] [PubMed] [Google Scholar]
- 69.Silverberg JI, Kleiman E, Lev-Tov H, et al. Association between obesity and atopic dermatitis in childhood: a case-control study. J Allergy Clin Immunol. 2011;127(5):1180–1186.:e1. doi: 10.1016/j.jaci.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 70.Weinmayr G, Forastiere F, Büchele G, et al. Overweight/obesity and respiratory and allergic disease in children: international study of asthma and allergies in childhood (ISAAC) phase two. PLoS ONE. 2014;9(12):e113996. doi: 10.1371/journal.pone.0113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am J Epidemiol. 2013;178(7):1120–1128. doi: 10.1093/aje/kwt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cepeda-Lopez AC, Aeberli I, Zimmermann MB. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res. 2010;80:263–270. doi: 10.1024/0300-9831/a000033. [DOI] [PubMed] [Google Scholar]
- 73.Paulis W, Silva S, Koes B, van Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obes Rev. 2014;15(1):52–67. doi: 10.1111/obr.12067. [DOI] [PubMed] [Google Scholar]
- 74.Pereira-Santos M, Costa PR, Assis A, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16(4):341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 75.Yao Y, Zhu L, He L, et al. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med. 2015;8:14977–14984. [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16(12):1081–1093. doi: 10.1111/obr.12323. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Dong G, Lin K, Lee Y. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14(3):222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 78.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013;13(1):121. doi: 10.1186/1471-2431-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. J Consult Clin Psychol. 2005;73(2):195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- 80.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychol. 2002;21(2):131–138. [PubMed] [Google Scholar]
- 81.Chen Z, Salam MT, Alderete TL, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. 2017;195(9):1181–1188. doi: 10.1164/rccm.201608-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121(5):1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Holderness H, Chin N, Ossip DJ, Fagnano M, Reznik M, Halterman JS. Physical activity, restrictions in activity, and body mass index among urban children with persistent asthma. Ann Allergy Asthma Immunol. 2017;118(4):433–438. doi: 10.1016/j.anai.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam K-M, Yang Y-H, Wang L-C, Chen S-Y, Gau B-S, Chiang B-L. Physical activity in school-aged children with asthma in an urban city of Taiwan. Pediatr Neonatol. 2016;57(4):333–337. doi: 10.1016/j.pedneo.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Steinbeck KS. The importance of physical activity in the prevention of overweight and obesity in childhood: a review and an opinion. Obes Rev. 2001;2(2):117–130. doi: 10.1046/j.1467-789x.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- 86.Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wamberg L, Christiansen T, Paulsen SK, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int J Obes (Lond) 2013;37(5):651–657. doi: 10.1038/ijo.2012.112. [DOI] [PubMed] [Google Scholar]
- 88.Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and metaanalysis of individual participant data. Lancet Respir Med. 2017;5(11):881–890. doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carrelli A, Bucovsky M, Horst R, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res. 2017;32(2):237–242. doi: 10.1002/jbmr.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jennings A, Welch A, van Sluijs EM, Griffin SJ, Cassidy A. Diet quality is independently associated with weight status in children aged 9-10 years. J Nutr. 2011;141(3):453–459. doi: 10.3945/jn.110.131441. [DOI] [PubMed] [Google Scholar]
- 91.Cleland V, Crawford D, Baur LA, Hume C, Timperio A, Salmon J. A prospective examination of children’s time spent outdoors, objectively measured physical activity and overweight. Int J Obes (Lond) 2008;32(11):1685–1693. doi: 10.1038/ijo.2008.171. [DOI] [PubMed] [Google Scholar]
- 92.Cepeda-Lopez AC, Zimmermann MB, Wussler S, et al. Greater blood volume and Hb mass in obese women quantified by the carbon monoxide-rebreathing method affects interpretation of iron biomarkers and iron requirements. Int J Obes (Lond) 2019;43(5):999–1008. doi: 10.1038/s41366-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown T, Moore TH, Hooper L, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2019;7(7) doi: 10.1002/14651858.CD001871.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]