Abstract
For decades, the study of memory has been neuron-centric, yet neurons do not function in isolation. Today we know that neuronal activity is modulated by the environment within which it occurs, and is subject to modulation by different types of glial cells. In this review we summarize recent findings on the functional roles of astrocytes and oligodendrocytes, two major types of glia cells in the adult brain, in memory formation and its cellular underpinnings across multiple time points. We will discuss the different methods that are being used to investigate the astrocytic and oligodendroglial involvement in memory. We shall focus on chemogenetics and optogenetics, which support genetically specificity and high spatiotemporal resolution, attributes that are particularly well suited to the investigation of the contribution of unique cell types at the different stages of memory formation.
Introduction
Memories are first acquired through different sensory modalities and encoded in various brain networks (e.g. hippocampus, amygdala) as fragile internal representations, which gradually stabilize through a process termed consolidation. This process occurs on different time scales following the acquisition stage: recent memory involves relatively fast processes, taking place during the first hours following learning on the synaptic level (synaptic consolidation). Remote memories, on the other hand, are consolidated over much longer time periods in a process termed ‘systems consolidation’, involving the interaction between multiple brain regions to enable the persistence of the memory [1].
Learning and memory rely on the coordinated neuronal activity within and between different brain regions. The activity level of neurons and the inter-regional communication are contingent on the environment within which they happen, regulated by glia cells like astrocytes and oligodendrocytes. Indeed, pioneering studies have shown that astrocytes monitor and directly modulate neuronal activity in the ‘tripartite synapse’, in which they do not merely encapsulate and insulate synapses, but also sense and actively modify synaptic transmission and plasticity [2–4]. Oligodendrocytes (OLs), responsible for wrapping axons in myelin sheaths in the CNS, were considered until recently to only insulate electrical axonal activity. However, recent evidence demonstrates that they are continuously generated in the adult brain from oligodendrocytes precursor cells (OPCs) [5–7], and this new myelination can create new sheaths, change sheath thickness and tune axonal conduction velocity, affecting spike-time arrival and synchronization in neuronal activity. Moreover, recent studies demonstrated that myelination shapes the function of neuronal circuits in an experience-dependent manner. Preventing adult myelination, either through conditional deletion of the transcription factor myrf or via epigenetic blockade of OLs differentiation, impaired motor skill learning and function a few weeks after manipulation [6,8]. On the contrary, inducing OPC proliferation in the premotor cortex via optogenetic stimulation of cortical layer V projection neurons, increased the amount of newly generated oligodendrocytes and myelin sheath thickness in the stimulated premotor circuit and also enhanced motor performance [6]. Taken together, these data motivate the study of astrocytes and OLs in memory as they suggest they both modulate neuronal activity through various memory-related mechanisms, such as synaptic plasticity and temporal coincidence of neuronal activity.
To study the role of astrocytes and OLs at different memory stages, we need to manipulate them within specific brain regions in a time-restricted manner. Many studies have examined the role of glia in memory (reviewed in Refs. [9,10]), employing either pharmacology (e.g. Ref. [11]), which can be reversible but not cell-type specific, or genetic manipulations (e.g. Ref. [12]) that target specific cell types but lack temporal and spatial precision. Chemogenetic and optogenetic tools are optimal for studying glial involvement in memory, as they allow reversible cell-type specific manipulation, as well as temporal and spatial precision [13]. For example, a commonly used chemogenetic approach employs engineered human muscarinic G-protein coupled receptors (GPCRs) which respond only to the designer drug Clozapine-N-Oxide (CNO) and termed Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) [14]. As it relies on the administration of a drug to activate the designer receptors, the temporal resolution of this technique is on the order of minutes to hours, making it less suitable for precise manipulations, but nonetheless advantageous when examining memory stages that are days apart. Optogenetics rely on light-sensitive proteins (opsins), which can be ion channels, ion pumps, or GPCRs, that are activated by a specific light wavelength [15]. As light delivery is controlled on a millisecond timescale, the length of activation depends on the dynamics of the opsin, ranging from milliseconds to minutes. Recently, these tools have been successfully integrated into the research of astrocytes, and have already advanced our understanding of how memories are formed. For OLs, such tools are missing and thus the study of their involvement in memory is limited. Still, recent innovative studies employed advanced techniques to gain time-dependent genetic control over OPCs or OLs, shedding light on the functional involvement of these cells in learning and memory. In this review we will focus on recent studies investigating the involvement of astrocytes and oligodendrocytes in orchestrating the spatiotemporal coordination of neuronal activity at the different stages of memory, from acquisition, through recent memory retrieval to remote recall.
Glia in short-term memory
Short-term memory (STM) is the ability to retain newly acquired information for short durations. The immediacy of STM demands rapid synaptic alterations and does not involve prolonged protein synthesis processes. Recent studies on glial involvement in STM shed light on its underpinnings.
Astrocytic effects on short-term memory
To investigate the direct astrocytic involvement in the acquisition of short-term memories, one must manipulate astrocytes during acquisition, and shortly after examine memory. Three recent studies employed chemogenetic and optogenetic tools to activate the Gq-pathway in CA1 astrocytes and found that this manipulation can enhance short-term memory [16• ,17• ,18• ]. Specifically, astrocytic activation with the Gq-DREADD hM3Dq improved the recognition of a novel arm in the T-maze test 5 min after the initial exposure, which took place 30 min after CNO injection [16• ]. Gq-coupled opsin melanopsin mediated activation in CA1 astrocytes during training also enhanced novel object discrimination 30 min later [17• ], demonstrating astrocytic activation-mediated STM enhancement. Astrocytes were also shown to affect working memory, the ability to retain and manipulate information for short durations. Specifically, photostimulation of melanopsin-expressing astrocytes in the medial prefrontal cortex (mPFC) resulted in an increased discrimination index in the object in place (OIP) preference test [18• ]. Consistently, Pinto-Duarte et al. showed that transgenic mice that completely lack the inositol 1,4,5-trisphosphate receptor type 2 (Ip3r2 −/− mice) suffered from chronic disruption of astrocytic calcium signaling and exhibited reduced performance in the Y-maze compared to wild-type mice [19• ]. In addition, genetic ablation of GABAb receptors (GABABRs) in mPFC astrocytes impaired performance in OIP test, providing an additional implication for the involvement of astrocytes in working memory [18• ]. The cortical manipulation seems to affect working memory through activation of astrocytic GABABRs by parvalbumin interneurons, tuning cortical gamma oscillations [18• ] (Figure 1a, lower panel). Interestingly, Lee et al. demonstrated that transgenic mice with disrupted vesicular release in astrocytes, showed reduced cortical gamma oscillations but maintained normal performance in the a spatial working memory task (i.e. Y maze) [20]. While Mederos et al. and Lee et al. results link astrocytes with gamma oscillations, further research is needed to resolve their contribution to working memory. To conclude, these studies exemplify the importance of integrating high spatiotemporal tools in research into the role of glia in memory, as they can uncover significant functional roles for these cells even at the brief, minutes-long process of memory acquisition.
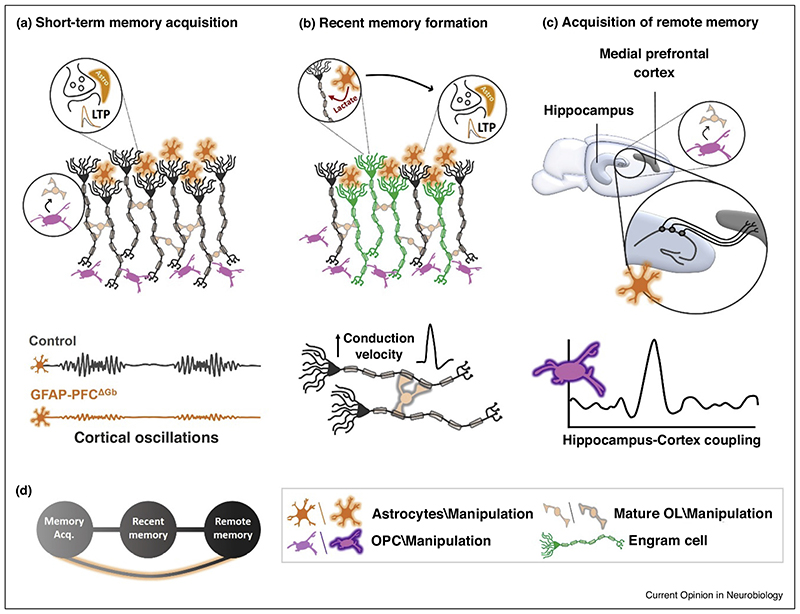
Figure 1. Overview of astrocytic and oligodendroglial contribution to memory formation.
(a) Upper panel: Chemogenetic hippocampal astrocytic activation (orange) induces LTP. Decreasing activity-dependent myelination via OPCs (purple) before acquisition impairs memory acquisition [5,7]. Lower panel: Genetic ablation of mPFC astrocytic GABABRs (GFAP-PFC∆Gb) reduces the power of low gamma oscillations (30−60 Hz) during forced alteration task and impairs working memory [18• ]. (b) Upper panel: Both chemogenetic and optogenetic hippocampal astrocytic manipulation (orange) induces LTP and augments the acquisition of recent contextual memory [16• ,24• ]. Moreover, the same manipulation during learning enhances memory allocation (green) and short-term memory [16• ,17• ]. Astrocytes provide lactate to neurons which is required for LTP and recent memory formation, based on chronic astrocytic manipulation (orange) in transgenic mice before acquisition [11,28–33]. Lower panel: sustained manipulation of mature OLs (glow grey) before acquisition increases axonal conduction velocity and enhances spatial and contextual memory [40]. (c) Upper panel: Gi-pathway activation of CA1 astrocytes (glow orange) during acquisition disrupts CA1-ACC communication and impairs remote, but not recent, memory [25• |. Lower panel: Steadman et al. found that cortical inhibition of oligodendrogenesis via manipulation of OPCs (purple) immediately after training blocked hippocampal ripple-cortical spindle coupling and impaired remote memory consolidation [38• |. Pan et al. [41 • | found that neuronal responses to conditioned context cues evolve over time in the mPFC, but not in animals that cannot form new myelin. (d) Scheme showing the gradual consolidation from acquisition to remote memory. The astrocytic experiment in Section (c) [25• | suggests that remote memories can stem directly from acquisition, and not only from recent memory as previously thought. Thus, a new path (marked in orange) can be added to the classic scheme from acquisition, through recent to remote memory.
Oligodendroglial effects on short-term memory
Oligodendrocytes were thought to just insulate axons, yet surprising recent studies have shown that adult oligodendrogenesis is correlated with the level of neuronal activity [5–7]. Hence, myelination can operate together with synaptic plasticity to assimilate new information in neuronal networks, and thus be especially relevant to memory acquisition. Consistently, two recent studies have found that chronically decreasing activity-regulated myelination and myelin content, either via the conditional removal of brain-derived neurotrophic factor (BDNF)-TrkB signaling pathway on OPCs or by inhibiting neurons at the mPFC using Gi-DEADDs, impaired short-term memory formation in adjusted novel object recognition test (i.e. with a short delay between the training and the testing phase) [5,7]. Yet, the direct and specific involvement of OLs in memory acquisition is still unclear as both studies started to manipulate activity-dependent myelination long before the memory was acquired (~1 month) (Figure 1a, top panel).
Glia in recent memory consolidation
After a memory is acquired it depends on a consolidation process to become stable. Synaptic consolidation transforms information into a long-term memory via changes in gene expression and protein synthesis at the relevant synapses in the neural circuits.
Astrocytic effects on recent memory consolidation and synaptic plasticity
Astrocytic involvement in recent memory formation was examined by both loss of function, as well as gain of function experiments. Mice with impaired astrocytic exocytosis exhibited defective spatial and recognition long-term memory [21,22]. These behavioral deficits were rescued by administration of exogenous gliotrans-mitters such as D-serine, immediately after memory acquisition but not one hour later, indicating early astrocytic involvement in the process of memory consolidation [21]. Interestingly, activating both chemogenetic GsPCR and optogenetic Channelrhodopsin-2 in astrocytes resulted in reduced spatial and contextual recent memory [23,47]. On the other hand, in experiments performed in our lab, in which we expressed hM3Dq in CA1 astrocytes, we observed that CNO administration before fear conditioning resulted in increased freezing one day later, when the mice were placed back in the conditioning context, indicating improved memory of the context. These findings suggest that astrocytes confer their cognition-enhancing effects during memory acquisition, and possibly early consolidation (as CNO can remain in the body several hours after administration), but not during memory recall. To demonstrate the involvement of astrocytes specifically at the acquisition stage, we employed the optoA1 opsin in astrocytes and administered light only during the 5-min training session. Remarkably, light administration during training resulted in 90% elevation in contextual freezing in OptoA1 mice tested a day later, suggesting astrocytic involvement in recent memory acquisition [16• ]. Astrocytic-mediated recent memory augmentation was also demonstrated by Nam et al., who found that chronic pharmacological activation of CA1 astrocytic μ-opioid receptor, a Gi-coupled receptor, drives the acquisition of contextual memory associated with conditioned place preference (CPP) [24• ]. Interestingly, acute chemogenetic activation of the Gi pathway in CA1 astrocytes via Gi-DREADDs during either fear conditioning or recall did not induce any significant effect on recent memory [25• ]. While these studies demonstrate astrocytic involvement in recent memory formation, they also emphasize the need of future research to decipher the interplay between astrocytic-mediated memory effects (i.e. impairment and enhancement) and the different signaling pathways in astrocytes.
Learning and memory are well associated with activity-dependent synaptic alterations and astrocytes were shown to be necessary for long-term synaptic potentiation (LTP) [26,27] (Figure 1b, upper panel). Accordingly, in most of the above astrocytic-induced memory impairments and enhancements, LTP was either impaired or enhanced, respectively ([16• ,17• ] Figure 1a upper panel, [21,24• ] Figure 1b upper panel). Moreover, astrocytic Gq-pathway activation via Gq-DREADDs induced de novo NMDA-dependent LTP [16• ], demonstrating that astrocytic activity is not only necessary but also sufficient to induce LTP.
Taken together, these studies indicate that astrocytes affect the acquisition and initial consolidation of recent memories. This effect was accompanied by astrocytic-mediated long-term changes in synaptic potentiation.
Astrocytic metabolic support of recent memory formation
Synaptic consolidation comprises sequential high-demanding energy processes, such as gene transcription, protein synthesis and post-translational modifications, that enable the stabilization of newly acquired information. To allow intact memory consolidation, neurons need to be provided with real-time metabolic support. Several recent studies showed that astrocytes supply lactate to neurons during various learning and memory tasks, such as context avoidance [11,28,29], spontaneous alteration [30] and novel object recognition [31], via either lactate monocarboxylate transporters (MCTs) or by the use of glycogen phosphorylase inhibitor [32]. On the other hand, reducing astrocytic supply of lactate, either by knocking down hippocampal MCT1 and MCT4 [11] or by continuous long administration of β2-adrenergic receptor agonist [31], suppresses LTP [11] and impairs memory recall [31]. Taken together, these studies suggest that astrocyte-neuron metabolic coupling is required for LTP and recent memory formation [33] (Figure 1b, upper panel).
Astrocytic effects on memory allocation
During memory allocation neuronal ensembles (also referred to as ‘engram cells’) are selected to serve as the physical basis of specific memories [34]. This selection process appears to be non-random and biased by the level of neuronal excitability. For example, when a small number of neurons are active during training, they are more likely to be allocated to the engram supporting an acquired memory [35]. Moreover, increasing the activity of a small neuronal population in the BLA before FC acquisition can improve fear memory [36,37]. Activity-dependent memory allocation raises the question of whether the previously described astrocytic-induced memory enhancement [16• ] could stem from a tailored response of astrocytes to the activity of their surrounding neurons, that is, can astrocytes affect memory allocation? We have shown that in vivo hippocampal astrocytic activation via Gq-DREADDs increased neuronal activity during memory acquisition only when coupled with learning but not in home-caged mice. Moreover, the same astrocytic manipulation enhanced recent memory recall [16• ]. Taken together, these results suggest a task-dependent enhancement of memory allocation, mediated by astrocytic activation (Figure 1b).
Oligodendrocytes affects recent memory formation
OLs were also found to affect recent memory formation, as demonstrated by Steadman et al. [38• ]. In this study, the authors examined the necessity of oligodendrogenesis for the different stages of spatial memory by using transgenic mice in which OPCs were prevented from maturing into oligodendrocytes in a temporally controlled manner, starting either at acquisition or during consolidation of spatial memory. Reduced adult oligodendrogenesis in the cerebral cortex and anterior corpus callosum during the training period resulted in impaired recent spatial and contextual memory [38• ]. Consistently, Wang et al. demonstrated that inhibiting adult oligodendrogenesis and myelination in the cortex can impair spatial memory [39• ]. Both studies manipulated OPCs to examine the effect of adult oligodendrogenesis and myelination on memory performance. Jeffries et al. tested the direct contribution of mature oligodendrocytes, instead of OPCs, to recent memory formation. They found that sustained activation of the extracellular signal-regulated kinases 1 and 2 in pre-existing OLs of adult mice for 5 or 10 consecutive days, before the start of the behavioral tasks, increased conduction velocity in the corpus callosum and spinal cord through expansion of myelin thickness and enhanced spatial and contextual memory [40] (Figure 1b, lower panel). To conclude, adult oligodendrogenesis and myelination are not only essential for recent memory but also capable of enhancing it.
Glia in remote memory
Systems consolidation gradually reorganizes long-term memories over distributed brain regions to store and retain remote memories. While it is known that this reorganization spans the hippocampus and frontal cortex, the exact temporal dynamics of this process is not clear. Exploring glial effects on remote memory formation may shed light on the long-term dynamics of systems consolidation.
Astrocytic effects on hippocampal-prefrontal interactions in remote memory formation
Recently, we showed, for the first time, that astrocytes can participate in the acquisition of remote memories. In this study, astrocytic Gi activation in the dorsal hippocampus during learning resulted in a specific impairment in remote, but not recent, memory recall accompanied by decreased neuronal activity in the anterior cingulate cortex (ACC) during retrieval [25• ]. Moreover, we revealed a massive recruitment of ACC-projecting CA1 neurons during memory acquisition, a process specifically inhibited by astrocytic manipulation (Figure 1c, upper panel). This surprising astrocytic capability to modulate inter-regional neuronal communication is also supported by previous findings demonstrating the necessity of astrocytic exocytosis for hippocampal–prefrontal synchronization [22]. So far, it was assumed that remote memory is the product of recent memory. However, this behavioral phenotype shows that the foundation of remote memory can be independently established during acquisition (Figure 1d). Astrocytes were also found to affect remote memory recall through local synaptic mechanisms. Lee et al. showed that impaired astrocytic exocytosis in transgenic mice decreased the power of cortical gamma oscillations, and impaired mice performance in the remote novel object recognition test [20]. Moreover, Pinto-Duarte et al. [19• ] showed that chronic disruption of calcium signaling in astrocytes did not affect recent memory recall but resulted in impairment in remote memory recall of fear, spatial and recognition memories. Yet, due to the implementation of chronic astrocyte manipulation in this study, temporal precision is not available, and the exact timing of astrocytic involvement cannot be determined (Figure 1c).
Oligodendroglial effects on prefrontal-hippocampal interactions in remote memory consolidation
As opposed to memory acquisition and early consolidation, systems consolidation spans long periods of time and thus is temporally compatible with the weeks-long maturation process of OPCs to myelinating OLs. Indeed, it was recently demonstrated that oligodendrogenesis occurs in frontal cortical regions during spatial and contextual memory consolidation [38• ,41• ], and that inhibiting it immediately after training impairs remote memory recall. Preventing the formation of new myelin before remote recall did not affect memory retrieval [38• ] suggesting that new oligodendrocytes are required for the acquisition and consolidation of remote memory. Furthermore, while Pan et al. [41•] showed that neuronal responses to cues associated with the conditioned context were found to evolve over time in the mPFC, this did not occur in animals that cannot form new myelin. This cortical involvement was also demonstrated by Steadman et al. [38• ], who showed that spatial learning promotes hippocampal ripple-cortical spindle coupling which can be blocked by decreased adult oligodendrogenesis (Figure 1c, lower panel). Together, these studies show that adult oligodendrogenesis shapes the activity of neuronal circuits, which in turn affects the acquisition and consolidation of remote memories.
The study of glia in memory and the dynamics of memory consolidation
Prominent theories based on human and animal studies suggest that the consolidation of remote memories is a dynamic process which first requires hippocampal activation, and later becomes independent of the hippocampus, instead relying on frontal cortical regions [42,43]. However, this temporal separation between hippocampal and frontal cortex in recent and remote memory is not rigid: We Ref. [44] and others Refs. [45,46] have shown that the hippocampus is still critically involved in remote retrieval, and frontal areas are required for recent memory. The accumulated evidence from the study of glial involvement in memory seem to support the notion that memory representation in both the hippocampus and frontal cortex changes as the memory ages, as do the connections between them, and this co-evolution of local ensembles and inter-region connectivity mediates the selection and persistence of recent and remote memories.
Future perspectives
The studies of the role of astrocytes in memory formation vastly outnumbers those focusing on oligodendrocytes (Figure 1). This imbalance might stem from technical reasons, as astrocyte research is blessed with a range of real-time spatiotemporal tools supporting their bi-directional manipulation, while OL research is relatively impoverished in this sense. Further development of tools supporting high spatiotemporal control will elaborate the study of glial involvement in memory formation. As the molecular mechanisms underlying astrocytes or oligodendroglial involvement in memory are still being discovered, adapting existing chemogenetic and optogenetic tools to OLs and OPCs and continuing their use in astrocytes is expected continue to advance our knowledge on their involvement in memory, in a time-restricted manner.
To conclude, much like a group of musicians in an orchestra who need to be coordinated to create a harmonic symphony, activity of neuronal ensembles must be precisely synchronized to form intact memory. Astrocytes and oligodendrocytes, which were considered irrelevant to learning and memory for a long time, may assist in conducting this synchronization via their recently discovered effects on synaptic plasticity and conduction velocity. Future investigations, utilizing astrocytic calcium imaging in awake rodents during acquisition and recall, as well as electrophysiological recordings together with astrocyte or OL manipulation during learning, memory consolidation and retrieval from memory-related brain areas (such as the hippocampus and ACC) are expected to further shed light on the underlying mechanisms. Lastly, after separately describing the astrocytic and oligodendroglial dynamics during memory acquisition and recall, the real-time crosstalk between these two cell populations should be examined as it is expected to enable substantial progress in our contemporary understanding of memory formation.
Acknowledgements
AK is supported by the JBC GOLD Scholarship. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 803589 to IG). IG is also supported by the Israel Science Foundation (ISF grant No. 1815/18), the Israeli Centers of Research Excellence Program (center No. 1916/12), and the Canada-Israel grants (CIHR-ISF, grant No. 2591/18). We thank Yaniv Ziv and Ami Citri, for the critical reading of the manuscript.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as: • of special interest
- 1.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu Rev Psychol. 2016;67:105–134. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 4.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Pasca SP, et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron. 2019;103:250–265.:e258. doi: 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teissier A, Le Magueresse C, Olusakin J, Andrade da Costa BLS, De Stasi AM, Bacci A, Imamura Kawasawa Y, Vaidya VA, Gaspar P. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol Psychiatry. 2020;25:1159–1174. doi: 10.1038/s41380-019-0493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 10.Adamsky A, Goshen I. Astrocytes in memory function: pioneering findings and future directions. Neuroscience. 2018;370:14–26. doi: 10.1016/j.neuroscience.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K, Abudara V, Dufour A, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Nagai J, Khakh BS. Improved tools to study astrocytes. Nat Rev Neurosci. 2020;21:121–138. doi: 10.1038/s41583-020-0264-8. [DOI] [PubMed] [Google Scholar]
- 14.Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18:222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018;174:59–71.:e14. doi: 10.1016/j.cell.2018.05.002. [• A paper using chemogenetics and optogenetics to show the effect of CA1 astrocytes during memory acquisition. The paper also suggests a role of astrocytes is memory allocation] [DOI] [PubMed] [Google Scholar]
- 17.Mederos S, Hernandez-Vivanco A, Ramirez-Franco J, Martin-Fernandez M, Navarrete M, Yang A, Boyden ES, Perea G. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia. 2019;67:915–934. doi: 10.1002/glia.23580. [• A paper demonstrating the effect of the Gq-coupled melanopsin in CA1 astrocytes on memory acquisition] [DOI] [PubMed] [Google Scholar]
- 18.Mederos S, Sánchez-Puelles C, Esparza J, Valero M, Ponomarenko A, Perea G. GABAergic signaling to astrocytes in prefrontal cortex sustains goal-directed behaviors. Nat Neurosci. doi: 10.1038/s41593-020-00752-x. [• A new work showing the neural circuit including astrocytes in memory, in the mPFC] [DOI] [PubMed] [Google Scholar]
- 19.Pinto-Duarte A, Roberts AJ, Ouyang K, Sejnowski TJ. Impairments in remote memory caused by the lack of Type 2 IP3 receptors. Glia. 2019;67:1976–1989. doi: 10.1002/glia.23679. [• A paper showing that chronic astrocytic modulation (lack of IP3R, type 2), impairs remote memory] [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Pina-Crespo JC, Roberts AJ, Verma IM, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 2014;111:E3343–3352. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, Bellocchio L, Soria-Gomez E, Papouin T, Varilh M, et al. Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron. 2018;98:935–944.:e935. doi: 10.1016/j.neuron.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Sardinha VM, Guerra-Gomes S, Caetano I, Tavares G, Martins M, Reis JS, Correia JS, Teixeira-Castro A, Pinto L, Sousa N, et al. Astrocytic signaling supports hippocampal-prefrontal theta synchronization and cognitive function. Glia. 2017;65:1944–1960. doi: 10.1002/glia.23205. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Li L, Wu J, Zhu Z, Feng X, Qin L, Zhu Y, Qiu Z, Duan S, Yu YQ. Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors. eLife. 2020;9 doi: 10.7554/eLife.57155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, Woo J, Kim J, Kwong E, Choi TY, et al. Activation of astrocytic mu-opioid receptor causes conditioned place preference. Cell Rep. 2019;28:1154–1166.:e1155. doi: 10.1016/j.celrep.2019.06.071. [• A demonstration that Gi in astrocytes causes improved contextual memory in CPP task] [DOI] [PubMed] [Google Scholar]
- 25.Kol A, Adamsky A, Groysman M, Kreisel T, London M, Goshen I. Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat Neurosci. 2020;23:1229–1239. doi: 10.1038/s41593-020-0679-6. [• A work showing Gi pathway in CA1 astrocytes impairs remote memory, but not recent one, via projection specific affect on ACC targeting neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A. 2012;109:E2832–E2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadi M, Allaman I, Lengacher S, Grenningloh G, Magistretti PJ. Learning-induced gene expression in the hippocampus reveals a role of neuron -astrocyte metabolic coupling in long term memory. PLoS One. 2015;10:e0141568. doi: 10.1371/journal.pone.0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM. Astrocytic beta2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Natl Acad Sci U S A. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong JH, Wang YJ, Cui M, Wang XJ, Zheng WS, Ma ML, Yang F, He DF, Hu QX, Zhang DL, et al. Adaptive activation of a stress response pathway improves learning and memory through Gs and beta-arrestin-1-regulated lactate metabolism. Biol Psychiatry. 2017;81:654–670. doi: 10.1016/j.biopsych.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezzoli E, Cali C, De Roo M, Ponzoni L, Sogne E, Gagnon N, Francolini M, Braida D, Sala M, Muller D, et al. Ultrastructural evidence for a role of astrocytes and glycogen-derived lactate in learning-dependent synaptic stabilization. Cereb Cortex. 2020;30:2114–2127. doi: 10.1093/cercor/bhz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman MQ, Gao V, Alberini CM. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci. 2016;10:10. doi: 10.3389/fnint.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Josselyn SA, Tonegawa S. Memory engrams: recalling the past and imagining the future. Science. 2020;367 doi: 10.1126/science.aaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA. Arc expression identifies the lateral amygdala fear memory trace. Mol Psychiatry. 2016;21:1153. doi: 10.1038/mp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 37.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron. 2020;105:150–164.:e156. doi: 10.1016/j.neuron.2019.10.013. [• An elegant demonstration of the role of adult myelination in the consolidation on recent and remote memory] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Ren SY, Chen JF, Liu K, Li RX, Li ZF, Hu B, Niu JQ, Xiao L, Chan JR, et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat Neurosci. 2020;23:481–486. doi: 10.1038/s41593-020-0588-8. [• A work showing that adult oligodedrogenesis impairs spatial memory] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL. ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J Neurosci. 2016;36:9186–9200. doi: 10.1523/JNEUROSCI.1444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan S, Mayoral SR, Choi HS, Chan JR, Kheirbek MA. Preservation of a remote fear memory requires new myelin formation. Nat Neurosci. 2020;23:487–499. doi: 10.1038/s41593-019-0582-1. [• A work nicely showing that the cues associated with the conditioned context evolve over time in the mPFC, only in the presence adult oligodendrogenesis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 43.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Vetere G, Kenney JW, Tran LM, Xia F, Steadman PE, Parkinson J, Josselyn SA, Frankland PW. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron. 2017;94:363–374.:e364. doi: 10.1016/j.neuron.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Gusev PA, Cui C, Alkon DL, Gubin AN. Topography of Arc/Arg3.1 mRNA expression in the dorsal and ventral hippocampus induced by recent and remote spatial memory recall: dissociation of CA3 and CA1 activation. J Neurosci. 2005;25:9384–9397. doi: 10.1523/JNEUROSCI.0832-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu G-Q, Adame A, et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18:423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]