Abstract
Background
Surgical approaches to the treatment of obesity and type 2 diabetes, most notably the Roux-en-Y gastric bypass (RYGB) procedure, have been shown to be renoprotective, reducing the incidence of albuminuria and end-stage kidney disease (ESKD) over 15-20 year follow-up in patients with obesity. The tissue level effects of metabolic surgery on the diabetic kidney are not easily interrogated in clinical samples. However, elucidation of the cellular and molecular basis for the renoprotective effects of metabolic surgery is now emerging from a body of pre-clinical work in rodent models of diabetic kidney disease (DKD).
Summary
Experimental metabolic surgery (RYGB, sleeve gastrectomy (SG), Roux-en-Y oesophagojejunotomy (RYEJ), and duodenojejunal bypass (DJB)) exert a pronounced albuminuria-lowering effect in rat models of DKD. Following RYGB in the Zucker Diabetic Fatty (ZDF) rat, glomerular histology is improved as demonstrated by reductions in podocyte stress, glomerulomegaly and glomerulosclerosis. Glomerular ultrastructure improves post-RYGB and post-SG, manifested by quantifiable reductions in podocyte foot process effacement. The transcriptional programme underpinning these structural improvements has been characterised at the pathway level using RNA-sequencing, and is associated with a significant reduction in the activation of inflammatory and fibrotic responses.
Key Messages
Experimental metabolic surgery reduces biochemical, histological and molecular indices of DKD. This pre-clinical data supports a growing interest in the potential utility of metabolic surgery as a therapeutic approach to slow renal function decline in patients with obesity and DKD.
Keywords: Obesity, Type 2 Diabetic Kidney Disease, Albuminuria, Metabolic Surgery, Zucker Diabetic Fatty Rats
Introduction
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease (ESKD) [1]. Although there have been recent advances in medical therapy for DKD, most notably sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists, it remains a progressive disease despite intensive outpatient management by nephrologists and diabetologists [2–4]. Most of the excess mortality attributable to diabetes occurs in people with kidney disease [5]. Cardiovascular mortality rates increase proportionally with DKD stage, and in particular, are unacceptably high in people with ESKD [6]. Therefore, prevention of progression of DKD to ESKD is of critical importance.
Obesity is common amongst people with chronic kidney disease (CKD); for example, the prevalence of obesity was 44.1% amongst adults with CKD in the United States during 2011-2014 and 35.3% in an Irish tertiary nephrology centre in 2018-2019 [7, 49]. Metabolic surgery is an effective means of inducing sustained weight loss and plays a central role in the management of patients with severe obesity (body-mass index (BMI) ≥40 kg/m2) with or without type 2 diabetes. However, given that less severe obesity (BMI <40 kg/m2) is more prevalent amongst patients with DKD and other microvascular complications of type 2 diabetes, and much of the beneficial end-organ impact of metabolic surgery occurs independently of weight loss, there is a growing interest in the role of metabolic surgery in patients with type 2 diabetes and less severe obesity [8]. Indeed, the Microvascular Outcomes after Metabolic Surgery randomised controlled trial, which demonstrated that Roux-en-Y gastric bypass (RYGB) surgery is a more effective means of achieving remission of albuminuria at 24 months than best medical treatment, selectively recruited patients with type 2 diabetes and a BMI 30-35 kg/m2[9].
Large-scale observational studies have demonstrated that metabolic surgery reduces the incidence of albuminuria, slows progressive renal functional decline, and reduces the incidence of ESKD in patients with obesity [10–13]. Improved control of body weight, blood pressure, dyslipidaemia, and glycaemia contribute to these findings [14]. However, in patients with type 2 diabetes, the anti-proteinuric effect of metabolic surgery occurs independently of improvements in body weight, blood pressure, and glycaemia [15]. Therefore, weight-independent renoprotective effects occur post-metabolic surgery in people with type 2 diabetes. Synergistic changes in visceral adipose tissue content and location, alterations in adipocytokine signalling, enhanced natriuresis, gut microbiota shifts, and reduced systemic and renal inflammation are purported to play a role [16, 13].
Limited access to human kidney tissue is a major limitation of studying human DKD, particularly post-metabolic surgery. Pre-clinical studies of metabolic surgery in experimental DKD thus offer a unique opportunity to investigate structural and molecular changes in the kidney postoperatively. In the present review, we aim to summarise the renoprotective effects and mechanisms observed in pre-clinical studies of metabolic surgery in rodents with obesity, type 2 diabetes, and kidney disease to date. We supplement the review with additional unpublished findings from our own research group.
Metabolic and Renal Biochemical Parameters
Table 1 provides an overview of metabolic and renal parameters assessed in pre-clinical studies of metabolic surgery for experimental DKD. Three studies evaluated the impact of RYGB surgery [17–19], two evaluated duodeno-jejunal bypass (DJB) surgery [20, 21], while one study each utilised Roux-en-Y esophagojejunostomy (RYEJ) and sleeve gastrectomy (SG) [22, 23]. All experiments studying RYGB were performed in the Zucker Diabetic Fatty (ZDF) rat model of obesity and DKD by our group. Studies evaluating DJB, RYEJ, and SG were performed in a high-fat diet (40% calories from fat) plus low-dose streptozotocin (STZ) model of obesity, diabetes and renal injury in Sprague Dawley or Wistar rats. Xiong et al. evaluated postoperative outcomes at 3 timepoints (4, 8, and 12 weeks); only data from the final timepoint (12 weeks) is presented in the Tables 1 and 2.
Table 1. Metabolic and renal biochemical parameters assessed in-preclinical studies of metabolic surgery for experimental diabetic kidney disease.a .
| Study | Obesity and DKD model | Surgery type | Postoperative follow-up | Body weight | Plasma glucose (mmol/L) | Plasma lipids (mmol/L) | Proteinuria | Glomerular filtration rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||
| Zhiqing et al., 2014 [20] | High-fat diet (40% fat) plus low-dose streptozotocin (35mg/kg) in Sprague Dawley rats | DJB | 8 weeks | 310±10g | 340±10g | 25±3 mmol/L (R) | 6±3 mmol/L (R) | N/A N/A |
1.5 ± 0.2 (TC) 1.2 (0.7 (TG) |
20±4 mg/24h (UAER) | 30±7 mg/24h (UAER) | 9±2 mL/g/day (CrCl) | 8±3 mL/g/day (CrCl) |
| Wang et al., 2016 [22] | High-fat diet (40% fat) plus low-dose streptozotocin (30mg/kg) in Sprague Dawley rats | RYEJ | 8 weeks | 375±20g | 300±20g | 18±2 mmol/L (F) | 7±1 mmol/L (F) | N/A | N/A | 15±2 mg/24h (UAER) | 20±3 mg/24h (UAER) | 10±2 mL/g/day CrCl) | 8±2 mL/g/day (CrCl) |
| Neff et al., 2017 [17] | Zucker diabetic fatty rat (fa/fa) | RYGB | 13 weeks | 430±10g | 390±10g | N/A, clamped <15mmol/L using insulin | N/A | N/A | 1.6±0.3g/mmol (UPCR) | 0.4±0.3g/mmol (UPCR) | N/A | N/A | |
| Wu et al., 2018 [21] | High-fat diet (40% fat) plus low-dose streptozotocin (35mg/kg) in Sprague Dawley rats | DJB | 8 weeks | 360±10g | 390±10g | 3000±200 (AUCOGTT) | 1500±200 (AUCOGTT) | N/A | N/A | 19±4 mg/24h (UAER) | 20±4 mg/24h (UAER) | 9±2 mL/g/day (CrCl) | 8±1 mL/g/day (CrCl) |
| Canney et al., 2019 [18] | Zucker diabetic fatty rat (fa/fa) | RYGB | 7 weeks | 387±5g | 285±7g | 30±0.9 mmol/L (R) | 8.3±1 mmol/L (R) | N/A | N/A | 650±1000 mg/g (UACR) | 68±21 mg/g (UACR) | N/A | N/A |
| Xiong et al., 2020 [23] | High-fat diet (40% fat) plus low-dose streptozotocin (35mg/kg) in Wistar rats | SG | 12 weeksb | 410±10g | 380±20g | 15±1 mmol/L (F) | 8.5±2 mmol/L (F) | N/A | N/A | 5±1 mg/g (UACR) | 4±1 mg/g (UACR) | N/A | N/A |
| Nair et al., 2020 [19] | Zucker diabetic fatty rat (fa/fa) | RYGB | 8 weeks | 390±10g | 324±24g | 25±2 mmol/L (F) | 7.2±2.7 mmol/L (F) | N/A N/A |
2.5 ±0.2 (TC) 4.2 [IQR 5.2-6] (TG) | 239.4±245.4 μg/mg (UACR) | 30.7±25.8 μg/mg (UACR) | N/A | N/A |
Where raw numerical data not reported, approximate values estimated from figures in manuscript.
This study evaluated outcomes at 3 timepoints (4, 8, and 12 weeks); data from the final timepoint (12 weeks) is presented.
AUCOGTT, glucose area under the curve derived from oral glucose tolerance test; CrCl, 24-hour urinary creatinine clearance; DJB, duodeno-jejunal bypass; F, fasting; IQR, interquartile range; N/A, not assessed; R, random; RYEJ, Roux-en-Y esophagojejunostomy; RYGB, Roux-en-Y gastric bypass surgery; SG, sleeve gastrectomy; TC, total cholesterol; TG, triglycerides; UACR, urinary albumin-to-creatinine ratio; UAER, urinary albumin excretion rate; UPCR, urine protein-to-creatinine ratio.
Table 2. Renal morphometric, immunohistochemical, and ultrastructural parameters in-preclinical studies of metabolic surgery for experimental DKD.a .
| Glomerular morphometry | Immunohistochemistry | Transmission electron microscopy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Obesity and DKD model | Surgery type (follow-up) | Glomerular area | Glomerular volume | Mesangial area/matrix fraction | GV/P (WT-1) | Synaptopodin | Desmin | CD68 | TGF-β1 | Nephrin | PFPF | PFPD | GBM thickness |
| Zhiqing et al., 2014 [20] | HFD (40%) plus low-dose STZ (SD rat) | DJB (8 weeks) | Unchanged | N/A | Decreased | N/A | Increased | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Wang et al., 2015 [22] | HFD (40%) plus low-dose STZ (SD rat) | RYEJ (8 weeks) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Decreased | N/A | N/A | N/A | N/A |
| Neff et al., 2017 [17] | ZDF rat | RYGB (13 weeks) | Decreased | N/A | N/A | N/A | N/A | N/A | Decreased | N/A | N/A | N/A | N/A | N/A |
| Wu et al., 2018 [21] | HFD (40%) plus low-dose STZ (SD rat) | DJB (8 weeks) | Decreased | N/A | Decreased | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Canney et al., 2019 [18] | ZDF rat | RYGB (7 weeks) | Decreased | Decreased | N/A | Decreased | N/A | Decreased | Decreasedb | N/A | N/A | Increased | Unchangedb | Unchanged |
| Xiong et al., 2020 [23] | HFD (40%) plus low-dose STZ (Wistar rat) | SG (12 weeks)c | Decreased | N/A | Decreased | N/A | N/A | N/A | N/A | N/A | Increased | N/A | Decreased | Decreased |
| Nair et al., 2020 [19] | ZDF rat | RYGB (8 weeks) | Decreased | Decreased | N/A | Unchangedb | N/A | Decreased | Unchangedb | N/A | N/A | Increased | Decreased | Unchanged |
Cell values indicate if morphometric parameter is increased, decreased, or unchanged in metabolic surgery-operated rats relative to SHAM-operated rats. Not all parameters were assessed in every study. Bold text emphasises parameters that were assessed in a given study.
Unpublished data from our group.
This study evaluated outcomes at 3 timepoints (4, 8, and 12 weeks); data from the final timepoint (12 weeks) is presented.
CD68, cluster of differentiation 68 (macrophage marker); DJB, duodeno-jejunal bypass; GBM, glomerular basement membrane; GV/P, glomerular volume served by podocyte (podocyte coverage); HFD, high-fat diet; N/A, not assessed; PFPD, podocyte foot process diameter; PFPF, podocyte foot process frequency; RYEJ, Roux-en-Y esophagojejunostomy; RYGB, Roux-en-Y gastric bypass surgery; SD, Sprague Dawley; SG, sleeve gastrectomy; STZ, streptozotocin; TGF-β1, transforming growth factor-beta 1; WT-1, Wilms’ tumour-1.
Body weight and metabolic control
RYGB, RYEJ, and SG achieved significant reductions in body weight at timepoints ranging from 7-13 weeks postoperatively [22, 17–19, 23]. Although rats undergoing DJB experienced weight gain postoperatively, body weight was reduced compared with SHAM-operated rats at 8 weeks postoperatively in both studies using this procedure [20, 21]. All metabolic surgeries effectively improved glycaemia, with greater than 70% reductions in plasma glucose post-RYGB reported by Canney et al. and Nair et al. [18, 19]. No studies have assessed glycaemic control post-metabolic surgery in experimental DKD using HbA1c or fructosamine. Significant reductions in total cholesterol and triglycerides compared with control rats were reported in a cross-sectional manner at study close post-DJB and post-RYGB by Zhiqing et al. and Nair et al., respectively [20, 19]. No studies to date have conducted longitudinal pre- and post-metabolic surgery profiling of plasma lipids.
Urinary Protein Excretion and Glomerular Filtration Rate
RYGB and SG are the only metabolic surgeries that have lowered proteinuria in pre-clinical studies of DKD to date [17–19, 23], although the magnitude of reduction in proteinuria has been greater with RYGB than SG. Human studies have indicated that RYGB may be a more effective than SG in terms of metabolic control [24]. Additionally, more observational and randomised human data exists for RYGB as a renoprotective intervention compared with SG [13]. Certain purported renoprotective effects of metabolic surgery, such as increased natriuresis activating tubuloglomerular feedback to combat glomerular hypertension, exists only for RYGB and not SG [25, 16]. Stable or increased levels of urinary albumin excretion rate at 8 weeks after DJB were reported by Wu et al. and Zhiqing et al., respectively [20, 21]. Conversely, Canney et al. reported an 86% reduction in urinary albumin-to-creatinine ratio compared with SHAM-operated rats at 7 weeks post-RYGB [18]. Similarly, Nair et al. reported an 87% reduction in preoperative urinary albumin-to-creatinine ratio values at 8 weeks post-RYGB [19]. While all studies evaluated urinary protein excretion on timed urinary collections using metabolic cages, there is variability in how results have been reported with researchers using urinary albumin excretion rate, urinary albumin-to-creatinine ratio, and urinary protein-to-creatinine ratio to quantify proteinuria.
Changes in renal function as assessed by measurement of 24-hour urinary creatinine clearance has been conducted post-RYEJ and post-DJB and compared against SHAM-operated animals. Reductions in creatinine clearance post-RYEJ and post-DJB relative to SHAM-operated animals is a consistent finding [20, 22, 21]. Limitations of estimating kidney function with serum creatinine post-metabolic surgery notwithstanding [26], these findings may reflect remission of glomerular hyperfiltration. Zhiqing et al. also measured serum cystatin C at 8 weeks post-DJB, finding that it was elevated relative to values in SHAM-operated rats [20]. To date, no studies have directly measured glomerular filtration rate using available methodologies including plasma clearance of iohexol and transcutaneous measurement of FITC-sinistrin clearance [27, 28].
Renal Morphometry and Immunohistochemistry
Table 2 outlines renal morphometric and immunohistochemical parameters assessed in pre-clinical studies of metabolic surgery for DKD to date. Studies have predominantly assessed changes in glomerular structure postoperatively to investigate the structural underpinnings of the pronounced anti-proteinuric effect of metabolic surgery. Reduced glomerular area has been demonstrated by 3 studies evaluating RYGB in ZDF rats [17–19], while Wu et al. and Xiong et al. also demonstrated reductions in glomerular area post-DJB and post-SG, respectively [21, 23]. Reduced glomerular volume has been exclusively demonstrated after RYGB in 2 studies [18, 19], while reduced mesangial matrix expansion has been shown in 2 separate studies of DJB and 1 study of SG but not RYGB [20, 21, 23]. No studies to date have directly assessed renal tubular morphology after metabolic surgery in experimental DKD, which should be a priority for future research in the field given the prominent role assigned to proximal tubular dysfunction in the onset and propagation of proteinuria in DKD.
Immunohistochemistry facilitates the investigation of molecular mechanisms and cell-specific responses within the kidney after metabolic surgery. Staining for Wilms’ tumour-1 protein (WT-1), a podocyte-specific nuclear antigen, highlights podocyte distribution and permits calculation of podocyte endowment within the glomerulus [29]. Canney et al. quantified WT-1 stained nuclei in the kidney after RYGB [18]. Although no absolute differences in podocyte number were observed, the smaller glomerular volume in RYGB-operated rats resulted in a decrease in the glomerular volume served per podocyte (reduced podocyte coverage). Similarly, Zhiqing et al. demonstrated increased renal expression of another podocyte-specific marker, synaptopodin, 8 weeks post-DJB [20]. Xiong et al. demonstrated increased renal expression of nephrin, a key structural protein located at the slit diaphragm area of podocytes, by both immunohistochemistry and western blotting up to 12 weeks post-SG [23]. De novo staining for desmin, a podocyte intermediate filament protein, is an early marker of podocyte mechanical stretch due to glomerular hypertension in the setting of DKD [29]. Canney et al. and Nair et al. both demonstrated significant reductions in the number of desmin-positive cells post-RYGB in ZDF rats [18, 19]. Together, these findings suggest that metabolic surgery opposes podocyte dedifferentiation in the setting of DKD.
Neff et al. demonstrated reduced renal expression of the macrophage marker CD68 post-RYGB in the ZDF model of DKD [17]. This occurred in parallel with decreased urinary excretion of monocyte chemotactic protein-1 (MCP-1), improvements in glomerular morphometry and proteinuria. This finding indicates reduced renal inflammation postoperatively, consistent with the observation of reduced urinary excretion of inflammatory cytokines at 1-year post-metabolic surgery in humans [30]. Notably, the RYGB-induced improvements observed by Neff et al. in ZDF rats were recapitulated (save for impact on proteinuria) in a parallel SHAM-operated group that underwent diet restriction to achieve RYGB-matched weight loss. Wang et al. demonstrated reduced renal expression of TGF-β1 in glomerular and renal tubular epithelial cells at 8 weeks post-RYEJ, indicative of an anti-fibrotic effect of the intervention [22].
Glomerular Ultrastructure
While most studies of metabolic surgery for experimental DKD to date have evaluated changes in glomerular morphometry postoperatively, 3 studies (2 post-RYGB and 1 post-SG) have assessed glomerular ultrastructure using transmission electron microscopy (Table 2) [18, 19, 23]. Injury-associated cytoskeletal rearrangements in podocytes result in the disruption and retraction of primary and secondary foot processes, a phenomenon referred to as foot process effacement. This pattern of glomerular ultrastructural disruption is mechanistically linked to the emergence of proteinuria in DKD through its impact on size-selective sieving properties of the glomerular filtration barrier [31].
Canney et al. demonstrated that RYGB restores normal podocyte foot process frequency (a marker of podocyte health) at 7 weeks post-RYGB [18]. Similarly, Nair et al. showed that RYGB restores normal podocyte foot process morphology by increasing foot process frequency and decreasing foot process diameter at 8 weeks post-RYGB [19]. Both studies highlighted that RYGB had no effect on glomerular basement membrane (GBM) thickness. As GBM thickness was not significantly elevated in SHAM-operated versus healthy control rats, power to detect reduced GBM thickening post-RYGB was significantly diminished. This latter finding, therefore, reflects a limitation of studying DKD in rodents as not all features of human diabetic nephropathy are reliably recapitulated in rats [32].
Conversely, GBM thickening to approximately 230-250 nm did develop in Xiong et al.’s study of SG conducted in high-fat diet Wistar rats treated with low-dose streptozotocin (STZ) [23]. Accordingly, GBM thickness was reduced by SG at 4, 8, and 12 weeks [23]. Similar to Nair et al.’s study of RYGB highlighted above [19], increased podocyte foot process width (analogous to diameter) which developed in SHAM-operated rats was reversed by SG out to 12-week follow-up [23].
Renal Cortical Transcriptome
Nair et al. interrogated changes in the renal cortical transcriptome (assessed using bulk RNA-sequencing) at 8 weeks post-RYGB in the ZDF rat model [19]. Downstream analysis focused on differentially expressed transcripts with an absolute fold-change ≥1.3 and p-value adjusted for multiplicity testing (Benjamini-Hochberg) <0.05. In total, 379 were genes differentially expressed between SHAM-operated ZDF rats compared with healthy fa/+ controls, while 942 genes were differentially expressed between RYGB-operated and SHAM-operated ZDF rats. This corresponded to a change in 2.1% (379/18,423) of the renal transcriptome in SHAM-operated ZDF rats and 5.1% (942/18,423) of the renal transcriptome in RYGB-operated rats, respectively. Inflammation, tubulopathy, and fibrosis-associated transcripts including Il24 (interleukin-24), Havcr1 (kidney-injury molecule-1), and Spp1 (osteopontin) were strongly increased from health to disease (SHAM-operated ZDF rats versus fa/+ rats) and markedly decreased with metabolic surgery (RYGB-operated versus SHAM-operated ZDF rats). Additionally, RYGB increased expression of several genes reflecting adaptive responses to postoperative micronutrient deficiency, including Epo and Cyp27b1, indicative of impaired iron and vitamin D homeostasis, respectively.
Pathway enrichment analyses performed using the Reactome database identified upregulation of renal inflammation and fibrosis pathways in SHAM-operated ZDF rats which was reversed by RYGB [33, 19]. Conversely, biological oxidation activity was decreased in SHAM-operated rats and restored by RYGB, reflecting restoration of renal tubular biotransformation capacity postoperatively. Using MCP-counter to estimate renal tissue-infiltrating immune and stromal cell populations [34], RYGB-operated animals were predicted to have decreased immune cell and fibroblast abundance compared with SHAM-operated ZDF rats.
Of the 379 transcripts differentially expressed from health to disease (SHAM-operated ZDF rats versus fa/+ rats), 144 (38.0%) of these genes were also changed by RYGB. The majority of these genes were changed in the opposite direction to disease-associated transcriptional shifts, emphasising the corrective impact of RYGB in experimental DKD. Of the 144 disease-associated transcripts corrected by RYGB, 22 were significantly differentially expressed in the glomeruli of patients with DKD [35], indicating the potential of RYGB to decrease DKD-associated inflammation (Csf1r, C4b), TGF-β1 mediated fibrosis (Vim, Fn1, Spp1), and adaptive cytoskeletal responses to mechanical stretch induced by glomerular hypertension (Tnnt, Tubb6), while also restoring tubular bone-morphogenetic protein-7 signalling (Id4). Indeed, disease-associated transcripts corrected by RYGB strongly and positively correlated with abnormal glomerular morphometry and negatively correlated with podocyte foot process frequency, a marker of glomerular health, suggesting that RYGB-induced corrections in renal inflammation and fibrosis signalling contribute to improved glomerular structure and ultrastructure postoperatively. In particular, TGF-β1 signalling pathway regulated genes including osteopontin (Spp1), vimentin (Vim), and fibronectin (Fn1) strongly correlated with altered glomerular structure. Reduced expression of these targets post-RYGB was confirmed by urinary ELISA (Spp1) and qPCR/western blotting of renal cortex (Vim/Fn1). Thus, reduced TGF-β1-mediated renal fibrosis emerged as a dominant transcriptomic response to RYGB which persisted through validation in a human DKD glomerular microarray dataset [35], findings which are consistent with reduced renal TGF-β1 expression by immunohistochemistry post-RYEJ demonstrated by Wang et al. [22].
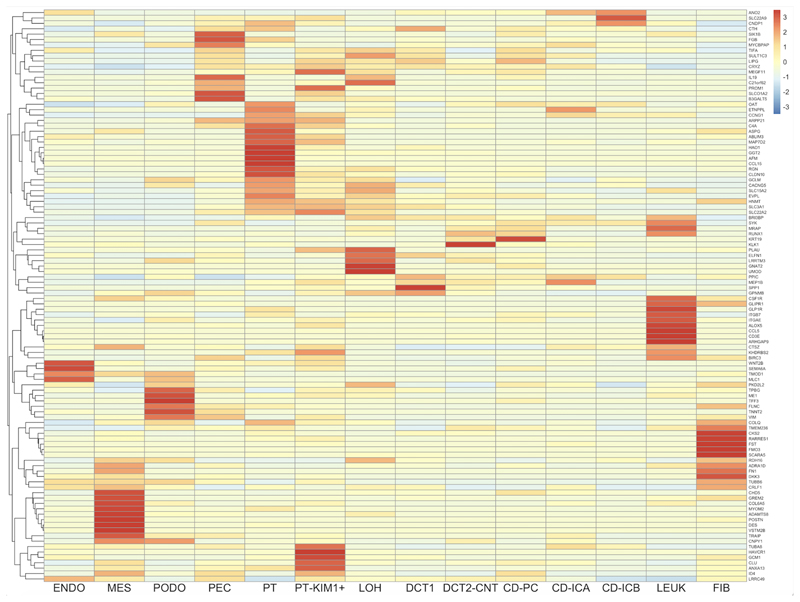
Elucidating cell-specific responses within the kidney post-RYGB has the potential to uncover new mechanisms governing renoprotection. Therefore, we performed a deconvolution analysis of our post-RYGB bulk renal cortical RNA-sequencing data in a publicly available single-cell RNA-sequencing dataset of the human diabetic kidney [36]. Figure 1 presents a heatmap of cell-specific expression patterns of 106 disease-associated transcripts with human orthologs changed by RYGB in our dataset. Interestingly, despite pronounced improvements in glomerular structure and ultrastructure post-metabolic surgery in rats, transcriptomic changes are more common in renal tubular segments (particularly proximal tubule) as well as leucocyte and fibroblast cell populations. This suggests that corrective gene expression changes induced by metabolic surgery in the kidney at sites distant from the glomerulus may contribute to the improved glomerular structure observed.
Figure 1. Deconvolution analysis of 106 DKD-associated transcripts which are corrected by RYGB in ZDF rats using publicly available human diabetic kidney single-cell RNA-sequencing data.
Columns represent 14 kidney cell types. Cell types include: ENDO, endothelial cells; MES, mesangial cells; PODO, podocytes; PEC, parietal epithelial cells; PT, proximal tubular cells; PT-KIM1+, proximal tubular cell cluster positive for kidney-injury molecule-1; LOH, loop of Henle; DCT1, distal convoluted tubule cluster 1; DCT2-CNT, distal convoluted tubule cluster 2-connecting tubule; CD-PC, collecting duct-principal cell; CD-ICA, collecting duct-intercalated cell type A; CD-ICB, collecting duct-intercalated cell type B; LEUK, leukocyte; FIB, fibroblast.
Rows indicate genes (official gene symbols of human orthologous genes displayed).
Cell colours indicate relative transcript expression levels in the human diabetic kidney: red – high expression in cell type; yellow – low expression in cell type; blue – very low/absent expression in cell type.
Translational Relevance of Rodent Models Employed in Pre-Clinical Studies of Metabolic Surgery for DKD
Rodent models of obesity and diabetes offer a unique opportunity to explore responses to metabolic surgery within the kidney and indeed at the level of the whole organism, although findings presented in this review must be interpreted within the context of limitations of pre-clinical modelling of DKD. Mice are the most widely used species in animal research because they breed quickly, are cheap to house, and are amenable to genetic manipulation [32]. However, mortality rates post-metabolic surgery in mice are very high, particularly with the RYGB procedure where mortality rates approach 100%, due to technical difficulty fashioning anastomoses [37]. Additionally, the murine forestomach lacks sufficient muscle to push nutrients through the anastomosis post-RYGB, resulting in mortality from gastric obstruction [37]. Rats have therefore been favoured for pre-clinical studies of metabolic surgery; postoperative mortality rates with the RYGB procedure in our group have ranged from 10-20%.
Rodent models of obesity and diabetes develop glomerular hyperfiltration, albuminuria, and reliably recapitulate histological features of early human diabetic nephropathy, but do not develop features of advanced human disease including nodular glomerulosclerosis, marked tubulo-interstitial fibrosis, and kidney failure [32]. Although the role of metabolic surgery in the treatment of advanced human DKD is an emerging research question, most human studies of metabolic surgery conducted to date have focused on reducing the incidence of or improving control of early-stage DKD [13]. The severity of kidney disease reiterated by rat models of obesity and diabetes is thus translationally relevant to ongoing human studies in the field.
The ZDF rat utilised in studies of RYGB by our group develops hyperphagia and insulin resistance as a consequence of monogenic obesity due to a homozygous recessive missense mutation in the fa gene encoding the leptin receptor [38]. Separate to the leptin receptor mutation, the ZDF rat harbours a genetic defect in pancreatic β-cell gene transcription which contributes to the emergence of type 2 diabetes in the setting of insulin resistance [39]. Importantly, due to the absence of an intact leptin signalling system, ZDF rats do not reliably manifest hypertension [40], which is a critical determinant of DKD progression in humans. ZDF rats also manifest hydronephrosis and progress rapidly to overt diabetes in the adolescent state prior to reaching maturity [40, 41]. Although ZDF rats manifest obesity, insulin resistance, and dyslipidaemia, they do not develop all features of the human metabolic syndrome and lack a significant period of pre-diabetes which is characteristic of human DKD.
High-fat diet and STZ-treated rats, which have been used by other groups in pre-clinical studies of metabolic surgery, also rapidly progress to overt diabetes without a prolonged period of pre-diabetes as a consequence of STZ toxicity to pancreatic β-cells [42]. Similar to the ZDF model, models of STZ-induced diabetes do not reliably manifest hypertension [42]. A distinct disadvantage of STZ is its non-specific renal cytotoxicity which can directly induce renal tubular injury [43]. Although all studies included in the current review utilised a single low-dose of STZ, STZ-induced renal tubular injury has been described even at low doses [44]. High-fat diet and low-dose STZ models may more readily develop manifestations of obesity-related glomerulopathy rather than diabetic nephropathy. However, given the overlap in pathophysiology and renal manifestations of obesity-related glomerulopathy and diabetic nephropathy [45], as well as the growing evidence for metabolic surgery for non-diabetic chronic kidney disease [13], the findings observed remain translationally relevant.
Several emerging pre-clinical models of obesity and diabetes overcome many of the aforementioned limitations of the rat models utilised in studies of metabolic surgery to date. The Zucker Diabetic Sprague Dawley (ZDSD) rat was developed by crossing lean homozygous ZDF rats with a substrain of Sprague Dawley rats that were selectively bred for high-fat diet-induced obesity [46]. Thus, the ZDSD rat combines the defect in pancreatic β-cell gene transcription characteristic of the ZDF rat with polygenic obesity of the Sprague Dawley rat to produce a model of obesity and diabetes with an intact leptin pathway [46]. The ZDSD rat is thus a more translationally relevant pre-clinical model which spontaneously develops type 2 diabetes and hypertension in the context of polygenic obesity and a prolonged pre-diabetic period. Renal manifestations of the model have also been characterised, and include glomerular hyperfiltration, glomerular and tubular injury, mesangial expansion, GBM thickening, and podocyte foot process effacement [47]. The renal physiology and structure of large animals more closely resembles that of the human kidney than smaller animals such as rats. The Iberian pig fed with high-fat diet is a promising model of obesity-related glomerulopathy and diabetic nephropathy, which develops renal histological manifestations that very closely resemble the human disease even in the absence of overt type 2 diabetes, including lipid deposits and some features of advanced glomerular disease such as nodular glomerulosclerosis [48]. These emerging models, which are more translationally relevant to human DKD, come at the expense of prolonged experimental timelines and increased animal husbandry costs.
Conclusions
All pre-clinical studies of metabolic surgery for experimental DKD have demonstrated pronounced improvements in glycaemia postoperatively. RYEJ, RYGB, and SG achieved postoperative weight loss [22, 17–19], while DJB slowed weight gain compared with SHAM-operated controls [20, 21]. RYGB exerted a potent proteinuria-lowering effect across 3 studies [17–19], while DJB and RYEJ slowed progression of proteinuria compared with SHAM-operated controls [20, 22, 21]. SG also lowered proteinuria in a single study, although reductions were lesser in magnitude compared with RYGB [23]. No studies to date have examined pre- and post-metabolic surgery changes in dyslipidaemia, blood pressure, and measured glomerular filtration rate and these should be prioritised by futures studies in the field.
Most pre-clinical studies have demonstrated improved glomerular morphometry post-metabolic surgery. Immunohistochemical interrogation of the kidney post-metabolic surgery has demonstrated improved podocyte endowment (WT-1, synaptopodin) and structural integrity of podocyte slit diaphragms (nephrin), decreased podocyte mechanical stretch (desmin), and reduced macrophage infiltration and fibrosis (CD68 and TGF-β1, respectively) [20, 22, 17–19, 23]. Two studies have used transmission electron microscopy to show that RYGB improves glomerular ultrastructure in ZDF rats [18, 19], while similar reductions in podocyte foot process effacement were observed in a single study of SG [23]. No studies have specifically examined changes in renal proximal tubular morphometry and ultrastructure post-metabolic surgery in experimental DKD. Such studies should be prioritised as RYGB-induced transcriptional changes are abundant in the proximal tubule and structural characterisation of this tubular segment may uncover new phenomena underpinning the anti-proteinuric effect of metabolic surgery.
Bulk RNA-sequencing of the renal cortex has highlighted that RYGB corrects DKD-associated alterations in multiple pathways including fibrosis, inflammation and biological oxidations. Pro-inflammatory and pro-fibrotic transcripts corrected by RYGB strongly correlate with glomerular structural integrity, providing mechanistic insight into the improved glomerular structure post-metabolic surgery. RYGB corrects DKD-associated transcriptomic alterations across all cell types in the kidney, with a predominant effect in glomerular cells, proximal tubular cells, leucocytes, and fibroblasts. Interrogating renal responses to metabolic surgery in experimental DKD using single-cell RNA-sequencing should add granularity to mechanisms underpinning its renoprotective effects.
Ultimately, studying renal responses to metabolic surgery in experimental DKD employs a reverse-translational approach whereby mechanisms underpinning the renoprotective effects of metabolic surgery observed in large-scale observational and emerging randomised human studies can be interrogated. Evidence accumulated in pre-clinical studies of metabolic surgery for experimental DKD to date support a growing role for metabolic surgery in the DKD treatment algorithm.
Acknowledgements
We acknowledge local support received in the realisation of studies described herein from the University College Dublin (UCD) Biomedical Facility and the Research Pathology and Genomics Core Facilities at the UCD Conway Institute. We thank Dr. Parker Wilson and Dr. Benjamin Humphreys for sharing their human kidney single-cell RNA-sequencing data with us.
Funding Sources
This work was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive National Doctors Training and Planning and the Health and Social Care, Research and Development Division, Northern Ireland. The relevant experimental studies conducted in our laboratories have been supported by generous funding from Science Foundation Ireland (12/YI/B2480) to CWleR, Swedish Medical Research Council (2015-02733) and European Foundation for the Study of Diabetes/Boehringer Ingelheim European Diabetes Research Programme (BI 2017_3) to CWleR and NGD.
Footnotes
Disclosure Statement
CWleR discloses personal fees outside of the submitted work from Novo Nordisk, GI Dynamics, Eli Lilly, Johnson and Johnson, Sanofi, Aventis, Astra Zeneca, Janssen, Bristol-Myers Squibb and Boehringer-Ingelheim. The authors have no conflicts of interest to declare.
Author Contributions
WPM wrote the manuscript with critical input from CWleR and NGD. All authors reviewed and approved the final manuscript.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(S1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017 Aug 31;377(9):839–48. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 3.Martin WP, Griffin TP, Lappin DW, Griffin DG, Ferguson JP, O'Brien T, et al. Influence of Referral to a Combined Diabetology and Nephrology Clinic on Renal Functional Trends and Metabolic Parameters in Adults With Diabetic Kidney Disease. Mayo Clin Proc Innov Qual Outcomes. 2017 Sep;1(2):150–60. doi: 10.1016/j.mayocpiqo.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–08. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim WH, Johnson DW, Hawley C, Lok C, Polkinghorne KR, Roberts MA, et al. Type 2 diabetes in patients with end-stage kidney disease: influence on cardiovascular disease-related mortality risk. Med J Aust. 2018 Nov 19;209(10):440–46. doi: 10.5694/mja18.00195. [DOI] [PubMed] [Google Scholar]
- 7.Chang AR, Grams ME, Navaneethan SD. Bariatric Surgery and Kidney-Related Outcomes. Kidney Int Rep. 2017 Mar;2(2):261–70. doi: 10.1016/j.ekir.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen RV, Pereira TV, Aboud CM, Caravatto PPdP, Petry TBZ, Correa JLL, et al. Microvascular Outcomes after Metabolic Surgery (MOMS) in patients with type 2 diabetes mellitus and class I obesity: rationale and design for a randomised controlled trial. BMJ Open. 2017;7(1) doi: 10.1136/bmjopen-2016-013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen RV, Pereira TV, Aboud CM, Petry TBZ, Lopes Correa JL, Schiavon CA, et al. Effect of Gastric Bypass vs Best Medical Treatment on Early-Stage Chronic Kidney Disease in Patients With Type 2 Diabetes and Obesity: A Randomized Clinical Trial. JAMA Surg. 2020:e200420–e20. doi: 10.1001/jamasurg.2020.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson LMS, Romeo S, Jacobson P, Burza MA, Maglio C, Sjöholm K, et al. The incidence of albuminuria after bariatric surgery and usual care in swedish obese subjects (SOS): a prospective controlled intervention trial. Int J Obes. 2014;39:169. doi: 10.1038/ijo.2014.72. 05/06/online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman AN, Wahed AS, Wang J, Courcoulas AP, Dakin G, Hinojosa MW, et al. Effect of Bariatric Surgery on CKD Risk. J Am Soc Nephrol. 2018;29(4):1289–300. doi: 10.1681/ASN.2017060707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shulman A, Peltonen M, Sjostrom CD, Andersson-Assarsson JC, Taube M, Sjoholm K, et al. Incidence of end-stage renal disease following bariatric surgery in the Swedish Obese Subjects Study. Int J Obes (Lond) 2018 Jun;42(5):964–73. doi: 10.1038/s41366-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin WP, White J, Lopez-Hernandez FJ, Docherty NG, le Roux CW. Metabolic Surgery to Treat Obesity in Diabetic Kidney Disease, Chronic Kidney Disease, and End-Stage Kidney Disease; What Are the Unanswered Questions? Front Endocrinol (Lausanne) 2020 doi: 10.3389/fendo.2020.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland JA, Martin WP, Docherty NG, le Roux CW. Impact of intentional weight loss on diabetic kidney disease. Diabetes Obes Metab. 2019 Oct;21(10):2338–41. doi: 10.1111/dom.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin WP, le Roux CW. Comment on: Metabolic surgery improves renal injury independent of weight loss: a meta-analysis. Surg Obes Relat Dis. 2019 Jun;15(6):1020–23. doi: 10.1016/j.soard.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin WP, Docherty NG, Le Roux CW. Impact of bariatric surgery on cardiovascular and renal complications of diabetes: a focus on clinical outcomes and putative mechanisms. Expert Rev Endocrinol Metab. 2018 Sep;19:1–12. doi: 10.1080/17446651.2018.1518130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff KJ, Elliott JA, Corteville C, Abegg K, Boza C, Lutz TA, et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis. 2017 Jan;13(1):21–27. doi: 10.1016/j.soard.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Canney AL, Cohen RV, Elliott JA, C MA, Martin WP, Docherty NG, et al. Improvements in diabetic albuminuria and podocyte differentiation following Roux-en-Y gastric bypass surgery. Diab Vasc Dis Res. 2019 Nov;14 doi: 10.1177/1479164119879039. 1479164119879039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair M, Martin WP, Zhernovkov V, Elliott JA, Fearon N, Eckhardt H, et al. Characterization of the renal cortical transcriptome following Roux-en-Y gastric bypass surgery in experimental diabetic kidney disease. BMJ Open Diabetes Res Care. 2020;8(1):e001113. doi: 10.1136/bmjdrc-2019-001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhiqing W, Jing W, Haili X, Shaozhuang L, Chunxiao H, Haifeng H, et al. Renal function is ameliorated in a diabetic nephropathy rat model through a duodenal-jejunal bypass. Diabetes Res Clin Pract. 2014 Jan;103(1):26–34. doi: 10.1016/j.diabres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Cheng YG, Huang X, Zhong MW, Liu SZ, Hu SY. Downregulation of lncRNA MALAT1 contributes to renal functional improvement after duodenal-jejunal bypass in a diabetic rat model. J Physiol Biochem. 2018 Aug;74(3):431–39. doi: 10.1007/s13105-018-0636-y. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, He B, Piao D, Han P. Roux-en-Y Esophagojejunostomy Ameliorates Renal Function Through Reduction of Renal Inflammatory and Fibrotic Markers in Diabetic Nephropathy. Obes Surg. 2016 Jul;26(7):1402–13. doi: 10.1007/s11695-015-1947-5. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y, Zhu W, Xu Q, Ruze R, Yan Z, Li J, et al. Sleeve Gastrectomy Attenuates Diabetic Nephropathy by Upregulating Nephrin Expressions in Diabetic Obese Rats. Obes Surg. 2020 Aug;30(8):2893–904. doi: 10.1007/s11695-020-04611-3. [DOI] [PubMed] [Google Scholar]
- 24.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes — 5-Year Outcomes. N Engl J Med. 2017;376(7):641–51. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Docherty NG, Fändriks L, le Roux CW, Hallersund P, Werling M. Urinary sodium excretion after gastric bypass surgery. Surg Obes Relat Dis. 2017 Sep;13(9):1506–14. doi: 10.1016/j.soard.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Martinez M, Luis-Lima S, Morales E, Navarro-Diaz M, Negrin-Mena N, Folgueras T, et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes (Lond) 2019 Oct 22; doi: 10.1038/s41366-019-0476-z. [DOI] [PubMed] [Google Scholar]
- 27.Schock-Kusch D, Xie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, et al. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 2011 Jun;79(11):1254–8. doi: 10.1038/ki.2011.31. [DOI] [PubMed] [Google Scholar]
- 28.Carrara F, Azzollini N, Nattino G, Corna D, Villa S, Cerullo D, et al. Simplified Method to Measure Glomerular Filtration Rate by Iohexol Plasma Clearance in Conscious Rats. Nephron. 2016;133(1):62–70. doi: 10.1159/000445843. [DOI] [PubMed] [Google Scholar]
- 29.Funk J, Ott V, Herrmann A, Rapp W, Raab S, Riboulet W, et al. Semiautomated quantitative image analysis of glomerular immunohistochemistry markers desmin, vimentin, podocin, synaptopodin and WT-1 in acute and chronic rat kidney disease models. Histochem Cell Biol. 2016 Mar;145(3):315–26. doi: 10.1007/s00418-015-1391-6. [DOI] [PubMed] [Google Scholar]
- 30.Fenske WK, Dubb S, Bueter M, Seyfried F, Patel K, Tam FW, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013 Jul-Aug;9(4):559–68. doi: 10.1016/j.soard.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Lin JS, Susztak K. Podocytes: the Weakest Link in Diabetic Kidney Disease? Curr Diab Rep. 2016;16(5):45–45. doi: 10.1007/s11892-016-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betz B, Conway BR. An Update on the Use of Animal Models in Diabetic Nephropathy Research. Curr Diab Rep. 2016;16(2):18–18. doi: 10.1007/s11892-015-0706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu G, He QY. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol Biosyst. 2016 Feb;12(2):477–9. doi: 10.1039/c5mb00663e. [DOI] [PubMed] [Google Scholar]
- 34.Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016 Oct 20;17(1):218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011 Sep;60(9):2354–69. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson PC, Wu H, Kirita Y, Uchimura K, Ledru N, Rennke HG, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci USA. 2019 Sep 24;116(39):19619–25. doi: 10.1073/pnas.1908706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin DP, Gao Q, Ma LL, Yan W, Williams PE, McGuinness OP, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254(1):73–82. doi: 10.1097/SLA.0b013e3182197035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996 May;13(1):18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 39.Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001 Jan;50(1):63–8. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- 40.Vora JP, Zimsen SM, Houghton DC, Anderson S. Evolution of metabolic and renal changes in the ZDF/Drt-fa rat model of type II diabetes. J Am Soc Nephrol. 1996 Jan;7(1):113–7. doi: 10.1681/ASN.V71113. [DOI] [PubMed] [Google Scholar]
- 41.Szöcs Z, Brunmair B, Stadlbauer K, Nowotny P, Bauer L, Luger A, et al. Age-dependent development of metabolic derangement and effects of intervention with pioglitazone in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2008 Jul;326(1):323–9. doi: 10.1124/jpet.108.136465. [DOI] [PubMed] [Google Scholar]
- 42.Kitada M, Ogura Y, Koya D. Rodent models of diabetic nephropathy: their utility and limitations. Int J Nephrol Renovasc Dis. 2016;9:279–90. doi: 10.2147/IJNRD.S103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tay Y-C, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DCH. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68(1):391–98. doi: 10.1111/j.1523-1755.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 44.Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, et al. Extent and Persistence of Streptozotocin-Induced DNA Damage and Cell Proliferation in Rat Kidney as Determined by in Vivo Alkaline Elution and BrdUrd Labeling Assays. Toxicol Appl Pharmacol. 1995;135(2):279–86. doi: 10.1006/taap.1995.1234. 1995/12/01/ [DOI] [PubMed] [Google Scholar]
- 45.D'Agati VD, Chagnac A, de Vries APJ, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–71. doi: 10.1038/nrneph.2016.75. 2016/08/01. [DOI] [PubMed] [Google Scholar]
- 46.Peterson RG, Jackson CV, Zimmerman K, de Winter W, Huebert N, Hansen MK. Characterization of the ZDSD Rat: A Translational Model for the Study of Metabolic Syndrome and Type 2 Diabetes. J Diabetes Res. 2015;2015:10. doi: 10.1155/2015/487816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson RG, Jackson CV, Zimmerman KM. The ZDSD rat: a novel model of diabetic nephropathy. Am J Transl Res. 2017;9(9):4236–49. [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez RR, González-Bulnes A, Garcia-Contreras C, Elena Rodriguez-Rodriguez A, Astiz S, Vazquez-Gomez M, et al. The Iberian pig fed with high-fat diet: a model of renal disease in obesity and metabolic syndrome. Int J Obes (Lond) 2020 Feb;44(2):457–65. doi: 10.1038/s41366-019-0434-9. [DOI] [PubMed] [Google Scholar]
- 49.Martin WP, Bauer J, Coleman J, Dellatorre-Teixeira L, Reeve JLV, Twomey PJ, et al. Obesity is common in chronic kidney disease and associates with greater antihypertensive usage and proteinuria: evidence from a cross-sectional study in a tertiary nephrology centre. Clin Obes. 2020 Dec;10(6):e12402457. doi: 10.1111/cob.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]