Abstract
In mammals and flies, only one cell in a multicellular female germline cyst becomes an oocyte, but how symmetry is broken to select the oocyte is unknown. Here we show that the microtubule minus end-stabilizing protein, Patronin/CAMSAP marks the future Drosophila oocyte and is required for oocyte specification. The spectraplakin, Shot, recruits Patronin to the fusome, a branched structure extending into all cyst cells. Patronin stabilizes more microtubules in the cell with most fusome. Our data suggest that this weak asymmetry is amplified by Dynein-dependent transport of Patronin-stabilized microtubules. This forms a polarized microtubule network, along which Dynein transports oocyte determinants into the presumptive oocyte. Thus, Patronin amplifies a weak fusome anisotropy to break symmetry and select one cell to become the oocyte.
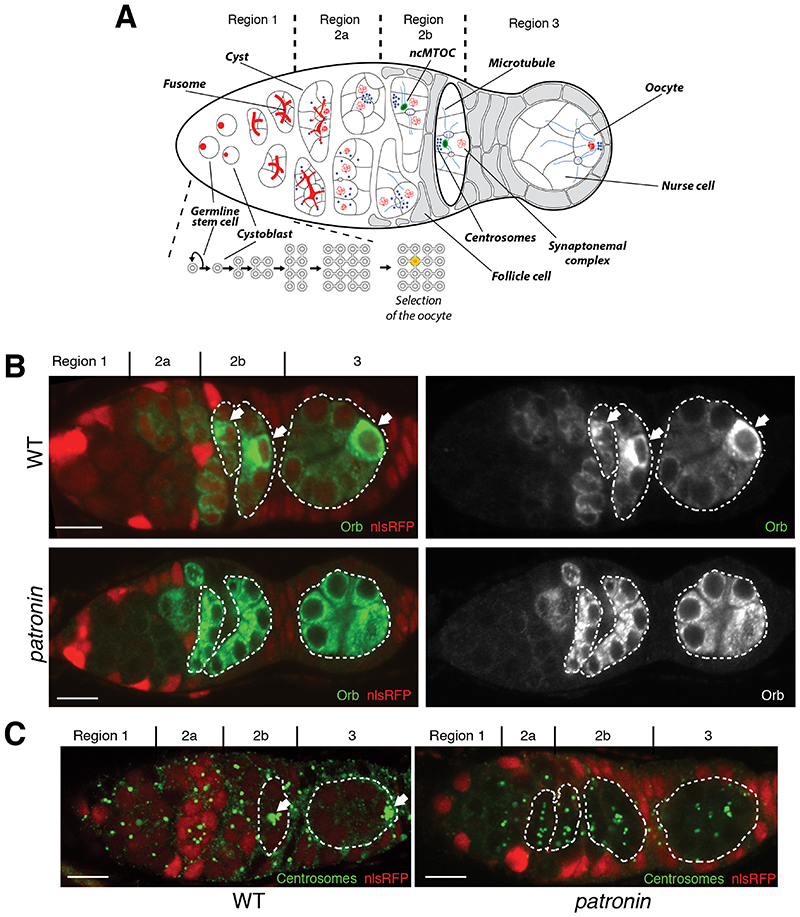
In many organisms, not all female germ cells develop into oocytes. Some cells become accessory cells that contribute material to the oocyte (1). Mouse female germ cells form cysts of up to 30 cells, but most cells undergo apoptosis after transferring cytoplasm and centrosomes to the small number of cells that become oocytes (2, 3). In Drosophila, germline cyst formation starts in the germarium, which has 3 regions. A stem cell produces a cystoblast, which then divides four times with incomplete cytokinesis to generate a cyst of 16 germ cells connected by intercellular bridges, “ring canals” (4, 5). As the cyst moves through regions 2a-b of the germarium, it is surrounded by epithelial follicle cells and then rounds up in region 3 to form a follicle. By this stage, one cell has been selected as the oocyte, whereas others become nurse cells (Fig. 1A). Oocyte selection depends on the formation of a noncentrosomal microtubule organizing center (ncMTOC) in the future oocyte that organizes a polarized microtubule network that directs the dynein-dependent transport of cell fate determinants and centrosomes into the pro-oocyte (6–8) (Fig. 1A). How symmetry is broken to specify which cell contains the ncMTOC and becomes the oocyte is unclear.
Fig. 1. Patronin is required for the oocyte specification.
(A) A schematic diagram of a Drosophila germarium showing germline cyst formation and oocyte selection. Distribution of the oocyte specification markers Orb (B) and centrosomes (C) in wild type (WT; top or left in C) and patronin mutant (bottom or right in C) cysts. For all figures: arrows point to the future oocyte; cysts are marked by dashed lines; mutant cysts are labeled by the absence of nlsRFP; regions of the germarium are indicated on the top; scale bars, 10μm.
Patronin and its vertebrate orthologues (CAMSAPs) are microtubule minus end binding proteins that have been recently found to be essential components of ncMTOCs (9–13). To investigate the role of Patronin in oocyte determination, we examined the distribution of oocyte markers in patroninc9-c5 mutant cysts (Fig. 1B-C and S1). In wild-type cysts, Orb and centrosomes accumulate in future oocytes in regions 2b-3 (14–16), but they are rarely localized in patronin mutants (24% and 3% of mutant cysts respectively) (Fig. 1B-C). Several germ cells enter meiosis in region 2a and accumulate the synaptonemal complex protein C(3)G. C(3)G becomes restricted to two cells in region 2b and to the oocyte in region 3 (17) (Fig. S1). C(3)G is not localized in region 3 of patronin cysts and 44% of the cysts in region 2b have 3 cells in meiosis (Fig. S1). Thus, Patronin is required for oocyte determination.
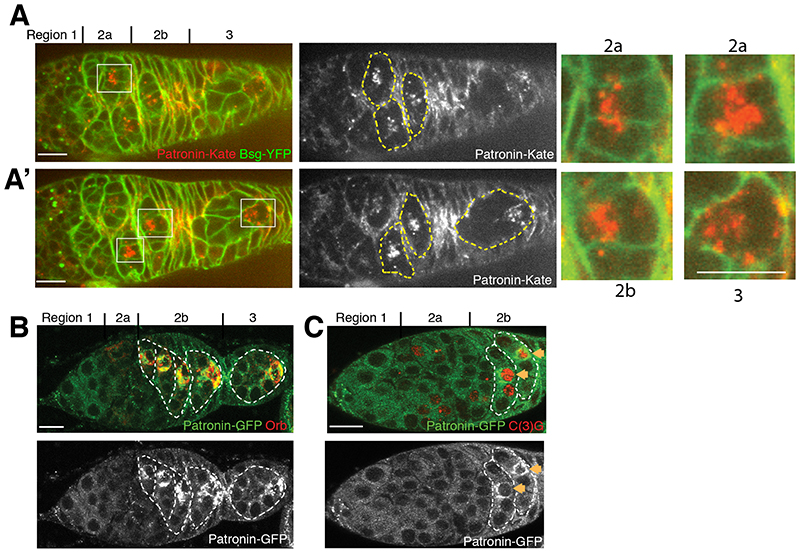
To examine whether Patronin is asymmetrically distributed in the cyst, we imaged germaria expressing endogenously tagged Patronin-Kate. Patronin starts to accumulate in a single cell in each cyst in region 2a, earlier than other markers for the presumptive oocyte, and remains in one cell in regions 2b-3, where it forms distinct foci in the cytoplasm (Fig. 2A-2A’). This cell will become the oocyte, as it is also labelled by Orb (Fig. 2B) and C(3)G (Fig. 2C). patronin mRNA is not localized within the cyst and Patronin expressed from a cDNA with heterologous UTRs and promoter shows a similar distribution to the endogenous protein, indicating that Patronin is localized as a protein and not through transcription in this cell or mRNA localization (Fig. 2B-C and Fig. S2).
Fig. 2. Patronin accumulates in the future oocyte.
(A-A’) Two different focal planes of a live germarium showing accumulation of endogenously tagged Patronin-Kate in one cell of the cyst. Regions 2a and 2b are shown as close-ups. Cell membranes are labelled by Basigin-YFP (Bsg-YFP). (B-C) Ectopically-expressed ubq>Patronin-GFP accumulates in future oocytes labelled by Orb (B) or C(3)G (C).
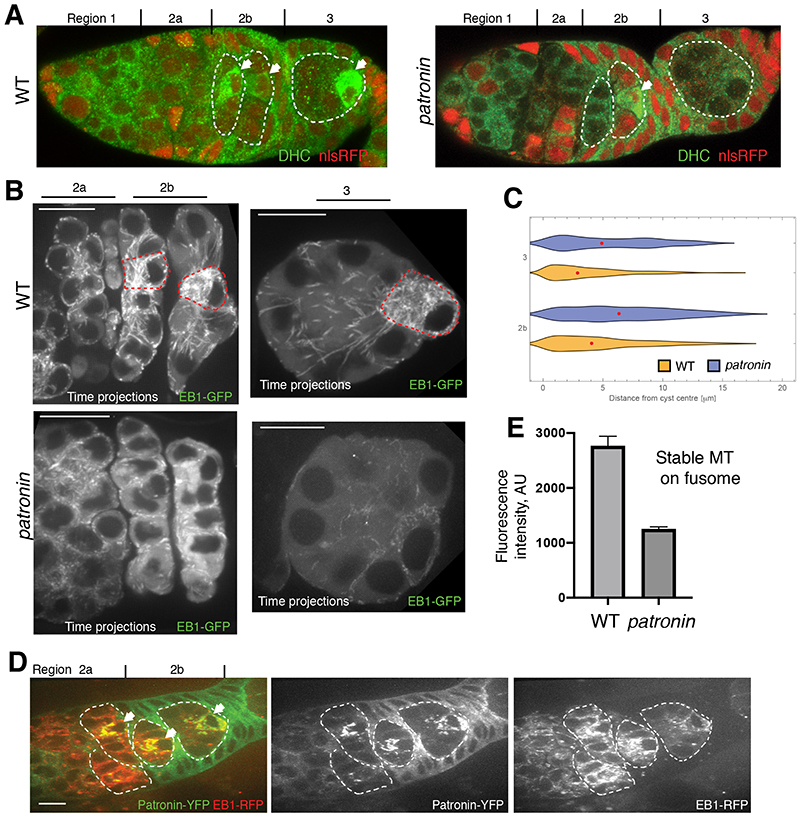
Dynein does not localize to the presumptive oocyte in patronin mutant cysts (Fig. 3A). This suggests that the loss of Patronin disrupts the formation of the MTOC in the pro-oocyte, leading to loss of the polarized microtubule network along which Dynein transports cargoes into one cell. As most of MT plus ends accumulate at the site of MT nucleation, we used the MT plus end-tracking protein EB1-GFP to visualize the putative MTOC in the cyst. The majority of EB1-GFP comets localize to one cell in regions 2b-3 (Fig. 3B-C, Movies S1-S2). Moreover, the densest EB1-GFP signal co-localizes with the Patronin foci in the same cell, suggesting that the latter are the MTOCs formed in the pro-oocyte (Fig. 3D). This asymmetric distribution of EB1-GFP is lost in patronin cysts, where EB1-GFP comets are distributed more homogeneously (Fig. 3B-C, Movies S3-S4). Patronin is therefore required for MTOC formation in the presumptive oocyte and the organization of a polarized MT network.
Fig. 3. Patronin is required for MT organisation in the cyst.
(A) Distribution of Dynein Heavy Chain (DHC) in wild type (WT) and patronin mutant cysts. (B-D) Patronin is required for MTOC formation in the presumptive oocyte. (B) EB-1 comet tracks in wild type (WT; top) and patronin mutant (bottom) cysts. The images are projections of several time points from Movies S1 (WT; region 2), S2 (WT; region 3), S3 (patronin; region 2) and S4 (patronin; region 3). The red dashed line marks cells with MTOCs. (C) Quantification of EB-1 comet distribution in wild type (WT) and patronin mutant cysts in region 3 and 2b of germarium. Red dots indicate median values. (D) Live germarium showing co-localization of Patronin-YFP foci with the microtubules plus end marker EB1-GFP in the presumptive oocyte. (E) Quantification of the mean fluorescence intensities of fusome associated acetylated microtubules in patronin mutant and WT cysts. Errors bars indicate the SEM.
Wild-type cysts contain a population of stable, acetylated MTs that form along the fusome, an ER, spectrin, and actin-rich structure that connects all cells of the cyst (16–19) (Fig. S3). In patronin mutant cysts, there is a 2.5 fold reduction in stable MTs (Fig. 3E and S3). Thus, in the absence of Patronin, the whole organization of MTs in the cyst is disrupted. Patronin binds MT minus ends and stabilizes MTs by protecting theirs minus ends against kinesin-13 induced depolymerization (11, 13). Our results suggest that early accumulation of Patronin in only one cell of the cyst stabilizes MT minus ends there, leading to dynein-dependent transport into this cell, the formation of MTOCs and the subsequent specification of the oocyte.
To examine whether centrosomes contribute to the formation of Patronin MTOCs, we imaged cysts expressing endogenously tagged Patronin-YFP and the centrosomal protein Asterless-Cherry. Although centrosomal clusters localize near Patronin foci, the Asterless and Patronin signals only partially overlap and most Patronin foci lie outside the centrosomal cluster, indicating that Patronin MTOCs are noncentrosomal (Fig. S4A). Centrosomes have been proposed to be inactive during their migration into the oocyte, and they lack crucial components of the PCM (8). To test whether centrosomes contribute to microtubule organization, we imaged cysts expressing EB1-GFP and Asterless-Cherry. The centrosomes show strong MT nucleating activity in region 1, where they organize the mitotic spindles (Fig. S4B and Movie S5). However, only some Asterless-Cherry labelled centrosomes in the presumptive oocyte produce EB1-GFP comets in region 2b (Fig. S4C and Movie S6). Thus, Patronin-dependent ncMTOCs create the initial asymmetry in MT organization that leads to the accumulation of centrosomes in the pro-oocyte, which may then be amplified by activation of some centrosomes in this cell. The close proximity of the active centrosomes to the ncMTOCs, raises the possibility that new MTs produced by these centrosomes are released and then captured and stabilized by Patronin in ncMTOCs, a mechanism described for CAMSAP proteins (20).
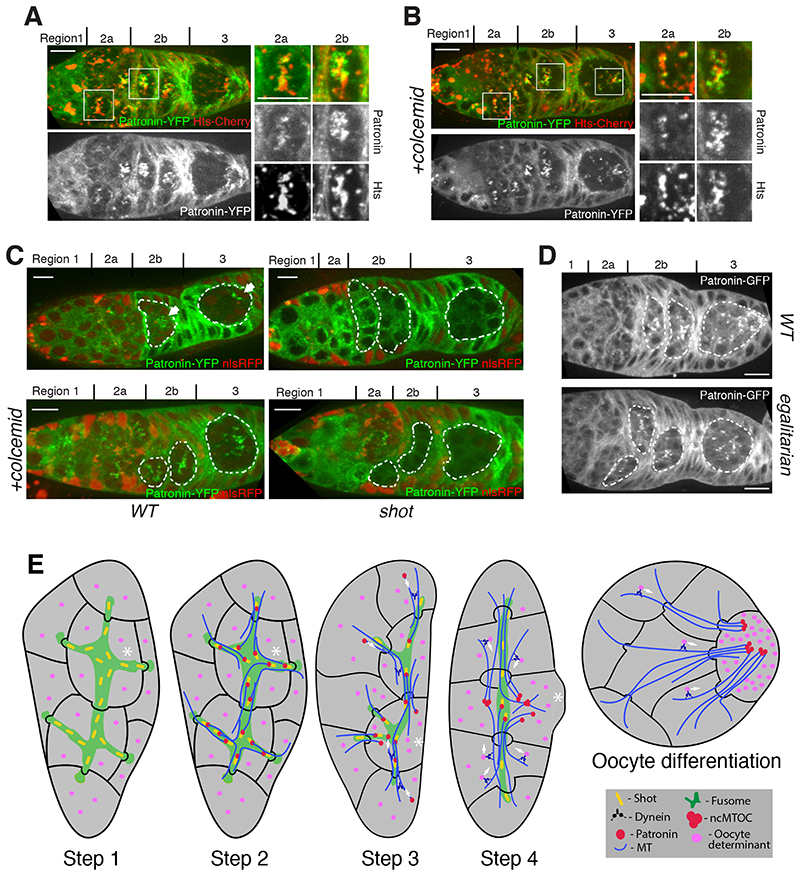
The observation that Patronin is the earliest known marker for the future oocyte raises the question of how symmetry is broken in the cyst to enrich Patronin in one cell. One proposed mechanism for symmetry-breaking is that the cell that inherits the most fusome becomes the presumptive oocyte (21). The fusome is asymmetrically partitioned during the mitoses in region 1, so that mother cells inherit more material than their daughters and one of the two cells with four ring canals has more fusome than the rest (19). To examine whether Patronin associates with the fusome, we imaged germaria expressing endogenously-tagged Patronin-YFP and the fusome marker, Hts-Cherry. Patronin localizes on the fusome in early region 2a, but becomes concentrated in one cell as the cyst progresses towards region 3 (Fig. 4A and S5A). When the MTs are depolymerized with colcemid, however, Patronin remains on the fusome in regions 2b-3 (Fig. 4B). Thus, the fusome determines the initial localization of Patronin in early region 2a, including its slight enrichment in the pro-oocyte, which is then amplified by a MT-dependent process.
Fig. 4. Patronin localisation is defined by fusome and by a positive feed back loop of Dynein mediated transport.
(A-B) Patronin associates with the fusome in a microtubule-dependent manner. Untreated (A) or colcemid-treated (B) live germaria expressing Patronin-YFP and Hts-Cherry. Regions 2a and 2b are shown as close-ups. (C) Shot links Patronin to the fusome. Live germaria containing wild type (WT; left) and shot mutant (right) cysts expressing Patronin-YFP either untreated (top) or treated with colcemid (bottom). (D) Patronin localisation depends on Dynein activity. Wild type (WT; top) and egalitarian mutant (bottom) live germaria expressing transgenic Patronin-GFP. (E) A diagram showing the 4 steps in cyst polarization that lead to the specification of the oocyte and its subsequent positioning at the posterior of the cyst in region 3. See text for details. Asterisk indicates the presumptive oocyte.
The spectraplakin Shot, localizes to the fusome, is required for the oocyte specification, and recruits Patronin to ncMTOCs in the oocyte later in oogenesis, making it a good candidate for a factor that links Patronin to the fusome (13, 17). In shot - cysts, Patronin does not accumulate in one cell and fails to form foci (Fig. 4C). Furthermore, loss of Shot prevents Patronin from associating with the fusome (Fig. 4C, S5B-C). Thus, Shot is required to recruit Patronin to the fusome, thereby transmitting fusome asymmetry to Patronin localization.
The MT-dependent enrichment of Patronin in one cell as the cyst moves through the germarium suggests its initial, weakly asymmetric distribution on the fusome is then amplified by Dynein-dependent transport towards the minus ends of the MT that have been stabilized by Patronin. We tested Dynein function by examining components of the Dynein/dynactin complex that are required for oocyte specification: egl, BicD and Arp1 (22–24), (Fig. 4D, S6A-6B). Like MT depolymerization, mutations in any of these genes disrupt the enrichment of Patronin foci in one cell. Deletion of the MT minus end-binding domain of Patronin, but not the CKK domain (25), also prevents Patronin accumulation in the pro-oocyte (Fig. S6C-D). Thus, Patronin localization depends on its binding to MT minus ends and on Dynein activity, suggesting that Dynein transports Patronin bound to MT minus ends towards the pro-oocyte.
Our observations lead us to propose a 4-step model of cyst polarization and oocyte selection (Fig. 4E). First, during cyst formation, the asymmetric segregation of the fusome leads to the one cell with more fusome material than the rest. Second, in region 2a, Patronin is recruited to the fusome by Shot. The cell with most fusome therefore contains more Patronin, leading to the stabilization of more MT minus ends in this cell and a weakly polarized MT network. Third, Patronin bound MTs in other cells of the cyst are then transported by Dynein along these MTs towards their minus ends in the pro-oocyte. Fourth, this creates a positive feedback loop: as Dynein transports more Patronin and MTs into the cell with most stabilized MT minus ends, more minus ends become stabilized in this cell, amplifying the MT polarity and leading to enhanced Dynein transport of oocyte determinants into this cell. In this way, the small original asymmetry in the fusome is converted into the highly polarized MT network that concentrates the oocyte determinants in one cell.
Patronin is a member of the conserved CAMSAP family, raising the possibility that the molecular mechanisms of oocyte selection in Drosophila could be conserved during the formation of mammalian oocytes. Although fusomes have not been observed in mammalian cysts (26), MT-dependent transport of organelles through intercellular bridges has been shown to play an important role in oocyte differentiation in mice (3).
Supplementary Material
One-Sentence Summary.
Patronin and Dynein form a positive feedback loop that amplifies a weak fusome asymmetry to specify the Drosophila oocyte.
Acknowledgments
We are grateful to R. Hawley, J. Raff, J. Scholey and the Bloomington Stock Center (NIH P40OD018537) for flies and reagents, the Gurdon Institute Imaging Facility for assistance with microscopy, N. Lowe and J. Overton for technical assistance.
Funding
Wellcome Principal Research Fellowship 080007 and 207496 (DSJ), Wellcome core support 092096 and 203144 (DSJ), Wellcome PhD studentship 109145 (MJ), Cancer Research UK core support A14492 and A24823 (DSJ), BBSRC grant BB/R001618/1 (DSJ, DN, IS).
Footnotes
Author contributions: Conceptualization: DN, DSJ; Methodology: DN, MJ. Investigation: DN, LB, MJ, IS, DSJ. Visualization: DN, MJ, DSJ. Funding acquisition: DN, DSJ. Project administration: DN, DSJ. Supervision: DN, DSJ. Writing – original draft: DN, DSJ. Writing – review & editing: DN, LB, MJ, IS, DSJ.
Competing interests: Authors declare that they have no competing interests.
Data and materials availability
All data are available in the main text or the supplementary materials.
References and Notes
- 1.Lu K, Jensen L, Lei L, Yamashita YM. Stay Connected: A Germ Cell Strategy. Trends Genet. 2017;33:971–978. doi: 10.1016/j.tig.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei L, Spradling AC. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 2013;140:2075–2081. doi: 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016;352:95–99. doi: 10.1126/science.aad2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Cuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–28. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- 5.Huynh J, StJohnston D. The Origin of Asymmetry: Early Polarisation of the Drosophila Germline Cyst and Oocyte. Current Biology. 2004;14:R438–R449. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Theurkauf WE, Alberts BM, Jan Y-N, Jongens TA. A central role for microtubules in the differentiation of Drosophila oocytes. Development. 1993;118:1169–1180. doi: 10.1242/dev.118.4.1169. [DOI] [PubMed] [Google Scholar]
- 7.McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- 8.Bolívar J, et al. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development. 2001;128:1889–1897. doi: 10.1242/dev.128.10.1889. [DOI] [PubMed] [Google Scholar]
- 9.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Baines AJ, et al. The CKK Domain (DUF1781) Binds Microtubules and Defines the CAMSAP/ssp4 Family of Animal Proteins. Molecular Biology and Evolution. 2009;26:2005–2014. doi: 10.1093/molbev/msp115. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin SS, Vale RD. Patronin Regulates the Microtubule Network by Protecting Microtubule Minus Ends. Cell. 2010;143:263–274. doi: 10.1016/j.cell.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka N, Meng W, Nagae S, Takeichi M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc Natl Acad Sci USA. 2012;109:20029–20034. doi: 10.1073/pnas.1218017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nashchekin D, Fernandes AR, Johnston D., St Patronin/Shot Cortical Foci Assemble the Noncentrosomal Microtubule Array that Specifies the Drosophila Anterior-Posterior Axis. Dev Cell. 2016;38:61–72. doi: 10.1016/j.devcel.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantz V, Chang JS, Horabin JI, Bopp D, Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev. 1994;8:598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- 15.Mahowald AP, Strassheim JM. Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. The Journal of Cell Biology. 1970;45:306–320. doi: 10.1083/jcb.45.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- 17.Roper K, Brown NH. A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr Biol. 2004;14:99–110. [PubMed] [Google Scholar]
- 18.Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 19.De Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- 20.Jiang K, et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014;28:295–309. doi: 10.1016/j.devcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Developmental Genetics. 1995;16:6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- 22.Navarro C, Puthalakath H, Adams JM, Strasser A, Lehmann R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat Cell Biol. 2004;6:427–435. doi: 10.1038/ncb1122. [DOI] [PubMed] [Google Scholar]
- 23.Suter B, Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991;67:917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwburg R, et al. Localised dynactin protects growing microtubules to deliver oskar mRNA to the posterior cortex of the Drosophila oocyte. Elife. 2017;6:e27237. doi: 10.7554/eLife.27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendershott MC, Vale RD. Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proceedings of the National Academy of Sciences. 2014;111:5860–5865. doi: 10.1073/pnas.1404133111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 27.Roper K, Brown NH. Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. Journal of Cell Biology. 2003;162:1305–1315. doi: 10.1083/jcb.200307089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ran B, Bopp R, Suter B. Null alleles reveal novel requirements for Bic-D during Drosophila oogenesis and zygotic development. Development. 1994;120:1233–1242. doi: 10.1242/dev.120.5.1233. [DOI] [PubMed] [Google Scholar]
- 29.Thibault ST, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nature Genetics. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 30.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Port F, Chen H-M, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proceedings of the National Academy of Sciences. 2014;111:E2967–76. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe N, et al. Analysis of the expression patterns, subcellular localisations and interaction partners of Drosophila proteins using a pigP protein trap library. Development. 2014;141:3994–4005. doi: 10.1242/dev.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gloor GB, Nassif NA, Johnson-Schlitz DM, Preston CR, Engels WR. Targeted gene replacement in Drosophila via P element-induced gap repair. Science. 1991;253:1110–1117. doi: 10.1126/science.1653452. [DOI] [PubMed] [Google Scholar]
- 34.Conduit PT, Wainman A, Novak ZA, Weil TT, Raff JW. Re-examining the role of Drosophila Sas-4 in centrosome assembly using two-colour-3D-SIM FRAP. Elife. 2015;4 doi: 10.7554/eLife.08483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao T, Graham OS, Raposo A, Johnston D., St Growing microtubules push the oocyte nucleus to polarize the Drosophila dorsal-ventral axis. Science. 2012;336:999–1003. doi: 10.1126/science.1219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Brust-Mascher I, Civelekoglu-Scholey G, Scholey JM. Patronin mediates a switch from kinesin-13-dependent poleward flux to anaphase B spindle elongation. The Journal of Cell Biology. 2013;4:1343. doi: 10.1083/jcb.201306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou TB, Perrimon N. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics. 1992;131:643–653. doi: 10.1093/genetics/131.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.