Abstract
Since Hamilton published his seminal papers in 1964, our understanding of the importance of cooperation for life on earth has evolved beyond recognition. Early research was focused on altruism in the social insects, where the problem of cooperation was easy to see. In more recent years, research into cooperation has expanded across the entire tree of life, and has been revolutionised by advances in genetic, microbiological, and analytical techniques. We highlight ten insights that have arisen from these advances, which have illuminated generalisations across different taxa, making the world simpler to explain. Furthermore, progress in these areas has opened up numerous new problems to solve, suggesting exciting directions for future research.
Introduction
Complex life is built by cooperation. Genes cooperate to produce organisms, cells cooperate to produce multicellular organisms, and multicellular animals cooperate to form complex social groups1. This cooperation poses an evolutionary problem. Cooperation provides a benefit to other individuals, and so, all else being equal, should reduce the relative fitness of individuals that cooperate2.
From a theoretical perspective, the evolutionary problem of “why cooperate?” has long been solved3–5. Inclusive fitness theory showed how cooperation can be favoured when it provides either a direct benefit to the individual performing the cooperation, or an indirect benefit to their relatives2,6 (kin selection; Box 1).
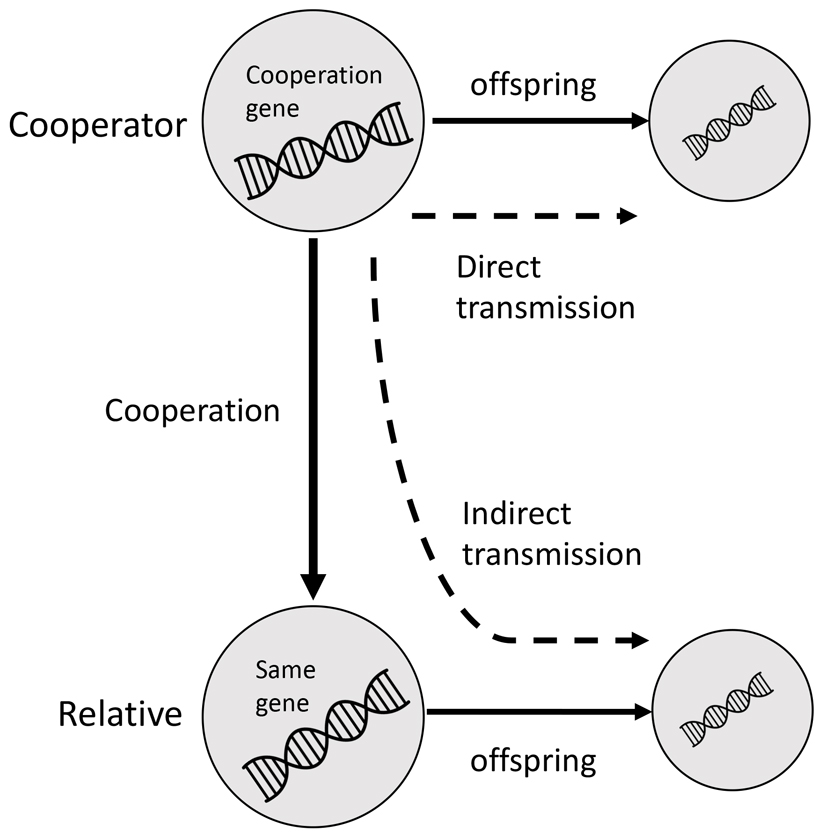
Box 1. Inclusive fitness and why cooperate?
A behaviour or trait is cooperative if it provides a benefit for another individual, and has evolved at least partially because of this benefit. Inclusive fitness theory provides two broad categories of theoretical explanation for cooperation: direct fitness benefits and indirect fitness benefits (kin selection) 3–5.
Box 1 Figure. Direct benefits and kin selection.
A gene can influence its transmission to the next generation by influencing the reproductive success of the individual carrier (direct), or by influencing the reproductive success of other individuals which carry copies (indirect). Hamilton’s rule provides an elegantly simple way of formalising this2.
Direct fitness benefits arise when cooperation increases the reproductive success of the actor that performs the cooperation 6,142. In such a case, cooperation is ‘mutually beneficial’, benefiting both the actor and the recipient 17. Direct benefits could arise as a simple consequence of cooperation. For example, the fitness of a symbiont could depend upon host reproduction, favouring cooperation with its host. Alternatively, direct benefits may depend on a mechanisms that enforce cooperation, such as rewarding cooperators, or punishing non-cooperators.
Indirect benefits arise when cooperation is directed towards other individuals that carry the gene for cooperation 2. This is usually termed ‘kin selection’ because the simplest and most common way this could occur is if cooperation is directed at relatives that share genes from a common ancestor. Genes don’t care where copies in future generations come from – so copies in the offspring of relatives are equally valuable as copies in direct descendants. Indirect benefits provide the only possible explanation for altruism: cooperative behaviours that are costly to the actor and beneficial to the recipient.
These explanations for cooperation are encapsulated in a simple way by Hamilton’s rule, which states that a behaviour or trait will be favoured by selection when RB-C>0, where C is the fitness cost to the actor in terms of number of offspring, B is the fitness benefit to the recipient, and R is the genetic relatedness of the recipient to the actor 2,184. Indirect fitness benefits can explain cooperation when the benefits to the recipient, weighted by relatedness (BR), outweigh the costs to the actor (C). Direct benefits can explain cooperation when the actor gains a direct benefit (C is negative).
The advantage of applying inclusive fitness to understand adaptations such as cooperation is that it has three complementary properties185. First, it provides a method for calculating the direction and potential endpoints of evolution2,186. Second, it provides a property which we expect natural selection to evolve towards maximising2,187. This provides a clear and simple link from evolutionary gene dynamics to the behaviour of individuals, and facilitates the identification of conflicts between individuals188. Third, it emphasises the special importance of common ancestry (kinship), because it leads to appreciable relatedness across the entire genome180,185. This unites the interest of genes across the genome, allowing complex multi-gene social adaptations to be constructed.
The major outstanding problem is not to explain “why cooperate?” at an abstract level, but rather to explain the distribution and diversity of cooperation across the natural world. Research has started to address this problem by asking more precise or nuanced questions. For example, how can we explain why only some species cooperate, the amount that particular individuals cooperate, or sex differences in cooperation (Box 2)?
Box 2. New questions and future directions.
Research on cooperation has advanced by asking more precise or nuanced questions about the distribution and form that cooperation takes in the natural world. For example:
Why does cooperation occur in some species, but not others?
Why does the effort or resources put into cooperation vary across species? For example, in birds, the amount of food provided by helpers varies across species1 from 20-160% the rate at which parent feed261.
Within a species or group, why do some individuals cooperate, but not others? Considering individuals that cooperate, why does the effort put into cooperation vary? For example, within groups of meerkats, different helpers give away between 0-58% of their food to pups77?
Which sex cooperates? Across different species, both the likelihood of helping and the effort put into helping varies between the sexes74,139.
Why do individuals preferentially direct help towards relatives (kin discrimination) in some species, such as long-tailed tits, but not others, such as superb fairy-wrens55?
Should cooperating individuals specialise to perform different tasks (division of labour)? For example, in the bacteria Bacillus subtillis, only a fraction of cells produce and release proteases to break down proteins189.
These questions have been addressed at all levels, from genes to individuals, to species and across the entire tree of life, from bacteria to birds, and in viruses. By examining the same questions at different levels, this research has illuminated several repeating or consistent patterns. Nonetheless, we have only just begun to explain the distribution and diversity of cooperation in the natural world. There are many outstanding problems and opportunities – here we provide a few examples, that follow from the insights we have discussed.
Kin Selection and Kin Discrimination
-
-
Relatedness is determined by the mechanism of group formation. What determines how groups form and why?
-
-
The importance of kin discrimination remains to be tested in variety of organisms, especially microorganisms.
-
-
Why haven’t advances in genetics led to the discovery of more genetic cues for kin discrimination?
-
-
Does Crozier’s paradox190 explain why genetic kin discrimination appears to be rare in animals?
-
-
To what extent do horizontal gene transfer and greenbeards interactions alter selection for social traits?
Costs and Benefits
-
-
Can we quantify, across species, how ecological variation leads to variation in the benefit (B) or cost (C) of cooperation?
-
-
How broadly can variation in the direct benefit of cooperation explain variation in the level of cooperation, either within or across species?
-
-
Can economic games help explain why cooperation evolved in humans, or do they just reveal proximate cognitive mechanisms (biases)?
-
-
How can we explain variation in the extent to which cooperative species divide labour, and the mechanism used to divide labour?
Conflict and Enforcement
-
-
Why is cheating observed in some systems, but not others?
-
-
Is it reasonable to think of cancer and similar ‘deleterious mutations’ as a form of cheating?
-
-
How often do organisms respond to cheating by ‘going private’? And how do we detect cooperation in the evolutionary history of a “privatised” trait?
-
-
Why are enforcement mechanisms, such as ‘sanctions’, important in some species, but not others?
-
-
To what extent do holobiome and microbiome interactions represent between species mutualisms, that can be explained with existing theory191,192?
-
-
Can cooperation and conflict help explain the evolution of brain size123? If so, do invertebrates and vertebrates differ in fundamental ways?
-
-
Why is cooperation lost from species and what are the consequences92,193?
-
-
What are the factors that led major evolutionary transitions, by effectively eliminating conflict and dividing labour to the point of mutual dependence (interdependence) 1,194,195
New Methods and New Applications
-
-
How can genetic and -omic methodologies be harnessed to help us understand the distribution of cooperation?
-
-
Can the phenotypic gambit survive progress in genomics?
-
-
What can be learnt from using genetic manipulations to follow the behaviour of individuals?
-
-
Can population genetic studies help us explain the factors driving selection for cooperation in natural populations?
-
-
How can we exploit novel opportunities for both experimental and comparative studies of cooperation offered by taxa such as bacteria and viruses?
Methodological and analytical advances have revolutionised how we study cooperation. Genetic manipulations have been used to identify and manipulate cooperation in bacteria and other microorganisms7. Genomic analyses have elucidated mechanisms of cooperation, identified new forms of cooperation, and revealed social dynamics in natural populations8–10. Analytical advances have allowed across species comparative studies to better investigate evolutionary causality, and phylogenetically based meta-analyses11–13.
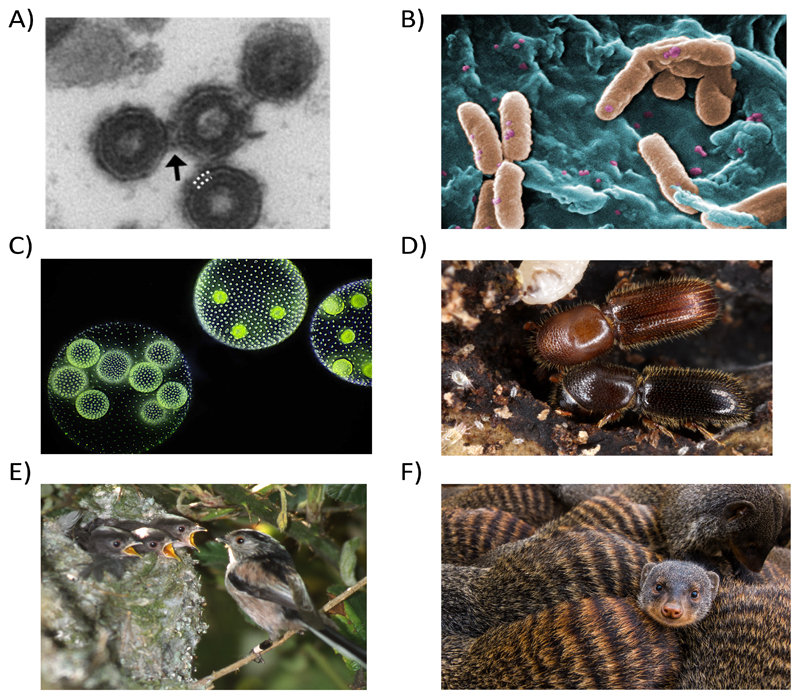
Taxonomically, the study of cooperation has expanded across the tree of life (Fig. 1). Major insights have arisen from previously neglected groups of animals, such as termites, beetles and shrimps14–17 Research on cooperation in microorganisms has exploded, following the demonstration of cooperation in slime moulds, bacteria and viruses7,14–16. This contrasts with the situation only 15 years ago, when the suggestion of cooperation in bacteria could cause a microbiologist to spit out their drink.
Figure 1. Cooperation across the tree of life.
Examples of cooperation include: (a) Vesicular Stomatitus Virus aggregates into multiple virus co-infection units (arrows) and produce proteins that help other viruses overcome host defences62,216 (Photo: Rafael Sanjuan); (b) Pseudomonas aeruginosa bacteria produce and secrete iron-scavenging siderophores that benefit neighbouring cells91 (Photo: CDC/Janice Haney Carr); (c) Volvox carteri algae form multicellular groups where some sterile helper cells beat their flagella to keep the colony afloat217 (Photo: Wikimedia Commons); (d) Xyleborinus saxesenii ambrosia beetles have sterile helpers218 (Photo: Gernot Kunz & Peter Biedermann); (e) long tailed tits are cooperative breeders, where individuals preferentially help closer relatives55 Photo: Andrew MacColl; (f) banded mongooses form cooperative groups, where the dominant will preferentially evict closer relatives58 (negative kin discrimination; Photo: Andy Young). Studies on cooperation in these species have used a mixture of methodologies: observational, experimental, comparative across-species, experimental evolution and genomic.
Our aim is to examine the impact of these recent advances in how we study cooperation. What biological insights have they provided? In addition, while we clearly know more about particular species, can we also identify big picture generalities?
Ten Recent Insights
We focus on identifying the major insights over approximately the last 15 years of research on cooperation. We focus on this time period because there has been a revolution in how we study cooperation. Inclusive fitness has provided a unifying framework for studying all forms of cooperation across the tree of life (Box 1). Methodological advances have allowed us to study cooperation in species and ways where this was not previously possible. The advantage of examining cooperation, with a single framework, across the entire tree of life, is that it we can move from anecdotes of what happens in particular species, to identifying general patterns. Progress prior to the last 15 years is reviewed elsewhere1,3–5,17–19
Insights 1-7
Our first seven insights are about the factors that favour cooperation, and how organisms respond to their variation.
1. Relatedness: from anecdotes to broad generalisations
Inclusive fitness theory shows how cooperation can be favoured by kin selection if it is directed towards relatives, who carry the gene for cooperation (relatives) 2 (Box 1). But how widespread is the importance of kin selection? And if it is important, how do we explain variation between individuals in the level of cooperation, or explain why cooperation is favoured in some species and not others?
Recent research has shown that the relatedness (R) between interacting individuals has a clear and consistent influence on the evolution of cooperation, with both theory and data suggesting that the same factors play analogous roles at all levels of biology, from simple replicators and viruses, to complex animal groups (see below; Supplementary Tables 1 & 2). This role of relatedness has been demonstrated with a combination of methodologies, including observational, experimental, experimental evolution, across-species comparisons, and genomic.
1a. Group formation
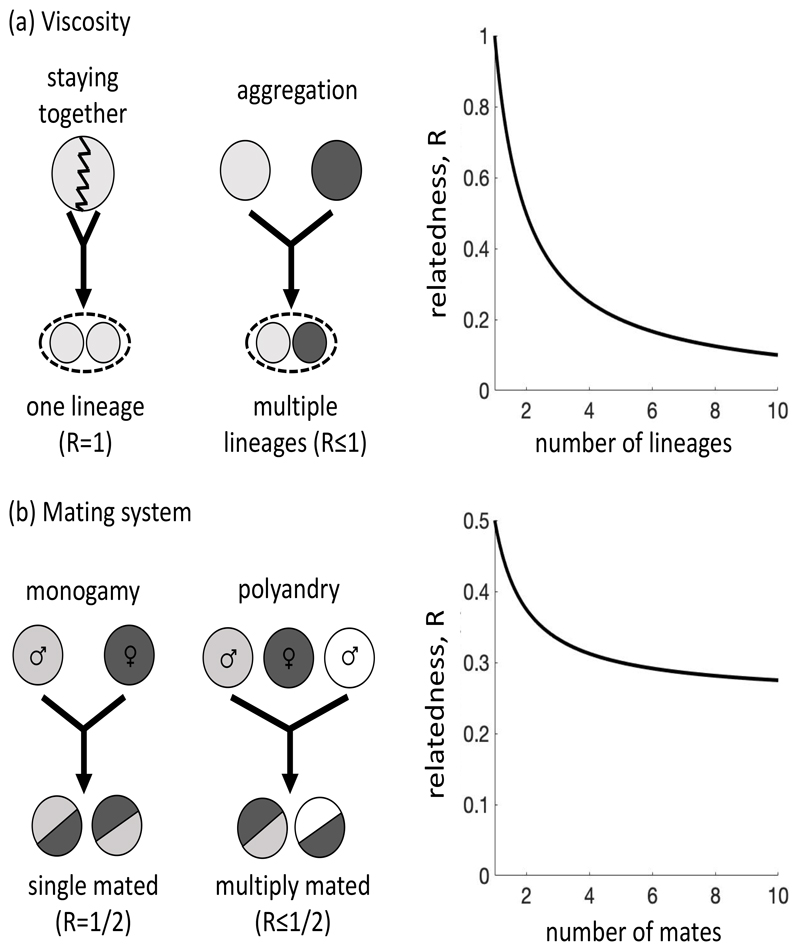
Consistent with kin selection favouring cooperation, there is considerable evidence that population structure and how groups form is a major determinant of whether cooperation is favoured2 (Fig. 2).
Figure 2. Group formation and relatedness.
The method of group formation determines relatedness within groups, and so influences whether cooperation is favoured. (a) Population viscosity: when offspring stay with their parent (staying together, in a family group) this leads to higher relatedness than when individuals aggregate together post-dispersal. Specifically, if a group is formed by n unrelated lineages, then the relatedness within that group will be R=1/n. An asexual species is shown, but the same pattern occurs in sexual species. (b) Mating system: when females mate with a single male (monogamy) this leads to a higher relatedness between her offspring (full-siblings), compared with when females mate with multiple males (polyandry; half-siblings). Specifically, if a diploid female mates with m males, then the average relatedness between her offspring will be R = 0.25 + 0.25/m. An analogous pattern applies in haplodiploids, where a female is related to her sisters by R = 0.25 + 0.5/m219.
-
(i)
Individuals are more cooperative in species where social groups form by staying together, compared with species where social groups form by aggregation, across a range of taxa, including bacteria, fungi, slime moulds, insects, and birds19–23.
-
(ii)
Individuals are more cooperative in species where females mate monogamously or with few males, in birds, mammals, insects and shrimps13,19,21,24–26.
-
(iii)
When population structure is manipulated experimentally, in a range of microorganisms, including bacteria, fungi and viruses, the conditions which lead to high relatedness favour greater cooperation15,27–32.
-
(iv)
The role of group formation has also been supported by sequence data. If a trait is favoured by kin selection, then lower relatedness will lead to weaker selection for that trait, and so the removal of deleterious mutations will be slower and less likely33–36. Consistent with kin selection playing an important role: (a) ant and bee species with either multiple mating or multiple queens, and hence reduced relatedness within family groups, showed greater polymorphism in genes upregulated in the worker caste (kin selected) compared with genes upregulated in the reproductive caste (directly selected) 35,37–39; (b) cooperative traits in bacteria and slime moulds show enhanced polymorphism10,34,40,41.
A number of other factors can help maintain high relatedness between interacting individuals. For example, in bacteria, clonal growth and limited movement will mean that clone mates will often be clustered42, and viscous mediums, biofilms and fibres can keep seccreted public goods locally, to be shared with clone mates43–46.
1b. Kin discrimination
Inclusive fitness theory predicts that individuals can be favoured to preferentially seek out closer relatives to cooperate with, and / or cooperate at higher levels when interacting with closer relatives. There is considerable evidence such kin discrimination across a range of organisms, including slime moulds, ants, mice and birds47–54. In some cases, kin discrimination is based on genetic cues, with individuals adjusting cooperation depending upon whether they match genotype at a ‘tag’ loci49. In other cases, environmental cues are used to recognise kin. For example, long-tailed tits appear to assess kinship on the basis of both vocal similarity and prior association55.
Inclusive fitness theory can also explain when kin discrimination is not observed. Across cooperative breeding vertebrates, the extent of kin discrimination is lower in species where the mean relatedness in groups is higher and shows less variation56. This pattern reflects that kin discrimination is not required when individuals tend to be highly related to all group members anyway57.
Kin selection theory has even explained when closer relatives are preferentially discriminated against (negative kin discrimination). In banded mongooses, dominant individuals are more likely to harass and evict subordinates in the group when they are closer relatives58. Resisting eviction is costly to the dominant attempting the eviction, and individuals are more likely to accept eviction when they are closer relatives of the dominant. Consequently, there is less to gain by trying to evict non-relatives, who will resist this eviction, and so dominants are favoured to preferentially evict closer relatives58. In addition, dispersal provides a kin selected benefit by reducing competition for those relatives left behind – this benefit will be greater for closer relatives59.
1c. Quantifying Hamilton’s rule
The above studies are qualitative, examining whether a higher relatedness is associated with greater cooperation. Other studies have used long-term field data, and detailed experiments, to quantify the parameters of Hamilton’s rule60 (Box 1). These studies have found that kin selection can completely explain cooperation in species ranging from long-tailed tits to vesicular stomatitis virus61,62.
2. Ecological determinants of cooperation can be hard to identify
While we have a good understanding of the cost (C) and benefit (B) of cooperation in particular species, we often lack broad ecological explanations for how the benefits of cooperation vary across species63. What ecological factors cause B/C to vary across species, and hence determine when cooperation is favoured? This contrasts with the numerous clear examples of how variation in relatedness (R) can explain which species cooperate and which do not (Insight 1).
In some cases, the role of ecological factors has even become less clear. In birds, cooperative breeding has long been known to be more common in harsh environments, where temperatures are hot and rainfall is unpredictable64–66. This led to the hypothesis that harsh environments favour cooperation, because they increased the relative benefit of cooperating (higher B/C). However, this pattern appears to be a consequence, not a driver of cooperation – the correlation arises because cooperative species are better able to colonise harsh environments, rather than cooperation being favoured in harsh environments 13. Other recent comparative analyses that raise questions about the direction of causality include studies of rodents and sponge shrimp63,67.
A possible complication is that the factors influencing the benefit (B) and cost (C) of cooperation vary across species, making it hard to quantify their influence in a comparable way. For example, in different species, the advantage of cooperation could depend upon breeding site availability, climate, competition with other species, or food availability68.
Another possibility is that the importance of variation across species in B and C is dwarfed by the consequences of variation in R. Relatedness shows considerable variation across species and so can have a large influence on whether cooperation is favoured (whether RB-C>0). In contrast, maybe cooperation usually provides some efficiency benefit, such that B/C is usually >1 and just doesn’t vary much across species. If R varies a lot more than B/C, then it will be much easier to detect an influence of R than B/C. We are not saying that relatedness is the only variable that matters, just that if it varies more, we should expect its influence to be easier to detect. The influence of B and C could perhaps be more easily detected by examining interactions with R – for example, not all monogamous species are cooperative 21,69,70.
3. Direct and indirect benefits disentangled
A number of animal studies have shown that the direct benefits of cooperation can still be substantial, even in species where helping appears to be primarily explained by kin selection. For example, in Polistes wasps, although cooperation appears to usually be explained by kin selection, some individuals help at nests where they are not related to the dominant female71. This appears to be because subordinate helpers can gain significant direct benefits from helping, by inheriting a nest after the death of the dominant72,73.
In birds, the sex that remains, and therefore has a chance of inheriting the nest, helps at higher levels than the sex that disperses, and so is less likely to gain a direct benefit from helping 74. A role of direct fitness benefits in birds is further supported by the observation that longer subordinate lifespans, which can increase the chance of inheriting a patch, have favoured the evolution of cooperative breeding, but especially in species where rates of polyandry are high, and so kin selection will be weaker 75. It is important to disentangle direct and indirect benefits of cooperation, to clarify the relevant theoretical predictions, and because evidence for one does not negate the other.
4. Microbes have sophisticated conditional behaviours
Studies on a range of animal species have shown how individuals adjust their level of cooperation in response to changes in the cost (C) and benefit (B) of cooperation48,76–79. Recent research has shown that microorganisms have similarly sophisticated conditional behaviours.
The costs and benefits of cooperating can depend upon the environment. A common form of cooperation in microorganisms is the production of some factor that is excreted from cells, and which then provides some ‘public good’ benefit to the local group of cells – for example, iron scavenging molecules, or an enzyme to digest protein15,27. In the bacteria Pseudomonas aeruginosa, a higher cell density allows cells to more easily share the benefit of producing public goods80. Cells use a form of signalling termed quorum sensing to upregulate the production of public goods at high density, and hence increase the level of cooperation when it provides greater benefits27. The increased benefits of cooperating at high-density can also select for the formation of multicellular cooperative groups, as appears to occur in yeast81,82.
The cost of producing public goods can depend upon resource availability83,84. P. aeruginosa increases the production of one factor, rhamnolipid biosurfactant, which aids movement, when there is more carbon available, making that rhamnolipid less costly to produce85.
Group formation can provide defence against predators, or aid in predation. By aggregating together into groups, algae make it harder for predators to eat them86. Clumping is costly in the absence of predators because competition for resources is increased; facultative clumping is favoured, therefore, where the behaviour is only triggered when predators are detected86. More generally, obligate multicellularity has only evolved when groups form clonally, with dividing cells sticking together22. Experimental studies on algae have shown that the mechanism of group formation is heritable and can evolve by selection87.
5. The invisible dynamics of cheating have been revealed
Scratch the surface of a cooperative society, and you will usually find conflict bubbling underneath. An extreme form of conflict is ‘cheating’ – where individuals that avoid the costs of cooperating can still gain the benefits of others cooperating88. Recent work has allowed the detection of the previously invisible dynamics of cheating across a diversity of organisms. In particular, genetic studies in microbes – slime moulds and bacteria - have allowed us to examine the occurrence of cheating, and the form that it takes, in natural populations10,40,41,89–92.
5a. Persistence of cheats
Theory suggests that restrictive conditions can be required for cheats to coexist with cooperators. When originally developing inclusive fitness theory, Hamilton2 found that the relative fitness of cooperators does not depend upon how common they are in a population. This lack of frequency dependence means that either cooperation is favoured and so cheats would be eliminated, or cooperation is not favoured, and so you couldn’t observe cheats exploiting cooperators. Given this result, which suggests that cheating will not be evolutionarily stable, how can we explain observations of cheating?
More recent theory has shown that a number of factors can lead to cheats having a higher fitness when rare (frequency dependence), allowing cheats and co-operators to coexist. For example, when cooperation provides some direct benefit, or when the benefits of cooperation are diminishing rather than increasing linearly93–95. These factors have been shown to explain coexistence of cheats and cooperators in bacteria and yeast94–96. Frequency dependence also requires non-additivity, where genes cause large variation in the level of cooperation (strong selection). In bacteria, viruses and other microorganisms, single ‘knock out’ mutations can eliminate cooperative behaviours, causing large variation in cooperation, which could help provide an explanation for why cheats are more commonly observed in these taxa95,97.
An alternative explanation for the observation of cheating is that cheats are maintained in a population at mutation-selection balance98,99. Mutation causes the loss of cooperation, and then this gives the appearance of cheating as those non-cooperators are selected out of the population. In this case, not cooperating would represent a deleterious mutation rather than a cheating adaptation. However, estimates from bacteria suggest that mutation-selection balance would lead to one non-cooperator in every 10,000-100,000 individuals, which is much lower than has been observed empirically98. Consequently, the observation of non-cooperators is more likely to be explained by frequency dependent cheating.
5b. Resistance to cheats
When cheats are maintained in a population, cooperators will be selected to avoid exploitation, or reduce the extent to which they can be exploited. An example of such a strategy has been observed in laboratory selection experiments on bacteria, and could also explain why many extracellular public goods and signalling molecules released by bacteria come in different types (specificity) 100–104 Another example can be seen in flowering plants, which reduce exploitation by nectar robbing cheats by restricting or altering the time of day at which they provide nectar rewards for pollinators105.
An even more extreme response to cheating has been observed in the bacteria P. aeruginosa, where individuals stopped cooperating and ‘went private’. In infections of the lung, in humans with cystic fibrosis, the invasion of cheats that did not produce iron scavenging siderophore molecules (a public good), led to the complete loss of siderophore production91. This was then followed by strains upregulating an alternative ‘private’ mechanism of acquiring iron, which is not cooperative and so cannot be exploited106.
6. Genetic architecture matters, sometimes
Most research on cooperation ignores the underlying genetics and assumes that all phenotypes are theoretically possible (phenotypic gambit107). Detailed characterisation of the genetics underlying some cooperative traits has helped us to understand where and why genetics can matter.
6a. Supergenes
Research on the fire ant, Solenopsis invicta, has shown how genetic architecture is key to the maintenance of different strategies within populations108. In this species, colonies form with either a single or multiple queens. The difference is driven by a supergene – a large inversion, covering 55% of an entire choromosome, with suppressed recombination. This supergene also controls traits where the optimum depends upon the number of queens, such as queen size and fecundity. Consequently, the supergene allows life history traits to be adaptively adjusted (linked) depending upon the social strategy (single or multiple queens). Supergenes appear to control social organisation in a number of ant species 109,110.
6b. Genetic red herrings
A range of genetic discoveries in bacteria and slime moulds have led to suggestions of novel mechanisms to explain cooperation. These include the discovery of pleiotropic links between cooperation and private traits, the regulation of cooperation by quorum sensing, and hitch hiking with advantageous mutations (Table 1).
Table 1. Maybe not explaining cooperation.
Discoveries about the genetic architecture and mechanisms underlying cooperation in microorganisms have led to a number of novel explanations for cooperation being suggested. However, there is a lack of evidence that these mechanisms provide explanations for cooperation. In some cases, the suggested explanations arise from over-interpretation of short-term evolutionary dynamics, and / or from implicitly assuming that the genetic architecture cannot evolve (mixing proximate and ultimate explanations of behaviour). Cooperation is usually more parsimoniously explained by standard explanations, such as kin selection.
| Suggested Explanation for Cooperation | Potential problems |
|---|---|
| A pleiotropic link between cooperation and a trait with a large personal (private) benefit (metabolic constraint / coregulation)196–202. The idea here is that if cooperation is lost, then the directly beneficial trait would also be lost, and that the direct benefit could outweigh the cost of cooperation. Similar arguments have been made for ‘hormonal pleiotropy’ in social insects203. | Pleiotropic links can favour any trait, and not preferentially cooperation. If the genetic architecture is not fixed, individuals could evolve to perform the directly beneficial trait, and not cooperate. The role of kin selection had already been established in these species, and so explaining cooperation was not a problem204. Causality can also go in both directions – in cases where some cooperation is favoured, pleiotropy can be selected to reduce cheat build up. |
| The down-regulation of cooperation at certain times (metabolic prudence or facultative cooperation)202,205. | Individuals will be selected to reduce their level of cooperation when the benefit is lower or cost is greater (Insight 4). However, this is not an answer to ‘why cooperate?’ - a mechanism such as kin selection is still required to explain when cooperation is carried out. The role of kin selection had already been established, and so explaining cooperation was not a problem. |
| The regulation of cooperation by quorum sensing in bacteria202. | Quorum sensing restricts cooperation to times when it appears to be more cost effective. An explanation is still required for why cooperate at those times. The role of kin selection had already been established, and so explaining cooperation was not a problem. |
| Genes for cooperation could hitch hike with mutations that are advantageous in a new environment (‘adaptive race’)206,207. | This hypothesis doesn’t favour cooperation per se, as cheats could also hitch hike207. Furthermore, any effects are only transient, as competition between cheats and cooperators can still take place within populations of locally adapted genomes. |
| Cooperation is favoured at greenbeard genes, which can identify copies of themselves in other individuals, and preferentially help those individuals208,209. | There are genes that have the properties of greenbeards. However, it is not clear if it is the greenbeard effect per se (linkage) that is favouring cooperation210,211. Cooperation may have been favoured anyway, due to kin selection, with the greenbeard affect just a mechanistic byproduct. |
| Horizontal gene transfer can increase relatedness for cooperative genes by ‘reinfecting cheats’212–214. | If there are cheat plasmids, that don’t cooperate, then horizontal gene transfer doesn’t necessarily favour cooperation215. While there is some evidence from E. coli that cooperative genes are more likely to be found on mobile elements213, there are alternative explanations216. |
However, there are potential problems with all these suggestions (Table 1). In some cases, the hypothesis relies on the assumption that the genetic architecture itself cannot evolve. In other cases, the genetic mechanism provides a way to vary the level of cooperation, but is not an answer to ‘why cooperate’. Furthermore, cooperation can usually be explained by some other mechanism, such as kin selection.
Overall, there is a lack of evidence that genetic architecture determines when cooperation is favoured (Table 1). We are not saying that it is not possible for genetic architecture to help explain cooperation, rather that the mechanisms suggested, so far, lack convincing support, and there are simpler explanations.
7. Humans aren’t special in that way
A number of experimental laboratory studies examining human behaviour in economic games have found that individuals cooperate more than expected from their own selfish interests111. This has led to a widely-held conclusion that humans are especially altruistic, and cooperate in ways that are not easily explained by evolutionary theory112.
This conclusion is based, however, on the assumption that laboratory economic games reflect evolutionary pressures, and that humans play ‘perfectly’. In addition, it has been pointed out that experiments using economic games often lack appropriate controls and null hypotheses113–115. More recent experiments have shown that human behaviour in economic games is more consistent with strategies for more natural settings, and shaped by the challenge of learning the rules of the game in a laboratory setting116,117. Furthermore, many instances of human cooperation are better explained as mutually beneficial cooperation, stabilised by enforcement mechanisms such as reciprocity, rather than altruistic cooperation118–121. What matters for natural selection is the average lifetime consequences of a behaviour in the natural environment, not short-term consequences in an unnatural environment.
In short, humans are not especially altruistic. Cells forming the fruiting body of a slime mould, where 20% of their cells sacrifice themselves as stalk cells, to help disperse others as spores, are more altruistic than a group of humans14. What appears to be special about humans is the extent to which enforcement mechanisms such as reciprocity are used to stabilise cooperation, and how we attempt to evade those mechanisms111,122. Indeed, it has even been suggested that brain size is a response to conflict in cooperative social groups123. Humans also stand out in their ability to learn from others. It is currently a matter of debate whether this potential for ‘cultural evolution’ favours increased or decreased cooperation124–128.
Insights 8 & 9
We are now in a position to start making big picture generalisations about why cooperation is favoured, both within (Insight 8) and between (Insight 9) species.
8. Indirect benefits play the major role in explaining cooperation within species
The relative importance of direct and indirect fitness benefits for explaining cooperation within species has long been debated129. Has recent research allowed us to move forward on this debate?
There has been growing evidence for indirect fitness benefits (kin selection) playing the major role in explaining cooperation within species. Considering animals, such as birds, mammals, and insects, the data suggests that relatedness is a key determinant of which species cooperate, and how much individuals cooperate (Insight 1). Furthermore, long term field studies on species such as long-tailed tits have shown cases where cooperation can be completely explained by kin selection60,61. Considering bacteria and other microbes, clonal growth means that cells will tend to interact with close relatives, and the forms of cooperation that are observed can be explained relatively easily by indirect benefits15,27,43,130.
We also have a clearer idea of the factors that lead to indirect fitness benefits. Population structure and group formation play similar roles in ensuring high relatedness, at all levels of biology (Insight 1; Fig. 2). The early stages in the evolution of life required cooperation between simple replicators, which may also have been favoured due to population structure keeping relatives together131,132. In contrast, while the possible role of haplodiploid genetics has attracted much attention, both theory and empirical data have led to many concluding that it was a red herring133–139.
We are not saying that there are never direct benefits to cooperation, or that enforcement never occurs. Insight 3 provides some elegant examples of how direct fitness benefits can be important for cooperation within species, and enforcement is reviewed elsewhere140. Instead, our point is that statistically speaking, direct fitness benefits seem much less important in explaining the distribution and diversity of within species cooperation. This makes sense, because the potential for indirect benefits can arise easily, whenever population structure keeps relatives together2. In contrast, mechanisms to provide or enforce direct fitness benefits to cooperation can be much harder to evolve initially, and/or require that individuals are already cooperating to some extent141–143. One of the clearest examples of enforcement favouring cooperation is policing in the social insects, which is favoured by indirect fitness benefits, and can be thought of as a form of kin discrimination144.
Direct fitness benefits may be more important in explaining less costly forms of cooperation. Evidence supporting a role for mechanisms such as reciprocity, tend to come from cooperative behaviours that are relatively low cost, such as grooming in primates145. Similarly, there are bird species where unrelated individuals form cooperative groups, but in those species, cooperation tends to be joint incubation or defence of territory, and does not involve giving up the chance to breed23. The fitness consequences of behaviours such as reciprocal grooming or joint territory defence are likely to be marginal relative to the sacrifice of personal reproduction to become a subordinate helper.
9. There are two ways to make a stable mutualism
Both theoretical and empirical work has suggested that there are two ways in which cooperation between different species (mutualisms) tend to be maintained: shared interests and enforcement.
9a. Shared Interests
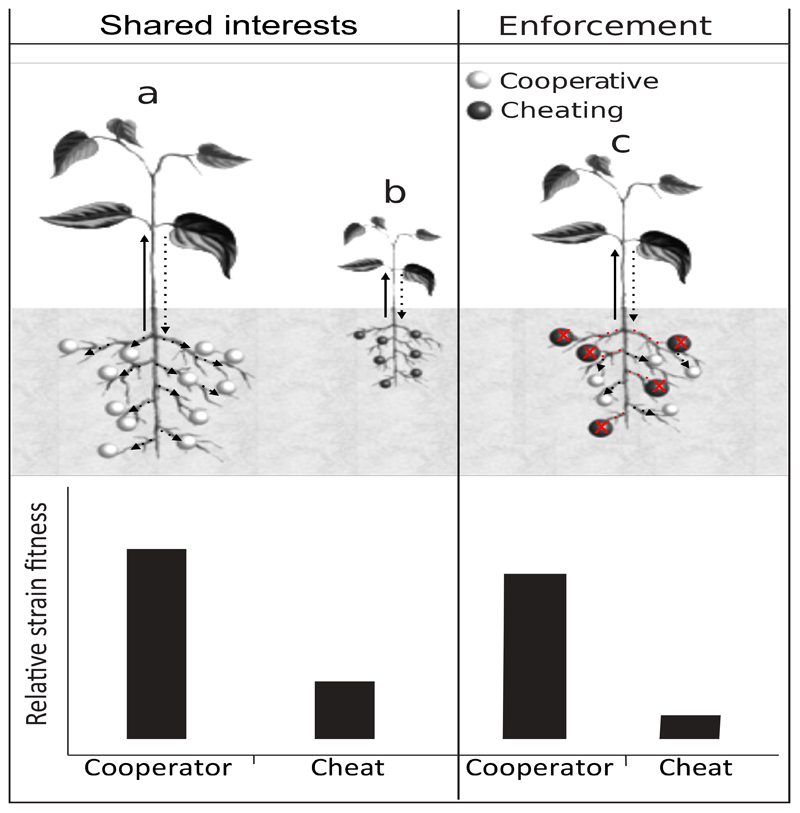
Cooperative mutualisms are favoured when the reproductive interests of different species are so entwined that each species gains from cooperating with the other (shared interests; Fig. 3a) 146. The easiest way for this to occur is when a small number of symbionts are transmitted vertically to each offspring of their host. In this case: (i) the symbionts within a host are highly related, possibly clonal, eliminating most within-host conflict; and (ii) the symbionts benefit from helping the host, because the host could then produce more offspring, who could carry more symbionts147. The hosts, in turn, are selected to help their symbionts, because those symbionts provide them a service. A classic example of this would be the Buchnera bacteria that synthesise amino acids for their aphid hosts148. The role of shared reproductive interests has been supported by a comparative study across species, and experimental studies. Across 38 bacterial symbioses, vertically transmitted symbionts provided an average of twice the fitness benefit to their host, relative to horizontally transmitted symbionts149. Theory suggests that the influence of transmission route is mainly via its influence on relatedness, and hence the level of within host conflict. Analogously, experimental evolution studies on interacting microbial species, have shown that vertical transmission and spatial structuring both favours increased cooperation between species150,151. This role of transmission route, via its influence on symbiont relatedness, is analogous to the role of group formation in insight 1.
Figure 3. Two ways to stable mutualism.
(a) Mutualistic cooperation is favoured when the reproductive interests of different species are so entwined that each species gains from cooperating with the other (shared interests). For example, when vertical transmission leads to a host containing a clonal population of symbionts, any benefits of cooperating with a host would be clone mates. Another way of looking at this is that cooperators (clear nodules) and non-cooperative cheats (filled nodules) would be in different hosts, and so cooperators gain the benefit of being in ‘helped’ hosts, whereas cheats do not. (b) Mutualistic cooperation can also be enforced, by rewarding cooperators (clear nodules) and / or stopping interactions with non-cooperative cheats (filled nodues). Figure inspiration220.
Symbiont transmission route also influences genome evolution. Vertically transmitted symbionts have smaller genomes than horizontally transmitted symbionts149. This is thought to reflect the usefulness of many traits becoming reduced when living entirely within a host, with relaxed selection then allowing inactivation and deletion of these non-essential genes152. The benefit that symbionts provide to their hosts is greater in species with smaller genomes – given that genomes reduce over time, this suggests that longer periods of host-symbiont coevolution lead to greater reliance on symbionts and/or more beneficial symbionts149. Some genes are also moved from symbionts to their hosts153.
9b. Enforcement
The second common explanation for cooperative mutualisms is when cooperation is enforced (Fig. 3b) 140,154. When members of one species interact with multiple partners of the other species, this can lead to conflict146. For example, if a host contains multiple symbiont strains, this will reduce the relatedness between symbionts and hence reduce the indirect fitness benefits gained from helping the host, and therefore helping the other symbionts within the host155. In this case, cooperation can be enforced, by rewarding cooperation and/or punishing non-cooperation (sanctions) 155. Leguminous plants cut off the oxygen supply to rhizobia bacteria that are relatively poor at fixing nitrogen156. Fig trees abort and reduce resource supply to fruits where the pollinator wasps did not pollinate sufficient flowers157,158.
Enforcement can also be bidirectional, such as when mycorrhizal fungi trade phosphorous with plants for carbohydrates159. Resource trading of this kind can lead to more elaborate strategies – fungi appear to move phosphorous across their mycelial network, to locations where phosphorous is more limiting, and so a better trade can be made160. The suppression of genes for selfish distorting behaviour by the “parliament of the genes” is analogous to this between-species enforcement161,162.
From a theoretical perspective, the finding that mutualisms tend to be stabilised by either shared interests or enforcement makes sense. The conditions that lead to conflict, and reduce shared interest, such as interacting with a large number of partners, are exactly the conditions which are expected to favour enforcement 163.
9c. Maintenance versus origin
It is useful to distinguish maintenance of cooperation from its evolutionary origin5. Our discussion in this section has focused on the maintenance of cooperative mutualisms. While enforcement can lead to higher levels of cooperation, it requires some initial level of cooperation to evolve141–143. It is, therefore, likely that mutualisms initially evolve via shared interests, before enforcement evolved to increase the level of cooperation164.
10. Cooperation can be exploited for applied purposes
Cooperation is fundamental to the success and growth of many organisms. Consequently, whenever we want to reduce the growth of damaging organisms, such as parasites, or increase the growth of beneficial organisms, manipulating cooperation could be a useful tool.
10a. Managing parasites and pathogens
Disrupting cooperation has at least three applications for managing parasites and pathogens. First, the level of cooperation has a large influence on parasite growth and virulence, the damage caused to the host. Indeed, traits termed ‘virulence factors’ by microbiologists appear to usually be cooperative (public goods) 165. Consequently, if cooperation can be disrupted, virulence can be reduced. Experiments on the bacteria P. aeruginosa have shown that the addition of noncooperative cheats to an infection reduced the mortality rates of rats by 50%166. The accumulation of defective interfering particles, which represent a form of cheating in viruses, lead to less severe outcomes of viral infections in humans, and is being developed as an antiviral therapy167,168.
Second, as well as disrupting infections, cheats could be used as a ‘Trojan horse’ to introduce medically beneficial alleles into a population, such as antibiotic resistance or antibacterial toxins169. For this, or virulence disruption to work, cheats need only have short-term success, spreading within a host – such therapies can work even when cheats cannot transmit successfully.
Third, it can be harder for parasites to evolve resistance against intervention techniques that disrupt cooperative behaviours, than against treatments such as antibiotics that directly kill individuals170. If cooperation is disrupted, this effectively makes all individuals cheats. Consequently, if there was a ‘resistance’ mutation that switched cooperation back on, the carrier of this mutation would be surrounded by cheats, making it harder to spread. This theory has been supported by an experimental evolution study on Salmonella, where resistance against standard antimicrobials rapidly evolves, but resistance to an inhibitor of cooperative biofilm formation did not evolve171. Nonetheless, we are a long way from convincing clinicians that it is useful to add more parasites (cheats), or that it is better to use interventions that don’t directly kill parasites, to avoid selecting for resistance in those parasites.
10b. Other applications
There are many other areas where we have not yet begun to realise the full potential for exploiting our understanding of cooperation. This includes optimising machine-machine cooperation in AI collectives, such as fleets of autonomous cars or interacting financial trading algorithms, as well as designing AI algorithms so as to minimise the potential for human-machine conflict172,173. The maintenance of cooperation is required for efficient bio-industry applications, including using bacteria to biodegrade pollutants and detoxify, or algae to produce biofuels174,175. Agricultural efficiency in crops or livestock can be increased by ‘social selection’ for increased cooperation or decreased competitiveness, rather than individual yield175,176.
Conclusion
While the inclusive fitness approach has provided the framework for the major insights made in the study of cooperation, it has also attracted controversy, which might suggest a field in disarray to an outside observer176–178. Welch argued that analogous controversy has arisen multiple times for research on adaptation, for reasons that do not reflect problems with the approach179. There is a repeated pattern where approaches such as inclusive fitness theory lead to major advances in our understanding of adaptation, and where the resulting attention generates charges of narrowness and oversimplification. These charges are however, misplaced, because it is the simplifications made by theory which facilitate its application to data107,179,180. In biology, theory is not an abstract enterprise, it is the interface between theory and data that matters. Scientific progress is not shaped by whether ideas are “liked” or “disliked”, but by making parsimonious predictions that hold up time and time again, generating new counterintuitive predictions that achieve additional confirmation. That is the achievement of inclusive fitness theory 181.
The existence of some controversy therefore reflects a productive and healthy field, where spectacular advances are being made. As well as explaining cooperation in particular species, these advances have illuminated broad generalisations, where the same factors appear to play analogous roles across the tree of life. This makes things pleasingly simpler for those of us studying cooperation – rather than a different explanation for every species, we can have a single explanatory framework – that is an amazing achievement! Furthermore, progress in the study of cooperation has demonstrated how the behavioural ecology approach, originally developed to study animals, can be successfully applied to any form of organism, from bacteria to viruses182,183. The recent progress summarised in this paper has opened up a great number of new problems to solve, and it is as exciting a time to be studying cooperation as it has ever been (Box 2).
Supplementary Material
Acknowledgments
We thank: Koos Boomsma, an anonymous referee, and the social evolution journal club for comments; Koos Boomsma for a sentence that was very useful in our conclusion; the ERC (AG, MG & SW) and St John’s College (GC) for funding; Peter Biedermann, Rafael Sanjuan & Andy Young for supplying photos.
Footnotes
Author Contributions
All authors conceived and wrote the manuscript (SAW, GAC, MBG & ASG). GAC & MBG constructed the figures.
Competing interests
The authors declare no competing interests.
References
- 1.Bourke AFG. Principles of Social Evolution. OUP; Oxford: 2011. [Google Scholar]
- 2.Hamilton WD. The genetical evolution of social behaviour. I & II. Journal of Theoretical Biology. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 3.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. The Quarterly Review of Biology. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann L, Keller L. The evolution of cooperation and altruism – a general framework and a classification of models. J Evol Biol. 2006;19:1365–1376. doi: 10.1111/j.1420-9101.2006.01119.x. [DOI] [PubMed] [Google Scholar]
- 5.West SA, Griffin AS, Gardner A. Evolutionary Explanations for Cooperation. Current Biology. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Trivers R. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 7.Velicer GJ, Kroos L, Lenski RE. Developmental cheating in the social bacterium Myxococcus xanthus. Nature. 2000;404:598–601. doi: 10.1038/35007066. [DOI] [PubMed] [Google Scholar]
- 8.McCutcheon JP, Moran NA. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proceedings of the National Academy of Sciences. 2007;104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spribille T, et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science. 2016;353:488–492. doi: 10.1126/science.aaf8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrowski EA, et al. Genomic Signatures of Cooperation and Conflict in the Social Amoeba. Current Biology. 2015;25:1–6. doi: 10.1016/j.cub.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadfield JD, Nakagawa S. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J Evol Biol. 2010;23:494–508. doi: 10.1111/j.1420-9101.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 12.Garamszegi LZ. Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. Springer; 2014. [Google Scholar]
- 13.Cornwallis CK, et al. Cooperation facilitates the colonization of harsh environments. Nat ecol evol. 2017;1:0057-10. doi: 10.1038/s41559-016-0057. [DOI] [PubMed] [Google Scholar]
- 14.Strassmann JE, Zhu Y, Queller DC. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature. 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- 15.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 16.Turner PE, Chao L. Prisoner’s dilemma in an RNA virus. Nature. 1999 doi: 10.1038/18913. [DOI] [PubMed] [Google Scholar]
- 17.West SA, Griffin AS, Gardner A. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol. 2007;20:415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 18.Queller DC, Strassmann JE. Kin selection and social insects. Bioscience. 1998;48:165–175. [Google Scholar]
- 19.Boomsma JJ. Kin Selection versus Sexual Selection: Why the Ends Do Not Meet. Current Biology. 2007;17:R673–R683. doi: 10.1016/j.cub.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Reeve HK, Keller L. Partitioning of Reproduction in Mother-Daughter Versus Sibling Associations - a Test of Optimal Skew Theory. Am Nat. 1995;145:119–132. [Google Scholar]
- 21.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science. 2008;320:1213. doi: 10.1126/science.1156108. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RM, Cornwallis CK, West SA. Group Formation, Relatedness,and the Evolution of Multicellularity. Current Biology. 2013;23:1120–1125. doi: 10.1016/j.cub.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Downing PA, Griffin AS, Cornwallis CK. Group formation and the evolutionary pathway to complex sociality in birds. Nat ecol evol. 2020;215:1–13. doi: 10.1038/s41559-020-1113-x. [DOI] [PubMed] [Google Scholar]
- 24.Promiscuity and the evolutionary transition to complex societies. 2011;466:969–972. doi: 10.1038/nature09335. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JE, Macdonald KS. Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis. Proceedings of the Royal Society B: Biological Sciences. 2009;277:575–584. doi: 10.1098/rspb.2009.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green JP, Hatchwell BJ. Inclusive fitness consequences of dispersal decisions in a cooperatively breeding bird, the long-tailed tit (Aegithalos caudatus) Proceedings of the National Academy of Sciences. 2018;115:12011–12016. doi: 10.1073/pnas.1815873115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diggle SP, West SA, Griffin AS, Campbell GS. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 28.Rumbaugh KP, et al. Kin selection, quorum sensing and virulence in pathogenic bacteria. Proceedings of the Royal Society B: Biological Sciences. 2012;279:3584–3588. doi: 10.1098/rspb.2012.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollitt EJG, West SA, Crusz SA, Burton-Chellew MN, Diggle SP. Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infection and Immunity. 2014;82:1045–1051. doi: 10.1128/IAI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzdzal-Fick JJ, Queller DC, Fox SA, Strassmann JE. High relatedness is necessary and sufficient to maintain multicellularity in Dictyostelium. Science. 2011;334:1548–1551. doi: 10.1126/science.1213272. [DOI] [PubMed] [Google Scholar]
- 31.Bastiaans E, Debets AJM, Aanen DK. Experimental evolution reveals that high relatedness protects multicellular cooperation from cheaters. Nature Communications. 2016;7:1–10. doi: 10.1038/ncomms11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost I, et al. Cooperation, competition and antibiotic resistance in bacterial colonies. ISME J. 2018;12:1582–1593. doi: 10.1038/s41396-018-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linksvayer TA, Wade MJ. Genes with social effects are expected to harbor more sequence variation within and between species. Evolution. 2009;63:1685–1696. doi: 10.1111/j.1558-5646.2009.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dyken JD, Wade MJ. Detecting the Molecular Signature of Social Conflict: Theory and a Test with Bacterial Quorum Sensing Genes. Am Nat. 2012;179:436–450. doi: 10.1086/664609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall DW, Goodisman MAD. The Effects of Kin Selection on Rates of Molecular Evolution in Social Insects. Evolution. 2012;66:2080–2093. doi: 10.1111/j.1558-5646.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- 36.Hall DW, Yi SV, Goodisman MAD. Kin selection, genomics and caste-antagonistic pleiotropy. Biology Letters. 2013;9:20130309. doi: 10.1098/rsbl.2013.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt BG, et al. Relaxed selection is a precursor to the evolution of phenotypic plasticity. Proceedings of the National Academy of Sciences. 2011;108:15936–15941. doi: 10.1073/pnas.1104825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt BG, et al. Sociality Is Linked to Rates of Protein Evolution in a Highly Social Insect. Molecular Biology and Evolution. 2010;27:497–500. doi: 10.1093/molbev/msp225. [DOI] [PubMed] [Google Scholar]
- 39.Warner MR, Mikheyev AS, Linksvayer TA. Genomic Signature of Kin Selection in an Ant with Obligately Sterile Workers. Molecular Biology and Evolution. 2017;34:1780–1787. doi: 10.1093/molbev/msx123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noh S, Geist KS, Tian X, Strassmann JE, Queller DC. Genetic signatures of microbial altruism and cheating in social amoebas in the wild. Proceedings of the National Academy of Sciences. 2018;12:201720324–6. doi: 10.1073/pnas.1720324115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira JL, et al. Conditional expression explains molecular evolution of social genes in a microbe. Nature Communications. 2019:10–12. doi: 10.1038/s41467-019-11237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadell CD, Drescher K, Foster KR. Spatial structure, cooperation and competition in biofilms. Nature Publishing Group. 2016;14:1–12. doi: 10.1038/nrmicro.2016.84. [DOI] [PubMed] [Google Scholar]
- 43.Xavier JB, Foster KR. Cooperation and conflict in microbial biofilms. of the National Academy of Sciences. 2007;104:876–881. doi: 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3531–3538. doi: 10.1098/rspb.2009.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. Solutions to the Public Goods Dilemma in Bacterial Biofilms. Current Biology. 2014;24:50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce JB, West SA, Griffin AS. Functional amyloids promote retention of public goods in bacteria. Proceedings of the Royal Society B: Biological Sciences. 2019;286:20190709–7. doi: 10.1098/rspb.2019.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehdiabadi NJ, et al. Kin preference in a social microbe. Nature. 2006;442:881–882. doi: 10.1038/442881a. [DOI] [PubMed] [Google Scholar]
- 48.Barve S, Koenig WD, Haydock J, Walters EL. Habitat Saturation Results in Joint-Nesting Female Coalitions in a Social Bird. The American Naturalist. 2019;193:830–840. doi: 10.1086/703188. [DOI] [PubMed] [Google Scholar]
- 49.Green JP, et al. The Genetic Basis of Kin Recognition in a Cooperatively Breeding Mammal. Current Biology. 2015;25:2631–2641. doi: 10.1016/j.cub.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 50.Lihoreau M, Rivault C. Kin recognition via cuticular hydrocarbons shapes cockroach social life. Behavioral Ecology. 2008;20:46–53. [Google Scholar]
- 51.Nam K-B, Simeoni M, Sharp SP, Hatchwell BJ. Kinship affects investment by helpers in a cooperatively breeding bird. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3299–3306. doi: 10.1098/rspb.2010.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madgwick PG, Stewart B, Belcher LJ, Thompson CRL, Wolf JB. Strategic investment explains patterns of cooperation and cheating in a microbe. Proceedings of the National Academy of Sciences. 2018;115:E4823–E4832. doi: 10.1073/pnas.1716087115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerrieri FJ, et al. Ants recognize foes and not friends. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2461–2468. doi: 10.1098/rspb.2008.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duffy E, Morrison C, Macdonald K. Colony defense and behavioral differentiation in the eusocial shrimp Synalpheus regalis. Behav Ecol Sociobiol. 2002;51:488–495. [Google Scholar]
- 55.Leedale AE, Lachlan RF, Robinson EJH, Hatchwell BJ. Helping decisions and kin recognition in long-tailed tits: is call similarity used to direct help towards kin? Philosophical Transactions of the Royal Society B: Biological Sciences. 2020;375:20190565–10. doi: 10.1098/rstb.2019.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornwallis CK, West SA, Griffin AS. Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J Evol Biol. 2009;22:2445–2457. doi: 10.1111/j.1420-9101.2009.01853.x. [DOI] [PubMed] [Google Scholar]
- 57.Duncan C, Gaynor D, Clutton-Brock TH, Dyble M. The Evolution of Indiscriminate Altruism in a Cooperatively Breeding Mammal. The American Naturalist. 2019;193:841–851. doi: 10.1086/703113. [DOI] [PubMed] [Google Scholar]
- 58.Thompson FJ, et al. Explaining negative kin discrimination in a cooperative mammal society. Proceedings of the National Academy of Sciences. 2017;114:5207–5212. doi: 10.1073/pnas.1612235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton WD, May RM. Dispersal in stable habitats. Nature. 1977;269:578–581. [Google Scholar]
- 60.Bourke AFG. Hamilton’s rule and the causes of social evolution. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130362. doi: 10.1098/rstb.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatchwell BJ, Gullett PR, Adams MJ. Helping in cooperatively breeding long-tailed tits: a test of Hamilton’s rule. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130565. doi: 10.1098/rstb.2013.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Domingo-Calap P, Segredo-Otero E, Duran-Moreno M, Sanjuán R. Social evolution of innate immunity evasion in a virus. Nature Microbiology. 2019;4:1006–1013. doi: 10.1038/s41564-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Firman RC, Rubenstein DR, Moran JM, Rowe KC, Buzatto BA. Extreme and Variable Climatic Conditions Drive the Evolution of Sociality in Australian Rodents. Curr Biol. 2020;30:691–697.:e3. doi: 10.1016/j.cub.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Arnold KE, Owens I. Cooperative breeding in birds: the role of ecology. Behavioral Ecology. 1999;10:465–471. [Google Scholar]
- 65.Jetz W, Rubenstein DR. Environmental Uncertainty and the Global Biogeography of Cooperative Breeding in Birds. Current Biology. 2010;21:1–7. doi: 10.1016/j.cub.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 66.Rubenstein DR, Lovette IJ. Temporal Environmental Variability Drives the Evolution of Cooperative Breeding in Birds. Current Biology. 2007;17:1414–1419. doi: 10.1016/j.cub.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 67.Brooks KC, Maia R, Duffy JE, Hultgren KM, Rubenstein DR. Ecological generalism facilitates the evolution of sociality in snapping shrimps. Ecol Lett. 2017;20:1516–1525. doi: 10.1111/ele.12857. [DOI] [PubMed] [Google Scholar]
- 68.Lin Y-H, Chan S-F, Rubenstein DR, Liu M, Shen S-F. Resolving the Paradox of Environmental Quality and Sociality: The Ecological Causes and Consequences of Cooperative Breeding in Two Lineages of Birds. The American Naturalist. 2019;194:207–216. doi: 10.1086/704090. [DOI] [PubMed] [Google Scholar]
- 69.Lukas D, Clutton-Brock TH. Cooperative breeding and monogamy in mammalian societies. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2151–2156. doi: 10.1098/rspb.2011.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornwallis CK, West SA, Davis KE, Griffin AS. Promiscuity and the evolutionary transition to complex societies. Nature. 2010;466:969–972. doi: 10.1038/nature09335. [DOI] [PubMed] [Google Scholar]
- 71.Queller DC, et al. Unrelated helpers in a social insect. Nature. 2000;405:784–787. doi: 10.1038/35015552. [DOI] [PubMed] [Google Scholar]
- 72.Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J. Nest Inheritance Is the Missing Source of Direct Fitness in a Primitively Eusocial Insect. Science. 2011;333:874–876. doi: 10.1126/science.1205140. [DOI] [PubMed] [Google Scholar]
- 73.Korb J, Hartfelder K. Life history and development - a framework for understanding developmental plasticity in lower termites. Biol Rev. 2008;83:295–313. doi: 10.1111/j.1469-185x.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 74.Downing PA, Griffin AS, Cornwallis CK. Sex differences in helping effort reveal the effect of future reproduction on cooperative behaviour in birds. Proceedings of the Royal Society B: Biological Sciences. 2018;285:20181164–8. doi: 10.1098/rspb.2018.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Downing PA, Cornwallis CK, Griffin AS. Sex, long life and the evolutionary transition to cooperative breeding in birds. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20151663–7. doi: 10.1098/rspb.2015.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komdeur J. Importance of Habitat Saturation and Territory Quality for Evolution of Cooperative Breeding in the Seychelles Warbler. Nature. 1992;358:493–495. [Google Scholar]
- 77.Clutton-Brock TH, et al. Contributions to cooperative rearing in meerkats. Animal Behaviour. 2001;61:705–710. [Google Scholar]
- 78.Field J, Cronin A, Bridge C. Future fitness and helping in social queues. Nature. 2006;441:214–217. doi: 10.1038/nature04560. [DOI] [PubMed] [Google Scholar]
- 79.Cant MA, Llop JB, Field J. Individual variation in social aggression and the probability of inheritance: Theory and a field test. Am Nat. 2006;167:837–852. doi: 10.1086/503445. [DOI] [PubMed] [Google Scholar]
- 80.Darch SE, West SA, Winzer K, Diggle SP. Density-dependent fitness benefits in quorum-sensing bacterial populations. 2012;109:8259–8263. doi: 10.1073/pnas.1118131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biernaskie JM, West SA. Cooperation, clumping and the evolution of multicellularity. Proceedings: Biological Sciences. 2015;282:1–9. doi: 10.1098/rspb.2015.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koschwanez JH, Foster KR, Murray AW. Improved use of a public good selects for the evolution of undifferentiated multicellularity. eLife. 2013;2:e00367. doi: 10.7554/eLife.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sexton DJ, Schuster M. Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nature Communications. 2017;8:1–8. doi: 10.1038/s41467-017-00222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Connelly BD, Bruger EL, McKinley PK, Waters CM. Resource abundance and the critical transition to cooperation. J Evol Biol. 2017:1–12. doi: 10.1111/jeb.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Molecular Microbiology. 2010;79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapsetaki SE, West SA. The costs and benefits of multicellular group formation in algae*. Evolution. 2019;73:1296–1308. doi: 10.1111/evo.13712. [DOI] [PubMed] [Google Scholar]
- 87.Ratcliff WC, et al. Experimental evolution of an alternating uni- and multicellular life cycle in Chlamydomonas reinhardtii. Nature Communications. 4:1–7. doi: 10.1038/ncomms3742. (1AD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghoul M, Griffin AS, West SA. Toward an evolutionary definition of cheating. Evolution. 2014;68:318–331. doi: 10.1111/evo.12266. [DOI] [PubMed] [Google Scholar]
- 89.Cordero OX, Ventouras L-A, DeLong EF, Polz MF. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proceedings of the National Academy of Sciences. 2012;109:20059–20064. doi: 10.1073/pnas.1213344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gano-Cohen KA, et al. Recurrent mutualism breakdown events in a legume rhizobia metapopulation. Proceedings of the Royal Society B: Biological Sciences. 2020;287:20192549–9. doi: 10.1098/rspb.2019.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersen SB, Marvig RL, Molin S, Krogh Johansen H, Griffin AS. Long-term social dynamics drive loss of function in pathogenic bacteria. Proceedings of the National Academy of Sciences. 2015;112:10756–10761. doi: 10.1073/pnas.1508324112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sachs JL, Ehinger MO, Simms EL. Origins of cheating and loss of symbiosis in wild Bradyrhizobium. J Evol Biol. 2010;23:1075–1089. doi: 10.1111/j.1420-9101.2010.01980.x. [DOI] [PubMed] [Google Scholar]
- 93.Patel M, Raymond B, Bonsall MB, West SA. Crystal toxins and the volunteer’s dilemma in bacteria. J Evol Biol. 2019;32:310–319. doi: 10.1111/jeb.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency Dependence and Cooperation: Theory and a Test with Bacteria. Am Nat. 2007;170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 96.Lujan AM, Gomez P, Buckling A. Siderophore cooperation of the bacterium Pseudomonas fluorescens in soil. Biology Letters. 2015;11:20140934. doi: 10.1098/rsbl.2014.0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meir M, et al. Competition between social cheater viruses is driven by mechanistically different cheating strategies. Science Advances. 2020;6:eabb7990. doi: 10.1126/sciadv.abb7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Dyken JD, Linksvayer TA, Wade MJ. Kin Selection–Mutation Balance: A Model for the Origin, Maintenance, and Consequences of Social Cheating. Am Nat. 2011;177:288–300. doi: 10.1086/658365. [DOI] [PubMed] [Google Scholar]
- 99.Jandér KC, Steidinger BS. Why mutualist partners vary in quality: mutation-selection balance and incentives to cheat in the fig tree-fig wasp mutualism. Ecol Lett. 2017;20:922–932. doi: 10.1111/ele.12792. [DOI] [PubMed] [Google Scholar]
- 100.Butaitė E, Baumgartner M, Wyder S, Kümmerli R. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nature Communications. 2017;8:1–12. doi: 10.1038/s41467-017-00509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kümmerli R, et al. Co-evolutionary dynamics between public good producers and cheats in the bacterium Pseudomonas aeruginosa. J Evol Biol. 2015;28:2264–2274. doi: 10.1111/jeb.12751. [DOI] [PubMed] [Google Scholar]
- 102.Bruce JB, Cooper GA, Chabas H, West SA, Griffin AS. Cheating and resistance to cheating in natural populations of the bacterium Pseudomonas fluorescens. Evolution. 2017;71:2484–2495. doi: 10.1111/evo.13328. [DOI] [PubMed] [Google Scholar]
- 103.Pollak S, et al. Facultative cheating supports the coexistence of diverse quorum-sensing alleles. Proceedings of the National Academy of Sciences. 2016;113:2152–2157. doi: 10.1073/pnas.1520615113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Even-Tov E, et al. Social Evolution Selects for Redundancy in Bacterial Quorum Sensing. PLoS Biol. 2016;14:1–18. doi: 10.1371/journal.pbio.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barker JL, Bronstein JL. Temporal Structure in Cooperative Interactions: What Does the Timing of Exploitation Tell Us about Its Cost? PLoS Biol. 2016;14:e1002371–16. doi: 10.1371/journal.pbio.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andersen SB, et al. Privatisation rescues function following loss of cooperation. eLife. 2018;7 doi: 10.7554/eLife.38594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grafen A. In: Behavioural Ecology An Evolutionary Approach. Krebs JR, Davies NB, editors. Behavioural ecology: an evolutionary approach; 1984. pp. 62–84. [Google Scholar]
- 108.Wang J, et al. A Y-like social chromosome causes alternative colony organization in fire ants. Nature. 2013;493:664–668. doi: 10.1038/nature11832. [DOI] [PubMed] [Google Scholar]
- 109.Avril A, Purcell J, Béniguel S, Chapuisat M. Maternal effect killing by a supergene controlling ant social organization. Proceedings of the National Academy of Sciences. 2020;117:17130–17134. doi: 10.1073/pnas.2003282117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan Z, et al. Evolution of a supergene that regulates a trans-species social polymorphism. Nat ecol evol. 2020;4:1–21. doi: 10.1038/s41559-019-1081-1. [DOI] [PubMed] [Google Scholar]
- 111.Fehr E, Schurtenberger I. Normative foundations of human cooperation. Nat hum behav. 2018;2:1–11. doi: 10.1038/s41562-018-0385-5. [DOI] [PubMed] [Google Scholar]
- 112.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 113.Burton-Chellew MN, El Mouden C, West SA. Conditional cooperation and confusion in public-goods experiments. Proceedings of the National Academy of Sciences. 2016;113:1291–1296. doi: 10.1073/pnas.1509740113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kümmerli R, Burton-Chellew MN, Ross-Gillespie A, West SA. Resistance to extreme strategies, rather than prosocial preferences, can explain human cooperation in public goods games. Proceedings of the National Academy of Sciences. 2010;107:10125–10130. doi: 10.1073/pnas.1000829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burton-Chellew MN, West SA. Prosocial preferences do not explain human cooperation in public-goods games. Proceedings of the National Academy of Sciences. 2013;110:216–221. doi: 10.1073/pnas.1210960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burton-Chellew MN, Nax HH, West SA. Payoff-based learning explains the decline in cooperation in public goods games. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20142678. doi: 10.1098/rspb.2014.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bayer R-C, Renner E, Sausgruber R. Confusion and learning in the voluntary contributions game. Exp Econ. 2013;16:478–496. [Google Scholar]
- 118.Barclay P. Reciprocity creates a stake in one’s partner, or why you should cooperate even when anonymous. Proceedings of the Royal Society B: Biological Sciences. 2020;287:20200819–6. doi: 10.1098/rspb.2020.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaeggi AV, Hooper PL, Beheim BA, Kaplan H, Gurven M. Reciprocal Exchange Patterned by Market Forces Helps Explain Cooperation in a Small-Scale Society. Current Biology. 2016;26:2180–2187. doi: 10.1016/j.cub.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 120.Kasper C, Mulder MB. Who Helps and Why? Current Anthropology. 2015;56:701–732. [Google Scholar]
- 121.Guala F. Reciprocity: Weak or strong? What punishment experiments do (and do not) demonstrate. Behav Brain Sci. 2012;35:15. doi: 10.1017/S0140525X11000069. [DOI] [PubMed] [Google Scholar]
- 122.Kurzban R, Burton-Chellew MN, West SA. The Evolution of Altruism in Humans. Annu Rev Psychol. 2015;66:575–599. doi: 10.1146/annurev-psych-010814-015355. [DOI] [PubMed] [Google Scholar]
- 123.Lukas D, Clutton-Brock TH. Social complexity and kinship in animal societies. Ecol Lett. 2018;21:1129–1134. doi: 10.1111/ele.13079. [DOI] [PubMed] [Google Scholar]
- 124.Molleman L, Quiñones AE, Weissing FJ. Cultural evolution of cooperation: The interplay between forms of social learning and group selection. Evolution and Human Behavior. 2013;34:342–349. [Google Scholar]
- 125.van den Berg P, Molleman L, Weissing FJ. Focus on the success of others leads to selfish behavior. Proceedings of the National Academy of Sciences. 2015;112:2912–2917. doi: 10.1073/pnas.1417203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burton-Chellew MN, El Mouden C, West SA. Social learning and the demise of costly cooperation in humans. Proceedings of the Royal Society B: Biological Sciences. 2017;284:20170067–9. doi: 10.1098/rspb.2017.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lehmann L, Feldman MW, Foster KR. Cultural Transmission Can Inhibit the Evolution of Altruistic Helping. Am Nat. 2008;172:12–24. doi: 10.1086/587851. [DOI] [PubMed] [Google Scholar]
- 128.Boyd R, Richerson PJ. Culture and the evolution of human cooperation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3281–3288. doi: 10.1098/rstb.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clutton-Brock TH. Breeding Together: Kin Selection and Mutualism in Cooperative Vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- 130.Gilbert OM, Foster KR, Mehdiabadi NJ, Strassmann JE, Queller DC. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proceedings of the National Academy of Sciences. 2007;104:8913–8917. doi: 10.1073/pnas.0702723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Higgs PG, Lehman N. The RNA World: molecular cooperation at the origins of life. Nature Publishing Group. 2014;16:7–17. doi: 10.1038/nrg3841. [DOI] [PubMed] [Google Scholar]
- 132.Levin SR, West SA. The evolution of cooperation in simple molecular replicators. Proceedings of the Royal Society B: Biological Sciences. 2017;284:20171967–8. doi: 10.1098/rspb.2017.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gardner A, Alpedrinha JAC, West SA. Haplodiploidy and the Evolution of Eusociality: Split Sex Ratios. The American Naturalist. 2012;179:240–256. doi: 10.1086/663683. [DOI] [PubMed] [Google Scholar]
- 134.Alpedrinha JAC, Gardner A, West SA. Haplodiploidy and the Evolution of Eusociality: Worker Revolution. Am Nat. 2014;184:303–317. doi: 10.1086/677283. [DOI] [PubMed] [Google Scholar]
- 135.Rautiala P, Helantera H, Puurtinen M. Unmatedness Promotes the Evolution of Helping More in Diplodiploids than in Haplodiploids. Am Nat. 2014;184:318–325. doi: 10.1086/677309. [DOI] [PubMed] [Google Scholar]
- 136.Rautiala P, Helantera H, Puurtinen M. The evolutionary dynamics of adaptive virginity, sex-allocation, and altruistic helping in haplodiploid animals. Evolution. 2017;72:30–38. doi: 10.1111/evo.13399. [DOI] [PubMed] [Google Scholar]
- 137.Quiñones AE, Pen I. A unified model of Hymenopteran preadaptations that trigger the evolutionary transition to eusociality. Nature Communications. 2017;8:1–13. doi: 10.1038/ncomms15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quiñones AE, Henriques GJB, Pen I. Queen–worker conflict can drive the evolution of social polymorphism and split sex ratios in facultatively eusocial life cycles. Evolution. 2019;74:15–28. doi: 10.1111/evo.13844. [DOI] [PubMed] [Google Scholar]
- 139.Ross L, Gardner A, Hardy N, West SA. Ecology, not the genetics of sex determination, determines who helps in eusocial populations. Current Biology. 2013;23:2383–2387. doi: 10.1016/j.cub.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 140.Ågren JA, Davies NG, Foster KR. Enforcement is central to the evolution of cooperation. Nat ecol evol. 2019;3:1–12. doi: 10.1038/s41559-019-0907-1. [DOI] [PubMed] [Google Scholar]
- 141.Kokko H, Johnstone RA, Clutton-Brock TH. The evolution of cooperative breeding through group augmentation. Proceedings of the Royal Society B: Biological Sciences. 2001;268:187–196. doi: 10.1098/rspb.2000.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 143.Gardner A, West SA. Cooperation and punishment, especially in humans. Am Nat. 2004;164:753–764. doi: 10.1086/425623. [DOI] [PubMed] [Google Scholar]
- 144.Wenseleers T, Ratnieks FLW. Enforced altruism in insect societies. Nature. 2006;444:50. doi: 10.1038/444050a. [DOI] [PubMed] [Google Scholar]
- 145.Schino G. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behavioral Ecology. 2006;18:115–120. [Google Scholar]
- 146.Frank SA. Kin Selection and Virulence in the Evolution of Protocells and Parasites. Proceedings of the Royal Society B: Biological Sciences. 1994;258:153–161. doi: 10.1098/rspb.1994.0156. [DOI] [PubMed] [Google Scholar]
- 147.Leeks A, dos Santos M, West SA. Transmission, relatedness, and the evolution of cooperative symbionts. J Evol Biol. 2019;32:1036–1045. doi: 10.1111/jeb.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moran NA, McCutcheon JP, Nakabachi A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 149.Fisher RM, Henry LM, Cornwallis CK, Kiers ET, West SA. The evolution of host-symbiont dependence. Nature Communications. 2017;8:1–8. doi: 10.1038/ncomms15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harcombe W. Novel cooperation experimentally evolved between species. Evolution. 2010;64:2166–2172. doi: 10.1111/j.1558-5646.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 151.Harcombe WR, Chacón JM, Adamowicz EM, Chubiz LM, Marx CJ. Evolution of bidirectional costly mutualism from byproduct consumption. Proceedings of the National Academy of Sciences. 2018;6:201810949-12004. doi: 10.1073/pnas.1810949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nature. 2011;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 153.Husnik F, McCutcheon JP. Functional horizontal gene transfer from bacteria to eukaryotes. Nature Publishing Group. 2017;16:67–79. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- 154.Frank SA. Repression of Competition and the evolution of cooperation. Evolution. 2003;57:693. doi: 10.1111/j.0014-3820.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 155.West SA, Kiers ET, Simms EL, Denison RF. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proceedings of the Royal Society B: Biological Sciences. 2002;269:685–694. doi: 10.1098/rspb.2001.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- 157.Jandér KC, Herre EA. Host sanctions and pollinator cheating in the fig tree-fig wasp mutualism. Proceedings of the Royal Society B: Biological Sciences. 2010;277:1481–1488. doi: 10.1098/rspb.2009.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jandér KC, Herre EA, Simms EL. Precision of host sanctions in the fig tree-fig wasp mutualism: consequences for uncooperative symbionts. Ecol Lett. 2012;15:1362–1369. doi: 10.1111/j.1461-0248.2012.01857.x. [DOI] [PubMed] [Google Scholar]
- 159.Kiers ET, et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science. 2011;333:880–882. doi: 10.1126/science.1208473. [DOI] [PubMed] [Google Scholar]
- 160.Whiteside MD, et al. Mycorrhizal Fungi Respond to Resource Inequality by Moving Phosphorus from Rich to Poor Patches across Networks. Current Biology. 2019;29:1–8. doi: 10.1016/j.cub.2019.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leigh EG., Jr . Adaptation and Diversity. Freeman, Cooper & Company; 1971. [Google Scholar]
- 162.Scott TW, West SA. Adaptation is maintained by the parliament of genes. Nature Communications. 2019;10:13. doi: 10.1038/s41467-019-13169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wyatt GAK, Kiers ET, Gardner A, West SA. A biological market analysis of the plant-mycorrhizal symbiosis. Evolution. 2014;68:2603–2618. doi: 10.1111/evo.12466. [DOI] [PubMed] [Google Scholar]
- 164.West SA, Kiers ET, Pen I, Denison RF. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J Evol Biol. 2002;15:830–837. [Google Scholar]
- 165.West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The Social Lives of Microbes. Annu Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- 166.Rumbaugh KP, et al. Quorum Sensing and the Social Evolution of Bacterial Virulence. Current Biology. 2009;19:341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 167.Vasilijevic J, et al. Reduced accumulation of defective viral genomes contributes to severe outcome in influenza virus infected patients. PLoS Pathog. 2017;13:e1006650–29. doi: 10.1371/journal.ppat.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tanner EJ, Kirkegaard KA, Weinberger LS. Exploiting Genetic Interference for Antiviral Therapy. PLoS Genet. 2016;12:e1005986–14. doi: 10.1371/journal.pgen.1005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown SP, West SA, Diggle SP, Griffin AS. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3157–3168. doi: 10.1098/rstb.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andre J-B, Letters BGE. Multicellular organization in bacteria as a target for drug therapy. Ecol Lett. 2005;85 [Google Scholar]
- 171.Dieltjens L, et al. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nature Communications. 2019;11:1–11. doi: 10.1038/s41467-019-13660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rahwan I, et al. Machine behaviour. Nature. 2019;568:477–486. doi: 10.1038/s41586-019-1138-y. [DOI] [PubMed] [Google Scholar]
- 173.Crandall JW, et al. Cooperating with machines. Nature Communications. 2018;9:1–12. doi: 10.1038/s41467-017-02597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hesse E, et al. Ecological selection of siderophore-producing microbial taxa in response to heavy metal contamination. Ecol Lett. 2017;21:117–127. doi: 10.1111/ele.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nikel PI, Silva-Rocha R, Benedetti I, de Lorenzo V. The private life of environmental bacteria: pollutant biodegradation at the single cell level. Environ Microbiol. 2014;16:628–642. doi: 10.1111/1462-2920.12360. [DOI] [PubMed] [Google Scholar]
- 176.Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality. Nature. 2010;466:1057–1062. doi: 10.1038/nature09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nowak MA, McAvoy A, Allen B, Wilson EO. The general form of Hamilton’s rule makes no predictions and cannot be tested empirically. Proceedings of the National Academy of Sciences. 2017;114:5665–5670. doi: 10.1073/pnas.1701805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nowak MA, Allen B. Inclusive Fitness Theorizing Invokes Phenomena That Are Not Relevant for the Evolution of Eusociality. PLoS Biol. 2015;13:e1002134–5. doi: 10.1371/journal.pbio.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Welch JJ. What’s wrong with evolutionary biology? Biol Philos. 2017;32:263–279. doi: 10.1007/s10539-016-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grafen A. A geometric view of relatedness. Oxford surveys in evolutionary biology. 1985:28–89. [Google Scholar]
- 181.Abbot P, et al. Inclusive fitness theory and eusociality. Nature. 2011;471:E1. doi: 10.1038/nature09831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Parker GA, Maynard Smith J. Optimality theory in evolutionary biology. Nature. 1990 [Google Scholar]
- 183.Davies NB, Krebs JR, West SA. An Introduction to Behavioural Ecology. Fourth Edition. 2012. [Google Scholar]
- 184.Hamilton WD. Evolution of Altruistic Behavior. Am Nat. 1963;97:354. [Google Scholar]
- 185.West SA, Gardner A. Adaptation and Inclusive Fitness Review. Current Biology. 2013;23:R577–R584. doi: 10.1016/j.cub.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 186.Taylor PD, Frank SA. How to make a kin selection model. Journal of Theoretical Biology. 1996;180:27–37. doi: 10.1006/jtbi.1996.0075. [DOI] [PubMed] [Google Scholar]
- 187.Grafen A. Optimization of inclusive fitness. Journal of Theoretical Biology. 2006;238:541–563. doi: 10.1016/j.jtbi.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 188.Trivers R, Hope H. Haplodiploidy and the evolution of the social insects. Science. 1976;191:249–263. doi: 10.1126/science.1108197. [DOI] [PubMed] [Google Scholar]
- 189.Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Molecular Microbiology. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crozier RH. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution. 1986;40:1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 191.Moran NA, Sloan DB. The Hologenome Concept: Helpful or Hollow? PLoS Biol. 2015;13:e1002311. doi: 10.1371/journal.pbio.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Werner GDA, et al. Symbiont switching and alternative resource acquisition strategies drive mutualism breakdown. Proceedings of the National Academy of Sciences. 2018;115:5229–5234. doi: 10.1073/pnas.1721629115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford University Press; 1995. [Google Scholar]
- 195.West SA, Fisher RM, Gardner A, Kiers ET. Major evolutionary transitions in individuality. Proceedings of the National Academy of Sciences. 2015;112:10112–10119. doi: 10.1073/pnas.1421402112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Foster KR, Shaulsky G, Strassmann JE, Queller DC. Pleiotropy as a mechanism to stabilize cooperation. Nature. 2004 doi: 10.1038/nature02894. [DOI] [PubMed] [Google Scholar]
- 197.Mitri S, Foster KR. Pleiotropy and the low cost of individual traits promote cooperation. Evolution. 2016;70:488–494. doi: 10.1111/evo.12851. [DOI] [PubMed] [Google Scholar]
- 198.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proceedings of the National Academy of Sciences. 2015;112:2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dandekar AA, Chugani S. Bacterial Quorum Sensing and Metabolic Incentives to Cooperate. Science. 2012;338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Majerczyk C, Schneider E. Quorum sensing control of Type VI secretion factors restricts the proliferation of quorum-sensing mutants. eLife. 2016;5:317. doi: 10.7554/eLife.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Frénoy A, Taddei F, Misevic D. Genetic Architecture Promotes the Evolution and Maintenance of Cooperation. 2013;9:e1003339–12. doi: 10.1371/journal.pcbi.1003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Özkaya Ö, Xavier KB, Dionisio F, Balbontín R. Maintenance of microbial cooperation mediated by public goods in single and multiple traits scenarios. Journal of Bacteriology. 2017;199:e00297–17. doi: 10.1128/JB.00297-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oliveira RC, et al. Hormonal pleiotropy helps maintain queen signal honesty in a highly eusocial wasp. Sci Rep. 2017;7:1–12. doi: 10.1038/s41598-017-01794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.dos Santos M, Ghoul M, West SA. Pleiotropy, cooperation, and the social evolution of genetic architecture. PLoS Biol. 2018;16:e2006671–25. doi: 10.1371/journal.pbio.2006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bruger E, Waters C. Sharing the sandbox: Evolutionary mechanisms that maintain bacterial cooperation. F1000Res. 2015;4:1–9. doi: 10.12688/f1000research.7363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Waite AJ, Shou W. Adaptation to a new environment allows cooperators to purge cheaters stochastically. Proceedings of the National Academy of Sciences. 2012;109:19079–19086. doi: 10.1073/pnas.1210190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Morgan AD, Quigley BJZ, Brown SP, Buckling A. Selection on non-social traits limits the invasion of social cheats. Ecol Lett. 2012;15:841–846. doi: 10.1111/j.1461-0248.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Queller DC, Ponte E, Bozzaro S, Strassmann JE. Single-gene greenbeard effects in the social amoeba Dictyostelium discoideum. Science. 2003;299:105–106. doi: 10.1126/science.1077742. [DOI] [PubMed] [Google Scholar]
- 209.Smukalla S, et al. FLO1 Is a Variable Green Beard Gene that Drives Biofilm-like Cooperation in Budding Yeast. Cell. 2008;135:726–737. doi: 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Madgwick PG, Belcher LJ, Wolf JB. Greenbeard Genes: Theory and Reality. Trends in Ecology & Evolution. 2019;34:1092–1103. doi: 10.1016/j.tree.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 211.West SA, Gardner A. Greenbeards. Evolution. 2010;64:25–38. doi: 10.1111/j.1558-5646.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 212.Smith J. The social evolution of bacterial pathogenesis. Proceedings of the Royal Society B: Biological Sciences. 2001;268:61–69. doi: 10.1098/rspb.2000.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nogueira T, et al. Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Current Biology. 2009;19:1683–1691. doi: 10.1016/j.cub.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McGinty SE, Lehmann L, Brown SP, Rankin DJ. The interplay between relatedness and horizontal gene transfer drives the evolution of plasmid-carried public goods. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20130400–8. doi: 10.1098/rspb.2013.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McGinty SE, Rankin DJ, Brown SP. Horizontal gene transfer and the evolution of bacterial cooperation. Evolution. 2010;65:21–32. doi: 10.1111/j.1558-5646.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ghoul M, Andersen SB, West SA. Sociomics: Using omic approaches to understand social evolution. Trends in Genetics. 2017;33:408–419. doi: 10.1016/j.tig.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 217.Hanschen ER, Ferris PJ, Michod RE. Early evolution of the genetic basis for soma in the Volvocaceae. Evolution. 2014;68:2014–2025. doi: 10.1111/evo.12416. [DOI] [PubMed] [Google Scholar]
- 218.Smith SM, Kent DS, Boomsma JJ, Stow AJ. Monogamous sperm storage and permanent worker sterility in a long-lived ambrosia beetle. Nat ecol evol. 2018;48:1–12. doi: 10.1038/s41559-018-0533-3. [DOI] [PubMed] [Google Scholar]
- 219.Hamilton WD. Altruism and related phenomena, mainly in social insects. Annual Review of Ecology and Systematics. 1972;3:193–232. [Google Scholar]
- 220.Kiers ET, Ratcliff WC, Denison RF. Single-strain inoculation may create spurious correlations between legume fitness and rhizobial fitness. New Phytol. 2013;198:4–9. doi: 10.1111/nph.12015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.