Abstract
Aedes mosquito-transmitted diseases such as dengue, Zika and chikungunya, are becoming major global health emergencies while old threats such as yellow fever are re-emerging. Traditional control methods, which have focused on reducing mosquito populations through the application of insecticides or preventing breeding through removal of larval habitat, are largely ineffective, as evidenced by the increasing global disease burden. Here, we review novel mosquito population reduction and population modification approaches with a focus on control methods that are based on the release of mosquitoes, including the release of Wolbachia-infected mosquitos and strategies to genetically modify the vector, that are currently under development and have the potential to contribute to a reversal of the current alarming disease trends.
Introduction
Aedes aegypti is exquisitely adapted to tropical and sub-tropical cities as its preferred habitat, living and breeding within people’s dwellings and the waste that accumulates around them. As tropical cities continue to grow, often outstripping the delivery of adequate infrastructure to manage either water delivery or waste removal, this mosquito has flourished. This rapid urban growth together with widespread global air travel, that enables human pathogens to travel as easily as their hosts, creates the perfect conditions for human disease to rapidly spread, as we are currently experiencing globally.
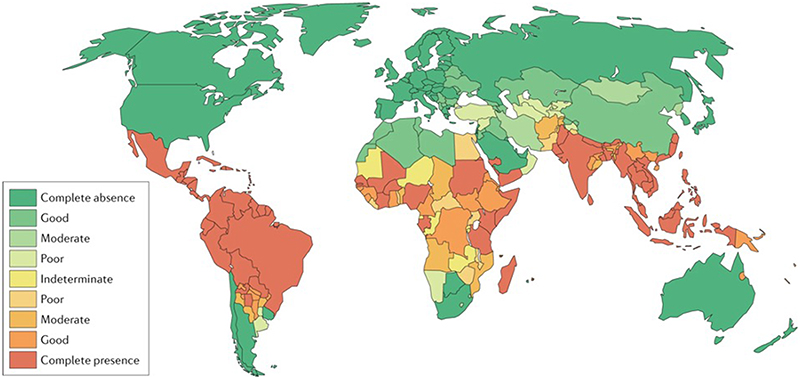
The burden of Aedes-transmitted disease has significantly increased over the past 50 years 1,2. The incidence of dengue, now the world’s most common mosquito-borne viral disease, grew more than 30-fold during this period 3. Dengue viruses are estimated to infect around 400 million people per year, and over half the world’s population is at risk of the disease (FIG. 1) 4.
Figure 1. The global distribution and burden of dengue.
Evidence consensus map showing the complete absence to complete presence of dengue. Green colours indicate evidence consensus towards absence of dengue, and orange and red colours indicate consensus towards presence of dengue. Darker colouring indicates more data supporting a conclusion about the presence or absence of dengue in a country. Figure adapted from ref 4.
More recently, chikungunya virus emerged from Africa in the mid-2000s, spreading first across India and Asia, and then into the Americas in 2013 5. Zika virus outbreaks occurred in the South Pacific in 2013 and the Americas in 2015 5. Infection with Zika virus in the Americas coincided with a surge in cases of microcephaly and other congenital abnormalities. Even yellow fever, for which an effective vaccine exists, is re-emerging. Recent outbreaks started in Angola in late 2015 and the virus quickly spread to the Democratic Republic of Congo, Kenya and China 6. In late 2016, hundreds of cases of yellow fever have been reported in Brazil 7.
This unprecedented global emergence of viruses that are transmitted by arthropod vectors (arboviruses) is thought to be caused by a combination of human population growth, increasing globalization, a rapid rise in population-dense cities in tropical areas and major expansion of the geographic range of A. aegypti 1,5,8. Existing methods that aim to reduce disease by suppressing mosquito populations through the physical removal of breeding sites or rely on the application of insecticides targeting either larvae or adults are unable to cope in this new global context. To effectively limit or prevent future outbreaks, novel public health interventions are desperately needed 3.
New vector control approaches that involve the release of mosquitoes currently fall into two broad classes: they either aim to reduce the vector population, or modify the vector to make it refractory to pathogen transmission. Reducing mosquito populations through suppression approaches is based on the intuitive assumption that as virus transmission is dependent on a bite from an infectious mosquito, reducing mosquitoes will lower transmission and disease. However, although this is clearly true if the mosquito population can be completely eliminated, the impact on disease if population suppression is only partial is much less clear. Currently, there is little experimental evidence (for example, randomised controlled trials with epidemiological endpoints) that indicates the effectiveness of imperfect mosquito suppression strategies 9,10.
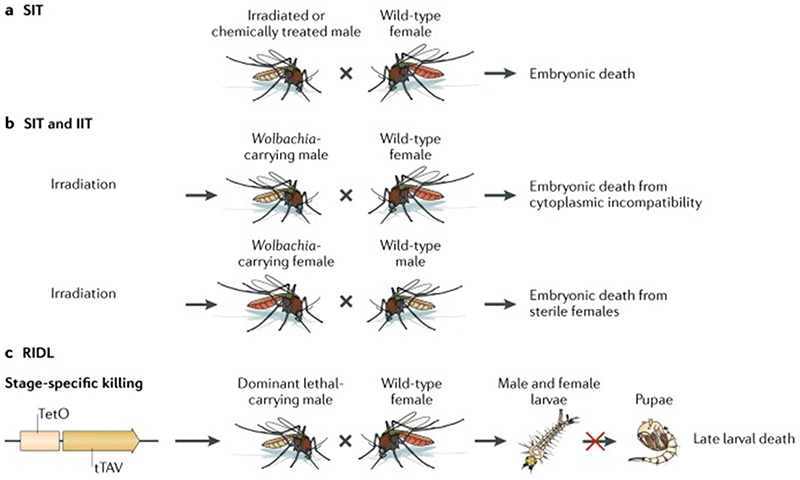
Novel population reduction approaches involve rearing and releasing large numbers of male mosquitoes that cannot produce viable offspring when they mate with wild females. Over the course of many generations of continual release of these males, the size of the vector population should be substantially reduced, which in turn should reduce disease transmission. These methods include the sterile insect technique (SIT), the incompatible insect technique (IIT) and various genetic modification strategies (FIG 2).
Figure 2. Modification of vectors for population reduction.
New vector control approaches that involve the release of mosquitoes aim to reduce the vector population. A) In the sterile insect technique (SIT) approach, male insects are exposed to either irradiation or sterilizing chemicals, causing large-scale random damage to the insect chromosomes or dominant-lethal mutations in the sperm. These males are then released in the wild population, and when they mate with wild females, viable offspring are rarely produced, eventually leading to a substantial decrease in vector population size. B) In the incompatible insect technique approach, a Wolbachia strain is stably introduced into a colony of a mosquito species. Only Wolbachia-infected males are released, which when mated to females that do not harbour the same Wolbachia strain or that do not carry Wolbachia, results in the death of their offspring due to CI. A combination of IIT and SIT could be used to suppress mosquito populations. During this approach, Wolbachia-infected mosquitoes are treated with low level irradiation. As in IIT alone, Wolbachia-males mating with wild females will not produce offspring. In the case of accidental female releases, these irradiated females are sterile and cannot reproduce with wild or Wolbachia-infected males. C) RIDL (Release of Insects carrying a Dominant Lethal) is a suppression strategy, whereby males that carry a transgene that causes late-acting lethality are released in the open field. These males mate with wild-type females, and the resulting offspring die before reaching the pupal stage.
By contrast, population modification approaches involve the release of both male and female mosquitoes that carry a heritable factor that reduces or blocks their ability to transmit viruses such as dengue or Zika viruses. As these mosquitoes mate with wild mosquitoes, the factor will spread through the population, effectively rendering the mosquitoes incapable of transmitting the pathogen without the need for population suppression. These approaches include the deployment of pathogen-blocking endosymbiotic bacteria Wolbachia pipientis (FIG. 3), and gene-drive mechanisms such as the CRISPR-Cas9 system coupled with transmission-blocking gene constructs (FIG. 4).
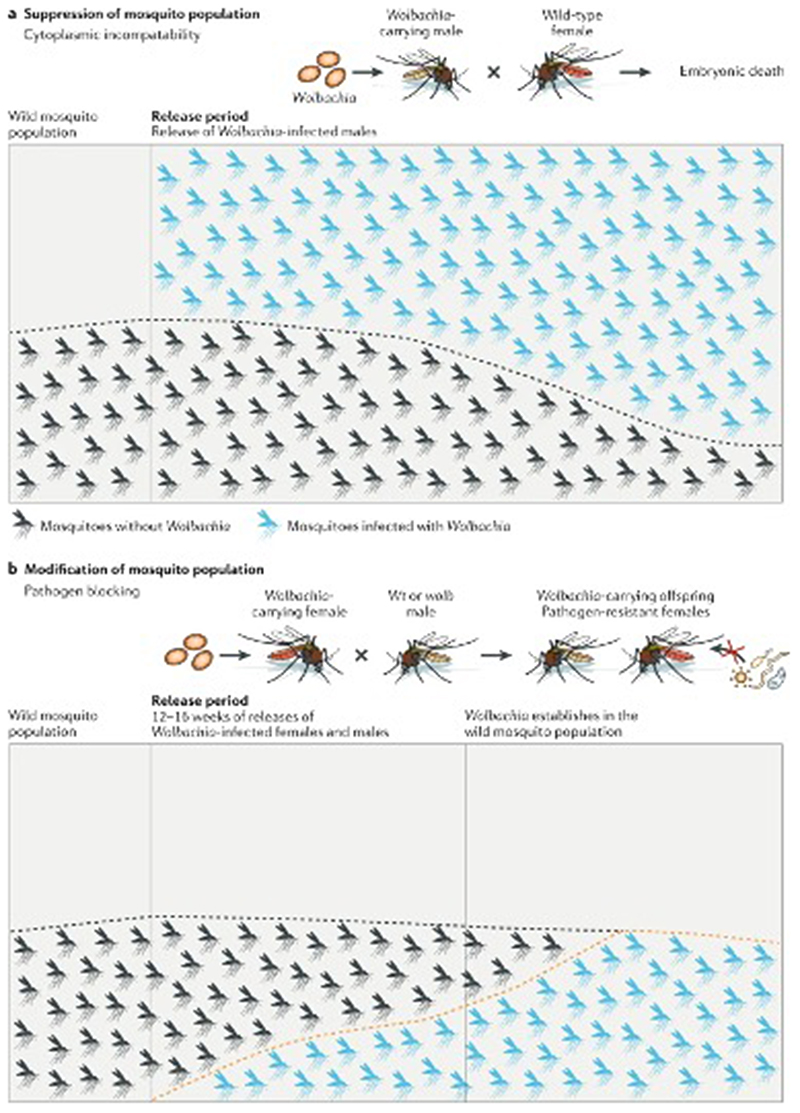
Figure 3. Wolbachia use for population reduction and modification approaches.
A) Using Wolbachia in the incompatible insect technique (IIT) approach, results in population reduction. In IIT, a Wolbachia strain is stably introduced into a colony of a mosquito species. Only Wolbachia-infected males are released, which when mated to females that do not harbour the same Wolbachia strain or that do not carry Wolbachia, results in the death of their offspring due to CI. Large numbers of males are released to increase the number of incompatible matings that are occurring. Over time, the population of disease-competent mosquitoes will decrease. B) Wolbachia can also be used to modify a mosquito population. Both Wolbachia-infected male and female mosquitoes are generally released over a 12-16 week period. CI provides a reproductive advantage to Wolbachia-infected females resulting in the spread and establishment of Wolbachia in the population. These Wolbachia-infected females are resistant to arboviruses such as dengue, Zika, and chikungunya.
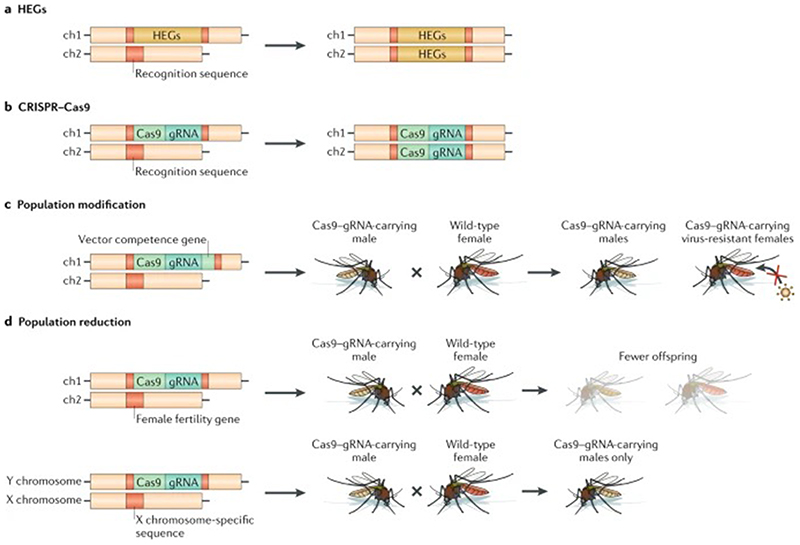
Figure 4. Gene-drive approaches for population modification and reduction.
A) Homing endonuclease genes (HEGs) encode endonuclease genes that recognize a specific DNA sequence and catalyse a break, which is then naturally repaired through homology-directed repair resulting in non-Mendelian inheritance. B) The CRISPR-Cas9 system is analogous to HEGs; however, a guide RNA (gRNA) provides sequence specificity for DNA cleavage by the Cas9 nuclease which is then repaired through homology-directed repair. Examples of CRISPR-Cas9 used in population modification and reduction approaches include C) the addition of vector competence genes with the Cas9-gRNA construct resulting in virus-resistant offspring 99, D) creating a gRNA to targeting female fertility genes resulting in sterile females 100, and creating a gRNA to target X-chromosome-specific sequences resulting in a reduction of female offspring 101. The Cas9-gRNA constructs inherited by any surviving offspring resulting in its continual spread.
Before any new vector control approach can be deployed at scale, it should progress from laboratory-based proof-of-concept experiments to semi-field and then open-field releases 11. To achieve this, researchers must adapt the vector-control methodologies for large-scale releases, satisfy regulatory requirements and commit to investing great effort in community engagement. Strong public support is critical for adoption of any new technology, as these stakeholders have the power to help “pull” a technology to the field – or alternatively prevent its implementation. Finally, if field releases are successful, the task of demonstrating epidemiological impact for a particular technology still remains. Effective epidemiological studies require the support and involvement of the community at the trial site, approval by the government, collaboration with the existing health system and robust financial support.
In this Review, we describe, evaluate and compare novel vector-control methodologies that are based on either the modification of the mosquito population or mosquito population suppression approaches and that require the active release of modified mosquitoes. We highlight knowledge gaps and discuss lessons learned from field releases that may aid in the success of these or other approaches going forward.
Population modification approaches
Wolbachia to target virus transmission
The endosymbiotic bacterium Wolbachia pipientis, referred to as Wolbachia from here on, naturally infects an estimated 40-60% of all insect species 12,13. It is vertically transmitted via the host egg, and many Wolbachia strains manipulate host reproduction to provide an advantage to infected females – most commonly by inducing cytoplasmic incompatibility (CI). Infected females can mate successfully with both infected and uninfected males, which enables the rapid spread of Wolbachia throughout a population (FIG. 3B). The expression of CI also provides a method to suppress insect populations, by releasing Wolbachia-infected males into a population of naturally uninfected female insects, thus effectively sterilizing those females (see below, also FIG. 3A).
Recently, it was discovered that in addition to inducing CI in insects Wolbachia can protect its natural host Drosophila melanogaster from pathogenic viruses such as Drosophila C virus 14,15. Since that initial observation, a number of different Wolbachia strains were shown to prevent the transmission of a range of viruses and parasites in laboratory studies 16–23 by preventing pathogen replication within the insect 16,24
The properties of CI coupled with the inhibition of virus replication provide the basis for a novel intervention strategy against mosquito-transmitted diseases. By releasing both male and female mosquitoes that are infected with Wolbachia into a wild population it should be possible for Wolbachia to invade that population. Wolbachia-infected females would have a reproductive advantage compared with wild-type females owing to the induction of CI, and Wolbachia would naturally spread throughout the population until nearly all mosquitoes carried it. The Wolbachia-infected females would then have greatly reduced ability to transmit a virus to humans, and disease should decline and potentially be eliminated from communities 25,26.
In contrast to many insects, including many mosquito species, A. aegypti is not a natural host for Wolbachia, and therefore to use Wolbachia to modify a population, the bacteria must be introduced into the mosquito through microinjection and a stable colony needs to be established 24,27. Subsequently, it needs to be determined if Wolbachia can reduce the vector competence of the mosquito. To date, eight different Wolbachia strains have been transinfected into A. aegypti: wMel, wMelPop-CLA, wMelCS, wRi, wAu, wAlbA, wAlbB and wPip 24,27–30. Importantly, it was shown that Wolbachia can limit the transmission of a range of human pathogens by A. aegypti, including dengue, Zika and chikungunya viruses 16,17,19,24, which suggests that this intervention could simultaneously target multiple diseases.
Using Wolbachia to reduce the ability of the mosquito population to transmit disease has a number of desirable attributes. The method requires the release of far fewer mosquitoes than population reduction methods such as SIT or IIT (Table I). Moreover, once Wolbachia are established in a population, they are expected to be maintained at a high frequency indefinitely 31. In Australia, initial releases of Wolbachia infected male and female insects were undertaken for 10 weeks, and Wolbachia infection has persisted in wild mosquito populations at frequencies above 90% 32. Therefore, mosquitoes infected with Wolbachia only need to be deployed once, which is in contrast to population suppression strategies (which requires the repeated deployments of modified mosquitoes as the natural vector population recovers). As a result, Wolbachia-based replacement strategies are cost effective and, moreover, as costs occur only for the initial deployment, donor fatigue’ might be less of a problem for this approach as no ongoing recurrent funding is needed to sustain the intervention. Finally, as this method involves the release of both female and male mosquitoes, there is no need for the laborious and error-prone process of sex sorting before release.
Table 1. Comparison of different vector control technologies that are currently being developed.
| Technology | Laboratory proof-of-concept | Field release | Scaled deployment beyond 50 km2 | Reapplication required | Approximate release rate (mosquitoes/ha/week) |
|---|---|---|---|---|---|
| Population modification | |||||
| Wolbachia | + | + | + | No | 10-100 75,109 |
| CRISPR-Cas9 | + | - | - | No | <1 |
| Population suppression | |||||
| SIT | + | + | - | Yes | 1,000* 67 |
| IIT (Wolbachia) | + | + | - | Yes | 1,000-10,000* 72,73,114 |
| RIDL | + | + | - | Yes | 25,000-50,000* 35,90 |
males/ha/week
Ongoing field trials
Currently, the World Mosquito Program (previously known as the Eliminate Dengue Program) 33 is undertaking deployments of A. aegypti infected with Wolbachia in five countries with strong community support. These studies have shown that the wMel strain of Wolbachia can quickly spread to near fixation in the wild mosquito population, and in the field sites in Australia, where this approach has been studied the longest, the frequency of the wMel strain in the mosquito population has remained stable since the initial deployment in 2011 at rates of around 90% or greater 32,34. Large-scale releases are now underway in Brazil (Niteroi and Rio de Janeiro), Colombia (Bello and Medellin) and Indonesia (Yogyakarta) 35 in the form of randomized cluster trials or large non-experimental deployments covering more than 2 million inhabitants each.
Work in Australia has shown that the method can be deployed successfully at low cost across small cities. Moreover, early time series observational data from these sites indicates no observations of local dengue transmission once Wolbachia is established in the local mosquito population 36. A randomised cluster trial of the method is currently in progress in Yogyakarta 33 (ClinicalTrials.gov, # NCT03055585). This trial, which is estimated to finish in 2019, is expected to provide high quality epidemiological evidence on the degree of disease reduction through the use of Wolbachia-infected mosquitoes.
Releasing female mosquitoes is not without issue. As female mosquitoes bite, their release can be a source of discomfort for individuals. During the period of active releases, the number of female mosquitoes present in the population will temporarily increase and the community may experience higher biting pressure. The released Wolbachia-carrying female mosquitoes are expected to decrease, rather than increase, the transmission of arboviruses such as dengue, Zika and chikungunya viruses 16,19,24; however, communities need to accept an approach that superficially seems to counter years of health-promotion messages advising to kill mosquitoes. This issue needs to be addressed with strong community engagement 37.
Publicly available risk analyses provide confidence about the safety of the method 33,38,39, but some concern has been raised that Wolbachia infection might enhance the transmission of other pathogens. For example, it has been suggested that transient Wolbachia infections (that is temporary infections of Wolbachia injected into the body of the mosquito followed by pathogen challenge) may enhance infection rates, although not dissemination or transmission rates, of West Nile virus in the mosquito Culex tarsalis 40. However, in stably-transmitted Wolbachia infections (where the Wolbachia infection is stable, infects germ line tissues and is maternally transmitted by the mosquito), no enhancement of transmission of any virus, including West Nile virus, has been shown 16–18,21,24,41–43. Similarly, transient Wolbachia infections in anopheline mosquitoes have suggested that in certain contexts vector competence for infections with Plasmodium spp. might be enhanced 44–46, whereas in naturally infected anophelines vector competence is reduced 47,48. By contrast, natural Wolbachia infections of Culex pipiens mosquitos have been shown to increase susceptibility to infections with Plasmodium relictum 49. Clearly, extensive testing is needed before release to ensure that the control measure does not inadvertently exacerbate disease.
Although Wolbachia is a vertically transmitted endosymbiont, comparisons of host and bacterial phylogenies suggest that horizontal transmission can occur 50,51, leading to the concern that the introduction of Wolbachia to a novel host such as A. aegypti could result in the horizontal transfer of the bacterium to predators or other insects. Laboratory- and field-based experimental testing for horizontal transfer of Wolbachia from A. aegypti have found no evidence of transfer 52,53. Moreover, 40-60% of all insect species are naturally infected with Wolbachia 13,54, and it is unlikely that introducing Wolbachia into one more species will increase the frequency of horizontal transmission, especially when closely related, Wolbachia-infected mosquitoes such as A. albopictus and A. notoscriptus already inhabit the same larval habitats as A. aegypti. Furthermore, the fact that A. aegypti is not a natural host for Wolbachia despite shared habitats with the aforementioned species provides further support that horizontal transmission is unlikely to occur.
It has also been suggested that viruses might develop mutations over time that render them less susceptible or resistant to Wolbachia 55. The mechanistic basis of Wolbachia-mediated pathogen blocking remains to be fully elucidated, but current data suggest that multiple pathways underlie this effect, which suggest that resistance will not evolve easily 56–58. Moreover, assessments of field-released mosquitoes suggest that if resistance does develop, it may not happen quickly 34. And even if resistance were to develop in the future, a great reduction in disease burden may have been afforded to communities in the intervening period.
Population reduction approaches
Sterile insect technique
Reducing mosquito populations has long been a focus of disease control programs with the underlying assumption that reducing the number of mosquitoes present in a population will limit the probability of transmission for viruses such as dengue or Zika. Population reduction approaches have been widely used, despite limited experimental evidence supporting the epidemiological effectiveness of these approaches 10. One such method of population reduction, the SIT, involves irradiating or chemically treating male mosquitos to sterilize them. When these males are released and mate with wild females, no offspring are produced, eventually leading to a substantial decrease in population size (FIG. 2A) 59. SIT has been used to reduce mosquito populations with some success, although it has generally been more successful in other agricultural pest species 60,61.
Work to explore the use of SIT in Aedes species has begun 62–66, and the results of a pilot field trial have been published. One study, trialing SIT in A. albopictus at four different sites, found that eggs collected in ovitraps from treated areas had induced egg sterility rates of 18-68% compared to eggs from untreated areas, with two sites showing a significant reduction (50-72%) 67.
The use of SIT has a number of advantages. As females will not be released, communities should experience no increase in biting rates and therefore the intervention may be more acceptable than population modification approaches described above. It is also aligned with existing health-promotion messaging of reducing mosquito population size. Finally, if the population can be suppressed then reductions in disease transmission are expected with little chance of pathogen resistance developing.
However, the temporary nature of vector population suppression has some disadvantages. Complete elimination of a vector population in an area would require large numbers of males to be released over a long time period (Table I) – especially considering the biology of A. aegypti (for example, mosquito eggs can withstand drying for many months) - and unless the population is completely eliminated it is expected to recover quickly if no further control measures are in place. Similarly, migration of mosquitoes from regions other than the treatment area would spark a population resurgence. This means that SIT releases need to be repeated regularly to maintain community protection from disease. It also requires that mosquitoes are sorted by sex before release, which is not straightforward, and any females that escape sorting may be a competent disease vector. Another difficulty in using SIT is the generation of sterile males that have high fitness and are reproductively competitive with wild-type males 65,68. Finally, as for all suppression methods, a likely scenario is that the mosquito population is only reduced and not eliminated. Unfortunately, there are no experimental studies available that robustly measure the impact of incomplete suppression on epidemiological endpoints, and therefore the effects on disease are currently unclear.
Incompatible insect technique
A modified version of SIT, termed the IIT, can overcome the fitness costs that are associated with irradiation or chemical treatment of males by using Wolbachia to effectively sterilize males 69. To implement IIT, a Wolbachia strain is stably introduced into a colony of a mosquito species. In contrast to population modification approaches, only male mosquitoes carrying Wolbachia are released into a wild population to mate with wild-type females; owing to the CI induced by Wolbachia, no offspring can be produced. If males are released in high enough numbers, more incompatible matings will occur, and ultimately, the population collapses (FIG 3A).
IIT has a long history of field trials. In 1967, Wolbachia was first used as a population reduction strategy to control Culex quinquefasciatus in Burma 70. Subsequently, semi-field and pilot field studies of IIT have been performed for A. albopictus and A. polynesiensis 71–73. Wolbachia-infected males of the lymphatic filiariasis vector, A. polynesiensis, were released over a thirty-week span in French Polynesia, leading to a significant decrease (17%) in egg brood-hatch success in the treated area relative to an untreated area 72. In Kentucky (USA), A. albopictus males transinfected with the wPip strain of Wolbachia were released over a 17 week period, causing a significant decrease in the mean number of females collected, as well as a reduction in egg hatch in treated compared to untreated areas 73.
IIT has many advantages as a method of vector population reduction. Using Wolbachia to ‘sterilize’ males is not associated with the fitness costs that can reduce male mating competitiveness in SIT approaches 68. Depending on the strain of Wolbachia used, females that escape sorting and are released may have greatly reduced ability to transmit pathogens. Finally, as Wolbachia is naturally occurring and already ubiquitous, the public may accept this technique more easily than genetic modification or irradiation.
By contrast, the IIT still shares many of the limitations of SIT. It requires the continual release of large numbers of males to suppress the population (Table I), and migration of mosquitos from surrounding (untreated) areas will limit long-term effectiveness of this method. As only males are introduced into the environment, an effective sex-sorting system is still required. If non-negligible numbers of females are also released, Wolbachia could spread through a population as in a replacement approach rather than suppress it – although the probability of this is dependent on the overall fitness effects that the Wolbachia strain has on the vector 31,74,75. Although the IIT approach was tested on a small scale in pilot studies more than 50 years ago, it has yet to be shown that this approach can be scaled-up sufficiently to be an effective operational tool for disease control.
Using a combination of IIT and SIT could further reduce the need to carefully sort females from males before release. In this method, Wolbachia-infected mosquitoes are treated with low level irradiation, which sterilizes females whereas males are unaffected. Females that escape sex sorting and are released into the wild cannot produce offspring and therefore would not interfere with the induced CI in the population (FIG. 2B) 76,77. The low dose of irradiation has minimal effects on male fitness in a laboratory setting, which suggests that this combined method could be effective in the field 76–79.
Genetically modified mosquitoes
Although a number of transgenic systems have been developed to suppress mosquito populations, few have progressed to field releases 80–83. Oxitec has developed a number of transgenic approaches that are based on their Release of Insects carrying a Dominant Lethal (RIDL) methodology 80,84. The OX513A mosquito strain has been used most successfully to date. This mosquito strain has a tetracycline-repressible transcriptional activator (tTAV) under the control of its own binding site (TetO) creating a positive-feedback loop in which the expression of tTAV results in late-larval lethality. 84. When the mosquitoes are reared on a diet supplemented with tetracycline, it binds tTAV preventing its binding to TetO, decreasing the production of tTAV allowing the mosquitoes to thrive. When OX513A males are released into the wild and mate with wild-type females, they pass on the transgene to their offspring; owing to the lack tetracycline in their diet, the transgene is expressed leading to late-larval death (FIG. 2C) 84–86.
Two field studies have compared the fitness of OX513A mosquitoes to that of wild-type mosquitoes for male competitiveness 87 as well as dispersal and longevity 88. The life expectancy and maximal dispersal distance of OX513A is similar to that of wild-type mosquitoes, but the mean distance travelled is significantly lower 88.
Field releases of these mosquitoes have been performed in the Cayman Islands and Bahia, Brazil. The release of OX513A in the Cayman Islands allowed researchers to perform real-time comparisons of the effective numbers of males required to achieve a significant decrease in mosquito populations. Under their highest release ratios, they found an 80% relative reduction in treated versus untreated areas over a 23-week period 89. In Brazil, mosquitoes were released over the course of one year. A 95% reduction in the local population of A. aegypti was observed based on adult trapping data, and an 81% reduction based on egg trapping data 90. Currently, Oxitec is performing releases in the Cayman Islands, Panama and Brazil, with plans for substantial expansion in their Brazil release sites 35.
Oxitec’s methodology has several advantages over traditional suppression methods. The radiation used in SIT generates dominant lethals in a non-specific manner – which can also lead to strong fitness effects and lowered mating competitiveness of males. The RIDL method specifically engineers a dominant lethal, thereby limiting off-target effects. Additionally, engineering allows for control of when the lethality is induced (that is, the presence of absence of tetracycline) 84. In contrast to the SIT, which induces lethality generally at the embryonic stage, lethality of OX513A is induced at late-larval stages 59, suggesting that although OX513A larvae ultimately die before adulthood, they still compete with wild-type larvae for food, possibly enhancing population suppression 84. Although the public may have concerns about the release of genetically modified insects, their concerns may be alleviated by the fact that the OX513A-based approach is a self-limiting technology. As the transgenic mosquitoes require tetracycline in their diet for survival, mosquitoes that carry the transgene cannot survive more than one generation in the field.
One of the biggest limitations of a RIDL technology such as the OX513A-based approach is that it requires large numbers of males to be released for successful suppression (Table I), and this can be technologically and financially difficult. For the release of OX513A in both the Cayman Island and Brazil, the planned field site sizes had to be decreased owing to rearing limitations and a requirement to maintain a mating fraction of 50% for genetically modified males 89,90. This suggests that large-scale releases could be difficult to maintain. As with SIT and IIT, accurate sex sorting is required for this RIDL method. Although sex-sorting methods have become more efficient, rates of accidental release of females were previously reported to be between 0.02-0.33% 88–90. For large scale releases, such as those planned in Brazil for which Oxitec estimates releases of 30-60 million males per week 35, this would result in the unintended daily release of thousands of females. In addition, a considerable community engagement effort to build sufficient trust for widespread deployment of genetically modified mosquitoes is required 91.
Emerging technologies
A number of developing technologies exist that have not yet progressed to field trials. Numerous laboratory-based studies have shown that the use of transgenes can be effective in limiting pathogen transmission through the expression of genes that target the pathogen, or that are effective in suppressing mosquito populations by targeting genes involved in reproduction or by using sex distortion systems. Although these systems show promise, the difficulty lies in how to spread the transgenes to all mosquitoes in a population. One of the most promising methods to solve this problem is the use of transgenes to generate a gene-drive system, a strategy that was first proposed nearly 15 years ago 92. Gene-drive systems alter normal Mendelian inheritance to greatly increase the odds that the drive system will be passed on to offspring. An effective gene-drive system could be used to establish disease inhibitors or population repressors into a population. Homing endonuclease genes (HEGs) were the initial inspiration for a gene drive system. HEGs encode proteins that recognize and cleave a 15-30 bp DNA sequence. By placing HEGs within their target sequences, the chromosome on which it was located would be resistant to cleavage. Cleavage of chromosomes that only contain the recognition site would occur, and owing to homology-directed repair (HDR) a heterozygote would be converted into a homozygote (FIG. 4A). HEGs have been developed in Anopheles and Aedes mosquitoes in proof-of-concept experiments 93–95.
The CRISPR-Cas9 system has been used in genome-editing for a number of years in diverse organisms 96,97. A study showed that placing the genes that encode Cas9 and a guide RNA (gRNA) into the template used for HDR generated a mutagenic ‘chain reaction’ capable of gene drive (FIG. 4B) 98. Subsequent work showed that in laboratory settings, CRISPR-Cas9 could be used to spread anti-Plasmodium falciparum effector genes into an Anopheles stephensi population 99, to target genes required for female fertility in Anopheles gambiae 100, and to create a sex distortion system that targets female A. gambiae 101, suggesting that that this system can be used for both population suppression and population modification (FIG. 4C, D). However, optimization of this methodology is still required before commencing field trials. The first two studies discussed above 99,100 used the regulatory regions of the germline-specific gene vasa to induce the expression of Cas9 in the germline thus causing only heritable mutations. However, one study found that the expression of Cas9 was not completely restricted to the germline resulting in somatic mutations 100. A second study found that maternal deposition of the Cas9 protein from the mother into the developing egg caused double-stranded DNA breaks during early embryonic development before a homologous chromosome was present as a repair template, resulting in an increase in non-homologous end joining repair (NHEJ) rather than HDR 99. Repair via NHEJ often results in mutations, or insertions or deletions of sequences which destroy the Cas9-recognition site and thus the generation of resistant alleles 99,100.
The CRISPR-Cas9 gene-drive method can potentially be extremely powerful. Only small numbers of the modified mosquitoes might need to be released (Table I) as the modification should drive itself throughout a population, 98, and nearly any sequence of interest can be targeted. However, similarly to HEGs, the CRISPR-Cas9 system is susceptible to developing resistance owing to mutations that can occur in the recognition site. As described above, multiple, laboratory-based studies using CRISPR-Cas9 for gene drive have reported the accumulation of mutations that led to CRISPR-resistant alleles 98–100, which halt the spread of any modifications throughout a population. Furthermore, based on theoretical modeling, evolution of resistance against the CRISPR-Cas9 system is inevitable 102,103. The emergence of resistance might be avoided, or at least prolonged, by targeting multiple sequences, by targeting conserved sequences that cannot tolerate disruption or by being more mindful of when releases occur in relation to seasonality of the vector population 102. Whereas the OX513A strain is self-limiting, the CRISPR-Cas9-drive is self-promoting. The potential for uncontrolled spread of genetic modifications has caused concern among the scientific community 104, resulting in the publication of guidelines not only pertaining to field releases of such modified organisms but also preventing the accidental release of the modified organisms from laboratories 104–107.
Lessons learned
Most of the different technologies described above are still at early developmental stages, with limited examples of field releases, and only Wolbachia-based population modification approaches, as undertaken by the World Mosquito Program (WMP) 33, being done at operational scales in medium-sized cities. A number of lessons are being learned that generally apply to all of the approaches.
Importance of field-cage studies
Advocates of phased testing approaches have stressed the importance of preliminary testing of technologies in semi-field cages prior to open field release 11. The construction of these facilities is expensive and time consuming, and evidence suggests that they may not actually provide data that are more useful than data collected from small laboratory cages in regards to evaluating an approach. Even very elaborate field cages 108 do not mimic the true field situation. For example, semi-field cage experiments demonstrated successful establishment of the wMelPop strain of Wolbachia in a mosquito population, but it was later shown that this Wolbachia strain could not be established following open field releases 24,109. Those findings together with the expense of such preliminary testing strategies indicate that field-cage studies should be carefully considered and relevant to the question being addressed and not automatically recommended.
When the WMP first started to undertake field releases there was some concern that Wolbachia might spread in an uncontrolled manner. There was good evidence documenting regional 110,111 and even global sweeps 112 of Wolbachia infections in naturally infected hosts, raising the prospect that once Wolbachia was released it might spread to locations that might not have approved its release. Initial field testing was done very carefully in Australia in geographically isolated areas to evaluate the ability of Wolbachia to spread 75. Over time it was realised that in A. aegypti, the spreading rates of the wMel strain of Wolbachia were very slow 113, and the initial concern was unfounded. The current controversy around the potential uncontrolled spread of CRISPR-Cas9 gene-drive technology is injecting an even greater sense of caution into this area 104,105,107. The theoretical ability of gene-drive systems to spread from very small numbers of released individuals and to alter an entire wild population is of concern as we do not fully understand possible adverse consequences of such a release and may not be able to assess it prior to release. Current gene-drive methodologies do not have reversibility built into the system, so if negative consequences are observed, it would be difficult to stop the intervention from spreading. However, the emerging issues of resistance with this technology suggest that similar to Wolbachia-based approaches, the power of the gene drive systems that are being developed might be similarly overstated. Although there is merit in a cautious framework to evaluate and test these methods, it must be balanced against the public health need of new technologies to protect people from ongoing disease outbreaks. Testing and regulatory frameworks need to be sufficiently flexible to be able to adapt to less stringent and time consuming testing procedures if empirical evidence shows that risks are likely to be overstated. Otherwise technology that is urgently needed may be unnecessarily impeded in its adoption and use.
A common feature of all of these new vector control tools is that they rely on the release of mosquitoes into the environment to control the diseases they transmit. This requires communities to have high levels of trust to willingly participate given that the health promotion messages of decades have been based on the dangerous nature of mosquitoes and the need to kill them to reduce disease risk. Even the most robust and elegant technology will fail to be implemented if communities will not accept it. Recently, this was exemplified by the difficulties Oxitec has faced in applying the RIDL methodology in open releases in Florida where deep issues of mistrust toward GM technology, government and industry have led to open protests and stalling of testing plans of a potentially robust and useful technology 91. Serious attention and resourcing is required for effective community engagement programs associated with these technologies so that trust and acceptance can be built with the communities that will be the end recipient of the technology 37. This engagement is costly and time consuming and needs to start early, even before a given technology is fully developed. Unfortunately, many of the scientists involved in the development of new technologies are laboratory focused specialists with little experience in field application or the principles of effective community engagement.
Conclusion
Existing vector control methods are clearly unable to cope with the unprecedented emergence and reemergence of arboviral diseases. A number of novel methods under development show promise in curbing the ability of A. aegypti mosquitoes to transmit pathogens. Within the next few years we expect that the evidence for the effectiveness of these new interventions will accumulate. Critical to wide scale adoption of any of these approaches will be rigorous epidemiological evidence showing the impact on disease, not just entomological indicators. Many of these technologies are being developed by scientists that are not located in disease endemic countries. Ultimately, collaborations between scientists and governments of affected countries are needed to test and apply the technology. This requires open and authentic partnerships to be developed very early with these collaborators so that they are active participants in the development and implementation of the technology in their countries. Without their full support and ownership there is no pathway to adoption. Just as important will be the sustainability and cost effectiveness of the different approaches for disease endemic countries with limited resources for control programs. Hopefully, at least some of these technologies will prove to be cost saving for health ministries in which case adoption pathways will be more straight forward. With solid epidemiological evidence and community support, their widespread implementation might reverse the current, alarming global disease trend.
Glossary.
Aedes aegypti
Aedes aegypti is the primary mosquito vector of epidemic transmission for viruses, such as dengue, zika and chikungunya. A. aegypti occurs primarily in tropical and sub-tropical regions of the world and is particularly adapted to urban habitats.
Wolbachia pipientis
A naturally occurring bacterial endosymbiont that is estimated to be present in 40-60% of all insect species. Commonly referred to as just Wolbachia.
Sterile insect technique
The radiation or chemical treatment of male mosquitos, which renders them sterile. When they are released in the field and they mate with wild-type females they cannot produce offspring.
incompatible insect technique
The release of Wolbachia-infected males, which when mated with wildtype females who contain no Wolbachia or a different, incompatible strain of Wolbachia, produce no offspring due to cytoplasmic incompatibility.
CRISPR-Cas9
A genome editing tool that was developed from adaptive immune systems found in bacteria and archaea. The system is composed of a nuclease, Cas9, and a guide RNA which targets the nuclease to a specific DNA sequence for cleavage.
Cytoplasmic incompatibility
When Wolbachia-infected male mosquitos mate with uninfected females, the resulting progeny die during early embryogenesis. If the female is also infected with the same Wolbachia strain, that infection can rescue the embryonic lethality resulting in viable progeny.
Vector competence
A measure of ability of arthropod vectors to acquire and transmit viruses in their saliva.
Ovitraps
Traps designed for the collection of mosquito eggs.
Homing endonuclease genes
Selfish genetic elements encoding endonucleases that recognize a specific DNA sequence and catalyse a break, which is then naturally repaired through homologous repair.
Homology-directed repair
A repair mechanisms of a DNA double-strand break, whereby the homologous chromosome is used as a template for repair.
Non-homologous end joining repair
A repair mechanism for DNA double-strand breaks, whereby the two DNA ends are ligated without the need for a homologous template, often resulting in small indels or the introduction of mutations.
Acknowledgements
The authors are grateful to M. Woolfit for reviewing the manuscript and acknowledge funding from The Foundation for the National Institutes of Health through the Vector-Based Transmission of Control: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative of the Bill & Melinda Gates Foundation and The Wellcome Trust Award No.102591.
Footnotes
Author contributions
H.A.F. researched data for the article. S.L.O’N. and H.A.F. made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests statement
Heather A. Flores and Scott L. O’Neill work for The World Mosquito Program.
References
- 1.Gubler DJ, Vasilakis N. Arboviruses: Molecular Biology, Evolution and Control. Caister Academic Press; 2016. pp. 1–6. [DOI] [Google Scholar]
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.Pang T, Mak TK, Gubler DJ. Prevention and control of dengue-the light at the end of the tunnel. Lancet Infect Dis. 2017;17:e79–e87. doi: 10.1016/S1473-3099(16)30471-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. A recent report estimating that dengue infections are three times higher than previously estimated by the World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagcchi S. Looking back at yellow fever in Angola. Lancet Infect Dis. 2017;17:269–270. doi: 10.1016/S1473-3099(17)30064-6. [DOI] [PubMed] [Google Scholar]
- 7.Zwizwai R. Infectious disease surveillance update. Lancet Infect Dis. 2017;17:270. doi: 10.1016/S1473-3099(16)00023-2. [DOI] [PubMed] [Google Scholar]
- 8.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson AL, et al. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31:380–390. doi: 10.1016/j.pt.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Bowman LR, Donegan S, McCall PJ. Is Dengue Vector Control Deficient in Effectiveness or Evidence?: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004551. doi: 10.1371/journal.pntd.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict M, et al. Guidance for Contained Field Trials of Vector Mosquitoes Engineered to Contain a Gene Drive System: Recommendations of a Scientific Working Group. 2008;8:127–166. doi: 10.1089/vbz.2007.0273. http://www.liebertpub.com/vbz. [DOI] [PubMed] [Google Scholar]
- 12.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? â□” a statistical analysis of current data. FEMS Microbiology Letters. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS biology. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. A demonstration that Wolbachia can provide viral protection to Drosophila melanogster. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702–702. doi: 10.1126/science.1162418. A demonstration that Wolbachia can provide viral protection to Drosophila melanogster. [DOI] [PubMed] [Google Scholar]
- 16.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. A demonstration that Wolbachia can provide pathogen protection to a transinfected Aedes aegypti host. [DOI] [PubMed] [Google Scholar]
- 17.Aliota MT, et al. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl Trop Dis. 2016;10:e0004677. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Scientific reports. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutra HLC, et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell host & microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. The first report that Wolbachia can reduce Zika virus transmission in transinfected Aedes aegypti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joubert DA, O’Neill SL. Comparison of Stable and Transient Wolbachia Infection Models in Aedes aegypti to Block Dengue and West Nile Viruses. PLoS Negl Trop Dis. 2017;11:e0005275. doi: 10.1371/journal.pntd.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux A-B, Sinkins SP. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl Trop Dis. 2013;7:e2152. doi: 10.1371/journal.pntd.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson NM, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra37-279ra37. doi: 10.1126/scitranslmed.3010370. A report modeling the impact of Wolbachia-infected Aedes aegypti mosquitoes on dengue virus transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorigatti I, McCormack C, Nedjati-Gilani G, Ferguson NM. Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol. 2017;34:102–113. doi: 10.1016/j.pt.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 28.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi Z, Khoo CCH, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 30.Fraser JE, et al. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017;13:e1006751. doi: 10.1371/journal.ppat.1006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turelli M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution. 2010;64:232–241. doi: 10.1111/j.1558-5646.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann AA, et al. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. [Accessed: 3rd September 2017];Eliminate Dengue Program. Available at: http://www.eliminatedengue.com.
- 34.Frentiu FD, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Servick K. Winged warriors. Science. 2016;354:164–167. doi: 10.1126/science.354.6309.164. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed: 9 April 2018];Queensland Health: Dengue outbreaks. Available at: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/diseases/mosquito-borne/dengue/dengue-outbreaks.
- 37.Kolopack PA, Parsons JA, Lavery JV. What makes community engagement effective?: Lessons from the Eliminate Dengue Program in Queensland Australia. PLoS Negl Trop Dis. 2015;9:e0003713. doi: 10.1371/journal.pntd.0003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Barro PJ, Murphy B, Jansen CC, Murray J. The proposed release of the yellow fever mosquito, Aedes aegypti containing a naturally occurring strain of Wolbachia pipientis, a question of regulatory responsibility. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2011;6:33–40. [Google Scholar]
- 39.Murray JV, Jansen CC, De Barro P. Risk Associated with the Release of Wolbachia-Infected Aedes aegypti Mosquitoes into the Environment in an Effort to Control Dengue. Front Public Health. 2016;4:43. doi: 10.3389/fpubh.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodson BL, et al. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis. 2014;8:e2965. doi: 10.1371/journal.pntd.0002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joubert DA, et al. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Micieli MV, Glaser RL. Somatic Wolbachia (Rickettsiales: Rickettsiaceae) levels in Culex quinquefasciatus and Culex pipiens (Diptera: Culicidae) and resistance to West Nile virus infection. J Med Entomol. 2014;51:189–199. doi: 10.1603/me13152. [DOI] [PubMed] [Google Scholar]
- 44.Hughes GL, Vega-Rodriguez J, Xue P, Rasgon JL. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl Environ Microbiol. 2012;78:1491–1495. doi: 10.1128/AEM.06751-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. Temperature alters Plasmodium blocking by Wolbachia. Scientific reports. 2014;4:3932. doi: 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes GL, Rivero A, Rasgon JL. Wolbachia can enhance Plasmodium infection in mosquitoes: implications for malaria control? PLoS Pathog. 2014;10:e1004182. doi: 10.1371/journal.ppat.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw WR, et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun. 2016;7:11772. doi: 10.1038/ncomms11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes FM, et al. Effect of naturally occurringWolbachiainAnopheles gambiae s.l.mosquitoes from Mali onPlasmodium falciparummalaria transmission. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:12566–12571. doi: 10.1073/pnas.1716181114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zélé F, et al. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proceedings Biological sciences. 2014;281:20132837-20132837. doi: 10.1098/rspb.2013.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol. 1999;16:1711–1723. doi: 10.1093/oxfordjournals.molbev.a026084. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed MZ, Breinholt JW, Kawahara AY. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC evolutionary biology. 2016;16:118. doi: 10.1186/s12862-016-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popovici J, et al. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Memorias do Instituto Oswaldo Cruz. 2010;105:957–964. doi: 10.1590/s0074-02762010000800002. [DOI] [PubMed] [Google Scholar]
- 53.Hurst TP, et al. Impacts of Wolbachia infection on predator prey relationships: evaluating survival and horizontal transfer between wMelPop infected Aedes aegypti and its predators. J Med Entomol. 2012;49:624–630. doi: 10.1603/me11277. [DOI] [PubMed] [Google Scholar]
- 54.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGraw EA, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 56.Caragata EP, et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rancès E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terradas G, Joubert DA, McGraw EA. The RNAi pathway plays a small part in Wolbachia-mediated blocking of dengue virus in mosquito cells. Scientific reports. 2017;7:43847. doi: 10.1038/srep43847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Black WC, Alphey L, James AA. Why RIDL is not SIT. Trends Parasitol. 2011;27:362–370. doi: 10.1016/j.pt.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Weidhaas DE, Breeland SG, Lofgren CS, Dame DA, Kaiser R. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. IV. Dynamics of the test population. Am J Trop Med Hyg. 1974;23:298–308. doi: 10.4269/ajtmh.1974.23.298. [DOI] [PubMed] [Google Scholar]
- 61.Dame DA, et al. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. II. Methods of rearing, sterilization, and distribution. Am J Trop Med Hyg. 1974;23:282–287. doi: 10.4269/ajtmh.1974.23.282. [DOI] [PubMed] [Google Scholar]
- 62.Boyer S, Gilles J, Merancienne D, Lemperiere G, Fontenille D. Sexual performance of male mosquito Aedes albopictus. Med Vet Entomol. 2011;25:454–459. doi: 10.1111/j.1365-2915.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- 63. [Accessed: 24 May 2017];IAEA FACTSHEET: The Zika Virus Mosquitoes How can the sterile insect technique help? Available at: https://www.iaea.org/sites/default/files/16/11/zika-virus-mosquitos-how-can-sterile-insect-technique-help.pdf.
- 64.Bellini R, et al. Dispersal and survival of Aedes albopictus (Diptera: Culicidae) males in Italian urban areas and significance for sterile insect technique application. J Med Entomol. 2010;47:1082–1091. doi: 10.1603/me09154. [DOI] [PubMed] [Google Scholar]
- 65.Bellini R, et al. Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J Med Entomol. 2013;50:94–102. doi: 10.1603/me11058. [DOI] [PubMed] [Google Scholar]
- 66.Balestrino F, et al. Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J Med Entomol. 2010;47:581–591. doi: 10.1093/jmedent/47.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013;50:317–325. doi: 10.1603/me12048. A report describing pilot field trials of SIT in Aedes albopictus populations in Italy. [DOI] [PubMed] [Google Scholar]
- 68.Atyame CM, et al. Comparison of Irradiation and Wolbachia Based Approaches for Sterile-Male Strategies Targeting Aedes albopictus. PLoS ONE. 2016;11:e0146834. doi: 10.1371/journal.pone.0146834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourtzis K, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132(Suppl):S150–63. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- 71.Chambers EW, Hapairai L, Peel BA, Bossin H, Dobson SL. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl Trop Dis. 2011;5:e1271. doi: 10.1371/journal.pntd.0001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor L, et al. Open release of male mosquitoes infected with a wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis. 2012;6:e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female Adult Aedes albopictus Suppression by Wolbachia-Infected Male Mosquitoes. Scientific reports. 2016;6:33846. doi: 10.1038/srep33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barton NH, Turelli M. Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of Allee effects. Am Nat. 2011;178:E48–75. doi: 10.1086/661246. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. A report showing that wMel Wolbachia can stably invade wild populations of Aedes aegypti. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D, Zheng X, Xi Z, Bourtzis K, Gilles JRL. Combining the sterile insect technique with the incompatible insect technique: I-impact of wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE. 2015;10:e0121126. doi: 10.1371/journal.pone.0121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brelsfoard CL, St Clair W, Dobson SL. Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasit Vectors. 2009;2:38. doi: 10.1186/1756-3305-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D, Lees RS, Xi Z, Gilles JRL, Bourtzis K. Combining the Sterile Insect Technique with Wolbachia-Based Approaches: II--A Safer Approach to Aedes albopictus Population Suppression Programmes, Designed to Minimize the Consequences of Inadvertent Female Release. PLoS ONE. 2015;10:e0135194. doi: 10.1371/journal.pone.0135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D, Lees RS, Xi Z, Bourtzis K, Gilles JRL. Combining the Sterile Insect Technique with the Incompatible Insect Technique: III-Robust Mating Competitiveness of Irradiated Triple Wolbachia-Infected Aedes albopictus Males under Semi-Field Conditions. PLoS ONE. 2016;11:e0151864. doi: 10.1371/journal.pone.0151864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wise de Valdez MR, et al. Genetic elimination of dengue vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu G, et al. Female-specific flightless phenotype for mosquito control. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinrich JC, Heinrich JC, Scott MJ, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8229–8232. doi: 10.1073/pnas.140142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labbé GMC, Scaife S, Morgan SA, Curtis ZH, Alphey L. Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus. PLoS Negl Trop Dis. 2012;6:e1724. doi: 10.1371/journal.pntd.0001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phuc HK, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Massonnet-Bruneel B, et al. Fitness of transgenic mosquito Aedes aegypti males carrying a dominant lethal genetic system. PLoS ONE. 2013;8:e62711. doi: 10.1371/journal.pone.0062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bargielowski I, Nimmo D, Alphey L, Koella JC. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti. PLoS ONE. 2011;6:e20699. doi: 10.1371/journal.pone.0020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris AF, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29:1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- 88.Lacroix R, et al. Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE. 2012;7:e42771. doi: 10.1371/journal.pone.0042771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harris AF, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol. 2012;30:828–830. doi: 10.1038/nbt.2350. A report that released Aedes aegypti mosquitoes that were engineered to have dominant lethal alleles can successfully mate with wild type females to suppress wild populations. [DOI] [PubMed] [Google Scholar]
- 90.Carvalho DO, et al. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bloss CS, Stoler J, Brouwer KC, Bietz M, Cheung C. Public Response to a Proposed Field Trial of Genetically Engineered Mosquitoes in the United States. JAMA. 2017;318:662–664. doi: 10.1001/jama.2017.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings Biological sciences. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Traver BE, Anderson MAE, Adelman ZN. Homing endonucleases catalyze double-stranded DNA breaks and somatic transgene excision in Aedes aegypti. Insect Mol Biol. 2009;18:623–633. doi: 10.1111/j.1365-2583.2009.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Windbichler N, et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212–215. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan Y-S, et al. The design and in vivo evaluation of engineered I-OnuI-based enzymes for HEG gene drive. PLoS ONE. 2013;8:e74254. doi: 10.1371/journal.pone.0074254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096-1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 97.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gantz VM, Bier E. Genome editing. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science. 2015;348:442–444. doi: 10.1126/science.aaa5945. A report describing the use of CRISPR-Cas as a method of gene-drive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gantz VM, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6736–43. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hammond A, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galizi R, et al. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Scientific reports. 2016;6:31139. doi: 10.1038/srep31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Unckless RL, Clark AG, Messer PW. Evolution of Resistance Against CRISPR/Cas9 Gene Drive. Genetics. 2017;205:827–841. doi: 10.1534/genetics.116.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reed FA. CRISPR/Cas9 Gene Drive: Growing Pains for a New Technology. Genetics. 2017;205:1037–1039. doi: 10.1534/genetics.116.198887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. eLife. 2014;3:20131071. doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akbari OS, et al. BIOSAFETY. Safeguarding gene drive experiments in the laboratory. Science. 2015;349:927–929. doi: 10.1126/science.aac7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okumu F, et al. Results from the Workshop ‘Problem Formulation for the Use of Gene Drive in Mosquitoes’. Am J Trop Med Hyg. 2017;96:530–533. doi: 10.4269/ajtmh.16-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.National Academies of Sciences, EngineeringMedicine. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. National Academies Press; 2016. [DOI] [PubMed] [Google Scholar]
- 108.Ritchie SA, et al. A Secure Semi-Field System for the Study of Aedes aegypti. PLoS Negl Trop Dis. 2011;5:e988. doi: 10.1371/journal.pntd.0000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen TH, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 111.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid Sequential Spread of Two Wolbachia Variants in Drosophila simulans. PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riegler M, Sidhu M, Miller WJ, O’Neill SL. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol. 2005;15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 113.Schmidt TL, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS biology. 2017;15:e2001894. doi: 10.1371/journal.pbio.2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. [Accessed: 1st September 2017];Debug Fresno, our first US field study. Available at: https://blog.verily.com/2017/07/debug-fresno-our-first-us-field-study.html.