Summary
Wiskott Aldrich syndrome (WAS) is a primary immunodeficiency disease resulting in recurrent infections, eczema and microthrombocytopaenia. In its classical form, significant combined immune deficiency, autoimmune complications and risk of haematological malignancy necessitate early correction with stem cell transplantation or gene therapy. A milder form, X-linked thrombocytopaenia (XLT), shares similar bleeding risk from thrombocytopaenia but is not associated with other significant clinical features and is generally managed conservatively. Here, we detail our approach to the diagnosis and treatment of classical WAS and XLT.
Keywords: Wiskott Aldrich syndrome, X-linked thrombocytopenia, immunodeficiency, haematopoietic stem cell transplant
Wiskott Aldrich syndrome (WAS) is a rare X-linked primary immunodeficiency disorder, with an incidence between 1 in 50 000 and 1 in 250 000 live births (Perry et al, 1980; Puck & Candotti, 2006). In its classical form, WAS presents early in life with a triad of recurrent infections, eczema and microthrombocytopenia caused by loss of function mutations in the WAS gene (Derry et al, 1994). Expressed only in haematopoietic cells, WAS encodes the Wiskott Aldrich syndrome protein (WASp), a key actin cytoskeletal regulator that coordinates assembly of actin filaments in response to cell signalling events (Machesky & Insall, 1998). Defects in WASp function have been shown to impair cellular processes in myeloid and lymphoid lineage cells, including cell adhesion and migration, phagocytosis, immune synapse assembly (reviewed in Thrasher & Burns, 2010) and, more recently, autophagy and inflammasome regulation (Lee et al, 2017). The pathogenesis of the platelet defect remains only partially understood and is thought to result from a combination of megakaryocyte dysfunction leading to small/abnormally formed platelets (Sabri et al, 2006; Ingrungruanglert et al, 2015) and increased platelet destruction in the spleen (Grottum et al, 1969). Megakaryocyte numbers in the bone marrow are typically normal (Grottum et al, 1969; Ochs et al, 1980).
Recognition of WAS is important because curative stem cell and gene therapies are available, without which median survival is reduced to 10–15 years as a result of infections, severe bleeding, autoimmune complications and haematological malignancies (Sullivan et al, 1994). Milder forms, known as X-linked thrombocytopenia (XLT), present with a similar bleeding phenotype but without other significant clinical features (Villa et al, 1995) and can generally be managed conservatively.
When we suspect WAS/XLT
Low number of platelets is a universal feature of WAS and XLT, usually presenting in the first year of life and typically causing petechiae, easy bruising, spontaneous or prolonged bleeding. Where no prior family history has impacted obstetric and neonatal care, cephalohematoma related to an instrumental delivery is not an uncommon first presentation, or prolonged bleeding if circumcision is undertaken (Ochs et al, 2009). In toddlers, bruising can be the presenting feature and may raise concerns about non-accidental injury. The degree of thrombocytopenia is variable and can be classified based on platelet count as severe (<20 × 109/l), moderate (20–50 × 109/l) or mild (>50 × 109/l). The finding of small platelets in the context of thrombocytopenia is pathognomonic for WAS/XLT (Andres et al, 2018) and the newly described- but functionally related ARPC1B deficiency (Kahr et al, 2017). Diagnosis can be achieved by an experienced haematologist on blood film, which we have found to be more reliable than routine full blood count parameters, where a normal haemocytometer mean platelet volume does not rule out the diagnosis. Occasionally, mild-to-moderate thrombocytopenia can present later in childhood, mimicking idiopathic thrombocytopenia (ITP) but without response to oral steroids. There are also reports of intermittent thrombocytopenia in XLT but these represent a rare subgroup (Notarangelo et al, 2002; Medina et al, 2017).
In contrast with thrombocytopenia, eczema and/or recurrent infections are variable features (Sullivan et al, 1994), but their presence in association with low platelet counts should prompt consideration of WAS/XLT (Fig 1). Autoimmunity and haematological malignancy are rarely the presenting features of classical WAS, but can complicate disease course (Fig 2).
Fig 1.
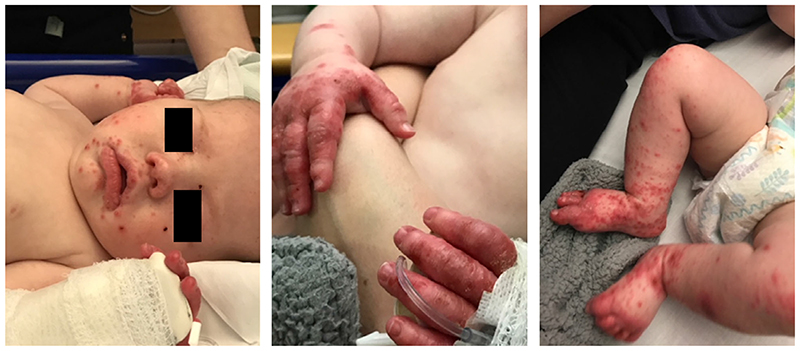
Classical presentation of WAS. Case presentation: Severe hand-foot-and-mouth disease on a background of eczema since birth, cow’s milk protein allergy and having a previous diagnosis of idiopathic thrombocytopaenia. Subsequently developed autoimmune haemolytic anaemia and thrombocytopenia. Initial investigations: Low CD8+ T-cells but otherwise normal lymphocyte numbers, with normal response to phytohaemagglutinin and vaccine response to tetanus. Platelet count was 27 × 109/l with normal mean platelet volume (automated). WAS protein expression was found to be absent and mutation in WAS gene [c.374G>A hemizygote, substitution of glycine for glutamic acid p.(Gly125Glu)] confirmed diagnosis. Treatment: matched unrelated donor haematopoietic stem cell transplantation at 9 months old.
Fig 2.
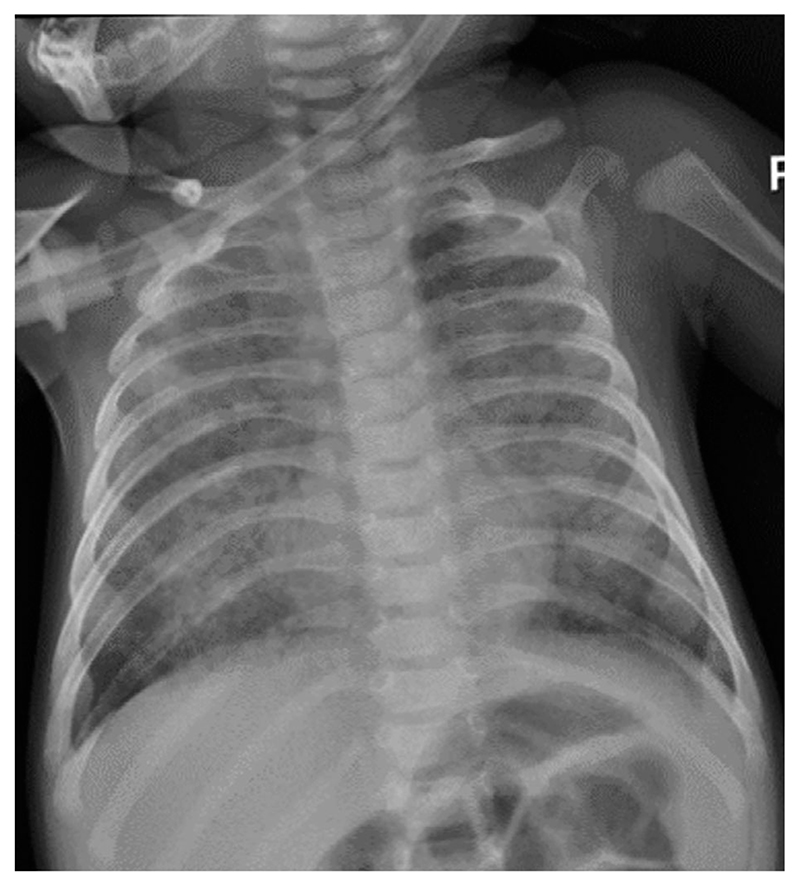
WAS and CMV infection. Case presentation: Monochorionic diamniotic twins presented with cytomegalovirus (CMV) pneumonitis at 5 months old on a background of persistent thrombocytopenia, infected eczema, reflux and colitis in the context of cow’s milk protein allergy. One twin subsequently developed pre-B infant acute lymphoblastic leukaemia. Initial investigations: normal lymphocyte numbers and response to phytohaemagglutinin stimulation with normal vaccine responses to tetanus and pneumococcus (conjugate vaccine) but absent response to CD3 stimulation. Platelet counts were low, at 37 × 109/l, with low mean platelet volume of 6·9. WAS protein expression was absent by flow and immunoblot, with mutation in WAS gene (c777+1G>A splice site mutation) confirming diagnosis. CMV viral loads were 302 888 and 122 834 copies/ml at presentation. Treatment: Haplo (T-cell receptor/CD19 depleted) haematopoietic stem cell transplantation at 21 months old.
How we diagnose WAS/XLT
While microthrombocytopenia is highly suggestive of WAS, genetic analysis is the gold standard for diagnostic confirmation and plays important roles in management decisions and family screening. Over 300 mutations are published (Burns et al, 2004) and this number is increasing with wider availability of genetic screening. Mutations of all types (nonsense, insertions, deletions, splice site and missense) occur throughout the whole gene, although clustering of missense mutations in the first four exons of the gene with a number of hot spots have been described (Schindelhauer et al, 1996; Jin et al, 2004).
Details of the genetic mutation alone are often insufficient to predict the severity of the clinical phenotype (although can be helpful if previously described), but a combination of information about the mutation plus its impact on WASp levels enable genotype-phenotype correlation (Imai et al, 2004; Jin et al, 2004; Liu et al, 2015) (Table I). Therefore, we perform analysis of WASp expression as part of our diagnostic work up. WASp quantitation by Western blotting has been superseded in our laboratory by flow cytometry, which has been shown to be a robust and rapid test (Chiang et al, 2018). It is important to note that protein expression alone cannot absolutely be relied on for diagnosis as missense mutations can sometimes preserve normal levels of functionally impaired WASp. Similarly, apparently absent WASp expression may arise from disturbance of the epitope recognised by the detecting antibody.
Table I. Typical characteristics of classical WAS and XLT patients.
| Classical WAS | XLT | |
|---|---|---|
| Clinical features | ||
| Thrombocytopenia | Yes | Yes |
| Eczema | Moderate/severe | None/mild |
| Infections | Yes | None/mild |
| Autoimmunity | Yes | No* |
| Malignancy | Yes | No |
| Typical WASp expression | Absent/low levels | Low/normal levels |
| Typical WAS mutation | Deletions/insertions/early stop codon/splice site | Missense/splice site |
A clinical scoring system is often used to aid classification of WAS patients, with one point assigned for each clinical feature (Zhu et al, 1995; Ochs et al, 2009). A score of ≥3, suggestive of a more severe phenotype, typically correlates with a diagnosis of classical WAS.
WAS, Wiskott-Aldrich syndrome; WASp, WAS protein; XLT, X-linked thrombocytopenia.
Some centres will consider a diagnosis of XLT with autoimmunity where this develops at a later stage, in the context of a mutation known to be associated with XLT and without other clinical features of classical WAS, such as severe/recurrent infections.
Female mutation carriers can also be detected using flow cytometry, confirmed by genetic sequencing. While WAS carriers are usually asymptomatic, clinical features have occasionally been reported in girls, where clinical manifestations occur as a result of non-random X-inactivation and extreme lyonisation, with preferential use of the mutated X chromosome (Andreu et al, 2003; Takimoto et al, 2015). A very rare phenocopy condition, caused by deficiency of the WASp regulating protein, WIP, is inherited in an autosomal recessive manner and can impact both boys and girls (Lanzi et al, 2012).
Classical WAS or XLT?
Assignment of classical WAS or XLT is ultimately a clinical classification. Scoring systems have been published (Zhu et al, 1995; Ochs et al, 2009) but in practice we consider the presence of severe infections, any autoimmunity or haematological malignancy to indicate classical WAS (Table I). As clinical features evolve over time, a diagnosis of XLT can only definitively be made after the age of 2 years. However, gene mutation details and protein levels can help to predict disease course in a young child in whom full clinical phenotype has yet to evolve. Patients with mutations that result in absence of WASp expression are predicted to have a severe clinical course (Imai et al, 2004) and we assign a diagnosis of classical WAS to these patients at an early stage to direct management (see below). Preservation of partial WASp expression (usually with missense or splice site mutations) is associated with a milder outcome in cohort analysis but not absolutely predictive for an individual (Imai et al, 2004). A number of common hotspot missense mutations are reasonably predictive of XLT but even within in this subset, some patients have been described to acquire additional complications, such as autoimmunity at a later stage (Albert et al, 2010). Finally, even within one family, the phenotype can be somewhat variable, presumably due to the influence of other genes, infections or epigenetic factors, although overall severity is generally consistent. As an example, we have looked after brothers who both required transplantation for classical WAS but whose grandfather had a mild course, effectively restricted to features of thrombocytopenia.
Typically, patients with classical WAS are described as having low numbers of CD8+ T-cells and dysgammaglobulinaemia, where IgG, IgA and IgM levels can be low or high as a result of altered humoral function. IgE levels are also typically raised. T-cell proliferative responses are usually normal to the mitogen phytohaemagglutinin but absent/reduced in response to anti-CD3 antibody stimulation, reflecting the fact that T-cell receptor signalling requires actin polymerisation. All of these parameters are less disturbed in patients with XLT, although anti-CD3 T-cell responses are also frequently impaired. Patients with classical WAS often have good initial vaccine responses to protein antigens but usually poor polysaccharide responses (for example to pneumococcal polysaccharides) and low levels of isohaemagluttinins. Given that polysaccharide responses are difficult to assess under the age of two, as a result of immunological immaturity, they are, in practice, rarely measured. In our experience, patients with XLT make normal responses to protein antigens found in regular childhood vaccines and do not show substantial increased risk of infection or susceptibility to opportunists, despite minor immunological abnormalities.
How we manage WAS
Definitive therapy
Arguably the most important management decision that needs to be made when a child is diagnosed with WAS, is whether definitive therapy is indicated; either haematopoietic stem cell transplantation (HSCT) or stem cell gene therapy (GT). Regardless of initial clinical presentation, we refer all children with absent WASp expression and a genetic mutation consistent with classical WAS for early consideration of definitive treatment and do not wait for emergence of a severe clinical phenotype. We aim for transplantation within the first 2 years of life with sub-myeloablative conditioning, with excellent outcomes (Elfeky et al, 2018). Outcomes for children with WAS undergoing HSCT are also excellent internationally, with survival rates over 97% (European cohort 1979–2001 97% (Ozsahin et al, 2008), UK experience 100% (Elfeky et al, 2018; Slatter et al, 2018)). Whilst use of sub-myeloablative conditioning regimens have reduced long-term effects, a number of post-transplant complications appear to be higher in WAS, including graft-versus-host disease (GvHD), infection in the context of prior splenectomy and autoimmunity. Although possible to preserve fertility with sub-myeloablative conditioning, infertility remains a substantial and, as yet, unquantified risk.
Gene therapy trials are in progress for management of classical WAS, at present restricted to patients without a fully matched donor. Although early WAS studies were hampered by late onset of haematological malignancy related to insertional mutagenesis (Braun et al, 2014) associated with gammaretroviral vectors, vector design modifications have improved safety. Recently reported studies have demonstrated good outcome data with no reported vector-related toxicity with resolution of eczema, infections and improved autoimmunity (Aiuti et al, 2013; Castiello et al, 2015; Hacein-Bey Abina et al, 2015).
Currently we do not recommend definitive treatment for XLT as medical management is available and definitive therapy can result in long-term complications including GvHD, infertility, secondary malignancy and death. Instead, we advise a wait and watch approach, with a low threshold for referral for HSCT or GT if disease severity progresses (e.g. development of autoimmunity). If parents are keen to explore definitive therapy even in the context of mild disease, they are referred for a HSCT discussion so that they have full information. In general, we do not recommend gene therapy for XLT where bleeding is the main clinical phenotype as correction of platelet numbers has been variable in clinical trials (Hacein-Bey Abina et al, 2015).
Definitive therapy for adults with WAS/XLT remains a management challenge. In practice, few patients with uncorrected classical WAS reach adulthood and HSCT is rarely offered in adult primary immunodeficiencies (PID) as outcomes were historically poor. However, we recently published excellent outcomes (85% long-term survival at 10 years post-HSCT) for a cohort of adults with different types of PID, making this a viable treatment option for carefully selected patients in specialist centres (Davila Saldana, 2018; Fox et al, 2018). We have also successfully treated one classical WAS adult with GT, which has achieved substantial clinical improvement and provided proof-of-principle that GT is a viable option even for adults (Kohn, 2017; Morris et al, 2017). Given the advantage of using autologous stem cells, thus avoiding GvHD, we view GT as a good option for adults with classical WAS and accumulated comorbidities. We also consider definitive therapy, mainly HSCT, in adult patients with an otherwise XLT phenotype who develop later onset autoimmunity or haematological malignancy. To date, we have considered the risk-benefit ratio of HSCT unfavourable for uncomplicated XLT in adults.
Supportive therapy
Supportive therapy for patients with classical WAS consists of prevention of infection, management of thrombocytopenia, autoimmune and autoinflammatory symptoms prior to definitive treatment (Table II). In our practice, patients with XLT require little supportive treatment, except for management of thrombocytopenia.
Table II. Supportive therapy in classical WAS and XLT.
| Prophylactic antibiotics | ||
| 1st line | Trimethoprim/sulfamethoxazole | Children <1 year: 30 mg/kg once daily; children >1 year: 450 mg/m2, rounded to nearest dose band; adults: 960 mg orally once daily (based on trimethoprim component) |
| 2nd line | Azithromycin | Children and adults: 10 mg/kg orally once daily for 3 consecutive days every 14 days, or 3 days per week (maximum 500 mg/day) |
| Immunoglobulin replacement therapy | ||
| 1st line | Subcutaneous immunoglobulin | Typically, weekly to achieve total dose of 300–500 mg/kg over 3 weeks |
| 2nd line | Intravenous immunoglobulin | 300-500 mg/kg intravenously once every 3 weeks |
| Eczema | ||
| 1st line | Topical emollient | Apply at least twice daily |
| 2nd line | Topical 1% hydrocortisone | Apply sparingly to the affected area(s) twice daily |
| or | Topical betamethasone valerate (0·1%) | Apply sparingly to the affected area(s) twice daily |
| or | Topical fluocinolone (0·025%) | Apply sparingly to the affected area(s) twice daily (>3 months of age) |
| or | Topical clobetasol (0·05%) | Apply sparingly to the affected area(s) twice daily (>12 years of age) |
| 3rd line | Oral prednisolone | 1 mg/kg/day orally given in 2 divided doses for 2–3 weeks, then taper gradually, maximum 60 mg od |
| or | Topical tacrolimus (0·03%) | Apply sparingly to the affected area(s) once or twice daily (children >2 years; for children >15 years 0·1% can be used) |
| Active bleeding | ||
| 1st line | Platelet transfusion | Children up to 10 kg: 10–15 ml/kg; children >15 kg and adults: 1 pool, repeated according to clinical response |
| +/− | Aminocaproic acid | Children: 100–200 mg/kg orally as a loading dose, followed by 100 mg/kg every 4–6 h; adults: 4–5 g orally as a loading dose, followed by 1 g/h for 8 h (maximum 30 g/day) |
| Minor nose bleeding | Tranexamic acid | Intravenous preparation used topically |
| ITP | ||
| 1st line | Oral prednisolone | Children and adults: 2 mg/kg/day orally for 1–2 weeks then taper gradually, maximum 60 mg/day |
| or | Methylprednisolone | Children and adults: 4 mg/kg/day intravenously for 4 days then taper gradually, maximum 60 mg/day |
| 1st/2nd line* | Intravenous immunoglobulin | Children and adults: 1 g/kg intravenously as a single dose; consult specialist for guidance on subcutaneous dose *Intravenous immunoglobulin and steroids given together as first line in children |
| 3rd line | Rituximab | 375 mg/m2 weekly for 4 weeks |
ITP, idiopathic thrombocytopenia; WAS, Wiskott-Aldrich syndrome; XLT, X-linked thrombocytopenia.
Non-autoimmune thrombocytopenia
Thrombocytopenia in WAS/XLT is universal and bleeding risk is a major management challenge for both groups of patients. Although life-threatening bleeding episodes, particularly gastrointestinal or intracranial bleeding, have been reported in 10–30% of patients (Albert et al, 2010; Mahlaoui et al, 2013), in our own cohort severe bleeding episodes requiring medical intervention were substantially lower (6% for classical WAS and 3% for XLT) (Rivers et al, 2018), possibly due to earlier access to definitive treatment for classical WAS and specific criteria for assigning a diagnosis of XLT. In our experience, severe bleeding in classical WAS is almost universally associated with the onset of autoimmune platelet consumption in addition to the intrinsic defect (see below).
As a result, our mainstay of thrombocytopenia management in classical WAS is early definitive therapy, typically within the first 2 years of life, to correct the platelet count and avoid emergence of autoimmunity. In the absence of active bleeding or a significant increase in petechiae/bruising, we do not actively support the platelet count with platelet transfusions (even when platelets <10 × 109/l) and intentionally minimise platelet transfusions to limit development of anti-platelet and anti-human leucocyte antigen antibodies, which can complicate HSCT.
Management of thrombocytopenia in XLT is a lifelong process in the absence of definitive therapy. Parents are given general advice about avoidance of high-risk activities, such as contact sports and to seek prompt medical assessment for any significant head injuries. While we do recommend appropriate use of helmets for activities, such as scooting and cycling, we do not generally recommend protective headgear for day-to-day activities or for toddlers learning to walk, partly because of compliance and stigma and partly because we consider this risk to be low. To date, we have not seen the emergence of autoimmune thrombocytopenia (AIT) in our XLT cohort, although this can rarely occur even in adulthood (Albert et al, 2010). Anxiety associated with thrombocytopenia usually results in significant restriction of activities which can have a substantial impact on quality of life of the child. For this reason, in recent years, we have advocated splenectomy for patients with XLT who have no significant infectious history and are at an age where polysaccharide vaccinations can be given. All patients receive pre-splenectomy booster vaccinations against pneumococcus, meningococcus (ACWY and B) and haemophilus influenzae type B, with protective vaccine responses ensured before proceeding. Although an increased incidence of sepsis in splenectomised patients is described in WAS (Lum et al, 1980; Albert et al, 2010), this risk can be significantly reduced with strict adherence to prophylactic antibiotics. We do not see a significant risk of infection post-splenectomy in our cohort (all receive antibiotic prophylaxis) and all patients with splenectomy for thrombocytopenia in XLT have shown an immediate and sustained platelet response with no episodes of thrombocytopenia relapse (Rivers et al, 2018).
Severe bleeding in WAS/XLT is a medical emergency and requires urgent assessment with fluid resuscitation and blood product support as needed. For minor prolonged nosebleeds, use of intravenous tranexamic acid topically may be of use in avoiding cautery or packing. Platelet agonists, such as Eltrombopag, have been reported to have some effect in elevating the platelet count in WAS/XLT (Gerrits et al, 2015) but to date we have not found these to be successful in children and have not utilised these in patients planned for definitive therapy. We do consider platelet agonists in adults with XLT if thrombocytopenia impacts quality of life and there is reluctance about splenectomy, but to date there is not sufficient experience to recommend this as first line treatment.
Autoimmune thrombocytopenia
Recognising the development of autoimmune platelet consumption (AIT) on top of baseline thrombocytopenia in WAS can be difficult, particularly due to the poor correlation with antiplatelet antibodies, but it is of particular importance as onset of AIT is associated with highest risk of significant bleeding (Rivers et al, 2018). We suspect AIT with the onset of significant increase in bruising/petechiae or spontaneous bleeding and acute drop in the platelet count (usually to <10 × 109/l) from baseline. To confirm AIT, we recommend platelet transfusion with 1-h and 24-h increment assessment. Where the platelet count has not incremented substantially at 1 h,or fallen significantly again by 24 h post-transfusion, we consider this to represent the onset of AIT and recommend treatment with high dose intravenous immunoglobulin (IVIg) and prednisolone (Table II). When assessing response to therapy, we consider the improvement in clinical symptoms separate to the rise in platelet count as a significant factor in guiding therapy, as medical management of AIT is unlikely to lead to sustained rise above the patient’s pre-AIT baseline. We have a low threshold for second line treatment with rituximab where there is failure of platelet control following IVIg and prednisolone, or for recurrence of thrombocytopenia. We consider splenectomy for AIT only in patients with severe thrombocytopenia refractory to first and second line treatments and where a delay in definitive treatment is likely.
Prevention of infection
Severe or recurrent infections are frequently seen in classical WAS, including bacterial, viral, fungal and opportunistic organisms, reflecting the broad functional immune defect (Table III and Fig 2).
Table III. Frequency of significant infections pre-transplant in our cohort of children with classical WAS (adapted from Elfeky et al, 2018).
| Organism | N | % |
|---|---|---|
| Viral | ||
| CMV | 6 | 17·65 |
| RSV | 2 | 5·88 |
| Unspecified respiratory viruses | 2 | 5·88 |
| EBV | 1 | 2·94 |
| Varicella | 1 | 2·94 |
| HPV | 1 | 2·94 |
| Molloscum | 1 | 2·94 |
| Coxsackie | 1 | 2·94 |
| Adenovirus | 0 | 0·00 |
| Parainfluenza | 0 | 0·00 |
| Fungal | ||
| Fungal pneumonia | 3 | 8·82 |
| Candida | 3 | 8·82 |
| Bacterial | ||
| Chronic otitis media | 1 | 2·94 |
| Recurrent perianal abscesses | 1 | 2·94 |
| Recurrent cellulitis | 1 | 2·94 |
| Atypical mycobacteria | 0 | 0·00 |
| Parasitic | ||
| Cryptosporidium | 1 | 2·94 |
CMV, cytomegalovirus; EBV, Epstein–Barr virus; HPV, human papilloma virus; RSV, respiratory syncytial virus; WAS, Wiskott-Aldrich syndrome.
All patients with classical WAS are commenced on immunoglobulin replacement treatment at diagnosis, even if total immunoglobulin levels and vaccine responses are in the normal range. Almost without exception, immunoglobulin is administered by the subcutaneous route to achieve a total monthly dose of approximately 0·4 g/kg (Table II). Parents are trained to deliver this at home on a weekly basis and despite severe thrombocytopenia, we have not encountered significant difficulties with bruising or haematomas. No further vaccinations are given once immunoglobulin is started, with the exception of annual inactivated flu vaccine. All live vaccinations are contraindicated because there is a risk of vaccine strain infection. In addition, patients with classical WAS are commenced on prophylactic antibiotics, typically co-trimoxazole, to provide broad-spectrum antibiotic cover and include protection against Pneumocystis jiroveci. Prophylactic antifungal or antiviral treatment is considered on a case-by-case basis, for recurrent candidiasis, prior cytomegalovirus viraemia or recurrent herpes simplex virus disease.
Patients classified as XLT at our centre do not have significant infections by definition and are therefore not commenced on immunoglobulin replacement, are rarely commenced on antibiotic prophylaxis and receive full routine vaccination including live vaccines and Bacillus Calmette-Guérin (BCG) where indicated. For adults with XLT who wish to travel overseas, we recommend standard travel vaccinations, with the exception of yellow fever for which there is no documented experience in mild forms of PID.
Eczema and atopy
Eczema is extremely common at presentation in classical WAS and is frequently extensive and difficult to manage. Atopy and food allergy are strongly associated, with cow’s milk protein allergy found almost universally in infants presenting with eczema (Tuano et al, 2015; Lexmond et al, 2016). Mild to moderate eczema can also be seen in XLT. We recommend standard treatment with emollients and topical steroids (Table II). Topical tacrolimus is an option as a steroid-sparing agent. In severe cases specialist input from dermatology should be sought and more intensive treatments, such as wet wraps or even oral steroids, considered. For significant or early onset eczema, we recommend early dietician input and trial of hydrolysed formula/cow’s milk exclusion diet.
Autoimmunity/Autoinflammatory features
Autoimmunity occurs frequently in classical WAS (over 40% in our cohort; Elfeky et al, 2018). In addition to AIT, other cytopenias (haemolytic anaemia and neutropenia) are common and managed with supportive care and immunosuppression. Arthritis, vasculitis and inflammatory bowel disease are other recognised but less common autoimmune features found in WAS and are managed with input from other specialties. Large vessel vasculitis can occasionally lead to aortic aneurysms and these are typically detected incidentally on radiological imaging for another purpose (Pellier et al, 2011). In our own practice, we do not routinely screen for the presence of large vessel vasculitis, because early definitive treatment should significantly reduce susceptibility.
IgA nephropathy is of particular interest as it is one of the more common autoimmune features associated with WAS mutations, appears to have a higher prevalence in patients with residual WASp expression and may present later in patients with an otherwise XLT phenotype (Imai et al, 2004; Albert et al, 2010; Shimizu et al, 2012; Liu et al, 2013). Increasing serum creatinine and proteinuria or episodes of haematuria in acute flares are presenting features (Fig 3). Diagnosis is confirmed on renal biopsy and treatment may require use of immunosuppression in the first instance. Progression is variable and may occur over years, but where renal transplantation is needed, careful discussions around appropriateness and timing of HSCT are warranted to balance the risks of nephrotoxicity from conditioning agents. We recommend screening for serum creatinine, blood pressure and proteinuria at routine follow-up appointments for all WAS/XLT patients and seeking specialist renal advice where appropriate.
Fig 3.
WAS and autoimmunity. Case presentation: Thrombocytopenia was noted following an upper respiratory tract infection at 8 months old, with small amounts of blood in the stool, mild eczema and suspicion of cow’s milk protein allergy. Subsequently developed molloscum contagiosum and warts but remained well until 8 years old with a phenotype otherwise consistent with attenuated Wiskott-Aldrich syndrome (WAS) (X-linked thrombocytopenia). Initial investigations: Normal lymphocyte numbers, response to phytohaemagglutinin and vaccine responses to tetanus and pneumococcus (conjugate vaccine), but absent response to CD3 stimulation. Platelet count was low at 40 × 109/l. Raised IgA and IgG (not on replacement immunoglobulin therapy). Normal WAS protein expression by flow cytometry and mutation in WAS identified as c.1498T>C, (p.Trp500Arg). Treatment: Splenectomy (age 3 years). Progress: At 8 years old, remains well from infection and inflammation point of view but developed cola-coloured urine (left) with subsequent episodes of frank haematuria (right), associated with hypertension and mildly elevated creatinine (56 μmol/l) consistent with IgA nephropathy (confirmed on biopsy). Normalisation of platelet number and size occurred post-splenectomy, with no relapse of thrombocytopenia.
In addition to classical autoimmunity, other inflammatory complications can be seen that resemble those seen in other autoinflammatory disorders, including intermittent rashes and arthralgia. We postulate these are related to inflammasome activation (Lee et al, 2017) and have used colchicine and the interleukin 1 receptor antagonist, anakinra, with marked benefit in some cases.
Malignancy
Haematological malignancy, specifically lymphomas and leukaemia, is estimated to occur in approximately 10–20% of patients with classical WAS over time in the absence of curative therapy (Sullivan et al, 1994; Imai et al, 2004). Epstein–Barr virus (EBV) infection is associated with development of lymphoproliferative disease and we have a low threshold for investigation of new lymphadenopathy, particularly where EBV viraemia has been documented. Ultrasound is useful as a first line investigation, with biopsy where abnormal architecture is found. In the context of normal lymph node architecture and absence of B symptoms (fever, nights sweats or weight loss), we recommend a two-week trial of Co-Amoxiclav in case of occult bacterial infection and re-consider biopsy if there is no improvement. Unusual infections, including mycobacteria, are seen in WAS and therefore biopsy samples should be sent for full microbiological assessment as well as for histology. Usual oncology protocols are used for treatment of malignancy, with a plan to move to definitive therapy in early remission.
Conclusions
Both supportive care and definitive treatment for Wiskott-Aldrich syndrome have improved substantially over the last two decades. Outcomes for HSCT are excellent and gene therapy is emerging as an attractive alternative option. The key decision for patient management is whether the combined genetic, protein and clinical phenotype indicate classical WAS or XLT. While supportive care remains the mainstay for patients with XLT, all patients with classical WAS should be referred early for definitive therapy before establishment of significant co-morbidities. Greater awareness of this rare disorder is the main challenge for improving diagnosis, with an ongoing need for education of paediatricians and haematologists, to whom patients are most likely to first present.
Acknowledgements
This work was supported by funding from The Wellcome Trust (090233/Z/09/Z AJT and 201250/Z/16/Z ER), National Institute for Health Research UCLH Biomedical Research Centre (SB), and National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. We would like to thank our patients and families for providing consent for photographs to be used in this review and Dr Kimberly Gilmour, Great Ormond Street Hospital for critical review of the manuscript.
Footnotes
Author contributions
The manuscript was written by Dr Rivers and Dr Burns, with content contribution and review by all authors.
References
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, Bosti-cardo M, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MH, Bittner TC, Nonoyama S, Notaran-gelo LD, Burns S, Imai K, Espanol T, Fasth A, Pellier I, Strauss G, Morio T, et al. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood. 2010;115:3231–3238. doi: 10.1182/blood-2009-09-239087. [DOI] [PubMed] [Google Scholar]
- Andres O, Henning K, Strauss G, Pflug A, Manukjan G, Schulze H. Diagnosis of platelet function disorders: a standardized, rational, and modular flow cytometric approach. Platelets. 2018;29:347–356. doi: 10.1080/09537104.2017.1386297. [DOI] [PubMed] [Google Scholar]
- Andreu N, Pujol-Moix N, Martinez-Lostao L, Oset M, Muniz-Diaz E, Estivill X, Volpini V, Fillat C. Wiskott-Aldrich syndrome in a female with skewed X-chromosome inactivation. Blood Cells, Molecules, & Diseases. 2003;31:332–337. doi: 10.1016/s1079-9796(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Braun CJ, Boztug K, Paruzynski A, Witzel M, Schwarzer A, Rothe M, Modlich U, Beier R, Gohring G, Steinemann D, Fronza R, et al. Gene therapy for Wiskott-Aldrich syndrome-long-term efficacy and genotoxicity. Science Translational Medicine. 2014;6:227ra233. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- Castiello MC, Scaramuzza S, Pala F, Ferrua F, Uva P, Brigida I, Sereni L, van der Burg M, Ottaviano G, Albert MH, Grazia Roncarolo M, et al. B-cell reconstitution after lentiviral vector-mediated gene therapy in patients with Wiskott-Aldrich syndrome. Journal of Allergy and Clinical Immunology. 2015;136:e692. doi: 10.1016/j.jaci.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SCC, Vergamini SM, Husami A, Neumeier L, Quinn K, Ellerhorst T, Sheppard L, Gifford C, Buchbinder D, Joshi A, Ifversen M, et al. Screening for Wiskott-Aldrich syndrome by flow cytometry. Journal of Allergy and Clinical Immunology. 2018;142:e338. doi: 10.1016/j.jaci.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Davila Saldana BJ. HSCT for PID: not just for children. Blood. 2018;131:843–844. doi: 10.1182/blood-2018-01-824896. [DOI] [PubMed] [Google Scholar]
- Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Elfeky RA, Furtado-Silva JM, Chiesa R, Rao K, Amrolia P, Lucchini G, Gilmour K, Adams S, Bibi S, Worth A, Thrasher AJ, et al. One hundred percent survival after transplantation of 34 patients with Wiskott-Aldrich syndrome over 20 years. Journal of Allergy and Clinical Immunology. 2018;142:1654–1656. doi: 10.1016/j.jaci.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Fox TA, Chakraverty R, Burns S, Carpenter B, Thomson K, Lowe D, Fielding A, Peggs K, Kottaridis P, Uttenthal B, Bigley V, et al. Successful outcome following allogeneic hematopoietic stem cell transplantation in adults with primary immunodeficiency. Blood. 2018;131:917–931. doi: 10.1182/blood-2017-09-807487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits AJ, Leven EA, Frelinger AL, 3rd, Brig-stocke SL, Berny-Lang MA, Mitchell WB, Revel-Vilk S, Tamary H, Carmichael SL, Barnard MR, Michelson AD, et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syn-drome/X-linked thrombocytopenia. Blood. 2015;126:1367–1378. doi: 10.1182/blood-2014-09-602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottum KA, Hovig T, Holmsen H, Abrahamsen AF, Jeremic M, Seip M. Wiskott-Aldrich syndrome: qualitative platelet defects and short platelet survival. British Journal of Haematology. 1969;17:373–388. doi: 10.1111/j.1365-2141.1969.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K, Picard C, Six E, Himoudi N, Gilmour K, McNicol AM, et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kaji-wara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- Ingrungruanglert P, Amarinthnukrowh P, Rungsiwiwut R, Maneesri-le Grand S, Sosothi-kul D, Suphapeetiporn K, Israsena N, Shotelersuk V. Wiskott-Aldrich syndrome iPS cells produce megakaryocytes with defects in cytoskeletal rearrangement and proplatelet formation. Thrombosis and Haemostasis. 2015;113:792–805. doi: 10.1160/TH14-06-0503. [DOI] [PubMed] [Google Scholar]
- Jin Y, Mazza C, Christie JR, Giliani S, Fiorini M, Mella P, Gandellini F, Stewart DM, Zhu Q, Nelson DL, Notarangelo LD, et al. Mutations of the Wiskott-Aldrich Syndrome Protein (WASP): hotspots, effect on transcription, and translation and phe-notype/genotype correlation. Blood. 2004;104:4010–4019. doi: 10.1182/blood-2003-05-1592. [DOI] [PubMed] [Google Scholar]
- Kahr WH, Pluthero FG, Elkadri A, Warner N, Drobac M, Chen CH, Lo RW, Li L, Li R, Li Q, Thoeni C, et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nature Communications. 2017;8:14816. doi: 10.1038/ncomms14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn DB. Gene therapy: WAS (not) just for kids. Blood. 2017;130:1278–1279. doi: 10.1182/blood-2017-08-798496. [DOI] [PubMed] [Google Scholar]
- Lanzi G, Moratto D, Vairo D, Masneri S, Del-monte O, Paganini T, Parolini S, Tabellini G, Mazza C, Savoldi G, Montin D, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. Journal of Experimental Medicine. 2012;209:29–34. doi: 10.1084/jem.20110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Lobato-Marquez D, Pramanik N, Siri-anni A, Daza-Cajigal V, Rivers E, Cavazza A, Bouma G, Moulding D, Hultenby K, Westerberg LS, et al. Wiskott-Aldrich syndrome protein regulates autophagy and inflammasome activity in innate immune cells. Nature Communications. 2017;8:1576. doi: 10.1038/s41467-017-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexmond WS, Goettel JA, Lyons JJ, Jacobse J, Deken MM, Lawrence MG, DiMaggio TH, Kotlarz D, Garabedian E, Sackstein P, Nelson CC, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. The Journal of Clinical Investigation. 2016;126:4030–4044. doi: 10.1172/JCI85129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Wu KH, Lin TY, Wei CC, Lin CY, Chen XX, Lee WI. Wiskott-Aldrich syndrome with IgA nephropathy: a case report and literature review. International Urology and Nephrology. 2013;45:1495–1500. doi: 10.1007/s11255-012-0178-0. [DOI] [PubMed] [Google Scholar]
- Liu DW, Zhang ZY, Zhao Q, Jiang LP, Liu W, Tu WW, Song WX, Zhao XD. Wiskott-Aldrich syndrome/X-linked thrombocytopenia in China: clinical characteristic and genotype-phenotype correlation. Pediatric Blood & Cancer. 2015;62:1601–1608. doi: 10.1002/pbc.25559. [DOI] [PubMed] [Google Scholar]
- Lum LG, Tubergen DG, Corash L, Blaese RM. Splenectomy in the management of the thrombocytopenia of the Wiskott-Aldrich syndrome. New England Journal of Medicine. 1980;302:892–896. doi: 10.1056/NEJM198004173021604. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insali RH. Scarl and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Current Biology. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Mahlaoui N, Pellier I, Mignot C, Jais JP, Bilhou-Nabera C, Moshous D, Neven B, Picard C, de Saint-Basile G, Cavazzana-Calvo M, Blanche S, et al. Characteristics and outcome of early-onset, severe forms of Wiskott-Aldrich syndrome. Blood. 2013;121:1510–1516. doi: 10.1182/blood-2012-08-448118. [DOI] [PubMed] [Google Scholar]
- Medina SS, Siqueira LH, Colella MP, Yamaguti-Hayakawa GG, Duarte BKL, Dos Santos Vilela MM, Ozelo MC. Intermittent low platelet counts hampering diagnosis of X-linked thrombocytopenia in children: report of two unrelated cases and a novel mutation in the gene coding for the Wiskott-Aldrich syndrome protein. BMC Pediatrics. 2017;17:151. doi: 10.1186/s12887-017-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EC, Fox T, Chakraverty R, Tendeiro R, Snell K, Rivat C, Grace S, Gilmour K, Workman S, Buckland K, Butler K, et al. Gene therapy for Wiskott-Aldrich syndrome in a severely affected adult. Blood. 2017;130:1327–1335. doi: 10.1182/blood-2017-04-777136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo LD, Mazza C, Giliani S, D’Aria C, Gandellini F, Ravelli C, Locatelli MG, Nelson DL, Ochs HD. Missense mutations of the WASP gene cause intermittent X-linked thrombocytopenia. Blood. 2002;99:2268–2269. doi: 10.1182/blood.v99.6.2268. [DOI] [PubMed] [Google Scholar]
- Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. [PubMed] [Google Scholar]
- Ochs HD, Filipovich AH, Veys P, Cowan MJ, Kapoor N. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biology of Blood and Marrow Transplantation. 2009;15:84–90. doi: 10.1016/j.bbmt.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, Schulz A, Thrasher AJ, Mazzolari E, Slatter MA, Le Deist F, Blanche S, Veys P, Fasth A, et al. Longterm outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111:439–445. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- Pellier I, Dupuis Girod S, Loisel D, Benabidallah S, Proust A, Malhlaoui N, Picard C, Najioullah F, de Saint Basile G, Blanche S, Rialland X, et al. Occurrence of aortic aneurysms in 5 cases of Wiskott-Aldrich syndrome. Pediatrics. 2011;127:e498–e504. doi: 10.1542/peds.2009-2987. [DOI] [PubMed] [Google Scholar]
- Perry GS, 3rd, Spector BD, Schuman LM, Mandel JS, Anderson VE, McHugh RB, Hanson MR, Fahlstrom SM, Krivit W, Kersey JH. The Wiskott-Aldrich syndrome in the United States and Canada (1892-1979) Journal of Pediatrics. 1980;97:72–78. doi: 10.1016/s0022-3476(80)80133-8. [DOI] [PubMed] [Google Scholar]
- Puck JM, Candotti F. Lessons from the Wiskott-Aldrich syndrome. New England Journal of Medicine. 2006;355:1759–1761. doi: 10.1056/NEJMp068209. [DOI] [PubMed] [Google Scholar]
- Rivers E, Worth A, Thrasher AJ, Burns SO. Bleeding and splenectomy in Wiskott-Aldrich syndrome: a single-centre experience. The Journal of Allergy and Clinical Immunology: In Practice. 2018;7:1042–1044. doi: 10.1016/j.jaip.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, Farndale RW, Jalil A, Blundell MP, Cramer EM, Louache F, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–140. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- Schindelhauer D, Weiss M, Hellebrand H, Golla A, Hergersberg M, Seger R, Belohrad-sky BH, Meindl A. Wiskott-Aldrich syndrome: no strict genotype-phenotype correlations but clustering of missense mutations in the amino-terminal part of the WASP gene product. Human Genetics. 1996;98:68–76. doi: 10.1007/s004390050162. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nikolov NP, Ueno K, Ohta K, Siegel RM, Yachie A, Candotti F. Development of IgA nephropathy-like glomerulonephritis associated with Wiskott-Aldrich syndrome protein deficiency. Clinical Immunology. 2012;142:160–166. doi: 10.1016/j.clim.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatter MA, Rao K, Abd Hamid IJ, Nademi Z, Chiesa R, Elfeky R, Pearce MS, Amrolia P, Worth A, Flood T, Abinun M, et al. Treosulfan and fludarabine conditioning for hematopoietic stem cell transplantation in children with primary immunodeficiency: UK experience. Biology of Blood and Marrow Transplantation. 2018;24:529–536. doi: 10.1016/j.bbmt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. Journal of Pediatrics. 1994;125:876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- Takimoto T, Takada H, Ishimura M, Kirino M, Hata K, Ohara O, Morio T, Hara T. Wiskott-Aldrich syndrome in a girl caused by heterozygous WASP mutation and extremely skewed X-chromosome inactivation: a novel association with maternal uniparental isodisomy 6. Neonatology. 2015;107:185–190. doi: 10.1159/000370059. [DOI] [PubMed] [Google Scholar]
- Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nature Reviews Immunology. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- Tuano KS, Orange JS, Sullivan K, Cunning-ham-Rundles C, Bonilla FA, Davis CM. Food allergy in patients with primary immunodeficiency diseases: prevalence within the US Immunodeficiency Network (USIDNET) Journal of Allergy and Clinical Immunology. 2015;135:273–275. doi: 10.1016/j.jaci.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, Strina D, Patrosso MC, Ramenghi U, Sacco MG, Ugazio A, et al. X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nature Genetics. 1995;9:414–417. doi: 10.1038/ng0495-414. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang M, Blaese RM, Derry JM, Junker A, Francke U, Chen SH, Ochs HD. The Wiskott-Aldrich syndrome and X-linked congenital thrombocytopenia are caused by mutations of the same gene. Blood. 1995;86:3797–3804. [PubMed] [Google Scholar]