Abstract
The Wiskott–Aldrich syndrome protein (WASP) participates in innate and adaptive immunity through regulation of actin cytoskeleton-dependent cellular processes, including immune synapse formation, cell signaling, migration and cytokine release. There is also emerging evidence for a direct role in nuclear transcription programmes uncoupled from actin polymerization. A deeper understanding of some of the more complex features of Wiskott Aldrich syndrome (WAS) itself, such as the associated autoimmunity and inflammation, has come from identification of defects in the number and function of anti-inflammatory myeloid cells and regulatory T and B cells, as well as defects in positive and negative B-cell selection. In this review we outline the cellular defects that have been characterized in both human WAS patients and murine models of the disease. We will emphasize in particular recent discoveries that provide a mechanistic insight into disease pathology, including lymphoid and myeloid cell homeostasis, immune synapse assembly and immune cell signaling.
Keywords: Autoimmunity, Immune synapse, Inflammation, Wiskott Aldrich syndrome, Wiskott Aldrich syndrome protein
Introduction
Wiskott Aldrich syndrome protein (WASP) is ubiquitously expressed in non-erythroid haematopoietic cells. Since identification of the WAS gene more than 20 years ago [1], there have been approximately 300 different mutations described, leading to a remarkably varied clinical phenotype including immunodeficiency, inflammatory symptoms, bleeding diathesis, autoimmunity and malignant potential. The clinical aspects of WAS and emerging treatments have been reviewed in detail recently [2] and will not be discussed here.
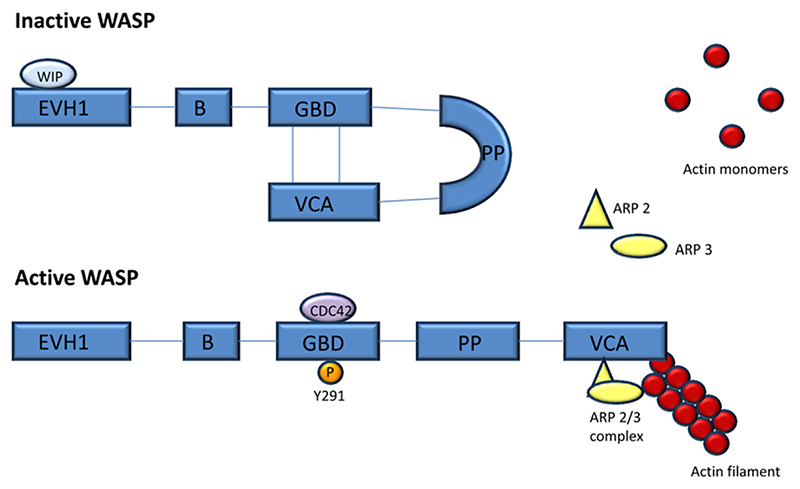
WASP is a cytosolic protein comprising 502 amino acids. It consists of an Ena-VASP homology domain (EVH1, also known as WH1) at the amino terminal, a short basic domain (B), a guanosine triphosphatase-binding domain (GBD), a large polyproline (PP) domain and verprolin homology/central/acidic (VCA) domain at the carboxyl terminal (Fig. 1). WASP is established as a key regulator of the actin cytoskeleton in haematopoietic cells, with important functional roles in lymphoid and myeloid cell migration, receptor signaling, cytotoxicity, and phagocytosis. More recently, cytoskeleton-independent functions of WASP have been identified at the level of nuclear transcription (Table 1). At rest, WASP exists in an autoinhibited state, in which the VCA domain associates with a hydrophobic pocket in the GBD domain [3] (Fig. 1, top). Binding of WASP with partners, such as the Rho family GTPase cell division cycle 42 (CDC42), and/or phosphorylation of a tyrosine residue (Y291 in human WASP) within the GBD hydrophobic region destabilizes the autoinhibited conformation (Fig. 1, bottom). This exposes the VCA region, thereby allowing actin related protein (ARP) 2/3 binding [4–6]. Subsequent localization and nucleation of actin filaments results in the formation of actin branches [7]. Several other proteins, particularly the SRC homology 3 (SH3) domain adaptor NCK, and a number of defined tyrosine kinases, also promote the activation of WASP through interaction with the basic or polyproline domains [8–11]. Phosphorylation of two serine residues in the VCA domain further regulate WASP activation through enhanced affinity to the ARP 2/3 complex [3, 5, 12, 13]. Evidence also exists for regulation of WASP activity through oligomerization, where active WASP forms complexes through VCA dimers or higher order oligomers, with much greater potency for ARP 2/3 stimulation [14, 15]. In addition to activation, phosphorylation of Y291 in the GBD domain is thought to mark WASP for degradation by calpain and protea-some proteolysis [6, 16–18]. WASP-interacting protein (WIP) stabilizes WASP by binding to the EVH1 domain, and protecting it from calpain and proteasomal degradation [16, 19] (Fig. 1, top), but also is important for localizing WASP to areas of actin polymerization [16, 20].
Figure 1.
Domain structure of WASP in its inactive and active forms. At rest, WASP exists in an autoinhibited state where the VCA region associates with the GBD region, the conformation of which is stabilised by WIP. WASP becomes activated through binding partners such as the GTPase CDC42 or phosphorylation of a tyrosine residue (Y291), which release the VCA domain and expose the ARP 2/3 binding domain. The ARP 2/3 complex recruits actin monomers resulting in the formation of branched actin filaments. ARP 2/3, actin-related protein; B, basic domain; CDC42, cell division cycle 42; EVH1, Ena-VASP homology domain; GBD, guanosine triphosphate binding domain; P, phosphate; PP, polyproline domain; VCA, verprolin homology/central/acidic domain; WASP, Wiskott Aldrich syndrome protein; WIP, WASP interacting protein.
Table 1. Actin cytoskeleton dependent and independent immune functions of WASP.
| Actin cytoskeleton-dependent functions of WASP | Actin cytoskeleton-independent functions of WASP |
|---|---|
| Lymphoid cell proliferation and homeostasis | T cell differentiation |
| Lymphoid and myeloid immune synapse assembly and signaling | Memory B cell activation, through transcription of B cell co-receptor CD19 |
| Lymphoid and myeloid cell cytokine polarization and release | Transcription of inflammatory cytokines |
| Myeloid cell protrusion activity and endothelial adhesion, through podosome formation | Transcriptional regulation of myeloid cells |
| Lymphoid and myeloid cell migration | |
| NK cytolytic activity, through perforin accumulation at NK-target cell contact | |
| Phagocytosis |
Early research identified WASP as an important regulator of the actin cytoskeleton. More recently, important cytoskeleton independent functions in nuclear transcription have been identified. NK, natural killer cell; WASP, Wiskott Aldrich syndrome protein.
The role of WASP in lymphoid lineages
WASP in T cells
WASP has been implicated in a number of intrinsic T-cell functions including cell proliferation, differentiation and survival, through both actin-dependent and -independent mechanisms (Table 1). In lymphoid lineages the development of early progenitors proceeds normally in both humans and mice bearing a WASP deficiency, but WASP is required for the survival and homeostasis of terminally differentiated cells [21–23]. Abnormal thymopoiesis in the absence of WASP has been suggested by evaluation of lymphocyte counts in WAS patients [24, 25], and by the observation of thymic hypoplasia at post mortem [26]. Evidence for a role of WASP in thymopoiesis has also been identified in murine models, in which subtle abnormalities such as a possible block in progression from double negative (CD4- CD8-) to double positive (CD4+ CD8+) T cells [22, 23] have been shown. The numbers of circulating naïve CD4+ T cells in human WAS are usually within the normal range, but the proportion of CD8+ T cells is usually low [2], often accompanied by an abundance of γδ T cells [27]. Age-dependent clonal skewing of T-cell receptor (TCR) β-chain repertoires has been found in human peripheral blood of WAS patients [28]. Interestingly, WASP has been found to be present in the T-cell nucleus and may play an important actin-independent role in regulating histone methylation at the TBX21 promoter [29] and in transcription of cytokines required for T helper (TH) 1 cell differentiation [30, 31]. The absence of WASP in human T helper cells is associated with detrimental effects on cytokine gene transcription required for TH1 differentiation resulting in skewing towards TH2 dominance (Fig. 2A). Additionally, WASP may play a role in lymphocyte survival. Activated T cells can undergo apoptosis in response to TCR stimulation in order to eliminate T cells responding to chronically expressed antigens, including autoantigens, through interactions with the tumour necrosis factor (TNF) family member Fas ligand (FasL). Murine wasp deficiency is associated with impaired TCR-induced FasL secretion in CD4+ T cells and reduced apoptosis with increased autoantibody production [32], which may go some way to explain the predisposition to autoimmunity. In contrast, attenuated B-cell lymphoma 2 (BCL2) and abnormal CD95 expression in human WAS have been associated with increased lymphocyte apoptosis [33, 34]. It is unclear whether this process is a result of dysregulated transcription or secondary to intracellular cytoskeletal events, but may reflect a compensatory mechanism in response to increased autoantigen expressing T cells.
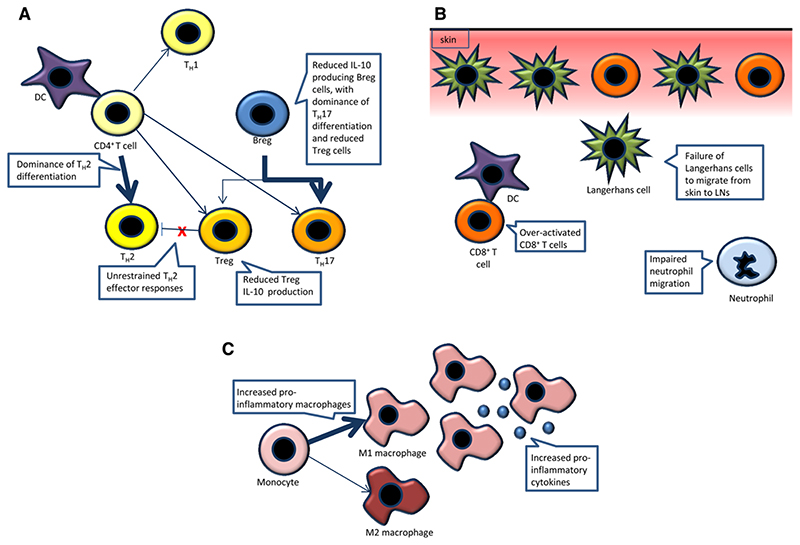
Figure 2. WASP deficiency and inflammation. Inflammatory symptoms are common in WAS and the role of WASP in inflammation is being increasingly defined.
(A) CD4+ T cells show impaired gene transcription required for TH1 differentiation, leading to TH2 dominance. Treg cells produce less of the anti-inflammatory cytokine IL-10 and fail to regulate TH2 effector responses, which has been associated with allergic intestinal inflammation. Reduced numbers of IL-10 producing Breg cells are associated with reduced Treg cell recruitment and increased pro-inflammatory TH17 cells. (B) Enhanced cross presentation leads to over activation of CD8+ T cells and is associated with skin inflammation, which is contributed to by allergen-laden Langerhans cells that fail to migrate from the skin to lymph nodes. Neutrophil migration to sites of sterile inflammation is also impaired, particularly through WASP-BTK interaction. (C) Increased numbers of pro-inflammatory macrophages are found, with increased production of pro-inflammatory cytokines. Breg cell, regulatory B cell; BTK, Bruton’s Tyrosine Kinase; DC, dendritic cell; LN, lymph node; TH1/2/17, T helper cells; Treg cell, regulatory T cell; WAS, Wiskott Aldrich syndrome; WASP, Wiskott Aldrich syndrome protein.
The immunological synapse (IS) is a highly dynamic interface between communicating immune cells. It is organized so that optimal antigen recognition and signal transduction may occur and relies on remodeling of the actin cytoskeleton to distribute proteins accordingly [35]. In wild-type cells, TCR ligation leads to IS assembly by clustering of receptor and signaling molecules into lipid rafts. Subsequent IS maturation results in a central concentration of TCRs and costimulatory molecules surrounded by a peripheral ring of adhesion molecules including lymphocyte function-associated antigen (LFA)-1 [36]. WASP is rapidly recruited to the TCR upon ligation and is required for efficient endocytic TCR internalization, which is impaired in WASP-deficient human and murine T cells [37–39]. WASP is recruited to the IS by WIP and is activated by CDC42 [40], but also through CD2 stimulation [41]. Murine and human WASP-deficient T cells have impaired actin polymerization at the T cell-antigen-presenting cell (APC) contact site, resulting in inefficient recruitment of other IS proteins in response to TCR stimulation [40–42], and lower numbers of lipid rafts that fail to cluster [42]. Disrupted formation of actin foci is found in murine wasp-deficient T cells upon T cell activation, with subsequent impaired calcium signaling, and may be critically important for focused signal integration and amplification of downstream signals [35, 43, 44]. Murine wasp-deficient T cells also demonstrate failure to polarize cytokines [45, 46], and exhibit abrogated chemokine-induced migration in transwell migration assays, with impaired homing to Peyer’s patches following adoptive T cell transfer [45–48].
The number and development of regulatory T (Treg) cells in WASP deficiency have been shown to be normal [49–51], but the peripheral homeostasis and function of these cells are disturbed [32, 49], and may therefore contribute to the predisposition of WAS patients to autoimmunity. A role for WASP in granzyme B-mediated B cell killing has been identified, with murine wasp-deficient Treg cells showing defective B-cell suppression [52]. Murine wasp-deficient Treg cells lack tissue-homing markers, including integrin α4β7 and chemokine receptors CCR4 and P and E selectin ligands, which may explain why they are almost entirely absent in inflamed peripheral tissues and found in decreased numbers in secondary lymphoid tissues [53]. Both human and murine WASP-deficient Treg cells exhibit impaired ability to suppress the proliferation of activated T effector cells [49–51], with relatively unrestrained TH2 effector responses driving inflammation in a mouse model of intestinal allergy [54] (Fig. 2A). WASP-deficient Treg cells also secrete less of the anti-inflammatory cytokine IL-10 [53], which may further predispose to pathological inflammation.
WASP in B cells
WASP plays a critical B-cell-specific role in immune homeostasis involved in development of the splenic marginal zone, regulation of lymph node germinal center interactions and prevention of autoimmunity by negative selection of autoreactive B-cell progenitors [55–57]. Murine wasp-deficient B cells demonstrate hyperproliferation associated with autoantibody production and enhanced differentiation into class switched plasmoblasts [55]. WAS patients, however, have normal or slightly reduced absolute numbers of circulating B cells, and normal numbers of class switched memory B cells [56]. In both humans and mice, transitional B cells exhibit enhanced proliferation in response to stimulation by antigen or myeloid differentiation primary response protein 88 (MYD88) [56, 58, 59], which, in addition to relaxed peripheral tolerance [59–61], results in the enrichment of autoreactive cells at the naïve B-cell stage.
Memory B-cell activation is disrupted in murine wasp deficiency by reduced transcription of the B-cell receptor (BCR) coreceptor CD19, and enhanced recruitment of the BCR’s negative regulators FcyIIB and SH2 inositol 5-phosphatase (SHIP) [62–65]. Impaired BCR and integrin signaling in human and murine WASP-deficient B cells also results in poorly formed immunological synapses, which may further impair B-cell activation, chemotaxis and subsequent signaling in memory B cells, but is not known to compromise class-switching [66]. Murine wasp-deficient immature B cells, however, appear to have enhanced BCR responsiveness, which promotes egress from the splenic marginal zone [57, 65] and may provide an explanation for the suboptimal T-independent antibody responses observed in WAS patients [55, 66]. Altered antibody production in WAS likely results from intrinsic B-cell dysfunction, but also through defective activity of follicular T (Tfh) cells, which proliferate poorly, and exhibit defective differentiation with increased apoptosis [67].
Recent studies have suggested that WASP is required for acquisition of normal regulatory B (Breg) cell number and function, which may have an important influence on the balance and recruitment of Treg cells and TH17 cells during inflammation [68, 69] (Fig. 2A). In particular, arthritic WAS knockout (KO) mice were shown to have reduced numbers of IL-10-producing Breg cells in association with reduced Treg cells and increased TH17 cells [68]. Interestingly, adoptive transfer of wild-type Breg cells ameliorated arthritis and restored the balance between Treg cells and TH17 cells, but selective deficiency of wasp in Breg cells did not lead to exacerbated arthritis or increase in TH17 cells despite reduced numbers of Breg and Treg cells. This suggests an element of compensation by other regulatory cell lineages.
WASP in NK and iNKT cells
WASP has previously been demonstrated to be one of a few cytoskeletal proteins responsible for the regulation of NK-cell killing [70]. Enriched human WASP-deficient NK cells demonstrate impaired actin polymerization and perforin accumulation at the NK-target contact point [71], resulting in significantly reduced NK-cell cytolytic activity. Expansion of NK-cell populations is often found in WAS patients [71], which may be compensatory for these functional defects.
WASP has also been implicated in the homeostasis and function of invariant NKT (iNKT) cells, which have roles in microorganism clearance, tumour surveillance and autoimmunity [72–75]. Circulating iNKT cells are almost absent in WAS, but interestingly are normal in patients with X-linked thrombocytopaenia (XLT), the milder form of disease where some residual WASP expression and function is retained [73]. This suggests that defective iNKT activity may contribute to disease pathology, but the degree to which this is important has not been defined. Murine studies have suggested that wasp is more important for peripheral homeostasis rather than thymic production, though iNKT-cell maturation in the wasp-deficient murine thymus shows retarded progression to mature phenotypes [72]. Murine wasp-deficient iNKT cells respond poorly to glycopeptide antigens, with defective activation, homing and retention within peripheral lymphoid tissues [72, 73], but the role of WASP in humans has not been properly explored.
The role of WASP in myeloid lineages
WASP has been shown to have an important role in myeloid cells, with profound abnormalities in actin distribution leading to impaired cell polarization and migration, protrusion activity and phagocytic cup formation in human and murine WASP-deficient monocytes, macrophages, dendritic cells (including Langerhans cells (LC)) and neutrophils [76–81]. WASP has also recently been shown to have a role in the transcriptional and epigenetic regulation of myeloid cells [82].
Cell migration requires adhesive interactions with substrata. The β-2 family of integrins is important in this process by linking the extracellular matrix to the actin cytoskeleton, necessary for transducing mechanical force and pulling on neighboring cells. Podosomes are specialized, highly dynamic structures found in many cells including macrophages and DCs. They contain an actin core surrounded by a ring of integrins, scaffold and actin-binding proteins, and are thought to be important for adhesion-dependent migration through digestion of the extracellular matrix [83]. There are many similarities between adhesive podosomes observed in myeloid cells and actin foci formed at the IS suggesting that this fundamental structure can be adapted for multiple tasks. In migrating human and murine polymorphonuclear (PMN) cells the absence of WASP leads to failure of integrin clustering at the leading edge [84]. Podosomes are completely absent in WASP-deficient human and murine myeloid cells, but are restored when a wild-type copy of the WAS gene is reintroduced, causally linking WASP with their formation [85]. Human and murine WASP deficiency results in impaired adhesion to endothelial adhesion molecule intercellular adhesion molecule 1 (ICAM-1), leading to defective migration, poor IS stabilization and degranulation, and abrogated activation of respiratory burst [83, 84, 86].
DC cytoskeletal remodeling by WASP is emerging as a key regulatory component of functional immune synapse formation, and consequently is important for directing T-cell responses [35, 87, 88]. Human and murine models have demonstrated that priming of wild-type T cells by WAS KO DCs is diminished [87, 89–91]. WASP-deficient DCs appear less able to support IL-12 and type 1 interferon secretion [92, 93] with abrogation of downstream events following TCR signaling. Such events include calcium flux, microtubule organizing center polarization, phosphorylation of zeta chain associated tyrosine kinase (ZAP)-70 and T-cell proliferation [87, 90]. WASP is also necessary for cytoskeletal remodeling during formation of the DC-NK cell immunostimulatory synapse and subsequent DC induction of NK-cell interferon gamma production [94].
One hypothesis for skin pathology in WAS is that decreased migration of LCs and DCs results in local potentiation of inflammatory T cells [95] (Fig. 2B). A recent study in mice subjected to skin challenge with allergens and parasitic infiltration revealed that in the absence of wasp, and thus impaired cdc42-mediated effector function, ras-related C3 botulinium toxin substrate 2 (rac2) activation was enhanced in DCs [96]. This led to enhanced cross-presentation of antigen through NADPH-oxidase mediated maintenance of neutral phagosome pH, and marked expansion of IFNγ producing CD8+ T cells at the expense of CD4+ T cells.
WASP has recently been implicated in the anti-inflammatory functions of macrophages (Fig. 2c), with an increased percentage of pro-inflammatory macrophages found in pre-colitic wasp-deficient mice [97]. Lipopolysaccharide (LPS) stimulation induced a much higher expression of pro-inflammatory cytokines in addition to enhanced CD4+ T-cell proliferation and decreased generation of Treg cells compared to wild-type mice.
WASP is important for neutrophil development. X-linked congenital neutropenia, resulting from gain of function mutations in the WASP GBD domain, results from destabilization of the autoinhibitory conformation and dysregulated actin polymerization [76]. Consequently, cytoplasmic viscosity of neutrophil precursors is increased, which impairs chromosomal separation during mitosis leading to premature apoptosis and relative failure of neutrophil differentiation [98]. WASP also plays a key role in neutrophil migration, with murine wasp-deficient PMNs migrating more slowly than wild-type through cell monolayers in parallel plate flow assays [84]. Although WASP-deficient PMNs adhered similarly to wild-type at low levels of shear stress in bead binding assays, attachments were lost when shear stress was increased to physiological levels [84], highlighting the additional importance of WASP in neutrophil adhesion.
A possible role of WASP in IgE-mediated mast cell cytoskeletal rearrangement has previously been identified. WASP-deficient mast cells exhibited defects in granule exocytosis and cytokine production, with decreased capacity to degranulate on FcεR1 triggering [99].
Activities of WASP family members and binding partners
WASP family proteins have recently been shown to have a number of important roles, particularly in autophagy, where the role of WASP itself is not yet known. WASH (WASP and SCAR homologue) has been implicated in downregulating autophagy by preventing ubiquitination of beclin 1 in murine embryonic fibroblasts [100]. WASH deficiency in Dictyostelium species and HeLa cells has more recently been linked with impaired autophagosome formation and lysosomal digestion of both phagocytic and autophagic cargo [101, 102]. WASH has additionally been shown to have an important role in ARP2/3-dependent endosomal sorting [101, 103]. Similarly, WAVE (WASP verprolin homologous proteins) and WHAMM (WASP homologue associated protein with actin membranes and microtubules) have now also been demonstrated to be important in endo/exocytosis and autophagosome formation [104, 105].
Defects in the WASP binding partners WIP [106], ARPC1B, a haematopoietic-restricted component of the ARP2/3 complex [107], and dedicator of cytokinesis 8 (DOCK8) [108–110] have recently been described to result in similar clinical phenotypes to WAS. The increased severity of DOCK8 deficiency may reflect an additional interaction with the WASP family protein neural WASP (N-WASP), which is more widely expressed [111]. DOCK8 forms a complex with WIP and WASP linking the TCR to the actin cytoskeleton, with actin polymerization occurring via DOCK8-mediated CDC42 activation of WASP following TCR ligation [112]. Combined DOCK8 and WASP deficiencies in mice show attenuated subcortical actin, with reduced filamentous (F) actin content, defective TCR-driven actin foci formation and mechanotransduction, resulting in impaired T-cell transendothelial migration and homing to lymph nodes [112]. The cytoskeletal adaptor and fes/CIP4 homology-bin/amphiphysin/rvs (F-BAR) protein proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) negatively regulates the transition from podosomes to filopodia in macrophages, through its interaction with WASP. Mutations in PSTPIP1 are causally associated with a number of autoinflammatory diseases, including PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum and acne), which shares some similar inflammatory pathologies to those of WAS. This further highlights the importance of actin cytoskeleton regulation in autoinflammation [113].
Bruton’s tyrosine kinase (BTK) has been shown to modulate inflammatory responses in macrophages through its interaction with WASP downstream of toll-like receptors (TLRs) [114, 115]. Inhibition of the WASP-BTK interaction in macrophages was shown to result in impaired phosphorylation of inhibitor of κB αβ (IKKαβ) and nuclear factor (NF)-kB with reduced transcription of the inflammatory cytokines TNF-α, IL-6 and IL-1β [114, 115]. How this plays into the wider picture of inflammation in WAS is not yet known. More recently, an interaction between WASP and BTK has also been demonstrated to be important for neutrophil migration in sterile inflammation [116] (Fig. 2B).
Additionally, neutrophil recruitment to sites of inflammation has been found to be dependent on WASP through its binding to SRC kinase-associated phosphoprotein 2 (SKAP 2), which appears to be necessary for regulating actin polymerization and β2 integrin activation [117]. Deletion of skap2 in mice results in failure of integrin activation and a leukocyte adhesion deficiency (LAD)-like phenotype.
Conclusion
Over the years, defects in WASP have been identified in many different lineages, but the challenge now is to work out how they operate together to create the complex identity of WAS. Interplay between defects of peripheral and central tolerance, and effector/regulatory cells that mediate balanced cytokine responses during inflammation is likely to be particularly important. Pursuit of understanding WAS has provided a unique opportunity to explore the role of the cytoskeleton during normal immune function from cellular homeostasis to evasion of infection, cancer and autoimmunity. Future studies may well lead to the emergence of targeted therapies for autoimmunity and inflammation extending beyond the realm of rare monogenic diseases such as WAS.
Acknowledgements
A.J.T. is supported by both the Wellcome Trust (104807/Z/14/Z) and by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. E.R. is supported by the Wellcome Trust (201250/Z/16/Z).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 2.Worth AJ, Thrasher AJ. Current and emerging treatment options for Wiskott-Aldrich syndrome. Expert Rev Clin Immunol. 2015;11:1015–1032. doi: 10.1586/1744666X.2015.1062366. [DOI] [PubMed] [Google Scholar]
- 3.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 4.Panchal SC, Kaiser DA, Torres E, Pollard TD, Rosen MK. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol. 2003;10:591–598. doi: 10.1038/nsb952. [DOI] [PubMed] [Google Scholar]
- 5.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J Biol Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 6.Blundell MP, Bouma G, Metelo J, Worth A, Calle Y, Cowell LA, Westerberg LS, et al. Phosphorylation of WASp is a key regulator of activity and stability in vivo. Proc Natl Acad Sci U S A. 2009;106:15738–15743. doi: 10.1073/pnas.0904346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–1011. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- 8.Blundell MP, Worth A, Bouma G, Thrasher AJ. The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function. Dis Markers. 2010;29:157–175. doi: 10.3233/DMA-2010-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasevic N, Jia Z, Russell A, Fujii T, Hartman JJ, Clancy S, Wang M, et al. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry. 2007;46:3494–3502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- 10.Rivero-Lezcano OM, Marcilla A, Sameshima JH, Robbins KC. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol Cell Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato M, Miki H, Imai K, Nonoyama S, Suzuki T, Sasakawa C, Takenawa T. Wiskott-Aldrich syndrome protein induces actin clustering without directbinding to Cdc42. J Biol Chem. 1999;274:27225–27230. doi: 10.1074/jbc.274.38.27225. [DOI] [PubMed] [Google Scholar]
- 12.Cory GO, Cramer R, Blanchoin L, Ridley AJ. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol Cell. 2003;11:1229–1239. doi: 10.1016/s1097-2765(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 13.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 14.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–735. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, et al. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou HC, Anton IM, Holt MR, Curcio C, Lanzardo S, Worth A, Burns S, et al. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–2344. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe Y, Sasahara Y, Ramesh N, Massaad MJ, Yeng Looi C, Kumaki S, Kure S, et al. T-cell receptor ligation causes Wiskott-Aldrich syndrome protein degradation and F-actin assembly downregulation. J Allergy Clin Immunol. 2013;132:648–655.:e641. doi: 10.1016/j.jaci.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Reicher B, Joseph N, David A, Pauker MH, Perl O, Barda-Saad M. Ubiquitylation-dependent negative regulation of WASp is essential for actin cytoskeleton dynamics. Mol Cell Biol. 2012;32:3153–3163. doi: 10.1128/MCB.00161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Fuente MA, Sasahara Y, Calamito M, Anton IM, Elkhal A, Gallego MD, Suresh K, et al. WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP) Proc Natl Acad Sci U S A. 2007;104:926–931. doi: 10.1073/pnas.0610275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh N, Anton IM, Hartwig JH, Geha RS. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc Natl Acad Sci USA. 1997;94:14671–14676. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 22.Cotta-de-Almeida V, Westerberg L, Maillard MH, Onaldi D, Wachtel H, Meelu P, Chung UI, et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci U S A. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 24.Park JY, Kob M, Prodeus AP, Rosen FS, Shcherbina A, Remold-O’Donnell E. Early deficit of lymphocytes in Wiskott-Aldrich syndrome: possible role of WASP in human lymphocyte maturation. Clin Exp Immunol. 2004;136:104–110. doi: 10.1111/j.1365-2249.2004.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotta-de-Almeida V, Dupre L, Guipouy D, Vasconcelos Z. Signal Integration during T Lymphocyte Activation and Function: Lessons from the Wiskott-Aldrich Syndrome. Front Immunol. 2015;6:47. doi: 10.3389/fimmu.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff JA. Wiskott-Aldrich syndrome: clinical, immunologic, and pathologic observations. J Pediatr. 1967;70:221–232. doi: 10.1016/s0022-3476(67)80417-7. [DOI] [PubMed] [Google Scholar]
- 27.Morio T, Takase K, Okawa H, Oguchi M, Kanbara M, Hiruma F, Yoshino K, et al. The increase of non-MHC-restricted cytotoxic cells (gamma/delta-TCR-bearing T cells or NK cells) and the abnormal differentiation of B cells inWiskott-Aldrich syndrome. Clin Immunol Immunopathol. 1989;52:279–290. doi: 10.1016/0090-1229(89)90179-7. [DOI] [PubMed] [Google Scholar]
- 28.Wada T, Schurman SH, Garabedian EK, Yachie A, Candotti F. Analysis of T-cell repertoire diversity in Wiskott-Aldrich syndrome. Blood. 2005;106:3895–3897. doi: 10.1182/blood-2005-06-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadhukhan S, Sarkar K, Taylor M, Candotti F, Vyas YM. Nuclear role of WASp in gene transcription is uncoupled from its ARP2/3-dependent cytoplasmic role in actin polymerization. J Immunol. 2014;193:150–160. doi: 10.4049/jimmunol.1302923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor MD, Sadhukhan S, Kottangada P, Ramgopal A, Sarkar K, D’Silva S, Selvakumar A, et al. Nuclear role of WASp in the pathogenesis of dysregulated TH1 immunity in human Wiskott-Aldrich syndrome. Sci Transi Med. 2010;2:37ra44. doi: 10.1126/scitranslmed.3000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, Guidotti L, Martino S, et al. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol. 2006;177:7451–7461. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- 32.Nikolov NP, Shimizu M, Cleland S, Bailey D, Aoki J, Strom T, Schwartzberg PL, et al. Systemic autoimmunity and defective Fas ligand secretion in the absence of the Wiskott-Aldrich syndrome protein. Blood. 2010;116:740–747. doi: 10.1182/blood-2009-08-237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlings SL, Crooks GM, Bockstoce D, Barsky LW, Parkman R, Weinberg KI. Spontaneous apoptosis in lymphocytes from patients with Wiskott-Aldrich syndrome: correlation of accelerated cell death and attenuated bcl-2 expression. Blood. 1999;94:3872–3882. [PubMed] [Google Scholar]
- 34.Rengan R, Ochs HD, Sweet LI, Keil ML, Gunning WT, Lachant NA, Boxer LA, et al. Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood. 2000;95:1283–1292. [PubMed] [Google Scholar]
- 35.Malinova D, Fritzsche M, Nowosad CR, Armer H, Munro PM, Blundell MP, Charras G, et al. WASp-dependent actin cytoskeleton stability at the dendritic cell immunological synapse is required for extensive, functional T cell contacts. J Leukoc Biol. 2016;99:699–710. doi: 10.1189/jlb.2A0215-050RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 37.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGavin MK, Badour K, Hardy LA, Kubiseski TJ, Zhang J, Siminovitch KA. The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J Exp Med. 2001;194:1777–1787. doi: 10.1084/jem.194.12.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N, Geha RS. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 41.Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, et al. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–154. doi: 10.1016/s1074-7613(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 42.Dupre L, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C, Roncarolo MG. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 2002;17:157–166. doi: 10.1016/s1074-7613(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 43.Kumari S, Depoil D, Martinelli R, Judokusumo E, Carmona G, Gertler FB, Kam LC, et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. Elife. 2015;4 doi: 10.7554/eLife.04953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvez R, Lafouresse F, De Meester J, Galy A, Valitutti S, Dupre L. The Wiskott-Aldrich syndrome protein permits assembly of a focused immunological synapse enabling sustained T-cell receptor signaling. Haematologica. 2011;96:1415–1423. doi: 10.3324/haematol.2011.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morales-Tirado V, Johannson S, Hanson E, Howell A, Zhang J, Siminovitch KA, Fowell DJ. Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J Immunol. 2004;173:726–730. doi: 10.4049/jimmunol.173.2.726. [DOI] [PubMed] [Google Scholar]
- 46.Morales-Tirado V, Sojka DK, Katzman SD, Lazarski CA, Finkelman FD, Urban JF, Fowell DJ. Critical requirement for the Wiskott-Aldrich syndrome protein in Th2 effector function. Blood. 2010;115:3498–3507. doi: 10.1182/blood-2009-07-235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallego MD, de la Fuente MA, Anton IM, Snapper S, Fuhlbrigge R, Geha RS. WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1alpha. Int Immunol. 2006;18:221–232. doi: 10.1093/intimm/dxh310. [DOI] [PubMed] [Google Scholar]
- 48.Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, Alt FW, Rosen FS, et al. WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J Leukoc Biol. 2005;77:993–998. doi: 10.1189/jlb.0804444. [DOI] [PubMed] [Google Scholar]
- 49.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, Baz Z, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adriani M, Aoki J, Horai R, Thornton AM, Konno A, Kirby M, Anderson SM, et al. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clin Immunol. 2007;124:41–48. doi: 10.1016/j.clim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, Nguyen T, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adriani M, Jones KA, Uchiyama T, Kirby MR, Silvin C, Anderson SM, Candotti F. Defective inhibition of B-cell proliferation by Wiskott-Aldrich syndrome protein-deficient regulatory T cells. Blood. 2011;117:6608–6611. doi: 10.1182/blood-2010-12-322834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, Nagler C, Bhan AK, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lexmond WS, Goettel JA, Lyons JJ, Jacobse J, Deken MM, Lawrence MG, Di Maggio TH, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest. 2016;126:4030–4044. doi: 10.1172/JCI85129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Recher M, Burns SO, de la Fuente MA, Volpi S, Dahlberg C, Walter JE, Moffitt K, et al. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–2828. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castiello MC, Bosticardo M, Pala F, Catucci M, Chamberlain N, van Zelm MC, Driessen GJ, et al. Wiskott-Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun. 2014;50:42–50. doi: 10.1016/j.jaut.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolhatkar NS, Scharping NE, Sullivan JM, Jacobs HM, Schwartz MA, Khim S, Notarangelo LD, et al. B-cell intrinsic TLR7 signals promote depletion of the marginal zone in a murine model of Wiskott-Aldrich syndrome. Eur J Immunol. 2015;45:2773–2779. doi: 10.1002/eji.201545644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon KL, Anderson SM, Garabedian EK, Moratto D, Sokolic RA, Candotti F. Molecular and phenotypic abnormalities of B lymphocytes in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2014;133:896–899.:e894. doi: 10.1016/j.jaci.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pala F, Morbach H, Castiello MC, Schickel JN, Scaramuzza S, Chamberlain N, Cassani B, et al. Lentiviral-mediated gene therapy restores B cell tolerance in Wiskott-Aldrich syndrome patients. J Clin Invest. 2015;125:3941–3951. doi: 10.1172/JCI82249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolhatkar NS, Brahmandam A, Thouvenel CD, Becker-Herman S, Jacobs HM, Schwartz MA, Allenspach EJ, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med. 2015;212:1663–1677. doi: 10.1084/jem.20150585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weill JC, Reynaud CA. The ups and downs of negative (and positive) selection of B cells. J Clin Invest. 2015;125:3748–3750. doi: 10.1172/JCI84009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Bai X, Wu J, Sharma S, Upadhyaya A, Dahlberg CI, Westerberg LS, et al. N-wasp is essential for the negative regulation of B cell receptor signaling. PLoS Biol. 2013;11:e1001704. doi: 10.1371/journal.pbio.1001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Miller H, Hui KL, Grooman B, Bolland S, Upadhyaya A, Song W. Abalance of Bruton’s tyrosine kinase and SHIP activation regulates B cell receptor cluster formation by controlling actin remodeling. J Immunol. 2011;187:230–239. doi: 10.4049/jimmunol.1100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burns SO, Zarafov A, Thrasher AJ. Primary immunodeficiencies due to abnormalities of the actin cytoskeleton. Curr Opin Hematol. 2017;24:16–22. doi: 10.1097/MOH.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 65.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westerberg L, Larsson M, Hardy SJ, Fernandez C, Thrasher AJ, Severinson E. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood. 2005;105:1144–1152. doi: 10.1182/blood-2004-03-1003. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, Dai R, Li W, Zhao H, Zhang Y, Zhou L, Du H, et al. Abnormalities of follicular helper T-cell number and function in Wiskott-Aldrich syndrome. Blood. 2016;127:3180–3191. doi: 10.1182/blood-2015-06-652636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bouma G, Carter NA, Recher M, Malinova D, Adriani M, Notarangelo LD, Burns SO, et al. Exacerbated experimental arthritis in Wiskott-Aldrich syndrome protein deficiency: modulatory role of regulatory B cells. Eur J Immunol. 2014;44:2692–2702. doi: 10.1002/eji.201344245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama T, Yoshizaki A, Simon KL, Kirby MR, Anderson SM, Candotti F. Age-Dependent Defects of Regulatory B Cells in Wiskott-Aldrich Syndrome Gene Knockout Mice. PLoS One. 2015;10:e0139729. doi: 10.1371/journal.pone.0139729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iizuka Y, Cichocki F, Sieben A, Sforza F, Karim R, Coughlin K, Isaksson Vogel R, et al. UNC-45 A Is a Nonmuscle Myosin IIA Chaperone Required for NK Cell Cytotoxicity via Control of Lytic Granule Secretion. J Immunol. 2015;195:4760–4770. doi: 10.4049/jimmunol.1500979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Astrakhan A, Ochs HD, Rawlings DJ. Wiskott-Aldrich syndrome protein is required for homeostasis and function of invariant NKT cells. J Immunol. 2009;182:7370–7380. doi: 10.4049/jimmunol.0804256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Locci M, Draghici E, Marangoni F, Bosticardo M, Catucci M, Aiuti A, Cancrini C, et al. The Wiskott-Aldrich syndrome protein is required for iNKT cell maturation and function. J Exp Med. 2009;206:735–742. doi: 10.1084/jem.20081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berzofsky JA, Terabe M. The contrasting roles of NKT cells in tumor immunity. Curr Mol Med. 2009;9:667–672. doi: 10.2174/156652409788970706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, Kempski H, Burns S, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108:2182–2189. doi: 10.1182/blood-2006-01-010249. [DOI] [PubMed] [Google Scholar]
- 77.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. [PubMed] [Google Scholar]
- 78.Leverrier Y, Lorenzi R, Blundell MP, Brickell P, Kinnon C, Ridley AJ, Thrasher AJ. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol. 2001;166:4831–4834. doi: 10.4049/jimmunol.166.8.4831. [DOI] [PubMed] [Google Scholar]
- 79.Tsuboi S, Meerloo J. Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J Biol Chem. 2007;282:34194–34203. doi: 10.1074/jbc.M705999200. [DOI] [PubMed] [Google Scholar]
- 80.Badolato R, Sozzani S, Malacarne F, Bresciani S, Fiorini M, Borsatti A, Albertini A, et al. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1998;161:1026–1033. [PubMed] [Google Scholar]
- 81.Zicha D, Allen WE, Brickell PM, Kinnon C, Dunn GA, Jones GE, Thrasher AJ. Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br J Haematol. 1998;101:659–665. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 82.Looi CY, Sasahara Y, Watanabe Y, Satoh M, Hakozaki I, Uchiyama M, Wong WF, et al. The open conformation of WASP regulates its nuclear localization and gene transcription in myeloid cells. Int Immunol. 2014;26:341–352. doi: 10.1093/intimm/dxt072. [DOI] [PubMed] [Google Scholar]
- 83.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 84.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, Hu Y, Wara D, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25:285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones GE, Zicha D, Dunn GA, Blundell M, Thrasher A. Restoration of podosomes and chemotaxis in Wiskott-Aldrich syndrome macrophages following induced expression of WASp. Int J Biochem Cell Biol. 2002;34:806–815. doi: 10.1016/s1357-2725(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 86.Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Thrasher AJ. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton. 2004;57:118–132. doi: 10.1002/cm.10163. [DOI] [PubMed] [Google Scholar]
- 87.Bouma G, Mendoza-Naranjo A, Blundell MP, de Falco E, Parsley KL, Burns SO, Thrasher AJ. Cytoskeletal remodeling mediated by WASp in dendritic cells is necessary for normal immune synapse formation and T-cell priming. Blood. 2011;118:2492–2501. doi: 10.1182/blood-2011-03-340265. [DOI] [PubMed] [Google Scholar]
- 88.Benvenuti F. The Dendritic Cell Synapse: A Life Dedicated to T Cell Activation. Front Immunol. 2016;7:70. doi: 10.3389/fimmu.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bouma G, Burns S, Thrasher AJ. Impaired T-cell priming in vivo resulting from dysfunction of WASp-deficient dendritic cells. Blood. 2007;110:4278–4284. doi: 10.1182/blood-2007-06-096875. [DOI] [PubMed] [Google Scholar]
- 90.Pulecio J, Tagliani E, Scholer A, Prete F, Fetler L, Burrone OR, Benvenuti F. Expression of Wiskott-Aldrich syndrome protein in dendritic cells regulates synapse formation and activation of naive CD8+ T cells. J Immunol. 2008;181:1135–1142. doi: 10.4049/jimmunol.181.2.1135. [DOI] [PubMed] [Google Scholar]
- 91.de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, Schulz O, Zwirner J, et al. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood. 2005;105:1590–1597. doi: 10.1182/blood-2004-06-2332. [DOI] [PubMed] [Google Scholar]
- 92.Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, Aiuti A, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med. 2013;210:355–374. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang PA, Shaabani N, Borkens S, Honke N, Scheu S, Booth S, Brenner D, et al. Reduced type I interferon production by dendritic cells and weakened antiviral immunity in patients with Wiskott-Aldrich syndrome protein deficiency. J Allergy Clin Immunol. 2013;131:815–824. doi: 10.1016/j.jaci.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- 95.Thrasher AJ, Jones GE, Kinnon C, Brickell PM, Katz DR. Is Wiskott-Aldrich syndrome a cell trafficking disorder? Immunol Today. 1998;19:537–539. doi: 10.1016/s0167-5699(98)01350-4. [DOI] [PubMed] [Google Scholar]
- 96.Baptista MA, Keszei M, Oliveira M, Sunahara KK, Andersson J, Dahlberg CI, Worth AJ, et al. Deletion of Wiskott-Aldrich syndrome protein triggers Rac2 activity and increased cross-presentation by dendritic cells. Nat Commun. 2016;7:12175. doi: 10.1038/ncomms12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biswas A, Shouval D, Goettel J, Field M, Griffith A, Pai SY, Notarangelo L, et al. O-008 Aberrant Anti-inflammatory Macrophage Function and Differentiation in Wiskott-Aldrich Syndrome Protein-Deficient Mice and Humans. Inflamm Bowel Dis. 2016;22(Suppl 1):S3 [Google Scholar]
- 98.Moulding DA, Moeendarbary E, Valon L, Record J, Charras GT, Thrasher AJ. Excess F-actin mechanically impedes mitosis leading to cytokinesis failure in X-linked neutropenia by exceeding Aurora B kinase error correction capacity. Blood. 2012;120:3803–3811. doi: 10.1182/blood-2012-03-419663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pivniouk VI, Snapper SB, Kettner A, Alenius H, Laouini D, Falet H, Hartwig J, et al. Impaired signalingviathe high-affinity IgE receptor in Wiskott-Aldrich syndrome protein-deficient mast cells. Int Immunol. 2003;15:1431–1440. doi: 10.1093/intimm/dxg148. [DOI] [PubMed] [Google Scholar]
- 100.Xia P, Wang S, Du Y, Zhao Z, Shi L, Sun L, Huang G, et al. WASH inhibits autophagy through suppression of Beclin 1 ubiquitina-tion. EMBO J. 2013;32:2685–2696. doi: 10.1038/emboj.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.King JS, Gueho A, Hagedorn M, Gopaldass N, Leuba F, Soldati T, Insall RH. WASH is required for lysosomal recycling and efficient autophagic and phagocytic digestion. Mol Biol Cell. 2013;24:2714–2726. doi: 10.1091/mbc.E13-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014;5:3828. doi: 10.1038/ncomms4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia P, Wang S, Huang G, Du Y, Zhu P, Li M, Fan Z. RNF2 is recruited by WASH to ubiquitinate AMBRA1 leading to downregulation of autophagy. Cell Res. 2014;24:943–958. doi: 10.1038/cr.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kast DJ, Zajac AL, Holzbaur EL, Ostap EM, Dominguez R. WHAMM Directs the Arp2/3 Complex to the ER for Autophagosome Biogenesis through an Actin Comet Tail Mechanism. Curr Biol. 2015;25:1791–1797. doi: 10.1016/j.cub.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z, Wu B, Chai W, Cao L, Wang Y, Yu Y, Yang L. Knockdown of WAVE1 enhances apoptosis of leukemia cells by downregulating autophagy. Int J Oncol. 2016;48:2647–2656. doi: 10.3892/ijo.2016.3446. [DOI] [PubMed] [Google Scholar]
- 106.Lanzi G, Moratto D, Vairo D, Masneri S, Delmonte O, Paganini T, Parolini S, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med. 2012;209:29–34. doi: 10.1084/jem.20110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuijpers TW, Tool AT, van der Bijl I, de Boer M, van Houdt M, de Cuyper IM, Roos D, et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol. 2016:273–277.:e10. doi: 10.1016/j.jaci.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–1302.:e1284. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H, Whittle B, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody pro-duction. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moulding DA, Record J, Malinova D, Thrasher AJ. Actin cytoskeletal defects in immunodeficiency. Immunol Rev. 2013;256:282–299. doi: 10.1111/imr.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janssen E, Tohme M, Hedayat M, Leick M, Kumari S, Ramesh N, Massaad MJ, et al. A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J Clin Invest. 2016;126:3837–3851. doi: 10.1172/JCI85774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Starnes TW, Bennin DA, Bing X, Eickhoff JC, Grahf DC, Bellak JM, Seroogy CM, et al. The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood. 2014;123:2703–2714. doi: 10.1182/blood-2013-07-516948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakuma C, Sato M, Takenouchi T, Chiba J, Kitani H. Critical roles of the WASP N-terminal domain and Btk in LPS-induced inflammatory response in macrophages. PLoS One. 2012;7:e30351. doi: 10.1371/journal.pone.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakuma C, Sato M, Takenouchi T, Kitani H. Specific binding of the WASP N-terminal domain to Btk is critical for TLR2 signaling in macrophages. Mol Immunol. 2015;63:328–336. doi: 10.1016/j.molimm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 116.Volmering S, Block H, Boras M, Lowell CA, Zarbock A. The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation. Immunity. 2016;44:73–87. doi: 10.1016/j.immuni.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boras M, Volmering S, Bokemeyer A, Rossaint J, Block H, Van Bardel B, Marck V, et al. Skap2 is required for beta2 integrin-mediated neutrophil recruitment and functions. J Exp Med. 2017;3:1–24.:157. doi: 10.1084/jem.20160647. [DOI] [PMC free article] [PubMed] [Google Scholar]