Abstract
Prostaglandin (PG) D2 has been in the focus of research for quite a long time, but its biological effects and its roles in human disease are still not fully characterized. When in 2001 a second major PGD2 receptor termed chemoattractant receptor homologue expressed on Th2 cells (CRTH2; alternative name DP2) was discovered, diverse investigations started to shed more light on the complex and often controversial actions of the prostaglandin. With various immunomodulating effects, such as induction of migration, activation, and cytokine release of leukocytes observed both in vivo and in vitro, CRTH2 has emerged as a promising target for the treatment of allergic diseases. However, with more and more research being performed on CRTH2, it has also become clear that its biological actions are far more diverse than expected at the beginning. In this review, we aim to summarize the roles that PGD2 – and CRTH2 in particular – might play in diseases of the central nervous system, kidney, intestine, lung, hair and skin, bone and cartilage, and in cancer. Based on current data we propose that blocking CRTH2 might be a potential therapeutic approach to numerous conditions beyond classical allergic diseases and asthma.
Keywords: CRTH2, DP2, DP1, PGD2, Inflammation pharmacological target
1. CRTH2 – history and clinical potential
Among prostaglandins (PG), PGD2 remained the most elusive species for a long time and was initially regarded as having negligible biological activity [1]. In 1974 its inhibitory effect on platelet aggregation was discovered by Smith et al. [2] and Mills & McFarlain [3], and both pressor and depressor actions were found in different smooth muscle preparations by Horton et al. [4]. In 1976, pro-inflammatory actions of PGD2 were described by Flower et al. in rat and human skin, causing erythema and edema, however, in the absence of pain [5]. In dog lung, PGD2 was observed to cause broncho- and vasoconstriction, while causing systemic hypotension [6] and renal vasodilation [7]. In contrast, guinea pig coronary arteries were constricted by PGD2 [8,9]. Later it was shown that it was the thromboxane receptor, TP, that mediated these constrictor effects, as PGD2 was found also to bind to TP at micromolar concentrations [10], whereas inhibition of platelet aggregation and vasodilation by PGD2 depended on its cognate D-type prostaglandin receptor, DP (also named DP1) [11]. In 1978, Anhut et al. [12] suggested that PGD2 was formed during anaphylactic reactions, which might contribute to broncho- and vasoconstriction during asthma attacks, as they hypothesized. Four years later, Lewis et al. demonstrated that mast cells were a major source of PGD2 [13]. Although Peskar & Brune already proposed in 1979 that PGD2 was the prevailing PG in acute inflammatory responses [14], its immune modulator mode of action still needed to be elucidated. In dogs, two studies indirectly suggested that PGD2 might be a chemoattractant for eosinophils, the first showing that intravenous PGD2 caused a transient drop in circulating eosinophil numbers [15], and the second that intratracheal PGD2 caused intra-luminal eosinophil accumulation [16]. In 1990, Woodward et al. described the ocular hypotensive effect of PGD2 and the selective DP1 agonist BW245c in guinea pigs [17]. However, they also found that PGD2–but not the DP1 agonist – induced ocular inflammation characterized by accumulation of eosinophils in the conjunctiva. Interestingly, the PGD2 metabolite PGJ2 was as effective as PGD2 in causing eosinophil accumulation, but was unable to decrease ocular pressure, which pointed to a yet unknown PGD2 receptor. Subsequently, PGD2 was shown to stimulate the migration of eosinophils towards zymosan-activated serum and induce calcium flux in human eosinophils [18,19], but it was only in 2001 that PGD2 was unequivocally shown to be a potent eosinophil chemoattractant acting through a novel receptor termed chemoattractant receptor homologue expressed on Th2 cells (CRTH2; alternative name: DP2) [20–22]. This receptor had previously been cloned as an orphan receptor (GPR44) that was expressed by eosinophils, basophils and Th2 lymphocytes [23]. In fact, CRTH2 was characterized as the most reliable surface marker for Th2 cells [24]. With these findings in mind, PGD2 and its receptor CRTH2 has become one of the most promising therapeutic targets in the field of allergy and asthma, which was also fueled by the discovery of indomethacin as a potent and selective CRTH2 agonist. This clinically used cyclooxygenase inhibitor subsequently served as a pharmacophore for the development of several CRTH2 antagonists [25], belonging to the family of indole-acetic acid derivatives. Some of those including OC000459, or AZD1981 have already been evaluated in clinical studies for the treatment of asthma, allergic rhinitis and eosinophilic esophagitis [26–30]. Although, major breakthroughs in the clinical usefulness of CRTH2 antagonists are still to be anticipated, recent studies in allergic asthma are showing promising results: Fevipriprant improved lung function in a subgroup of patients suffering from severe air flow limitation [31] and timapriprant (OC000459) beneficially altered asthma control as well as lung function in atopic eosinophilic asthmatics [32]. Timapiprant and another CRTH2 antagonist, BI 671800, also successfully reduced nasal and ocular symptoms in allergic subjects exposed to grass pollen [27,33]. For a detailed review of PGD2 receptor antagonists in the treatment of asthma, please refer to the recent review by Santus and Radovanovic [25].
Ironically, the purported TP antagonist ramatroban (BAY u 3405) which had already been marketed in Japan as a treatment of allergic rhinitis, was also revealed to be a potent CRTH2 antagonist [34].
2. CRTH2 beyond allergy and asthma
Meanwhile, CRTH2 has been found to be expressed on several additional cell types and in different tissues suggesting that the PGD2/CRTH2 axis might be of potential relevance beyond allergy and asthma. Although the role of PGD2 in a Th2-biased inflammation is well established, investigation of its function in other groups of inflammatory reactions in experimental mouse models is confounded by differential expression patterns of CRTH2 in mice and humans: While CRTH2 can be used as an exclusive marker for Th2 cells in humans, CRTH2-positive Th1 cells as well as neutrophils are present in mice. These differences have to be taken into consideration when drawing conclusions from studies exclusively based on mouse data. A detailed summary of the presence or absence of CRTH2 on various cells types can be found in Table 1.
Table 1. Reported presence (or reactivity) and absence of CRTH2/DP2 on human and murine structural and immune cells.
| Human | |||
|---|---|---|---|
| Cell type | Reported by | Reference | |
| CRTH2/DP2 positive | bronchial epithelium | immunostaining | [35,36] |
| mast cells | immunohistochemistry, flow cytometry | [38] | |
| basophils | flow cytometry, mRNA expression | [39] | |
| eosinophils | flow cytometry, mRNA expression | [21,39] | |
| macrophages | flow cytometry, immunohistochemistry | [40] | |
| monocytes | flow cytometry, mRNA expression | [41] | |
| innate lymphoid cells type 2 | flow cytometry | [42] | |
| Th2 cells | flow cytometry, mRNA expression, western blotting | [23] | |
| dendritic cells | flow cytometry, mRNA expression | [41] | |
| CD8 + T cells (positive in same cases) | flow cytometry, mRNA expression, western blotting | [23] | |
| CRTH2/DP2 negative | Th1 cells | flow cytometry, mRNA expression | [23] |
| NK cells | flow cytometry, mRNA expression | [23] | |
| B cells | flow cytometry, mRNA expression | [23] | |
| neutrophils | mRNA expression | [22,43] | |
| Mouse | |||
|---|---|---|---|
| Cell type | Reported by | Reference | |
| CRTH2/DP2 positive/reactive | epithelium | reactive to CRTH2 agonism | [44] |
| mast cells | mRNA expression, western blotting | [45] | |
| eosinophils | mRNA expression | [46] | |
| macrophages | mRNA expression | [47] | |
| monocytes | mRNA expression | [47] | |
| Innate lymphoid cells type 2 | flow cytometry, mRNA expression | [48] | |
| Th2 cells | mRNA expression | [49] | |
| Th1 cells | mRNA expression | [49] | |
| CD8+ T cells | mRNA expression | [49] | |
| neutrophils | mRNA expression | [50] | |
2.1. Respiratory tract
In the human lung, the majority of structural (epithelium and endothelium) and immune cells (including macrophages, monocytes, mast cells, Th2 cells and eosinophils) express CRTH2 receptors. Interestingly, CRTH2 expression levels as well as the ratio of CRTH2-positive vs CRTH2-negative cells have been reported to correlate with disease activity. In scleroderma, an increased ratio of CCR5- vs CRTH2-expressing cells in the circulating T lymphocyte population was associated with a persistent involvement of the lung vasculature manifested as pulmonary arterial hypertension. This state of a high CCR5/CRTH2 ratio was associated with a poorer prognosis and a profibrotic phenotype in scleroderma patients [51]. In experimental fibrosis induced by bleomycin application, hematopoietic PGD synthase-deficient mice exhibited a more severe phenotype. Although the authors did not assess the specific receptors involved, a protective role of PGD2 in fibrosis was proposed [52]. It is reasonable to at least partially attest the protective role of PGD2 in pulmonary fibrosis to both direct anti-proliferative effect on fibroblasts [53] and anti-fibrotic effects mediated by inhibition of TGF-beta-induced collagen production by DP1 receptor activation [54]. In addition, an involvement of CRTH2 receptors seems also likely, as earlier studies using indomethacin found reduced collagen content and improved lung histopathology after intratracheal administration of bleomycin (primarily inducing lung damage and fibrosis) [55] as well as after systemic bleomycin administration (causing multiple organ fibrosis) [56]. In support of these findings, preliminary reports also suggested that bleomycyin-induced pulmonary fibrosis was aggravated in CRTH2-knockout mice, displaying higher mortality rate, reduced pulmonary compliance and increased inflammation and collagen deposition [57,58]. This notion of an anti-fibrotic action of PGD2 was further substantiated by the ability of CRTH2/PGD2 to inhibit epithelial-to-mesenchymal transition, a process observed during development of fibrosis [59]. Unfortunately, the involvement of CRTH2 receptors has not been assessed in human pulmonary fibrosis thus far. Given the differential expression of CRTH2 receptors in mice and humans, the antifibrotic effects in experimental fibrosis may not be directly transferable to human disease. Indeed, at variance with the murine studies, Zhou and colleagues described a profibrotic role of CRTH2 in the inherited disorder Hermansky-Pudlak syndrome. This disease can present with pulmonary fibrosis as a leading cause of mortality. The authors here described a functional interaction of CRTH2 and chitinase 3-like-1 (CHI3L1) resulting in increased pro-fibrotic signaling [60]. Hence, these data suggest that CRTH2 can be associated both with anti- and pro-fibrotic events.
CRTH2 has further been found to contribute to acute lung inflammation. In a murine model of endotoxin-induced acute lung injury,we found that CRTH2 activation led to an early-phase polarization of alveolar macrophages resulting in a lung milieu favoring neutrophil recruitment and, therefore, inducing a more severe phenotype with regard to lung histo-pathology as well as lung function. In this study, activation of CRTH2 on macrophages induced a pro-inflammatory phenotype leading to elevated levels of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), monocyte chemotactic protein-1 and keratinocyte-derived cytokine in the bronchoalveolar lavage fluid (BALF) which – in turn – stimulated neutrophils. Accordingly, a measurable increase in endogenous PGD2 levels was detected in the BALF of endotoxin-treated animals and pharmacological blockade of CRTH2 ameliorated alveolar neutrophil influx into the lungs. Although murine neutrophils are known to express CRTH2 receptors (as mentioned in the previous section), then pro-inflammatory actions on neutrophils were not due to direct activation by PGD2 but via macrophage activation. [40]. In a more severe form of LPS-induced acute lung injury, PGD2 was found to play a protective role, which seemed to depend on the DP1 receptor rather than CRTH2 [37]. Therefore, in acute inflammation, CRTH2 activation is likely to induce a proinflammatory signature in the lung.
The prominent role of CRTH2 in the lung prompted investigations to evaluate the potential of the CRTH2 antagonist AZ11805131 in tobacco smoke-induced airway inflammation, modelling chronic obstructive pulmonary disease (COPD) [61]. The decreased levels of bronchoalveolar lavage neutrophils, macrophages and lymphocytes as well as an improved lung mucosal pathology upon CRTH2 antagonism showed promising results in this mouse study. In the same year another study provided further support for the therapeutic potential of CRTH2 antagonism in both acute as well as sub-chronic murine models of cigarette-induced airway inflammation. In this study, the potent CRTH2 antagonists AM156 and AM206 inhibited neutrophil and lymphocyte recruitment, and additionally also ameliorated airway inflammation by reduction of airway epithelial thickening and mucus cell metaplasia [62]. With these promising results from murine experimental studies, the CRTH2 antagonist AZD1981 was tested in COPD patients. Unfortunately, the positive effects observed in murine models were not replicated in a phase II trial of the CRTH2 antagonist in COPD patients [63]. In consideration that COPD patients present with a Th1 skewing, it cannot be excluded that the beneficial effect in the murine models resulted from an antagonistic action on Th1 cells and neutrophils, which might not be the case in human pathology.
2.2. Kidney
In the renal system, increased expression of the lipocalin-like PGD synthase (L-PGDS) has been reported in early stage diabetic nephropathy in rats [64] and adriamycin-induced nephropathy in mice [65], suggesting a possible contribution of PGD2 in chronic kidney disease. To our knowledge, so far only one study investigated the functional role of CRTH2-mediated PGD2 effects in kidney disease. Here, the authors corroborated the previous findings of increased L-PGDS expression in another model of chronic kidney disease induced by ureteral obstruction. Furthermore, genetic as well as pharmacological blockade of CRTH2 signaling strongly reduced renal fibrosis and inflammation via suppression of the interleukin (IL)-4/IL-13 axis[66]. Hence, there is a clear involvement of PGD2 in the renal system, with elevated levels of PGD2 after induction of various forms of kidney pathology, and a profibrotic role of CRTH2 activation. Therefore, although data in humans are still lacking, CRTH2 antagonists might also be a promising approach to kidney disease.
2.3. Gastrointestinal tract
Increasing evidence further suggests that CRTH2 might evolve as a promising therapeutic target in inflammatory bowel diseases. In patients suffering from Crohn’s disease, which can affect the entire gastrointestinal tract, we found increased serum levels of PGD2 and its metabolite Δ(12)-PGJ2, and in a corresponding mouse model of colitis induced by 2,4,6-trinitrobenzenesulfonic acid, the CRTH2 antagonist timapiprant ameliorated inflammation via inhibition of pro-inflammatory mediators TNF-α, IL-1β and IL-6 [67]. In ulcerative colitis, where inflammatory reactions are limited to the colon, we investigated CRTH2 expression in peripheral blood cells and observed an inverse correlation of CRTH2 expression on peripheral blood eosinophils and disease activity in affected patients. We also found that CRTH2 antagonism in a murine model of dextran sulfate sodium-induced colitis improved disease activity with regard to inflammation score, myeloperoxidase levels and weight loss [68]. Previously it was noted that the numbers of CRTH2-positive cells, most likely CD4-positive lymphocytes, were increased in mildly inflamed mucosa and at the margins of more severely inflamed areas in patients with ulcerative colitis [69]. These findings suggest that both in mice and humans the involvement of a Th2-dominated immune response may be possible in the early pathogenesis of inflammatory bowel disease. Peripheral blood eosinophils of patients with eosinophilic esophagitis showed enhanced CRTH2 expression, among other markers [70,71]. Supporting this pro-inflammatory role of CRTH2 in IBD, timapiprant significantly reduced eosinophil infiltration in the tissue and induced some clinical improvement in eosinophilic esophagitis patients [28].
2.4. Bone and cartilage
Interestingly, PGD2 can potently modulate bone metabolism with its capacity to induce collagen synthesis during the process of calcification [72] and IL-6 secretion by osteoblasts [73]. In 2005, Gallant and colleagues described both the production of PGD2 by, and the presence of both DP1 and CRTH2 receptors on, human osteoblasts. Selective CRTH2 activation in osteoblasts resulted in an increased production of osteoprotegerin, suggesting an autocrine and/or paracrine function of the PGD2-CRTH2 axis in bone anabolism [74]. In human differentiated osteoclasts, CRTH2 stimulation induced lamellipodia formation via actin reorganization, a process crucial for motility and bone resorption. Consequently, CRTH2 antagonism inhibited vitamin D3-induced bone resorption and osteoclastogenesis [75]. In addition, CRTH2 has been proposed as an inducer of apoptosis in osteoclasts via the intrinsic pathway, depending on caspase 9 activity [76] as a consequence of Erk1/2 and Akt signaling [77]. Osteoclast activation also plays a role in arthritis. Interestingly, a murine model of adjuvant-induced joint inflammation revealed that CRTH2-deficient mice develop a more severe phenotype with increased levels of paw swelling and infiltration of inflammatory cells, particularly CD68+ macrophages, which appeared to accelerate the inflammatory response [78]. Noteworthy, this model does not involve T-cell infiltration in the affected joints, which is a clear limitation when compared to the adaptive autoimmune response observed in human rheumatoid arthritis. Interestingly, treatment of mice with a CRTH2 antagonist did not modify disease severity in a different experimental model of rheumatoid arthritis, i.e. collagen-induced arthritis, while selective activation of DP1 proved beneficial [79]. Together, the role of PGD2 receptors in bone disorders and arthritis still needs to be clarified.
2.5. Nervous system
Inflammation, especially if inappropriately controlled, cannot only lead to a chronic state, but also induce signaling pathways in the brain that influence behavior, emotion and cognitive function. PGD2 signaling via DP1 is known to regulate crucial CNS-related functions such as food intake [80] and the sleep–wake cycle [81,82]. Increasing numbers of studies are now also addressing a link between PGD2 and CRTH2, and the modulation of cognitive function. A role for the PGD2 metabolite, 15-deoxy-PGJ2 in the central nervous system was first described in 1999 as an enhancer of nerve growth factor-mediated neurite outgrowth, a function which appeared to be independent from PPARγ and DP1 receptors [83,84], but involved CRTH2 receptors [85]. Additionally, PGD2 produced by astrocytes carrying the amyotrophic lateral sclerosis-causing gene SOD1 was identified to contribute to the devastating process of motor-neuron degeneration conferred by glial cells, but blockade of the DP1 receptor only slightly reversed motor neuron loss, tentatively suggesting a potential role for CRTH2 [86–88]. In the peripheral nervous system the PGD2eCRTH2 axis contributes to myelination, as both genetic deletion and pharmacological inhibition of L-PGDS as well as genetic ablation of CRTH2/DP2 caused myelin damage and hypomyelination [89].
A direct link between CRTH2 activation and cognitive dysfunction was proposed recently in mice. LPS-induced sickness behavior, social impairment as well as induction of c-Fos expression in the hypothalamic paraventricular nucleus and central amygdala were dependent on the presence of CRTH2 and were reversed by CRTH2 antagonists. Similar effects were observed with regards to social impairment after tumor inoculation [90]. In a model of cognitive dysfunction induced by the N-methyl-D-aspartate receptor antagonist, MK-801, both pharmacological inhibition and genetic deletion of CRTH2 were shown to be beneficial [91]. Thus, while CRTH2 is essential/involved in myelination and neurite outgrowth, it might also contribute to sickness-induced changes in cognitive function and behavior.
2.6. Skin
Prostaglandins have long been implicated in skin homeostasis [92]. Human and mouse keratinocytes produce PGD2 and express both PGD2 receptors [93,94]. Stimulation of CRTH2 leads to release of the anti-microbial factor beta-defensin-3 from human keratinocytes [93], suggesting a protective effect of the prostaglandin. However, several mouse models have shown that PGD2 and it receptor CRTH2 are actively involved in allergic skin inflammation [95,50,96–100]. Moreover, peripheral blood eosinophils and CD4-positive T cells of patients with allergic skin disease have been shown to express higher levels of CRTH2 as compared to healthy controls [101,102]. In a model of chronic skin inflammation, transgenic mice overexpressing lypocalin-type PGD synthase exhibited a complex phenotype: While PGD2 acting via DP1 ameliorated the early phase of croton oil-induced skin-inflammation due to its barrier-enhancing properties, PGD2 acting via CRTH2 prolonged and worsened the later phase of the inflammatory response by promoting neutrophil activation [103]. CRTH2 seemed to outweigh DP1-mediated responses which – in this specific model – resulted in an overall exaggerated inflammatory response mediated by CRTH2.
CRTH2 might also play a role in eosinophilic pustular folliculitis, which is a chronic pruritic skin disease characterized by massive eosinophil infiltrates of sebaceous glands. One treatment option for the disease is systemic administration of the COX inhibitor and CRTH2 agonist, indomethacin. In addition to abrogating prostaglandin synthesis, indomethacin was found to reduce CRTH2 expression in peripheral blood eosinophils and lymphocytes, probably thereby preventing their recruitment to inflamed skin [104,105].
Bimatoprost, a PGF2α analogue used to decrease ocular pressure in glaucoma, stimulates the growth of eyelash hair as a side effect [106]. In 2012, PGD2 and lipocalin-type PGD synthase were found at abundant levels in male scalp tissue of balding areas as compared to non-balding areas [107]. The authors found a direct inhibitory effect of PGD2 on hair growth that could be attributed to its action on CRTH2. Moreover, PGD2 inhibited hair follicle regeneration in a mouse model of dermal injury in a CRTH2-dependent manner [108]. Previously both DP1 and CRTH2 were found to be present in hair follicles [109]. Another study described that 15-deoxy-PGJ2 induces keratinocyte apoptosis, thereby contributing to PGD2-induced inhibition of hair growth [110]. Setipiprant, an orally available CRTH2 antagonist, is purportedly investigated in the treatment of androgenic alopecia in a phase II study.
2.7. CRTH2 in cancer
Inflammation is a two-edged sword, on the one hand fighting pathogens to limit tissue damage and promote healing, but if inappropriate in nature and degree on the other hand, inflammation itself can drive tissue damage. This is not only the case in allergy and autoimmune disorders but also in cancer [111]. It is now well established that there is both cancer-related inflammation as well as inflammation-induced cancer [112,113]. This interaction is established both via direct cell-to-cell interaction as well as communication by inflammatory mediators such as cytokines or prostanoids. Some of the pro-apoptotic properties of PGD2 and its metabolites such as 15-deoxy-PGJ2 can be attributed to both PPARγ activation and a receptor-independent mechanism, such as modulation of intracellular redox potential in osteosarcoma cells [114], but a clear contribution of CRTH2-mediated effects is also given: CRTH2 activation can induce apoptosis via autocrine stimulation of both reactive oxygen species and TNF-α production in a MAPK pathway-dependent manner in cardiomyocytes [115], and via Erk1/2 and Akt signaling in human osteoclasts [77]. Although, these in-vitro data suggest anti-tumorigenic properties, the exact role of CRTH2 in cancer is still unclear: CRTH2 expression on circulating CD4 positive cells was elevated in the late stage of non-small cell lung cancer [116], and in an experimental model using Lewis lung carcinoma cells implanted on the back of mice, CRTH2 expression was detected in vascular cells and the growing tumor [117]. Furthermore, in 277 samples of human gastric cancer, 17% of cases showed cancer cells positive for CRTH2 [118] and polarized group 2 innate lymphoid cells (ILC2) with increased levels of CRTH2 were found in the peripheral blood of gastric cancer patients [119]. These data point to a potential implication of PGD2 and CRTH2 in cancer, but whether beneficial or deleterious still needs to be elucidated.
3. Conclusion
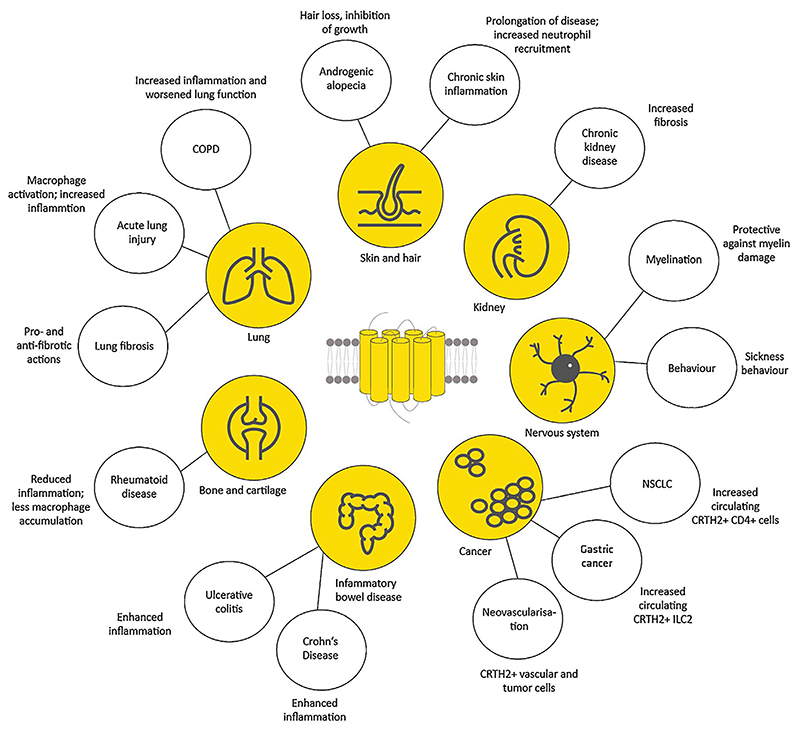
With a plethora of actions, CRTH2-mediated effects are apparent in almost every tissue of the human body (Fig. 1). There is growing evidence that CRTH2 plays important roles in allergic inflammation of the respiratory tract and the skin; however, this does not exclude it from being a potential therapeutic target in other conditions, too. These might comprise inflammatory bowel disease, mood disturbances or even cognitive dysfunction on the one hand, and autoimmune disease such as rheumatoid arthritis, and lung and kidney fibrosis, on the other hand. In male-type baldness, CRTH2 antagonists might already be on the crossroads to becoming available for patients, soon.
Fig 1. CRTH2/DP2-mediated effects beyond allergic inflammation and asthma; COPD (chronic obstructive pulmonary disease), NSCLC (non-small cell lung carcinoma).
Acknowledgements
We are indebted to Bernhard A. Peskar and Rufina Schuligoi for their helpful comments on this manuscript.
Funding
Supported by the Austrian Science Fund FWF (grant P22521-B18 to A.H.) and the Austrian National Bank (grant 14263 to A.H.). K.J. was funded by the PhD Program DK-MOLIN (FWF-W1241).
References
- [1].Nugteren DH, Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- [2].Smith JB, Silver MJ, Ingerman CM, Kocsis JJ. Prostaglandin D2 inhibits the aggregation of human platelets. Thromb Res. 1974;5(3):291–299. doi: 10.1016/0049-3848(74)90168-6. [DOI] [PubMed] [Google Scholar]
- [3].Mills DC, Macfarlane DE. Stimulation of human platelet adenylate cyclase by prostaglandin D2. Thromb Res. 1974;5(3):401–412. doi: 10.1016/0049-3848(74)90176-5. [DOI] [PubMed] [Google Scholar]
- [4].Horton EW, Jones RL. Proceedings Biological activity of prostaglandin D2 on smooth muscle. Br J Pharmacol. 1974;52(1):110P–111P. [PMC free article] [PubMed] [Google Scholar]
- [5].Flower RJ, Harvey EA, Kingston WP. Inflammatory effects of prostaglandin D2 in rat and human skin. Br J Pharmacol. 1976;56(2):229–233. doi: 10.1111/j.1476-5381.1976.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wasserman MA, DuCharme DW, Griffin RL, DeGraaf GL, Robinson FG. Bronchopulmonary and cardiovascular effects of prostaglandin D2 in the dog. Prostaglandins. 1977;13(2):255–269. doi: 10.1016/0090-6980(77)90007-7. [DOI] [PubMed] [Google Scholar]
- [7].Bolger PM, Eisner GM, Shea PT, Ramwell PW, Slotkoff LM. Effects of PGD2 on canine renal function. Nature. 1977;267(5612):628–630. doi: 10.1038/267628a0. [DOI] [PubMed] [Google Scholar]
- [8].Taube C, Förster W. The effect of prostaglandin D2 on isolated perfused guineapig hearts. Acta Biol Med Ger. 1978;37(5–6):773–775. [PubMed] [Google Scholar]
- [9].Schrör K. Prostaglandin D2 (PGD2)–a potent coronary vasoconstrictor agent in the guinea pig isolated heart. Naunyn Schmiedebergs Arch Pharmacol. 1978;302(1):61–62. doi: 10.1007/BF00586598. [DOI] [PubMed] [Google Scholar]
- [10].Coleman RA, Sheldrick RL. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br J Pharmacol. 1989;96(3):688–692. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989;96(2):291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Anhut H, Peskar BA, Bernauer W. Release of 15-keto-13, 14-dihydro-thromboxane B2 and prostaglandin D2 during anaphylaxis as measured by radioimmunoassay. Naunyn Schmiedebergs Arch Pharmacol. 1978;305(3):247–252. doi: 10.1007/BF00498818. [DOI] [PubMed] [Google Scholar]
- [13].Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982;129(4):1627–1631. [PubMed] [Google Scholar]
- [14].Peskar BA, Brune K. Prostaglandin D2: the prevailing prostaglandin in an acute inflammation. Agents Actions. 1979;(Suppl. (4)):260–266. [PubMed] [Google Scholar]
- [15].Marsden KA, Rao PS, Cavanagh D, Spaziani E. The effect of prostaglandin D2 (PGD2) on circulating eosinophils. Prostaglandins Leukot Med. 1984;15(3):387–397. doi: 10.1016/0262-1746(84)90137-9. [DOI] [PubMed] [Google Scholar]
- [16].Emery DL, Djokic TD, Graf PD, Nadel JA. Prostaglandin D2 causes accumulation of eosinophils in the lumen of the dog trachea. J Appl Physiol. 1989;67(3):959–962. doi: 10.1152/jappl.1989.67.3.959. [DOI] [PubMed] [Google Scholar]
- [17].Woodward DF, Hawley SB, Williams LS, Ralston TR, Protzman CE, Spada CS, et al. Studies on the ocular pharmacology of prostaglandin D2. Invest Ophthalmol Vis Sci. 1990;31(1):138–146. [PubMed] [Google Scholar]
- [18].Raible DG, Schulman ES, DiMuzio J, Cardillo R, Post TJ. Mast cell mediators prostaglandin-D2 and histamine activate human eosinophils. J Immunol. 1992;148(11):3536–3542. [PubMed] [Google Scholar]
- [19].Pr B, Cj V. The effect of prostanoids on the function of human eosinophils. Agents Actions. 1990;(Suppl. 31):103–112. doi: 10.1007/978-3-0348-7379-6_12. [DOI] [PubMed] [Google Scholar]
- [20].Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98(6):1942–1948. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- [21].Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108(6):982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- [22].Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162(3):1278–1286. [PubMed] [Google Scholar]
- [24].Cosmi L, Annunziato F, Galli MIG, null, Maggi RME, null, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30(10):2972–2979. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [25].Santus P, Radovanovic D. Prostaglandin D2 receptor antagonists in early development as potential therapeutic options for asthma. Expert Opin Investig Drugs. 2016;25(9):1083–1092. doi: 10.1080/13543784.2016.1212838. [DOI] [PubMed] [Google Scholar]
- [26].Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2012;42(1):38–48. doi: 10.1111/j.1365-2222.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- [27].Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67(12):1572–1579. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- [28].Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–385. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- [29].Kuna P, Bjermer L, Tornling G. Two Phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des Devel Ther. 2016;10:2759–2770. doi: 10.2147/DDDT.S105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schmidt JA, Bell FM, Akam E, Marshall C, Dainty IA, Heinemann A, et al. Biochemical and pharmacological characterization of AZD1981, an orally available selective DP2 antagonist in clinical development for asthma. Br J Pharmacol. 2013;168(7):1626–1638. doi: 10.1111/bph.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, et al. The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in un-controlled allergic asthma. Pulm Pharmacol Ther. 2016;39:54–63. doi: 10.1016/j.pupt.2016.06.005. [DOI] [PubMed] [Google Scholar]
- [32].Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69(9):1223–1232. doi: 10.1111/all.12451. [DOI] [PubMed] [Google Scholar]
- [33].Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133(2):414–419. doi: 10.1016/j.jaci.2013.10.013. [DOI] [PubMed] [Google Scholar]
- [34].Sugimoto H, Shichijo M, Iino T, Manabe Y, Watanabe A, Shimazaki M, et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther. 2003;305(1):347–352. doi: 10.1124/jpet.102.046748. [DOI] [PubMed] [Google Scholar]
- [35].Stinson SE, Amrani Y, Brightling CE. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol. 2015;135(2):395–406. doi: 10.1016/j.jaci.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shirasaki H, Kikuchi M, Kanaizumi E, Himi T. Accumulation of CRTH2-positive leukocytes in human allergic nasal mucosa. Ann Allergy Asthma Immunol. 2009;102(2):110–115. doi: 10.1016/S1081-1206(10)60239-6. [DOI] [PubMed] [Google Scholar]
- [37].Murata T, Aritake K, Tsubosaka Y, Maruyama T, Nakagawa T, Hori M, et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc Natl Acad Sci U S A. 2013;110(13):5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moon TC, Campos-Alberto E, Yoshimura T, Bredo G, Rieger AM, Puttagunta L, et al. Expression of DP2 (CRTh2), a prostaglandin D2 receptor, in human mast cells. PLoS One. 2014;9(9):e108595. doi: 10.1371/journal.pone.0108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459(2):195–199. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- [40].Jandl K, Stacher E, Bálint Z, Sturm EM, Maric J, Peinhaupt M, et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J Allergy Clin Immunol. 2016;137(3):833–843. doi: 10.1016/j.jaci.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel A-B, et al. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. J Immunol. 2003;170(10):4943–4952. doi: 10.4049/jimmunol.170.10.4943. [DOI] [PubMed] [Google Scholar]
- [42].Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- [43].Sawyer N, Cauchon E, Chateauneuf A, Cruz RPG, Nicholson DW, Metters KM, et al. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137(8):1163–1172. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316(4):1009–1014. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- [45].Boehme SA, Franz-Bacon K, Chen EP, Ly TW, Kawakami Y, Bacon KB. Murine bone marrow-derived mast cells express chemoattractant receptor-homologous molecule expressed on T-helper class 2 cells (CRTh2) Int Immunol. 2009;21(6):621–632. doi: 10.1093/intimm/dxp031. [DOI] [PubMed] [Google Scholar]
- [46].Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174(6):3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- [47].Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J Pharmacol Exp Ther. 2008;326(2):493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- [48].Wojno EDT, Monticelli LA, Tran SV, Alenghat T, Osborne LC, Thome JJ, et al. The prostaglandin D2receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8(6):1313–1323. doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abe H, Takeshita T, Nagata K, Arita T, Endo Y, Fujita T, et al. Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene. 1999;227(1):71–77. doi: 10.1016/s0378-1119(98)00599-x. [DOI] [PubMed] [Google Scholar]
- [50].Takeshita K, Yamasaki T, Nagao K, Sugimoto H, Shichijo M, Gantner F, et al. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol. 2004;16(7):947–959. doi: 10.1093/intimm/dxh096. [DOI] [PubMed] [Google Scholar]
- [51].Boin F, De Fanis U, Bartlett SJ, Wigley FM, Rosen A, Casolaro V. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis Rheum. 2008;58(4):1165–1174. doi: 10.1002/art.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kida T, Ayabe S, Omori K, Nakamura T, Maehara T, Aritake K, et al. Prostaglandin D2 attenuates bleomycin-induced lung inflammation and pulmonary fibrosis. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167729. [cited 15) January 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].van den Brule S, Wallemme L, Uwambayinema F, Huaux F, Lison D. The,D prostanoid receptor agonist BW245C [(4S)-(3-[(3R,S)-3-cyclohexyl-3-hydroxypropyl]-2,5-dioxo)-4-imidazolidineheptanoic acid] inhibits fibroblast proliferation and bleomycin-induced lung fibrosis in mice. J Pharmacol Exp Ther. 2010;335(2):472–479. doi: 10.1124/jpet.110.169250. [DOI] [PubMed] [Google Scholar]
- [54].Ayabe S, Kida T, Hori M, Ozaki H, Murata T. Prostaglandin D2 inhibits collagen secretion from lung fibroblasts by activating the DP receptor. J Pharmacol Sci. 2013;121(4):312–317. doi: 10.1254/jphs.12275fp. [DOI] [PubMed] [Google Scholar]
- [55].Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- [56].Mall G, Zimmermann P, Siemens I, Burkhardt A, Otto HF. Prevention of bleomycin-induced fibrosing alveolitis with indomethacin: stereological studies on rat lungs. Virchows Arch A: Pathol Anat Histopathol. 1991;419(4):339–347. doi: 10.1007/BF01606525. [DOI] [PubMed] [Google Scholar]
- [57].Asano K, Ueda S, Ishii M, Suzuki Y, Fukunaga K, Oguma T, et al. Prostaglandin D2 receptor, CRTH2, is critical for gamma-Delta t cell-Mediated suppression of pulmonary Inflammation/Fibrosis induced by bleomycin, D14 Insights into Pathogenesis of Lung Fibrosis and Granulomas; American Thoracic Society International Conference Abstracts; 2011. A5579-A5579. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A5579 [Internet]. [cited 5 May 2017] [Google Scholar]
- [58].Ueda S, Asano K, Tomomatsu K, Ogura H, Horiuchi N, Kodama M, et al. A role of ProstagrandinD2 and its receptor CRTH2, in bleomycin-Induced lung fibrosis model,D30 The Role of Inflammation in Chronic Interstitial Lung Disease; American Thoracic Society International Conference Abstracts; 2009. A5662. [Internet]. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccmconference.2009.179.1_MeetingAbstracts.A5662 [cited 5 May 2017] [Google Scholar]
- [59].Yoon Y-S, Lee Y-J, Choi Y-H, Park YM, Kang JL. Macrophages programmed by apoptotic cells inhibit epithelial-mesenchymal transition in lung alveolar epithelial cells via PGE2, PGD2, and HGF. Sci Rep. 2016;6 doi: 10.1038/srep20992. [Internet]. [cited 5 May 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhou Y, He CH, Herzog EL, Peng X, Lee C-M, Nguyen TH, et al. Chitinase 3-like-1 and its receptors in Hermansky-Pudlak syndrome-associated lung disease. J Clin Invest. 2015;125(8):3178–3192. doi: 10.1172/JCI79792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sargent C, Stinson S, Schmidt J, Dougall I, Bonnert R, Paine S. The Effect of a Selective CRTh2 Antagonist on Tobacco Smoke (TS) Induced Airway Inflammation and Remodelling in the Mouse. 2009 Available from: https://www.scienceopen.com/document?vid=c9a119e2-f803-4fea-b06c-333dcb3514f6 [cited January 15 2017] [Google Scholar]
- [62].Stebbins KJ, Broadhead AR, Baccei CS, Scott JM, Truong YP, Coate H, et al. Pharmacological blockade of the DP2 receptor inhibits cigarette smoke-induced inflammation, mucus cell metaplasia, and epithelial hyperplasia in the mouse lung. J Pharmacol Exp Ther. 2010;332(3):764–775. doi: 10.1124/jpet.109.161919. [DOI] [PubMed] [Google Scholar]
- [63].Snell N, Foster M, Vestbo J. Efficacy and safety of AZD1981, a CRTH2 receptor antagonist, in patients with moderate to severe COPD. Respir Med. 2013;107(11):1722–1730. doi: 10.1016/j.rmed.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [64].Hirawa N, Uehara Y, Ikeda T, Gomi T, Hamano K, Totsuka Y, et al. Urinary prostaglandin D synthase (beta-trace) excretion increases in the early stage of diabetes mellitus. Nephron. 2001;87(4):321–327. doi: 10.1159/000045937. [DOI] [PubMed] [Google Scholar]
- [65].Tsuchida T, Eguchi N, Eguchi Y, Numabe A, Nakajima H, Oda H, et al. Lipocalin-type prostaglandin D synthase in urine in adriamycin-induced nephropathy of mice. Nephron Physiol. 2004;96(2):42–51. doi: 10.1159/000076407. [DOI] [PubMed] [Google Scholar]
- [66].Ito H, Yan X, Nagata N, Aritake K, Katsumata Y, Matsuhashi T, et al. PGD2-CRTH2 pathway promotes tubulointerstitial fibrosis. J Am Soc Nephrol JASN. 2012;23(11):1797–1809. doi: 10.1681/ASN.2012020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Radnai B, Sturm EM, Stančić A, Jandl K, Labocha S, Ferreirós N, et al. Eosinophils contribute to intestinal inflammation via chemoattractant receptor-homologous molecule expressed on Th2Cells, CRTH2, in experimental crohn’s disease. J Crohns Colitis. 2016;10(9):1087–1095. doi: 10.1093/ecco-jcc/jjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sturm EM, Radnai B, Jandl K, Stančić A, Parzmair GP, Högenauer C, et al. Opposing roles of Prostaglandin D2 receptors in ulcerative colitis. J Immunol. 2014;193(2):827–839. doi: 10.4049/jimmunol.1303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Matsuzaki K, Hokari R, Kato S, Tsuzuki Y, Tanaka H, Kurihara C, et al. Differential expression of CCR5 and CRTH2 on infiltrated cells in colonic mucosa of patients with ulcerative colitis. J Gastroenterol Hepatol. 2003;18(9):1081–1088. doi: 10.1046/j.1440-1746.2003.03088.x. [DOI] [PubMed] [Google Scholar]
- [70].Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, et al. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J Innate Immun. 2011;3(6):594–604. doi: 10.1159/000331326. [DOI] [PubMed] [Google Scholar]
- [71].Lingblom C, Käppi T, Bergquist H, Bove M, Arkel R, Saalman R, et al. Differences in eosinophil molecular profiles between children and adults with eosinophilic esophagitis. Allergy. 2017;72(9):1406–1414. doi: 10.1111/all.13140. [DOI] [PubMed] [Google Scholar]
- [72].Tasaki Y, Takamori R, Koshihara Y. Prostaglandin D2 metabolite stimulates collagen synthesis by human osteoblasts during calcification. Prostaglandins. 1991;41(4):303–313. doi: 10.1016/0090-6980(91)90001-v. [DOI] [PubMed] [Google Scholar]
- [73].Tokuda H, Kozawa O, Harada A, Uematsu T. Prostaglandin D2 induces inter-leukin-6 synthesis via Ca2+ mobilization in osteoblasts: regulation by protein kinase C. Prostaglandins Leukot Essent Fatty Acids. 1999;61(3):189–194. doi: 10.1054/plef.1999.0089. [DOI] [PubMed] [Google Scholar]
- [74].Gallant MA, Samadfam R, Hackett JA, Antoniou J, Parent J-L, de Brum-Fernandes AJ. Production of prostaglandin D(2) by human osteoblasts and modulation of osteoprotegerin, RANKL, and cellular migration by DP and CRTH2 receptors. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20(4):672–681. doi: 10.1359/JBMR.041211. [DOI] [PubMed] [Google Scholar]
- [75].Durand M, Gallant MA, de Brum-Fernandes AJ. Prostaglandin D2 receptors control osteoclastogenesis and the activity of human osteoclasts. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23(7):1097–1105. doi: 10.1359/jbmr.080228. [DOI] [PubMed] [Google Scholar]
- [76].Yue L, Durand M, Lebeau Jacob MC, Hogan P, McManus S, Roux S, et al. Prostaglandin D2 induces apoptosis of human osteoclasts by activating the CRTH2 receptor and the intrinsic apoptosis pathway. Bone. 2012;3:338–346. doi: 10.1016/j.bone.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [77].Yue L, Haroun S, Parent J-L, de Brum-Fernandes AJ. Prostaglandin D(2) induces apoptosis of human osteoclasts through ERK1/2 and Akt signaling pathways. Bone. 2014;60:112–121. doi: 10.1016/j.bone.2013.12.011. [DOI] [PubMed] [Google Scholar]
- [78].Tsubosaka Y, Nakamura T, Hirai H, Hori M, Nakamura M, Ozaki H, et al. A deficiency in the prostaglandin D2 receptor CRTH2 exacerbates adjuvant-induced joint inflammation. J Immunol. 2014;193(12):5835–5840. doi: 10.4049/jimmunol.1303478. [DOI] [PubMed] [Google Scholar]
- [79].Maicas N, Ibáñez L, Alcaraz MJ, Úbeda A, Ferrándiz ML. Prostaglandin D2 regulates joint inflammation and destruction in murine collagen-induced arthritis. Arthritis Rheum. 2012;64(1):130–140. doi: 10.1002/art.30656. [DOI] [PubMed] [Google Scholar]
- [80].Ohinata K, Takagi K, Biyajima K, Fujiwara Y, Fukumoto S, Eguchi N, et al. Central prostaglandin D(2) stimulates food intake via the neuropeptide Y system in mice. FEBS Lett. 2008;582(5):679–684. doi: 10.1016/j.febslet.2008.01.050. [DOI] [PubMed] [Google Scholar]
- [81].Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98(20):11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Qu W-M, Huang Z-L, Xu X-H, Aritake K, Eguchi N, Nambu F, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci. 2006;103(47):17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Satoh T, Furuta K, Suzuki M, Watanabe Y. Prostaglandin J2 and its metabolites promote neurite outgrowth induced by nerve growth factor in PC12 cells. Biochem Biophys Res Commun. 1999;258(1):50–53. doi: 10.1006/bbrc.1999.0587. [DOI] [PubMed] [Google Scholar]
- [84].Jung KM, Park KS, Oh JH, Jung SY, Yang KH, Song YS, et al. Activation of p38 mitogen-activated protein kinase and activator protein-1 during the promotion of neurite extension of PC-12 cells by 15-deoxy-delta12,14-prostaglandin J2. Mol Pharmacol. 2003;63(3):607–616. doi: 10.1124/mol.63.3.607. [DOI] [PubMed] [Google Scholar]
- [85].Hatanaka M, Shibata N, Shintani N, Haba R, Hayata A, Hashimoto H, et al. 15d-prostaglandin J2 enhancement of nerve growth factor-induced neurite out-growth is blocked by the chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells (CRTH2) antagonist CAY10471 in PC12 cells. J Pharmacol Sci. 2010;113(1):89–93. doi: 10.1254/jphs.10001sc. [DOI] [PubMed] [Google Scholar]
- [86].Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [87].Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, et al. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci Off J Soc Neurosci. 2006;26(16):4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pirooznia SK, Dawson VL, Dawson TM. Motor neuron death in ALS: programmed by astrocytes? Neuron. 2014;81(5):961–963. doi: 10.1016/j.neuron.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, et al. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat Neurosci. 2014;17(12):1682–1692. doi: 10.1038/nn.3857. [DOI] [PubMed] [Google Scholar]
- [90].Haba R, Shintani N, Onaka Y, Kanoh T, Wang H, Takenaga R, et al. Central CRTH2, a second prostaglandin D2 receptor,mediates emotional impairment in the lipopolysaccharide and tumor-Induced sickness behavior model. J Neurosci. 2014;34(7):2514–2523. doi: 10.1523/JNEUROSCI.1407-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Onaka Y, Shintani N, Nakazawa T, Kanoh T, Ago Y, Matsuda T, et al. Prostaglandin D2 signaling mediated by the CRTH2 receptor is involved in MK-801-induced cognitive dysfunction. Behav Brain Res. 2016;1(314):77–86. doi: 10.1016/j.bbr.2016.07.050. [DOI] [PubMed] [Google Scholar]
- [92].Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):131–138. doi: 10.1016/j.plefa.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [93].Kanda N, Ishikawa T, Watanabe S. Prostaglandin D2 induces the production of human beta-defensin-3 in human keratinocytes. Biochem Pharmacol. 2010;79(7):982–989. doi: 10.1016/j.bcp.2009.11.012. [DOI] [PubMed] [Google Scholar]
- [94].Black AT, Gray JP, Shakarjian MP, Mishin V, Laskin DL, Heck DE, et al. UVB light upregulates prostaglandin synthases and prostaglandin receptors in mouse keratinocytes. Toxicol Appl Pharmacol. 2008;232(1):14–24. doi: 10.1016/j.taap.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matsushima Y, Satoh T, Yamamoto Y, Nakamura M, Yokozeki H. Distinct roles of prostaglandin D2 receptors in chronic skin inflammation. Mol Immunol. 2011;49(1–2):304–310. doi: 10.1016/j.molimm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- [96].Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177(4):2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- [97].He R, Oyoshi MK, Wang JYT, Hodge MR, Jin H, Geha RS. The prostaglandin D2 receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. J Allergy Clin Immunol. 2010;126(4):784–790. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Boehme SA, Chen EP, Franz-Bacon K, Sásik R, Sprague LJ, Ly TW, et al. Antagonism of CRTH2 ameliorates chronic epicutaneous sensitization-induced inflammation by multiple mechanisms. Int Immunol. 2009;21(1):1–17. doi: 10.1093/intimm/dxn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Boehme SA, Franz-Bacon K, Chen EP, Sásik R, Sprague LJ, Ly TW, et al. A small molecule CRTH2 antagonist inhibits FITC-induced allergic cutaneous inflammation. Int Immunol. 2009;21(1):81–93. doi: 10.1093/intimm/dxn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Oiwa M, Satoh T, Watanabe M, Niwa H, Hirai H, Nakamura M, et al. CRTH2-dependent,STAT6-independent induction of cedar pollen dermatitis. Clin Exp Allergy. 2008;38(8):1357–1366. doi: 10.1111/j.1365-2222.2008.03007.x. [DOI] [PubMed] [Google Scholar]
- [101].Yahara H, Satoh T, Miyagishi C, Yokozeki H. Increased expression of CRTH2 on eosinophils in allergic skin diseases. J Eur Acad Dermatol Venereol. 2010;24(1):75–76. doi: 10.1111/j.1468-3083.2009.03267.x. [DOI] [PubMed] [Google Scholar]
- [102].Hijnen D, Nijhuis E, Bruin-Weller M, Holstege F, Koerkamp MG, Kok I, et al. Differential expression of genes involved in skin homing, proliferation, and apoptosis in CD4+ T cells of patients with atopic dermatitis. J Invest Dermatol. 2005;125(6):1149–1155. doi: 10.1111/j.0022-202X.2005.23932.x. [DOI] [PubMed] [Google Scholar]
- [103].Sarashina H, Tsubosaka Y, Omori K, Aritake K, Nakagawa T, Hori M, et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J Immunol. 2014;192(1):459–465. doi: 10.4049/jimmunol.1302080. [DOI] [PubMed] [Google Scholar]
- [104].Satoh T, Shimura C, Miyagishi C, Yokozeki H. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90(1):18–22. doi: 10.2340/00015555-0759. [DOI] [PubMed] [Google Scholar]
- [105].Kataoka N, Satoh T, Hirai A, Saeki K, Yokozeki H. Indomethacin inhibits eosinophil migration to prostaglandin D2: therapeutic potential of CRTH2 desensitization for eosinophilic pustular folliculitis. Immunology. 2013;140(1):78–86. doi: 10.1111/imm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Khidhir KG, Woodward DF, Farjo NP, Farjo BK, Tang ES, Wang JW, et al. The prostamide-related glaucoma therapy,bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27(2):557–567. doi: 10.1096/fj.12-218156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu LAY Garza, Yang Z, Alagesan B, Lawson JA, Norberg SM, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4(126):126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J Invest Dermatol. 2013;133(4):881–889. doi: 10.1038/jid.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Colombe L, Michelet J-F, Bernard BA. Prostanoid receptors in anagen human hair follicles. Exp Dermatol. 2008;17(1):63–72. doi: 10.1111/j.1600-0625.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- [110].Joo HW, Kang YR, Kwack MH, Sung YK. 15-deoxy prostaglandin J2, the nonenzymatic metabolite of prostaglandin D2, induces apoptosis in keratinocytes of human hair follicles: a possible explanation for prostaglandin D2-mediated inhibition of hair growth. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(7):809–813. doi: 10.1007/s00210-016-1257-z. [DOI] [PubMed] [Google Scholar]
- [111].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [112].Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- [113].Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- [114].Koyani CN, Kitz K, Rossmann C, Bernhart E, Huber E, Trummer C, et al. Activation of the MAPK/Akt/Nrf2-Egr1/HO-1-GCLc axis protects MG-63 osteosarcoma cells against 15d-PGJ2-mediated cell death. Biochem Pharmacol. 2016;15(104):29–41. doi: 10.1016/j.bcp.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Koyani CN, Windischhofer W, Rossmann C, Jin G, Kickmaier S, Heinzel FR, et al. 15-deoxy-Δ12,14-PGJ2promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFα axis. Int J Cardiol. 2014;173(3):472–480. doi: 10.1016/j.ijcard.2014.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Karagöz B, Bilgi O, Kandemir EG, Erikçi AA, Sayan Ö, Özgün A, et al. Peripheral blood CD4 + CRTH2+ cells in advanced stage non small cell lung cancer. Cent Eur J Med. 2010;5(4):431–436. [Google Scholar]
- [117].Murata T, Lin MI, Aritake K, Matsumoto S, Narumiya S, Ozaki H, et al. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc Natl Acad Sci U S A. 2008;105(50):20009–20014. doi: 10.1073/pnas.0805171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fukuoka T, Yashiro M, Morisaki T, Kinoshita H, Hasegawa T, Kasashima H, et al. The role of type d prostanoid receptors and PPARγ in gastric cancer progression. Anticancer Res. 2014;34(6):2771–2778. [PubMed] [Google Scholar]
- [119].Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu Y, et al. Polarization of ILC2 s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J Immunol Res. 2014 doi: 10.1155/2014/923135. (2014)[Internet]. [cited January 15 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]