summary
Background & aims
Stunting in children is a comorbid condition in undernutrition that may be ameliorated by the provision of high-quality foods that provide protein and micronutrients. Addressing this problem in lower social economic environments requires, in part, affordable and scalable food-based solutions with efficacious food products. Towards this end, biochemical/metabolic indicators for fastthroughput screening of foods and their components are desired.
A highly acceptable and economical micronutrient-fortified food product with different levels of legume protein was provided to stunted Indian children for one month, to determine change in their linear growth and explore associated biochemical, metabolomic and microbiome indicators.
Methods
A randomized controlled pilot trial was conducted with 100 stunted children (6e10 years of age) to elucidate metabolic and microbiome-based biomarkers associated with linear growth. They were randomized into 4 groups receiving 6, 8, 10 or 12 g of legume-based protein for one month. Anthropometry, blood biochemistry, aminoacidomics, acylcarnitomics and fecal microbiome were measured before and after feeding.
Results
No significant differences were observed between groups in height, height-for-age Z-score (HAZ) or BMI-for-age Z-score (BAZ); however, 38 serum metabolites were altered significantly (Bonferroni adjusted P < 0.1) in response to the interventions. IGF-1 (Insulin like Growth Factor-1) was positively (ρ > 0.2, P = 0.02), while serine and ornithine (ρ < −0.2, P = 0.08) were negatively associated with change in height. Leucine, isoleucine and valine positively correlated (P = 0.011, 0.023 and 0.007 respectively) with change in BAZ. Three Operational Taxonomic Units belonging to Bacteroidetes and Firmicutes (VIP score > 1.5) were significantly correlated with change in height.
Conclusions
In this pilot trial, a number of fasting serum metabolomic and fecal microbiome signatures were associated with linear growth after a short-term dietary intervention. The alterations of these markers should be validated in long-term dietary intervention trials as potential screening indicators towards the development of food products that favor growth. This trial was registered at www.ctri.nic.in as CTRI/2016/12/007564.
Keywords: Stunting, Intervention, Legume protein, Metabolome, Microbiome, Linear growth
1. Introduction
Stunting affects about one-quarter of children under five years of age worldwide, with 75% of them in South Asian and Sub-Saharan African countries [1]. A poor-quality diet, particularly with regard to its protein and micronutrient content, is thought to be important in the etiology of stunting, since linear growth has been associated with high-quality dietary protein intakes, and interventions with high-quality animal proteins have shown positive effects on height [2]. However, animal source proteins are not well accepted in many populations, can have a high environmental cost, and can be less sustainable than plant proteins [3]. Recent studies have shown that micronutrient fortified legume protein sources can be equally effective as animal sources for the treatment of severe and moderate acute malnutrition [4,5]. Grain legumes can contribute significantly towards sustainability by generating income and food security for poor farmers, and sustain agriculture through relative drought tolerance and ease of integration into multi-cropping systems [6,7].
The establishment of optimal, affordable and scalable feeding strategies can be facilitated by the use of biomarkers that rapidly reflect growth [8]. Mass spectrometry (MS)-based techniques allow high-throughput profiling of metabolic intermediates. For example, severe acute malnutrition (SAM)-associated plasma metabolomic patterns in Nigerian children have been discerned by untargeted multi-platform analysis [9]. Similarly, targeted metabolomic analysis of serum biomarkers such as amino acids, C3-acylcarnitine, adipo-nectin and leptin, showed significant changes in children recovering from SAM with ready-to-use therapeutic food (RUTF) [10]. Malnutrition-induced changes in the plasma metabolome and gut microbiome of piglets included significant changes in amino acids, choline metabolites and products of microbiome metabolites [11]. Other investigators have reported different patterns of maturity of the gut microbiome after nutritional intervention in Malawian and Bangladeshi children with SAM, relative to similar healthy controls [12,13]. Furthermore, recent reports have observed positive effects on the growth of school children and stunted infants, with egg or egg and legume-based interventions [14–16]. However, the above studies did not conduct an in-depth measurement of intervention induced changes in metabolic intermediates or gut microbiota that could lead to the discovery of potential biomarkers associated with growth or illuminate the underlying mechanisms involved.
To our knowledge there are no published studies describing intervention feeding trials in moderately stunted children that evaluate the optimal dose of protein required with respect to growth, or evaluate changes in the targeted measurement of metabolic intermediates and signaling molecules, along with changes in the gut microbiome. The current pilot study was designed to use a multi-disciplinary approach to first evaluate the sensory and acceptability measures of a novel and economical micronutrient-fortified food product containing high-quality legume protein, in stunted, low socioeconomic Indian primary school children, and then to assess the effects of its consumption over a one-month period on growth and changes in the fasting serum metabolome and the fecal microbiome.
2. Methods
2.1. Study design
Two trials were conducted from January–March 2017: first, a double-blind acceptability trial of a legume-based, micronutrient-fortified protein food product (in the form of a snack) that was used in the subsequent intervention study. A randomized double-blind intervention trial with four parallel arms tested the effects of four levels of protein offered through a legume-based intervention product on changes in linear growth, metabolome and the microbiome in mildly stunted children. For ethical reasons in these stunted children, control arms with either no intervention or intervention with non-micronutrient fortified product were not considered.
2.1.1. Food product acceptability trial
A product acceptability trial was conducted in 44 children from a low socio-economic status (SES) school in Bangalore City, Karnataka, India (Supplemental Fig. 1). The Institutional Ethics Review Board (IERB) of St. John’s Medical College approved the study protocol. Each child received a 26 g package of legume-based and micronutrient-supplemented product (alternating between chilli and tomato flavors) twice a day in the morning and afternoon, for 5 days consecutively providing either 6 or 12 g of protein/day. An interviewer obtained a daily intake survey from each participant. Both children and investigators were blinded to the level of protein and the flavor of the food products. The children were monitored to ensure that the product was not shared between them, and to note down wastage, if any. The daily intake of the product and feedback or any other comment were also recorded. Visual Analogue Scales were used to quantitate the child’s response to appearance, taste, texture, aroma, mouthfeel and overall impression of the food product. An exit survey that captured domains such as sufficiency of quantity in each serving, the ability to eat the food product twice for an extended period of time, any discomfort or nausea that was felt after eating, and whether the food product affected the child’s appetite for the school provided lunch, was administered at the end of the feeding period.
2.1.2. Protein dose–response intervention trial
Stunted Indian school children age 6–10 years of both sexes (in equal proportion) were recruited for the study from a school located in North Karnataka region, which caters to children from low SES. The IERB of St. John’s Medical College approved the study protocols and the trial was registered at the www.ctri.nic.in as CTRI/2016/12/007564. All parents provided informed consent, and the children were asked for their assent to the study. The background to the study was also provided to the school authorities.
Children with a recent history (in the last 3 months) of serious infections, injuries and/or surgeries, or those using any prescription medications, consuming nutritional supplements and/or health food drinks on a regular basis, or who had food allergies were excluded from the study. Moderately stunted children (height-for-age Z-scores < −2.0 SD and >−3.0 SD) were included in the study. Prior to enrollment, the research assistants explained the study and associated procedures in simple and understandable terms in their local language before obtaining informed consent from the parents and assent from the children. The targeted sample size was 100 children (boys and girls, 50:50) in total, such that there were a minimum 25 children in each study arm.
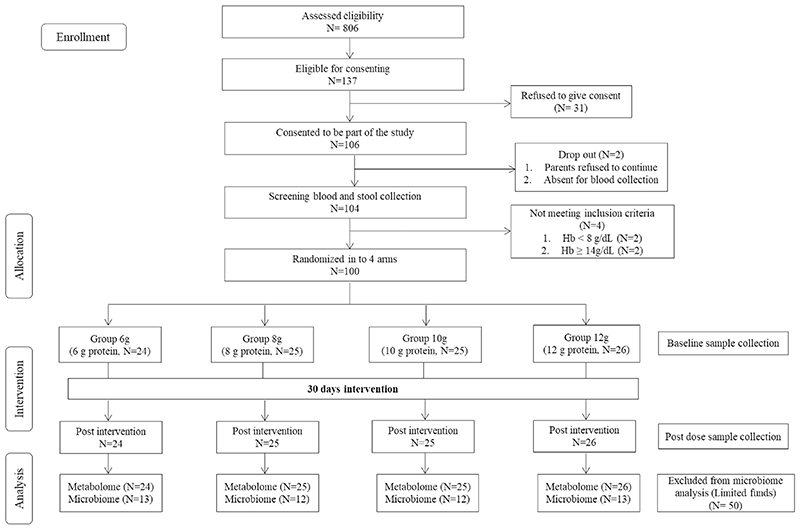
After screening and consenting,104 children were recruited into the study. Four participants were excluded, as they had hemoglobin (Hb) concentrations <8 and ≥ 14 g/dL. The detailed screening flow chart is provided in Fig. 1. All study investigators, except the biostatistician (who generated the participant randomization list), were blinded to the protein levels of each product type. All the selected participants (n = 100), were randomly assigned to four different intervention groups, who received the intervention products containing 6, 8,10 or 12 g of protein per day as described below.
Fig. 1. Trial flow diagram for the doseeresponse intervention study.
After randomization of the participants based on their baseline height, before the initiation of feeding, fasting baseline samples of blood and feces were collected from each child in the morning. Height was measured with a precision of 0.1 cm without shoes using a floor model stadiometer with the head in the Frankfort plane (Indosurgicals Delhi, India), and body weight was measured in light indoor clothes without shoes on a standard weighing scale with a precision of 0.1 kg (2005D, Tian Shan, China). HAZ and weight- or BMI-for-age (BAZ) were calculated using the WHO AnthroPlus software and the World Health Organization (WHO) growth reference data for 5–19 year old [17]. Mid upper arm circumference (MUAC) and waist circumference were also measured with a non-elastic tape; medical history, vital signs (blood pressure and heart rate), age and SES were recorded by the field staff. On each study day, the participants received a 26 g package of a legume-based and micronutrient-supplemented product (containing 6, 8, 10 and 12 g of protein/day) twice in the morning and afternoon for 30 days as per the randomization schedule generated by https://www.sealedenvelope.com/simple-randomiser/v1/lists. The intake of the product was recorded daily. Information on the habitual food intakes of all the study participants (as reported by mothers) was collected by 24-h recalls once in the middle of the intervention period. The nutrient composition of the intakes was calculated based on a food database developed at the Division of Nutrition, St. John’s Research Institute, Bangalore [18]. At the end of the study period, all anthropometric measurements were repeated along with blood and fecal samples, on day 30, after an overnight fast. Sample collections at the start and end of the study were performed at the same time of day in each child, to avoid circadian effects.
2.1.3. Intervention food product
The intervention food product was a micronutrient fortified ready-to-eat snack product with extruded yellow pea as the primary ingredient and source of protein in the product (manufactured by Mars International India Pvt. Ltd). This product was offered in two flavors (tomato and chilli) for each of the four levels of protein and stored at 23 °C. The product composition was similar for all four protein levels (Supplemental Table 1) except for protein, carbohydrate and vitamin D content. Each flavor/protein product type was provided in a color-coded package.
2.2. Sample collection, storage and assays
Fasting blood samples (10 mL) were collected into EDTA and plain vacutainers (Becton Dickinson, NJ, USA) for plasma and serum separation respectively. Blood samples collected in plain vacutainers were kept at room temperature for 30 min for serum separation, and were then centrifuged for 10 min at 3,500 r.p.m. Serum samples were aliquoted (100 μL each) into pre-labeled screw cap cryovials. Hb was measured at room temperature by a hematology analyzer (ABX Pentra 60 C+, HORIBA ABX Diagnostics, Japan). For plasma, blood samples were kept on ice, and then cold-centrifuged for 10 min at 3,500 r.p.m. 100 μL aliquots were stored in pre-labeled screw cap cryovials, and both serum and plasma samples were stored on dry ice until their shipment back to the laboratory, where they were stored at −80 °C until analysis. Fecal samples (4 g) were collected in screw cap cryovials and stored at −80 °C until analysis.
For all the analyses samples were brought to room temperature and mixed by vortexing. Concentrations of vitamin B12, ferritin, cortisol, insulin, folate and human growth hormone (hGH) were measured by an automated electrochemiluminescence system (e411, Roche Diagnostics, Switzerland). Biorad lyphocheck trilevel immunoassay controls were used as quality controls and inter- and intra-assay CVs were 5.0% and 4.1% respectively. C-reactive protein (CRP) concentration was measured by immunoturbidometry. The inter-assay CVs for the high, medium and low concentrations of quality control (QC) samples were 5.1%, 4.9% and 5.2% respectively. For the measurement of interleukin (IL)-6, IGF-1, adiponectin and leptin, individual aliquots of 100 μL of serum sample were used. A Quantikine ELISA kit (R&D Systems, USA) based analysis was performed on these samples. Pooled serum was used as the quality control with an inter- and intra-assay CVs of 2.8% and 2.0% respectively. Plasma zinc concentrations were estimated by flame atomic absorption spectrometer (AAS iCE 3000 series, Thermo Fisher Scientific, USA) according to an established method [19]. Seronorm Trace Elements (Sero, Norway) level I and II were used as the quality control material with an intra-assay CV of 1.0% and 1.2% respectively, and inter-assay CV 1.3% and 1.6% for level I and II respectively.
2.2.1. Aminoacidomics and acylcarnitomics
Serum samples (100 mL) were thawed on ice for 45 min. Free amino acids were then extracted and derivatised using a commercially available Amino Acid Biological Fluids LC-MS/MS Analysis Kit (Zivak technologies, Kagithane, Istanbul, Turkey). 3 μL of the derivatised sample was injected in a Liquid ChromatographyeMass Spectrometer (LCeMS/MS, 6460 Triple Quadrupole, Agilent, CA, USA), equipped with Agilent Jet Stream technology and 1290 Infinity binary pump, autosampler, and a thermostat-based temperature-controlled column compartment. Separation was achieved on a reverse phase HPLC column (2 x 250 mm ID, 5 micron, C18, Zivak Technologies, Kagithane, Istanbul, Turkey) at a flow rate of 0.25 mL/min at 25 °C with aqueous solvent A and organic solvent B. The autosampler tray was set to operate at 4 °C. The elution program started with 38% B (62% A) and then increased to 65% (35% A) over 12 min. At 12.01 min B was 95% (05% A) and this was held until 14 min. A Dynamic Multiple Reaction Monitoring (DMRM) based method was set and MS was operated in positive ionization mode (capillary voltage 3,500 V, nozzle voltage 0 V, capillary gas temperature 300 ° C, capillary gas flow 7 L/min, sheath gas temperature 350 °C, sheath gas flow 11 L/min, nebulizer 30 psi, Fragmentor voltage 135 V and cell accelerator voltage 7 V) with nitrogen as a collision gas. Quantitative analysis of 40 amino acids in serum was achieved in a 20 min run time. Samples were run with external calibrators and QC at the beginning, middle and end of each analytical queue. The intra- and interassay CV of the assay was 2.0% and 7.0% respectively.
For the measurement of acylcarnitines, analytical standards (unlabeled and 2H-labeled) were procured (NSK-B, NSK-B-G1, NSK-B-US and NSK-B-G1-US, Cambridge Isotope Laboratories, Andover, MA). All the standards were reconstituted with 1 mL water: methanol (50:50) for the assay. 2H labeled acylcarnitines were used as internal standard (IS) for the quantification. Serum from 10 volunteers was pooled to make in-house QC samples. 100 μL serum aliquots were thawed on ice for 45 min. Serum samples were spiked with 4 μL of IS mix (NSK-B and NSK-B-G1) and then deproteinised with 800 μL of ice-cold methanol and vortexed thoroughly for 20 s. Further, these samples were centrifuged at 14,000 r.p.m for 20 min at 4 °C. The supernate was transferred to LC injection vials and dried in a vacuum concentrator (Labconco, MO, USA) at 45 °C for 150 min. Dried samples were then derivatised with 100 μL of 0.2 M O-benzylhydroxylamine hydrochloride (OBA, Sigma Aldrich, Germany) in 50% methanol and 50% 10 mM ammonium acetate (Sigma Aldrich, Netherlands) (pH 5.5) and 10 μL of 2 M, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC, Sigma Aldrich, UK) in milli Q water. Samples were vortex mixed for 30 s, and left at room temperature for 10 min for the derivatisation reaction to complete. Samples were then transferred to 150 μL glass inserts in injection vials, and 5 μL injected in a Liquid ChromatographyeMass Spectrometer (LCeMS/MS, 6495 Triple iFunnel Quadrupole, Agilent, CA, USA), equipped with Agilent Jet Stream technology and 1290 Infinity binary pump, autosampler, and a thermostat-column compartment with a ZORBAX Extend C18 column (2.1 x 100 mm ID, 1.8 micron particle size, Agilent, CA, USA) maintained at 55 °C. The autosampler tray was set at 4 °C and the binary pump was operated at 0.4 mL/min flow rate of mobile phase A composed of 0.1% formic acid in water and B containing 0.1% formic acid in acetonitrile. The elution program started with 1% B (99% A), and increased to 20% (80% A) over 3 min and 30%(70%A) over 5.00 min. At12.50 min Bwas increased to 95% (05% A) and held for 3 min. ADMRM based method was set and MS was operated in positive ionization mode (capillary voltage 3500 V, nozzle voltage 0 V, capillary gas temperature 250 °C, capillary gas flow 11 L/min, sheath gas temperature 400 °C, sheath gas flow 11 L/min, nebulizer 30 psi, Fragmentor voltage 380 and iFunnel High/low pressure RF 200/100 V) for analysis of acylcarnitines with nitrogen as collision gas. Quantitative analysis of 13 acylcarnitines in serum was achieved in an 18 min run time. External calibrators and QC samples were analyzed with each batch of study samples at the beginning, middle and end of each analytical sequence run. The intra- and inter-assay CV of the assay was 2.0% and 8.0% respectively.
Peaks were automatically integrated using Agilent MassHunter Qualitative analysis software (version B 07.00). Chromatograms were checked manually for confirming the integration parameters and areas. Missing values from undetected peaks due to low intensity were assigned manually. Amino acids and acylcarnitine concentrations were calculated as follows:
((Peak area of sample × Peak area of IS in calibrator)/(Peak area of calibrator × Peak area of IS in sample)) × Concentration of calibrator
2.2.2. Microbiome analyses
Fecal samples (approximately 250 mg) were taken in a sterile 1.5 mL vial and 300 mL (5 mg/mL) of lysozyme (Sigma# L6876) was added. The sample tubes were invert-mixed and incubated at room temperature for 30 min at 37 °C. 200 μL of lysis buffer was added to samples and vortexed. Samples were subjected to Proteinase-K treatment at 56 °C for 2 h followed by RNase treatment at 65 °C for 20 min. The lysate was mixed well with 100% alcohol and loaded onto a Qiagen DNeasy blood and tissue column (#69506). DNA was purified by following the manufacturer’s instructions. DNA was eluted in 1X TE buffer and stored at 20 °C. DNA was quantified by Nanodrop2000 and analyzed on a 0.8% agarose gel. The V4 region of 16s rDNA was amplified with region specific primers. 50 ng of genomic DNA was amplified for 26 cycles using KAPA HiFi HotStart PCR Kit (KAPA Biosystems Inc., Boston, MA USA) in a reaction containing 0.02 μM of each primer. 1 μL of the product was amplified for 10 cycles with Illumina sequencing barcoded adaptors (Nextera XT v2 Index Kit, Illumina, USA) to enable multiplexing of samples. Libraries were pooled in equimolar concentrations and sequenced on Miseq platform, 275 Paired end chemistry.
The Illumina paired-end reads having dual indexed barcodes specific to the sample were de-multiplexed using bcl2fastq tool allowing 1 mismatch in the index sequence. The generated paired end reads were quality checked using FastQC tool. The reads were processed using in-house ABLT script to filter the adapter and low quality bases. The high quality paired end reads (70% of the bases having phred score (Q > 30)) with primer sequences were stitched using fastqjoin tool. The stitched reads were analyzed using QIIME v 1.9.0 software. The query sequences were mapped against the curated chimera free 16s rRNA database (Greengenes v 13.8) using UCLUST method and OTUs were identified at ≥ 97% sequence similarity. The generated biome was rarefied at a depth of 75,000 sequences/sample with a step size (10 defaults) and alpha diversity indexes were calculated using various metrics. The rarefied biome was used for beta diversity distance matrix calculation using a weighted and unweighted UniFrac method. Rare OTUs (relative abundance < 0.1% within each sample) were removed and then relative abundance of OTUs was calculated as % of total OTUs per sample. The effect of the intervention was analyzed by estimating the ‘fold’ change between baseline and end of intervention for each OTU. These analyses were performed at Genotypic Technology Pvt. Ltd. Bangalore, India.
2.3. Statistical analyses
Exploratory data analysis was performed and reported by using means and SD when data were normally distributed and otherwise by median and quartiles. Histograms and boxplots were used to visually examine the distribution of the serum metabolites in the different protein groups. Normally distributed data were compared by ANOVA followed by post-hoc Bonferroni adjusted test. The difference in height and HAZ was compared between the protein groups using KruskaleWallis test. The missing metabolite data were imputed with the mean values. The values below detectable limits were imputed with the minimum detected values. The metabolite data were preprocessed by mean-centering and pareto-scaling. The fold change in metabolites from baseline to end of intervention was compared between the four protein groups using heat maps and between baseline and the end of intervention by the Wilcoxon sign rank test. Change in prevalence of micronutrient deficiencies between baseline and end of intervention was compared using McNemar’s test. Deficiency cut-offs used for vitamin B12 and folate were <200 pg/mL and <4.4 mg/L respectively [20,21]. The metabolites were compared between the four protein intervention groups using KruskaleWallis test, followed by post-hoc ManneWhitney U-test with Bonferroni adjusted P-values. The association of metabolite fold change with height change was examined using partial least squares regression (PLS-R). Metabolites with variable importance projection (VIP) scores >1.5 were further considered in a correlation analysis. A false discovery rate correction using Bonferroni adjustment for Type I error was employed for the metabolite analysis. The Bonferroni adjustment was for 4 general classes, namely micronutrients, amino acids, hormones and acylcarnitines.
The number of OTUs and the Shannon diversity index were compared between the 4 groups using Kruskal Wallis test at baseline and end of intervention. The %OTUs abundance for the microbiome were compared between the 4 intervention groups using PLS-Discriminant analysis (PLS-DA). The association of the fold change with height change was examined using PLS-R and Spearman rank correlation coefficients.
Statistical significance was considered at P < 0.05 for all analysis other than the metabolite and microbiome analysis, where P < 0.1 with Bonferroni adjustment was considered. All analyses were performed using R software, and all figures were generated using the ggplot program.
3. Results
3.1. Food product acceptability trial
Children (n = 44) who participated in the acceptability study (Supplemental Fig. 1) were randomized to receive products containing either 6 or 12 g of protein/day for 5 consecutive days, in two different flavors. Nutrient compositions of the products are summarized in Supplemental Table 1. 98% of the children completed the trial and reported that the products were acceptable for consumption twice daily for a month (Supplemental Table 2).
3.2. Protein doseeresponse pilot intervention trial
3.2.1. Participants and baseline measures
A total of 100 participants were recruited for the study after screening 806 subjects. In the screened population, the prevalence of moderate and severe stunting was 17 and 3% respectively with the balance non-stunted [height-for-age Z-score median (quartile 1, quartile 3): −0.5 (−0.8, −0.1)]. The moderately stunted participants were randomized into one of four intervention groups (flowchart of recruitment in Fig. 1) corresponding to food products that provided increasing amounts of protein, 6, 8, 10 and 12 g of protein/day respectively (nutrient composition of the products in Supplemental Table 1).
The baseline demographic and anthropometric characteristics of the study population are presented in Table 1A. The four intervention groups were comparable in their age and hemoglobin (Hb) concentrations, and the proportion of girls was 50% for the whole group. All anthropometric characteristics other than HAZ (significantly lower in Group 6 g compared to Group 8 g, using Bonferroni adjusted p-value) were comparable between the four intervention groups. Habitual dietary intakes of the children were obtained 2 weeks into the trial and are summarized in Supplemental Table 3. In Group 10 g, energy (compared to Groups 6 g, 8 g and 12 g, P < 0.05) and macronutrient [carbohydrate (compared to only Group 6 g, P < 0.05), protein and fat (compared to Group 6 g and 8 g but not 12 g, P < 0.05)] intakes were significantly lower. On average, the intervention provided 228 kcal energy, and increased the protein: energy (PE) ratio from 9.5 to 10.7% per day. The proportion of protein supplemented from the intervention products contributed to 21–47% of the total protein intake of the subjects, while carbohydrate supplementation contributed to 9–13% of the total carbohydrate intake. For the micronutrients, vitamin B12 intake increased from an average of 0.47–2.1 mg/day, with the other micronutrient intakes doubled on average.
Table 1A. Demographic and anthropometric characteristics of the study participants at baseline.
| Variables | Group 6 g (n = 24) | Group 8 g (n = 25) | Group 10 g (n = 25) | Group 12 g (n = 26) | P-value |
|---|---|---|---|---|---|
| Girls (%)* | 50 | 48 | 52 | 50 | 0.99 |
| Age (years) | 8.3 ± 1.3 | 8.2 ± 1.4 | 8.0 ± 0.9 | 8.2 ± 1.3 | 0.77 |
| Hemoglobin (g/dL) | 12.2 ± 0.5 | 12.2 ± 0.9 | 12.1 ± 0.7 | 11.9 ± 0.9 | 0.49 |
| Height (cm) | 117.8 ± 6.5 | 116.2 ± 6.9 | 115.7 ± 4.8 | 116.3 ± 6.8 | 0.69 |
| Weight (cm) | 19.8 ± 3.5 | 18.8 ± 3.2 | 18.4 ± 2.0 | 19.1 ± 3.3 | 0.44 |
| BAZ | –1.4 ± 1.1 | –1.6 ± 1.1 | –1.6 ± 0.8 | –1.5 ± 0.9 | 0.78 |
| HAZ | –2.2 ± 0.2a | –2.5 ± 0.4b | –2.3 ± 0.3ab | –2.4 ± 0.3ab | 0.026 |
| MUAC (cm) | 16.7 ± 1.6 | 17.1 ± 1.8 | 17.2 ± 1.2 | 16.8 ± 1.7 | 0.60 |
| Waist circumference (cm) | 52.1 ± 3.7 | 52.7 ± 3.4 | 52.7 ± 2.5 | 52.2 ± 3.6 | 0.87 |
P-value from ANOVA comparing four intervention groups. Values are mean ± SD. Different superscripts indicate statistical signi ficance using post-hoc Bonferroni adjusted test. BAZ: BMI for Age, HAZ: Height for Age and MUAC: Middle Upper Arm Circumference.
P-value from Chi-square test comparing four intervention groups.
3.2.2. Effect of the protein doseeresponse intervention on anthropometry, fasting serum metabolome and fecal microbiome
Table 1B presents the changes in anthropometric measures during the intervention period. There were no significant differences in the change in height, HAZ, weight or BAZ from baseline to end of intervention, either between the four protein dose-intervention groups or when the lowest and highest protein groups were compared. Fold changes between baseline and the end of the intervention for the measured nutritional biomarkers and metabolites were not significantly different except for hydroxyisovalerylcarnitine, which was greater for Group12 g compared to Group 6 g (Kruskal Wallis test; P = 0.052, post-hoc ManneWhitney U test, Bonferroni adjusted P < 0.05, Supplemental Fig. 2c). Supplementary Fig. 3 summarizes the correlation matrix of metabolite fold change.
Table 1B. Changes in hemoglobin and anthropometric characteristics of the study participants during the intervention period.
| Variables | Group 6 g (n = 24) | Group 8 g (n = 25) | Group 10 g (n = 25) | Group 12 g (n = 26) | P-value |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | –0.02 (0.11) | 0.08 (0.10) | 0.02 (0.11) | 0.16 (0.09) | 0.64 |
| Height (cm) | 0.94 (0.10) | 1.06 (0.19) | 0.85 (0.08) | 0.95 (0.16) | 0.78 |
| Weight (cm) | 0.57 (0.12) | 0.28 (0.25) | 0.45 (0.06) | 0.58 (0.09) | 0.45 |
| BAZ | 0.08 (0.06) | –0.01 (0.13) | 0.08 (0.04) | 0.10 (0.05) | 0.78 |
| HAZ | 0.04 (0.02) | 0.05 (0.03) | 0.02 (0.01) | 0.04 (0.02) | 0.82 |
| MUAC (cm) | 0.48 (0.14) | 0.45 (0.11) | 0.53 (0.12) | 0.47 (0.10) | 0.96 |
| Waist circumference (cm) | 0.12 (0.28) | 0.35 (0.17) | 0.32 (0.20) | 0.43 (0.15) | 0.72 |
| Stunted children (%)* | 79 | 88 | 92 | 92 | 0.46 |
Values are mean (Standard Error). P-value from ANOVA comparing four intervention groups. P-value from Chi-square test comparing four intervention groups. BAZ: BMI for Age, HAZ: Height for Age and MUAC: Middle Upper Arm Circumference.
Stunted children at end of intervention.
The fecal microbiome was assessed in a subset of participants (N = 50). The demographic and anthropometric characteristics of these participants at baseline and at the end of intervention were comparable with those of the participants not included in this analysis (Supplemental Table 4). Clean sequences obtained per sample were 182,316 ± 63,642 (mean ± SD). Sampling was assumed to have reached saturation based on rarefaction curves (Supplemental Fig. 4). Fecal microbiome diversity measured by either the Operational Taxonomic Units number (OTUs: taxonomically related groups of bacteria), or the Shannon’s index, was not different between baseline and the end of intervention in any of the protein dose-intervention groups (Supplemental Fig. 5).
Using PLS-DA, taxa associated with the intervention groups were identified (Supplemental Fig. 6). Based on a VIP score cut-off of >1.5, nine OTUs contributed to changes in the microbiome across the intervention groups and a single OTU (OTU ID: 580087, VIP score 2.060) corresponding to genus Blautia contributed maximally to the protein dose-intervention group difference changes in the microbiome (Supplemental Table 5A).
3.3. Combined analyses
To evaluate the overall effect of the intervention, data from the four protein dose-intervention groups were pooled for analysis.
3.3.1. Effect of the intervention on anthropometry and fasting serum metabolites
There were significant increases in height, HAZ, weight and weight-for-age Z-score (WAZ) from baseline to end of intervention (n = 100; height and weight: P < 0.001; HAZ: P = 0.001; BAZ: P = 0.005). The median (quartile1, quartile3) height change in the single month of intervention was 0.9 (0.5, 1.2) cm. This was significantly (P < 0.001) higher than the 4.8 cm/y (0.4 cm/mo) increase in height based on the cross-sectional change by age measured in the baseline data.
From baseline to the end of the intervention, 38 metabolites changed (increased or decreased) significantly (Bonferroni adjusted P < 0.1, Wilcoxon sign rank test). Out of these, a significant increase was observed in fasting serum levels of 21 metabolites including vitamin B12, folate, and ferritin as a marker of iron status, micronutrients that were provided in the intervention product (Supplemental Tables 6 and 7). There was also a significant decrease in the prevalence of deficiencies of vitamin B12 and folate (from 27% to 3% for vitamin B12 and from 21% to 4% for folate) (Supplemental Table 7).
3.3.2. Association of fold-changes in the fasting serum metabolome and fecal microbiome with the linear growth response
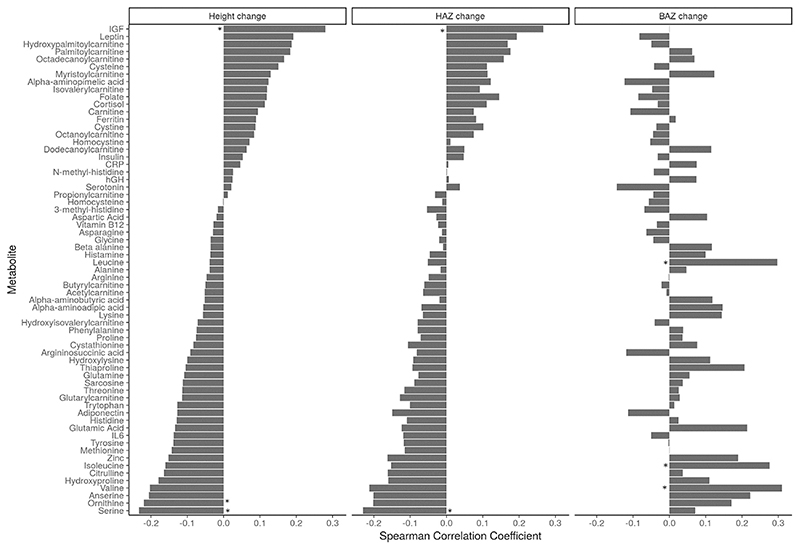
The associations of fold change of metabolites between baseline and the end of intervention were next explored with the change in height, HAZ and BAZ (Fig. 2). Fold change in the concentrations of micronutrients was not significantly associated with the change in height. There was a weak positive correlation (ρ > 0.2) between fold changes in some metabolites, such as leptin, palmytoylcarnitine and IGF-1 with height or HAZ change, and the correlation coefficient of IGF-1 alone was statistically significant (P = 0.02). Moreover, in the 95 participants for whom data on IGF-1 levels was available, fold change in IGF-1 between baseline and the end of intervention was significantly higher for the 79 participants with greater than expected increase in height (≥0.4 cm/mo) compared to the 16 participants with ≤0.4 cm change in height [median (quartile 1, quartile 3): 1.11 (0.89, 1.45) versus 0.83 (0.67, 1.13) respectively; P = 0.029 Mann–Whitney U Test].
Fig. 2.
Spearman rank correlation of change in metabolites with change in anthropometric measures. CRP: C-reactive protein, hGH: human growth hormone, IGF: insulin-like growth factor 1, IL-6: interleukin-6. Statistical significance with Bonferroni adjusted P value < 0.1 indicated with *.
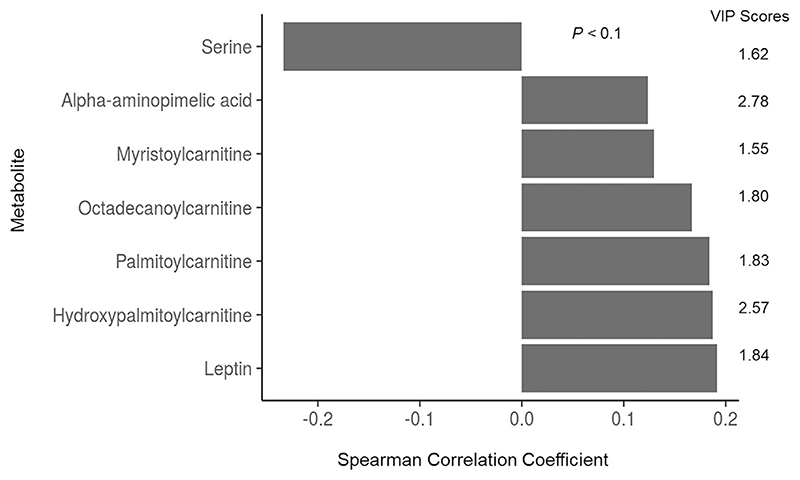
A negative trend between the fold changes of serine and ornithine and height change and for serine with HAZ change was observed (all r < −0.2, P = 0.08). The fold changes of leucine, isoleucine and valine were positively correlated with change in BAZ (P = 0.011, 0.023 and 0.007 respectively). Further, associations of fold changes with height change were explored by PLS-R and those metabolites with a VIP score greater than 1.5 are listed in Fig. 3 with their corresponding correlation coefficient. Among these, serine alone had a significant correlation coefficient (P = 0.08).
Fig. 3. VIP score (>1.5) of metabolite fold change in PLS-R with height change and the Spearman rank correlation coefficients.
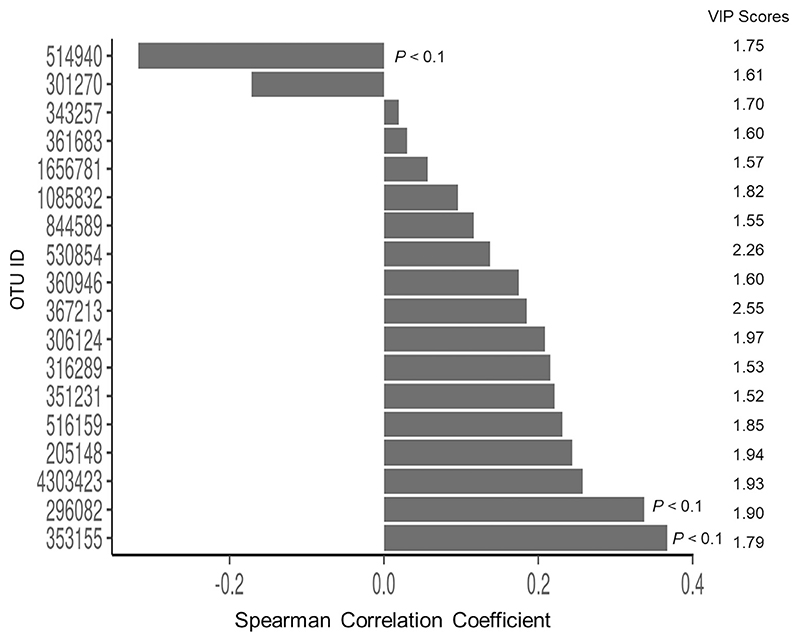
The change in fecal microbiome diversity as measured by the Shannon’s index between baseline and the end of intervention was not correlated with the change in height. Using PLS-R, microbiota % abundance fold changes associated with change in height were evaluated (Fig. 4). Based on a VIP score cut-off of >1.5 and the Spearman’s rank correlation, three OTUs (k_Bacteria; p_Bacteroidetes; c_Bacteroidia; o_Bacteroidales; f_Prevotellaceae; g_Pre-votella; s, k_Bacteria; p_Firmicutes; c_Clostridia; o_Clostridiales; f_Ruminococcaceae; g_Oscillospira; s and k_Bacteria; p_Firmicutes; c_Clostridia; o_Clostridiales; f_Ruminococcaceae; g_Faecalibacterium; s_prausnitzii) were significantly correlated with height change (Supplemental Table 5B).
Fig. 4. VIP score of fold change of relative % abundance of OTUs in PLS-R (VIP score >1.5) with height change and the Spearman rank correlation coefficient.
4. Discussion
The principal finding of this study was that the daily provision of an energy- and micronutrient-fortified protein supplement to stunted, primary school children for one month led to significant changes in a number of metabolites that were associated with improved linear growth. Importantly, in pooled analyses, the positive effect on linear growth after 4 weeks of product intake was similar and not significantly different across the four protein levels and suggests that the lowest level of protein content, in the form of a micronutrient-fortified, culturally acceptable food product, may potentially be adequate to stimulate growth. This is reasonable, as the lowest level of protein intervention (6 g protein/day) met 21% of the daily safe requirement for protein for this age group [22]. The prevalence of vitamin B12 and folate deficiency was reduced significantly in the participants, and while it is possible that these micronutrients were also drivers for the increase in height gain, there were no specific correlations between the changes in the status of these micronutrients with change in height.
In pooled participant responses, there was a significant relationship between the plasma IGF-1 fold change and the change in height of the children, which is consistent with the provision of more protein. Animal studies and human clinical studies have demonstrated that change in protein intake can affect IGF-1 concentrations. In a refeeding study of under-nourished Bangladeshi children,a significantly higher increase in IGF-1 was reported in the group receiving a high protein diet, compared to the group receiving normal protein diets [23]. On the opposite end of the spectrum, long term calorie restriction combined with reduced protein intake results in significant reductions in total IGF-1 concentrations [24]. Though IGF-1 levels increase with age in childhood, peaking at puberty, the positive relationship observed in the current study between the plasma IGF-1 and the change in height was likely due to the effect of the intervention regimen for the following reasons [23,25]. First, the average plasma IGF-1 levels did not increase significantly during the course of intervention, possibly reflecting the range of change in height. Second, the fold change in the plasma IGF-1 concentration was significantly higher in the group of participants that grew more than expected compared to the rest of the participants.
A significantly positive association of leptin and palmitoylcarnitine with height was also observed. Leptin is an adi-pokine neuromodulator in connection with the GHRF-GH-IGF (Hypothalamic GH Releasing Factor, Growth Hormone) hormonal system [26], where the catabolic actions of GH are modulated between the energy demands of the body and growth [27,28]. In protein-energy malnourished children, a 10% weight gain was associated with an increase in the levels of leptin and IGF-1, along with a significantly positive correlation between IGF-1 and leptin [29]. The present study results also converge with those of Bartz et al., where significant increments in serum IGF-1, leptin and branched chain amino acid (BCAA) concentrations were associated with a significant increase in the weight for height Z-score as part of the recovery in children with SAM, after two weeks of nutritional rehabilitation with RUTF [10]. In 6-month old infants fed milk formula with different levels of protein along with breast feeding, serum BCAAs and long-chain acylcarnitines (C10-C18) levels increased in the low protein group, with the interpretation that elevated levels of leucine suppressed β-oxidation in the high protein group, thereby reducing acylcarnitine levels [30].
Serine and ornithine (fold change between baseline and end of intervention) were negatively associated with linear growth. An important function of serine is to act as a precursor for the one-carbon pool, for the synthesis of nucleotides during cell proliferation and rapid growth [31]. A negative relationship between maternal plasma serine concentrations and birth weight has been observed [32]. Though both Bartz et al. and Giovanni et al. have reported an increase in ornithine concentration in children post-nutritional intervention, there are important differences between these studies and the current study [10,33]. The participants of these studies were younger children, with an average age of 2 and 1.5 years respectively, who had been hospitalized for nutritional rehabilitation of SAM, and whose metabolome changes had been assessed over a period of 2 weeks and 3 days respectively. It is possible that the reported increase in ornithine in these studies was an immediate read-out of nutritional stabilization of SAM in younger children that may or may not have been linked to improvement in linear growth [10,33].
Another interesting finding in the present study was that the increase in the plasma concentration of all three BCAAs (leucine, isoleucine and valine) were positively and significantly correlated with the intervention-associated change in BAZ. Plasma BCAAs concentrations could be an early indicator of anabolism and growth as Giovanni et al. also found elevated BCAAs concentrations after just 3 days of nutritional stabilization with RUTF and feeding formula, in malnourished Malawi children [33]. However, these concentrations could also be indicators of thrifty growth and associated metabolic derangements in later life, as BCAAs have been reported to be significantly associated with incident T2DM as well as with a future diagnosis of diabetes mellitus and cardiovascular diseases in the Framingham Offspring Study [34–36].
In terms of the microbiome, lower abundance of Faecalibacterium prausnitzii was observed to significantly associate with increase in height in all children post-intervention. F. prausnitzii has been associated previously with increased risk of inflammatory bowel disease (IBD), characterized by an inappropriate mucosal inflammatory response [37]. It is reasonable to suggest that lower F. prausnitzii abundance reduced gut inflammation in these children, thereby improving nutrient absorption and linear growth. Increase in two OTUs, phyla Firmicutes, family Ruminococcaceae, genus Oscillospira and phyla Bacteroidetes, family Prevotellaceae, genus Prevotella, associated significantly with increased height in this study. While Oscillospira has been associated with leanness or lower BMI in children and adults [38], both Oscillospira and Pre-votella have been reported to be significantly reduced in patients with IBD, likely implicating these as risk-reduction genera for IBD [39,40]. The ‘Prevotella enterotype’ has been associated with vegetarian diets high in carbohydrates, simple sugars as well as high fiber [41,42].
A strength of the current study is the use of a multi-omic approach in the setting of a randomized controlled trial of protein supplement doseeresponse, to assess the intermediary metabolomic and microbiome changes associated with linear growth in stunted Indian children. Unlike cross-sectional studies, this translates to the ability to analyze the multi-omic changes as cause-effect relationships instead of associations. However, our study has some limitations. There was no control group for ethical reasons and the children had not been randomized based on their pre-intervention habitual protein intakes since all of them were from a single school, with similar socioeconomic and cultural backgrounds with presumably similar habitual dietary patterns. Due to the lack of pre- and post-intervention dietary intake data, the effects of the intervention on the habitual intakes of the children could not be assessed. Further, only fasted metabolomic profiles were measured and postprandial metabolomic signatures, which would relate acutely to the intervention, were also not evaluated.
In conclusion, the daily provision to stunted, Indian school children for one month, of a novel, economical, micronutrientfortified food product, containing different doses of high-quality legume protein, resulted in an overall better than expected linear growth. However, there was no difference in linear growth by feeding graded dose (6–12 g/day) of protein. The children grew significantly taller than predicted from baseline data and more than the WHO growth reference values for age during the intervention period [17]. This study also identified intervention mediated growth-associated signatures of the serum metabolome and fecal microbiome that correlated with linear growth responses. It therefore provides the basis for defining a legume-based micronutrient-fortified food designed to meet the daily gap in affordable nutrition programs,to potentially reduce stunting in resource-poor settings. Future short-term feedings studies will provide insights into whether further lowering of protein content in the food product is feasible for maintaining the stimulatory effect on growth as well as to improve the affordability of the product. Further, longer-term trials would be needed for validation of the metabolome and microbiome signatures of growth identified in the current study.
Supplementary Material
Acknowledgement
The authors acknowledge the school and the children for their participation in the study. The enthusiastic work of Mr. Arun Das, clinical coordinator of the study, his team of social workers who supported the study during the intervention period, and Ms. Beena Bose who coordinated with biochemical analysis is also acknowledged. Our thanks also go to the laboratory technicians Mr. Kashiraya D Bansode and Mr. Vincent Devraj for their help provided during the execution of the study.
Financial support
This study was supported by the Tata Trusts, India, a philanthropic organization, which along with Mars International India Private Limited, participated in the development of the food products. The food products utilized in this study were an unrestricted gift by Mars International India Private Limited. This work was also supported by the Wellcome Trust/DBT India Alliance fellowship (IA/M/14/1/501681) awarded to AVK.
Abbreviations
- BAZ
BMI for age Z-score
- BCAA
branched chain amino acids
- CRP
C-reactive protein
- HAZ
height for age Z-score
- Hb
hemoglobin
- IGF-1
insulin like growth factor-1
- IL-6
interleukin-6
- MS
mass spectrometry
- MUAC
mid upper arm circumference
- OTU
operational taxonomic units
- QC
quality control
- RUTF
ready-to-use therapeutic food
- SAM
severe acute malnutrition
- VIP score
variable importance projection score.
Footnotes
Author contributions
AVK, CLK, LHA, and TT: conceived the project and its design; AV, SD, MP, AM and TT: conducted the project; AVK, TT, NGK, AM, AV, SD, LHA, CLK, MD and RH: analyzed the data; AV, SD, AM, TT and AVK: wrote the manuscript and all authors read and edited the manuscript.
Conflicts of interest
MD has a consultant relation with MARS, Inc., USA. CLK is the Mars Chair in Developmental Nutrition. AVK is an advisor to the Tata Trusts, India. All other authors report no conflicts of interest.
References
- [1].Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Heal. 2013;1:e26–36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gat-Yablonski G, Yackobovitch-Gavan M, Phillip M. Which dietary components modulate longitudinal growth? Curr Opin Clin Nutr Metab Care. 2017;20:211–6. doi: 10.1097/MCO.0000000000000364. Available from: http://insights.ovid.com/crossref?an=00075197-201705000-00012[Internet] [DOI] [PubMed] [Google Scholar]
- [3].Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515:518–22. doi: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- [4].Fabiansen C, Yaméogo CW, Iuel-Brockdorf A-S, Cichon B, Rytter MJH, Kurpad A, et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: a randomised 2 x 2 x 3 factorial trial in Burkina Faso. PLoS Med. 2017;14:e1002387. doi: 10.1371/journal.pmed.1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bahwere P, Balaluka B, Wells JCK, Mbiribindi CN, Sadler K, Akomo P, et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr. 2016;103:1145–61. doi: 10.3945/ajcn.115.119537. [DOI] [PubMed] [Google Scholar]
- [6].Ebert AW. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustain MDPI AG. 2014;6:319–35. [Google Scholar]
- [7].Considine MJ, Siddique KHM, Foyer CH. Nature’s pulse power: legumes, food security and climate change. J Exp Bot. 2017;68:1815–8. doi: 10.1093/jxb/erx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].USDA. (Report of the dietary guidelines advisory committee dietary guidelines for Americans, 2010).Nutr Rev. 2010;53:376–9. doi: 10.1111/j.1753-4887.1995.tb01493.x. [DOI] [PubMed] [Google Scholar]
- [9].McMillan A, Orimadegun AE, Sumarah MW, Renaud J, da Encarnacao MM, Gloor GB, et al. Metabolic derangements identified through untargeted metabolomics in a cross-sectional study of Nigerian children with severe acute malnutrition. Metabolomics. 2017;13 [Google Scholar]
- [10].Bartz S, Mody A, Hornik C, Bain J, Muehlbauer M, Kiyimba T, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab. 2014;99:2128–37. doi: 10.1210/jc.2013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang P, Stanstrup J, Thymann T, Sangild PT, Dragsted LO. Progressive changes in the plasma metabolome during malnutrition in Juvenile pigs. J Proteome Res. 2016;15:447–56. doi: 10.1021/acs.jproteome.5b00782. [DOI] [PubMed] [Google Scholar]
- [12].Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for Kwashiorkor. Science. 2013;339:548–54. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baum JI, Miller JD, Gaines BL. The effect of egg supplementation on growth parameters in children participating in a school feeding program in rural Uganda: a pilot study. Food Nutr Res. 2017;61:1330097. doi: 10.1080/16546628.2017.1330097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iannotti LL, Lutter CK, Waters WF, Gallegos Riofrío CA, Malo C, Reinhart G, et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: a randomized controlled trial in Ecuador. Am J Clin Nutr. 2017;106:1482–9. doi: 10.3945/ajcn.117.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Martinez B, Webb MF, Gonzalez A, Douglas K, del Pilar Grazioso M, Rohloff P. Complementary feeding intervention on stunted Guatemalan children: a randomised controlled trial. BMJ Paediatr open. 2018;2:e000213. doi: 10.1136/bmjpo-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].World Health Organization. Growth reference data for 5-19 years. World Health Organization; 2006. [cited 2017 Dec 11]. Available from: http://www.who.int/growthref/en/ [Google Scholar]
- [18].Bharathi AV, Kurpad AV, Thomas T, Yusuf S, Saraswathi G, Vaz M. Development of food frequency questionnaires and a nutrient database for the Prospective Urban and Rural Epidemiological (PURE) pilot study in South India: methodological issues. Asia Pac J Clin Nutr. 2008;17:178–85. [PubMed] [Google Scholar]
- [19].Tinoco-Veras CM, Bezerra Sousa MS, da Silva BB, Franciscato Cozzolino SM, Viana Pires L, Coelho Pimentel JA, et al. Analysis of plasma and erythrocyte zinc levels in premenopausal women with breast cancer. Nutr Hosp. 2011;26:293–7. doi: 10.1590/S0212-16112011000200008. [DOI] [PubMed] [Google Scholar]
- [20].Alayne MA, Rushdia A, Mahbub Latif AHM, Sabrina R, Sumon Das K, Enamul H, et al. Impact of fortified biscuits on micronutrient deficiencies among primary school children in Bangladesh. PloS One. 2017;12 doi: 10.1371/journal.pone.0174673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(Suppl. 2):238–44. doi: 10.1177/15648265080292S129. [DOI] [PubMed] [Google Scholar]
- [22].Joint WHO/FAO/UNU Expert Consultation. (World Health Organization technical report series).Protein and amino acid requirements in human nutrition. 2007 [PubMed]
- [23].Pucilowska JB, Davenport ML, Kabir I, Clemmons DR, Thissen JP, Butler T, et al. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab. 1993;77:1516–21. doi: 10.1210/jcem.77.6.7505287. [DOI] [PubMed] [Google Scholar]
- [24].Clayton PE, Hall CM. Insulin-like growth factor I levels in healthy children. Horm Res. 2004:2–7. doi: 10.1159/000080752. [DOI] [PubMed] [Google Scholar]
- [25].Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–7. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].LaPaglia N, Steiner J, Kirsteins L, Emanuele M, Emanuele N. Leptin alters the response of the growth hormone releasing factor- growth hormone–insulin-like growth factor-I axis to fasting. J Endocrinol. 1998;159:79–83. doi: 10.1677/joe.0.1590079. [DOI] [PubMed] [Google Scholar]
- [27].Soliman AT, ElZalabany MM, Salama M, Ansari BM. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism. 2000;49:819–25. doi: 10.1053/meta.2000.6745. [DOI] [PubMed] [Google Scholar]
- [28].Llopis MA, Granada ML, Cuatrecasas G, Formiguera X, Sanchez-Planell L, Sanmarti A, et al. Growth hormone-binding protein directly depends on serum leptin levels in adults with different nutritional status. J Clin Endocrinol Metab. 1998;83:2006–11. doi: 10.1210/jcem.83.6.4894. [DOI] [PubMed] [Google Scholar]
- [29].Palacio AC, Pérez-Bravo F, Santos JL, Schlesinger L, Monckeberg F. Leptin levels and IgF-binding proteins in malnourished children: effect of weight gain. Nutrition. 2002;18:17–9. doi: 10.1016/s0899-9007(01)00690-6. [DOI] [PubMed] [Google Scholar]
- [30].Kirchberg FF, Harder U, Weber M, Grote V, Demmelmair H, Peissner W, et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metab. 2015;100:149–58. doi: 10.1210/jc.2014-3157. [DOI] [PubMed] [Google Scholar]
- [31].Cook RJ. Defining the steps of the folate one-carbon shuffle and homocysteine metabolism. Am J Clin Nutr. 2000;72:1419–20. doi: 10.1093/ajcn/72.6.1419. [DOI] [PubMed] [Google Scholar]
- [32].Kalkhoff RK, Kandaraki E, Morrow PG, Mitchell TH, Kelber S, Borkowf HI. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism. 1988;37:234–9. doi: 10.1016/0026-0495(88)90101-1. [DOI] [PubMed] [Google Scholar]
- [33].Di Giovanni V, Bourdon C, Wang DX, Seshadri S, Senga E, Versloot CJ, et al. Metabolomic changes in serum of children with different clinical diagnoses of malnutrition. J Nutr. 2016;146:2436–44. doi: 10.3945/jn.116.239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tillin T, Hughes AD, Wang Q, Würtz P, Ala-Korpela M, Sattar N, et al. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall and Brent REvisited) Study. Diabetologia. 2015;58:968–79. doi: 10.1007/s00125-015-3517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engström G, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Hear J. 2013;34:1982–9. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dubinsky M, Braun J. Diagnostic and prognostic microbial biomarkers in inflammatory bowel diseases. Gastroenterology. 2015;149:1265–e74.e3. doi: 10.1053/j.gastro.2015.08.006. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0016508515011385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Konikoff T, Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24:523–4. doi: 10.1016/j.tim.2016.02.015. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0966842X16000524. [DOI] [PubMed] [Google Scholar]
- [39].Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–33. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Santoru ML, Piras C, Murgia A, Palmas V, Camboni T, Liggi S, et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci Rep. 2017;7:9523. doi: 10.1038/s41598-017-10034-5. Available from: http://www.nature.com/articles/s41598-017-10034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.