Abstract
Anti-CD19 chimeric antigen receptor (CAR) T cells showed significant anti-leukemic activity in B-precursor acute lymphoblastic leukemia (ALL). Allogeneic, HLA-mismatched off-the-shelf 3rd-party donors may offer ideal fitness of the effector cells but carry the risk of graft-versus-host disease. Knockout of the endogenous T-cell receptor (TCR) in CD19-CAR-T cells may be a promising solution. Here, we induced a CRISPR/Cas9-mediated knockout of the TCRβ-chain in combination with a 2nd-generation retroviral CAR transduction including a 4-1BB costimulatory domain in primary T cells. This tandem engineering led to a highly functional population of TCR-KO-CAR-T cells with strong activation (CD25, IFN-γ), proliferation and specific killing upon CD19 target recognition. TCR-KO-CAR-T cells had a balanced phenotype of central memory and effector memory T cells. KO of the endogenous TCR in T cells strongly ablated alloreactivity in comparison to TCR-expressing T cells. In a patient-derived xenograft model of childhood ALL, TCR-KO-CAR-T cells clearly controlled CD19+ leukemia burden and improved survival in vivo. However, coexpression of endogenous TCR plus CAR led to superior persistence of T cells and significantly prolonged leukemia control in vivo, confirmed by a second in vivo model using NALM6 leukemia cells. These results point towards an essential role of the endogenous TCR for longevity of the response at the price of alloreactivity. In conclusion, anti-CD19 CAR-T cells with a CRISPR/Cas9-mediated TCR-KO are promising candidates for non-matched third-party adoptive T-cell transfer with high anti-leukemic functionality in the absence of alloreactivity, but long-term persistence in vivo is better in the presence of the endogenous TCR.
Introduction
Treatment with autologous anti-CD19 chimeric antigen receptor (CAR) T cells has shown high initial complete response rates in patients with relapsed and refractory acute lymphoblastic B-precursor leukemia (BCP-ALL). 1,2 Nevertheless, up to 50% of pediatric patients experience relapse due to loss of CAR-T cells or escape variants of leukemic blasts. 3,4 Moreover, insufficient lymphocyte numbers for leukapheresis, chemotherapy pre-treatment and failure of CAR-T-cell production remain unsolved challenges in various CAR production approaches. 4,5,6 Insufficient T-cell function has also been attributed to leukemia-induced T-cell exhaustion. 7 Administration of allogeneic off-the-shelf products from healthy HLA-mismatched donors may overcome these hurdles but carry the risk of severe graft-versus-host disease (GvHD). Current CAR-T-cell protocols use lymphodepleting regimens eliminating the host’s T-cell pool for the time of CAR-T-cell infusion and expansion, minimizing the risk of rejection of CAR-T-cells. 3,6 T-cell receptor (TCR) knockout (KO) of the CAR-T-cell product would be supposed to prevent GvHD. Two patients were reported previously that were treated with anti-CD19-CAR-T cells, engineered with transcription activator-like effector nucleases (TALENs) to knock-out the endogenous TCR. 8 In these cases, allogeneic T cells derived from a HLA-mismatched healthy third-party donor. TALEN-mediated TCR-KO was combined with lentiviral transduction to produce TCR-/CAR+ T cells, which were administered to two pediatric BCP-ALL patients with good initial response rates. However, TCR+ cells from the CAR infusion expanded in vivo and induced GvHD.
The relevance of the TCR was also analyzed in a mouse model using knock-in of an anti-CD19 CAR into the locus of the endogenous constant T-cell receptor alpha chain (TRAC) by combination of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology with adeno-associated viral (AAV) transfer. 9 The CAR in the TRAC locus resulted in increased CAR functionality in preclinical mouse models, but was not yet investigated in clinical phase I/II trials. Nevertheless, recently published clinical data with virus-specific T cells showed, that additional TCR stimulation enhances the expansion and function of CD19-CAR-T cells. 10
Here, we combine CRISPR/Cas9-mediated TCR-KO with retroviral transduction of a 2nd generation anti-CD19 CAR with 4-1BB-based co-stimulation to analyze the relevance of the endogenous T-cell receptor for functionality of TCR-/CAR+ T cells. TCR-KO-CAR-T cells exert excellent anti-CD19 activity and significantly decreased alloreactivity. However, TCR+ CAR-T cells showed significantly improved persistence in vivo indicating an essential role of the endogenous TCR for sustained CAR T-cell function in vivo. Nevertheless, highly functional allogeneic TCR- CARs might be promising treatment candidates as bridge to transplant.
Materials and methods
Isolation of peripheral blood mononuclear cells (PBMCs), T-cell activation, cell lines, flowcytometry, intracellular staining, histology, proliferation, cytotoxicity assays, CAR-enrichment and depletion of CD3+ T cells are described in the Supplements.
Retroviral anti-CD19 CAR transduction
For retroviral transduction, 2nd generation anti-CD19 CAR sequence containing FMC63, 11 CD8 transmembrane and spacer domains and 4-1BB costimulatory domain (based on patent WO2015187528A1) was cloned into pMP71 (kindly provided by Christopher Baum, Medizinische Hochschule Hannover, Hannover, Germany) via EcoRI and NotI (pMP71_CAR). A myc tag was included into the CAR for detection and purification. pMP71_CAR was transfected into 293Vec-Galv cells (kindly provided by BioVec Pharma Inc, Quebec, Canada) using TransIT-293 Transfection Reagent (Mirus Bio, Madison, Wisconsin, USA) according to the supplier’s information. Retroviral supernatant was used for transduction of 293-Vec-RD114 cells (kindly provided by BioVec Pharma Inc, Quebec, Canada) to create a stable producer system. Retroviral supernatant of 293-Vec-RD114 cells was used to transduce primary T cells from healthy donors. Therefore, 24-well plates were coated with 2.5μg RetroNectin Reagent (Takara Bio, Kusatsu, Japan) per well at 37°C for 2 hours. Plates were blocked with 2% Albumin Fraction V (Carl Roth, Karlsruhe, Germany) in PBS (Gibco, Thermo Fisher Scientific, Waltham, Massachusetts, USA) for 30 min and washed with a 1:40 dilution of HEPES 1M (Thermo Fisher Scientific, Waltham, Massachusetts, USA) in PBS. Virus supernatant was harvested and filtered (0.45μm). 1ml virus supernatant was transferred in each well of the plate and centrifuged 3000g for 90min at 32°C. Virus supernatant was discarded and 1x10 6 T cells in 1ml TexMACS GMP medium (Miltenyi Biotec, Bergisch Gladbach, Germany)/2.5% human AB serum (Institute for Clinical Transfusion Medicine, Ulm, Germany) + 12.5ng/ml human IL-7 and IL-15, premium grade (Miltenyi Biotec, Bergisch Gladbach, Germany) + 2μg/ml Protamine sulfate (Sigma-Aldrich, Taufkirchen, Germany) were added. Plates were centrifuged for 10min at 450g, 32°C. Transduction was performed on day 2 after T-cell activation.
CRISPR/Cas9-mediated TCR knockout
CRISPR/Cas9-mediated TCR KO was performed one day after CAR transduction. The gRNA targeting the T-cell receptor constant β-chain was published previously. 12,13 For CRISPR/Cas9-mediated TCR KO, Alt-R CRISPR-Cas9 tracrRNA and Alt-R CRISPR-Cas9 crRNA (both from Integrated DNA Technologies, Coralville, Iowa, USA) were mixed 1:1 and heated 5min at 95°C. Alt-R S.p. Cas9 Nuclease 3NLS (Integrated DNA Technologies, Coralville, Iowa, USA) was mixed with the gRNA complex and Alt-R Cas9 Electroporation Enhancer (Integrated DNA Technologies, Coralville, Iowa, USA) and incubated 15 min at room temperature. For electroporation, buffer 1M 14 was used on a Nucleofector 2b Device according to the manufacturer’s instructions (Lonza, Basel, Switzerland). After electroporation, T cells were immediately transferred to fresh medium. T cells treated with a non-binding gRNA were used as control (Integrated DNA Technologies, Coralville, Iowa, USA). T cells were further expanded as described above.
Alloreactivity assay
PBMCs were isolated from six different healthy donors, irradiated with 20Gy and pooled. To distinguish between (TCR KO) T cells and allogeneic donor-derived PBMCs, T cells were labeled with CellTrace CFSE Cell Proliferation Kit and PBMCs were labeled with CellTrace Violet Cell Proliferation Kit (both Thermo Fisher Scientific, Waltham, Massachusetts, USA). After co-culturing the cells at a 1:5 E:T ratio for one to five days, TCR-KO vs wildtype T cells were analyzed for their activation marker profile (CD69, CD137, CD25) and their proliferation by flow cytometry.
In vivo experiments
All animal trials were performed in accordance with the current ethical standards of the official committee on animal experimentation (written approval by Regierung von Oberbayern, tierversuche@regob.bayern.de; ROB-55.2Vet-2532.Vet_02-16-7). Mice were maintained under specific pathogen-free conditions, had free access to food and water, and were housed with a 12h light/dark cycle and constant temperature. NALM6 cells and ALL-265 PDX cells 15 were genetically modified by lentiviral transduction to express enhanced firefly luciferase and eGFP as selection marker (see Addgene vector 104834). 16 1x105 luciferase positive NALM6 or 2x106 BCP-ALL PDX cells were transplanted i.v. into NSG mice (NOD-scid IL2Rgammanull; The Jackson Laboratory, Bar Harbour, ME, USA). Three days after injection, CAR-T cells were thawed, counted, and 2x107 cells in 200μl PBS were injected i.v. into mice. This high number of T cells was chosen to increase risk of graft-versus-host disease (GvHD). Early onset of GvHD clarify differences between TCR+ CAR T cells and TRBC KO CAR T cells, in terms of alloreactivity and tumor growth control. Leukemia burden was monitored once or twice per week by bioluminescence in vivo imaging (BLI) as described previously. 15,17 Furthermore, peripheral blood was analyzed regularly to detect presence of human T and CAR-T cells. Mice were monitored daily for signs of graft-versus-host-disease (GvHD) like weight loss, hair loss, altered posture, or reduced mobility. When BLI reached values above 1x1010P/sec, or if mice showed clinical signs of illness or GvHD, mice were sacrificed and blood was analyzed for presence of NALM6 or PDX cells and human T / CAR-T cells.
Statistics
Statistical analyses were performed using Graphpad Prism 7. A two-tailed paired t-test was performed to analyze statistical significance between experimental conditions of at least 3 independent experiments. When more than two groups were compared with each other, statistical significance was tested with a one-way ANOVA. Overall survival of mice was calculated using Kaplan-Meier method. Two-tailed Mann-Whitney test was used to compare presence of T cells in the peripheral blood of mice. * means p<0.05, ** means p <0.01, *** means p<0.001, **** means p<0.0001.
Results
CAR-T cells with knockout of the TCRβ chain show comparable transduction rates and expansion compared to conventional CAR-T cells
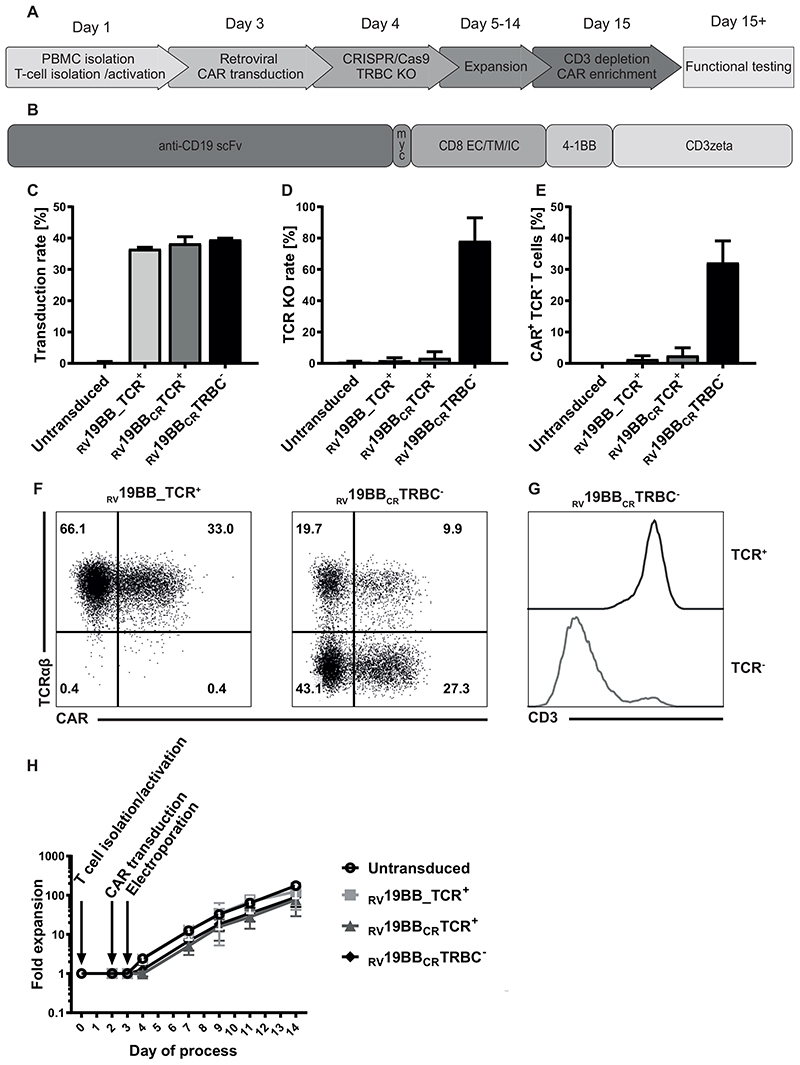
T cells were isolated, activated (CD3/CD28-stimulation; Fig. 1A), retrovirally transduced with a second generation CAR (Fig. 1B) and one day after transduction, CRISPR/Cas9-mediated TCR-KO was performed by electroporation of the ribonucleoprotein (RNP) complex including a guide RNA (gRNA) targeting the TCR-β-chain (TRBC). Non-electroporated conventional CARs (RV19BB_TCR+), CAR-T cells electroporated with Cas9 and a non-binding gRNA (RV19BBCRTCR+) and untransduced, non-electroporated T cells served as comparator for CAR-T cells with knockout of the TCRβ chain (RV19BBCRTRBC-). The gRNA targeting the TCRβ chain was published before and showed high on-target and low off-target effects. 18 As the TCR beta constant region comprises two different genes, TRBC1 and TRBC2, we made sure that the selected gRNA targets both TRBC loci. RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs showed comparable mean transduction rates of 36.6%, 38.3% and 39.6% respectively (Fig. 1C). RV19BBCRTRBC- CAR-T cells reached a mean TCR-KO rate of 78.2% determined by flow cytometry (Fig. 1D). 32.2% of all T cells had both a CAR on the surface as well as a TCR-KO after expansion (Fig. 1E). Exemplary flow cytometry plots to determine transduction and TCR-KO rates are shown in Fig. 1F. Defective TCR assembly upon KO of the TCRβ chain is confirmed by simultaneous CD3 downregulation (Fig. 1G). After magnetic CAR enrichment (c-myc tag) and CD3 depletion, a high purity of TCR-/CAR+ T cells was achieved (Supplementary Fig. 1A). Untransduced T cells and RV19BB_TCR+ CARs showed an expansion greater than 120-fold (175-fold and 124 -fold), whereas RV19BBCRTCR+ and RV19BBCRTRBC-CARs showed slightly reduced proliferative capacity (77-fold and 89-fold) (Fig. 1H). As expansion impairment was seen in RV19BBCRTCR+ and RV19BBCRTRBC- CARs, this effect was mediated by electroporation process itself rather than by loss of the TCR.
Fig. 1. Generation of TCR KO CAR-T cells.
(A) Schematic overview of the time points for retroviral transduction, CRISPR/Cas9-mediated TCR KO, duration of cultivation and purification of the final CAR-T-cell product. (B) Structure of the CAR construct: the 2nd generation CAR consists out of the anti-CD19 scFv region, followed by the myc tag, CD8 extra-, trans- and intracellular domain, as well as a costimulatory 4-1BB domain and T-cell activating CD3zeta chain. (C) A mean CAR transduction rate of more than 35% could be reached for the RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC-CARs as assessed by flow cytometry (3 independent experiments). (D) The mean TCR KO rate within CD4+CD8+ cells was 78.20 % and (E) the proportion of cells expressing the CAR and lacking the TCR reached around 32.2% (3 independent experiments). (F) Exemplary flow cytometry plots to determine transduction rate and TCR KO efficacy and (G) correlation of CD3 and TCR expression in TCR+ and TCR- T cells within RV19BBCRTRBC-CARs is shown in this histogram. (H) Fold expansion of the untransduced T cells, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC-CARs after T-cell isolation and activation, transduction and electroporation (n≥4). PBMC=peripheral blood mononuclear cell, PB=peripheral blood, KO=knockout, TRBC=constant T-cell receptor β-chain, SSC=side scatter, RV19BB_TCR+=conventional CAR-T cells without electroporation, RV19BBCRTCR+=CAR-T cells electroporated with nonsense gRNA, RV19BBCRTRBC-=CAR-T cells electroporated with TRBC-targeting gRNA.
In vitro CAR-T-cell function is independent of presence or absence of endogenous TCR
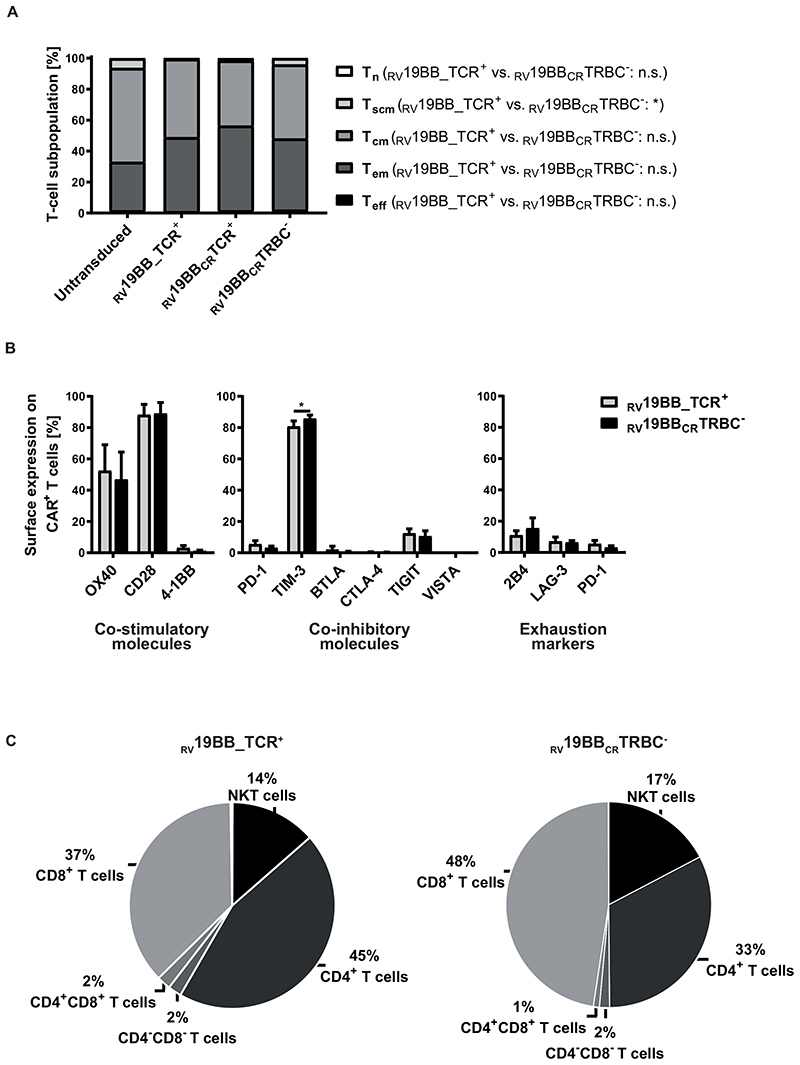
T-cell characteristics with or without TCR were analyzed (CD62L/CD95/CD45RO expression; Fig. 2A). 19 After expansion (14 days IL-7/IL-15), no naïve T cells and only a minor fraction (<2.2%) of terminally differentiated effector T cells (Teff) were detectable in untransduced, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CAR-T cells. Thus, the final T-cell product consisted mainly of central memory (Tcm) and effector memory (Tem) T cells. T-cell subpopulations were distributed equally between RV19BB_TCR+ CAR-T cells with a mean of 50.6% Tcm and 47.8% Tem and the RV19BBCRTRBC- CARs with a mean of 47.1% Tcm and 46.0% Tem cells. Expression of co-stimulatory and co-inhibitory molecules as well as exhaustion markers 2B4, LAG-3 and PD-1 was analyzed by flow cytometry at the end of expansion phase (Fig. 2B). RV19BBCRTRBC- CAR-T cells shared a comparable profile of co-stimulatory and -inhibitory molecules with RV19BB_TCR+ CAR-T cells. RV19BB_TCR+ and RV19BBCRTRBC- CARs showed high surface expression levels of OX40 (52.4% vs. 46.9%) and CD28 (88.2% vs 89.0%), whereas 4-1BB was expressed to a very low amount at the end of the expansion protocol. Both RV19BB_TCR+ and RV19BBCRTRBC- CARs displayed low expression of co-inhibitory molecules like PD-1 (5.5% vs 3.2%), BTLA (2.7% vs 0.6%), CTLA-4 (0.4% vs. 0.2%), TIGIT (12.4% vs 10.6%) and VISTA (0.1% vs 0.1%). Only TIM-3 was highly expressed on RV19BB_TCR+ as well as even slightly increased on RV19BBCRTRBC- CART cells (80.8% vs. 85.8%; p=0.0222). Besides that, the TCR-KO on CAR-T cells had no effect on the expression of typical exhaustion markers like 2B4 (11.1% vs 15.5%), LAG-3 (7.1% vs 6.4%) and PD-1 (5.5% vs 3.2%). At the end of the expansion protocol, different T-cell subsets were present in the final product, but no monocytes, B cells or NK cells (Fig. 2C). RV19BBCRTRBC- CARs and RV19BB_TCR+ CARs showed a similar cellular composition of CD8+ T cells (47.5% vs 37.1%) and NKT cells (17.4% vs 13.5%), with minor differences in the CD4+ T-cell population (32.6% vs 44.8%, p=0.0222).
Fig. 2. Cellular characteristics of the final CAR-T-cell product.
(A) The final product of CAR-expressing T cells showed mainly central memory and effector memory T cells regardless of TCR expression after expansion (n=3). (B) Surface expression profile of several co-stimulatory (OX40, CD28, 4-1BB) and co-inhibitory molecules (PD-1, TIM-3, BTLA, CTLA-4, TIGIT, VISTA) as well as commonly used exhaustion markers (2B4, LAG-3, PD-1) was determined by flow cytometry and compared between CAR-T cells with or without TCR KO (n=3). (C) Cellular composition of CAR-T cells was determined on day 14 (n=4). A two-tailed paired t test was performed to determine statistical significance. Tn: naïve T cells, Tscm: stem cell-like memory T cells, Tcm: central memory T cells, Tem: effector memory T cells, Teff: Effector T cells; Tn: CD62L+, CD45RO-, CD95-; Tscm: CD62L+, CD45RO-, CD95+; Tcm: CD62L+, CD45RO+, CD95+; Tem: CD62L-, CD45RO+, CD95+; Teff: CD62L-, CD45RO-, CD95+, RV19BB_TCR+=conventional CAR-T cells without electroporation, RV19BBCRTCR+=CAR-T cells electroporated with nonsense gRNA, RV19BBCRTRBC-=CAR-T cells electroporated with TRBC-targeting gRNA, n.s.=not significant, NKT cells=natural killer T cells.
CAR-T cells with knockout of the TCRβ chain are highly functional in vitro and prevent alloreactivity
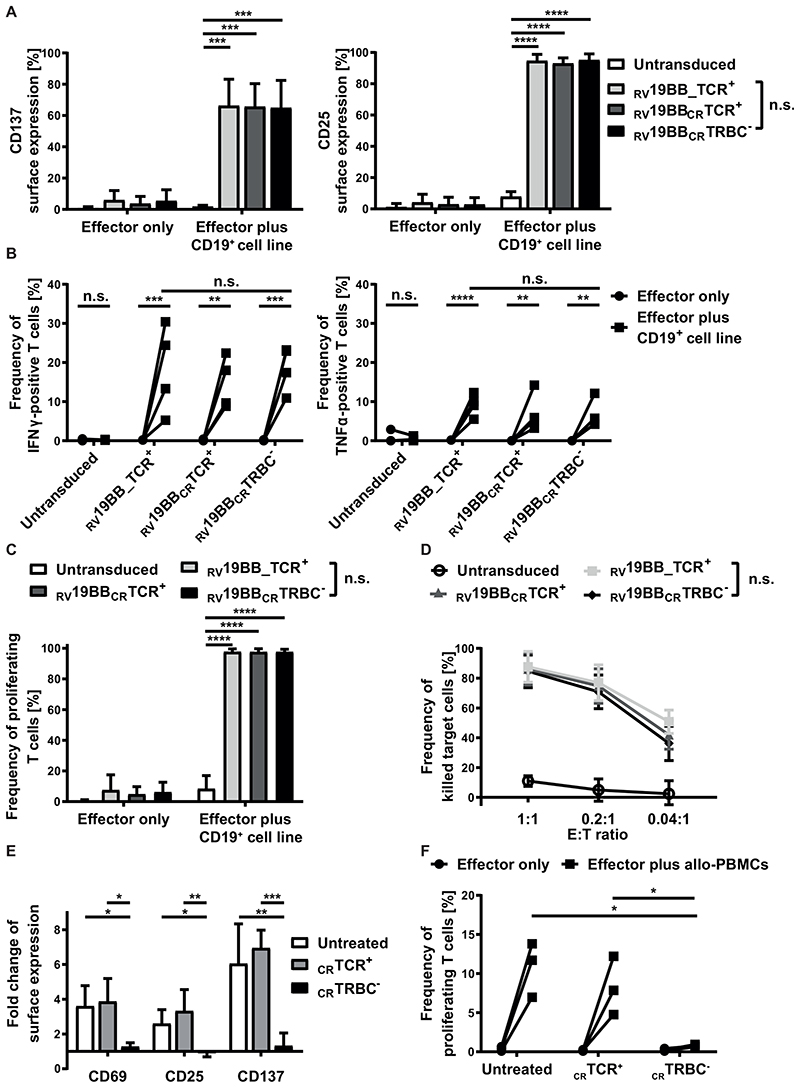
Activation potential of TCR-KO-CAR-T cells was tested (Fig. 3A). Untransduced T cells, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs were co-cultured with a CD19+ target cell line for 24 hours and then stained for the activation markers CD137 and CD25. The baseline surface expression of both markers was very low ranging from 0.8% to 6.0% for CD137 and from 1.2% to 4.2% for CD25. Upon antigen contact, the CAR expressing T cells showed a significant upregulation of CD137 and CD25 compared to untransduced T cells. RV19BBCRTRBC- CARs displayed a similar target-specific activation compared to RV19BB_TCR+ CAR-T cells with a mean CD137 expression of 65.0% vs. 66.3% and a mean CD25 expression of 95.2% vs. 94.7%. To determine the lytic potential of RV19BBCRTRBC- CAR-T cells, cytokine secretion after 24 hours of co-culturing with CD19+ target cells was analyzed (Fig. 3B). Notably, interindividual variability in cytokine secretion was compensated by analysis of four different individual donors. Without antigen contact untransduced T cells, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs showed less than 0.5% of IFNγ positive cells. After co-culturing with target cells RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs showed a significantly increased percentage of IFNγ positive cells ranging from 5.3% to 30.4% of RV19BB_TCR+ CARs, 8.8% to 22.4% of RV19BBCRTCR+ CARs and 10.9% to 23.2% of RV19BBCRTRBC- CARs. A similar pattern was observed for TNFα secretion upon stimulation. RV19BB_TCR+ CAR-T cells as well as RV19BBCRTRBC- CARs showed a significant upregulation of TNFα positive cells upon antigen stimulation (5.5% up to 12.3% vs. 4.3% up to 12.1%). Therefore, loss of the TCR on RV19BBCRTRBC- CARs showed no disadvantage regarding secretion of IFNγ and TNFα compared to RV19BB_TCR+ CARs. The proliferative characteristics of RV19BBCRTRBC- CARs showed comparable results to RV19BB_TCR+ CARs after 72 hours of antigen contact (Fig. 3C). More than 97.60% of RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs proliferated. To analyze the cytotoxic capacity, the different CAR approaches were co-cultured with CD19+ target cells for 48 hours (Fig. 3D). RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs showed high anti-CD19 cytotoxicity at various E:T ratios independent of TCR expression. At an E:T ratio of 1:1, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs showed a mean killing of 87.6%, 86.1% and 84.6%, respectively. Even at a very low E:T ratio of 0.04:1 more than 36% of the CD19-expressing target cells were killed by the CAR-T cells, indicating strong efficacy even at low T-cell concentrations.
Fig. 3. Functionality of RV19BBCRTRBC-CAR-T cells and alloreactive potential of TCR+ T cells in vitro.
(A-D) The functionality of RV19BBCRTRBC-CAR-T cells was compared to RV19BB_TCR+, RV19BBCRTCR+CARs and untransduced T cells (UTs) in different functionality assays. (A) 24 hours after co-culturing the CAR-T cells with CD19+ target cells in a 1:1 E:T ratio the cells were harvested and analyzed for expression of activation markers CD137 and CD25 (n=6). (B) Intracellular staining of IFNγ and TNFα was performed after 24 hours of co-culturing effector cells with CD19+ cells at an E:T of 1:1 (n=4). (C) CFSE labeled T cells were used to analyze the frequency of proliferating effector cells after contact with CD19+ target cells for 72 hours (n=3). (D) CD19+ target-cell killing was determined by flow cytometry after target cells were co-cultured for 48 hours with CAR-T cells in different E:T ratios (n=4). (E,F) Untransduced, CRTCR+, CRTRBC- T cells were co-cultured with allogeneic PBMCs (allo-PBMCs) pooled from six different donors and co-cultured at an E:T ratio of 1:5. (E) After 48 hours of co-culture, T cells were analyzed for surface expression of the activation markers CD69, CD25 and CD137 (n=3). (F) Percentage of proliferating T cells after contact with allogeneic PBMCs was analyzed after five days (n=3). A two-tailed paired t test or one-way ANOVA was performed to determine statistical significance. RV19BB_TCR+=conventional CAR-T cells without electroporation, RV19BBCRTCR+=CAR-T cells electroporated with nonsense gRNA, RV19BBCRTRBC-=CAR-T cells electroporated with TRBC-targeting gRNA, n.s.=not significant, E:T ratio=effector to target ratio.
For analysis of alloreactivity in vitro, CD19 expressing B cells are necessary for sufficient induction of alloreactivity and anti-CD19-reactivity becomes indistinguishable from alloreactivity for CAR-T cells in a mixed lymphocyte reaction. Instead, wildtype T cells with (CRTCR-) or without a TCR KO (CRTCR+) were analyzed for alloreactivity. Untreated T cells, CRTCR+ and CRTCR- T cells were co-cultured with irradiated PBMCs pooled from six different donors. After 48 hours of co-culture, expression of activation markers CD69, CD25 and CD137 was analyzed (Fig. 3E). Untreated and CRTCR+ showed a significant upregulation of CD69 (3.6-fold and 3.9-fold), CD25 (2.6-fold and 3.3-fold) and CD137 (6.1-fold and 7.0-fold) upon contact with allogeneic PBMCs (allo-PBMCs) compared to CRTCR- T cells, which showed no response to allo-PBMCs (CD69: 1.3-fold; CD25: 0.9-fold; CD137: 1.3-fold). A similar picture was seen for the proliferative response five days after allo-PBMC stimulation (Fig. 3F). Untreated T cells and CRTCR+ T cells showed elevated levels of proliferating cells ranging from 7.0% to 13.8% and 4.8% to 12.2% upon stimulation, whereas CRTCR- T cells showed almost no response after contact with allo-PBMCs (0.7% to 0.9% proliferating cells), pointing towards significantly reduced alloreactivity of TCR-deficient T cells.
Patient derived xenografts of CD19+ childhood ALL induce sufficient activation and target-cell killing of anti-CD19-CAR-T cells
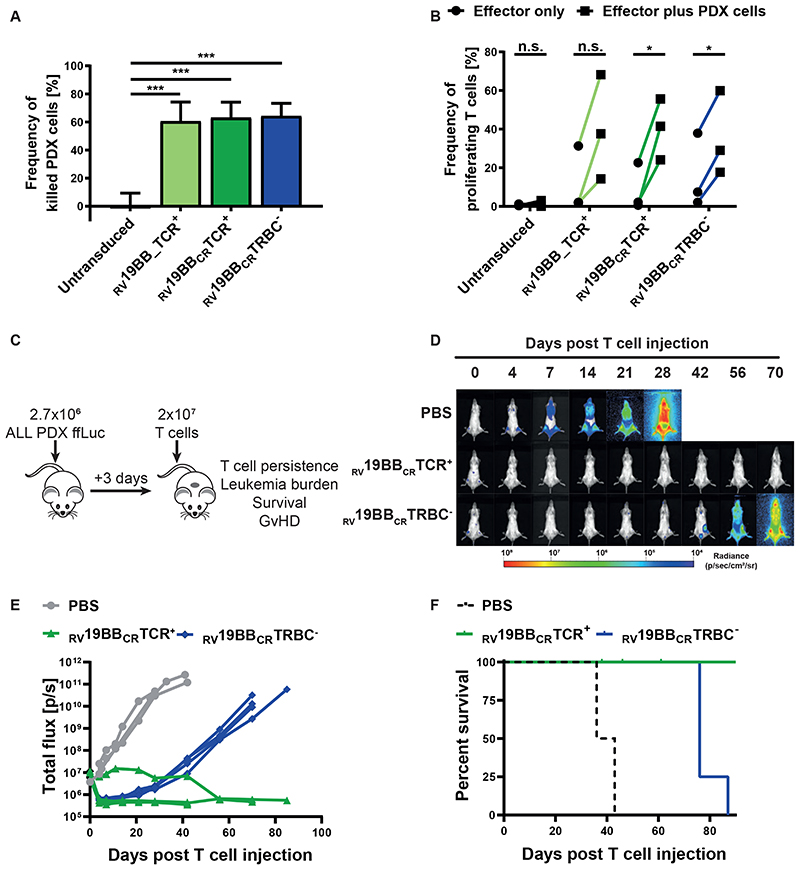
To investigate whether RV19BBCRTRBC- CAR-T cells can eliminate allogeneic, patient-derived tumor cells in vitro, patient-derived xenograft (PDX) leukemic cells were expanded in a murine model. Leukemic cells from a pediatric BCP-ALL patient, were transferred to NOD-scid IL2Rgammanull (NSG) mice, expanded over several passages and genetically modified to express enhanced firefly luciferase and eGFP as selection marker. 16,17 Passage 3 and passage 5 PDX cells were extracted from the bone marrow of NSG mice and expression of CD19 was confirmed by flow cytometry (Supplementary Fig. 1B). To determine the killing capacity of CAR-T cells, in vitro cytotoxicity assays at a 0.2:1 E:T ratio were performed (Fig. 4A). RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC-CARs showed a significantly increased killing (60.5%, 63.1% and 64.3%) of ALL-265 PDX cells compared to untransduced T cells (0.2%). Cytotoxicity of RV19BBCRTRBC- CAR-T cells was comparable to conventional CAR-T cells. We next investigated the proliferative capacity of RV19BBCRTRBC- CAR-T cells upon contact with ALL-265 PDX cells for 72 hours (Fig. 4B). RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC- CARs proliferated upon contact with ALL-265 PDX cells ranging from 14.2% to 68.2%, 24.1% to 55.6% and 17.0% to 59.9% of proliferating cells.
Fig. 4. CAR T cells against patient-derived xenograft (PDX) cells in vitro and long-lasting leukemia control in vivo.
(A) Untransduced T cells and CAR-T cells were co-cultured with CD19+ ALL-265 PDX cells at an E:T ratio of 0.2:1. Cytotoxicity was investigated 48 hours later (n=3). (B) Untransduced T cells, RV19BB_TCR+, RV19BBCRTCR+ and RV19BBCRTRBC-CARs were co-cultured with CD19+ ALL-265 PDX cells at an E:T ratio of 1:1. Proliferation was analyzed after 72 hours by flow cytometry (n=3). A two-tailed paired t test or one-way ANOVA was performed to determine statistical significance. (C) NSG mice were injected with ALL-265 PDX cells, followed by i.v. T-cell injection (2x107 cells) of RV19BBCRTCR+ CAR-T cells or RV19BBCRTRBC-CAR-T cells or PBS three days after. (D,E) At indicated time points after T-cell injection PDX leukemia burden was monitored by bioluminescence in vivo imaging. (D) Bioluminescence pictures and (E) quantification of leukemia burden are shown for mice suffering of ALL-265 PDX cell induced leukemia and treated with PBS (n=3), RV19BBCRTCR+ (n=5) or RV19BBCRTRBC- (n=4; see also Supplemental Figures). (F) Kaplan–Meier analysis of survival of mice treated with PDX cells. RV19BB_TCR+=conventional CAR-T cells without electroporation, RV19BBCRTCR+=CAR-T cells electroporated with nonsense gRNA, RV19BBCRTRBC-=CAR-T cells electroporated with TRBC-targeting gRNA, n.s.=not significant. A two-tailed paired t test was performed to determine statistical significance.
To analyze CAR-T-cell functionality in vivo, ALL-265 PDX cells were injected i.v. into NSG mice, followed by T-cell injection three days later (Fig. 4C). Mice were monitored for leukemia burden by bioluminescence. RV19BBCRTCR+ and RV19BBCRTRBC- CARs were able to immediately control growth of PDX ALL cells within 4 days below detection threshold (<1x106 P/sec) (Fig. 4D, E, Supplementary Fig. 2A). While PBS control mice had to be sacrificed between day 36 and 43 after T-cell injection due to endstage leukemia, RV19BBCRTRBC- CARs increased survival to at least 76 days (Fig. 4F). In contrast, mice treated with RV19BBCRTCR+ CARs completely controlled leukemia burden, but four mice had to be sacrificed between day 38 and 61 due to clinical signs of GvHD, such as skin rash and ruffled fur (pictures not shown) and an eye infection.
Presence of endogenous TCR significantly improves persistence of CAR-T cells in vivo
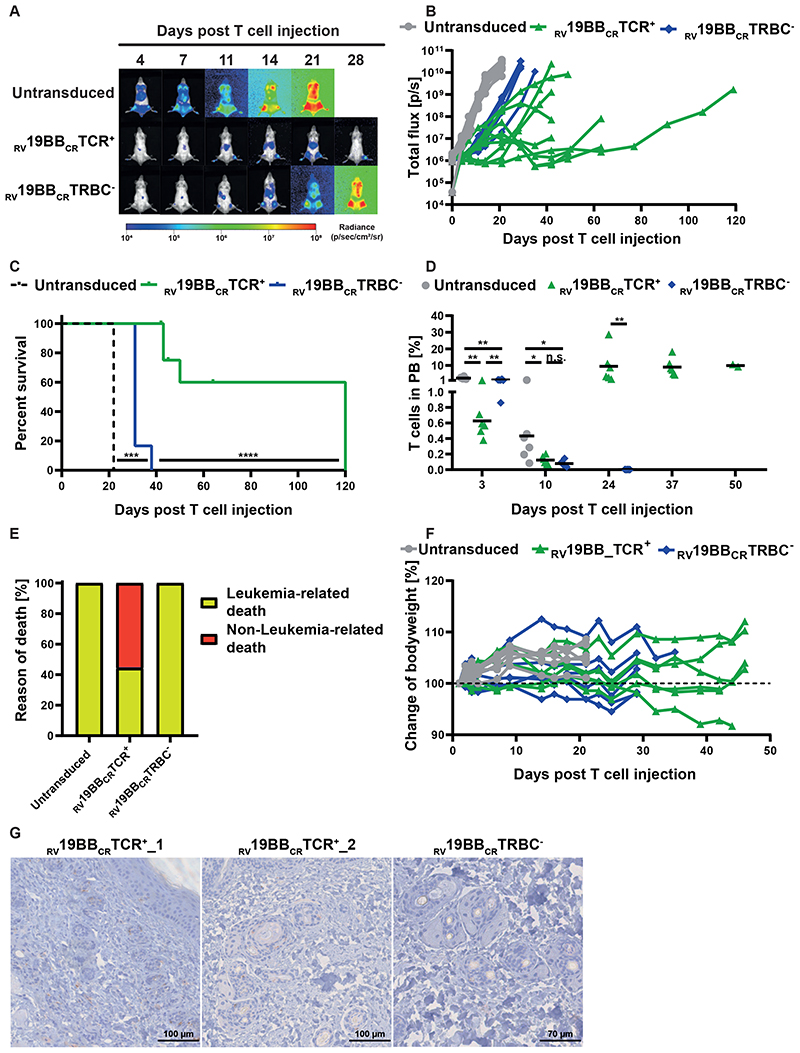
To further analyze CAR-T-cell functionality and the relevance of the endogenous TCR in vivo, we used the more aggressive NALM6 in vivo model and injected those i.v. in NSG mice, followed by T-cell injection three days later. Mice treated with untransduced T cells showed a fast increase in leukemia burden and had to be sacrificed 22 days after T-cell injection (Fig. 5A-C). In contrast, mice receiving RV19BBCRTRBC- or RV19BBCRTCR+ CARs showed a clear and comparable control of leukemia growth until day 14 post T-cell injection (average 2.4x107 P/sec vs. 1.8x107 P/sec) (Fig. 5A, B, Supplementary Fig. 2B). After day 14, treatment with RV19BBCRTCR+CARs led to an improved prevention of leukemia re-growth compared to RV19BBCRTRBC- CAR-T cells. Moreover, administration of RV19BBCRTCR+ CAR-T cells resulted in prolonged survival rates compared to RV19BBCRTRBC- CAR-T-cell treatment (Fig. 5C).
Fig. 5. Functionality of RV19BBCRTCR+ and RV19BBCRTRBC-CAR-T cells in vivo.
(A-H) NSG mice were injected i.v. with 1x105 NALM6, followed by i.v. T-cell injection (2x107 cells) of untransduced T cells (n=6), RV19BBCRTCR+ (n=9) and RV19BBCRTRBC- CAR-T cells (n=6) three days after. (A,B) At indicated time points after T-cell injection NALM6 leukemia burden was monitored by bioluminescence in vivo imaging. (A) Exemplary bioluminescence pictures are shown (B) as well as changes in NALM6 leukemia load for each mouse. (C) Kaplan–Meier analysis of survival of mice treated with 1x105 NALM6 cells (n=6 per group) is shown. A log-rank Mantel-Cox test was used to test for statistical significance. (D) T-cell persistence in peripheral blood was measured by FACS. A two-tailed Mann-Whitney test was performed to determine statistical significance. (E) Overview of incidence of leukemia- or non-leukemia related death in mice. (F) Bodyweight was monitored at several time points after T-cell injection. (G) Exemplary micrographs of skin tissue stained for cleaved caspase 3 (CC3) by IHC are shown for mice treated with RV19BBCRTCR+ or RV19BBCRTRBC- CARs. PB=peripheral blood, RV19BBCRTCR+=CAR-T cells electroporated with nonsense gRNA, RV19BBCRTRBC-=CAR-T cells electroporated with TRBC-targeting gRNA, n.s.=not significant.
Peripheral blood was regularly taken to analyze T-cell persistence (Fig. 5D). Untransduced T cells, RV19BBCRTCR+ CARs and RV19BBCRTRBC- CAR-T cells showed differences in engraftment three days after injection (mean of 2.4% vs. 0.6% vs 1.3%) and a reduction by day ten, with comparable T-cell amounts in mice treated with RV19BBCRTCR+ CARs and RV19BBCRTRBC- CAR-T cells (0.1% vs. 0.1%). The majority of analyzed T cells were CD4+, independent of time point and genotype (Supplementary Fig. 2C). In the further course, T cells showed strong expansion in RV19BBCRTCR+ CAR-T cell group, which was stable from day 24 on, whereas no T cells were detectable anymore in mice with RV19BBCRTRBC- CAR-T cell treatment after day 24. While all mice receiving untransduced T cells or RV19BBCRTRBC- CART cells had to be sacrificed due to high leukemia burden (6 out of 6 mice each), this only occurred in 44.4 % of the RV19BBCRTCR+ CAR-T cell group (4 out of 9 mice) (Fig. 5E). Instead, four mice were sacrificed as they showed signs of GvHD in absence of high tumor burden upon day 45 and 64 after T-cell injection, with slight weight loss (Fig. 5F), skin rash and ruffled fur (pictures not shown) and one mouse died during anesthesia. Cleaved Caspase 3 (CC3) staining of skin tissue was performed to monitor further signs of GvHD (Fig. 5G). In some mice treated with TCR+ CARs (RV19BBCRTCR+_1, RV19BBCRTCR+_2) several apoptotic cells surrounding hair follicles were detectable, while leukemia load was comparably low at the time point of sacrifice (data not shown). However, this could not be seen in all mice that had to be sacrificed due to weight loss or clinical signs of discomfort.
Taken together, TCR KO does not impair anti-leukemia activity of CAR T-cells in the xenograft mouse model in vivo. T-cell persistence depends on TCR-mediated alloreactivity, leading to long-term leukemia control with relevant risk of GvHD.
Discussion
In this study, we address the hurdles for off-the-shelf CD19-CAR-T cells against ALL using allogeneic donors. Relevance of the endogenous TCR is investigated using CRISPR/Cas9 knockout of the constant TCR β-chain. TCR-/CAR+ T cells were highly functional and showed no alloreactivity in vitro and in vivo compared to CAR-T cells with an endogenous TCR. CAR-T cells with or without a TCR demonstrated both improved survival rates in an ALL patient-derived xenograft mouse model as well as in a NALM6 leukemia bearing xenogeneic mouse model in comparison with mice treated with untransduced T cells. However, only in the presence of endogenous TCRs, the CAR-T cells showed prolonged and sustained persistence in vivo.
The TCR is essential for T-cell activation upon recognition of pathogens or tumor cells. Both chains, the α- and β-chain, are required for assembly of a TCRαβ and hence both knockout strategies targeting the α- or β-chain result in complete absence of the TCR. 12,18,20 Pilot experiments with TRAC and TRBC knockout, resulted in slightly higher TCR KO efficacy when targeting the TRBC locus (data not shown). Therefore, a TRBC-specific gRNA was used for further evaluation in this study. For this TRBC-specific gRNA, high on-target and low off-target events were previously confirmed. 18 Besides that, most of the trials investigated CARs with a TCR knockout are targeting the α-chain, e.g. with zinc-finger nucleases, TALEN or CRISPR/Cas9, so additional data for targeting the β-chain is of great interest. 8,9,20 Using TCR-deficient T cells, potentially impaired cellular function has to be excluded. Characteristics and functionality of TCR-deficient CARs were well preserved compared to conventional 2nd generation CAR-T cells in vitro with a balanced CD4/CD8-ratio and a promising phenotype of mainly Tcm and Tem, which are known to have high proliferative capacity as well as functionality in vivo. 1,21 Both TCR- - as well as TCR+ - CAR T cells showed a comparable fraction of NKT cells in the final cell product. In a previously published study, we could show that this is not mediated by the T-cell-isolation method, as both CD4/CD8 as well as an untouched T cell enrichment includes subpopulations of NKT-cells. 5 TCR-/CAR+ T cells highly expressed co-stimulatory molecules, which enhance CAR-T-cell functionality in the presence of their ligands. TCR-deficient CAR-T cells did not express relevant numbers of co-inhibitory molecules. Only TIM-3 was highly expressed on the final CAR-T-cell product, which is most likely mediated by anti-CD3/CD28-stimulation and IL-7/IL-15 supplemented cell culture media and could thus be a sign of activation, as shown before. 22 Based on recent data, the minor differences in expression of TIM-3 on TCR-deficient CARs compared to conventional CARs, are not expected to be of functional relevance. 23 Furthermore, exhaustion markers were analyzed and showed almost no surface expression, underlining the promising cellular characteristics of the TCR-deficient CAR-T cells. While conventional CAR-T cells showed high alloreactive potential, no alloreactivity of TCR-KO T cells was seen in vitro and in vivo.
In the presence of CD19+ target cells in vitro, TCR-deficient CAR-T cells displayed high functionality, with similar upregulation of activation markers, cytokine secretion, proliferative capacity and cytotoxicity compared to conventional CAR-T cells. This confirms that early CAR-T-cell activation is not dependent on endogenous TCR signals, but is rather mediated by the CAR-intrinsic CD3ζ and 4-1BB signaling domains. This was also supported through data from our in vivo model, where leukemia-bearing mice treated with RV19BBCRTRBC- or RV19BBCRTCR+ CARs showed comparable initial control of NALM6 and ALL265-PDX leukemia burden. Durable persistence of CAR-T cells in clinical studies has been described in patients with successful leukemia control 24 emphasizing that persistence of CAR-T cells is essential to control leukemia in the long term. Due to lack of persistence of RV19BBCRTRBC- CAR-T cells, mice treated with those T cells showed minor longterm leukemia control than mice receiving RV19BBCRTCR+ CAR-T cells. An association of CAR-T-cell persistence with alloimmune- or autoimmune T-cell activation through the TCR has not been described until now. However, TCR engagement negatively affected CD8 but not CD4 CAR-T-cell expansion and leukemic clearance in an immunocompetent syngeneic murine model. 25 Therefore, the endogenous TCR might play a role in activation or stabilization of the T cell and thereby prolong in vivo persistence and expansion. Specificity of these responses might be infectious or allogeneic (xenogeneic in case of mouse tissue), since several studies have shown induction of GvHD when human T cells are injected into NSG mice. 26–28 Furthermore, in patients treated with HLA-mismatched allogeneic TALEN-engineered TCR-deficient CAR-T cells, only a small fraction of contaminating TCR+-CAR-T cells persisted and induced GvHD. 8 Lapteva et al. could show that additional stimulation through a virus-specific TCR on CAR-T cells led to enhanced proliferation and functionality in patients, underlining the important role of the TCR. 10 Another study could show that CARs with CD3ζ transmembrane domains dimerize with endogenous TCR/CD3 complexes, probably resulting in phosphorylation of endogenous ITAMs and thereby strengthening CAR activation. 29 Constitutive tonic signaling in the absence of ligand is an increasingly recognized complication of engineered T cells and can be a cause of poor anti-tumor efficacy, impaired survival and reduced persistence in vivo. 30 This constitutive or chronic cell signaling may have a substantial deleterious impact on CAR-T-cell effector function and survival and may lead to a significant disparity between in vitro cytolytic capacity and in vivo anti-tumor efficacy. In our in vivo data, long-term control of ALL over >120 days through an RV19BBCRTCR+ CAR excludes predominance of this deleterious signaling and demonstrates capacity for sustained in vivo responses. However, impaired in vivo persistence of TCR-deficient CARs in mice, might be different in humans, as the human cytokine mileu and lymphoid tissue are different. 26,31 Given the diversity of human cytokine profiles in pretreated leukemia patients, 32 and the differences in stromal cells and secondary lymphoid organs, mouse models hardly reflect completely the situation in patients. Despite these limitations of the animal model, differences of in vivo persistence and leukemia control both reflect T-cell functionality.
In conclusion, we present a novel and efficient tandem engineering approach to generate anti-CD19 CART cells with a CRISPR/Cas9 mediated knockout of the TCRβ chain. TCR knockout leads to significant reduction of alloreactivity both in vitro and in vivo. TCR knockout in CAR-T cells is associated with a reduction of in vivo persistence. As allogeneic TCR-/CAR+ T cells show efficient early responses against leukemia cells, they might serve as temporary therapy until a successful generation of autologous CARs or bridge to transplant. The role and specificity of the endogenous TCR for the in vivo expansion of CAR-T cells remains to be investigated in clinical studies and opens new options to improve longevity of remissions after CAR-T-cell therapy in the future.
Supplementary Material
Significance.
CRISPR/Cas9-mediated T-cell-receptor knockout with anti-CD19-CAR expression enables allo-CAR-T-cell therapy
Co-expression of endogenous TCR and CD19-CAR prolong in vivo persistence of T cells
Acknowledgements and author contribution
We thank Tanja Weißer, Nicola Habjan, Nadine Stoll and Stefanie Stein for excellent technical assistance and Maike Fritschle, Annette Frank and Liliana Mura for mouse handling. This work was supported by the Kinderkrebshilfe Ebersberg e.V., Bettina Bräu Stiftung, Gesellschaft für Kinderkrebsforschung, Dr. Sepp und Hanne Sturm Gedaechtnisstiftung, Gertrud und Hugo Adler Stiftung and Gottfried Kieser-Stiftung. SW was supported by the Else-Kröner-Fresenius Stiftung and DS was supported by the German Cancer Research Center/German Cancer Consortium (DKTK). SK is supported by the European Research Council (Starting Grant 756017). IJ is funded by ERC Consolidator Grant 681524 and a Mildred Scheel Professorship from German Cancer Aid. The laboratory of TGPG is supported by grants from the Bettina Bräu Stiftung, the German Cancer Aid (DKH-70112257), and the Gert und Susanna Mayer Foundation. Experiments were performed by DS, TS, TK, SW and FB. Concept development, data analysis and manuscript preparation were done by DS, FB and TF. FR, SK, DS, TF, SB, FR and FB designed CAR constructs and performed retroviral transduction. KS, DHB and TS designed gRNAs and established the CRISPR/Cas9 protocol. BV and IJ performed in vivo experiments and established Luciferase-transgenic PDX mouse models. Irradiation was performed by BW, histology and immunohistochemistry by TGPG. The Visual Abstract was created with BioRender.com. The manuscript was reviewed by all authors. None of the authors has a relevant conflict of interest.
Literature
- 1.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary MC, Lu X, Huang Y, et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clinical Cancer Research. 2018 doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 5.Blaeschke F, Stenger D, Kaeuferle T, et al. Induction of a central memory and stem cell memory phenotype in functionally active CD4(+) and CD8(+) CAR T cells produced in an automated good manufacturing practice system for the treatment of CD19(+) acute lymphoblastic leukemia. Cancer immunology, immunotherapy : CII. 2018;67(7):1053–1066. doi: 10.1007/s00262-018-2155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. The New England journal of medicine. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feucht J, Kayser S, Gorodezki D, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Science translational medicine. 2017;9(374) doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 9.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapteva N, Gilbert M, Diaconu I, et al. T Cell Receptor Stimulation Enhances the Expansion and Function of CD19 Chimeric Antigen Receptor-Expressing T Cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019 doi: 10.1158/1078-0432.CCR-18-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson IC, Lenton KA, Little DJ, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34(16-17):1157–1165. doi: 10.1016/s0161-5890(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 12.Schober K, Müller TR, Gökmen F, et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nature Biomedical Engineering. 2019 doi: 10.1038/s41551-019-0409-0. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(9):2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chicaybam L, Barcelos C, Peixoto B, et al. An Efficient Electroporation Protocol for the Genetic Modification of Mammalian Cells. Front Bioeng Biotechnol. 2016;4:99. doi: 10.3389/fbioe.2016.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vick B, Rothenberg M, Sandhöfer N, et al. An Advanced Preclinical Mouse Model for Acute Myeloid Leukemia Using Patients’ Cells of Various Genetic Subgroups and In Vivo Bioluminescence Imaging. PLOS ONE. 2015;10(3):e0120925. doi: 10.1371/journal.pone.0120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heckl BC, Carlet M, Vick B, et al. Frequent and reliable engraftment of certain adult primary acute lymphoblastic leukemias in mice. Leukemia & Lymphoma. 2019;60(3):848–851. doi: 10.1080/10428194.2018.1509314. [DOI] [PubMed] [Google Scholar]
- 17.Ebinger S, Özdemir EZ, Ziegenhain C, et al. Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer cell. 2016;30(6):849–862. doi: 10.1016/j.ccell.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clinical Cancer Research. 2017;23(9):2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell–like properties. Nature medicine. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torikai H, Reik A, Liu P-Q, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graef P, Buchholz Veit R, Stemberger C, et al. Serial Transfer of Single-Cell-Derived Immunocompetence Reveals Stemness of CD8+ Central Memory T Cells. Immunity. 2014;41(1):116–126. doi: 10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Mujib S, Jones RB, Lo C, et al. Antigen-Independent Induction of Tim-3 Expression on Human T Cells by the Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Is Associated with Proliferation and Is Dependent on the Phosphoinositide 3-Kinase Pathway. The Journal of Immunology. 2012;188(8):3745–3756. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 23.Blaeschke F, Willier S, Stenger D, et al. Leukemia-induced dysfunctional TIM-3(+)CD4(+) bone marrow T cells increase risk of relapse in pediatric B-precursor ALL patients. Leukemia. 2020 doi: 10.1038/s41375-020-0793-1. [DOI] [PubMed] [Google Scholar]
- 24.Grupp SA, Maude SL, Shaw PA, et al. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019) Blood. 2015;126(23):681–681. [Google Scholar]
- 25.Yang Y, Kohler ME, Chien CD, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Science translational medicine. 2017;9(417) doi: 10.1126/scitranslmed.aag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B, Ren J, Luo Y, et al. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell reports. 2017;20(13):3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevanović S, Nijmeijer BA, van Schie MLJ, et al. Donor T Cells Administered Over HLA Class II Barriers Mediate Antitumor Immunity without Broad Off-Target Toxicity in a NOD/Scid Mouse Model of Acute Leukemia. Biology of Blood and Marrow Transplantation. 2013;19(6):867–875. doi: 10.1016/j.bbmt.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One. 2012;7(8):e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. Journal of immunology (Baltimore, Md : 1950) 2010;184(12):6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 30.Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurton LV, Singh H, Najjar AM, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences. 2016;113(48):E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazur B, Mertas A, Sońta-Jakimczyk D, Szczepański T, Janik-Moszant A. Concentration of IL-2, IL-6, IL-8, IL-10 and TNF-alpha in children with acute lymphoblastic leukemia after cessation of chemotherapy. Hematological Oncology. 2004;22(1):27–34. doi: 10.1002/hon.725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.