Abstract
Blood biomarkers have great potential to advance clinical care and accelerate trials in Alzheimer’s disease (AD). Plasma phospho-tau181 (p-tau181) is a promising blood biomarker however, it is unknown if levels increase in presymptomatic AD. Therefore, we investigated the timing of p-tau181 changes using 153 blood samples from 70 individuals in a longitudinal study of familial AD (FAD). Plasma p-tau181 was measured, using an in-house Single molecule array assay. We compared p-tau181 between symptomatic carriers, presymptomatic carriers, and non-carriers, adjusting for age and sex. We examined the relationship between p-tau181 and neurofilament light and estimated years to/from symptom onset (EYO), as well as years to/from actual onset in a symptomatic subgroup. Additionally, we studied associations between p-tau181 and clinical severity, as well testing for differences between genetic subgroups. Twenty-four were presymptomatic carriers (mean baseline EYO -9·6 years) while 27 were non-carriers. Compared with non-carriers, plasma p-tau181 concentration was higher in both symptomatic (p<0·001) and presymptomatic mutation carriers (p<0·001). Plasma p-tau181 showed considerable intra-individual variability but individual values discriminated symptomatic (AUC 0·93 [95% CI 0·85−0·98]) and presymptomatic (EYO ≥ -7 years) (AUC 0·86 [95% CI 0·72−0·94]) carriers from non-carriers of the same age and sex. From a fitted model there was evidence (p=0·050) that p-tau181 concentrations were higher in mutation carriers than non-carriers from 16 years prior to estimated symptom onset. Our finding that plasma p-tau181 concentration is increased in symptomatic and presymptomatic FAD suggests potential utility as an easily accessible biomarker of AD pathology.
Introduction
Historically, a definitive diagnosis of Alzheimer’s disease (AD) required histopathological confirmation of amyloid-β plaques and neurofibrillary tangles (NFT) comprised of hyperphosphorylated tau (1). Cerebrospinal fluid (CSF) biomarkers and positron emission tomography (PET) ligands for amyloid-β and tau have transformed this approach: facilitating a more secure diagnosis in clinical practice, in life and before dementia (2,3); improving screening for trials (4); and allowing earlier intervention (5). However, the invasiveness and cost of lumbar punctures and PET scans limits the availability of these biomarkers, especially in low-resource settings.
Blood-based biomarkers have advantages of ease, cost, acceptability and potential global applicability (6,7). However, blood is a more challenging matrix than CSF with lower analyte concentrations, as well as extra-cerebral analyte production and metabolism. Despite these challenges huge progress has occurred. Plasma neurofilament light (NfL) is now recognised as a robust, albeit non-specific, biomarker of neuronal damage (6). More recently tau phosphorylated at threonine 181 (p-tau181) has emerged as a promising blood biomarker of amyloid pathology (8). However, the ability of plasma p-tau181 to detect AD presymptomatically, and when and how concentrations change over time and in relation to NfL are still unclear.
Familial AD (FAD), caused by mutations in Presenilin 1/2 (PSEN1/2) and Amyloid Precursor Protein (APP), is highly penetrant with a reasonably consistent age at symptom onset within families (9). FAD provides a unique opportunity to better understand disease pathophysiology by allowing biomarker changes to be studied from presymptomatic stages through to clinical decline.
Using a longitudinal cohort study of FAD we assessed plasma biomarker changes testing whether p-tau181 concentration is altered in mutation carriers relative to non-carriers and if changes occur presymptomatically (10). We examined when changes in p-tau181 and NfL first discriminate between carriers and non-carriers. We examined the relationship between p-tau181 and clinical decline, and the influence of FAD genotype.
Methods
Study design and participants
We studied 70 participants within a longitudinal study of FAD at the Dementia Research Centre, UCL between 2010 and 2019; cohort details have been described previously (10). Eligibility was either (i) a clinical diagnosis of FAD or (ii) an FAD affected parent. At enrolment 19 participants were symptomatic, with pathogenic mutations in PSEN1 or APP genes; 51 individuals were asymptomatic but at 50% risk of inheriting a mutation and thereby of developing symptoms at a similar age to their affected parent.
Ethical approval was provided by the local Research Ethics Committee. Written informed consent was obtained from all participants or from a consultee if cognitive impairment precluded informed consent.
Procedures
FAD mutation status was determined using Sanger sequencing; results were provided only to statisticians, ensuring participants and clinicians assessing participants remained blind to mutation status. All individuals identified an informant who was interviewed separately for a collateral history. Estimated years from symptom onset (EYO) was calculated by subtracting the age at which the participant’s affected parent first developed progressive cognitive symptoms from the participant’s age at blood sampling.
Participants underwent a semi-structured health questionnaire (to rule out confounding illness) and a neurological examination. Participants were assessed using the mini mental state examination (MMSE) and the Clinical Dementia Rating (CDR) scale (11,12), which incorporates information from participant and informant on day-to-day cognition. Global CDR and CDR Sum of Boxes (CDR-SOB) scores were calculated. Individuals were defined as symptomatic if: i) cognitive decline was reported by participant and/or their informant; ii) the clinical impression was that the participant was experiencing progressive decline; iii) the global CDR was >0; and iv) an alternative cause of cognitive impairment was not identified. Neuropsychology tests performed included Recognition Memory (RMT) and Digit Symbol tests (13,14).
P-tau181 and neurofilament light quantification in plasma
Non-fasting plasma samples were collected throughout the day in 10ml ethylenediaminetetraacetic acid coated tubes. Samples were processed, aliquoted, and frozen at –80°C according to standardised procedures and shipped frozen to the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Gothenburg for analysis blinded to participants’ mutation status and clinical diagnosis. Samples from all time points were randomised and processed concurrently using the same kit and batch of reagents to maximise consistency. P-tau181 concentration was measured using an in-house Single molecule array (Simoa) method on the Simoa HD-1 instrument (Quanterix, Billerica, MA, USA). The assay is based on a mouse monoclonal antibody specific for the threonine-181 phosphorylation site (AT270, Invitrogen, Waltham, MA, USA) coupled to paramagnetic beads, in combination with an N-terminal anti-tau mouse monoclonal antibody for detection, and full-length recombinant tau 441 phosphorylated in vitro by GSK-3β as the calibrator, described in detail elsewhere (15). Intra-assay coefficients of variation (CoV) of a high (17·9 pg/mL) and a low (4·0 pg/mL) concentration quality control sample, each measured in duplicates at the start and end of each run, were below 10%. One result was identified as an unusually high (128 pg/mL) outlier and excluded, without knowledge of the individual’s mutation status. Plasma NfL was measured using the commercially available Simoa NfL assay on the HD-1 instrument following the manufacturer’s instructions: intra-assay and inter-assay CoVs of high (51·2 pg/mL) and low (6·8 pg/mL) quality control samples were < 7%.
Statistical analysis
Baseline (first visit) summary statistics and box plots of p-tau181 concentrations were separately produced for each group (symptomatic carriers, presymptomatic carriers, non-carriers). All other plots and analyses used data from all visits. Several participants exhibited large within-person changes in p-tau181 over short time intervals, making fitting mixed models problematic. Specifically, models for p-tau181 with a random intercept (at actual or expected onset, depending on the model) and a (correlated) random slope either didn’t converge or converged with the estimated correlation between slope and intercept being unity. Instead, linear regression models with robust Huber-White standard errors that allowed for clustering within individuals and families were fitted to the repeated p-tau181 measures (16,17). Clusters comprised individuals from the same family and mutation group. P-tau181 was log-transformed so normality assumptions concerning residuals were not materially violated; estimated coefficients were back-transformed and expressed as multiplicative effects. Three initially presymptomatic individuals became symptomatic during follow-up, with their group membership changed accordingly.
Age and sex adjusted differences in p-tau181 between groups were estimated, as were differences by genotype (PSEN1 vs APP, among all carriers) and by mutation position (pre- vs post-codon 200, among PSEN1 participants); these analyses were performed separately in presymptomatic and symptomatic carriers, the former models additionally adjusting for EYO, and the latter for actual years since symptom onset (as well as age and sex). For non-carriers only, models assessed associations between p-tau181 and, separately, age and sex.
To investigate p-tau181’s ability to discriminate i) non-carriers from symptomatic carriers and ii) non-carriers from presymptomatic carriers within 7 years of expected symptom onset, age- and sex-adjusted receiver operating characteristic (ROC) curves were plotted and areas under the curve (AUC) calculated, with bias corrected bootstrapped 95% confidence intervals (1 000 replications, cluster resampling).
The age and sex adjusted relationship between log p-tau181 and EYO was modelled. Given prior experience and earlier findings that CSF p-tau181 decreases after estimated age at symptom onset (18,19), we pre-specified the inclusion of mutation status and EYO, plus their interaction, as explanatory variables in the model, and additionally that we would investigate the inclusion of quadratic and cubic terms for EYO, plus their interaction with mutation status. In fact, there was no evidence to include these terms. The estimated geometric mean longitudinal p-tau181 concentration profiles for mutation carriers and non-carriers (and 95% confidence intervals) were plotted against EYO, standardised to a female aged 38·1 years (mean age of non-carriers). The curvature of the fitted relationship is a consequence of the assumed linear relationship with log-transformed values, which is no longer linear after back-transformation. In order to estimate the timepoint at which there is evidence of divergence between carriers and non-carriers we calculated the estimated difference in geometric mean p-tau181 between carriers and non-carriers, after adjusting for age and sex, for integer values of EYO between –20 and 10 years. The point when this estimated difference was statistically significantly different from zero (p≤0·05) was interpreted cautiously as an indication of where there was evidence that the estimated trajectory of p-tau181 over EYO for mutation carriers diverged from non-carriers. Separately, for participants with known symptom onset, p-tau181 was modelled as a function of years to/from onset, although here the p-value for the inclusion of a quadratic term was 0·057 and this was included. The modelled geometric mean longitudinal p-tau181 concentration profile (and its 95% confidence interval) for symptomatic carriers was plotted against actual years to/from onset, with the same age and sex standardisation as in the EYO graphs. In a sensitivity analysis we re-fitted this model omitting one participant with a large outlier value for known years to/from onset; the quadratic term was no longer statistically significant (p=0·264) but the estimated trajectory was similar.
For comparison we modelled the age and sex adjusted relationship between log NfL and i) EYO and ii) actual years to/from onset for participants with known symptom onset. For the former analysis the quadratic term gave a p-value of 0·057, while for the latter p-values for polynomial terms and their interactions with mutation status were far from 0·05, results consistent with earlier findings (18).
Finally, to investigate the relationship between log-transformed p-tau181 and cognition over time, age and sex adjusted linear regression models were fitted with MMSE, CDR-SOB, Digit Symbol and RMT-average (separately) as predictors, using data from all visits, first in all mutation carriers and then separately for the symptomatic and presymptomatic groups with clusters comprising individuals from the same family; the models with Digit Symbol and RMT-average additionally adjusted for years in education. Bias corrected and accelerated bootstrapped 95% confidence intervals were computed here.
All analyses used Stata v16.
Results
Baseline demographic and clinical characteristics are presented in Table 1. Fifty-one participants were asymptomatic (24 mutation carriers and 27 non-carriers). The mean age of the presymptomatic carriers, 37·2 years, was similar to the mean age of the control group, 38·1 years. Presymptomatic carriers were, as expected, younger than the symptomatic carrier group: the mean EYO of the presymptomatic carriers was - 96 years. There was no clinically relevant difference in MMSE score between non-carriers and presymptomatic carriers.
Table 1. Baseline characteristics (N=70).
| Non-carrier N=27 | Presymptomatic N=24 | Symptomatic N=19 | P-value | |
|---|---|---|---|---|
| Sex, n (%) Men Women | 11 (41%) 16 (59%) | 13 (54%) 11 (46%) | 12 (63%) 7 (37%) | 0·308 * |
| Age, years (mean (SD)) | 38·1 (10·7) | 37·2 (6·7) | 50·7 (10·0) | <0·001** |
| EYO, years (mean (SD)) | NA | -9·6 (7·2) | 4·5 (3·6) | <0·001** |
| MMSE (Median [IQR]) | 30 [30, 30] | 30 [29, 30] | 23 [16, 25] | <0·001** |
| CDR Global (Median [IQR] | 0 [0, 0] | 0 [0, 0] | 0·5 [0·5, 0·8] (3 missing values) | <0·001** |
| CDR SOB (Median [IQR]) | 0 [0, 0] | 0 [0, 0] | 3·5 [1·8, 4·3] (3 missing values) | <0·001** |
| Samples per participant (mean (SD)) | 2·2 (1·4) | 2·5 (1·1) | 1·7 (1·3) | NA |
| Interval 1 , years between p-tau181 samples (mean (SD)) | 1·7 (1·1) | 1·6 (1·0) | 1·4 (1·0) | NA |
| Duration of follow-up, years (mean (SD)) | 2·0 (2·3) | 2·5 (2·2) | 1·1 (2·0) | NA |
| P-tau181 (pg/ml) (mean (SD)) | 9·7 (9·3) | 16·9 (11·0) | 23·7 (10·5) | <0·0001‡ |
| NfL (pg/ml) (mean (SD)) 2 | 5·8 (2·0) | 8·7 (3·5) | 18·9 (10·1) | <0·0001‡‡ |
NA=not applicable.
Pearson Chi-squared test.
P-value for Kruskal-Wallis test across the three groups; post-hoc Dunn’s pairwise tests where appropriate.
Symptomatic carriers were: older than presymptomatic carriers (p<0·001) and non-carriers (p<0·001); had lower MMSE than presymptomatic carriers (p<0·0001) and non-carriers (p<0·0001); had higher CDR Global and CDR SOB than presymptomatic carriers (both comparisons p<0·0001) and non-carriers (both comparisons p<0·0001).
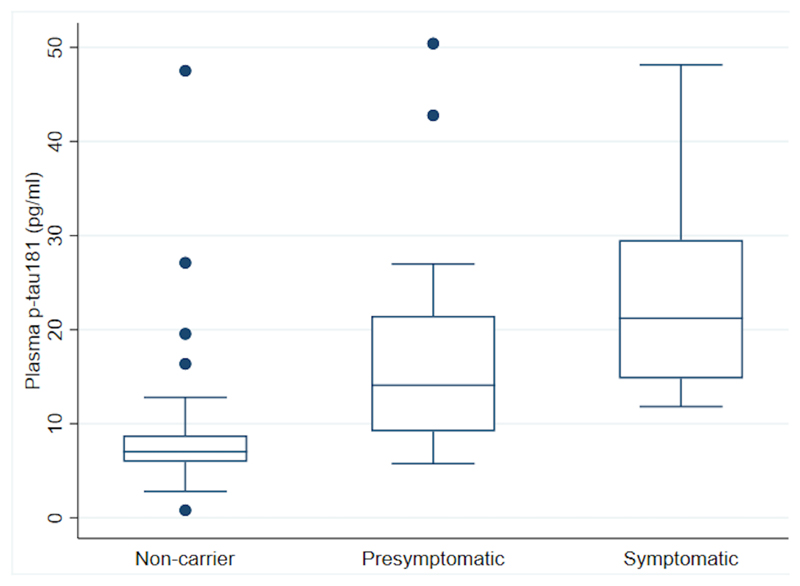
At baseline, symptomatic carriers had higher p-tau181 concentrations than presymptomatic carriers and non-carriers (p=0·005 and p<0·001, respectively); presymptomatic carriers had higher p-tau181 concentration than non-carriers (p=0·004); unadjusted comparisons carried out using a linear regression of log-transformed p-tau181 concentrations on participant group, with robust standard errors.
At baseline, symptomatic carriers had higher NfL concentrations than presymptomatic carriers and non-carriers (p<0·001 for both comparisons); presymptomatic carriers had higher NfL concentration than non-carriers (p=0·001); unadjusted comparisons carried out using a linear regression of log-transformed NfL concentrations on participant group, with robust standard errors.
The 32 participants with only one visit are not included (11 non-carriers; 7 presymptomatic mutation carriers; 14 symptomatic mutation carriers).
Thirty-one individuals only had NfL data for one visit. One ’At risk’ participant did not have a sufficient volume sample for a baseline NfL measure but did have a baseline p-tau181 value; their group is not identified to preserve blinding to genetic status.
Adjusting for age at visit and sex, concentrations of p-tau181 were significantly higher in both symptomatic and presymptomatic carriers compared with non-carriers (observed baseline data shown in Figure 1). The estimated percentage difference in mean p-tau181 between symptomatic carriers and non-carriers was 204% higher (95% CI:115%, 330%, p<0·001), and 73% higher (95% CI:22%, 145%, p<0·001) between presymptomatic carriers and non-carriers. Additionally, p-tau181 concentration was elevated in symptomatic compared to presymptomatic carriers (76% higher, 95% CI:20%, 158%, p<0·001). Within the control group a one-year increase in age was associated with a non-significant 0·6% increase in p-tau181 (95% CI:1·1% decrease, 2·4% increase; p=0·477). In this group there was no significant sex difference in p-tau181 with men having an estimated 19·2% higher concentration (95%CI:11·1% lower, 59·7% higher; p=0·224).
Figure 1. Box and whisker plots for observed baseline plasma p-tau181 concentration across the 3 groups.
The measured unadjusted plasma p-tau181 concentrations at baseline are shown. Mutation carriers have been divided into those who are symptomatic and those who are presymptomatic. A box shows the median, and 25th and 75th percentiles; the whiskers extend to the largest value less than or equal to the upper quartile + 1.5 times the interquartile range, and the smallest value greater than or equal to the lower quartile - 1.5 times the interquartile range; values outside this range are shown individually.
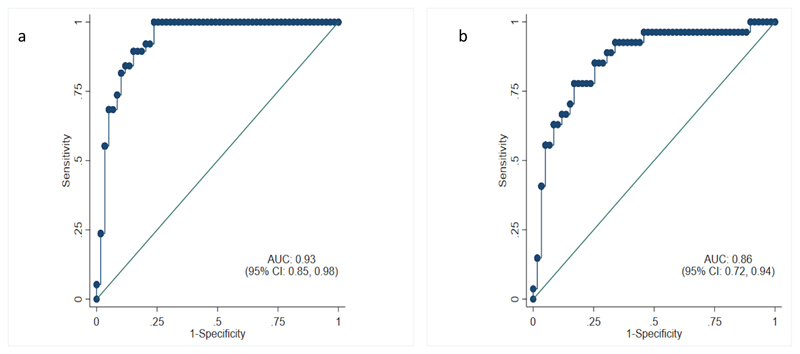
ROC analyses of p-tau181 (Figure 2) showed the ability of individual measures of p-tau181 to discriminate between symptomatic carriers and non-carriers of the same age and sex with an adjusted AUC of 0·93 (95% CI:0·85, 0·98); and discriminated between presymptomatic individuals within 7 years of estimated symptom onset and non-carriers of the same age and sex with an adjusted AUC of 0·86 (95% CI:0·72, 0·94).
Figure 2. Age- and sex-adjusted receiver operating characteristic (ROC) curves.
2a Symptomatic mutation carriers versus non-carriers. 2b Presymptomatic mutation carriers within 7 years of expected symptom onset versus non-carriers. Area under the curve (AUC) values are the probability that a randomly selected ’case’ (here, a symptomatic mutation carrier or a presymptomatic carrier within 7 years of expected onset, respectively) is ranked as being at greater risk of being a ’case’ than a randomly selected control of the same age and sex.
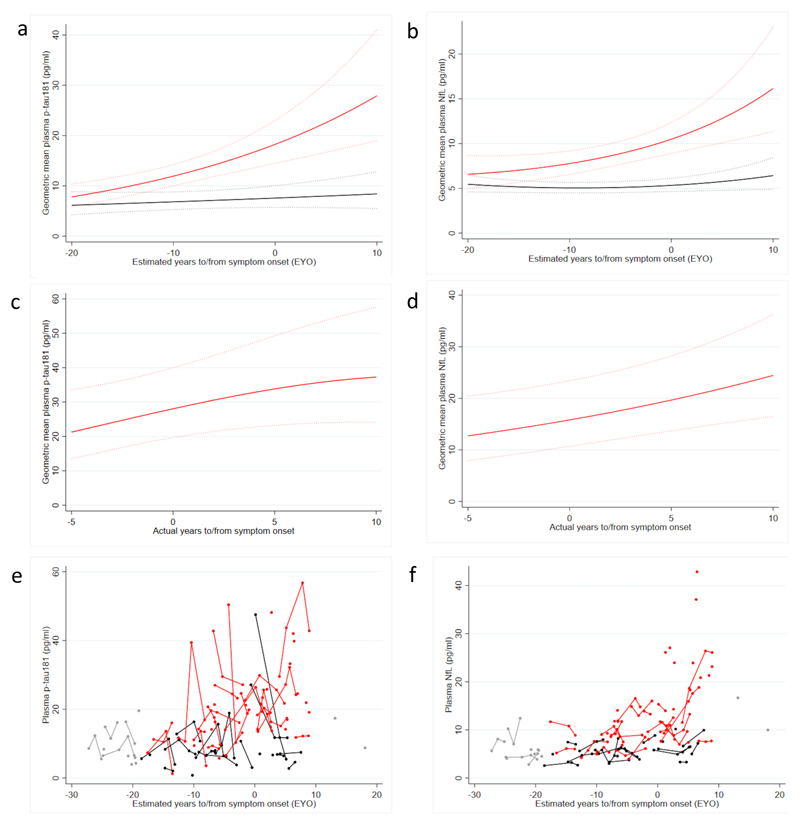
The difference in mean p-tau181 concentrations between all mutation carriers and non-carriers estimated from our model was first significant (p=0·050) 16 years prior to estimated symptom onset (Figure 3a, supplementary Table 1S), after adjusting for age and sex. Plasma NfL levels in carriers began to diverge from non-carrier levels at a similar time, becoming statistically significant at EYO of -17 years (p=0·033) (Figure 3b, supplementary Table 1S).
Figure 3. Trajectory of plasma biomarkers against estimated and actual years to/from onset.
Mutation carriers represented in red; non-carriers in black. Modelled changes in geometric mean plasma p-tau181 (a) and plasma NfL (b), for a hypothetical female aged 38·1 years (the average age of non-carriers), against estimated years to/from onset. Modelled changes in geometric mean plasma p-tau181 (c) and plasma NfL (d) against actual years to/from symptom onset for a hypothetical female carrier aged 38·1. There were 24 symptomatic subjects where actual age at onset was known, 19 of whom were already symptomatic at time of first plasma sampling and five subjects who became symptomatic after their baseline sample; for three of these plasma sampling took place before and after symptom onset. Dotted lines indicate 95% confidence intervals (a-d). Observed plasma p-tau181(e) and plasma NfL(e) concentrations against estimated years to/from symptom onset. To preserve blinding to genetic status of all observed values for timepoints more than 19 years before expected symptom onset and more than 10 years after expected symptom onset are shown in grey; some timepoints have been removed for at risk individuals who could be identified by their number of visits.
For 24 mutation carriers, age at symptom onset was known; modelling p-tau181 and NfL changes in this group showed broadly consistent results with their respective EYO models (Figure 3 c-d). We did not find any evidence of continuing progressive increases in p-tau181: directionally our analysis suggested deceleration on a logarithmic scale, although this was not formally statistically significant (p=0·057).
For several participants, there were large within-person changes in p-tau181 concentrations over relatively short time intervals; also seen, to a lesser extent, in NfL levels (Figure 3 e-f).
An analysis including all mutation carriers and all visit data found evidence of an association between p-tau181 and each of CDR-SOB, MMSE and RMT-average (Table 2). However, when the presymptomatic group was analysed separately only the association between p-tau181 and RMT-average remained statistically significant. In contrast, p-tau181 in the symptomatic group showed a statistically significant association with CDR-SOB and no evidence of an association with RMT-average. Investigating an association with MMSE in symptomatic carriers resulted in a bootstrap 95% confidence interval with an upper limit that was close to, but just included, zero. There was no evidence of association between p-tau181 and the Digit Symbol in any mutation group.
Table 2. Associations between p-tau181 plasma concentration and cognitive measures for mutation carriers.
Percentage (%) increase/decrease (95% CI) in p-tau181 for a one unit increase in the cognitive test score. All analyses adjust for age and sex; models for RMT-average and Digit Symbol additionally adjust for years of education.
| MMSE | CDR SOB | Digit Symbol | RMT-average | |
|---|---|---|---|---|
| N | MC: 43 PMC: 24 SMC: 19 |
MC: 40 PMC: 24 SMC: 16 |
MC: 30 PMC: 20 SMC: 10 |
MC: 35 PMC: 23 SMC: 12 |
| All mutation carriers (MC) | 4·5% decrease (2·6% decrease, 7·5% decrease)* |
16·2% increase (6·8% increase, 27·6% increase)* |
0·7% decrease (1·7% decrease, 0·1% increase) |
3·1% decrease (6·5% decrease, 0·8% decrease)* |
| Presymptomatic (PMC) | 12·0% decrease (31·5% decrease, 4·7% increase) |
Not applicable 1 | 0·1% decrease (1·6% decrease, 1·3% increase) |
3·4% decrease (12·8% decrease, 0·02% decrease)* |
| Symptomatic (SMC) | 1·6% decrease (3·2% decrease, 0·2% increase) |
10·5% increase (4·5% increase, 15·0% increase)* |
0·1% decrease (1·6% decrease, 1·3% increase) |
1·8% increase, (2·6% decrease, 5·4% increase) |
Significant associations i.e. confidence intervals do not include zero.
CDR SOB equal to zero for all but one observation in this group
N: Number with data for the cognitive variable; MC: mutation carrier; SMC: symptomatic mutation carrier; PMC: presymptomatic mutation carrier
To analyse the influence of genotype and mutation position on p-tau181, symptomatic and presymptomatic carriers were analysed separately. In the presymptomatic group (details Table 2S Supplementary), after adjusting for age, sex and EYO, there was no significant difference in p-tau181 concentration between APP and PSEN1 mutation carriers (p=0·284), nor between PSEN1 individuals with mutations pre- and post-codon 200 (p=0·844). Within the symptomatic group, after adjusting for age, sex and actual years since onset, there was no difference between APP and PSEN1 carriers (p=0·921). There was evidence (p=0·010) that symptomatic PSEN1 individuals with mutations beyond codon 200 had higher p-tau181 concentrations (55% higher; 95% CI:13%, 113%) than those with mutations before codon 200 (Figure 1S supplementary).
Discussion
In this longitudinal study of FAD, we showed that plasma p-tau181 concentration was significantly increased in symptomatic and in presymptomatic mutation carriers compared to non-carriers. Additionally, the symptomatic group had higher p-tau181 than the presymptomatic group suggesting that concentrations increase as the disease progresses across these stages.
We found a divergence in plasma p-tau181 concentration prior to the onset of cognitive decline, with a significant difference between mutation carriers and non-carriers from 16 years before estimated symptom onset. Changes in plasma NfL levels began at a similar time (EYO -17 years). The timing of these biomarker changes is in line with previous studies in FAD as well as being consistent with findings in sporadic AD where changes in plasma p-tau181 and NfL begin soon after changes in CSF amyloid-β (18–22).
We found an association between plasma p-tau181 and global cognition in symptomatic carriers. This association was not seen presymptomatically, and this is perhaps expected given that these individuals include those many years from onset with little or no decline in CDR-SOB and MMSE scores (23,24). However, we did see a relationship between p-tau181 concentration and presymptomatic performance on a measure of memory function (RMT-average), suggesting that the p-tau181 levels reflect pathological changes with relevant cognitive consequences.
When actual age at onset data was used we did not find evidence of continuing progressive increases in p-tau181 throughout the symptomatic period. This must be treated cautiously given the small numbers of symptomatic participants but it is consistent with previous CSF studies in sporadic and familial AD which report that p-tau181 does not continue to increase after symptom onset (19,25,26).
Elevated plasma p-tau181 concentrations distinguished symptomatic carriers from non-carriers with reasonable diagnostic accuracy (AUC >0·90). Studies in sporadic AD similarly found higher plasma p-tau181 concentrations in AD dementia suggesting this effect is not limited to FAD (8,27). P-tau181 increases may be relatively specific to AD as they have not been seen in frontotemporal dementia (28). In addition, we found that p-tau181 discriminated between presymptomatic carriers nearing symptom onset and non-carriers (AUC 0·86 [95% CI:0·72, 0·94]); this accords with reports of elevated p-tau181 levels in blood in cognitively normal amyloid positive individuals (8). Taken together, these findings suggest plasma p-tau181 may prove to be an accessible marker of AD pathology with potential for widespread use even in low resource settings. Given global efforts to improve diagnosis in dementia this is needed now but will be even more important with the advent of disease modifying therapies.
Symptomatic carriers had plasma p-tau181 concentrations on average about three times that of non-carriers with the presymptomatic group having a mean concentration between control and symptomatic groups, after adjusting for age and sex. These are remarkable ratios given this is a blood-based assay. Nonetheless, the overlap between groups, allied to the variability in concentration within individuals, suggests that plasma p-tau181 may be most useful in combination with blood biomarkers of amyloid, as seen in CSF where tau/amyloid-β ratios proved most useful in clinical practice.
We found plasma p-tau181 demonstrated considerable intra-individual variability; variability has previously been reported in NfL levels in the same cohort (18). It is unlikely that disease-related processes are solely responsible for these within-subject variations as changes were also seen in non-carriers. Timing of sampling may account for some volatility as intra-individual fluctuations have been shown in same-day CSF p-tau181 levels (29). Research is needed to understand and reduce intra-individual variability in plasma p-tau181 before it can be an effective screening measure.
The timing of changes in plasma p-tau181 contrasts with the timing of changes reported with tau PET. A study of FAD found that tau PET standardised uptake value ratios increased at the time of symptom onset rather than before (30). Similarly, in sporadic AD, CSF p-tau181 changes preceded tau PET increases (31). This may be due to differences in sensitivity of the techniques or alternatively tau-PET and p-tau181 changes may reflect different aspects of AD pathology. CSF and plasma p-tau181 may represent an amyloid response; increased soluble tau release has been reported in the presence of amyloid pathology (32). The timing of p-tau181 changes in this study supports the concept of alterations in tau processing being related to, or synergistic with, changes in cerebral amyloid (24).
In light of previously reported clinical, imaging and neuropathologic differences between APP and PSEN1 mutations carriers (10,33,34), we tested for the influence of genetic group on p-tau181 levels. After adjusting for age at visit, sex and disease stage, we did not find a difference in p-tau181 between APP and PSEN1 carriers, suggesting changes in p-tau181 are not gene-specific. We did find some evidence that symptomatic PSEN1 post-codon 200 carriers had higher p-tau181 concentrations than pre-codon 200 carriers. Although it is important to avoid over-interpreting this finding in such small numbers, it is interesting to note that carriers with mutations after codon 200 have more severe amyloid angiopathy and higher rates of neurofibrillary tangle accumulation (34,35). Heterogeneity in pathobiology may contribute to differences in p-tau181 concentration; further studies are, of course, needed.
Our study has limitations. First, the sample size, due to the rarity of FAD mutations, was relatively small and replication, especially of sub-group analyses, is needed. Secondly, we used parental age at symptom onset to estimate timing of future cognitive decline in carriers. While this provides a reasonable estimate of future age at onset (9), it is not without error due both to variability in age at onset between family members and to imprecision in determining the time of cognitive decline in a preceding, now often deceased, generation (10). However, we did perform an analysis of mutation carriers for whom an actual age at onset was known. Reassuringly the results of this subgroup analysis are consistent with changes in plasma p-tau181 occurring presymptomatically. Thirdly, we did not use PET or CSF data to confirm diagnosis or subgroup cases by amyloid or tau status. However, FAD is a well characterised disease in terms of its pathological changes and the diagnosis in affected individuals is relatively secure (24,36). Finally, this study is retrospective; prospective studies are needed to assess diagnostic accuracy and to investigate the physiological and pathophysiological drivers of variability in biomarker concentration (37,38). It will also be important to examine the impact of different genetic modifiers and in particular APOE. Studies in sporadic AD have shown that APOE4 is associated with higher CSF p-tau181 levels (39), however APOE has not been found to have a major impact on biomarker levels in FAD (19). The contribution of analytical factors will be important to investigate, however are unlikely to account for all the variability seen here as all samples were analysed in the same laboratory using the same batch of reagents. Given the relative ease of acquiring blood samples these prospective studies are likely to follow soon.
FAD provides a remarkable opportunity to examine and understand presymptomatic AD. The near 100% penetrance of these mutations and the lack of comorbidities or mixed pathology, means that biomarker changes can reliably be attributed to AD. Non-carriers offer a suitable control group with much in common with mutation carriers in terms of environmental and social backgrounds and they and the researchers are blind to mutation status. Nonetheless, findings in FAD may not necessarily generalise to sporadic disease in older individuals.
This paper provides insights into the timing of blood biomarker changes in AD. Plasma biomarkers may accelerate clinical trials however a greater understanding of variability is needed. Reliable and reproducible blood-based measures, including plasma p-tau181, would transform clinical practice and research in dementia.
Supplementary Material
Supplementary information is available at MP’s website.
Acknowledgements
AOC is supported by an Alzheimer’s Society clinical research training fellowship. T.K.K. was supported by the Swedish Alzheimer Foundation, the Swedish Dementia Foundation, Gamla Tjänarinnor, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, and the Anna Lisa and Brother Björnsson’s Foundation. NJA is funded by the Wallenburg Centre for Molecular and Translational. NSR is supported by a University of London Chadburn Academic Clinical Lectureship. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931) and the UK Dementia Research Institute at UCL. KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. This work was supported by the NIHR UCLH/UCL Biomedical Research Centre, the Rosetrees Trust, the MRC Dementia Platform UK and the UK Dementia Research Institute at UCL which receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK.
Footnotes
Contributors : AOC, NCF did the literature search. AOC, TKK, KB, HZ and NCF designed the study. AOC, PSJW, NSR, IP, and AK contributed to recruitment. Data were collected by AOC, PSJW and NSR. Blood samples were processed and analysed by AJH, EA, EC, IS, NJA, JLR, TKK and HZ. TP and CF carried out the statistical analysis. SM and JP contributed to the genetic analysis. TP created the figures. All authors were involved in the interpretation of results and writing the report.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the NINCDS-ADRDA work group★ under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984 Jul;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014 Jun 1;13(6):614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018 Apr 1;14(4):535–62. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman DS, Petersen RC, Jack CR. Neurology. Vol. 92. Lippincott Williams and Wilkins; 2019. A brief history of “Alzheimer disease”: Multiple meanings separated by a common name; pp. 1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: A new lexicon. The Lancet Neurology. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H, O’Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. 2018 Oct 8;:1. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zetterberg H, Bendlin BB. Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol Psychiatry. 2020 Apr 6;:1–13. doi: 10.1038/s41380-020-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020 Mar 2;26(3):379–86. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 9.Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014 Jul 15;83(3):253–60. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan NS, Nicholas JM, Weston PSJ, Liang Y, Lashley T, Guerreiro R, et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurol. 2016 Dec;15(13):1326–35. doi: 10.1016/S1474-4422(16)30193-4. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov 1;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Warrington E. Recognition Memory Test: Rmt.(Words) Test Booklet 1. 1984 [Google Scholar]
- 14.Weschler D. Wechsler Adult Intelligence Scale - Fourth Edition. Stat Solut. 2008:1–3. [Google Scholar]
- 15.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Phosphorylated tau 181 as a biomarker for Alzheimer’s disease : a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(May):1–12. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 16.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980 May;48(4):817. [Google Scholar]
- 17.Huber P. Behavior of maximum likelihood estimates under nonstandard conditions. Proc 5th Berkeley Sympt Math Statist Prob; 1967. [Google Scholar]
- 18.Weston PSJ, Poole T, O’Connor A, Heslegrave A, Ryan NS, Liang Y, et al. Longitudinal measurement of serum neurofilament light in presymptomatic familial Alzheimer’s disease. Alzheimers Res Ther. 2019 Dec 20;11(1):19. doi: 10.1186/s13195-019-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TLS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014 Mar 5;6(226) doi: 10.1126/scitranslmed.3007901. 226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019 Feb 21;25(2):277–83. doi: 10.1038/s41591-018-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmqvist S, Insel PS, Stomrud E, Janelidze S, Zetterberg H, Brix B, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol Med. 2019 Nov 11;:e11170. doi: 10.15252/emmm.201911170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller JT, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. 2020 Jun 1;19(6):513–21. doi: 10.1016/S1474-4422(20)30137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TL, Buckles V, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease CME Course. Neurol ® . 2018;91:1295–306. doi: 10.1212/WNL.0000000000006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 Aug 30;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013 Nov 29;126(5):659–70. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007 May 23;419(1):18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 27.Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. 2018;14(8):989–97. doi: 10.1016/j.jalz.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020 Mar 2;:1–11. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthélemy NR, Liu H, Lu W, Kotzbauer PT, Bateman RJ, Lucey BP. Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid. Ann Neurol. 2020 Feb 27; doi: 10.1002/ana.25702. ana.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon BA, Blazey TM, Christensen J, Dincer A, Flores S, Keefe S, et al. Tau PET in autosomal dominant Alzheimer’s disease: relationship with cognition, dementia and other biomarkers. Brain. 2019;142(4):1063–76. doi: 10.1093/brain/awz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson N, Scholl M, Strandberg O, Smith R, Palmqvist S, Insel PS, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med. 2017;9(9):1212–23. doi: 10.15252/emmm.201707809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018 Mar 21;97(6):1284–1298.e7. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scahill RI, Ridgway GR, Bartlett JW, Barnes J, Ryan NS, Mead S, et al. Genetic Influences on Atrophy Patterns in Familial Alzheimer’s Disease: A Comparison of APP and PSEN1 Mutations. J Alzheimer’s Dis IOS Press RI Scahill al / Genet Influ Atrophy FAD. 2013;35:199–212. doi: 10.3233/JAD-121255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringman JM, Monsell S, Ng DW, Zhou Y, Nguyen A, Coppola G, et al. Neuropathology of Autosomal Dominant Alzheimer Disease in the National Alzheimer Coordinating Center Database. J Neuropathol Exp Neurol. 2016 Mar 1;75(3):284–90. doi: 10.1093/jnen/nlv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan NS, Biessels G-J, Kim L, Nicholas JM, Barber PA, Walsh P, et al. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial Alzheimer’s disease. Neurobiol Aging. 2015 Dec;36(12):3140–51. doi: 10.1016/j.neurobiolaging.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006 doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 37.Boccardi M, Gallo V, Yasui Y, Vineis P, Padovani A, Mosimann U, et al. Neurobiology of Aging. Vol. 52. Elsevier Inc; 2017. The biomarker-based diagnosis of Alzheimer’s disease. 2—lessons from oncology; pp. 141–52. [DOI] [PubMed] [Google Scholar]
- 38.Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 2017 Aug 1;16(8):661–76. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 39.Toledo JB, Zetterberg H, van Harten AC, Glodzik L, Martinez-Lage P, Bocchio-Chiavetto L, et al. Alzheimer’s disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain. 2015 Sep;138(Pt 9):2701–15. doi: 10.1093/brain/awv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.