Summary
Background
Dislocation following total hip replacement (THR) is associated with repeated hospitalisations and substantial costs to the health system. Factors influencing dislocation following primary THR are not well understood. We aimed to assess the associations of patient-, surgery-, implant- and hospital-related factors with dislocation risk following primary THR.
Methods
We did a systematic review and meta-analysis of all longitudinal studies reporting these associations. We searched MEDLINE, Embase, Web of Science, and Cochrane Library to March 8, 2019. Summary measures of association were calculated using relative risks (RRs) (with 95% confidence intervals, CIs). The review is registered on PROSPERO, number CRD42019121378.
Findings
We identified 149 articles based on 125 unique studies with data on 4 633 935 primary THRs and 35 264 dislocations. The incidence rates of dislocation ranged from 0·12% to 16·13%, with an overall pooled rate of 2·10% (1·83-2·38) over a weighted mean follow-up duration of 6 years. Using median year of data collection, there was a significant decline in dislocation rates from 1971 to 2015. Comparing males vs females, age ≥70 vs <70 years, and high vs low income, RRs (95% CIs) for dislocation were 0·97 (0·88-1·08), 1·27 (1·02-1·57), and 0·79 (0·74-0·85) respectively. White ethnicity, drug use disorder, and social deprivation were each associated with an increased dislocation risk. Comparing body mass index (BMI) ≥30 vs. <30 kg/m2, the RR (95% CI) for dislocation was 1·38 (1·03-1·85). Medical and surgical history-related factors associated with dislocation risk included neurological disorder, psychiatric disease, comorbidity indices, previous surgery including spinal fusion, and surgical indications including avascular necrosis, rheumatoid arthritis, inflammatory arthritis, and osteonecrosis. Surgical factors such as the anterolateral, direct anterior, or lateral approach and posterior with short external rotator and capsule repair were each associated with reduced dislocation risk. At the implant level, larger femoral head diameters, elevated acetabular liners, dual mobility cups, cemented fixations and standard femoral neck lengths reduced the risk of dislocation. Hospital-related factors such as experienced surgeons and high surgeon procedure volume each reduced the risk of dislocation.
Interpretation
Dislocation following primary THR is on a temporal decline. Surgical approaches that reduce dislocation risk can be used by clinicians when performing primary THR. Alternative bearings such as dual mobility can be used in individuals at high risk of dislocation. Modifiable risk factors such as high BMI and comorbidities may be amenable to optimisation prior to surgery.
Funding
National Institute for Health Research
Keywords: risk factor, dislocation, instability, primary total hip replacement, systematic review, meta-analysis
Introduction
Total hip replacement (THR) is a common, successful and cost-effective intervention for improving pain and disability associated with advanced hip joint disease such as osteoarthritis. 1–4 Despite high success rates, some replacements inevitably fail. Common indications for revision after primary THR include aseptic loosening, dislocation, prosthetic joint infection, fracture, and adverse reaction to particulate debris. 5 In 2017, of the 8,589 revision procedures recorded in the National Joint Registry of England, Wales and Northern Ireland and the Isle of Man, 44% were for aseptic loosening, 16% for dislocation, 16% for infection and 15% for periprosthetic fracture. 5 The incidence of dislocation has been reported to range from less than 1% to as high as 22%. 6–11 More than half of dislocations occur in the first three months following primary THR. 12–14 As the population ages and with growing volumes of primary THR, it is estimated that there will be a proportionate rise in the number of complications and revisions, 15,16 including revision for dislocations. Dislocation can result in severe pain, restriction of mobility, recurrent dislocation, and poor quality of life. 17 The associated consequences of dislocation include repeated hospitalisations and substantial financial burden to both patient and healthcare system. 11,18 It has been reported that that an early dislocation increased the cost of a primary THR by 342% 19 and a recurrent dislocation increased costs by 300% compared with an isolated episode of dislocation. 17
There is therefore a need to maximise the delivery of efficient and cost-effective healthcare. Hence, it is imperative to identify relevant factors which influence the risk of dislocation in order to counsel patients, guide surgeons and healthcare providers to plan effectively and mitigate risks. Data from revision THRs suggest that several patient-, surgery- and implant-related factors influence dislocation rates. 20,21 Given the differing risk profiles, it is uncertain if these factors also apply to primary THR. Individual studies are not always definitive, as some are often poorly powered to adequately quantify the nature and magnitude of the associations and findings are not always inconsistent. Previous reviews on the topic have often only focused on single or a few risk factors, used narrative approaches to summarise the data or did not conduct detailed exploration of potential sources of bias such as heterogeneity and publication bias. 22–26 Furthermore, several relevant individual studies have been published since the publication of these reviews. There is therefore a need for a comprehensive quantitative synthesis of the evidence in one single investigation, which will bring together the results of many different individual studies as well as findings of reviews based on single risk factors to disentangle any inconsistencies in the evidence. Given the existing evidence, we hypothesise that a wide range of patient-, surgery-, implant- and hospital-related factors will influence the risk of dislocation following primary THR.
In the absence of data from a carefully designed and adequately powered large-scale study, we aimed to comprehensively assess the nature and magnitude of the longitudinal associations of patient-, surgery-, implant- and hospital-related factors with the risk of dislocation following primary THR by conducting a systematic meta-analysis of published studies. Given the variable incidence rates of dislocation reported in the literature, 6–11 a secondary aim was to pool and characterise temporal trends in incidence rates of dislocation across identified studies.
Methods
This review was based on a pre-defined protocol which was registered in the prospective register of systematic reviews, PROSPERO (CRD42019121378) and was conducted in accordance with PRISMA and MOOSE guidelines 27,28 (appendix pp 2-4). Detailed description of data sources and search strategy, eligibility criteria, and data extraction and quality assessment methods are reported in appendix pp 5-6.
Statistical analyses
We pooled the incidence rate for dislocation (estimated from the number of dislocations within average follow-up period/total number of participants or procedures as reported) with 95% confidence intervals (CIs). Temporal trends in dislocation rates were evaluated using the median year of data collection/surgery reported by studies, as previously reported. 29 Relative risks (RRs) with 95% CIs were used as summary measures of associations. Detailed statistical analyses are described in appendix pp 9. The statistical analyses employed Stata MP 16 (Stata Corp, College Station, Texas, USA).
Role of the funding source
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. SKK had full access to all the data in the study and had final responsibility for the decision to submit for publication
Results
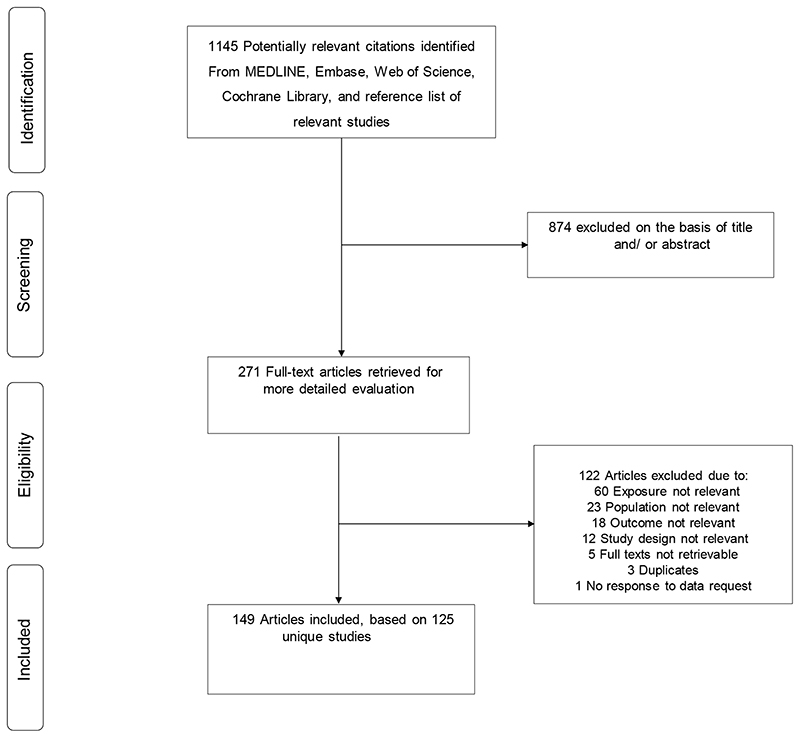
The literature search strategy and manual screening of references identified 1145 potential articles relevant to the review. After screening of titles and abstracts, 271 articles remained for detailed full text evaluation. Following evaluation, 122 articles were excluded because (i) the exposure was not relevant or was assessed after THR (n=60); (ii) the population was not relevant (n=23); (iii) the outcome was not relevant (n=18); (iv) study design was not relevant (n=12); (v) full texts could not be retrieved (n=5); (vi) duplicate of another study already included in review (n=3); and (vii) no response to data request (n=1). The remaining 149 articles corresponding to 125 unique studies were eligible to be included in the review (figure 1; table 1; appendix pp 11-20).
Figure 1. PRISMA flow diagram.
Table 1. Summary characteristics of the 125 unique studies.
| Characteristics | |
|---|---|
| Participants | |
| Total number of total hip replacements | 4 633 935 |
| Total number of dislocation cases | 35 264 |
| Study characteristics | |
| Location | Number of studies (%) (Number of THRs) |
| North America | 52 (41.6%) (3 216 656) |
| Asia | 15 (12.0%) (14 691) |
| Europe | 53 (42.4%) (1 000 150) |
| Pacific | 5 (4.0%) (402 438) |
| Study design | Number of studies (%) (Number of THRs) |
| Retrospective cohorts | 82 (65.6%) (4 151 752) |
| Prospective cohorts | 28 (22.4%) (476 674) |
| Nested case-control studies | 6 (4.8%) (4148) |
| Randomised controlled trials | 9 (7.2%) (1361) |
| Weighted mean follow-up (SD; min-max), years | 5·96 (4.25; 0·05-14·00) |
| Median (IQR) study quality score for observational studies | 7 (6-8) |
| Study level participant characteristics | |
| Weighted mean age (SD; min-max), years | 66·4 (1.7; 42·8-78·0) |
| Median (IQR) % males | 44·0% (37·3-47·0) |
IQR=interquartile range; SD, standard deviation; THR, total hip replacement
The 125 unique studies comprised of 116 (92.8%) observational designs and 9 (7.2%) RCTs. Publication dates of included studies ranged from 1975 to 2019. A summary of the key characteristics of eligible studies is presented in table 1. Relevant baseline characteristics and quality assessment scores (for observational studies) of the individual articles/studies are summarised in appendix pp 21-25. Overall, the 125 studies involved 4 633 935 primary THRs and 35 264 dislocations. The 116 observational studies included 4 632 574 primary THRs and 35 223 dislocations. Altogether, the 9 RCTs comprised 1361 primary THRs and 41 dislocations. Overall, 53 (42.4%) studies were conducted in Europe (Denmark, Finland, Norway, Sweden, France, Germany, Italy, Lithuania, Switzerland, The Netherlands, Spain, and UK), 52 (41.6%) in North America (USA and Canada), 15 (12.0%) in Asia (China, Japan, South Korea, and Taiwan), and 5 (4.0%) from the Pacific region (Australia). The average baseline age of participants in the included studies ranged from 42·8 to 78·0 years and the weighted mean age was 66·4 years. The average duration of follow-up for dislocation outcomes reported by studies ranged from 5 days to 14 years, with a weighted mean follow-up duration of 6 years. Methodological quality of observational studies ranged from 5-8 using NOS. Using the Cochrane risk of bias tool, 7 of 9 trials demonstrated a high risk of bias within 1-4 areas of study quality (blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other bias). All trials had a low risk of bias for selective reporting. Four trials had an unclear risk of bias in random sequence generation and allocation concealment. Two trials had a low risk of bias in all but one area of study quality (appendix pp 26).
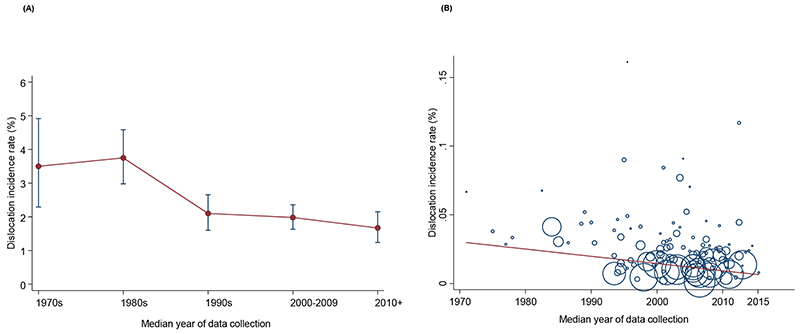
Across 112 studies with relevant data over a weighted mean follow-up duration of 6 years, the incidence rates of dislocation ranged from 0·12% to 16·13%. The pooled random effects incidence rate (95% CI) over this follow-up duration was 2·10% (1·83-2·38) (appendix pp 27). The 95% prediction interval for the summary incidence rate was 0·25 to 5·41%, suggesting that the true incidence rate for any single new study will usually fall within this range. Comparing reported dislocation endpoints by case series and registries, the incidence rate of dislocation was 2·28% (1·93-2·66) over a weighted mean follow-up duration of 5·8 years and that for revision for dislocation was 0·88% (0·66-1·12) over a weighted mean follow-up duration of 6·5 years. The pooled incidence rate of dislocation at specific average follow-up periods reported by studies was 0·00% (0·00-0·00) at < 30 days (inpatient stay), 1·32% (1·14-1·50) at 30 days, 1·21% (0·97-1·48) at 3 months, 1·91% (1·39-2·49) at 6 months, 2·12% (1·19-3·28) at 1 year, 1·99% (1·57-2·45) at 2 years, 1·76 (1·52-2·02) at 5 years, and 3·60% (2·42-4·99) at 10 years (appendix pp 28). There was a decrease in dislocation rates from 1971 to 2015, based on the median year of data collection/surgery (figure 2A) and the decline was significant in a meta-regression analysis (p=0·016) (figure 2B).
Figure 2. Temporal trends in dislocation rates following primary total hip replacement.
A, Incidence of dislocation by median year of data collection; B, Meta-regression bubble plot of incidence of dislocation against median year of study data collection; capped vertical bars represent 95% confidence intervals
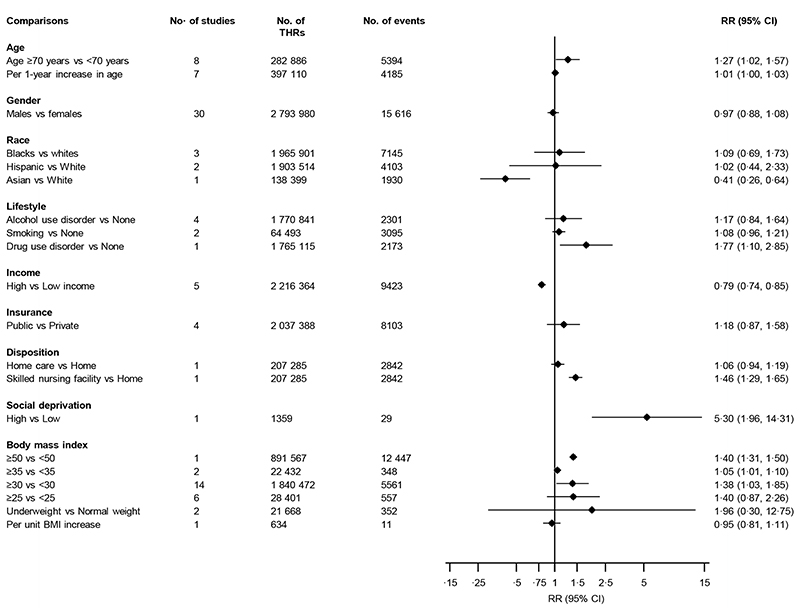
Figure 3 provides a summary of the associations of several sociodemographic characteristics and body mass index (BMI) with the risk of dislocation. Older age was associated with an increased risk of dislocation: RRs (95% CIs) of 1·27 (1·02-1·57) and 1·01 (1·00-1·03) comparing age ≥70 years vs <70 years (8 studies) and per one-year increase (7 studies) respectively. Comparing males with females in 30 studies, the pooled RR (95% CI) for dislocation was 0·97 (0·88-1·08) (figure 3; appendix pp 29). There was evidence of substantial heterogeneity between studies contributing gender data (I 2 =80%; 95% CI 73, 86%; p<0·001), which was partly explained by degree of adjustment (p for meta-regression=0·044) and study methodological quality (p for meta-regression<0·001) (appendix pp 30). Comparing high to low income (5 studies), the pooled RR (95% CI) for dislocation was 0·79 (0·74-0·85). There was no difference in the risk of dislocation comparing public with private insurance/funding (4 studies); RR (95% CI) of 1·18 (0·87-1·58). Alcohol use disorder and smoking status were not associated with the risk of dislocation. Results from single reports showed that White race/Caucasians (compared to Asian race), social deprivation, drug use disorder and care in a skilled nursing facility (compared to care at home) after the surgery, were each associated with an increased risk of dislocation (figure 3).
Figure 3. Associations of sociodemographic characteristics and body mass index comparisons with risk of dislocation.
BMI, body mass index; CI, confidence interval (bars); RR, relative risk; THR, total hip replacement
The associations of BMI by specified categories with the risk of dislocation were reported in 22 studies (figure 3; appendix pp 31). In pooled analysis of 14 studies, the RR (95% CI) for dislocation comparing BMI ≥30 vs <30 kg/m2 was 1·38 (1·03-1·85). There was evidence of heterogeneity between these contributing studies (I 2 =64%; 95% CI 36, 80%; p=0·001), which was partly explained by the sample size (p for meta-regression=0·02) and methodological quality of studies (p for meta-regression<0·001) (appendix pp 32). The pooled RR (95% CIs) for dislocation in two studies comparing BMI ≥35 vs <35 kg/m2 was 1·05 (1·01-1·10). Comparing BMIs ≥25 vs <25 and underweight vs normal weight, there were no significant associations. Findings from single reports showed that assessment of BMI as a continuous variable was not associated with an increased risk of dislocation per unit BMI increase, whereas comparing BMI ≥50 vs <50 kg/m2 was associated with an increase in dislocation risk RR (95% CI) of 1·40 (1·31-1·50). A single study assessed the association between height and dislocation risk and reported a RR (95% CI) of 1·03 (0·98-1·07) per 1 cm increase in height.
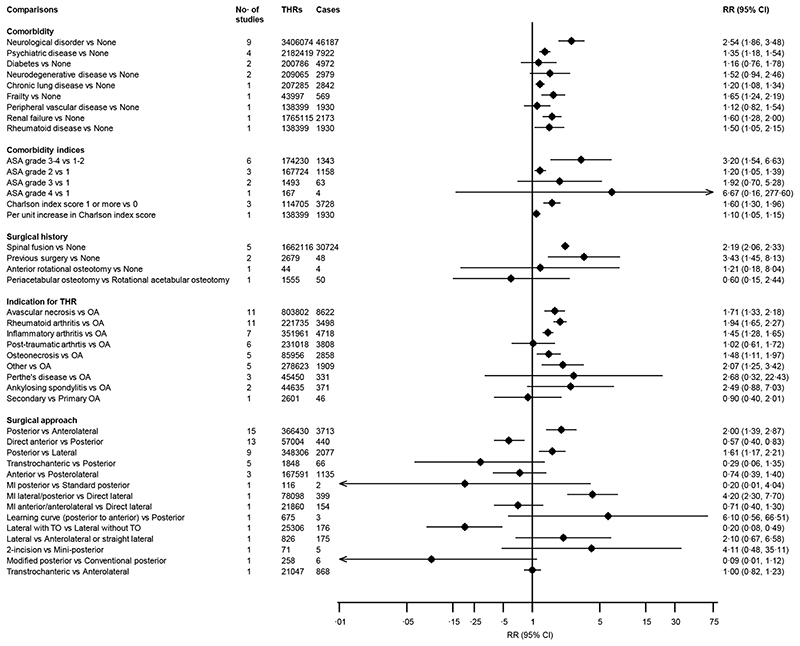
The associations of several medical and surgical history characteristics as well as surgical approaches with the risk of dislocation are reported in figure 4. In pooled analysis of 9 studies, a history of a neurological disorder was associated with an increased risk of dislocation RR (95% CI) 2·54 (1·86-3·48) (figure 4; appendix pp 33). In pooled analysis of 4 studies, a history of psychiatric disease was associated with an increased risk of dislocation, RR (95% CI) 1·35 (1·18-1·54) (figure 4). In single reports, there was evidence of statistically significant associations of dislocation with histories of frailty, chronic lung disease, renal failure, and rheumatoid disease whereas diabetes, neurodegenerative disease, and peripheral vascular disease were not associated. The following comorbidity indices comparisons: American Society of Anesthesiologists (ASA) grade 3-4 vs 1-2; ASA grade 2 vs 1; Charlson Comorbidity Index (CCI) 1 or more vs 0; and per unit increase in CCI, were each associated with an increase in risk of dislocation (figure 4). Comparing a previous spinal fusion vs none and previous surgery of the hip vs none, the RRs (95% CIs) for dislocation were 2·19 (2·06-2·33) and 3·43 (1·45-8·13) respectively. In evaluation of surgical indications for primary THR, avascular necrosis (11 studies), rheumatoid arthritis (11 studies), inflammatory arthritis (7 studies), and osteonecrosis (5 studies) were each associated with an increased risk of dislocation when compared with osteoarthritis: RRs (95% CIs) of 1·71 (1·33-2·18), 1·94 (1·65-2·27), 1·45 (1·28-1·65) and 1·48 (1·11-1·97) respectively (figure 4; appendix pp 34-35). Between-study heterogeneity values for studies contributing data for avascular necrosis and rheumatoid arthritis were (I 2 =72%; 95% CI 49, 85%; p<0·001) and (I 2 =19%; 95% CI 0, 59%; p=0·26) respectively. Study level characteristics such as geographical location, endpoint (dislocation vs revision for dislocation), and sample size seemed to be partly responsible for the substantial heterogeneity among studies contributing data for avascular necrosis (appendix pp 36).
Figure 4. Associations of medical and surgical history comparisons and surgical approaches with risk of dislocation.
ASA, American Society of Anaesthesiologists; CI, confidence interval (bars); OA, osteoarthritis; RR, relative risk; THR, total hip replacement; TO, trochanteric osteotomy
In pooled analyses of the following surgical approach comparisons: posterior vs anterolateral (15 studies); direct anterior vs posterior (13 studies); and posterior vs lateral (9 studies), RRs (95% CIs) of dislocation were 2·00 (1·39-2·87); 0·57 (0·40-0·83); and 1·61 (1·17-2·21) respectively (figure 4). Between-study heterogeneity estimates for studies contributing data for surgical approach comparisons: posterior vs anterolateral and direct anterior vs posterior were (I 2 =90%; 95% CI 86, 93%; p<0·001) and (I 2 =25%; 95% CI 0, 61%; p=0·20) respectively (appendix pp 37-38). None of the study-level characteristics explored explained the substantial heterogeneity between studies contributing data for posterior vs anterolateral surgical approaches (appendix pp 39). Summary associations of other surgery-related factors with the risk of dislocation are reported in appendix pp 40. In pooled analysis of 12 studies that compared a posterior approach with short external rotator and capsule repair with no repair, the RR (95% CIs) for dislocation was 0·28 (0·12-0·66) (appendix pp 40-41). There was evidence of heterogeneity between these contributing studies (I 2 =60%; 95% CI 25, 79%; p=0·004), which was not explained by any of the study level characteristics evaluated (appendix pp 42). The following binary factors related to acetabular cup position were all associated with an increased risk of dislocation: cup abduction >50 or >55 degrees; cup abduction window 35 to 50 degrees / cup anteversion window 5 to 25 degrees; cup anteversion window 10 to 30 degrees; and cup anteversion window 0 to 20 degrees (appendix pp 40). For combined stem and cup position, pooled analysis of two studies showed a combined window of 40 to 60 degrees was associated with an increased risk of dislocation RR (95% CIs) of 6·59 (1·98-21·93). Other factors such as femoral stem position, type of anaesthesia employed during surgery, and computer-assisted techniques were each not associated with the risk of dislocation (appendix pp 40).
Fifty studies reported on associations between implant-related factors and the risk of dislocation (appendix pp 43). Comparing femoral head diameters 28mm vs 32mm; 22mm vs 32mm; 36mm vs 28mm; 36mm vs 32mm; >36mm vs 28mm; 26mm vs 30mm; and 28mm vs 30mm, RRs (95% CIs) of dislocation were 1·67 (1·28-2·18); 1·88 (1·51-2·33); 0·45 (0·26-0·78); 0·64 (0·52-0·78); 0·09 (0·05-0·17); 3·24 (2·40-4·40); and 1·76 (1·32-2·36) respectively. There was evidence of moderate heterogeneity between studies contributing data for the femoral head diameter comparison 28mm vs 32mm (12 studies; appendix pp 43-44) (I 2 =61%; 95% CI 27, 79%; p=0·003), which was not explained by any of the study level characteristics evaluated (appendix pp 45). In single reports that evaluated acetabular cup outer diameter, RRs (95% CIs) of dislocation were 0·42 (0·21-0·86) and 3·43 (1·27-9·29) respectively for the comparisons: ≥56mm vs <56mm and ≥62mm vs ≤60mm. In pooled analysis of 4 studies, comparing an elevated acetabular liner with a standard rim, the RR (95% CIs) of dislocation was 0·49 (0·36-0·66). Six studies assessed the association between acetabular cup design and the risk of dislocation: compared to a conventional cup, a dual mobility cup was associated with a reduced risk of dislocation RR (95% CI) 0·15 (0·08-0·29). Cemented fixations were associated with a reduced risk of dislocation compared to uncemented fixations RR (95% CI) of 0·72 (0·57-0·91). There was no evidence of associations of other fixation types (hybrid and reverse hybrid) with the risk of dislocation (appendix pp 43). In separate evaluation of acetabular and femoral fixation types, a cemented acetabular component fixation (vs uncemented) was associated with a reduced risk of dislocation RR (95% CI) of 0·63 (0·47-0·84); whereas a cemented femoral component fixation (vs uncemented) was not associated with dislocation risk. On the role of bearing types on the risk of dislocation, comparing metal-on-metal (MoM) vs metal-on-polyethylene (MoPE); ceramic-on-cross-linked polyethylene (CoXLPE) vs metal-on-cross-linked polyethylene (MoXLPE); and ceramic-on-ceramic (CoC) vs metal-on-cross-linked polyethylene (MoXLPE), RRs (95% CIs) were 2·44 (1·23-4·85); 0·79 (0·65-0·96); and 0·69 (0·54-0·88) respectively. On the influence of femoral neck length on dislocation risk, both long and short necks were each associated with an increased risk of dislocation when compared to standard neck; RRs (95% CIs) of 3·55 (1·83-6·86) and 2·38 (1·02-5·56) respectively. Other factors relating to femoral stem type and design were each not associated with the risk of dislocation (appendix pp 43).
A total of 13 studies reported on the associations of hospital-related factors and the risk of dislocation (appendix pp 46-47). There was a decreased risk of dislocation comparing experienced surgeons with less experienced surgeons in pooled analysis of 4 studies RR (95% CI) 0·66 (0·48-0·92). In pooled analysis of two studies, hospitals with high surgeon procedure volume were associated with a decreased risk of dislocation compared to low volume RR (95% CI) 0·31 (0·25-0·39). In a single study that assessed the influence of preoperative patient education on the risk of dislocation, participants who participated in these sessions had a reduced risk of dislocation compared to nonparticipants RR (95% CI) 0·36 (0·14-0·92). Other assessed factors such as hospital location (urban vs rural), hospital type (teaching vs nonteaching), hospital procedure volume (high vs low), and hospital stay (short vs long stay) were each not associated with the risk of dislocation.
Funnel plots for all comparisons that involved 10 or more studies were all symmetrical under visual examination (appendix pp 48). All results were consistent with Egger’s regression tests showing little evidence of publication bias.
Discussion
Over an average follow-up of 6 years, the incidence rates for dislocation following primary THR ranged from 0·12 to 16·13% across individual studies and averaged approximately 2·10% in pooled analysis. Our results also showed a temporal decline in dislocation rates following primary THR. On the role of patient-related factors and their associations with the risk of dislocation, older patients, White ethnicity, high BMI, low income, drug use disorder, and social deprivation each had an increased risk of dislocation. Comorbidities that increased the risk of dislocation included neurological disorders, psychiatric disease, frailty, chronic lung disease, renal failure, and rheumatoid disease. High ASA grade or CCI was associated with an increased risk of dislocation. Patients with previous surgery such as spinal fusion and previous hip surgery also had an increased risk of dislocation. Patients undergoing THR for avascular necrosis, rheumatoid arthritis, inflammatory arthritis, or osteonecrosis were at increased risk of dislocation. At the surgical level, surgical approaches such as anterolateral, direct anterior, lateral or lateral with trochanteric osteotomy was associated with reduced risk of dislocation compared to the posterior approach. However, performing short external rotator and capsule repair with the posterior approach led to a lower risk of dislocation for this approach. At the implant level, larger femoral head diameters, elevated acetabular liners, dual mobility cups, cemented fixations, bearing types (MoPE, CoXLPE, CoC) or standard femoral neck lengths reduced the risk of dislocation. Hospital-related factors such as experienced surgeons, high surgeon procedure volume or preoperative patient education reduced the risk of dislocation.
A number of previous reviews have attempted to investigate potential risk factors for dislocation in THR, but these have either been based on single or selected risk factors, revision THR, a mixture of both primary and revision THR or were summarised using a narrative approach. 20–23,25,30,31 Haverkamp and colleagues in their review sought to evaluate the influence of obesity; in pooled analysis of 10 out of 15 studies, they demonstrated obesity (BMI >30 vs <30 kg/m2) to be associated with an increased risk of dislocation. 22 In pooled analysis of 14 studies (of which 12 estimates were actually pooled), Jia and colleagues compared dislocation risk between direct anterior and posterior surgical approaches and reported no statistical significant difference in risk and their estimate was imprecise because of the low event rate. 23 In our pooled analysis of 13 studies, the direct anterior approach was associated with a substantial risk reduction in dislocation compared with the posterior approach. However, the summary effect estimate seemed to be driven by the largest study included in the analysis; 32 hence, further studies are required to confirm this finding. In pooled analysis of seven clinical trials using a posterior approach for primary THR, use of a soft tissue repair was associated with a reduced risk of dislocation. 31 Consistent with our findings of reduced risk of dislocations with dual mobility bearing surfaces (compared with standard bearings) and larger femoral head sizes, a recently published network meta-analysis demonstrated similar findings but was based on primary or revision THRs. 30 To our knowledge, our review represents the first attempt at evaluating and synthesising evidence on the relationships of patient-, surgery-, implant- and hospital-related factors with dislocation risk in one single investigation and in more detail than ever before using a systematic meta-analytic approach.
Several plausible underlying pathways may explain some of the associations demonstrated. The role of gender in relation to complications and implant failure following THR is a controversial topic. Similarly, evidence on the role of gender in influencing dislocation has been inconsistent; whereas some studies have reported increased dislocation risk for females, 6,33 others have reported opposite findings. 34,35 Overall, our findings suggest that gender may not be an independent risk factor for dislocation following primary THR. The increased risk of dislocation associated with low income and social deprivation is likely to be multifactorial. However, there is a possibility that these patients have lower levels of education, have poorer access to specialised care and are more likely to be referred to less-experienced surgeons. Whether obesity (compared to normal weight) as measured by BMI, influences the risk of dislocation following THR has also been a subject of controversy, 22 as findings from studies have been contradictory and plausible explanations have been proposed for these inconsistent findings. For example, it has been suggested that obesity is associated with limited mobility and hence less risk of complications such as dislocation, since these complications are functions of use. 36 However, putting the overall findings together suggest that obesity may indeed be associated with an increased risk of dislocation. Plausible pathways underlying this relationship include the presence of more comorbidities, component malpositioning and less stability in the early postoperative period; the latter arising from greater soft tissue damage and the longer duration of surgery associated with a larger soft tissue envelope. 22,37,38 Extraarticular impingement created by thigh-on-thigh soft tissue contact during adduction and flexion, is also more prevalent in higher BMIs leading to the femoral head lifting out of the acetabular cup. 39 Several comorbidities such as neurological disorders, psychiatric diseases, frailty and renal failure were associated with increased dislocation risk; which are likely due to factors associated with these conditions such as muscle weakness, imbalance, and inability to comply with activity restrictions and are known to increase the risk of dislocation. 40–42 It has been postulated that the increased dislocation risk associated with spinal fusion is because these patients often have less pelvic roll when seated with more hip flexion, which leads to less acetabular component anteversion and increased dislocation when they stand from sitting. 43 Though computer-assisted surgery has been developed to help orthopaedic surgeons achieve better accuracy of implant placement (e.g., cup placement) and to improve functional outcomes and reduce complications such as dislocation, risk estimates from the one study that evaluated this 33 found no significant difference in dislocation risk comparing computer-assisted THR with conventional THR. These findings are consistent with two other studies, 44,45 though they could not be included in our pooled analysis because risk estimates for dislocation risk were not available. Though computer-assisted surgical techniques improve cup position in THR surgery 46 and are increasingly being adopted by surgeons, 47 little evidence exists to show they actually improve outcomes and reduce complication rates in total joint replacement. 44,45,48,49
In the United States, it has been estimated that there will be an increase in the demand of primary THRs by 174% between 2005 and 2030. 15 Dislocations, which are also common causes of revision surgery, 5 are therefore expected to rise proportionately. The social, health and economic costs associated with dislocations are substantial and potentially devastating. 11,18 Our review has demonstrated that the aetiology of dislocation is multifactorial and can be attributed to patient-, surgery-, implant- and hospital-related factors, the majority of which are modifiable or there are strategies available to ameliorate the associated risks. Recognition of these factors prior to surgery with careful planning before and after surgery may represent an implementable strategy by which dislocations can be prevented or minimised among patients at high risk.
In addition to several strengths already mentioned, our review is the first comprehensive assessment of the incidence of dislocation following primary THR with characterisation of temporal trends. Included studies were based on patients from many continents which made results generalisable. We employed appropriate meta-analytic approaches in our analyses; these included standardisation and harmonisation of some reported associations to a common scale to ensure consistency before pooling, quantification and exploration of heterogeneity, and evaluation of small study effects. Finally, given that some of the articles were based on the same database or study and therefore presented the potential for participant overlap, we employed comprehensive data checks to ensure the uniqueness of each study in contributing data to the pooled analysis and therefore it is unlikely any patients would have been double counted during the pooling process. Several limitations deserve consideration; (i) inability to characterise the shape of any dose-response associations due to lack of appropriate data; (ii) lack of consistent reporting precluded exploration of associations by whether dislocation was early or late; (iii) the majority of studies were retrospective in design and also did not adjust for confounding; (iv) given the use of published data, we were unable to account for temporal changes in risk factors due to recent innovations in THR or adoption of strategies to mitigate the risk of dislocation; (v) our findings on the temporal trends in dislocation rates were based on median year of data collection reported by studies, which may not accurately capture specific periods of surgery and follow-up; (vi) our incidence data at specific time points were based on average follow-up periods reported by eligible studies, hence these findings may be underestimates and finally (vii) some of our findings were based on single reports, hence they need interpretation with caution and also require replication in further studies.
In conclusion, this aggregate review demonstrates that though the incidence of dislocation following primary THR is variable, the average incidence is less than 3% over an average follow-up period of 6 years and there is a temporal decline in rates. The risk of dislocation following primary THR has a multifactorial aetiology. Surgical approaches that reduce dislocation risk should be preferred in primary THR. For patients at high risk of dislocation, use of cemented fixations, large femoral head sizes, elevated acetabular liners or dual mobility bearings may be considered. Modifiable risk factors such as high BMI and comorbidities should be recognised and may be amenable to optimisation prior to surgery.
Supplementary Material
Research in context.
Evidence before this study
Dislocation is a common indication for revision following primary total hip replacement (THR) and is associated with severe pain, restriction of mobility, recurrent dislocation, poor quality of life and substantial healthcare costs. There is therefore an urgent need to identify relevant factors which influence the risk of dislocation in order to counsel patients, guide surgeons and healthcare providers to plan effectively and mitigate risks. Data from several individual longitudinal studies conducted in revision THRs as well as their aggregate analyses, suggest that several patient- (demographics and comorbidity), surgery- (approach) and implant-related factors (bearing size and component position) influence dislocation rates. It is uncertain if these factors also apply to primary THR. In a preliminary literature search, we searched MEDLINE, Embase and Web of Science from inception to February 5, 2019, for systematic reviews and meta-analyses reporting on associations of patient-, surgery-, implant- or healthcare system-related factors with risk of dislocation following primary THR. We used search terms related to the population (e.g., “primary total hip replacement”), exposures (e.g, “risk factor”, “body mass index”, “comorbidity”), and outcome (e.g., “dislocation”, “instability”). Majority of reviews identified were based on revision THRs. Five relevant reviews attempted to investigate potential risk factors for dislocation in primary THR, but these were based on single or selected risk factors (mostly comorbidities), included a mixture of both primary and revision THRs, summarised the evidence using a narrative approach or did not explore for potential sources of bias. No review has comprehensively and quantitatively evaluated evidence on the role of patient-, surgery-, implant- or healthcare system-related factors on dislocation risk following primary THR. Variable incidence rates of dislocation are also reported in the literature.
Added value of this study
To our knowledge, this is the first aggregate analysis to evaluate the associations of several patient-, surgery-, implant- and hospital-related factors with dislocation risk and assess the incidence of and temporal trends in dislocation following primary THR in one single comprehensive investigation. In contrast to previous individual studies and reviews, these findings provide a detailed picture of the multifactorial aetiology of dislocation risk following primary THR. Though multifactorial, it appears most of the risk is driven by patient factors (such as sociodemographic characteristics, high body mass index, and comorbidities), surgery-related factors (surgical approaches) and several implant-related factors. Except for surgeon experience and high surgeon procedure volume which decrease the risk of dislocation, the majority of hospital-related factors do not seem to influence the risk of dislocation. Over an average follow-up period of 6 years, the average incidence of dislocation following primary THR is less than 3%. Furthermore, there appears to be an ongoing worldwide temporal decline in dislocation rates following primary THR, which likely reflects recent innovations in surgical practice.
Implications of all the available evidence
As life expectancy continues to increase with a growing burden due to osteoarthritis, there will be an increase in demand for primary THRs and dislocations are expected to increase proportionately. The social, health and economic costs associated with dislocations are substantial and potentially devastating. This study demonstrates that the risk of dislocation can be attributed to several patient-, surgery-, implant- and hospital-related factors, the majority of which are modifiable or there are strategies available to ameliorate the associated risks. Recognition of these factors prior to surgery with careful planning before and after surgery and using a multidisciplinary approach may represent an implementable strategy by which dislocations can be prevented or minimised among patients at high risk. Furthermore, data on incidence rates and temporal trends of dislocation is a valuable resource for clinicians and policy makers, as it enables quantification of the societal impact of dislocations and assists in planning purposes.
Acknowledgements
This study was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol (BRC-1215-20011). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Footnotes
Contributors
SKK, MCB, ADB, AJ, AWB, VW, and MRW contributed to study conception and design. SKK, MCB, and MRW contributed to literature searches, screening and data collection. SKK conducted the combined statistical analysis and wrote the first draft. SKK, MCB, ADB, AJ, AWB, VW, and MRW contributed to data interpretation, further drafting and critical revision of the manuscript for important intellectual content
Declaration of Interests
MRW and AWB undertake teaching on basic sciences for Orthopaedic trainees preparing for the Fellowship of the Royal Colleges of Surgeons (FRCS); their institution receives market rate payment for this teaching from Heraeus and DePuy. VW and AWB are co-applicants on a grant from Stryker investigating the outcome of the Triathlon total knee replacement.
References
- 1.Fortin PR, Clarke AE, Joseph L, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis and rheumatism. 1999;42(8):1722–8. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Ritter MA, Albohm MJ, Keating EM, Faris PM, Meding JB. Comparative outcomes of total joint arthroplasty. J Arthroplasty. 1995;10(6):737–41. doi: 10.1016/s0883-5403(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 3.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84-A(2):171–7. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Soderman P, Malchau H, Herberts P. Outcome after total hip arthroplasty: Part I. General health evaluation in relation to definition of failure in the Swedish National Total Hip Arthoplasty register. Acta orthopaedica Scandinavica. 2000;71(4):354–9. doi: 10.1080/000164700317393330. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed on 17 July 2019];15th Annual Report 2018. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2015th%20Annual%20Report%202018.pdf .
- 6.Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456–63. doi: 10.2106/JBJS.D.02860. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134–42. [PubMed] [Google Scholar]
- 8.Meek RM, Allan DB, McPhillips G, Kerr L, Howie CR. Late dislocation after total hip arthroplasty. Clin Med Res. 2008;6(1):17–23. doi: 10.3121/cmr.2008.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 10.Gwam CU, Mistry JB, Mohamed NS, et al. Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplasty. 2017;32(7):2088–92. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Bozic KJ, Kamath AF, Ong K, et al. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin Orthop. 2015;473(6):2131–8. doi: 10.1007/s11999-014-4078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295–306. [PubMed] [Google Scholar]
- 13.Fessy MH, Putman S, Viste A, et al. What are the risk factors for dislocation in primary total hip arthroplasty? A multicenter case-control study of 128 unstable and 438 stable hips. Orthop Traumatol Surg Res. 2017;103(5):663–8. doi: 10.1016/j.otsr.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Tamaki T, Oinuma K, Miura Y, Higashi H, Kaneyama R, Shiratsuchi H. Epidemiology of Dislocation Following Direct Anterior Total Hip Arthroplasty: A Minimum 5-Year Follow-Up Study. J Arthroplasty. 2016;31(12):2886–8. doi: 10.1016/j.arth.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89-A(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 16.Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598–605. doi: 10.2106/JBJS.H.00067. [DOI] [PubMed] [Google Scholar]
- 17.Abdel MP, Cross MB, Yasen AT, Haddad FS. The functional and financial impact of isolated and recurrent dislocation after total hip arthroplasty. Bone Joint J. 2015;97-B(8):1046–9. doi: 10.1302/0301-620X.97B8.34952. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290–4. doi: 10.2106/JBJS.D.02799. [DOI] [PubMed] [Google Scholar]
- 19.de Palma L, Procaccini R, Soccetti A, Marinelli M. Hospital cost of treating early dislocation following hip arthroplasty. Hip international : the journal of clinical and experimental research on hip pathology and therapy. 2012;22(1):62–7. doi: 10.5301/HIP.2012.9059. [DOI] [PubMed] [Google Scholar]
- 20.Faldini C, Stefanini N, Fenga D, et al. How to prevent dislocation after revision total hip arthroplasty: a systematic review of the risk factors and a focus on treatment options. J Orthop Traumatol. 2018;19(1):17. doi: 10.1186/s10195-018-0510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Yang Y, An B, et al. Risk factors for dislocation after revision total hip arthroplasty: A systematic review and meta-analysis. Int J Surg. 2017;38:123–9. doi: 10.1016/j.ijsu.2016.12.122. [DOI] [PubMed] [Google Scholar]
- 22.Haverkamp D, Klinkenbijl MN, Somford MP, Albers GH, van der Vis HM. Obesity in total hip arthroplasty--does it really matter? A meta-analysis. Acta Orthop. 2011;82(4):417–22. doi: 10.3109/17453674.2011.588859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip international : the journal of clinical and experimental research on hip pathology and therapy. 2018:1120700018820652. doi: 10.1177/1120700018820652. [DOI] [PubMed] [Google Scholar]
- 24.Romagnoli M, Grassi A, Costa GG, Lazaro LE, Lo Presti M, Zaffagnini S. The efficacy of dualmobility cup in preventing dislocation after total hip arthroplasty: a systematic review and meta-analysis of comparative studies. Int Orthop. 2019;43(5):1071–82. doi: 10.1007/s00264-018-4062-0. [DOI] [PubMed] [Google Scholar]
- 25.Rowan FE, Benjamin B, Pietrak JR, Haddad FS. Prevention of Dislocation After Total Hip Arthroplasty. J Arthroplasty. 2018;33(5):1316–24. doi: 10.1016/j.arth.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;441:262–7. doi: 10.1097/01.blo.0000194308.23105.f4. [DOI] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of Observational Studies in Epidemiology. JAMA: The Journal of the American Medical Association. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berstock JR, Beswick AD, Lopez-Lopez JA, Whitehouse MR, Blom AW. Mortality After Total Knee Arthroplasty: A Systematic Review of Incidence, Temporal Trends, and Risk Factors. J Bone Joint Surg Am. 2018;100(12):1064–70. doi: 10.2106/JBJS.17.00249. [DOI] [PubMed] [Google Scholar]
- 30.Pituckanotai K, Arirachakaran A, Tuchinda H, et al. Risk of revision and dislocation in single, dual mobility and large femoral head total hip arthroplasty: systematic review and network meta-analysis. Eur J Orthop Surg Traumatol. 2018;28(3):445–55. doi: 10.1007/s00590-017-2073-y. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Chen L, Peng K, Xing F, Wang H, Xiang Z. Effectiveness and safety of the posterior approach with soft tissue repair for primary total hip arthroplasty: a meta-analysis. Orthop Traumatol Surg Res. 2015;101(1):39–44. doi: 10.1016/j.otsr.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Sheth D, Cafri G, Inacio MC, Paxton EW, Namba RS. Anterior and Anterolateral Approaches for THA Are Associated With Lower Dislocation Risk Without Higher Revision Risk. Clin Orthop. 2015;473(11):3401–8. doi: 10.1007/s11999-015-4230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gausden EB, Parhar HS, Popper JE, Sculco PK, Rush BNM. Risk Factors for Early Dislocation Following Primary Elective Total Hip Arthroplasty. J Arthroplasty. 2018;33(5):1567–71.:e2. doi: 10.1016/j.arth.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Zijlstra WP, De Hartog B, Van Steenbergen LN, Scheurs BW, Nelissen R. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty. Acta Orthop. 2017;88(4):395–401. doi: 10.1080/17453674.2017.1317515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menendez ME, Ring D, Barnes CL. Inpatient Dislocation After Primary Total Hip Arthroplasty. J Arthroplasty. 2016;31(12):2889–93. doi: 10.1016/j.arth.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 36.McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18(1):35–9. doi: 10.1002/jor.1100180106. [DOI] [PubMed] [Google Scholar]
- 37.Elson LC, Barr CJ, Chandran SE, Hansen VJ, Malchau H, Kwon YM. Are morbidly obese patients undergoing total hip arthroplasty at an increased risk for component malpositioning? J Arthroplasty. 2013;28(8 Suppl):41–4. doi: 10.1016/j.arth.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi S, Nishiyama T, Fujishiro T, et al. Obese patients may have more soft tissue impingement following primary total hip arthroplasty. Int Orthop. 2012;36(12):2419–23. doi: 10.1007/s00264-012-1701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Morshed S, Joseph T, Bozic K, Ries MD. Clinical impact of obesity on stability following revision total hip arthroplasty. Clin Orthop. 2006;453:142–6. doi: 10.1097/01.blo.0000238874.09390.a1. [DOI] [PubMed] [Google Scholar]
- 40.Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169–78. [PubMed] [Google Scholar]
- 41.Woolson ST, Rahimtoola ZO. Risk factors for dislocation during the first 3 months after primary total hip replacement. J Arthroplasty. 1999;14(6):662–8. doi: 10.1016/s0883-5403(99)90219-x. [DOI] [PubMed] [Google Scholar]
- 42.Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278(2):E219–25. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- 43.Esposito CI, Miller TT, Kim HJ, et al. Does Degenerative Lumbar Spine Disease Influence Femoroacetabular Flexion in Patients Undergoing Total Hip Arthroplasty? Clin Orthop. 2016;474(8):1788–97. doi: 10.1007/s11999-016-4787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoude AA, Aldebeyan SA, Nooh A, Weber MH, Tanzer M. Thirty-Day Complications of Conventional and Computer-Assisted Total Knee and Total Hip Arthroplasty: Analysis of 103,855 Patients in the American College of Surgeons National Surgical Quality Improvement Program Database. J Arthroplasty. 2016;31(8):1674–9. doi: 10.1016/j.arth.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 45.Parratte S, Ollivier M, Lunebourg A, Flecher X, Argenson JN. No Benefit After THA Performed With Computer-assisted Cup Placement: 10-year Results of a Randomized Controlled Study. Clin Orthop. 2016;474(10):2085–93. doi: 10.1007/s11999-016-4863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdier N, Billaud A, Masquefa T, Pallaro J, Fabre T, Tournier C. EOS-based cup navigation: Randomised controlled trial in 78 total hip arthroplasties. Orthop Traumatol Surg Res. 2016;102(4):417–21. doi: 10.1016/j.otsr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Deep K, Shankar S, Mahendra A. Computer assisted navigation in total knee and hip arthroplasty. SICOT J. 2017;3:50. doi: 10.1051/sicotj/2017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237–45. doi: 10.1016/j.knee.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261–9. doi: 10.2106/JBJS.F.00601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.