Summary
Sequence-specific interactions between RNA stem-loops and coat protein (CP) subunits play vital roles in the life cycles of the RNA bacteriophages, e.g., by allowing translational repression of their replicase cistrons and tagging their own RNA genomes for encapsidation. The CPs of bacteriophages Qβ and MS2 each discriminate in favor of their cognate translational operators, even in the presence of closely related operators from other phages in vivo. Discrete mutations within the MS2 CP have been shown to relax this discrimination in vitro. We have determined the structures of eight complexes between such mutants and both MS2 and Qβ stem-loops with X-ray crystallography. In conjunction with previously determined in vivo repression data, the structures enable us to propose the molecular basis for the discrimination mechanism.
Introduction
Packaging of cognate nucleic acid genomes is one of the principal functions of viral capsids. We have been studying the molecular basis of such packaging events by using the RNA bacteriophages MS2 and Qβ as model systems. MS2 and Qβ are both T = 3 icosahedral RNA bacteriophages that package single-stranded genomes. The structures of both bacteriophages have been solved by X-ray crystallography: MS2 has been solved to a resolution of 2.8 Å (Valegård et al., 1990; Golmohammadi et al., 1993), and Qβ has been solved to a resolution of 3.5 Å (Golmohammadi et al., 1996), allowing for a detailed comparison of their atomic structures. Although Qβ and MS2 coat protein (CP) subunits only share about 20% sequence identity, their overall folds are very similar (Golmohammadi et al., 1996), and subunits associate to form dimers within the capsid shells.
The two bacteriophages utilize a similar mechanism for translational repression of their replicase cistrons. In vivo, a single CP dimer binds to a short RNA stem-loop operator within the viral genome that encompasses the start codon for the viral replicase, leading to inhibition of translation. The translational repression complex of the RNA phage MS2 has become a major paradigm for understanding RNA-protein interactions at the atomic level as a result of extensive biochemical studies of these recognition events and, more recently, the availability of many crystal structures of the complexes formed (Valegård et al., 1994, 1997; Rowsell et al., 1998; van den Worm et al., 1998; Grahn et al., 2000, 2001; Helgstrand et al., 2002; Horn et al., 2004). Several amino acid residues that are involved in RNA-protein interactions in MS2 are conserved in Qβ (Golmohammadi et al., 1996), and mutational studies have demonstrated that most of the key residues important for the RNA affinity in Qβ correspond to their structural equivalents involved in RNA binding in MS2 (Lim et al., 1996). The conserved residues of the Qβ RNA binding site are also oriented in a similar way to those of MS2. This suggests that, although the CPs of MS2 and Qβ discriminate against the binding of each other’s RNA operator, a similar general mechanism of operator binding may occur in both phages (Golmohammadi et al., 1996).
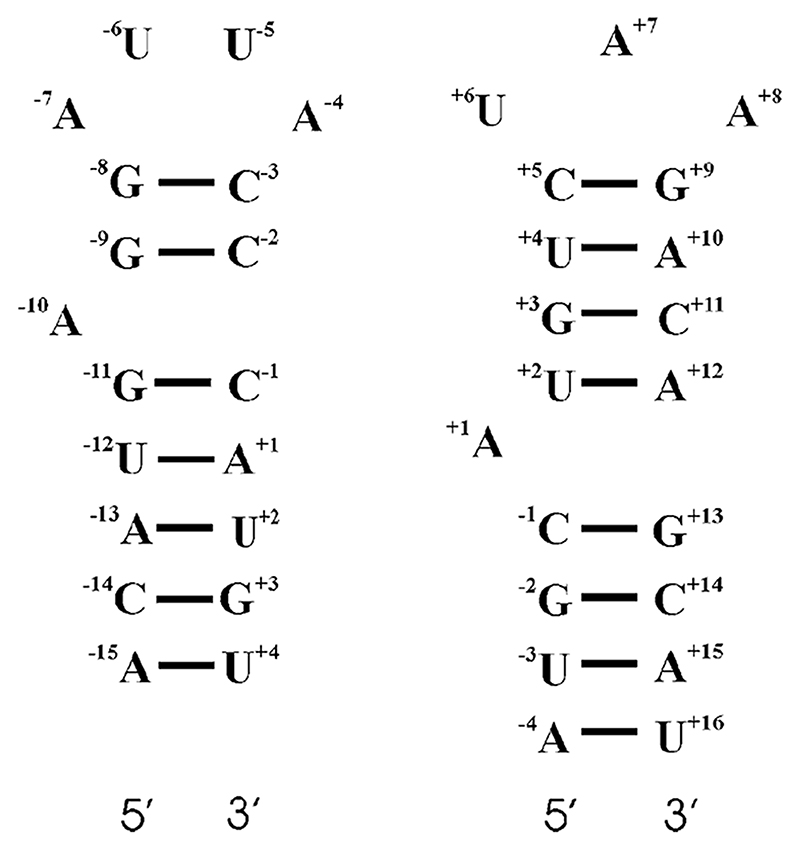
While the protein surfaces of the RNA-protein complexes of MS2 and Qβ are similar, significant differences exist between the secondary, and presumably tertiary, structures of Qβ and MS2 stem-loop operators (Figure 1). The Qβ RNA stem-loop has a three nucleotide (3 nt) loop, as opposed to the tetra-loop characteristic of the MS2 operator. Both operators have unpaired adenosines within the stem, although they are separated by different numbers of base pairs (bp) from the loop: 2 bp in MS2 and 4 bp in Qβ.
Figure 1. Secondary Structures of the MS2 and Qβ RNA Stem-Loop Operators.
MS2 structure is shown on the left, and the Qβ structure on the right. The numbering in each case is relative to the first nucleotide (+1) of the replicase start codon.
Although an atomic resolution structure of the Qβ stem-loop operator complexed with its cognate CP has not yet been determined, biochemical experiments have established the relative importance of stem-loop elements for binding affinity (Lim et al., 1996; Witherell and Uhlenbeck, 1989). In order to achieve high-affinity binding to Qβ CP, the Qβ stem-loop must encompass a 3 nt loop with an 8 bp stem (Witherell and Uhlenbeck, 1989). The only nucleotide within this sequence whose identity is essential for this interaction, provided that the base pairing within the stem is maintained, is the A+8 nucleotide; replacement with either guanine or uracil results in a 100-fold decrease in affinity. The other two nucleotides of the loop can be freely substituted with no significant effects on affinity. The importance of the unpaired A+1 is unclear; deletion of this base leads to either a 1.5-fold (Witherell and Uhlenbeck, 1989) or 5-fold (Lim et al., 1996) loss of affinity for Qβ CP. In vitro selection of operator-like aptamers against Qβ CP resulted in a majority of RNA sequences with no base equivalent to A+1 (Hirao et al., 1999), suggesting that it does not contribute significantly to affinity. The introduction of a single mismatched base pair in the stem region above the bulged A+1 also has little, if any, effect on binding affinity (Spingola et al., 2002). Truncation of the stem causes a reduction in affinity, however, which would be consistent with RNA-protein contacts throughout the length of this region (Witherell and Uhlenbeck, 1989).
Although there are close structural similarities between the Qβ and MS2 stem-loop binding sites of their respective CPs, each phage preferentially discriminates in favor of its cognate operator, even in mixed infections in the same cell (Ling et al., 1970). Affinity studies with CP mutants of MS2 and Qβ have identified specific mutations that overcome this discrimination, allowing the binding of the Qβ RNA operator to MS2 mutant CPs and vice versa (Lim et al., 1996; Spingola and Peabody, 1997). The side chain of Asn87 of the MS2 CP is an important recognition element for the MS2 operator; an Asn87Ser substitution in the MS2 CP reduces affinity for the MS2 operator (Lim et al., 1994) while enhancing that for the Qβ stem-loop (Spingola and Peabody, 1997). Similarly, changing Asp91 of the Qβ CP (the structural equivalent of Asn87 in MS2) to Asn enables binding to the MS2 stem-loop (Lim et al., 1996). The crystal structure of the wild-type MS2 CP bound to its cognate operator shows that a hydrogen bond forms between the O2 of the U-5 base and Asn87 (Valegård et al., 1994), consistent with these observations. A second discriminatory residue within the MS2 CP is Glu89, which appears to disfavor binding of the Qβ stem-loop. Substitution of this residue with aspartic acid leads to a small increase in the binding affinity for the Qβ target, but replacement with lysine leads to significantly (~35-fold) increased affinity, with little deleterious effect on binding for the MS2 stem-loop (Spingola and Peabody, 1997). A doubly mutant MS2 CP (Asn87Ser, Glu89Lys) shows a slight increase in affinity for the Qβ stem-loop over the single Glu89Lys substitution, both in vitro and in vivo, to a level similar to that of Qβ CP to its cognate stem-loop (Spingola and Peabody, 1997).
In order to understand the sequence specificity and hence the RNA stem-loop discrimination mechanisms more fully, we have determined the X-ray crystal structures of eight complexes between wild-type MS2 and Qβ stem-loop operators and MS2 CP mutants at residues 87 and 89. The results are consistent with the hypothesis that the side chains of residues Asn87 and Glu89, together with the differing secondary and hence tertiary structures of the RNA operators, regulate specificity via a combination of steric clashes and electrostatic repulsion.
Results
Details of all structure determinations are shown in Table 1 and are described below. To aid clarity, MS2 mutant CPs, in the form of T = 3 capsids, complexed with RNA stem-loops are given the following designations throughout the rest of the text: the Asn87Ser mutant complexed with either MS2 or Qβ stem-loops is designated 87SerMS2 or 87SerQβ, respectively; and the Asn87Ser, Glu89Lys double mutant complexed with MS2 or Qβ stem-loops is designated 87Ser89-LysMS2 or 87Ser89LysQβ, respectively. The corresponding names are used for the Asn87Ala and Asn87Ala, Glu89Lys double mutant complexes.
Table 1.
Statistics from Data Collection, Scaling, and Refinement for the 87SerMS2, 87AlaMS2, 87Ser89LysMS2, 87Ala89LysMS2, 87SerQβ, 87Ser89LysQβ, 87Ala89LysQβ, and 87Ala89LysQβU+12 Complexes
| Complex | 87SerMS2 | 87AlaMS2 | 87Ser89LysMS2 | 87Ala89LysMS2 | 87SerQβ | 87Ser89LysQβ | 87Ala89LysQβ | 87Ala89LysQβU+12 |
|---|---|---|---|---|---|---|---|---|
| Data Collection | ||||||||
| Space group | R32 | R32 | R32 | C2 | R32 | R32 | P1 | P1 |
| Cell dimensions | ||||||||
| a, b, c (Å) | 288, 288, 653 | 288, 288, 653 | 288, 288, 652.8 | 467, 285.6, 273.3 | 288, 288, 653 | 287.8, 287.8, 652.6 | 273.1, 273.5, 273.4 | 274.2, 273, 273.9 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 122.23, 90 | 90, 90, 120 | 90, 90, 120 | 63.26, 63.11, 63.31 | 63.06, 62.98, 63.28 |
| Resolution (Å) | 30–3.0 | 30–3.0 | 30–3.15 | 30–2.9 | 30–3.0 | 30–2.9 | 30–2.8 | 30–2.45 |
| Rmerge (%) | 19.3 (34.2) | 12.8 (23.2) | 22.0 (48.7) | 19.6 (49.9) | 20.5 (38.1) | 21.2 (46.1) | 5.3 (32.6) | 4.4 (30.3) |
| I/σI | 8.2 (1.9) | 7.3 (2.3) | 6.8 (1.3) | 7.2 (1.4) | 8.2 (2.7) | 9.1 (2.3) | 15.3 (4.3) | 13.1 (2.2) |
| Completeness (%) | 80.7 (30.6) | 49.4 (13.8) | 62.4 (45.8) | 33.5 (6.8) | 75.6 (68.4) | 65.2 (14.3) | 23.5 (1 0.1) | 19.6 (5.6) |
| Redundancy | 1.6 (1.0) | 1.5 (1.1) | 2.1 (1.2) | 1.5 (1.0) | 1.7 (1.2) | 1.6 (1.1) | 1.3 (1.0) | 1.3 (1.0) |
| Synchrotron station, beamline and detector |
SRS: 14.1, ADSC Quantum 4R CCD |
Max-II:711, Mar345IP |
SRS: 14.2, ADSC Quantum 4R CCD |
Max-II:711, Mar345IP |
SRS: 14.1, ADSC Quantum 4R CCD |
SRS: 14.2, ADSC Quantum 4R CCD |
ESRF: ID14.1, ADSC Quantum 4R CCD |
ESRF:ID14.1, ADSC Quantum 4RCCD |
| Collection temperature (K) | 298 | 100 | 298 | 100 | 298 | 298 | 100 | 100 |
| Refinement | ||||||||
| Resolution (Å) | 30–3.0 | 30–3.0 | 30–3.15 | 30–2.9 | 30–3.0 | 30–2.9 | 30–2.8 | 30–2.45 |
| Number reflections | 105,792 | 102,199 | 75,223 | 221,184 | 141,000 | 148,000 | 347,537 | 460,526 |
| Rwork/Rfree | 0.202/0.209 | 0.202/0.211 | 0.227/0.229 | 0.293/0.298 | 0.225/0.232 | 0.198/0.205 | 0.245/0.248 | 0.245/0.246 |
| Number of atoms | ||||||||
| Protein | 2,889 | 2,886 | 2,889 | 2,886 | 2,889 | 2,889 | 2,886 | 2,886 |
| RNA | 661 | 590 | 636 | 760 | 257 | 259 | 485 | 441 |
| Water | 207 | 81 | 0 | 0 | 103 | 125 | 156 | 178 |
| B factors (Å2) | ||||||||
| Protein | 32.4 | 39.5 | 29.3 | 31.6 | 32.5 | 32.7 | 48.1 | 29.9 |
| RNA | 70.1 | 77.5 | 65.1 | 59.6 | 58.7 | 71.4 | 87.2 | 77.4 |
| Water | 50.5 | 47.7 | N/A | N/A | 38.3 | 39.0 | 54.7 | 32.0 |
| Rms deviations | ||||||||
| Bond lengths (Å) | 0.007 | 0.006 | 0.008 | 0.007 | 0.008 | 0.010 | 0.007 | 0.006 |
| Bond angles (°) | 1.3 | 1.3 | 1.4 | 1.4 | 1.5 | 1.6 | 1.4 | 1.3 |
| PDB code | 2B2G | 2BNY | 2B2E | 2BQ5 | 1ZSE | 2B2D | 2BS1 | 2BS0 |
Numbers in parentheses correspond to the highest-resolution bin.
Structures of the Mutant CPs Complexed with the MS2 Stem-Loop
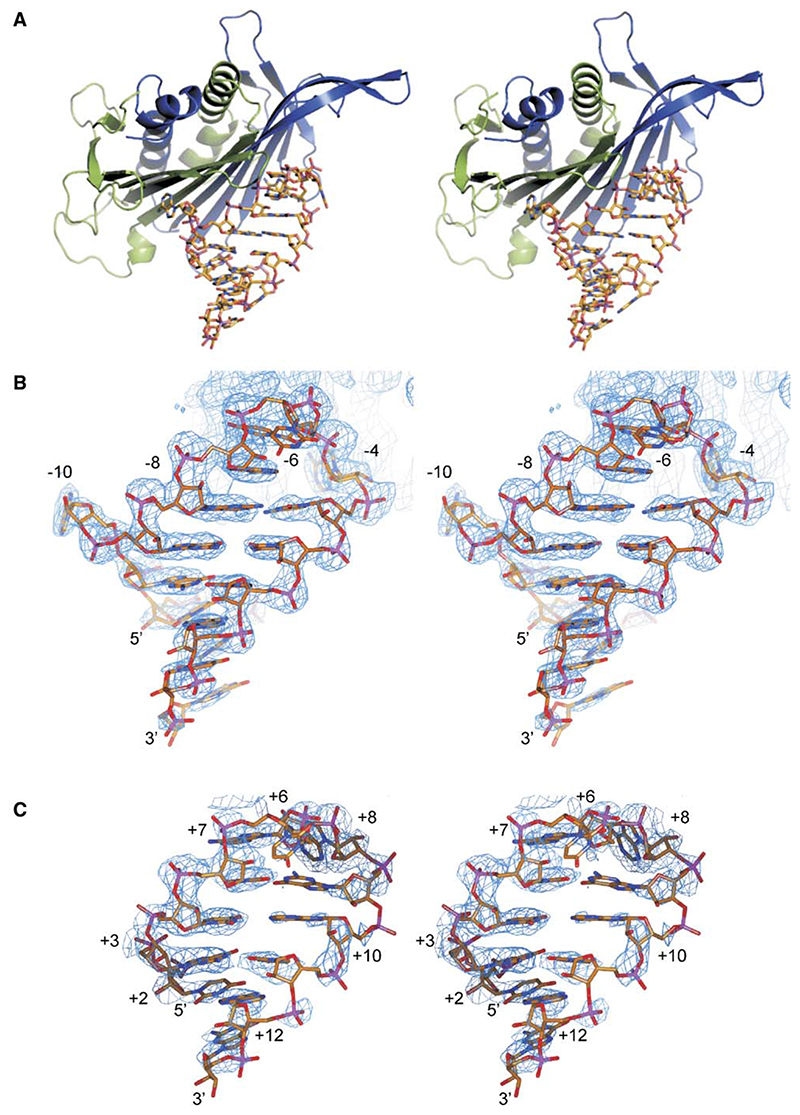
The structures of all of the CPs in complex with MS2 stem-loops are essentially identical to those seen in wild-type phage capsids, with small differences in the conformations of flexible lysine and arginine side chains on the inner surface of the capsid (Figure 2A). Binding sites for RNA stem-loops are present on both AB and CC protein dimers within the capsid shell, and the conformations of complexed RNA at each site are identical (Valegård et al., 1997). Due to the 2-fold symmetry of the CC site, the RNA binds in two orientations, making interpretation of the electron density problematic. All complexed RNA structures discussed below are thus modeled at the asymmetric AB site, where only a single binding orientation occurs.
Figure 2. Stereoimages of the Wild-Type MS2 RNA Stem-Loop Coat Protein Dimer Complex and the 87SerMS2 and 87SerQβ Complexes.
(A) Stereoimage of the complex between the wild-type MS2 RNA stem-loop and an MS2 AB protein dimer (Valegård et al., 1997). Subunit A is shown in blue, subunit B is shown in green, and the RNA is shown in stick format.
(B and C) Stereoimages of the RNA stem-loops in the same orientation as in (A) modeled into the 2Fo – Fc electron density, shown in blue contoured at 1 rms, in the capsids for (B) 87SerMS2 and (C) 87SerQβ complexes.
2Fo – Fc electron density maps clearly show interpretable density for all or most of the stem-loops (Figure 2B). Electron density for the unpaired U-6 in the MS2 stem-loop is weak or absent, precluding definitive modeling. This is also true for the majority of MS2 stem-loop complexes that have been studied (Grahn et al., 2001). The base is, however, clearly pointing away from the protein and makes no interactions with other parts of the RNA. The tertiary structures of the RNAs are also very similar to that observed in the wild-type complex (Figure 2A; [Valegård et al., 1997]). The rms deviation of the 16 common phosphorous atoms of the structures, calculated with the program LSQMAN (Kleywegt and Jones, 1994), is 0.3−0.6 Å. All ribose sugars were modeled in the C3′ endo conformation, with the exception of the sugars at the −4, −5, and −6 positions, which were modeled in the C2′ endo conformation (Valegård et al., 1997).
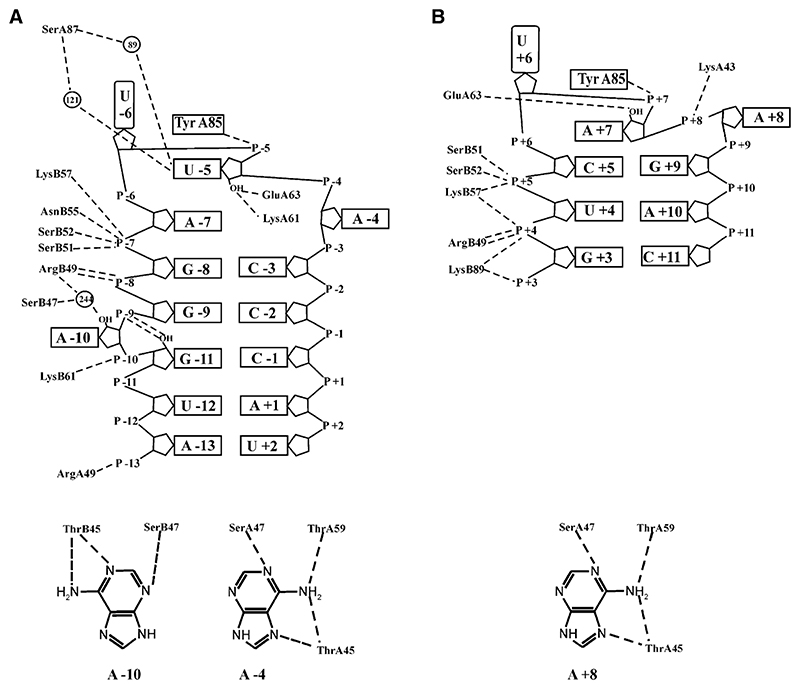
As expected, there are changes in the RNA-protein interactions at the sites of substitution compared to the wild-type complex (Valegård et al., 1997). In the 87SerMS2 complex, the SerA87 side chain is too far from U-5 to form the direct hydrogen bond observed with Asn87. However, two water molecules are in close proximity to both the Oγ of SerA87 and the −5 uracil, allowing for the formation of two RNA-protein water-mediated hydrogen bonds between the N3 and the O2 of U-5 and the Oγ of SerA87 (Figure 3A and Table 2). In addition, the base has moved closer (~ 0.3 Å) to SerA87. In the 87AlaMS2 and 87Ala89LysMS2 complexes, the 87Ala has no polar group for interaction with a water molecule. Although a water molecule is bound to O2 of the −5 base in the 87AlaMS2 complex, it does not form any hydrogen bonds with protein atoms. In both of these complexes, the base has moved slightly away from Ala87 compared to its position in the wild-type complex.
Figure 3. Schematic Showing RNA-Protein Hydrogen Bond Interactions in the 87SerMS2 and 87Ser89LysQβ Complexes.
(A and B) (A) 87SerMS2. (B) 87Ser89LysQβ. Putative hydrogen bond contacts are shown as black, dashed lines where atoms lie within 3.3 Å of each other. Ordered water molecules are shown as black circles. The details of the interactions at the adenine binding pockets are shown below the main diagram.
Table 2. RNA-Protein Contact Distances between Atoms Forming Hydrogen Bonds or Salt Links.
| Protein | Wild-Type | 87Ser | 87Ala | 87Ser89Lys | 87Ala89Lys | 87Ser | 87Ser89Lys | 87Ala89Lys | 87Ala89Lys | |
|---|---|---|---|---|---|---|---|---|---|---|
| RNA | MS2 | MS2 | MS2 | MS2 | MS2 | Qβ | Qβ | Qβ | QβU+12 | |
| RNA atom | Protein atom | |||||||||
| O2P −13/* | Nη1 ArgA49 | 3.1 | D | D | D | D | D | D | ||
| O2P −13/* | Nη2 ArgA49 | 2.6 | D | D | 3.2 | D | D | D | D | |
| O2P −11/* | Nζ LysB61 | 2.9 | 3.4 | 3.2 | 3.0 | 3.2 | D | D | D | D |
| O1P −10/* | Nζ LysB89 | — | — | — | 3.3 | 3.6 | — | D | D | D |
| O2P −10/* | Nζ LysB61 | 2.8 | 2.5 | 2.7 | 2.4 | 2.5 | D | D | D | D |
| O2P −9/+3 | Nζ LysB89 | — | — | — | — | 3.2 | 3.3 | 3.3 | ||
| O2P −8/+4 | Nϵ ArgB49 | 2.6 | 2.7 | 3.3 | 2.9 | |||||
| O2P −8/+4 | Nη1 ArgB49 | 2.5 | 2.5 | 2.7 | ||||||
| O2P −8/+4 | Nη2 ArgB49 | 2.6 | 2.7 | 2.5 | 2.8 | 2.8 | ||||
| O1P −8/+4 | Nη2 ArgB49 | 2.7 | 3.1 | 2.9 | ||||||
| O1P −8/+4 | Nζ LysB89 | — | — | — | — | 3.3 | 3.3 | 3.3 | ||
| O1P −8/+4 | Nζ LysB57 | 3.5 | 2.9 | 2.9 | 3.7 | 3.1 | 3.4 | 3.6 | ||
| O1P −7/+5 | Nζ LysB57 | 3.0 | 3.2 | 3.4 | 3.2 | 3.0 | 2.7 | 2.7 | 2.8 | 2.8 |
| O2P −7/+5 | Oγ SerB51 | 3.0 | 2.9 | 3.3 | 3.0 | 2.7 | 2.8 | 2.8 | 2.7 | 2.8 |
| O2P −7/+5 | Oγ SerB52 | 2.6 | 2.7 | 2.5 | 2.9 | 2.5 | 2.9 | 2.8 | 2.8 | 2.7 |
| O2P −7/+5 | Nδ2 AsnB55 | 2.9 | 3.2 | 3.1 | 3.2 | |||||
| O1P −6/+6 | Nδ2 AsnB55 | 3.1 | ||||||||
| O2P −6/+6 | Nδ2 AsnB55 | 3.2 | ||||||||
| O1P −5/+7 | Oη TyrA85 | 2.6 | 3.1 | 3.0 | 3.0 | 2.9 | 2.8 | |||
| O2′ −5/+7 | Oϵ2 GluA63 | 3.0 | 3.0 | 2.8 | 3.1 | 3.0 | 2.8 | 3.2 | 3.2 | 3.0 |
| O2′ −5/+7 | Nζ LysA61 | 2.9 | 2.6 | |||||||
| O3′ −5/+7 | Nζ LysA61 | 3.2 | 3.0 | |||||||
| O1P −4/+8 | Nζ LysA43 | 3.6 | 3.6 | 2.8 | 3.1 | 3.8 | 3.1 | 3.0 | 3.8 | |
| N6 Ade −10/− | Oγ1 ThrB45 | 2.9 | 3.0 | 3.0 | ||||||
| N1 Ade −10/− | Oγ1 ThrB45 | 2.6 | 2.6 | 2.6 | 2.7 | 2.6 | ||||
| N3 Ade −10/− | Oγ SerB47 | 2.5 | 2.6 | 2.9 | 2.8 | 2.7 | ||||
| O2 Ura −5/+7 | Nδ2 AsnA87 | 3.0 | — | — | — | — | — | — | — | — |
| N1 Ade −4/+8 | Oγ SerA47 | 2.9 | 2.9 | 2.6 | 2.8 | 2.6 | 2.9 | 2.8 | 2.8 | 2.8 |
| N6 Ade −4/+8 | Oγ1 ThrA45 | 3.1 | 3.1 | 3.1 | 3.0 | 3.1 | 3.1 | 2.9 | 3.0 | 3.0 |
| N6 Ade −4/+8 | O ThrA59 | 2.9 | 2.9 | 2.9 | 2.9 | 2.8 | 2.8 | 2.9 | 2.9 | 2.9 |
| N7 Ade −4/+8 | Oγ1 ThrA45 | 2.9 | 2.6 | 2.6 | 2.6 | 2.7 | 2.6 | 2.5 | 2.6 | 2.7 |
In the RNA column, the nucleotide number (Figure 1) is given for the MS2 and Qβ RNA molecules, respectively, when the contact is present in both cases. An asterisk replaces the number when only one of the RNA molecules has this interaction. In the table, a “D” indicates that one of the involved atoms is completely disordered and not modeled, a “—” means that one of the atoms is missing, and an empty space indicates that the distance is larger than 3.3 Å (3.8 Å for salt links). The contacts to the RNA backbone are listed first, and the base interactions are at the end.
The side chain of ArgB49 forms a salt link with GluB89 and the phosphate at −8 in the wild-type MS2 complex (Valegård et al., 1994, 1997). In all of the complexes with a lysine at position 89, ArgB49 has a different conformation. Although it still interacts with the phosphate at −8, it is farther away from the lysine. The LysB89 side chain is not well ordered in any of the complexes, but it has the potential to form hydrogen bond interactions with either phosphates −8 or −10 (see Discussion). Residues LysA89 and Ser/Ala B87 are too far from the RNA to make any contact.
Structures of Mutant MS2 CPs Complexed with Qβ Stem-Loops
In these four complexes with Qβ RNA, the protein has essentially the same conformation as in the complexes with MS2 RNA, but the RNA is less well ordered. Electron density for the Qβ RNA was observed for the loop and part of the stem regions of the RNA, allowing for unambiguous modeling of these portions of the stem-loop (Figure 2C). Strong density was apparent for the +8 adenine within the MS2 −4 binding pocket of the CP, and there was also density that confirmed the presence of a stacking interaction between TyrA85 and the +7 adenine of the loop region of the RNA (Figure 3B). Note, the protein binding site is referred to by using the MS2 numbering system. No electron density was observed for the base of U+6, suggesting that this base is projecting away from the loop and makes no contacts with the protein, analogous to the base at −6 in the MS2 RNA complexes. The Qβ RNA thus maintains its 3 nt loop conformation upon complex formation. There is also density for up to 4 bp, corresponding to the upper stem of the RNA (Figure 2C). There is no convincing electron density that could correspond to a base in the −10 binding pocket, allowing the backbone to form an A-type helix that, in turn, allows for contacts between phosphates +3/+4 and residue Lys89 (Figure 4A; see the Discussion). There is no interpretable density for A+1, nor any base below it apart from G+13. A Qβ variant RNA, synthesized deliberately to facilitate a possible base insertion at the −10 pocket, also showed no such contacts in the crystal structure of the complex (87Ala89Lys QβU+12).
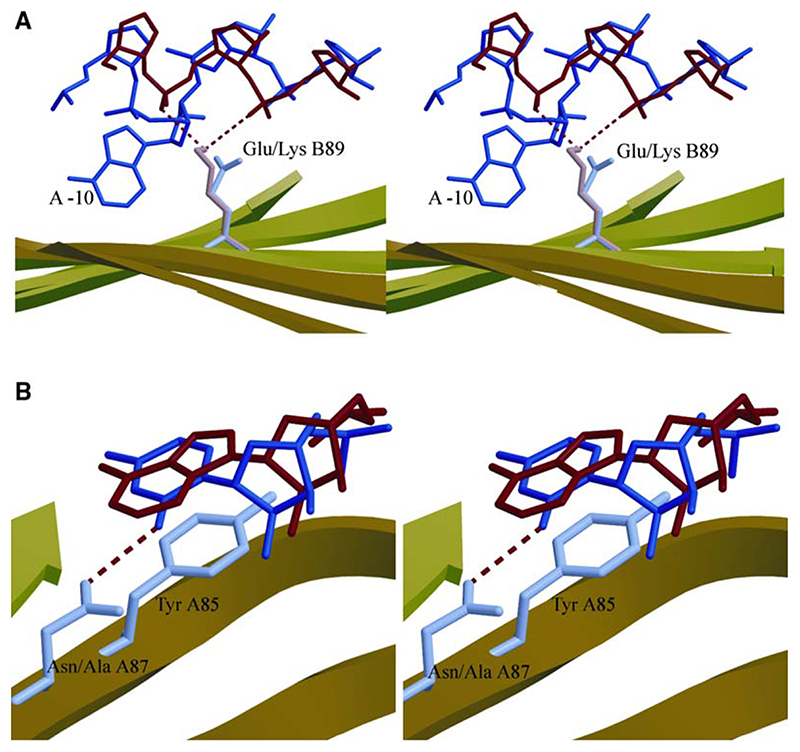
Figure 4. Stereodiagrams.
(A) Stereodiagram depicting the orientation of the GluB89 side chain (purple) relative to the wild-type MS2 stem-loop backbone (blue), including the base at −10, and the interaction between the side chain of LysB89 (pink) with the phosphate backbone of the Qβ RNA stem-loop (red).
(B) Stereodiagram depicting the stacking of the A+7 base of the 87Ala89LysQβ complex (red) and the U-5 base from the MS2 stem-loop (blue) onto TyrA85. The AsnA87 side chain and its orientation relative to the U-5 base are shown; however, the mutated AlaA87 side chain is omitted for clarity. Hydrogen bond interactions are shown as red, dashed lines in both panels.
The backbone conformation between nucleotides +4 to +8 of the Qβ RNA (the loop and the two 5′ nucleotides) is very similar to the corresponding fragment of the MS2 operator (−8 to −4), and there are numerous contacts between the P+4 and P+5 phosphates and the side chains of ArgB49, SerB51, SerB52, and LysB57, similar to those observed in the MS2 wild-type complex. The main difference between the structure of the 3 nt loop in Qβ and the 4 nt loop in the MS2 stem-loops is the absence of the unpaired A-7, which in the MS2 complexes is stacked between U-5 and G-8 (Figure 3). In the Qβ stem-loop complexes, it is C+5 that forms a stacking interaction with A+7, the equivalent of U-5 in the MS2 stem-loop. There is a significant change in the position of the +9 phosphate, compared to the −3 position in MS2, to accommodate the formation of the base pair between G+9 and C+5 that closes the 3 nt loop. The conformation of the Qβ loop is very similar to that seen previously for an MS2 aptamer, F6, that also contained a three base loop (Convery et al., 1998). Numerous hydrogen bond interactions between the CP and the Qβ stem-loop are evident from the structures (Figure 3 and Table 2), several of which are also observed in the MS2 wild-type complex (Valegård et al., 1997). The A+8 base, located within the −4 binding pocket, makes four hydrogen bonds with CP, and GluA63 makes a hydrogen bond to the 2′ hydroxyl of A+7. In the Asn87Ser and Asn87Ala mutant CP complexes, the smaller side chains preclude hydrogen bond contacts to A+7.
Discussion
The results described above list the gross structural changes in the RNA and protein ligands as they accommodate sequence and structural differences. In each case, there are a number of differences from the conformations that are observed in the MS2 wild-type complex. It is impossible to decide on the basis of the crystallography alone the relative importance of each of these changes for operator discrimination, and whether there are single key interactions or whether discrimination arises via an ensemble of small effects. An additional complication is that the complexes seen in the protein shells are formed under conditions in which the RNA ligand is in high molar excess, allowing us to see interactions with RNA fragments that have intrinsically low affinity for the coat protein. In general, however, solution binding assays reflect the expected sensitivity to changes in amino acid side chains, RNA sequence, and functional groups based on the crystal structures, implying that such low-affinity complexes do form in solution (Grahn et al., 1999, 2001; Helgstrand et al., 2002; Rowsell et al., 1998).
In order to establish the relative importance of the structural changes seen here, we have analyzed them in concert with repression efficiencies (Spingola and Peabody, 1997) obtained in in vivo reporter assays (Table 3). These data are all drawn from one set of experiments for comparability, although not all combinations of protein and RNA studied here were used.
Table 3. In Vivo Operator Binding Data.
| Coat Protein | In Vivo Repression β-Galactosidase Activity (%) a | Structural Interpretation | |
|---|---|---|---|
| MS2 Operator | Qβ Operator | ||
| MS2 wild-type | 2.4 | 50 | Qβ RNAA+7 causes a steric clash with Asn87. |
| Qβ wild-type | 100 | 3.6 | |
| MS2 87Ser | 25 | 20 | Creates more room to allow the Qβ RNA A+7 stacking interaction |
| MS2 87Ser, 89Lys | 25 | 5.3 | The improved affinity for Qβ RNA arises from the formation of the lysine backbone contact seen in the crystal structure. |
| MS2 89Lys | 50 | 25 | |
The translational repressor activities of the various proteins were measured by their ability to inhibit β-galactosidase expression from a reporter plasmid (Spingola and Peabody, 1997) containing either the MS2 (pRZ5) or the Qβ operator(pRZQ5). The completely unrepressed state gives a β-galactosidase activity of 100%, while full repression gives values in the range of 2%–4%.
It is clear that the wild-type coat proteins discriminate strongly against the noncognate operators. The steric clash with Asn87 caused by the A+7 base in the Qβ stem-loop, together with its lack of a bulged adenine in a position to insert in the −10A binding pocket, would account for its low MS2 CP affinity (Figure 4). Presumably the details of the fully cognate Qβ complex differ, allowing it similarly to disfavor binding to the MS2 RNA. Note that we have determined the X-ray crystal structure of an A-5 variant of the MS2 stem-loop that shows that the Asn87 side chain must alter its conformation to accommodate the larger base (Grahn et al., 2001). Substitution of Asn87 with the smaller Ser side chain permits the formation of two water-mediated hydrogen bonds to the base. However, this is clearly deleterious for binding the MS2 stem-loop, presumably, in part, due to the entropic cost of trapping the water molecules. Conversely, the repression data show that elimination of the steric clash with A+7 allows the mutant to recognize Qβ operators in vivo. The same mutation thus both favors noncognate binding and disfavors the cognate interaction. The magnitude of these effects implies that the Asn87 contact is the principal molecular recognition event leading to discrimination in favor of MS2 operator binding and disfavoring binding of Qβ RNA.
Glu89 makes no direct contact with MS2 RNA, and therefore its substitution might be expected to have no effect on affinity. However, it is involved in an indirect contact via its interaction with Arg49, which does contact MS2 RNA at phosphate −8. The Lys89 data are consistent with this contact to the backbone being important for the MS2 cognate interaction. In contrast, this substitution produces an improved repression of the Qβ operator in vivo that increases still further in the double mutant in which the steric clash with AsnA87 is also removed. Substitution of Glu89 favors Qβ RNA binding by eliminating a potentially unfavorable electrostatic interaction and replacing it with the favorable interactions to phosphates +3 and +4 (Figure 4A). A similar favorable contact to the MS2 RNA cannot be made due to differences in phosphodiester conformations in this region. In MS2, this difference is a consequence of the bulged adenine (A-10) and its extrusion from the A helical stem to make the contact with the protein. This interaction appears to be a secondary barrier discriminating against noncognate binding and arises from a combination of the presence of the wild-type Glu89 side chain in the protein and the secondary structure of the MS2 operator.
The structures described here explain the observed packaging discrimination of these two RNA phages in vivo. Previous studies with this system, however, show that the RNA-protein interface can adapt to make interactions in response to changes in protein side chains and RNA functional groups, in one case leading to a dramatic conformational rearrangement (Grahn et al., 2000) and in several cases to effects that have no obvious structural explanation (Grahn et al., 2001). The picture that emerges is of a generic RNA binding site that can be tailored to accommodate differing detailed RNA-protein contacts, and we are exploring further details of this fascinating system.
Experimental Procedures
Expression, Crystallization, and RNA Synthesis
Site-directed mutants of the MS2 expression plasmid were generated by overlap extension mutagenesis (Higuchi et al., 1988; Ho et al., 1989) by using Pfu Turbo DNA polymerase (Stratagene). To construct double mutants, the template used was a plasmid expressing the appropriate single mutant MS2. Primers were synthesized by Interactiva/Hybaid. Plasmid purifications and extraction of DNA fragments after agarose gel electrophoresis were performed by using Qiagen kits (Qiagen GmbH). The final product was gel purified and ligated to the XbaI/EcoRI fragment of vector pTAC-ACP′-λB. Plasmids purified from transformed ampicillin-resistant XL10-gold (Stratagene) colonies were screened by gel electrophoresis of XhoI digests. The DNA sequences of plasmids were verified on both strands of the insert region (DBS Genomics, University of Durham).
Mutant and wild-type MS2 CP were overexpressed in E. coli and were purified by using previously described methods (Mastico et al., 1993; Stonehouse and Stockley, 1993). Crystals of MS2 capsids, largely free of RNA, were grown by using the hanging drop technique under crystallization conditions used previously (Valegård et al., 1986). The MS2 and Qβ RNA stem-loops were synthesized via solid-phase phosphoramidite chemistry and were purified as described (Murray et al., 1994). Crystals were up to 1.5mm in size and were soaked with RNA at a final concentration of 2 mg/ml for at least 5 days prior to mounting in loops or glass capillaries for data collection (Valegård et al., 1994).
Data Collection and Processing
Crystals are of space group R32 with cell dimensions a = b = 288.0 Å and c = 653.0 Å. For freezing, crystals were soaked in mother liquor containing 30% (v/v) glycerol. Upon freezing, the packing of the particles changes slightly, resulting in different cell dimensions and space groups. Data sets collected from these frozen crystals are often very difficult to scale to each other due to small changes in cell dimensions and thus in the intensities of the reflections. Diffraction data for the complexes were collected at the synchrotrons at SRS, Daresbury Laboratories, Warrington, United Kingdom; ESRF, Grenoble, France; and Max-lab, Lund, Sweden, at the beam-lines indicated in Table 1. Data collected at SRS (87SerQβ, 87Ser89LysQβ, 87SerMS2, and 87Ser89LysMS2 complexes) were processed with MOSFLM and scaled with SCALA (CCP4, 1994). The rest of the data were processed with DENZO and scaled by using SCALEPACK (Otwinowski and Minor, 1996). The program TRUNCATE (CCP4, 1994) was used to derive structure factor amplitudes from the scaled data. Statistics from the data collection, scaling, and refinement are shown in Table 1.
Structural Determination and Refinement
Initial phases for the data were derived by using the published coordinates of the MS2 capsid (Golmohammadi et al., 1993) (PDB entry 2MS2) as a phasing model. The CNS program suite (Brünger et al., 1998) was utilized for the refinement of each structure, with stereochemical restraints applied throughout the refinement for both the protein (Engh and Huber, 1991) and the RNA (Parkinson et al., 1996). In the cases in which the space group and cell dimensions were changed, the rotation function was used to determine the orientation of the particle in relation to the axes. For this, the locked rotation function in program GLRF (Tong and Rossmann, 1990) was used. In each case, an initial round of rigid body refinement was applied to the capsid crystallographic asymmetric unit, 2Fo – Fc and Fo – Fc electron density maps were calculated, and cyclic real space averaging over the 10-fold (R32), 30-fold (C2), or 60-fold (P1) noncrystallographic symmetry was carried out to improve the quality of the maps by using the RAVE package (Kleywegt and Jones, 1994). For the P1 crystals, the cell dimensions obtained from postrefinement in SCALEPACK were not sufficiently accurate. They were improved by using RAVE; the same map was averaged with cell dimensions that were changed stepwise until the optimal correlation coefficient between calculated and observed structure factor amplitudes was obtained.
Refinement of atomic positions was alternated with the refinement of the individual atomic temperature factors (20 cycles per refinement). Strict noncrystallographic symmetry, with all subunits of the same type constrained to be identical, and a bulk-solvent correction were applied throughout the refinements.
Molecular envelopes for the icosahedral asymmetric unit, containing the three protein capsid subunits A, B, and C and the regions corresponding to the bound RNA, were generated by using the coordinates of the wild-type complex (Valegård et al., 1997) (PDB entry 1ZDI). Envelopes were calculated by using the program MAMA within the RAVE package (Kleywegt and Jones, 1994). In each case, the electron density for the bound RNA was clearly defined, and the RNA at the AB and CC binding sites was modeled with the program O (Jones et al., 1991).
After both the AB and CC RNA had been built into the models, water molecules were added at positions at which peaks in the electron density were within range of a hydrogen bond donor/acceptor. A summary of the refinement statistics for the complexes is given in Table 1.
Analysis of the stereochemistry was carried out by using PRO-CHECK (Laskowski et al., 1993). The Ramachandran plots showed that there were only two residues in disallowed or generously allowed regions. Both residues (AsnC36 and SerB2) have been previously observed to have unusual backbone angles in several other MS2 RNA complexes (Valegård et al., 1997; Grahn et al., 2000). The RNA geometries of the complexes were checked with the program CURVES (Lavery and Sklenar, 1988). The atomic coordinates for the complexes have been deposited in the Protein Data Bank (PDB) with identification codes as indicated in Table 1.
Graphics
All figures except Figure 1A were prepared with SPOCK (http://mackerel.tamu.edu/spock/) and MOLSCRIPT (Kraulis, 1991). Figure 1A was created with PYMOL (DeLano, 2002).
Acknowledgments
This work was supported by The Leverhulme Trust, the United Kingdom Biotechnology and Biological Sciences Research Council and The Wellcome Trust, and the Swedish Research Council. W.T.H. was supported by a UK BBSRC studentship. Fariborz Nasertorabi contributed to data collection and processing at early stages of this work.
Footnotes
References
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS. Crystallography & NMR System: a new software suite for macromolecular structural determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- CCP4 (Collaborative Computational Project, Number 4) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Convery MA, Rowsell S, Stonehouse NJ, Ellington AD, Hirao I, Murray JB, Peabody DS, Phillips SEV, Stockley PG. Crystal structure of an RNA aptamer-protein complex at 2.8 Å resolution. Nat Struct Biol. 1998;5:133–139. doi: 10.1038/nsb0298-133. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. 2002. http://www.pymol.org .
- Engh RA, Huber R. Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr A. 1991;47:392–400. [Google Scholar]
- Golmohammadi R, Valegård K, Fridborg K, Liljas L. The refined structure of bacteriophage MS2 at 2.8 Å resolution. J Mol Biol. 1993;234:620–639. doi: 10.1006/jmbi.1993.1616. [DOI] [PubMed] [Google Scholar]
- Golmohammadi R, Fridborg K, Bundule M, Valegård K, Liljas L. The crystal structure of bacteriophage Qβ at 3.5Å resolution. Structure. 1996;4:543–554. doi: 10.1016/s0969-2126(96)00060-3. [DOI] [PubMed] [Google Scholar]
- Grahn E, Stonehouse NJ, Murray JB, van den Worm S, Valegård K, Fridborg K, Stockley PG, Liljas L. Crystallographic studies of RNA hairpins in complexes with recombinant MS2 capsids: implications for binding requirements. RNA. 1999;5:131–138. doi: 10.1017/s1355838299981645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn E, Stonehouse NJ, Adams CJ, Fridborg K, Beigelman L, Matulic-Adamic J, Warriner SL, Stockley PG, Liljas L. Deletion of a single hydrogen bonding atom from the MS2 RNA operator leads to dramatic rearrangements at the RNA-coat protein interface. Nucleic Acids Res. 2000;28:4611–4616. doi: 10.1093/nar/28.23.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn E, Moss T, Helgstrand C, Fridborg K, Sundarum M, Tars K, Lago H, Stonehouse NJ, Davis DR, Stockley PG, Liljas L. Structural basis of pyrimidine specificity in the MS2 RNA hairpin-coat-protein complex. RNA. 2001;7:1616–1627. [PMC free article] [PubMed] [Google Scholar]
- Helgstrand C, Grahn E, Moss T, Stonehouse NJ, Tars K, Stockley PG, Liljas L. Investigating the structural basis of purine specificity in the structures of MS2 coat protein RNA translational operator hairpins. Nucleic Acids Res. 2002;30:2678–2685. doi: 10.1093/nar/gkf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro and preparation for specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao I, Spingola M, Peabody D, Ellington AD. The limits of specificity: an experimental analysis with RNA aptamers to MS2 coat protein variants. Mol Divers. 1999;4:75–89. doi: 10.1023/a:1026401917416. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain-reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horn WT, Convery MA, Stonehouse NJ, Adams CJ, Liljas L, Phillips SEV, Stockley PG. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: further challenges in the modelling of ligand-RNA interactions. RNA. 2004;10:1776–1782. doi: 10.1261/rna.7710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowen SW, Kjeldgaard M. Improved methods for building models in electron density maps and locations of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kleywegt GJ, Jones TA. In: From First Map to Final Model. Bailey S, Hubbard R, Waller D, editors. EPSRC Daresbury Laboratory; Warrington, UK: 1994. Halloween…masks and bones; pp. 59–66. [Google Scholar]
- Kraulis PJ. Molscript: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- Laskowski RA, Macarthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- Lavery R, Sklenar H. The definition of generalised heli-coidal parameters and of axis curvature for irregular nucleic acids. J Biomol Struct Dyn. 1988;6:63–91. doi: 10.1080/07391102.1988.10506483. [DOI] [PubMed] [Google Scholar]
- Lim F, Spingola M, Peabody DS. Altering the RNA binding specificity of a translational repressor. J Biol Chem. 1994;269:9006–9010. [PubMed] [Google Scholar]
- Lim F, Spingola M, Peabody DS. The RNA binding site of bacteriophage Qβ coat protein. J Biol Chem. 1996;271:31839–31845. doi: 10.1074/jbc.271.50.31839. [DOI] [PubMed] [Google Scholar]
- Ling CM, Hung PP, Overby LR. Independent assembly of Qβ and MS2 phages in doubly infected E.coli . Virology. 1970;40:920–929. doi: 10.1016/0042-6822(70)90138-8. [DOI] [PubMed] [Google Scholar]
- Mastico RA, Talbot SJ, Stockley PG. Multiple presentation of foreign peptides on the surface of an RNA-free spherical bacteriophage capsid. J Gen Virol. 1993;74:541–548. doi: 10.1099/0022-1317-74-4-541. [DOI] [PubMed] [Google Scholar]
- Murray JB, Collier AK, Arnold JRP. A general purification procedure for chemically synthesised oligoribonucleotides. Anal Biochem. 1994;218:177–184. doi: 10.1006/abio.1994.1157. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. In: Methods in Enzymology. Carter CW Jr, Sweet RM, editors. Academic Press; New York: 1996. Processing of X-ray diffraction data collected in oscillation mode; pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Parkinson G, Vojtechovsky J, Clowney L, Brügner AT, Berman HM. New parameters for the refinement of nucleic-acid containing structures. Acta Crystallogr D Biol Crystal-logr. 1996;52:57–64. doi: 10.1107/S0907444995011115. [DOI] [PubMed] [Google Scholar]
- Rowsell S, Stonehouse NJ, Convery MA, Adams CJ, Ellington AD, Hirao I, Peabody DS, Stockley PG, Phillips SEV. Crystal structures of a series of RNA aptamers complexed to the same protein target. Nat Struct Biol. 1998;5:970–975. doi: 10.1038/2946. [DOI] [PubMed] [Google Scholar]
- Spingola M, Peabody DS. MS2 coat protein mutants which bind Qβ RNA. Nucleic Acids Res. 1997;25:2808–2815. doi: 10.1093/nar/25.14.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spingola M, Lim F, Peabody DS. Recognition of diverse RNAs by a single protein structural framework. Arch Biochem Biophys. 2002;405:122–129. doi: 10.1016/s0003-9861(02)00334-x. [DOI] [PubMed] [Google Scholar]
- Stonehouse NJ, Stockley PG. Effects of amino acid substitution on the thermal stability of MS2 capsids lacking genomic DNA. FEBS Lett. 1993;334:355–389. doi: 10.1016/0014-5793(93)80711-3. [DOI] [PubMed] [Google Scholar]
- Tong L, Rossmann MG. The locked rotation function. Acta Crystallogr A. 1990;46:783–792. doi: 10.1107/s0108767390005530. [DOI] [PubMed] [Google Scholar]
- Valegård K, Unge T, Montelius I, Strandberg B. Purification, crystallisation and preliminary X-ray data of the bacteriophage MS2. J Mol Biol. 1986;190:587–591. doi: 10.1016/0022-2836(86)90244-5. [DOI] [PubMed] [Google Scholar]
- Valegård K, Liljas L, Fridborg K, Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Valegård K, Murray JB, Stockley PG, Stonehouse NJ, Liljas L. Crystal structure of a bacteriophage RNA coat protein operator system. Nature. 1994;371:623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- Valegård K, Murray JB, Stonehouse NJ, van den Worm S, Stockley PG, Liljas L. The three dimensional structures of two complexes between recombinant MS2 capsids and RNA operator fragments reveal sequence specific protein-RNA interactions. J Mol Biol. 1997;270:724–738. doi: 10.1006/jmbi.1997.1144. [DOI] [PubMed] [Google Scholar]
- van den Worm S, Stonehouse NJ, Valegård K, Murray JB, Walton C, Stockley PG, Liljas L. Crystal structures of MS2 coat protein mutants in complex with wild-type RNA operator fragments. Nucleic Acids Res. 1998;26:1345–1351. doi: 10.1093/nar/26.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherell GW, Uhlenbeck OC. Specific RNA binding by Qβ coat protein. Biochemistry. 1989;28:71–76. doi: 10.1021/bi00427a011. [DOI] [PubMed] [Google Scholar]