Abstract
Background
Associations between sleep duration and Type 2 diabetes (T2D) risk markers in childhood have been little studied. We examined associations between self-reported sleep duration and T2D risk markers in children.
Methods
Cross-sectional study of 4525 multi-ethnic UK children aged 9-10 years. Sleep time was calculated from self-reported usual time of going to bed and getting up on a school day. Sleep duration was validated in a sub-set using accelerometers. Fasting blood samples provided levels of serum lipids and insulin, plasma glucose, HbA1c. Physical measures included height, weight, bioimpedance and blood pressures. Multilevel linear regression models of anthropometric, T2D and cardiovascular risk markers with sleep duration were adjusted for sex, age, month, ethnicity, socioeconomic position, observer (physical measures only) and random effect of school.
Results
On average children slept 10.5 hours/night (95% range 8.0-12.0 hours). There were strong inverse graded relationships between sleep duration, adiposity and diabetes risk markers. In adjusted models, a one hour greater sleep duration was associated with 0.19kg/m2 lower body mass index (95%CI 0.09,0.28kg/m2), 0.03 kg/m5 lower fat-mass index (95%CI 0.00,0.05kg/m5), 2.9% lower HOMA insulin resistance (95%CI 1.2,4.4%), and 0.24% lower fasting glucose (95%CI 0.03,0.44%); there was no association with HbA1c or with cardiovascular risk. Associations with insulin and glucose remained after additional adjustment for adiposity markers.
Conclusions
The finding of an inverse association between sleep duration and T2D risk markers in childhood is novel. Intervention studies are needed to establish the causality of these associations, which could provide a simple strategy for early T2D prevention.
Keywords: Children, Sleep duration, Adiposity, Type 2 Diabetes
Introduction
The prevalence of type 2 diabetes (T2D), overweight and obesity have been rising in the UK and in many other countries, 1,2 not only in adults but also in adolescents and children. 3 Understanding the early determinants of adiposity and T2D risk in young people could be particularly important for reducing risks of T2D and obesity across the life course.
There has been particular interest in the importance of sleep for T2D and adiposity risks.
Sleep duration has complex prospective relations with adiposity and T2D in adults, with both short and long sleep durations associated with higher risk. 4–6 In contrast, studies in childhood have shown graded inverse associations between sleep duration and levels of adiposity, with increased sleep duration associated with lower levels of obesity; there has been little evidence that longer sleep duration is associated with increased adiposity. 5,7 However, little is known about the effects of sleep on other T2D risk markers in childhood, particularly glycaemic blood markers and insulin resistance. Such associations could be of public health importance; while sleep durations have appeared relatively stable in adulthood, 8 recent evidence suggests that average sleep duration has declined in children and adolescents over time, particularly over the last 15 years where actual sleep duration has declined by 0.73 minutes per year. 9 The ramifications of these reduced sleep durations on future health are yet to be established. However, if shorter sleep duration is related to emerging T2D risk in childhood, evidence on optimal sleep duration for protecting against T2D risk could underpin efforts to prevent T2D risk at an early stage. 10 Moreover, early ethnic differences in T2D precursors, particularly where South Asians have a worse metabolic profile compared to children of white European ancestry, 11 might be partially explained by differences in sleep duration. If so, this may offer a further strategy to reduce ethnic differences in T2D risk in early life.
We therefore examined the association between self-reported sleep duration, T2D and cardiovascular risk factors, in a large-scale multi-ethnic population based study of 9 to 10 year old children.
Patients and Methods
The Child Heart and Health Study in England (CHASE) was a cross-sectional investigation of the cardiovascular and type 2 diabetes risk profiles of UK primary school children aged 9-10 years of white European, South Asian and black African-Caribbean origin. Ethical approval was obtained from the relevant multicentre research ethics committee. Full details of study methods have been published. 11 The study was based in 200 state primary schools in London, Birmingham and Leicester, half with a high prevalence of UK South Asian children (stratified by Indian, Pakistani and Bangladeshi origin) and half with a high prevalence of UK black African-Caribbean children (stratified by black African and black Caribbean origin). Informed parental consent and child assent were obtained.
Measurements of body composition
A single survey team of three trained Research Nurses made all measurements between October 2004 and February 2007; to limit ethnic differences in observer bias, each observer measured approximately one-third of children in each ethnic group. Height and weight were measured and body mass index (BMI) calculated as kg/m2. To provide an objective measure of adiposity, fat mass was determined from arm-to-leg bioelectrical impedance, measured on the right side using the Bodystat 1500 bioelectrical impedance monitor (Bodystat Ltd, Isle of Man, UK); fat mass was derived using equations derived specifically for UK children of this age group which were sex and ethnic group specific. 12 Fat mass was height-standardized (fat mass index = fat mass (kg)/height (m)5). Skinfold thicknesses were also measured to provide a subcutaneous measure of adiposity, at the biceps, triceps, subcapsular and suprailiac locations Skinfold thickness provide a better predictor of body fatness, compared to weight-for-height measures. Seated blood pressure (BP) was measured twice in the right arm after a 5 minute rest using an Omron HEM-907 (Omron Electronics Ltd., Milton Keynes, UK) with the appropriate cuff size; the mean of the two values was used in analysis after adjustment for cuff size. 13
Blood measurements
A blood sample was obtained after an overnight fast; children were asked not to eat on the morning of the examination and those who reported having eaten breakfast were excluded from analysis. Serum for insulin assay was separated and frozen on dry ice immediately after collection. All other samples were shipped to a central laboratory within 48 hours. Insulin, glucose, glycated haemoglobin (HbA1c) and blood lipids were measured as described previously. The homeostasis model assessment (HOMA) model equations were used to provide an estimate of insulin resistance. 14 Serum urate was assayed using an enzymatic method. 15
Questionnaire data
Children were asked two questions on sleeping habits on a school day; “what time do you usually go to bed on schooldays?” and “what time do you usually get up in the morning on schooldays?”. The difference between these two times was used to define sleep duration. Ethnic origin of the child was based on self-defined parental ethnicity (coded using a classification similar to the 2001 UK Census) or (if not available) parental report of the ethnic origin of the child or using information on the report of parental and grand-parental place of birth as previously described. 11 In the present analyses, ‘white European’ includes children whose ethnic origin was defined as ‘white British’, ‘white Irish’ and ‘white European’ (or a combination of these) and excludes ‘white Other’. ‘South Asian’ includes ‘Indian’, ‘Pakistani’, ‘Bangladeshi’ and ‘Sri Lankan’ (or a combination of these). Remaining Asian children were classified as ‘Asian Other’ and included Asian-mixed ethnicity, Chinese and Middle-Eastern ethnic groups. ‘Black African-Caribbean’ includes ‘Black African’, ‘Black Caribbean’, ‘Black British’ and ‘Black Other’ (or combination of these). Children of other ethnic groups and mixed ethnic origin (except Asian) were allocated to a separate ‘Other’ group. Parental socio-economic position was based on self-reported parental occupation and coded using UK National Statistics Socioeconomic Classification (NS-SEC) for the parent with the highest NS-SEC grade. 16 The three class version was used in all analyses (professional and managerial, intermediate, routine and manual) as previously described. 17 Self-reported pubertal status was measured in girls only, using the Tanner development score. 18
Physical activity assessment
In a subset of children, recruited from 79 schools in the latter phase of the study (January 2006 to February 2007), objective assessment of physical activity was carried out using a waist-worn accelerometer (ActiGraph GT1M, ActiGraph, LLC, Pensacola, FL). Details of the physical activity assessment have been published previously. 19 In brief, children were asked to wear the monitor (worn at the waist above the left hip using an elasticated belt) during waking hours for seven whole days. On return of the instrument a dedicated software program was used to determine activity outcomes, including steps by hour of the day, allowing activities before and after self-reported bed times to be examined. It also allowed a comparison of monitor non-wear time and reported sleep duration.
Statistical Analysis
Statistical analyses were carried out using STATA/SE software (Stata 13 for Windows, StataCorp LP, College Station, TX, USA). All diabetes and cardiovascular risk markers and body composition variables were examined for normality and log transformed where necessary. Multilevel linear regression models adjusted for age in quartiles, sex, month, ethnicity, social position and random effect for school were used to calculate adjusted means for measures of body composition and diabetes / cardiovascular risk markers by five categories of sleep duration (less than 9 hours, 9 to 9.9 hours, 10 to 10.9 hours, 11 to 11.9 hours and 12 hours or more). Continuous linear associations with sleep duration in hours were determined using the same adjustments; for outcomes that were log transformed associations are quantified as the percentage difference per extra hour of sleep. Associations with sleep duration were also examined in boys and girls separately and by ethnicity. Effects of adjustment for pubertal status (girls only) were also explored. Linear associations between sleep duration, T2D and cardiovascular risk markers which were statistically significant were further adjusted for measures of adiposity including fat mass index and fat free mass index. The contribution of sleep duration to previously reported ethnic differences in diabetes and cardiovascular risk markers 11,20 was also examined.
Results
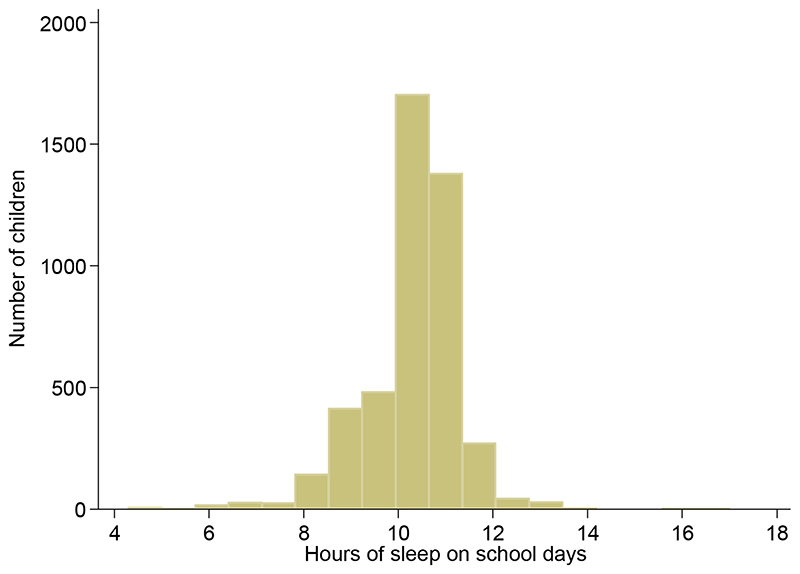
Of 8641 children invited to participate in CHASE, 5887 (68%) took part. Among 5681 singleton children, 4525 (80%) provided a fasting blood sample, had complete data for measures of body composition and self-reported data for bedtime and getting up time on a school day. On average children were 10.0 years old (SD 0.4 years, reference range 9.2 to 10.7 years). Sleep duration was on average 10.5 hours per night on a school day (95% central range 8.0 to 12.0 hours – Figure 1). In a subset of 1766 children who wore an accelerometer during waking hours, a mean of 600 steps per hour were recorded during the hours of 8am to 7pm on a school day but only 54 steps in the hour after reported bedtime, compared with 234 steps in the hour before bedtime. One hour before wake up time the number of steps recorded was zero compared with 435 steps on average one hour after wake time. In the subset that wore an accelerometer, daily non-wear time was 10.2 hours, and reported sleep duration 10.3 hours (equivalent to a mean difference of 7 minutes, 95% CI 4 to 10 minutes).
Figure 1. Distribution of hours of sleep on school days.
Table 1 shows demographic characteristics of the children in relation to sleep duration on a school day. Children who had longer sleep duration were on average slightly younger and were more likely to be girls (Table 1). Sleep duration differed marginally by ethnicity; white European children had the longest mean sleep duration and Black African Caribbean children the shortest. There was no clear evidence of any trend in sleep duration by parental social position.
Table 1. Demographic characteristics for 4525 children by duration of sleep with adjusted average sleep duration.
| Hours of self-reported sleep on a school night $ | |||||||
|---|---|---|---|---|---|---|---|
| Less than 9 hours |
9-9.9hrs | 10-10.9hrs | 11-11.9hrs | 12hrs or more | Adjusted mean sleep |
P value† | |
| N = | 279 (6.2%) | 841 (18.6%) |
2014 (44.5%) |
1166 (25.8%) |
225 (5%) | hours (95%CI) | |
| Average age years (SD) | 10.0 (0.4) | 10.0 (0.4) | 10.0 (0.4) | 9.0 (0.4) | 9.8 (0.4) | 10.3 (10.2, 10.3) | 0.05 |
| Males | 180(8.2) | 431(19.7) | 963(44.1) | 501(22.9) | 110(5.0) | 10.2 (10.1, 10.2) | |
| Females | 99(4.2) | 410(17.5) | 1051(44.9) | 665(28.4) | 115(4.9) | 10.4 (10.3, 10.4) | <0.0001 |
| Ethnic group | |||||||
| White European | 43(3.9) | 179(16.4) | 493(45.3) | 316(29.0) | 58(5.3) | 10.4 (10.3, 10.5) | <0.0001 |
| Black African- | |||||||
| Caribbean | 87(7.6) | 245(21.4) | 508(44.4) | 252(22.0) | 51(4.5) | 10.1 (10.1, 10.2) | |
| South-Asian | 89(7.2) | 217(17.5) | 551(44.4) | 335(27.0) | 50(4.0) | 10.2 (10.2, 10.3) | |
| Asian Other | 17(6.1) | 56(20.1) | 121(43.5) | 71(25.5) | 13(4.7) | 10.2 (10.1, 10.3) | |
| Other | 43(5.6) | 144(18.6) | 341(44.1) | 192(24.8) | 53(6.9) | 10.3 (10.2, 10.4) | |
| Parental social economic position | |||||||
| Managerial/Professional | 55(4.3) | 220(17.4) | 629(49.6) | 316(24.9) | 47(3.7) | 10.3 (10.2, 10.4) | 0.04 |
| Intermediate | 75(6.5) | 239(20.6) | 511(44.1) | 290(25.0) | 45(3.9) | 10.2 (10.1, 10.3) | |
| Routine/Manual | 96(6.9) | 254(18.3) | 586(42.3) | 361(26.1) | 88(6.4) | 10.3 (10.2, 10.3) | |
| Economically Inactive | 33(7.2) | 77(16.9) | 171(37.5) | 143(31.4) | 32(7.0) | 10.3 (10.2, 10.4) | |
| Unclassified/missing | 20(7.8) | 51(19.8) | 117(45.5) | 56(21.8) | 13(5.1) | 10.2 (10.1, 10.3) | |
values are mean and SD for age; for all categorical variables values are N (row %)
p-value from likelihood ratio test for heterogeneity from the same multilevel model
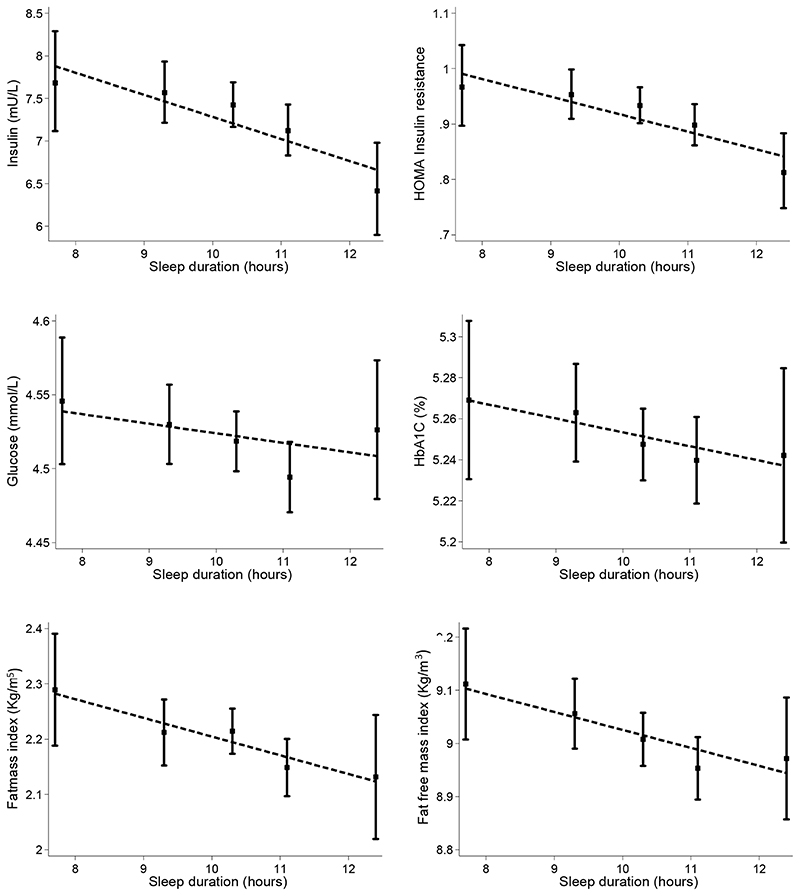
There were strong inverse linear relationships between sleep duration and all measures of body size and fatness (Figure 2, Table 2 and Supplementary Figure S1). Children who slept longer were on average shorter, had lower body weight, fat free mass and lower levels of fat mass index and skinfold thickness; effect sizes for adiposity measures, including BMI, sum of skinfolds, and leptin, were statistically significant and between 1-3% per extra hour of sleep. Sleep duration was also inversely related to insulin, insulin resistance and glucose. Per extra hour of sleep insulin levels were lower by nearly 3% (95%CI from 1.2% to 4.5% reduction) and a similar association was seen for insulin resistance (Table 2). The size of the association with glucose was smaller and there was no clear evidence of an association with HbA1c (Table 2). There was no evidence of associations with lipids or blood pressure. Very similar strengths of associations were found in analyses stratified by ethnicity and gender. (Supplementary Table S1). There was no consistent evidence of effect modification by gender (likelihood ratio test (LRT) for interaction with gender p-value >0.1 for all measures of body size and cardio-metabolic risk markers) or ethnicity (LRT p value for interaction >0.2 in all instances).
Figure 2.
Adjusted means for type 2 diabetes risk markers and body fatness indices by sleep duration. Means are adjusted for sex, age quartiles, month, ethnicity, social position, observer (physical measures only) and random effect for school.
Table 2. Adjusted mean values of type 2 diabetes and cardiovascular risk markers and difference in risk markers per hour increase in sleep.
| Adjusted means (95% confidence intervals) | |||||||
|---|---|---|---|---|---|---|---|
| Hours of self-reported sleep on a school night | |||||||
| Less than 9 hours |
9-9.9hrs | 10-10.9hrs | 11-11.9hrs | 12hrs or more |
Difference per hour |
||
| Body size | 279 | 841 | 2014 | 1166 | 225 | of sleep (95%CI) ** |
p-value† |
| Height (cm) | 140.1 (139.3,140.9) |
141.0 (140.6,141.5) |
140.2 (139.9,140.5) |
140.0 (139.6,140.4) |
139.0 (138.2,139.9) |
-0.26 (-0.44,-0.08) |
0.005 |
| Weight (Kg) | 37.8 (36.7,38.9) |
38.2 (37.6,38.8) |
37.2 (36.8,37.6) |
36.5 (36.0,37.0) |
35.4 (34.2,36.6) |
-0.51 (-0.76,-0.26) |
<0.0001 |
| Body mass index (kg/m2) |
19.1 (18.7,19.5) |
19.0 (18.8,19.3) |
18.7 (18.6,18.9) |
18.4 (18.2,18.6) |
18.2 (17.7,18.6) |
-0.19 (-0.28,-0.09) |
<0.0001 |
| Sum skin folds (mm)* | 42.0 (39.6,44.5) |
42.8 (41.3,44.2) |
41.2 (40.3,42.1) |
39.9 (38.8,41.1) |
38.8 (36.4,41.3) |
-1.79 (-3.08,-0.49) |
0.007 |
| Fat mass (Kg) | 12.6 (12.0,13.3) |
12.7 (12.3,13.1) |
12.3 (12.0,12.5) |
11.8 (11.5,12.2) |
11.2 (10.5,12.0) |
-0.27 (-0.42,-0.11) |
0.001 |
| Fat free mass (Kg) | 25.2 (24.7,25.7) |
25.5 (25.2,25.8) |
25.0 (24.8,25.2) |
24.7 (24.5,24.9) |
24.2 (23.7,24.8) |
-0.24 (-0.35, -0.13) |
<0.0001 |
| Fat mass index (Kg/m5) |
2.29 (2.19,2.39) |
2.21 (2.15,2.27) |
2.21 (2.17,2.26) |
2.15 (2.10,2.20) |
2.13 (2.02,2.24) |
-0.03 (-0.05,0.00) |
0.03 |
| Fat-free mass index (Kg/m3) |
9.11 (9.01,9.22) |
9.06 (8.99,9.12) |
9.01 (8.96,9.06) |
8.95 (8.89,9.01) |
8.97 (8.86,9.09) |
-0.04 (-0.06,-0.01) |
0.002 |
| Cardiometabolic risk markers | |||||||
| Insulin (mU/L)* ‡ | 7.68 (7.12,8.29) |
7.57 (7.22,7.93) |
7.42 (7.17,7.69) |
7.12 (6.83,7.43) |
6.42 (5.90,6.98) |
-2.88 (-4.50, -1.23) |
0.001 |
| Insulin resistance* ‡ | 0.97 (0.90,1.04) |
0.95 (0.91,1.00) |
0.93 (0.90,0.97) |
0.90 (0.86,0.94) |
0.81 (0.75,0.88) |
-2.81 (-4.41, -1.17) |
0.001 |
| Glucose (mmol/L)* | 4.55 (4.50,4.59) |
4.53 (4.50,4.56) |
4.52 (4.50,4.54) |
4.49 (4.47,4.52) |
4.53 (4.48,4.57) |
-0.24 (-0.44, -0.03) |
0.03 |
| HbA1c (%) | 5.27 (5.23,5.31) |
5.26 (5.24,5.29) |
5.25 (5.23,5.26) |
5.24 (5.22,5.26) |
5.24 (5.20,5.28) |
-0.01 (-0.02, 0.00) |
0.11 |
| HbA1c (mmol/mol) | 34 (34,35) |
34 (34,34) |
34 (34,34) |
34 (34,34) |
34 (34,34) |
0.11 | |
| Leptin (ng/mL)* ‡ | 9.2 (8.2,10.3) |
9.7 (9.1,10.4) |
9.5 9.1,9.9) |
8.6 (8.1,9.1) |
8.0 (7.1,9.0) |
-3.03 (-5.42, -0.59) |
0.02 |
| Total cholesterol (mmol/L) |
4.6 (4.5,4.7) |
4.6 (4.5,4.6) |
4.6 (4.5,4.6) |
4.6 (4.5,4.6) |
4.5 (4.4,4.6) |
-0.01 (-0.03, 0.01) |
0.32 |
| LDL cholesterol (mmol/L) |
2.7 (2.6,2.8) |
2.7 (2.6,2.7) |
2.7 (2.6,2.7) |
2.7 (2.7,2.7) |
2.7 (2.6,2.8) |
-0.01 (-0.02, 0.01) |
0.57 |
| HDL cholesterol (mmol/L) |
1.5 (1.5,1.5) |
1.5 (1.5,1.5) |
1.5 (1.5,1.5) |
1.5 (1.5,1.5) |
1.5 (1.5,1.5) |
0.00 (-0.01, 0.01) |
0.70 |
| Triglyceride (mmol/L)* |
0.8 (0.8,0.8) |
0.8 (0.8,0.8) |
0.8 (0.8,0.8) |
0.8 (0.8,0.8) |
0.8 (0.7,0.8) |
-0.35 (-1.37, 0.67) |
0.50 |
| Urate (mmol/L)* | 0.2 (0.2,0.2) |
0.2 (0.2,0.2) |
0.2 (0.2,0.2) |
0.2 (0.2,0.2) |
0.2 (0.2,0.2) |
0.20 (-0.48, 0.89) |
0.56 |
| Systolic blood pressure (mmHg) |
104.1 (102.9,105.4) |
105.1 (104.3,105.8) |
104.9 (104.4,105.4) |
104.5 (103.8,105.1) |
104.3 (102.9,105.7) |
0.03 (-0.25, 0.31) |
0.84 |
| Diastolic blood pressure (mmHg) |
62.9 (61.8,64.1) |
63.5 (62.8,64.2) |
63.0 (62.4,63.5) |
62.8 (62.2,63.4) |
62.4 (61.2,63.6) |
0.00 (-0.25, 0.25) |
0.97 |
Adjusted means of outcome variables by sleep category from multilevel model adjusted for sex, age quartiles, month, ethnicity, social position, observer (physical measures only) and random effect for school
geometric means for log transformed variables
insulin and insulin resistance missing data for 76 children, leptin missing data for 97 children
regression coefficients from multilevel model for continuous association with hours of sleep adjusted for sex, age quartiles, month, ethnicity, social position, observer (physical measures only) and random effect for school. For log transformed outcomes these represent % differences
p-values for regression coefficient from the multilevel model assuming a linear continuous association with sleep duration
In addition to factors adjusted for in Table 2, Table 3 shows associations with insulin and insulin resistance were slightly attenuated, with further adjustment for height (Table 3; model 1). Additional adjustment for fat mass index (model 2) or fat free mass index (model 3) or both (model 4) attenuated the associations further for insulin and insulin resistance, and were no longer statistically significant after adjustment for fat free mass index. In the fully adjusted (model 4) coefficients were approximately halved when compared with coefficients in Table 2 that did not adjust for height or adiposity. Associations between sleep duration and glucose were little affected by adjustment for height or body fatness. Adjustment for pubertal status (recorded in girls only) also made no difference to the findings, as did exclusion of girls who had entered puberty. Physical activity was recorded objectively over a week in a subset of children (n=1492) using Actigraph monitors. Adjustment for the average level of physical activity on a school day did not alter the results, but there was a loss in power due to the analyses being based on reduced numbers.
Table 3. Adjusted difference in type 2 diabetes risk markers per hour increase in sleep.
| % difference in metabolic risk marker per 1 hr increase in sleep (95% confidence interval); P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | Insulin | Insulin resistance | Glucose | HbA1c | ||||
| 1 | -2.22 (-3.77, -0.65) | 0.006 | -2.16 (-3.70, -0.60) | 0.007 | -0.21 (-0.42, -0.01) | 0.04 | -0.01 (-0.01, 0.00) | 0.14 |
| 2 | -1.49 (-2.89, -0.07) | 0.04 | -2.16 (-3.70, -0.60) | 0.007 | -0.22 (-0.42, -0.01) | 0.04 | 0.00 (0.00, 0.00) | 0.31 |
| 3 | -1.09 (-2.52, 0.37) | 0.14 | -1.04 (-2.47, 0.41) | 0.16 | -0.19 (-0.39, 0.02) | 0.08 | -0.01 (-0.01, 0.00) | 0.20 |
| 4 | -1.15 (-2.54, 0.26) | 0.11 | -1.04 (-2.47, 0.41) | 0.16 | -0.20 (-0.41, 0.00) | 0.05 | -0.01 (-0.01, 0.00) | 0.14 |
Model 1 includes adjustment for sex, age quartiles, month, ethnicity, social position, anthropometric observer, height and random effect for school
Model 2 as in Model 1 plus fat mass index
Model 3 as in Model 1 plus fat free mass index
Model 4 as in Model 1 plus fat mass index and fat free mass index
Insulin and insulin resistance missing data for 76 children
We examined ethnic differences in T2D precursors before and after adjustment for sleep duration. Compared with white Europeans, black African Caribbean children had 8.5% higher levels of insulin (95% CI 3.2% to 14.0%) after adjustment for age quartiles, sex, height, month, social position; this difference reduced to 7.6% higher (2.3% to 13.3%) with additional adjustment for sleep duration. In South Asian children the difference in insulin levels compared to White European was 30.3% (23.9% to 36.9%) before adjustment for sleep duration and 29.5% (23.3% to 36.3%) after adjustment for sleep duration.
Discussion
Main findings
The present study is novel in showing inverse associations between reported sleep duration and T2D risk factors in early life, which are independent of adiposity, and observed in different ethnicities. It also shows strong inverse associations between reported sleep duration and adiposity, including detailed measures of body fatness, confirming findings from earlier studies. Given the rising prevalence of diabetes worldwide, and especially in low to middle income countries, we believe our findings will help motivate further simple pragmatic trials in this area.
Relation to earlier studies
Both short and long sleep duration have been linked to adiposity and T2D in adulthood. 4–6 Previous cross sectional observations in childhood have shown inverse association between sleep duration, levels of BMI and obesity, with BMI being 0.75 kg/m2 lower for every additional hour of sleep, 21 and pooled estimates suggest short durations of sleep are associated with a near doubling of obesity prevalence compared to long durations. 5,22 These associations have also been confirmed in analysis of prospective studies in childhood, which have shown slightly smaller associations with 0.5 kg/m2 lower BMI per hour of sleep. 23 These observations concur with adult observations, where pooled findings suggest inverse associations between sleep duration, BMI and obesity. 5 The present study confirms the inverse association with BMI and extends these observations by demonstrating strong graded inverse associations with more detailed measures of adiposity, including sum of skin folds and bioelectrical impedance derived measures of fat mass. Inverse associations between height and sleep duration may well be explained by maturation, with more mature children tending to have later weekday bedtimes. The inverse association with leptin is of particular interest, as this may allude to a biological mechanism of effect, by which increased sleep may alter appetite, by down regulation of leptin production, 24 although we accept circulating leptin levels generally reflect fat mass in stable weight individuals. This agrees with earlier work suggesting that short sleep durations in childhood are associated with higher intake of energy dense and sugary foods. 25
The present study is also novel in demonstrating inverse associations between reported sleep duration and T2D risk markers, including insulin, insulin resistance and blood glucose (although associations with HbA1c were marginal). These associations appear to be partially independent of the detailed adiposity measures. One previous small study (sample size of 245 participants) has shown an inverse association between sleep duration and HOMA measures of insulin resistance in adolescence. 26 However, we are not aware of any other population based studies that have reported similar associations earlier in childhood, except amongst high-risk obese children. 27 Sleep duration did not appear to explain early ethnic differences in T2D risk previously reported, particularly the higher levels of insulin resistance and glycaemia among South Asian children. 11
The present study also provided the opportunity to examine sleep durations and cardiovascular risk factors, including blood lipids and blood pressure, where there was no consistent evidence of an association. These null findings are in agreement with a limited number of earlier observations in childhood, 28,29 suggesting that sleep duration does not alter other cardiovascular risk in early life, other than by increased obesity and metabolic risks which, if sustained or accentuated, take time to accelerate cardiovascular risks.
Strengths and limitations
The present study has a number of strengths and limitations which require further consideration. The study was large, and included multi-ethnic child population. Although ethnic differences in sleep duration were observed, where white European children slept the longest and black Africans the shortest, associations with adiposity measures and T2D risk were consistent across ethnic groups. Moreover, associations were not materially altered by adjustment for socioeconomic position, pubertal status (in girls only), and remained after adjustment for other potential confounders (i.e., sleep-T2D associations remained after adjustment for measures of adiposity). The study was cross sectional, which should not be judged as a disadvantage given the plausible short-term effects of sleep duration on cardiometabolic risk. The possibility of reverse causality, where metabolic dysregulation alter sleep patterns, seems unlikely given the child population. 30 Reassuringly findings were consistent with observations from longitudinal studies, as outlined above. One potential weakness was that sleep duration was derived from weekday self-reported bed-times, not weekend reported bed-times, which may underestimate total weekly sleep duration. 31 However, reported mean and distribution of sleep time was in keeping with other studies of similarly aged children (i.e., mean 10.3 SD 1.1 hours versus 10.5, SD 0.7 hours in a large cohort of children aged 9 years). 32 Accelerometer assessment in a subset 19 provided a further validation of sleep time, showing similar monitor non-wear time and reported sleep duration, and that on average children recorded very few steps 1 hour before compared to 1 hour after reported wake time, and far fewer steps 1 hour after compared to 1 before reported bed time. However, a reduction or absence of steps may not fully reflect sleep, only rest, as other sedentary activities could be taking place. Moreover, waking time accelerometer, as opposed to sleep time accelerometry, does not allow sleep quality to be assessed, which could plausibly exert metabolic effects. 33
Biological mechanisms by which sleep may alter T2D risk have been proposed, including the dysregulation of neuroendocrine control of appetite. 24,34 This lends weight to a potentially causal association. If the association is indeed causal, it would be important to establish evidence based sleep time recommendations, which would be particularly relevant, given trends towards decreasing sleep time in contemporary children. 9 However, robust experimental evidence (i.e. trials) on the association between sleep duration and T2D risk is needed before causality can be inferred. Unfortunately, evidence from a small number of experimental studies both in childhood and adulthood examining the effects of changing sleep duration on adiposity has so far been inconclusive, largely because the effects of interventions on sleep duration have been modest. 35,36 Hence, interventions which are more effective in altering sleep duration are needed. Establishing causality is a priority, as increasing sleep duration could offer a simple, cost effective approach to reducing adiposity and T2D risk from early life.
Conclusions
In the present study, increasing the mean weekday sleep duration (10.5 hrs) by half an hour, could be associated with 0.1 kg/m2 lower BMI, and a 0.5% reduction in insulin resistance. These differences should be considered in relation to the largest observed ethnic difference in BMI and insulin resistance within this study population, where children of South Asian origin had 30% higher insulin resistance, 11 and 0.4 kg/m2 lower BMI compared to children of white European ancestry. 37 If experimental evidence were to corroborate the associations observed between sleep duration and Type 2 diabetes precursors, allowing a causal association to be inferred, these effects could plausibly persist into later life. Levels of insulin resistance in childhood have been shown to impact on T2D risk over a 10 year period and may magnify with increasing age. 38 Hence, reducing levels, even by modest amounts in childhood, may have longer-term implications for reduced T2D in later life. 39 Furthermore, greater weight gain trajectories in childhood associate with greater risks for adolescent non-alcoholic fatty liver disease, a well-accepted precursor to diabetes risks. 40
Supplementary Material
Table of Contents Summary
In this study of 4525 children a one hour longer sleep duration was associated with lower levels of adiposity, insulin resistance and fasting glucose.
What’s Known on This Subject
Shorter sleep duration has been associated with type 2 diabetes (T2D) in adults, and with obesity both in adults and children. However, associations between sleep duration and T2D risk markers in childhood have been little studied.
What This Study Adds
This study demonstrates a novel graded association between short sleep duration and elevated type 2 diabetes risk markers in a large multi-ethnic population of 9-10 year-old children. The report confirms the association between short sleep duration and body fatness.
Acknowledgements
We are grateful to the members of the CHASE study team and to all participating schools, pupils and parents.
Funding source
Data collection was supported by grants from the Wellcome Trust (068362/Z/02/Z), the British Heart Foundation (PG/06/003) and by the National Prevention Research Initiative (NPRI). The Funding Partners for this NPRI award were: British Heart Foundation; Cancer Research UK; Department of Health; Diabetes UK; Economic and Social Research Council; Medical Research Council; Research and Development Office for the Northern Ireland Health and Social Services; Chief Scientist Office, Scottish Executive Health Department; and Welsh Assembly Government. Additional analytical support was provided by Diabetes prevention research at St George’s, University of London is supported by the NIHR. Collaboration for Leadership in Applied Health Research and Care (CLAHRC-2013-10022) South London. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
Contributors’ statement:
All authors conceptualized and designed the study, coordinated and supervised data collection, interpreted data, critically reviewed and revised the manuscript and approved the final manuscript as submitted. All authors agree to be accountable for all aspects of the work. Dr Rudnicka carried out the initial analyses and drafted the initial manuscript.
Reference list
- 1.Gatineau M, Hancock C, Holman N, et al. Adult obesity and type 2 diabetes. 2014. [Accessed March 2016]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/338934/Adult_obesity_and_type_2_diabetes_.pdf .
- 2.IDF Diabetes Atlas. 6th. International Diabetes Federation; Brussels, Belgium: 2013. [Accessed March 2016]. https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf . [Google Scholar]
- 3.Davies SC, Barlow J. Our Children Deserve Better: Prevention Pays. Annual Report of the Chief Medical Officer 2012; 2013. [Google Scholar]
- 4.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 7.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 8.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev. 2012;16:223–230. doi: 10.1016/j.smrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129:548–556. doi: 10.1542/peds.2011-2039. [DOI] [PubMed] [Google Scholar]
- 10.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Whincup PH, Nightingale CM, Owen CG, et al. Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the Child Heart And health Study in England (CHASE Study) PLoS Med. 2010;7:e1000263. doi: 10.1371/journal.pmed.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nightingale CM, Rudnicka AR, Owen CG, et al. Are ethnic and gender specific equations needed to derive fat free mass from bioelectrical impedance in children of South asian, black african-Caribbean and white European origin? Results of the assessment of body composition in children study. PLoS One. 2013;8:e76426. doi: 10.1371/journal.pone.0076426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas C, Nightingale CM, Donin AS, et al. Ethnic and socioeconomic influences on childhood blood pressure: the Child Heart and Health Study in England. J Hypertens. 2012;30:2090–2097. doi: 10.1097/HJH.0b013e32835837c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 15.Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980;26:227–231. [PubMed] [Google Scholar]
- 16.Office for National Statistics. The National Statistics Socio-economic Classification User Manual. Palgrave Macmillan; Basingstoke, Hampshire, UK: 2005. [Google Scholar]
- 17.Thomas C, Nightingale CM, Donin AS, et al. Socio-economic position and type 2 diabetes risk factors: patterns in UK children of South Asian, black African-Caribbean and white European origin. PLoS One. 2012;7:e32619. doi: 10.1371/journal.pone.0032619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and Perinatal Epidemiology. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 19.Owen CG, Nightingale CM, Rudnicka AR, et al. Physical activity, obesity and cardiometabolic risk factors in 9- to 10-year-old UK children of white European, South Asian and black African-Caribbean origin: the Child Heart And health Study in England (CHASE) Diabetologia. 2010;53:1620–1630. doi: 10.1007/s00125-010-1781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donin AS, Nightingale CM, Owen CG, et al. Ethnic differences in blood lipids and dietary intake between UK children of black African, black Caribbean, South Asian, and white European origin: the Child Heart and Health Study in England (CHASE) Am J Clin Nutr. 2010;92:776–783. doi: 10.3945/ajcn.2010.29533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 23.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjeldsen JS, Hjorth MF, Andersen R, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond) 2014;38:32–39. doi: 10.1038/ijo.2013.147. [DOI] [PubMed] [Google Scholar]
- 26.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35:1353–1358. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flint J, Kothare SV, Zihlif M, et al. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150:364–369. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 28.Bayer O, Neuhauser H, von K R. Sleep duration and blood pressure in children: a cross-sectional study. J Hypertens. 2009;27:1789–1793. doi: 10.1097/HJH.0b013e32832e49ef. [DOI] [PubMed] [Google Scholar]
- 29.Kong AP, Wing YK, Choi KC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011;12:659–665. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Cappuccio FP, Miller MA. Is prolonged lack of sleep associated with obesity? BMJ. 2011;342:d3306. doi: 10.1136/bmj.d3306. [DOI] [PubMed] [Google Scholar]
- 31.Williams JA, Zimmerman FJ, Bell JF. Norms and trends of sleep time among US children and adolescents. JAMA Pediatr. 2013;167:55–60. doi: 10.1001/jamapediatrics.2013.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair PS, Humphreys JS, Gringras P, et al. Childhood sleep duration and associated demographic characteristics in an English cohort. Sleep. 2012;35:353–360. doi: 10.5665/sleep.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutson KL, Van CE. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoong SL, Chai LK, Williams CM, Wiggers J, Finch M, Wolfenden L. Systematic review and meta-analysis of interventions targeting sleep and their impact on child body mass index, diet, and physical activity. Obesity (Silver Spring) 2016;24:1140–1147. doi: 10.1002/oby.21459. [DOI] [PubMed] [Google Scholar]
- 36.Capers PL, Fobian AD, Kaiser KA, Borah R, Allison DB. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes Rev. 2015;16:771–782. doi: 10.1111/obr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study) Int J Epidemiol. 2011;40:33–44. doi: 10.1093/ije/dyq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison JA, Glueck CJ, Horn PS, Wang P. Childhood predictors of adult type 2 diabetes at 9- and 26-year follow-ups. Arch Pediatr Adolesc Med. 2010;164:53–60. doi: 10.1001/archpediatrics.2009.228. [DOI] [PubMed] [Google Scholar]
- 39.Landhuis CE, Poulton R, Welch D, Hancox RJ. Childhood sleep time and long-term risk for obesity: a 32-year prospective birth cohort study. Pediatrics. 2008;122:955–960. doi: 10.1542/peds.2007-3521. [DOI] [PubMed] [Google Scholar]
- 40.Anderson EL, Howe LD, Fraser A, et al. Weight trajectories through infancy and childhood and risk of non-alcoholic fatty liver disease in adolescence: the ALSPAC study. J Hepatol. 2014;61:626–632. doi: 10.1016/j.jhep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.