Abstract
Healthy aging requires the coordination of numerous stress signaling pathways that converge on the protein homeostasis network. The Integrated Stress Response (ISR) is activated by diverse stimuli, leading to phosphorylation of the eukaryotic translation initiation factor elF2 in its α-subunit. Under replete conditions, elF2 orchestrates 5’ cap-dependent mRNA translation and is thus responsible for general protein synthesis. elF2α phosphorylation, the key event of the ISR, reduces global mRNA translation while enhancing the expression of a signature set of stress response genes. Despite the critical role of protein quality control in healthy aging and in numerous longevity pathways, the role of the ISR in longevity remains largely unexplored. ISR activity increases with age, suggesting a potential link with the aging process. Although decreased protein biosynthesis, which occurs during ISR activation, have been linked to lifespan extension, recent data show that lifespan is limited by the ISR as its inhibition extends survival in nematodes and enhances cognitive function in aged mice. Here we survey how aging affects the ISR, the role of the ISR in modulating aging, and pharmacological interventions to tune the ISR. Finally, we will explore the ISR as a plausible target for clinical interventions in aging and age-related disease.
Abbreviations
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ATF4
activating transcription factor 4
- BiP
Endoplasmic reticulum chaperone BiP
- CHOP/DDIT-3
C/EBP-homologous protein, DNA damage-inducible transcript 3
- eIF2
eukaryotic translation initiation factor 2
- GCN2
general control nonderepressible 2 (EIF2AK4)
- HRI
Heme-regulated inhibitor (EIF2AK1)
- ISR
integrated stress response
- ISRIB
integrated stress response inhibitor
- MEHMO
mental deficiency, epilepsy, hypogenitalism, microcephaly and obesity)
- PERK
PKR-like endoplasmic reticulum kinase (EIF2AK3)
- PKR
protein kinase R (EIF2AK2)
- PPP1R15A/PP1
protein phosphatase 1
- PPP1R15B/GADD34
Growth Arrest and DNA Damage-Inducible Protein
- CReP
Constitutive Repressor of eIF2a Phosphorylation
- TM
tunicamycin
- TOR
target of rapamycin
- uORF
upstream open reading frame
- UPR
unfolded protein response
- VWM
vanishing white matter disease
Introduction
Aging predisposes to numerous diseases and confronts us with major economic and social challenges. Defined as a pathological process 1 , aging leads to a progressive loss of physiological integrity, accompanied by reduced cellular, organ, and systemic performance. Aging is characterized by cellular hallmarks including genomic instability, deregulated nutrient sensing, and loss of protein homeostasis 2 . During aging, stress signaling pathways such as the Heat Shock Response (HSR) or the ER Unfolded Protein Response (UPR) become dysfunctional leading to an imbalance of the protein homeostasis network 3–5 . The ISR responds to internal and external stimuli and controls numerous outputs including amino acid metabolism, apoptosis, and protein homeostasis via the regulation of the eukaryotic initiation factor 2 (eIF2) and mRNA translation. Thus, the ISR plays a critical role in organismal resilience.
The ISR controls mRNA translation initiation
The ISR is an evolutionarily conserved pathway in eukaryotic cells whose function is to restore cellular homeostasis in response to stress 6–8 (Figure 1). The key node of the ISR is the GTPase eIF2 that controls global protein synthesis as the central regulator of mRNA translation initiation 9 . During translation initiation, the ternary complex composed of eIF2, GTP, and the initiator Met-tRNA, binds to the 40S ribosomal subunit with eIF1, eIF1A, eIF3, and eIF5 to form the 43S preinitiation complex 10 . The preinitiation complex scans an mRNA and a stable mRNA codon-tRNA anticodon interaction upon reaching an AUG triggers GTP hydrolysis and Pi release. eIF2· GDP has a lower affinity to Met-tRNA, releasing eIF2· GDP. Next, eIF5B mediates binding of the large 60S subunit to form the 80S ribosome ready to start translation elongation. eIF2B is the guanine nucleotide exchange factor of eIF2, contributing to ternary complex recycling for a new round of translation initiation 11 .
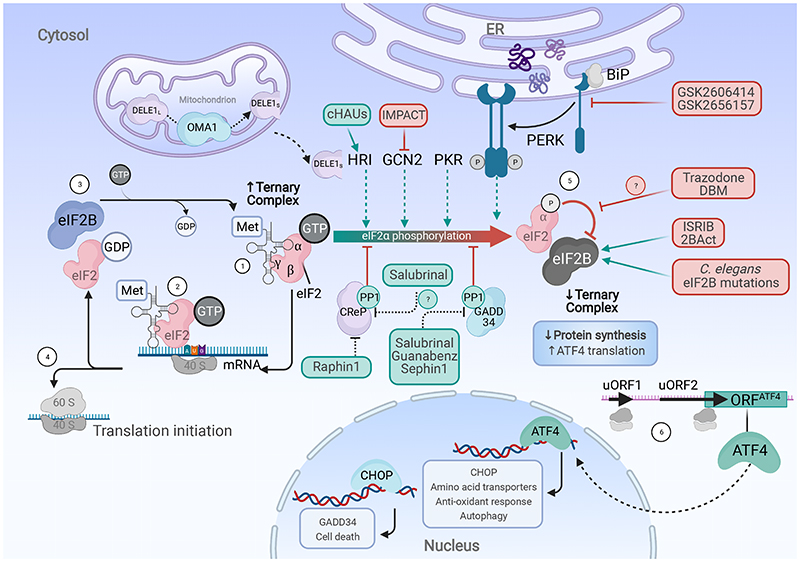
Figure 1. The ISR signaling pathway.
Translation initiation is controlled by the abundance of the eIF2· GTP· Met-tRNAi ternary complex (1). The ternary complex and the 40S ribosome scan along mRNAs until AUG start codon recognition (2). GTP hydrolysis releases eIF2 and other initiation factors from the mRNA-40S-complex (3), allowing the 60S ribosomal subunit to bind and proceed to elongation (4). The guanine nucleotide exchange factor eIF2B catalyzes the GTP exchange to recycle the ternary complex ensuring further rounds of translation initiation. Several stimuli (ER and mitochondrial stress, heme deprivation, viral infection, and amino acid starvation) activate the eIF2 kinases PERK, HRI, PKR and GCN2, respectively, leading to eIF2α phosphorylation at Ser51 (5). p-eIF2 inhibits its own activator eIF2B, leading to ternary complex depletion, decreasing cap-dependent translation an elevating the selective translation of uORF regulated genes, such as ATF4 (6). ISR termination is accomplished by the dephosphorylation of p-eIF2α by protein phosphatase 1 (PP1) complexed with either the constitutively expressed CReP or the ISR-inducible GADD34 regulatory subunits. Pharmacological modulation of the ISR occurs at different levels: ISR activators (in green boxes) target the PP1:CReP complex or HRI while ISR inhibitors (in red boxes) target eIF2B, GCN2 or PERK.
Four stress sensors, the eIF2 kinases, activate the ISR: heme-regulated inhibitor (HRI) 7 , protein kinase R (PKR) 12 , general control nonderepressible 2 (GCN2) 6 , and protein kinase R-like endoplasmic reticulum kinase (PERK) 13 . The kinases respond to distinct inputs such as iron deficiency and mitochondrial stress (HRI) 14,15 , viral infection (PKR), amino acid deprivation (GCN2), and accumulation of misfolded protein in the ER (PERK). Activation of the ISR first requires autophosphorylation of the kinases 16 . The substrate of the four ISR kinases is serine 51 of eIF2α, providing an elegant mechanism that integrates the various upstream stress signals through one specific chemical modification. Phosphorylated eIF2α (p-eIF2α) is a potent noncompetitive inhibitor of its own activator eIF2B, leading to reduced ternary complex formation and attenuated global protein synthesis 17–19 . Paradoxically, several mRNAs that contain short upstream open reading frames (uORFs), such as the activating transcription factors 4 (ATF4) and 5 (ATF5), C/EBP homologous protein (CHOP), and PPP1R15A/GADD34, are selectively translated when eIF2α is phosphorylated 20–22 . eIF2α phosphorylation is further controlled by protein phosphatase 1 (PP1), as a catalytic core, in complex with either the constitutively expressed PPP1R15B/CReP 23 or the ISR-inducible PPP1R15A/GADD34 24 . GADD34 contributes to a feedback loop to antagonize the ISR and is responsible for termination of the ISR by promoting p-eIF2α dephosphorylation. Depending on stress intensity and duration, ISR signaling is adaptive to promote homeostasis, or it can lead to apoptotic cell death 25 .
It is important to note that the ISR kinases directly act on the key node of the translation initiation machinery without the involvement of a bona fide signal transduction pathway. Thus, the ISR acts through the eIF2 pathway and the activity of the ISR kinases favors certain states of translation initiation: the ISR is a tuning mechanism of translation initiation. In line with this, the ISR contributes to maintaining tight regulation of global translation rates. Forced expression of ATF4 and CHOP, for instance, leads to increased protein synthesis, ATP depletion, oxidative stress, and cell death 26 . In turn, loss of the CHOP-GADD34 feedback loop promotes eIF2α phosphorylation, reduces ER protein aggregation and counters ER stress-induced apoptosis in vivo 27 .
There is extensive cross talk between the ISR and other signaling pathways. The UPR specifically monitors the luminal ER milieu and PERK is one of its stress sensors, along with IRE1 and ATF6 28 . Thus, PERK-induced signaling represents an overlap between the ISR and the UPR. Additionally, the transcription factor NRF2 is a PERK substrate and critical for PERK-mediated cell survival during stress 29 . Downstream ISR effectors also cross-talk with other pathways. ATF4, for example, mediates metabolic effects of mTORC1 signaling without eIF2α phosphorylation 30,31 . Given the function of the mTORC1 pathway in growth, this appears counter-intuitive, but ATF4 controls amino acid synthesis and uptake which are downstream of both mTORC and ISR signaling.
Deregulation of the ISR is observed in a wide variety of diseases such as cancer, neurodegenerative disorders, and metabolic syndrome 32–42 . Mutations in any eIF2B subunit can cause vanishing white matter (VWM) disease, an autosomal recessive leukoencephalopathy characterized by myelin loss and ataxia 43–45 . Mutations in HRI and PKR have been detected in individuals presenting developmental delay and leukoencephalopathy, supporting the role of eIF2 in neuronal homeostasis 46 . eIF2 mutations have been linked to MEHMO syndrome, a rare X-linked intellectual disability 47–49 . Moreover, mutations in the eIF2α phosphatase regulator CReP causes diabetes, short stature, and microcephaly 50,51 . Mutations in PERK result in the rare Wolcott-Rallison syndrome that is characterized by insulin-requiring diabetes and impaired neuro-physiological development 52,53 . Mutations in GCN2 are linked to pulmonary veno-occlusive disease (PVOD), a form of pulmonary hypertension 54 . These diseases demonstrate the essential role of the ISR in cellular resilience and health.
Does aging affect the ISR?
Pathways that affect aging often undergo dramatic changes with age, and the causes need to be carefully disentangled from consequences. To first delineate a possible link between aging and the ISR, here we refer to studies that are sufficiently powered and that use truly aged animals that, in the case of mice and rats, are over 18 months of age. The eIF2α kinases constitute the first layer of ISR regulation and several studies using rodents report age-related changes in their expression. PERK phosphorylation is increased in the pancreas of old male mice compared to young adult animals 55 . A comparison of adult and old male mice demonstrated an increase of PKR protein levels in all tested tissues, including kidney, liver, colon, brain, testes, pancreas, lung, and heart 56 . Human muscle biopsies from donors ranging between 20 and more than 80 years of age also show increasing PKR abundance with age 56,57 . Further studies analyzed the phosphorylation status of GCN2, finding that it is increased in the brain of old (aged 19 months, n=3) male mice compared to mature adults (3-6 months of age, n=5) 58 . To our knowledge, no studies reported results on HRI activity during aging.
At the level of eIF2α phosphorylation, there is evidence for age-related ISR induction in multiple species. In very old male mice, the p-eIF2α:eIF2 ratio in skeletal muscle is 2-fold higher compared to mature adult animals 59 . Mice also show an increase in eIF2α phosphorylation in aged liver and kidney, albeit only two animals were used per condition in this study 56 . In male rats, the p-eIF2α:eIF2 ratio is increased in the aged cortex 60 . Clearly, rodent studies using females are yet lacking to delineate possible sex differences in age-dependent ISR changes. In the fruit fly, eIF2α phosphorylation is increased when comparing animals aged 9 to 12 days with animals aged 8 weeks 61 . In Caenorhabditis elegans, we demonstrated a strong increase in eIF2α phosphorylation beginning early in adulthood 62 .
While the ISR attenuates global protein synthesis, it enhances translation of select uORF-regulated mRNAs. GCN4/ATF4 is conserved from yeast to mammals and it is considered one of the main ISR effectors 20 . A study in rat substantia nigra neurons showed elevated ATF4 in aged females 63 . Furthermore, ATF4 was found increased in the brain of old male mice 58 . Long-lived mice subjected to caloric or methionine restriction, or after treatment with the lifespan extending drugs rapamycin and acarbose, showed elevated ATF4 and CHOP. This suggests an adaptive ISR in long-lived animals 64,65 .
Together, these observations suggest that the ISR is activated in aged animals, which goes hand in hand with the reduction in protein synthesis observed during aging 66–69 (Table 1). However, from these studies it remains open if the ISR might promote health in old animals or if it might instead contribute to age-related dysfunction. There likely exists cell type heterogeneity regarding ISR kinase expression and ISR tuning with age in different tissues. Studying this relationship at the single-cell level and using existing ATF4 reporter mice 70 , or the recently engineered in vivo ISR reporter 71 , would constitute an important next step.
Table 1. Changes of ISR activity during aging.
Effects of aging on the ISR in indicated organisms and tissues. y: year, m: months, w: week, d: day. Data represent protein level changes.
| Name | Organism | Age | n | Sex | Tissue | Change with aging | Reference |
|---|---|---|---|---|---|---|---|
| p-PERK | Mouse | 22-27 m vs 10 w | 8 | M | Pancreas | Increased | Naidoo 2014 |
| PKR | Mouse | 20 m vs 8 w | 5 | M | Kidney, liver, colon, brain, testes, pancreas, lung and heart | Ladiges 2000 | |
| Human | 20 y to >80 y | n≥10 | M/F | Muscle (biopsies) | Ubaida Mohien 2019 | ||
| p-GCN2 | Mouse | 19 m vs 3-6 m | 3/5 | M | Brain lysate | Krukowski 2020 | |
| p-elF2a | Mouse | 20 m vs 8 w | 2 | M | Kidney and liver | Ladiges 2000 | |
| Mouse | 26 m vs 8 m | 8 | M | Skeletal muscle | Chalil 2015 | ||
| Rat | 26 m vs 8 w | 4 | M | Brain cortex | Segev 2013 | ||
| Fruit fly | 8 w vs 9 d | 4 | F | Fly heads | Brown 2014 | ||
| Worm | d 6 vs d 1 | 4 | - | Worm lysate | Derisbourg 2021 | ||
| ATF4 | Rat | 24 m vs 2 m | 10 | M/F | Substantia nigra neurons | Salganik 2015 | |
| Mouse | 19 m vs 3-6 m | 3/5 | M | Brain lysate | Krukowski 2020 |
Beyond its function as a central hub of protein homeostasis, the ISR is implicated in other hallmarks of aging as well 2 . UV irradiation leads to ribosome collisions that activate a GCN2-mediated stress response 72 , an elegant mechanism to resolve the stress through translational attenuation. DNA damage also leads to eIF2 phosphorylation through PKR, inducing cell death 73 . Furthermore, altered nutrient sensing and nutritional stress have been linked to ISR activation. Dietary deficiency of essential amino acids activates the neuronal GCN2-p-eIF2α axis in mice, affecting foraging behavior and food selection for complementary amino acid sources 74 . Obesity is characterized by chronically elevated glucose, free fatty acids, and inflammatory cytokines, which trigger ER stress 75 . ATF4 null mice resist diet- or age-induced obesity, they are hypoglycemic, and have increased energy expenditure, mimicking some effects of mTORC inhibition, again linking downstream effects of ISR and TOR signaling 76 . Finally, nutrient limitation in solid tumors is linked to the GCN2-p-eIF2α-ATF4 axis promoting cancer cell survival and proliferation through expression of asparagine synthase 77 whereas the PERK-p-eIF2α-ATF4 axis is critical for hypoxia adaptation of tumor cells 78 . In conclusion, a variety of cellular stimuli mediate ISR activation during aging or age-related pathologies, contributing to physiological changes at the organismal level.
Genetic manipulation of the ISR affects aging
As the previously mentioned studies suggest a link between aging and ISR signaling, we will next discuss a causal involvement of the ISR in the aging process. In yeast, translation rates decrease during replicative aging as a consequence of GCN2 activation and eIF2α phosphorylation but without induction of the ATF4 homolog GCN4. Experimental activation of GCN4, however, extends yeast lifespan by activating autophagy 79 . An excellent recent review further covers the links between ISR activation and lifespan extension in S. cerevisiae 80 .
The use of C. elegans to investigate the ISR has been fairly limited although it is optimally suited to study fundamental aspects of aging and longevity. The genome of C. elegans contains orthologues of key ISR genes including two eIF2α kinases pek-1/PERK and gcn-2/GCN2 81,82 . At the amino acid level, eIF2α shares 50% identity with the human protein and the residues surrounding Ser51 are completely conserved. Knock-down eIF2Bδ reduces overall mRNA translation and extends lifespan 83 . Genetic analysis of the 5’UTR of C. elegans atf-4 demonstrated the presence of two uORFs. A translational reporter containing the atf-4 5’UTR and uORFs GFP expression was built in David Ron’s lab, and ER stress increases its expression. Thus, as in mammals, worm ATF-4 is induced by cellular stress 84 . Similarly, as in yeast or mammals, amino acid limitation increases ATF-4, and this is gcn-2-dependent, demonstrating the conservation of key players of the ISR 85 .
pek-1 deletion mutants are hypersensitive to TM, functionally supporting the role of pek-1 as the PERK orthologue 79 . Several studies highlight the role of pek-1 in nematode survival during regular or stressed conditions. pek-1 is partially required to ensure normal larval development 86 . In the absence of ER stress, the UPR is an important regulator of ER homeostasis and pek-1 is required to promote larval survival upon bacterial infection 81 . In response to crowding, elevated temperature, or starvation, C. elegans enter into an alternative developmental dauer state that is protected from the effects of aging 87 . The phosphorylation of eIF2α by PEK-1 in specific chemosensory neurons is a key mediator of the transition into the dauer state to promote survival 88,89 .
Several studies focused on the role of gcn-2 in C. elegans physiology. Increased lifespan mediated by mitochondrial dysfunction depends on gcn-2 and eIF2α phosphorylation, suggesting that in C. elegans, the kinase plays a role as a mediator of the mitochondrial stress response pathway 90 . In mammalian cells, in contrast, HRI links mitochondrial stress with the ISR 14,15 . Translation attenuation and protein aggregation caused by hypertonic stress are mediated by gcn-2 and eIF2α phosphorylation 91,92 . Moreover, gcn-2 plays an important role in the lifespan extension mediated by dietary restriction or TOR inhibition 85 . GCN1 is a scaffold protein required for GCN2 activation 93 and this interaction is modulated by IMPACT that prevents GCN2 from interacting with GCN1 94 . IMPACT suppression thus activates the ISR and increases worm lifespan and stress resistance 95 . Of note, a partial atf-4 deletion does not affect C. elegans lifespan 85 . Recently, we showed that pharmacological or genetic inhibition of the ISR leads to longevity 62 . In an unbiased point mutagenesis screen in C. elegans for longevity, we found dominant mutations in eIF2Bγ as well as loss-of-function mutations in PEK-1 and GCN-2. An eIF2αS51A mutant confirmed that ISR inhibition leads to longevity. In line with ISR inhibition this occurred without reduction in global mRNA translation, but through translational changes of selected mRNAs 62 . This work revealed a novel longevity mechanism triggered by ISR inhibition in addition to known longevity paradigms that require GCN-2 and ATF-4 activation. Longevity caused by ISR inhibition suggests that ISR modulation and translational reprogramming, in addition to a role in specific diseases, impinges on the aging process itself.
In D. melanogaster, as in C. elegans, the main actors of the ISR including GCN2 (dGCN2), PERK (dPERK), and eIF2B are conserved and they are essential for development and homeostasis 96–98 . The fruit fly also possesses an eIF2α phosphatase regulatory subunit dPPP1R15, which is functionally homologous to the mammalian CReP, and, as in mammals, its expression is controlled by uORFs 99 . Over-expression of the eIF2α kinase dPERK in photoreceptor neurons leads to eye defects and this phenotype can be rescued by over-expressing the eIF2α phosphatase 100 . dPERK is a crucial regulator of intestinal homeostasis, promoting regeneration 101 . Prolonged ISR activation, however, is detrimental in later age and a partial dPERK knockdown in intestinal stem cells improves gut homeostasis, barrier function, and extends lifespan 101 . This strikingly mirrors our findings in the worm that link ISR inhibition to prolonged survival. As in worms and yeast, dietary restriction mediated longevity in D. melanogaster is dGCN2 dependent 102 . Concluding, genetic manipulation of the ISR in yeast, worms, and flies has provided considerable knowledge about its role in development and health, but more studies are required to understand how the ISR controls aging. One possible direction is an exploration of tissue-specific effects of ISR manipulation.
As detailed above, the ISR plays a crucial role in mammalian development and physiology. The homozygous eIF2αS51A substitution, which disrupts phosphorylation, is lethal in neonatal mice 103 . Similarly, deletion of PERK leads to prenatal death in 40% of pups and surviving newborns display severe growth retardation and hyperglycemia 104 . Constant ISR activation mediated by the genetic deletion of both GADD34 and CReP is lethal as well 105 . Whether genetic manipulation of the ISR affects mammalian aging remains unknown, but potent pharmacological ISR modulators have emerged, as detailed next.
Pharmacological ISR modulation in aging and age-related diseases
Genetic and pharmacological ISR inhibition enhance memory in aged mice and increase survival in C. elegans 58,62 . It is thus tempting to speculate that small-molecule ISR inhibitors could increase health- and lifespan. ISR inhibition ameliorates pathology in mouse models of the progeroid Down syndrome 38 and Alzheimer’s disease (AD) 40 . However, beneficial effects of ISR activation have been demonstrated in Huntington’s disease 106 ,ALS 42,107,108 and multiple sclerosis 109 . Thus, depending on disease context, the ISR appears to be protective or maladaptive and during aging the consequences of chronic ISR modulation remain unclear. We will next discuss ISR activator and inhibitor compounds and their role in age-related diseases (summarized in Table 2).
Table 2. Consequences of pharmacological ISR modulation in age-related diseases.
ISR activators and inhibitors used in rodents or cell culture models of neurodegeneration with the main observations reported in the cited studies. y: year, m: months, w: week, d: day, M: male, F: female, doses are per body weight.
| Compound | Disease | Animal model | Main outcomes | Dose/Age/Time intervention/Sex | ISR manipulation | Reference |
|---|---|---|---|---|---|---|
| Salubrinal | Parkinson’s disease | Mice over-expressing a-synuclein with PD-associated mutation A53T |
|
Presymptomatic mice (10-14 m), Salubrinal concentration not indicated | Activation is beneficial | Colla 2012 |
| Guanabenz | TBI | Injured rats |
|
30 min post-injury, treatment with 5 mg/kg. Rats of 275-300 g (M) | Activation is beneficial | Dash 2015 |
| Guanabenz | VWM | VWM eIF2Be-R191H mice |
|
8 m treatment (2-10 m of age) with 10 mg/kg (F) | Activation is beneficial | Dooves 2018 |
| Guanabenz | ALS | SOD1G93A mice |
|
4 mg/kg starting at 40 d of age until disease onset (F) | Activation is beneficial | Jiang 2014 |
| Guanabenz | ALS | SOD1G93A mice |
|
8 mg/kg starting at 60 d of age until disease onset (F) | Activation is beneficial | Wang 2014 |
| Guanabenz | Multiple Sclerosis | GFAP/tTA;TRE/IFN-γ mice |
|
20 d treatment with 4.8 and 16 mg/kg (F) | Activation is beneficial | Way 2015 |
| Raphin1 | Huntington’s disease | HD82Q mice |
|
4 w treatment with 2 mg/kg, starti ng at 4-10 w of age (M) | Activation is beneficial | Krzyzosiak 2018 |
| Sephin1 | CMT1 | Myelin protein zero mutant mice |
|
28 d old mice, treatment bi-daily with 1 mg/kg Sehpin1 for 150 d (M) | Activation is beneficial | Das 2015 |
| Sephin1 | Multiple Sclerosis | Subcutaneous administration of myelin oligodendrocyte glycoprotein (MOG35-55) peptide |
|
Daily treatment 7 d after immunization (F) | Activation is beneficial | Chen 2019 |
| Sephin1 | ALS | SOD1G93A mice |
|
7 w treatment with 5 mg/kg, daily from 4 w of age (M) | Activation is beneficial | Das 2015 |
| GSK2606414 | Dementia | rTg4510 mice, that expresses the tauP301 L mutant in forebrain |
|
2 m treatment with 50 mg/kg starting at ~6 m of age (M) | Inhibition is beneficial | Radford 2015 |
| ISRIB | Tauopathy | PS19 mice |
|
9 w treatment with 5 mg/kg starting at 8-9 m of age (M) | Inhibition has no effect | Briggs 2017 |
| 2BAct | VWM | VWM eIF2Be-R191H mice |
|
21 w treatment with 30 mg/kg starting at 6-11 w of age (M/F) | Inhibition is beneficial | Wong 2019 |
| ISRIB | TBI | Injured mice |
|
Mice injured at ~12 w of age, ISRIB 2.5 mg/kg treated during training period 27 days post injury (M) | Inhibition is beneficial | Chou 2017 |
| ISRIB | Down syndrome | Ts65Dn mice |
|
1 w treatment (once every 2 d) with 2.5 mg/kg, starting ~3-5 m of age | Inhibition is beneficial | Zhu 2019 |
| ISRIB | Prostate cancer | Mice over-expressing MYC and down-regulating PTEN |
|
6 w treatment with 2.5 mg/kg ISRIB, starting ~6 m of age | Inhibition is beneficial | Nguyen 2018 |
| Trazodone and DBM | Prion disease | Prion-infected mice |
|
T reatment with 40 mg/kg trazodone or diet containing 0.5% DBM, starting ~8 m of age | Inhibition is beneficial | Halliday 2017 |
| GSK2606414 |
|
Treatment with 50 mg/kg twice daily, from 7 and 9 w post infection | Inhibition is beneficial | Moreno 2013 | ||
| ISRIB |
|
ISRIB was administered at 0.25 mg/kg once daily from 7 w post infection | Inhibition is beneficial | Halliday 2015 | ||
| ISRIB | Alzheimer’s disease | Intracerebroventricular infusion of β-amyloid oligomers |
|
0.25 mg/kg ISRIB, 3 m of age (M/F) | Inhibition is beneficial | Oliveira 2021 |
| Mice overexpressing amyloid precursor protein (APP) together with deleted presinilin-1 gene in exon 9 |
|
0.25 mg/kg ISRIB, 10-13 m of age (M/F) | Inhibition is beneficial | Oliveira 2021 |
The HRI-activating compounds 1-((1,4-trans)-4-aryloxycyclohexyl)-3-arylureas (cHAUs) increase p-eIF2α, inhibit mRNA translation and slow cancer cell proliferation 110 . At this point, they have not been widely explored in further disease contexts. Salubrinal was first identified as an inhibitor of CreP and GADD34 111 but direct evidence supporting this conclusion is missing. Nonetheless, it is a potent activator of the ISR 112 and is successfully used for in vivo studies 40 . In a mouse model of Parkinson’s disease, salubrinal attenuates motor dysfunction and extends lifespan 41 . Guanabenz and its derivative Sephin1 protect against toxic protein misfolding and ER stress 113 . Guanabenz and Sephin1 bind to GADD34 to counter p-eIF2α dephosphorylation 107 . In vitro evidence supports the role of Guanabenz and Sephin1 as selective inhibitors of GADD34 114,115 . Other studies, however, did not detect a direct effect of Guanabenz or Sephin1 on p-eIF2α dephosphorylation and cells lacking GADD34 or carrying the phospho-defective eIF2αS51A mutation remain responsive to Sephin1 116,117 . In rodents, Guanabenz improves memory and reduces cortical tissue loss after traumatic brain injury 118 and it ameliorates myelin pathology caused by a VMW disease mutation 119 . Sephin1 is protective in a mouse model for multiple sclerosis and this effect depends on GADD34 120 . Despite the debate regarding their molecular mechanism, Guanabenz and Sephin1 have therapeutic potential and they are tested in clinical trials in the context of Charcot-Marie-Tooth disease and ALS, where Guanabenz is showing encouraging results 121 . Raphin1, also derived from Guanabenz, binds the constitutive PP1 regulatory subunit CReP 106 . In HeLa cells, Raphin1 induces a transient increase in eIF2α phosphorylation with a concomitant increase in ATF4, GADD34, and translational attenuation; effects that are lost in the CReP-/- genetic background 106 . Administered in vivo, Raphin1 does not show any measurable adverse effects on body weight, pancreatic and liver function, or memory 106 . Instead, it attenuates neurological decline in a mouse model for Huntington’s Disease 106 , making it an attractive candidate to study consequences of ISR activation in aging and other age-related diseases.
ISR inhibitors target the eIF2 kinases or desensitize cells to the effects of eIF2α phosphorylation. GSK2606414 and derivatives inhibit PERK activation 122 , they are effective in cultured cells 123 but have not been used in aging experiments. However, they might not be suitable due to side effects: GSK2606414 can lead to pancreatic toxicity 124 , weight loss, and mild hyperglycemia 125 , as a possible consequence of on target toxicity through pancreatic PERK inhibition. ISRIB (ISR inhibitor) decreases ATF4 translation during ER stress 126 and cells treated with ISRIB are resistant to eIF2α phosphorylation. ISRIB counters mRNA translation changes caused by TM stress and eIF2α phosphorylation, it exacerbates TM toxicity, and it attenuates the formation of stress granules 21,126 . In striking similarity to eIF2+/S51A heterozygous mice, ISRIB treatment increases long-term memory 126 . eIF2B is the target of ISRIB 112,113 that acts as a molecular staple promoting assembly of the hetero-octameric eIF2B(βδγε)2 structure and enhancing its affinity to the eIF2B(α2) dimer 124,125 . On the eIF2B surface, the binding sites for p-eIF2 and ISRIB are ~50 Å apart and indeed ISRIB allosterically antagonizes the inhibitory binding of p-eIF2 to eIF2B 127,128 . ISRIB antagonizes the inhibitory binding of p-eIF2 to eIF2B. On the eIF2B surface, the binding sites for p-eIF2 and ISRIB are ~50 Å apart, suggesting allosteric interaction between ISRIB and p-eIF2 binding 121,122 . Although there is no evidence indicating that ISRIB might affect lifespan, a recent study showed that ISRIB reverses age-related memory decline in mice: ISRIB reverses age-related deficits in spatial, working, and episodic memory by improving both neuronal structure and function 58 . 2BAct was developed to enhance pharmacokinetic properties and solubility of ISRIB 129 . Trazodone and dibenzoylmethane (DBM) inhibit the ISR as measured using a CHOP::luciferase reporter 130 . Trazodone and DBM do not prevent eIF2α phosphorylation but decrease ATF4 protein levels and partially rescue translation, placing these compounds downstream of p-eIF2α but unlike ISRIB, trazodone or DBM do not affect eIF2B dimerization 130 .
ISR inhibition by compounds that desensitize cells to the inhibitory effects of p-eIF2α is beneficial in the context of multiple diseases. ISRIB and 2BAct ameliorate defects in mouse models of human disorders affecting protein synthesis through eIF2B mutations, for example in (VWM) 123,125 , Down syndrome 38 , and in traumatic brain injury 37 . Similarly, various ISR inhibitors have been successfully used in the context of age-related diseases. Administration of GSK2606414 in a mouse model for frontotemporal dementia restores protein synthesis and reduces the levels of p-PERK, p-eIF2α, and ATF4 that are increased in the vehicle controls 131 . On the other hand, the use of ISRIB did not alleviate the behavioral impairments or neuropathology observed in the PS19 tauopathy mouse model 132 .
In a cellular ALS model, ISRIB decreases SOD1G93A-dependent neuronal death and ER-stress 39 . In prion disease, ISR inhibition by ISRIB, trazodone, or DBM prevent neurological symptoms and increase survival 130 oral administration of GSK2606414 to prion-infected mice limits p-PERK, p-eIF2α, ATF4, and CHOP levels, and eliminates the signs of prion disease, preventing neurodegeneration 125 . Additionally, ISRIB provides a valuable therapeutic approach in a mouse model of AD reversing memory impairment, dendritic spine loss and defective hippocampal protein synthesis induced by Aβ oligomers 40 . In contrast, ISRIB did not rescue spatial learning nor memory in the hAPP-J20 mouse model of AD 133 .
In a mouse model of prostate cancer, characterized by PERK activation and translational attenuation, ISRIB not only restores protein synthesis but also induces tumor regression after a 3-week treatment: ISRIB extends survival and exhibits tumor regression in xenograft studies 35 , making ISRIB and ISR inhibitors promising approaches in cancer therapy.
Concluding remarks
ISR inhibition or activation have the potential to counter pathology, depending on the disease context (Table 2), suggesting that ISR manipulation might be a therapeutic way forward 39,42,134,135 . How should we approach future studies addressing the ISR in disease and aging? We propose the parallel experimental use of ISR activators and inhibitors with known molecular mechanisms. This will reduce potential biases stemming from strains, genotypes, timing and dosing of interventions, analyzed tissues, age, and enhance reproducibility.
While many experiments have been performed to explore the consequences of ISR modulation in various diseases, it remains unknown whether pharmacological interventions modulating the ISR could affect aging. Our recent data indicate that pharmacological and genetic inhibition of the ISR extend lifespan in worms 62 . Of note, ISR inhibition initiated in mid-life had the same beneficial effect on survival as treatment throughout adult life, suggesting that relatively late intervention might increase survival also in other species. Leveraging the deep knowledge about molecular targets and their mechanisms of action, the small molecule ISR modulators Raphin1, ISRIB, and 2Bact should be tested for their potential to modulate the aging process. The clinical molecule Sephin1 as well as Salubrinal should also be tested for their effect on survival in higher organisms while more work is needed to understand the molecular mechanisms of action the latter. ISRIB was found in screens using mammalian cells and the drug-target interaction is extremely specific 136 , hence its use in lifespan experiments in non-vertebrates is likely to fail. We did not detect any biochemical or physiological effects of ISRIB in C. elegans; consequently, although lifespan experiments in rodents are time-consuming, they are needed to study how these compounds might impact the aging process. Lifespan studies in mice supplemented with ISRIB/2BAct, in parallel with ISR activators like Raphin1, will provide definitive data regarding the role of ISR tuning in mammalian aging. It would further be important to include late-life interventions. Given the complex nature of ISR signaling and outputs, a combination of proteomic, phosphoproteomic, and (single-cell) RNA sequencing experiments should be done to define the regulation of the ISR with aging.
The canonical view in geroscience predicts that longevity can emerge when stress response pathways are activated, or by inhibition of growth signals. The observation that translational reprogramming mediated by ISR inhibition leads to lifespan extension surprisingly goes against this concept. Nematode longevity occurs in eIF2Bγ mutants that are likely to mimic the effect of ISRIB 35 . Together with the beneficial effects of ISR inhibition in numerous age-related diseases, this strongly suggests that ISR inhibition modulates the aging process itself. With this, it does not only expand the theoretical framework of geroscience but provides a promising avenue in the prevention and treatment of aging and age-related diseases.
Acknowledgements
We thank all Denzel laboratory members for helpful discussions about this manuscript. We further thank Peter Walter, Mauro Costa-Mattioli, and Anne Bertolotti for valuable comments on the manuscript. Figure 1 was created with BioRender.com. This work was supported by the European Commission (ERC-2014-StG-640254-MetAGEn) and by the Max Planck Society.
Footnotes
Author contributions:
M.J.D., M.D.H., and M.S.D. wrote and edited the manuscript.
Competing Interest Statement:
M.S.D. is cofounder of Acus Laboratories GmbH and scientific advisor to JLP Health GmbH. All other authors declare no competing interests.
References
- 1.The Lancet Diabetes Endocrinology. Opening the door to treating ageing as a disease. Lancet Diabetes Endocrinol. 2018;6:587. doi: 10.1016/S2213-8587(18)30214-6. [DOI] [PubMed] [Google Scholar]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyriakakis E, Princz A, Tavernarakis N. Stress responses during ageing: molecular pathways regulating protein homeostasis. Methods Mol Biol. 2015;1292:215–234. doi: 10.1007/978-1-4939-2522-3_16. [DOI] [PubMed] [Google Scholar]
- 5.Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2018;217:51–63. doi: 10.1083/jcb.201709072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dever TE, et al. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 7.Brostrom CO, Prostko CR, Kaufman RJ, Brostrom MA. Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2alpha kinase. J Biol Chem. 1996;271:24995–25002. doi: 10.1074/jbc.271.40.24995. [DOI] [PubMed] [Google Scholar]
- 8.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 9.Merrick WC, Pavitt GD. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb Perspect Biol. 2018;10:a033092. doi: 10.1101/cshperspect.a033092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomakin IB, Steitz TA. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature. 2013;500:307–311. doi: 10.1038/nature12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavitt GD, Ramaiah KV, Kimball SR, Hinnebusch AG. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 1998;12:514–526. doi: 10.1101/gad.12.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar KU, Srivastava SP, Kaufman RJ. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol Cell Biol. 1999;19:1116–1125. doi: 10.1128/mcb.19.2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, et al. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature. 2020;579:427–432. doi: 10.1038/s41586-020-2078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fessler E, et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature. 2020;579:433–437. doi: 10.1038/s41586-020-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniuchi S, Miyake M, Tsugawa K, Oyadomari M, Oyadomari S. Integrated stress response of vertebrates is regulated by four eIF2α kinases. Sci Rep. 2016;6:32886. doi: 10.1038/srep32886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimball SR, Fabian JR, Pavitt GD, Hinnebusch AG, Jefferson LS. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2alpha. Role of the alpha- and delta-subunits of eiF2b. J Biol Chem. 1998;273:12841–12845. doi: 10.1074/jbc.273.21.12841. [DOI] [PubMed] [Google Scholar]
- 18.Adomavicius T, et al. The structural basis of translational control by eIF2 phosphorylation. Nat Commun. 2019;10:2136. doi: 10.1038/s41467-019-10167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordiyenko Y, Llácer JL, Ramakrishnan V. Structural basis for the inhibition of translation through eIF2α phosphorylation. Nat Commun. 2019;10:2640–11. doi: 10.1038/s41467-019-10606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidrauski C, McGeachy AM, Ingolia NT, Walter P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife. 2015;4:R106. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y-Y, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem. 2009;284:6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jousse C, et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frakes AE, Dillin A. The UPRER: Sensor and Coordinator of Organismal Homeostasis. Mol Cell. 2017;66:761–771. doi: 10.1016/j.molcel.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Cullinan SB, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–733. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrence ME, et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife. 2021;10 doi: 10.7554/eLife.63326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConkey DJ. The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 2017;482:450–453. doi: 10.1016/j.bbrc.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moortgat S, et al. Two novel EIF2S3 mutations associated with syndromic intellectual disability with severe microcephaly, growth retardation, and epilepsy. Am J Med Genet A. 2016;170:2927–2933. doi: 10.1002/ajmg.a.37792. [DOI] [PubMed] [Google Scholar]
- 34.Skopkova M, et al. EIF2S3 Mutations Associated with Severe X-Linked Intellectual Disability Syndrome MEHMO. Hum Mutat. 2017;38:409–425. doi: 10.1002/humu.23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen HG, et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci Transl Med. 2018;10:eaar2036. doi: 10.1126/scitranslmed.aar2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tameire F, et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat Cell Biol. 2019;21:889–899. doi: 10.1038/s41556-019-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou A, et al. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci USA. 2017;114:E6420–E6426. doi: 10.1073/pnas.1707661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu PJ, et al. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science. 2019;366:843–849. doi: 10.1126/science.aaw5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bugallo R, et al. Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis. Cell Death Dis. 2020;11:397–16. doi: 10.1038/s41419-020-2601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira MM, et al. Correction of eIF2-dependent defects in brain protein synthesis, synaptic plasticity, and memory in mouse models of Alzheimer’s disease. Sci Signal. 2021;14:eabc5429. doi: 10.1126/scisignal.abc5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colla E, et al. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J Neurosci. 2012;32:3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang H-Q, et al. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience. 2014;277:132–138. doi: 10.1016/j.neuroscience.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 43.Scali O, Di Perri C, Federico A. The spectrum of mutations for the diagnosis of vanishing white matter disease. Neurol Sci. 2006;27:271–277. doi: 10.1007/s10072-006-0683-y. [DOI] [PubMed] [Google Scholar]
- 44.Hanefeld F, et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics. 1993;24:244–248. doi: 10.1055/s-2008-1071551. [DOI] [PubMed] [Google Scholar]
- 45.Schiffmann R, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol. 1994;35:331–340. doi: 10.1002/ana.410350314. [DOI] [PubMed] [Google Scholar]
- 46.Mao D, et al. De novo EIF2AK1 and EIF2AK2 Variants Are Associated with Developmental Delay, Leukoencephalopathy, and Neurologic Decompensation. Am J Hum Genet. 2020;106:570–583. doi: 10.1016/j.ajhg.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeLozier-Blanchet CD, Haenggeli CA, Bottani A. MEHMO, a novel syndrome: assignment of disease locus to Xp21.1-p22.13. Mental retardation, epileptic seizures, hypogonadism and genitalism, microcephaly, obesity. Eur J Hum Genet. 1999;7:621–622. doi: 10.1038/sj.ejhg.5200364. [DOI] [PubMed] [Google Scholar]
- 48.Hunter JM, et al. Review of X-linked syndromes with arthrogryposis or early contractures-aid to diagnosis and pathway identification. Am J Med Genet A. 2015;167A:931–973. doi: 10.1002/ajmg.a.36934. [DOI] [PubMed] [Google Scholar]
- 49.Gregory LC, et al. Impaired EIF2S3 function associated with a novel phenotype of X-linked hypopituitarism with glucose dysregulation. EBioMedicine. 2019;42:470–480. doi: 10.1016/j.ebiom.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdulkarim B, et al. A Missense Mutation in PPP1R15B Causes a Syndrome Including Diabetes, Short Stature, and Microcephaly. Diabetes. 2015;64:3951–3962. doi: 10.2337/db15-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kernohan KD, et al. Homozygous mutation in the eukaryotic translation initiation factor 2alpha phosphatase gene, PPP1R15B, is associated with severe microcephaly, short stature and intellectual disability. Hum Mol Genet. 2015;24:6293–6300. doi: 10.1093/hmg/ddv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolcott CD, Rallison ML. Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J Pediatr. 1972;80:292–297. doi: 10.1016/s0022-3476(72)80596-1. [DOI] [PubMed] [Google Scholar]
- 53.Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis. 2010;5:29–13. doi: 10.1186/1750-1172-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyries M, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 55.Naidoo N, et al. Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell. 2014;13:131–141. doi: 10.1111/acel.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ladiges W, Morton J, Blakely C, Gale M. Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech Ageing Dev. 2000;114:123–132. doi: 10.1016/s0047-6374(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 57.Ubaida-Mohien C, et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8:852. doi: 10.7554/eLife.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krukowski K, et al. Small molecule cognitive enhancer reverses age-related memory decline in mice. Elife. 2020;9:1671. doi: 10.7554/eLife.62048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalil S, et al. Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem Biophys Res Commun. 2015;468:702–707. doi: 10.1016/j.bbrc.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Segev Y, Michaelson DM, Rosenblum K. ApoE ε4 is associated with eIF2α phosphorylation and impaired learning in young mice. Neurobiol Aging. 2013;34:863–872. doi: 10.1016/j.neurobiolaging.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Brown MK, et al. Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging. 2014;35:1431–1441. doi: 10.1016/j.neurobiolaging.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derisbourg MJ, Wester LE, Baddi R, Denzel MS. Mutagenesis screen uncovers lifespan extension through integrated stress response inhibition without reduced mRNA translation. Nat Commun. 2021;12:1678–14. doi: 10.1038/s41467-021-21743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salganik M, et al. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (α-syn) toxicity to rat nigral neurons. Neurobiol Aging. 2015;36:2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Li X, Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. 2014;13:1012–1018. doi: 10.1111/acel.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Miller RA. Elevated ATF4 function in fibroblasts and liver of slow-aging mutant mice. J Gerontol A Biol Sci Med Sci. 2015;70:263–272. doi: 10.1093/gerona/glu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makrides SC. PROTEIN SYNTHESIS AND DEGRADATION DURING AGING AND SENESCENCE. Biological Reviews. 1983;58:343–422. doi: 10.1111/j.1469-185x.1983.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 67.Johnson TE, McCaffrey G. Programmed aging or error catastrophe? An exmination by two-dimensional polyacrylamide gel electrophoresis. Mech Ageing Dev. 1985;30:285–297. doi: 10.1016/0047-6374(85)90118-6. [DOI] [PubMed] [Google Scholar]
- 68.Rattan SI, Clark BF. Intracellular protein synthesis, modifications and aging. Biochem Soc Trans. 1996;24:1043–1049. doi: 10.1042/bst0241043. [DOI] [PubMed] [Google Scholar]
- 69.Tavernarakis N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 2008;18:228–235. doi: 10.1016/j.tcb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Iwawaki T, et al. Transgenic mouse model for imaging of ATF4 translational activation-related cellular stress responses in vivo. Sci Rep. 2017;7:46230–9. doi: 10.1038/srep46230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helseth AR, et al. Cholinergic neurons constitutively engage the ISR for dopamine modulation and skill learning in mice. Science. 2021;372 doi: 10.1126/science.abe1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu CC-C, Peterson A, Zinshteyn B, Regot S, Green R. Ribosome Collisions Trigger General Stress Responses to Regulate Cell Fate. Cell. 2020;182:404–416.:e14. doi: 10.1016/j.cell.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peidis P, Papadakis AI, Muaddi H, Richard S, Koromilas AE. Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 2011;18:145–154. doi: 10.1038/cdd.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao S, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 75.Özcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 76.Seo J, et al. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–2573. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye J, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rozpedek W, et al. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr Mol Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu Z, et al. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. Elife. 2018;7:4443. doi: 10.7554/eLife.35551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Postnikoff SDL, Johnson JE, Tyler JK. The integrated stress response in budding yeast lifespan extension. Microb Cell. 2017;4:368–375. doi: 10.15698/mic2017.11.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richardson CE, Kinkel S, Kim DH. Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet. 2011;7:e1002391. doi: 10.1371/journal.pgen.1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horsman JW, Miller DL. Mitochondrial Sulfide Quinone Oxidoreductase Prevents Activation of the Unfolded Protein Response in Hydrogen Sulfide. J Biol Chem. 2016;291:5320–5325. doi: 10.1074/jbc.M115.697102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tohyama D, Yamaguchi A, Yamashita T. Inhibition of a eukaryotic initiation factor (eIF2Bdelta/F11A3.2) during adulthood extends lifespan in Caenorhabditis elegans. FASEB J. 2008;22:4327–4337. doi: 10.1096/fj.08-112953. [DOI] [PubMed] [Google Scholar]
- 84.Horn M, et al. Hexosamine Pathway Activation Improves Protein Homeostasis through the Integrated Stress Response. iScience. 2020;23:100887. doi: 10.1016/j.isci.2020.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rousakis A, et al. The general control nonderepressible-2 kinase mediates stress response and longevity induced by target of rapamycin inactivation in Caenorhabditis elegans. Aging Cell. 2013;12:742–751. doi: 10.1111/acel.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 87.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 88.Kulalert W, Kim DH. The unfolded protein response in a pair of sensory neurons promotes entry of C. elegans into dauer diapause. Curr Biol. 2013;23:2540–2545. doi: 10.1016/j.cub.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kulalert W, Sadeeshkumar H, Zhang YK, Schroeder FC, Kim DH. Molecular Determinants of the Regulation of Development and Metabolism by Neuronal eIF2α Phosphorylation in Caenorhabditis elegans. Genetics. 2017;206:251–263. doi: 10.1534/genetics.117.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 2012;8:e1002760. doi: 10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee EC-H, Strange K. GCN-2 dependent inhibition of protein synthesis activates osmosensitive gene transcription via WNK and Ste20 kinase signaling. American Journal of Physiology-Cell Physiology. 2012;303:C1269–77. doi: 10.1152/ajpcell.00294.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim H, Strange K. Changes in translation rate modulate stress-induced damage of diverse proteins. American Journal of Physiology-Cell Physiology. 2013;305:C1257–64. doi: 10.1152/ajpcell.00176.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Barrio M, Dong J, Ufano S, Hinnebusch AG. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J. 2000;19:1887–1899. doi: 10.1093/emboj/19.8.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pereira CM, et al. IMPACT, a protein preferentially expressed in the mouse brain, binds GCN1 and inhibits GCN2 activation. J Biol Chem. 2005;280:28316–28323. doi: 10.1074/jbc.M408571200. [DOI] [PubMed] [Google Scholar]
- 95.Ferraz RC, et al. IMPACT is a GCN2 inhibitor that limits lifespan in Caenorhabditis elegans. BMC Biol. 2016;14:87. doi: 10.1186/s12915-016-0301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santoyo J, Alcalde J, Méndez R, Pulido D, de Haro C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2alpha) kinase from Drosophila melanogaster. Homology To yeast GCN2 protein kinase. J Biol Chem. 1997;272:12544–12550. doi: 10.1074/jbc.272.19.12544. [DOI] [PubMed] [Google Scholar]
- 97.Olsen DS, Jordan B, Chen D, Wek RC, Cavener DR. Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2alpha kinase. Genetics. 1998;149:1495–1509. doi: 10.1093/genetics/149.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pomar N, et al. Functional characterization of Drosophila melanogaster PERK eukaryotic initiation factor 2alpha (eIF2alpha) kinase. Eur J Biochem. 2003;270:293–306. doi: 10.1046/j.1432-1033.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- 99.Malzer E, et al. Coordinate regulation of eIF2α phosphorylation by PPP1R15 and GCN2 is required during Drosophila development. J Cell Sci. 2013;126:1406–1415. doi: 10.1242/jcs.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malzer E, et al. Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. J Cell Sci. 2010;123:2892–2900. doi: 10.1242/jcs.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang L, Ryoo HD, Qi Y, Jasper H. PERK Limits Drosophila Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress. PLoS Genet. 2015;11:e1005220. doi: 10.1371/journal.pgen.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang M-J, et al. 4E-BP is a target of the GCN2-ATF4 pathway during Drosophila development and aging. J Cell Biol. 2017;216:115–129. doi: 10.1083/jcb.201511073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 104.Zhang P, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harding HP, et al. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci USA. 2009;106:1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krzyzosiak A, et al. Target-Based Discovery of an Inhibitor of the Regulatory Phosphatase PPP1R15B. Cell. 2018;174:1216–1228.:e19. doi: 10.1016/j.cell.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Das I, et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348:239–242. doi: 10.1126/science.aaa4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L, Popko B, Tixier E, Roos RP. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol Dis. 2014;71:317–324. doi: 10.1016/j.nbd.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Way SW, et al. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun. 2015;6:6532–13. doi: 10.1038/ncomms7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen T, et al. Explorations of substituted urea functionality for the discovery of new activators of the heme-regulated inhibitor kinase. J Med Chem. 2013;56:9457–9470. doi: 10.1021/jm400793v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boyce M, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 112.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 114.Carrara M, Sigurdardottir A, Bertolotti A. Decoding the selectivity of eIF2α holophosphatases and PPP1R15A inhibitors. Nat Struct Mol Biol. 2017;24:708–716. doi: 10.1038/nsmb.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dedigama-Arachchige PM, Acharige NPN, Pflum MKH. Identification of PP1-Gadd34 substrates involved in the unfolded protein response using K-BIPS, a method for phosphatase substrate identification. Mol Omics. 2018;14:121–133. doi: 10.1039/c7mo00064b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crespillo-Casado A, Chambers JE, Fischer PM, Marciniak SJ, Ron D. PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. Elife. 2017;6:209. doi: 10.7554/eLife.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crespillo-Casado A, et al. A Sephin1-insensitive tripartite holophosphatase dephosphorylates translation initiation factor 2α. J Biol Chem. 2018;293:7766–7776. doi: 10.1074/jbc.RA118.002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dash PK, et al. Inhibition of Eukaryotic Initiation Factor 2 Alpha Phosphatase Reduces Tissue Damage and Improves Learning and Memory after Experimental Traumatic Brain Injury. J Neurotrauma. 2015;32:1608–1620. doi: 10.1089/neu.2014.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dooves S, et al. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol Appl Neurobiol. 2018;44:391–403. doi: 10.1111/nan.12411. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y, et al. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain. 2019;142:344–361. doi: 10.1093/brain/awy322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bella ED, et al. The unfolded protein response in amyotrophic later sclerosis: results of a phase 2 trial. Brain. 2021 doi: 10.1093/brain/awab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Axten JM, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK. J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 123.Rozpędek W, et al. Inhibition of PERK-dependent pro-adaptive signaling pathway as a promising approach for cancer treatment. Pol Przegl Chir. 2017;89:7–10. [PubMed] [Google Scholar]
- 124.Halliday M, et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015;6:e1672. doi: 10.1038/cddis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moreno JA, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138-206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 126.Sidrauski C, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schoof M, et al. eIF2B conformation and assembly state regulate the integrated stress response. Elife. 2021;10 doi: 10.7554/eLife.65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zyryanova AF, et al. ISRIB Blunts the Integrated Stress Response by Allosterically Antagonising the Inhibitory Effect of Phosphorylated eIF2 on eIF2B. Mol Cell. 2021;81:88–103.:e6. doi: 10.1016/j.molcel.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong YL, et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife. 2019;8:1867. doi: 10.7554/eLife.42940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Halliday M, et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain. 2017;140:1768–1783. doi: 10.1093/brain/awx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Radford H, Moreno JA, Verity N, Halliday M, Mallucci GR. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 2015;130:633–642. doi: 10.1007/s00401-015-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Briggs DI, et al. Role of Endoplasmic Reticulum Stress in Learning and Memory Impairment and Alzheimer’s Disease-Like Neuropathology in the PS19 and APPSwe Mouse Models of Tauopathy and Amyloidosis. eNeuro. 2017;4:ENEURO.0025-17.2017. doi: 10.1523/ENEURO.0025-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson ECB, Kang J. A small molecule targeting protein translation does not rescue spatial learning and memory deficits in the hAPP-J20 mouse model of Alzheimer’s disease. PeerJ. 2016;4:e2565. doi: 10.7717/peerj.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vieira FG, et al. Guanabenz Treatment Accelerates Disease in a Mutant SOD1 Mouse Model of ALS. PLoS ONE. 2015;10:e0135570. doi: 10.1371/journal.pone.0135570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luh LM, Bertolotti A. Potential benefit of manipulating protein quality control systems in neurodegenerative diseases. Curr Opin Neurobiol. 2020;61:125–132. doi: 10.1016/j.conb.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 136.Anand AA, Walter P. Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response. FEBS J. 2020;287:239–245. doi: 10.1111/febs.15073. [DOI] [PubMed] [Google Scholar]