Abstract
Morphine and other mu-opioid receptor (MOR) agonists remain the mainstay treatment of acute and prolonged pain states worldwide. The major limiting factor for continued use of these current opioids is the high incidence of side effects that result in loss of life and loss of quality of life. The development of novel opioids bereft, or much less potent, at inducing these side effects remains an intensive area of research, with multiple pharmacological strategies being explored. However, as with many G protein-coupled receptors (GPCRs), translation of promising candidates from in vitro characterisation to successful clinical candidates still represents a major challenge and attrition point. This review summarises the preclinical animal models used to evaluate the key opioid-induced behaviours of antinociception, respiratory depression, constipation and opioid-induced hyperalgesia and tolerance. We highlight the influence of distinct variables in the experimental protocols, as well as the potential implications for differences in receptor reserve in each system. Finally, we discuss how methods to assess opioid action in vivo and in vitro relate to each other in the context of bridging the translational gap in opioid drug discovery.
Keywords: Opioids, Antinociception, Respiratory depression, Constipation, Opioid-induced hyperalgesia, Tolerance
1. Introduction
Opioids are the gold standard for analgesic medications, both for acute severe pain and chronic pain management. Chronic pain in particular is a major health issue across the world today. Estimates of the prevalence of chronic pain range from 20 to 40% of the population in both developing (Sá et al., 2019) and developed (Dahlhamer et al., 2018) countries, representing a significant societal and economic cost.
Opioids still remain mainstay analgesics despite the suite of on-target side effects mediated by activation of the μ-opioid receptor (MOR), the primary target of most opioid pain medications (Matthes et al., 1996). Two major side effects include respiratory depression and addiction. These side effects are, in many ways, the “face” of opioids (Okie, 2010), with respiratory depression being the cause of fatality in an overdose situation and opioid addiction being present in most, if not all, countries worldwide. In 2017, fatal opioid overdoses totalled over 60,000 in the USA (Hedegaard & Warner, 2017) and the number and rate of increase in overdose deaths was the highest in the UK since records began (Office of National Statistics, UK, 2019). Additional side effects include constipation, due to opioid-induced reduction in gut motility, and hyperalgesia, a condition where opioid use paradoxically induces a heightened sensitivity to pain (Benyamin et al., 2008). These are still significant side effects that affect the quality of life and decrease patient compliance (Camilleri, 2011; Katz, 2002; Panchal, Mueller-Schwefe, & Wurzelmann, 2007).
Intense research is currently addressing the urgent need for improved and safer analgesics, either through the discovery and validation of new targets such as other cell surface receptors e.g. cannabinoid receptors (Vučković, Srebro, Vujovic, Vučetić, & Prostran, 2018), voltagegated sodium channels (Bennett, Clark, Huang, Waxman, & Dib-Hajj, 2019), targeted toxins (Yaksh, Woller, Ramachandran, & Sorkin, 2015) or through the development of new opioid receptor ligands with new mechanisms of action (Crombie et al., 2015; Dekan et al., 2019; DeWire et al., 2013; Gassaway, Rives, Kruegel, Javitch, & Sames, 2014; Koblish et al., 2017; Schmid et al., 2017).
Early data from arrestin-3 KO mice suggested that with the removal of arrestin-3 morphine induced enhanced antinociception, with a reduced degree of respiratory depression (Raehal, Walker, & Bohn, 2005). Since then, focus has been centred in the development of G protein-biased agonists to potentially retain the antinociceptive properties while avoiding the respiratory depressant effects (Dekan et al., 2019; DeWire et al., 2013; Gillis et al., 2020; Manglik et al., 2016; Schmid et al., 2017; Varadi et al., 2016). Some novel opioids generated based on this hypothesis have resulted in marginally improved separation between the antinociceptive and respiratory depressant dose response curves (DeWire et al., 2013; Gillis, Gondin, et al., 2020; Schmid et al., 2017). However, the mechanisms responsible for this wider therapeutic windows are still unclear (Azevedo Neto et al., 2020; Bachmutsky et al., 2021; Haouzi, McCann, & Tubbs, 2021; Kliewer et al., 2019; Kliewer et al., 2020).
Other strategies to develop improved opioid analgesics target other opioid receptor subtypes. κ-Opioid receptor (KOR) agonists have been shown to provide effective antinociception without inducing respiratory depression. However, the dysphoria associated with KOR agonism limits their clinical utility (Chavkin & Koob, 2016). Several reports suggest that dysphoria is induced predominantly by activation of an arrestin-mediated signalling pathway (Brust et al., 2017; Lovell et al., 2015) suggesting the development of G-protein biased KOR agonists as a potential strategy to develop new analgesics. Unfortunately, attempts to obviate KOR-induced dysphoria whilst producing an appreciable level of antinociception have not translated into therapies as yet (Mores, Cummins, Cassell, & van Rijn, 2019). There has also been an increasing body of evidence suggesting that the δ-opioid receptor (DOR) may be an attractive target for specific modalities of pain such as chronic inflammatory or neuropathic pain (Pradhan, Befort, Nozaki, Gavériaux-Ruff, & Kieffer, 2011). However, interest in DOR therapeutics has been limited due to pro-convulsive activity exhibited by certain DOR agonists (Broom et al., 2002), as well as an apparent greater liability for tolerance (Pradhan et al., 2010). Attempts to alleviate these side effects are ongoing (Audet et al., 2012; Charfi, Audet, Bagheri Tudashki, & Pineyro, 2015; Conibear et al., 2020). Additionally, beyond the three classical MOR, KOR and DOR subtypes, the nociceptin opioid peptide receptor (NOP) is actively pursued as a potential target for novel analgesic therapeutics (Zaveri, 2016); the widespread expression of NOP and its upregulation in chronic pain states suggest a potential role for NOP antagonists in pain therapy (Calo & Lambert, 2018; Zaveri, 2016). Indeed, molecules that combine a MOR and NOP partial agonist mode of action have been shown to provide antinociception in the absence of side effects in nonhuman primates (Ding et al., 2018).
The paucity of novel opioid-based analgesics reaching the bedside reflects a general attrition in G protein-coupled receptors (GPCR) drug discovery programmes. Translation of candidate molecules from in vitro pharmacological characterisation into pre-clinical and clinical settings still represents a significant hurdle. Differences in experimental conditions, both in vitro and in animal models, are likely to underlie this translational challenge.
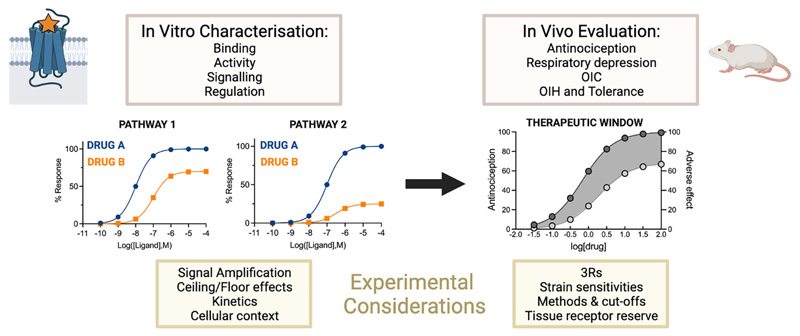
This review examines the preclinical animal models used to evaluate key opioid-induced behaviours. We consider the influence of distinct variables in the experimental protocols, as well as the potential implications for differences in receptor reserve in each system. Finally, we discuss how the methods to assess opioid action in vivo and in vitro relate to each other in the context of bridging the translational gap in opioid drug discovery (Fig. 1). In particular, we focus on the methods used to assess opioid-induced antinociception, respiratory depression, constipation, hyperalgesia and tolerance in the drug discovery setting. While the rewarding, addictive and consequent withdrawal effects associated with opioid use are major side effects, their complex and multi-faceted nature as well as the myriad of approaches used to investigate them are outside the scope of this review. The mechanisms underlying these behaviours, as well as the methods used to study them in preclinical models are elegantly reviewed elsewhere (Banks & Negus, 2017; Charbogne, Kieffer, & Befort, 2014; Kreek et al., 2012; Negus & Moerke, 2019; Rodríguez-Arias, Aguilar, Manzanedo, & Miñarro, 2010; Sadee, Oberdick, & Wang, 2020; Swain, Gewirtz, & Harris, 2021).
Fig. 1.
Experimental considerations for the assessment of opioid pharmacology in vitro and in vivo. In vitro and in vivo characterisations of opioid action are both essential in drug discovery. However, important factors need to be considered to provide a consistent and systematic framework of opioid action. In vitro, compounds are tested for their binding to MOR, and for their ability to activate downstream signalling. These assays need to take into account distinct variables than can affect the measured outcome. In vivo, candidates are usually first assessed for their antinociceptive efficacy, and then for their ability to elicit adverse effects. OIC: Opioid-induced constipation, OIH: Opioid-induced hyperalgesia.
2. Assessment of opioid-induced antinociception
Antinociception refers to the inhibition of the detection of a painful stimulus by nociceptive (pain) neurons. In contrast, the term analgesia is used when referring to the alleviation of the experience of pain, which includes not only the inhibition of nociceptive signalling but also a subjective component that cannot be assessed in animal models. As such, the term antinociception is used to indicate the reductions in pain state observed in nonhuman animals, where an assessment of the subjective component is not possible. The most widely used in vivo models of antinociception involve a pain stimulus (physical activation of nociceptive fibre) being delivered to the animal to elicit a pain behaviour and the subsequent measurement of the reduction of such pain behaviours by analgesic agents. Thermal nociception tests are commonly used in the discovery and characterisation of opioid compounds with the hot plate assay and tail flick (or tail withdrawal) tests being regularly used as the first in vivo measurement of antinociceptive efficacy (Table 1). Other mechanical (e.g Von Frey, Hargreaves) and chemical (e.g formalin, complete Freund’s adjuvant) nociception assays are also used to evaluate antinociceptive efficacy of opioids in conditions such as inflammatory and neuropathic pain.
Table 1. Opioid-induced antinociception assays used in MOR drug discovery.
| Compound | Antinociception assay – species | Strain | Temp & cut-off | Reference |
|---|---|---|---|---|
| TRV130 | Hot plate – mouse | C57/BL6J | 56 °C, 30 s | (DeWireet al., 2013) |
| C57/BL6 | 54 °C, 20 s | (Gillis, Gondin, et al., 2020) | ||
| ICR | 55 °C, 30 s | (Mori et al., 2021) | ||
| Tail flick – mouse | Swiss Webster | 52 °C,10 s | (Altarifi et al., 2017) | |
| C57/BL6 | Variable, 10 s | (Liang, Li, Nwaneshiudu, Irvine, & Clark, 2019) | ||
| Hot plate – rat | Sprague-Dawley | 52 °C, 30 s | (DeWireet al., 2013) | |
| Tail flick – rat | Sprague-Dawley | Variable, 15 s | (DeWireet al., 2013) | |
| Sprague-Dawley | 50 °C, 20 s | (Schwienteck et al., 2019) | ||
| TRV0109101 | Hot plate – mouse | C57/BL6 | 56 °C, 30 s | (Koblish et al., 2017) |
| Hot plate – rat | Sprague-Dawley | 52 °C, 30 s | ||
| Tail flick – rat | Sprague-Dawley | Variable, 15 s | ||
| Von Frey filaments – mouse | C57/BL6 | 0.4g | ||
| PZM21 | Hot plate – mouse | C57/BL6J | 55 °C, 30 s | (Mangliket al., 2016) |
| C57/BL6J | 52.5 °C, 30 s | (iKudla et al., 2019) | ||
| C57/BL6 | 54 °C, 20 s | (Gillis, Gondin, et al., 2020) | ||
| CD-1 | 52.5 °C, 30 s | (Hill et al., 2018) | ||
| Tail flick – mouse | C57/BL6J | 56 °C,10 s | (Mangliket al., 2016) | |
| C57/BL6J | Variable, 9 s | (iKudla et al., 2019) | ||
| Affective vs reflexive – hot plate – mouse | C57/BL6J | 52.5 °C, 30 s | (Mangliket al., 2016) | |
| Formalin chemical assay – mouse | C57/BL6J | 1% formalin | (Mangliket al., 2016) | |
| SR-17018 | Hot plate – mouse | C57/BL6J | 52 °C, 20 s | (Grim et al., 2020; Schmid et al., 2017) |
| C57/BL6 | 54 °C, 20 s | (Gillis, Gondin, et al., 2020) | ||
| Tail flick – mouse | C57/BL6J | 49 °C, 30 s | (Grim et al., 2020; Schmid et al., 2017) | |
| Mitragynine | Tail flick – mouse | CD-1 | Variable, 3× baseline | (Varadi et al., 2016) |
| C57/BL6 | Variable, 3× baseline | |||
| C57/BL6J | 48 °C, 30 s | (Wilson et al., 2021) | ||
| 129S1 | Variable, 10 s | (Kruegel et al., 2019) | ||
| 7-OH Mitragynine | Tail flick – mouse | CD-1 | Variable, 3× baseline | (Varadi et al., 2016) |
| 129S1 | Variable, 10 s | (Kruegel et al., 2019) | ||
| Mitragynine Pseudoindoxyl | Hot plate – mouse | CD-1 | 55 °C, 30 s | (Varadi et al., 2016) |
| Tail flick – mouse | CD-1 | Variable, 3× baseline | (Varadi et al., 2016), (Wilson et al., 2021) | |
| C57/BL6 | Variable, 3× baseline | |||
| 129Sv6 | Variable, 3× baseline | |||
| C57/BL6J | 48 °C, 30 s | |||
| Tianeptine | Hot plate – mouse | C57/BL6 | 50 or 55 °C, 30 s | (Samuels et al., 2017) |
| MP-1208 & MP1207 | Tail flick – mouse | C57BL/6 | 55 °C,15 s | (Uprety et al., 2021) |
| CYM51010 | Tail flick – mouse | C57/BL6 | Heat intensity | (Gomes et al., 2013) |
| 10 (IITC LifeScience), 20 s | (Faouzi et al., 2020) | |||
| C57/BL6 | Variable, 10 s | |||
| CD-1 | Variable, 10 s | |||
| 129 | Variable, 10 s | |||
| MP135 | Tail flick – mouse | C57/BL6 | Variable, 10 s | (Faouzi et al., 2020) |
| CD-1 | Variable, 10 s | |||
| 129 | Variable, 10 s | |||
| Hargreaves – rat | Long Evans | not given | ||
| AT-201 | Tail flick – mouse | ICR | Radial beam | (Khroyan et al., 2007; Toll et al., 2009) |
| (Stoelting, Wood Day, IL), 15 s | ||||
| Endomorphin analogues | Tail flick – mouse | CD-1 | Variable, 9 s | (Zadina et al., 2016) |
| Tail flick – rat | Sprague-Dawley | Variable, 9 s |
Summary of the antinociception assay, species, strain and cut-offs used for the evaluation of novel opioids.
2.1. Tail flick assay
The tail flick assay measures the latency required for an animal to remove or flick its tail from a heat source. It is typically used in rodents that are usually restrained (by scruffing, cupping, wrapping, or placed in a tube) when a heat stimulus is applied to the distal portion of the tail (D’Amour & Smith, 1941; Hardy, 1953; Hardy, Stoll, Cunningham, Benson, & Greene, 1957; Hardy, Wolff, & Goodell, 1940). The noxious heat stimulus can be immersion of the tail in warm water or application of radiant heat to a smaller section of the tail with greater heat intensity (i.e. non radiant heat) resulting in shorter reaction times (Carroll, 1959; Carstens & Wilson, 1993; Granat & Saelens, 1973; Levine, Murphy, Seidenwurm, Cortez, & Fields, 1980). Baseline tail-flick latency is typically 2–4 s (Raffa et al., 1992). Importantly, this assay measures the lower cervical reflex arc responsible for the tail flick. It therefore monitors the response to a spinal reflex, rather than an indication of pain behaviours involving higher brain centres.
2.2. Hot plate assay
The hot plate assay measures behaviours that require central processing and are considered to integrate supraspinal pathways. It consists of the application of a thermal stimulus to the paws, tail and the dorsal flank of the animal and measures the latency for the animal to elicit a pain behaviour (Eddy & Leimbach, 1953; Ocallaghan & Holtzman, 1975; Woolfe & Macdonald, 1944). Typically unrestrained rodents are placed in a metal surface maintained at a constant temperature and manifestations of pain are seen as fluttering of the feet, rearing and licking forepaws/hindpaws, squirming to dissipate heat, and jumping (Carter, 1991).
While both the tail flick and the hot plate assay provide useful information on the antinociceptive actions of opioids, there are obvious ethical considerations integral to the experiment that prevent unnecessary animal suffering, and yet ensure that the data remain valid and useable. Limits in the maximum temperature of the noxious stimulus (the thermal limit - typically 48-55 °C) and maximum latency cut-offs (i.e maximum time until response is observed, typically 10–30 s) prevent damage to the tissues of the experimental animal (Table 1). It is also important to note that increased temperatures are associated with not just decreased latencies (Carroll, 1959; Carstens & Wilson, 1993; Ren & Han, 1979) but also associated reduced antinociceptive efficacy, namely, analgesics are less efficacious the higher the temperature (Ankier, 1974; Hunskaar, Berge, & Hole, 1986; Zimet, Wynn, Ford, & Rudo, 1986). In addition, ethical considerations also limit the frequency at which the test can be repeated in the same animal. This usually implies that data is acquired in 15–30 min bins, which impacts the temporal resolution of the assay and can affect determinations of peak effects and of pharmacokinetic profiles.
The necessary cut-offs in antinociceptive assays have significant implications in estimations of in vivo agonist efficacy. Antinociception is calculated as a percentage of the maximum possible effect (%MPE) which is calculated as follows:
where “test latency” is the latency measured for the test drug, “control latency” is the baseline latency and “Max latency” is the upper temporal limit established to prevent tissue damage. It follows that when the latency measured for the test drug equals or exceeds the maximum latency cut-off, the test compound will display 100% MPE. Thus, detection of differences of opioid antinociceptive efficacy beyond a certain threshold will be prevented.
The above-mentioned cut-offs also have an impact on the determinations of antinociceptive potency, which in turn are important in the determination of therapeutic windows, where ED50s (the effective dose eliciting a 50% of the maximal response) between antinociception and other side effects are compared. Similarly, they should be taken into account when different efficacies are observed between measurements of antinociception and of other opioid-induced side effects. Thus, different temperatures and latency cut-offs being used can impact interpretations of effects across different studies (Table 1).
There is significant accumulated evidence that shows that inbred strains of mice can vary significantly in their nociceptive sensitivity (Table 2) (Homanics, Quinlan, & Firestone, 1999; Mogil et al., 1996). Differences across strains have been generally observed with regards to the baseline latencies and the antinociceptive responses of classical opioids such as morphine (Crain & Shen, 2000; Kest, Hopkins, Palmese, Adler, & Mogil, 2002; Mogil & Wilson, 1997). While such differences can be related to the different pharmacokinetics of opioid drugs in different strains, this remains to be systematically investigated. Thus, when comparing reports of antinociceptive efficacy of novel opioid drugs as well as when designing new compound evaluation strategies, the choice of mouse strain should also be taken into account.
Table 2. Strain differences in opioid-induced behaviours.
| Drug | Assay | Strains | Observations | References |
|---|---|---|---|---|
| Morphine | Hot water tail flick – 49 °C and 15 s cutoff Four day escalating dose ofmorphine (10-40 mg/kg) used to induce tolerance. |
Strain – baseline (s)
129P3-2.4 ± 0.1 A – 2.4 ± 0.1 AKR – 2.9 ± 0.2 ABLB/c – 3.1 ± 0.2 C3H/He – 1.8 ± 0.1 C57BL/6-2.2 ± 0.1 CBA – 1.9 ±0.1 DBA/2-2.5 ± 0.1 LP – 2.8 ± 0.1 SJL – 2.2 ± 0.1 SWR – 1.8 ± 0.1 |
|
(Kestet al., 2002) |
| Morphine | Hot water tail flick – 55 °C and 10 s cutoff |
Strain – baseline (s)
SW – 2.7 ± 0.6 129/SvEv – 3.5 ± 0.6 |
|
(Crain and Shen, 2000) |
| Morphine | Hot water tail flick – 49 °C and 15 s cutoff |
Strain – baseline (s)
129/J – 4.7 ± 0.2 129/SvJ – 4.5 ± 0.1 B6-2.6 ± 0.1 |
|
(Mogil & Wilson, 1997) |
| Morphine | LD50 determination by percent survival – measured by return to regular breathing |
Strain and sex – LD50 (mg/kg)
129S1/SvlmJ F – 631.3 129S1/SvlmJ M -664.2 A/J F – 212.2 A/J M – 225.2 C57BL/6 J F – 311.6 C57BL/6 J M – 254.3 CAST/EiJ F – 882.2 CAST/EiJ M – 429.9 NOD/ShiLtJ F – 811.0 NOD/ShiLtJ M – 588.8 NZO/HILtJ F – 333.9 NZO/HILtJ M – 324.4 PWK/PhJ F – 261.0 PWK/PhJ M – 359.1 WSB/EiJ F – 526.4 WSB/EiJ M – 695.6 |
|
(Bubier et al., 2020) |
| Morphine | Measurement of respiration (whole body plethysmography) and constipation (Charcoal transit) | C57BL/6 J A/J |
|
(Young et al., 2018) |
Summary of the assays, strains and results reported in the studies addressing strain differences to opioid sensitivity.
3. Assessment of opioid-induced respiratory depression
Opioid-induced respiratory depression is a significant, potentially lethal, adverse effect of opioid agonists. MOR is expressed throughout the respiratory network of the brainstem, and recent studies have demonstrated a key role of MOR expressed in nuclei including; the preBötzinger complex (preBötC), Kölliker-Fuse (KF), post-inspiratory complex, ventral respiratory column and the retrotrapezoid/parafacial nucleus (Bachmutsky et al., 2021; Ramirez et al., 2021; Varga, Reid, Kieffer, & Levitt, 2020). The relative importance of each respiratory brain nuclei to respiratory rhythmogenesis as well as their role in the response to exogenous opioids is currently debated (Ramirez et al., 2021). While it is clear that the preBötC is an important site of action for opioid depression of respiration (Bachmutsky, Wei, Kish, & Yackle, 2020; Ramirez et al., 2021), reports suggest both, an essential and a non-essential role of the preBötC for breathing rhythmogenesis (Montandon & Horner, 2014) (Lalley, Pilowsky, Forster, & Zuperku, 2014). Further investigation is required to elucidate the importance of each nuclei both in the persistent control of respiration as well as in the effect of opioids on respiration.
Due to its acute onset and relative ease of measurement, respiratory depression is one of the first adverse effects to be assessed in the characterisation of novel opioid agonists (Gillis et al., 2020a; Gillis, Gondin, et al., 2020; Hil let al., 2018; Manglik et al., 2016; Schmid et al., 2017). Several non-invasive methods can be used to monitor respiration in rodents and determine respiratory parameters (for extended review of these methods see (Hoymann, 2007).
3.1. Whole body plethysmography
In whole body plethysmography (WBP), freely moving animals are placed in a closed chamber and the pressure fluctuations that occur during the breathing cycle are recorded. A pressure transducer monitors the pressure differences between the experimental chamber of the ple-thysmograph where the animal is placed and a reference chamber. Both chambers have a regulated flow of room air or a controlled combination of gas and the system is calibrated with known air volume changes. The three primary parameters derived from this technique are tidal volume (TV), the volume of each breath; respiratory frequency, the number of breaths per minute (BPM); and minute volume (MV), the composite of breath volume and frequency (Hill et al., 2016). It is important to consider all three parameters as changes in MV may be due to a decrease in either BPM, TV or both. For example, fentanyl-like drugs are thought to induce a decrease in TV through a separate action of muscle stiffness not commonly seen in other opioids (Hill, Santhakumar, Dewey, Kelly, & Henderson, 2020), which may contribute to their lethality.
As respiration can be altered by stressed and quiescent states, measurements of respiration in WBP systems need to minimise the effect of these variables. Stress can induce a heightened respiratory baseline, and therefore, changes in respiration may indirectly be assessing the anxiogenesis and anxiolytic effects of the compounds tested (Lynch 3rd et al., 2019). Minimising stress can be achieved with sufficient habituation of the animal to the WBP chamber (ideally the day prior to the experiment) (Hill et al., 2016). As animals are unrestrained in the WBP chamber, it is possible for them to curl up and either sleep or enter a quiescent state. This can often be detected in vehicle administered groups (e.g. saline) when a significant reduction in respiration is observed while the treatment is known to have no effect on respiration. This effect appears to be mitigated by using larger WBP chambers such as those in comprehensive lab animal monitoring systems (CLAMS) (Reilley et al., 2010) which provide a greater area for normal locomotor behaviour.
Timing of experiments and housing conditions that maximise the activity of rodents can also help mitigate these confounds in smaller WBP chambers. These usually involve conducting measurements in the most active phases of the night cycle (Bains etal., 2018) and housing the rodents on a reverse lit day-night cycle that allows experimentation during the night cycle of the animal, when they are naturally more active. Additionally, a mild hypercapnic stimulus can help maintain steady respiratory rates of mice without a significant induction of stress (as measured by corticosterone release (Hill et al., 2016) without apparent changes in the ability or sensitivity of opioids to induce respiratory depression (Hill et al., 2018).
3.2. Head-out plethysmography
In contrast to WPB, head-out plethysmography restrains the experimental animal in a plethysmograph chamber supplied with a controlled flow of air (Hoymann, 2007) but leaves the head free through a sealed neck ring. As movement restraint is a significant stressor (Lynch 3rd et al., 2019), it is likely that the anxiolytic effect of opioids, in addition to their respiratory depressant effects, contribute significantly to the measurements using this approach. However, it may be the method of choice when additional more invasive methods are used for the study of lung and airway pathology.
3.3. Pulse-oximetry
Unlike plethysmography, pulse-oximetry does not measure the mechanical actions of breathing in rodents but instead measures changes in blood oxygen levels. A decrease in blood oxygen saturation is used as a proxy for respiratory depression. The non-invasiveness and nonstressful properties of this method are advantages for the use of this approach to characterise the respiratory depressant effects of opioids in rodents (DeWire et al., 2013; Faouzi, Varga, & Majumdar, 2020; Schmid et al., 2017). It is however unclear how the respiratory parameters obtained using pulse oximetry relate to those obtained with the more established systems of whole body plethysmography and head out plethysmography. As mentioned above, minute volume, tidal volume as well as breathing frequency can be altered to differing extents by different opioids (Hill et al., 2020).
It is important to note that experimental assessments of respiratory depression do not have the same cut-offs as antinociception assays. This allows for estimates of efficacy and potency that are not subject to a defined maximal effect. Moreover, it allows the assessment of the respiratory depressant potential of all novel opioids at higher doses than those providing effective antinociception, which defines the therapeutic window. Assessing the potential to induce respiratory depression is of relevance as this side effect underlies most opioid-overdose fatalities in humans.
The impact of strain sensitivity on the respiratory depressant effects of opioids has recently been described (Table 2) (Bubier et al., 2020; Young et al., 2018). Of note, the reduced sensitivity of the 129Sv1J mouse line to opioidergic responses has been suggested to underlie the arrestin-3 knock-out data (Bubier et al., 2020; Kliewer et al., 2020).
4. Assessment of opioid-induced constipation
Opioid-induced constipation (OIC) is one of the most common side effects of opioid use in the clinic (McNicol et al., 2003). Severe OIC has been reported in 40-95% of patients following the onset of a prescribed opioid regime (Pappagallo, 2001; Prichard & Bharucha, 2015; Swegle & Logemann, 2006), impacting the patient’s quality of life, decreasing compliance and often resulting in heightened pain (Hjalte, Berggren, Bergendahl, & Hjortsberg, 2010; Katz, 2002; Trescot et al., 2008).
Opioids act to inhibit acetylcholinergic neurons preventing the release of neurotransmitters and increasing longitudinal smooth muscle tone while decreasing propulsive activity (Brock et al., 2012; Wood & Galligan, 2004). These effects are thought to be primarily mediated by the MOR as the effects are absent in MOR knock-out mice (Roy, Liu, & Loh, 1998). Importantly, both in animals and in humans, tolerance does not seem to develop within the GI tract actions of opioids, unlike antinociceptive tolerance, that requires increasing doses of opioids to provide the same level of analgesia (Müller-Lissner et al., 2017; Prichard & Bharucha, 2015). This was first identified in dogs (Plant & Miller, 1926) and has since been replicated across different species (Ling, Paul, Simantov, & Pasternak, 1989; Matsumoto et al., 2016;). Lack of tolerance to morphine-induced constipation has been shown from short (≤72 h) (Ross, Gabra, Dewey, & Akbarali, 2008) to longer (up to 10 days) administration periods. The use of peripherally restricted opioid antagonists, such as methylnaltrexone (Anissian et al., 2012; Bader, Durk, & Becker, 2013; Michna et al., 2011) or combination therapies of oxycodone and naloxone can be used to effectively treat OIC whilst retaining pain relief (Lowenstein et al., 2009; Simpson et al., 2008). Pre-clinically, the most common methods to measure OIC are faecal boli accumulation and glass bead expulsion.
Faecal boli accumulation measures the overall production of faecal matter over time (typically 2-6 h) following administration of opioids. This is usually done in under controlled feeding in order to measure food consumption against expulsion, and additionally a dye (e.g blue ink, charcoal) may be introduced into the diet to aid tracking of the bolus (Anand et al., 2018).
The glass bead expulsion assay requires brief anaesthesia of the animal in order to insert a small glass bead into a defined distance in the colon (Matsumoto et al., 2016). Following recovery from the anaesthetic, the time taken for the bead expulsion is measured. In this assay the opioid treatment can be administered before or after insertion of the glass bead. Whilst this assay is reliable and reproduceable, it is limited in its assessment of overall gut activity, as it only measures colonic transit effects. Comparatively, the faecal boli accumulation assay measures the effect of opioid inhibition throughout the entire gut, does not require the use of anaesthetics and is less invasive. However, it must also be considered that opioids can induce tolerance to differing degrees in colonic versus small and large intestinal transit in rodents (Matsumoto et al., 2016; Mori et al., 2013). Given these differences, the use of both assays in parallel may provide valuable information into regional differences in OIC.
While the literature surrounding mouse strain differences in relation to OIC is sparse, a recent study compared two inbred mouse strains (A/J and C57BL/6J) and found that C57BL/6 J mice were significantly more constipated following doses of morphine exceeding 40 mg/kg (Table 2)(Young et al., 2018).
5. Assessment of opioid-induced hyperalgesia and antinociceptive tolerance
Chronic opioid use can lead to opioid-induced antinociceptive tolerance and hyperalgesia. Analgesic or antinociceptive tolerance corresponds to a progressive decrease of analgesia produced by a given opioid dose. This results in the need to increase the dose of opioids to provide a similar analgesic effect. Cellular and molecular mechanisms that underlie MOR regulation have been comprehensively reviewed (Williams et al., 2013). Whilst some mechanisms of MOR regulation such as phosphorylation (Doll et al., 2011) and desensitization (Bailey et al., 2009) have been thoroughly studied, there are still significant gaps in our understanding of the molecular processes responsible for loss of MOR function after chronic exposure to opioids. In particular the role of arrestins, whilst clearly important in the development of opioid tolerance through MOR desensitization, is still not fully understood (Bohn, Lefkowitz, & Caron, 2002; Kliewer et al., 2019; Williams et al., 2013).
Opioid induced hyperalgesia (OIH) is characterised by a paradoxical increase in pain perception following the onset of opioid medication (Lee, Silverman, Hansen, Patel, & Manchikanti, 2011; Roeckel, Le Coz, Gaveriaux-Ruff, & Simonin, 2016). While most opioid drugs appear to induce OIH regardless of their intrinsic efficacy and mechanism of action (Angst & Clark, 2006; Araldi, Ferrari, & Levine, 2018; Compton, Canamar, Hillhouse, & Ling, 2012), the molecular mechanisms underlying this state of nociceptive sensitisation are complex and still not fully understood. The most important proposed mechanisms for OIH have been reviewed elsewhere (Lee et al., 2011; Roeckel et al., 2016). These mechanisms involve the NMDA-glutamatergic system, transient receptor potential channels V1 and M8 (TRPV1 and TRPM8), and are influenced by several factors including genetic background and sex differences of experimental animals (Roeckel et al., 2016).
Experimentally, OIH and antinociceptive tolerance are measured through antinociception assays upon chronic administration of opioids, either by repeated administration or through osmotic minipumps (Hill et al., 2016; Hill et al., 2018; Koblish et al., 2017). The most common antinociception assays used in this context are thermal stimulation (hot plate and tail flick) for antinociceptive tolerance and OIH, and mechanical stimulation for OIH assessment only (Koblish et al., 2017; D.-Y. Liang et al., 2006; Roeckel et al., 2016). These allow the detection of significant changes in opioid-induced responses, and baseline latencies differences of naïve versus chronically treated animals are readily observed (in particular for OIH). However, when assessing for hyperalgesia, higher temperatures or applied forces are an impediment to obtaining clear, meaningful data; the baseline nociceptive latency of the animal decreases as the thermal or physical intensity increases (Carroll, 1959; Carstens & Wilson, 1993; Ren & Han, 1979), therefore the ability to detect a decrease in baseline threshold is also reduced at higher temperatures.
The same inherent ethical limitations apply for assessment of hyperalgesia and tolerance as they do for antinociception assays. Moreover, another limiting factor is the amplitude of the baseline nociceptive latency as an increase in the severity of the noxious stimulus will compromise the detection window even further upon the development of OIH (see above for the thermal example) (Jensen & Finnerup, 2014; Yalcin, Charlet, Freund-Mercier, Barrot, & Poisbeau, 2009). It is also of particular importance that multiple pain modalities are tested in the context of OIH as the development of OIH in patients is usually related to both chronic use of opioids as well as an underlying pain states (Burma, Leduc-Pessah, & Trang, 2017; Marrone et al., 2017). Other modalities include mechanical stimulation of pain responses (e.g. Von Frey filaments) (Koblish et al., 2017), that can be performed in conjunction with pro-inflammatory treatments (e.g. carrageenan or capsaicin injections) (Luo et al., 2008; Yalcin et al., 2009) as well as neuropathic models of pain (e.g. nerve ligation assays) (Chen et al., 2020).
6. In vitro measurements of opioid pharmacology and their relationship with opioid-induced effects in vivo
The antinociceptive, respiratory and gastrointestinal effects of opioid therapeutics are mainly caused by the activation of the MOR (Bachmutsky et al., 2021; Matthes et al., 1996; Roy et al., 1998; Varga et al., 2020). The MOR signals predominantly through the activation of Gαi/o and βγ proteins. MOR activation alters neurotransmitter release through presynaptic inhibition of voltage-gated calcium channels (VGCC) and inhibits neuronal activity through hyperpolarisation caused by post-synaptic activation of G protein-coupled inwardly rectifying potassium (GIRK) channels. Moreover, G protein activation by MOR also results in inhibition of adenylate cyclase, resulting in decreased levels of the second messenger cAMP. As with most GPCRs, the G protein signalling of MORs is regulated by phosphorylation of intracellular domains, arrestin binding and internalisation. Numerous in vitro assays are available for the detection of MOR signalling and regulation in cell lines. These assays include sensors to monitor receptor, G protein and GIRK channel activation, measurements of cAMP levels, recruitment of GRK and arrestins and receptor internalisation (Table 3). However, with the exception of electrophysiological approaches (Birdsong & Williams, 2020), application of these approaches for the detection of MOR signalling in neuronal cultures remains slightly more challenging.
Table 3. In vitro signalling assays used in MOR drug discovery.
| Compound | Response | Cell type | Assays | References |
|---|---|---|---|---|
| TRV130 (Oliceridine) | β-Arrestin recruitment Nb33 recruitment mGsi recruitment G protein activation GIRK/VGC channel modulation Inhibition of Fsk-induced cAMP accumulation Internalisation MOPr phosphorylation |
HEK293 | DiscoverX PathHunter, BRET, FRET BRET BRET BRET, FRET Membrane potential assay, patch-clamp electrophysiology cAMP HiRange Kit, BRET, GloSensor DiscoverX PathHunterBRET, TR-FRET, imaging Western blot |
(DeWire et al., 2013, Gillis, Gondin, et al., 2020, Pedersen et al., 2020, Mori et al., 2021, Ehrlich et al., 2019, Yudin et al., 2019) |
| TRV0109101 | β-Arrestin recruitment Inhibition of Fsk-induced cAMP accumulation Internalisation MOPr phosphorylation |
HEK293 | DiscoverX PathHunter cAMP HiRange Kit DiscoverX PathHunter pSer375 Western blot |
(Koblish et al., 2017) |
| PZM21 | β-Arrestin recruitment Nb33 recruitment mGsi recruitment G protein activation GIRK/VGC channel modulation Calcium release Inhibition of Fsk-induced cAMP accumulation Internalisation MOPr phosphorylation |
HEK293 | DiscoverX PathHunter, BRET, FRET BRET BRET BRET, GTPγS binding, FRET Membrane potential assay, patch-clamp electrophysiology FLIPR (Fluo-4 dye) BRET, GloSensor DiscoverX PathHunterBRET Western blot |
(Manglik et al., 2016) (Gillis, Gondin, et al., 2020) (Hill et al., 2018) (Yudin et al., 2019) |
| SR-17018 | β-Arrestin recruitment Nb33 recruitment mGsi recruitment G protein activation GIRK channel activation Inhibition of Fsk-induced cAMP accumulation Internalisation MOPr phosphorylation |
CHO-K1, U2OS, HEK293 | DiscoverX PathHunter, BRET, FRET, imaging BRET BRET BRET, GTPγS binding Membrane potential assay BRET, cAMP HiRange Kit BRET, imaging Western blot |
(Grim et al., 2020; Schmid et al., 2017) (Gillis, Gondin, et al., 2020) |
| Mitragynine | G protein activation β-Arrestin recruitment |
CHO | GTPγS binding DiscoverX PathHunter |
(Varadi et al., 2016) (Wilson et al., 2021) |
| 7-OH Mitragynine | G protein activation β-Arrestin recruitment |
CHO | GTPγS binding DiscoverX PathHunter |
(Varadi et al., 2016) |
| Mitragynine Pseudoindoxyl | G protein activation β-Arrestin recruitment |
CHO | GTPγS binding DiscoverX PathHunter |
(Varadi et al., 2016) |
| Tianeptine | G protein activation Inhibition of Fsk-induced cAMP accumulation |
HEK293T | BRET BRET |
(Gassaway et al., 2014) |
| MP-1208&MP1207 | β-Arrestin recruitment G protein activation Inhibition of Fsk-induced cAMP accumulation |
HEK293, HTLA | Tango, BRET BRET, GTPγS binding GloSensor |
(Uprety et al., 2021) |
| CYM51010 | β-Arrestin recruitment G protein activation |
U2OS |
DiscoverX PathHunter BRET, GTPγS binding |
(Gomes et al., 2013) (Faouzi et al., 2020) |
| MP135 | β-Arrestin recruitment G protein activation |
U2OS | DiscoverX PathHunter BRET, GTPγS binding |
(Faouzi et al., 2020) |
Summary of the cell signalling assays commonly used for opioid drug discovery. Fsk; forskolin, ORs; opioid receptors, BRET: Bioluminescence Resonance Energy Transfer, FRET; Förster Resonance Energy Transfer, TR-FRET; Time-Resolve FRET; GIRK channel, G protein inwardly rectifying channel; VGCC, voltage-gated calcium channel.
Despite the initial reports, recent evidence suggests that a clear separation of the signalling responsible for antinociceptive versus deleterious side effects of opioids is unlikely (Bachmutsky, Wei, Durand, & Yackle, 2021; Kliewer et al., 2019; Kliewer et al., 2020). Antinociception, respiratory depression and constipation are all mediated by G protein activation by MOR and retained in arrestin KO mice (Bachmutsky et al., 2021; Benyamin et al., 2008; Conibear & Kelly, 2019; Gillis et al., 2020b; Kliewer et al., 2019; Matthes et al., 1996; Montandon et al., 2016; Valentino & Volkow, 2018). However, this should not preclude the development of improved opioids that minimise adverse effects and provide wider therapeutic windows through other mechanisms or pharmacological properties different than biased agonism. For example, the role of ligand binding kinetics, as well as kinetic of effect has been proposed to underlie potential signalling differences among GPCR ligands (Klein Herenbrink et al., 2016; van der Velden, Heitman, & Rosenkilde, 2020) although this does not seem to be the case for some novel opioids (Pedersen et al., 2020).
It has also been proposed that low intrinsic efficacy may provide an alternative explanation to the improved therapeutic profiles (Azevedo Neto et al., 2020; Benredjem et al., 2019; Gillis, Gondin, et al., 2020; Kelly, 2013). This is supported by a recent study of clinically used opioids which found that intrinsic efficacy, rather than any G protein/β-arrestin bias, predicted the rate of reported adverse events (Benredjem et al., 2019), and by the actions of the opioid buprenorphine, an extremely low efficacy MOR agonist with a ceiling effect in respiratory depression and reduced overdose risk (Dahan et al., 2006; Walsh, Preston, Stitzer, Cone, & Bigelow, 1994). In this context, the response of partial agonists, compounds with low intrinsic efficacy, is very sensitive to the presence of receptor reserve (also known as spare receptors). As such, partial agonists will display robust responses in systems with high receptor reserve, whilst in systems with lower receptor reserve they will produce a partial maximum response even at full receptor occupancy. As receptor reserve is a property of the tissue and of the agonist (Buchwald, 2019; S. J. Hill, 2006), it therefore follows that the same drug may elicit full or partial responses depending on the tissue receptor reserve. Although difficult to prove experimentally, these differences may explain the effects of low efficacy opioids (A. E. Conibear & Kelly, 2019; Gillis, Sreenivasan, & Christie, 2020; Pineyro & Nagi, 2021; Uprety et al., 2021). The presence of receptor reserve in the responses of opioid agonists is a pharmacological concept that has been thoroughly explored in cultured cell lines (Carliss et al., 2009; Kelly, 2013) and utilised in electrophysiology (Lowe & Bailey, 2015). This is commonly assessed using irreversible antagonists (such as β-funaltrexamine (Takemori, Larson, & Portoghese, 1981), β-chlornaltrexamine (Portoghese, Larson, Jiang, Takemori, & Caruso, 1978) or methacinnamox (Broadbear et al., 2000) or genetic strategies to decrease receptor levels (Mizoguchi et al., 1999; Singleton et al., 2021; Sora et al., 2001).
As mentioned above, measurements of antinociceptive efficacy are limited by the imposed cut-offs. However, given that low efficacy agonists such as buprenorphine or oliceridine provide effective antinociception, it may be suggested that some receptor reserve is present in this system. Similarly, as tail flick and hot plate assays do not measure identical responses, differences in receptor reserve may also be relevant to consider. The relationship between tolerance and receptor reserve as received particular attention (Chavkin & Goldstein, 1984; Mjanger & Yaksh, 1991; Williams et al., 2013). A recent study, investigated the effect of irreversible antagonism in vitro and of reduced receptor expression in MOR+/- mice to interrogate the antinociceptive tolerance of oliceridine. Although a clear effect was observed in terms of ligand efficacy, oliceridine was still able to induce tolerance in mice expressing 50% less MOR than wild type (Singleton et al., 2021).
Despite the potential to explain the different actions of opioids in different tissues, the impact of receptor reserve in the other physiological responses of opioids such as respiratory depression and constipation have not as yet been studied and compared with the effect of receptor reserve on antinociception. Future studies addressing the relevance of receptor reserve for the effects of opioids in vivo are likely to provide a much-needed framework of efficacy to drug discovery programs that may suggest some new strategies for the development of improved analgesics.
7. Concluding remarks
The inherent limitations in the experimental design of antinociception assays can influence determinations of efficacy of opioid drugs. When characterising novel opioid agonists, consideration should be taken into how these limitations affect the data.
Differential strain sensitivity to the effects of opioids (including the potential for differences in drug pharmacokinetics) needs to be thoroughly studied. This is not only important when comparing actions of opioids across laboratories, but also when considering the use of genetically modified mice.
Adverse side effects limit the therapeutic potential of novel opioid analgesics. It is important that all opioidergic behaviours induced by novel agonists are assessed at doses above those providing effective antinociception in order to understand the liabilities and risks associated with deliberate or accidental abuse.
Whilst early in vitro assessment of novel opioids provides valuable insight into their actions, these assays are limited in their ability to predict side effect liabilities. Early ex vivo characterisation of novel opioids in native tissue may provide better indications for side effect liability. Recent measurements of respiratory depression in zebra fish provides an example of an innovative higher throughput screen for novel opioids (Zaig, da Silveira Scarpellini, & Montandon, 2021).
Further assessments of the impact of receptor reserve and relative receptor expression levels across different tissues and animal strains are necessary to understand the relationship between efficacy and opioid-induced responses in vivo and provide a framework for the development of new opioid-based analgesics.
Acknowledgments
This work was supported by the Academy of Medical Sciences. Fig. 1 was made using Biorender (www.biorender.com). We would like to acknowledge the authors of studies that, due to space limitations, are not cited in this manuscript.
Abbreviations
- GPCR
G protein-coupled receptor
- MOR
mu-opioid receptor
- OIH
opioid-induced hyperalgesia
- OIC
opioid-induced constipation
- WBP
whole body plethysmography
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest.
References
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. Journal of Psychopharmacology. 2017;31:730–739. doi: 10.1177/0269881116689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Kochan KE, Nastase AF, Montgomery D, Griggs NW, Traynor JR, et al. Jutkiewicz EM. In vivo effects of μ-opioid receptor agonist/δ-opioid receptor antagonist peptidomimetics following acute and repeated administration. British Journal of Pharmacology. 2018;175:2013–2027. doi: 10.1111/bph.14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Anissian L, Schwartz HW, Vincent K, Vincent HK, Carpenito J, Stambler N, Ramakrishna T. Subcutaneous methylnaltrexone for treatment of acute opioid-induced constipation: Phase 2 study in rehabilitation after orthopedic surgery. Journal of Hospital Medicine. 2012;7:67–72. doi: 10.1002/jhm.943. [DOI] [PubMed] [Google Scholar]
- Ankier SI. New hot plate tests to quantify antinociceptive and narcotic antagonist activities. European Journal of Pharmacology. 1974;27:1–4. doi: 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Mu-opioid receptor (MOR) biased agonists induce biphasic dose-dependent hyperalgesia and analgesia, and hyperalgesic priming in the rat. Neuroscience. 2018;394:60–71. doi: 10.1016/j.neuroscience.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Doré A-J, Millecamps M, et al. Pineyro G. Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of delta opioid receptor agonists. The Journal of Neuroscience. 2012;32:4827–4840. doi: 10.1523/JNEUROSCI.3734-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo Neto J, Costanzini A, De Giorgio R, Lambert DG, Ruzza C, Calo G. Biased versus partial agonism in the search for safer opioid analgesics. Molecules. 2020:25. doi: 10.3390/molecules25173870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. Elife. 2020;9:e52694. doi: 10.7554/eLife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Durand A, Yackle K. ß2-Arrestin germline knockout does not attenuate opioid respiratory depression. Elife. 2021;10:e62552. doi: 10.7554/eLife.62552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S, Durk T, Becker G. Methylnaltrexone for the treatment of opioid-induced constipation. Expert Review of Gastroenterology & Hepatology. 2013;7:13–26. doi: 10.1586/egh.12.63. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, et al. Henderson G. Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. British Journal of Pharmacology. 2009;158:157–164. doi: 10.1111/j.1476-5381.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains RS, Wells S, Sillito RR, Armstrong JD, Cater HL, Banks G, Nolan PM. Assessing mouse behaviour throughout the light/dark cycle using automated in-cage analysis tools. Journal of Neuroscience Methods. 2018;300:37–47. doi: 10.1016/j.jneumeth.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends in Pharmacological Sciences. 2017;38:181–194. doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD. The role of voltage-gated sodium channels in pain signaling. Physiological Reviews. 2019;99:1079–1151. doi: 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- Benredjem B, Gallion J, Pelletier D, Dallaire P, Charbonneau J, Cawkill D, et al. Pineyro G. Exploring use of unsupervised clustering to associate signaling profiles of GPCR ligands to clinical response. Nature Communications. 2019;10:4075. doi: 10.1038/s41467-019-11875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Birdsong WT, Williams JT. Recent progress in opioid research from an electrophysiological perspective. Molecular Pharmacology. 2020;98:401–409. doi: 10.1124/mol.119.119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. The Journal of Neuroscience. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR. Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: Comparison with clocinnamox, beta-funaltrexamine, and beta-chlornaltrexamine. The Journal of Pharmacology and Experimental Therapeutics. 2000;294:933–940. [PubMed] [Google Scholar]
- Brock C, Olesen SS, Olesen AE, Frokjaer JB, Andresen T, Drewes AM. Opioid-induced bowel dysfunction pathophysiology and management. Drugs. 2012;72:1847–1865. doi: 10.2165/11634970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague-Dawley rats. Psychopharmacology. 2002;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, et al. Bohn LM. A G protein-biased ligand of the kappa opioid receptor is antinociceptive and antipruritic but does not cause sedation or dysphoria. Faseb Journal. 2017:31. [Google Scholar]
- Bubier JA, He H, Philip VM, Roy T, Hernandez CM, Bernat R, et al. Chesler EJ. Genetic variation regulates opioid-induced respiratory depression in mice. Scientific Reports. 2020;10:14970. doi: 10.1038/s41598-020-71804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald P. A receptor model with binding affinity, activation efficacy, and signal amplification parameters for complex fractional response versus occupancy data. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma NE, Leduc-Pessah H, Trang T. Genetic deletion of microglial Panx1 attenuates morphine withdrawal, but not analgesic tolerance or hyperalgesia in mice. Channels (Austin, Tex) 2017;11:487–94. doi: 10.1080/19336950.2017.1359361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Lambert DG. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. British Journal of Anaesthesia. 2018;121:1105–1114. doi: 10.1016/j.bja.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Opioid-induced constipation: Challenges and therapeutic opportunities. American Journal of Gastroenterology. 2011;106:835–842. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- Carliss RD, Keefer JF, Perschke S, Welch S, Rich TC, Weissman AD. Receptor reserve reflects differential intrinsic efficacy associated with opioid diastereomers. Pharmacology, Biochemistry, and Behavior. 2009;92:495–502. doi: 10.1016/j.pbb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Carroll MN. The effect of injury in nociceptive tests employed in analgetic assays. Archives Internationales De Pharmacodynamie Et De Therapie. 1959;123:48–57. [PubMed] [Google Scholar]
- Carstens E, Wilson C. Rat tail-flick reflex – magnitude measurement of stimulus-response function, suppression by morphine and habituation. Journal of Neurophysiology. 1993;70:630–639. doi: 10.1152/jn.1993.70.2.630. [DOI] [PubMed] [Google Scholar]
- Carter RB. Differentiating analgesic and non-analgesic drug activities on rat hot plate – effect of behavioral end-point. Pain. 1991;47:211–220. doi: 10.1016/0304-3959(91)90207-E. [DOI] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfi I, Audet N, Bagheri Tudashki H, Pineyro G. Identifying ligand-specific signalling within biased responses: Focus on δ opioid receptor ligands. British Journal of Pharmacology. 2015;172:435–48. doi: 10.1111/bph.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. Opioid receptor reserve in normal and morphine-tolerant guinea pig ileum myenteric plexus. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:7253–7257. doi: 10.1073/pnas.81.22.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Koob GF. Dynorphin, dysphoria, and dependence: The stress of addiction. Neuropsychopharmacology. 2016;41:373–374. doi: 10.1038/npp.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Moutal A, Navratilova E, Kopruszinski C, Yue X, Ikegami M, et al. Porreca F. The prolactin receptor long isoform regulates nociceptor sensitization and opioid-induced hyperalgesia selectively in females. Science Translational Medicine. 2020;12 doi: 10.1126/scitranslmed.aay7550. Article eaay7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Canamar CP, Hillhouse M, Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. The Journal of Pain. 2012;13:401–409. doi: 10.1016/j.jpain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear AE, Asghar J, Hill R, Henderson G, Borbely E, Tekus V, et al. Kelly E. A novel G protein-biased agonist at the <em>δ</em> opioid receptor with analgesic efficacy in models of chronic pain. Journal of Pharmacology and Experimental Therapeutics. 2020;372:224–236. doi: 10.1124/jpet.119.258640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear AE, Kelly E. A biased view of mu-opioid receptors? Molecular Pharmacology. 2019;96:542–549. doi: 10.1124/mol.119.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen K. Enhanced analgesic potency and reduced tolerance of morphine in 129/SvEv mice: Evidence for a deficiency in GM1 ganglioside-regulated excitatory opioid receptor functions. Brain Research. 2000;856:227–235. doi: 10.1016/s0006-8993(99)02446-4. [DOI] [PubMed] [Google Scholar]
- Crombie A, Arezzo J, Cowan C, DeWire S, Gowen-MacDonald W, Hawkins M, et al. Violin J. TRV250: A novel biased ligand at the delta receptor for the potential treatment of migraine. Cephalalgia. 2015;35:1225–1226. [Google Scholar]
- Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Buprenorphine induces ceiling in respiratory depression but not in analgesia. BJA: British Journal of Anaesthesia. 2006;96:627–632. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morbidity and Mortality Weekly Report. 2018;67:1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. Journal of Pharmacology and Experimental Therapeutics. 1941;72:74–79. [Google Scholar]
- Dekan Z, Sianati S, Yousuf A, Sutcliffe KJ, Gillis A, Mallet C, et al. Christie MJ. A tetrapeptide class of biased analgesics from an Australian fungus targets the microopioid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:22353–22358. doi: 10.1073/pnas.1908662116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, et al. Violin JD. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. The Journal of Pharmacology and Experimental Therapeutics. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, et al. Ko M-C. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Science Translational Medicine. 2018;10 doi: 10.1126/scitranslmed.aar3483. Article eaar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S. Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. British Journal of Pharmacology. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. 2. Dithienylbutenylamines and dithienylbutylamines. Journal of Pharmacology and Experimental Therapeutics. 1953;107:385–393. [PubMed] [Google Scholar]
- Ehrlich AT, Semache M, Gross F, Da Fonte DF, Runtz L, Colley C, Mezni A, Le Gouill C, Lukasheva V, Hogue M, Darcq E, et al. Biased Signaling of the Mu Opioid Receptor Revealed in Native Neurons. iScience. 2019 Apr 26;14:47–57. doi: 10.1016/j.isci.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi A, Varga BR, Majumdar S. Biased opioid ligands. Molecules. 2020;25:4257. doi: 10.3390/molecules25184257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Translational Psychiatry. 2014;4 doi: 10.1038/tp.2014.30. Article e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, et al. Canals M. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Science Signaling. 2020;13 doi: 10.1126/scisignal.aaz3140. [DOI] [PubMed] [Google Scholar]
- Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, Canals M. Critical assessment of g protein-biased agonism at the mu-opioid receptor. Trends in Pharmacological Sciences. 2020a;41(12):947–959. doi: 10.1016/j.tips.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, Canals M. Critical assessment of G protein-biased agonism at the mu-opioid receptor. Trends in Pharmacological Sciences. 2020b;41:947–959. doi: 10.1016/j.tips.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Gillis A, Sreenivasan V, Christie MJ. Intrinsic efficacy of opioid ligands and its importance for apparent bias, operational analysis, and therapeutic window. Molecular Pharmacology. 2020;98:410–424. doi: 10.1124/mol.119.119214. [DOI] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, et al. Devi LA. Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity. Proceedings ofthe NationalAcademy ofSciences ofthe United States of America. 2013;110:12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granat FR, Saelens JK. Effect of stimulus intensity on potency of some analgetic agents. Archives Internationales De Pharmacodynamie Et De Therapie. 1973;205:52–60. [PubMed] [Google Scholar]
- Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho JH, Acevedo-Canabal A, et al. Bohn LM. A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuro psychopharmacology. 2020;45:416–425. doi: 10.1038/s41386-019-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, McCann M, Tubbs N. Respiratory effects of low and high doses of fentanyl in control and β-arrestin 2 deficient mice. Journal of Neurophysiology. 2021 Apr 1;125(4):1396–1407. doi: 10.1152/jn.00711.2020. Epub 2021 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JD. Thresholds of pain and reflex contraction as related to noxious stimulation. Journal of Applied Physiology. 1953;5:725–739. [Google Scholar]
- Hardy JD, Stoll AM, Cunningham D, Benson WM, Greene L. Responses of the rat to thermal radiation. American Journal of Physiology. 1957;189:1–5. doi: 10.1152/ajplegacy.1957.189.1.1. [DOI] [PubMed] [Google Scholar]
- Hardy JD, Wolff HG, Goodell H. Studies on pain.A new method for measuring pain threshold: Observations on spatial summation of pain. Journal of Clinical Investigation. 1940;19:649–657. doi: 10.1172/JCI101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner MA. Drug overdose deaths in the United States, 1999-2018. Hyattsville, MD: National Center for Health Statistics; 2017. (NCHS Data Brief, no 356) [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, et al. Henderson G. The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. British Journal of Pharmacology. 2018;175:2653–2661. doi: 10.1111/bph.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, et al. Henderson G. Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuro psychopharmacology. 2016;41(3):762–773. doi: 10.1038/npp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G. Fentanyl depression of respiration: Comparison with heroin and morphine. British Journal of Pharmacology. 2020;177:254–265. doi: 10.1111/bph.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ. G-protein-coupled receptors: Past, present and future. British Journal of Pharmacology. 2006;147(Suppl. 1):S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalte F, Berggren A-C, Bergendahl H, Hjortsberg C. The direct and indirect costs of opioid-induced constipation. Journal of Pain and Symptom Management. 2010;40:696–703. doi: 10.1016/j.jpainsymman.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacology, Biochemistry, and Behavior. 1999;63:21–26. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Hoymann HG. Invasive and noninvasive lung function measurements in rodents. Journal of Pharmacological and Toxicological Methods. 2007;55:16–26. doi: 10.1016/j.vascn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Berge OG, Hole K. A modified hot-plate test sensitive to mild analgesics. Behavioural Brain Research. 1986;21:101–108. doi: 10.1016/0166-4328(86)90088-4. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. The Lancet Neurology. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- Katzhang N. The impact of pain management on quality of life. Journal of Pain and Symptom Management. 2002;24:S38–S47. doi: 10.1016/s0885-3924(02)00411-6. [DOI] [PubMed] [Google Scholar]
- Kelly E. Ligand bias at the μ-opioid receptor. Biochemical Society Transactions. 2013;41:218–224. doi: 10.1042/BST20120331. [DOI] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: A survey of 11 inbred mouse strains. Pharmacology, Biochemistry, and Behavior. 2002;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L. SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: Analgesic and rewarding properties in mice. The Journal of Pharmacology and Experimental Therapeutics. 2007;320:934–943. doi: 10.1124/jpet.106.111997. [DOI] [PubMed] [Google Scholar]
- Klein Herenbrink C, Sykes DA, Donthamsetti P, Canals M, Coudrat T, Shonberg J, et al. Lane JR. The role of kinetic context in apparent biased agonism at GPCRs. Nature Communications. 2016;7:10842. doi: 10.1038/ncomms10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, et al. Schulz S. Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. British Journal of Pharmacology. 2020;177:2923–2931. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, et al. Schulz S. Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nature Communications. 2019;10:367. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblish M, Carr R, Siuda ER, Rominger DH, Gowen-MacDonald W, Cowan CL, et al. Lark MW. TRV0109101, a G protein-biased agonist of the mu-opioid receptor, does not promote opioid-induced mechanical allodynia following chronic admin-istration. Journal of Pharmacology and Experimental Therapeutics. 2017;362:254–262. doi: 10.1124/jpet.117.241117. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: Underlying molecular neurobiology and genetics. The Journal of Clinical Investigation. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, et al. Sames D. 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Central Science. 2019;5:992–1001. doi: 10.1021/acscentsci.9b00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla L, Bugno R, Skupio U, Wiktorowska L, Solecki W, Wojtas A, et al. Przewlocki R. Functional characterization of a novel opioid, PZM21, and its effects on the behavioural responses to morphine. British Journal of Pharmacology. 2019;176:4434–4445. doi: 10.1111/bph.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. CrossTalk opposing view: The pre-Botzinger complex is not essential for respiratory depression following systemic administration of opioid analgesics. Journal of Physiology (London) 2014;592:1163–1166. doi: 10.1113/jphysiol.2013.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- Levine JD, Murphy DT, Seidenwurm D, Cortez A, Fields HL. A study of the quantal (all-or-none) change in reflex latency produced by opiate analgesics. Brain Research. 1980;201:129–141. doi: 10.1016/0006-8993(80)90780-5. [DOI] [PubMed] [Google Scholar]
- Liang DY, Li WW, Nwaneshiudu C, Irvine KA, Clark JD. Pharmacological characters of oliceridine, a μ-opioid receptor G-protein-biased ligand in mice. Anesthesia and Analgesia. 2019;129:1414–1421. doi: 10.1213/ANE.0000000000003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D-Y, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sciences. 1989;45:1627–1636. doi: 10.1016/0024-3205(89)90272-5. [DOI] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, et al. Bohn LM. Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK 1/2 phosphorylation while preserving G protein over βarrestin2 signaling bias. ACS Chem Neurosci. 2015 Aug 19;6(8):1411–1419. doi: 10.1021/acschemneuro.5b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JD, Bailey CP. Functional selectivity and time-dependence of μ-opioid receptor desensitization at nerve terminals in the mouse ventral tegmental area. British Journal of Pharmacology. 2015;172:469–481. doi: 10.1111/bph.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein O, Leyendecker P, Hopp M, Schutter U, Rogers PD, Uhl R, et al. Reimer K. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: A randomised controlled trial. Expert Opinion on Pharmacotherapy. 2009;10:531–543. doi: 10.1517/14656560902796798. [DOI] [PubMed] [Google Scholar]
- Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. The Journal of Pharmacology and Experimental Therapeutics. 2008;325:267–275. doi: 10.1124/jpet.107.132167. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Rossignol E, Moehrle JJ, Van Vleet TR, Marsh KC, Parman T, et al. Mittelstadt SW. Increased stress associated with head-out plethysmography testing can exacerbate respiratory effects and lead to mortality in rats. Journal of Pharmacological and Toxicological Methods. 2019;99:106580. doi: 10.1016/j.vascn.2019.106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Le Rouzic V, Varadi A, Xu J, Rajadhyaksha AM, Majumdar S, et al. Pasternak GW. Genetic dissociation of morphine analgesia from hyperalgesia in mice. Psychopharmacology. 2017;234:1891–1900. doi: 10.1007/s00213-017-4600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Umemoto H, Mori T, Akatsu R, Saito S, Tashima K, et al. Horie S. Differences in the morphine-induced inhibition of small and large intestinal transit: Involvement of central and peripheral μ-opioid receptors in mice. European Journal of Pharmacology. 2016;771:220–228. doi: 10.1016/j.ejphar.2015.12.033. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Carr D. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. The Journal of Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- Michna E, Blonsky ER, Schulman S, Tzanis E, Manley A, Zhang H, et al. Randazzo B. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: A randomized controlled study. The Journal of Pain. 2011;12:554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Narita M, Oji DE, Suganuma C, Nagase H, Sora I, et al. Tseng LF. The mu-opioid receptor gene-dose dependent reductions in G-protein activation in the pons/medulla and antinociception induced by endomorphins in mu-opioid receptor knockout mice. Neuroscience. 1999;94:203–207. doi: 10.1016/s0306-4522(99)00298-5. [DOI] [PubMed] [Google Scholar]
- Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. The Journal of Pharmacology and Experimental Therapeutics. 1991;258:544–550. [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3048–3055. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG. Nociceptive and morphine antinociceptive sensitivity of 129 and C57BL/6 inbred mouse strains: Implications for transgenic knock-out studies. European Journal of Pain. 1997;1:293–297. doi: 10.1016/s1090-3801(97)90038-0. [DOI] [PubMed] [Google Scholar]
- Montandon G, Horner R. CrossTalk proposal: The preBötzinger complex is essential for the respiratory depression following systemic administration of opioid analgesics. The Journal of Physiology. 2014;592:1159–1162. doi: 10.1113/jphysiol.2013.261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology. 2016;124:641–650. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mores KL, Cummins BR, Cassell RJ, van Rijn RM. A review of the therapeutic potential of recently developed g protein-biased kappa agonists. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, et al. Suzuki T. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: Differential involvement of μ-opioid receptor sites and responsible regions. The Journal of Pharmacology and Experimental Therapeutics. 2013;347:91–99. doi: 10.1124/jpet.113.204313. [DOI] [PubMed] [Google Scholar]
- Mori T, Takemura Y, Arima T, Iwase Y, Narita M, Miyano K, et al. Narita M. Further investigation of the rapid-onset and short-duration action of the G protein-biased μ-ligand oliceridine. Biochemical and Biophysical Research Communications. 2021;534:988–994. doi: 10.1016/j.bbrc.2020.10.053. [DOI] [PubMed] [Google Scholar]
- Müller-Lissner S, Bassotti G, Coffin B, Drewes AM, Breivik H, Eisenberg E, et al. Morlion B. Opioid-induced constipation and bowel dysfunction: A clinical guideline. Pain Medicine. 2017;18:1837–1863. doi: 10.1093/pm/pnw255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ. Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides. 2019;112:23–31. doi: 10.1016/j.peptides.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocallaghan JP, Holtzman SG. Quantification of analgesic activity of narcotic-antagonists by a modified hot-plate procedure. Journal of Pharmacology and Experimental Therapeutics. 1975;192:497–505. [PubMed] [Google Scholar]
- Office of National Statistics, UK. 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2018registrations .
- Okie S. A flood of opioids, a rising tide of deaths. The New England Journal of Medicine. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Panchal SJ, Mueller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: Prevalence, pathophysiology and burden. International Journal of Clinical Practice. 2007;61:1181–1187. doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. American Journal of Surgery. 2001;182:11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- Pedersen MF, Wróbel TM, Märcher-Rørsted E, Pedersen DS, Møller TC, Gabriele F, et al. Bräuner-Osborne H. Biased agonism of clinically approved μ-opioid receptor agonists and TRV130 is not controlled by binding and signaling kinetics. Neuropharmacology. 2020;166:107718. doi: 10.1016/j.neuropharm.2019.107718. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Nagi K. Signaling diversity of mu- and delta- opioid receptor ligands: Re-evaluating the benefits of μ-arrestin/G protein signaling bias. Cellular Signalling. 2021;80:109906. doi: 10.1016/j.cellsig.2020.109906. [DOI] [PubMed] [Google Scholar]
- Plant OH, Miller GH. Effects of morphine and some other opium alkaloids on the muscular activity of the alimentary canal I. Action on the small intestine in unanesthetized dogs and man. Journal of Pharmacology and Experimental Therapeutics. 1926;27:361. [Google Scholar]
- Portoghese PS, Larson DL, Jiang JB, Takemori AE, Caruso TP. 6beta-[N, N-Bis(2-chloroethyl)amino]-17-(cyclopropylmethyl)-4,5alpha-epoxy-3,14-dihydroxymorphinan(chlornaltrexamine) a potent opioid receptor alkylating agent with ultralong narcotic antagonist actitivty. Journal of Medicinal Chemistry. 1978;21:598–599. doi: 10.1021/jm00205a002. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. The delta opioid receptor: An evolving target for the treatment of brain disorders. Trends in Pharmacological Sciences. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AAA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, et al. Kieffer BL. Ligand-directed trafficking of the μ-opioid receptor <em>in vivo</em>: Two paths toward analgesic tolerance. The Journal of Neuroscience. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard D, Bharucha A. Management of opioid-induced constipation for people in palliative care. International Journal ofPalliative Nursing. 2015;21:272–280. doi: 10.12968/ijpn.2015.21.6.272. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism ofaction of tramadol, an atypical opioid analgesic. Journal of Pharmacology and Experimental Therapeutics. 1992;260:275–285. [PubMed] [Google Scholar]
- Ramirez JM, Burgraff NJ, Wei AD, Baertsch NA, Varga AG, Baghdoyan HA, et al. Levitt ES. Neuronal mechanisms underlying opioid-induced respiratory depression: Our current understanding. Journal of Neurophysiology. 2021;125:1899–1919. doi: 10.1152/jn.00017.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilley KJ, Giulianotti M, Dooley CT, Nefzi A, McLaughlin JP, Houghten RA. Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library. The AAPS Journal. 2010;12:318–329. doi: 10.1208/s12248-010-9191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren MF, Han JS. Rat tail flick acupuncture analgesia model. Chinese Medical Journal. 1979;92:576–582. [PubMed] [Google Scholar]
- Rodríguez-Arias M, Aguilar MA, Manzanedo C, Miñarro J. Preclinical evidence of new opioid modulators for the treatment of addiction. Expert Opinion on Investigational Drugs. 2010;19:977–994. doi: 10.1517/13543784.2010.500612. [DOI] [PubMed] [Google Scholar]
- Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience. 2016;338:160–182. doi: 10.1016/j.neuroscience.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and colon. The Journal of Pharmacology and Experimental Therapeutics. 2008;327:561–572. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Liu HC, Loh HH. mu-Opioid receptor-knockoutmice: The role of mu-opioid receptor in gastrointestinal transit. Molecular Brain Research. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- Sá KN, Moreira L, Baptista AF, Yeng LT, Teixeira MJ, Galhardoni R, de Andrade DC. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Reports. 2019;4:e779. doi: 10.1097/PR9.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadee W, Oberdick J, Wang Z. Biased opioid antagonists as modulators ofopioid dependence: Opportunities to improve pain therapy and opioid use management. Molecules. 2020;25 doi: 10.3390/molecules25184163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, Gassaway MM, et al. Hen R. The behavioral effects of the antidepressant tianeptine require the mu-opioid receptor. Neuropsychopharmacology. 2017;42:2052–2063. doi: 10.1038/npp.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, et al. Bohn LM. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171:1165–1175. doi: 10.1016/j.cell.2017.10.035. Article e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, et al. Banks ML. Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology. 2019;150:200–209. doi: 10.1016/j.neuropharm.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson K, Leyendecker P, Hopp M, Muller-Lissner S, Lowenstein O, De Andres J, et al. Reimer K. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Current Medical Research and Opinion. 2008;24:3503–3512. doi: 10.1185/03007990802584454. [DOI] [PubMed] [Google Scholar]
- Singleton S, Baptista-Hon DT, Edelsten E, McCaughey KS, Camplisson E, Hales TG. TRV130 partial agonism and capacity to induce anti-nociceptive tolerance revealed through reducing available μ-opioid receptor number. British Journal of Pharmacology. 2021;178:1855–1868. doi: 10.1111/bph.15409. [DOI] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: Evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Swain Y, Gewirtz JC, Harris AC. Behavioral predictors of individual differences in opioid addiction vulnerability as measured using i.v. self-administration in rats. Drug and Alcohol Dependence. 2021;221 doi: 10.1016/j.drugalcdep.2021.108561. Article 108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. American Family Physician. 2006;74:1347–1354. [PubMed] [Google Scholar]
- Takemori AE, Larson DL, Portoghese PS. The irreversible narcotic antagonistic and reversible agonistic properties of the fumaramate methyl ester derivative of naltrexone. European Journal of Pharmacology. 1981;70:445–451. doi: 10.1016/0014-2999(81)90355-1. [DOI] [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: Implications for therapeutic applications. The Journal of Pharmacology and Experimental Therapeutics. 2009;331:954–964. doi: 10.1124/jpet.109.157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trescot A, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11:S181–S200. [PubMed] [Google Scholar]
- Uprety R, Che T, Zaidi SA, Grinnell SG, Varga BR, Faouzi A, et al. Majumdar S. Controlling opioid receptor functional selectivity by targeting distinct subpockets of the orthosteric site. Elife. 2021;10 doi: 10.7554/eLife.56519. Article e56519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43:2514–2520. doi: 10.1038/s41386-018-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, et al. Majumdar S. Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit beta-arrestin-2. Journal of Medicinal Chemistry. 2016;59:8381–8397. doi: 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential impact oftwo critical respiratory centres in opioid-induced respiratory depression in awake mice. The Journal of Physiology. 2020;598:189–205. doi: 10.1113/JP278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden WJC, Heitman LH, Rosenkilde MM. Perspective: Implications of ligand-receptor binding kinetics for therapeutic targeting of G protein-coupled receptors. aCs Pharmacology & Translational Science. 2020;3:179–189. doi: 10.1021/acsptsci.0c00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic S, Srebro D, Vujovic KS, Vucetic C, Prostran M. Cannabinoids and pain: New insights from old molecules. Frontiers in Pharmacology. 2018;9:1259. doi: 10.3389/fphar.2018.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clinical Pharmacology and Therapeutics. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Christie MJ. Regulation of mu-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacological Reviews. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LL, Chakraborty S, Eans SO, Cirino TJ, Stacy HM, Simons CA, et al. McLaughlin JP. Kratom alkaloids, natural and semi-synthetic, show less physical dependence and ameliorate opioid withdrawal. Cellular and Molecular Neurobiology. 2021;41(5):1131–1143. doi: 10.1007/s10571-020-01034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterology and Motility. 2004;16:17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- Woolfe G, Macdonald AD. The evaluation of the analgesic action of Pethidine hydrochloride (Demerol) Journal of Pharmacology and Experimental Therapeutics. 1944;80:300–307. [Google Scholar]
- Yaksh TL, Woller SA, Ramachandran R, Sorkin LS. The search for novel analgesics: Targets and mechanisms. F1000Prime Reports. 2015;7:56. doi: 10.12703/P7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. The Journal of Pain. 2009;10:767–773. doi: 10.1016/j.jpain.2009.01.325. [DOI] [PubMed] [Google Scholar]
- Young A, Viswanath A, Kalladka M, Khan J, Eliav E, Diehl SR. Mouse model demonstrates strain differences in susceptibility to opioid side effects. Neuroscience Letters. 2018;675:110–115. doi: 10.1016/j.neulet.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Yudin Y, Rohacs T. The G-protein-biased agents PZM21 and TRV130 are partial agonists of mu-opioid receptor-mediated signalling to ion channels. Br J Pharmacol. 2019 Sep;176(17):3110–3125. doi: 10.1111/bph.14702. Epub 2019 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Nilges MR, Morgenweck J, Zhang X, Hackler L, Fasold MB. Endomorphin analog analgesics with reduced abuse liability, respiratory depression, motor impairment, tolerance, and glial activation relative to morphine. Neuropharmacology. 2016;105:215–227. doi: 10.1016/j.neuropharm.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Zaig S, da Silveira Scarpellini C, Montandon G. Respiratory depression and analgesia by opioid drugs in freely-behaving larval zebrafish. Elife. 2021;10 doi: 10.7554/eLife.63407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT. Nociceptin opioid receptor (NOP) as a therapeutic target: Progress in translation from preclinical research to clinical utility. Journal of Medicinal Chemistry. 2016;59:7011–7028. doi: 10.1021/acs.jmedchem.5b01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimet PO, Wynn RL, Ford RD, Rudo FG. Effect of hot plate temperature on the antinociceptive activity of mixed opioid agonist-antagonist compounds. Drug Development Research. 1986;7:277–280. [Google Scholar]