Abstract
Background
Here, we explore the association between excess weight during early to mid-adulthood and survival in patients diagnosed with breast and colorectal cancer, using a pooled analysis of five cohort studies and study participants from 11 countries.
Methods
Participant-level BMI trajectories were estimated by fitting a growth curve model using over 2 million repeated BMI measurements from close to 600,000 cohort participants. Cumulative measures of excess weight were derived. Data from over 23,000 breast and colorectal cancer patients were subsequently analyzed using time-to-event models for death with the date of diagnosis as start of follow-up. Study-specific results were combined through a random effect meta-analysis.
Results
We found a significant dose-response relationship (p-trend=0.013) between the average BMI during early and mid-adulthood and death from breast cancer, with a pooled hazard ratio of 1.31 (1.07, 1.60) and the time to death shortened by 16% for average BMI above 25 kg/m2 compared with average BMI less or equal to 22.5 kg/m2, respectively. Similar results were found for categories of cumulative time spent with excess weight. There was no association between excess body fatness during early to mid-adulthood and death in colorectal cancer patients.
Conclusions
Excess body fatness during early to mid-adulthood is not only associated with an increased risk of developing cancer, but also with a lower survival in breast cancer patients.
Impact
Our results emphasize the importance of public health policies aimed at reducing overweight during adulthood and inform future studies on the relationship between excess weight and cancer outcomes.
Keywords: breast cancer, cohort studies, colorectal cancer, life course, obesity duration
Introduction
Excess body fatness, herein referred to as excess weight, is an established risk factor for several cancer sites and other non-communicable diseases and accounts for at least 4% of the global cancer burden.(1) The prevalence of excess weight has continued to increase and recent birth cohorts spend a greater proportion of their lives with overweight or obesity than any generation before.(2) In 2015, approximately two billion adults globally were estimated to be overweight (defined as body mass index [BMI] ≥25 kg/m2), with more than a quarter of them being obese (BMI≥30 kg/m2); trends show a continuing increase, especially in children and adolescents.(3) These trends affect the obesity-related disease burden and may explain an increasing number of cancer cases in successively younger generations born since around 1950(4) in high-income countries, such as the United States.
The effect of excess weight on the occurrence of health-related outcomes is usually investigated based on height and weight assessments at only one time point (e.g., at study entry or baseline). Although a single BMI assessment informs on the severity of an individual’s overweight at some point in time, it fails to reflect the temporality of the exposure as well as its cumulative impact. Indeed, the impact of excess weight on health outcomes might not only depend on its magnitude but also on its duration,(5,6) the biological mechanisms mediating the effect of excess weight on health probably act through long-term processes such as chronic exposure to elevated insulin levels, chronic inflammation, and increased levels of estrogens in post-menopausal women.(7)
While the association between excess weight and cancer incidence is well-established(8) and has been shown to be dose-dependent with increasing duration and intensity leading to higher risks(9,10), its impact on the survival of cancer patients is less clear. Previous studies demonstrated a detrimental effect of overweight and obesity on all-cause mortality and cancer-specific mortality in the general population;(11–13) however, important questions remain concerning the impact of excess weight during adulthood on prognosis after a cancer diagnosis. In a recent study, we examined the association between excess weight during adulthood and survival in post-menopausal women with breast or colorectal cancer, two of the most commonly diagnosed cancers that have been causally related with body fatness. We found that the intensity and duration of excess weight was related to lower survival in breast cancer but not in colorectal cancer patients.(14) However, the size of the study population was limited so that our results needed to be confirmed.
In the present study, we conducted a pooled analysis of five prospective cohort studies with participants from 11 countries, enabling us to explore the association between excess weight during early to mid-adulthood, hereafter defined as the period between 20 and 50 years of age, and the survival of patients diagnosed with breast (women only) or colorectal cancer after the age of 50 years.
Material and Methods
Study design and participants
This study is part of the SurvPool project aiming to assess the cumulative impact of lifestyle-related risk factors, in particular overweight and obesity, on cancer incidence and mortality using data from international population-based prospective cohort studies [http://survival.iarc.fr/Survpool/en/]. Inclusion criteria were the availability of individual-level data on breast and colorectal cancer occurrence and mortality, demographic and lifestyle-related variables from the baseline questionnaire, as well as a minimum of two assessments of BMI (or separate assessments of height and weight) per study participant. The corresponding participant flow chart is shown in Supplementary Figure 1.
Five cohorts that met these criteria were included: the Cancer Prevention Study II (CPS-II) (data access was granted to the authors for breast cancer only) (15); the European Prospective Investigation into Cancer and Nutrition (EPIC) study (16); the Japan Public Health Center-based Prospective Study, Cohort I (JPHC-I)(17); the Japan Public Health Center-based Prospective Study, Cohort II (JPHC-II)(17); and the Women’s Lifestyle and Health (WLHS) study(18). A summary table of the characteristics of the included cohorts with 783,338 participants, is presented in Table 1. The Institutional Review Board of each cohort as well as the International Agency for Research on Cancer (IARC) Ethics Committee approved the study, which was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the respective cohort study.
Table 1. Characteristics of the cohorts included in the cohort consortium assessing the relationship between BMI and mortality in breast and colorectal cancer patients.
| Study cohort a | CPS-II Nutrition Survey | EPIC PANACEA | JPHC I | JPHC II | WLHS |
|---|---|---|---|---|---|
| Country | United States | Denmark, France, Germany, Italy, Norway, Spain, Sweden, The Netherlands, United Kingdom | Japan | Japan | Sweden |
| Years of recruitment | 1992-1993 | 1992-1999 | 1990 | 1993-1994 | 1991-1992 |
| Age at recruitment, years | 40-80 | 35-70 | 40-59 | 40-69 | 30-49 |
| Original population size | 184,194 | 450,112 | 43,140 | 56,634 | 49,258 |
| Anthropometric assessments | Height at baseline Weight at baseline, in 1992, and every two years from 1997 to 2013 | Height at baseline Weight at age 20 (18 for Norway), baseline and at 5 years of follow-up | Height and weight at baseline, 5 year- and 10 year- follow-up | Height at baseline, 5 year- and 10 year- follow-up Weight at age 20, baseline, 5 years and 10 years of follow-up | Height at baseline Weight at age 18, baseline, and 10 years of follow-up |
| Population included for BMI b trajectory estimation | 78,936 | 389,123 | 38,611 | 52,478 | 33,031 |
| Total number of BMI assessments (mean number per participant) | 711,352 (9.01) | 920,241 (2.36) | 107,491 (2.78) | 174,694 (3.33) | 95,670 (2.90) |
| Censoring date | 31/12/2014 | 31/12/2013 | 31/12/2016 | 31/12/2016 | 31/12/2012 |
| Cancer ascertainment | Cancer and national death registries | Cancer registries (Denmark, Italy, Norway, Spain, Sweden, The Netherlands, United Kingdom), combination of methods in France, Germany, Naples-Italy | Cancer registries and death certificates | Cancer registries and death certificates | Cancer and national death registries |
Abbreviations for study names are as follows: CPS-II, Cancer Prevention Study II; EPIC, European Prospective Investigation into Cancer and nutrition; JPHC I, Japanese Public Health Center-based study cohort I; JPHC II, Japanese Public Health Center-based study cohort II; WLHS, Women’s Lifestyle and Health Study;
BMI: Body Mass Index.
Identification and follow-up of cancer cases
Incident cases of invasive breast and colorectal cancer (respectively, codes C50 and C18-C20 of the International Classification of Diseases for Oncology, 3rd edition) and deaths were identified by active patient notification from major local hospitals and data linkage with population-based cancer registries or national death registries. In EPIC, for France, Germany, and Naples-Italy, a combination of methods was used to identify cases and vital status, including health insurance records, cancer and pathology registries, and an active follow-up of study participants. In CPS-II, deaths were identified based on linkages with the U.S. National Death Index.
Statistical analyses
For each participating cohort, the impact of BMI on all-cause mortality in cancer patients was assessed through a two-step approach:(14,19) first, we used repeated BMI assessments to create individual-specific BMI trajectories among all cohort participants. Then, these trajectories were used to construct BMI-related variables reflecting cumulative exposure, which were subsequently used as predictors of death in individuals diagnosed with breast or colorectal cancer in survival models adjusted for potential confounding factors.
Cohort-specific BMI-related variables construction
For the first step, we used BMI values (computed from height and weight measurements or self-reports) taken at or after the age of 18 years and, subject to data availability, verifying the following criteria: height greater than 130 cm, BMI greater than 16 kg/m2, and waist circumference between 40 and 160 cm. Individuals were included if they contributed at least two valid BMI assessments before the end of the study follow-up, death, or at most one year before a diagnosis of cancer, whichever came first.
We used a growth curve model to describe the trajectory of BMI as a function of age. More precisely, the BMI for individual i assessed on occasion j, BMI ij was modelled using the following equation:
This model describes BMI as a quadratic polynomial function of age with random intercepts and slopes (respectively u0i and u1i) assumed to be multivariate normally distributed. In the EPIC study, the intercept as well as the linear and quadratic effects of age were allowed to vary between countries.
Using the estimates from the growth curve model, it was thus possible to construct for each individual a model-based BMI trajectory corresponding to a quadratic polynomial of age with individual-specific intercept and slope. These model-based BMI trajectories were then used to to compute the average BMI during early and mid-adulthood (this cumulative measure corresponds to the area under the BMI trajectory from age 20 to 50 years divided by the length of the period, i.e., 30 years) and the time spent with overweight or obesity during early and mid-adulthood (defined as the interval of time between age 20 and 50 years during which the model-based BMI was equal to or greater than 25 kg/m2). It should be noted that the average BMI between age 20 and 50, as a cumulative measure of excess weight, cannot be interpreted as a single BMI assessment and consequently, as such this should not be directly interpreted or associated with the standard definitions of ‘overweight’ and ‘obesity’, based on BMI measured at a given point in time. Nonetheless, to allow comparison with studies using single point-in-time BMI, sensitivity analyses using the most recent pre-diagnostic BMI assessments obtained at least one year preceding cancer diagnosis were also performed. For analysis and presentation purposes, average BMI across this 30-year time span was then categorised into less or equal than 22.5 kg/m2, greater than 22.5 and less or equal 25 kg/m2, and more than 25 kg/m2, and time spent with overweight was categorised into never, less than or equal to 15 years and more than 15 years.
Cohort-specific time-to-event analyses
The second step was restricted to individuals diagnosed with breast (women only) or colorectal cancer after the age of 50 years and with information on vital status at the end of follow-up. The analysis was restricted to patients diagnosed after the age of 50 years so that the cumulative BMI-related variables for each participant could be defined on the same exposure window of 30 years. Follow-up time for each participant was defined from the date of cancer diagnosis to the date of death or end of follow-up (ranging between the 31st of December 2012 and the 31st of December 2016 depending on the cohort, see Table 1), whichever came first. We used Cox proportional regression models with time since diagnosis as the time scale. We adjusted for the following potential confounders of the association between excess weight and cancer survival assessed at study entry: smoking status, alcohol consumption, vigorous physical activity, education, and history of diabetes. All models were stratified on cancer stage at diagnosis (localized, regional and distant tumour), age at diagnosis (using five-year age categories), and for the EPIC study, a further stratification on the country was made. Although each study was analysed separately, we harmonised the definition of the adjusting and stratifying variables between cohorts in order to facilitate the analysis: the correspondance between the original and harmonised definitions can be found in Supplementary Table 1. The proportionality of hazards assumption for the other variables included in the model was tested using the Grambsch-Therneau test. When there was evidence of non-proportionality for some of the adjusting factors, these were introduced in the model as strata. The proportion of missing values in the adjusting factors considered in the model ranged from 12.4% (JPHC I cohort) and 62.9% (EPIC cohort) for breast cancer and from 14.1% (JPHC I cohort) and 72.3% (EPIC cohort) for colorectal cancer. High proportions of missing values in the EPIC study were largely due to missing information on stage at diagnosis. The distribution of the confounding factors, including missing values, in each cohort is shown in Supplementary Tables 2-6. Multiple imputation was used to account for missing values: for each combination of cancer and BMI-related variable, fifty datasets were imputed using the multiple imputation by chained equations method(20) and results combined using Rubin’s rule.(21)
We also conducted survival analyses using the same procedure as above but replacing the Cox proportional hazard model by a Weibull accelerated failure time model to provide time ratios that quantify how much faster individuals in a higher risk group die compared to the unexposed group.(22,23) For example, if a given proportion of deaths is reached after time t in the unexposed group, the same proportion of deaths will be reached after time αt in the group exposed to the risk factor, where α is the time ratio. This means that if the time ratio is smaller than 1, exposure to the risk factor will accelerate the occurrence of deaths while a time ratio greater than 1 corresponds to a slowing down of death occurrence.
Finally, to allow comparison with previous studies, we also performed the above-mentioned analyses using a single BMI assessment (namely, the most recent BMI assessed at least one year before cancer diagnosis).
Pooled analysis
The cohort-specific results were pooled through a random effect model.(24,25) As described above, multiple imputation for covariates was performed at the study level, before aggregating the results, as suggested by Burgess et al.(26)
All analyses were performed using R statistical software (version 3.6.0; R Development Core Team, 2019).
Results
A total of 592,179 cohort participants contributing 2,009,448 BMI assessments were included in the estimation of cohort-specific BMI trajectories across time. The pooled analysis included data on 16,072 women diagnosed with breast cancer, as well as 3,350 men and 3,708 women diagnosed with colorectal cancer at the age of 50 years or above. In breast cancer patients, 3,485 deaths were observed during 137,455 person-years and in colorectal cancer patients, 3,106 deaths were observed during 40,106 person-years (Table 2). The median value of average BMI between ages 20 and 50 years among women diagnosed with breast cancer ranged from 22.1 kg/m2 in the WHLS cohort to 22.9 kg/m2 in the JPHC II cohort. Among patients diagnosed with colorectal cancer, the median value of average BMI between ages 20 and 50 years ranged from 22.3 kg/m2 in the WLHS cohort to 23.0 kg/m2 in the EPIC cohort for women; and from 22.8 kg/m2 in the JPHC I cohort to 25.1 kg/m2 in the EPIC cohort for men (see Table 2). A large proportion of breast cancer patients was diagnosed with localized breast cancer (between 58.2% and 75.8%) while for colorectal cancer these proportions varied from 21.0% and 49.1% for women and between 48.9% and 54.2% for men.
Table 2. Characteristics of breast and colorectal cancer patients in the international cohort consortium according to cohort study and cancer types.
| Study (cohort) | Cases | Deaths | Person-years | Estimated average BMI during in early and mid-adulthood a,b | Missing stage information | Stage at diagnosis | ||
|---|---|---|---|---|---|---|---|---|
| Localized | Regional | Distant | ||||||
| Breast cancer (women) | ||||||||
| CPS-II | 5292 | 1802 | 56,135 | 22.8 (21.5, 24.6) | 63 (1.2) | 3963 (75.8) | 1170 (22.4) | 96 (1.8) |
| EPIC | 9171 | 1414 | 69,767 | 22.5 (20.8, 24.3) | 3921 (42.8) | 3651 (69.6) | 1414 (26.9) | 185 (3.5) |
| JPHC I | 363 | 89 | 3263 | 22.5 (20.8, 24.2) | 25 (6.9) | 215 (63.6) | 102 (30.2) | 21 (6.2) |
| JPHC II | 383 | 115 | 2948 | 22.9 (21.6, 24.2) | 48 (12.5) | 203 (60.6) | 112 (33.4) | 20 (6.0) |
| WLHS | 863 | 65 | 5342 | 22.1 (20.9, 23.5) | 275 (31.9) | 342 (58.2) | 234 (39.8) | 12 (2.0) |
| Colorectal cancer (women) | ||||||||
| EPIC | 2635 | 1025 | 14,580 | 23.0 (21.5, 25.0) | 1371 (52.0) | 555 (43.9) | 271 (21.4) | 438 (34.7) |
| JPHC I | 402 | 146 | 2714 | 22.4 (20.7, 24.2) | 48 (11.9) | 154 (43.5) | 143 (40.4) | 57 (16.1) |
| JPHC II | 494 | 234 | 3155 | 22.9 (21.9, 24.1) | 81 (16.4) | 203 (49.1) | 135 (32.7) | 75 (18.2) |
| WLHS | 177 | 40 | 688 | 22.3 (21.3, 23.8) | 63 (35.6) | 24 (21.0) | 62 (54.4) | 28 (24.6) |
| Colorectal cancer (men) | ||||||||
| EPIC | 2029 | 943 | 10,617 | 25.1 (23.6, 26.9) | 1185 (58.4) | 436 (51.7) | 135 (16.0) | 273 (32.3) |
| JPHC I | 567 | 302 | 3634 | 22.8 (21.3, 24.7) | 60 (10.6) | 248 (48.9) | 149 (29.4) | 110 (21.7) |
| JPHC II | 754 | 416 | 4718 | 23.2 (22.1, 24.4) | 99 (13.1) | 355 (54.2) | 205 (31.3) | 95 (14.5) |
BMI: Body Mass Index;
Median (25th, 75th percentiles).
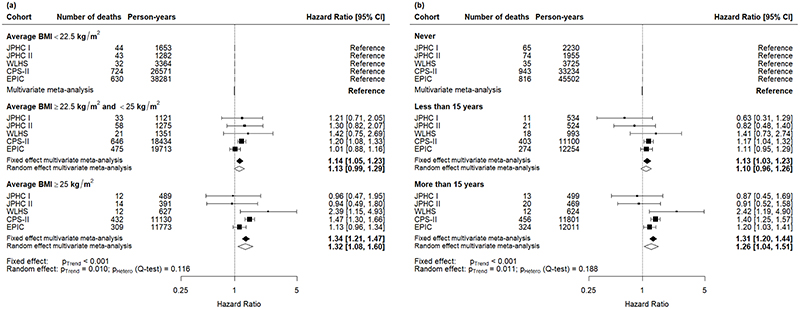
The pooled hazard ratios by BMI groups and stratified by stage at diagnosis and age group are presented in Figures 1-3. Among women diagnosed with breast cancer after the age of fifty years, the pooled hazard ratio increased in a dose-response manner (p-trend=0.01) in patients who experienced a higher average BMI during early and mid-adulthood: 1.13 (95% confidence intervals: 0.99, 1.29) in the category of average BMI between 22.5 kg/m2 to 25 kg/m2; and 1.32 (95% CI: 1.08, 1.60) in the category of average BMI greater than 25 kg/m2 (Figure 1a). A significant dose-response relationship (p-trend=0.011) was also found when we investigated the time spent with excess weight during early or mid-adulthood and its impact on death among breast cancer patients. Compared to women who were never in excess weight, women with less than 15 years with excess weight had a hazard ratio of 1.10 (95% CI: 0.96, 1.26) of dying and reached 1.26 (95% CI: 1.04, 1.51) for women who spent more than 15 years with excess weight (Figure 1b).
Figure 1.
Cohort-specific and pooled effect (measured in terms of hazard ratios) of mortality among women diagnosed with breast cancer after the age of 50 years (a) by categories of average body mass index (BMI); (b) by categories of cumulative time spent with overweight (body mass index, BMI ≥ 25 kg/m2) during early and mid-adulthood. The model was adjusted for smoking status, alcohol consumption, vigorous physical activity, education, and history of diabetes at study entry, and stratified on cancer stage at diagnosis, age at diagnosis, and country (for the EPIC study only).
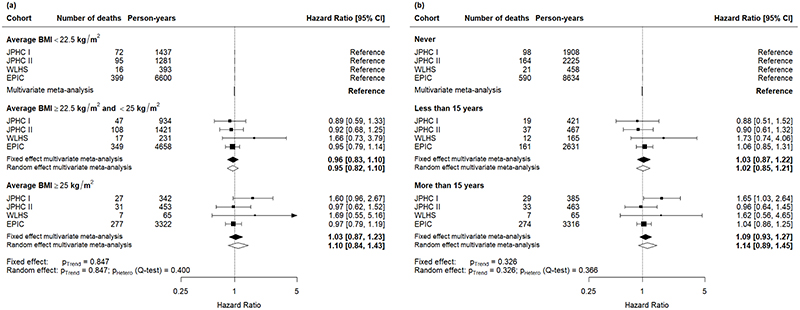
Figure 3.
Cohort-specific and pooled effect (measured in terms of hazard ratios) of mortality among men diagnosed with colorectal cancer after the age of 50 years (a) by categories of average body mass index (BMI); (b) by categories of cumulative time spent with overweight (body mass index, BMI ≥ 25 kg/m2) during early and mid-adulthood. The model was adjusted for smoking status, alcohol consumption, vigorous physical activity, education, and history of diabetes at study entry, and stratified on cancer stage at diagnosis, age at diagnosis, and country (for the EPIC study only).
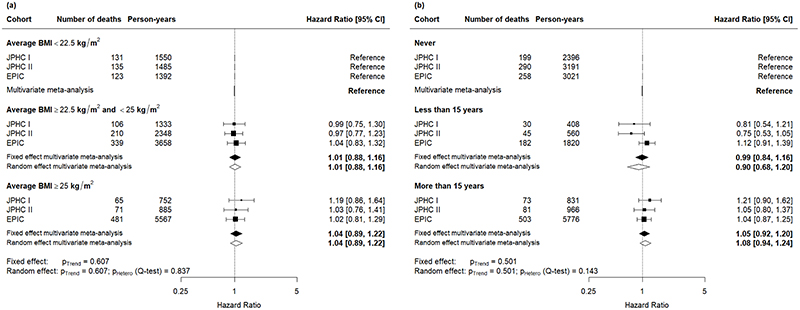
Among women diagnosed with colorectal cancer after the age of 50 years, there was no significant dose-response association between mean BMI during early and mid-adulthood and mortality (Figure 2a). Likewise, no significant association was found for time spent with excess weight during early and mid-adulthood (Figure 2b). For men diagnosed with colorectal cancer after the age of fifty years, there was also no significant dose-response association between average BMI during early and mid-adulthood nor of time spent with excess weight and mortality (Figure 3).
Figure 2.
Cohort-specific and pooled effect (measured in terms of hazard ratios) of mortality among women diagnosed with colorectal cancer after the age of 50 years (a) by categories of average body mass index (BMI); (b) by categories of cumulative time spent with overweight (body mass index, BMI ≥ 25 kg/m2) during early and mid-adulthood. The model was adjusted for smoking status, alcohol consumption, vigorous physical activity, education, and history of diabetes at study entry, and stratified on cancer stage at diagnosis, age at diagnosis, and country (for the EPIC study only).
Similar results were obtained when we used accelerated failure time models (see Supplementary Figures 2-4). The pooled time ratios for women diagnosed with breast cancer after the age of 50 years showed a significant dose-response effect for average BMI during early and mid-adulthood (p-trend=0.032), as well as for time spent with excess weight (p-trend<0.001). The time ratio for women in the category of average BMI in early and mid-adulthood greater than 25 kg/m2 compared to the reference category was 0.84 (95% CI: 0.73, 0.96) and the time ratio for women who spent more than 15 years with excess weight during early and mid-adulthood was 0.85 (95% CI: 0.78, 0.93), indicating that time to death was shortened by up to 15% in these groups when compared to their reference. For colorectal cancer, we found no significant association between the BMI-related variables and mortality among men nor women.
Results obtained when using a single BMI assessment were qualitatively similar (Supplementary Figures 5-7). In particular, BMI was found to have a significant detrimental effect on survival in women diagnosed with breast cancer (p-trend=0.001), although hazard ratios were of lower magnitude than those based on the average BMI during early and mid-adulthood: 0.97 (95% confidence intervals: 0.89, 1.06) in the category of BMI between 22.5 kg/m2 to 25 kg/m2; and 1.18 (95% CI: 1.07, 1.30) in the category of average BMI greater than 25 kg/m2 (Supplementary Figure 5a).
Discussion
In this study, we provide novel evidence on the impact of pre-diagnostic excess weight duration during early and mid-adulthood on mortality in patients diagnosed with breast and colorectal cancer after the age of 50, based on a pooled analysis from an international cohort consortium. We found that an average BMI greater than 25 kg/m2 during early and mid-adulthood and more than 15 years spent with excess weight between age 20 and 50 years were positively associated with mortality in women diagnosed with breast cancer. The time to death after a breast cancer diagnosis was shortened by up to 15% in a dose-response manner for women with an average BMI greater than 25 kg/m2 compared to women with an average BMI lower than 22.5 kg/m2 during early and mid-adulthood. For colorectal cancer, we found no statistically significant evidence for a positive association between cumulative measures of excess weight duration and mortality. These findings reinforce public health recommendations for preventing overweight and obesity at any age.
The relationship between excess weight and the survival of cancer patients has already been the subject of several studies.(27–29) A meta-analysis on the effect of obesity on survival of women diagnosed with breast cancer(27) showed a poorer overall and disease-specific survival of obese women with hazard ratios of 1.33 (95% CI: 1.21, 1.47) and 1.33 (95% CI: 1.19, 1.50), respectively. In another meta-analysis by Chan et al.,(28) women diagnosed with breast cancer who were overweight or obese before cancer diagnosis had a relative risk of 1.07 (95% CI: 1.02, 1.12) and 1.41 (95% CI: 1.29, 1.53) respectively for dying compared to the reference group of normal weight women. When looking at breast cancer-specific mortality, the relative risk estimates for overweight and obese women were 1.11 (95% CI: 1.06, 1.17) and 1.35 (95% CI: 1.24, 1.47), respectively. For colorectal cancer, a meta-analysis showed poorer overall and cancer-specific survival of obese patients with hazard ratios of 1.14 (95% CI: 1.07-1.21) and 1.14 (95% CI: 1.05-1.24), respectively, but no association with overweight.(29) These results were based on single BMI assessements before diagnosis, hence they are not completely comparable to those of the present study. However, they support our finding of a negative impact of excess weight on breast cancer prognosis.
A number of mechanisms have been postulated to be responsible for the association between excess weight and cancer development.(30–34) However, the association between excess weight and survival of cancer patients is less well understood. From a general point of view, there are three broad classes of hypotheses that might explain the role of excess weight on the survival of cancer patients.
Firstly, excess weight has been associated with an increased tumour size, higher tumour grades, and an increased risk of metastasis.(35) Consequently, the poor survival of overweight cancer patients might be partly explained by tumour characteristics at diagnosis, and these might be caused by biological mechanisms similar to those associated with cancer incidence. Indeed, it has been shown that excess body fatness impacts several signalling pathways associated with dysregulated cell growth and inhibition of apoptosis, resulting in more aggressive tumours.(36) In particular, overweight is associated with insulin resistance and subsequent increase in levels of insulin and free (unbound) insulin-like growth factor-1 (IGF-1) is associated with tumour growth and inhibition of apoptosis of micrometastases, resulting in poorer survival, particularly for breast and colorectal cancers.(37,38) Adipose tissue also stimulates the production of inflammatory factors such as interleukin-6 (IL6), interleukin 1β and Tumor Necrosis Factor α (TNFα), and inhibits apoptosis.(39) Increased levels of estrogens due to excess weight in postmenopausal women may also play a role.(7) Yet, in the present study, we found an effect of excess weight on survival even after stratifying the analyses on stage at diagnosis, suggesting that tumour characteristics are not the only mediator of poor survival of cancer patients with excess weight.
Secondly, excess weight is also known to be associated with several comorbidities such as diabetes or cardiovascular diseases which might further increase the risk of dying among cancer patients. It has indeed recently been shown that specific adverse lifestyle factors, including excess weight, are associated with an increased risk of multimorbidity of cancer and cardiovascular diseases,(40) suggesting that the effect of excess weight on survival of cancer patients is indirectly mediated by these. With the exception of past history of diabetes mellitus, information on comorbidities such as cardiovascular diseases, was not available from all included cohorts and therefore could not be accounted for in the analyses. Similarly, cause of death information was not consistently available across cohorts, meaning that it was not possible to analyse cause-specific mortality.
Thirdly, another mechanism that might explain our results is the fact that excess weight might influence the choice of treatment and consequently, there might be an increased risk of adverse consequences of cancer treatment in patients with excess weight.(41) For example, severe obesity might contra-indicate surgery and complicate the adjustment of chemotherapeutic treatment doses in overweight patients, potentially leading to suboptimal therapeutic concentrations and increased dose-related adverse effects such as cardiotoxicity.(42,43) Moreover, obese patients with reduced mobility might experience a higher risk of thrombosis and pulmonary complications such as pulmonary embolism and pneumonia after surgery.(44,45)
Although the above-mentioned mechanisms point to general explanations of the association between excess weight and survival in cancer patients, it should be noted that in our study, we found no significant association between excess weight and survival for colorectal cancer patients. A subgroup analysis restricted to colon cancer patients only equally showed a lack of association between excess weight and survival. It is possible that the association between excess weight and survival in colorectal cancer patients is weaker than for breast cancer. Indeed, in the meta-analysis by Doleman et al.(29), the association was limited to obese patients, with no significant effect for overweight patients, contrary to results for breast cancer.(27,28)
Our study has several strengths. First, by using data from five cohorts spanning three continents, we were able to assess the association between lifetime BMI and death in cancer patients using a large-scale database, including exposure information from close to 600,000 study participants at over 2 million occasions and follow-up for all-cause death from over 23,000 breast and colorectal cancer survivors. Second, as opposed to previous studies with only baseline BMI, we assessed lifetime overweight using multiple assessments of body weight across adulthood and modelled BMI trajectories, which were then translated into indicators summarizing cumulative exposure such as time spent overweight and average BMI during early and mid-adulthood. Moreover, by defining average BMI between ages 20 and 50 years and looking at cancer cases that occurred after the age of 50 years, we ensured that the exposure was defined in a uniform way across subjects and was not conditional on the age at cancer diagnosis.
Also, we presented two different measures to express the impact of lifetime overweight on survival in cancer patients. Although they represent the most standard way of assessing the impact of risk factors in time-to-event analyses, hazard ratios quantify how exposure to the risk factor modifies the mortality hazard rate, an instantaneous conditional probability. As such, they cannot be easily interpreted on the absolute risk scale, i.e., in terms of probabilities of survival at a given time. They consequently have a limited interest in clinical practice.(46) Time ratios derived from accelerated failure time models provide an altogether different measure of impact of the risk factor: they quantify by how much the time until occurrence of the event is shortened (time ratio below 1) or prolonged (time ratio above 1) compared to the reference group.(22) In this study, both approaches provided consistent and complementary results.
However, we also note some limitations. First, as frequent and regular BMI assessments during follow-up and for all years of age of all cohort participants are generally unavailable, we needed to use modelling procedures to estimate cumulative BMI-related variables. We mitigated the uncertainty by limiting our study to participants with information on BMI (or body height and weight) on at least two occasions before cancer diagnosis. Previous studies on the impact of lifetime excess weight on cancer risk showed that this is a robust methodology that allows for important insights into this relationship.(9,10) The self-reported (prospective and retrospective) assessments may have led to an underestimation of true BMI in overweight individuals contributing to some exposure misclassification, resulting in potential bias in our risk estimates. Secondly, as corresponding data were unavailable, we were unable to investigate associations across cancer-specific subgroups, for example by hormone receptor status for breast cancer or by tumor microsatellite instability status. Thirdly, a large degree of heterogeneity existed between studies, particularly when comparing the Japanese cohorts to their US and European counterparts. While this may partly be driven by differences in sample size, it needs to be taken into account when interpretating the results. While different cut-off points to define overweight have been suggested for Asian populations, for the purpose of international comparisons we retained the traditional definitions of overweight and obesity.(47) For the same reason, the impact of social determinants of health, such as socioeconomic status, could not be explored in this study. Lastly, the evidence of an association between excess weight, used as a proxy for body fatness, and survival in cancer patients presented in this study is based only on BMI assessments. These are subjects to measurement error, especially as in most cohort, some of the BMI assessments were based on retrospectively self-reported measures. Moreover, BMI is an imperfect indicator of body fatness. In particular, the relationship between the percentage of body fat and BMI varies with age, sex and ethnicity(48) and it might not appropriately reflect metabolic functions.(41) This emphasizes the need to develop combined indicators taking account of various aspects of body fatness (BMI, waist and hip circumference, or quantification of the volume of adipose compartments based on imaging(41)) in order to better understand the complex biological mechanisms between adiposity and survival of cancer patients.
To our knowledge, this is the first large-scale study on the effects of excess weight in early and mid-adulthood on the survival of breast and colorectal cancer patients. It highlights the negative impact of excess weight and time spent with excess weight before the age of 50 on the risk of dying among women diagnosed with breast cancer after the age of 50 years. These results emphasize the importance of public health policies aimed at preventing excess weight early in life. Future work should aim to understand in more detail how overweight and obesity are related to the prognosis of cancer, including the impact of post-diagnostic weight change and disentangling its complex relationships with treatment, comorbidities and across cancer subtypes.
Supplementary Material
Acknowledgements
The authors would like to thank all study participants and study staff for their valuable contribution to this research. The authors also would like to thank the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, for their contribution and ongoing support to the EPIC Study.
Funding
This study was funded by the World Cancer Research Fund International [grant number 2016/1636 awarded to I. Soerjomataram]. The coordination of EPIC is financially supported by the European Commission (DG-SANCO); and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer; Institut Gustave Roussy; Mutuelle Générale de l’Education Nationale; and Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), and Federal Ministry of Education and Research (BMBF) (Germany); Italian Association for Research on Cancer (AIRC); National Research Council; and Associazione Iblea per la Ricerca Epidemiologica (AIRE-ONLUS) Ragusa, Associazione Volontari Italiani Sangu (AVIS) Ragusa, Sicilian Government (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS); Netherlands Cancer Registry (NKR); LK Research Funds; Dutch Prevention Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); and Statistics Netherlands (the Netherlands); Health Research Fund (FIS); Regional Governments of Andalucía, Asturias, Basque Country, Murcia (No. 6236) and Navarra; and the Centro de Investigación Biomédica en Red en Epidemiología y Salud Pública and Instituto de Salud Carlos II (ISCIII RETIC) (RD06/0020) (Spain); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology - ICO (Spain); Swedish Cancer Society; Swedish Scientific Council; and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK; Medical Research Council; Stroke Association; British Heart Foundation; Department of Health; Food Standards Agency; and the Wellcome Trust (UK). Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 and C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom). The EPIC-Norfolk study (DOI 10.22025/2019.10.105.00004) has received funding from the Medical Research Council (MR/N003284/1 and MC-UU_12015/1) and Cancer Research UK (C864/A14136). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support
World Cancer Research Fund International [grant number 2016/1636] (HC, MA, IS)
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Authorship contribution: HC, HF, IS, and MA conceived and designed the study; HC performed the statistical analysis and has primary responsibility for final content; HC, HF, IS, and MA wrote the manuscript; and all authors assisted with data interpretation and read and approved the final manuscript.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
References
- 1.Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e47. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 5.Abdullah A, Amin FA, Stoelwinder J, Tanamas SK, Wolfe R, Barendregt J, et al. Estimating the risk of cardiovascular disease using an obese-years metric. BMJ open. 2014;4(9):e005629. doi: 10.1136/bmjopen-2014-005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdullah A, Wolfe R, Stoelwinder JU, de Courten M, Stevenson C, Walls HL, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–96. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 7.Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. Int J Cancer. 2010;126(2):490–9. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States. PLoS medicine. 2016;13(8):e1002081. doi: 10.1371/journal.pmed.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold M, Freisling H, Stolzenberg-Solomon R, Kee F, O’Doherty MG, Ordonez-Mena JM, et al. Overweight duration in older adults and cancer risk: a study of cohorts in Europe and the United States. Eur J Epidemiol. 2016;31(9):893–904. doi: 10.1007/s10654-016-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38.:348/17/1625 [pii] doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Bodymass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold M, Charvat H, Freisling H, Noh H, Adami HO, Soerjomataram I, et al. Adult Overweight and Survival from Breast and Colorectal Cancer in Swedish Women. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1518–24. doi: 10.1158/1055-9965.EPI-19-0075. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 16.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 17.Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777–82. doi: 10.1093/jjco/hyu096. [DOI] [PubMed] [Google Scholar]
- 18.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort Profile: The Swedish Women’s Lifestyle and Health cohort. Int J Epidemiol. 2017;46(2):e8. doi: 10.1093/ije/dyv089. [DOI] [PubMed] [Google Scholar]
- 19.Noh H, Charvat H, Freisling H, Olafsdottir GH, Olafsdottir EJ, Tryggvadottir L, et al. Cumulative exposure to premenopausal obesity and risk of postmenopausal cancer: A population-based study in Icelandic women. Int J Cancer. 2020;147(3):793–802. doi: 10.1002/ijc.32805. [DOI] [PubMed] [Google Scholar]
- 20.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. Br J Cancer. 2003;89(3):431–6. doi: 10.1038/sj.bjc.6601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei LJ. The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med. l992;11(14-15):1871–9. doi: 10.1002/sim.4780111409. [DOI] [PubMed] [Google Scholar]
- 24.Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med. 2012;31(29):3821–39. doi: 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 26.Burgess S, White IR, Resche-Rigon M, Wood AM. Combining multiple imputation and meta-analysis with individual participant data. Stat Med. 2013;32(26):4499–514. doi: 10.1002/sim.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 28.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20(8):517–35. doi: 10.1007/s10151-016-1498-3. [DOI] [PubMed] [Google Scholar]
- 30.Wada K, Nagata C, Tamakoshi A, Matsuo K, Oze I, Wakai K, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol. 2014;25(2):519–24. doi: 10.1093/annonc/mdt542. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo K, Mizoue T, Tanaka K, Tsuji I, Sugawara Y, Sasazuki S, et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan. Ann Oncol. 2012;23(2):479–90. doi: 10.1093/annonc/mdr143. [DOI] [PubMed] [Google Scholar]
- 32.Freisling H, Arnold M, Soerjomataram I, O’Doherty MG, Ordonez-Mena JM, Bamia C, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116(11):1486–97. doi: 10.1038/bjc.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, et al. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status--the Japan public health center-based prospective study. Int J Cancer. 2011;129(5):1214–24. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A, et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Blair CK, Wiggins CL, Nibbe AM, Storlie CB, Prossnitz ER, Royce M, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5:33. doi: 10.1038/s41523-019-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–59. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68(24):10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 38.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27(2):176–85. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30(2):164–71. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 40.Freisling H, Viallon V, Lennon H, Bagnardi V, Ricci C, Butterworth AS, et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020;18(1):5. doi: 10.1186/s12916-019-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slawinski CGV, Barriuso J, Guo H, Renehan AG. Obesity and Cancer Treatment Outcomes: Interpreting the Complex Evidence. Clin Oncol (R Coll Radiol) 2020;32(9):591–608. doi: 10.1016/j.clon.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz NS, Wright AA. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol. 2015;138(1):201–6. doi: 10.1016/j.ygyno.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabore EG, Guenancia C, Vaz-Luis I, Di Meglio A, Pistilli B, Coutant C, et al. Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study. PLoS medicine. 2019;16(12):e1002989. doi: 10.1371/journal.pmed.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Movahed MR, Khoubyari R, Hashemzadeh M, Hashemzadeh M. Obesity is strongly and independently associated with a higher prevalence of pulmonary embolism. Respir Investig. 2019;57(4):376–9. doi: 10.1016/j.resinv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–46. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 46.Saad ED, Zalcberg JR, Peron J, Coart E, Burzykowski T, Buyse M. Understanding and Communicating Measures of Treatment Effect on Survival: Can We Do Better? J Natl Cancer Inst. 2018;110(3):232–40. doi: 10.1093/jnci/djx179. [DOI] [PubMed] [Google Scholar]
- 47.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 48.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789–96. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.