Abstract
Radiation-attenuated sporozoites induce sterilizing immunity and remain the ‘gold standard’ for malaria vaccine development. Despite practical challenges in translating these whole sporozoite vaccines to large-scale intervention programmes, they have provided an excellent platform to dissect the immune responses to malaria pre-erythrocytic (PE) stages, comprising both sporozoites and exoerythrocytic forms. Investigations in rodent models have provided insights that led to the clinical translation of various vaccine candidates—including RTS,S/AS01, the most advanced candidate currently in a trial implementation programme in three African countries. With advances in immunology, transcriptomics and proteomics, and application of lessons from past failures, an effective, long-lasting and wide-scale malaria PE vaccine remains feasible. This review underscores the progress in PE vaccine development, focusing on our understanding of host-parasite immunological crosstalk in the tissue environments of the skin and the liver. We highlight possible gaps in the current knowledge of PE immunity that can impact future malaria vaccine development efforts.
Keywords: exoerythrocytic forms; liver; malaria immunology; Plasmodium; pre-erythrocytic; RTS,S; skin; sporozoite
1. Background
Malaria remains an intractable global public health problem with an estimated 228 million cases and 405 000 deaths in 2018 alone. 1 A vast majority of these deaths occur in sub-Saharan Africa, where malaria is associated with a 24% prevalence and 94% of the malaria-associated deaths globally. 1,2 Recent advances in malaria control including improved diagnostic approaches, artemisinin-combination treatments (ACTs), intermittent preventive treatment (IPT) in pregnancy and vector control saw a 48% decrease in mortality rates between 2000 and 2015. 1 Whilst these strategies have unquestionably contributed to reduction in incidence and mortality rates, an effective vaccine would provide the ultimate solution to malaria elimination and should be an urgent public health priority.
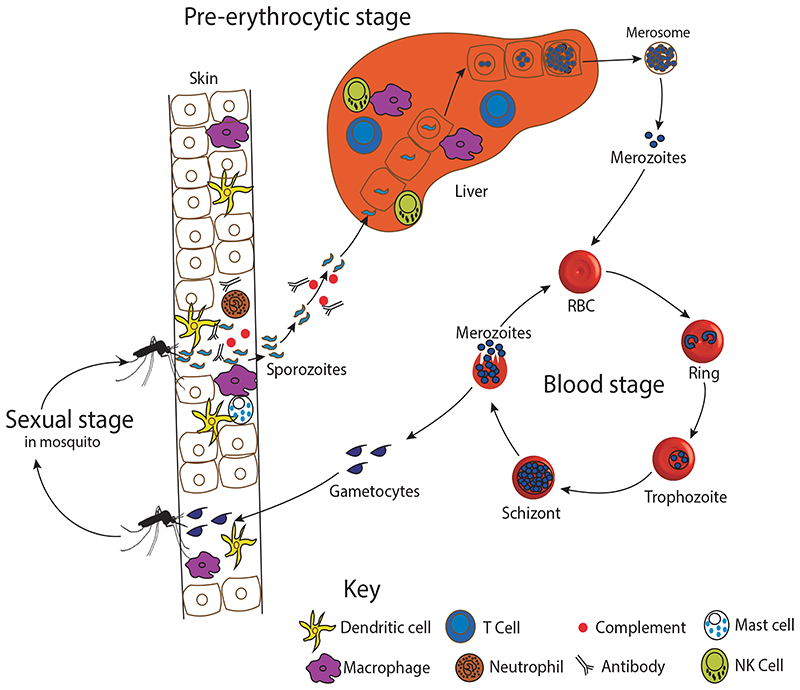
Malaria biology is complex. Our understanding of the pre-erythrocytic (PE) stage infections is based on model systems with Plasmodium berghei (Pb) and P. yoelii (Py), with limited information on P. falciparum (Pf). The PE stage begins when an infected female Anopheles mosquito inoculates a few (typically <100) infective sporozoites into the host skin. 3,4 Quantitative studies with Pb and Py indicate that a large proportion (~60%) lose motility and remain localized at the site of inoculation where they can develop into skin exoerythrocytic forms (EEF) and initiate an immune response. 5–8 Some sporozoites ‘trickle out’ of the skin into the blood (~25%) and the lymphatic drainage (~15%). 6,9 Most of the sporozoites that enter the bloodstream reach and invade the liver, where they traverse through several hepatocytes in a transient vacuole. The sporozoites then invade a final hepatocyte and form parasitophorous vacuoles (PV), where the liver EEFs develop. 6,10 The circumsporozoite protein (CSP), which is the major antigen on the sporozoite surface, and thrombospondin-related anonymous protein (TRAP), a micronemal protein, are thought to facilitate invasion into the hepatocytes. 11,12 In the liver, the parasites undergo asexual development for a number of days depending on the Plasmodium species (ie 7-10 days for human malaria vs 42-44 hours for Pb infection in mice), pre-existing immunity and concomitant malaria prophylaxis. 13 They differentiate into multinucleated schizonts that form thousands of merozoites via nuclear division. In the late stages of development, the PV membrane is lysed, and the merozoites become packaged together inside merosomes. 14,15 These merosomes egress out of the liver, circulate through the heart and reach the lung microvasculature where merozoites are released to invade erythrocytes. 16 This initiates bloodstage cycle of development amongst ring, trophozoite and schizont forms (Figure 1). Exponential expansion of the parasite during the blood-stage stage and concomitant immune responses result in malaria-related symptoms (as reviewed elsewhere). 17
Figure 1.
The malaria life cycle. An infected mosquito deposits motile infective sporozoites into the dermis of a susceptible host. Some sporozoites migrate to the liver, where they invade hepatocytes, multiply asexually to produce thousands of merozoites which egress in merosomes and rupture inside microvasculature of lungs. The merozoites invade the red blood cells (RBC), and undergo multiple cycles of ring, trophozoite and schizont stages, to initiate the clinical phase of the disease. Some parasites differentiate into male and female gametocytes, which are taken up mosquitoes during their next blood meal. Different immune cells interact with the malaria sporozoites during its journey from the skin to the liver and may be exploited in the development of an effective and long-lasting vaccine. NK denotes natural killer cells
The PE stages form a bottleneck for the malaria parasite and can be targeted in developing an effective malaria vaccine. Once thought to be immunologically quiescent, accumulating evidence shows that the PE stages provoke immune responses. 8,18–21 The sporozoites are exposed to antibodies in the bloodstream and in the skin and hepatic extracellular fluids. It is only during the PE stages where Plasmodium parasites invade nucleated cells of humans and rodent models, which can present parasite antigens via major histocompatibility complex (MHC) I. This gives a wide array of innate and adaptive immune effector mechanisms that can be exploited in developing an effective malaria vaccine. A vaccine targeting the clinically ‘silent’ PE stages will not only block symptomatic blood-stage infections and associated complications, but it would also halt further transmission of the parasite. Nonetheless, the host-parasite crosstalk during the PE stages is intricate and remains inadequately studied. In this review, we systematically explore the current knowledge on vaccine development and immune responses to malaria PE stages, and highlight some of the existing gaps.
2. Progress With Malaria Pre-Erythrocytic Stage Vaccines
2.1. Whole sporozoite vaccines
Seminal studies in the late 1960s on mice immunized with radiation-attenuated sporozoites (RAS) demonstrated sterile immune protection against malaria reinfections. 22,23 The sterile protection was later observed in nonhuman primates and challenge human malaria infection (CHMI) trials with efficacy levels of >80%. 24–27 RAS are currently in clinical trials across the world (Table 1). Due to the development of sterile immunity, RAS became the ‘gold standard’ for a malaria vaccine development. Nonetheless, translation of RAS to a wide-scale applicable human vaccine remains challenging. Extremely large numbers of dissected parasites (up to 6.75 × 105 given at five doses or 9 × 105 given at three doses), which are delivered intravenously, are required to induce sterile immunity. 28,29 Increasing the dose to 1.8 × 106 parasites greatly reduces vaccine efficacy. The sterile immunity induced by RAS is not long-lasting, but the durability of protection can be extended by booster immunizations. 26
Table 1. The status of current malaria pre-erythrocytic stage vaccine candidates (adapted from the World Health Organization tables of malaria vaccine projects globally—’Rainbow Tables’) 172 .
| Whole Sporozoite | |||||||
| PfSPZ | NCT02215707 | Sanaria Inc | RAS | USA | I | 2014 | 51 |
| PfSPZ | NCT02627456 | Sanaria Inc | RAS | Mali | II | 2016 | |
| PfSPZ | NCT02613520 | Sanaria Inc | RAS | Tanzania | I | 2015 | 27,173 |
| PfRAS | NCT01994525 | USAMRDC | RAS | USA | I | 2013 | |
| PfSPZ-CVac | NCT02115516 | Sanaria Inc | CPS (SPZ-CQ) | Germany | I | 2014 | 54 |
| PfGAP3KO | NCT03168854 | NIAID | GAP | USA | I | 2017 | |
| PfSPZ | NCT02663700 | NIAID | RAS | Burkina Faso, USA | I | 2016 | |
| PfSPZ-CVac | NCT02773979 | NIAID | CPS (SPZ-CQ) | USA | I | 2016 | |
| Sub-unit | |||||||
| RTS,S/AS01E | NCT02374450 | GSK | CSP | Kenya, Burkina Faso, Ghana | IV | 2015 | 174 |
| RTS,S/AS01 fractional dose | NCT01857869 | GSK | CSP | Kenya, Gambia, Burkina Faso | II | 2013 | 61 |
| R21/AS01B | NCT02600975 | University of Oxford | CSP | United Kingdom | I | 2015 | |
| R21/Matrix – M1 | NCT02925403 | University of Oxford | CSP | Burkina Faso | I | 2016 | |
| R21/ME-TRAP | NCT02905019 | University of Oxford | CSP/TRAP | United Kingdom | II | 2016 | 175 |
| CS-Vac | NCT01450280 | University of Oxford | CSP | Ireland | I | 2011 | 65 |
| PfCelTOS FMP012/AS01B | NCT02174978 | USAMRMC | CelTOS | USA | I | 2014 | |
| ChAd63/MVA ME-TRAP | NCT01635647 | University of Oxford | ME-TRAP | Burkina Faso, Kenya, Gambia | II | 2012 | 72-74 |
| ChAd63/MVA ME-TRAP + Matrix M™ | NCT01663512 | University of Oxford | ME-TRAP | United Kingdom | I | 2012 | 176 |
Note: RAS denotes radiation-attenuated sporozoites.
Abbreviations: Adjuv, adjuvant; CelTOS, cell-traversal protein for ookinetes and sporozoites; ChAd, chimpanzee adenovirus; CPS, chemoprophylaxis following sporozoite infection; CQ, chloroquine; CSP, circumsporozoite protein; GAP, genetically attenuated parasites; GSK, GlaxoSmithKline; KO, knockout; MVA, modified vaccinia Ankara; NIAID, National Institute of Allergy and Infectious Diseases; Pf, Plasmodium falciparum; SPZ, sporozoites; TRAP, thrombospondin-related anonymous protein; USAMRDC, United States Army Medical Research and Development Command.
During the past two decades, there has been a renaissance of approaches to develop whole sporozoite vaccines. Pre-clinical evidence suggests that invasion and development in the liver are required for sterile PE immunity. 30,31 RAS vaccines successfully invade the liver, but their development is arrested early in EEF development. Administration of sporozoites followed by antimalarial chemoprophylaxis with chloroquine or mefloquine (CPS vaccines), which acts on blood stage but not liver-stage parasites, yields comparable efficacy levels to RAS and confers protection against PE stages in both rodent models and humans. 32–36 CPS vaccines may provide more robust immunity as the sporozoites undergo complete liver-stage development. Alternative CPS approaches involve using antibiotics, such as clindamycin and azithromycin, which allow full parasite development in the liver, but lead to delayed death in the resulting merozoites. 37 In rodent models, CPS vaccines have been shown to induce robust, long-lived immunity that not only protects against PE stages, but also against blood stages. 38,39 This apparent cross-stage immunity induced by CPS vaccines needs to be further explored.
Genetically attenuated parasite (GAP) vaccines rely on targeted gene deletion technology that arrests the development of sporozoites at either early- or late-stage EEFs. Studies initially targeted the attenuation of the upregulated in infective sporozoite (uis) genes, which attenuates sporozoite development in the early stages. 40,41 Pb parasite lines with uis3 - and uis4 - knockout genes arrest their development after completion of sporozoite development in the early EEF stages. 42 Studies using Pb found a stage-specific durable sterile protection against reinfection after immunization with three doses of uis3– sporozoites. 41 GAP vaccines targetting other Plasmodium genes have produced varied results (as reviewed by Kreutzfeld et al 43 ). A clinical trial using PfGAP lacking two genes (p36 - p52 -) reported favourable anti-sporozoite immune responses. 44 The triple gene knockout (PfGAP3KO: p36 - p52 - sap1 -) PfGAP was reported to fully attenuate sporozoite development in the early liver stages in in vitro and humanized mice studies. 45,46 PfGAP3KO was reported to be safe and immunogenic in human volunteers after 150-200 mosquito bites, but is yet to complete clinical trials. 47 Other GAP vaccine development efforts include targeting the late EEF stages, such as deletion of fabb/f, PlasMei2, and liver-specific protein 2 (LISP2) genes. 31,48 GAP vaccines targeting the late EEF stages may be efficacious at lower doses, induce a larger breadth of immune responses and protect against blood-stage infections. 49 PfSPZ-GA1 vaccine, a Pf identical double knockout (b9 - slarp -), which attenuate early in EEF development, presented safety profile and elicited immune responses. 50 The pre-clinical findings of PfSPZ-GA1 are promising, as they have shown optimal immunogenicity and some indication of protection.
Sterile and cryopreserved sporozoite vaccines (PfSPZ), injected intravenously, conferred up to 100% sterile protection after CHMI with homologous strains, and ~80% protection against heterologous strains. 51–54 A comparable outcome is obtained with CPS vaccines (using chloroquine as the antimalarial drug) where only modest protection was obtained with heterologous challenge. 55 A challenge for whole sporozoite vaccines is to increase the diversity of strains represented in the vaccine. Of particular interest, the inoculation of PfSPZ intradermally, mimicking the natural route of sporozoite infection, was not protective. 54 Additionally, PfSPZ efficacy was greatly reduced in a setting of seasonal transmission, showing about 30% protection at 6 months in Mali adults. 56 The low efficacy has been associated with hypo-responsiveness to PfSPZ in malaria-exposed adults. A study on adult males from Equatorial Guinea reported lower antibody responses to PfSPZ compared to US adults receiving a similar dosage regimen. 57 Additional studies are required on dosage optimization for participants in malaria-endemic areas, 29 and particularly for children who are most affected by severe malarial disease in sub-Saharan Africa. The need for liquid nitrogen storage to maintain PfSPZ vaccines may be a logistical challenge in malaria-endemic areas. Future efforts should focus on developing a thermal-stable PfSPZ vaccine, which can reduce delivery challenges to remote areas.
2.2. Sub-unitvaccines
Sporozoites are covered with a dense coat, and CSP—a 40-66 kDa protein, with ~40 NANP repeats in the central region of PfCSP — is the major surface protein. 58 Inadvertently, many approaches have been explored to target and improve immune responses to CSP. RTS,S/AS01 (Mosquirix™), the most advanced malaria vaccine to date, contains a section of the CSP central repeat region (18 NANP repeats with B-cell epitopes) and C-terminal (with T-cell epitopes). In a large phase III study involving 15 459 infants (6-12 weeks old) and young children (5-17 months old) at 11 sites, RTS,S showed moderate vaccine-induced protection at 18 months (26% and 45%, respectively) which waned on follow-up. 59 In subjects receiving a booster at 20 months, the vaccine efficacy was ~36% in children (vs 28% in controls without the booster) and ~25% in infants (vs 18% in controls) at the end of a 48-month study period. 60 Fractional dosing of the third dose may increase the vaccine efficacy up to ~86%, 61 but this remains to be seen in endemic areas where efficacy in adult declined with an increase in malaria transmission. 62 After a positive review by the European Medicines Agency, RTS,S was recently rolled out for implementation in three African countries (Malawi, Kenya and Ghana). 63 Assuring earlier concerns that CSP diversity may impact vaccine efficiency, it is noteworthy that in the above large phase III trials, <10% of the parasites corresponded the CSP alleles used in the RTS,S. 64
Prime-boost viral vectored delivery platforms using chimpanzee adenoviruses (eg ChAd63) prime and a modified vaccinia strain Ankara (MVA) have been explored as alternative approaches to improve the efficacy of CSP-based vaccines. ChAd63-MVA CSP vaccine candidate induced high levels of antigen-specific antibodies and T-cell responses. 65 Nevertheless, its efficacy in a CHMI trial was poor, protecting only 1/15 subjects. 66 In vitro and rodent studies have suggested that CSP is dispensable in achieving sterile immunity and low levels of anti-CSP antibodies may aid in sporozoite invasion. 58,67,68 Other studies reported that the CSP repeat region, but not the C-terminal domain, induced antibody-dependent phagocytic activity that was protective against infection. 69 Thus, the modest protection induced by CSP-based vaccines, as compared to the sterile immunity observed in RAS, calls for exploration of alternative adjuvants, antigens and/or CSP epitopes as vaccine targets and increased focus on antibody functionality rather than quantity.
The genome of Pf reference strain 3D7 contains ~5400 genes. 70 Some of these genes encode for proteins that are essential for cell traversal (sporozoite microneme protein essential for cell traversal [SPECT], phospholipase [PL], cell-traversal protein for ookinetes and sporozoites [CelTOS], gamete egress and sporozoite traversal protein [GEST] and perforin-like protein [PLP1 also known as SPECT2]); liver invasion (TRAP and apical membrane antigen [AMA] 1) and hepatic development (liver surface antigens [LSA1, LSA2 and LSA3] and sporozoite threonine and asparagine-rich protein [STRAP]). Most of these proteins have the potential of becoming vaccine targets, but only a few are in current clinical trials (Table 1). ChAd63-MVA ME-TRAP, which primarily targets TRAP but also contains multiple epitopes of CSP, LSA1, LSA3, STARP, EXP1, has been reported to have high immunogenicity and safety levels in human studies even when administered concurrently with the expanded program on immunization. 71–74 Combination vaccines of ME-TRAP and CSP have so far yielded varying results depending on vaccine regimen and routes of administration. 75–77
3. Immune Responses To Malaria Pre-Erythrocytic Stages In The Skin And The Liver
3.1. Innate host responses in the skin and the liver
The skin is the first defence layer against the malaria parasites. Apart from being a physical barrier, the skin harbours a diverse range of phenotypically and functionally distinct dendritic cells (DCs) and macrophages that interact with sporozoites, as described in mouse malarias (Figure 1). 5,6 The contribution of these cells is challenging to study in humans considering the ‘silent’ clinical nature of malaria PE stages. Neutrophils and monocytes infiltrate the site of sporozoite inoculation, and mast cells have been reported to induce DCs and T-cell recruitment. 78,79 Remarkably, a rodent study reported that neutrophils and monocytes may not be critical in the development of sterile immunity. 78 Further work is needed to dissect the roles of neutrophils and monocytes in PE stage immunity.
Whilst the liver is known to be an autonomous and competent priming site for naïve CD8+ T cells, 80 the role of hepatocytes and other liver cells in antigen presentation during PE stages remain poorly understood. Liver cells including hepatocytes, liver sinusoidal endothelial cells, Kupffer cells, hepatic DCs and hepatic stellate cells interact with the parasite during the liver invasion process (as reviewed by Hafalla et al 81 ). Rodent studies have shown that CD11c+ DCs found in the spleen, liver and liver-draining lymph nodes are required to present antigens to CD8+ T cells, and their depletion abrogates CD8+ T-cell responses. 5,82–84 It is thought that these DCs directly present sporozoite antigens to CD8+ T cells through antigen cross-presentation. 5,8,83 Blocking the ability of the DCs to cross-present antigens represses CD8+ T-cell responses. 85,86 CD4+ T cells play a role in ‘licensing’ these antigen-presenting DCs. 83,87 How antigens that are expressed exclusively during EEF development prime CD8+ T-cell responses remains inadequately characterized. Recent studies have implicated a subset of liver-infiltrating monocyte-derived CD11c+ cells, which acquire rodent parasites after parasite invasion but before merozoite release. 82 Consistent with the presentation of sporozoite-derived antigens, these monocyte-derived CD11c+ cells were found to prime CD8+ T-cell responses in the liver-draining lymph nodes.
Infected hepatocytes can become ‘stressed’ (express heat shock proteins) and/or apoptotic. 21 This induces inflammatory responses and recruitment of effector immune cells to the site of EEF infection. Plasmodial dsRNA accessing hepatocytic cytosol induces release of type I interferons (IFN-α and IFN-β) that recruit natural killer (NK) and CD3+CD49b+ natural killer T (NKT) cells. 88,89 NK cells are a highly enriched effector cell population that respond to invading sporozoites, as they account for up to 50% of liver-resident lympho-cytes. 90 NK and NKT cells are potent producers of IFN-γ, 18,20 which activates the nitric oxide pathway in macrophages. 18,91 In RTS,S CHMI studies, concentrations of serum IFN-γ and transcriptional signatures related to IFN-γ production were linked to protection from infection. 92,93 It is also conceivable that NK and NKT cells participate in IFN-γ-independent killing of infected hepatocytes. Recently, serological profiling studies suggested that NK cells may inhibit sporozoite invasion through antibody-mediated interactions. 94 On the other hand, NKT cells may be dispensable in the development of sterile immunity. 95
Nutritional immunity may play a role in protection against Plasmodium infections. In endemic settings, children with iron deficiency are protected against malaria. 96,97 The hepatic hormone hepcidin has been reported to increase across the malaria season in these settings. 98,99 Hepcidin restricts iron availability in the liver hence denying Plasmodium parasites a vital nutrient, and may protect against secondary liver-stage infections. 100 Supplementing children with iron in a malaria-endemic region was associated with increased malaria incidences and mortality. 101 Accordingly, targeting the nutritional requirements of the parasite is an alternative innate response to malaria infections.
3.2. Antibody responses, including targeting the parasites whilst in the skin
Antibodies are correlates of protection for most approved vaccines in clinical use. Their effector pathways include neutralization of pathogens, antibody-dependent cytotoxicity, antibody-dependent complement deposition and antibody-dependent phagocytosis. Mechanistically, humoral responses begin when a naïve B-cell encounters an antigen at the interface of the T and B regions of secondary lymphoid organs. Depending on the existing signals, these antigenically stimulated B cells may undergo (a) rapid proliferation in the extrafollicular foci to produce short-lived isotype-switched antibody-secreting plasmablasts (SLPCs), (b) interact with CD4+ T follicular helper (TFH) cells in a germinal-centre (GC)—dependent or GC-independent process to produce long-lived memory cells or (c) an anergic response. The B cells that interact with TFH-dependent differentiate into long-lived plasma cells (LLPC) or circulating memory B cells (MBCs) (as reviewed by Nutt et al 102 ). LLPCs migrate to the bone marrow and continuously secrete neutralizing antibodies, whilst MBCs form a ready-to-respond antigen-specific B-cell pool.
Early malaria vaccine studies reported increased production of anti-CSP antibodies in response to RAS, and these antibodies are associated with protection against reinfection. 18,22,24,103 In field and CHMI studies, antibody responses to other PE antigens such as LSA-1, TRAP and STARP have also been reported 104–106 and protected individuals may have higher antibody titres. 105–107 Passive transfer of monoclonal anti-sporozoite antibodies delayed patency of Pb infection in mice. 108 The effector activity of these antibodies may include blocking sporozoite motility, dermal exit and subsequent invasion of hepatocytes. 78,109 Antibodies may remove the surface coat protein of sporozoites in the skin and expose the parasites to their own pore-forming proteins. 110 Beyond inhibiting sporozoite mobility, antibodies also aid in sporozoite destruction through activation of the complement system, phagocytosis and Fc-mediated innate cell functions. 94,111–113
Various field studies have reported that high antibody levels against sporozoites are required for effective and long-term protection. 105,114,115 RTS,S vaccines induce high anti-PfCSP antibodies titres with moderate CD4+ T-cell responses, 116–118 yet none of them have been recognized as an unequivocal correlate of protection. It remains poorly understood if protection against sporozoites is dependent on immunoglobulin sub-class, but high levels of antigen-specific IgG3 and IgG1 in participants receiving RTS,S have been observed. 111,119 Although individuals with higher antibodies against sporozoite antigens have better protection against infection, 105–107 antibody titres have generally performed poorly as correlates of protection in malaria vaccine studies. 94,120 The modest efficacy of RTS,S in endemic regions suggests that the functionality and avidity of the antibodies, rather than the antibody titres, is a better correlate of immune protection to malaria. 94,113 In recent serological profiling studies, the functionality of antibodies was reported to be a better predictor of protection. 94 These antibodies were reported to induce NK cell effector functions, including activation and phagocytosis.
The hurdle with malaria infections is the inability to generate long-lasting protective immunity. This is compounded by the lack of appropriate surrogates of protection in field and CHMI studies. Malaria-specific MBCs are elicited at levels comparable to conventional licensed vaccines 121 and can persist in naturally infected and travellers to endemic regions. 122 Like antibodies, malaria MBCs appear to increase with age and exposure. 123 Studies have demonstrated that Pf-specific MBCs target PE stage antigens, and existing antibodies to CSP, LSA-1 and TRAP may protect against clinical malaria in an endemic setting. 105,124 Current literature does not indicate the magnitude of humoral reaction to other malaria PE antigens or if PE-specific MBCs are linked to protective immunity.
How antibody and MBC responses are regulated during malaria infections is poorly defined. TH1 responses have also been implicated in the regulation and function of MBCs after malaria infections in humans and mice. 125–127 These studies reported that TH1-polarized PD-1+CXCR5+CXCR3+ TFH cells are preferentially elevated during malaria infections and may play a role in impaired GC responses. How these responses influence LLPC and MBC responses to PE stages remain poorly characterized. Recently, a group of atypical MBCs (CD19+CD21-CD27-) expressing high levels of FcRL5 has been suggested to play a role in the incomplete anti-Plasmodium immunity. 128,129 Whether or not atypical MBCs are induced during PE stage natural and vaccine responses remains to be described. However, the dynamics behind the MBC development and the roles of atypical MBCs in de novo malaria infections remain an open question.
3.3. CD4+ T-cell effector mechanisms
CD4+ T cells have multiple effector functions ranging from regulation of immune responses and activation of CD 8+ T cells, B cells, innate immune cells and other nonimmune cells. 130 CD4+ T cells play a critical role in response to malaria PE stages and maintenance of immunity both independently and in conjunction with other cells. 131–133 In model studies, CD4+ T cells were reported to confer protection against Pb and Py in β2-microglobulin knockout mice (CD8+ T cells deficient) immunized with RAS, 131 probably through direct killing of infected hepatocytes. 134 Field and CHMI studies have also reported high CD4+ T-cell numbers after RTS,S or whole sporozoite infection, 35,116,135 including high serum levels of CD4+ T cell–associated cytokines (IFN-γ, tumour necrosis factor [TNF] and IL-2). 32,136 In modelling and CPS vaccine studies, T cells 133 and IFN-γ 92,93 have been reported as correlates of immune protection against malaria infection. Detailed investigations are required to determine the longevity of CD4+ T cells in response to PE stages and their ability to serve as surrogates of immune protection.
The functional roles of CD4+ T cells are not limited to direct activity. As discussed before, CD4+ T cells may be involved in the licensing of the antigen-presenting DCs that prime effector CD8+ T cells. The cytokines generally produced by CD4+ T cells may influence other immune cells involved in response to malaria and development of immunity. IL-4–producing CD4+ T cells sustain and expand the effector and memory Py-specific CD8+ T-cell pool. 87,137,138 In the absence of CD4+ T cells, the sporozoite-specific memory CD8+ T cells fail to protect against challenge infections in mice. 137 Some of the cytokines produced by CD4+ T cells, such as IFN-γ, IL-4, IL-5 and IL-10, enable B cells to undergo immunoglobulin class-switching. 102 A subset of CD4+ T cells, FOXP3+ regulatory T cells (TREGs), has been associated with poor development of CPS vaccine-induced immunity. 139 A recent study implicated a subset of TFH CD4+ T cells in the poor response of participants receiving RTS,S and ME-TRAP combinations. 77 Nonetheless, further studies are required to elucidate induction, regulation, maintenance and tissue requirements of CD4+ T cells in malaria PE stage immunity.
3.4. CD8+ T-cell effector mechanisms, including liver-resident memory CD8+ T cells
CD8+ T cells are the primary effector cells against PE stages as seen in rodent, non-human primate and human studies. 140–144 As observed in Py, the responses by CD8+ T cells begin after they are primed by mature CD11c+ DCs in the skin-draining lymph nodes. 8 Naïve CD8+ T cells do not exert antiparasitic activity, unless previously primed by antigen-presenting cells. 145 The CD8+ T cells with cognate receptors to the antigens presented by the DCs will differentiate to short-lived effector cells (SLEC) or memory precursor effector cells (MPEC) depending on the local cytokine environment and transcriptional factor profile. 146–148 Activated CD8+ T then undergo clonal expansion, which requires the presence of IL-2/IL-4 produced by CD4+ T cells. 87 The numbers of CD8+ T cells have been shown to increase rapidly after sporozoite inoculation. 86,145,149,150 The activation and proliferation of naïve CD8+ T cells are dose-dependent, and a successful response requires viable sporozoites. 5,53,151 The SLEC migrate to the liver to exert their effector properties whilst MPEC further differentiate to memory cells. 152,153
CD8+ T cells confer sterile immunity against Pb-independent of B cells or CD4+ T cells. 18 In rodent and nonhuman primate models, depletion of CD8+ T cells abrogates sterile immunity after RAS immunization, whilst their restoration reinstates the protection. 140,143 However, the effector mechanisms of these malaria PE-specific CD8+ T cells are not well characterized. In vivo imaging studies report that CD8+ T cells recognize cognate epitopes on the infected hepatocyte MHC-I and cluster around these cells. 154 Murine and vaccine studies have reported elevated CD8+ T-cell effector mediators including cytokines (IFN-γ and TNF) and/or proteins involved in contact-mediated cytotoxicity (perforin, TRAIL, FAS ligand and granzyme). 18,35,134,151,155 Surprisingly, CD8+ T cells lacking perforin, FAS ligand and perforin can still eliminate Py- and Pb-infected hepatocytes. 156,157
Malaria memory T cells are involved in patrolling, surveillance and rapid recruitment to the site of infection. 34,155,158 This enables a fast, effective, specific and durable protection against subsequent malaria infections. Pre-clinical and CHMI trials have shown induction and persistence of Pf-specific CD4+159,160 and CD8+ T cells. 144 In Pb and Py, CD8+ T memory cells have been described as CXCR3hiCXCR6hi CD62L–CD69+ liver-resident (TRM), CXCR3loCXCR6lo CD44+CD62L– CD122– circulating effector (TEM), and CD44+CD62L+CD122+ central memory (TCM) cells, 157,161,162 and their effector immune responses are species-specific. 157 Nonetheless, the epitope signatures and correlates of CD8+ T memory cell protection are yet to be characterized.
Majority of the circulating CD8+ T memory cells in mouse studies are TEM but a small proportion of TCM has also been observed. 150,162 A large population of TEM cells is required for effective and longterm protection. 150,163 Whilst TEM rapidly induce effector functions, TCM has been shown to respond to malaria challenge with delay and short-lived IFN-γ responses. 145,162 TRM, on the other hand, are the non-circulating phenotype. TRM cells have reduced expression of sphingosine 1 phosphate (S1P) receptor and CCR7, and have been associated with protection to sporozoite reinfection. 161 In vitro studies suggest that the patrolling and effector activity of Plasmodiumspecific TRM is dependent upon LFA1-ICAM1 interactions. 164 Consequently, TRM cells important in first-line responses including being able to recruit other cells despite the reduced ability to recirculate. Current efforts are underway to harness these TRM for improved vaccines against PE stages.
3.5. Perspectives on immune responses to PE stages
Naturally acquired immunity in endemic areas is short-lived and non-sterilizing, and wanes over time without repetitive exposures. This suggests a defect in the development of immunological memory after natural malaria infections. The exact reason for this impaired immune memory has not been adequately described. Indeed, the induction, maintenance and regulation of effector and memory responses have emerged as crucial stumbling blocks in malaria PE stage vaccine development.
It is widely appreciated that an effective and long-lasting malaria vaccine will need to induce robust antibody and T-cell responses. This may require further investigations on the specificities and correlates of immune protection induced by vaccine and CHMI trials, as well how to maintain large frequencies of effector and memory responses. Studies from animal models and humans reiterate the need for extremely high titres of functional antibodies and elevated frequencies of CD8+ T cells for sterile protective immunity. 105,114,115,150,163 There is paucity of data on the quantity of CD4+ T cells required to induce sterile immunity. More work is also needed to understand how trained immunity of innate cells, which has recently been described, 165,166 may contribute to immune protection in PE stages. Various adjuvants including alum, ASO1 and viral vectors have been employed as immunostimulants and/or delivery systems for the existing vaccine candidates. 167 Adjuvants have the potential to induce and maintain large numbers of effector and memory immune cells, and the appropriate choice or combination of adjuvants may be the key to unlocking a malaria vaccine that confers sterile and long-lasting protection.
Very little is known regarding the regulation of immune responses to PE stages—the possible roles for regulatory T cells, cytokines and TH1/TFH have been thoroughly explored in malaria blood stages. 168 Additionally, malaria blood-stage infections have been reported to downregulate PE stage immunity. 169,170 Checkpoint blockade has been explored in cancer and malaria blood-stage research, 171 and it is possible that some answers to the regulation of frequencies of anti-PE stage immune responses lie here. The contribution of inhibitory and other regulatory proteins, and their tissue-specific regulation, has not been widely studied in the context of malaria PE stages, but it is plausible that they are involved in a complex web of factors influencing protection against malaria.
4. Conclusion
Delivery of an efficient and long-lasting vaccine protection remains an ambitious goal that requires sustained efforts of all stakeholders. Gaps in the existing parasite-host immunological crosstalk in both the skin and the liver during malaria PE stages need to be addressed first. Quantification and characterization of immune mechanisms have only started to emerge recently despite decades of research into an efficient malaria vaccine. Nonetheless, the identification of correlates of protection and protective malaria PE stage epitopes remain a work in progress. In the current review, we highlighted how protection to malaria sporozoites may rely on a fine, yet to be adequately described, balance between innate and adaptive immune responses. Utilizing advances in other fields such as systems biology and bioinformatics can inform the study of more immunological processes, which have proven challenging to study in the setting of a natural infection. Alternative efforts should include targeting novel sporozoite proteins, a multi-stage and multi-antigen vaccine, or a ‘nutritional’ vaccine that targets metabolic requirements of sporozoites.
Funding information
KMA was funded by the Wellcome Trust (212600/Z/18/Z) and the DELTAS Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from Wellcome [107769/Z/10/Z] and the UK government. JCRH was funded by grants from The Royal Society (University Research Fellowship UF0762736/UF120026, Project Grant RG130034 and Challenge Grant CH160018) and the National Centre for the Replacement, Refinement & Reduction of Animals in Research (Project Grant NC/ L000601/1). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
KMA and JCRH conceptualized the study and wrote the original draft of the manuscript. KMA, WJW and JCRH revised subsequent drafts and approved the final draft for publication.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
References
- 1.World Health Organization. World malaria report 2019. Geneva, Switzerland: 2019. [Accessed October 03, 2020]. https://www.who.int/publications-detail/world-malaria-report-2019 . [Google Scholar]
- 2.Snow RW, Sartorius B, Kyalo D, et al. The prevalence of Plasmodium falciparum in sub-Saharan Africa since 1900. Nature. 2017;550(7677):515–518. doi: 10.1038/nature24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg R, Wirtz RA, Schneider I, Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 1990;84(2):209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- 4.Hopp CS, Kanatani S, Archer NK, et al. Quantitative intravital imaging of Plasmodium falciparum sporozoites: a novel platform to test malaria intervention strategies. bioRxiv. 2019:716878 [Google Scholar]
- 5.Plebanski M, Hannan CM, Behboudi S, et al. Direct processing and presentation of antigen from malaria sporozoites by professional antigen-presenting cells in the induction of CD8 T-cell responses. Immunol Cell Biol. 2005;83(3):307–312. doi: 10.1111/j.1440-1711.2005.01325.x. [DOI] [PubMed] [Google Scholar]
- 6.Amino R, Thiberge S, Martin B, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12(2):220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 7.Voza T, Miller JL, Kappe SH, Sinnis P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infect Immun. 2012;80(6):2158–2164. doi: 10.1128/IAI.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13(9):1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9(5):1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risco-Castillo V, Topcu S, Marinach C, et al. Malaria sporozoites traverse host cells within transient vacuoles. Cell Host Microbe. 2015;18(5):593–603. doi: 10.1016/j.chom.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Akhouri RR, Sharma A, Malhotra P, Sharma A. Role of Plasmodium falciparum thrombospondin-related anonymous protein in host-cell interactions. Malar J. 2008;7:63. doi: 10.1186/1475-2875-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultan AA, Thathy V, Frevert U, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90(3):511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 13.Prudencio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4(11):849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 14.Sturm A, Graewe S, Franke-Fayard B, et al. Alteration of the parasite plasma membrane and the parasitophorous vacuole membrane during exo-erythrocytic development of malaria parasites. Protist. 2009;160(1):51–63. doi: 10.1016/j.protis.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Graewe S, Rankin KE, Lehmann C, et al. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 2011;7(9):e1002224. doi: 10.1371/journal.ppat.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007;3(11):e171. doi: 10.1371/journal.ppat.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: biology and disease. Cell. 2016;167(3):610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 19.Khan ZM, Ng C, Vanderberg JP. Early hepatic stages of Plasmodium berghei: release of circumsporozoite protein and host cellular inflammatory response. Infect Immun. 1992;60(1):264–270. doi: 10.1128/iai.60.1.264-270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7(2):436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Leiriao P, Mota MM, Rodriguez A. Apoptotic Plasmodium-infected hepatocytes provide antigens to liver dendritic cells. J Infect Dis. 2005;191(10):1576–1581. doi: 10.1086/429635. [DOI] [PubMed] [Google Scholar]
- 22.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 23.Nussenzweig R, Vanderberg J, Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. dose response, specificity and humoral immunity. Mil Med. 1969;134(10):1176–1182. [PubMed] [Google Scholar]
- 24.Clyde DF, McCarthy VC, Miller RM, Hornick RB. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266(6):398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Gwadz RW, Cochrane AH, Nussenzweig V, Nussenzweig RS. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57(Suppl 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 27.Jongo SA, Church LWP, Mtoro AT, et al. Safety and differential antibody and T-Cell responses to the Plasmodium falciparum Sporozoite malaria vaccine, PfSPZ vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg. 2019;100(6):1433–1444. doi: 10.4269/ajtmh.18-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richie TL, Billingsley PF, Sim BK, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–7461. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jongo SA, Church LWP, Mtoro AT, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ Vaccine in Tanzanian adults. Clin Infect Dis. 2019:ciz1152. doi: 10.1093/cid/ciz1152. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S, François G, Druilhe P, Timperman G, Wéry M. Immunity to Plasmodium berghei exoerythrocytic forms derived from irradiated sporozoites. Parasitol Res. 1996;82(4):297–303. doi: 10.1007/s004360050117. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan AM, Sack BK, Dankwa D, et al. A Plasmodium parasite with complete late liver stage arrest protects against preerythrocytic and erythrocytic stage infection in mice. Infect Immun. 2018;86(5):e00088–18. doi: 10.1128/IAI.00088-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 33.Mordmuller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roestenberg M, Teirlinck AC, McCall MB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377(9779):1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 35.Belnoue E, Costa FT, Frankenberg T, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172(4):2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 36.Bijker EM, Bastiaens GJ, Teirlinck AC, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci USA. 2013;110(19):7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friesen J, Silvie O, Putrianti ED, Hafalla JC, Matuschewski K, Borrmann S. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med. 2010;2(40):40ra49. doi: 10.1126/scitranslmed.3001058. [DOI] [PubMed] [Google Scholar]
- 38.Nahrendorf W, Spence PJ, Tumwine I, et al. Blood-stage immunity to Plasmodium chabaudi malaria following chemoprophylaxis and sporozoite immunization. eLife. 2015;4:e05165. doi: 10.7554/eLife.05165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll KL, Butler NS, Harty JT. CD8 T cell independent immunity after single dose infection-treatment-vaccination (ITV) against Plasmodium yoelii. Vaccine. 2014;32(4):483–491. doi: 10.1016/j.vaccine.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J Biol Chem. 2002;277(44):41948–41953. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- 41.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 42.Mueller AK, Camargo N, Kaiser K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102(8):3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreutzfeld O, Müller K, Matuschewski K. Engineering of genetically arrested parasites (GAPs) for a precision malaria vaccine. Front Cell Infect Microbiol. 2017;7:198. doi: 10.3389/fcimb.2017.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spring M, Murphy J, Nielsen R, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of anopheles mosquitoes to adult volunteers. Vaccine. 2013;31(43):4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 45.van Schaijk BC, Ploemen IH, Annoura T, et al. A genetically attenuated malaria vaccine candidate based on P falciparum b9/slarp gene-deficient sporozoites. eLife. 2014;3:03582. doi: 10.7554/eLife.03582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikolajczak SA, Lakshmanan V, Fishbaugher M, et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther. 2014;22(9):1707–1715. doi: 10.1038/mt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kublin JG, Mikolajczak SA, Sack BK, et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med. 2017;9(371):eaad9099. doi: 10.1126/scitranslmed.aad9099. [DOI] [PubMed] [Google Scholar]
- 48.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9(6):451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sack BK, Keitany GJ, Vaughan AM, Miller JL, Wang R, Kappe SH. Mechanisms of stage-transcending protection following immunization of mice with late liver stage-arresting genetically attenuated malaria parasites. PLoS Pathog. 2015;11(5):e1004855. doi: 10.1371/journal.ppat.1004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roestenberg M, Walk J, van der Boor SC, et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med. 2020;12(544):eaaz5629. doi: 10.1126/scitranslmed.aaz5629. [DOI] [PubMed] [Google Scholar]
- 51.Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI insight. 2017;2(1):e89154. doi: 10.1172/jci.insight.89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA. 2017;114(10):2711–2716. doi: 10.1073/pnas.1615324114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 54.Bastiaens GJ, van Meer MP, Scholzen A, et al. Safety, immunogenicity, and protective efficacy of intradermal immunization with aseptic, purified, cryopreserved Plasmodium falciparum sporozoites in volunteers under chloroquine prophylaxis: a randomized controlled trial. Am J Trop Med Hyg. 2016;94(3):663–673. doi: 10.4269/ajtmh.15-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walk J, Reuling IJ, Behet MC, et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med. 2017;15(1):168. doi: 10.1186/s12916-017-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olotu A, Urbano V, Hamad A, et al. Advancing global health through development and clinical trials partnerships: a randomized, placebo-controlled, double-blind assessment of safety, tolerability, and immunogenicity of PfSPZ vaccine for malaria in healthy equatoguinean men. Am J Trop Med Hyg. 2018;98(1):308–318. doi: 10.4269/ajtmh.17-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar KA, Sano G-I, Boscardin S, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444(7121):937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 59.RTSS Clinical Trials Partnership. Efficacy and safety of the RTS,S/ AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Medicine. 2014;11(7):e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.RTSS Clinical Trials Partnership. Efficacy and safety of RTS,S/ AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214(5):762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 62.Bejon P, White MT, Olotu A, et al. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13(4):319–327. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxmen A. First proven malaria vaccine rolled out in Africa - but doubts linger. Nature. 2019;569(7754):14–15. doi: 10.1038/d41586-019-01342-z. [DOI] [PubMed] [Google Scholar]
- 64.Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med. 2015;373(21):2025–2037. doi: 10.1056/NEJMoa1505819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Barra E, Hodgson SH, Ewer KJ, et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS One. 2014;9(12):e115161. doi: 10.1371/journal.pone.0115161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodgson SH, Ewer KJ, Bliss CM, et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis. 2015;211(7):1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mauduit M, Tewari R, Depinay N, et al. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect Immun. 2010;78(5):2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nudelman S, Renia L, Charoenvit Y, et al. Dual action of anti-sporozoite antibodies in vitro. J Immunol. 1989;143(3):996–1000. [PubMed] [Google Scholar]
- 69.Chaudhury S, Ockenhouse CF, Regules JA, et al. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J. 2016;15:301. doi: 10.1186/s12936-016-1348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aurrecoechea C, Brestelli J, Brunk BP, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. Database. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilbert SC, Plebanski M, Harris SJ, et al. A protein particle vaccine containing multiple malaria epitopes. Nat Biotechnol. 1997;15(12):1280–1284. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- 72.Ogwang C, Kimani D, Edwards NJ, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med. 2015;7(286):286re5. doi: 10.1126/scitranslmed.aaa2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bliss CM, Drammeh A, Bowyer G, et al. Viral vector malaria vaccines induce high-level T cell and antibody responses in West African children and infants. Mol Ther. 2017;25(2):547–559. doi: 10.1016/j.ymthe.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mensah VA, Roetynck S, Kanteh EK, et al. Safety and immunogenicity of malaria vectored vaccines given with routine expanded program on immunization vaccines in Gambian infants and neonates: a randomized controlled trial. Front Immunol. 2017;8:1551. doi: 10.3389/fimmu.2017.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rampling T, Ewer KJ, Bowyer G, et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia ankara vectored vaccines expressing ME-TRAP. J Infect Dis. 2016;214(5):772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rampling T, Ewer KJ, Bowyer G, et al. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ vaccines. 2018;3:49. doi: 10.1038/s41541-018-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowyer G, Grobbelaar A, Rampling T, et al. CXCR3(+) T Follicular helper cells induced by co-administration of RTS,S/AS01B and viral-vectored vaccines are associated with reduced immunogenicity and efficacy against malaria. Front Immunol. 2018;9:1660. doi: 10.3389/fimmu.2018.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mac-Daniel L, Buckwalter MR, Berthet M, et al. Local immune response to injection of Plasmodium sporozoites into the skin. J Immunol. 2014;193(3):1246–1257. doi: 10.4049/jimmunol.1302669. [DOI] [PubMed] [Google Scholar]
- 79.Demeure CE, Brahimi K, Hacini F, et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol. 2005;174(7):3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 80.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 81.Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol Rev. 2011;240(1):297–316. doi: 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 82.Kurup SP, Anthony SM, Hancox LS, et al. Monocyte-derived CD11c(+) cells acquire plasmodium from hepatocytes to prime CD8 T cell immunity to liver-stage malaria. Cell Host Microbe. 2019;25(4):565–77.:e6. doi: 10.1016/j.chom.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parmar R, Patel H, Yadav N, et al. Infectious sporozoites of plasmodium berghei effectively activate liver CD8alpha(+) dendritic cells. Front Immunol. 2018;9:192. doi: 10.3389/fimmu.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cockburn IA, Tse SW, Radtke AJ, et al. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo. PLoS Pathog. 2011;7(3):e1001318. doi: 10.1371/journal.ppat.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hafalla JC, Morrot A, Sano G, Milon G, Lafaille JJ, Zavala F. Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J Immunol. 2003;171(2):964–970. doi: 10.4049/jimmunol.171.2.964. [DOI] [PubMed] [Google Scholar]
- 87.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8(2):166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 88.Liehl P, Zuzarte-Luis V, Chan J, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014;20(1):47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roland J, Soulard V, Sellier C, et al. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol. 2006;177(2):1229–1239. doi: 10.4049/jimmunol.177.2.1229. [DOI] [PubMed] [Google Scholar]
- 90.Mikulak J, Bruni E, Oriolo F, Di Vito C, Mavilio D. Hepatic natural killer cells: organ-specific sentinels of liver immune homeostasis and physiopathology. Front Immunol. 2019;10:946. doi: 10.3389/fimmu.2019.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krzych U, Schwenk R, Guebre-Xabier M, et al. The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites. Immunol Rev. 2000;174:123–134. doi: 10.1034/j.1600-0528.2002.00013h.x. [DOI] [PubMed] [Google Scholar]
- 92.Vahey MT, Wang Z, Kester KE, et al. Expression of genes associated with immunoproteasome processing of major histocompatibility complex peptides is indicative of protection with adjuvanted RTS,S malaria vaccine. J Infect Dis. 2010;201(4):580–589. doi: 10.1086/650310. [DOI] [PubMed] [Google Scholar]
- 93.van den Berg RA, Coccia M, Ballou WR, et al. Predicting RTS,S vaccine-mediated protection from transcriptomes in a malaria-challenge clinical trial. Front Immunol. 2017;8:557. doi: 10.3389/fimmu.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suscovich TJ, Fallon JK, Das J, et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/ AS01 vaccination. Sci Transl Med. 2020;12(553):eabb4757. doi: 10.1126/scitranslmed.abb4757. [DOI] [PubMed] [Google Scholar]
- 95.Romero JF, Eberl G, MacDonald HR, Corradin G. CD1d-restricted NK T cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Parasite Immunol. 2001;23(5):267–269. doi: 10.1046/j.1365-3024.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- 96.Muriuki JM, Mentzer AJ, Kimita W, et al. Iron status and associated malaria risk among african children. Clin Infect Dis. 2018;68(11):1807–1814. doi: 10.1093/cid/ciy791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gwamaka M, Kurtis JD, Sorensen BE, et al. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54(8):1137–1144. doi: 10.1093/cid/cis010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abuga KM, Rockett KA, Muriuki JM, et al. Interferon-gamma polymorphisms and risk of iron deficiency and anaemia in Gambian children. Wellcome Open Res. 2020;5:40. doi: 10.12688/wellcomeopenres.15750.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atkinson SH, Armitage AE, Khandwala S, et al. Combinatorial effects of malaria season, iron deficiency, and inflammation determine plasma hepcidin concentration in African children. Blood. 2014;123(21):3221–3229. doi: 10.1182/blood-2013-10-533000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Portugal S, Carret C, Recker M, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17(6):732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 102.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 103.Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969;222(5192):488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- 104.Fidock DA, Pasquetto V, Gras H, et al. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur J Immunol. 1997;27(10):2502–2513. doi: 10.1002/eji.1830271007. [DOI] [PubMed] [Google Scholar]
- 105.John CC, Tande AJ, Moormann AM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197(4):519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreira-da-Cruz MF, Deslandes DC, Oliveira-Ferreira J, et al. Antibody responses to Plasmodium falciparum sporozoite-, liver- and blood-stage synthetic peptides in migrant and autochthonous populations in malaria endemic areas. Parasite. 1995;2(1):23–29. doi: 10.1051/parasite/1995021023. [DOI] [PubMed] [Google Scholar]
- 107.White MT, Bejon P, Olotu A, et al. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med. 2014;12:117. doi: 10.1186/s12916-014-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34(9):991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 110.Aliprandini E, Tavares J, Panatieri RH, et al. Cytotoxic anti-circumsporozoite antibodies target malaria sporozoites in the host skin. Nature microbiology. 2018;3(11):1224–1233. doi: 10.1038/s41564-018-0254-z. [DOI] [PubMed] [Google Scholar]
- 111.Kurtovic L, Agius PA, Feng G, et al. Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 2019;17(1):45. doi: 10.1186/s12916-019-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Behet MC, Kurtovic L, van Gemert GJ, et al. The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect Immun. 2018;86(7):e00920–17. doi: 10.1128/IAI.00920-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dobaño C, Sanz H, Sorgho H, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun. 2019;10(1):2174. doi: 10.1038/s41467-019-10195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.John CC, Moormann AM, Pregibon DC, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73(1):222–228. [PubMed] [Google Scholar]
- 115.Offeddu V, Olotu A, Osier F, Marsh K, Matuschewski K, Thathy V. High sporozoite antibody titers in conjunction with microscopically detectable blood infection display signatures of protection from clinical malaria. Front Immunol. 2017;8:488. doi: 10.3389/fimmu.2017.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lalvani A, Moris P, Voss G, et al. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180(5):1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 117.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S malaria vaccine evaluation group. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 118.Horowitz A, Hafalla JC, King E, et al. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol. 2012;188(10):5054–5062. doi: 10.4049/jimmunol.1102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ubillos I, Ayestaran A, Nhabomba AJ, et al. Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children. BMC Med. 2018;16(1):197. doi: 10.1186/s12916-018-1186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/ AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15(12):1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nogaro SI, Hafalla JC, Walther B, et al. The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PLoS One. 2011;6(10):e25582. doi: 10.1371/journal.pone.0025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ndungu FM, Lundblom K, Rono J, Illingworth J, Eriksson S, Farnert A. Long-lived Plasmodium falciparum specific memory B cells in naturally exposed Swedish travelers. Eur J Immunol. 2013;43(11):2919–2929. doi: 10.1002/eji.201343630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weiss GE, Traore B, Kayentao K, et al. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6(5):e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wipasa J, Suphavilai C, Okell LC, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6(2):e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obeng-Adjei N, Portugal S, Tran TM, et al. Circulating Th1-cell-type tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 2015;13(2):425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryg-Cornejo V, Ioannidis LJ, Ly A, et al. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep. 2016;14(1):68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 127.Portugal S, Tipton CM, Sohn H, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. eLife. 2015;4:e07218. doi: 10.7554/eLife.07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weiss GE, Clark EH, Li S, et al. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS One. 2011;6(1):e15983. doi: 10.1371/journal.pone.0015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muellenbeck MF, Ueberheide B, Amulic B, et al. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210(2):389–399. doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oliveira GA, Kumar KA, Calvo-Calle JM, et al. Class II-restricted protective immunity induced by malaria sporozoites. Infect Immun. 2008;76(3):1200–1206. doi: 10.1128/IAI.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reece WH, Pinder M, Gothard PK, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10(4):406–410. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 133.White MT, Bejon P, Olotu A, et al. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One. 2013;8(4):e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renia L, Marussig MS, Grillot D, et al. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc Natl Acad Sci USA. 1991;88(18):7963–7967. doi: 10.1073/pnas.88.18.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moncunill G, Mpina M, Nhabomba AJ, et al. Distinct helper T cell Type 1 and 2 responses associated with malaria protection and risk in RTS,S/AS01E vaccinees. Clin Infect Dis. 2017;65(5):746–755. doi: 10.1093/cid/cix429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bijker EM, Teirlinck AC, Schats R, et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis. 2014;210(10):1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Overstreet MG, Chen YC, Cockburn IA, Tse SW, Zavala F. CD4+ T cells modulate expansion and survival but not functional properties of effector and memory CD8+ T cells induced by malaria sporozoites. PLoS One. 2011;6(1):e15948. doi: 10.1371/journal.pone.0015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300(5617):337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 139.Bijker EM, Schats R, Visser LG, Sauerwein RW, Scholzen A. Ex vivo lymphocyte phenotyping during Plasmodium falciparum sporozoite immunization in humans. Parasite Immunol. 2015;37(11):590–598. doi: 10.1111/pim.12276. [DOI] [PubMed] [Google Scholar]
- 140.Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One. 2012;7(2):e31247. doi: 10.1371/journal.pone.0031247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156(9):3374–3381. [PubMed] [Google Scholar]
- 142.Malik A, Egan JE, Houghten RA, Sadoff JC, Hoffman SL. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA. 1991;88(8):3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mueller AK, Deckert M, Heiss K, Goetz K, Matuschewski K, Schluter D. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am J Pathol. 2007;171(1):107–115. doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, Zavala F. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med. 2001;194(2):173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 147.Obar JJ, Jellison ER, Sheridan BS, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187(10):4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rutishauser RL, Martins GA, Kalachikov S, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chandele A, Mukerjee P, Das G, Ahmed R, Chauhan VS. Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology. 2011;132(2):273–286. doi: 10.1111/j.1365-2567.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmidt NW, Podyminogin RL, Butler NS, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hafalla JC, Rai U, Morrot A, Bernal-Rubio D, Zavala F, Rodriguez A. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur J Immunol. 2006;36(5):1179–1186. doi: 10.1002/eji.200535712. [DOI] [PubMed] [Google Scholar]
- 152.Radtke AJ, Tse SW, Zavala F. From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells. Semin Immunopathol. 2015;37(3):211–220. doi: 10.1007/s00281-015-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reyes-Sandoval A, Wyllie DH, Bauza K, et al. CD8+ T effector memory cells protect against liver-stage malaria. J Immunol. 2011;187(3):1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cockburn IA, Amino R, Kelemen RK, et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc Natl Acad Sci USA. 2013;110(22):9090–9095. doi: 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J Immunol. 2009;183(9):5870–5878. doi: 10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 156.Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun. 2008;76(8):3628–3631. doi: 10.1128/IAI.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Butler NS, Schmidt NW, Harty JT. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P yoelii sporozoites. J Immunol. 2010;184(5):2528–2538. doi: 10.4049/jimmunol.0903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zarling S, Berenzon D, Dalai S, Liepinsh D, Steers N, Krzych U. The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria. J Immunol. 2013;190(10):5128–5141. doi: 10.4049/jimmunol.1203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zander RA, Vijay R, Pack AD, et al. Th1-like Plasmodium-specific memory CD4(+) T cells support humoral immunity. Cell Rep. 2018;23(4):1230–1237. doi: 10.1016/j.celrep.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moris P, Jongert E, van der Most RG. Characterization of T-cell immune responses in clinical trials of the candidate RTS,S malaria vaccine. Hum Vaccin Immunother. 2018;14(1):17–27. doi: 10.1080/21645515.2017.1381809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernandez-Ruiz D, Ng WY, Holz LE, et al. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45(4):889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 162.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol. 2003;171(4):2024–2034. doi: 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 163.Spencer AJ, Longley RJ, Gola A, Ulaszewska M, Lambe T, Hill AV. The threshold of protection from liver-stage malaria relies on a fine balance between the number of infected hepatocytes and effector CD8(+) T cells present in the liver. J Immunol. 2017;198(5):2006–2016. doi: 10.4049/jimmunol.1601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McNamara HA, Cai Y, Wagle MV, et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol. 2017;2(9):eaaj1996. doi: 10.1126/sciimmunol.aaj1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schrum JE, Crabtree JN, Dobbs KR, et al. Cutting edge: Plasmodium falciparum induces trained innate immunity. J Immunol. 2018;200(4):1243–1248. doi: 10.4049/jimmunol.1701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mata E, Salvador A, Igartua M, Hernández RM, Pedraz JL. Malaria vaccine adjuvants: latest update and challenges in preclinical and clinical research. Biomed Res Int. 2013;2013:282913. doi: 10.1155/2013/282913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kurup SP, Obeng-Adjei N, Anthony SM, et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat Med. 2017;23(10):1220–1225. doi: 10.1038/nm.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ndungu FM, Urban BC, Marsh K, Langhorne J. Regulation of immune response by Plasmodium-infected red blood cells. Parasite Immunol. 2005;27(10-11):373–384. doi: 10.1111/j.1365-3024.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 170.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400(6739):73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 171.Wykes MN, Horne-Debets JM, Leow CY, Karunarathne DS. Malaria drives T cells to exhaustion. Front Microbiol. 2014;5:249. doi: 10.3389/fmicb.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.World Health Organization. Tables of malaria vaccine projects globally. 2017. [Accessed October 03, 2020]. http://www.who.int/immunization/research/development/Rainbow_tables/en/
- 173.Schindler T, Jongo S, Studer F, et al. Two cases of long-lasting, sub-microscopic Plasmodium malariae infections in adults from coastal Tanzania. Malar J. 2019;18(1):149. doi: 10.1186/s12936-019-2787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moncunill G, De Rosa SC, Ayestaran A, et al. RTS,S/AS01E malaria vaccine induces memory and polyfunctional T cell responses in a pediatric african phase III trial. Front Immunol. 2017;8:1008. doi: 10.3389/fimmu.2017.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill AVS. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 2017;7:46621. doi: 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Venkatraman N, Anagnostou N, Bliss C, et al. Safety and immunogenicity of heterologous prime-boost immunization with viral-vectored malaria vaccines adjuvanted with Matrix-M. Vaccine. 2017;35(45):6208–6217. doi: 10.1016/j.vaccine.2017.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.