Abstract
Background
Acetaminophen is widely used as first-line therapy for chronic pain due to its perceived safety and the assumption that, unlike non-steroidal anti-inflammatory drugs, it has little or no effect on blood pressure (BP). Although observational studies suggest that acetaminophen may increase BP, clinical trials are lacking. We therefore studied the effects of regular acetaminophen dosing on BP in individuals with hypertension.
Methods
In this double-blind, placebo-controlled, crossover study, 110 individuals were randomized to receive acetaminophen 1g four times daily or matched placebo for 2 weeks followed by a 2-week washout period before crossing over to the alternate treatment. 24-hour ambulatory BP was measured at the beginning and end of each treatment period. The primary outcome was a comparison of the change in mean daytime systolic BP from baseline to end of treatment between placebo and acetaminophen arms.
Results
103 patients completed both arms of the study. Regular acetaminophen, compared to placebo, resulted in a significant increase in mean daytime systolic BP (132.8±10.5 to 136.5±10.1 mmHg vs. 133.9±10.3 to 132.5±9.9, p<0.0001) with a placebo-corrected increase of 4.7 mmHg (95% confidence interval [CI] 2.9-6.6) and mean daytime diastolic BP (81.2±8.0 to 82.1±7.8 mmHg vs. 81.7±7.9 to 80.9±7.8, p=0.005) with a placebo-corrected increase of 1.6 mmHg (95% CI 0.5-2.7). Similar findings were seen for 24-hr ambulatory and clinic BP.
Conclusion
Regular acetaminophen intake of 4g daily increases systolic BP in individuals with hypertension by around 5 mmHg when compared to placebo, increasing cardiovascular risk and calling into question the safety of regular acetaminophen use in this situation.
Funding
British Heart Foundation grant (PG/13/26/3012 8)
Introduction
Acetaminophen (paracetamol in the UK) is the most widely used analgesic globally and generally the initial drug of choice for the treatment of chronic pain 1 . Recent evidence, however, suggests that its role in the management of chronic pain has probably been overstated 2-5 . As evidence grows to suggest regular acetaminophen use has at best a limited benefit in chronic pain, greater emphasis on determining the harms of acetaminophen will allow more informed decision making by clinicians and patients. The significant risks of acetaminophen in overdose are well known 6 . However, considerable uncertainty remains regarding the safety of chronic acetaminophen use at therapeutic doses, owing to a reliance on observational data and cohort studies 1 , often with conflicting results. One key area of study has been acetaminophen’s effect on blood pressure (BP). Many observational studies suggest that acetaminophen increases BP. However, interventional data remain limited to small largely-underpowered trials that have not affected clinical practice 7 . To address this knowledge gap, we performed a randomized double-blind crossover study comparing the effects of regular acetaminophen and matched placebo on BP in individuals with hypertension [the PAraceTamol in Hypertension - Blood Pressure (PATH-BP) trial].
Methods
Study design
This single-center, randomized, double-blind, placebo-controlled, investigator-initiated crossover study funded by the British Heart Foundation analyzed the impact of regular acetaminophen treatment over two weeks on BP in individuals with treated and untreated hypertension. The study was performed in the University of Edinburgh’s Clinical Research Centre, Western General Hospital, Edinburgh, United Kingdom and was overseen by the Academic and Clinical Central Office for Research Development (ACCORD), a partnership between the University of Edinburgh and NHS Lothian Health Board. The study protocol was approved by the East of Scotland Research Ethics Service (13/ES/0087) and the Medicines and Healthcare products Regulatory Agency (2013-003204-40). It was registered with the US National Institutes of Health (ClinicalTrials.gov; identifier NCT01997112) & European Union Drug Regulating Authorities Clinical Trials Database (Number: 2013-003204-40).
Study Population
To meet inclusion criteria for enrolment, individuals had to be aged ≥18 years and hypertensive. They either had to be (i) treated for hypertension with an average daytime ambulatory BP of <150/95 mmHg on stable doses of one or more antihypertensive medication or (ii) untreated with an average daytime ambulatory BP ≥135/85 mmHg but <150/95 mmHg. Individuals were excluded if they had a history of ischemic heart disease, heart failure, cerebrovascular disease, liver impairment (alanine aminotransferase >50 IU/L), stage 3-5 chronic kidney disease or suicidal ideation. Individuals were also excluded if they weighed <55kg or were regularly taking acetaminophen, non-steroidal anti-inflammatory drugs, corticosteroids or oral anticoagulants. Participants were recruited from local ambulatory BP clinics, general practices (with support from NHS Research Scotland (NRS) Primary Care Network), and SHARE (an NRS register of people interested in participating in health research). All study participants provided written informed consent before participation.
Protocol
After screening and recruitment, participants were randomly assigned to receive either acetaminophen 1g four times daily (the maximum recommended daily dose and a commonly prescribed dose for chronic pain in the UK 8 ) or matched placebo for 2 weeks. Following a 2-week washout, patients crossed over to the other treatment arm for a further 2 weeks of treatment (supplemental Fig 1). Treatment order was randomized, with concealed allocation, using a random block design and participants were assigned to receive drug then placebo, or placebo then drug, in a 1:1 ratio. All researchers and participants were blinded to treatment throughout the study.
Participants attended for 4 visits during each arm of the study, 2 long visits at days 0 (pretreatment) and 14, and 2 short visits at days 4 and 7. During the long visits, clinic BP was recorded, a 24-hour ambulatory BP monitor (ABPM) fitted, and blood samples taken. On short visits, only clinic BP and blood samples were taken. Blood samples were taken for measurement of urea & electrolytes, liver function tests (bilirubin, alkaline phosphatase and alanine aminotransferase (ALT)) and acetaminophen concentration.
The study drug and placebo were both prepared in identical hard gelatine capsules (Swedish Orange, Size 00, Capsugel®) by Investigational Supplies Group, University of Edinburgh to ensure identical appearance of both formulations for blinding purposes. The study drug contained 500mg acetaminophen (product license number PL17907/0057, Bristol Laboratories Ltd, Berkhamsted, UK) and had negligible sodium content (0.04mg sodium per capsule). Placebo contained maize starch. No changes to background antihypertensive therapy were allowed during the study.
Blood pressure monitoring
During each visit clinic BP measurements were taken after subjects had been sitting for a minimum of 10 minutes. Three serial clinic BP measurements were taken in the non-dominant arm using a calibrated Microlife Watch BP recorder (Microlife AG Swiss Corporation, Switzerland) 9 . The average of the second and third readings was recorded.
At the beginning and end of each phase of the study ABPM was obtained over a 24-hour period using the Spacelabs Healthcare 90207 Ambulatory BP recorder (Spacelabs, Washington, USA). This was done in accordance with current UK guidelines 10 . The monitors were set to record BP every 30 minutes during the day and hourly at night.
Laboratory Analysis
Urea & electrolytes, liver function tests and serum acetaminophen were analyzed by NHS Lothian laboratories (UK Accreditation Service Laboratory No. 8699) in accordance with International Standard ISO 15189:2012 using Abbott Architect c16000 & ci16200 analyzers.
Study Outcomes
The primary outcome was defined as the change in mean daytime systolic ambulatory BP after 2 weeks of treatment with acetaminophen compared with placebo. The pre-specified secondary endpoints were change in mean daytime diastolic BP, systolic 24-hour BP, diastolic 24-hour BP and clinic BP after 2 weeks of treatment with acetaminophen compared to placebo.
Sample size and statistical analysis
We estimated that a total of 110 patients would need to be recruited in order to detect a 1.6 mm Hg difference in the change in systolic BP between acetaminophen and placebo arms using a two-sided, paired Student’s t-test with 5% level of significance and 90% power, assuming a standard deviation of the difference of 4.9mmHg 11 and a dropout rate of 10%.
The statistical analysis was pre-defined in the statistical analysis plan which was finalized and signed before the data were unblinded. The ABPM analyses were based on a modified intention to treat population (MITT), consisting of all randomized participants who had valid ABPM data at all time points, thus excluding participants with missing ABPM data. In order to account for the potential impact of treatment order the primary and secondary BP data were analysed using a mixed model where treatment, period and baseline BP were fitted as fixed effects and the participant as a random effect with results presented in the form of least square means. Each of the comparisons was considered significant if the p value was < 0.05. In addition, a per-protocol analysis was performed based on compliance with treatment, where compliance was based on serum acetaminophen levels. Compliance was defined as an undetected acetaminophen level (<3mg/L) throughout the placebo phase and at baseline of the acetaminophen phase as well as a detectable acetaminophen level at the final measurement (when ABPM was assessed) and at least one of the other two timepoints during the treatment period. Blood results were analyzed using paired Student’s t-tests. Each of the comparisons was considered significant if the p value was < 0.05.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study Population
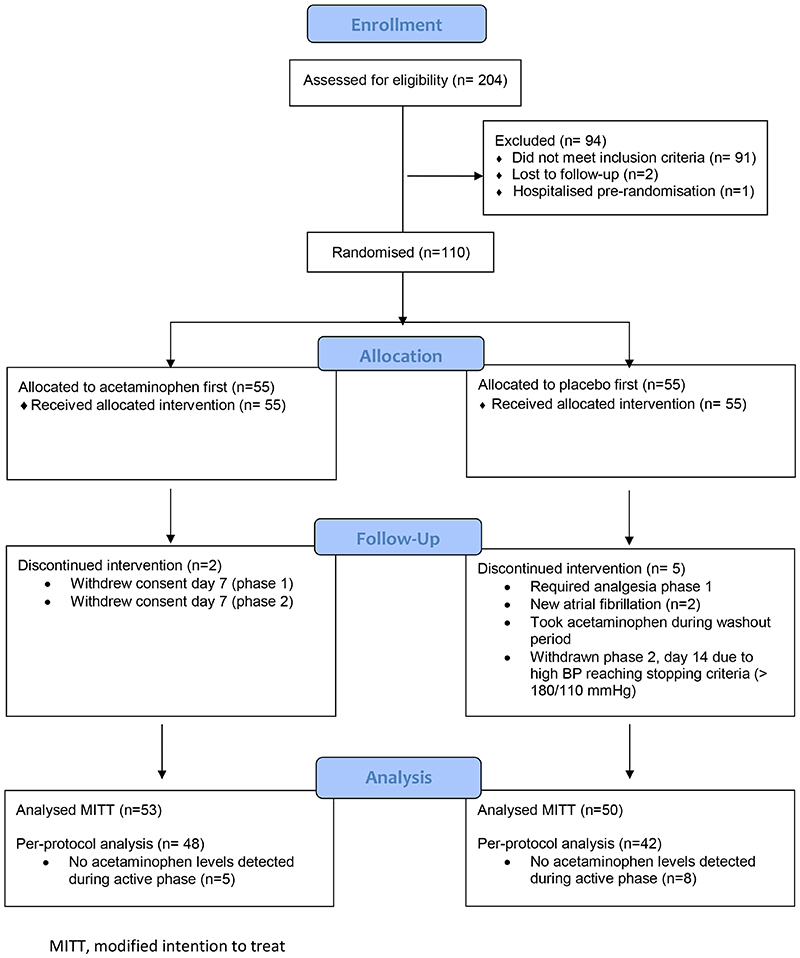
A total of 204 local participants were screened and 110 Caucasian subjects were randomized onto the study between September 2014 and June 2019 (Figure 1). Seven participants did not complete both arms of the study (drop-out < 10%), so 103 participants were included in the MITT analysis. The study group was balanced on all baseline characteristics (Table 1 & Table S1). Based on acetaminophen assays, 90 participants were included in the per-protocol analysis (Figure 1).
Figure 1. Flow diagram of study.
Table 1. Baseline characteristics.
|
Placebo-first
(n = 55) |
||
|---|---|---|
| Age (yrs.) (± SD) | 60.9 (7.8) | 62.5 (7.8) |
| Male sex – no. (%) | 40 (73) | 44 (80) |
| Smoking Status – no. (%) | ||
| • Current | 2 (4) | 2 (4) |
| • Ex-smoker | 17 (31) | 22 (40) |
| • Never smoked | 36 (65) | 31 (56) |
| On treatment for hypertension – no. (%) | 39 (71) | 35 (64) |
| Antihypertensive treatment – no. (%) | ||
| ACE Inhibitor | 19 (35) | 15 (27) |
| Angiotensin receptor blocker | 18 (33) | 16 (29) |
| Calcium channel blocker | 10 (18) | 14 (25) |
| Diuretic | 13 (24) | 17 (31) |
| Beta-blocker | 4 (7) | 4 (7) |
| Number of Antihypertensives – no. (%) | ||
| No antihypertensives | 16 (29) | 19 (35) |
| 1 Drug | 21 (38) | 14 (25) |
| 2 Drugs | 11 (20) | 14 (25) |
| 3 Drugs | 7 (11) | 8 (15) |
| Statin therapy – no. (%) | 15 (27) | 13 (24) |
Data for the patients in the modified intention to treat (MITT) group are shown in Supplementary Table S1.
Primary endpoint
Using a mixed model to account for period effect there was an increase in mean daytime systolic ambulatory BP of 4.7 mmHg (95% confidence interval [CI] 2.9 to 6.6; p<0.0001) with acetaminophen compared to placebo (Table 2).
Table 2. Change in blood pressure following acetaminophen and placebo: modified intention to treat (MITT) analysis.
| p-value | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Daytime systolic BP
(mmHg) |
132.8 ± 10.5 | 136.5 ± 10.1 | 3.7 ± 7.4 | 133.9 ± 10.3 | 132.5 ± 9.9 | -1.4 ± 7.6 | 4.7 (2.9 to 6.6) |
<0.0001 |
|
24-hr systolic BP
(mmHg) |
126.5 ± 9.8 | 130.0 ± 9.9 | 3.5 ± 7.1 | 127.4 ± 9.6 | 126.4 ± 9.9 | -1.0 ± 7.3 | 4.2 (2.4 to 6.0) |
<0.0001 |
|
Daytime diastolic BP
(mmHg) |
81.2 ± 8.0 | 82.1 ± 7.8 | 0.9 ± 4.2 | 81.7 ± 7.9 | 80.9 ± 7.8 | -0.8 ± 4.4 | 1.6 (0.5 to 2.7) |
0.005 |
|
24-hr diastolic BP
(mmHg) |
76.8 ± 7.5 | 77.8 ± 7.3 | 0.9 ± 4.2 | 77.3 ± 7.0 | 76.7 ± 7.0 | -0.5 ± 4.3 | 1.4 (0.2 to 2.5) |
0.017 |
|
Clinic systolic BP
(mmHg) |
137.4 ± 11.0 | 140.5 ± 12.2 | 3.15 ± 10.3 | 136.6 ± 10.3 | 135.6 ± 10.9 | -1.1 ± 9.2 | 4.6 (2.4 to 6.7) |
<0.0001 |
|
Clinic diastolic BP
(mmHg) |
85.9 ± 8.5 | 86.5 ± 9.1 | 0.6 ± 6.6 | 85.7 ± 8.8 | 84.8 ± 8.9 | -0.9 ± 6.1 | 1.6 (0.1 to 3.1) |
0.031 |
The modified intention to treat (MITT) analysis included all subjects with valid ambulatory blood pressure recordings for each time period and included the primary endpoint: placebo corrected change in daytime systolic blood pressure. P values were derived from a mixed model with treatment, period, and baseline blood pressure as fixed effects and participant as a random effect. Data are shown as mean ± SD. Estimate (95% CI) of difference in change from the mixed model is presented as least square means.
Secondary endpoints
Ambulatory Blood Pressure
Compared with placebo treatment, acetaminophen treatment was associated with an increase in mean 24-hr systolic BP of 4.2 mmHg (95% CI 2.4 to 6.0; p<0.0001), mean daytime diastolic BP of 1.6 mmHg (95% CI 0.5 to 2.7; p =0.005) and mean 24-hr diastolic BP of 1.4 mmHg (95% CI 0.3 to 2.5; p= 0.017) (Table 2). Similar findings were seen in the per-protocol analysis with increases in mean daytime systolic BP of 4.5 mmHg (95% CI 2.5 to 6.5; p<0.0001), mean 24-hr systolic BP of 4.2 mmHg (95% CI 2.3 to 6.1; p <0.0001), mean daytime diastolic BP of 1.5 mmHg (95% CI 0.3 to 2.7; p = 0.015), and mean 24-hr diastolic BP of 1.3 mmHg (95% CI 0.2 to 2.5; p = 0.021) (Table 3). Post-hoc analysis showed no evidence of a statistical difference in the change in daytime systolic BP between participants with treated or untreated hypertension (Fig. S2 in the Supplementary Appendix).
Table 3. Change in blood pressure following acetaminophen and placebo: per protocol analysis.
| p-value | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Daytime systolic BP
(mmHg) |
133.1 ± 10.6 | 136.7 ± 10.2 | 3.6 ± 6.9 | 134.1 ± 10.5 | 132.9 ± 10.2 | -1.2 ± 7.6 | 4.5 (2.5 to 6.5) |
<0.0001 |
|
24-hr systolic BP
(mmHg) |
126.7 ± 9.9 | 130.3 ± 9.9 | 3.6 ± 6.6 | 127.5 ± 9.7 | 126.7 ± 9.3 | -0.9 ± 7.0 | 4.2 (2.3 to 6.1) |
<0.0001 |
|
Daytime diastolic BP
(mmHg) |
80.8 ± 7.8 | 81.8 ± 7.7 | 1.0 ± 3.9 | 81.4 ± 7.7 | 80.8 ± 7.9 | -0.5 ± 4.4 | 1.5 (0.3 to 2.7) |
0.015 |
|
24-hr diastolic BP
(mmHg) |
76.5 ± 7.5 | 77.5 ± 7.4 | 1.0 ± 4.0 | 77.0 ± 7.0 | 76.5 ± 7.1 | -0.4 ± 4.1 | 1.4 (0.2 to 2.5) |
0.021 |
|
Clinic systolic BP
(mmHg) |
137.4 ± 11.0 | 140.1 ± 11.8 | 2.7 ± 10.2 | 137.0 ± 10.3 | 135.5 ± 10.4 | -1.5 ± 9.0 | 4.4 (2.1 to 6.7) |
0.0002 |
|
Clinic diastolic BP
(mmHg) |
85.6 ± 8.6 | 85.9 ± 8.9 | 0.3 ± 6.3 | 85.3 ± 8.4 | 84.3 ± 8.6 | -1.0 ± 6.2 | 1.5 (-0.1 to 3.0) |
0.059 |
Per protocol analysis included all patients with appropriately detectable acetaminophen during the study. P values were derived from a mixed model with treatment, period, and baseline blood pressure as fixed effects and participant as a random effect. Data are shown as mean ± SD. Estimate (95% CI) of difference in change from the mixed model is presented as least square means.
Clinic Blood Pressure
An increase in clinic BP was seen in the acetaminophen arm when compared to placebo, with a systolic BP change of 4.6 mmHg (95% CI 2.4 to 6.7; p<0.0001) and diastolic BP change of 1.6 mmHg (95% CI 0.1 to 3.0; p = 0.031) (Table 2). In the per protocol analysis there was an increase in systolic BP of 4.4 (95% CI 2.1-6.7; p<0.001) with no significant change in diastolic BP (1.5 mmHg 95% CI -0.1-3.0; p = 0.059) (Table 3). The rise in BP was seen by day 4 and sustained at day 14 (Fig. S3 in the Supplementary Appendix).
Biochemical parameters
Biochemical parameters are shown in Table 4. No significant changes were seen except for a modest but statistically significant rise in ALT activity with acetaminophen therapy, which normalized within 2 weeks of stopping acetaminophen.
Table 4. Laboratory values before and after acetaminophen and placebo.
| Placebo Week 2 | ||||
|---|---|---|---|---|
|
Urea – mmol/L
(n=103) |
5.6 ± 1.4 | 5.7 ± 1.6 | 5.7 ± 1.4 | 5.6 ± 1.6 |
|
Sodium – mmol/L
(n=103) |
139.8 ± 2.0 | 139.7 ± 2.5 | 140.0 ± 1.8 | 139.9 ± 2.1 |
|
Potassium – mmol/L
(n=103) |
4.3 ± 0.3 | 4.4 ± 0.4 | 4.3 ± 0.3 | 4.4 ± 0.3 † |
|
Creatinine – (μmol/L)
(n=103) |
77.2 ± 12.0 † | 76.3 ± 11.9 | 77.2 ± 11.9 | 77.6 ± 12.3 |
|
Serum Bicarbonate – mmol/L
(n=103) |
25.7 ± 2.0 | 25.4 ± 2.0 | 25.5 ± 1.9 † | 25.6 ± 2.1 |
|
Alk Phos – U/L
(n=100) |
74.2 ± 18.4 | 70.9 ± 16.2 | 73.1 ± 16.6 | 72.2 ± 15.4 |
|
ALT – U/L
(n=100) |
24.3 ± 18.7 | 36.2 ± 20.7 * | 23.5 ± 10.5 | 22.4 ± 9.6 |
|
Bilirubin – μmol/L
(n=100) |
10.4 ± 4.3 | 9.8 ± 4.1 | 10.3 ± 4.8 | 9.8 ± 4.4 |
Alk Phos, alkaline phosphatase; ALT, alanine aminotransferase
Data are shown as mean ± SD
single data point missing n=102
Statistically significant difference (p <0.0001), acetaminophen versus placebo.
Serious Adverse Events
Two serious adverse events were recorded during the study. The first was a case of atrial fibrillation requiring the participant to be admitted to hospital. This occurred during the active phase of the study but was not considered to be related to acetaminophen. The second serious adverse event, a myocardial infarction, occurred prior to dosing of any study medications and was, therefore, not related to either acetaminophen or placebo.
One participant had to be withdrawn from the study after exceeding the predefined safety stopping criteria for BP, defined as having a clinic BP >180/110 mmHg. This occurred on day 14 of acetaminophen treatment. The participant’s clinic BP measured 185/76 mmHg and after a further 10 minutes rest period remained elevated at 183/85 mmHg. Following discontinuation of acetaminophen, the participant’s BP normalized. As this patient did not complete all 4 ABPM recordings, their data were not included in the MITT or per protocol analysis.
Discussion
This randomized placebo-controlled crossover study provides clear evidence that acetaminophen raises BP over a 2-week period when compared with placebo in people with hypertension. The effects are robust for systolic BP measured by ABPM (the ‘gold standard’ for BP measurement 10 ) and in the clinic. The increase in systolic BP, when compared to placebo, was around 4.7 mmHg and that in diastolic BP around 1.6 mmHg, both significant when compared to placebo. This effect on BP was similar in those with either treated or untreated hypertension. Owing to the established continuous relationship between BP and cardiovascular and cerebrovascular disease, even a small change in BP can have important effects on clinical outcomes. Indeed, the 4.7 mmHg difference in BP, rather greater than the study was powered to detect, might be expected to translate to around 20% more cardiovascular events during any period of chronic treatment 12,13 .
Acetaminophen is the most widely used over-the-counter and prescription analgesic worldwide 1 . In Scotland alone over 500,000 patients (from a total population of 5.4 million) received three or more prescriptions for acetaminophen-containing medications in 2018, consistent with regular use and predominantly to treat chronic pain [NHS National Services Scotland prescribing data 2018]. In the USA it is estimated that between 3-5% of the adult population take regular acetaminophen 14 , increasing to ~8% in those newly diagnosed with hypertension 15 . Given the large number of people taking acetaminophen regularly in the
USA and worldwide the 4.7 mmHg placebo corrected rise in systolic BP, as seen in our study, could have considerable consequences on the population as a whole.
Many observational studies have suggested that long-term acetaminophen use is associated with an increased risk of developing hypertension 7 . The prospective Nurses’ Health Study II, which included 80,030 participants, found an association between regular acetaminophen use and hypertension with a relative risk of developing hypertension of 2.0 (95% confidence interval (CI) 1.5 – 2.6). This was near identical to that of non-steroidal anti-inflammatories (NSAIDs), which had a relative risk of 1.9 (95% CI 1.5-2.3) 16 . Further analysis of the Nurses’ Health Studies I and II also suggested a possible dose-response relationship with increasing doses of acetaminophen independently increasing the risk of hypertension in women 17 . In contrast, however, a recent retrospective observational study, with propensity matching, in 2,754 participants, showed no association between regular acetaminophen use and hypertension 18 . With many possible confounders, not all of which are likely to be identified, drawing any reliable conclusions from these observational studies is difficult. Prospective interventional trials, however, have been generally limited by small size and poor design 7 . The previous largest and best designed such study involved 33 participants with known coronary artery disease. The results showed that acetaminophen 3g per day significantly increased BP after 2 weeks, with a rise in systolic BP of around 3 mmHg compared to placebo 11 . These results are in keeping with our current study. Unfortunately, the study’s relatively small sample size and its very specific patient population has limited its generalizability.
The findings of our study further call into question current guidelines suggesting that acetaminophen is a safe alternative to NSAIDs. Indeed, the rise in BP seen in this study matches that with NSAIDs 19-22 and may well explain the finding that self-reported frequent acetaminophen use in women is associated with an increase in cardiovascular events similar to that seen with frequent NSAID use 23 . While the precise mechanism of actions of acetaminophen remain unclear it is believed to involve cyclo-oxygenase-2 inhibition which may, at least in part, explain the these similarities 1 . These findings would suggest that caution should be used when encouraging or prescribing regular use of acetaminophen, particularly in those with hypertension, and otherwise at risk of ischemic heart disease and stroke. Additionally, acetaminophen should no longer be thought of as a ‘safe’ alternative analgesic to an NSAID, at least with respect to hypertension.
Some limitations of our study should be taken into account. Firstly, the study was performed in individuals with a diagnosis of hypertension. It is, therefore, not clear whether the findings can be extrapolated to individuals who are not hypertensive. In general, however, like hypertension, rates of chronic pain increase with age so it would be expected that a substantial proportion of patients with chronic pain will also have a diagnosis of hypertension. The second limitation is study duration with it being unclear whether the increase in BP with acetaminophen use over two weeks is sustained in people taking longer-term acetaminophen therapy. However, the clinic BP data shows that BP rises by day 4 and remains stably elevated at day 14 (Supplemental Figure 3), making the effect likely to be long term, in keeping with the findings of the largest observational study 16 and other studies examining the effects of NSAIDs on BP 19,20 . A third limitation of the study was that it was performed in a group of individuals who did not suffer from chronic pain and would not normally be taking regular acetaminophen. The study was designed in this way to remove pain as a possible confounder due to its known effects on BP. With increasing evidence that acetaminophen has limited, if any, effect on chronic pain 2-5 , it is likely, in many patients, that reducing the dose or even stopping acetaminophen would reduce BP, and its associated cardiovascular risk, without worsening chronic pain. Finally, the study was only performed in a Caucasian population and it is, therefore, unclear whether these differences would be observed in other populations.
Conclusion
The current study shows that acetaminophen increases BP in people with hypertension and adds to concerns regarding the safety of regular acetaminophen treatment, particularly in those at risk of developing ischemic heart disease and stroke.
Supplementary Material
Clinical Perspective.
What is new?
Regular acetaminophen use increases both systolic and diastolic BP in individuals with hypertension, with an effect similar to that of non-steroidal intiinflammatories.
This rise in BP is seen both in those taking and not taking antihypertensive therapy
Clinical Implications
Acetaminophen is widely prescribed for the management of chronic pain but has limited evidence of efficacy.
Due to the established continuous relationship between BP and cardiovascular and cerebrovascular disease, and acetaminophen’s widespread use, this rise in BP may contribute to an increase in cardiovascular morbidity and mortality.
Caution should be taken when prescribing acetaminophen, particularly in those with increased cardiovascular risk and opportunities to stop acetaminophen or reduce the dose should be considered.
Acknowledgements
We would like to thank all those involved in making the PATH-BP study a success, and particularly all of the participants and local GP practices who supported the study.
Recruitment to this study was facilitated by SPCRN (Scottish Primary Care Research Network) and SHARE (Scottish Health Research Register), both of which are supported by NHS Research Scotland and the Scottish Government’s Chief Scientist Office.
Funding
This study was funded by a grant (PG/13/26/3012 8) from the British Heart Foundation. The authors had complete control over design, analysis, and the decision to submit the article for publication. The sponsor had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Footnotes
Competing Interests
There are no competing interests to declare.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol - a review. Br J Clin Pharmacol. 2018;84:2218–30. doi: 10.1111/bcp.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Abdel Shaheed C, Maher CG. Paracetamol for low back pain. Cochrane Database Syst Rev. 2016:CD012230. doi: 10.1002/14651858.CD012230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791–803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 5.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384:1586–96. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 6.Pettie JM, Caparrotta TM, Hunter RW, et al. Safety and efficacy of the SNAP 12-hour acetylcysteine regimen for the treatment of paracetamol overdose. EClinicalMedicine. 2019;11:11–7. doi: 10.1016/j.eclinm.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turtle EJ, Dear JW, Webb DJ. A systematic review of the effect of paracetamol on blood pressure in hypertensive and non-hypertensive subjects. Br J Clin Pharmacol. 2013;75:1396–405. doi: 10.1111/bcp.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.What dose of paracetamol for older people? Drug Ther Bull. 2018;56:69–72. doi: 10.1136/dtb.2018.6.0636. [DOI] [PubMed] [Google Scholar]
- 9.Stergiou GS, Giovas PP, Gkinos CP, Patouras JD. Validation of the Microlife WatchBP Home device for self home blood pressure measurement according to the International Protocol. Blood Press Monit. 2007;12:185–8. doi: 10.1097/MBP.0b013e3280b083ce. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. [last accessed 01 Oct 2021]. [online]. Available at https://www.nice.org.uk/guidance/ng136 . [PubMed]
- 11.Sudano I, Flammer AJ, Periat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122:1789–96. doi: 10.1161/CIRCULATIONAHA.110.956490. [DOI] [PubMed] [Google Scholar]
- 12.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 13.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 14.Paulose-Ram R, Hirsch R, Dillon C, Gu Q. Frequent monthly use of selected non-prescription and prescription non-narcotic analgesics among U.S. adults. Pharmacoepidemiol Drug Saf. 2005;14:257–66. doi: 10.1002/pds.983. [DOI] [PubMed] [Google Scholar]
- 15.Hwang AY, Dave CV, Smith SM. Use of prescription medications that potentially interfere with blood pressure control in new-onset hypertension and treatment-resistant hypertension. Am J Hypertens. 2018;31:1324–31. doi: 10.1093/ajh/hpy118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204–8. doi: 10.1001/archinte.162.19.2204. [DOI] [PubMed] [Google Scholar]
- 17.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500–7. doi: 10.1161/01.HYP.0000177437.07240.70. [DOI] [PubMed] [Google Scholar]
- 18.Dawson J, Fulton R, McInnes GT, et al. Acetaminophen use and change in blood pressure in a hypertensive population. J Hypertens. 2013;31:1485–90. doi: 10.1097/HJH.0b013e328360f6f8. [DOI] [PubMed] [Google Scholar]
- 19.Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–84. [PubMed] [Google Scholar]
- 20.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Aw TJ, Haas SJ, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–6. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 22.Ruschitzka F, Borer JS, Krum H, et al. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: the PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement) Trial. Eur Heart J. 2017;38:3282–92. doi: 10.1093/eurheartj/ehx508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan AT, Manson JE, Albert CM, et al. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113:1578–87. doi: 10.1161/CIRCULATIONAHA.105.595793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.