Abstract
Emerging infectious diseases (EIDs) threaten the health of people, animals, and crops globally, but our ability to predict their occurrence is limited. Current public health capacity and ability to detect and respond to EIDs is typically weakest in low- and middle-income countries countries (LMICs). Many known drivers of EID emergence also converge in LMICs. Strengthening capacity for surveillance of diseases of relevance to local populations can provide a mechanism for building the cross-cutting and flexible capacities needed to tackle both the burden of existing diseases and EID threats. A focus on locally relevant diseases in LMICs and the economic, social, and cultural contexts of surveillance can help address existing inequalities in health systems, improve the capacity to detect and contain EIDs, and contribute to broader global goals for development.
For an increasingly interconnected planet, emerging infectious diseases (EIDs) pose profound threats to human health, the animals and crops we depend upon, and ultimately the economies and societies that sustain us. Although there is widespread recognition of the need for effective surveillance, there is less clarity about how to achieve this. Approaches that promise the ability to predict and thus prevent disease outbreaks have obvious attraction (1). However, despite important insights into the dynamics of cross-species transmission (2, 3), risk factors associated with EID events (4–6), and determinants of spread and persistence (3, 7), we still have limited ability to identify when and where new disease events will occur.
The current state of global capacity for EID surveillance shows geographic variation in outbreak distribution, detection, and reporting times. More than half (53%) of all outbreaks reported to the World Health Organization (WHO) from 1996 to 2009 were from Africa, the region with the longest delays in detection and public communication (8). Globally, delays are inversely related to the Human Development Index (9). Despite the implementation of the International Health Regulations (IHR) (10), which mandate countries to develop surveillance systems and response capacities to contain epidemics, fewer than 20% of United Nations member states have achieved the required standards (11).
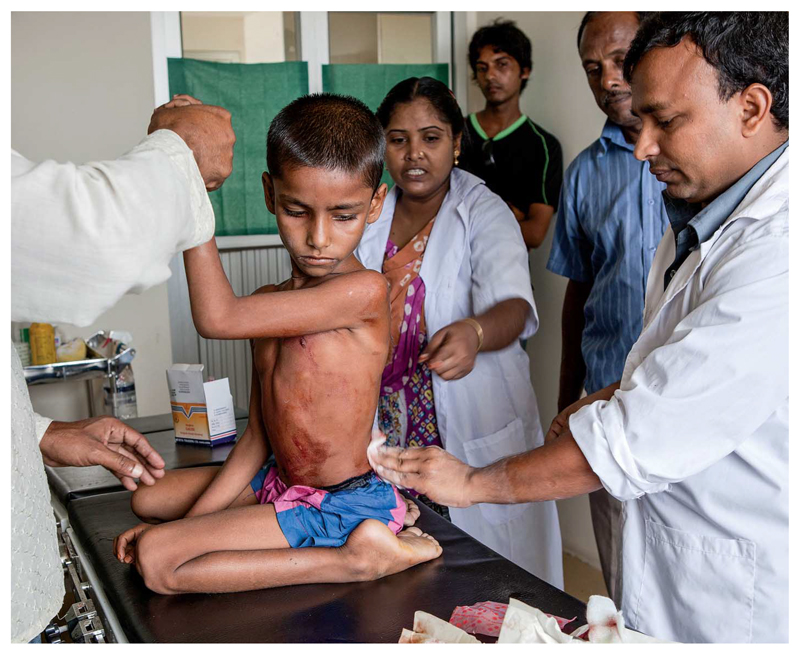
Trained health care professionals treat a boy aboard a hospital ship that provides responsive medical outreach to isolated communities along the Brahmaputra River, Bangladesh.
The absence and/or breakdown of public health measures has been identified as by far the most important factor underlying the occurrence of infectious disease outbreaks of international concern (12). Although the precise location of the next EID event cannot be predicted, regions where public health capacity is currently weak are readily identifiable: typically in low- and middle-income countries (LMICs), where many of the known drivers of EID emergence [deforestation, rapid urbanization, and agricultural intensification (13)] also converge. For wildlife-associated EIDs, emergence events occur most often where dense human populations intersect with areas of high species richness (5), again, mostly in LMICs. There is thus a clear rationale for strengthening disease surveillance in LMICs.
Surveillance systems and responsive interventions are often designed for a specific disease; however, many components of surveillance and response systems are not disease-specific. Numerous surveillance systems and organizations, established originally with a focus on a specific locally relevant disease, have subsequently leveraged their capacities to tackle other threats, including EIDs. For example, laboratories established through the Global Polio Eradication Initiative have expanded to cover pathogens including hemorrhagic fevers, Japanese encephalitis, severe acute respiratory syndrome, H5N1 influenza, and, most recently, Ebola virus disease (14, 15). The International Centre for Diarrhoeal Disease Research Bangladesh, established as a cholera research laboratory, now conducts research into diverse public health threats across a wider region. The predecessor organization of the U.S. Centers for Disease Control and Prevention (CDC) was established to control malaria, and cholera control was the initial impetus for the creation of today’s Pan American Health Organization and WHO. In the veterinary sector, the infrastructures established through the Pan African Rinderpest Campaign are now central to the Food and Agriculture Organization’s Emergency Prevention System for Transboundary Animal and Plant Pests and Disease (EMPRES). The participatory epidemiology approaches and improved animal health delivery systems for marginalized communities developed for Rinderpest have since been applied to control peste des petit ruminants, Rift Valley fever, highly pathogenic avian influenza, and foot-and-mouth disease (16). Specific advantages of eradication programs, in terms of legacy effects, emerge from their broad geographic scope and networks of trained health workers. The need to demonstrate freedom from disease requires verifiable and rigorous targets to be met even in the most hard-to-reach communities (16).
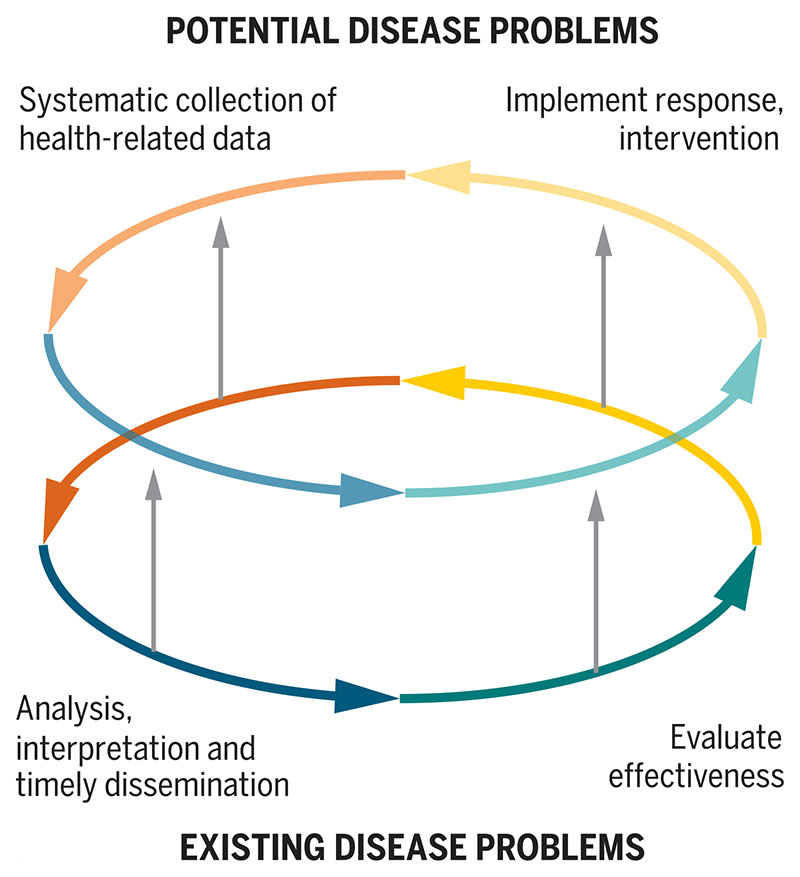
EID surveillance requires a network of trained reporters who can recognize and communicate disease events and systems to receive, collate, and disseminate reports. As the majority of EIDs are zoonoses, integration of surveillance capacities across human and animal health sectors is needed (1, 5, 11). Crucially, disease surveillance and the implementation and evaluation of interventions to contain the disease threat must be linked. Surveillance data inform control measures, and useful responses incentivize future data collection and reporting (Fig. 1) (1). Investments in sustainable surveillance programs must ultimately be linked to tangible health improvement outcomes (17).
Fig. 1. Surveillance cycles for current and potential disease problems.
The lower cycle illustrates the self-reinforcing nature of effective surveillance processes for existing disease problems. The upper cycle illustrates the equivalent processes for potential disease problems. Because it is difficult to implement interventions for potential disease threats that provide a useful response, and thus motivate further grassroots data collection, this cycle is less intrinsically sustainable, limiting the long-term effectiveness of approaches that focus on EIDs and potential threats alone. The vertical arrows show how capacity for potential disease problems and EID surveillance overall can be built by “borrowing” critical capacities (shown by the vertical arrows) from approaches that focus on existing diseases.
Prioritizing action to tackle existing diseases
A focus on strengthening capacity for surveillance of existing diseases that are of relevance to local populations can provide a mechanism for building the adaptable surveillance capacities that are also needed to tackle EID threats. Our rationale is that the act of focusing on locally relevant and ongoing disease threats enables greater local engagement and improves the chances of successful disease control by recognizing the social determinants of health (17–19). “Soft” organizational capacities, including communication, trust building, diplomacy, networking, political advocacy, and leadership are critical for health systems improvement (19). Aligning capacity strengthening for EIDs with locally relevant disease control efforts can overcome many of the challenges inherent in building de novo systems for EIDs. Additional benefits to such a pragmatic approach include synergies with multiple sustainable development goals (SDGs) (12, 17).
There are several existing frameworks that can be used to evaluate surveillance capacities and identify capacity-strengthening needs for both EIDs and existing disease challenges. These include the Performance of Veterinary Services pathway of the World Organisation for Animal Health, IHR targets (10), tools defining core competencies for surveillance at the human-animal interface (20), and tools for zoonotic disease prioritization developed through the Global Health Security Agenda (21). One Health approaches are important for zoonotic disease risks, but equally, tackling nonzoonotic human diseases or economically important livestock diseases may be the most effective way to build the capacity needed in many cases. No single approach to the selection of locally relevant diseases (be they infectious, noncommunicable, endemic, or emerging) will be appropriate in all settings. A case study illustrating how actions to control rabies—a locally relevant zoonosis in many LMICs—contribute to strengthening of surveillance capacities for EIDs and global goals for sustainable development is given in table S1.
Understanding surveillance in context
Surveillance is costly; hence, any perceived lack of benefits is particularly discouraging for stake-holders, including patients, livestock keepers, health-care providers, and governments. Aligning private incentives with local and global public benefits is key, and a focus on locally relevant disease problems can help achieve this. Surveillance costs to livestock keepers, for example, may include time contacting a private- or public-sector animal health official and paying for their services, actions that require a clear private benefit to that farmer. In the absence of effective veterinary responses, surveillance relying on individual livestock keepers will collapse. This is all too often the case for animal (and human) health surveillance initiatives in many LMIC settings, with the unsurprising result that endemic diseases are chronically underreported. Where surveillance is focused on potential disease threats, and the nature of the appropriate response to a novel threat is unknown, the potential benefits of participation are impossible to measure, thus compounding this problem.
The potential benefits of disease-reporting actions by a livestock keeper range from private benefits, when early detection and reporting minimize income loss from sick animals, to the spillover benefits to other local households from reduced disease incidence. Further, there will be national benefits, such as human health risk reductions of zoonoses, economic benefits of a healthier livestock sector, and reduced transmission from and to wildlife (22). Finally, there will be advantages from the increased capacity to detect EIDs rapidly and minimize their spread and impact worldwide. The wider-ranging benefits are less tangible to individual livestock keepers and of little incentive. Despite these far-reaching benefits, the major surveillance costs fall on local stakeholders, who will only partially gain benefits. For national or global programs to be able to rely on grassroots disease monitoring, it is important to ensure that individuals benefit by subsidizing or rewarding participation in surveillance activities—for example, via incentive payments or through involvement with institutions created for coordination and benefit-sharing across communities. These could include, for example, cooperatives of livestock farmers acting together on surveillance and disease control behavior to generate greater benefits in total than if each person acted according to individual interests and providing a mechanism for sharing these benefits. It is important to identify who should fund such programs and how best to design cooperative institutions (23).
If the long-term outcome is improved global capacity to detect and respond to EIDs, the entire global population benefits. A key question, therefore, is whether richer beneficiaries should pay for surveillance by the individuals who supply this public good. If so, investments in tackling diseases in LMICs that will improve global EID surveillance capacity should be funded by Western governments, development banks, or aid agencies. The design of systems to tackle misalignments of costs and benefits must also consider the best form in which incentives can be provided (1). Theoretical models that identify when it is best to subsidize actions rather than outcomes, or a combination of the two, provide compelling solutions to analogous questions in biodiversity conservation (24).
New technologies, including mobile phones, have potential to facilitate real-time communication and improve surveillance capacity if adopted for routine use. For example, a recent large-scale mobile-phone–based system in southern Tanzania improved rabies surveillance data quality and time-liness at low cost in comparison to paper-based surveillance (25). Regular phone communications and feedback supported users, particularly in isolated areas, and incentivized surveillance. Importantly, this system was designed by government representatives, community members, and system users. Integrating these technologies within widely used surveillance platforms such as DHIS2 could facilitate their uptake and simplify demands on health and veterinary workers (26).
Public health interventions often fail because of a lack of attention to their social, cultural, and historical contexts and engagement with the people they are designed to benefit. Effective community engagement has been crucial for successful control of Ebola in West Africa (27), rinderpest eradication (16), and the success of many neglected tropical disease (NTD) programs (28). Indeed, the need to tailor delivery strategies to local contexts is a common finding from social science studies of NTD programs (18), and there is no reason to think that this is any less necessary for other infectious disease challenges, including surveillance (17, 19).
Research is vital to understand the relative importance of disease alongside other priorities for individual households, perceptions of ability to effect change, and the situations required for action to be taken. Even in the midst of epidemics, attitudes toward disease risk and management, for instance, may not be focused around one pathogen, or even on avoiding disease at all, but on providing food, income, education, or investment for the future. Local understanding of disease, relationships to government officials and the state, and past experiences with development projects play important mediating roles in community engagement with disease control and capacity-strengthening programs (18, 29). The causes of social difference are complex. It is not always possible to address these through individual behavior change and, instead, wider social, economic, and political processes must be understood. One Health approaches offer an advantage here because they can offer a broader understanding of the interdependencies of human, animal, environmental, and socioeconomic health in the decision-making of households, in addition to the likely effects of disease control interventions. However, interventions are unlikely to be adopted if individuals perceive that they lack the agency to enact change. Focusing on tangible and tractable current problems can help to empower individuals.
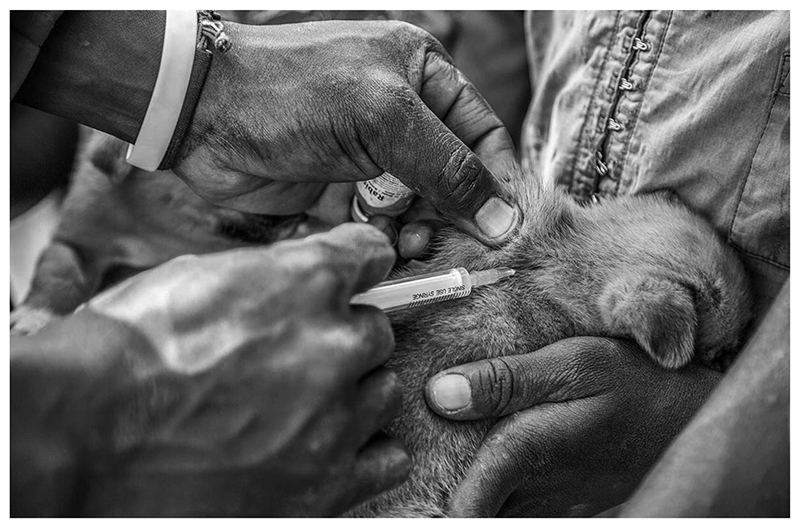
Canine rabies vaccination campaigns achieve community engagement to improve disease control and surveillance in Siaya County, Kenya.
Maximizing synergies and sustainability
By focusing on detecting and reporting rare EID events independently of other disease problems, we miss important opportunities for broader-reaching benefits and synergies. Appropriately targeted improvements in surveillance capacity can be viewed as investments in a country’s “inclusive wealth,” argued to be a key factor in deter-mining a country’s ability to achieve sustained improvements in well-being (30). Our proposed strategy reflects a growing global interest in optimizing interactions across the SDGs (31). Progress toward the SDG3.d target relating to EIDs—to strengthen capacity of all countries, in particular developing countries, for early warning, risk reduction, and management of national and global health risks—depends on several other SDG3 targets, including access to good-quality essential health-care services. Efforts to manage a locally relevant disease can also contribute to ending neglected tropical diseases (SDG3.3) and achieving universal health coverage (SDG3.8). Interventions that support targets for sustainable cities and communities (SDG11) could be designed with a view to detecting and mitigating emerging vector-borne threats, such as Zika, chikungunya, and yellow fever viruses, which are all transmitted by urban-adapted species of Aedes mosquito vectors. Interventions to support sustainable and resilient agricultural practices that help maintain diverse ecosystems (SDG2) could be developed to mitigate against the land-use changes that have been linked with spillover transmission and disease emergence from wildlife reservoirs.
The components and requirements of effective global EID surveillance are known, but there has been less focus on the mechanisms that can be employed to strengthen the capacities needed. This is particularly true in LMICs with the weakest human and animal health systems, where many diseases have emerged and where the consequences of EID events are likely to be most serious. Many of the I capacities required for EID surveillance are identical to those required to tackle existing diseases of ongoing local importance. We argue for an approach whereby gaps in EID surveillance capacity are filled by responding to existing local health challenges rather than through a focus on EIDs exclusively. The investments required to achieve comparable capacity gains through an EID-only strategy are likely to be greater and ultimately unsustainable. In contrast, approaches to EID capacity strenghthening that address locally relevant disease problems can capitalize on positive reinforcement processes that will sustain the capacities and collaborative networks that make up a functional surveillance system. By addressing ongoing disease problems, greater understanding of the social determinants and context of disease response capability in low-resource settings can be built, generating insights that apply far beyond the initial target disease. This approach provides a mechanism for achieving the necessary improvements in global EID surveillance capacity while also contributing to broader global goals for development.
Supplementary Material
Acknowledgments
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC) BB/J010367/1 (J.E.B.H., J.P.S., D.T.H., and S.C.), the UK Zoonoses and Emerging Livestock Systems Initiative grants BB/L018845/1 (J.E.B.H. and D.T.H.) and BB/L018926/1 (J.P.S. and S.C.), the Wellcome Trust 095787/Z/11/Z (K.H.), the Economic and Social Research Council (ESRC) RES-070-27-0039 (J.P.S.), and a Leverhulme-Royal Society Africa Award (AA130131). J.E.B.H., D.T.H., and S.C. conceptualized the project. J.E.B.H., N.H., J.P.S., and SC wrote the original draft. J.E.B.H., K.H., N.H., T.L., J.P.S., D.T.H., and S.C wrote, reviewed and edited the manuscript. The authors declare that they have no competing interests. All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplementary materials.
References and Notes
- 1.Keusch GT, Pappaioanou M, Conzalez MC, Scott KA, Tsai P, editors. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 2.Streicker DG, et al. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Smith JO, et al. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolhouse MEJ, Gowtage-Sequeria S. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones KE, et al. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse SS, et al. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolhouse MEJ, Haydon DT, Antia R. Trends Ecol Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan EH, et al. Proc Natl Acad Sci USA. 2010;107:21701–21706. doi: 10.1073/pnas.1006219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kluberg SA, et al. Emerg Infect Dis. 2016;22:E1–E6. doi: 10.3201/eid2210.151956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. International health regulations. Third edition. 2005. [accessed 11 April 2017]. http://apps.who.int/iris/bitstream/10665/246107/1/9789241580496-eng.pdf?ua=1 . [Google Scholar]
- 11.Burkle FM., Jr Disaster Med Public Health Prep. 2015;9:568–580. doi: 10.1017/dmp.2015.26. [DOI] [PubMed] [Google Scholar]
- 12.Bogich TL, et al. PLOS Med. 2012;9:e1001354. doi: 10.1371/journal.pmed.1001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patz JA, et al. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitchcock P, Chamberlain A, Van Wagoner M, Inglesby TV, O’Toole T. Biosecur Bioterror. 2007;5:206–227. doi: 10.1089/bsp.2007.0041. [DOI] [PubMed] [Google Scholar]
- 15.Vaz RG, et al. J Infect Dis. 2016;213(suppl 3):S140–S146. doi: 10.1093/infdis/jiv581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariner JC, et al. Science. 2012;337:1309–1312. doi: 10.1126/science.1223805. [DOI] [PubMed] [Google Scholar]
- 17.Frenk J. PLOS Med. 2010;7:e1000089. doi: 10.1371/journal.pmed.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardosh K. Infect Dis Poverty. 2014;3:35. doi: 10.1186/2049-9957-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson RC, et al. Global Health. 2015;11:5. [Google Scholar]
- 20.World Health Organization. World Organization for Animal Health, Handbook for the assessment of capacities at the human-animal interface. [accessed 11 April 2017]. http://www.who.int/ihr/publications/handbook_OMS_OIE/en/
- 21.Global Health Security Agenda. [accessed 11 April 2017]. https://www.ghsagenda.org/
- 22.Horan RD, Fenichel EP. Am J Agric Econ. 2007;89:1232–1238. [Google Scholar]
- 23.Cason TN, Sheremeta RM, Zhang JJ. Games Econ Behav. 2012;76:26–43. [Google Scholar]
- 24.White B, Hanley N. Environ Resour Econ. 2016;63:765–787. [Google Scholar]
- 25.Mtema Z, et al. PLOS Med. 2016;13:e1002002. doi: 10.1371/journal.pmed.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chisha Z, et al. Malar J. 2015;14:222. doi: 10.1186/s12936-015-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Ebola response: What needs to happen in 2015. [accessed 01 April 2017]. http://www.who.int/csr/disease/ebola/one-year-report/response-in-2015/en/
- 28.Kabatereine NB, et al. PLOS Negl Trop Dis. 2010;4:e755. doi: 10.1371/journal.pntd.0000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmer P. Infections and Inequalities: The Modern Plagues. University of California Press; Berkeley; London: 2001. ed Updated ed with a new preface. [Google Scholar]
- 30.UNU-IHDP, UNEP. Inclusive Wealth Report 2014. Measuring Progress Toward Sustainability. Summary for Decision-Makers. UNU-IHDP; Delhi: 2014. [Google Scholar]
- 31.Nilsson M, Griggs D, Visbeck M. Nature. 2016;534:320–322. doi: 10.1038/534320a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.