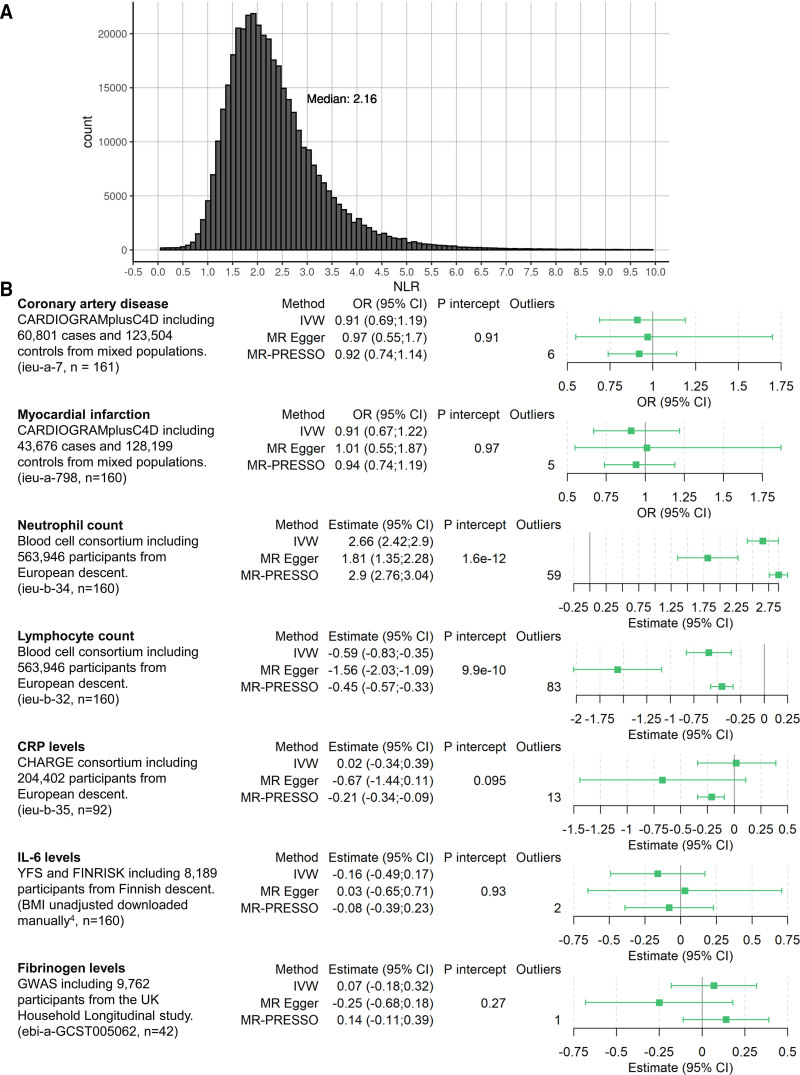
Inflammation contributes to atherosclerosis and coronary artery disease (CAD). To help identify therapeutic targets, it is important to ascertain whether biomarkers associated with CAD risk are causal. In a recent meta-analysis of clinical trials, neutrophil-to-lymphocyte ratio (NLR) was associated with increased cardiovascular risk.1 We investigate a potential causal nature of this relationship by performing Mendelian randomization (MR) analyses.
All participants provided prior consent for each study included, and the studies were approved by the relevant review committees. Nonpublic estimates are available from the corresponding author upon reasonable request. To identify genetic variants associated with NLR, we obtained published genome-wide association study summary data for neutrophil count and lymphocyte count in 361 194 European ancestry participants from http://www.nealelab.is/uk-biobank/, which were selected based on self-reported ancestry and genetic principal components. The propagation of error method was used to estimate the association of all available single-nucleotide polymorphisms (SNPs) with lymphocyte count subtracted from neutrophil count, resulting either in a positive value (which represents an increasing NLR) or a negative value (representing a decreasing NLR). Instruments were selected for MR by clumping all common SNPs (minor allele frequency, >0.01) at genome-wide significance (P<5×10−8) to pairwise linkage disequilibrium threshold r2 <0.01 (using European participants from the 1000Genomes project as reference). We estimated the association of each candidate instrument with NLR in individual participant data on 396 020 UK Biobank participants of White British descent with similar genetic ancestry (as reported by the UK Biobank resource), for whom neutrophil count, lymphocyte count, and data on all selected SNPs were available and had no extreme NLR value (defined as either NLR=0 or NLR=infinity).2 To estimate the association of each SNP with NLR, we used log-linear regression with adjustment for age, sex, the first 10 principal components of genetic ancestry, and measurement batch. We chose log-linear regression because NLR can only be positive, with some values close to zero, rendering normal linear regression inappropriate. The Figure (A) shows the distribution of NLR, which is similar to the distributions from various clinical trials including CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study).1
Figure.
Neutrophil-to-lymphocyte ratio (NLR) distribution and forest plot.
A, Distribution of NLR in the UK Biobank. Seven hundred sixty-seven values above 10 are not displayed for clarity purposes. B, Overview of included data and estimates for the Mendelian randomization association scaled to a 1-unit increase in genetically predicted log-transformed NLR. Estimates for coronary artery disease and myocardial infarction are depicted as odds ratio (OR); neutrophil and lymphocyte counts as percentage of total white blood cell count; CRP (C-reactive protein) as natural-log(mg/L); IL-6 (interleukin-6) as SD unit; and fibrinogen as log(g/L). n: number of single-nucleotide polymorphisms of the instrument available in the outcome data set and thus used for analysis; P intercept: Egger intercept P; outliers: outliers removed by MR-PRESSO. ieu-X-XXX: MRC Integrative Epidemiology Unit open genome-wide association study (GWAS) project data set code for use in TwoSampleMR package (https://gwas.mrcieu.ac.uk/). BMI indicates body mass index; and IVW, inverse-variance weighted. CHARGE indicates Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium; MR-PRESSO, Mendelian randomization-Pleiotropy Residual Sum and Outlier; and YFS, The Cardiovascular risk in Young Finns Study.
Statistical power calculations for the minimum detectable odds ratio were performed for a power of 80% and type 1 error rate of 0.05.3 We performed 2-sample MR analyses, where we considered the following outcomes: CAD, myocardial infarction, circulating CRP (C-reactive protein), IL-6 (interleukin-6), and fibrinogen levels (Figure). CRP, IL-6, and fibrinogen were considered to investigate the effect of NLR on inflammatory biomarkers.4 Finally, to investigate the validity of the NLR instruments, we performed analyses with neutrophil count and lymphocyte count as outcomes. If data on a SNP were unavailable in one specific genome-wide association study, we searched for an available proxy in high linkage disequilibrium (r2 >0.9), and if unavailable, we omitted the SNPs for that specific analysis. All analyses were performed using the package TwoSampleMR in R v4.0.3. Results are presented per 1-unit increase in genetically predicted log-transformed NLR.
Power calculations showed that we had 80% power to detect a minimum odds ratio of 1.07 for CAD. For myocardial infarction, power calculations showed 80% power to detect an odds ratio of 1.08. After clumping and omitting multiallelic SNPs, a total of 182 uncorrelated SNPs were selected as potential instruments in MR analyses given their genome-wide significant association with lymphocyte count subtracted from neutrophil count. In primary analyses (Figure [B]), we observed strong evidence of an association between genetically predicted NLR and neutrophil count (2.66% of white blood cell count [95% CI, 2.42–2.90]) and lymphocyte count (−0.59% of white blood cell count [95% CI, −0.83 to −0.35]). We did not observe evidence supporting a causal effect of NLR on CAD (0.91 [95% CI, 0.69–1.19]), myocardial infarction (0.91 [95% CI, 0.67–1.22]), CRP (0.02 natural-log(mg/L) units [95% CI, −0.34 to 0.39]), IL-6 (−0.16 SD units [95% CI, −0.49 to 0.17]), or fibrinogen (0.07 log(g/L) [95% CI, −0.18 to 0.32]). In sensitivity analyses, we observed similar results using the MR Egger method. Excluding outliers, MR-Pleiotropy Residual Sum and Outlier showed similar results to the primary inverse-variance weighted results, with the exception of CRP (Figure [B]).
This MR study did not identify evidence to support that NLR is causally related to risk of CAD and myocardial infarction. Moreover, we did not find consistent evidence of a causal association of NLR on CRP, IL-6, or fibrinogen levels. This contrasts with previous reports that have suggested a potential causal role for NLR in CAD, given the finding that both neutrophils and lymphocytes are involved in atherogenesis.1,5 The discrepancy may be attributable to the associations identified in epidemiological studies arising due to confounding and reverse causation. However, even if not causally related to CAD, NLR could still be used as a predictive measure for cardiovascular disease risk. To what extent NLR has added value in future cardiovascular risk prediction over and above CRP levels remains to be fully explored.
Most data are retrievable from the public domain. Summary data for the genetic instrument are available from the corresponding author.
Article Information
Sources of Funding
Dr Cupido reports grants from the Prince Bernhard Culture Fund and Stichting de Drie Lichten. Dr Burgess is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z) and supported the National Institute for Health Research Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care. Dr Asselbergs is supported by University College London Hospitals National Institute for Health Research Biomedical Research Centre. Dr Hovingh reports research grants from the Netherlands Organization for Scientific Research (ViDi 016.156.445), het Klinkerpad fonds and the European Union (transcard). Dr Gill is supported by the British Heart Foundation Research Centre of Excellence (RE/18/4/34215) at Imperial College London and by a National Institute for Health Research Clinical Lectureship (CL-2020-16-001) at St. George's, University of London.
Disclosures
Dr Hovingh has received funding from Regeneron, Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Kowa, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, The Medicines Company, and Ionis and is part-time employed by Novo Nordisk, Copenhagen, Denmark. Dr Gill is employed part-time by Novo Nordisk. The other authors report no conflicts.
Footnotes
For Sources of Funding and Disclosures, see page 80.
G.K. Hovingh and D. Gill contributed equally.
Contributor Information
Jordan M. Kraaijenhof, Email: j.kraaijenhof@amsterdamumc.nl.
Stephen Burgess, Email: sb452@medschl.cam.ac.uk.
Folkert W. Asselbergs, Email: f.asselbergs@ucl.ac.uk.
G. Kees Hovingh, Email: g.k.hovingh@amc.uva.nl.
Dipender Gill, Email: dipender.gill@imperial.ac.uk.
References
- 1.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, Tabas IA, Mehta NN, Ridker PM. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021; 42:896–903. doi: 10.1093/eurheartj/ehaa1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018; 562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014; 43:922–929. doi: 10.1093/ije/dyu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaoja M, Corbin LJ, Tan VY, Ahola-Olli AV, Havulinna AS, Santalahti K, Pitkänen N, Lehtimäki T, Lyytikäinen LP, Raitoharju E, et al. The role of inflammatory cytokines as intermediates in the pathway from increased adiposity to disease. Obesity (Silver Spring). 2021; 29:428–437. doi: 10.1002/oby.23060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021; 20:589–610. doi: 10.1038/s41573-021-00198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]