Abstract
There is increasing evidence for a cerebellar contribution to cognitive processing, but the specific input pathways conveying this information remain unclear. We probed the role of climbing fiber inputs to Purkinje cells in generating and evaluating predictions about associations between motor actions, sensory stimuli and reward. We trained mice to perform a visuomotor integration task to receive a reward and interleaved cued and random rewards between task trials. Using two-photon calcium imaging and Neuropixels probe recordings of Purkinje cell activity, we show that climbing fibers signal reward expectation, delivery and omission. These signals map onto cerebellar microzones, with reward delivery activating some microzones and suppressing others, and with reward omission activating both reward-activated and reward-suppressed microzones. Moreover, responses to predictable rewards are progressively suppressed during learning. Our findings elucidate a specific input pathway for cerebellar contributions to reward signaling and provide a mechanistic link between cerebellar activity and the creation and evaluation of predictions.
The cerebellum is thought to facilitate smooth behavioral execution and learning by generating expectations about the sensory consequences of actions and using sensory input to inform future motor output—that is, forming internal models of how we interact with the world 1,2 . Purkinje cells, the output neurons of the cerebellar cortex, are crucial for the construction and updating of internal models 3 . These neurons receive thousands of inputs from parallel fibers carrying contextual sensory and motor information, and a single but exceptionally strong input from a climbing fiber. These climbing fibers, which generate complex spikes in Purkinje cells, carry supervisory instructive signals and modify the synaptic weights of parallel fiber inputs to Purkinje cells 4–6 .
Climbing fiber activation triggers complex spikes in Purkinje cells at low rates (~0.5–2 Hz) and yet exerts a powerful influence on cerebellar function at the level of Purkinje cell populations. This is due to the anatomical and functional relationships between inferior olive neurons, the source of climbing fibers, and Purkinje cells 7,8 . Olivary neurons are gap-junction coupled and exhibit subthreshold oscillations 9,10 , and neighboring olivary neurons, which innervate neighboring Purkinje cells, fire action potentials synchronously and consequently trigger synchronous complex spikes in neighboring Purkinje cells. In this way, functional clusters of Purkinje cells, known as microzones, experience climbing fiber activation coherently and coordinate cerebellar output via synchronous output to neurons of the cerebellar nuclei 11 . Population recording methods have recently made it possible to address how olivary neurons engage Purkinje cell populations during behavior 12–20 .
In well-studied tasks that engage the cerebellum, the instructive signals conveyed by climbing fibers are usually considered as error signals in an extrinsic framework; for example, retinal slip during visual tracking 21,22 . However, there is increasing evidence for the cerebellum’s involvement in a higher-order processing 23 including spatial navigation 24 , language processing 25 and, notably for our study, reward 15,26 . We therefore examined whether the climbing fiber inputs to Purkinje cells may carry internally generated instructive signals and tested this possibility directly by studying how reward context is represented by climbing fiber inputs to Purkinje cell populations.
We demonstrate topographically organized encoding of reward context in the complex spiking patterns of Purkinje cell populations in the lobule simplex of the cerebellar cortex, a region traditionally thought to modulate forelimb movements 15,27 . We recorded dendritic calcium signals (a proxy for climbing fiber input and complex spikes) using two-photon microscopy and made direct recordings of complex spikes using Neuropixels probes while mice received rewards with varying degrees of predictability: after performing a trained motor action, after a tone cue that preceded reward by a fixed delay and randomly without prompting. Population activity of Purkinje cells represented reward context in a diverse but predictable manner organized spatially into microzones: some microzones exhibited elevated activity at reward delivery (‘reward-activated microzones’), while other microzones were inhibited (‘reward-suppressed microzones’). Some of these microzones also exhibited an elevated rate of complex spiking in anticipation of upcoming reward, with this behavior preferentially expressed in reward-suppressed microzones. When rewards were omitted on motor trials, both reward-activated and reward-suppressed microzones exhibited omission-related feedback error signals. Omitting tone-cued rewards also triggered feedback error signals and these signals occurred just after the time of expected reward. Finally, the degree of reward predictability modulated reward-related sensory responses in a graded fashion: the more predictable the reward, the smaller the sensory response it triggered. Combined with the recent demonstration that cerebellar granule cells also encode reward context 26 , our data demonstrate that the cerebellar cortex has access to the information streams necessary to create and evaluate expectations about higher-order variables.
Results
Population Purkinje cell complex spike recordings during a sensorimotor task
We trained mice to perform a visually guided sensorimotor integration task to study the variety of climbing fiber signals conveyed to Purkinje cells during behavior. Mice were head-fixed in front of an array of monitors and trained to use a steering wheel placed in front of their forepaws to control a virtual object (Fig. 1a, left). The object appeared at an eccentric visual position ~45° from the visual midline and mice had to move it into the center of the environment to receive a delayed water reward. After ~10 days of pre-training aimed to establish an association between steering wheel turns and virtual object movement, mice were transitioned to a more difficult version of the task. Here, the object always appeared on the same side (left) at a fixed position and the mice were required to make a single, continuous turn of the steering wheel to translate the object/wheel to a visible target region ±15° from the visual midline (Fig. 1a, right). Each trial was initiated by the appearance of the object and well-trained mice-initiated movements as soon as the object appeared. After the mouse initiated a wheel movement, trial outcome was assessed by the position of the object when the mouse had, for the first time since trial start, stopped moving the wheel for 100 ms continuously. If the object was positioned within the central target region, a delayed reward was given 500 ms after the object was stopped (400 ms from trial evaluation). Mice performed 214±8 trials per session (mean ± s.e.m., n = 61 sessions from six mice) and there were three possible outcomes of each trial: undershoots, correct (rewarded) trials and overshoots (Fig. 1b). Behavioral performance plateaued after < 1 week on this final task version and resulted in the following breakdown of performance: 28 ± 4% undershoots, 59 ± 2% correct trials and 13 ± 3% overshoots (mean ± s.e.m., n = 6 mice, averaged within mouse for sessions ≥5 of the final task version; Fig. 1c). By comparison, performance on days 1 and 2 of the final task version was significantly lower (46 ± 3% correct trials, mean ± s.e.m., n = 6 mice, P < 0.05).
Fig. 1. Population Purkinje cell complex spike imaging during a sensorimotor task.
a, Behavioral setup: mice were head-fixed in front of three monitors and trained to use a steering wheel to translate a virtual object from an eccentric visual position (45° left of midline) to the midline (±15° target) to obtain a delayed reward. b, Example behavioral trials: single undershoot (left), correct (middle) and overshoot (right) wheel trajectories, along with reward time and licking behavior. c, Behavioral performance in well-trained mice. Colored lines represent performance of individual mice (averaged across sessions), and thick black line represents average performance across mice. Data are shown as mean±s.e.m. (n = 6 mice). d, GCaMP6f-labeled Purkinje cells in lobule simplex and adjacent vermis. A field of view (FOV) in a typical lobule simplex recording location is shown in cyan. Scale bar, 300 μm. e, Extracted Purkinje cell dendritic regions of interest (ROIs) from field highlighted in d. Scale bar, 100 μm. f, Six example Purkinje cell dendrite fluorescence traces (black) and extracted dendritic events (blue). The thickness of the blue line denotes event amplitude. g, Top: trial-averaged Ca2+ responses in Purkinje cell population aligned to wheel movement onset for undershoot, correct and overshoot trials. Cells are sorted by the first coefficient of principal component analysis (PCA) performed over the interval ±500 ms from movement onset on correct trials. Middle: trial-averaged steering wheel position. Bottom: trialaveraged licking. Position and licking traces are shown as mean±s.e.m. (n = 97 undershoots, 156 corrects and 18 overshoots). h, Same as g, but aligned to reward delivery and sorted by over the interval ±500 ms. Scale bar, 500 ms. Purkinje cell dendritic responses were sorted independently in g and h. i, Mean time course of fluorescence responses (top) and detected events (bottom) aligned to movement onset (vertical dashed line). Mean response for statistical comparisons was computed on detected events over an interval of −300 to 0 ms from movement onset (bar above traces). Data are shown as mean±s.e.m. (n = 1,101 neurons from 6 FOVs in 6 mice). No group was significantly different from any other group (NS; Kruskal-Wallis test, H = 4.6, d.f. = 2, P = 0.1). j, Same as i, but aligned to reward time (500 ms after wheel stop). Mean response for statistical comparisons was computed on detected events over the interval of 0 to +100 ms post-reward (bar above traces). Data are shown as mean ± s.e.m. (n = 1,101 neurons from 6 FOVs in 6 mice). Response on correct trials was significantly different from response on undershoot and overshoot trials (Kruskal–Wallis test, H = 22.7, d.f. = 2, P = 1×10−5, significance values for Bonferroni-corrected individual comparisons: correct versus undershoot trials, P = 0.004; correct versus overshoot trials, P=1×10−5; undershoot versus overshoot, P = 0.5). Statistics summary: n.s., not significant, **P<0.01.
To image Purkinje cell populations during our task, we expressed Cre-dependent GCaMP6f virus in Pcp2(L7)-cre mice. Injections were targeted to the left lobule simplex and adjacent vermis (Fig. 1d), regions known to be involved in forelimb movements 15,27 . Purkinje cell population activity was recorded using resonant scanning two-photon microscopy to measure dendritic calcium signals, faithful indicators of climbing fiber input and complex spiking in Purkinje cells 28–31 . Our FOVs yielded 219 ± 27 (mean ± s.e.m., n = 13 different fields from nine mice) distinct dendritic ROIs corresponding to individual Purkinje cells (Fig. 1e). Individual dendritic ROIs exhibited fast calcium transients indicative of complex spikes and we extracted the size and timing of these events for each recorded neuron (Fig. 1f).
As a first step toward understanding how Purkinje cell activity is related to different aspects of the behavior, we aligned the calcium responses of our neurons to two important time points in the task across trial outcomes: (1) movement initiation and (2) movement termination and reward delivery (which occurred with a fixed time interval), and sorted neurons by their response during these epochs on correct trials. Subsets of Purkinje cells exhibited elevated dendritic calcium signals in the interval immediately before movement initiation and during the movement itself (Fig. 1g). Because animals usually initiated wheel movements immediately on object appearance, these signals may reflect either object appearance (a sensory signal) or the wheel movement (a motor signal). To distinguish these (not mutually exclusive) possibilities, we compared activity in trials where mice reacted rapidly from those in which they did not and wheel movement during trials to those made during inter-trial intervals (Supplementary Fig. 1). We found that while the object appearance itself could evoke responses in our recorded neurons, movement-aligned activity was similar for trials in which animals reacted quickly or slowly and also similar for wheel movements initiated within and outside trials. Furthermore, trial-by-trial analysis of population activity as a function of reaction time showed a tighter linkage between activity and movement onset than object appearance. Thus, movement onset-aligned activity is preferentially related to movement.
Many Purkinje cells also exhibited elevated calcium signals in the interval between the end of wheel movements and the reward, and at the time of the reward delivery itself (Fig. 1h). Overall, we found that movement onset-related activity was not predictive of trial outcome (Fig. 1i), while reward delivery on correct trials modulated our recorded populations potently (Fig. 1j). In subsequent experiments, we explored how reward-related signals were organized in Purkinje cell populations and how they could be modulated by reward context.
Topographic organization of reward-related signals
Microzones constitute a fundamental unit of cerebellar processing and are defined by the relationship between Purkinje cells and the climbing fibers that innervate them 7,8,28,30,32 . We asked whether the functional segregation of Purkinje cells activated by reward delivery maps onto microzones. To begin, instead of sorting ROIs on the basis of response magnitude, we sorted them on the basis of anatomy: orthogonally to the parasagittal axis of Purkinje cell dendrites. This sorting revealed groups of reward delivery-activated and reward delivery-suppressed Purkinje cells (Fig. 2a). To classify individual Purkinje cells into microzones systematically in all our recorded FOVs, we used PCA to reduce the dimensionality of each dataset and performed k-means clustering followed by a series of validation steps to identify microzonal clusters (see Methods and Supplementary Fig. 2). The clustering results for our example FOV are plotted for the first three components in Fig. 2b. These functionally defined clusters mapped onto anatomically clustered Purkinje cell populations, revealing almost perfect mediolateral segregation into microzones (Fig. 2c and Supplementary Fig. 3). This method yielded similar results to previously established correlation-based methods for identifying microzones, as evident from the blockdiagonal correlation matrix structure of our sorted Purkinje cells (see Methods and Supplementary Fig. 3). FOVs (670 μm × 670 μm) contained 5.3 ± 0.3 microzones (mean ± s.e.m., n = 6 fields from six mice). Microzones were 170 ± 10 μm wide (~17 dendrites wide) and contained 34 ± 3 dendrites (n = 1,101 dendrites, 32 microzones), consistent with reported microzonal widths on the order of 100–200 μm 15,28,30 (Supplementary Fig. 3).
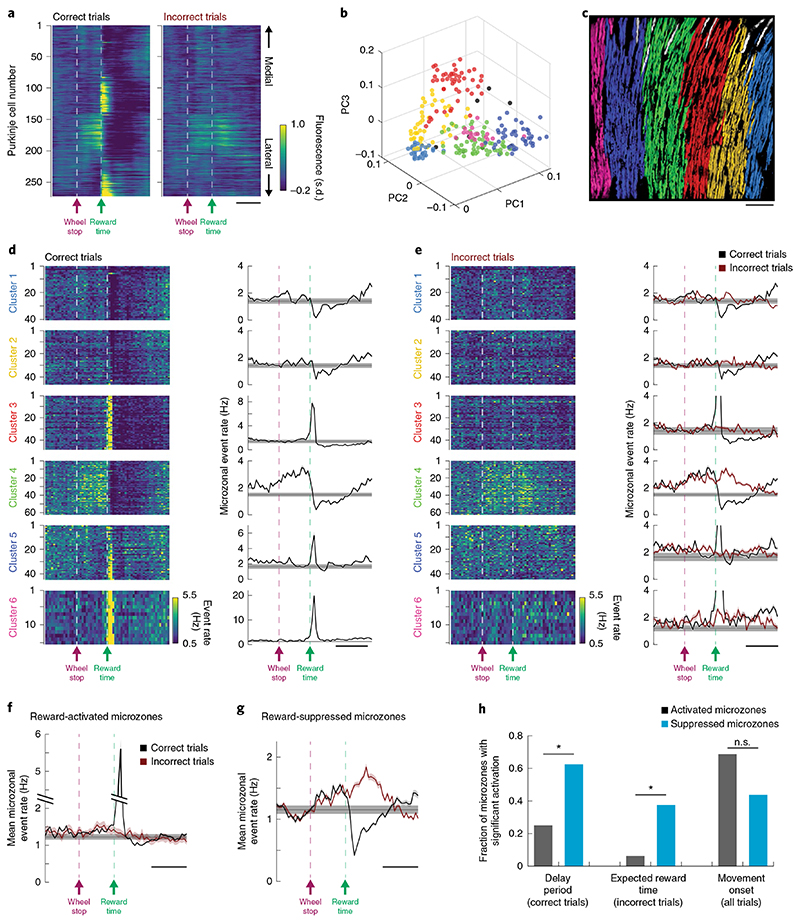
Fig. 2. Reward-activated and suppressed Purkinje cells segregate to distinct cerebellar microzones.
a, Trial-averaged Ca2+ responses in example FOV aligned to reward delivery on correct trials (left) or reward delivery time on incorrect trials (right), sorted anatomically from medial to lateral and warped by the local curvature of our recorded Purkinje cell ROIs. Scale bar, 500 ms. b, PCA projection of z-scored Purkinje cell dendritic Ca2+ activity (spontaneous activity only) onto first three components. Individual ROIs are colored on the basis of k-means clustering (k = 6) of neuronal projections onto first six principal components (p = 6). Outlier ROIs shown in gray; n = 273 neurons. c, Anatomical mapping of functionally identified clusters. Colors correspond to those in b (outlier ROIs shown in gray). Scale bar, 100 μm. d, Trial-averaged dendritic events plotted separately for neurons within a cluster (left) and as the average microzone response (right) on correct trials. Data in right panels are shown as mean ± s.e.m. across trials. Group correspondence is denoted by color of y axis labels. e, Same as d for incorrect trials. Note that correct trial responses are cropped to better illustrate responses on incorrect trials. f, Time course of mean microzonal event rates on correct trials (black) and incorrect trials (red) for reward-activated microzones. Data are shown as mean ± s.e.m. across microzones (n = 16 microzones, 6 mice). g, Same as f for reward-suppressed microzones (n = 16 microzones, 6 mice). d–g, Scale bars, 500 ms. Gray bars indicate mean ± 2 s.d. of baseline event rate. h, Fraction of reward-activated (gray) and reward-suppressed (cyan) microzones that show elevated activity during the delay period on correct trials (assessed using two-sided Wilcoxon signed-rank test with Bonferroni correction), at the time of expected reward on incorrect trials (assessed using two-sided Wilcoxon signed-rank test with Bonferroni correction) and during the movement onset period on all trials (assessed using two-sided Wilcoxon signed-rank test). Statistical significance between proportions was assessed using a Chi-squared test (n = 16 reward-activated and 16 reward-suppressed microzones, significance values for individual comparisons: delay period (correct trials), P = 0.03; expected reward time (incorrect trials), P = 0.03; movement onset (all trials), P=0.15). Statistics summary: n.s.,not significant, *P<0.05.
We used our microzonal groupings to ask how functionally related groups of Purkinje cells encoded reward-related activity in their complex spiking patterns. Most Purkinje cells within a given microzone exhibited similar patterns of reward-related activity (Fig. 2d) and microzones segregated into two groups—those that increased their activity on reward delivery (‘reward-activated’, Fig. 2d, Clusters 3, 5 and 6 and Fig. 2f) and those that decreased their activity on reward delivery (‘reward-suppressed’, Fig. 2d, Clusters 1, 2 and 4 and Fig. 2g). Across our six FOVs, we found an equal proportion of reward-activated and reward-suppressed microzones (16 of each). These reward-related groupings were not strictly related to movement onset-related activity, with both reward-activated (11 of 16) and reward-suppressed (7 of 16) microzones showing significant activation at the time of movement onset (assessed across trials for intervals −300–0 ms before movement onset and compared to baseline firing rates; Wilcoxon signed-rank test). We also aligned a subset of our FOVs (four of six) to coarser anatomical maps of the cerebellar surface. These coarse maps showed a gross level of stereotypy between animals with alternating groups of reward-activated and reward-suppressed neurons that could contain multiple functionally identified microzones (Supplementary Fig. 4).
We next asked whether complex spikes in Purkinje cells, at the level of microzones, may encode upcoming reward predictively and whether they may signal lack of reward on incorrect trials. We found that both reward-activated and reward-suppressed microzones could exhibit elevated activity in the delay period between movement offset and reward (assessed across trials for delay intervals 0–200 ms and 200–400 ms after movement offset, and compared to interval 500 ms before movement offset; Wilcoxon signed-rank test, P<0.025 with Bonferroni correction), with a higher fraction of reward-suppressed microzones showing significant modulation (Fig. 2h). Activity during the delay period was similarly elevated on correct and incorrect trials (Fig. 2e–g), suggesting that mice used reward delivery as the ultimate signal reflecting trial outcome. Indeed, mice that licked predictively during the delay period (three of six mice) did so similarly for correct and incorrect trials. To rule out the possibility that delay-period activation was simply a reflection of this licking motor program, we correlated the level of activation in reward-predictive microzones to the level of predictive licking that each animal exhibited and found no relationship (Supplementary Fig. 5). Thus, predictive licking cannot explain the elevated activity observed during the delay period.
Subsets of activated and suppressed microzones also exhibited elevated activity in the period after expected reward time on incorrect trials (assessed across trials for post-reward intervals 100–300 ms and 300–500 ms after expected reward time compared to delay period 500–0 ms before reward time; Wilcoxon signed-rank test, P < 0.025 with Bonferroni correction), with a higher proportion of reward-suppressed microzones showing significant modulation (Fig. 2h). Thus, complex spikes in Purkinje cell populations collectively encode reward-related information in our task, including putatively predictive signals, bidirectionally modulated reactive signals and error-like signals associated with lack of reward on incorrect trials.
Predictability modulates reward-related sensory responses in trained mice
In some experiments, mice were occasionally provided with random rewards during inter-trial intervals of the motor task to maintain their motivation. When we analyzed these experiments and compared reward-related responses during the task to those given randomly during inter-trial intervals, we noticed that random rewards triggered significantly larger responses than rewards earned during correct trials of the task (Supplementary Fig. 6). We reasoned that this difference may reflect an expectation-dependent modulation of the reward-related sensory cue (solenoid sound), similarly to the suppression of climbing fiber responses to predicted periocular air puffs during eye-blink conditioning 33 .
To test this directly, most of the mice in our study (five of six mice from Fig. 2) were trained to perform the motor task with interleaved random or tone-cued rewards on a subset (10% each) of inter-trial intervals (Fig. 3a). Thus, we could compare how climbing fiber inputs to Purkinje cells convey information about random (not predictable), operant and tone-cued (fully predictable) rewards (Fig. 3b). Consistent with tone-cued rewards being more predictable (and carrying a greater degree of expectation) than operant rewards, all mice exhibited greater predictive licking during the delay between the tone cue and reward than during the delay between a correctly executed operant trial and reward (Supplementary Fig. 7). Predictive lick was, by definition, not present in the random reward condition. The level of reward predictability had a clear influence on reward-related sensory responses: random reward evoked the largest signals, operant rewards evoked signals of intermediate size and tone-cued rewards exhibited strong suppression of the sensory response typically associated with reward delivery (Fig. 3c,d). To further validate that reward predictability exerted a suppressive effect on reward-related sensory signals, we also analyzed data from two mice that were trained on an easier version of our task, where all vigorous wheel movements toward the midline produced a correct trial and were rewarded (see Methods). In these mice, we found that reward responses were suppressed even more than those mice trained on our normal task (Supplementary Fig. 8).
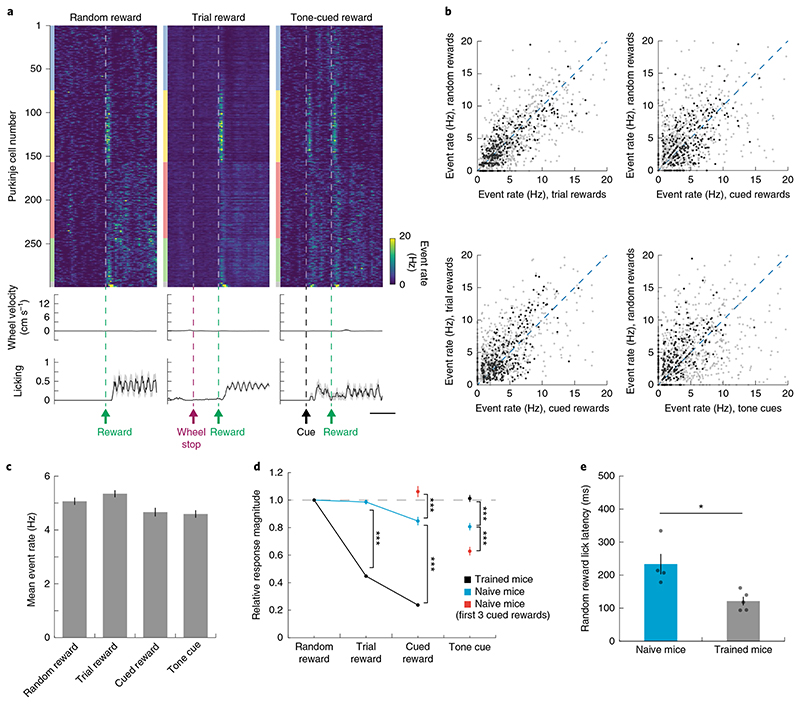
Fig. 3. Predictability modulates reward responses in trained mice.
a, Schematic of reward perturbation experiments: during each behavioral session, we randomly interspersed random rewards (10% of inter-trial intervals) or tone-cued rewards (also 10% of inter-trial intervals; 500 ms delay between cue onset and reward). b, Top: trial-averaged population response of a representative FOV (same as Fig. 2) to random, operant and tone-cued rewards. ROIs are sorted first by mediolateral position of identified microzones, then mediolaterally within each microzone. Color blocks adjacent to each heatmap denote microzonal designation, following the color scheme of Fig. 2 (gray, unclustered). Middle: trial-averaged steering wheel velocity. Bottom: trialaveraged licking. Velocity and licking are shown as mean ± s.e.m. across trials (n = 30 random rewards, 156 trial rewards and 30 tone-cued rewards). Scale bar, 500 ms. c, Scatter plots showing pairwise comparisons of response amplitude (computed as mean over 0 to +100 ms after each event) across different reward conditions; n = 891 neurons from 5 FOVs in 5 mice. Data points from representative FOV (b) are shown in darker gray. d, Cell-wise average of Purkinje cell dendritic response to each reward-related event. Data are shown as mean ± s.e.m. (n = 891 neurons from 5 FOVs in 5 mice, Kruskal-Wallis test, H = 460, d.f. = 3, P = 2×10−99, significance values for Bonferroni-corrected individual comparisons: random versus trial reward, P = 2×10−18; random versus cued reward, P = 3×10−33; trial versus cued reward, P= 0.009; trial reward versus tone cue, P = 1×10−57; cued reward versus tone cue, P= 5×10−82). e, Summary of Pearson’s correlations between pairs of reward-related events. Data are shown as box plots: center line, median; box edges, interquartile range; whiskers, range without outliers; gray points, outliers (n = 891 neurons from 5 FOVs in 5 mice, Kruskal-Wallis test, H = 237, d.f. = 3, P = 5×10−51, significance values for Bonferroni-corrected individual comparisons: random and trial reward versus random and cued reward, P= 7×10−32; random and trial reward versus trial and cued reward, P = 3×10−35; random and cued reward versus trial and cued reward, P>0.9; random and cued reward versus random reward and tone cue, P = 1×10−17; trial and cued rewards versus random reward and tone cue, P = 4×10−20). f, Time course of mean responses across reward conditions for Purkinje cells in reward-activated microzones (top, n = 361 neurons) and reward-suppressed microzones (bottom, n = 470 neurons). Scale bar, 250 ms. Note that 60 neurons were not clustered into a microzone and excluded from this analysis. Data are shown as mean ± s.e.m. Statistics summary: n.s., not significant, **P<0.01, ***P<0.001.
How does the predictability of reward alter the patterns of activity displayed by populations of Purkinje neurons? To answer this question, we computed correlations between the mean activity response vectors in each Purkinje cell over the interval 0–500 ms post-reward in our three reward conditions. Random and operant rewards triggered highly correlated activity patterns, confirming that similar subsets of Purkinje cell dendrites were activated in these two reward conditions. In contrast, the correlation between activity patterns recruited by either random or operant rewards with those recruited by tone-cued rewards was lower than between random and operant rewards (Fig. 3e), demonstrating that reward predictability modulated these responses in a similar manner. To test whether representations of reward predictability varied continuously or whether they were categorically different across our reward conditions, we performed trial-by-trial analysis of the reward responses in individual neurons for trials with different amounts of predictive licking. This analysis did not show any obvious trend of greater suppression of the reward response in trials with stronger predictive licking for either tone-cued or operant rewards (Supplementary Fig. 7). Thus, while predictive licking was categorically different across our different reward conditions, it was not sufficient to explain the differences in reward responses across different reward categories.
We also analyzed responses to random and cued rewards separately for Purkinje cells in reward-activated and reward-suppressed microzones (defined during the operant motor task; Fig. 3f). We found that, on average, Purkinje cells from both groups were activated by the predictive tone cue and exhibited little modulation at the time of reward. In contrast, random reward delivery could activate not only those Purkinje cells that were activated by the reward cue in the operant task, but also Purkinje cells that were suppressed by operant rewards. Thus, the level of predictability exerts a bidirectional influence on reward-related activity across Purkinje cell populations, modulating responses when there is ambiguity in the outcome and remaining neutral when there is no ambiguity.
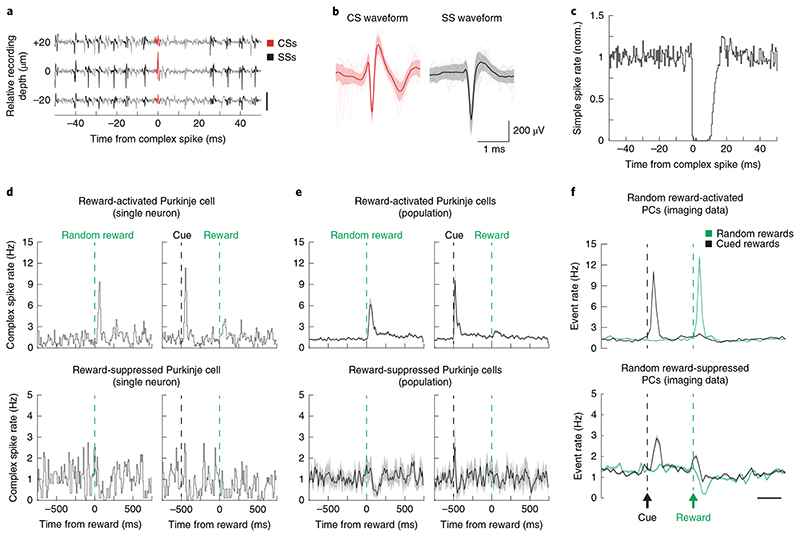
To validate that the reward-related modulation of Purkinje cell dendritic calcium signals does indeed reflect modulation in complex spiking and not some other process (for example, modulation of dendritic calcium signals by molecular layer interneurons 34 ), we complemented our imaging experiments with direct electrophysiological recordings of complex spikes in Purkinje cells (Fig. 4a) using Neuropixels probes. We performed these experiments in a minimal behavioral task in which we presented mice with tone-cued and random rewards (without a motor task), and processed the electrophysiological recordings using automated spike sorting methods combined with post hoc manual curation (Supplementary Fig. 9 and see Methods). Recordings from Purkinje cells were readily identifiable by a range of criteria, including the presence of complex spikes (Fig. 4b, left) and high-frequency simple spikes (Fig. 4b, right), which exhibited characteristic pauses in firing after complex spikes (Fig. 4c). Most recorded neurons (56/61 cells, n = 3 mice) exhibited an increase in complex spikes on delivery of random rewards (Fig. 4d). In agreement with our imaging experiments, the response to tone-cued rewards in these Purkinje cells was significantly suppressed (Fig. 4e,f, top). Furthermore, the minority of Purkinje cells in our recordings that exhibited suppressed complex spike response to random rewards also were activated by the tone cues and exhibited minimal modulation at reward time when rewards were cued (Fig. 4e,f). Thus, the results of our imaging experiments are highly consistent with those observed using direct electrophysiological recordings of complex spikes.
Fig. 4. Electrophysiological recordings of complex spikes during cued and random reward presentation.
a, Example raw traces (gray) recorded on three adjacent vertically consecutive sites (20 μm vertical separation) of a Neuropixels probe within a Purkinje cell layer. The simple spikes and complex spikes of a single Purkinje cell are highlighted in black and red, respectively. Scale bar, 500 μV. Several other Purkinje cells were identified in this recording but are not highlighted. b, Examples of waveforms of complex spikes (CS waveform, left) and simple spikes (SS waveform, right) recording using Neuropixels probes (same recording as in a). Each panel shows detected spike waveform (mean ± s.d.) and 20 overlaid raw traces. c, Normalized histogram of simple spike firing rate (same neuron as a and b) aligned to time of complex spikes, demonstrating the characteristic post-CS pause. d, Peristimulus time histogram (bin size = 10 ms) of complex spikes in example units that were activated (top) and suppressed (bottom) by random reward delivery on random reward trials (left) and cued reward trials (right); n = 146 random rewards and 154 cued rewards. e, Same as d but for all recorded units that showed activation (top, n = 56 neurons from three mice) and suppression (bottom, n = 5 neurons from three mice) to random reward delivery. Data are shown as mean±s.e.m. f, Random and tone-cued reward responses (imaging data) in Purkinje cells (PCs) activated by random reward (top, n = 280 neurons, 236 of 361 from trial reward-activated microzones and 44 of 470 from trial reward-suppressed microzones) and Purkinje cells suppressed by random reward (bottom, n = 273 neurons, 28 of 361 from trial reward-activated microzones and 245 of 470 from trial reward-suppressed microzones). Modulation of individual Purkinje cells was assessed by comparison of response in post-reward period (33–133 ms post-reward) to pre-reward withhold period (1 s). Scale bar, 250 ms. Data are shown as mean ± s.e.m.
Modulation of reward-related responses develops with training
Reward expectation must, by definition, be associated with the development of trained behavior and expectation signals should be absent in naïve mice. To test this, we analyzed recordings from the first day of training, when mice could begin to form associations between rewards and the tone cues, wheel turns or solenoid clicks that preceded rewards. Naïve mice learned to lick to rewards over the course of this first session, but the majority of ‘within-trial’ rewards on this first day were given as auto-rewards (see Methods). In naïve mice, reward delivery evoked dendritic calcium events in Purkinje cells that were similar across all conditions (Fig. 5a–c). We observed the development of suppression of tone-cued rewards even on this first day of training. While random and within-trial rewards evoke similar responses, the response to tone-cued rewards was slightly reduced when averaging all tone-cued rewards (on average, ten) given on this first day. However, when we analyzed only the first three tone-cued rewards given in each session, we saw no difference in the response when we compared them to random rewards (Fig. 5d). Thus, the suppression of responses for predictable rewards was learned and could develop rapidly during training. To further support the idea that mice learned to associate task parameters and reward, we compared the latency to the first lick for random rewards in naïve and trained mice. The lick latency in trained mice was significantly shorter than in naïve mice, consistent with a learned association that developed with training (Fig. 5e).
Fig. 5. Modulation of reward-related responses develops with training.
a, Top: trial-averaged population response of a representative FOV (same as Fig. 2) to random, operant and tone-cued rewards in naïve mice (first training session). ROIs are sorted first by mediolateral position of identified microzones, then mediolaterally within each identified microzone. Color blocks adjacent to each heatmap denote microzonal designation, following the color scheme of Fig. 2 (gray, unclustered). Middle: trial-averaged steering wheel velocity. Bottom: trial-averaged licking. Velocity and licking are shown as mean ± s.e.m. across trials; n = 8 random rewards, 70 trial rewards and 10 tone-cued rewards. Scale bar, 500 ms. b, Scatter plots showing pairwise comparisons of response amplitude (computed as mean over 0 to + 100 ms after each event) across different reward conditions; n = 1,187 neurons from 5 FOVs in 5 mice. Data points from a representative FOV (a) are shown in darker gray. c, Cell-wise average of Purkinje cell dendritic response to each reward-related event, pooled over the same 1,187 cells in 5 mice. Data are shown as mean ± s.e.m. d, Relative response magnitude in neurons responsive to random reward (mean response over 0–100 ms after random reward >2 s.d. above baseline) in trained (black), naïve mice (whole first session, cyan) and naïve mice (first three trials only, red). Data are shown as mean ± s.e.m.; n = 400 neurons (of 891) in trained mice and n = 710 neurons (of 1,187) in naïve mice (Kruskal-Wallis test, H = 1857, d.f. = 11, P<1×10−99, significance values for Bonferroni-corrected individual comparisons: trained versus naïve mice (trial reward), P = 1×10−73; trained versus naïve mice (cued reward), P = 1×10−59; trained versus naïve mice (tone cue), P = 3×10−15; naïve mice (all trials) versus naïve mice (first three trials) (cued reward), P = 2×10−91; naïve mice (all trials) versus naïve mice (first three trials) (tone cue), P = 7×10−43). e, Comparison of latency to first lick in trained mice (gray) and naïve mice (cyan). For naïve mice, trials in which mice did not produce a lick to reward delivery (typically the first 5–10 rewards) were excluded (n = 5 trained mice and 4 naïve mice; licks were not registered for one naïve mouse). Data are shown as mean ± s.e.m., P = 0.02 (two-side Wilcoxon rank-sum test). Statistics summary: *P<0.05, ***P<0.001.
Fictive reward on operant trials triggers error signals across microzones
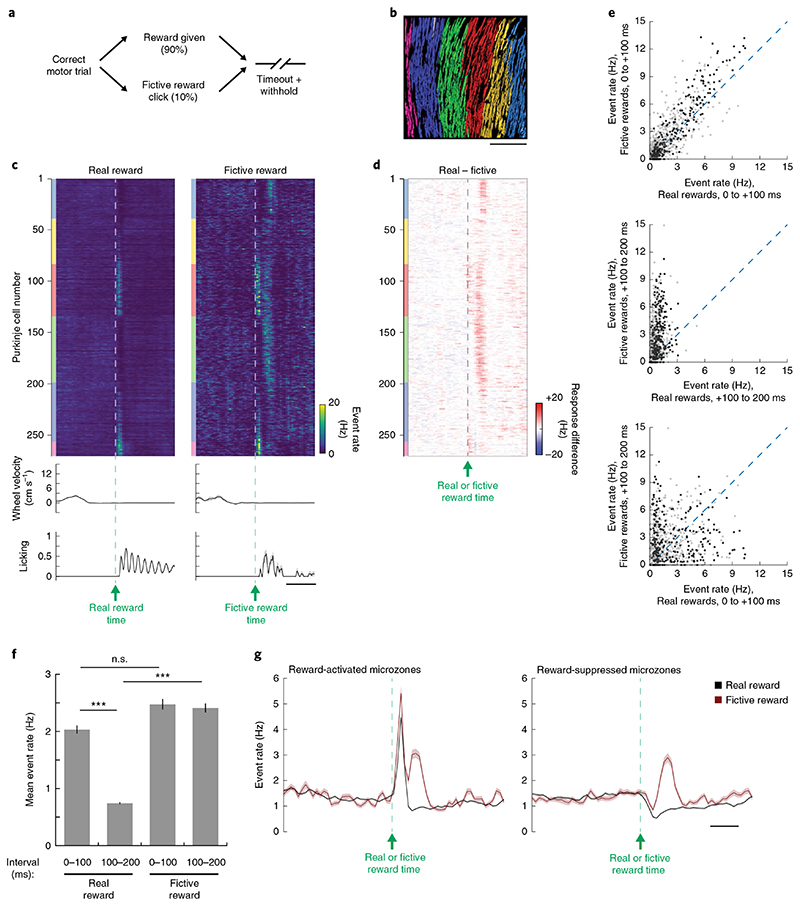
We next tested how omission of reward could produce an error response, similar to that observed at reward time on incorrect motor trials and to the reward-omission response recently reported by Heffley and colleagues 15 . We took advantage of the fact that the solenoid valve-associated sensory cue that was audible at reward time represents the most immediate signal that reward would be delivered across reward conditions in our task. We introduced perturbation trials on 10% of correct trials in our motor task in which we triggered an identical solenoid valve to the one that normally delivered our reward but was not coupled to reward: that is we gave a fictive reward (Fig. 6a). In five of our six mice, we recorded from FOVs that showed reward-related activity (Fig. 6b compared to Fig. 2c) and measured the differences in neural activity and behavior between real and fictive reward presentation (Fig. 6c,d). Responses on trials with real and fictive reward were similar during the pre-reward delay period and the immediate post-reward period (Fig. 6c–g), demonstrating that mice could not distinguish between the sound of real and fictive reward. However, Purkinje cells exhibited strong activation in the later post-reward period (+100 to +200 ms) 15 , presumably when mice realized the lack of reward delivery (Fig. 6c–f). This reward-related error signal was present across our two groups of Purkinje cells (Fig. 6g). Thus, reward-related error signals transcend microzone boundaries: both reward-activated and suppressed microzones can convey these signals.
Fig. 6. Fictive rewards on operant trials trigger error signals across microzones.
a, Schematic of fictive reward experiments: on 10% of correct motor trials, correct trials triggered a second solenoid that mimicked the reward sound. b, Anatomical mapping of functionally identified microzones from an example FOV (same as Fig. 2 on different recording day). Outlier ROIs shown in gray. Scale bar, 200 μm. c, Top: population response heatmap (trial-averaged events) of FOV from b to real (left) and fictive (right) rewards. ROIs are sorted first by mediolateral position of identified microzones, then mediolaterally within each identified microzone. Color blocks adjacent to each heatmap denote microzonal designation, following the color scheme in b. Middle: trial-averaged steering wheel velocity. Bottom: trial-averaged licking. Velocity and licking are shown as mean ± s.e.m. across trials; n = 141 real rewards and 16 fictive rewards. Scale bar, 500 ms. d, Mean difference image (smoothed over three frames) comparing responses to real and fictive rewards. e, Pairwise comparisons of reward-related responses at different time intervals after delivery of real and fictive rewards. Data pooled from 832 Purkinje cell dendritic ROIs from 5 FOVs in 5 mice (1 FOV per mouse). Data points from a representative FOV (b) are shown in darker gray. f, Cell-wise average of Purkinje cell dendritic response to each reward-related event. Data are shown as mean ± s.e.m. (n = 832 neurons from 5 FOVs in 5 mice, Kruskal-Wallis test, H = 333, d.f. = 3, P = 8×10−72, significance values for Bonferroni-corrected individual comparisons: real reward (0–100 ms) versus real reward (100–200 ms), P = 3×10−40; real reward (0–100 ms) versus fictive reward (0–100 ms), P = 0.7; real reward (100–200 ms) versus fictive reward (100–200 ms), P = 4×10−56). g, Time course of mean responses on real reward trials (black) and fictive reward trials (red) for Purkinje cells in reward-activated microzones (left, n = 368 neurons) and reward-suppressed microzones (right, n = 405 neurons). Scale bar, 250 ms. Note that 59 neurons were not clustered into a microzone and excluded from this analysis. Data are shown as mean ± s.e.m.; n.s., not significant, ***P<0.001.
Feedback error signals caused by omission of tone-cued reward
Modulation of reward-related activity on tone-cued rewards is drastically reduced in trained animals. Given the cerebellum’s crucial role in motor timing 3,12,33,35 , we reasoned that mice may learn the delay interval between the cue and reward for tone-cued rewards and wondered how violations of this expectation would be represented in the climbing fiber input to Purkinje cells. To test this directly, we introduced tone cues in our task that were not followed by rewards (Fig. 7a). To obtain enough repetitions for each condition, we altered the likelihood of the reward event types during inter-trial intervals of our operant task, such that 30% of intervals contained a cued reward and 10% of intervals contained a cue but no reward (3:1 reward-to-omission ratio) and recorded from the same five mice subjected to fictive reward (Fig. 7b). In two of these mice, we also recorded video of orofacial movements during these experiments (Supplementary Fig. 10). Cued omission of reward evoked responses in many Purkinje cells (Fig. 7c,d) at the time of expected reward (computed over 0–200 ms post expected reward, Fig. 7e,f). These error signals were related to the expectation based on the tone cue, because we omitted any sensory signal at the time of the reward itself. Responses to the tone cue were similar for rewarded and unrewarded cues (Fig. 7e,f). We again asked if Purkinje cells defined as reward-activated and reward-suppressed in our motor task encoded this reward omission differently and found that, as for our analysis of incorrect trials during the motor task, these error responses were expressed more strongly (but not exclusively) by neurons in reward-suppressed microzones (Fig. 7g). We also validated that these omission responses were present in our electrophysiological recordings of Purkinje cells by omitting cued rewards in our simple conditioning task. We found neurons with significant increases in complex spike rates at expected reward time (Fig. 7h), confirming that omission-related error signals identified in our imaging experiments are reflected in the underlying complex spike patterns of Purkinje cells.
Fig. 7. Omission of cued rewards triggers feedback error signals.
a, Schematic of cued reward omission: during each behavioral session, we randomly interspersed random rewards as in previous experiments (10% of inter-trial intervals), tone-cued rewards (30% of inter-trial intervals) or tone cues with reward omitted (10% of inter-trial intervals). b, Anatomical mapping of functionally identified microzones from an example FOV (same as Fig. 2 but on a different recording day). Outlier ROIs shown in gray. Scale bar, 200 pm. c, Top: trial-averaged population response of a representative FOV to tone-cued rewards and omissions. ROIs are sorted first by mediolateral position of identified microzones, then mediolaterally within each identified microzone. Color blocks adjacent to each heatmap denote microzonal designation. Middle: trial-averaged steering wheel velocity. Bottom: trial-averaged licking. Velocity and licking are shown as mean±s.e.m. across trials; n = 59 cued rewards and 20 cued omissions. Scale bar, 500 ms. d, Mean difference image (smoothed over three frames) comparing responses to real and fictive rewards. e, Pairwise comparisons of reward-related responses at different time intervals after delivery of real and fictive rewards. Data pooled from 765 Purkinje cell dendritic ROIs from 4 FOVs in 4 mice (1 FOV per mouse). Data points from a representative FOV (b) are shown in darker gray. f, Cell-wise average of Purkinje cell dendritic response to each reward-related event measured over interval 0–200 ms after each event. Data are shown as mean ± s.e.m. and statistical significance between cued rewards and cued omissions was assessed using the two-sided Wilcoxon signed-rank test (n = 765 neurons from 4 FOVs in 4 mice, P = 2×10−42). g, Time course of mean event rates (from imaging experiments) on real reward trials (black) and fictive reward trials (red) for Purkinje cells in reward-activated microzones (left, n = 349 neurons) and reward-suppressed microzones (right, n = 362 neurons). Note that 54 neurons were not clustered into a microzone and excluded from this analysis. h, Time course of mean complex spike rates (from electrophysiology experiments) on real reward trials (black) and fictive reward trials (red) (n = 7 neurons from 3 mice). Electrophysiological complex spike recordings were acquired without a motor task. g,h, Scale bars, 250 ms. Data are shown as mean ± s.e.m. (P=0.02, two-sided Wilcoxon signed-rank test). Statistics summary: *P<0.05, ***P<0.001.
Discussion
The nature and variety of signals conveyed to Purkinje cell populations by climbing fibers has been vigorously debated. This debate has centered on whether climbing fibers carry feedback error signals or timing signals to sculpt ongoing and future actions 36 . Here we show that when mice learn to associate multiple parameters— operant wheel movements, tone cues and solenoid clicks—with reward, this reward context is encoded in climbing fiber input to Purkinje cells. Specifically, climbing fiber signals encode parameters related to internally generated expectations, namely those relating to reward expectation, delivery and evaluation. In this way, the cerebellum can use all relevant signals—be they self-generated or sensed—to make predictions about the future, evaluate these predictions and relay them to the rest of the brain.
Microzonal organization of reward signals in Purkinje cells
Our results demonstrate that climbing fiber inputs signal reward bidirectionally, as activation and suppression, via distinct but adjacent groups of microzones 7,32 . Purkinje cells in microzones that were suppressed by reward delivery were more likely to exhibit reward-predictive activity, while Purkinje cells that exhibited reward-related sensory responses exhibited expectation-dependent modulation of these responses. However, when reward was expected but not delivered, both groups could exhibit error signals in response to this violated expectation. Notably, these error signals were strongest (that is, strongly engaged in both reward-activated and reward-suppressed microzones) in our fictive reward condition, when mice both made the correct action and were provided with the reward-associated sensory signal, and less prominent on incorrect motor trials and when tone-cued rewards were omitted. Mice also made larger, less stereotyped orofacial movements on omission of expected reward, presumably in search of the reward they were expecting. The generality of these error signals, which manifest both on a neural and behavioral level, suggests that when expectations are violated, climbing fibers may be activated in a heterogeneous manner to destroy previously created associations, since the outcomes of these expectations were not fulfilled.
Learned, temporally specific suppression of sensory responses to predictable rewards
The degree of predictability of upcoming reward exhibited a profound influence on reward-related signals in our trained mice. The greater the likelihood of upcoming reward, the greater the suppression of responses to reward. Reward delivery elicited large climbing fiber responses in Purkinje cells when reward was delivered randomly, moderate responses when reward was delivered in a motor trial context in which success was not guaranteed and virtually no responses when reward was cued with a fully predictive tone. These reward-related expectations developed with training: responses to reward were similar at the very beginning of training and suppression of predictively reward-related signals developed rapidly (during the first training session for fully predictable rewards). The mechanism of this suppression is unclear, but a potential source may be the cerebellum itself, whose output could exert either an indirect excitatory or direct inhibitory influence over the inferior olive 37 . These expectation signals were also temporally specific: omission of reward on tone-cued trials evoked omission-related activation of Purkinje cells specifically at the time of expected reward.
Relationship between reward-related signals in the two input streams to Purkinje cells
Cerebellar granule cells have recently also been shown to encode reward 26 , presumably driven by mossy fiber input of unknown origin. Assessing the similarity of these granule cell signals with reward-related climbing fiber signals will require a careful comparison of the reward contingencies of these signals, ideally using the same behavioral task. Specifically, a spatial organization of reward-related signals (Fig. 2a–d) and activity suppressed by reward (Fig. 2g) have not yet been observed in granule cells. If the granule cell and climbing fiber-mediated reward signals indeed exhibit similar behavioral contingencies, it will be interesting to examine whether these signals converge on the same Purkinje cells, as might be expected from microzonal functional organization 7 . Simultaneous encoding of reward-related signals by granule cell and climbing fiber inputs to Purkinje cells parallels the acquisition of predictive signals in these inputs during delayed eye-blink conditioning 33,38 . Robust representation of reward signals in these two input pathways, which can drive plasticity mechanisms in Purkinje cells, may be crucial for the role of the cerebellar cortex in guiding learned behavior.
Relationship with reward signals elsewhere in the brain
Our data highlight the diversity of information about reward expectation and delivery provided by climbing fiber inputs to Purkinje cells. Reward-related complex spike responses are inversely scaled by reward predictability in both reward-activated and reward-suppressed microzones, consistent with temporal-difference prediction error models 39 invoked in studies of the midbrain dopaminergic system 40,41 and for Purkinje cells during eye-blink conditioning 33 . In this framework, unexpected stimuli should evoke stronger responses than predictable ones. However, in contrast to the predictions of temporal-difference models, in which neurons activated by reward delivery would be suppressed by omission of reward (and vice versa), we observed that reward omission was signaled as an increase in the climbing fiber input in both reward-activated and reward-suppressed Purkinje cells 15 .
The ramp-like increase in climbing fiber activity observed in some Purkinje cells in anticipation of reward (Fig. 2d–h) represents a non-canonical mode of firing for climbing fibers, which typically have been reported to exhibit brief changes in firing rates locked to sensory and motor events. The mechanism of this steady activation is not clear, but it may reflect a change in excitability of olivary neurons triggered by descending inputs from the cerebellum itself 37,42–44 . These patterns of activation are similar to those of GABAergic neurons in the ventral tegmental area 41 and serotonergic neurons in the dorsal raphe nucleus 45 , which progressively increase their activity in anticipation of upcoming reward.
Understanding how cerebellar circuits engage with processing of reward in other parts of the brain is an important avenue for future research. The afferent inputs to the inferior olive arise from a variety of cortical and subcortical sources 37,46 . Cerebellar outputs target the midbrain dopaminergic system 47 and can influence both premotor 48,49 and basal ganglia 50 circuits via the thalamus. Thus, the olivocerebellar system may interact with the canonical reward circuitry of the brain through these reciprocal connections. Overall, our findings lend further support to the idea that the cerebellum coordinates with the rest of the brain to process a range of cognitive functions.
Methods
Animals
All animal procedures were approved by the local Animal Welfare and Ethical Review Board at University College London and performed under license from the UK Home Office in accordance with the Animals (Scientific Procedures) Act 1986. We used male Pcp2(L7)-Cre mice (line Jdhu—B6.Cg-Tg(Pcp2-Cre)3555Jdhu/J) 51 aged between three and six months. Male mice were preferred in our task because they were larger and more willing to initiate wheel movements at the beginning of training, facilitating more rapid learning in our task. Mice were group housed before surgery, single-housed after surgery and maintained on a 12/12 day-night cycle. In total, data from 12 mice (nine imaging and three electrophysiology) were used in this study.
Headplating, virus injection and chronic window installation
A minimum of 2 h before surgery, mice were injected with dexamethasone to reduce swelling during surgery. A single procedure, during which mice were maintained under 1.5–2% isoflurane anesthesia, was performed on each mouse lasting approximately 2 h to install a headplate over the cerebellar cortex, infect Purkinje cells with GCaMP6f and install a chronic window for chronic imaging experiments. Buprenorphine (1 mg kg−1, subcutaneous, Vetergesic) was administered peri-operatively for analgesia. Once mice were anesthetized, custom headplates with an oval inner opening 7 mm long and 9 mm wide were installed over the forelimb regions of the cerebellar cortex on the left side of each mouse (lobule simplex and adjacent paravermis lobules V and VI) and secured with dental cement (Super-Bond C&B, Sun-Medical). This corresponded to the posterior tip of the interparietal bone, 1.8 mm displaced from the midline (approximately 6 mm caudal and 1.8 mm lateral from bregma). Mice to be used for imaging experiments were next injected with virus and implanted with a cranial window, while mice used for electrophysiology experiments were allowed to recover at this point.
For mice used in imaging experiments, we performed a 3 mm craniotomy, centered in the middle of the headplate hole, to expose the cerebellar cortex for virus injection and window installation. We then injected Cre-dependent GCaMP6f 52 virus (AAV1.CAG.Flex.GCaMP6f.WPRE.SV40) diluted 1:12 from stock titer in three locations spanning paravermis and intermediate lobule simplex. At each location, ~100 nl of virus solution was pressure-injected at depths of 500, 375 and 250 μm below the cerebellar surface at 2 min intervals. We waited ~5 min after the final of set of three injections before retracting the injection pipette. In total, ~1 μl of diluted virus was injected per mouse. Finally, a 3 mm single-paned coverslip was press-fit in to the craniotomy, sealed to the skull by a thin layer of cyanoacrylate (VetBond) and fixed in place by dental cement. The conical portion of a nitrile rubber seal (RS Components, stock number 749-581) was then glued to the headplate with dental cement and filled with Kwik-Cast to protect the window preparation during recovery and between recording sessions. Mice were allowed to recover for a minimum of 7 days before beginning water restriction, during which time they were given post-operative analgesia as needed.
After mice had recovered from surgery, they were placed under water restriction for at least 5 days during which time they were acclimated to the recording setup and expression-checked. All mice were maintained at 80–85% of their initial weight over the course of recording experiments. Trained mice typically received all their water for the day from rewards during the behavioral task, while naïve mice were supplemented to 1 g water per day with Hydrogel.
On the day of electrophysiology experiments, a small craniotomy (<1 mm diameter) was performed over the proximal part of lobule simplex under brief anesthesia (<20 min), a nitrile rubber seal was affixed to the headplate to act as a recording chamber and the chamber was filled with Kwik-Cast. Mice were allowed to recover for >2 h before experiments began.
Behavior
Motor task training protocol
Mice were head-fixed in front of an array of three monitors with screens arranged at 135° relative to each other and the central screen directly in front of the mouse (creating three sides of an octagon). Below their forepaws was a Lego rubber tire that could be rotated left and right and whose angle was measured using a rotary encoder coupled to the wheel’s axle. We used the MATLAB-based software ViRMEn 53 to construct and operate the virtual reality environment. The rotation of the steering wheel translated the virtual object (a revolving black and white beach ball) displayed on the screens during each operant motor trial.
Mice were initially trained to translate the virtual object, which appeared in the middle of either the left or right screens (at +45° or −45°), toward the visual midline to receive a reward (inspired by the visual decision-making task of Burgess and colleagues 54 ), at a high wheel gain (9° per mm wheel rotation). On the first few days of training, the virtual object drifted toward the midline and triggered an auto-reward after a long delay (60–180 s). These auto-reward sessions were useful to allow mice to make the initial associations necessary to perform the more difficult versions of the task. The data from naïve mice shown in Fig. 5 come from the first day of these auto-reward sessions. After several days (~1 week of training), mice learned to make wheel turns on their own accord to receive rewards. At this point, they were switched to a unilateral version of the task (left trials only) and increased the difficulty in multiple steps. We decreased the gain to 6° per mm and rewarded all trials in which the mice moved the object past the visual midline. This simplified task version facilitated training mice to react rapidly to object appearance and to make vigorous movements. The data shown in Supplementary Fig. 8 come from this task version. We then made the task slightly harder by decreasing the gain to 2.25° per mm, so that mice had to make more than one movement (typically two) to get the wheel to the target region (±15° from the visual midline) and only reward trials in which the object was left unmoved in the target region for 500 ms. After mice learned to do this consistently (on >70% of trials), we analyzed the wheel movements for each mouse and identified a gain for each mouse that was most likely to produce a correct trial in a single movement—defined as one where the wheel is stopped in the target region for 100 ms. The mean gain across the mice used in this study was 3.3 ° per mm corresponding to a 13.6 mm translation of the wheel to hit the center of the target (range 2.5–4° per mm). On the first day that mice were trained on this final task version, their performance was 30–50% and plateaued at ~60% after about 1 week of training on this final task version. All recordings in ‘trained’ mice were performed after behavior had plateaued.
Rewards on correct trials consisted of ~3 μl of a sugar water solution (5% sucrose) and were delivered through a solenoid valve (NResearch, part number 225PNC1-21) whose click was audible to the mouse. Reward delivery on correct motor trials was delivered 400 ms after trial evaluation (500 ms after the wheel stopped moving) and were followed by short (0–2 s) timeout, while incorrect motor trials were followed by a long (5–7 s) timeout. After completion of the timeout, a variable withhold period (1.5–2.5 s) was enforced, in which time mice were obligated to not lick or turn the steering wheel. Licks were detected using an electrical lick circuit 55 . As indicated in the main text, random or tone-cued reward were administered on the completion of these withhold periods. Tone cues for reward trials consisted of a 100 ms long, 4 kHz tone followed by 400 ms of silence before reward delivery. The timing of these cued reward was designed to mimic those of the operant motor rewards, which required the wheel to be stopped for 100 ms to trigger a reward 400 ms later (same 500 ms total delay). Random rewards were given immediately on the completion of the withhold period. In all mice used for the analyses in this study, random and tone-cued reward were included throughout training with 10% probability of each extra reward type being given on any single inter-trial interval, except in perturbation experiments as indicated. Behavioral parameters and task-related triggers were fed back to the virtual reality system through an Arduino and National Instruments DAQ card (NI USB-6212).
Pavlovian conditioning protocol
Mice used for Neuropixels electrophysiology experiments were trained on a Pavlovian conditioning protocol consisting of an equal mixture of cued and random rewards during training (nine training sessions). The same tone cues, timing intervals and solenoid valves were used for these experiments as for the tone-cued and random reward imaging experiments. On the day of recording (session 10), mice were presented with 50 baseline trials of cued and random rewards (equal probability), after which 20% of rewards were randomly omitted.
Data acquisition
Two-photon calcium imaging
Imaging experiments were performed through a 16×/0.8 NA objective (Nikon) using a Sutter MOM microscope equipped with the Resonant Scan box module. A Ti:Sapphire laser tuned to 930 nm (Mai Tai, Spectra Physics) was raster scanned using a resonant scanning galvanometer (8 kHz, Cambridge Technologies) and images were collected at 512 × 512 pixel resolution over FOVs of 670 μm × 670 μm at 30 Hz. Sample plane power used for recordings ranged from 30 to 70 mW and recordings were performed midway between the pial surface and the Purkinje cell body layer, at depths of ~75 μm. The microscope was controlled using ScanImage (v.2015, Vidrio Technologies) and tilted to ~10° so that the objective was orthogonal to the surface of the brain and coverglass. Blood vessel landmarks were used to approximately find the same FOV across imaging sessions and fine scale adjustments were made to maximize day-to-day overlap by taking short imaging movies (10 s) and aligning them to the previous day’s recordings.
Electrophysiological recordings
Electrophysiological recordings were made using Neuropixels (‘Phase 3A’) electrode arrays 56 mounted on a custom three-dimensionally printed plastic piece and affixed to a three-axis micromanipulator with one axis tilted to be perpendicular to stereotaxic coordinates in the sagittal plane. This manipulator axis was used to lower the probe into the cerebellum at ~8 μm.s−1 to a final depth of ~3 mm. Electrodes were allowed to settle for a minimum of 20 min before beginning experiments. Signals were recorded from the distal 384 channels (covering ~3.84 mm of linear distance). Recordings were made in external reference mode with gain of 500 for the action potential band (300 Hz high-pass filter) and acquired at 30 kHz using SpikeGLX software (http://billkarsh.github.io/SpikeGLX/). Electrodes were coated with a lipophilic dye (DiI) to facilitate histological identification of electrode tracks.
Video analysis of orofacial movements
Frontal video of mice on omission trials was recorded at 100 Hz using an Allied Vision Mako U-130B camera. To analyze orofacial movements, the brightness of a region of interest surrounding each mouse’s mouth (~4 × 8 mm) was averaged, baseline-subtracted (eighth percentile of a 2 s rolling average surrounding each data time point) and aligned to behavior. Because the mice tongues appeared bright in these videos, we could use the brightness value at each time point as a proxy for tongue movements.
Anatomical mapping and histology
The anatomical maps shown in Supplementary Fig. 4 were made by taking tiled z-stacks of the exposed portions of the cerebellum (in live mice) and stitching them to create a panoramic image of the cerebellar cortical surface. Imaging FOVs were manually aligned to these reference images.
For histological experiments requiring post-mortem histology, mice were deeply anesthetized with ketamine/xylazine then transcardially perfused with PBS then 4% paraformaldehyde in PBS. Brains were removed and post-fixed overnight in 4% paraformaldehyde in PBS.
Data analysis
Extraction of Purkinje cell dendritic ROIs and identification of putative complex spikes
ROIs corresponding to single Purkinje cells were extracted using a combination of Suite2p software in MATLAB 57 for initial source extraction and custom-written software to merge over-segmented dendrites. For each recorded FOV, we identified individual dendrites using the following protocol:
-
(1)
After running initial segmentation using Suite2p, all dendritic segments corresponding to a fluorescent portion of a Purkinje cell dendrite in the mean fluorescence image were selected for further processing using Suite2p’s built-in user interface.
-
(2)
Correlations of the baseline-subtracted (eighth percentile of a 2 s rolling average surrounding each data time point) fluorescence traces of all selected dendritic segments were computed. Dendritic segments that did not exhibit correlations above 0.5 with any other dendritic segments were classified as unique Purkinje cell ROIs.
-
(3)
The dendritic segments that did exhibit correlations above 0.5 with any other segment were classified into non-redundant groups. These groups were then visualized in a custom-written MATLAB graphical user interface that displayed each segment in a different color and overlaid it and its correlated partners on the mean fluorescence image. Segments that originated from the same single dendrite (that is, had highly correlated fluorescence traces and were aligned in the axis of Purkinje cell dendrites) were merged. A weighted average of the fluorescence trace of each group of merged dendritic segments was computed on the basis of the number of pixels in each segment.
An event detection algorithm, MLspike 58 , was used to identify fast dendritic calcium transients, faithful indicators of complex spiking activity in Purkinje cells 28–30,59–61 , in each dendritic ROI. As input to MLspike, we used baseline-subtracted fluorescence traces (ΔF) to which we added the maximum value of each trace (input values near zero are problematic for MLspike). The baseline fluorescence parameter (F 0) was set as the 25th percentile of each fluorescence trace, the sampling rate (dt) was set at 1/30 (30 Hz) and the indicator decay parameter (tau) was set to 0.15. The output of MLspike is an event time, as well as an amplitude (an integer multiple of the unitary event size detected of each trace). Events detected in consecutive bins, which are very likely to reflect a large dendritic event corresponding to a single complex spike rather than multiple separate complex spikes at our imaging rates (30 Hz), were summed and binned in to the first time point of each sequence. Event amplitudes for each ROI were normalized by the mean amplitude of detected events for that ROI. The absolute event rate across all recorded Purkinje cell dendritic ROIs in this study was 1.4 ± 0.4 Hz (mean ± s.d., n = 2,854 ROIs from 13 FOVs in nine mice), consistent with previously reported rates of complex spiking during behavior 62 . Event amplitudes were converted into rates by multiplying by the imaging frequency (30 Hz), creating a complex spike firing rate weighted by event amplitude. Treating all detected complex spike events the same (that is, setting their magnitude equal to 1) produced very similar results (Supplementary Fig. 11).
Synchronization of behavior and recordings
All behavioral parameters—trial onset and offset triggers, wheel translation, reward deliveries, tone cues, virtual reality frame update times, two-photon imaging frame times and video frame times— were acquired simultaneously and digitized at 5 kHz using a National Instruments (NI USB-6212) and saved using PackIO software 63 . Subsequent analysis was conducted off-line using custom-written scripts in MATLAB (v.2017a or 2018a).
Recorded dendritic fluorescence traces and extracted events (complex spikes) were aligned to different behavioral events of interest at the first frame whose acquisition began after each event and averaged across occurrences of each of these behavioral events. Wheel movement initiation was defined as the first time on each motor trial that wheel velocity exceeded 1 mm s−1. Binary licking traces, whose value was one when the mouse’s tongue contacted the lickport and was zero otherwise, were averaged in their raw format in all plots and quantifications.
Identification of Purkinje cell microzones
Initial spatial sorting of Purkinje cells was by (1) fitting a line through the pixels comprising each ROI and using this line to create a local direction vector for each ROI, (2) binning these ROI vectors at a density of 32 × 32 pixels—creating a 16 × 16 grid from our 512 × 512 pixel images with one mean vector per square, (3) fitting local contour lines to this grid using MATLAB’s ‘streamline’ function and (4) grouping ROIs by their closest local contour line, sorting ROIs orthogonally to this contour line and concatenating groups closest to each contour line. ROIs were organized and indexed from medial to lateral, by convention. This analysis demonstrated clearly that parasagittal clusters of Purkinje cells exhibited uniformity in their responses to reward.
To systematically identify functional clusters of Purkinje cells (microzones) in our recordings, we devised the following analysis pipeline:
-
(1)
We normalized our recordings by z-scoring our baseline-normalized fluorescence data matrices and performed PCA on both our whole baseline data matrix (‘all data’) as well as just spontaneous activity obtained by concatenating the withhold periods before the start of each trial (‘spontaneous only’).
-
(2)
To determine the relevant principal component subspace in which to cluster our data, we performed 1,000 shuffles of our ‘all data’ matrix where each neuron’s activity was jittered in time over the interval ±400 ms (±12 imaging frames). We computed a mean and standard deviation of the variance explained by the principal components of these temporally jittered data and took the first n principal components of our real data whose variance explained exceeded the mean + 2 s.d. of the shuffled data. The number of principal components with significant information varied between four and seven, depending on the FOV.
-
(3)
The coefficients associated with this number of principal components (p) were used for k-means clustering of the ‘all data’ matrix and ‘spontaneous only’ matrix. The number of clusters in this p-dimensional subspace of our data was chosen programmatically using silhouette criterion values to identify the optimal number from a range 1–12. To optimize clustering, centroid positions were re-seeded 1,000 times and the solution yielding the lowest within-cluster distances was used for further analysis.
-
(4)
Identified clusters were mapped on to anatomy and all further analysis was conducted using the ‘spontaneous only’ matrix to align with the original conception of microzones, but results from the ‘all data’ matrix were used as comparison.
-
(5)The following criteria were applied sequentially to refine identified microzones:
-
(a)Clusters with fewer than five members were merged with their closest neighbor.
-
(b)Clusters with clear multipeaked spatial distributions in the mediolateral axis were split into separate clusters. To identify multipeaked distributions, the mediolateral coordinates of ROI centroids were binned at ~80 μm per bin (64 pixels per bin) and normalized to the peak bin. Secondary peaks were defined as those containing counts greater than 40% of the largest bin of the histogram.
-
(c)ROIs that were spatial outliers along the mediolateral axis of a given cluster were excluded from further analysis. These outliers were defined as having a mediolateral centroid position greater than three scaled median absolute deviations from the median mediolateral centroid of the cluster.
-
(a)
-
(6)
We sorted Purkinje cells within each cluster’s spatial as described above and also sorted microzones relative to each other on the basis of median ROI position. Thus, we sorted ROIs inside each microzone on the basis of their mediolateral position and also sorted microzones relative to each other on the basis of their mediolateral position.
Electrophysiological analysis
Data from Neuropixels recordings were automatically spike sorted with Kilosort 2 (https://github.com/MouseLand/Kilosort2) 64 and manually curated using the ‘Phy’ GUI (https://github.com/kwikteam/phy). Given the foliation of the cerebellar cortex, recordings typically yielded multiple crossings of the Purkinje cell layer and we were usually able to isolate 4–5 Purkinje cell units from each layer. Purkinje cells were identified by their characteristic electrophysiological signature 65,66 , including the presence of complex spikes and simple spikes. The rate of complex spikes was 1.4 ± 0.5 Hz (mean ± s.d., n = 61 units, three recordings from three mice). As shown in Supplementary Fig. 9, complex spikes exhibited either a narrow waveform followed by spikelets if the recording site was perisomatic, or a broader waveform when the recording site was dendritic, i.e., in the molecular layer 66 . All recording sites were confirmed by post-hoc histology, in which recording tracks (labeled with DiI coating the recording electrode) were identified in 100 μm coronal cerebellar sections in brains fixed after recording and counterstained using Neurotrace 435/455 (Supplementary Fig. 9a). Recording tracks were aligned to the Allen Mouse Common Coordinate Framework (CCF 67,68 ) using ‘Allen CCF tools’, a custom GUI for three-dimensional alignment of electrode tracks to histology 69 . Spike sorting analysis and complex spike identification were performed with the experimenter blind to task conditions. After these sorting procedures, units were aligned to behavior and grouped into reward-activated and reward-suppressed categories on the basis of responses to random reward. Units activated at reward omission were identified by inspection.
Statistical analysis
No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous publications 15,20,26 . No randomization of experimental subjects was necessary as all mice were trained and recorded under the same conditions. Behavioral events in each training session were randomized on a trial-by-trial basis within the temporal ranges and incidence rates described in the text. Data collection and analysis were not performed blind to the conditions of the experiment, but analysis relied on code that was standardized for all experimental conditions.
Categorical comparisons between proportions were made using the Chi-squared test. Data distributions were not assumed to be normally distributed and all statistical comparisons between groups of continuous variables were performed using non-parametric tests—the Wilcoxon rank-sum test and sign test were used to study differences between two groups of unpaired and paired data, respectively, and the Kruskal–Wallis test was used when more than two groups were compared. Bonferroni correction was applied for multiple comparisons. In general, 95% confidence intervals (P < 0.05) were used to define statistical significance.
Supplementary Material
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Acknowledgements
We are grateful to P. Dayan, M. Fisek, S. Tsutsumi, C. Buetfering, B. Clark, Y. Chung and the members of the Hausser laboratory for discussions and comments on the manuscript. We would like to thank N. Steinmetz for help with Neuropixels recordings, M. Pachitariu for generously providing us access to Kilosort2 before general distribution and N. Smith for illustrations. This work was supported by the Wellcome Trust (M.H., PRF 201225), ERC (M.H., AdG 695709) and EMBO (D.K., ALTF 914–2015).
Footnotes
Online content
Any methods, additional references, Nature Research reporting summaries, source data, statements of data availability and associated accession codes are available at https://doi.org/10.1038/s41593-019-0381-8.
Author contributions
D.K. and M.H. conceived the project and wrote the manuscript. D.K., M.B. and M.B.P. performed experiments and analysis.
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The custom analysis code used in this study is available from the corresponding authors upon reasonable request.
References
- 1.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 2.Kawato M, Furukawa K, Suzuki R. A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern. 1987;57:169–185. doi: 10.1007/BF00364149. [DOI] [PubMed] [Google Scholar]
- 3.Medina JF. The multiple roles of Purkinje cells in sensori-motor calibration: to predict, teach and command. Curr Opin Neurobiol. 2011;21:616–622. doi: 10.1016/j.conb.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albus JA. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 6.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 7.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–8785. doi: 10.1523/JNEUROSCI.1961-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathy A, et al. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 11.Tang T, Blenkinsop TA, Lang EJ. Complex spike synchrony dependent modulation of rat deep cerebellar nuclear activity. eLife. 2019;8:e40101. doi: 10.7554/eLife.40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 13.Ozden I, Dombeck DA, Hoogland TM, Tank DW, Wang SS. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS One. 2012;7:e42650. doi: 10.1371/journal.pone.0042650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh KK, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heffley W, et al. Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci. 2018;21:1431–1441. doi: 10.1038/s41593-018-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Gruijl JR, Hoogland TM, De Zeeuw CI. Behavioral correlates of complex spike synchrony in cerebellar microzones. J Neurosci. 2014;34:8937–8947. doi: 10.1523/JNEUROSCI.5064-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogland TM, De Gruijl JR, Witter L, Canto CB, De Zeeuw CI. Role of synchronous activation of cerebellar purkinje cell ensembles in multi-joint movement control. Curr Biol. 2015;25:1157–1165. doi: 10.1016/j.cub.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Najafi F, Giovannucci A, Wang SS, Medina JF. Coding of stimulus strength via analog calcium signals in Purkinje cell dendrites of awake mice. eLife. 2014;3:e03663. doi: 10.7554/eLife.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deverett B, Koay SA, Oostland M, Wang SS. Cerebellar involvement in an evidence-accumulation decision-making task. eLife. 2018;7:e36781. doi: 10.7554/eLife.36781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–532. doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 24.Rochefort C, et al. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–389. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- 25.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544:96–100. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KH, et al. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron. 2015;86:529–540. doi: 10.1016/j.neuron.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozden I, Sullivan MR, Lee HM, Wang SS. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29:10463–10473. doi: 10.1523/JNEUROSCI.0967-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura K, Häusser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J Neurosci. 2011;31:10847–10858. doi: 10.1523/JNEUROSCI.2525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Häusser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 2009;29:8005–8015. doi: 10.1523/JNEUROSCI.4919-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaffield MA, Bonnan A, Christie JM. Conversion of graded presynaptic climbing fiber activity into graded postsynaptic Ca2+ signals by Purkinje cell dendrites. Neuron. 2019 doi: 10.1016/j.neuron.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oscarsson O. Functional units of the cerebellum - sagittal zones and microzones. Trends Neurosci. 1979;2:143–145. [Google Scholar]
- 33.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci. 2015;18:1798–1803. doi: 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowan MJM, et al. Graded control of climbing-fiber-mediated plasticity and learning by inhibition in the cerebellum. Neuron. 2018;99:999–1015.:e6. doi: 10.1016/j.neuron.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 36.Lang EJ, et al. The roles of the olivocerebellar pathway in motor learning and motor control. A consensus paper. Cerebellum. 2017;16:230–252. doi: 10.1007/s12311-016-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ten Brinke MM, Boele HJ, De Zeeuw CI. Conditioned climbing fiber responses in cerebellar cortex and nuclei. Neurosci Lett. 2019;688:26–36. doi: 10.1016/j.neulet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci A, et al. Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat Neurosci. 2017;20:727–734. doi: 10.1038/nn.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton RS. Learning to predict by methods of temporal differences. Mach Learn. 1988;3:9–44. [Google Scholar]
- 40.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 41.Watabe-Uchida M, Eshel N, Uchida N. Neural circuitry of reward prediction error. Annu Rev Neurosci. 2017;40:373–394. doi: 10.1146/annurev-neuro-072116-031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turecek J, et al. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron. 2014;81:1375–1388. doi: 10.1016/j.neuron.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathy A, Clark BA, Häusser M. Synaptically induced long-term modulation of electrical coupling in the inferior olive. Neuron. 2014;81:1290–1296. doi: 10.1016/j.neuron.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefler Y, Yarom Y, Uusisaari MY. Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron. 2014;81:1389–1400. doi: 10.1016/j.neuron.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki K, Miyazaki KW, Doya K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J Neurosci. 2011;31:469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garden DLF, Rinaldi A, Nolan MF. Active integration of glutamatergic input to the inferior olive generates bidirectional postsynaptic potentials. J Physiol. 2017;595:1239–1251. doi: 10.1113/JP273424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363:eaav0581. doi: 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Z, et al. A cortico-cerebellar loop for motor planning. Nature. 2018;563:113–116. doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chabrol FP, Blot A, Mrsic-Flogel TD. Cerebellar contribution to preparatory activity in motor neocortex. biorXiv. 2018 doi: 10.1101/335703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci. 2014;17:1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XM, et al. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis. 2004;40:45–51. doi: 10.1002/gene.20062. [DOI] [PubMed] [Google Scholar]
- 52.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aronov D, Tank DW. Engagement of neural circuits underlying 2D spatial navigation in a rodent virtual reality system. Neuron. 2014;84:442–456. doi: 10.1016/j.neuron.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess CP, et al. High-yield methods for accurate two-alternative visual psychophysics in head-fixed mice. Cell Rep. 2017;20:2513–2524. doi: 10.1016/j.celrep.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slotnick B. A simple 2-transistor touch or lick detector circuit. J Exp Anal Behav. 2009;91:253–255. doi: 10.1901/jeab.2009.91-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jun JJ, et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551:232–236. doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pachitariu M, et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at biorXiv. 2017 doi: 10.1101/061507. [DOI] [Google Scholar]
- 58.Deneux T, et al. Accurate spike estimation from noisy calcium signals for ultrafast three-dimensional imaging of large neuronal populations in vivo. Nat Commun. 2016;7:12190. doi: 10.1038/ncomms12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozden I, Lee HM, Sullivan MR, Wang SS. Identification and clustering of event patterns from in vivo multiphoton optical recordings of neuronal ensembles. J Neurophysiol. 2008;100:495–503. doi: 10.1152/jn.01310.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsutsumi S, et al. Structure-function relationships between aldolase C/zebrin II expression and complex spike synchrony in the cerebellum. J Neurosci. 2015;35:843–852. doi: 10.1523/JNEUROSCI.2170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramirez JE, Stell BM. Calcium imaging reveals coordinated simple spike pauses in populations of cerebellar Purkinje cells. Cell Rep. 2016;17:3125–3132. doi: 10.1016/j.celrep.2016.11.075. [DOI] [PubMed] [Google Scholar]
- 62.Streng ML, Popa LS, Ebner TJ. Climbing fibers control Purkinje cell representations of behavior. J Neurosci. 2017;37:1997–2009. doi: 10.1523/JNEUROSCI.3163-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson BO, Yuste R, Packer AM. PackIO and EphysViewer: software tools for acquisition and analysis of neuroscience data. Preprint at biorXiv. 2016 doi: 10.1101/054080. [DOI] [Google Scholar]
- 64.Pachitariu M, Steinmetz NA, Kadir SN, Carandini M, Harris KD. Fast and accurate spike sorting of high-channel count probes with Kilosort. Adv Neural Inf Process Syst. 2016;29:4448–4456. [Google Scholar]
- 65.Armstrong DM, Rawson JA. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao H, Solages Cd, Lena C. Tetrode recordings in the cerebellar cortex. J Physiol Paris. 2012;106:128–136. doi: 10.1016/j.jphysparis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Dong H-W. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse. Wiley; 2008. [Google Scholar]
- 68.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shamash P, Carandini M, Harris K, Steinmetz N. A tool for analyzing electrode tracks from slice histology. Preprint at biorXiv. 2018 doi: 10.1101/447995. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
The custom analysis code used in this study is available from the corresponding authors upon reasonable request.