One of the final frontiers for neuroscience is explaining how the brain is capable of storing experiences, facts, skills and knowledge and in what form they are stored. In mammals, long-term memories reside in the cerebral cortex and are generally considered to be embodied in the weights of widely distributed synaptic inputs. Long-term memory relating to facts and knowledge (semantic memory) is thought to reside in the six-layered neocortex, becoming progressively independent of the hippocampus over time, whereas experiences (episodic memory) remain dependent on the medial temporal lobe structures including the hippocampus (1). Other kinds of memory, such as skills (procedural memory) require additional brain structures like the basal ganglia. Recently, there has been growing evidence from several laboratories showing that inputs from outside the neocortex that influence memory predominantly target neocortical layer 1. This leads to the hypothesis that layer 1 serves as the locus of memory formation and storage in the neocortex, presumably enabling the selection and activation of engram cortical neurons – those neurons whose changes in firing encode new memories (2). Here we will discuss the hypothesis that semantic memories are encoded via synaptic inputs onto the pyramidal cell tuft dendrites shaped by local inhibitory circuits in layer 1 or the resultant effects of these synaptic inputs on the firing of pyramidal neurons in the column.
Traditionally, experimental and theoretical studies on memory have focused on the hippocampus and surrounding areas that are associated specifically with formation of new memories. To explain the time-limited role of the hippocampus in long-term memory formation, several models proposed that the fast-learning hippocampus instructs slow-learning neocortex to stabilize long-term distillation of experiences, either by repetitively co-activating or indexing the memory traces distributed across different neocortical areas. However, it has proven extremely difficult to pinpoint the locus of formation, storage and retrieval of long-term memory in the neocortex due to its hierarchical and distributed organization. Recent advances in the field might overcome this challenge by identifying specific anatomical substrates of neocortical memory. The emerging picture is that neocortical layer 1 might be a key site for long-term plasticity (3) and learning (4, 5). There is converging evidence that this layer is crucial for sensory, motor and fear memory encoding and involves the regulation of activity in layer 1 interneurons and the distal tuft dendrites of pyramidal neurons (4, 5). More recently, it was demonstrated that the hippocampus also targets neocortical layer 1 via parahippocampal structures and is crucial for associative learning (6).
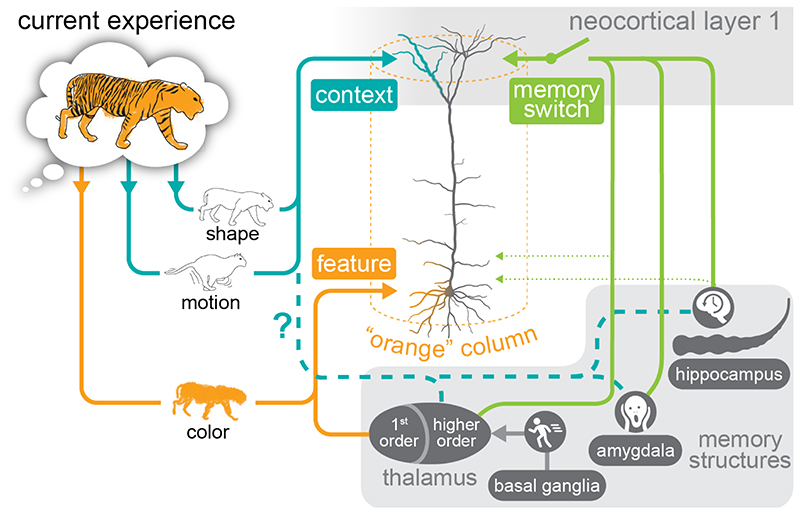
Layer 1 of the neocortex is enigmatic and there is still no consensus about its function. It stands out as the only layer almost devoid of cell bodies which makes it even more surprising that it is the target of, as Ramón y Cajal in 1894 observed, “an almost infinite number of long-range terminal nerve fibers”. These fibers tend to come from higher cortical areas and higher-order thalamic nuclei, suggesting that they convey feedback about associated information to the tuft dendrites of pyramidal neurons in layer 1. Neocortical pyramidal neurons have been shown to associate simultaneous input to the tuft and basal dendrites via the generation of explosive dendritic calcium spikes that dominate the output of the neuron (7). This mechanism allows the cortex to integrate feature-related, feed-forward information influencing the basal dendritic compartment with relevant feedback information from elsewhere in the brain converging on the apical dendritic compartment (Figure). Indeed, it suggests a useful, functional definition of feature and associated context, where a feature is defined as the primary output of a module (‘column’) of cortex during a perceptual experience, and context is defined as relevant information from across the brain that arrives in layer 1. Therefore, ‘Shape’ can be context for a column coding for Color, and simultaneously a feature coded in a column elsewhere (Figure).
Figure. A schematic description of the role of layer 1 in memory formation.
Neocortical layer 1 receives a convergence of information from different memory-related structures outside the neocortex (including hippocampus, amygdala and the basal ganglia) that act as a switch that gates plasticity in layer 1 (note: some projections involve auxiliary inputs to deeper layers, dotted green lines). Alternatively, these inputs might directly provide contextual memory information (dashed blue lines). The diagram represents a hypothesis about memory formation based on the role of pyramidal neurons and their ability to integrate feed-forward, feature-specific information with feedback, context-related information (7). Here, a feature is defined as the primary output of a module (‘column’) of cortex during a perceptual experience. These columns receive feed-forward, feature-specific information in the throughout the cortical layers primarily influencing the basal dendritic compartment of pyramidal neurons (orange lines). Context, in this hypothesis, is defined as information from other cortical columns converging on layer 1 and primarily influencing the apical dendritic compartment of pyramidal neurons (blue lines). (Note, for simplicity this schematic diagram ignores a parallel feedback stream that targets mostly layer 6.)
Given these observations, it is intriguing that multiple memory structures from outside the neocortex also converge on layer 1 and therefore the context-related compartment of pyramidal neurons. For instance, top-down neocortical inputs to layer 1 have been shown to correlate with learning (8, 9). Moreover, higher-order thalamic input targets layer 1 in sensory cortex where it has also been associated with learning (10) and enhanced activity in the tuft dendrites of pyramidal neurons (3). Similarly, it was shown that the amygdala also projects to layer 1 and is crucial for fear memory formation (11). This suggests that such memory-related structures might modulate or gate context-related input to layer 1. In this scenario, experience would involve the short-term association of context with features and memory would involve encoding and stabilization of this association. For example, recognizing a tiger should evoke distributed neuronal activity around the cortex according to the features accessible via the senses (Figure, orange line) and the context derived from previously learned associations (Figure, blue lines). Seeing a tiger for the first time would involve temporary associations that would need to be formed or stabilized. The recent findings of Doron and colleagues (6) may imply that hippocampal input via the medial temporal lobe structures to layer 1 is prerequisite for this stabilization (Figure, green lines). We speculate that different memory-related brain structures projecting to layer 1 provide different criteria for stabilization of context association (i.e., novelty detection, importance or emotional significance, or deviation from expectation etc.). This implies associative memory is the convergence of different types of information from various cortical and subcortical sources to layer 1, and is therefore intrinsically distributed even in a given cortical area. In summary, we hypothesize that semantic memory is the longterm association of different contexts with particular features in neocortical layer 1.
The alternative scenario that memory structures convey contextual input directly to layer 1 (Figure, dashed blue lines) entails certain predictions. Firstly, that these inputs constitute memory content themselves. Secondly, that they show long-term plasticity, and lastly that they require the source structure that must be activated during retrieval. Since it has already been established that semantic memory in the cortex can operate without the hippocampus and medial temporal lobe, we claim that this system operates at least via the gating principle. On the other hand, it has been established that the hippocampus mediates spatio-temporal context that is an important aspect of episodic memory. With the hypothesis we are proposing, it is possible, that these inputs provide both a criterion for consolidating associated context (Figure, ‘memory switch’ and green lines) and a constitutive part of the context itself (e.g., an autobiographical component). Furthermore, evidence for the memory content scenario has been observed directly for thalamic projections to layer 1 (10) (Figure; dashed blue lines).
As yet, the precise mechanisms of memory stabilization in layer 1 remain unknown. It may involve the up- or down-regulation of synaptic inputs to the densely packed tuft dendrites of pyramidal neurons in layer 1 (8), or possibly more complex circuit refinements involving heterosynaptic plasticity induction including other cortical layers (3). There is also evidence that local inhibition mechanisms in layer 1 can shape and modulate long-range contextual inputs (10). Layer 1 interneurons are optimally positioned to determine synaptic plasticity via calcium-dependent signaling in distal dendrites. Indeed, previous studies found several classes of inhibitory neurons reside in or influence layer 1 (12). These inhibitory sources may have complementary roles and competing influences on the associative properties of pyramidal neurons, with some controlling dendritic calcium spikes via direct dendritic inhibition and others controlling the firing of the interneurons to release dendrites from inhibition (disinhibition). Moreover, inhibition in layer 1 has itself been found to be plastic and undergo experience dependent changes (4, 12). Such modulation may form the basis of selection and activation of engram pyramidal cortical neurons (2). On the other hand, stabilization could also involve the regulation of postsynaptic dendritic excitability (5). Modulation of the intrinsic excitability of the tuft dendrites could, in principle, have a large effect on the output of the neuron because they are known to generate powerful local dendritic NMDA and calcium spikes. Other open questions include the extent to which local dendrite-specific protein synthesis in the apical dendritic compartment is required for memory stabilization and how the influence of memory-related projections to layer 1 is mediated (i.e., via specific transmitters or neuromodulators and whether they are spatially targeted or widely distributed).
At the other end of the scale, the hypothesis that associative, context-related memories are stored in layer 1 raises the interesting questions about the psychology of learning. We presume that features are learnt during early development in a critical window within which statistical learning dominates (13). For instance, children typically absorb new motor skills and low-level features such as the accent of a new language with great ease, which we hypothesize is independent of layer 1 and the tuft dendrites of pyramidal neurons. Learning features would therefore involve the synapses distributed throughout the other layers of the cortical column. Thus, we posit that there are two phases of learning. The first phase involves the grounding of the cortical network in the statistical features most prevalent in the external world which take a long time (many repetitions) to establish but are then relatively stable over time. The second phase involves a more dynamic learning of context and high-level concepts that establish an internal model using the putative predictive role of the tuft dendrites (7). For example, adults can more quickly and explicitly learn the higher-level structure of a language (e.g. grammar), while never completely acquiring the detailed features of the accent. Consistent with this, the distal dendritic calcium spike in pyramidal neurons is absent during the critical period and only begins to form during adolescence (14).
The shape of the pyramidal neurons would facilitate this two-phase model of learning by allowing plasticity mechanisms to be segregated and separately modulated in the different dendritic trees at the top and the bottom of the neuron. The physical segregation of learning would also allow independent access to the different classes of memory. This segregation would also allow top-down information to be deployed flexibly, which might serve as a major advantage over the hard-wired plasticity of bottom-up wiring, enabling the encoding and retrieval of associations without compromising feed-forward information.
The modeling of information segregation using multi-compartment neurons may also inform the field of machine learning (15). Despite their successes, modern artificial networks still perform very poorly in tasks requiring inference from very little information which is a distinctive feature of adult learning in intelligent animals. We hypothesized that the shape of pyramidal neurons facilitates the integration of feed-forward and feedback input and allows the segregation of the learning rules associated with each stream. Moreover, Doron and colleagues (6) also found that after learning, neurons more frequently emitted bursts of action potentials that were more salient to memory retrieval why has since been hypothesized to facilitate correct credit assignment for learning in a feedback system (15). It is therefore possible that the mammalian brain uses a ternary rather than binary system of outputs that can signal the type of information (statistical versus associative) to downstream neurons. We speculate that the recent discoveries about mammalian learning and memory discussed here may offer inspiration in the design of more robust and intuitive machine learning principles.
In conclusion, recent evidence suggests that layer 1 is the locus of semantic memory in the cortex. Identifying this fact promises to accelerate our understanding of learning and memory in the human brain and provide insights into the development of novel treatments for memory disorders and the design of architectures for biologically-inspired artificial intelligence.
References
- 1.Dudai Y, Karni A, Born J. The Consolidation and Transformation of Memory. Neuron. 2015;88:20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambino F, Pagès S, Kehayas V, Baptista D, Tatti R, Carleton A, Holtmaat A. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature. 2014;515:116–119. doi: 10.1038/nature13664. [DOI] [PubMed] [Google Scholar]
- 4.Abs E, Poorthuis RB, Apelblat D, Conzelmann K-K, Spiegel I, Letzkus JJ. Learning-Related Plasticity in Dendrite-Targeting Layer 1 Interneurons. Neuron. 2018;100:684–699. doi: 10.1016/j.neuron.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cichon J, Gan W-B. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doron G, Shin JN, Takahashi N, Bocklisch C, Skenderi S, Drüke M, de Mont L, Toumazo M, von Heimendahl M, Brecht M, Naud R, et al. Perirhinal input to neocortical layer 1 controls learning. Science. 2020 doi: 10.1101/713883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkum M. A cellular mechanism for cortical associations: An organizing principle for the cerebral cortex. Trends in Neurosciences. 2013;36:141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Makino H, Komiyama T. Learning enhances the relative impact of top-down processing in the visual cortex. Nature Neuroscience. 2015;18:1116–1122. doi: 10.1038/nn.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manita S, Suzuki T, Homma C, Matsumoto T, Odagawa M, Yamada K, Ota K, Matsubara C, Inutsuka A, Sato M, Ohkura M, et al. A Top-Down Cortical Circuit for Accurate Sensory Perception. Neuron. 2015;86:1304–1316. doi: 10.1016/j.neuron.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Pardi MB, Vogenstahl J, Dalmay T, Spanò T, Pu D-L, Naumann LB, Kretschmer F, Sprekeler H, Letzkus JJ. A thalamocortical top-down circuit for associative memory. Science. 2020;370:844–848. doi: 10.1126/science.abc2399. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Liu DQ, Huang W, Deng J, Sun Y, Zuo Y, Poo MM. Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nature Neuroscience. 2016;19:1348–1355. doi: 10.1038/nn.4370. [DOI] [PubMed] [Google Scholar]
- 12.Schuman B, Dellal S, Prönneke A, Machold R, Rudy B. Neocortical Layer 1: An Elegant Solution to Top-Down and Bottom-Up Integration. Annual Review of Neuroscience. 2021;44:221–252. doi: 10.1146/annurev-neuro-100520-012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blakemore C, Cooper GF. Development of the brain depends on the visual environment. Nature. 1970;228:477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: Amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. Journal of Physiology. 2000;526:571–587. doi: 10.1111/j.1469-7793.2000.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payeur A, Guerguiev J, Zenke F, Richards BA, Naud R. Burst-dependent synaptic plasticity can coordinate learning in hierarchical circuits. Nat Neurosci. 2021;24:1010–1019. doi: 10.1038/s41593-021-00857-x. [DOI] [PubMed] [Google Scholar]