Abstract
Background
Maternal cardiovascular risk factors have been associated with adverse maternal and fetal outcomes. Given the difficulty in establishing causal relationships using epidemiological data, we applied Mendelian randomization to explore the role of cardiovascular risk factors on risk of developing pre-eclampsia or eclampsia, and low fetal birthweight.
Methods
Uncorrelated single nucleotide polymorphisms associated systolic blood pressure, body mass index, type 2 diabetes mellitus, low-density lipoprotein with cholesterol, smoking, urinary albumin-to-creatinine ratio and estimated glomerular filtration rate at genome-wide significance in studies of 298,957 to 1,201,909 European ancestry participants were selected as instrumental variables. A two-sample Mendelian randomization study was performed with primary outcome of pre-eclampsia or eclampsia (PET). Risk factors associated with PET were further investigated for their association with low birthweight.
Results
Higher genetically-predicted systolic blood pressure was associated increased risk of PET [odds ratio (OR) per 1-SD systolic blood pressure increase 1.90 (95% confidence interval (CI)1.45-2.49;p=3.23x10-6 and reduced birthweight (OR=0.83; 95%CI=0.79-0.86;p=3.96x10-18), and this was not mediated by PET. Body mass index and type 2 diabetes were also associated with PET (respectively, OR per 1-SD body mass index increase=1.67 95%CI=1.44-1.94,;p=7.45x10-12; and OR per logOR increase type 2 diabetes=1.11 95%CI=1.04-1.19p;=1.19x10-3), but not with reduced birthweight.
Conclusions
Our results provide evidence for causal effects of systolic blood pressure, body mass index and type 2 diabetes on PET, and identify that systolic blood pressure is associated with reduced birthweight independently of PET. The results provide insight into the pathophysiological basis of PET and identify hypertension as a potentially modifiable risk factor amenable to therapeutic intervention.
Keywords: pre-eclampsia, fetal birthweight, body mass index, type 2 diabetes, hypertension
Non-standard abbreviations and acronyms
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- GWAS
genome-wide association study
- InSIDE
Instrument Strength Independent of Direct Effects
- IVW
Inverse variance weighted
- LD
linkage disequilibrium
- LDL-c
low density lipoprotein cholesterol
- MR
Mendelian randomization
- MR-Egger
Mendelian randomization-Egger
- MR-PRESSO
Mendelian Randomization Pleiotropy RESidual Sum and Outlier
- OR
odds ratio
- PET
pre-eclampsia or eclampsia
- SBP
systolic blood pressure
- SNP
Single nucleotid polymprphism
- STROBE-MR
Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization
- T2DM
type 2 diabetes
- uACR
urinary albumin-to-creatinine ratio
Introduction
Cardiovascular risk factors such as elevated blood pressure, dyslipidemia and type 2 diabetes (T2DM) increase in prevalence with age. With the steady increase in maternal age in the past four decades, combined with the global rise in obesity, there has been an increase in pregnancies among women with pre-existing cardiovascular risk factors 1 . For this reason, investigating the effect of maternal cardiovascular risk profiles on outcomes of pregnancy, both in terms of maternal and fetal outcomes, is becoming increasingly relevant 2–4 .
Hypertensive disorders of pregnancy are the leading cause of maternal and fetal morbidity globally. Among the hypertensive disorders of pregnancy, pre-eclampsia and eclampsia (PET) are especially harmful. Not only do they adversely affect maternal health leading to risk of death and long-term organ damage, they are also associated with preterm birth and low birthweight, which in turn are negative predictors of the child’s future health and cardiovascular risk 5,6 .
Cardiovascular risk factors such as elevated body mass index (BMI) 7,8 , blood pressure 7,8 , low density lipoprotein cholesterol (LDL-C) 8 , T2DM 7,8 and renal dysfunction 9,10 have all been observationally identified as risk factors for hypertensive disorders of pregnancy. Cardiovascular risk factors themselves are also associated with adverse fetal outcomes, including higher risk of preterm birth 11,12 , low birthweight, neonatal morbidity 13,14 and, in the long-term, worse cardiometabolic profiles 15 . However, despite the wealth of retrospective evidence, it is difficult to establish a causal relationship between these cardiovascular risk factors and adverse maternal and fetal outcomes. Conclusive causal relationships are difficult to establish on the basis of observational evidence alone, due to the potential for residual confounding, due to unmeasured or unmeasurable factors, in these study designs. Even with careful adjustment for measured confounders, several unmeasurable factors such as health education, engagement with healthcare and lifestyle behaviours may bias causal estimates.
The Mendelian randomization (MR) paradigm leverages genetic variants predicting variation in an exposure to explore causal effects of that exposure on an outcome. At a practical level, the approach explores associations of ‘genetically-predicted’ levels of an exposure with the outcome. Since alleles are randomly distributed during meiosis and conception, this helps eliminate confounding from environmental factors, similar to randomization in a clinical trial. We therefore performed MR to investigate the effect of traditional cardiovascular risk factors on the development of PET and its downstream effect child birthweight.
Methods
Ethical approval, data availability and reporting
All data used in this study are publicly available. All original studies obtained written, informed participant consent for use of the presented data. The paper is reported on the basis of recommendations by the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) Guidelines 16 . All statistical analyses were performed using R version 4.0.4 (2021-02-15) 17 using the TwoSampleMR 18 and Mendelianrandomization packages 19 .
Data sources
For the primary analyses, genetic association estimates for BMI were obtained from Pulit et al.’s genome-wide association study (GWAS) including 806,834 patients of European ancestry 20 . Genetic association estimates for systolic blood pressure (SBP) and low-density lipoprotein cholesterol (LDL-C) were obtained from the Neale lab second release analysis of UK Biobank data, respectively on 340,159 and 343,621 patients of European ancestry (http://www.nealelab.is/uk-biobank/). Genetic associations for T2DM were obtained from Mahajan et al.’s investigation on 289,957 individuals of European ancestry (48,286 cases and 250,671 controls) 21 . Smoking genetic association estimates were obtained from Wootton et al.’s investigation of 462,690 European ancestry individuals on whom a smoking index was calculated to quantify lifetime exposure to smoking 22 . Genetic association estimates for urinary albumin-to-creatinine ratio (uACR) and estimated glomerular filtration rate estimated by creatinine (eGFR) were obtained respectively from Teumer et al.’s study on 547,361 individuals 23 and Stanzick et al.’s GWAS on 1,201,909 individuals 24 , both of European ancestry. The genetic association estimates for the primary outcome of PET were extracted from the analysis of the FinnGen consortium data’s third release (https://finngen.gitbook.io/documentation) using the phenotype of “pre-eclampsia or eclampsia”, which included 3,903 cases and 114,735 controls of Finnish ancestry. The secondary outcome of birthweight was investigated using genetic association estimates for weight of the first-born child from the Neale lab second release analysis of UK Biobank data (http://www.nealelab.is/uk-biobank/) on 155,202 individuals from the UK Biobank. Details of population characteristics for each of these studies are available in the original publications and websites. Studies were chosen such that there is no sample overlap between the exposure and outcome datasets. A summary table for all the data used for the analyses is outlined in Supplementary Table S1.
Instrumental variable selection
Instrumental variable single nucleotide polymorphisms (SNPs) were selected if they were associated with the exposure of interest in the respective GWAS at genome-wide significance (p<5x10-8) and if they were in pair-wise linkage disequilibrium (LD) at r2 <0.001. The SNPs explained 15.1% of the variance for SBP, 32.7% of the variance for BMI, 8.3% of the variance for LDL-C, 18.0% of the variance for T2DM, 2% of the variance for smoking index, 4.3% of the variance for uACR, and 9.8% of the variance for eGFR. Estimates of variance explained were obtained from the respective original study publications for BMI, smoking, T2DM, uACR and eGFR, and from the Neale lab heritability browser (https://nealelab.github.io/UKBB_ldsc/) for SBP and LDL-C. These heritability estimates were used in a power calculation using the mRnd online power calculator (https://shiny.cnsgenomics.com/mRnd/) 25 . The analyses had a power of 80% to detect a true MR estimate for the outcome of PET, smaller and greater the following ORs respectively: BMI 0.88 and 1.22, SBP 0.82 and 1.18, LDL-C 0.76 and 1.24, T2DM 0.84 and 1.17, smoking 0.50 and 1.51, uACR 0.89 and 1.11, and eGFR 0.95 and 1.05.
Statistical analysis
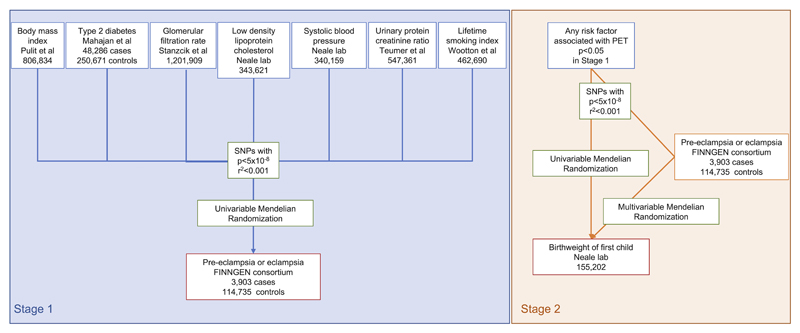
The flowchart for the statistical analysis plan is displayed in Figure 1. For the variants selected as genetic instruments, genetic association summary statistics were used to investigate the causal association between the exposure and the outcome, in a two-sample Mendelian randomization design. Inverse-variance weighted (IVW) MR with multiplicative random effects 26 was used as the primary analysis method for all models to estimate the association between the genetically-predicted risk factors and PET risk 27 . Results are presented as odds ratios (OR) with respective 95% confidence intervals (CI).
Figure 1. Data acquisition and analysis flowchart.
The IVW MR approach assumes that instrumental variables are not associated with confounder traits of the association between the risk factor and the outcome, and that the instrumental variables are only associated with the outcome through their association with the risk factor. In situations where genetic variants have effects multiple parallel biological pathways and subsequent phenotypes, these assumptions are violated. This is termed horizontal pleiotropy. Three sensitivity analyses, including MR-Egger regression 28 , the weighted median 29 and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) 30 , were performed to explore this. We opted for these three analyses as they operate in different ways and rely on different assumptions for valid inferences to assess the reliability of MR analyses 31 . MR-Egger regression produces an estimate of MR effects that accounts for directional pleiotropy by introducing an intercept in the weighted regression model. The intercept can detect pleiotropy by a p-value. After correcting for pleiotropic effects under the Instrument Strength Independent of Direct Effects (InSIDE) assumption, MR-Egger provides robust effect estimates at the cost of statistical power 28 . The weighted median method can provide consistent estimates assuming at least half the weight is derived from valid SNPs. While the two previous methods rely on different consistency assumptions, the MR-PRESSO analysis can detect outlying SNPs and is able to provide consistent casual estimates after removing the (possible) outliers assuming the remaining SNPs are valid.
For any cardiovascular risk factors that were significantly associated with PET risk in our MR analyses, we further performed analysis investigating birthweight of the first child as an outcome. If the risk factor was also associated with lower birthweight in MR, we investigated the proportion of this that is mediated by PET using a summary data multivariable MR 32 . In this analysis, the variant-birthweight genetic association estimates were regressed on the variant-exposure and variant-PET estimates weighted for the precision of the variant-birthweight association, with the intercept fixed to zero. If the effect estimate is attenuated after adjustment for PET, the proportion of this relationship mediated by PET can be calculated as the difference between the total unadjusted effect estimate of the risk factor on birthweight and the direct effect estimate of the risk factor on birthweight adjusted for PET, and dividing this by the total unadjusted effect estimate of the risk factor on birthweight 33 .
Results
Cardiovascular risk factors and pre-eclampsia or eclampsia
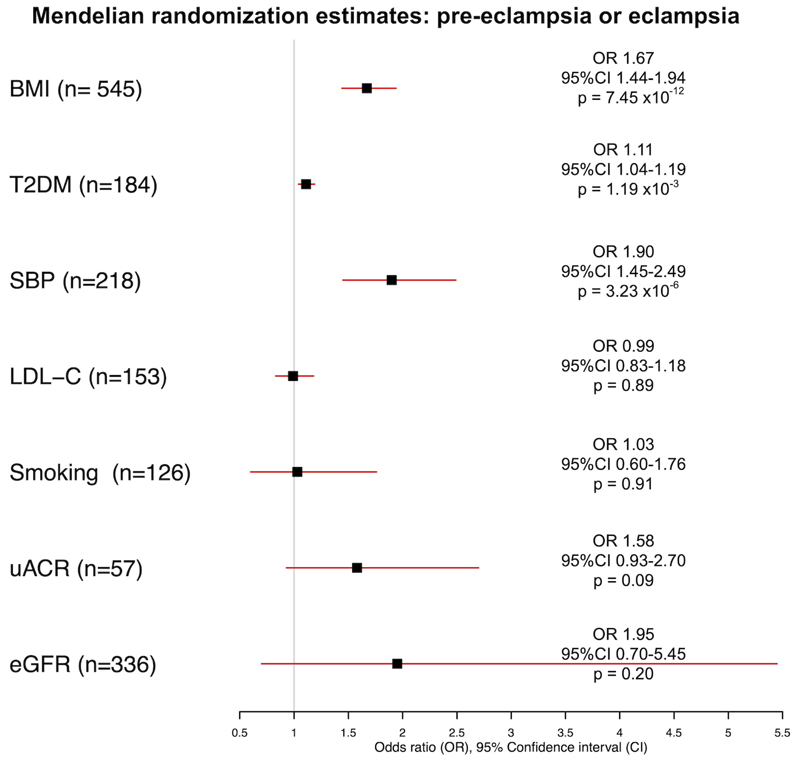
The risk of PET was greater in patients with higher genetically-predicted BMI (odds ratio (OR) per 1-SD increase in BMI: 1.67, 95% confidence interval (95%CI), 1.44-1.94, p=7.45 x10-12), higher genetically-predicted T2DM (OR per logOR increase in T2DM risk 1.11, 95%CI 1.04-1.19, p=1.19 x10-3) and higher genetically-predicted SBP (OR per 1-SD increase in SBP 1.90 95%CI 1.45-2.49, p=3.23 x10-6) on the primary analysis, as shown in Figure 2.
Figure 2.
Mendelian randomization inverse-variance weighted estimates for effect of genetically-predicted body mass index (BMI), type 2 diabetes (T2DM), systolic blood pressure (SBP), low density lipoprotein cholesterol (LDL-C), smoking, urinary albumin-to-creatinine ratio (uACR) and estimated glomerular filtration rate (eGFR) on the outcome of pre-eclampsia or eclampsia.
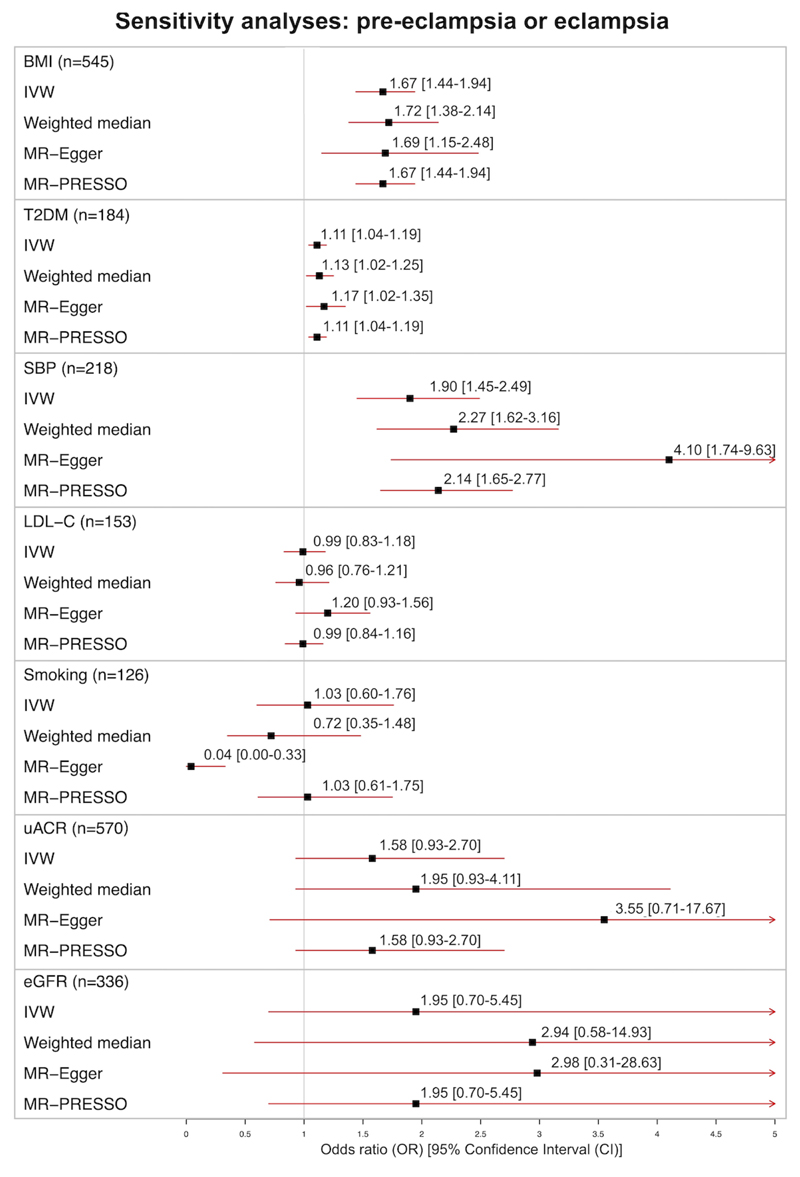
Sensitivity analyses with weighted median MR, MR-Egger and MR-PRESSO produced consistent results for all three exposures, identifying no evidence of significant pleiotropy or outliers: BMI (weighted median estimate OR 1.72 95%CI 1.38-2.14; MR-Egger intercept 0.00 95%CI -0.01 to 0.01 p=0.96; MR-PRESSO outliers identified=0), T2DM (weighted median estimate OR 1.13 95%CI 1.02-1.25; MR-Egger intercept -0.00 95%CI -0.01 to 0.01 p=0.42; MR-PRESSO outliers identified=0) and SBP (weighted median estimate 2.27 95%CI 1.62-3.16; MR-Egger intercept -0.02 95%CI -0.03 to 0.00 p=0.06; MR-PRESSO outliers identified=3, p distortion=0.50). The effect estimates from the sensitivity analyses are displayed in Figure 3.
Figure 3.
Sensitivity analyses for effect of genetically-predicted body mass index (BMI), systolic blood pressure (SBP), low density lipoprotein cholesterol (LDL-C), type 2 diabetes (T2DM), smoking, urinary albumin-to-creatinine ratio (uACR) and estimated glomerular filtration rate (eGFR) on the outcome of pre-eclampsia or eclampsia: Mendelian randomization Egger (MR-Egger), weighted median, and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) estimates. Odds ratios and 95% confidence intervals (OR [95% CI]) are presented for every 1-SD increase genetically-predicted BMI, SBP, LDL-C, Smoking index, uACR and eGFR; and for every logOR increase in T2DM liability.
As illustrated in Figure 2 and Table 1, genetically-predicted LDL-C, smoking, uACR or eGFR were not associated with PET: OR per 1-SD increase in LDL-C: 0.99, 95%CI 0.83-1.18, p=0.89; OR per 1-SD increase in lifetime smoking index: 1.03, 95%CI 0.60-1.76, p=0.91; OR per 1-SD increase in uACR: 1.58, 95%CI 0.93-2.70, p=0.09 and OR per 1-SD increase in eGFR 1.95, 95%CI 0.70-5.45, p=0.20. Sensitivity analyses for each risk factor are displayed in Figure 3.
Table 1. Mendelian randomization estimates for effect of body mass index (BMI), type 2 diabetes mellitus (T2DM), systolic blood pressure (SBP), low density.
| Method | SNPs | OR | LCI 95% | UCI 95% | p-val | |
|---|---|---|---|---|---|---|
| BMI (1-SD kg/m2 ↑) | IVW | 545 | 1.67 | 1.44 | 1.94 | 7.45 x10-12 |
| Weighted median | 1.72 | 1.38 | 2.14 | 1.94 x10-6 | ||
| MR Egger | 1.69 | 1.15 | 2.48 | 0.01 | ||
| MR-PRESSO | 1.67 | 1.44 | 1.94 | 2.02 x10-11 | ||
| T2DM (log OR ↑) | IVW | 184 | 1.11 | 1.04 | 1.19 | 1.19 x10-3 |
| Weighted median | 1.13 | 1.02 | 1.25 | 0.03 | ||
| MR Egger | 1.17 | 1.02 | 1.35 | 0.03 | ||
| MR-PRESSO | 1.11 | 1.04 | 1.19 | 1.42 x10-3 | ||
| SBP (1-SD mmHg ↑) | IVW | 218 | 1.90 | 1.45 | 2.49 | 3.23 x10-6 |
| Weighted median | 2.27 | 1.62 | 3.16 | 1.49 x10-6 | ||
| MR Egger | 4.10 | 1.74 | 9.63 | 1.21 x10-3 | ||
| MR-PRESSO | 2.14 | 1.65 | 2.77 | 3.00 x10-8 | ||
| LDL-C (1-SD mg/dL ↑) | IVW | 153 | 0.99 | 0.83 | 1.18 | 0.89 |
| Weighted median | 0.96 | 0.76 | 1.21 | 0.73 | ||
| MR Egger | 1.20 | 0.93 | 1.56 | 0.16 | ||
| MR-PRESSO | 0.99 | 0.84 | 1.16 | 0.85 | ||
| Smoking (1-SD lifetime smoking ↑) | IVW | 126 | 1.03 | 0.60 | 1.76 | 0.91 |
| Weighted median | 0.72 | 0.35 | 1.48 | 0.37 | ||
| MR Egger | 0.04 | 0.00 | 0.33 | 3.27 x10-3 | ||
| MR-PRESSO | 1.03 | 0.61 | 1.75 | 0.91 | ||
| uACR (1-SD mg/g ↑) | IVW | 57 | 1.58 | 0.93 | 2.70 | 0.09 |
| Weighted median | 1.95 | 0.93 | 4.11 | 0.08 | ||
| MR Egger | 3.55 | 0.71 | 17.67 | 0.12 | ||
| MR-PRESSO | 1.58 | 0.93 | 2.70 | 0.10 | ||
| eGFR (1-SD ml/ min/ 1.73m2 ↑) | IVW | 336 | 1.95 | 0.70 | 5.45 | 0.20 |
| Weighted median | 2.94 | 0.58 | 14.93 | 0.19 | ||
| MR Egger | 2.98 | 0.31 | 28.63 | 0.34 | ||
| MR-PRESSO | 1.95 | 0.70 | 5.45 | 0.20 |
lipoprotein cholesterol (LDL-C), smoking index, urinary albumin-to-creatinine ratio (uACR) and estimated glomerular filtration rate (eGFR) investigated on the primary outcome of pre-eclampsia or eclampsia.
IVW = inverse variance weighted with mixed random effects, MR-Egger = Mendelian randomization Egger, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier, SNP = single nucleotide polymorphism, SD = standard deviation, LCI= lower confidence interval, UCI = upper confidence interval, p-val = p-value
Birthweight of the first child
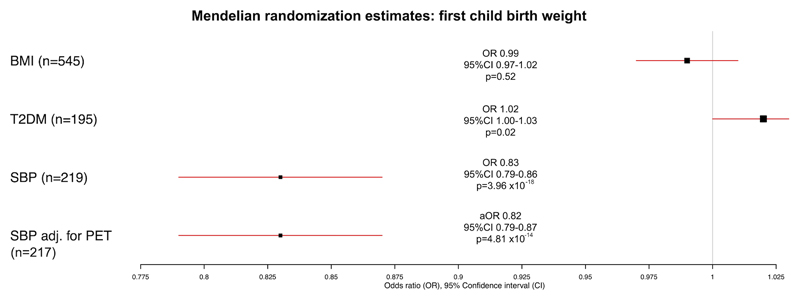
The relationship between genetically-predicted BMI, T2DM and SBP and birthweight was investigated as these were the risk factors significant in the primary outcome analysis for PET. Genetically-predicted BMI was not associated with birthweight of the first child (OR per 1-SD increase in BMI: 0.99, 95%CI 0.97-1.02, p=0.52). Genetically-predicted T2DM was associated with higher birthweight of the first child (OR per increase in logOR T2DM: 1.02, 95%CI 1.00-1.03, p=0.02). Genetically-predicted SBP was inversely associated with birthweight of the first child (OR per 1-SD increase in SBP: 0.83, 95%CI 0.79-0.86, p=3.96 x 10-18), as displayed in Table 2 and Figure 4.
Table 2. Mendelian randomization estimates for effect of body mass index (BMI), type 2 diabetes (T2DM) and systolic blood pressure (SBP), alone and with adjustment for pre-eclampsia or eclampsia (PET), on the outcome of birthweight of the first child.
| Method | SNP number | OR | LCI 95% | UCI 95% | p-val | |
|---|---|---|---|---|---|---|
| BMI (1-SD mmHg ↑) | IVW | 545 | 0.99 | 0.97 | 1.02 | 0.52 |
| Weighted median | 0.99 | 0.96 | 1.03 | 0.66 | ||
| MR-Egger | 1.00 | 0.95 | 1.06 | 0.89 | ||
| MR-PRESSO | 0.99 | 0.97 | 1.01 | 0.43 | ||
| T2DM (logOR ↑) | IVW | 195 | 1.02 | 1.00 | 1.03 | 0.02 |
| Weighted median | 1.01 | 1.00 | 1.02 | 0.10 | ||
| MR-Egger | 1.03 | 1.00 | 1.06 | 0.06 | ||
| MR-PRESSO | 1.02 | 1.01 | 1.03 | 3.95 x10-3 | ||
| SBP (1-SD mmHg ↑) | IVW | 219 | 0.83 | 0.79 | 0.86 | 3.96 x10-18 |
| Weighted median | 0.83 | 0.80 | 0.87 | 1.84 x10-18 | ||
| MR-Egger | 0.84 | 0.73 | 0.96 | 0.01 | ||
| MR-PRESSO | 0.85 | 0.82 | 0.88 | 1.23 x10-16 | ||
| Multivariable MR adjusted for PET | 217 | 0.82 | 0.79 | 0.87 | 4.81 x10-14 |
IVW = inverse variance weighted with mixed random effects, MR-Egger = Mendelian randomization Egger, MR-PRESSO = Mendelian Randomization Pleiotropy RESidual Sum and Outlier, Multivariable MR = multivariable Mendelian randomization, SNP = single nucleotide polymorphism, SD = standard deviation, LCI= lower confidence interval, UCI = upper confidence interval, p-val = p-value.
Figure 4.
Univariable and multivariable Mendelian randomization estimates for effect of body mass index (BMI), type 2 diabetes (T2DM) and systolic blood pressure (SBP), alone and with adjustment for pre-eclampsia or eclampsia (PET) on the outcome of birthweight of first child for mediation analysis.
Odds ratios and 95% confidence intervals (OR [95% CI]) are presented for every 1-SD increase genetically-predicted BMI and SBP and for every logOR increase in T2DM liability. Adjusted odds ratio (aOR) and 95% CI is presented for every 1-SD increase genetically-predicted SBP adjusted for the effect of every logOR increase in PET risk on birthweight.
The findings for SBP were consistent when using weighted median MR (OR 0.83, 95%CI 0.80-0.87, p=1.84 x 10-18) and MR-Egger (OR 0.84, 95%CI 0.73-0.96, p=0.01), where no significant pleiotropy was identified (MR-Egger intercept: 0.00, 95%CI 0.00-0.00, p=0.84). Results using MR-PRESSO were also consistent (OR 0.85, 95%CI 0.82-0.88, p=1.23 x 10-16) after elimination of 11 outlier SNPs (distortion test p=0.08). When adjusting for PET, the relationship between SBP and birthweight remained similar (OR 0.82 95%CI 0.79-0.87 p=4.81 x 10-14), indicating no mediation by PET.
Discussion
In this study, we explored the relationship between cardiovascular risk factors and maternal and fetal outcomes of pregnancy (PET and first child’s birthweight) using MR. Our results demonstrate that genetically-predicted BMI, T2DM and SBP are associated with a higher risk of developing PET. Genetically-predicted SBP demonstrated the strongest effect and was also associated with lower birthweight of the child. Multivariable MR did not identify PET as a mediator in this relationship, indicating that genetically-predicted SBP reduces birthweight through pathways independent of PET.
The pathophysiology of PET continues to be debated. Current knowledge supports a disease process that is initiated by abnormal placentation 34 where failure of spiral artery transformation leads to high oxidative stress, hypoperfusion and ischaemia at the materno-fetal interface. In the second stage, an imbalance in circulating angiogenic factors and soluble fms-like tyrosine kinase 1, a ligand trap antagonising vascular endothelial growth factor 35 , results in widespread maternal endothelial lesions, glomerular endotheliosis and dysfunction 36 , leading to the characteristic pattern of progressive thrombo-microangiopathic organ damage. However, not all PET are the same and two phenotypes have been described: early (<34 weeks) and late onset (>34 weeks). Early-onset PET is characterized by abnormal uterine artery Dopplers, fetal growth restriction, lower cardiac output 37 and adverse maternal and neonatal outcomes 38 . By contrast, late-onset PET is usually associated with normal or mildly abnormal uterine artery Doppler, a lower risk of fetal involvement, and generally more favourable maternal and child outcomes 38,39 .
Impact of SBP on maternal and fetal outcomes
The results of our study corroborate previous observational evidence on the association between maternal SBP and PET risk 8 , whilst providing evidence for a causal link using the MR paradigm. We also show an inverse association between genetically-predicted SBP and birthweight of the first child. This finding is in line with the higher risk of PET we identified in this study. Previous studies have shown that PET, especially if early-onset, is associated with higher rates of growth restriction and therefore lower birthweight 40 . However, in our study, we found that PET was not a major mediator in the relationship between SBP and low birthweight. This is of clinical significance, as it implies that women with high genetically-predicted SBP (as indicated by personal or family history) are at risk of low birthweight regardless of whether they develop PET.
The causal association between higher SBP and risk of PET that was identified in this study may be explained by a common genetic predisposition to higher vascular reactivity, propensity for atherosclerosis and endothelial dysfunction that underlies both pathologies. Women who suffer from PET are known to develop hypertension at higher rates and younger ages (~30–40 vs. ~50–60) than women who have a normal pregnancy 41 , and to be at higher risk of developing metabolic syndrome 42 . Concordant with this, multiple studies have identified higher rates of long-term major cardiovascular events in women who have a background of PET when compared to those with normal pregnancy 43–45 .
Maternal hypertension has been recognised as a risk factor for low birthweight in previous studies 46 .There are a number of possible mechanisms for this. First, higher maternal pre-pregnancy SBP has been associated with higher risk of preterm birth, which may explain a part of the association with low birthweight 47 . Second, for women with already manifest hypertension at the time of the pregnancy, high blood pressure is known to increase endothelial dysfunction and decrease placental perfusion, and this may contribute to low birthweight due to poor placental blood flow in the early stages of pregnancy 48 . However, it is important to note that some studies have failed to identify an association between pre-pregnancy blood pressure and birthweight 49 . This gives rise to an important possibility. In view of the naturally young age of the cohort at risk of PET, typically in the range of 20-45 years, many may not have manifest hypertension despite having a genetic risk for higher SBP. It is therefore possible that low birthweight of the child, even in women without diagnosed hypertension at the time of pregnancy, is an ‘early’ manifestation of the endothelial and vascular dysfunction which underlies the pathophysiology of systemic arterial hypertension. This is an important possibility, especially in view of previous studies that have identified that delivery of an infant of low birthweight is associated with higher risk of maternal major cardiovascular events and cardiovascular death in later life 50 . This could imply that women who have child that is born with a low birthweight could be an important cohort to target for primary prevention of cardiovascular disease.
Tight blood pressure control in hypertensive disorders of pregnancy has been shown to reduce the risk of maternal end-organ damage and adverse perinatal outcomes for the baby 51,52 . However, it remains unclear whether a tight approach to maternal blood pressure control actually reduces PET risk 53 , and whether it benefits birthweight. Paradoxically, observational evidence has shown an association between tight pharmacological blood pressure reduction in pregnancy and decreased birthweight of the baby 54,55 , even in the setting of gestational hypertension 56 . In the Control of Hypertension in Pregnancy Study (CHIPS) trial, randomization of women with non-severe gestational hypertension to the ‘tighter’ blood pressure control group (diastolic blood pressure aim <85mmHg vs <100mmHg) did not reduce the risk of PET, and when randomized before 24 weeks of gestation led to increased risk of <10th centile birthweight of the baby. This does not refute the benefits of adequate blood pressure control, but it identifies that there may be a U-shaped, and not linear, association between blood pressure and birthweight. This is a conceivable hypothesis since excessive reductions in blood pressure may reduce placental blood flow. Of further interest, in the study, this association was not present in women randomized after 24 weeks of gestation 57 , highlighting a possible time-dependent effect. Overall, the results of this trial are the only randomized evidence providing information on the targets and approach to blood pressure control in pregnancy. Our results highlight the requirement for further research to characterise the optimal treatment, timing and targets for the management of hypertension both prior to, and during pregnancy.
Impact of BMI on maternal and fetal outcomes
The association between BMI and PET that we found in this study is in line with past observational evidence from cohort studies and their meta-analyses 11,58,59 . For example, in the recent Cardiac Disease in Pregnancy (CARPREG) cohort study, as many as 8% of women with obesity developed PET 58 . The pathophysiology behind this risk increase is likely multifactorial, with contributions from metabolic, inflammatory and placentation factors that differ with higher BMI 60 . In contrast, high BMI was not associated with birthweight of the first child in this study. The lack of association has multiple potential explanations. First, as well as causing PET, high BMI is associated with gestational diabetes, which is a major cause of increased birthweight. It is possible that this association, which is not accounted for in our study, compensates for any potential reduction in birthweight that may be mediated by the association between BMI and PET. Second, genetic predisposition to higher BMI is likely to contribute to higher birthweight if the weight is not standardized to the expected weight of the baby when considering parental BMI. Finally, high BMI is more commonly associated with late-onset PET, in which risk of fetal growth restriction is lower, perhaps explaining the lack of evidence in our analyses exploring the association between BMI and birthweight.
Impact of T2DM on maternal and fetal outcomes
We observed a positive association between genetically predicted T2DM and PET, in line with past observational evidence 7 . We also observed a positive association between genetically predicted T2DM and birthweight. This is biologically plausible, as maternal diabetes (both pre-existing and gestational) is known to cause high birthweight 61,62 . It is important to consider that despite the overall association between higher T2DM liability and increased birthweight, this represents an overall estimate that balances factors that may contribute to low birthweight as well as those that contribute to high birthweight in women with high T2DM risk. For example, women with high genetic liability to T2DM are both at risk of PET (which is associated with reduced birthweight) and gestational diabetes (which is associated with increased birthweight). The predisposition for gestational diabetes may outweigh the one for PET, tilting the overall balance towards high birthweight and masking potential opposite effects of PET. Due to the lack of individual-level data, this could not be further explored in this study. Overall, our results indicate that T2DM was not associated with reduced birthweight, but this should not be interpreted to indicate that women with T2DM who develop PET are not at risk of delivering a baby with low birthweight. Similar to BMI, the impact of T2DM liability on birthweight specifically in women who develop PET should be further studied.
LDL-C, smoking, uACR and eGFR
Contrary to previous observational studies, we did not find significant associations between genetically-predicted LDL-C, smoking, uACR, and eGFR with the risk of developing PET 7,8,11,58,59 . It is important to note, however, that the power for these analyses was limited. The results of this study therefore do not exclude an association between these risk factors and PET that are smaller than the specific thresholds that we had power to detect. Furthermore, it is important to note that the MR paradigm assumes a linear dose-response relationship, which may not be valid for variables such as eGFR and uACR as it may be that only below a certain threshold eGFR (or above a certain threshold uACR) clinical sequelae are observed.
Study limitations
Unfortunately, we could not explore some clinically important questions due to limitations in data availability. First, due to lack of publicly available GWAS summary data, we could not assess the association between the cardiovascular risk factors and preterm birth, which would be important to explain the mechanisms of low fetal birthweight. Second, our summary data MR study design does not allow for detailed clinical characterization of study participants, because it does not consider individual participant data. As a result, we were not able to perform stratified analyses that separately considered early- and late-onset PET. Similarly, we could not make a distinction between pre-eclampsia and eclampsia in our analyses. Finally, the sources of data for this study mainly concentrate on European cohorts, and this may impair the generalisability of the study results to women of other ethnicities.
Conclusion
We explored possible causal associations between cardiovascular risk factors with adverse maternal and fetal outcomes using MR. Higher genetically-predicted BMI, T2DM and SBP were associated with higher risk of PET. SBP was also associated with lower birthweight of the first-born child, supporting a causal association between these factors. PET was not a major mediator of the association between genetically-predicted SBP and lower birthweight of the first-born child.
Perspectives
The results of this study should be considered when assessing and managing women with a personal or family history of obesity, T2DM and hypertension who are planning a pregnancy. Targeted control of elevated blood pressure has an especially important potential to reduce the risk of complications for both the mother and the child 4,63 .
Supplementary Material
Pathophysiologic Novelty and Significance.
What is new
Past research has identified observational associations between body mass index (BMI), systolic blood pressure (SBP), low density lipoprotein cholesterol, smoking, type 2 diabetes (T2DM), urinary albumin-to-creatinine ratio and glomerular filtration rate and risk of pre-eclampsia or eclampsia (PET). This study uses Mendelian randomization to explore the causal pathways underlying these observational associations. We found evidence to support that genetically-predicted BMI, T2DM and SBP were causally associated with a higher risk of developing PET. Genetically-predicted SBP was also associated with lower birthweight, though multivariable MR did not identify PET as a mediator in this relationship.
What is relevant
The results are of clinical significance, as they imply that women with high genetically-predicted BMI, T2DM risk and SBP (as indicated by personal or family history) are at greater risk of PET. Furthermore, children of women with personal or family history of hypertension are at risk of low birthweight, regardless of whether the mother develops PET. This causal association may be explained by a common genetic predisposition to higher vascular reactivity and endothelial dysfunction that underlies both pathologies. Further study on the impact of blood pressure lowering on PET and child birthweight is warranted.
What are the pathophysiologic implications
We explored the previously reported associations between cardiovascular risk factors with adverse maternal and fetal outcomes using MR. Our results show that higher genetically-predicted BMI, T2DM and SBP were associated with higher risk of PET. SBP was also associated with lower birthweight of the first-born child, and this relationship was not mediated by PET.
Funding
MA received funding from the Medical Research Council and University of Oxford. EAWS is funded by the NIHR Cambridge BRC. KHKP was supported by an NIHR BRC project grant to FSN. DKR received funding from the George’s Academic Training Small Grant Fund. DG was supported by the British Heart Foundation Centre of Research Excellence at Imperial College London (RE/18/4/34215) and by a NIHR Clinical Lectureship at St. George’s, University of London (CL-2020-16-001). FSN was supported by the British Heart Foundation (RG/16/3/32175) and the NIHR Imperial Biomedical Research Centre (BRC). All other authors report no relevant funding.
Footnotes
Disclosures
DG is employed part-time by Novo Nordisk. All other co-authors have no relevant disclosures, and all authors declare there are no conflicts of interest in relation to this work.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final Data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final Data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47. doi: 10.1097/EDE.0b013e318225c960. [DOI] [PubMed] [Google Scholar]
- 3.Sharma G, Ying W, Silversides CK. The Importance of Cardiovascular Risk Assessment and Pregnancy Heart Team in the Management of Cardiovascular Disease in Pregnancy. Cardiol Clin. 2021;39(1):7–19. doi: 10.1016/j.ccl.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang P, Sharma G. The Potential for Pregnancy Heart Teams to Reduce Maternal Mortality in Women With Cardiovascular Disease. J Am Coll Cardiol. 2020;76(18):2114–2116. doi: 10.1016/j.jacc.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti D, Tikkanen E, Gustafsson S, Priest JR, Burgess S, Ingelsson E. Birthweight, Type 2 Diabetes Mellitus, and Cardiovascular Disease. Circ Genomic Precis Med. 2018;11(6) doi: 10.1161/CIRCGEN.117.002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Osmond C, Winter P, Margetts B, Simmonds S. WEIGHT IN INFANCY AND DEATH FROM ISCHAEMIC HEART DISEASE. Lancet. 1989;334(8663):577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 7.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.High Risk of Pre-eclampsia Identification Group. Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garovic VD, White WM, Vaughan L, et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol. 2020;75(18):2323–2334. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayaballa M, Sood S, Alahakoon I, Padmanabhan S, Cheung NW, Lee V. Microalbuminuria is a predictor of adverse pregnancy outcomes including preeclampsia. Pregnancy Hypertens. 2015;5(4):303–307. doi: 10.1016/j.preghy.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Chatzi L, Plana E, Daraki V, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009;170(7):829–836. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 12.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive Disorders in Pregnancy and Subsequently Measured Cardiovascular Risk Factors. Obstet Gynecol. 2009;114(5):961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 13.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 14.Persson M, Razaz N, Edstedt Bonamy A-K, Villamor E, Cnattingius S. Maternal Overweight and Obesity and Risk of Congenital Heart Defects. J Am Coll Cardiol. 2019;73(1):44–53. doi: 10.1016/j.jacc.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Bhatta L, Moen G-H, et al. Investigating a Potential Causal Relationship Between Maternal Blood Pressure During Pregnancy and Future Offspring Cardiometabolic Health. Hypertension. 2021 January;:1–8. doi: 10.1161/HYPERTENSIONAHA.121.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith Davey G, Davies N, Dimou N, et al. STROBE-MR: Guidelines for strengthening the reporting of Mendelian randomization studies. 2019 doi: 10.7287/peerj.preprints.27857. [DOI] [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. R Foundati; 2021. Computing. [Google Scholar]
- 18.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulit SL, Stoneman C, Morris AP, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28(1):166–174. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan A, Wessel J, Willems SM, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50(4):559–571. doi: 10.1038/s41588-018-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wootton RE, Richmond RC, Stuijfzand BG, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. 2020;50(14):2435–2443. doi: 10.1017/S0033291719002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teumer A, Li Y, Ghasemi S, et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat Commun. 2019;10(1):4130. doi: 10.1038/s41467-019-11576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanzick KJ, Li Y, Schlosser P, et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat Commun. 2021;12(1):4350. doi: 10.1038/s41467-021-24491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brion MJA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. doi: 10.1097/EDE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Smith Davey G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186. doi: 10.12688/wellcomeopenres.15555.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313–329. doi: 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess S, Thompson SG. Multivariable Mendelian Randomization: The Use of Pleiotropic Genetic Variants to Estimate Causal Effects. Am J Epidemiol. 2015;181(4):251–260. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting Causal Pathways Using Mendelian Randomization with Summarized Genetic Data: Application to Age at Menarche and Risk of Breast Cancer. Genetics. 2017;207(2):481–487. doi: 10.1534/genetics.117.300191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht JL, Zsengeller ZK, Spiel M, Karumanchi SA, Rosen S. Revisiting decidual vasculopathy. Placenta. 2016;42(2016):37–43. doi: 10.1016/j.placenta.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a Disease of the Maternal Endothelium. Circulation. 2011;123(24):2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18(8):2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 37.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and Late Preeclampsia. Hypertension. 2008;52(5):873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 38.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 39.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 40.Corrigan L, O’Farrell A, Moran P, Daly D. Hypertension in pregnancy: Prevalence, risk factors and outcomes for women birthing in Ireland. Pregnancy Hypertens. 2021 February;24:1–6. doi: 10.1016/j.preghy.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-Eclampsia and Future Cardiovascular Risk Among Women. J Am Coll Cardiol. 2014;63(18):1815–1822. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 42.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y-S, Tang C-H, Yang C-YC, et al. Effect of Pre-Eclampsia–Eclampsia on Major Cardiovascular Events Among Peripartum Women in Taiwan. Am J Cardiol. 2011;107(2):325–330. doi: 10.1016/j.amjcard.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 44.Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129 290 births. Lancet. 2001;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 45.Kestenbaum B, Seliger SL, Easterling TR, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42(5):982–989. doi: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Xiong X. Impact of Preeclampsia and Gestational Hypertension on Birth Weight by Gestational Age. Am J Epidemiol. 2002;155(3):203–209. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, He Y, Li Q, et al. Preconception blood pressure and risk of preterm birth: a large historical cohort study in a Chinese rural population. Fertil Steril. 2015;104(1):124–130. doi: 10.1016/j.fertnstert.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Everett TR, Lees CC. Beyond the placental bed: Placental and systemic determinants of the uterine artery Doppler waveform. Placenta. 2012;33(11):893–901. doi: 10.1016/j.placenta.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Li N, Li Z, Ye R, et al. Preconception Blood Pressure and Risk of Low Birth Weight and Small for Gestational Age: A Large Cohort Study in China. Hypertension. 2016;68(4):873–879. doi: 10.1161/HYPERTENSIONAHA.116.07838. [DOI] [PubMed] [Google Scholar]
- 50.Bonamy A-KE, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth Characteristics and Subsequent Risks of Maternal Cardiovascular Disease. Circulation. 2011;124(25):2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 51.Ramlakhan KP, Johnson MR, Roos-Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17(11):718–731. doi: 10.1038/s41569-020-0390-z. [DOI] [PubMed] [Google Scholar]
- 52.Shawkat E, Mistry H, Chmiel C, et al. The effect of labetalol and nifedipine MR on blood pressure in women with chronic hypertension in pregnancy. Pregnancy Hypertens. 2018 November;11:92–98. doi: 10.1016/j.preghy.2017.12.007. 2017. [DOI] [PubMed] [Google Scholar]
- 53.Magee LA, von Dadelszen P, Rey E, et al. Less-Tight versus Tight Control of Hypertension in Pregnancy. N Engl J Med. 2015;372(5):407–417. doi: 10.1056/nejmoa1404595. [DOI] [PubMed] [Google Scholar]
- 54.Steer PJ, Little MP, Kold-Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. BMJ. 2004;329(7478):1312. doi: 10.1136/bmj.38258.566262.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Dadelszen P, Ornstein M, Bull S, Logan A, Koren G, Magee L. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355(9198):87–92. doi: 10.1016/S0140-6736(98)08049-0. [DOI] [PubMed] [Google Scholar]
- 56.Bánhidy F, Ács N, Puhó EH, Czeizel AE. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population-based study. Hypertens Res. 2010;33(5):460–466. doi: 10.1038/hr.2010.17. [DOI] [PubMed] [Google Scholar]
- 57.Pels A, Mol BWJ, Singer J, et al. Influence of Gestational Age at Initiation of Antihypertensive Therapy. Hypertension. 2018;71(6):1170–1177. doi: 10.1161/HYPERTENSIONAHA.117.10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfaller B, Siu SC, D’Souza R, et al. Impact of Obesity on Outcomes of Pregnancy in Women With Heart Disease. J Am Coll Cardiol. 2021;77(10):1317–1326. doi: 10.1016/j.jacc.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Grieger JA, Bianco-Miotto T, Grzeskowiak LE, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med. 2018;15(12):1–16. doi: 10.1371/journal.pmed.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poston L, Chappell L, Seed P, Shennan A. Biomarkers of oxidative stress in pre-eclampsia. Pregnancy Hypertens. 2011;1(1):22–27. doi: 10.1016/j.preghy.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of Maternal Diabetes and Body Mass Index With Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019;173(4):371. doi: 10.1001/jamapediatrics.2018.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleinberger J, Maloney K, Pollin T. The Genetic Architecture of Diabetes in Pregnancy: Implications for Clinical Practice. Am J Perinatol. 2016;33(13):1319–1326. doi: 10.1055/s-0036-1592078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma G, Zakaria S, Michos ED, et al. Improving Cardiovascular Workforce Competencies in Cardio-Obstetrics: Current Challenges and Future Directions. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.119.015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.