Abstract
Background
SARS‐CoV‐2 has infected a large number of pregnant women.
Objective
To compare clinical, perinatal outcomes of women with COVID‐19 from high‐income countries (HICs) and low‐ to middle‐income countries (LMICs).
Search strategy
Online databases were searched.
Selection criteria
Original studies on pregnant women with COVID‐19 were included.
Data collection and analysis
Information on clinical presentation, co‐morbidities, pregnancy outcomes, neonatal outcomes, and SARS‐CoV‐2 infection in neonates was extracted.
Main results
The pooled estimate of SARS‐CoV‐2 positive neonates is 3.7%. Symptomatic presentations are less common in LMICs compared to HICs (odds ratio [OR] 0.38). Diabetes (OR 0.5), hypertension (OR 0.5), and asthma (OR 0.14) are commonly reported from HICs; hypothyroidism (OR 2.2), anemia (OR 3.2), and co‐infections (OR 6.0) are commonly reported in LMICs. The overall risk of adverse pregnancy outcomes is higher in LMICs compared to HICs (OR 2.4). Abortion (OR 6.2), stillbirths (OR 2.0), and maternal death (OR 7.8) are more common in LMICs. Preterm births and premature rupture of membranes are comparable in both groups. Neonatal deaths (OR 3.7), pneumonia (OR 7.5), and neonatal SARS‐CoV‐2 infection (OR 1.8) are commonly reported in LMICs.
Conclusions
In LMICs, pregnant women and neonates are more vulnerable to adverse outcomes due to COVID‐19.
PROSPERO registration no: CRD42020198743.
Keywords: coronavirus, COVID‐19, death, newborn, pregnancy, SARS‐CoV‐2, stillbirth, vertical transmission
Synopsis
Pregnant women and newborns in low‐ and middle‐income countries are more vulnerable and will require a rigorous clinical evaluation and proper follow‐up to minimize the adverse impact of COVID‐19.
1. INTRODUCTION
Adverse maternal and neonatal outcomes and birth defects are associated with certain viral infections during pregnancy. Maternal infections with rubella, cytomegalovirus, Zika virus, chickenpox, vaccinia, and poliomyelitis are associated with adverse pregnancy outcomes including birth defects such as microcephaly or even fetal death. 1 , 2 The novel coronavirus SARS‐CoV‐2, causing the respiratory disease COVID‐19, has rapidly spread worldwide and infected a large number of individuals including pregnant women. Adverse pregnancy outcomes are documented in a limited number of women infected with other coronaviruses such as the SARS‐Co‐V and Middle East Respiratory Syndrome. 3 Thus, there is a need to study the effects of SARS‐CoV‐2 infections in pregnancy. The study protocol was exempted from review by the institutional ethics committee of ICMR‐National Institute for Research in Reproductive Health (NIRRH) (D/ICEC/Sci‐49/52/2020).
To address the issue of COVID‐19 and pregnancy outcomes, case reports, case series, observational studies, and cohort and case‐control studies are published that describe the maternal and fetal presentation of SARS‐CoV‐2 infection in pregnant women. However, these reported studies have yielded conflicting findings leading to contradictory conclusions. Some reports indicate that there are no major adverse outcomes of SARS‐CoV‐2 infection in pregnancy, 4 , 5 others have reported serious pregnancy complications such as preterm births, neurological disorders, miscarriages, and maternal deaths in women infected with SARS‐COV2 during pregnancy. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Multiple systematic reviews have been conducted that have majorly described the risk of vertical transmission and maternal presentation associated with COVID‐19. These studies have indicated that there are higher odds of having severe disease, low birth weight infants, and preterm delivery in pregnant women with COVID‐19; the neonates born to these mothers also have a higher risk of hospitalization. 6 , 14 , 15 , 16 Thus pregnant women with COVID‐19 will need special medical attention.
The scale of the COVID‐19 outbreak has resulted in the disruption of maternal health services and is predicted to adversely impact pregnancy outcomes, specifically in low‐ and middle‐income countries (LMICs). 17 However, the understanding of the impact of COVID‐19 disease on obstetric outcomes of women in LMICs is limited as much of the evidence is based on pooled estimates of the global data or registry data from high‐income countries (HICs). 14 , 15 , 16 A single study has compared the maternal presentation of COVID‐19 by country of origin. 18 However, the comparisons are mainly done from studies reported from Spain, France, and China. It is believed that there is no comparative information on maternal presentations and pregnancy outcomes in women with COVID‐19 from LMICs and HICs.
Herein, the maternal presentations and outcomes of pregnant women with COVID‐19 globally were systematically assessed and the data obtained from LMICs and HICs were compared. The incidence of vertical transmission of SARS CoV‐2 was also assessed.
2. METHODS
2.1. General overview
For this review, the Preferred Reporting Items for Systematic reviews and Meta‐analysis (PRISMA) guidelines were followed. The study protocol was registered at PROSPERO (CRD42020198743).
2.2. Eligibility criteria, information sources, and search strategy
The PubMed, Embase, Web of Science, Scopus, Google Scholar literature database, ScienceDirect, preprint servers and specialized COVID resources were searched for articles on pregnant women. The keywords and MeSH terminology used were a combination of “coronavirus,” “2019 n‐COV,” “SARS‐CoV‐2,” “SARS virus” or “COVID‐19,” “corona virus disease 2019,” “pregnancy,” “maternal,” “mothers,” “neonates,” and “infants.” Snowballing strategy and Ripe‐tomato.org, a resource site for data on pregnant women and COVID‐19 (https://ripe‐tomato.org/covid‐19/), was also searched manually to identify any missed articles. Articles in non‐English languages that could be translated into English using Google Translate were also included.
2.3. Study selection
The PRIMSA flowchart for the present systematic review is presented in Figure 1. Articles published between March 1 to December 31, 2020, were reviewed. Two authors shortlisted original studies on pregnant women with SARS‐CoV‐2 infection. Only those studies that reported the data on the laboratory‐confirmed diagnosis of SARS‐CoV‐2 were included. Duplicate articles, reviews, narrative articles, abstracts, and gray literature such as media reports, blogs, and information from unverified sources were excluded. Articles that did not have the primary outcome as maternal presentations or pregnancy outcomes were also excluded. The extracted data were verified independently and the inconsistencies in the data entries were sorted. It was not possible to rank the quality of the studies included due to the inherent nature of the data. Data were sorted based on country of origin and the information was pooled for HICs and LMICs (as per the World Bank Data of 2020).
FIGURE 1.
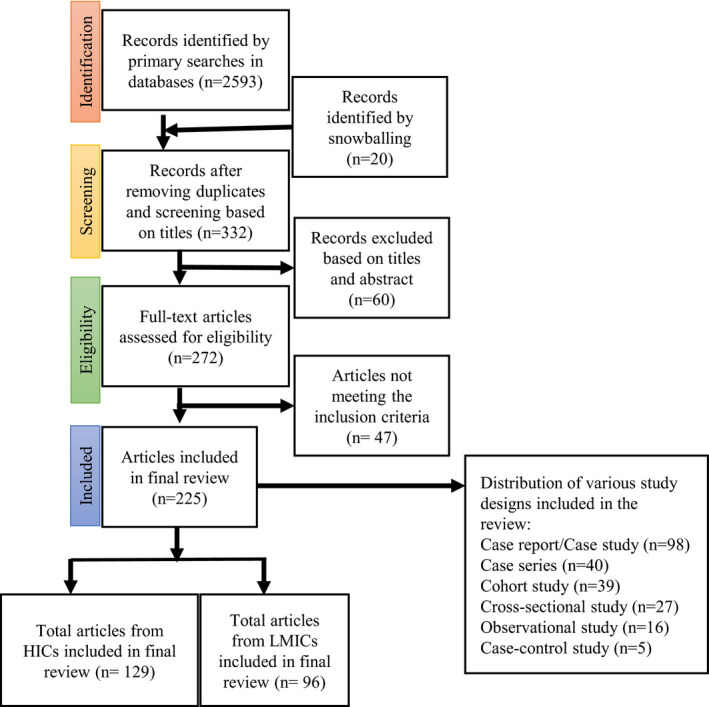
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart of included studies describing the sequential inclusion and exclusion of the studies reporting COVID‐19 and pregnancy. The number of articles included from HICs and LMICs are shown, as well as the various study designs included in the systematic review. Abbreviations: HIC, high‐income country; LMIC, low‐ and middle‐income country.
2.4. Outcome measures
Information was collected on maternal presentations, that is, the proportion of women presenting with fever, cough, myalgia, dyspnea, chest pain, or headache. The information on pregnancy outcomes included type of delivery (cesarean or vaginal), preterm and term delivery, premature rupture of membranes (PROM), abortion, stillbirths, and maternal deaths. The neonatal outcomes included the numbers of neonates positive for SARS‐CoV‐2, the numbers with respiratory distress, and neonatal deaths.
2.5. Data extraction and meta‐analysis
Individual patient data or pooled data from papers were recorded in a table format. Pooled data were used with a random‐effects meta‐analysis to estimate the incidence of outcomes as proportions with 95% confidence intervals (CIs). Heterogeneity was reported as I 2 statistics and analysis was performed using Stata 15 package (StataCorp., College Station, TX, USA).
3. RESULTS
Through database searches and snowballing, 2593 articles were identified. After screening and assessment of eligibility (Fig. 1), 225 studies reporting the data of 10 582 women were found eligible for inclusion (Table S1). These studies are from 35 countries (Fig. 2) with the majority of the data from the United States (24%), India (11%), Brazil (9%), Colombia (9%), China (7%), Spain (7%), Iran (7%), France (6%), and the United Kingdom (5%).
FIGURE 2.
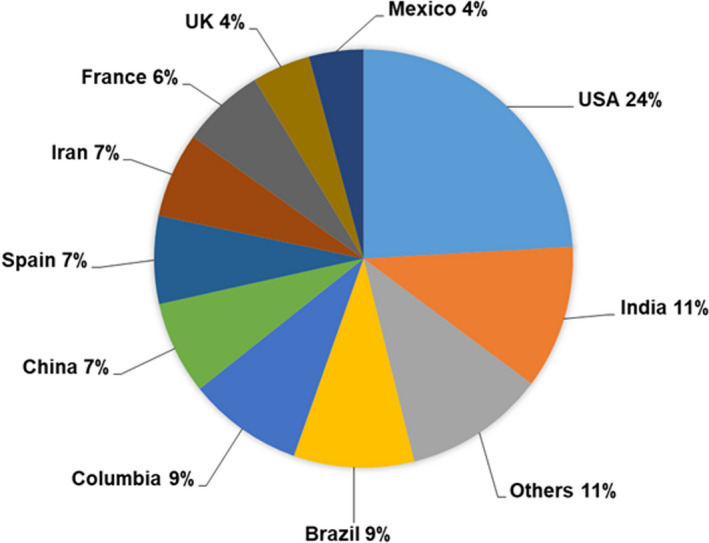
Country‐wise breakup of the 225 studies from which data on 10 582 pregnant women with COVID‐19 included in this systematic review were derived. The percentage of pregnant women reported from each country are given.
3.1. Maternal presentations, co‐morbidities, and pregnancy outcomes
Figure 3 gives the clinical presentations and pregnancy outcomes synthesized from the included studies. The most common maternal presentations (Figure 3a) were fever (23%, 95% CI 22.19–24.04) and cough (25%, 95% CI 23.71–25.60). The other symptoms were less common and reported in less than 10% of cases.
FIGURE 3.

Pooled estimates of (a) the maternal presentations, (b) co‐morbidities, and (c) pregnancy and neonatal outcomes in pregnant women with COVID‐19. Abbreviations: DM, diabetes mellitus; GDM, gestational diabetes mellitus; GH/HT, Gestational hypertension/Hypertension; PCR, polymerase chain reaction; PROM, premature rupture of membrances; RDS, respiratory distress syndrome.
The major co‐morbidities (Fig. 3b) were diabetes mellitus (9.3%, 95% CI 8.72–10.02) and hypertensive disorders (8.4%, 95% CI 7.79–9.04). Asthma and hypothyroidism were the other reported co‐morbidities. Placental disorders such as placenta previa, placenta accreta, and placental abruption were reported in less than 1% of women with COVID‐19. There were some reports of co‐infections that included hepatitis B, influenza, dengue, malaria, tuberculosis (TB), Legionella pneumophila, and mycoplasma.
Among the pregnant women with COVID‐19 who had delivered, the cumulative incidence of vaginal delivery was 48.5% (2352/4841, 95% CI 47–1–50.0) and 51.5% (2489/4841, 95% CI 50.0–52.8) for cesarean delivery. Among the adverse pregnancy outcomes (Fig. 3c), preterm birth was the most common (22%, 95% CI 20.81–23.15). Fetal distress was the most common reason for cesarean delivery. There were 140 reported instances of fetal loss, of which 79 of 9054 were cases of abortions (0.87%, 95% CI 0.70–1.09) and 61 of 9054 were stillbirths (0.67%, 95% CI 0.52–0.86). The overall risk of pregnancy loss in women with COVID‐19 was 1.46%. The overall incidence of maternal death was 1.79% (188/10 492, 95% CI 1.56–2.06).
3.2. Complications in infants born to mothers with COVID‐19
The infant data of mothers with COVID‐19 are shown in Figure 3c. Of the data on 4797 newborns, there were 23 neonatal deaths (0.49%, 95% CI 0.33–0.73). Respiratory distress syndrome (RDS) was reported in 1.68% of cases (95% CI 1.34–2.12) and pneumonia was reported in 1.07% of neonates born to mothers with COVID‐19 (95% CI 0.80–1.43). Testing for SARS‐CoV‐2 was carried on 4112 newborns, of which 3.78% (95% CI 3.24–4.40) neonates born to mothers with COVID‐19 were positive for SARS‐CoV‐2.
3.3. Comparison of data on HICs and LMICs
Figure 4 gives the comparisons of clinical presentations, co‐morbidities, and pregnancy and neonatal outcomes in women with COVID‐19 in HICs (n = 5080) versus LMICs (n = 5502). The actual values and the statistical comparisons are given in Table S2. Symptomatic presentations are less commonly reported in studies from LMICs compared to HICs (overall OR 0.38, I 2 = 93.5%, P = 0.000). The proportion of women reporting fever (HIC vs LMIC 30.3% vs 16.7%), cough (HIC vs LMIC 37.0 % vs 13.7%), dyspnea (HIC vs LMIC 14.4% vs 5.7%), myalgia (HIC vs LMIC 8.7% vs 2.9%), and headache (3.8% vs 1.6%) were significantly higher (P < 0.01) in women from HICs compared to those from LMICs (Fig. 4a, Table S2). Although diarrhea was reported as common in both groups (HIC vs LMIC 3.9% vs 3.2%), the marginal reduction was not statistically significant (P > 0.05).
FIGURE 4.
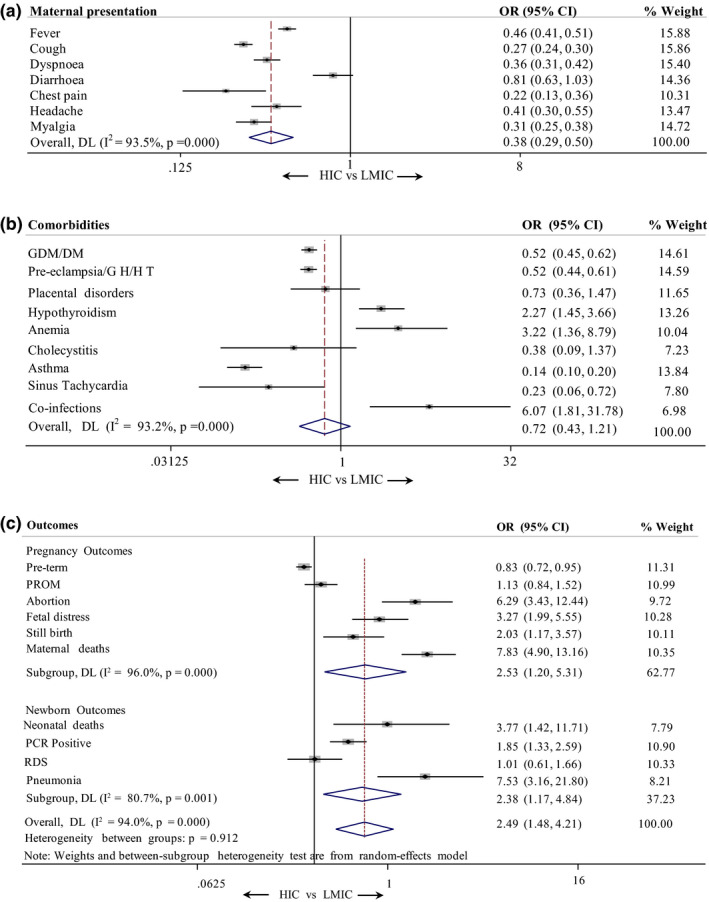
Comparison of (a) maternal presentations, (b) co‐morbidities, and (c) pregnancy and newborn outcomes in women with COVID‐19 from HICs and LMICs. The events, total values, percentages, and P values are given in Table S2. Abbreviations: DM, diabetes mellitus; GDM, gestational diabetes mellitus; GH/HT, Gestational hypertension/Hypertension; HIC, high‐income country; LMIC, low‐ and middle‐income country; PCR, polymerase chain reaction; PROM, premature rupture of membrances; RDS, respiratory distress syndrome.
Among the co‐morbidities (Fig. 4b, Table S2), diabetes mellitus, including gestational diabetes mellitus (HIC vs LMIC 12.3% vs 6.8%), and hypertension, including pre‐eclampsia (HIC vs LMIC 11.0% vs 6.1%), were more commonly reported in pregnant women in from HICs compared to those from LMICs. Asthma was almost six times more commonly reported in women from HICs compared to LMICs (HIC vs LMIC 6.3% vs 1.0%). The differences were statistically significant (P < 0.01). In LMICs, hypothyroidism (HIC vs LMIC 0.8% vs 1.8%), anemia (HIC vs LMIC 0.2% vs 0.6%), and co‐infections (HIC vs LMIC 0.1% vs 0.5%) were more commonly reported compared to HICs, and these differences were statistically significant (P < 0.01).
Adverse pregnancy outcomes (Fig. 4c, Table S2) were more commonly seen in LMICs compared to HICs (overall OR 2.49, I 2 = 94.0%, P = 0.000). Women in LMICs were more likely to experience pregnancy losses. The odds of abortion were 6.2 times higher (HIC vs LMIC 0.3% vs 1.6%) and stillbirth was twice as likely (HIC vs LMIC 0.5% vs 0.9)% in LMICs compared to HICs. These differences are statistically significant (P < 0.01) The risk of death was almost eight times higher in LMICs compared to HICs (HIC vs LMIC 0.4% vs 3.1%). This difference is statistically significant (P < 0.01). However, preterm births and PROM are comparable in both groups.
Adverse neonatal outcomes (Fig. 4c, Table S2) were also more common in women in LMICs compared to those in HICs. The rates of neonatal deaths were four times more common (HIC vs LMIC 0.2% vs 0.8%) and pneumonia was 7.5 times more common (HIC vs LMIC 0.3% vs 2.0%) in LMICs compared to HICs. These differences are statistically significant (P < 0.01). However, RDS was not significantly different between the two groups.
In LMICs, the neonates born to mothers with COVID‐19 were twice as likely to be positive for SARS‐CoV‐2 compared to those in HICs (HIC vs LMIC 2.8% vs 5.1%). This difference was statistically significant (P < 0.01).
4. DISCUSSION
Data from 10 582 pregnant women with COVID‐19 from 35 countries were analyzed in the present systematic review. The major emphasis was to evaluate the clinical presentations, co‐morbidities, and pregnancy outcomes of pregnant women with COVID‐19. Most of the data are mainly of hospitalized pregnant women near term, and there is very little information on the outcomes of first‐ and second‐trimester infections separately.
Several studies are published globally that have assessed the outcomes of SARS‐CoV‐2 infection in pregnancy. The highest proportion of these data is from the USA followed by India, Brazil, Columbia, and China. Although there are comparable data from the HICs and LMICs (5080 and 5502 women, respectively), these are mainly from India, Brazil, Columbia, China, Spain, and Iran. There is a dearth of data from Far East and southeast Asian LMICs (such as Malaysia, Myanmar, Bangladesh, and Sri Lanka) and almost negligible data from African countries. Thus there is a need to increase data reporting from underrepresented countries to have a clear global picture of COVID‐19 and pregnant women.
The initial identification of COVID‐19 was based on a diagnosis of severe presentation of respiratory distress. It was eventually evident that most cases of laboratory‐confirmed SARS‐COV‐2 infection were asymptomatic. 19 In an Indian cohort of 1169 pregnant women, 20 the prevalence of symptomatic cases was 11.5% while that of asymptomatic cases was 88.5%. In the present systematic review, the clinical manifestations of COVID‐19 in the symptomatic cases were highly heterogeneous where fever (23%) and cough (24%) were the most commonly reported symptoms. These numbers are comparable to those reported in other systematic reviews. 4 , 15 Some studies have shown that some ethnicities are more likely to have a severe presentation of COVID‐19 during pregnancy 15 , 21 To address whether socioeconomic status has had an impact on the clinical presentation of COVID‐19 during pregnancy, data based on country of origin were analyzed and it was observed that symptomatic presentations were more commonly reported from studies in HICs compared to LMICs. Although there was a lot of heterogeneity across the studies, this was unexpected as it was anticipated that most of the data were out of tertiary referral centers and only symptomatic women may present to the hospital in LMICs. It will be important to address the LMIC population and determine why these women have presented with less severe symptoms.
The commonality of asymptomatic and less severe presentations of COVD‐19 in pregnant women in LIMCs is a matter of concern. With the scarcity of resources for testing, many women without their COVID‐19 status would be hospitalized and these could be a source of SARS‐CoV‐2 infection in the community and among healthcare workers. The results of the present study further emphasize the need for the strict implementation of universal screening of SARS‐CoV‐2 in an obstetric population and the LMICs must implement these more rigorously to prevent outbreaks in hospitals.
Epidemiological evidence suggests that hypertension and diabetes are risk factors for poor outcomes of COVID‐19. In addition, in pregnant women hypertensive disorders, diabetes, and asthma were the major co‐morbidities associated with COVID‐19. Intriguingly, the prevalence of co‐morbidities was very different in LMICs versus HICs. Pregnant women with COVID‐19 in HICs were more likely to be diabetic or hypertensive or asthmatic compared to those in LMICs. Hypothyroidism or anemia or co‐infections are commonly reported in women in LMICs compared to those in HICs. Globally, the estimated prevalence of hypothyroidism in pregnancy is in the range of 2%–3%. In the present study, it was observed that the number is approximately 1.3% in women with COVID‐19. A higher chance of developing hypothyroxinemia is observed in pregnant Chinese women with COVID‐19. 22 However, the incidence of hypothyroidism in pregnant women with COVID‐19 in LMICs is 1.8% versus 0.8% for those in HICs. However, the reasons for such differences are unclear. Similar to hypothyroidism, women with COVID‐19 in LMICs were more likely to suffer from anemia (OR 3.2). This could be due to a higher incidence of anemia among pregnant women in LMICs 23 ; however, anemia itself is identified to be an independent risk factor for COVID‐19 in the general population. 24
Further, in women with COVID‐19, adverse pregnancy outcomes were preterm deliveries (21.9%), PROM (4%), and fetal distress (0.93%). These numbers are comparable to those reported in recent systematic reviews. 4 , 15 , 16 A higher incidence of preterm delivery is reported in pregnant women with COVID‐19 compared to matched controls. 25 , 26 Whether the increased risk of preterm birth is iatrogenic or directly as a consequence of COVID‐19 is presently debatable. Comparison of the rates of preterm births and PROM between HIC and LMICs showed a surprising trend. Although LMICs are likely to have a higher general incidence of preterm births, 27 the rates of preterm births and PROM in women with COVID‐19 are comparable between HICs and LMICs. Decreased rates of institutional deliveries during the pandemic due to limited travel access to reach the hospitals during the lockdown, mainly in LMICs, might skew the outcome measure. 28 Systematic case controls and cohort studies are required to address the incidence of COVID‐19–associated preterm births and PROM in LMICs.
Beyond SARS‐CoV‐2, many countries are endemic to other infections such as TB, malaria, chikungunya, and dengue. It was observed that although the overall proportions of women with COVID‐19 presenting with co‐infections are quite low, it is six times more common in LMICs than HICs. The presenting symptoms of TB, malaria, chikungunya, and dengue often overlap with SARS‐CoV‐2 infection and there is a clinical dilemma. In a small case series from India, 29 it was observed that symptoms of dengue or malaria overlapped with those of COVID‐19 in pregnancy and the co‐infection did not aggravate the symptoms, making the clinical diagnosis and management challenging. Further, coinfections of TB and SARS‐CoV‐2 during pregnancy are reported to have severe complications and adverse outcomes, and SARS‐CoV‐2 enhances the symptoms of previously undetected TB. 30 Thus obstetricians must remain vigilant about the other common conditions in endemic regions and investigate for them in symptomatic cases of COVID‐19 to avoid complications.
The incidence of maternal deaths in COVID‐19 is a matter of controversy. Some studies reported negligible death rates of pregnant women with COVID‐19, while others reported a high proportion. Herein, it was estimated that a maternal death rate of 2% (188/10 492 women) related to COVID‐19. This is comparable to those observed in systematic reviews. 15 , 16 Although the numbers of maternal deaths concerning COVID‐19 are not high as those reported for influenza, 31 a high incidence of COVID‐19–related maternal deaths is reported from Brazil (13%, 125/981). Indeed, the present analysis revealed that eight times more pregnant women are likely to die of COVID‐19 in LMICs (3.1%) compared to HICs (0.4%). This is not due to more severe presentations (in fact, women in LMICs often have less severe or asymptomatic presentations) or higher prevalence of co‐morbidities. Poor access to healthcare services, inadequate infrastructure, and the impact of lockdown are possible factors that may lead to a higher risk of deaths in LMICs. 17 Thus, more data on pregnant women with COVID‐19 from LMICs are required to identify the risk factors for maternal mortality.
Infection with influenza A virus during pregnancy is associated with poor neonatal outcomes and there is a higher risk of delivering low birth weight infants. Low birth weight was observed in almost 8% of neonates born to mothers with COVID‐19 (data not shown). Other complications were respiratory distress, pneumonia, and neonatal deaths. In the present analysis, stillbirth rates were more common in LMICs compared to HICs (OR 2.03). Further, this was not due to higher preterm birth rates as the numbers of studies reporting preterm births and PROM were higher in LMICs. Thus, like maternal deaths, loss of a pregnancy or newborn (due to abortion, stillbirth, or newborn death) is more likely in LMICs due to COVID‐19.
SARS‐CoV‐2 infection in the newborns of mothers with COVID‐19 is a matter of concern. Some isolated case reports and small case series could not detect the presence of viral RNA in fetal tissues such as the placenta, amniotic fluid, cord blood, and in the neonatal respiratory tract. 32 This led to the notion that there is minimal risk of newborns acquiring maternal SARS‐CoV‐2 infection. However, SARS‐COV‐2 receptors are detected in the placenta and there is evidence of the presence of viral RNA in the placenta of women with COVID‐19. 10 , 33 , 34 The cumulative data in the present study indicate that nearly 3.7% of the neonates born had SARS‐CoV‐2 infection in their nasopharyngeal swabs. This number is comparable to those reported in other systematic reviews. 34 Intriguingly, the incidence of neonates born to mothers with COVID‐19 acquiring SARS‐CoV‐2 is higher in LMICs compared to HICs (OR 1.85). This is despite the fact that nearly the same numbers of neonates are tested in both groups. Furthermore, it is important to note that neonatal pneumonia was also more commonly observed in children born to mothers with COVID‐19 in LMICs versus those in HICs (OR 7.5). Thus, neonates born to pregnant women in LMICs will require a rigorous clinical evaluation and proper follow‐up to minimize the adverse impact of COVID‐19.
4.1. Study limitations
The present systematic review is based on almost all the data published from infections occurring around the time of delivery and there is limited information on the presentations and outcomes of SARS‐CoV‐2 infection in earlier trimesters. There was no uniformity in testing the newborns across various studies and the data are largely underreported. Thus the incidence of vertical transmission of SARS‐CoV‐2 cannot be accurately estimated. There is heterogeneity in the reported incidence of multiple parameters across studies and this is largely due to variations in sample size, reporting criteria, and study designs.
5. CONCLUSION
In pregnant women with COVID‐19 at term, there is evidence of adverse pregnancy and neonatal outcomes. Well‐defined studies will be required to generate clear evidence on vertical transmission of SARS‐CoV‐2. The data in the present study suggest that pregnant women and their neonates in LMICs are more vulnerable to adverse outcomes due to COVID‐19, although there is considerable heterogeneity across the reported studies. High‐quality systematic reporting from registries will be required to sort these issues. Coupled with compromised health services, COVID‐19 itself will jeopardize the roadmap toward achieving the Sustainable Development Goals in maternal and child health in the LMICs. With the availability of the vaccine, high priority must be given to pregnant women to at least partially rescue the damage done by COVID‐19 to maternal health in LMICs.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
RG conceived the study. RG and DM designed the study. MS, PK, RG, and DM screened the abstracts for inclusion in the study. MS, PK, SC, RKP, and AP analyzed the data. SM coordinated the discussions and helped in data interpretation. RG and DM drafted the manuscript, which was then critically revised by all authors. All authors approved the final manuscript.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
The laboratories of RG, DM, and SM are funded by grants from the Indian Council of Medical Research (ICMR). RG is an awardee of the DBT Wellcome India Alliance Clinical and Public Health Intermediate Fellowship (Grant no. IA/CPHI/18/1/503933). The manuscript bears ICMR‐NIRRH ID RA/896/04‐2020.
Gajbhiye RK, Sawant MS, Kuppusamy P, et al. Differential impact of COVID‐19 in pregnant women from high‐income countries and low‐ to middle‐income countries: A systematic review and meta‐analysis. Int J Gynecol Obstet. 2021;155:48–56. 10.1002/ijgo.13793
REFERENCES
- 1. Racicot K, Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127(5):1591‐1599. 10.1172/JCI87490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams Waldorf KM, Nelson BR, Stencel‐Baerenwald JE, et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med. 2018;24(3):368‐374. 10.1038/nm.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2(2): 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papapanou M, Papaioannou M, Petta A, et al. Maternal and neonatal characteristics and outcomes of COVID‐19 in pregnancy: an overview of systematic reviews. Int J Environ Res Public Health. 2021;18(2):596. 10.3390/ijerph18020596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vizheh M, Muhidin S, Aghajani F, et al. Characteristics and outcomes of COVID‐19 pneumonia in pregnancy compared with infected nonpregnant women. Int J Gynaecol Obstet. 2021;153(3):462‐468. 10.1002/ijgo.13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lokken EM, Huebner EM, Taylor GG, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225(1):77.e1–77.e14. 10.1016/j.ajog.2020.12.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS‐CoV‐2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):eabc3582. 10.1126/sciimmunol.abc3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haye MT, Cartes G, Gutiérrez J, et al. Maternal and perinatal outcomes in pregnant women with confirmed severe and mild COVID‐19 at one large maternity hospital in Chile. The Journal of Maternal‐Fetal & Neonatal Medicine. 2021;1‐6. 10.1080/14767058.2021.1902498 [DOI] [PubMed] [Google Scholar]
- 9. Ayed A, Embaireeg A, Benawadh A, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID‐19 in Kuwait. BMC Pregnancy Childbirth. 2020;20(1):754. 10.1186/s12884-020-03461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shende P, Gaikwad P, Gandhewar M, et al. Persistence of SARS‐CoV‐2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod. 2021;36(4):899‐906. 10.1093/humrep/deaa367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahajan NN, Ansari M, Gaikwad C, et al. Impact of SARS‐CoV‐2 on multiple gestation pregnancy. Int J Gynaecol Obstet. 2021;152(2):220‐225. 10.1002/ijgo.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tilve A, Mahajan NN, Pandey A, et al. Impact of COVID‐19 on pregnant women with Rheumatic heart disease or Peripartum cardiomyopathy. Eur J Obstet Gynecol Reprod Biol. 2021;258:459‐461. 10.1016/j.ejogrb.2021.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bachani S, Arora R, Dabral A, et al. Clinical profile, viral load, maternal‐fetal outcomes of pregnancy with COVID‐19: 4‐week retrospective, tertiary care single‐centre descriptive study. J Obstet Gynaecol Can. 2021;43(4):474‐482. 10.1016/j.jogc.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jafari M, Pormohammad A, Sheikh Neshin SA, et al. Clinical characteristics and outcomes of pregnant women with COVID‐19 and comparison with control patients: a systematic review and meta‐analysis. Rev Med Virol. 2021;e2208. 10.1002/rmv.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, Newborn complications and maternal‐fetal transmission of SARS‐CoV‐2 in women with COVID‐19: a systematic review. MedRxiv. 2020. 10.1101/2020.04.11.20062356 [DOI] [Google Scholar]
- 17. Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID‐19 pandemic on maternal and child mortality in low‐income and middle‐income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901‐e908. 10.1016/S2214-109X(20)30229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuñarro‐López Y, Pintado‐Recarte P, Cueto‐Hernández I, et al. The profile of the obstetric patients with SARS‐CoV‐2 infection according to country of origin of the publication: a systematic review of the literature. J Clin Med. 2021;10(2):360. 10.3390/jcm10020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singhal T. A review of Coronavirus Disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waghmare R, Gajbhiye R, Mahajan NN, Modi D, Mukherjee S, Mahale SD. Universal screening identifies asymptomatic carriers of SARS‐CoV‐2 among pregnant women in India. Eur J Obstet Gynecol Reprod Biol. 2021;256:503‐505. 10.1016/j.ejogrb.2020.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin T‐T, Zhang C, Zhang H‐Q, et al. Thyroid hormone changes in early pregnancy along with the COVID‐19 pandemic. Front Endocrinol (Lausanne). 2020;11:606723. 10.3389/fendo.2020.606723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Athe R, Dwivedi R, Pati S, Mazumder A, Banset U. Meta‐analysis approach on iron fortification and its effect on pregnancy and its outcome through randomized, controlled trials. J Family Med Prim Care. 2020;9(2):513‐519. 10.4103/jfmpc.jfmpc_817_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hariyanto TI, Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID‐19) infection. Transfus Apher Sci. 2020;59(6):102926. 10.1016/j.transci.2020.102926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez‐Perez O, Prats Rodriguez P, Muner Hernandez M, et al. The association between SARS‐CoV‐2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021;21(1):273. 10.1186/s12884-021-03742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cruz‐Lemini M, Ferriols Perez E, de la Cruz Conty M, et al. Obstetric outcomes of SARS‐CoV‐2 infection in asymptomatic pregnant women. Viruses. 2021;13(1):112. 10.3390/v13010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chawanpaiboon S, Vogel JP, Moller A‐B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37‐e46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman MA, Halder HR, Islam SMS. Effects of COVID‐19 on maternal institutional delivery: fear of a rise in maternal mortality. J Glob Health. 2021;11:3041. 10.7189/jogh.11.03041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahajan NN, Kesarwani SN, Shinde SS, et al. Co‐infection of malaria and dengue in pregnant women with SARS‐CoV‐2. Int J Gynaecol Obstet. 2020;151(3):459‐462. 10.1002/ijgo.13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gajbhiye RK, Mahajan NN, Kamath N, et al. Clinical presentations, pregnancy complications, and maternal outcomes in pregnant women with COVID‐19 and tuberculosis: a retrospective cohort study. Int J Gynaecol Obstet. 2021;153(1):176‐179. 10.1002/ijgo.13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhalerao‐Gandhi A, Chhabra P, Arya S, Simmerman JM. Influenza and pregnancy: a review of the literature from India. Infect Dis Obstet Gynecol. 2015;2015:1‐8. 10.1155/2015/867587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335‐1336. 10.3201/eid2606.200287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashary N, Bhide A, Chakraborty P, et al. Single‐cell RNA‐seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS‐CoV‐2. Front Cell Dev Biol. 2020;8:783. 10.3389/fcell.2020.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2021;224(1):35‐53.e3. 10.1016/j.ajog.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


