Abstract
Background
Prenatal exposure to ZIKV has potential teratogenic effects with a wide spectrum of clinical presentation called congenital Zika syndrome (CZS). There are limited data on survival of children with CZS, we estimated mortality comparing live births with and without CZS.
Methods
A population-based cohort study using linked routinely collected data in Brazil, from January 2015 to December 2018. Kaplan-Meier and survival analyses were performed adjusted for confounding and stratified by gestational age, birth weight and small for gestational age.
Results
We followed 11,737,554 live births for up to 36 months. The mortality rate among live births with CZS was 52.6 (95% confidence interval [CI] 47.6-58.0) and among those without CZS it was 5.6 (5.6-5.7) per 1000 person-years. The mortality rate ratio was 11.3 (95%CI 10.2-12.4) times higher among live births with CZS than those without CZS up to the age of 36 months. For infants born before 32 weeks' gestation or with birth weight less than 1500g, the risk of death was similar regardless of CZS. Infants with CZS born at term (mortality rate with and without CZS—38.4 vs 2.7) or with birth weight greater than 2499g (mortality rate with and without CZS—32.6 vs 2.5) were 14.3 (95%CI 12.4-16.4) and 12.9 (95%CI 10.9-15.3) times more likely to die than those without CZS. The burden of congenital anomalies, diseases of the nervous system and infectious diseases, as recorded causes of deaths, were higher among the CZS group.
Conclusion
There is a higher mortality risk in live births with CZS than live births without CZS that persists throughout the first three years of life.
Zika virus (ZIKV) infections during pregnancy can be transplacentally transmitted to developing fetuses with potential teratogenic effects, including severe central nervous system anomalies 1 . Although most fetuses with prenatal ZIKV exposure present no detectable clinical anomalies, a subset of congenitally infected offspring can have mild, moderate or severe adverse outcomes 2 collectively referred to as congenital Zika syndrome (CZS) 3,4 . The CZS phenotype encompasses a wide spectrum of structural anomalies (e.g., cortical atrophy with microcephaly 5 ), functional impairments (e.g., dysphagia 6 ), and clinical sequelae (e.g., epilepsy 7,8 ) that may manifest either at birth or in early life 9 . The prognosis for live births with CZS is not fully described; however, the most severe phenotype seems to be associated with first-trimester exposure 10 and one important prognostic factor is the severity of microcephaly 11 . Preliminary evidence indicates that affected children may experience a case fatality rate of 10% in the first years of life 12 . Nevertheless, the relative risk of mortality among live-born children with CZS compared to live-born children without CZS and the role of important predictors of child mortality such as gestational age at birth and birth weight remains unknown.
Using national population-based linked data on more than 11.7 million live births born between 2015 and 2018 in Brazil, a country with a significant burden of CZS in the recent ZIKV epidemic, this study aimed to: (i) investigate mortality risk and causes of deaths among live births with CZS compared with those without CZS, (ii) compare mortality rates in live births with CZS with and without microcephaly, and by the timing of maternal rash, and (iii) examine the role of gestational age at birth and birth weight by calculating stratified mortality rate ratios.
Methods
Study design
We conducted a retrospective population-based cohort study, including all singletons live births in Brazil from January 1, 2015 to December 31, 2018. These live births were followed-up from birth until December 31, 2018, death, or age of 36 months.
Data source
We obtained information about CZS from the Public Health Event Record 13 , which registered information from all cases with suspected microcephaly and/or central nervous system (CNS) alterations possibly associated with congenital ZIKV infection since 2015. From the Public Health Event Record, we retained data on final disease classification (confirmed or excluded), maternal rash during pregnancy and live birth head circumference.
The Live Births Information System-SINASC 14 , an information system with 100% coverage of the Brazilian territory 15 , records data from the Declaration of Live Births, a legal document completed by the health worker who assists the delivery. From this system, we obtained information about the mother (maternal age, education, marital status, ethnicity); the pregnancy (antenatal appointments, length of gestation, multiple fetuses); and the newborn (birth weight, sex).
Death-related information was obtained from the Mortality Information System, which records the Death Certificates. The death certificate is a legal document that must be completed by the physician responsible for clinical care, an assistant or another practician from the institution who attests to the cause of death. In cases when the death occurs without medical assistance, the death certificate will be provided by a pathologist. We obtained information on the date and the underlying cause of death by International Classification of Diseases/ICD 10. As of 2011, Mortality Information System was estimated to cover 96.1% of deaths in Brazil 16 .
Linkage process
Live births records from Live Births Information System were linked with the Public Health Event Record and Mortality Information System. Name, date of birth or age, and maternal residence were used as matching variables. The linkage was performed with the Center of Data and Knowledge Integration for Health -RL-Record Linkage; a novel record-linkage tool developed to link large-scale administrative datasets at Center of Data and Knowledge Integration for Health applying the combination of indexing and searching algorithms approach 17 . All data were extracted in 2020 and made available by the Brazilian Ministry of Health. Linkage procedures were conducted at Center of Data and Knowledge Integration for Health in a strict data protection environment and according to ethical and legal rules 18 .
Procedures
We included all singleton live births who contributed records during the study period. We excluded records registered in the Public Health Event Record as suspected of CZS but ruled out after epidemiological investigation and those classified as cases under investigation or inconclusive.
In Brazil, live births who meet one or more of the following criteria should be reported and investigated as suspected cases of CZS: 1) microcephaly defined as an head circumference more than 2 standard deviations below the mean for age and sex (according to INTERGROWTH 21st standards 19 for those born with less than 37 gestation weeks, or World Health Organization (WHO) standards for those born with 37 gestation weeks or more); 2) craniofacial disproportion (microcrania in relation to the face); CNS changes suggestive of congenital infection detected from neuro-imaging tests (accepted imaging were cranial computed tomography, brain magnetic resonance, or transfontanellar ultrasound); two or more neurological, visual or auditory manifestations; 3) newborns or fetuses from mothers who reported a fever and/or skin rash during pregnancy, likely or confirmed for ZIKV infection, regardless of the identification of congenital malformations at birth 20,21 . After notification, all suspected cases were investigated by the epidemiological surveillance teams and classified as confirmed, probable, inconclusive or excluded cases (Figure S2).
Suspected cases were considered confirmed when they had signs and symptoms consistent with CZS (Table S2), and laboratory evidence of ZIKV infection (from molecular or serological testing) or their mother reported fever and/or rash during pregnancy. Probable cases showed clinical changes compatible with CZS, and tested negative for other congenital infections, but the specific laboratory diagnosis for ZIKV infection was not available, and the mother was asymptomatic during pregnancy. Suspected cases were considered excluded if they had compatible clinical symptoms that, after clinical and epidemiological investigation, were attributed to having another cause, for example, microcephaly related to restricted intrauterine growth or genetic diseases. Other cases were inconclusive due to insufficient information for proper classification or remained under investigation 20 .
We defined CZS cases as all live births classified in the Public Health Event Record as confirmed or probable cases and had a register linked with a Live Births Information System record. We then classified live births with CZS into two categories: with microcephaly (head circumference more than 2 standard deviations below the mean for age and sex) or without microcephaly, according to INTERGROWTH 21st standards. We also classified CZS cases according to the time of maternal rash during pregnancy (first trimester, second trimester, third trimester, or no report of rash during pregnancy). Live births that died during the study period were identified by linking the Live Births Information System with Mortality Information System.
Statistical analyses
Descriptive statistics are presented for maternal sociodemographic data and newborn characteristics. Mortality rates (deaths/1,000-person-year (PY) and crude hazard ratios (HRs) with 95% CIs comparing live births with CZS to live births without CZS were estimated using Cox proportional hazards models. We also conducted a sensitivity analysis with confirmed cases only. Kaplan-Meier curves were plotted, and we compared live births with and without CZS in total, CZS according to head circumference (classified as with microcephaly and without microcephalic) and CZS according to maternal rash during pregnancy compared with live births without CZS. Finally, we fitted penalized Cox proportional hazards regression models using restricted maximum likelihood with frailty terms corresponding to random effects from a Gaussian distribution to account for within-cluster (maternal residency region) homogeneity in outcomes 22 . We used these analyses to obtain the adjusted HR. The adjusted models were controlled for maternal age, education, marital status, ethnicity, number of prenatal appointments, newborn sex and year of birth and stratified by gestational age (< 32, 32-36, ≥37 weeks), birth weight (<1500, 1500-2499, ≥2500 grams) and small for gestational age (smaller than the 10th centile according to the INTERGROWTH 21st standards). For the time scale in our survival analyses, we used age attained within the study, neonatal mortality (up to 27 days), infant mortality (up to 364 days) and mortality up to 36 months. Data analyses were performed in Stata version 15.0.
This study analyzed de-identified data and was approved by the Institute of Collective Health, Federal University of Bahia Research Ethics Committee (CAAE registration no. 70745617 2 0000 5030).
Results
We followed 11,737,554 live births from birth up to 36 months (mean 23 [range 0–36]) (Figure S1). The characteristics of our study population are reported in Table 1. In general, live births with CZS (n=3,308) had mothers who were younger and less educated. Nearly 20% of the live births with CZS were born preterm, 36% were low birth weight (LBW), and 37% were small for gestational age (SGA) compared with 10.2% preterm births, 7.3% LBW and 6.9% SGA among live births without CZS (n=11,477,907).
Table 1. Baseline characteristics (n and %) of singleton live births in the cohort-linked data by congenital Zika syndrome status, Brazil 2015-2018.
| Characteristics | With CZS | Without CZS | ||
|---|---|---|---|---|
| n | % | N | % | |
| Maternal age (years) | ||||
| < 20 | 769 | 23.2 | 1,958,784 | 17.1 |
| 20-34 | 2,179 | 65.9 | 7,922,774 | 69.0 |
| 35+ | 360 | 11.9 | 1,596,199 | 13.9 |
| Maternal marital status | ||||
| Single/Widow/Divorced | 1,736 | 53.1 | 5,046,501 | 44.0 |
| Married/union | 1,532 | 46.9 | 6,303,447 | 54.9 |
| Maternal education (Years) | ||||
| none | 25 | 0.8 | 54,922 | 0.5 |
| 1 - 3 | 106 | 3.3 | 264,514 | 2.3 |
| 4 – 7 | 749 | 23.0 | 1,932,786 | 16.8 |
| 8 – 12 | 2,011 | 61.8 | 6,834,566 | 59.5 |
| 12 + | 365 | 11.2 | 2,225,266 | 19.4 |
| Maternal race/ethnicity | ||||
| White | 584 | 18.8 | 3,987,857 | 36.4 |
| Black, Mixed and others | 2,517 | 81.2 | 6,973,896 | 63.6 |
| Number of prenatal appointments | ||||
| None | 51 | 1.6 | 64,992 | 0.6 |
| 0-3 | 307 | 9.7 | 683,661 | 6.0 |
| 4 – 6 | 923 | 29.1 | 2,609,078 | 22.7 |
| 7+ | 1,895 | 59.7 | 7,824,331 | 68.2 |
| Year of birth | ||||
| 2015 | 1.215 | 36.7 | 2,945,913 | 25.7 |
| 2016 | 1538 | 46.5 | 2,794,266 | 24.3 |
| 2017 | 364 | 11.0 | 2,857,930 | 24.9 |
| 2018 | 191 | 5.8 | 2,879,798 | 25.1 |
| Sex of the newborn | ||||
| Female | 1,757 | 53.2 | 5,591,949 | 48.7 |
| Male | 1,543 | 46.8 | 5,883,957 | 51.3 |
| Birth region | ||||
| North | 181 | 5.5 | 1,237,863 | 10.8 |
| Northeast | 1,986 | 60.0 | 3,224,654 | 28.1 |
| Southeast | 801 | 24.2 | 4,508,232 | 39.3 |
| South | 73 | 2.2 | 1,553,930 | 13.5 |
| Midwest | 265 | 8.0 | 949,767 | 8.3 |
| Gestational age at birth (weeks) | ||||
| <32 | 110 | 3.4 | 155,151 | 1.4 |
| 32-36 | 535 | 16.7 | 988,036 | 8.8 |
| 37 + | 2,552 | 79.8 | 10,085,776 | 89.8 |
| Birth weight (g) | ||||
| < 1500 | 155 | 4.7 | 127,967 | 1.1 |
| 1500-2499 | 1,048 | 31.7 | 706,939 | 6.2 |
| 2500+ | 2,103 | 63.6 | 10,632,209 | 92.7 |
| Small for gestational age | ||||
| No | 2,000 | 63.4 | 10,317,503 | 89.9 |
| Yes | 1,156 | 36.6 | 794,313 | 6.9 |
896,987 missing data
By the end of the study period, 398 live births who met the study case definition criteria of CZS and 120,629 live births without CZS had died. The overall mortality rate up to 36 months in live births with CZS was 11.3 times higher (95% CI, 10.2-12.4) than in live births without CZS. The increased risk of death persisted throughout the observation period with no suggestion of attenuation by the age of three years in children with CZS. The highest mortality rate ratio, 21.9 (95% CI, 17.3-27.6), was found after the first year of life when the mortality in children without CZS was 0.7 deaths/1000 PY (Table 2). The analysis restricted to confirmed CZS cases showed similar results (Table S1).
Table 2. Mortality risk by age group among singleton live births in the cohort-linked data, Brazil, 2015-2018.
| Live births | Live births with CZS | Live births without CZS | HR for mortality (95% CI) |
|---|---|---|---|
| Neonatal mortality (up to 27 days) | |||
| Neonatal deaths | 163 | 80,006 | 7.2 (6.2-8.4) |
| Deaths per 1,000 PY | 696.5 (597.3 -812.0) | 95.7 (95.0 - 96.3) | |
| Post-neonatal mortality (28-364 days) | |||
| Post-neonatal deaths | 163 | 32,175 | 17.4 (14.9-20.3) |
| Deaths per 1,000 PY | 59.4 (51.0 - 69.3) | 3.5 (3.5 - 3.6) | |
| Infant mortality (up to 364 days) | |||
| Infant deaths | 326 | 112,181 | 10.2 (9.1-11.3) |
| Deaths per 1,000 PY | 109.2 (98.0 - 121.8) | 11.3 (11.2 - 11.3) | |
| Mortality after one year (12-36 months) | |||
| Deaths after 1 year | 72 | 8,448 | 21.9 (17.3-27.6) |
| Deaths per 1,000 PY | 15.7(12.4-19.7) | 0.7 (0.7-0.8) | |
| Total mortality (up to 36 months) | |||
| Total Deaths | 398 | 120,629 | 11.3 (10.2-12.4) |
| Deaths per 1,000 PY | 52.6 (47.6-58.0) | 5.6 (5.6-5.7) | |
PY= person years
CZS = congenital Zika syndrome
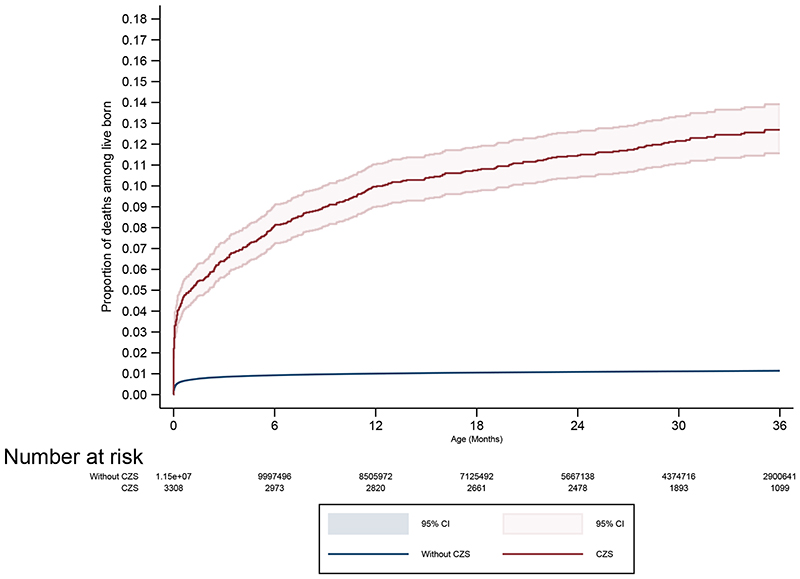
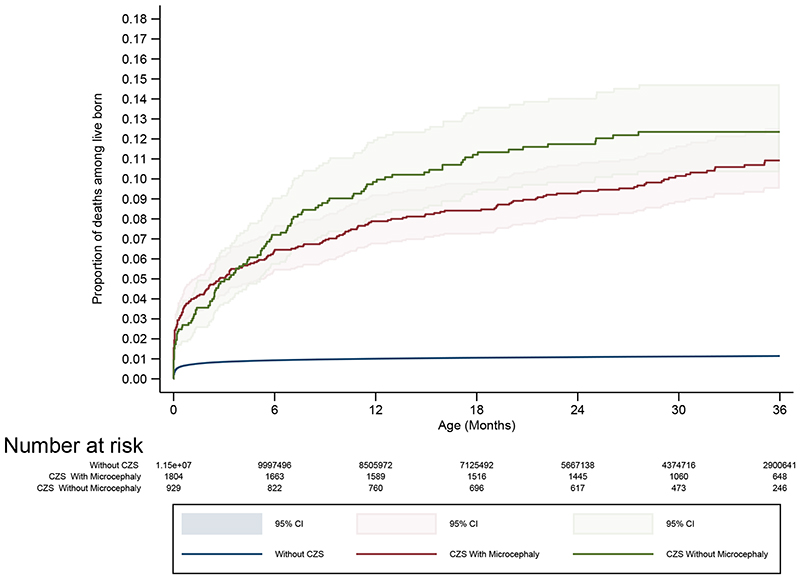
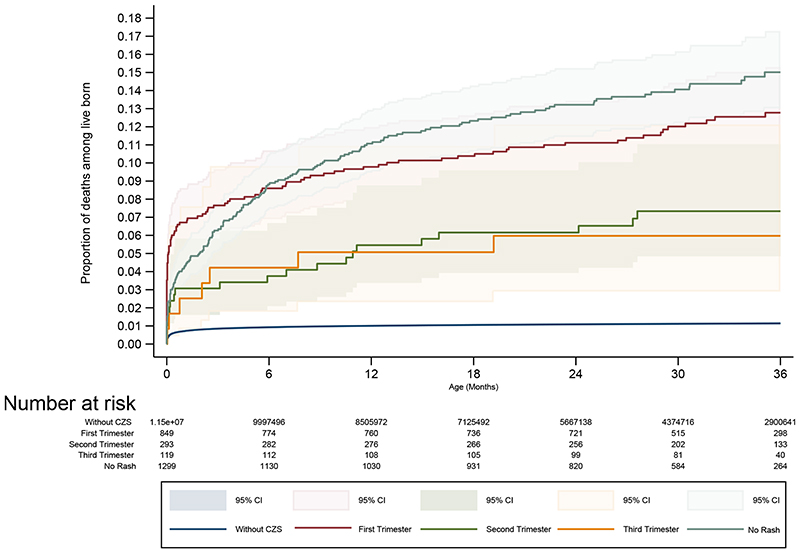
The likelihood of death in the study population from birth up to 36 months is seen in Figure 1. Among live births with CZS the probability of death was greater than in live births without CZS, and the difference continued to increase during the study period. Information on head circumference was available for 82.6% (2,733/3,308) of live births with CZS: 66.0% had microcephaly at birth. Among live births with CZS and complete information on maternal rash, 51.2% (1,364/2,663) reported this symptom during pregnancy, with 62.2% of exanthems reported in the first trimester, 21.5% the second, 8.7% the third and 7.6% with unspecified trimester. The association of CZS on mortality up to 36 months did not materially differ for live births with and without microcephaly or for whether mothers reported a rash during pregnancy and the timing of any rash. However, numbers were relatively small, and we may have lacked the power to identify sub-group differences (Figure 1).
Figure 1. Kaplan-Meier curves showing survival comparing CZS births with non-CZS births up to 36 months of age (A), stratified by head circumference (B) and maternal rash (C).
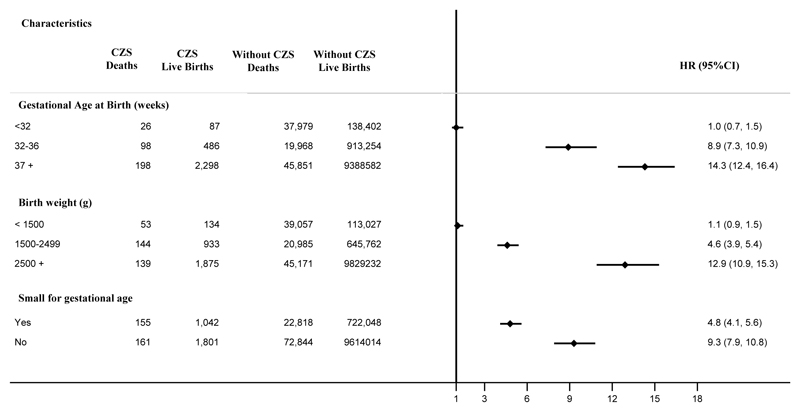
Mortality rates differ across gestational age, birth weight and small for gestational age categories. Live births with and without CZS had similar absolute mortality rates if they were born before 32 weeks or weighed less than 1500g. If they were born after 32 weeks, live births with CZS were more likely to die than live births without CZS, and the highest ratios were observed among children born term and with normal birth weight; in these groups, live births with CZS were 14.3 (95% CI, 12.4-16.4) and 12.9 (95% CI, 10.9-15.3) times more likely to die when compared with their counterpart live births without CZS, respectively. Small for gestational age live births with CZS were almost five times more likely to die, while appropriate for gestational age children were nine times more likely to die than their counterpart live births without CZS (Figure 2).
Figure 2. Cox proportional hazards for mortality risk comparing CZS births with non-CZS births up to 36 months of age stratified by gestational age at birth, birth weight and small for gestational age.
*CZS: Congenital Zika Syndrome; HR: Hazard Ratio; CI: Confidence Interval
* adjusted by maternal age, education, and marital status, ethnicity, number of prenatal appointments, new born sex and year of birth
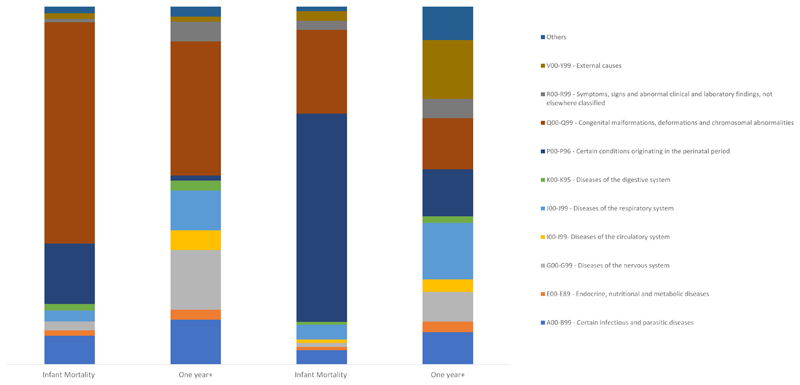
Among infants with CZS, the causes of deaths coded under ICD-10 certain infectious and parasitic diseases (A00-B99), diseases of the nervous system (G00-G99), and congenital malformations (Q00-Q99) were two times more common than among infant without CZS. The leading causes were sepsis, unspecified organism; hydrocephalus unspecified; and microcephaly, respectively. After the first year of life, the nervous system diseases and congenital anomalies continue to be leading chapters. However, for the G00-G99 chapter, the most common cause of death in this age group was cerebral palsy. The circulatory system diseases (I00-I99) were responsible for 58% more deaths in the CZS group than live birth without CZS (Figure 3). The causes of deaths were cardiomyopathy, other cardiac arrhythmias, and heart failure.
Figure 3. Causes of death using ICD-10 chapter according to age group by CZS status Brazil, 2015-2018.
CZS: Congenital Zika Syndrome
Discussion
Analyses of Brazilian national, registry-based data showed that the mortality rates up to 3 years old were more than eleven times greater in live births with CZS than live births without CZS overall. Among live births with CZS, mortality risk by microcephaly status and maternal reported rash did not materially differ. The risk of death among the smallest infants was similar regardless of CZS status; however, term and normal birth weight live births with CZS were over 12 times more likely to die than their counterparts without CZS. Finally, we observed that conditions classified as congenital malformations, diseases of the nervous system and certain infectious diseases were more common causes of deaths among live births with CZS than those without CZS.
Data on mortality associated with CZS are scant. In a series of confirmed CZS cases in Brazil, mortality risk up to 8 days was estimated at 41.1 per 1000 live births 23 . In a population-based surveillance study conducted among all infants and fetuses with congenital abnormalities potentially related to Zika virus infection in the USA, the neonatal mortality risk was 45.9 per 1000 live births 24 . A similar risk was observed in our study. However, only a longer follow-up could have revealed that after the neonatal period the absolute mortality rates in the live births with CZS did not decrease as dramatically over time as they did among live births without CZS.
Our understanding of the impact of CZS on the brain is still emerging; the effects of CNS dysfunction with or without microcephaly are expected to result in a wide variety of outcomes 4,25 . Although some studies have shown that the most severe phenotype appears to be associated with exposure during the first trimester 25,26 , neither early exposure (using rash as a proxy) nor microcephaly status showed difference in the mortality risk, potentially due to the small number of events. Therefore, the role of head circumference and timing of maternal symptoms of ZIKV infection as risk factors for death among children with CZS cannot be fulled assessed.
Previous studies have shown that live births with CZS had greater frequencies of LBW 27 and SGA 28,29 , features compatible with higher child mortality. However, the absolute mortality risks in the smallest infants did not differ according to CZS status. Moreover, in the CZS group, term normal-weight infants, who would have had a high chance of thriving without the impairments resulting from CZS, are at strikingly elevated mortality risk. Children with CZS have multiple neurological complications and long-term sequelae, such as cerebral palsy that was one of the main causes of deaths identified in this study and epilepsy, estimated in 67% in this group 30 , that confer an increased risk of death. However, a better understanding of the causal mortality chain is needed.
A strength of our study was the large sample size, including all confirmed and probable CZS cases notified in the country. We also included a population-representative comparison group and were able to control for confounding. The sensitivity analyses (including only confirmed cases) showed consistency of our findings. There are, however, limitations. First, the present study was based on registry data, and relevant clinical data were not available. Second, at the beginning of the epidemic, the health services network did not have specific diagnostic tests for ZIKV infections. Therefore, there may have been underreporting in the Public Health Event Record, mainly among those fetuses prenatally exposed to ZIKV, but without detectable malformations at birth. Third, the linkage process could have introduced classification bias due to a linkage error. However, if an error occurred in the linkage that enabled the exposure assessment (Public Health Event Record) it would have likely underestimated the measure of association. If the error occurred in the linkage that enabled the outcome assessment (Live Births Information System - Mortality Information System) it is probably non-differential and unlikely to introduced bias in the measure of association, although the absolute measures of risk may be underestimated. There was a slight variation in data completeness by CZS status. However, data on all variables were more than 80% complete in both groups.
Our findings draw attention to the importance of primary prevention of infection in women (of childbearing age and pregnant women) against Aedes aegypti bites.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Supplementary Material
Acknowledgments
We thank the data production team Center of Data and Knowledge Integration for Health /FIOCRUZ collaborators for their work linking these data and for providing information on data quality. We also thank the IT team for making all the efforts to help us access the data in as smooth away as possible. We also thank Tiago Mendes Cruz and Kerry Wong for their help with the figures.
Funding
This study is part of the "Long-term surveillance platform for Zika and microcephaly under the Unified Health System" and it is funded by the Secretary of Health Surveillance, Ministry of Health of Brazil. EPS is funded by the Wellcome Trust [Grant number 213589/Z/18/Z]. Wellcome Trust & the UK Department for International Development (205377/Z/16/Z). ESP is funded by the Wellcome Trust Grant number 213589/Z/18/Z. EBB received funding from the British Council Newton Fund (527418645), European Union's Horizon 2020 Research and Innovation programme under ZikaPLAN (734584);The funders had no role in study design, analysis, decision to publish or preparation of the manuscript.
This research was funded in part by the Wellcome Trust. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
CDC disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declarations
We declare no competing interests.
References
- 1.Musso D, Ko AI, Baud D. zika Virus Infection — After the Pandemic. N Engl J Med. 2019 doi: 10.1056/nejmra1808246. [DOI] [PubMed] [Google Scholar]
- 2.Pomar L, et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: Prospective cohort study in French Guiana. BMJ. 2018 doi: 10.1136/bmj.k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Barros Miranda-Filho D, et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health. 2016 doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CA, et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatrics. 2017 doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Campo M, et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet Part A. 2017 doi: 10.1002/ajmg.a.38170. [DOI] [PubMed] [Google Scholar]
- 6.da Oliveira DMS, et al. Comparison of Oropharyngeal Dysphagia in Brazilian Children with Prenatal Exposure to zika Virus, With and Without Microcephaly. Dysphagia. 2020 doi: 10.1007/s00455-020-10173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho MDCG, et al. Early epilepsy in children with Zika-related microcephaly in a cohort in Recife, Brazil: Characteristics, electroencephalographic findings, and treatment response. Epilepsia. 2020 doi: 10.1111/epi.16444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quilião ME, et al. Neurological development, epilepsy, and the pharmacotherapy approach in children with congenital Zika syndrome: Results from a two-year followup study. Viruses. 2020 doi: 10.3390/v12101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Oliveira Melo AS, et al. Congenital Zika virus infection: Beyond neonatal microcephaly. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen SA, Jamieson DJ. Teratogen update: Zika virus and pregnancy. Birth Defects Res. 2020;112 doi: 10.1002/bdr2.1781. [DOI] [PubMed] [Google Scholar]
- 11.Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clinical Microbiology Reviews. 2016 doi: 10.1128/CMR.00014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N Costa MC, et al. Case Fatality Rate Related to Microcephaly Congenital zika Syndrome and Associated Factors: A Nationwide Retrospective Study in Brazil †. Viruses. 2020 doi: 10.3390/v12111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministério da Saúde do Brasil. RESP-Registro de Eventos Em Saúde Pública. 2017. http:%0A//www.resp.saude.gov.br/microcefalia#/painel .
- 14.Paulo S, de S de S SM. Manual de Preenchimento da Declaração de Nascido Vivo Prefeito do Município de São Paulo. 2011:1–24. [Google Scholar]
- 15.de Bonilha EA, et al. Cobertura, completude e confiabilidade das informações do Sistema de Informações sobre Nascidos Vivos de maternidades da rede pública no município de São Paulo, 2011. Epidemiol e Serv saude Rev do Sist Unico Saude do Bras. 2018 doi: 10.5123/s1679-49742018000100011. [DOI] [PubMed] [Google Scholar]
- 16.Ministério da Saúde. SIM. Sistema de Informações sobre Mortalidade. Datasus. 2017 [Google Scholar]
- 17.Barbosa GCG, et al. CIDACS-RL: a novel indexing search and scoring-based record linkage system for huge datasets with high accuracy and scalability. BMC Med Inform Decis Mak. 2020 doi: 10.1186/s12911-020-01285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto ML, et al. The Center for Data and Knowledge Integration for Health (CIDACS) Int J Popul Data Sci. 2019 doi: 10.23889/ijpds.v4i2.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papageorghiou AT, et al. International standards for fetal growth based on serial ultrasound measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014 doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 20.Ministério da Saúde (BR) Orientações integradas de vigilância e atenção à saúde no âmbito da Emergência de Saúde Pública de Importância Nacional. Ministério da Saúde; 2017. [Google Scholar]
- 21.BRASIL. emergência de saúde pública de importância internacional-ESPII. 2a edição. 2016. Protocolo de Vigilância e resposta à ocorrência de Microcefalia e/ou Alteraçõe do sistema nervoso central central (SNC) p. 55. 10/03/2016. [Google Scholar]
- 22.Austin PC. A tutorial on multilevel survival analysis: Methods, models and applications. Int Stat Rev. 2017 doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.França GVA, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016 doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 24.Smoots AN, et al. Population-Based Surveillance for Birth Defects Potentially Related to zika Virus Infection — 22 States and Territories, January 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6903a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler AC. Development of infants with congenital zika syndrome: What do we know and what can we expect? Pediatrics. 2018 doi: 10.1542/peds.2017-2038D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molnár Z, Kennedy S. Neurodevelopmental disorders: Risks of Zika virus during the first trimester of pregnancy. Nature Reviews Neurology. 2016 doi: 10.1038/nrneurol.2016.71. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho-Sauer R, da Costa MCN, Barreto FR, Teixeira MG. Congenital Zika Syndrome: Prevalence of low birth weight and associated factors. Bahia, 2015-2017. Int J Infect Dis. 2019 doi: 10.1016/j.ijid.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016 doi: 10.1056/nejmoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper HJ, et al. Maternal zika Virus Infection: Association with Small-for-Gestational-Age Neonates and Preterm Birth. Obstet Gynecol. 2019 doi: 10.1097/AOG.0000000000003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Linden H, et al. Epilepsy Profile in Infants with Congenital zika Virus Infection. N Engl J Med. 2018 doi: 10.1056/nejmc1716070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.