Abstract
The clinical value of the polygenetic component of blood pressure (BP) is commonly questioned. We evaluated a genetic risk score for BP (BP-GRS858), based on the most recently published genome-wide association studies variants that were significantly associated with either systolic BP or diastolic BP, for prediction of hypertension and cardiovascular end points. The genotyping was performed in 2 urban-based prospective cohorts: the Malmö Diet and Cancer (n=29 295) and the Malmö Preventive Project (n=9367) and a weighted BP-GRS858 based on 858 SNPs was calculated. At baseline, we found a difference of 9.0 mm Hg (systolic BP) and 4.8 mm Hg (diastolic BP) between the top and the bottom quartile of BP-GRS858. In Malmö Preventive Project, the top versus bottom quartile of BP-GRS858 was associated with a doubled risk of incident hypertension (odds ratio, 2.05 [95% CI, 1.75–2.39], P=1.4×10−21), a risk higher than that of body mass index, as evaluated in quartiles. In Malmö Diet and Cancer, significant association was found between the age and sex-adjusted BP-GRS858 and the incidence of total cardiovascular events, stroke, coronary artery disease, heart failure, atrial fibrillation, and total mortality. Most of these associations remained significant after adjusting for traditional risk factors, including hypertension. BP-GRS858 could contribute predictive information regarding future hypertension, with an effect size comparable to other well-known risk factors such as obesity, and predicts cardiovascular events. Given that the exposure to high polygenetic risk starts at birth, we suggest that the BP-GRS858 might be useful to identify children or adolescents who would benefit from early hypertension screening and treatment.
Keywords: atrial fibrillation, blood pressure, coronary artery disease, genotype, hypertension
Hypertension affects 1.13 billion people worldwide, is a leading cause of cardiovascular events, and accounts for almost 10 million deaths annually. 1 Blood pressure (BP) and hypertension are highly heritable traits, and their regulation depend on a typically polygenic contribution 2 in which each of the genes gives a small contribution to the phenotypes. Understanding the genetics of hypertension and the underlying pathophysiological mechanisms can be important even to develop an effective risk prediction model. 3
Genetic risk score (GRS) is a common tool used to aggregate the effect of many genetic variants identified through genome-wide association studies (GWAS), into a single score. 4 Its importance relies on its potential usage as a clinical tool for the identification of high-risk segments of the population to a certain complex disease. 5 GWAS consortia have continuously increased power to identify new BP loci by expanding numbers of studies included in meta-analyses, leading to increasing number of single nucleotide polymorphisms (SNPs) associated with BP-related traits. In the latest meta-analyses of GWAS, more than 900 loci and more than 1000 variants have been associated with BP traits. 6,7 Common concerns have been that the majority of BP genetic variants identified by GWAS, especially those detected after the first comprehensive meta-analyses 8 have so small individual effect sizes that they have no clinical meaning even when added together into a score. Results based on UK biobank data among ≈360 000 individuals, clearly argued against such concerns by showing clinically meaningful differences in systolic BP (SBP; ≈13 mm Hg) between top and bottom deciles of GRS. 6 Before clinical implementation of these findings can be considered, they need to be replicated using individual level data in population-based settings outside the UK biobank. Moreover, even if the GRS is unchanged throughout life, its value in the prospective setting is unknown, that is, what does it add in predicting future hypertension among nonhypertensive individuals.
In this work, we constructed a GRS for BP (BP-GRS858) using 858 genetic variants significantly associated to BP traits derived from the recent meta-analyses. 6,7 We investigated its association to BP traits, hypertension incidence, and cardiovascular end points in 2 large Swedish urban-based cohorts (Malmö Preventive Project [MPP] and Malmö Diet and Cancer [MDC]) to test its performance as a predictive tool, also in comparison with other well-known risk factors. Finally, we estimated how much additional information the BP-GRS858 contributed with on cross sectional BP on top of a previously reported GRS including the 29 top hits for BP (BP-GRS-29). 9
Nonstandard Abbreviation and Acronyms
- BMI
body mass index
- BP
blood pressure
- BP-GRS
genetic risk score for blood pressure traits made up by 858 SNP
- DBP
diastolic BP
- GRS
genetic risk score
- GWAS
genome-wide association studies
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MDC
Malmö Diet and Cancer
- MPP
Malmö Preventive Project
- SBP
systolic BP
- SNP
single nucleotide polymorphism
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Populations, Genotyping
Malmö Preventive Project
Hypertension prevalence and incidence was studied in the MPP, an urban-based prospective study including BP measurement both at a baseline exam of Malmö citizens between 1974 and 1992 and at a re-examination between 2002 and 2006 in 18 240 individuals. 9 Of those, 9367 had undergone GWAS genotyping.
In MPP at baseline BP was measured after 1-minute rest in the supine position followed by another measurement after 1 minute in an upright standing position. This procedure was repeated also after 10 minutes of resting. For those subjects with a least 3 valid measurements, BP values were averaged and used for the analysis. At reinvestigation 2 measures in supine position were recorded. For those subjects with at least 2 valid measures, we used the averaged BP values.
Malmö Diet and Cancer
Cross sectional BP, hypertension prevalence, and incidence of cardiovascular events were studied in the MDC, a large-scale urban population-based cohort consisting of 30 447 individuals (58±7.6 years) from Malmö, Sweden. Of these, 29 386 were genotyped for GWAS. Men aged 46 to 73 and women aged 45 to 73 included between 1991 and 1996 were included. 10,11 At the baseline exam, BP was measured once in the supine position after 5 minutes rest. Measurement of other covariates are explained in the Data Supplement. Cardiovascular diseases (the sum of coronary artery diseases and stroke), stroke, coronary artery disease (consisting of coronary events, coronary artery bypass graft, or percutaneous coronary intervention), heart failure, atrial fibrillation, and total mortality were used as end points in the survival analysis. Details about the definitions of cardiovascular events and their codes are presented in the Data Supplement. End points referred to a follow-up time until 31 December 2016. Mean follow-up time across the population was 19.7±5.6 years.
Anthropometric and Metabolic Measures
Laboratory analyses were performed according to standard methods. Whole blood glucose and lipids were determined after an overnight fast and analyzed at the Department of Clinical Chemistry, Malmö University Hospital. Glomerular filtration rate was computed applying the Chronic Kidney Disease Epidemiology Collaboration equation for creatinine in mg/dL. Information about medical history, anthropometric data, lifestyle and socio-economic factors, and family history of hypertension in first-degree relatives was derived from the baseline questionnaire. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). BMI categories were defined depending on BMI values <18.5 kg/m2 or BMI >18.5 kg/m2 <25 kg/m2 or BMI >25<30 or BMI >30 kg/m2
Definition of Hypertension
In both cohorts, hypertension was defined as either being on treatment with antihypertensive drugs or having either SBP ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg according to current European guidelines. 1 In the cross sectional BP analyses in MDC, BP was adjusted using a stepped addition method that took into account the number of antihypertensive drugs as previously described, 9,12 that is, 8/4, 14/10, 20/16, and 26/22 mm Hg were added to SBP/DBP when individuals were treated by 1, 2, 3, and 4 drugs, respectively. In the MPP, since the number of antihypertensive drugs was not available, we adjusted SBP/DBP, respectively, for 15 and 10 mm Hg in presence of antihypertensive therapy.
Genotyping of both cohorts was performed using the Illumina GWAS chip (GSA v1 array) Information about genotyping is provided in the Data Supplement.
Genetic Risk Score
A weighted score, BP-GRS858, was constructed adding up all risk alleles (from previous GWAS studies) of the SNPs that resulted GWAS-significant association hypertension, SBP and DBP. 6,7 β-coefficients used for individual variant weighting and effect allele were derived from recent meta-analyses. 6,7 The genome analysis tool-set PLINK (v 1.9; http://pngu.mgh.harvard.edu/purcell/plink/) and the web-based application LDLink were used to compute the score and to assess SNPs independency, respectively. 13,14
Further details and the list of variants, the weights, and the effect allele used to derive the score are provided in the Data Supplement.
Population has been stratified based on quartiles and deciles of the distribution of BP-GRS858.
Statistics
GRS and Cross-Sectional BP Analyses in MDC
BP-GRS858 was standardized as well as categorized in quartiles and deciles. Continuous variables are presented as mean±SD. In MDC, a linear regression model was used to test the association of the GRS with cross-sectional BP-related traits. Two models were tested for the analysis: model (A) adjusting for age, sex, and BMI; model (B) model A plus adjustment for the BP-GRS we previously constructed including 29 SNPs (BP-GRS29). 9 This last test was done to evaluate the improvement added by the BP-GRS858 with respect to the BP-GRS29. The r2 and the partial r2 for each model is presented allowing a comparison of the effect size of different risk factors. In particular, it is possible to appreciate the effect size of being in extreme quartiles of either BMI or BP with respect to the effect size of the BP-GRS858.
One-way ANOVA was used to evaluate differences among quartiles and deciles in terms of SBP and DBP.
Hypertension Prevalence and Incidence
A logistic regression model was used to assess the association of the BP-GRS858 with the prevalence of hypertension at baseline both in MDC and MPP and with hypertension incidence at reinvestigation, after a mean follow-up time of 23.0±4.7 years, in the MPP. For the analysis about the prevalence of hypertension in MPP, 9156 among the 9367 had data sufficient for a correct classification both at baseline and at reinvestigation. For the analysis about hypertension incidence, among the 9156 subjects, 3135 who were already hypertensive at baseline were excluded, leaving 6021 individuals for the analysis. In the MDC cohort, among the 29 292 who have complete data for BP classification at baseline, 11 325 were normotensive.
Since different covariates were available for the 2 cohorts, different adjustment models were adopted. For MPP, we used the following 3 models: (A) age, sex, age×sex, age2, heart rate; (B) as in model A plus BMI, diabetes (fasting blood glucose ≥6.1 mmol/L), hypercholesterolemia (total serum cholesterol >5.17 mmol/L or specific treatment), hypertriglyceridemia (serum tryglicerydes ≥1.7 mmol/L), HDL (high-density lipoprotein)-cholesterol, smoking status, family history of hypertension (first relative); (C) as in model B plus SBP, glomerular filtration rate, physical activity level, and civil status. Follow-up time from baseline was also added as a covariate to the models in the longitudinal analyses. The area under the curve was evaluated before and after adding the BP-GRS858 to model C.
Incidence of Cardiovascular End Points in MDC
For MDC the following models were constructed: (A) age, sex, age2, age×sex; (B) as model A plus obesity, diabetes, APOA (apolipoprotein A), APOB (apolipo-protein B), smoking status; (C) model B plus SBP or DBP. Survival analysis using COX proportional hazard models were used to evaluate the role of the BP-GRS858 in the incidence of cardiovascular end points in MDC. All subjects that already encountered cardiovascular events before baseline evaluation were excluded from the analysis. Three models were used for adjustment. Covariates in the models were respectively: (A) age and sex, (B) as in model A plus obesity, diabetes, APOA, APOB, smoking, (C) as in model B plus hypertension. Both continuous and quartiles of GRS were analyzed both in longitudinal and in survival analysis. Heart failure and mortality end points were also adjusted for cardiovascular disease and coronary artery disease.
Statistical analyses were performed by SPSS software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) and R software (R Core Team, 2018, v 3.5.1). P<0.05 was considered significant.
Results
Genetic Risk Score
Eight hundred fifty-eight SNPs were included in the BP-GRS858. The list of genetic variants, effect alleles, and weights used to construct the BP-GRS858 are reported in the Data Supplement (Tables S1 and S2 in the Data Supplement), as well as general characteristics of the populations and distribution of the BP-GRS858 (Tables S3 and S4; Figures S1 and S2).
Cross Sectional Association of BP-GRS858 to BP in MDC
In the MDC, the BP-GRS858 (model A) was highly significantly associated with all tested BP traits, as shown in Table S5. One SD increase in the BP-GRS858 corresponded to an increment of 3.3 mm Hg of SBP (β, 3.3 [95% CI, 3.1–3.5]; P=7.46×10−200) and 1.7 mm Hg of DBP (β, 1.7 [95% CI, 1.6–1.9]; P=5.07×10−206). The results remained significant also after adjustment for the BP-GRS29 9 both for SBP (β, 3.1 [95% CI, 2.9–3.4]; P=2.4×10−195) and for DBP (β, 1.7 [95% CI, 1.5–1.8]; P=1.8×10−147) as also shown in the Table S5 (model B). The association is maintained also when using fixed adjustment for both traits (results not shown). Results for the analysis replicated in the MPP are presented in the Table S6.
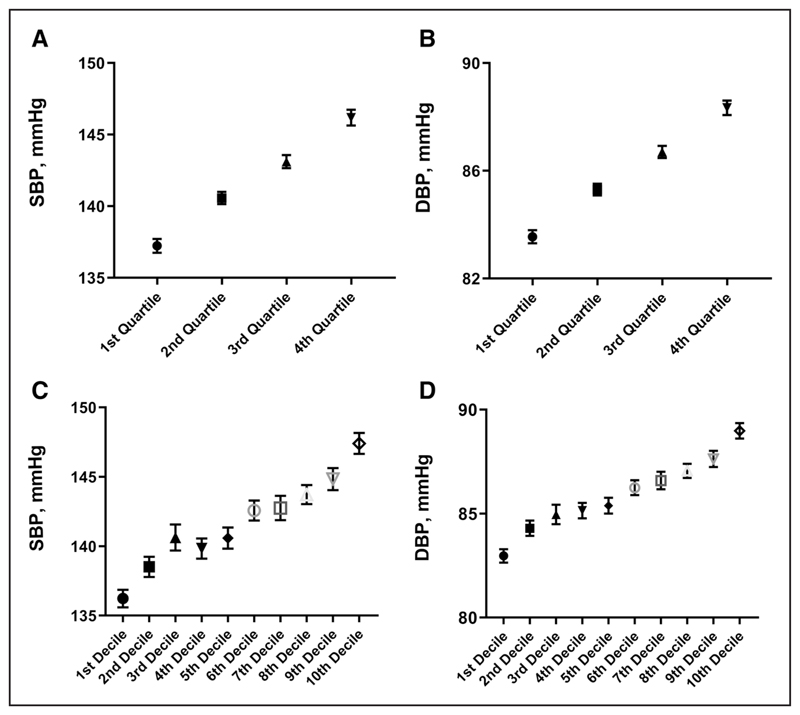
When comparing the top versus the bottom quartiles and deciles of BP-GRS858, respectively, we found a difference of 9.0/11.2 mm Hg for SBP (Figure 1A and 1C) and 4.8/6.0 mm Hg for DBP (Figure 1B and 1D).
Figure.
Difference in mmHg between quartiles and deciles of BP-GRS858. A, C: difference in systolic blood pressure; B, D: difference in diastolic blood pressure
Prevalence of Hypertension in MDC and MPP
Analyzing the prevalence of hypertension in the MDC, we found that a single SD increase in the BP-GRS858 was highly significantly associated with the prevalence of hypertension (odds ratio [OR], 1.45 [95% CI, 1.41–1.49]; P=1.1×10−171 for model A). When comparing the highest versus the lowest quartiles of the BP-GRS858, we found an odds ratio of 2.7 (OR, 2.78 [95% CI, 2.56–3.01], P=1.1×10−138) that increased to 3.7 when comparing extreme deciles (3.71 [3.32–4.14], P=4.22×10−118), in model A. The association was significant also in the MPP where the BP-GRS858, resulted associated with the prevalence of hypertension, when analyzed either as SD increase (OR, 1.35 [95% CI, 1.29–1.41], P=1.4×10−37), or as extreme quartiles (2.14 [1.88–2.44], P=3.22×10−30) or deciles (2.95 [2.39–3.64], P=7.50×10−24), respectively. The effect size of the associations was attenuated but remained significant when adjusting for other covariates in different models (Table S7).
Incidence of Hypertension in the MPP
The BP-GRS858 was significantly associated with the incidence of hypertension in the MPP cohort (Table 1) in all the tested models. In the first model, subjects in the top quartile and top decile of BP-GRS858 showed a more than doubled incidence of hypertension respect to the ones in the bottom quartile or decile (OR, 2.05 [95% CI, 1.75–2.39], P=1.4×10−21); (2.13, [1.67–2.71] P=2.2×10−8), respectively (Table 1). In the statistical model including all covariates except the BP-GRS858 for the prediction of hypertension incidence, the area under the curve was 0.71 (0.69–0.72), which had a nonstatistically significant increase to 0.72 (0.70–0.73) when adding the BP-GRS858 (Figure S3).
Table 1. Association of BP-GRS858 With Incidence of Hypertension in MPP.
| Incidence of hypertension | ||||||
|---|---|---|---|---|---|---|
| Variables | Model A, n=5971* | Model B, n=5819* | Model C, n=5639* | |||
| OR (95% CI) | P value† | OR (95% CI) | P value† | OR (95% CI) | P value† | |
| BP-GRS858 ‡ | 1.31 (1.24–1.38) | 3.7×10−22 | 1.32 (1.25–1.40) | 3.14×10−22 | 1.26 (1.19–1.34) | 1.09×10−14 |
| Fourth vs first quartile (68.8% vs 53.1% HT) | 2.05 (1.75–2.39) | 1.4×10−21 | 2.09 (1.78–2.46) | 2.24×10−21 | 1.90 (1.60–2.25) | 1.36×10−14 |
| Tenth vs first decile (68.6 vs 52.1% HT) | 2.13 (1.67–2.71) | 2.2×10−18 | 2.19 (1.71–2.81) | 3.84×10−18 | 1.83 (1.40–2.38) | 1.63×10−11 |
BMI indicates body mass index; GFR, glomerular filtration rate; HDL, high-density lipoprotein; HT, Hypertension; and OR, odds ratio.
Model A: age, sex, age2, age×sex, heart rate, follow-up time; model B: as model A plus BMI, diabetes, hypercholesterolemia, hypertriglyceridemia, HDL, smoking status, family history of hypertension; and model C: as model 2 plus systolic or diastolic blood pressure, GFR, physical activity level and civil status.
P value for trend.
BP-GRS858 is expressed as continuous variable for SD increment.
Comparison With BMI and Other Traditional Risk Factors
In the Table 2, the contribution of each covariate included in model C for incident hypertension in the MPP is shown. Stratification in quartiles of other important factors traditionally associated with hypertension such as either BMI or baseline BP and the BP-GRS858, allows the comparison of the effect size in the fully adjusted model. In the MPP, the contribution of the BP-GRS858 is fairly similar to that of the other well-known factors (see table). We also evaluated the area under the curve after the addition of either BMI or diabetes to the fully adjusted model (without BP-GRS858), and we obtain no increment in area under the curve: 0.71 (0.70–0.72) before and after adding BMI or diabetes, respectively.
Table 2. Contribution That Each Variable Included in Model C Gives to the Incidence of Hypertension in the MPP.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age, y | 1.15 | 1.02–1.29 | 0.022 |
| Sex | 8.97 | 2.91–2768 | 0.0001 |
| BP-GRS858 | |||
| Second vs first quartile | 1.28 | 1.10–1.50 | 0.001 |
| Third vs first quartile | 1.67 | 1.42–1.96 | 3.75×10−10 |
| Fourth vs first quartile | 1.94 | 1.64–2.29 | 1.07×10−14 |
| BMI | |||
| Second vs first quartile | 1.31 | 1.13–1.53 | 0.001 |
| Third vs first quartile | 1.38 | 1.17–1.82 | 0.0001 |
| Fourth vs first quartile | 1.51 | 1.25–1.82 | 1.32×10−5 |
| SBP, mm Hg | |||
| Second vs first quartile | 1.90 | 1.67–2.18 | 2.55×10−21 |
| Third vs first quartile | 3.28 | 2.78–3.88 | 1.61×10−44 |
| Fourth vs first quartile | 5.68 | 3.76–8.58 | 1.71×10−16 |
| Diabetes | 1.63 | 1.06–2.51 | 0.03 |
| Family history of hypertension | 1.19 | 1.05–1.36 | 0.01 |
| Smoking status | 1.66 | 1.46–1.88 | 6.01×10−15 |
| Heart rate, bpm | 1.01 | 1.00–1.02 | 0.02 |
| Follow-up time | 1.12 | 1.10–1.15 | 5.04×10−19 |
| Hypercholesterolemia | 1.07 | 94–1.21 | ns |
| Hypertrygliceridemia | 1.02 | 84–1.23 | ns |
| GFR, mL/min per 1.73 m2 | 1.00 | 1.00–1.00 | ns |
| HDL-cholesterol, mmoL/L | 0.69 | 0.59–0.80 | 6.16×10−7 |
| Level of physical activity | 1.04 | 0.90–1.19 | 0.62 |
| Civil state | 0.97 | 0.85–1.11 | ns |
BMI indicates body mass index; BP-GRS858, genetic risk score for blood pressure traits made up by 858 SNPs; GFR, estimate glomerular filtration rate; HDL, high-density lipoprotein; MPP Malmö Preventive Project; OR, odds ratio; and SBP systolic blood pressure.
Cardiovascular End Points in the MDC
We found that the BP-GRS858 was significantly associated to the risk of cardiovascular disease, stroke, coronary artery disease, heart failure, atrial fibrillation, and total mortality in the MDC (Table 3). The risk of cardiovascular events was significantly higher comparing the top and the bottom quartile or decile of BP-GRS858 as shown in Table 3. Most of the associations were maintained also in the models adjusted for traditional risk factors. In particular, in the model A and B (Table 3), the BP-GRS858 remained associated to all events, while in the third model, after adjustment for hypertension, the association with heart failure, atrial fibrillation, and with total mortality were no longer statistically significant (Table 3).
Table 3. Role of BP-GRS858 in Cardiovascular End Points.
| Events | Model A | Model B | Model C | |||
|---|---|---|---|---|---|---|
| BP-GRS858 (for 1 SD increase) | ||||||
| n (model A/model B/model C) | HR (95% CI) | P value* | HR (95% CI) | P value* | HR (95% CI) | P value* |
| CVD (n=22 579/n=20 543/n=20 524) | 1.12 (1.10–1.15) | 1.9×10−18 | 1.11 (1.08–1.14) | 6.6×10−14 | 1.08 (1.05–1.1 1) | 1.0×10−7 |
| Stroke (n=28 962/n=26 222/n=26 202) | 1.14 (1.10–1.19) | 7.5×10−14 | 1.13 (1.09–1.18) | 3.3×10−11 | 1.10 (1.06–1.14) | 1.2×10−6 |
| Coronary artery disease (n=28 552/n=25 857/n=25 837) | 1.13 (1.10–1.17) | 2.3×10−15 | 1.12 (1.08–1.15) | 3.2×10−11 | 1.09 (1.05–1.12) | 7.6×10−7 |
| Heart failure (n=29 207/n=24 791/n=24 7221) | 1.13 (1.08–1.19) | 3.2×10−5 | 1.13 (1.08–1.19) | 4.1×10−7 | 1.09 (1.04–1.15) | 0.0005 |
| Atrial fibrillation (n=24 120/n=21 784/n=21 763) | 1.06 (1.03–1.09) | 3.2×10−5 | 1.06 (1.03–1.09) | 0.0001 | 1.03 (1.003–1.065) | 0.03 |
| Total mortality (n=29 295/n=26 512/n=1 7 307) | 1.05 (1.03–1.07) | 4.0×10−6 | 1.04 (1.02–1.07) | 7.8×10−5 | 1.02 (1.00–1.04) | 0.046 |
| Fourth vs first quartile of BP-GRS858 | ||||||
| n (model A-model B-model C) | HR (95% CI) | P value* | HR (95% CI) | P value* | HR (95% CI) | P value* |
| CVD (n=22579/n=20543/n=20 524) | 1.42 (1.31–1.54) | 2.0×10−16 | 1.38 (1.27–1.50) | 2.8×10−12 | 1.27 (1.17–1.39) | 5.2×10−7 |
| Stroke (n=28 962/n=26 222/n=26 202) | 1.44 (1.29–1.60) | 1.1×10−9 | 1.41 (1.26–1.58) | 3.0×10−9 | 1.29 (1.15–1.45) | 1.4×10−5 |
| Coronary artery disease (n=28 552/n=25 857/n=25 837) | 1.46 (1.33–1.60) | 2.0×10−13 | 1.40 (1.27–1.54) | 3.5×10−11 | 1.30 (1.17–1.43) | 3.5×10−7 |
| Heart failure (n=29 207/n=24 791/n=24 7221) | 1.37 (1.19–1.57) | 1.1×10−5 | 1.37 (1.19–1.59) | 0.0002 | 1.24 (1.070–1.44) | 0.031 |
| Atrial fibrillation (n=24 120/n=21 784/n=21 763) | 1.15 (1.05–1.25) | 0.002 | 1.13 (1.04–1.24) | 0.006 | 1.06 (0.968–1.16) | ns |
| Total mortality (n=29 295/n=26 512/n=1 7 307) | 1.12 (1.06–1.19) | 0.0002 | 1.10 (1.03–1.17) | 0.007 | 1.04 (0.978–1.1 1) | ns |
| 10th vs first quartile of BP-GRS858 | ||||||
| n (model A-model B-model C) | HR (95% CI) | P value* | HR (95% CI) | P value* | HR (95% CI) | P value* |
| CVD (n=22 579/n=20 543/n=20 524) | 1.48 (1.33–1.65) | 1.3×10−13 | 1.41 (1.26–1.59) | 3.6×10−10 | 1.28 (1.14–1.44) | 1.8×10−5 |
| Stroke (n=28 962/n=26 222/n=26 202) | 1.57 (1.36–1.82) | 3.2×10−8 | 1.52 (1.30–1.77) | 1.8×10−7 | 1.35 (1.16–1.58) | 0.0002 |
| Coronary artery disease (n=28 552/n=25 857/n=25 837) | 1.56 (1.369–1.77) | 1.3×10−11 | 1.48 (1.30–1.70) | 1.1×10−8 | 1.35 (1.18–1.54) | 1.6×10−5 |
| Heart failure (n=29 207/n=24 791/n=247 221) | 1.53 (1.27–1.86) | 0.002 | 1.57 (1.28–1.92) | 0.006 | 1.38 (1.12–1.69) | ns |
| Atrial fibrillation (n=24 120/n=21 784/n=21 763) | 1.21 (1.08–1.37) | 0.001 | 1.23 (1.09–1.39) | 0.018 | 1.14 (1.04–1.28) | ns |
| Total mortality (n=29 295/n=26 512/n=1 7 307) | 1.13 (1.37–1.22) | 0.005 | 1.10 (1.01–1.20) | 0.034 | 1.02 (0.94–1.12) | ns |
BP-GRS858 indicates genetic risk score for blood pressure traits made up by 858 SNP; CVD, cardiovascular diseases; and HR, hazard ratio.
P value for trend.
Discussion
We constructed a new GRS for BP (BP-GRS858) using the most recent SNPs consistently associated with BP traits in published meta-analyses 6,7 to evaluate its performance with respect to previous score that used fewer SNPs. In particular, we tested the association of the BP-GRS858 with BP phenotypes, especially the incidence of hypertension that was not assessed in previous studies, and cardiovascular end points in 2 urban-based Swedish cohorts, the MPP and the MDC.
We included 858 independent variants associated either to SBP or DBP, a considerably larger number compared with the previous 29 SNPs-based score (BP-GRS29) that we tested in the same cohorts. 9,12 The BP-GRS858, as expected, was highly significantly associated to BP traits and 1 SD increase of age-sex adjusted BP-GRS858 corresponded to an increase of 3.3 mm Hg for SBP and 1.7 mm Hg for DBP in MDC. These associations are almost 3× higher than the ones found using our previous score in which 1 SD increasing of BP-GRS29 corresponded to 1 and 0.7 mm Hg for SBP and DBP respectively. 9 Supported further by quartile and decile analyses, this clearly shows that adding SNPs discovered more recently on top of the 29 initial top hits add significant information despite very low effect sizes of the individual variants.
We then investigated the role of BP-GRS858 on hypertension incidence, an information not available in previous studies. Individuals in the top quartile of BP-GRS858 showed a risk 2× higher to become hypertensive respect to the one in the bottom one, independently from the other main risk factors of hypertension such as family history of hypertension, baseline BP, obesity, and diabetes. Moreover, the effect size is even higher than that of BMI, when quartiles of the BP-GRS858 were compared with quartiles of BMI in the same model. This shows that BP-GRS858 adds more predictive information regarding future risk of hypertension than one of the most established clinically used risk factors for prediction of hypertension, that is, BMI or family history of hypertension. In particular, about family history of hypertension, even if it is generally true that offspring of hypertensive parents have a higher risk to have high BP, not all hypertensive have a positive family history. 15 This is due to the fact that hypertension is a highly polygenic disease as demonstrated by the fact that almost 1000 SNPs are recognized as associated with BP/hypertension. Thus, the family history of hypertension is a too row marker of the possible development of hypertension with respect to considering all these variants in a GRS.
Moreover, it can be a possibility that some confounding comes from methodological issues arising in the familiarity of hypertension measurement and studying. 16 Finally, in the MDC, we tested the effect of the BP-GRS858 on cardiovascular end points and found an association of the age and sex-adjusted BP-GRS858 with total cardiovascular events, stroke, coronary artery disease, heart failure, atrial fibrillation, and total mortality, as in previous studies. 6 Most of the associations remained significant also in the fully adjusted model in which also hypertension was included.
Our results are in line with previous studies: that is, in the Airwave cohort a difference of 6.0/9.3 mm Hg was found for SBP between highest and lowest quintile of a BP-GRS (267 variants) in individuals of <50/≥50 years, respectively, and 3.7 and 4.6 mm Hg for DBP. 17 Warren et al reported a difference of 10.4/12.9 mm Hg of SBP and a difference and 6/7.5 mm Hg comparing top and bottom quintile/decile of a sex-adjusted GRS, built by 901 SNPs. 6 In the Airwaive cohort, a 2.0 OR higher for hypertension prevalence in individuals in the top quintile of sex-adjusted BP-GRS267 was identified 17 ; whereas in the UK Biobank the risk of hypertension was 3.3 higher between the top and bottom decile. 6
Our findings confirm that adding novel genetic variants into a BP-GRS, despite the very low effect size of individual variants, increases its predictive performance. The BP-GRS858 is able to explain a meaningful variation in terms of mm Hg, since it is demonstrated that a reduction of ≈1 to 5 mm Hg is associated with lower cardiovascular events. 18,19 Based on our results, we can state that the newer BP-GRS858 shows a relevant ability of prediction of the individual risk. In particular, the score could be important in identifying those individuals that have a higher risk to become hypertensive, more likely to develop cardiovascular disease due to their genetic predisposition. Those individuals could potentially benefit from early identification of the risk, even during young adulthood or adolescence, by implementing a healthy behavior. As screening of measured BP is usually not applied routinely until middle-age, another potential clinical implication is introduction of screening with BP measurement in adolescence and early adulthood in subjects with high genetic risk, that is, belonging to the top quartile or decile of the BP-GRS858. However, before any benefit of such strategies can be judged, the performance of the BP-GRS should be confirmed and evaluated also in this age span, even if some evidence, at least for the BP-GRS29 is already available. 20 Moreover, in previous studies, other GRS tailored to cardiovascular events seems to work better in younger individuals, 21 and it has been already shown that a favorable lifestyle based on no smoking, no obesity, regular physical activity, and healthy diet can counteract the effects of genetic susceptibility to coronary artery disease. 22
Only randomized clinical trials could give definitive information about the feasibility and consistency of tailored interventions and their subsequent benefit. A couple of pilot trials, mainly focused on cholesterol, were conducted in a small group of people with cardiovascular risk factors with encouraging results. In the first study, it was found a significant decrease of LDL (low-density lipoprotein)-cholesterol level in subjects who were randomized to have the incorporation of a cardiovascular GRS in the estimate of risk for coronary heart disease in addition to the assessment of traditional risk factors. 23 The second trial evaluated the effect of including the GRS for coronary artery disease as a motivator to obtain an improvement in the control of cardiovascular risk factors in randomized patients. Contrary to the previous study, the authors did not find a significant improvement in the LDL-cholesterol level in the GRS arm (n=49), but found a beneficial effect on weight loss and physical activity. 24 Both these studies did not find an association between the level of anxiety and the genetic test, but disclosure of genetic information about future risk of disease bring lots of ethical problems, and it is potentially accompanied also by the risk of harm leading to anxiety in patients with high risk. 23
The BP-GRS858 resulted also to be, at least partially, independent from the other traditional risk factors that could manifest during life, suggesting that the contribution of the genetic component on the development of the diseases is not eliminated by other risk factors and can bring additional information of a comparable effect size. Of note, even if this speculation holds true for hypertension incidence, coronary artery disease and stroke, our data did not support an effect of the GRS that is independent of measured BP for other important diseases such as atrial fibrillation and congestive heart failure.
Moreover, this genetic information is potentially available during periods of life more prone to the change in lifestyle being significantly modified by educational interventions such as childhood, adolescence, or early adulthood. 25,26 However, the translation into clinical context has to take into consideration ethical and social concerns 27,28 ; moreover, the cost-effectiveness of this strategy has not been proved yet.
Our study has limitations: the generalizability to other ethnicities or age groups except Swedish middle-aged individuals is not known. We could use only APOA and APOB plasma levels as substitutes for LDL and HDL-cholesterol. It is likely that other SNPs associated to BP-related traits could have been included in the GRS to increase its performance or that the use of a polygenic risk score based on all the genetic variants genotyped in an individual, including rare variants (<1% minor allele frequency), regardless even to the specific association with BP/hypertension could give even better results, as recently shown in the UK Biobank sample for the risk of coronary artery disease, atrial fibrillation, type 2 diabetes, inflammatory bowel disease, and breast cancer. 29 Additional studies would need to address these questions. Among these, functional analyses that investigates quantitative trait loci involved in BP regulation could be useful to uncover how the association found by GWAS could contributes to pathophysiology of hypertension. 30,31
Perspectives
In conclusion, a BP-GRS based on 858 independent SNPs, chosen because previously associated at genome-wide level with BP phenotypes, is associated with all the BP-associated traits, including hypertension incidence, and with CV events, even after adjustment for traditional risk factors. The magnitude of the association and the capacity to predict quite large differences in BP, and events can render the BP-GRS858 potentially useful for clinical practice, being available even during childhood, adolescence, and young adulthood. Before implementing this strategy, other important issues, both from a technical and ethical point of view, should be solved.
Supplementary Material
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.15449.
Novelty and Significance.
What Is New?
A new genetic risk score for blood pressure (BP-GRS858) was constructed including 858 variants associated, in previous studies, to BP-related traits. Its association with hypertension and cardiovascular events was tested in two Swedish cohorts.
What Is Relevant?
The strength of the association of the BP-GRS858 with the incidence of hypertension and cardiovascular diseases was comparable to that of other traditional risk factors such as BMI, making it potentially useful for clinical practice.
Summary
Our results show that adding more variants to a BP-GRS increases its performance to predict incident hypertension and cardiovascular events.
Sources of Funding
The study was supported by grants from the Knut and Alice Wallenberg Foundation, the Göran Gustafsson Foundation, the European research Council (AdG-885003), the Swedish Heart- and Lung Foundation, the Swedish Research Council, the Novo Nordisk Foundation, Region Skåne and Skåne University Hospital and the Swedish Foundation for Strategic Research for IRC15-0067.
Footnotes
Disclosures
None.
Contributor Information
Alice Giontella, Department of Medicine, University of Verona, Verona, Italy; Department of Clinical Sciences, Clinical Research Center, Lund University, Malmö, Sweden.
Marketa Sjögren, Department of Clinical Sciences, Clinical Research Center, Lund University, Malmö, Sweden.
Luca A. Lotta, Regeneron Genetics Center, Tarrytown, NY
John D. Overton, Regeneron Genetics Center, Tarrytown, NY
Aris Baras, Regeneron Genetics Center, Tarrytown, NY.
Pietro Minuz, Department of Medicine, University of Verona, Verona, Italy.
Cristiano Fava, Department of Medicine, University of Verona, Verona, Italy; Department of Clinical Sciences, Clinical Research Center, Lund University, Malmö, Sweden.
Olle Melander, Department of Clinical Sciences, Clinical Research Center, Lund University, Malmö, Sweden; Department of Emergency and Internal Medicine, Skåne University Hospital, Malmö, Sweden.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Blencowe M, Shi X, Shu L, Levian C, Ahn IS, Kim SK, Huan T, Levy D, Yang X. Integrative genomics analysis unravels tissue-specific pathways, networks, and key regulators of blood pressure regulation. Front Cardiovasc Med. 2019;6 doi: 10.3389/fcvm.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Wang J-G. Genome-wide association studies of hypertension and several other cardiovascular diseases. Pulse. 2018;6:169–186. doi: 10.1159/000496150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28:R133–R142. doi: 10.1093/hmg/ddz187. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CM, Vassos E. Prospects for using risk scores in polygenic medicine. Genome Med. 2017;9:96. doi: 10.1186/s13073-017-0489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Million Veteran Program. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. doi: 10.1038/s41588-018-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, Torstenson ES, Kovesdy CP, Sun YV, Wilson OD, et al. Understanding Society Scientific Group; International Consortium for Blood Pressure; Blood Pressure-International Consortium of Exome Chip Studies; Million Veteran Program. Transethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51:51–62. doi: 10.1038/s41588-018-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fava C, Sjögren M, Montagnana M, Danese E, Almgren P, Engström G, Nilsson P, Hedblad B, Guidi GC, Minuz P, et al. Prediction of blood pressure changes over time and incidence of hypertension by a genetic risk score in Swedes. Hypertension. 2013;61:319–326. doi: 10.1161/HYPERTENSIONAHA.112.202655. [DOI] [PubMed] [Google Scholar]
- 10.Berglund G, Elmstähl S, Janzon L, Larsson SA. The malmo diet and cancer study Design and feasibility. J Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö diet and cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 12.Fava C, Ohlsson T, Sjögren M, Tagetti A, Almgren P, Engström G, Nilsson P, Hedblad B, Minuz P, Melander O. Cardiovascular consequences of a poly-genetic component of blood pressure in an urban-based longitudinal study: the Malmö diet and cancer. J Hypertens. 2014;32:1424–1428. doi: 10.1097/HJH.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 13.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandi AM, Gaudio G, Fachinetti A, et al. Hyperinsulinemia, family history of hypertension, and essential hypertension. Am J Hypertens. 1996;9:732–738. doi: 10.1016/0895-7061(96)00095-7. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein IB, Shapiro D, Guthrie D. Ambulatory blood pressure and family history of hypertension in healthy men and women. Am J Hypertens. 2006;19:486–491. doi: 10.1016/j.amjhyper.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B, Ntalla I, Surendran P, Liu C, Cook JP, et al. International Consortium of Blood Pressure (ICBP) 1000G Analyses, The CHD Exome+ Consortium, The ExomeBP Consortium, The T2D-GENES Consortium, The GoT2DGenes Consortium, The Cohorts for Heart and Ageing Research in Genome Epidemiology (CHARGE) BP Exome Consortium, The International Genomics of Blood Pressure (iGEN-BP) Consortium; UK Biobank CardioMetabolic Consortium BP working group Corrigendum: genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49:1558. doi: 10.1038/ng1017-1558a. [DOI] [PubMed] [Google Scholar]
- 18.Verdecchia P, Gentile G, Angeli F, Mazzotta G, Mancia G, Reboldi G. Influence of blood pressure reduction on composite cardiovascular endpoints in clinical trials. J Hypertens. 2010;28:1356–1365. doi: 10.1097/HJH.0b013e328338e2bb. [DOI] [PubMed] [Google Scholar]
- 19.Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PR, Folsom AR, Heiss G, MacLehose RF, Matsushita K, Avery CL. Reducing the blood pressure-related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. 2015;4:e002276. doi: 10.1161/JAHA.115.002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zafarmand MH, Spanjer M, Nicolaou M, Wijnhoven HAH, van Schaik BDC, Uitterlinden AG, Snieder H, Vrijkotte TGM. Influence of dietary approaches to stop hypertension-type diet, known genetic variants and their interplay on blood pressure in early childhood: ABCD study. Hypertens. 2020;75:59–70. doi: 10.1161/HYPERTENSIONAHA.118.12292. [DOI] [PubMed] [Google Scholar]
- 21.Tada H, Melander O, Louie JZ, Catanese JJ, Rowland CM, Devlin JJ, Kathiresan S, Shiffman D. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur Heart J. 2016;37:561–567. doi: 10.1093/eurheartj/ehv462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullo IJ, Kullo IJ, Jouni H, Austin EE, Brown SA, Kruisselbrink TM, Isseh IN, Haddad RA, Marroush TS, Shameer K, Olson JE, et al. Incorporating a genetic risk score into coronary heart disease risk estimatesCLINICAL PERSPECTIVE. Circulation. 2016;133:1181–1188. doi: 10.1161/CIRCULATIONAHA.115.020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowles JW, Zarafshar S, Pavlovic A, Goldstein BA, Tsai S, Li J, McConnell MV, Absher D, Ashley EA, Kiernan M, et al. Impact of a genetic risk score for coronary artery disease on reducing cardiovascular risk: a pilot randomized controlled study. Front Cardiovasc Med. 2017;4:53. doi: 10.3389/fcvm.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krosnick JA, Alwin DF. Aging and susceptibility to attitude change. J Pers Soc Psychol. 1989;57:416–425. doi: 10.1037//0022-3514.57.3.416. [DOI] [PubMed] [Google Scholar]
- 26.Siegel BS, Dobbins MI, Earls MF, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into life-long health. Pediatrics. 2012;129 doi: 10.1542/peds.2011-2662. [DOI] [PubMed] [Google Scholar]
- 27.Palk AC, Dalvie S, de Vries J, Martin AR, Stein DJ. Potential use of clinical polygenic risk scores in psychiatry - ethical implications and communicating high polygenic risk. Philos Ethics Humanit Med. 2019;14:4. doi: 10.1186/s13010-019-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19:581–590. doi: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]
- 29.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng AY. Genetic basis of polygenic hypertension. Hum Mol Genet. 2007;16(2):R195–R202. doi: 10.1093/hmg/ddm126. [DOI] [PubMed] [Google Scholar]
- 31.Deng AY, Ménard A. Biological convergence of three human and animal model quantitative trait loci for blood pressure. J Hypertens. 2020;38:322–331. doi: 10.1097/HJH.0000000000002267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.