Abstract
Blood vessels form a versatile transport network that is best known for its critical roles in processes such tissue oxygenation, metabolism, and immune surveillance. In addition, the vasculature provides local, often organ-specific molecular signals that control the behaviour of other cell types in their vicinity during development, homeostasis, and regeneration but also in disease processes. In the skeletal system, the local vasculature is an active player both in bone formation and resorption. In addition, blood vessels participate in inflammatory processes and contribute to the pathogenesis of rheumatoid arthritis and osteoarthritis. This review summarizes the current understanding of the architecture, angiogenic growth and functional properties of the bone vasculature. We also discuss the impact of ageing and several pathological conditions.
Introduction
Blood vessels represent an extensively branched and hierarchically organized system of endothelial tubules, which, with a few exceptions such as cartilage and the lens of the eye, extends into every tissue in the body. The transport of a wide range of different cargoes, including hormones, gases, nutrients, waste products, and circulating cells, is the main function of this vascular network. Meeting physiological demands in the majority of organs requires cooperation with a second endothelial system, the lymphatic vasculature, which mediates liquid homeostasis, nutrient uptake, and immune surveillance 1,2 . In the skeletal system, lymphatic vessels are normally absent and the emergence of ectopic lymphatics is associated with massive osteolysis and progressive bone loss in human disorders such as Gorham–Stout disease 3,4 . Likewise, changes affecting the blood vessel network, which is the focus of this article, are associated with the progression of bone diseases including cancer and osteoporosis 5,6 . Disruption of the vascular supply to bone, which can be caused by a variety of conditions including bone fracture, joint dislocation, accidentally during surgery, or high-dose corticosteroid treatment, will trigger osteonecrosis characterized by massive local cell death 7–9 . Conversely, ectopic angiogenic blood vessel growth and inflammation of the synovial membrane, a layer of connective tissue lining the inner surface of articular joint capsules, are closely integrated processes in the pathogenesis of osteoarthritis (OA) 10,11 . These few examples illustrate why it is critical to understand fundamental features of the vasculature in the skeletal system, its crosstalk with other cell types, and the molecular signals controlling bone homeostasis and repair but also pathobiological processes.
Endothelial cell (EC) networks in different organs exhibit specialized morphological features and gene expression profiles, which reflect different functional roles 12–15 . While the vasculature in lung, for example, is specialized for gas exchange, it participates in blood ultrafiltration in kidney, supports metabolic processes in liver, or is part of the blood-brain barrier protecting the central nervous system against potentially toxic substances and immune cells from the circulation. In addition, ECs are frequently a source of paracrine (so-called “angiocrine”) acting molecular signals, which control the behaviour of other cell types in the surrounding tissue 16,17 . EC-derived instructive signals have been shown to regulate endodermal cells during the liver and pancreas development in the early mouse embryo 18,19 . In addition to crucial roles in directing hepatic and pulmonary development, angiocrine signals control the regeneration of these organs after tissue injury 20–22 . Moreover, vascular endothelium provides protective and nurturing niches for multiple adult stem cell populations such as neural stem cells 23,24 , spermatogonial stem cells 25 , muscle stem cells 26 , and hepatic progenitors 27 . In the skeletal system, ECs and vessel-associated reticular cells provide niche microenvironments for hematopoietic stem cells with great implications for lifelong blood formation in the healthy organism but also hematologic diseases like leukaemia 28–31 . Similarly, ECs communicate with osteoprogenitor cells during bone development and fracture healing 32–34 . The identification of different capillary subtypes with distinct locations and functional roles in long bone has further enhanced our understanding of the heterogeneity and specialization of the bone vasculature. These findings also shed new light on bone development and homeostasis but also on osteoporosis, osteoarthritis, ageing and fracture healing, which we will discuss in this review article.
Vessels in skeletal development
Blood vessels in endochondral ossification
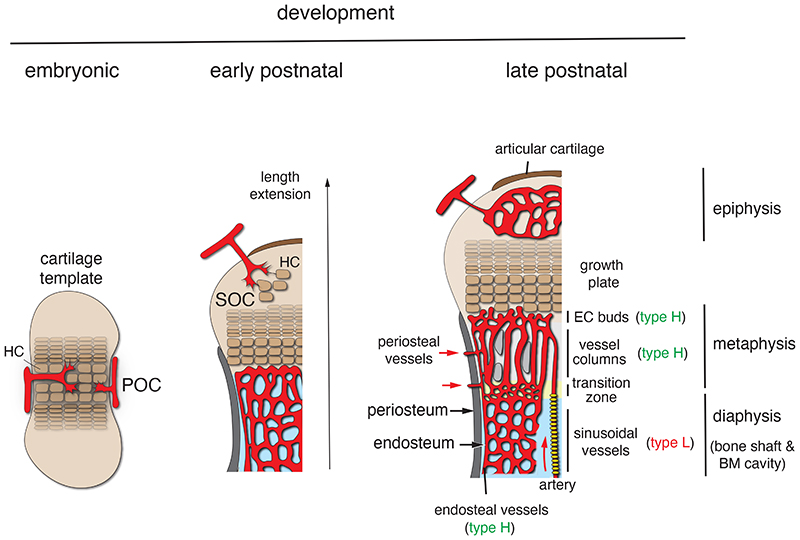
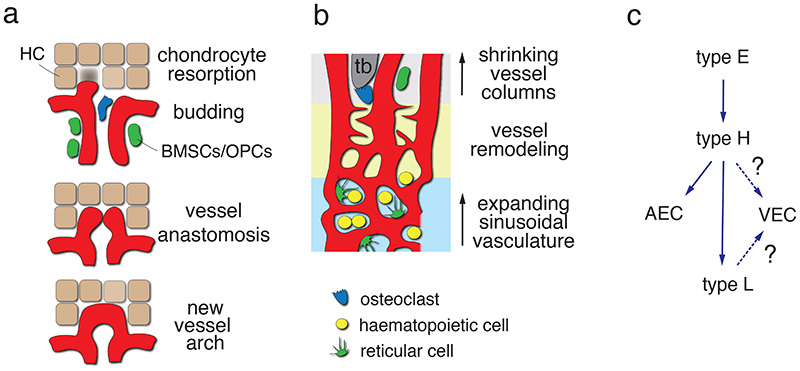
The generation of skeletal elements during development can involve two distinct modes of ossification. Flat bones such as cranium and ilium are generated through the direct conversion of mesenchymal cells into bone forming cells (osteoblasts) in a process termed intramembranous ossification. In contrast, endochondral ossification, which involves the formation of an intermediate cartilage template that is subsequently converted into calcified tissue, generates the majority of the skeletal structures including the appendicular skeleton and vertebrae 35,36 . These processes have been predominantly studied in animal models and this article refers to findings in mice unless mentioned otherwise. The invasion of growing blood vessels is an important step in all modes of osteogenesis and is triggered by extracellular matrix and signals such as vascular endothelial growth factor A (VEGF-A). VEGF-A is a known master regulator of angiogenesis, which activates signalling through VEGFR2, a receptor tyrosine kinase expressed by endothelial cells (ECs) but also by osteoprogenitors and other cell populations 37–39 . Conversely, hypertrophic chondrocytes and osteogenic progenitors are major sources of VEGF-A and thereby regulate angiogenesis in bone 38,40–42 . Angiogenesis involves EC proliferation and, in most developing and regenerating organs, the emergence of endothelial sprouts from pre-existing vessels 43 . Pointed, filopodia-extending endothelial protrusions extend from the periosteal vasculature during the vascularization of the femoral cartilage shaft in the embryo and lead to formation of a first vessel plexus. This process is coupled to ossification and formation of the primary ossification centre (POC) 38,40,44,45 and, later, the secondary ossification centre in the epiphysis (Fig. 1). In contrast, extension of the POC in postnatal femur or tibia involves a different mode of angiogenesis, namely the extension of blunt vessel buds from vessel loops (arches) in close proximity of hypertrophic growth plate chondrocytes 33,46 (Fig. 1). Historic experiments using ink injection or corrosion casting in combination with electron microscopy had already indicated the existence of bulb-shaped terminal vessel structures near the growth plate but lacked insight into the organization and behaviour of the ECs surrounding the vessel lumen 47–49 . Modern static and dynamic microscopic imaging confirm that distal vessel buds are fully lumenized and show that they are formed by multiple ECs, which interact with the surrounding chondrocyte matrix through short filopodia. Vessel buds protrude into the space released by the apoptosis of growth plate chondrocytes and new vessel arches are generated by the anastomosis of two adjoining buds 46 (Fig. 2). At their proximal end, the distal arches are connected to relatively straight, column-shaped capillaries that are strongly associated with perivascular bone mesenchymal stromal cells (BMSCs) and osteoprogenitors.
Fig. 1. Organisation of the bone vasculature during development.
During endochondral osteogenesis in the developing embryo (left), signals provided by hypertrophic chondrocytes (HC) trigger the invasion of blood vessels into an initially avascular cartilage template (left). This process coincides with the onset of osteogenesis and the formation of the primary ossification centre (POC). Similarly, vessel ingrowth into hypertrophic cartilage of the distal end of long bone, which occurs postnatally in mice (centre), triggers secondary ossification centre (SOC) formation (centre). Postnatal growth and bone length extension in late postnatal and adolescent mice (right) is accompanied by the establishment of morphologically and molecularly distinct capillary subpopulations. CD31hi Emcnhi (type H) ECs include vessel buds in direct proximity of the growth plate, metaphyseal vessel columns and endosteal capillaries, whereas sinusoidal (type L) ECs in bone marrow (BM) show comparably low expression of CD31 and Emcn. In the transition zone at the metaphyseal-diaphyseal interface, type H columns are progressively remodelled into sinusoidal vessels during expansion of the BM cavity. Red arrows indicate perfusion through arteries and periosteal vessels.
Fig. 2. Vessel formation, remodelling and EC heterogeneity in bone.
(a) Resorption of hypertrophic chondrocytes (HC) in the growth plate enables the invasion of type H vessel buds, which emerge from distal vessel arches (top). Anastomotic fusion of contiguous buds (centre) leads to the formation of new arch-shaped vessels (bottom) from which new buds can emerge subsequently. Osteoclasts (blue), bone mesenchymal stromal cells (BMSCs; green) and osteoprogenitor cells (OPCs; green) are associated with metaphyseal type H vessels. (b) Reduction of the metaphysis after the decline of developmental growth is accompanied by expansion of the BM cavity. BM contains haematopoietic cells and reticular cells, whereas immature bone mesenchymal cells are mostly confined to the metaphysis and endosteum. BM expansion involves remodelling of type H vessel columns (top) into sinusoidal (type L) vessels (bottom) at the metaphyseal-diaphyseal interface (yellow area) through endothelial sprouting. (c) Hierarchy of EC subpopulations in bone. Type E ECs are abundant in embryonic long bone and give rise to type H cells, which, subsequently, can generate arterial and venous ECs but also type L sinusoidal ECs. While sinusoidal vessels directly connect to the large central vein, the lineage relationship between the CD31lo Emcnlo subpopulation and venous ECs is unclear.
EC subpopulations in the skeletal system
The ECs of all three substructures, buds, arches and columns, share high expression of the cell adhesion molecule CD31/PECAM1 (platelet and endothelial cell adhesion molecule 1) and the sialoglycoprotein Endomucin (Emcn). High expression of these two markers and association with osteoprogenitors are also defining features of capillaries in the endosteum lining the inner surface of compact bone. Accordingly, we have previously summarised these capillaries and their ECs under the term CD31hi Emcnhi or type H 32,33 . Endosteal type H vessels connect to the highly branched and relatively irregular sinusoidal vasculature of the bone marrow cavity, which is formed by ECs expressing comparably low levels of CD31 and Emcn (CD31lo Emcnlo or type L) (Fig. 1) 32 . The base of the type H capillary columns in the metaphysis is also connected to the BM vasculature at the metaphyseal-diaphyseal interface (Fig. 2). Another, transiently existing EC population with strong osteoprogenitor association and high expression of CD31 and Emcn, termed type E (for embryonic), was discovered in embryonic and early postnatal long bone 50 . Type E ECs show particularly high expression of angiogenic and pro-osteogenic genes, induce osteogenic differentiation of mesenchymal cells in 3D spheroid cultures, and, as genetic lineage tracing shows, give rise to type H and type L ECs in postnatal life (Fig. 2) 50 . While this review does not cover the function of the bone vasculature in blood formation, it is important to mention that sinusoidal (type L) ECs of the BM play important roles in the trafficking of haematopoietic cells 51–53 and include specialized vessels serving as vascular niches for myelopoiesis 54 .
Molecular pathways driving bone angiogenesis
Apart from VEGF-A signalling, several pathways have found to control bone angiogenesis and type H vessel formation (Textbox 1) with strong implications for osteogenesis. Endothelial Notch signalling is known to inhibit EC proliferation, sprouting and vessel growth in many different organs and experimental conditions. In bone, however, Notch activation in ECs promotes angiogenesis, type H vessel formation and osteogenesis 33 . The basis for these organ-specific differences in endothelial Notch function remain unknown. Hypoxia-inducible factor (HIF) signalling upregulates VEGF-A expression in hypoxic tissues many different cell types including chondrocytes. In bone ECs, HIF-1α controls type H vessel formation and increases endochondral angiogenesis and osteogenesis 32,55 . Osteoblasts regulate angiogenesis and type H vessels in a paracrine fashion through the secretion of soluble SLIT3 and activation of Robo receptors in ECs 56–58 . Bone morphogenetic proteins (BMPs) are well known for their ability to promote osteogenesis, but some of the ligands can also activate ECs and stimulate blood vessel growth, providing another molecular link between angiogenesis and osteogenesis 59,60 . The transcriptional co-regulators Yap1 and Wwtr1/Taz, components of the Hippo signalling pathway, were found to suppress bone angiogenesis. Postnatal EC-specific loss-of-function mutant mice show augmented angiogenesis, higher expression of HIF pathway target genes and increased bone formation 61 .
Box 1. Signalling pathways controlling bone angiogenesis.
VEGF-A
The growth factor is member of the VEGF family and generated in different isoforms, some of which critical lack sequence motifs required for retention at the cell surface and matrix binding. Several cell surface receptors and co-receptors for VEGF-A (VEGFR1, VEGFR2, Neuropilin-1) are known. VEGF-A is also an important regulator of vascular permeability. Apart from its important function in ECs, VEGF-A is known to control osteoblast lineage cells and inflammatory cells 39,62 .
Hypoxia-inducible factor
HIF heterodimers are transcription factors composed of one α (HIF-1α, HIF-2α and HIF-3α) and a common β subunit (HIF-1β). HIF proteins are unstable under high oxygen conditions, which involves HIF hydroxylation by prolyl hydroxylases (PHDs), ubiquitination by the von-Hippel Lindau (VHL) ubiquitin ligase and proteasomal degradation.
Notch
ECs predominantly express the Notch receptors 1 and 4 as well as the ligand Dll4. Dll4 levels are increased by Notch signalling and in response to VEGF-A, which generates feedback loops because Notch also suppresses VEGFR2 signalling. Notch has important cell-autonomous roles in many different cell types and is also critically required for the maintenance of osteoprogenitors 63,64 .
Slit-Robo
Slit ligands are secreted proteins that were originally identified as regulators of axon guidance in invertebrates. In mammals, three known family members (Slit1, Slit2, and Slit3) and their Robo (Roundabout) transmembrane receptors (Robo1-4) are involved in numerous processes including neuronal wiring and angiogenesis 65,66 .
Hippo pathway
Yap1 and Taz are transcriptional co-activators that promote gene expression and growth through interactions with DNA-binding TEAD (Transcriptional Enhanced Associate Domain) transcription factors (TEAD1-4) but also other transcriptional regulators. In response to phosphorylation by an upstream signalling cascade involving the Stk3/4 (Hippo; Mst1/2) and Lats1/2 protein kinases, Yap1 and Taz are retained in the cytoplasm and subjected to proteasomal degradation 67 .
Bone Morphogenetic Proteins (BMPs)
Large family of ligands belonging to the Transforming Growth Factor β (TGFβ) superfamily. BMP signalling involves binding to type I and type II heterotetrameric TGFβ family serine/threonine kinase receptor complexes followed by phosphorylation of SMAD proteins (Smad1/5), which are transferred from the cytoplasm into the nucleus to control gene expression 68,69 .
Vascular architecture in bone
Blood vessel organisation and hydrodynamics
Insight into the heterogeneity of ECs in the skeletal system is critical for understanding functional roles and regional specialisation. In recent years, single cell RNA sequencing data has been used to establish cell atlases for many different species, organs and conditions. The analysis of adult bone stromal cells, however, has so far uncovered a surprisingly limited number of EC subpopulations, which correspond to arterial and arteriolar, sinusoidal and mitotic cells 70–72 . An independent approach, namely single cell protein expression mapping by CyTOF mass cytometry, uncovered 28 distinct stromal cell subsets with 3 endothelial populations including arterial and sinusoidal ECs. A third and CD31+ population was found in the bone fraction 73 and might represent type H ECs or arterioles located in the proximity of osteoblast lineage cells.
Morphologically, the vasculature of long bone displays the classical hierarchical arrangement of arteries, veins and interconnecting capillaries. In femur, which has been studied most extensively, multiple different sources of blood supply have been described 74–76 . A so-called nutrient artery enters the diaphysis through the cortex, extends over considerable distance through the marrow cavity, and branches out in the metaphysis. The epiphysis and associated cartilage are supplied by epiphyseal arteries and vessels of the ring of La Croix, a perichondral structure that surrounds the growth plate laterally 77–80 . Small periosteal vessels, recently redescribed as transcortical vessels, cross the cortex at numerous locations along the bone shaft and contribute substantially to both afferent and efferent blood flow 76,81,82 (Fig. 1; Fig. 3). Periosteal vessels may facilitate the direct access of cells from the BM to nearby tissues, as has been shown for the migration of skull bone marrow-derived myeloid cells towards the surface of the adjacent brain 83 .
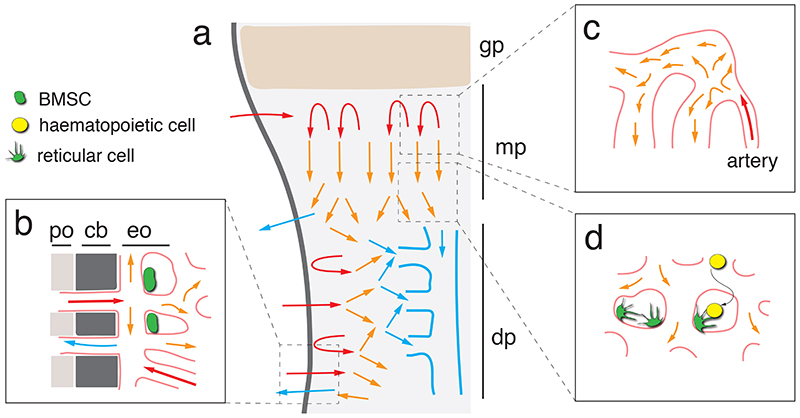
Fig. 3. Blood flow in long bone.
(a) Overview image showing arterial (red arrows) flow in the metaphysis and endosteum. Perfused blood flow at low speed through capillaries and sinusoidal vessels (orangearrows) before entering draining veins (blue). Growth plate (gp), metaphysis (mp) and diaphysis (dp) are indicated. (b) Periosteal (po) vessels penetrate through cortical bone (cb) and supply the endosteum, which harbours a fraction immature bone mesenchymal stromal cells (green). (c) While the relatively narrow arteries and arterioles in bone permit laminar perfusion, flow slows down substantially and becomes turbulent after entry into capillaries. (d) Flow is very slow in the sinusoidal vasculature, which facilitates transendothelial migration of homing leukocytes (yellow). Reticular cells associated with sinusoidal ECs are shown in green.
In long bone, both periosteal vessels and arteriolar branches emerging from the nutrient artery feed into the type H capillaries of the endosteum, which, in turn, drains into the sinusoidal vasculature of bone marrow 84 . Similarly, the distalmost arterioles in the metaphysis connect to type H capillaries near the growth plate and thereby provide flow that will reach the BM through the metaphyseal-diaphyseal interface (Fig. 1; Fig. 3). The type L sinusoidal capillaries in the femoral BM drain into a large central vein, which is a major route for outbound flow. While arterioles have few side branches and are relatively narrow with a diameter of about 10 μm or less, capillaries in the metaphysis and diaphysis are much wider and have numerous interconnections. Accordingly, arterial laminar flow becomes turbulent and slows down rapidly after entry into the capillary network 46,51 (Fig. 3). These features together with the spatial distribution of arterial-capillary connections also generate distinct metabolic zones characterized by high hypoxia in BM and higher levels of oxygenation in the metaphysis and endosteum 32,46,84 . Slow flow in sinusoidal vessels may also facilitate the transendothelial migration of blood cells and, as mentioned above, leukocyte trafficking is indeed confined to sinusoidal vessels in adult mice 51–53 (Fig. 3).
Role of mechanical forces
Mechanical forces and, in particular, increased loading can promote bone formation in the adult organism. Chondrocytes and osteocytes, fully differentiated osteoblast lineage cells that are embedded in calcified bone, play important roles in mechanosensing 85,86 . The osteocyte lacuno-canalicular network is connected to adjacent blood vessels and, similar to osteoblasts, osteocytes may be a source of VEGF to control bone angiogenesis and EC behaviour 87,88 . Interestingly, hindlimb unloading-induced bone loss is accompanied by reduction of type H capillaries, whereas mechanical loading stimulates bone angiogenesis and type H vessel formation 89,90 .
Osteoporosis and osteoarthritis
Special relationship between ECs and osteoclasts
Bone is a surprisingly dynamic tissue that undergoes livelong renewal and remodelling, which involve the balanced activity of bone-forming osteoblasts and bone-degrading osteoclasts. Impairment of this balance results in reduced bone mineral density (osteopenia) or osteoporosis, a disease characterized by bone weakness, increased risk of fracturing, loss of mobility, and chronic pain. Osteoporosis is very common in aged people and especially in postmenopausal women. Bone loss also occurs during rheumatoid arthritis (RA) and osteoarthritis and is based in part on higher activity of bone resorbing osteoclasts. Whereas interaction of bone forming osteoblasts and vasculature during bone loss during aging is discussed below, we focus in this section here on the unique role of bone resorbing osteoclasts in vascular growth and its implication in bone remodelling and osteoporosis.
Osteoclasts are unique myeloid cells derived from monocyte precursors that fuse and thereby generate polynuclear and strongly polarized cells. Cytoskeletal protrusions enable osteoclasts to build a sealing zone and generate an acidic compartment for bone resorption. A landmark study discovered that osteoclasts in bone emerge from tissue-resident erythro-myeloid progenitors (EMPs) but undergo fusion with circulation monocytes throughout life and in response to pathological challenges 91 (Fig. 4). Apart from monocyte precursors, dendritic cells (DCs) were also shown to fuse to osteoclasts or contribute to their generation in pathological bone conditions, including arthritis, periodontitis and osteopetrosis, but also in other inflammatory diseases including inflammatory bowel disease 92 . Depending on whether osteoclasts were derived from monocytes or from DCs, they were found for either immunologically tolerogenic or pro-inflammatory, respectively 92 .
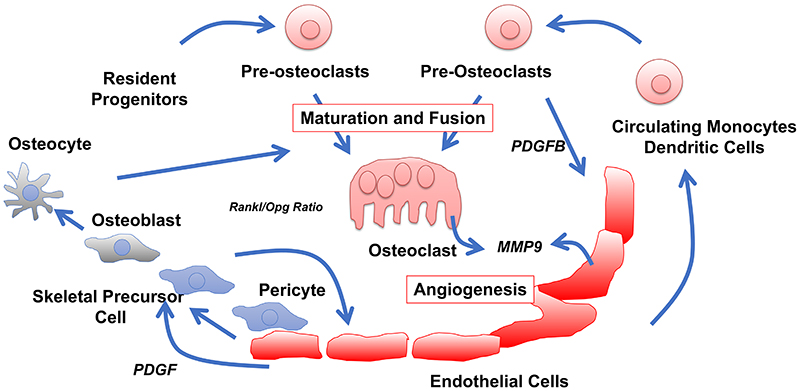
Fig. 4. Osteoclast-EC crosstalk.
Osteoclasts are generated by maturation and fusion of myeloid resident and circulating precursor cells, the latter derived from the circulation. The rate-limiting signalling molecule important for fusion is RANKL released mainly by osteocytes and osteoblasts, derived from skeletal precursor cells that are often perivascular. RANKL has to exceed the antagonistic acting osteoprotegerin (Opg). Non-fused pre-osteoclasts are essential triggers of angiogenesis via PDGF-B, whereas mature osteoclasts were suggested to facilitate angiogenesis by degradation of extracellular matrix. Recent data challenge this concept by demonstrating EC-derived MMP9 is required for angiogenic processes in areas with non-degradative osteoclasts.
Osteoclast lineage cells in the regulation of bone angiogenesis
Osteoclasts and their progenitors have been implicated in the regulation of vascular growth in bone by providing various pro-angiogenic factors (summarized in ref. 93 ). In addition, osteoclasts were identified as a source of the matrix metallopeptidase 9 (MMP9), which is important for angiogenesis both in explants and in vivo 94 (Fig. 4). The conclusion that osteoclasts stimulate angiogenesis through MMP9 was challenged by another study describing a subgroup of vessel-associated osteoclasts (VAOs) 95 . VAOs are reportedly involved in the anastomoses of type H vessels but not in the resorption of the hypertrophic cartilage. The same study used genetic experiments to show that MMP9 provided by ECs, but not by osteoclasts, is essential for essential for cartilage resorption and directional bone growth 95 (Fig. 4).
Preosteoclasts, but not monocytes or mature osteoclasts, were found to induce angiogenesis, type H vessel formation and osteogenesis via the secretion of platelet-derived growth factor B (PDGF-B) 96 . Ovariectomy-induced osteoporosis in mice leads to a reduction in serum and BM levels of PDGF-B with a concomitant decrease of type H vessels in long bone. Treatment with exogenous PDGF-B or administration of cathepsin K, which increases the number of preosteoclasts and thereby the endogenous levels of PDGF-B, stimulates type H vessel formation and osteogenesis in ovariectomized mice 96 .
CD31hi Emcnhi vessels have been implicated in OA development by several studies 97–99 . Preosteoclast-mediated release of PDGF-B contributes to OA development and CD31hi Emcnhi vessels are induced in the DMM model (destabilization of the medial meniscus) in subchondral bone and start to invade the joint cartilage. Conditional ablation of PDGF-B expression in preosteoclasts attenuates aberrant subchondral bone angiogenesis and joint damage, whereas transgenic overexpression of PDGF-B in preosteoclasts results in spontaneous osteoarthritis 99 . In all the studies above, the exact mechanism of PDGF-B function remains to be elucidated. Expression of the corresponding receptor, the tyrosine kinase PDGFRβ, is absent in ECs but found in various mesenchymal stromal cell populations, including skeletal stem and progenitor cells, as well as committed osteoblast lineage cells and synoviocytes 100–102 .
Effect of glucocorticoids and anti-resorptive drugs
Glucocorticoids (GCs) are used for the treatment of inflammatory diseases such as RA, asthma or skin conditions, but adverse side effects include GC-induced osteoporosis (GIO), which is very frequent due to the abundant use of steroids 103–105 . Conditional mutations impairing glucocorticoid signalling revealed a pivotal role for GC action in osteoblasts 106 , osteoclasts 107–109 and osteocytes 110,111 .
Only recently, the impact of the pharmacological effects of GCs on the bone vasculature was considered. In the femoral head, but less in distal femur, GC administration decreases the local vascularization, accompanied by decreased HIF-1α and VEGF expression 112 . In juvenile mice, bone angiogenesis and type H vessel were disrupted by GC administration, which was linked to reduced PDGF-B expression in preosteoclasts 113 . This effect of GC relies, in part, on cathepsin K. Inhibition of cathepsin K blocked Prednisolone-induced effects in the secondary spongiosa, the region where newly formed bony trabeculae are remodelled into mature trabeculae, and even enhanced H type vessels in the primary spongiosa, the site near the growth plate where trabecular bone formation is initiated 114 . Direct regulation of Pdgfb, the gene encoding PDGF-B, via transrepression of p65-NFkB was suggested, but effects were only observed at very high doses and the putative NF-kB binding site was not functionally evaluated 113 .
While numerous studies have linked preosteoclasts to the regulation of vessels, mature resorbing osteoclasts seem less important in this context. In tail vertebrae of mice treated with clodronate, an anti-osteoporotic drug belonging to the group of bisphosphonates, vessels are present despite blocked osteoclast-mediated bone resorption 115 . Similarly, lack of osteoclasts in osteopetrotic knockout mice lacking the transcription factor c-Fos does not lead to the absence of blood vessels. On the other hand, improved bone sample processing and imaging revealed that the treatment of mice with another bisphosphonate, namely Alendronate, leads to an increase of type H vessels in long bone 46 .
Bone inflammation
Interactions between vessels and fibroblast-like synoviocytes
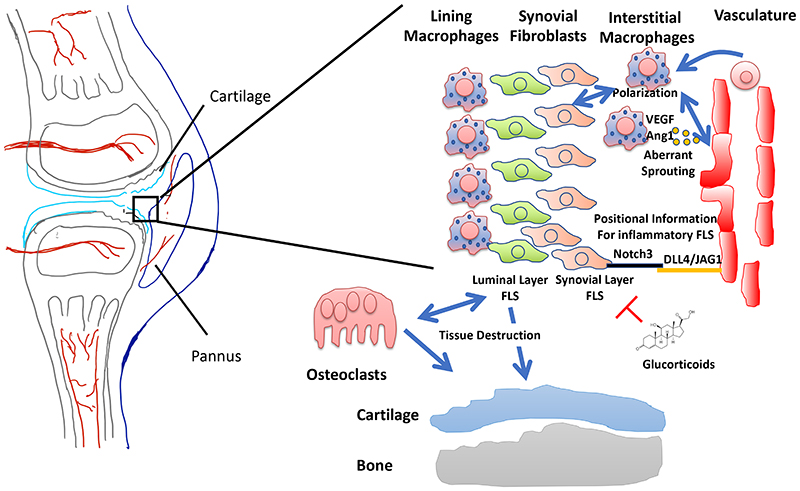
During inflammatory bone disease bone destruction is a consequence of chronic inflammation. For this inflammatory process, the cross-talk between vessels, leukocytes, but also stromal cells are major driving forces. One major example is rheumatoid arthritis. RA is a complex bona fide autoimmune disease that affects bone and joint integrity with unclear aetiology. Chronic inflammation, synovial swelling and pannus formation with subsequent bone and cartilage degradation are hallmarks of RA. Aberrant angiogenesis is observed in the subchondral area and in the pannus itself (reviewed in ref. 116 ) (Fig. 5). Tissue-resident macrophages and fibroblast-like synoviocytes (FLS) are presumably the first trigger of inflammation-induced angiogenesis. This is likely to occur in concert with hypoxia-controlled signalling pathways (via HIF), pro-inflammatory mediators and pro-angiogenic factors (such as VEGF-A or angipoietin-1) to induce vessel sprouting (reviewed in ref. 116 ). On the other hand, the presence of vessels appears to define the degree of inflammatory potential of FLS by inducing a position-dependent gene expression program 117 (Fig. 5). This spans from FLS lining the synovial membrane up to those that are in close vicinity of blood vessels. The closer FLS are located to vessels the more they express the pro-inflammatory marker Thy1 (CD90) and resemble active pro-inflammatory cells. The “lining” Thy1-negative FLS at the synovial luminal side are supposed to play a larger part in tissue destruction, as revealed by cell ablation and transplantation experiments 118 (Fig. 5). Instructive signals, such as expression of the Notch ligand Dll4 by ECs, leading to the activation of Notch3 on FLS promotes the Thy1+ pro-inflammatory signature 117 . Thus, vessels support the pro-inflammatory phenotype of FLS during the arthritic process and, accordingly, genetic and pharmacological inhibition of Notch signalling ameliorates inflammation.
Fig. 5. Interplay of vasculature, fibroblast like synoviocytes (FLS) and macrophages during inflammation of rheumatoid arthritis.
The pannus consists of recently discovered lining macrophages, luminal FLS located more distant from vasculature and synovial layer FLS close to the vasculature, interstitial resident and BM monocyte derived macrophages and freshly recruited macrophages and other immune cells (not shown). Tissue resident macrophages and FLS trigger aberrant angiogenesis. The vasculature itself provides positional information for FLS, whether they belong to the inflammatory active synovial layer FLS or the more tissue destructive luminal layer FLS. Luminal layer FLS promote tissue destruction presumably via inducing osteoclastogenesis and degradative enzyme release. Synovial layer FLS promote pro-inflammatory macrophage polarization which in turn triggers angiogenesis. Anti-inflammatory acting glucocorticoids suppress inflammation via action on FLS in arthritis.
Crosstalk between bone vessels and macrophages
Apart from the interactions between FLS and the endothelium in RA models, there are also important roles of macrophages. In the context of joint inflammation, macrophages can be subdivided by their functional phenotype. Recently identified subsets of resident macrophages are providing a barrier in the synovium, thus protecting against excessive inflammation, while recruited monocyte-derived macrophages in the synovial cavity actively contribute to joint inflammation 119 (Fig. 5). Epithelial cell-like macrophages at the synovial lining may be derived from self-renewing resident macrophages located in the synovial tissue. During inflammation, this barrier becomes disrupted and allows infiltration of inflammatory cells, including poly-morphonuclear granulocytes (PMNs) and macrophages, from the circulation, involving trans-endothelial migration.
In addition, it is very likely that vessels communicate with macrophages also indirectly during arthritis. Vessels polarize FLS towards Thy1+ pro-inflammatory phenotype via DLL4/Notch signalling 117 . In turn, these pro-inflammatory FLS could lead to pro-inflammatory polarization of macrophages. This scenario is supported by a study that demonstrate that immune suppressive glucocorticoids decrease inflammation via FLS leading to anti-inflammatory polarization of macrophages 120 (Fig. 5). Thus, an EC-FLS-macrophage interaction axis is driving inflammation in arthritis, with different FLS subpopulations driving inflammation and bone destruction, respectively. This axis might also promote the resolution of inflammation and could therefore provide an unexploited therapeutic target.
Bone repair and ageing
Role of vessels in regenerative osteogenesis
Bone development and fracture repair share many features and both processes rely on angiogenesis 40,121 . The entry of osteoblast precursors correlates with blood vessel ingrowth into cartilage during the developmental formation of the POC, but simultaneous entry of vessels and osteoblastic cells is also observed during fracture healing 34 . Treatment of mice with a soluble, neutralizing VEGF receptor not only decreases angiogenesis during repair of femoral fractures but also impairs osteogenesis, callus mineralization and bone healing. Conversely, exogenous VEGF-A can enhance blood vessel formation, ossification and callus remodelling 122 . Osteoblast lineage cells are an important source VEGF-A and thereby contribute to different phases of bone repair. During the repair of drilled lesions in tibia, VEGF-A provided by osteoblasts was found to promote macrophage recruitment and angiogenesis in the inflammation phase, which initiates the repair process 39 . Later in regeneration, in the endochondral ossification stage, osteoblast- and hypertrophic chondrocyte–derived VEGF-A stimulates vessel growth, osteoclast recruitment and cartilage resorption at the repair site. The role of osteoblasts as source of VEGF-A extends into the final remodelling phase of the repair process 39 . Perivascular bone mesenchymal stromal cells expressing Gli1 (glioma-associated oncogene homolog 1) were reported to interact with type H capillaries during bone development and defect healing. While defect healing involves the expansion of type H ECs, this increase and bone repair are impaired by genetic ablation of Gli1+ cells 123 . It was also shown that both osteoprogenitors and macrophages show VEGF-A immunosignal and are closely associated with type H vessels in the forming and maturing callus in a mouse osteotomy model 124 . In addition to the well-established function of VEGF-A, other molecular signals will mediate the crosstalk between different cell populations in growing and regenerating bone. In a mouse model of augmented postnatal bone formation, an increase in type H vessels precedes the appearance of the high bone mass phenotype. Effects on the vasculature were mediated osteoblast-derived SLIT3, which activates the receptor Robo1 on ECs. Remarkably, administration of recombinant SLIT3 improves bone fracture healing and suppresses OVX-induced bone loss 56 .
Vessels as a potential target in anti-osteoporotic treatments
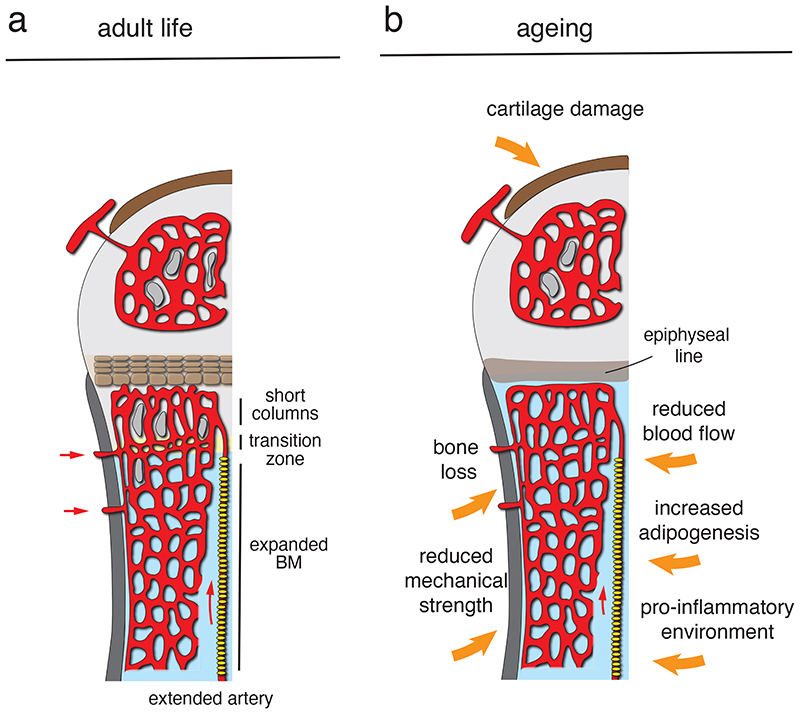
Ageing is associated with a loss of mineralised bone and increased fracture risk 125 , which are further enhanced in osteoporosis (Fig. 6). These conditions are associated with reduced skeletal blood flow both in human patients 126,127 and in animal models 46,128,129 , which might affect a range of physiological features including nutrient delivery, tissue metabolism, or the influx of calcium and phosphate 130,131 . Surgical or pharmacological interference with normal blood flow alters EC behaviour and reduces the abundance of type H vessels in murine femur 46 . Like in the OVX-induced osteoporosis model, normal ageing results in a profound diminishment of type H vessels and associated osteoprogenitors but also of arteries and arterioles in femur (Fig. 6), which is likely to contribute to reduced perfusion and impaired bone homeostasis 32,46,55 . In mice, enhanced HIF activity in ECs via tissue-specific inactivation of VHL leads to increases in type H vasculature and perivascular osteoprogenitors, resulting in augmented trabecular bone formation. Treatment of aged mice with deferoxamine mesylate (DFM), which enhances HIF1α stability and activity, also increases type H vasculature and mineralized trabecular bone 32 . Likewise, EC-specific genetic approaches enhancing Notch signalling lead to the growth of type H vessels, increase in osteoprogenitor and trabecular bone formation in ageing animals 46,55 . These proof-of-principle experiments indicate skeletal blood vessels are not only responding to ageing processes in the surrounding tissue but might also potentially represent a therapeutic target for the treatment of osteoporosis either alone or in combination with anabolic or anti-resorptive drugs.
Fig. 6. Remodelling of the bone vasculature in adult life and ageing.
(a) Type H columns are progressively remodelled into sinusoidal vessels in the transition zone at the metaphyseal-diaphyseal interface during development, resulting in a substantial expansion of the BM cavity in adult mice. Accordingly, the abundance of type H ECs and length of vessel columns declines with age. (b) In aged mice, type H ECs are scarce and the bone shaft is largely remodelled into a large marrow cavity. Features of bone ageing include the gradual loss of mineralized bone, reduced mechanical strength, increased adipogenesis, increased baseline inflammation, and damage in the articular cartilage. The number of arteries and blood flow are also reduced.
Relevance for human ageing and osteoporosis
While future research will undoubtedly enhance our understanding of the roles of the bone vasculature in health and disease further, several reports already suggest that certain key findings might be relevant for human subjects. Human bone ECs (termed hRECs) expressing the cell surface protein Endoglin/CD105, are associated with skeletal development and regeneration, share critical features with murine type H endothelium 132 . Human type H vessels have been reported to be a sensitive biomarker of bone mass in ageing subjects and osteoporotic patients 133 . Likewise, CD31hi Emcnhi EC abundance is positively associated with bone mineral density in human femur neck and total hip BMD but not lumbar vertebra. Moreover, CD31hi Emcnhi EC percentage in postmenopausal subjects was found to be significantly lower relative to premenopausal women 134 . Taken together, the existing evidence is encouraging and indicates that at least some of the fundamental findings made in mice are of broader relevance and might be translatable to humans. At the same time, it has to be considered that the number of published reports is still rather limited and more research is needed to get a better understanding of the processes in the healthy and diseased human skeletal system.
Conclusions
Even though it is unquestionable that future work will have to provide more insight into the role of the vasculature and capillary subpopulations in bone development, homeostasis, regeneration, healthy ageing, and disease, it is increasingly evident that vascular cells are more than just building blocks of a transport network and, instead, actively control critical processes through communication with a variety of other cell types. Such interactions include the crosstalk with chondrocytes and perivascular osteoblasts lineage cells, which is bidirectional and results in a coupling of angiogenesis and osteogenesis. Osteoclasts are also associated with bone vessels and the abundance of type H ECs and bone angiogenesis are controlled by signals provided by preosteoclasts. In contrast, there is relatively limited evidence for a potential direct regulation of osteoclasts by EC-derived signals 95,135,136 . Thus, it remains to be addressed whether bone ECs directly control osteoclastogenesis and thereby bone turnover and fracture healing but also conditions such as osteopenia and osteoporosis. Furthermore, vessels are likely to play important roles in OA and other inflammatory conditions not just through their role in immune cell signalling but also through interaction with synoviocytes and other cell populations in the joint. The identification of relevant molecular signals and potential therapeutic relevance require investigation.
Taken together, it is clear that the vasculature in the skeletal system is much more than a passive conduit system and learning more about its function, dynamic modulation, the molecular crosstalk with other cell types the local microenvironment, offers great opportunities. Insight into the role of bone vessels is highly relevant for fundamental physiological processes in healthy bone but might also reveal new potential targets for the treatment of diseases such as osteoporosis or OA, which currently impose huge burdens on our ageing population.
Key points.
The vascular system is essential for bone development and growth.
Capillary endothelial cells represent multiple subpopulations with distinct molecular and functional properties.
The type H endothelial subpopulation communicates with chondrocytes and perivascular osteoblast lineage cells.
The endothelium is closely intertwined with inflammatory cells during bone inflammation.
Preosteoclasts secret factors affecting bone angiogenesis and the abundance of type H endothelial cells.
Interdependent cross-talk of endothelial cells and other cell populations in bone might provide novel entry points for therapy of bone erosion.
Acknowledgements
RHA is supported by the Max Planck Society (RHA) and the European Research Council (AdG 786672, PROVEC) and the Deutsche Forschungsgemeinschaft (Tu220/12, Tu220/14-1, Ci 216/2).
Footnotes
Competing interests: RHA is investigator on patent EP 2 860 243 A1 (Reprogramming bone endothelial cells for bone angiogenesis and osteogenesis). JT declares that he has no competing interests.
References
- 1.Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic System in Cardiovascular Medicine. Circ Res. 2016;118:515–530. doi: 10.1161/CIRCRESAHA.115.306544. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell. 2020;182:270–296. doi: 10.1016/j.cell.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, et al. Lymphatic Endothelial Cells Produce M-CSF, Causing Massive Bone Loss in Mice. J Bone Miner Res. 2017;32:939–950. doi: 10.1002/jbmr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hominick D, et al. VEGF-C promotes the development of lymphatics in bone and bone loss. Elife. 2018;7 doi: 10.7554/eLife.34323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carulli C, Innocenti M, Brandi ML. Bone vascularization in normal and disease conditions. Front Endocrinol (Lausanne) 2013;4:106. doi: 10.3389/fendo.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadomski S, et al. Id1 and Id3 Maintain Steady-State Hematopoiesis by Promoting Sinusoidal Endothelial Cell Survival and Regeneration. Cell Rep. 2020;31:107572. doi: 10.1016/j.celrep.2020.107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews AH, Davis DD, Fish MJ, Stitson D. StatPearls. 2020. [PubMed]
- 8.Gadinsky NE, et al. Femoral Head Vascularity: Implications Following Trauma and Surgery About the Hip. Orthopedics. 2019;42:250–257. doi: 10.3928/01477447-20190723-03. [DOI] [PubMed] [Google Scholar]
- 9.Trueta J. Blood supply and the rate of healing of tibial fractures. Clin Orthop Relat Res. 1974:11–26. [PubMed] [Google Scholar]
- 10.Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20:573–580. doi: 10.1097/BOR.0b013e3283103d12. [DOI] [PubMed] [Google Scholar]
- 12.Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcu R, et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience. 2018;4:20–35. doi: 10.1016/j.isci.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleuren ACA, et al. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc Natl Acad Sci U S A. 2019;116:23618–23624. doi: 10.1073/pnas.1912409116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potente M, Makinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol. 2017;18:477–494. doi: 10.1038/nrm.2017.36. [DOI] [PubMed] [Google Scholar]
- 16.Augustin HG, Koh GY. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. 2017;357 doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 17.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 19.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 20.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 23.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 26.Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acar M, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte D, et al. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell. 2018;22:64–77.:e66. doi: 10.1016/j.stem.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maes C, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarkin C, Olsen BR. On bone-forming cells and blood vessels in bone development. Cell Metab. 2010;12:314–316. doi: 10.1016/j.cmet.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–348. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- 37.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 38.Duan X, et al. Vegfa regulates perichondrial vascularity and osteoblast differentiation in bone development. Development. 2015;142:1984–1991. doi: 10.1242/dev.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maes C, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 41.Zelzer E, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 42.Thompson TJ, Owens PD, Wilson DJ. Intramembranous osteogenesis and angiogenesis in the chick embryo. J Anat. 1989;166:55–65. [PMC free article] [PubMed] [Google Scholar]
- 43.Wacker A, Gerhardt H. Endothelial development taking shape. Curr Opin Cell Biol. 2011;23:676–685. doi: 10.1016/j.ceb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Maes C, et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010;29:424–441. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maes C, et al. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramasamy SK, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7:13601. doi: 10.1038/ncomms13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trueta J, Morgan JD. The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br. 1960;42-B:97–109. doi: 10.1302/0301-620X.42B1.97. [DOI] [PubMed] [Google Scholar]
- 48.Aharinejad S, et al. Microvascular pattern in the metaphysis during bone growth. Anat Rec. 1995;242:111–122. doi: 10.1002/ar.1092420115. [DOI] [PubMed] [Google Scholar]
- 49.Skawina A, Litwin JA, Gorczyca J, Miodonski AJ. The vascular system of human fetal long bones: a scanning electron microscope study of corrosion casts. J Anat. 1994;185(Pt 2):369–376. [PMC free article] [PubMed] [Google Scholar]
- 50.Langen UH, et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 2017;19:189–201. doi: 10.1038/ncb3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bixel MG, et al. Flow Dynamics and HSPC Homing in Bone Marrow Microvessels. Cell Rep. 2017;18:1804–1816. doi: 10.1016/j.celrep.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo Celso C, Lin CP, Scadden DT. In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat Protoc. 2011;6:1–14. doi: 10.1038/nprot.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itkin T, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature. 2021 doi: 10.1038/s41586-021-03201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kusumbe AP, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–384. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu R, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24:823–833. doi: 10.1038/s41591-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li N, et al. Osteoclasts are not a source of SLIT3. Bone Res. 2020;8:11. doi: 10.1038/s41413-020-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ignatius A, Tuckermann J. New horizons for osteoanabolic treatment? Nat Rev Endocrinol. 2018;14:508–509. doi: 10.1038/s41574-018-0069-2. [DOI] [PubMed] [Google Scholar]
- 59.Bautch VL. Bone morphogenetic protein and blood vessels: new insights into endothelial cell junction regulation. Curr Opin Hematol. 2019;26:154–160. doi: 10.1097/MOH.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 60.Larrivee B, et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sivaraj KK, et al. YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells. Elife. 2020;9 doi: 10.7554/eLife.50770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shibuya M. VEGF-VEGFR System as a Target for Suppressing Inflammation and other Diseases. Endocr Metab Immune Disord Drug Targets. 2015;15:135–144. doi: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- 63.Hilton MJ, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engin F, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blockus H, Chedotal A. Slit-Robo signaling. Development. 2016;143:3037–3044. doi: 10.1242/dev.132829. [DOI] [PubMed] [Google Scholar]
- 66.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 68.Dyer LA, Pi X, Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol Metab. 2014;25:472–480. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramel MC, Hill CS. Spatial regulation of BMP activity. FEBS Lett. 2012;586:1929–1941. doi: 10.1016/j.febslet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 70.Tikhonova AN, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baryawno N, et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell. 2019;177:1915–1932.:e1916. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baccin C, et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Severe N, et al. Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping. Cell Stem Cell. 2019;25:570–583.:e577. doi: 10.1016/j.stem.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brookes M, Harrison RG. The vascularization of the rabbit femur and tibio-fibula. J Anat. 1957;91:61–72. [PMC free article] [PubMed] [Google Scholar]
- 75.Shim SS, Copp DH, Patterson FP. Measurement of the rate and distribution of the nutrient and other arterial blood supply in long bones of the rabbit. A study of the relative contribution of the three arterial systems. J Bone Joint Surg Br. 1968;50:178–183. [PubMed] [Google Scholar]
- 76.de Saint-Georges L, Miller SC. The microcirculation of bone and marrow in the diaphysis of the rat hemopoietic long bones. Anat Rec. 1992;233:169–177. doi: 10.1002/ar.1092330202. [DOI] [PubMed] [Google Scholar]
- 77.Fenichel I, Evron Z, Nevo Z. The perichondrial ring as a reservoir for precartilaginous cells. In vivo model in young chicks’ epiphysis. Int Orthop. 2006;30:353–356. doi: 10.1007/s00264-006-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trueta J, Harrison MH. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B:442–461. doi: 10.1302/0301-620X.35B3.442. [DOI] [PubMed] [Google Scholar]
- 79.Crock HV. A Revision of the Anatomy of the Arteries Supplying the Upper End of the Human Femur. J Anat. 1965;99:77–88. [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez JI, Delgado E, Paniagua R. Changes in young rat radius following excision of the perichondrial ring. Calcif Tissue Int. 1985;37:677–683. doi: 10.1007/BF02554930. [DOI] [PubMed] [Google Scholar]
- 81.Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. J Anat. 1996;188(Pt 3):611–621. [PMC free article] [PubMed] [Google Scholar]
- 82.Gruneboom A, et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab. 2019;1:236–250. doi: 10.1038/s42255-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herisson F, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–1217. doi: 10.1038/s41593-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Z, Quarles LD. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord. 2015;16:115–129. doi: 10.1007/s11154-015-9313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH. Aging, Osteocytes, and Mechanotransduction. Curr Osteoporos Rep. 2017;15:401–411. doi: 10.1007/s11914-017-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prasadam I, et al. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. Mol Cell Biochem. 2014;386:15–25. doi: 10.1007/s11010-013-1840-2. [DOI] [PubMed] [Google Scholar]
- 88.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang S, et al. The coupling of reduced type H vessels with unloading-induced bone loss and the protection role of Panax quinquefolium saponin in the male mice. Bone. 2021;143:115712. doi: 10.1016/j.bone.2020.115712. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, et al. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J. 2021;35:e21150. doi: 10.1096/fj.202001080RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacome-Galarza CE, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568:541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madel MB, et al. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front Immunol. 2019;10:1408. doi: 10.3389/fimmu.2019.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cackowski FC, Roodman GD. Perspective on the osteoclast: an angiogenic cell? Ann N Y Acad Sci. 2007;1117:12–25. doi: 10.1196/annals.1402.073. [DOI] [PubMed] [Google Scholar]
- 94.Cackowski FC, et al. Osteoclasts are important for bone angiogenesis. Blood. 2010;115:140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romeo SG, et al. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat Cell Biol. 2019;21:430–441. doi: 10.1038/s41556-019-0304-7. [DOI] [PubMed] [Google Scholar]
- 96.Xie H, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu J, et al. Positive-Feedback Regulation of Subchondral H-Type Vessel Formation by Chondrocyte Promotes Osteoarthritis Development in Mice. J Bone Miner Res. 2018;33:909–920. doi: 10.1002/jbmr.3388. [DOI] [PubMed] [Google Scholar]
- 98.Cui Z, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75:1714–1721. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su W, et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohm AM, et al. Activation of Skeletal Stem and Progenitor Cells for Bone Regeneration Is Driven by PDGFRbeta Signaling. Dev Cell. 2019;51:236–254.:e212. doi: 10.1016/j.devcel.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Charbonneau M, et al. Platelet-Derived Growth Factor Receptor Activation Promotes the Prodestructive Invadosome-Forming Phenotype of Synoviocytes from Patients with Rheumatoid Arthritis. J Immunol. 2016;196:3264–3275. doi: 10.4049/jimmunol.1500502. [DOI] [PubMed] [Google Scholar]
- 102.Brun J, et al. PDGF Receptor Signaling in Osteoblast Lineage Cells Controls Bone Resorption Through Upregulation of Csf1 Expression. J Bone Miner Res. 2020;35:2458–2469. doi: 10.1002/jbmr.4150. [DOI] [PubMed] [Google Scholar]
- 103.McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20:131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 104.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 105.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 106.Rauch A, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Kim HJ, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jia D, O’Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conaway HH, Henning P, Lie A, Tuckermann J, Lerner UH. Activation of dimeric glucocorticoid receptors in osteoclast progenitors potentiates RANKL induced mature osteoclast bone resorbing activity. Bone. 2016;93:43–54. doi: 10.1016/j.bone.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 110.Piemontese M, Xiong J, Fujiwara Y, Thostenson JD, O’Brien CA. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am J Physiol Endocrinol Metab. 2016;311:E587–593. doi: 10.1152/ajpendo.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartmann K, et al. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol Rev. 2016;96:409–447. doi: 10.1152/physrev.00011.2015. [DOI] [PubMed] [Google Scholar]
- 112.Weinstein RS, et al. The Pathophysiological Sequence of Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology. 2017;158:3817–3831. doi: 10.1210/en.2017-00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng Y, et al. Glucocorticoids Disrupt Skeletal Angiogenesis Through Transrepression of NF-kappaB-Mediated Preosteoclast Pdgfb Transcription in Young Mice. J Bone Miner Res. 2020;35:1188–1202. doi: 10.1002/jbmr.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang P, et al. Preservation of type H vessels and osteoblasts by enhanced preosteoclast platelet-derived growth factor type BB attenuates glucocorticoid-induced osteoporosis in growing mice. Bone. 2018;114:1–13. doi: 10.1016/j.bone.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deckers MM, et al. Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Miner Res. 2002;17:998–1007. doi: 10.1359/jbmr.2002.17.6.998. [DOI] [PubMed] [Google Scholar]
- 116.Balogh E, Biniecka M, Fearon U, Veale DJ, Szekanecz Z. Angiogenesis in Inflammatory Arthritis. Isr Med Assoc J. 2019;21:345–352. [PubMed] [Google Scholar]
- 117.Wei K, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 2020;582:259–264. doi: 10.1038/s41586-020-2222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Croft AP, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Culemann S, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572:670–675. doi: 10.1038/s41586-019-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koenen M, et al. Glucocorticoid receptor in stromal cells is essential for glucocorticoid-mediated suppression of inflammation in arthritis. Ann Rheum Dis. 2018;77:1610–1618. doi: 10.1136/annrheumdis-2017-212762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stegen S, van Gastel N, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27. doi: 10.1016/j.bone.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 122.Street J, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen J, et al. Gli1(+) Cells Couple with Type H Vessels and Are Required for Type H Vessel Formation. Stem Cell Reports. 2020;15:110–124. doi: 10.1016/j.stemcr.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stefanowski J, et al. Spatial Distribution of Macrophages During Callus Formation and Maturation Reveals Close Crosstalk Between Macrophages and Newly Forming Vessels. Front Immunol. 2019;10:2588. doi: 10.3389/fimmu.2019.02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith DM, Khairi MR, Johnston CC., Jr The loss of bone mineral with aging and its relationship to risk of fracture. J Clin Invest. 1975;56:311–318. doi: 10.1172/JCI108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen WT, et al. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001;220:213–218. doi: 10.1148/radiology.220.1.r01jl32213. [DOI] [PubMed] [Google Scholar]
- 127.Shih TT, et al. Correlation of MR lumbar spine bone marrow perfusion with bone mineral density in female subjects. Radiology. 2004;233:121–128. doi: 10.1148/radiol.2331031509. [DOI] [PubMed] [Google Scholar]
- 128.Prisby RD, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 129.Bloomfield SA, Hogan HA, Delp MD. Decreases in bone blood flow and bone material properties in aging Fischer-344 rats. Clin Orthop Relat Res. 2002:248–257. doi: 10.1097/00003086-200203000-00036. [DOI] [PubMed] [Google Scholar]
- 130.McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88(Suppl 3):4–9. doi: 10.2106/JBJS.F.00890. [DOI] [PubMed] [Google Scholar]
- 131.Tomlinson RE, Silva MJ. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013;1:311–322. doi: 10.4248/BR201304002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kenswil KJG, et al. Characterization of Endothelial Cells Associated with Hematopoietic Niche Formation in Humans Identifies IL-33 As an Anabolic Factor. Cell Rep. 2018;22:666–678. doi: 10.1016/j.celrep.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, et al. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017;8:e2760. doi: 10.1038/cddis.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu Y, et al. The association between CD31(hi)Emcn(hi) endothelial cells and bone mineral density in Chinese women. J Bone Miner Metab. 2019;37:987–995. doi: 10.1007/s00774-019-01000-4. [DOI] [PubMed] [Google Scholar]
- 135.Alam AS, et al. Endothelin inhibits osteoclastic bone resorption by a direct effect on cell motility: implications for the vascular control of bone resorption. Endocrinology. 1992;130:3617–3624. doi: 10.1210/endo.130.6.1597159. [DOI] [PubMed] [Google Scholar]
- 136.Zaidi M, et al. Role of the endothelial cell in osteoclast control: new perspectives. Bone. 1993;14:97–102. doi: 10.1016/8756-3282(93)90234-2. [DOI] [PubMed] [Google Scholar]