Abstract
Background
Two-thirds of children with tuberculosis have non-severe disease.
Method
SHINE was an open-label treatment-shortening non-inferiority trial in children with non-severe, symptomatic, presumed drug-susceptible, smear-negative tuberculosis, in Uganda, Zambia, South Africa and India. Children aged <16 years were randomised to 16- versus 24-week standard first-line anti-tuberculosis treatment using WHO-recommended paediatric fixed-dose-combinations and a non-inferiority margin of 6% was used. The primary efficacy outcome was a composite of treatment failure, anti-tuberculosis treatment changes/restarts, on-treatment loss-to-follow-up, TB recurrence or death by 72 weeks, excluding children not reaching 16 weeks follow-up (modified-intent-to-treat). Primary safety outcome was on-treatment grade ≥3 adverse events.
Results
1204 children (602 in each group) were enrolled between July 2016 and July 2018; median age 3.5 years (range 2 months-15 years), 52% male, 11% HIV-infected, 14% bacteriologically-confirmed tuberculosis. Retention by 72 weeks and adherence to allocated anti-tuberculosis treatment were 95% and 94%, respectively. Sixteen (3%) versus 18 (3%) children reached the primary efficacy outcome in 16- versus 24-week arms respectively: unadjusted difference -0.4%, 95% CI (-2.2, 1.5). Non-inferiority of 16-weeks was consistent across intention-to-treat, per-protocol and key secondary analyses including when restricting analysis to the 958 (80%) children independently adjudicated to have tuberculosis at baseline. 95 (8%) children experienced grade ≥3 adverse events, including 17 adverse reactions (11 hepatic, all except three occurred within first 8 weeks, when treatment arms were the same).
Conclusions
4-months anti-tuberculosis treatment was non-inferior to 6 months for children treated for drug-susceptible non-severe smear-negative tuberculosis.
(Supported by University College London; Trial Registration: ISRCTN 63579542)
An estimated 1.2 million children develop tuberculosis disease (TB) annually, and almost 20% die,(1, 2) but children have historically been excluded from clinical efficacy trials of anti-tuberculosis treatment. This is in part due to low rates of bacteriological confirmation in children, because of high rates of paucibacillary disease and difficulties collecting respiratory specimens. Paediatric treatment recommendations are therefore extrapolated from trials in adults whose criteria for treatment entry often include smear-positive respiratory disease.
In contrast to adults, most children have non-severe TB disease.(3)(4) Although spontaneous resolution has been described,(5) it is generally agreed that mild forms of paediatric TB require treatment because of the risk of disease progression and dissemination, particularly in the youngest children or with concurrent HIV.(6, 7) It is likely that non-severe forms of disease could be treated with shorter durations of therapy yet no randomised trials have evaluated treatment shortening for drug-susceptible TB in children. Current international guidelines recommend six months of antituberculosis treatment, as for adults.
Early pharmacokinetic studies of first-line anti-tuberculosis treatment showed low drug exposures in young children compared to adults and led to increased dosing recommendations from the World Health Organization (WHO) in 2010. New fixed-dose-combination dispersible formulation tablets were developed to enable revised dosing and became available in 2015.(8, 9)
The SHINE trial aimed to determine whether 4 months of anti-tuberculosis treatment would be as good as 6 months in children with presumed non-severe, drug-susceptible TB, using the new fixed-dose-combination formulations. In addition, the SHINE trial evaluated cost-effectiveness of the 4-month approach.
Methods
SHINE was a multicentre, open-label, parallel-group, non-inferiority, randomised controlled, two-arm trial comparing 4-month (16 weeks) vs standard 6-month (24 weeks) anti-tuberculosis treatment using WHO-recommended paediatric doses.(9)
All national/local ethics committees and University College London research ethics committee approved the trial. Carers and children gave written informed consent and/or assent as appropriate.
Children under 16 years, with symptomatic non-severe and respiratory-sample smear-negative TB, due to start first-line anti-tuberculosis treatment, were eligible for enrolment. Non-severe TB included pulmonary TB confined to one lobe with no cavities (<1 lobe), no signs of miliary TB and no complex pleural effusion, intra-thoracic lymph node TB with no significant airway obstruction and no bilateral airway narrowing, and peripheral lymph node TB (Appendix-Protocol)).(10)
Children were seen at screening, enrolment (randomisation) and at weeks 2, 4, 8, 12, 16, 20, 24, 28, 36, 48, 60 and 72. Screening procedures included TB contact history and symptom check, Mantoux or interferon gamma-release assays (IGRA), where available, chest x-ray (CXR) and at least two respiratory samples (gastric aspirate, expectorated or induced sputum) for smear microscopy, Xpert MTB/RIF© (Xpert; Cepheid, Sunnydale, CA, USA), culture and drug susceptibility testing. In children with peripheral lymphadenopathy, a fine-needle aspirate was collected. A baseline CXR, read by site clinicians, was assessed for severe respiratory TB.(3) Blood for biochemistry, haematology (all children) and, HIV-1 viral load and CD4 count (HIV-infected children), was obtained at screening and scheduled follow-up visits. Full study details can be seen in the protocol and SAP at nejm.org.
Children with confirmed drug resistance or known exposure to an adult with any drug-resistant (including mono-resistant) TB were excluded. Eligible children were randomly allocated 1:1 to 16- or 24-week anti-tuberculosis treatment using minimisation (with random element) by centre, age (over/under 3 years), HIV status and ethambutol use. All children received 8 weeks of standard isoniazid, rifampicin and pyrazinamide (fixed-dose-combination formulation) with/without ethambutol as per local guidelines, followed by standard isoniazid/rifampicin fixed-dose-combination for 16 weeks in the control arm, and 8 weeks in the intervention arm. All antituberculosis treatment was administered seven days a week according to WHO TB weight-bands using child-friendly formulations,(9) found to be acceptable by trial participants and caregivers.(11) Health worker DOT was not used.
A symptom checklist and clinical examination were performed at each visit to detect TB-associated symptoms and adverse events. Repeat respiratory samples were collected if previous respiratory specimens were microbiologically positive, clinically indicated (to assess recurrence or treatment failure) or a new drug-resistant TB source case was identified.
CXRs were retrospectively reviewed centrally by two independent experts. CXRs with discordant interpretations at the primary reading were reviewed by a third expert and the majority opinion was used. This was conducted, blind to treatment allocation and clinical information, using a standardised approach (Appendix-Protocol). TB status at enrolment (confirmed, unconfirmed or unlikely TB) was adjudicated by an independent expert committee based on all available clinical, radiological and laboratory data.(12, 13) An endpoint review committee, blind to treatment allocation, reviewed clinical events suggestive of TB treatment failure/recurrence and all deaths. Grade 3/4/5 clinical/laboratory adverse events were defined using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (DAIDS).(14) Additional reported notable events included suspected bacterial infections requiring hospitalisation, ocular toxicity and pregnancy.
Adherence was assessed by pill count at each visit during treatment and questions on missed doses were collected twice (at the end of the intensive and continuation phases). Treatment extensions due to excessive missed doses were reconciled against pill count data.
The primary efficacy outcome was unfavourable status by 72 weeks, defined as TB events (treatment failures, including treatment extensions beyond replacement of missed doses, anti-tuberculosis treatment drug changes or restarts due to suspected treatment failure, and TB recurrences adjudicated by endpoint review committee blind to arm), loss to follow-up on treatment or death (all-causes) by 72 weeks. The primary safety outcome was grade ≥3 adverse events on or 30 days post-treatment. Secondary outcomes were mortality, adverse drug events possibly, probably or definitely related to study drug (adverse reactions), bacterial infections requiring hospitalisation, adherence and acceptability.
The trial was powered on a key secondary subgroup of children adjudicated to have TB at enrolment (assumed 80% of total). Assuming 10% loss to follow-up (post-treatment), a composite unfavourable outcome rate of 8% in the control arm,(15, 16) a non-inferiority margin of 6%, and 90% power, 5% 2-sided significance, the planned sample size was 1200 children. (Appendix-Protocol/SAP)
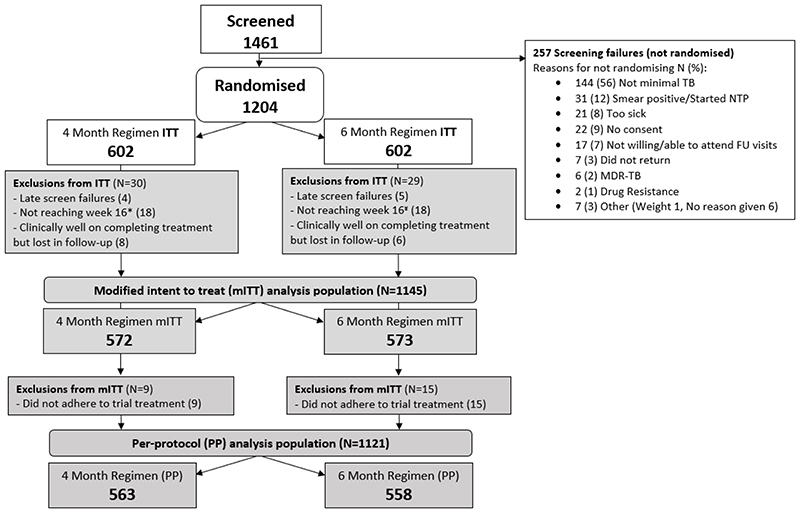
The primary modified intent-to-treat (mITT) population included all children except those not reaching week 16 (when both arms were the same) or late exclusions (based on data collated prior to randomisation) and children who were clinically well after completing treatment but subsequently lost to follow-up. Additional exclusions from the per-protocol (PP) analysis were those taking medications on fewer than 80% of daily doses within 120% of allocated treatment duration (set in advance). (Figure 1)
Figure 1. SHINE CONSORT diagram.
* Did not reach week 16 (18): Death from any cause (5), TB event (3), Withdrawn (4) Lost-to-follow-up and not unfavourable (3), Treatment change intensive phase (3)
¥ Did not reach week 16 (18): Death from any cause (6), TB event (2), Withdrawn (4) Lost-to-follow-up and not unfavourable (4), Treatment change intensive phase (2)
The primary efficacy analysis was based on the absolute difference in proportions of unfavourable outcomes between 16- and 24-week strategies in the mITT analysis population, adjusted for minimisation factors using Cochran Mantel-Haenszel weights. Time-to-unfavourable outcome and time-to-death were compared using log rank tests and Cox proportional hazards models. Analyses were performed in Stata version 15.1 or later and SAS version 10.1.
Economic analyses were performed to estimate costs and health outcomes in life years and quality-adjusted life years (QALYs) during the 72-week trial using data on resources used in the trial and published unit costs for each country. Costs were estimated from a health sector perspective and QALYs were estimated by combing health-related quality of life scores, estimated using the EQ-5D, and survival. Costs and outcomes were discounted at 3% per annum (Appendix-S7).
An Independent Data Monitoring Committee (IDMC) reviewed data by treatment arm four times during the trial.
Results
1,461 children were screened and 1,204 randomised between July 2016 and July 2018 (Uganda 376, Zambia 364, South Africa 315, India 149). Principal reasons for ineligibility were smear-positive respiratory samples and presence of severe TB on CXR (Figure 1).
Fifty-nine children were excluded from the mITT analysis (30 in the 16-week arm; 29 in 24-week arm): 36 did not reach week 16 (i.e. had protocol defined unfavourable outcome before week 16) (18 on 16-week arm and 18 on 24 week arm); 14 were lost to follow-up after successfully completing treatment (6 on 16-week arm; 8 on 24-week arm). The most frequent reason for further exclusion for the PP analysis was non-adherence to allocated treatment strategy (n=24: 9 on 16-week arm and 15 on 24-week arm). (Figure 1).
Demographic and clinical characteristics of the children were similar in the two groups (Table 1, Appendix-S1). Median age was 3.5 years (range 2 months-15 years), 52% male, 88% African, 12% Indian, 11% HIV-infected, 67% pulmonary, 3% peripheral lymph node TB, 29% mixed TB (intra-thoracic disease and peripheral LN) and 14% were bacteriologically confirmed, defined as positive for M. tuberculosis by culture or Xpert. Xpert semi-quantitative results are presented in Appendix-S1, showing that all positive Xpert values were low or very low.
Table 1. Baseline characteristics, baseline clinical presentation and AFB smear and culture of children in SHINE.
| 16 weeks (N=602) |
24 weeks (N=602) |
Total (N=1204) |
||
|---|---|---|---|---|
| Sex N (%) | Female | 297 (49) | 286 (48) | 583 (48) |
| Age (Years) | Median (IQR), (Min,Max) | 3.4 (1.5, 6.9), (2m, 15y) | 3.5 (1.5, 7.1), (2m, 15y) | 3.5 (1.5, 7.0), (2m, 15y) |
| Site country | ||||
| South Africa | 156 (26) | 159 (26) | 315 (26) | |
| HIV status, N (%) | Positive | 65 (11) | 62 (10) | 127 (11) |
| WHO weight band (kg), N (%) | 3-3.9 | 0 | 3 (1) | 3 (<1) |
| Clinical Presentation N (%) | Respiratory | 398 (66) | 406 (67) | 804 (67) |
| MTB culture and Xpert MTB/RIF results θ N (%) | Total (culture positive for MTB (MGIT or LJ) OR Xpert MTB/RIF positive) | 85 (14) | 80 (13) | 165 (14) |
Data presented as number of participants (%), unless otherwise stated. IQR=interquartile range, LJ=Lowenstein Jensen solid culture medium, MGIT =mycobacteria growth indicator tube liquid culture system, y=years, m=months, RIF=rifampicin, Xpert=GeneXpert.
_Microbiological confirmation was from respiratory samples (gastric aspirate/washing, induced or expectorated sputum) and fine needle aspiration of enlarged lymph nodes and was defined as positive for M. tuberculosis by culture or Xpert MTB/RIF assay.
Participants did not have a cough for more than 2 weeks or peripheral lymph node(s) suggestive of TB.
Retention based on attendance at final study visit at week 72 was 95% of those expected (excluding formal withdrawals (n=8) and deaths (n=31)), similar across the arms (Appendix-S2).
Adherence to allocated treatment duration was similar between arms with 94% children taking at least 80% of daily doses within 120% of allocated days (Appendix-Figure S1). CXRs with discordant interpretations at the primary (baseline) reading were reviewed by a third expert in 37% (435/1174) of cases (30 baseline CXRs could not be located).
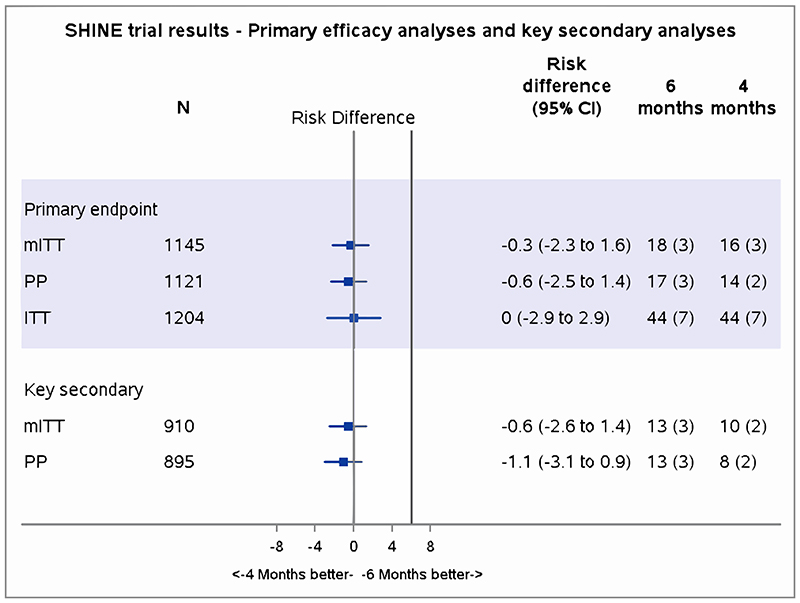
In the mITT analysis, 21 TB events occurring before week 16 were excluded (11 deaths, 5 TB progression, 5 treatment extensions/drug changes (Figures 1,2). In the primary mITT analysis, the number (proportion) with unfavourable outcomes were 16 (3%) versus 18 (3%) in 16- versus 24-week arms respectively, adjusted absolute difference -0.4% (95% CI -2.2 to 1.5; Figure 2, Table 2bi). Results from ITT and PP populations gave similar results (Appendix-S9, Figure 2), with no significant differences in time-to-unfavourable outcome (secondary outcomes) (hazard ratio 0.88, 95% CI 0.45 to 1.74) or time-to-death (hazard ratio 0.63, 95% CI 0.31 to 1.30) between randomised arms (Appendix-Figures S2,S3).
Table 2bi. Primary efficacy analysis results (mITT).
| 16 weeks | 24 weeks | Total | |
|---|---|---|---|
| Randomised | 602 | 602 | 1204 |
| Unassessable | 30 | 29 | 59 |
| Favourable | 556 (97) | 555 (97) | 1111 (97) |
| Total included in analysis (Assessable) | 572 | 573 | 1145 |
| Difference from control in unfavourable rate (CMH weights, Adjusted) |
-0.4% | ||
| Difference from control in unfavourable rate (Unadjusted)* | -0.3% |
Data presented as number of participants (%), unless otherwise stated.
Unadjusted is displayed in figure 2. CMH: Cochran–Mantel–Haenszel.
Adjusted for centre, age (over/under 3 years), HIV status and ethambutol use.
The most common reason for an unfavourable outcome (after week 16) was death from any cause (7 in 16-week and 12 in 24-week arm), followed by TB treatment failure (9 in 16-week; 5 in 24-week). Among treatment failures, 2 were treatment extensions (both in 16-week), 2 were drug changes (1 in 16-week; 1 in 24-week) and 10 were TB recurrences (6 in 16-week; 4 in 24-week (Table 2a).
Table 2a. Components of the primary mITT analysis.
| 16 weeks | 24 weeks | Total | |||
|---|---|---|---|---|---|
| Randomised | 602 | 602 | 1204 | ||
| Included in analysis (assessable) | 572 | 573 | 1145 | ||
| Favourable | Completed treatment and clinically well (without retreatment or otherwise unfavourable) at 72 weeks | 556 | 555 | 1111 | |
| 556 (97) | 555 (97) | 1111 (97) | |||
| Unfavourable | Death from any cause after week 16 | 7 | 12 | 19 | |
| TB events | TB recurrence | ||||
| Total unfavourable (% of assessable) | 16 (3) | 18 (3) | 34 (3) | ||
Data presented as number of participants (%). mITT = modified intention to treat.
Both participants had treatment restarted after defaulting on treatment.
In the key secondary analysis among 958 children independently adjudicated to have TB at baseline (477 (79%) in 16-week; 481 (80%) in 24-week) the adjusted absolute difference from the control arm was -0.6% (95% CI -2.6 to 1.4); in the PP analysis it was -1.1% (95% CI -3.1 to 0.9; Figure 2).
Results for the mITT efficacy endpoint by pre-specified subgroup analyses (HIV status, region, sex, age, weight band, TB type, bacteriological confirmation and ethambutol given at baseline) all gave consistent findings to the primary result (Appendix-S4,S8).
There were 115 grade≥3 adverse events on treatment or for up to 30 days post-treatment. in 95 (8%) children, 47 (8%) in 16-week and 48 (8%) in 24-week arms, most common being pneumonia or other chest infection (29 (25%)) or liver-related events (11 (10%)), similar across arms (Table 3, Appendix-S3).
Table 3. Primary safety endpoint events* serious adverse events (SAEs), deaths and suspected bacterial infections requiring hospitalisation.
| Timing | Randomised | 16 weeks (N=602) |
24 weeks (N=602) |
Total (N=1204) |
|---|---|---|---|---|
| Overall | DAIDS grade 3, 4 or 5 adverse events | 49 | 66 | 115 |
| Before week 16 | Grade 3, 4 or 5 adverse events | 35 | 52 | 87 |
| After week 16 | Grade 3, 4 or 5 adverse events | 14 | 14 | 28 |
| Overall | SAEs | 88 | 104 | 192 |
| Before week 16 | SAEs | 35 | 50 | 85 |
| After week 16 | SAEs | 53 | 54 | 107 |
| Total | No. of Deaths | 12 | 19 | 31 |
| Before week 16 | Deaths | 5 | 6 | 11 |
| After week 16 | Deaths | 7 | 13 | 20 |
| Overall | No. of adverse drug reactions ¥ on treatment and within 30 days of completing treatment | 6 (1) | 11 (2) | 17 (1) |
| Overall | Events described as bacterial infection requiring hospitalisation | 40 | 40 | 80 |
Data presented as number of events or number of participants with at least one event (%) as indicated. DAIDS = Division of AIDS table for grading the severity of adult and paediatric adverse events; SAE=serious adverse event, defined using International Conference on Harmonisation-Good Clinical Practice (ICH-GCP) definitions as adverse event resulting in death, is life-threatening, requires hospitalisation or prolongs existing hospitalisation, results in persistent or significant disability or incapacity, consists of a congenital anomaly or birth defect, or considered to be another important medical condition.
Primary safety endpoint events include on-treatment grade 3 or higher adverse events up to 30 days after the last dose of study drugs.
Participants can appear in both before and after 16 week breakdown.
Adverse drug reactions were defined as being possibly, probably or definitely related to the trial drugs as assessed by the site investigator. Further information on these can be found in the listing in the supplementary material and include event type and information on treatment discontinuation.
A total of 192 serious adverse events occurred in 150 (12%) children, including 31 deaths (12 in 16-week; 19 in 24-week). Twenty deaths (7 in 16-week; 13 in 24-week) occurred after week 16. Thirteen deaths were considered related to TB (5 in 16-week; 8 in 24-week; Table 3). Twenty-five out of thirty-one deaths were in children aged <2 years (Appendix-S5). There were 115 ≥grade 3 adverse events (49 in 16-week and 66 in 24-week; Table 3a). Sixty-six (5%) children were hospitalised due to a respiratory bacterial infection; forty-five were after week 16 (26 in 16-week; 19 in 24-week; Table 3, Appendix-S3).
There were 17 grade 3 or 4 adverse reactions (considered possibly, probably or definitely) related to trial drugs by study investigators, including 11 hepatic events; all adverse reactions except 3 occurred in the first 8 weeks of therapy (Appendix-S4). Two children permanently discontinued treatment following interruption for an adverse reaction and none led to death.
A cost effectiveness analysis showed that at 72 weeks, children treated for 16 weeks had similar health outcomes with reduced healthcare costs compared with those treated for 24 weeks. A regression analysis controlling for chance differences in demographic characteristics and symptom severity estimated that quality-adjusted life years were improved by 0.003 (95% CI -0.009 to 0.0144) and healthcare costs reduced by $17.34 (95% CI $3.77 to $30.91, 2019 USD) per child (Appendix-S5).
Discussion
SHINE evaluated duration of anti-tuberculosis treatment in children with non-severe drug-susceptible TB and was conducted in high TB burden countries, where nearly 90% of paediatric TB occurs.(17) The trial demonstrated non-inferiority of 4 months compared to standard 6 months of treatment with the upper CI bound below the pre-specified 6% margin. Consistency of results across all analyses including the key subgroup of children adjudicated to have TB at baseline was observed. Children responded well to treatment with few drug adverse events, which mostly occurred before 16 weeks when both trial arms were the same.
Shortening treatment for drug-susceptible TB is a key goal for both adults and children. Early trials showed it was possible to shorten treatment duration in adults with culture-negative disease.(18–20) A recent meta-analysis of treatment duration trials in adults reported that 4-month drug regimens were efficacious in adults with paucibacillary TB who have <2+ sputum smear grade or non-cavity disease.(21) Recently, results of TBTC Study 31 have reported non-inferiority of 4-month high-dose rifapentine/moxifloxacin containing regimens compared to 6-month standard therapy, for all forms of drug-susceptible TB in adults and adolescents, including cavity disease.(22) Challenges remain in terms of drug availability, dosing data in children, child friendly formulations and cost. However, our results show that a new regimen with new drugs and formulation is not needed for treatment shortening for the majority of children with drug-susceptible TB, as this can be accomplished with already available and affordable child-friendly fixed-dose-combinations.(23)
SHINE demonstrated the feasibility of identifying children with non-severe disease. We used a pragmatic approach to non-severe TB following routine screening procedures and the use of local CXR review to assess for severe respiratory TB. Despite perceived difficulties of respiratory sample collection in children, such challenges were overcome with appropriate training and samples were successfully obtained for TB testing in all 1,204 randomised children. We included children living both with and without HIV, with consistent results.
Most children with TB have paucibacillary and non-severe disease with low rates of microbiological TB confirmation in routine care. To ensure applicability of our results to clinical practice and the spectrum of disease that is most prevalent in children, we did not limit the trial to bacteriologically confirmed TB. We adapted the paediatric consensus algorithm for diagnosis of intrathoracic TB(12) to both intrathoracic TB and peripheral lymph node TB, and used independent expert review and central reading of CXRs, both blind to treatment allocation, to ensure objective categorisation of TB status. The endpoint review committee, also blind to randomised arm, reviewed TB endpoints to minimise the effect of treatment allocation on adjudication.
Our trial had several strengths. It was well powered and we observed 94% adherence (to more than 80% of doses) in allocated arms and 95% retention, increasing confidence in the results. We assumed 8% of children would have unfavourable outcomes in the sample size calculation and observed 7% overall in the trial. However, 3% of these occurred after week 16, when the trial arms received different durations of continuation-phase treatment, which we had not anticipated when we designed the study
A limitation of the trial is that it was open label, which had potential to result in more frequent treatment extensions in the 16-week arm, contributing to more unfavourable treatment outcomes in this arm. Despite this possible disadvantage for the 16-week arm, the results consistently demonstrated that 4 months was as good as 6 months treatment. Another limitation relates to generalisability of our results to settings where CXRs are not available to characterise non-severe TB.
Our inclusion criteria for the trial required that smear microscopy was undertaken to rule out more severe forms of pulmonary TB. With current roll out of rapid molecular diagnostic tests replacing smear microscopy,(24) this may pose a challenge to the trial results implementation. However, smear grade and Xpert semi-quantitative results have been shown to be correlated.(25) In our study most Xpert results from respiratory samples were negative and the few positive Xpert samples had low or very low semi-quantitate results, suggesting trial findings can be extrapolated to settings where Xpert is replacing smear and children with negative, low or very low positive Xpert values can be categorised as having non-severe TB. Future implementation studies should explore treatment shortening in all children treated for drug-susceptible non-severe TB, regardless of smear and/or Xpert results.
SHINE suggests that a stratified medicine approach alternative to the ‘one-size-fits-all’ of first-line treatment for presumptive drug-susceptible TB could be implemented in children with non-severe TB.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Supplementary Material
Funding and acknowledgments
We thank all the SHINE children and their carers; the nursing, pharmacy and laboratory staff; all those who advised, volunteered, or otherwise supported community engagement at the SHINE trial sites; the Trial Steering Committee (TSC) members, Endpoint Review Committee (ERC) and Independent Data Monitoring Committee (IDMC) for their contributions including oversight of the safety of the trial.
SHINE is funded by UKRI MRC DfID Wellcome NIHR Joint Global Health Trials grant (Ref: MR/L004445/1) and UKRI COVID 19 Grant Extension Allocation Award; TB Alliance provided support for drug purchase. AMC and AT are supported by UKRI MRC grant MC_UU_00004/04; JAS is supported by a Clinician Scientist Fellowship jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (MR/R007942/1).
References
- 1.WHO. Global Tuberculosis Report 2020. World Health Organization; Geneva: 2020. Licence: CC BY-NC-SA 3.0 IGO 2020. [Google Scholar]
- 2.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health. 2017;5(9):e898–e906. doi: 10.1016/S2214-109X(17)30289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman CA, Gie RP, Starke JR, Schaaf HS, Donald PR, Cotton MF, et al. A proposed comprehensive classification of tuberculosis disease severity in children. The Pediatric infectious disease journal. 2012;31(4):347–52. doi: 10.1097/INF.0b013e318243e27b. [DOI] [PubMed] [Google Scholar]
- 4.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2006;10(7):732–8. [PubMed] [Google Scholar]
- 5.Loveday M, Sunkari B, Marais BJ, Master I, Brust JC. Dilemma of managing asymptomatic children referred with ‘culture-confirmed’ drug-resistant tuberculosis. Archives of disease in childhood. 2016;101(7):608–13. doi: 10.1136/archdischild-2015-310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. The Journal of infectious diseases. 2012;206(12):1809–15. doi: 10.1093/infdis/jis608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez L, Cords O, Horsburgh CR, Andrews JR, Pediatric TBCSC. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet. 2020;395(10228):973–84. doi: 10.1016/S0140-6736(20)30166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham SM, Grzemska M, Gie RP. The background and rationale for a new fixed-dose combination for first-line treatment of tuberculosis in children. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(Suppl 1):3–8. doi: 10.5588/ijtld.15.0416. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2014. [PubMed]
- 10.Chabala C, Turkova A, Thomason MJ, Wobudeya E, Hissar S, Mave V, et al. Shorter treatment for minimal tuberculosis (TB) in children (SHINE): a study protocol for a randomised controlled trial. Trials. 2018;19(1):237. doi: 10.1186/s13063-018-2608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wademan DT, Busakwe L, Nicholson TJ, van der Zalm M, Palmer M, Workman J, et al. Acceptability of a first-line anti-tuberculosis formulation for children: qualitative data from the SHINE trial. Int J Tuberc Lung Dis. 2019;23(12):1263–8. doi: 10.5588/ijtld.19.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin Infect Dis. 2015;61Suppl 3(Suppl 3):S179–87. doi: 10.1093/cid/civ581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wills GH, Graham SM, Seddon JA, Welch SB, McGowan C, Turkova A, et al., editors. A method for baseline adjudication of tuberculosis diagnosis in children in a therapeutic clinical trial: experience from SHINE 51st World Conference on Lung Health; 2020. Virtual. [Google Scholar]
- 14.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 20. 2014. Nov, [accessed 16 August 2020]. https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf .
- 15.WHO. Geneva: 2012. (Global Tuberculosis Report). [Google Scholar]
- 16.Kekitiinwa A, Cook A, Nathoo K, Mugyenyi P, Nahirya-Ntege P, Bakeera-Kitaka S, et al. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet. 2013;381(9875):1391–403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Geneva: 2019. (Global tuberculosis report 2019). [Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 . [Google Scholar]
- 18.Teo SK, Tan KK, Khoo TK. Four-month chemotherapy in the treatment of smear-negative pulmonary tuberculosis: results at 30 to 60 months. Annals of the Academy of Medicine, Singapore. 2002;31(2):175–81. [PubMed] [Google Scholar]
- 19.Hong Kong Chest Service/Madras Tuberculosis Rearsrch Centre/British MRC. A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 5 years. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. The American review of respiratory disease. 1989;139(4):871–6. doi: 10.1164/ajrccm/139.4.871. [DOI] [PubMed] [Google Scholar]
- 20.Hong Kong Chest Service/Madras Tuberculosis Rearsrch Centre/British MRC. A controlled trial of 2-month, 3-month, and 12-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 60 months. The American review of respiratory disease. 1984;130(1):23–8. doi: 10.1164/arrd.1984.130.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Imperial MZ, Nahid P, Phillips PPJ, Davies GR, Fielding K, Hanna D, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708–15. doi: 10.1038/s41591-018-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. The New England journal of medicine. 2021;384(18):1705–18. doi: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alliance T. Global Alliance for TB drug development (TB Alliance) New milestone for children with tuberculosis reached as one million treatments of child-friendly medicines are ordered. 2019. [Available from: https://www.tballiance.org/news/one-million-child-friendly-tuberculosis-medicines .
- 24.WHO. WHO consolidated guidelines on tuberculosis Module 3: Diagnosis-Rapid diagnostics for tuberculosis detection. 2020. [Available from: https://www.who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-module-3-diagnosis---rapid-diagnostics-for-tuberculosis-detection . [PubMed]
- 25.Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, Munsamy V, et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. American journal of respiratory and critical care medicine. 2011;184(9):1076–84. doi: 10.1164/rccm.201103-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.