Abstract
We demonstrate the in-droplet separation and enrichment of molecules, from small organic molecules to long nucleic acids (lambda DNA). Electric potentials are applied via two parallel three-dimensional electrodes, which interface the nanodroplets through polydimethylsiloxane (PDMS)-carbon composite membranes. These membranes enable the generation of uniform electric fields inside the droplets, while simultaneously preventing the formation of electrolytic byproducts. Biomolecules of different sizes migrate towards one side of the droplets, according to their net charge, when exposed to the electric field. Directly afterwards, a Y-junction promotes droplet splitting, resulting in the generation of biomolecules-enriched daughter droplets. Biomolecules were fluorescently labelled, and fluorescence microscopy was employed to assess their electrophoretic separation and enrichment. Experimental results demonstrate how the enrichment of biomolecules is influenced by their size, charge and concentration, by the ionic strength, viscosity and pH of the suspending medium, and by the in-droplet flow profile.Enrichments above 95% were observed for small molecules and highly-charged species at velocities over 10 mm/s (13 droplets per second). Moreover, the enrichment performance asymptotically approached a value of 38% for velocities as high as 50 mm/s, demonstrating the potential of this technique for the high-throughput separation of charged species. The applicability of the system was demonstrated by cleaving a peptide and selectively separating the cleaved fragments in different daughter droplets based on their net charge.
Controlling the motion of biomolecules and colloidal particles is a fundamental component in many microfluidic bioanalytical platforms. Of special interest is droplet microfluidics, where a continuous flow is segmented in discrete droplets with volumes of pico- or nanoliters surrounded by an immiscible fluid. 1 Droplet-based microfluidics has evolved into one of the most versatile and powerful microfluidic platforms for cell-based screening and biochemical assays. 2,3 Along with the short processing time of analysis and low sample consumption that characterize microfluidic devices, droplets serve as microreactors with well-defined environments. Droplets can be generated at frequencies of tens of kHz, 4,5 enabling the development of high-throughput assays for a wide spectrum of applications, including molecular assays, 4 enzyme evolution, 6 drug screening, 2 single-cell analysis 3,7 and sequencing. 8,9 Thus, is not surprising that many benchtop processes have been adapted to droplet microfluidic platforms. 10 Examples include the mixing of solutions during droplet formation, 11,12 their fusion 13–15 and fission, 16 the storage and incubation of droplets, 17,18 and their sorting triggered by a readout parameter such as fluorescence intensity or absorbance. 19–21
In contrast, other basic and routine biochemical procedures are less commonly implemented to date, like the manipulation of particles and biomolecules. Recent years have witnessed the application of techniques well-established in continuous microfluidics for the in-droplet enrichment of microscale particles.For instance, Kurup and Basu 22 exploited the sedimentation tendency of glass beads (38 μm) to hydrodynamically enrich them in the rear of moving droplets. Employing the same hydrodynamic effect, Hein et al. 23 accumulated poly(methyl methacrylate) beads (8 μm) at both sides of moving droplets at velocities around 0.75 mm/s.Han et al. 24 proposed a device with a pair of planar electrodes that was able to dielectrophoretically focus polystyrene particles (5 μm) towards one side of droplets at a maximum velocity of 2 mm/s. Magnetic forces were used by Lombardi and Dittrich 25 to separate target compounds binding to functionalized beads (1 μm) from solution. In a related study, Brouzes et al. 26 developed a microfluidic chip that retained super-paramagnetic particles (1 μm) at a maximum droplet velocity of 6 mm/s. Recently, Fornell et al. 27,28 employed acoustic forces to actively control the final position of polystyrene particles inside dropletsat velocities as high as 8 mm/s. 29
These studies have made significant contributions for the in-droplet manipulation of analytes. However, the application of these fields is limited to the focusing and enrichment of micrometer-sized particles, like beads or cells, where the exerted force magnitude is large enough to overcome the flow inside droplets. In contrast to the laminar flow profile usually observed in continuous microfluidics, droplets confined in microchannels develop a three-dimensional recirculative flow that tends to distribute particles over the whole droplet volume once a critical velocity is reached. 22,23,30 While biomolecules can be harvested on the functionalized surface of these particles,no technique has been reported for the direct separationand enrichment of biomolecules inside moving droplets.Such a technique would allow the separation of nanometric species by exploiting, for instance, differences in the isoelectric point of peptides and proteins with a similar size.
In this contribution, small biomolecules (i.e., organic molecules, peptides, proteins and nucleic acids) wereseparated inside droplets by exploiting their net charge using an electrophoretic-based approach. Droplets were then split in two pieces, one of them enriched with biomolecules. The biomolecules were labeled to track the fluorescence intensity of both daughter droplets at varying electric fields and droplet velocities, reflecting the performance of the separation and enrichment processes. Biomolecules of different sizes and charge magnitudes, and buffers with varying ionic strength and viscosity, were studied to assess the limitations of the proposed technique. The separation of peptide fragments after a proteolytic cleavage and their enrichment into individual daughter droplets was used to demonstrate how differences in the isoelectric point can be exploited with this technique.A key component for the functioning of the device was the integration of thin, conductive membranes between the parallel electrodes and the droplet displacement channel, as seen in Figure 1a. These membranes, fabricated by doping polydimethylsiloxane (PDMS) with carbon nanotubes, enabled the generation of electrical currents inside the droplets and simultaneously decreased the formation of electrolytic byproducts. 31,32 Previous efforts for coupling electrophoresis and droplet microfluidics focused on de-emulsifying droplets to extract their content intoa secondary separation channel. 33–36 In contrast, the technique proposed here constructs towards the use of electrophoretic-based approaches directly inside droplets to separate and enrich charged molecules at velocities over 10 mm/s (13 droplets/sec).
Figure 1.
(a) Conceptual schematic of the microfluidic device, comprising a droplet generator, a flow channel, two parallel liquid electrodes, and a Y-junction for droplet splitting. The carbon-based membranes, located between the electrodes and flow channel, are colored in light grey. The image shows the evolution of the enrichment process as the droplet moves through the region between the parallel electrodes and conductive membranes. The process evolution is experimentally shown (i) immediately after droplet generation, at (ii) 1 mm, (iii) 3 mm and (iv) 4 mm into the region with parallel electrodes. (v) Downstream, directly after the enrichment, the droplet splits into two, one containing most of the molecules. The enrichment is visualized with the fluorescent dye dichlorofluorescein. (b) Molecules migrate inside the droplets, according to their charge, once an electric field is induced. (c) Photograph of the microfluidic device. The flow channel is filled with green food dye and the channels for the liquid electrodes are filled with red food dye.
Experimental Section
Device design and fabrication
The microfluidic devices were made from PDMS (Sylgard 184, Dow Corning) and fabricated using standard soft-lithography techniques. 37,38 Each microfluidic device contained a flow-focusing droplet generator, a flow channel (200 μm wide), two parallel channels that serve as liquid electrodes (500 μm wide, 5 mm long), and a Y-junction for droplet splitting (Fig. 1a). The electrodes and droplets are interfaced by means of conductive membranes (100 μm wide) generated from a mixture of PDMS and (8 w%) 39 carbon nanotubes (Fig. 1a). The thickness of the device was 85 μm. The fabrication details of the microfluidic device, including the generation of the integrated conductive membranes, are extensively described in the Supporting Information, SI.
Biomolecules sample preparation
Solutions containing different biomolecules were prepared to assess their separation and enrichment as a function of their size and charge. All biomolecules were suspended in a buffer solution containing 10 mM HEPES, 20 mM Bis-Tris and 0.1% v/v Tween 20. The pH was adjusted to 7.5 using sodium hydroxide, resulting in a final ionic strength of 5.3 mM. Further details of the biomolecules (e.g., size, charge, fluorescent label, and vendor) can be found in the SI. The molecule concentration of all samples was 0.01 μg/μl, which is in the concentration range of other biochemical assays performed in microfludics. 40,41
Experimental procedure
Experiments started with a clean microchannel that was filled with Fluorinert FC-40 oil (3M), supplied with a 0.5% of 008-FluoroSurfactant (Ran Biotechnologies). This oil-surfactant combination served as the continuous phase of the discretized samples. High-precision syringe pumps (neMESYS, Cetoni) were used to deliver both the sample and continuous phase through the channel inlets. A 3 M potassium chloride solution was injected in the electrode channels to serve as liquid electrodes. 42 Platinum wire electrodes were then placed at the electrode channel reservoirs and a DC electric potential was applied using a source meter (2612A, Keithley). The electrical current flowing through the microfluidic device was monitored and recorded by the source meter. An EMCCD camera (iXon DV887, Andor) was used to capture fluorescent microscopy images and videos.
Fluorescence measurements
The fluorescence intensity of the droplets before and after splitting was tracked during experiments, and was used to estimate the biomolecule concentration by means of linear calibration curves (Table S3 in SI). An enrichment factor (E) was defined to assess the capacity of the system to separate biomolecules into one side of droplets and retain them in one of the daughter droplets after splitting. This factor computed the difference in concentration in the daughter droplets before (C O ) and after (C E ) an electric potential was applied, normalized by C O :
| (1) |
This enrichment factor is relative to the case where no electric field is induced, ranging from 0% (both daughter droplets have the same concentration) to 100% (all biomolecules are retained in one of the daughter droplets). A full description of the fluorescence measurement process can be found in the SI.
Peptide cleavage
A peptide (sequence R9G7, Peptide Specialty Laboratories) was suspended in a buffer solution containing 1 mM HEPES and 2 mM Bis-Tris (pH of 7.2), to a final concentration of 20 ng/μL. Trypsin (mass spectrometry grade, Promega) was then added to the solution vial (2 ng/μL), vortexed, and incubated at 37 °C for 12 hours.Sodium hydroxide was then added to increase the pH to 11, so that positively and negatively charged fragments could be separated from each other. The solution was introduced into the device, discretized in droplets, and subjected to the separation and enrichment process at a constant current of 10 μA. The daughter droplets were progressively collected in an Eppendorf tube attached to the chip outlets. After an adequate volume was collected, the emulsion was broken by placing the tubes in the freezer for two hours, and putting them back at room temperature.
Mass spectrometry analysis
Samples extracted from the coalesced daughter droplets were pipetted in 5 μL droplets on a stainless-steel target. Before the droplet dried, 5 μL of a DHB matrix solution (30 mg/ml of 2,5-dihydroxybenzoic acid in water) was added on top of the sample droplets, and mixed using the pipette. The mixture was left to dry at room temperature for 20 minutes. Next, the slide was inserted into a rapifleX MALDI Tissuetyper (Bruker) to generate the mass spectra of the daughter droplets. Further details on the mass spectrometry analysis can be found in the SI.
Results and Discussion
Working mechanism
Individual solutions containing organic molecules, peptides, proteins, and nucleic acids were segmented in droplets using a flow-focusing approach (Fig. 1a). After droplet generation, the molecules were uniformly distributed in the whole droplet volume, as shown in Figure 1a, panel i, for the case of dichlorofluorescein.
When droplets arrived at the region between the parallel electrodes, however, an electric field established inside them. The molecules then migrated towards one side of the droplets by the resulting electrophoretic force. All negatively-charged molecules were attracted towards the anode, and progressively accumulated in the upper half of the droplets as they moved in this region (Fig. 1b).Note that upper half refers here to the side closest to the anode in the schematic drawings and experimental images of Figure 1. The separation and progressive accumulation of molecules is shown in Figure 1a, panels ii-iv, for droplets at different positions within the parallel-electrodes region. Any positively-charged molecule would migrate towards the opposite direction, accumulating in the lower half, while neutral species would remain unaffected in the whole droplet volume. Since molecules accumulate in one-half of the droplets, a Y-junction was used to split droplets in two identical pieces, one of them enriched with the negatively-charged molecules (Fig. 1a, panel v, and Video V1). A photograph of the microfluidic device is shown in Figure 1c.
Enrichment performance of the system: effect of the size and charge of biomolecules
The electrophoretic force exerted on molecules is a function of their size and charge, the applied electric potential, and the properties of the surrounding buffer. 43 The enrichment factor for all molecules as a function of the induced electric field is shown in Figures 2a (for the non-nucleic acid species) and 2b (for the nucleic acids). The profile of such factor follows a similar concave behavior for all molecules: the enrichment performance increases with the electric field and reaches a maximum value before decreasing. The decrease in performance is caused by an increasing proportion of biomolecules being redirected towards the lower daughter droplet during splitting (Fig. 1a, panel v). An analysis of the fluorescence intensity distribution prior to droplet splitting (Figure S2 in SI) revealed that the electric field promotes the accumulation of biomolecules at the interface between the upper and lower halves (i.e., at the centerline of droplets). Thus, once a critical electric field is reached, biomolecule accumulation at this interface is large enough to redirect some biomolecules into the lower droplet, decreasing the enrichment factor.
Figure 2.
Enrichment factor (E) for (a) the non-nucleic acid species and (b) the nucleic acids as a function of the induced electric field. Droplet velocity was the same (7 mm/s) for all experiments. The dotted lines and shaded regions represent the mean enrichment factor and 95% confidence intervals, respectively, as evaluated using spline functions. Each point represents the average of over 140 droplets (n > 140).
As observed in Figure 2, the proposed in-droplet technique is able to separate and enrich all the studied molecules, from the small dichlorofluorescein molecule to the largest strand of nucleic acids. The maximum enrichment factor and critical electric field, however, vary for the different species, as illustrated by the height and position of the peaks in Figures 2a and 2b. Dichlorofluorescein, the smallest studied molecule, shows the highest enrichment factor among all the non-nucleic acid species, with an average value of 96% at an applied electric field of 270 V/cm. Videos V2-V4 show the splitting and resulting fluorescence of the daughter droplets when no electric field is induced (V2), at the critical field (V3), and beyond the critical field (V4). The enrichment of dichlorofluorescein is followed by that of biotin, showing an average enrichment of 63% when an electric field of 240 V/cm is induced. Although both dichlorofluorescein and biotin have a similar charge (-1, Table S1 in SI), the larger size of biotin seems to be responsible for the observed decrease in the maximum enrichment factor. The (Arg)9 polypeptide, whose size is comparable to that of biotin, exhibits a similar enrichment performance (59%). The critical electric field, however, increases to 310 V/cm, partially driven by a lower charge magnitude (-0.85). A similar behavior is observed for the green fluorescent protein (rGFP), where the high charge magnitude (-8.81) reduces the critical electric field to 200 V/cm. Despite the significantly larger size of rGFP, the maximum enrichment (88%) is comparable to that of the small dichlorofluorescein molecule, where the size increase is outweighed by its high charge magnitude. These results evidence the influence of the size and charge of the biomolecules on their in-droplet separation and enrichment. In general, biomolecules with a similar charge magnitude show a better separation and enrichment as size decreases, while the critical electric field decreases with an increase in charge. An outlier behavior is observed for streptavidin, a protein whose size is roughly twice the size of rGFP. Although the net charge of streptavidin (-3.30) is only 25% of the charge owned by rGFP, the critical electric field decreases to 180 V/cm. The maximum enrichment is, however, limited to 50%, which is significantly lower than the enrichments exhibited by the other molecules.
The profile of the enrichment factor for the nucleic acids (Fig. 2b) reflects a similar interplay between their size and charge. Nucleic acids are (negatively) highly-charged molecules, owing to their deoxyribose backbone with phosphate groups, which translates into high enrichment factors. On average, 80% of the shortest DNA strand (10 base pairs) can be separated and enriched in one daughter droplet when 300 V/cm are applied through the electrodes. Although an increase in size to 1,000 base pairs is accompanied by a slight increase in the electrophoretic mobility of the molecule (Table S2 in SI), it is enough to increase the maximum enrichment to 98% at a slightly lower electric field (280 V/cm). A further increase in size to 20,000 base pairs, which does not increase the electrophoretic mobility of the molecule, 46,5 has little effect on the enrichment performance (94%). The increase in size, however, scales the critical field to 320 V/cm. This behavior is more drastic when size is increased to 48,500 base pairs (lambda DNA), which scales the critical field to 420 V/cm for a similar enrichment (95%) to take place. The nucleic acid results indicate that highly-charged biomolecules can be enriched indistinctly of their size, as long as any size increase is compensated by a stronger electric field. This observation indicates that size and other molecular properties (e.g., viscous drag, rigidity, and structure) affect the migration of biomolecules between both sides of the droplets, adding a hydrodynamic component to their net migration.
Dynamics of the separation and enrichment process
At low velocities, the flow profile inside droplets has two main recirculating regions, located at the upper and lower halves. 23,30 Once an electric field is established, biomolecules recirculating in one side are forced to escape into the other side by the resulting electrophoretic force; and are retained there by the recirculation in that region and the electrophoretic force itself.Figure 3 shows the evolution of the accumulation process, which can be described using the cumulative distribution function of a log-normal distribution. 44 A minimal electrode length of 0.75 mm is needed to promote the electromigration of dichlorofluorescein into the upper half. The transport rate is initially high, with an effective electrode length of 1.0 mm (accumulation of 50% of the molecules), and 81% of the molecules can be enriched 1.7 mm into the electrodes/membranes region. After this length, the transport rate slows down and the enrichment asymptotically approaches a final value of 96%. These results evidence the requirement for a careful selection of the electrode length, since electrodes shorter than required may not result in a complete enrichment. In contrast, electrodes longer than needed increase the risk of electrolysis with little to no improvement on the enrichment factor, as observed when the electrodes length is doubled from 2.5 to 5.0 mm.
Figure 3.
Enrichment factor (E) of dichlorofluorescein as a function of the electrodes length. The dotted line represents the cumulative distribution function of a log-normal distribution (residual standard error of 7.05 and 3 degrees of freedom). The fitted parameters are {b, c, d, e} = {3.97, 4.06, 89.46, 0.99}, which represent the steepness of the enrichment curve (b), the initial and maximum asymptotic enrichments (c and d, respectively), and the effective electrode length (e). The shaded regions represent the 95% confidence interval for the mean enrichment. Droplet velocity was the same (7 mm/s) for all experiments. Each point represents the average of 175 droplets (n = 175).
Effect of the ionic strength, viscosity and concentration
The electromigration rate of molecules is heavily influenced by the surrounding buffer. Figure 4a illustrates the dependence of the in-droplet separation and enrichment of molecules for buffers with ionic strengths ranging from 0.5 to 10.6 mM, all with the same pH (7.5). As observed, an increase in the ionic strength decreases the separation and retention of dichlorofluorescein, as shown by a declining enrichment factor (relative to the ionic strength of 5.3 mM).This decline is significant at 10.6 mM, driven by a reduction of the electrophoretic mobility at higher ionic strengths. 43,45
Figure 4.
Relative enrichment factor (with respect to the referenced value) for dichlorofluorescein as functions of (a) the ionic strength and (b) HPMC content of the buffer, and (c) the initial analyte concentration. Droplet velocity was the same (7 mm/s) for all experiments. Each bar represents the average of over 145 droplets (n > 145).
High viscosities suppress the recirculation regions inside droplets, 30 and simultaneously retard the molecular electromigration. To study these effects on the separation and enrichment of biomolecules, 0.05% w/v hydroxypropyl methylcellulose (HPMC)was added to the suspension, which increased the viscosity by 11%. 46 Dichlorofluorescein exhibited a slower separation and retention, as reflected by a significant decrease in the enrichment factor, from 96% to 68% (Figure 4b). Higher concentrations of HPMC (0.10% and 0.25%) result in a further increase in viscosity (25% and 76%), reducing the enrichment factor to 40%.
During the separation and retention process, the local concentration of molecules increases in one side of the droplets. As described earlier, once a critical field strength is reached, a large proportion of the molecules is redirected towards the lower daughter droplet (Video V5). This critical point, observed as a decrease in the enrichment factor, is concentration-dependent, as shown in Figure 4c. The maximum enrichment factor decreases exponentially with the initial concentration (R 2 of 0.991).
The results presented so far show the dependence of the separation and retention dynamics on parameters beyond the electrophoretic mobility of species. Properties such as the diffusivity, viscous drag, rigidity, shape, and structure, affect the molecular flux between both sides of the droplets, and contribute to the net migration. Moreover, concentration-depending parameters (e.g., local rheology changes, colloidal interactions, and molecular charge-transport mechanisms) 22,47,48 may also play a role on the separation and retention process.
Throughput evaluation: droplet velocity and size
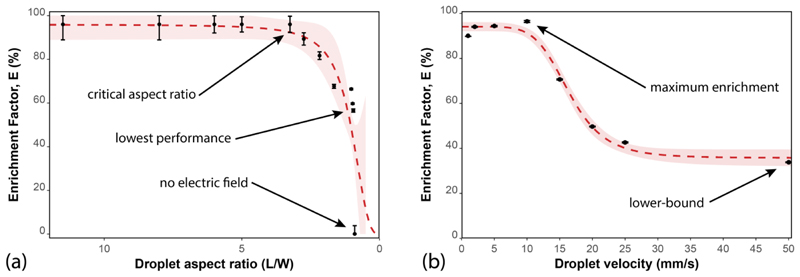
The size of droplets, represented here as the aspect ratio, defines the area of droplets in contact with the channel walls and electrodes, as well as the area between the upper and lower halves. Droplets with an aspect ratio above 3.2 show a high and stable enrichment factor, as observed in Figure 5a. Aspect ratios as high as 50 were studied, showing a similarly high enrichment performance (data not shown). In contrast, droplets with low aspect ratios (below 3.2) exhibited a sharp decay in their enrichment performance, reaching a value of 60% for an aspect ratio close to 1. Smaller droplets lacked a direct contact with the conductive membranes, which resulted in enrichment factors of zero (same molecule concentration in both daughter droplets).
Figure 5.
(a) Enrichment factor (E) of dichlorofluorescein as a function of the droplet aspect ratio (droplet length over droplet width). Droplet velocity was the same (5 mm/s) for all experiments. (b) Enrichment factor (E) of dichlorofluorescein as a function of droplet velocity. Droplet size was approximately the same (3.5 times droplet width) for all experiments. In both sub-figures, the dotted lines represent cumulative distribution functions of the log-logistic distribution, while the shaded regions represent the 95% confidence interval for the mean enrichment. Each point represents the maximum achievable enrichment, averaged over more than 235 droplets (n > 235).
The behavior of the enrichment factor at several droplet velocities is illustrated in Figure 5b. Once an electric field is induced inside droplets, the negatively-charged dichlorofluorescein migrates into the upper half and is successfully retained there at velocities below 10 mm/s, as reflected by an average enrichment factor of 95%.When droplet velocity exceeds 10 mm/s, however, the enrichment factor shows an initial exponential decay, and the performance rapidly drops to 50% at 20 mm/s. At higher velocities (above 20 mm/s), the decay rate decreases, and the enrichment performance asymptotically approaches a final value of 38% at 50 mm/s. The manipulation of micrometer-sized beads, in contrast, shows a complete redistribution of particles at velocities ranging from 0.75 to 10.00 mm/s, 29 depending on the target particles and applied technique.
In-droplet separation and enrichment of digested peptide fragments
Finally, we demonstrate the applicability of our system for the in-droplet separation of reaction products by cleaving a peptide with sequence R9G7 using trypsin. Trypsin has a preference for cleaving at the carboxyl side of arginine (R), producing fragments with all sequence combinations in the set {R1, R1G7, R2G7, …, R9G7}. After digestion, the pH was adjusted to 11, the resulting fragments were enriched in different daughter droplets based on their charge, and analyzed using MALDI-MS. Figure 6 shows the mass spectra, from 100 to 2,000 m/z, for the anode-sided (Figs. 6a, 6c and 6e) and cathode-sided (Figs. 6b, 6d and 6f) daughter droplets. The peak at m/z = 195.3 (Figs. 6a and 6b) is associated with the DHB matrix, 49 and is used as a reference peak to determine the relative abundance of the digested fragments. 50 At a pH of 11, the charge of R1 is slightly negative (-0.1), and the amino acid tends to be transported and retained in the anode-sided daughter droplets. Although the net charge of the amino acid is low, its relatively small size (174.6 Da) benefits the electromigration process, and a larger abundance in the anode-sided droplets is obtained (peaks difference in Figs. 6a and 6b). When the G7 sequence is part of the fragment (R1G7), the net charge of the amino acid remains unaffected, since glycine (G) does not own ionizable side chains. The size of the fragment, however, scales to 573.6 Da, slowing its migration and decreasing its relative abundance in the anode-sided droplets (Figs. 6a and 6b). The addition of one R amino acid (R2G7) increases the size of the fragment in 156 Da, and reverses the charge polarity (+0.8). Thus, a slightly larger abundance in the cathode-sided droplets is observed (Figs. 6c and 6d). The addition of further R amino acids increases logarithmically the charge-to-mass ratio of the fragment, promoting a faster electromigration and a larger abundance on the cathode-sided droplets for the fragments in {R3G7, R3G7, …, R9G7} (Figs. 6c-6f). These results demonstrate that operation at pH levels between 7 and 11 are compatible with the device, and pH can be adjusted to modify the net charge of different biomolecules. This opens a venue for performing customized in-droplet separations.
Figure 6.
MALDI-MS mass spectra for the (a, c, e) anode-sided and (b,d,f) cathode-sided daughter droplets. The spectra is divided in three m/z ranges: (a,b) 100 to 500, (c,d) 500 to 1,000, and (e,f) 1,000 to 2,000. The highlighted squares represent the peaks associated with the digested fragments in the set {R1, R1G7, R2G7, R3G7, …, R9G7}. The abundance of the fragment species is relative 50 to the peak at m/z = 195.3, which is associated with the employed DHB matrix. 49 The estimated m/z value for the molecular ions expected in the positive ionization mode (M+H)+is displayed in brackets []. The net charge of the fragments at a pH of 11, calculated based on the composition of ionizable residues, is displayed in parenthesis ().
Conclusions
We demonstrated the separation and enrichment of molecules with sizes ranging from 401 Da (dichlorofluorescein) to 31.6 MDa (lambda DNA) inside droplets. The microfluidic device contained two parallel three-dimensional electrodes, which interfaced the droplets through carbon-based PDMS membranes. These membranes decrease the generation of electrolytic byproducts that would otherwise alter these paration and enrichment process in the small droplet volumes. By applying an electric potential through the parallel electrodes, uniform electric fields were induced inside droplets. It was found that the separation and enrichment increase when the biomolecules are either small or highly-charged, in agreement with other studies. Enrichments of over 90% were observed for dichlorofluorescein (the smaller molecule) and for nucleic acids (highly-charged) at velocities over 10 mm/s (13 droplets per second).
The internal flow topology of droplets induces the formation of two axisymmetric recirculation regions. Parameters intrinsic to biomolecule properties (e.g., viscous drag, rigidity and structure) and to their local concentration (e.g., rheology changes) influence the separation and retention of molecules. However, further work is required to elucidate and describe the separation and retention dynamics inside droplets. Nevertheless, the described in-droplet technique has been shown to be suitable for high-throughput separations of charged species.It can be applied for a broad range of molecules and conditions, and is straightforward to integrate into existing microfluidic devices.We therefore believe that the technique is a valuable tool to perform a complete droplet reaction and separation workflow, which will allow for screening multiple conditions directly on-chip. Since the products are enriched in daughter droplets, this versatile method also improves the detection of droplet content, e.g. by mass spectrometry.
Supplementary Material
The Supporting Information contains all details of the fabrication process, biomolecules properties, fluorescence measurements and mass spectrometry analysis, and is available free of charge on the ACS Publications website (PDF).
Supplementary videos of (V1) droplet splitting after the electro-phoretic separation and enrichment; (V2) droplet splitting when no electric potential is applied; droplet splitting when (V3) 270 V/cm and (V4) 320 V/cm are induced; and (V5) accumulation of analytes at the centerline at increasing electric fields (AVI).
Acknowledgment
Funding from the Swiss National Science Foundation (NCCR Molecular Systems Engineering) and the European Research Council (ERC Consolidator Grant No. 681587 “HybCell”) is gratefully acknowledged. The authors would like to thank Dr. Darius G. Rackus for proofreading the manuscript.
Footnotes
Author Contributions
M.A.S and P.S.D. developed the concept. M.A.S. performed the experiments and analyzed the data. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Contributor Information
Mario A. Saucedo-Espinosa, Department of Biosystems Science and Engineering, ETH Zürich, Mattenstrasse 26, 4058 Basel, Switzerland.
Petra S. Dittrich, Department of Biosystems Science and Engineering, ETH Zürich, Mattenstrasse 26, 4058 Basel, Switzerland.
References
- (1).Teh SY, Lin R, Hung LH, Lee AP. Droplet Microfluidics. Lab Chip. 2008;8(2):198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- (2).Dressler OJ, Maceiczyk RM, Chang SI, Demello AJ. Droplet-Based Microfluidics: Enabling Impact on Drug Discovery. J Biomol Screen. 2014;19(4):483–496. doi: 10.1177/1087057113510401. [DOI] [PubMed] [Google Scholar]
- (3).Hümmer D, Kurth F, Naredi-Rainer N, Dittrich PS. Single Cells in Confined Volumes: Microchambers and Microdroplets. Lab Chip. 2016;16(3):447–458. doi: 10.1039/c5lc01314c. [DOI] [PubMed] [Google Scholar]
- (4).Guo MT, Rotem A, Heyman JA, Weitz DA. Droplet Microfluidics for High-Throughput Biological Assays. Lab Chip. 2012;12(12):2146–2155. doi: 10.1039/c2lc21147e. [DOI] [PubMed] [Google Scholar]
- (5).Clark IC, Abate AR. Microfluidic Bead Encapsulation above 20 KHz with Triggered Drop Formation. Lab Chip. 2018;18(23):3598–3605. doi: 10.1039/c8lc00514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sjostrom SL, Bai Y, Huang M, Liu Z, Nielsen J, Joensson HN, Andersson Svahn H. High-Throughput Screening for Industrial Enzyme Production Hosts by Droplet Microfluidics. Lab Chip. 2014;14(4):806–813. doi: 10.1039/c3lc51202a. [DOI] [PubMed] [Google Scholar]
- (7).Joensson HN, Andersson Svahn H. Droplet Microfluidics—A Tool for Single-Cell Analysis. Angew Chemie Int Ed. 2012;51(49):12176–12192. doi: 10.1002/anie.201200460. [DOI] [PubMed] [Google Scholar]
- (8).Eastburn DJ, Huang Y, Pellegrino M, Sciambi A, Ptácek LJ, Abate AR. Microfluidic Droplet Enrichment for Targeted Sequencing. Nucleic Acids Res. 2015;43(13):e86. doi: 10.1093/nar/gkv297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lan F, Demaree B, Ahmed N, Abate AR. Single-Cell Genome Sequencing at Ultra-High-Throughput with Microfluidic Droplet Barcoding. Nat Biotechnol. 2017;35(7):640–646. doi: 10.1038/nbt.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shang L, Cheng Y, Zhao Y. Emerging Droplet Microfluidics. Chem Rev. 2017;117(12):7964–8040. doi: 10.1021/acs.chemrev.6b00848. [DOI] [PubMed] [Google Scholar]
- (11).Wang J, Wang J, Feng L, Lin T. Fluid Mixing in Droplet-Based Microfluidics with a Serpentine Microchannel. RSC Adv. 2015;5(126):104138–104144. [Google Scholar]
- (12).Yesiloz G, Boybay MS, Ren CL. Effective Thermo-Capillary Mixing in Droplet Microfluidics Integrated with a Microwave Heater. Anal Chem. 2017;89(3):1978–1984. doi: 10.1021/acs.analchem.6b04520. [DOI] [PubMed] [Google Scholar]
- (13).Yuan H, Pan Y, Tian J, Chao Y, Li J, Cheung Shum H. Electricity-Free Picoinjection Assisted Droplet Microfluidics. Sensors Actuators, B Chem. 2019;298 126766. [Google Scholar]
- (14).Li S, Zeng M, Gaule T, McPherson MJ, Meldrum FC. Passive Picoinjection Enables Controlled Crystallization in a Droplet Microfluidic Device. Small. 2017;13(41) doi: 10.1002/smll.201702154. 1702154. [DOI] [PubMed] [Google Scholar]
- (15).Guzman AR, Kim HS, de Figueiredo P, Han A. A Three-Dimensional Electrode for Highly Efficient Electrocoalescence-Based Droplet Merging. Biomed Microdevices. 2015;17(35):35. doi: 10.1007/s10544-014-9921-x. [DOI] [PubMed] [Google Scholar]
- (16).Chaudhuri J, Timung S, Dandamudi CB, Mandal TK, Bandyopadhyay D. Discrete Electric Field Mediated Droplet Splitting in Microchannels: Fission, Cascade, and Rayleigh Modes. Electrophoresis. 2017;38(2):278–286. doi: 10.1002/elps.201600276. [DOI] [PubMed] [Google Scholar]
- (17).Shih SCC, Goyal G, Kim PW, Koutsoubelis N, Keasling JD, Adams PD, Hillson NJ, Singh AK. A Versatile Microfluidic Device for Automating Synthetic Biology. ACS Synth Biol. 2015;4(10):1151–1164. doi: 10.1021/acssynbio.5b00062. [DOI] [PubMed] [Google Scholar]
- (18).Kaminski TS, Scheler O, Garstecki P. Droplet Microfluidics for Microbiology: Techniques, Applications and Challenges. Lab Chip. 2016;16(12):2168–2187. doi: 10.1039/c6lc00367b. [DOI] [PubMed] [Google Scholar]
- (19).Gielen F, Hours R, Emond S, Fischlechner M, Schell U, Hollfelder F. Ultrahigh-Throughput-Directed Enzyme Evolution by Absorbance-Activated Droplet Sorting (AADS) Proc Natl Acad Sci U S A. 2016;113(47):E7383–E7389. doi: 10.1073/pnas.1606927113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Qin Y, Wu L, Wang J, Han R, Shen J, Wang J, Xu S, Paguirigan AL, Smith JL, Radich JP, Chiu DT. A Fluorescence-Activated Single-Droplet Dispenser for High Accuracy Single-Droplet and Single-Cell Sorting and Dispensing. Anal Chem. 2019;91(10):6815–6819. doi: 10.1021/acs.analchem.9b01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hasan S, Geissler D, Wink K, Hagen A, Heiland JJ, Belder D. Fluorescence Lifetime-Activated Droplet Sorting in Microfluidic Chip Systems. Lab Chip. 2019;19(3):403–409. doi: 10.1039/c8lc01278d. [DOI] [PubMed] [Google Scholar]
- (22).Kurup GK, Basu AS. Field-Free Particle Focusing in Microfluidic Plugs. Biomicrofluidics. 2012;6(2) doi: 10.1063/1.3700120. 022008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hein M, Moskopp M, Seemann R. Flow Field Induced Particle Accumulation inside Droplets in Rectangular Channels. Lab Chip. 2015;15(13):2879–2886. doi: 10.1039/c5lc00420a. [DOI] [PubMed] [Google Scholar]
- (24).Han SI, Soo Kim H, Han A. In-Droplet Cell Concentration Using Dielectrophoresis. Biosens Bioelectron. 2017;97(15):41–45. doi: 10.1016/j.bios.2017.05.036. [DOI] [PubMed] [Google Scholar]
- (25).Lombardi D, Dittrich PS. Droplet Microfluidics with Magnetic Beads: A New Tool to Investigate Drug-Protein Interactions. Anal Bioanal Chem. 2011;399(1):347–352. doi: 10.1007/s00216-010-4302-7. [DOI] [PubMed] [Google Scholar]
- (26).Brouzes E, Kruse T, Kimmerling R, Strey HH. Rapid and Continuous Magnetic Separation in Droplet Microfluidic Devices. Lab Chip. 2015;15(3):908–919. doi: 10.1039/c4lc01327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Fornell A, Ohlin M, Garofalo F, Nilsson J, Tenje M. An Intra-Droplet Particle Switch for Droplet Microfluidics Using Bulk Acoustic Waves. Biomicrofluidics. 2017;11(3) doi: 10.1063/1.4984131. 031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Fornell A, Nilsson J, Jonsson L, Periyannan Rajeswari PK, Joensson HN, Tenje M. Controlled Lateral Positioning of Microparticles Inside Droplets Using Acoustophoresis. Anal Chem. 2015;87(20):10521–10526. doi: 10.1021/acs.analchem.5b02746. [DOI] [PubMed] [Google Scholar]
- (29).Tenje M, Fornell A, Ohlin M, Nilsson J. Particle Manipulation Methods in Droplet Microfluidics. Anal Chem. 2018;90(3):1434–1443. doi: 10.1021/acs.analchem.7b01333. [DOI] [PubMed] [Google Scholar]
- (30).Ma S, Sherwood JM, Huck WTS, Balabani S. On the Flow Topology inside Droplets Moving in Rectangular Microchannels. Lab Chip. 2014;14(18):3611–3620. doi: 10.1039/c4lc00671b. [DOI] [PubMed] [Google Scholar]
- (31).Erlandsson PG, Robinson ND. Electrolysis-Reducing Electrodes for Electrokinetic Devices. Electrophoresis. 2011;32(6–7):784–790. doi: 10.1002/elps.201000617. [DOI] [PubMed] [Google Scholar]
- (32).Fu X, Mavrogiannis N, Ibo M, Crivellari F, Gagnon ZR. Microfluidic Free-Flow Zone Electrophoresis and Isotachophoresis Using Carbon Black Nano-Composite PDMS Sidewall Membranes. Electrophoresis. 2017;38(2):327–334. doi: 10.1002/elps.201600104. [DOI] [PubMed] [Google Scholar]
- (33).Edgar JS, Pabbati CP, Lorenz RM, He M, Fiorini GS, Chiu DT. Capillary Electrophoresis Separation in the Presence of an Immiscible Boundary for Droplet Analysis. Anal Chem. 2006;78(19):6948–6954. doi: 10.1021/ac0613131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Roman GT, Wang M, Shultz KN, Jennings C, Kennedy RT. Sampling and Electrophoretic Analysis of Segmented Flow Streams Using Virtual Walls in a Microfluidic Device. Anal Chem. 2008;80(21):8231–8238. doi: 10.1021/ac801317t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wang M, Roman GT, Perry ML, Kennedy RT. Microfluidic Chip for High Efficiency Electrophoretic Analysis of Segmented Flow from a Microdialysis Probe and in Vivo Chemical Monitoring. Anal Chem. 2009;81(21):9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Niu X, Pereira F, Edel JB, De Mello AJ. Droplet-Interfaced Microchip and Capillary Electrophoretic Separations. Anal Chem. 2013;85(18):8654–8660. doi: 10.1021/ac401383y. [DOI] [PubMed] [Google Scholar]
- (37).Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane) Anal Chem. 1998;70(23):4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- (38).Armbrecht L, Müller RS, Nikoloff J, Dittrich PS. Single-Cell Protein Profiling in Microchambers with Barcoded Beads. Microsystems Nanoeng. 2019;5(5):55. doi: 10.1038/s41378-019-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kim JH, Hwang JY, Hwang HR, Kim HS, Lee JH, Seo JW, Shin US, Lee SH. Simple and Cost-Effective Method of Highly Conductive and Elastic Carbon Nanotube/Polydimethylsiloxane Composite for Wearable Electronics. Sci Rep. 2018;8(1) doi: 10.1038/s41598-017-18209-w. 54853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Henzler C, Schomaker M, Yang R, Lambert AP, LaRue R, Kincaid R, Beckman K, Kemmer T, Wilson J, Yohe S, Thyagarajan B, Nelson AC. Optimization of a Microfluidics-Based next Generation Sequencing Assay for Clinical Oncology Diagnostics. Ann Transl Med. 2018;6(9):162–162. doi: 10.21037/atm.2018.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Datinská V, Vorácová I, Berka J, Foret F. Preparative Concentration of Nucleic Acids Fragments by Capillary Isotachophoretic Analyzer. J Chromatogr A. 2018;1548:100–103. doi: 10.1016/j.chroma.2018.03.021. [DOI] [PubMed] [Google Scholar]
- (42).Sciambi A, Abate AR. Generating Electric Fields in PDMS Microfluidic Devices with Salt Water Electrodes. Lab Chip. 2014;14(15):2605–2609. doi: 10.1039/c4lc00078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Li D, Fu S, Lucy CA. Prediction of Electrophoretic Mobilities. 3. Effect of Ionic Strength in Capillary Zone Electrophoresis. Anal Chem. 1999;71(3):687–699. doi: 10.1021/ac980843x. [DOI] [PubMed] [Google Scholar]
- (44).Ritz C, Baty F, Streibig JC, Gerhard D. Dose-Response Analysis Using R. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0146021. e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bahga SS, Bercovici M, Santiago JG. Ionic Strength Effects on Electrophoretic Focusing and Separations. Electrophoresis. 2010;31(5):910–919. doi: 10.1002/elps.200900560. [DOI] [PubMed] [Google Scholar]
- (46).Andonova V, Zagorchev P, Katsarov P, Kassarova M. Eye Drops with Nanoparticles as Drug Delivery Systems. Int J Pharm Pharm Sci. 2015;7(2):431–435. [Google Scholar]
- (47).Belloni L. Colloidal Interactions. J Phys Condens Matter. 2000;12(46):R549. [Google Scholar]
- (48).Ribeiro WC, Gonçalves LM, Liébana S, Pividori MI, Bueno PR. Molecular Conductance of Double-Stranded DNA Evaluated by Electrochemical Capacitance Spectroscopy. Nanoscale. 2016;8(16):8931–8938. doi: 10.1039/c6nr01076h. [DOI] [PubMed] [Google Scholar]
- (49).Gill EL, Yost RA, Vedam-Mai V, Garrett TJ. Precast Gelatin-Based Molds for Tissue Embedding Compatible with Mass Spectrometry Imaging. Anal Chem. 2017;89(1):576–580. doi: 10.1021/acs.analchem.6b04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang P, Giese RW. Journal of Chromatography A. Elsevier B.V; 2017. Feb 24, Recommendations for Quantitative Analysis of Small Molecules by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry; pp. 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.