Abstract
Two genetic variants that alter alcohol metabolism, ALDH2‐rs671 and ADH1B‐rs1229984, can modify oesophageal cancer risk associated with alcohol consumption in East Asians, but their associations with other cancers remain uncertain. ALDH2‐rs671 G>A and ADH1B‐rs1229984 G>A were genotyped in 150 722 adults, enrolled from 10 areas in China during 2004 to 2008. After 11 years' follow‐up, 9339 individuals developed cancer. Cox regression was used to estimate hazard ratios (HRs) for site‐specific cancers associated with these genotypes, and their potential interactions with alcohol consumption. Overall, the A‐allele frequency was 0.21 for ALDH2‐rs671 and 0.69 for ADH1B‐rs1229984, with A‐alleles strongly associated with lower alcohol consumption. Among men, ALDH2‐rs671 AA genotype was associated with HR of 0.69 (95% confidence interval: 0.53‐0.90) for IARC alcohol‐related cancers (n = 1900), compared to GG genotype. For ADH1B‐rs1229984, the HRs of AG and AA vs GG genotype were 0.80 (0.69‐0.93) and 0.75 (0.64‐0.87) for IARC alcohol‐related cancers, 0.61 (0.39‐0.96) and 0.61 (0.39‐0.94) for head and neck cancer (n = 196) and 0.68 (0.53‐0.88) and 0.60 (0.46‐0.78) for oesophageal cancer (n = 546). There were no significant associations of these genotypes with risks of liver (n = 651), colorectal (n = 556), stomach (n = 725) or lung (n = 1135) cancers. Among male drinkers, the risks associated with higher alcohol consumption were greater among ALDH2‐rs671 AG than GG carriers for head and neck, oesophageal and lung cancers (P interaction < .02). Among women, only 2% drank alcohol regularly, with no comparable associations observed between genotype and cancer. These findings support the causal effects of alcohol consumption on upper aerodigestive tract cancers, with ALDH2‐rs671 AG genotype further exacerbating the risks.
Keywords: ADH1B, alcohol, ALDH2, cancer, China
What's new?
Alcohol consumption has been increasing among men in China, and is a major contributor to the total cancer burden. Two genetic variants that alter alcohol metabolism are associated with esophageal cancer risk in East Asians. Do these variants also play a role in other cancers, or influence the effect of alcohol on cancer risk? In this large Chinese study, the authors found that certain genotypes were associated with reduced upper aero‐digestive tract cancer risk, and that one of the variants may exacerbate the effects of alcohol on several cancers.
Abbreviations
- ADH1B
alcohol dehydrogenase 1B
- ALDH2
aldehyde dehydrogenase 2
- BMI
body mass index
- CI
confidence interval
- CKB
China Kadoorie Biobank
- HBV
hepatitis B virus
- HR
hazard ratio
- IARC
International Agency for Research on Cancer
- ICD‐10
International Classification of Diseases, 10th Revision
- MR
Mendelian randomisation
- SNP
single nucleotide polymorphism
1. INTRODUCTION
Cancer is a leading cause of premature mortality and disability globally, accounting for an estimated 19.3 million new cancer cases and 10 million deaths in 2020. 1 Worldwide, 24% of total cancer cases, including 37% of lung cancer and 47% of digestive tract (oesophagus, stomach and liver) cancers, occurred in China. 1 Based mainly on observational studies, the International Agency for Research on Cancer (IARC) reported that there is sufficient evidence that alcohol consumption is causally related to development of cancers in the head and neck, oesophagus, liver, colon‐rectum and female breast, but causal evidence remains inconclusive for other cancer sites including lung and stomach due to other possible confounders (eg, smoking, diet). 2 Worldwide, it was estimated that 3 million deaths could be attributed to alcohol consumption, including >0.4 million from cancer. 3
Alcohol consumption has been increasing over recent decades in China, almost exclusively in men, 4 and is a major contributor to the total cancer burden in Chinese men. 5 , 6 In China and other East Asian populations, two common genetic variants affect alcohol tolerability and are strongly associated with lower alcohol intake. 7 An East Asian‐specific loss‐of‐function variant in the aldehyde dehydrogenase 2 (ALDH2) gene (rs671 G>A) substantially decreases the breakdown of acetaldehyde, which is a Group 1 human carcinogen classified by IARC and a toxic metabolite produced during alcohol metabolism, causing the characteristic East Asian alcohol flushing response. 2 , 8 Another variant in the alcohol dehydrogenase 1B (ADH1B) gene (rs1229984 G>A) accelerates acetaldehyde formation from alcohol. 9 These genetic variants, which are randomly allocated at conception and usually independent of other lifestyle exposures, can be used as instruments for alcohol intake to help assess the likely causal effects of alcohol consumption on disease risks. 7 , 10 Importantly, appropriate understanding of the interplay between these genetic variants and between genetic variants and alcohol consumption may provide insight into the involvement of alcohol‐derived acetaldehyde in the carcinogenesis of certain site‐specific cancers. Previous studies have shown that the ALDH2‐rs671 AA and ADH1B‐rs1229984 AA and AG genotypes were associated with lower oesophageal cancer risk compared to the GG genotype, 11 , 12 and that ALDH2‐rs671 genotype may modify the relationship between alcohol intake and oesophageal cancer risk. 12 , 13 , 14 However, there is limited evidence on the associations of these genotypes with risk of cancer at other sites, 11 , 15 and for the potential interactions between genotype and alcohol intake on cancer risks. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 A comprehensive assessment of the interplay between ALDH2‐rs671, ADH1B‐rs1229984 and alcohol consumption on risks of different cancer types in a large‐scale population‐based cohort study may provide valuable insights into the aetiological role of alcohol on different cancers.
Using data from the prospective China Kadoorie Biobank (CKB), we investigated the associations of ALDH2‐rs671 and ADH1B‐rs1229984 with total and common site‐specific cancers in 151 000 Chinese adults. In addition, we investigated possible gene‐alcohol and gene‐gene interactions on cancer risks.
2. METHODS
2.1. Study population
Details of the CKB study design and methods have been previously reported. 24 Briefly, 512 726 adults aged 30 to 79 years were recruited from 10 rural and urban areas across China during 2004 to 2008. Trained health workers administered a laptop‐based questionnaire recording sociodemographic factors, lifestyles (eg, alcohol drinking, smoking, diet, physical activity) and medical history; undertook physical measurements (eg, blood pressure, anthropometry); and collected a blood sample for long‐term storage. Two resurveys of ~5% randomly selected surviving participants were conducted using similar procedures in 2008 and 2013 to 2014.
2.2. Assessment of alcohol consumption
Detailed questionnaire assessment of alcohol consumption has been described previously. 25 , 26 , 27 Based on their past and current drinking history, participants were classified into: abstainers; ex‐regular drinkers; occasional drinkers and current regular drinkers (ie, had drunk alcohol in most weeks in the past year). Current regular drinkers were asked further questions about their drinking patterns including drinking frequency, beverage type and amount consumed for each type on a typical drinking day, age started drinking and experience of alcohol flushing response after drinking. Level of alcohol consumption was calculated as grams (g) of alcohol per week based on frequency, beverage type and amount consumed. Further details of alcohol assessment are reported in the Supporting Information Methods.
2.3. Follow‐up and main outcome measures
The vital status of participants was obtained periodically from local death registries, supplemented by annual active confirmation through local residential, health insurance and administrative records. Incident cancers were collected through linkage with cancer registries and the national health insurance system (>98% coverage across the 10 study areas), supplemented by active follow‐up approach (see Table S1 and Supporting Information Methods for further details on completeness and quality of cancer outcome measures). 28 All events were coded with International Classification of Diseases, 10th Revision (ICD‐10), blinded to the baseline information.
The main cancer outcomes investigated in our study were total cancer; IARC alcohol‐related cancers (defined as cancers with convincing causal relevance with alcohol as concluded by IARC 2 ) which were cancers of the head and neck (included cancers of the lip and oral cavity, pharynx and larynx; ICD‐10: C00‐C14, C32), oesophagus (C15), colon‐rectum (C18‐C20), liver (C22) and female breast (C50); and certain other site‐specific cancers including lung cancer (C33‐C34) and stomach cancer (C16). Other cancers, apart from ill‐defined neoplasms (C76‐C80, C97), were combined as “other cancers of known sites.” Upper aerodigestive tract (UADT) cancers were defined as cancers of the head and neck and oesophagus. By 1 January 2018, 49 459 (9.7%) deaths were recorded among the 512 726 CKB participants, with 5302 (1.0%) lost to follow‐up.
2.4. Genotyping
The two variants of interest, ALDH2‐rs671 and ADH1B‐rs1229984, were both genotyped in 151 035 randomly selected participants from the CKB cohort using the Affymetrix Axiom 800K‐single nucleotide polymorphism (SNP) array (n = 100 168) or 384‐SNP Illumina GoldenGate array (n = 92 958) at BGI (Shenzhen, China). Genotyping concordance for the studied variants was high between the two arrays (>99.9% among ~25 400 participants genotyped with both arrays). 7
2.5. Statistical analysis
Participants with missing data on genomic principal components (n = 313) were excluded from the analyses, leaving 150 722 participants in the study (see Figure S1). Means and percentages of baseline characteristics were calculated by genotype, standardised to the age and study area structure of the genotyped study population. Cox proportional hazard models, stratified by age‐at‐risk and study area and adjusted for 12 genomic principal components, were used to estimate hazard ratios (HRs) for cancers reported during follow‐up associated with ALDH2‐rs671 and ADH1B‐rs1229984 genotypes, in men and women separately. The genotypic associations with cancer risk were further examined separately by drinking status.
Potential effect modification of the associations between amount of alcohol consumption and cancer risks by genotype were investigated among current regular drinkers. The joint effects of alcohol consumption and ALDH2‐rs671 were assessed by estimating the HRs associated with four categories defined by baseline alcohol intake (<280, 280+ g/wk in men; <70, 70+ g/wk in women) and genotype (GG, AG), excluding AA individuals as few of them drank. The models were stratified by age‐at‐risk and study area and adjusted for 12 genomic principal components, education, household income, smoking, fruit intake, physical activity, body mass index (BMI) and family history of cancer. Likelihood ratio test was used to test for interaction between alcohol consumption and genotype by comparing two models with and without the interaction term. As tobacco smoking also produces acetaldehyde, subgroup analyses by smoking status were conducted to assess potential residual confounding from smoking. The joint effects of alcohol and ADH1B‐rs1229984 were assessed using similar methods. Alcohol intake was also modelled as a continuous variable to estimate adjusted HRs of cancers associated with a 280 g/wk higher usual alcohol intake by genotype, with heterogeneity in effect sizes assessed by χ 2 tests.
The joint effects of ALDH2‐rs671 and ADH1B‐rs1229984 were examined by estimating the HRs associated with the nine groups defined by the combination of genotypes for both variants (from GG/GG to AA/AA [ALDH2‐rs671/ADH1B‐rs1229984], which represented the highest to lowest mean alcohol intake) in men, stratified by age‐at‐risk and study area and adjusted for 12 genomic principal components.
Various sensitivity analyses were performed, including: (a) additional adjustments for socioeconomic status and major lifestyle risk factors for cancer; (b) area‐stratified analysis by combining within‐area genotypic effects using inverse‐variance‐weighted meta‐analysis to investigate potential residual confounding by population stratification (given the differences in allele frequencies between study areas) and (c) excluding individuals with a prior history of cancer at baseline from the gene‐alcohol interaction analysis to reduce potential reverse causation due to changes in drinking habits for health reasons.
As few women drank alcohol regularly in CKB, 25 , 26 the main analyses were focused among men, with genotypic analyses among women conducted to assess the presence of pleiotropic effects (ie, genotypic associations that are not mediated by alcohol consumption). For analyses involving more than two exposure categories, the floating absolute risk method was used to compute the group‐specific 95% confidence intervals (CIs) derived from the variance of the log hazard of each category, such that each HR (including the one for the reference group) has a group‐specific 95% CI that facilitates comparisons between any two categories, as described previously. 7 , 29 , 30 For comparisons of two groups (ie, an exposure category with the reference group), conventional 95% CIs were reported. Repeat alcohol measures for participants who attended both subsequent resurveys were used to correct for regression dilution bias. 31 Further details of the statistical analysis are reported in the Supporting Information Methods. All analyses used SAS (version 9.4) and R (version 4.0.4).
3. RESULTS
Among the 150 722 study participants, the mean age was 52.1 (SD 10.7) years, 40% were men and 56% lived in rural areas. The overall A‐allele frequency was 0.21 (range by area from 0.13 to 0.29) for ALDH2‐rs671 and 0.69 (from 0.64 to 0.74) for ADH1B‐rs1229984, with a generally higher frequency in southern than northern areas for both variants (Table S2).
Among men, ALDH2‐rs671 was strongly associated with the prevalence of current regular drinking (46%, 17% and 1% for GG, AG and AA, respectively) and mean alcohol intake (143, 35, 2 g/wk, respectively; all P trend < .0001) (Table 1). ADH1B‐rs1229984 genotype was also associated with current regular drinking prevalence (43%, 34% and 32%) and mean alcohol intake (146, 99 and 91 g/wk). Among male current regular drinkers, ALDH2‐rs671 was strongly associated with the alcohol flushing response (11%, 56% and 62%) and age at drinking onset (28, 32 and 40 years); the correlations with the alcohol flushing response were consistent directionally for A alleles of both variants, but the effects were weaker with ADH1B‐rs1229984 (15%, 18% and 20%) (P trend < .0001 for all above). In women, similar patterns of associations between drinking patterns and genotype were observed as in men, but with very low prevalence of regular drinking (2%) the differences were small in magnitude (Table S3). There were no material effects of these genotypes on smoking or other lifestyle characteristics in men or women, except a slightly higher prevalence of daily fresh fruit intake and lower physical activity in male ALDH2‐rs671 A‐allele carriers, and slightly lower mean BMI in A‐allele carriers for both variants in both sexes.
TABLE 1.
Baseline characteristics of participants by ALDH2‐rs671 and ADH1B‐rs1229984 genotypes, in men
| Overall (N = 60 835) | ALDH2‐rs671 | ADH1B‐rs1229984 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GG (N = 38 247) | AG (N = 19 827) | AA (N = 2761) | P trend a | GG (N = 29 078) | AG (N = 26 025) | AA (N = 5732) | P trend a | ||
| Sociodemographic characteristics | |||||||||
| Mean age, years | 52.9 | 52.9 | 52.9 | 53.0 | .11 | 52.6 | 53.0 | 53.0 | .11 |
| Education >6 years, % | 57.7 | 57.8 | 57.4 | 56.5 | .053 | 57.0 | 57.8 | 57.6 | .65 |
| Household income >20 000 yuan/yr, % | 44.6 | 44.6 | 44.8 | 44.3 | .84 | 44.5 | 44.3 | 44.9 | .23 |
| Lifestyle risk factors | |||||||||
| Current regular smokers, % | 60.9 | 60.9 | 61.1 | 59.8 | .38 | 61.1 | 60.7 | 61.1 | .54 |
| Nondaily fresh fruit intake, % | 85.1 | 86.1 | 83.4 | 83.4 | <.0001 | 85.6 | 85.2 | 85.0 | .17 |
| Physical activity, mean MET‐h/d | 22.1 | 22.2 | 21.9 | 21.4 | .030 | 21.8 | 22.2 | 22.0 | .68 |
| Mean body mass index, kg/m2 | 23.5 | 23.5 | 23.3 | 23.2 | <.0001 | 23.7 | 23.5 | 23.4 | <.0001 |
| Self‐reported health and medical history, % | |||||||||
| Poor self‐rated health status | 9.0 | 9.0 | 8.9 | 10.0 | .79 | 9.3 | 9.1 | 8.8 | .11 |
| Prior chronic disease b | 23.0 | 23.0 | 23.1 | 24.0 | .40 | 22.9 | 23.4 | 22.7 | .19 |
| Prior cancer b | 0.5 | 0.4 | 0.5 | 0.4 | .32 | 0.5 | 0.5 | 0.4 | .52 |
| Family history of cancer | 16.9 | 16.9 | 16.9 | 16.7 | .63 | 17.0 | 16.7 | 17.1 | .50 |
| Alcohol drinking, % | |||||||||
| Abstainers, % | 20.3 | 9.8 | 31.2 | 71.2 | 15.7 | 20.4 | 20.9 | ||
| Ex‐regular drinkers, % | 8.6 | 10.8 | 5.8 | 1.2 | 9.1 | 8.2 | 8.8 | ||
| Occasional drinkers, % | 37.2 | 33.3 | 46.5 | 26.4 | 32.1 | 37.5 | 38.0 | ||
| Current regular drinkers, % | 34.0 | 46.0 | 16.5 | 1.2 | <.0001 | 43.2 | 33.9 | 32.3 | <.0001 |
| Mean intake in current drinkers, g/wk | 286.4 | 302.7 | 200.3 | 89.9 | <.0001 | 331.9 | 286.5 | 275.1 | <.0001 |
| Mean age at drinking onset in current drinkers, year | 28.6 | 28.0 | 32.1 | 40.3 | <.0001 | 28.4 | 28.7 | 28.6 | .70 |
| Flushing response in current drinkers, % | 18.4 | 11.4 | 55.6 | 61.8 | <.0001 | 14.6 | 18.1 | 19.6 | <.0001 |
| Mean intake overall c , g/wk | 99.4 | 142.7 | 35.1 | 2.4 | <.0001 | 145.6 | 99.3 | 91.0 | <.0001 |
Note: Prevalences and means are adjusted for age (in 10‐year intervals) and study areas as appropriate.
Abbreviation: MET‐h/d, metabolic equivalent of task per hour per day.
Associations between genotype and baseline characteristics were assessed using multinomial logistic regression for drinking status, logistic regression for binary variables and linear regression for continuous variables, adjusted for age and area where appropriate.
Based on participants' self‐reported prior disease history at baseline. Prior chronic disease included self‐reported history of coronary heart disease, stroke, transient ischaemic attack, diabetes, cancer, tuberculosis, rheumatoid arthritis, peptic ulcer, emphysema/chronic bronchitis, gallstone/gallbladder disease and kidney disease.
The overall mean alcohol intake was calculated across all categories of drinking status. Calculations assigned an intake of 0 g/wk to baseline nondrinkers and 5 g/wk to baseline occasional drinkers.
During a median of 11.2 (interquartile range: 10.3‐12.2) years of follow‐up, 9339 participants (4509 men, 4830 women) developed cancer. Among men, those with ALDH2‐rs671 AA genotype had 14% lower risk of any cancer (HR = 0.86 [95% CI: 0.73‐1.00]) and 31% (0.69 [0.53‐0.90]) lower risk of IARC alcohol‐related cancers than those with GG genotype (Figure 1A). The associations were directionally consistent, albeit with wide CIs due to the small number of cases involved, for individual IARC alcohol‐related cancer sites and for stomach cancer, but not for lung cancer. There were no clear differences in cancer risks between ALDH2‐rs671 AG and GG genotypes at the overall level, however, the associations appeared to differ by drinking status (Table S4). Compared to ALDH2‐rs671 GG genotype, AG genotype was associated with significantly higher risks of IARC alcohol‐related cancers (1.30 [1.11‐1.52]) and oesophageal cancer (2.07 [1.58‐2.71]) in male ever‐regular drinkers but not in never‐regular drinkers. A higher risk of lung cancer was observed in those with AG vs GG genotype among male never‐regular drinkers, but not among ever‐regular drinkers (Table S4).
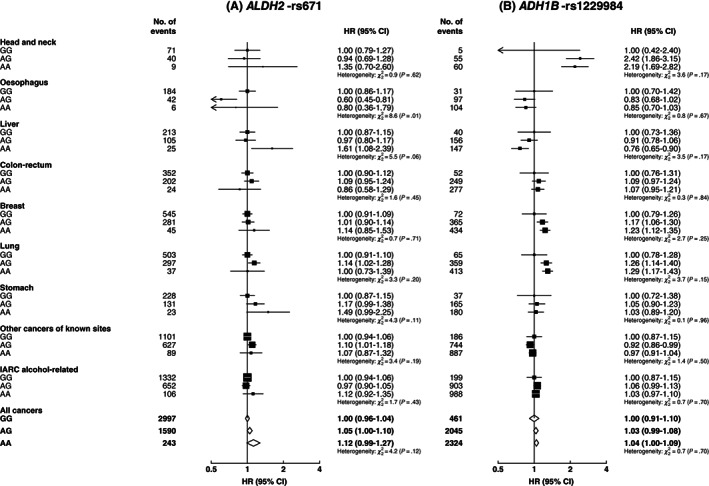
FIGURE 1.
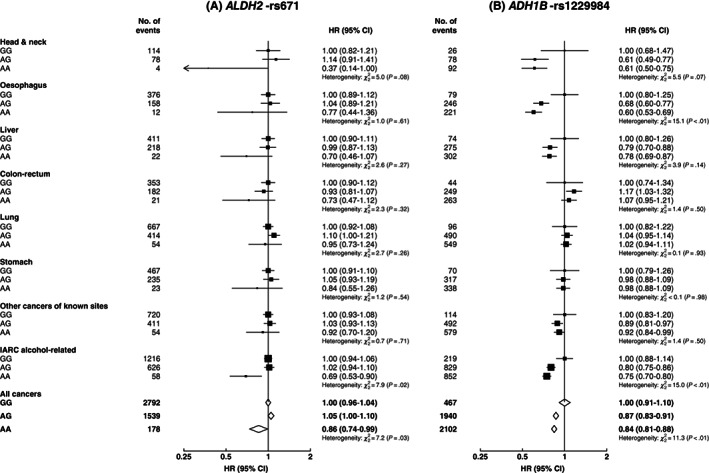
Associations of genotypes for ALDH2‐rs671 (A) and ADH1B‐rs1229984 (B) with risks of total and selected site‐specific cancers, in men. Cox models were stratified by age‐at‐risk and study area, and adjusted for 12 genomic principal components. Each solid square represents HR with the area inversely proportional to the variance of the group‐specific log hazard. The horizontal lines indicate group‐specific 95% CIs. Open diamonds represent the overall HRs for all cancers. IARC alcohol‐related cancers included cancers of the head and neck, oesophagus, liver and colon‐rectum. CI, confidence interval; HR, hazard ratio; IARC, International Agency for Research on Cancer
For ADH1B‐rs1229984, compared to men with GG genotype, men with AG or AA genotypes had 13% to 25% lower risks of overall cancer (0.87 [0.78‐0.96], AG vs GG; 0.84 [0.76‐0.93], AA vs GG) and IARC alcohol‐related cancers (0.80 [0.69‐0.93]; 0.75 [0.64‐0.87]), mainly driven by head and neck cancer (0.61 [0.39‐0.96]; 0.61 [0.39‐0.94]) and oesophageal cancer (0.68 [0.53‐0.88]; 0.60 [0.46‐0.78]; Figure 1B). Men with AG or AA genotypes also tended to have lower, although nonsignificant, risks of liver cancer, but not of other cancers. The associations of ADH1B‐rs1229984 with overall cancer, IARC alcohol‐related cancers and oesophageal cancer were directionally consistent across different drinking groups, and were more apparent among ever‐regular drinkers (Table S5).
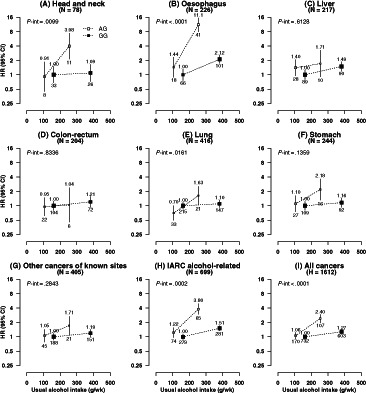
Among male current regular drinkers, there was evidence of interactions between alcohol consumption and ALDH2‐rs671, especially the AG genotype, on risks of overall and IARC alcohol‐related cancers. For total cancer, the adjusted HRs were 1.00 (0.92‐1.08) for GG and 1.06 (0.91‐1.23) for AG drinkers consuming <280 g/wk alcohol and were 1.27 (1.17‐1.39) for GG and 2.40 (1.98‐2.92) for AG drinkers consuming at least 280 g/wk (P interaction < .0001); for IARC alcohol‐related cancers, the corresponding HRs were more extreme (P interaction = .0002). Similar significant interactions between alcohol and ALDH2‐rs671 were also observed for site‐specific cancers, especially oesophageal cancer (P interaction < .0001) and head and neck cancer (P interaction < .01) and less so for lung cancer (P interaction = .016) (Figure 2). The associations between these cancers and joint alcohol‐ALDH2‐rs671 groups were broadly similar in never‐regular smokers and in ever‐regular smokers (Figure 3). There were no clear interactions between alcohol consumption and ALDH2‐rs671 for liver or colorectal cancers, but the dose‐response association between alcohol and stomach cancer appeared stronger in ALDH2‐rs671 AG drinkers than in GG drinkers (HR = 3.36 [1.73‐6.54] vs 1.02 [0.73‐1.41], per 280 g/wk; P heterogeneity = .002; Figure S2). For ADH1B‐rs1229984, no clear interactions with alcohol consumption on cancer risks were observed among male current regular drinkers (Figures S3 and S4).
FIGURE 2.
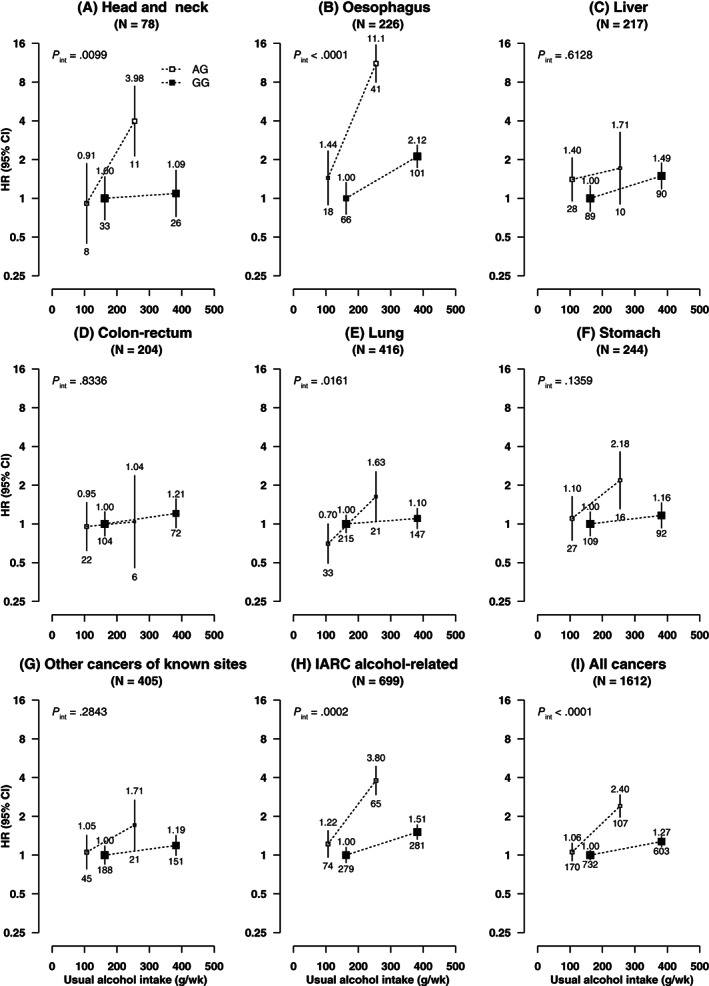
(A‐I) Associations of ALDH2‐rs671 genotypes with risks of total and selected site‐specific cancers at different usual intake levels of alcohol, in male current regular drinkers. Cox models were stratified by age‐at‐risk and study area and adjusted for 12 genomic principal components, education, household income, smoking status, physical activity, fresh fruit intake, body mass index and family history of cancer. Each box represents HR with the area inversely proportional to the variance of the group‐specific log hazard. The vertical lines indicate group‐specific 95% CIs. The numbers above the error bars are point estimates for HRs, and the numbers below are number of events. Solid boxes denote ALDH2‐rs671 GG genotype and open boxes denote ALDH2‐rs671 AG genotype. Alcohol intake, separately in ALDH2‐rs671 AG and GG drinkers, was classified based on baseline consumption of <280 and ≥280 g/wk. AA individuals were excluded as few of them drank (n = 28). IARC alcohol‐related cancers included cancers of the head and neck, oesophagus, liver and colon‐rectum. CI, confidence interval; HR, hazard ratio; IARC, International Agency for Research on Cancer
FIGURE 3.
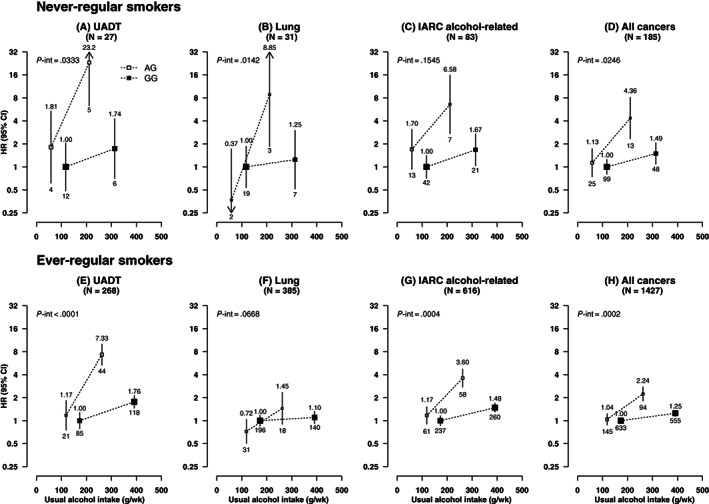
(A‐H) Associations of ALDH2‐rs671 genotypes with risks of selected cancers at different usual intake levels of alcohol, in male never and ever‐regular smokers. UADT cancers included cancers of the head and neck and oesophagus. IARC alcohol‐related cancers included cancers of the head and neck, oesophagus, liver and colon‐rectum. CI, confidence interval; HR, hazard ratio; IARC, International Agency for Research on Cancer; UADT, upper aerodigestive tract. Conventions are as in Figure 2
Examination of the joint effects of the two genetic variants in men showed that the risks of IARC alcohol‐related cancers and of UADT cancers were highest for the combination of ALDH2‐rs671 AG with ADH1B‐rs1229984 GG genotypes, followed by the combination of GG/GG genotypes, and were lowest for the combinations of ALDH2‐rs671 AA with ADH1B‐rs1229984 AG or AA genotypes (Table S6). However, these modest gene‐gene interactions were not significant.
In men the genotypic associations with cancers were unaltered with additional adjustment for other cancer risk factors (education, household income, smoking, fresh fruit intake, physical activity, BMI, family cancer history, hepatitis B virus [HBV] infection status; Figure S5) or in area‐stratified as opposed to area‐adjusted analyses (Figure S6). Similar ALDH2‐rs671‐alcohol interactions were observed as in the main analyses after excluding individuals with prior cancer or further adjusting for HBV infection status (Figures S7 and S8).
Among women, there were no clear associations of these two genetic variants with risks of overall or IARC alcohol‐related cancers (Figure 4). For site‐specific cancers, compared to ALDH2‐rs671 GG genotype, AG genotype was associated with a lower oesophageal cancer risk, while AA genotype was associated with a higher liver cancer risk, but the numbers of cases involved were small. When comparing the associations of these genotypes with cancer risks between men and women, heterogeneity of the associations was seen for several IARC alcohol‐related cancers (Table S7). Among women, no clear gene‐alcohol interactions were observed (Tables S8‐S10).
FIGURE 4.
Associations of genotypes for ALDH2‐rs671 (A) and ADH1B‐rs1229984 (B) with risks of total and site‐specific cancers, in women. IARC alcohol‐related cancers included cancers of the head and neck, oesophagus, liver, colon‐rectum and breast. CI, confidence interval; HR, hazard ratio; IARC, International Agency for Research on Cancer. Conventions are as in Figure 1
4. DISCUSSION
In this large genetic study of Chinese adults, two common genetic variants, ALDH2‐rs671 G>A and ADH1B‐rs1229984 G>A, which strongly reduced alcohol consumption, were associated with lower risks of overall and IARC alcohol‐related cancers, especially UADT cancers, in men among whom over a third drank alcohol regularly. Among male drinkers, ALDH2‐rs671 genotype significantly modified the effects of alcohol consumption on certain cancers, with greater excess risks in men with the AG than GG genotype for a given level of alcohol consumption, especially for UADT cancers and potentially for lung cancer, regardless of smoking status. Among women, very few drank alcohol regularly and these variants were not associated with overall or IARC alcohol‐related cancer risk.
Previous case‐control studies and meta‐analyses in East Asian populations have reported associations of ALDH2‐rs671 genotypes with risks of oesophageal cancer 10 , 12 , 32 and head and neck cancer, 33 and that the relationships may be modified by alcohol consumption. Compared to GG genotype, AA genotype was associated with an overall lower risk, while AG genotype was associated with a higher risk among drinkers but not among never drinkers. 10 , 12 , 33 The increased risks among ALDH2‐rs671 AG drinkers, who have markedly elevated acetaldehyde levels after consuming alcohol, but not among never drinkers suggest that acetaldehyde may be the underlying mechanism through which alcohol consumption increases UADT cancer risk. In the present prospective study of Chinese population, we observed broadly similar effect modifications of ALDH2‐rs671 genotypes on risks for IARC alcohol‐related and oesophageal cancers associated with alcohol consumption, as in previous studies. 10 , 12 , 14 , 33 For other cancers sites, existing genotypic evidence has been inconclusive. 15 , 16 , 19 , 22 , 34 , 35 , 36 , 37 , 38 A previous meta‐analysis of case‐control studies found no clear associations of ALDH2‐rs671 with cancers in the liver (1522 cases, 7 studies), colon‐rectum (2356 cases, 10 studies), stomach (5558 cases, 8 studies) or lung (1105 cases, 2 studies). 15 In contrast, recent large case‐control studies (>1000 cases each) in South Korea and Japan showed that ALDH2‐rs671 AA and AG genotypes were associated with 22% to 30% lower risk of colorectal cancer, 16 but approximately 23% to 30% higher risk of stomach cancer, 19 , 38 compared to GG genotype. In CKB, there were generally lower risks of liver, colorectal and stomach cancers comparing ALDH2‐rs671 AA vs GG genotype among men, but precision was low due to the small numbers of AA carriers, and no clear genotypic associations were observed for lung cancer.
For ADH1B‐rs1229984, a meta‐analysis of case‐control studies involving mainly East Asian populations showed that both AG and AA genotypes were associated with reduced risks of oesophageal cancer (9117 cases, 23 studies) and head and neck cancer (6646 cases, 16 studies) compared to GG genotype. 11 However, most previous studies found no significant associations of ADH1B genotype with other cancer sites. 11 , 16 , 19 , 35 , 39 Similarly, in our study we found clear inverse associations of the ADH1B‐rs1229984 A‐allele with risks of overall and IARC alcohol‐related cancers, mainly driven by UADT cancers and potentially also by liver cancer, but there were no clear associations with other cancer sites.
Using genetic variants as proxy for alcohol intake, the inverse associations between genotypes that predict lower alcohol consumption (ALDH2‐rs671 AA, ADH1B‐rs1229984 AA and AG) and IARC alcohol‐related cancers among men in CKB, especially UADT cancers, supported the causal effects of alcohol consumption on these cancers, as consistent with existing observational evidence. 2 , 5 , 40 Observational studies have also linked heavy drinking to excess risks of liver and colorectal cancers, and less consistently of stomach and lung cancers. 2 , 40 Taking together our findings for both genetic variants, the nonsignificant lower risks of liver cancer consistently observed for A‐alleles of both variants might suggest causal effects of alcohol consumption, but there was no clear evidence supporting causal relationships of alcohol consumption with colorectal, stomach or lung cancers. Our findings were broadly consistent with a Mendelian randomisation (MR) study in European ancestry populations using data from ~90 SNPs (without ALDH2‐rs671) associated with alcohol consumption. 41 That MR study showed nonsignificant positive associations of genetically predicted alcohol consumption with oesophageal cancer and head and neck cancer risk but otherwise found no evidence supporting a causal role of alcohol consumption on other site‐specific cancers, which may be due in part to the relatively weak genetic instruments used (variance in alcohol consumption explained ~0.3%). 41 In our study, the effects of ALDH2‐rs671 genotype on UADT cancers in different drinking groups were influenced by a potential gene‐environment interaction between alcohol intake and genotype. This demonstrated the limitations of applying ALDH2‐rs671 directly in MR studies of alcohol and cancer without careful consideration of possible gene‐alcohol interactions. Nevertheless, the strong effect of ALDH2‐rs671 on alcohol consumption indicates that ALDH2‐rs671 is a much stronger genetic instrument for alcohol intake in East Asians than those available for MR studies in European ancestry populations. Importantly, appropriate analysis and interpretation of ALDH2‐rs671‐alcohol interactions offers the opportunity to assess the causality of alcohol and to investigate the role of alcohol‐derived acetaldehyde in the aetiology of certain cancers.
Previous studies have reported interactions between ALDH2‐rs671 and alcohol consumption on upper aerodigestive cancer risk in East Asian populations. 12 , 13 , 14 , 20 , 23 , 33 A meta‐analysis of 31 case‐control studies involving 8510 cases showed that the ORs of oesophageal cancer comparing ALDH2‐rs671 AG vs GG genotype increased from 1.21 (0.95‐1.73) in non‐/rare drinkers to 3.79 (3.05‐4.72) in light drinkers and 6.50 (5.34‐7.92) in heavy drinkers. 12 Similar findings have also been reported for head and neck cancer in a meta‐analysis of six Japanese case‐control studies (945 cases). 33 In contrast, most existing studies found no evidence of interaction between ALDH2‐rs671 and alcohol consumption for colorectal cancer, 16 , 18 , 23 , 35 , 42 whereas findings for liver cancer, 22 , 43 , 44 stomach cancer, 19 , 23 , 38 , 45 , 46 , 47 and lung cancer 36 , 48 have been inconclusive. In the present prospective study, in addition to the significant ALDH2‐rs671‐alcohol interactions on head and neck cancer and oesophageal cancer which supported findings from previous studies, 12 , 13 , 14 , 20 , 33 there was also suggestive evidence of an ALDH2‐rs671‐alcohol interaction on lung cancer risk, which was concordant with a previous Japanese case‐control study (505 cases) 36 and our previous report of a stronger dose‐response association of alcohol with lung cancer among male drinkers reporting the alcohol flushing response. 5 While we found no clear evidence of interactions between ALDH2‐rs671 and alcohol consumption for colorectal or liver cancers, the somewhat stronger dose‐response association of alcohol intake with stomach cancer among male ALDH2‐rs671 AG drinkers was consistent with case‐control studies in Japan (1375 cases). 19 In contrast to the ALDH2‐rs671‐alcohol interactions observed, we found no clear interactions between alcohol consumption and ADH1B‐rs1229984, or between the two genetic variants, on cancer risks, which were largely consistent with previous studies. 16 , 17 , 18 , 19 , 20 , 21 , 44 , 49 , 50 , 51
The biological pathways via which alcohol consumption may cause cancers are not fully understood and likely vary by cancer site. A major pathway proposed is via local exposure to alcohol‐formed acetaldehyde, especially in the upper gastrointestinal tract where, in contrast to the liver, the mucosa has limited capacity to eliminate acetaldehyde. 2 , 52 After drinking, local acetaldehyde exposure in the upper digestive tract mucosa starts instantly, mainly due to microbial acetaldehyde formation from alcohol in saliva, followed by long‐term acetaldehyde formation from alcohol that is diffused back to saliva from blood circulation. 53 Particularly in ALDH2‐deficient individuals, excess salivary acetaldehyde may be produced through human ethanol metabolism in the salivary glands, resulting in excess long‐term acetaldehyde exposure in the upper digestive tract mucosa. 52 , 53 This is supported by our findings of increased UADT cancer risks only among ALDH2‐rs671 AG drinkers but not among ALDH2‐rs671 AG never‐regular drinkers, and the greater excess risks in ALDH2‐rs671 AG drinkers than GG drinkers for a given amount of alcohol consumed. For a given level of alcohol consumption, ALDH2‐deficient individuals were reported to be exposed to 2‐fold to 3‐fold (salivary) and 5‐fold to 6‐fold (gastric juice) higher acetaldehyde concentrations than those with active ALDH2 enzyme, 52 supporting the putative involvement of local alcohol‐derived acetaldehyde in upper gastrointestinal tract carcinogenesis. In addition to increased alcohol consumption, the ADH1B‐rs1229984 GG genotype is associated with slower ethanol oxidation such that ethanol remains in the blood and saliva for longer, which may result in prolonged exposure to salivary acetaldehyde due to oral microbial acetaldehyde production from ethanol and consequently increased risks of UADT cancers. 54 , 55 This prolonged salivary acetaldehyde exposure would be greater in the presence of ALDH2‐rs671 AG genotype. 53 Alcohol may also increase risks of cancers of the upper digestive and respiratory tract by acting as a solvent for tobacco carcinogens. 40 Moreover, smoking and heavy drinking combined may also modify the oral microflora to produce higher acetaldehyde levels in saliva. 53 It is possible that the observed ALDH2‐rs671‐alcohol interactions might be partly related to acetaldehyde from smoking rather than from alcohol consumption alone, especially for lung cancer for which no causality of alcohol has been inferred by genotypic associations. This is however unlikely, as similar ALDH2‐rs671‐alcohol interactions were observed in never‐regular smokers, and there were no clear additional excess risks among smokers when we examined the joint effects of alcohol consumption, ALDH2‐rs671 and smoking on cancer risks, although the number of cases was small (Table S11). It is plausible that in addition to potential interactions between ALDH2‐rs671 genotype and alcohol in lung cancer risk, these genotypes may interact with other exogenous sources of aldehyde (eg, air pollution) which could influence risk of lung cancer. Furthermore, experimental studies in mice have shown that ALDH2 expression was detected at various levels in multiple organs other than liver, including lung, 56 , 57 and that liver ALDH2 was responsible for clearing only half of circulating acetaldehyde after alcohol intake, 56 suggesting that multiple ALDH2‐expressing organs contribute to systemic acetaldehyde clearance. Future studies are warranted to elucidate the potential roles of alcohol and ALDH2 deficiency in the carcinogenesis of lung and other sites. On the other hand, the potential lack of ALDH2‐rs671‐alcohol interaction for liver cancer and colorectal cancer might suggest that other alcohol‐induced pathways are more important for carcinogenesis in these sites, for example, alcohol‐induced oxidative stress, changes in folate metabolism and intestinal inflammation. 2 , 58
The chief strengths of our study include the prospective study design, large community‐based study population, reliable alcohol consumption data 5 , 7 and extensive information on lifestyle risk factors, and reasonably large numbers of incident events for various common cancer sites traced via comprehensive and complete follow‐up. We were also able to minimise population stratification bias with adjustments for study area and genomic principal components. Nevertheless, several limitations also warrant consideration. Although the two genetic variants were strong instruments for alcohol intake and were not associated with smoking, they were weakly associated with other cancer risk factors (eg, fresh fruit intake, physical activity, BMI) in CKB. However, the differences were extremely small in magnitude and might have been the consequences of alcohol consumption. Importantly, additional adjustments for these risk factors did not alter the main findings. Also, the two enzymes affected by the studied genetic variants are involved in many biochemical pathways, 59 , 60 which might potentially affect carcinogenesis independent of alcohol consumption. Nonetheless, among women who rarely drank alcohol despite their genotype, there were no clear genotypic associations with IARC alcohol‐related cancers or most site‐specific cancers. Although ALDH2‐rs671 was associated with oesophageal cancer and liver cancer among women, which might be partly related to acetaldehyde exposure from other sources (eg, air pollution, cooking oil fumes, passive smoking) or endogenous aldehyde exposure, 2 , 59 the associations were not directionally consistent to those observed in men. These findings suggest the genotypic results in men were likely to be driven chiefly by alcohol consumption rather than by pleiotropic pathways. While further adjustment for HBV infection status did not materially alter our findings, other major risk factors, for example, Helicobacter pylori infection for stomach cancer and hepatitis C infection for liver cancer were not available to be included in our analysis. Although these infectious agents are unlikely to be confounding factors in the associations between the studied genetic variants and cancer risks, whether they may interact with alcohol consumption and ALDH2 deficiency remains to be elucidated. Finally, our study may be underpowered to detect any weak causal effects of alcohol intake on site‐specific cancers other than UADT cancers.
In conclusion, in Chinese men ALDH2‐rs671 G>A and ADH1B‐rs1229984 G>A genotypes were associated with lower risks of overall and IARC alcohol‐related cancers, mainly UADT cancers. Furthermore, ALDH2‐rs671 genotype may modify the effects of alcohol consumption on certain cancers, especially UADT cancers. These findings support the causal role of alcohol consumption in the aetiology of UADT cancers, which is exacerbated in individuals with inherited low alcohol tolerability. The study reinforces the need to lower population‐levels of alcohol consumption for cancer prevention, especially in China where alcohol consumption is increasing despite the low alcohol tolerability among a subset of the population.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Pek Kei Im analysed the data and drafted the manuscript. Pek Kei Im, Iona Y Millwood, Ling Yang and Zhengming Chen contributed to the conception of this article, interpretation of the results and the revision of article. Liming Li and Zhengming Chen designed the study. Liming Li, Zhengming Chen, Iona Y Millwood, Ling Yang, Christiana Kartsonaki, Yiping Chen, Yu Guo, Huaidong Du, Kuang Lin, Rene Kerosi, Alex Hacker, Jingchao Liu, Canqing Yu, Jun Lv and Robin G Walters, contributed to data acquisition. All authors critically reviewed the article and approved the final submission.
ETHICS STATEMENT
Ethical approval was obtained from the Ethical Review Committee of the Chinese Centre for Disease Control and Prevention (Beijing, China, 005/2004) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK, 025‐04) and all participants provided written informed consent.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
The chief acknowledgment is to the participants, the project staff and the China National Centre for Disease Control and Prevention (CDC) and its regional offices for assisting with the fieldwork. We thank Judith Mackay in Hong Kong; Yu Wang, Gonghuan Yang, Zhengfu Qiang, Lin Feng, Maigeng Zhou, Wenhua Zhao, Yan Zhang and Zheng Bian in China CDC; Lingzhi Kong, Xiucheng Yu and Kun Li in the Chinese Ministry of Health; and Garry Lancaster, Sarah Clark, Martin Radley, Mike Hill, Hongchao Pan and Jill Boreham in the CTSU, Oxford, for assisting with the design, planning, organisation and conduct of the study. Members of the China Kadoorie Collaborative Group are listed in the Supporting Information Material.
Im PK, Yang L, Kartsonaki C, et al. Alcohol metabolism genes and risks of site‐specific cancers in Chinese adults: An 11‐year prospective study. Int. J. Cancer. 2022;150(10):1627‐1639. doi: 10.1002/ijc.33917
Zhengming Chen and Iona Y. Millwood are joint senior authors.
China Kadoorie Biobank (CKB) Collaborative Group includes members listed in the Supporting Information Material.
Funding information British Heart Foundation, Grant/Award Number: CH/1996001/9454; Cancer Research UK, Grant/Award Numbers: C16077/A29186, C500/A16896; GlaxoSmithKline; Kadoorie Charitable Foundation; National Key Research and Development Program of China, Grant/Award Numbers: 2016YFC0900500, 2016YFC0900501, 2016YFC0900504, 2016YFC1303904; National Natural Science Foundation of China, Grant/Award Number: 91843302; Nuffield Department of Population Health, University of Oxford, Early Career Research Fellowship; UK Medical Research Council, Grant/Award Numbers: MC‐PC‐13049, MC‐PC‐14135, MC_U137686851, MC_UU_00017/1, MC_UU_12026/2; Wellcome Trust, Grant/Award Numbers: 088158/Z/09/Z, 104085/Z/14/Z, 202922/Z/16/Z, 212946/Z/18/Z
Contributor Information
Ling Yang, Email: ling.yang@ndph.ox.ac.uk.
Iona Y. Millwood, Email: iona.millwood@ndph.ox.ac.uk.
DATA AVAILABILITY STATEMENT
The China Kadoorie Biobank (CKB) is a global resource for the investigation of lifestyle, environmental, blood biochemical and genetic factors as determinants of common diseases. The CKB study group is committed to making the cohort data available to the scientific community in China, the UK and worldwide to advance knowledge about the causes, prevention and treatment of disease. For detailed information on what data is currently available to open access users and how to apply for it, visit: http://www.ckbiobank.org/site/Data+Access.
Researchers who are interested in obtaining the raw data from the China Kadoorie Biobank study that underlines this paper should contact ckbaccess@ndph.ox.ac.uk. A research proposal will be requested to ensure that any analysis is performed by bona fide researchers and—where data is not currently available to open access researchers—is restricted to the topic covered in this article.
Further information is available from the corresponding authors upon request.
REFERENCES
- 1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I & Bray F Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today/home. Accessed April 14, 2021.
- 2. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1‐538. [PMC free article] [PubMed] [Google Scholar]
- 3. Shield K, Manthey J, Rylett M, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5(1):e51‐e61. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Global Status Report on Alcohol and Health 2018. Geneva: WHO; 2018. [Google Scholar]
- 5. Im PK, Millwood IY, Kartsonaki C, et al. On behalf of the China Kadoorie biobank collaborative group. Alcohol drinking and risks of total and site‐specific cancers in China: a 10‐year prospective study of 0.5 million adults. Int J Cancer. 2021;149(3):522‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W, Xia C, Zheng R, et al. Disparities by province, age, and sex in site‐specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health. 2019;7(2):e257‐e269. [DOI] [PubMed] [Google Scholar]
- 7. Millwood IY, Walters RG, Mei XW, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. 2019;393:1831‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Mukherjee N, Soundararajan U, et al. Geographically separate increases in the frequency of the derived ADH1B*47His allele in eastern and western Asia. Am J Hum Genet. 2007;81(4):842‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis SJ, Smith GD. Alcohol, ALDH2, and esophageal cancer: a meta‐analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1967‐1971. [DOI] [PubMed] [Google Scholar]
- 11. Tan B, Ning N. Association of ADH1B Arg47His polymorphism with the risk of cancer: a meta‐analysis. Biosci Rep. 2019;39(4):BSR20181915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao T, Wang C, Shen L, et al. Clinical significance of ALDH2 rs671 polymorphism in esophageal cancer: evidence from 31 case‐control studies. Onco Targets Ther. 2015;8:649‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu C, Guo Y, Bian Z, et al. Association of low‐activity ALDH2 and alcohol consumption with risk of esophageal cancer in Chinese adults: a population‐based cohort study. Int J Cancer. 2018;143(7):1652‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuo K, Hamajima N, Shinoda M, et al. Gene‐environment interaction between an aldehyde dehydrogenase‐2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis. 2001;22(6):913‐916. [DOI] [PubMed] [Google Scholar]
- 15. Zuo W, Zhan Z, Ma L, Bai W, Zeng S. Effect of ALDH2 polymorphism on cancer risk in Asians: a meta‐analysis. Medicine (Baltimore). 2019;98(13):e14855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi CK, Shin MH, Cho SH, et al. Association between ALDH2 and ADH1B polymorphisms and the risk for colorectal cancer in Koreans. Cancer Res Treat. 2021;53(3):754‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin G, Hamajima N, Morita M, Tajima O, Tabata S, Kono S. Lack of influence of the ADH1B Arg47His genetic polymorphism on risk of colorectal adenoma in middle‐aged Japanese men. Asian Pac J Cancer Prev. 2011;12(1):297‐302. [PubMed] [Google Scholar]
- 18. Yin G, Kono S, Toyomura K, et al. Alcohol dehydrogenase and aldehyde dehydrogenase polymorphisms and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98(8):1248‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishioka K, Masaoka H, Ito H, et al. Association between ALDH2 and ADH1B polymorphisms, alcohol drinking and gastric cancer: a replication and mediation analysis. Gastric Cancer. 2018;21(6):936‐945. [DOI] [PubMed] [Google Scholar]
- 20. Wu M, Chang SC, Kampman E, et al. Single nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and esophageal cancer: a population‐based case‐control study in China. Int J Cancer. 2013;132(8):1868‐1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka F, Yamamoto K, Suzuki S, et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut. 2010;59(11):1457‐1464. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Yang HI, Lee MH, et al. Alcohol drinking mediates the association between polymorphisms of ADH1B and ALDH2 and hepatitis B‐related hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25(4):693‐699. [DOI] [PubMed] [Google Scholar]
- 23. Koyanagi YN, Suzuki E, Imoto I, et al. Across‐site differences in the mechanism of alcohol‐induced digestive tract carcinogenesis: an evaluation by mediation analysis. Cancer Res. 2020;80(7):1601‐1610. [DOI] [PubMed] [Google Scholar]
- 24. Chen Z, Chen J, Collins R, et al. China Kadoorie biobank of 0.5 million people: survey methods, baseline characteristics and long‐term follow‐up. Int J Epidemiol. 2011;40(6):1652‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Millwood IY, Li L, Smith M, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio‐demographic and health‐related correlates. Int J Epidemiol. 2013;42(3):816‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Im PK, Millwood IY, Guo Y, et al. Patterns and trends of alcohol consumption in rural and urban areas of China: findings from the China Kadoorie Biobank. BMC Public Health. 2019;19(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Im PK, Millwood IY, Chen Y, et al. Problem drinking, wellbeing and mortality risk in Chinese men: findings from the China Kadoorie Biobank. Addiction. 2020;115(5):850‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan R, Zhu M, Yu C, et al. Cancer incidence and mortality: a cohort study in China, 2008‐2013. Int J Cancer. 2017;141(7):1315‐1323. [DOI] [PubMed] [Google Scholar]
- 29. Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case‐control analysis avoiding an arbitrary reference group. Stat Med. 1991;10(7):1025‐1035. [DOI] [PubMed] [Google Scholar]
- 30. Im PK, Millwood IY, Kartsonaki C, et al. Alcohol drinking and risks of liver cancer and non‐neoplastic chronic liver diseases in China: a 10‐year prospective study of 0.5 million adults. BMC Med. 2021;19(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol. 1999;150(4):341‐353. [DOI] [PubMed] [Google Scholar]
- 32. Yokoyama A, Muramatsu T, Ohmori T, Higuchi S, Hayashida M, Ishii H. Esophageal cancer and aldehyde dehydrogenase‐2 genotypes in Japanese males. Cancer Epidemiol Biomarkers Prev. 1996;5(2):99‐102. [PubMed] [Google Scholar]
- 33. Boccia S, Hashibe M, Gallì P, et al. Aldehyde dehydrogenase 2 and head and neck cancer: a meta‐analysis implementing a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2009;18(1):248‐254. [DOI] [PubMed] [Google Scholar]
- 34. Chen J, Pan W, Chen Y, Wen L, Tu J, Liu K. Relationship of ALDH2 rs671 and CYP2E1 rs2031920 with hepatocellular carcinoma susceptibility in east Asians: a meta‐analysis. World J Surg Oncol. 2020;18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seol JE, Kim J, Lee BH, et al. Folate, alcohol, ADH1B and ALDH2 and colorectal cancer risk. Public Health Nutr. 2021;24(4):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minegishi Y, Tsukino H, Muto M, et al. Susceptibility to lung cancer and genetic polymorphisms in the alcohol metabolite‐related enzymes alcohol dehydrogenase 3, aldehyde dehydrogenase 2, and cytochrome P450 2E1 in the Japanese population. Cancer. 2007;110(2):353‐362. [DOI] [PubMed] [Google Scholar]
- 37. Joo Kang S, Shin CM, Sung J, Kim N. Association between ALDH2 polymorphism and gastric cancer risk in terms of alcohol consumption: a meta‐analysis. Alcohol Clin Exp Res. 2021;45(1):6‐14. [DOI] [PubMed] [Google Scholar]
- 38. Yang S, Lee J, Choi IJ, et al. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget. 2017;8(4):6630‐6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Govind P, Pavethynath S, Sawabe M, Arai T, Muramatsu M. Association between rs1229984 in ADH1B and cancer prevalence in a Japanese population. Mol Clin Oncol. 2020;12(6):503‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Cancer Research Fund/American Institute for Cancer Research . Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018, 2018. [Google Scholar]
- 41. Larsson SC, Carter P, Kar S, et al. Smoking, alcohol consumption, and cancer: a mendelian randomisation study in UKbiobank and international genetic consortia participants. PLoS Med. 2020;17(7):e1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirose M, Kono S, Tabata S, et al. Genetic polymorphisms of methylenetetrahydrofolate reductase and aldehyde dehydrogenase 2, alcohol use and risk of colorectal adenomas: self‐defense forces health study. Cancer Sci. 2005;96(8):513‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding J, Li S, Wu J, et al. Alcohol dehydrogenase‐2 and aldehyde dehydrogenase‐2 genotypes, alcohol drinking and the risk of primary hepatocellular carcinoma in a Chinese population. Asian Pac J Cancer Prev. 2008;9(1):31‐35. [PubMed] [Google Scholar]
- 44. Sakamoto T, Hara M, Higaki Y, et al. Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int J Cancer. 2006;118(6):1501‐1507. [DOI] [PubMed] [Google Scholar]
- 45. Hidaka A, Sasazuki S, Matsuo K, et al. Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center‐based prospective study. Carcinogenesis. 2015;36(2):223‐231. [DOI] [PubMed] [Google Scholar]
- 46. Shin CM, Kim N, Cho SI, Kim JS, Jung HC, Song IS. Association between alcohol intake and risk for gastric cancer with regard to ALDH2 genotype in the Korean population. Int J Epidemiol. 2011;40(4):1047‐1055. [DOI] [PubMed] [Google Scholar]
- 47. Cao HX, Li SP, Wu JZ, et al. Alcohol dehydrogenase‐2 and aldehyde dehydrogenase‐2 genotypes, alcohol drinking and the risk for stomach cancer in Chinese males. Asian Pac J Cancer Prev. 2010;11(4):1073‐1077. [PubMed] [Google Scholar]
- 48. Park JY, Matsuo K, Suzuki T, et al. Impact of smoking on lung cancer risk is stronger in those with the homozygous aldehyde dehydrogenase 2 null allele in a Japanese population. Carcinogenesis. 2010;31(4):660‐665. [DOI] [PubMed] [Google Scholar]
- 49. Crous‐Bou M, Rennert G, Cuadras D, et al. Polymorphisms in alcohol metabolism genes ADH1B and ALDH2, alcohol consumption and colorectal cancer. PLoS One. 2013;8(11):e80158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferrari P, McKay JD, Jenab M, et al. Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphisms, alcohol intake and the risk of colorectal cancer in the European prospective investigation into cancer and nutrition study. Eur J Clin Nutr. 2012;66(12):1303‐1308. [DOI] [PubMed] [Google Scholar]
- 51. Huang C‐C, Hsiao J‐R, Lee W‐T, et al. Investigating the association between alcohol and risk of head and neck cancer in Taiwan. Sci Rep. 2017;7(1):9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nieminen MT, Salaspuro M. Local acetaldehyde‐an essential role in alcohol‐related upper gastrointestinal tract carcinogenesis. Cancer. 2018;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salaspuro M. Local acetaldehyde: its key role in alcohol‐related oropharyngeal cancer. Visc Med. 2020;36(3):167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsai ST, Wong TY, Ou CY, et al. The interplay between alcohol consumption, oral hygiene, ALDH2 and ADH1B in the risk of head and neck cancer. Int J Cancer. 2014;135(10):2424‐2436. [DOI] [PubMed] [Google Scholar]
- 55. Yokoyama A, Mizukami T, Yokoyama T. Genetic polymorphisms of alcohol dehydrogense‐1B and aldehyde dehydrogenase‐2, alcohol flushing, mean corpuscular volume, and aerodigestive tract neoplasia in Japanese drinkers. Adv Exp Med Biol. 2015;815:265‐279. [DOI] [PubMed] [Google Scholar]
- 56. Guillot A, Ren T, Jourdan T, et al. Targeting liver aldehyde dehydrogenase‐2 prevents heavy but not moderate alcohol drinking. Proc Natl Acad Sci. 2019;116(51):25974‐25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stagos D, Chen Y, Brocker C, et al. Aldehyde dehydrogenase 1B1: molecular cloning and characterization of a novel mitochondrial acetaldehyde‐metabolizing enzyme. Drug Metab Dispos. 2010;38(10):1679‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut‐derived inflammation. Alcohol Res: Curr Rev. 2017;38(2):163‐171. [PMC free article] [PubMed] [Google Scholar]
- 59. Chen C‐H, Ferreira JCB, Gross ER, Mochly‐Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94(1):1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Polimanti R, Gelernter J. ADH1B: from alcoholism, natural selection, and cancer to the human phenome. Am J Med Genet B Neuropsychiatr Genet. 2018;177(2):113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The China Kadoorie Biobank (CKB) is a global resource for the investigation of lifestyle, environmental, blood biochemical and genetic factors as determinants of common diseases. The CKB study group is committed to making the cohort data available to the scientific community in China, the UK and worldwide to advance knowledge about the causes, prevention and treatment of disease. For detailed information on what data is currently available to open access users and how to apply for it, visit: http://www.ckbiobank.org/site/Data+Access.
Researchers who are interested in obtaining the raw data from the China Kadoorie Biobank study that underlines this paper should contact ckbaccess@ndph.ox.ac.uk. A research proposal will be requested to ensure that any analysis is performed by bona fide researchers and—where data is not currently available to open access researchers—is restricted to the topic covered in this article.
Further information is available from the corresponding authors upon request.


