Abstract
Background
Escherichia coli bloodstream infections (EC-BSIs), particularly antibiotic-resistant EC-BSIs, are increasing in the UK and internationally. The evidence base to guide interventions against this major public health concern is limited. We aimed to investigate possible drivers of changes in EC-BSI incidence and antibiotic susceptibilities in Oxfordshire over the last two decades, while stratifying for hospital-exposure.
Methods
EC-BSIs and E. coli urinary tract infections (EC-UTIs) incidence in one UK region (Oxfordshire) were estimated from anonymised linked microbiological and hospital electronic health records, and modelled using negative binomial regression based on microbiological, clinical and healthcare exposure risk factors. Infection severity, 30-day all-cause mortality, and community and hospital co-amoxiclav use were also investigated.
Findings
From 1998-2016, 5706 EC-BSIs occurred in 5215 patients, and 228376 EC-UTIs in 137075 patients. 1365(24%) EC-BSIs were nosocomial (onset >48h post-admission), 1863(33%) were community (>365 days post-discharge), 1346(24%) quasi-community (31-365 days post-discharge), and 1132(20%) quasi-nosocomial (≤30 days post-discharge). 1413(20%) EC-BSIs and 36270(13%) EC-UTIs were co-amoxiclav-resistant (41% and 30%, respectively, in 2016). Increases in EC-BSIs were driven by increases in community (10%/year (95% CI:7%-13%)) and quasi-community (8%/year (95% CI:7%-10%)) cases. Changes over time in EC-BSI-associated 30-day mortality were at most modest in the nosocomial (rate ratio=0·98 (95% CI 0·96,1·00), p=0·03) and quasi-nosocomial (0·98 (0·95,1·00), p=0·06) groups, with no evidence for changes in mortality in the quasi-community (0·99 (0·96,1·01), p=0·32) and community (0·99 (0·96,1·01), p=0·21) groups. Mortality was, however, substantial (14-25% across groups). By contrast, co-amoxiclav-resistant EC-BSIs increased in all groups (by 11%-19%/year, significantly faster than susceptible EC-BSIs, pheterogeneity<0·0001), as did co-amoxiclav-resistant EC-UTIs (by 13%-29%/year, pheterogeneity<0·0001). Co-amoxiclav use in primary-care facilities was associated with subsequent co-amoxiclav-resistant EC-UTIs (p=0·03) and all EC-UTIs (p=0·002).
Interpretation
Current increases in EC-BSIs in Oxfordshire are primarily community-associated, with high rates of co-amoxiclav resistance; nevertheless, there was little or no change in mortality. Focussing interventions on primary-care facilities, particularly with high co-amoxiclav usage may be most effective, in this region and more generally.
Funding
National Institute for Health Research.
Introduction
Escherichia coli is a major cause of bloodstream infection (BSI) 1 and a critical antimicrobial resistance (AMR) concern; 2 rates are rising across Europe.3–5 E. coli bloodstream infections (EC-BSIs) reported (voluntarily) to Public Health England rose by 44% between 2003-2011. 6 After introducing mandatory reporting in July 2011, a further 28% increase occurred by July-September 2016, to 78·8 cases/100,000 population. 7
As elsewhere, most (>70%) EC-BSIs in England are identified within two days of admission. 7 However, the impact of previous hospital-exposure on trends in EC-BSI has not been comprehensively investigated, with only two relevant previous studies, one in the Calgary Health Region 2000-2006, 8 and another in Oxfordshire in 1999-2011 9 ; further, the relevance of such older studies to current trends is unclear. EC-BSI source may also differ by hospital-exposure. In a recent study, ~50% of EC-BSIs in England were considered most likely due to urinary tract infections (UTIs); 10 gastrointestinal foci are more common in inpatients. 7
30-day all-cause mortality following EC-BSI is ~16%; 11 and could rise given the impact of increasing AMR on outcomes. 2 In Oxfordshire, EC-BSI incidence rises through 2011 were essentially confined to ciprofloxacin-, co-amoxiclav-, cefotaxime- and/or aminoglycoside-resistant organisms. 9 The reasons for rising resistant EC-BSI, and EC-BSI more generally, are unclear, with increased antibiotic usage or resistance implicated in some, but not all, studies; 5,12–18 an aging population is also hypothesised to contribute. 5 Although individual hospital and primary-care guidelines vary, in England co-amoxiclav is commonly used as empiric treatment, particularly for community-acquired pneumonia and undifferentiated sepsis in hospitals, 19 as well as for prophylaxis, making it one of the most commonly used antibiotics in England. 20 Hence, trends in co-amoxiclav resistance are particularly important. We therefore aimed to investigate possible drivers of changes in EC-BSI incidence and antibiotic susceptibilities in Oxfordshire over the last two decades, while stratifying for hospital-exposure. We hypothesized that increases may be due to features of the at-risk population (therefore exploring demographics, recurrent infections, increased ascertainment), healthcare-history (previous urine cultures, catheter specimens, admission diagnoses, antibiotic usage), and/or the bacteria (exploring mortality/severity, AMR burden).
Methods
The Infections in Oxfordshire Research Database (IORD) 21 records all admissions to the Oxford University Hospitals National Health Service Foundation Trust (OUH), Oxfordshire, UK, from April 1997, linked by patient with microbiology and biochemistry/haematology results. The four OUH hospitals provide all acute care, microbiology and pathology services in the region (~680,000 individuals). Out-of-hospital mortality was determined by updates from a national information system. IORD has Research Ethics Committee and Confidentiality Advisory Group approvals (14/SC/1069, ECC5-017(A)/2009). Data on antibiotic prescribing and numbers of registered patients for each primary-care facility were obtained from the Health and Social Care Information Centre (available January 2011-December 2016 only).
The primary study outcome was EC-BSI, defined as E. coli isolated from blood cultures taken 01/Jan/1998-31/Dec/2016 inclusive, including polymicrobial cultures (13%), without age restriction and de-duplicated within 14-days of each index positive following mandatory reporting guidelines. 22 For context we also analysed E. coli UTIs (EC-UTIs), defined as pure culture from urine of >104 colony-forming-units/ml, de-duplicated within 90-days to avoid over-counting ongoing infections. 23 We classified EC-BSIs/EC-UTIs as ‘nosocomial’ if samples were taken >48h post-admission until discharge. 24 All other EC-BSIs/EC-UTIs were classified as ‘community’, ‘quasi-community’ or ‘quasi-nosocomial’ if the last hospital discharge was >1 year, 31-365 days, or 0-30 days previously. 8,25–27 We also calculated incidences of first ever and recurrent EC-BSIs. See Supplementary Methods for further details.
To assess demographic changes such as ageing and population growth, we standardised incidence against the 1998 Oxfordshire age-sex population (estimates from the UK Office for National Statistics). 28 To assess ascertainment, we considered the incidence of blood/urine cultures, regardless of result, and also additionally standardised outcome for culture rates. Both standardisations were done using inverse probability weighting.
As a proxy for changes in bacterial virulence, we considered separately 30-day mortality after sample collection, and levels of monocytes, neutrophils, lymphocytes, C-reactive protein (CRP), creatinine and urea at sample collection (closest value within [-2,+2] days). To assess the impact of AMR, which might also affect treatment outcomes, we investigated EC-BSI reported by the diagnostic laboratory as resistant to amoxicillin, co-amoxiclav, trimethoprim, gentamicin, ciprofloxacin, ceftriaxone, ceftazidime, piperacillin-tazobactam and meropenem, and EC-UTI reported as resistant to amoxicillin, co-amoxiclav, trimethoprim, ciprofloxacin, nitrofurantoin and cefalexin (the only drugs consistently tested throughout the study period). Susceptibility testing was performed using disk-diffusion to 31/Jan/2013, then by microbroth dilution (BD Phoenix™ Automated Microbiology System, Beckton Dickinson, Franklin Lakes, NJ, USA) (see Supplementary Methods).
Guidelines recommend empirical treatment for uncomplicated UTIs and for urine samples to be sent for microbiological testing only from individuals with clinical treatment failure, frequent or recurrent UTI or with possibly resistant infections. 20 To investigate this group, we first classified EC-BSIs by whether the patient had ever had an EC-UTI ≥3 days previously. To investigate the contribution of UTI around EC-BSIs, including symptomatic UTIs where E. coli was not isolated, we classified EC-BSIs as ‘likely urine-associated’ (urine sample taken 3-30 days previously; EC-UTI or mixed growth/negative but UTI suspected clinically from request codes), ‘urosepsis’ (defined as ‘likely urine-associated’ but urine samples within (-3,+2] days of the EC-BSI), ‘unlikely urine-associated’ (UTI with non-E. coli pathogen or no urine sample), or ‘unknown’ (other) (details in Supplementary Methods). To investigate the contribution of catheters, we classified EC-BSIs by whether a catheter urine specimen had been submitted up to and including the day of blood collection (regardless of result).
To investigate the contribution of previous admission characteristics, we classified quasi-nosocomial EC-BSIs by whether the primary diagnostic code of the antecedent admission was infection-related, or any diagnostic code (primary/secondary) included UTI (Supplementary Methods and Results).
Statistical analysis
Counts of EC-BSI/EC-UTI per month were modelled using negative-binomial regression (incorporating overdispersion), assuming the same underlying population (no offset). 30-day mortality following EC-BSI and CRP≥156mg/L at EC-BSI (binary) were modelled using poisson regression (to estimate analogous rate ratios), and absolute values of other test results were modelled using median quantile regression, both against sample date and adjusted for age and sex. Changes in trends in all outcomes were estimated using iterative sequential regression, 29 and compared between outcomes using stacked regression. 30 Bivariate cross-correlations summarised univariable associations between hospital antimicrobial usage and nosocomial co-amoxiclav-resistant EC-BSIs. To estimate associations with primary care co-amoxiclav usage, co-amoxiclav defined-daily-doses (DDDs) per 1000 registered patients in the previous or current year and primary-care facility were included as explanatory variables with the number of patients per primary-care facility per year as an offset in negative-binomial regression models for yearly co-amoxiclav resistant EC-UTIs, EC-UTIs and all urines regardless of result. Full details, including missing data, are provided in Supplementary Methods.
Analyses were conducted using R 3.2.2, 31 and STATA 14.1 for stacked regression and probability weighted analyses.
Role of the funding source
The study sponsor had no role in design, data collection, analysis, interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
After 14-day de-duplication, from 1998-2016 5706 EC-BSIs occurred in 5215 patients (i.e. 9% recurrences (relapse and/or reinfection)). Recurrences occurred a median(IQR) 144(39-577) days apart: of 391 patients with recurrences, 324(83%) had one and 52(13%) had two (range 1-8). Overall incidence increased year-on-year (annual incidence rate ratio (IRR)=1·06 (95% CI 1·05-1·06)). Most (5393(95%)) EC-BSI cases were admitted to OUH before or within 24h following blood culture (remainder mostly sampled in emergency departments/community hospitals). Only 1365(24%) EC-BSIs were ‘nosocomial’ (≥48h post-admission). A further 1132(20%) were ‘quasi-nosocomial’ (discharged up to 30 days previously), 1346(24%) were ‘quasi-community’ (discharged 31-365 days previously) and 1863(33%) were ‘community’ cases (discharged >1 year previously or never previously admitted to OUH). The 1132 quasi-nosocomial EC-BSI were most commonly previously admitted for malignancy (395,35%), gastrointestinal disorders (177,16%), and renal/urological disorders (164,14%) (Supplementary Table 1), with no major temporal variability (Supplementary Figure 1A).
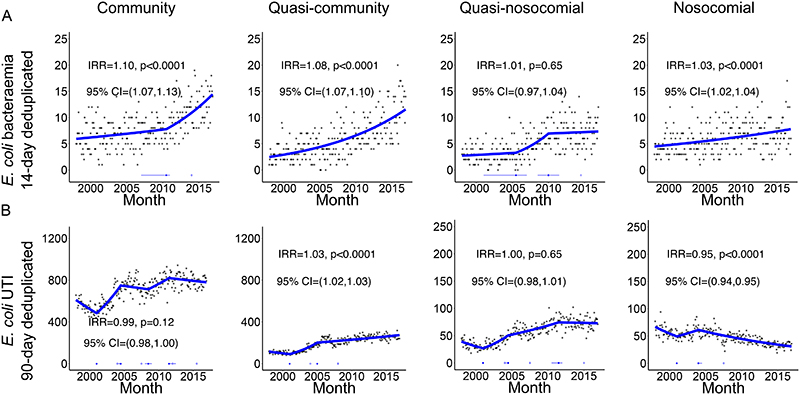
Incidence trends for EC-BSIs varied substantially with hospital-exposure (Figures 1A&2A, Supplementary Table 2), with overall increases clearly driven by community and quasi-community hospital-exposure groups, and no evidence of different incidence trends between these two groups in 2016 (pheterogeneity=0·27). By contrast, quasi-nosocomial and nosocomial EC-BSIs increased more slowly. Considering only the first EC-BSI per patient or subsequent EC-BSIs (Figure 2A, Supplementary Figure 2) gave broadly similar results. Year-on-year increases in the incidence of first EC-BSI became smaller (but still significant) the more recent the hospital exposure. Quasi-community recurrent EC-BSI increased faster than first EC-BSIs (pheterogeneity<0·0001) and the stable current trend in the incidence of all quasi-nosocomial EC-BSIs was driven by reduced recurrences in this group.
Figure 1. Monthly (A) EC-BSI and (B) EC-UTI according to recent hospital-exposure (first and recurrent infections).
Footnote: only counting EC-BSI recurrences occurring >14 days after an index positive, and EC-UTI recurrences occurring >90 days after an index positive. Thick blue line represents the estimated incidence by iterative sequential regression (ISR). Blue lines at the base of the graph represent 95% CI around the breakpoints estimated by the ISR model. IRR=annual incidence rate ratio in 2016, that is the relative increase in rate per year as estimated in 2016.
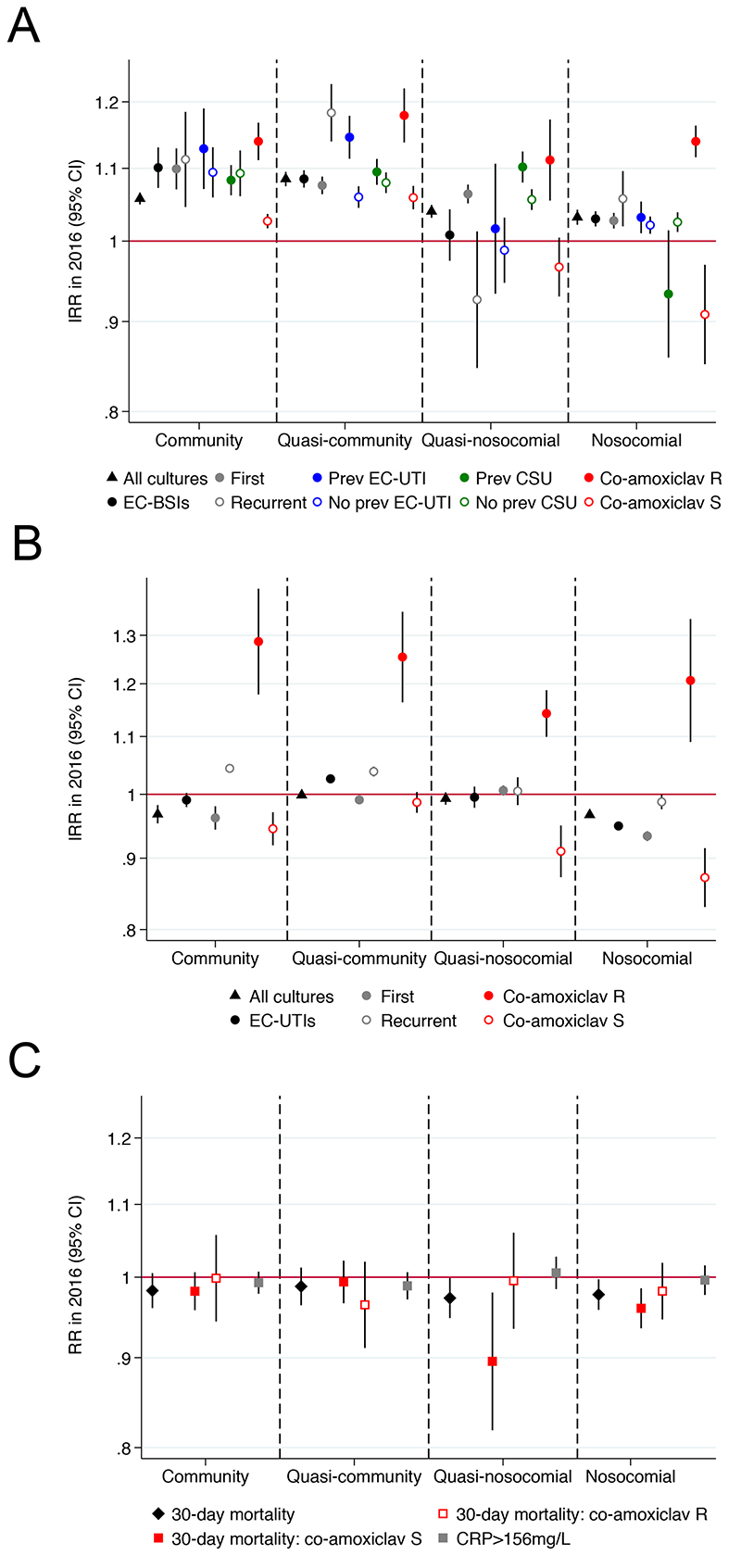
Figure 2. Summary of incidence trends in 2016 for (A) EC-BSIs, (B) EC-UTIs, and (C) severity of co-amoxiclav resistant and sensitive EC-BSIs.
Footnote: IRR=annual incidence rate ratio in 2016, that is the relative increase in rate per year as estimated in 2016. See Supplementary Table 1 for numbers and heterogeneity tests.
After 90-day de-duplication, 228376 EC-UTIs occurred in 137075 patients (i.e. 40% recurrences (relapse/re-infection)). Recurrences occurred a median(IQR) 457(200-1119) days apart: of the 41371(30%) patients with recurrences, 22011(53%) had one and 8742(21%) had two (range 1-33). 12898(9%) patients had two EC-UTI within six months. EC-UTIs were predominantly community (160359,70%), and less commonly quasi-community (44283,19%), quasi-nosocomial (12764,6%) or nosocomial (10970,5%). Incidence of EC-UTI increased over 1998-2016 in community, quasi-community and quasi-nosocomial groups, although current trends were fairly stable, but declined significantly in the nosocomial group (Figure 1B&2B). Furthermore, increases were accounted for entirely by substantial increases in recurrent EC-UTI episodes, with decreasing overall trends in first EC-UTI per patient (Supplementary Figure 3).
In 2016, therefore, recurrences accounted for at least half of community, quasi-community and quasi-nosocomial EC-UTIs, and around a fifth of quasi-community and quasi-nosocomial EC-BSIs (Supplementary Table 3).
Impact of population and sampling on EC-BSI
Blood culture submission rates increased substantially from 1998-2016 for community/quasi-community/quasi-nosocomial groups (Figure 2A, Supplementary Figure 4), raising the possibility that observed increases in EC-BSIs were driven by increases in the use of blood cultures as a diagnostic test. However, there was no suggestion that the indications for blood culture changed with time: changes in neutrophils and CRP when cultures were taken were small and not clinically meaningful and 30-day mortality post blood culture sampling was stable (Supplementary Figure 5). Further, increases in community blood culture submission rates were significantly smaller than increases in community EC-BSIs (p=0·0006, Figure 2A). Standardising for age and sex explained only 10-26%, and standardising additionally for blood cultures taken only 9-28%, of the increase in overall or first-per-patient EC-BSIs, with the greatest percentage explained in nosocomial EC-BSIs and the least in community EC-BSIs (Supplementary Tables 4,5). In contrast, urine sample submission was more stable over time (Supplementary Figure 6).
Disease severity of EC-BSIs
30-day mortality following EC-BSI declined slightly in the nosocomial (IRR=0·98 (95% CI 0·96,1·00), p=0·03) and quasi-nosocomial (IRR=0·98 (0·95,1·00), p=0·06) groups, but there was no evidence for changes in quasi-community (IRR=0·99 (0·96,1·01), p=0·32) and community (IRR=0·99 (0·96,1·01), p=0·21) groups (Supplementary Figure 7, adjusting for age and sex). Mortality was substantial at 25% (340/1363), 19% (217/1128), 16% (219/1344) and 14% (254/1784) across the groups, respectively (Supplementary Table 6). Changes in haematology/biochemistry test results over time were small and/or non-significant (Supplementary Figure 7), and did not indicate that less severe infections were being identified, or that there were changes in pathogen virulence.
Impact of previous illness on EC-BSI
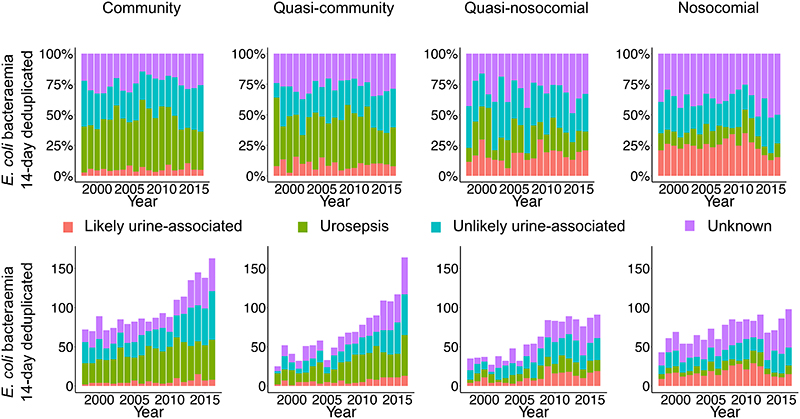
1755(31%) EC-BSI occurred in patients with an EC-UTI ≥3 days previously (median(IQR) 213(43-918) days previously). However, incidence trends were broadly similar for EC-BSIs with or without EC-UTIs ≥3 days previously, although quasi-community EC-BSIs were rising particularly fast in those with previous EC-UTIs (pheterogeneity<0·0001, Figure 2A, Supplementary Figure 8). We next explored whether EC-BSI increases were associated with past symptomatic UTIs, including those without positive urine cultures. Considering urine samples/results taken within 30 days before the EC-BSI, and incorporating information on mixed growth and request codes, only 760(13%) EC-BSIs were ‘likely urine-associated’, with 1613(28%) ‘urosepsis’, 1613(28%) ‘unlikely urine-associated’ (of which 181[11%] had a contemporaneous urine specimen positive for another pathogen), and 1720(30%) unknown. However, the relative proportions of these did not vary substantially over time (Figure 3), suggesting no specific subgroup was associated with incidence increases. Percentages of EC-BSIs with a previous catheter urine specimen (CSU) increased across hospital-exposure groups, being present in 365(20%) community, 364(32%) quasi-community, 541(40%), quasi-nosocomial, 584(43%) nosocomial cases. However, incidence trends were broadly similar for EC-BSIs with or without a previous CSU (Figure 2A, Supplementary Figure 9), although quasi-nosocomial EC-BSIs were rising particularly fast in those with previous CSUs (pheterogeneity=0·0002), while increases in nosocomial EC-BSIs were restricted to those without previous CSUs (pheterogeneity=0·03).
Figure 3. Annual EC-BSI according to recent hospital-exposure and urine sample submission/results.
Footnote: See Supplementary Methods for definitions.
Antimicrobial susceptibility
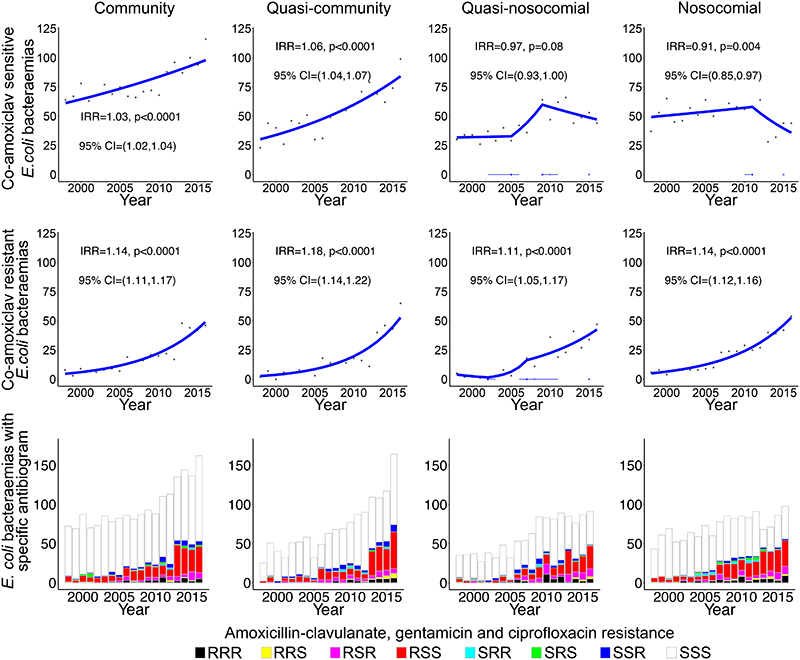
Exploring the possibility that EC-BSI increases were associated with AMR, the only EC-BSI antibiotic-resistant phenotype that consistently increased across all hospital-exposure groups was co-amoxiclav (p<0·0001; Figures 2A&4), with 212(41%) of 515 EC-BSIs in 2016 being co-amoxiclav resistant (Supplementary Tables 6,7). Co-amoxiclav-resistant EC-BSIs increased significantly faster than co-amoxiclav-susceptible EC-BSIs (pheterogeneity<0·0001), but community and quasi-community co-amoxiclav-susceptible EC-BSIs were still increasing significantly in 2016 (p<0·0001) (Figure 4). Most (942/1412, 67%) co-amoxiclav-resistant EC-BSIs remained susceptible to gentamicin and ciprofloxacin (Figure 4).
Figure 4. Annual EC-BSI susceptible and resistant to co-amoxiclav, with and without resistance to gentamicin and ciprofloxacin, according to recent hospital-exposure.
Footnote: IRR=annual incidence rate ratio in 2016, that is the relative increase in rate per year as estimated in 2016.
Increases in other antibiotic-resistant EC-BSIs were most notable in the community and quasi-community groups, with significant year-on-year increments in all but trimethoprim-resistant EC-BSIs, which remained stable in these groups (Supplementary Figure 10). Co-amoxiclav-resistant EC-UTIs also rose consistently and significantly regardless of healthcare-exposure, but trends were more variable for other antibiotics (Supplementary Figure 11). In 2016, 3921/13792(28%) EC-UTIs were co-amoxiclav-resistant.
Given the substantial increase in co-amoxiclav resistant EC-BSIs, we investigated whether severity differed in susceptible versus resistant cases. There was no strong evidence that co-amoxiclav-resistant EC-BSIs were associated with higher neutrophil counts in any hospital-exposure group (p>0·04, adjusting for age and sex), or that neutrophil counts were changing differently over time in co-amoxiclav-resistant versus co-amoxiclav-susceptible EC-BSI (pheterogeneity>0·67; Supplementary Figure 12). Mortality was higher for co-amoxiclav-resistant vs co-amoxiclav-susceptible nosocomial EC-BSIs (unadjusted 30% (117/395) vs 23% (222/967) respectively; rate ratio adjusting for age and sex =1.32 (1.13-1.46) p=0·002). However, there was no evidence of higher mortality in co-amoxiclav-resistant community/quasi-community/quasi-nosocomial EC-BSIs (p>0·48), and mortality did not change differently over time in any group (pheterogeneity>0·35; Supplementary Figure 12, Figure 2C) (Supplementary Table 6).
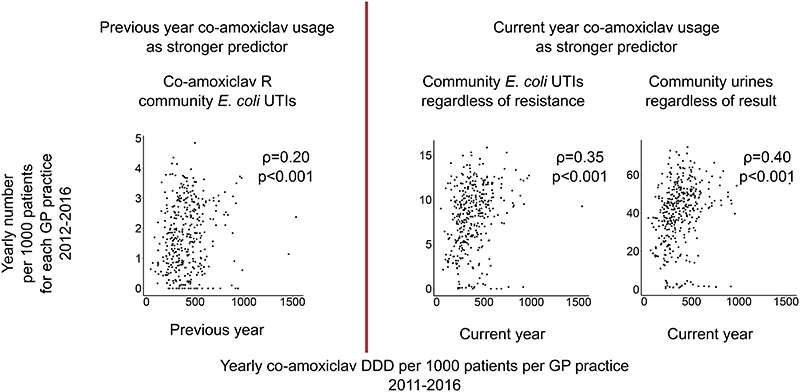
The strongest associations with nosocomial co-amoxiclav-resistant EC-BSIs were with hospital co-amoxiclav (cross-correlation 0·75) and third-generation cephalosporin (0·80) use (Supplementary Table 8; available only financial years 2003-2014). Community prescribing data was only available from 2011, and co-amoxiclav-resistant EC-BSIs were too few to consider relationships with co-amoxiclav use. However, from 2012-2016, primary care facilities prescribing more co-amoxiclav in the previous year had higher rates of subsequent co-amoxiclav-resistant-community-EC-UTIs (IRR (per 100DDD higher)=1·05 (95% CI 1·02-1·08) p=0·003, Figure 5), co-amoxiclav use in the current year did not add any predictive value (p=0.12 adjusted for previous year vs p=0.64 alone). In contrast, facilities prescribing more co-amoxiclav in the current year had higher rates of community-EC-UTIs and urine specimen submission (IRR=1·02 (1·00-1·04) p=0·01 and 1.02 (1·01-1·03) p=0·0001 respectively); co-amoxiclav use in the previous year did not add any predictive value (p=0.58 adjusted for current year vs p=0.11 alone, respectively p=0.21 adjusted for current year vs p=0.006 alone).. Results were seen across all samples regardless of hospital-exposure group (Supplementary Figure 13), and also when adjusting instead for the proportion aged over 65 and male in 2017 per practice. There was no association between current/prior quinolone use and any of these outcomes (p>0.9).
Figure 5. Community co-amoxiclav-resistant EC-UTIs (A), community EC-UTIs (B) and community urine samples submitted regardless of result (C) per 1000 patients per primary-care facility 2012-2016 compared with co-amoxiclav usage.
Footnote: showing one record per year per primary-care facility. For (A) the strongest predictor was co-amoxiclav DDD per 1000 patients per general practice in the previous year; for (B) and (C) the strongest predictor was co-amoxiclav DDD per 1000 patients per general practice in the current year. Spearman rho (and models) for each panel excludes 5 facilities which submitted less than 151 samples over 2011-2016 (all others submitted over 300). Spearman rho for univariable associations with previous vs current co-amoxiclav usage for the 3 outcomes left to right ρ=0.20 vs ρ=0.04, ρ=0.33 vs ρ=0.35, ρ=0.37 vs ρ=0.40 respectively.
Discussion
We have explored potential explanations for continuing increases in EC-BSI in Oxfordshire over 19 years using extensive, routinely-collected data, including laboratory/microbiology results. Incidence varied dramatically by hospital-exposure, with increases being driven by community/quasi-community cases. This is important given the National Health Service ambition to reduce Gram-negative BSIs by targeting ‘healthcare-associated’ cases (although this definition incorporates recent community antibiotic use). Previous successful campaigns to reduce methicillin-resistant Staphylococcus aureus (MRSA) BSI and Clostridium difficile infections also focussed on nosocomial risk factors. Our data suggest that defining appropriate strategies aiming to reduce community/quasi-community associated EC-BSIs, such as improved catheter care and improved quality of antibiotic use for UTI management in the community, might have a greater impact. Given that recent antibiotic use is the greatest risk factor for subsequent resistant UTIs, 32 many people prescribed antibiotics may not have bacterial UTIs, 33 and many bacterial UTIs may resolve in a similar timeframe without antibiotics, 34 better point-of-care tests that predict benefit from antibiotics are urgently needed to guide prescribing decisions. Co-amoxiclav-resistant EC-BSIs rose significantly faster than co-amoxiclav-susceptible EC-BSIs, regardless of hospital-exposure, with the greatest number of co-amoxiclav-resistant EC-BSIs in 2016 being community/quasi-community EC-BSIs. Primary-care facilities with higher co-amoxiclav prescribing rates in the previous year had more co-amoxiclav resistant EC-UTIs in the subsequent year. Co-amoxiclav is one of the most commonly prescribed antibiotics nationally in both the community and hospitals in England, 20,35 and our findings are consistent with this exerting selection pressure for co-amoxiclav resistant EC-UTI and EC-BSI. Despite co-amoxiclav being used for empiric BSI treatment, there were no clinically important changes in mortality.
EC-BSI is generally considered ‘community-acquired’ although there are differing definitions of healthcare-associated BSI. 7,26 By linking to previous hospital admissions, one major study strength is that we could identify that incidence trends for non-nosocomial EC-BSIs varied significantly by time since discharge. Blood sample submission also increased significantly over time, potentially increasing ascertainment of ‘mild’ cases. However, blood cultures are key to the assessment of unwell patients whenever infection is suspected, and there were no clinically important changes in EC-BSI-associated severity at presentation or mortality, despite substantially increasing incidence, suggesting major ascertainment bias is unlikely. As standardising for age/sex using crude data available had at most modest effects, main analyses did not use this.
The increasing trend in nosocomial EC-BSI was significantly smaller than for community/quasi-community EC-BSI in Oxfordshire, as observed nationally. 10 Multiple infection control interventions were rolled out in UK hospitals from 2005-2010 36,37 in response to MRSA/C. difficile, and horizontal components could have contributed to lowering nosocomial rates. Consistent with this, increases in hospital-onset Gram-negative BSI reversed after introducing a MRSA Prevention Initiative in the US, while community-acquired incidence remained unchanged. 38
Whereas MRSA and C. difficile are predominantly hospital-associated pathogens, differences in EC-BSI epidemiology highlight the need for different interventions, particularly in primary care. 7 In particular, recurrences explain relatively little of the ongoing increases in EC-BSIs, and both co-amoxiclav-resistant and co-amoxiclav-susceptible EC-BSI are rising. Overall, 42% of EC-BSI appeared to be more likely amenable to urinary-focussed intervention, similar to an England-wide study that found 51% of EC-BSIs had an underlying urogenital tract focus, with UTI treatment in the prior four weeks the largest independent risk factor. 10 In our study, 13% of EC-BSIs were likely urine-associated and 28% presented as urosepsis; the first group may be most tractable for prevention but was smallest in both community and quasi-community EC-BSI, whereas urosepsis was the largest. One key limitation is lack of data on visits to primary-care facilities, meaning our assessment of urinary sources relied on samples being submitted for microbiological testing, and may therefore underestimate the true burden of urinary-associated EC-BSI, since some patients may have had UTI symptoms and either did not present to primary-care facilities or were treated empirically without a urine sample being submitted. Changes in patient health-seeking behaviour or sample submission over time could lead to bias in attributing EC-BSIs to different sources. However, successfully treated UTIs, and those resolving without intervention, should not cause bacteraemia, and bacteraemias due to UTI treatment failure should be ascertained within our data since guidelines recommend urine samples be submitted from individuals with clinical treatment failure, frequent or recurrent UTI or with a possibly resistant infection. 20 . Much of the burden of EC-BSIs, and especially the rising incidence, is hypothesized to arise from poor urinary catheter care. However, only 20% and 30% of the community and quasi-community groups, where incidence is increasing fastest, had a previous CSU, and there was no evidence that incidence was increasing faster in those with a previous CSU versus without. One key limitation is that we did not have records of the presence of a catheter, but only urine specimens recorded as being taken from a catheter, arguing that if a catheter was present and causing infection, a specimen would likely have been taken from it at some time. Similarly, we did not have direct information on the source of each individual EC-BSI. Interestingly, there was strong evidence that quasi-nosocomial EC-BSIs with UTI or infectious diagnostic codes in the previous admission were rising faster than those without (Supplementary Figure 1). This may reflect underlying predisposition to infection (e.g. chronic illnesses), or that prior antibiotic use adversely affects a patient’s microbiota potentially leading to colonisation/overgrowth by more pathogenic E. coli, thus predisposing to EC-BSI.
A limitation of surveillance studies is changes in antimicrobial susceptibility testing methodology (here in February 2013). Whilst testing protocol can affect results, 39,40 crucially changes in co-amoxiclav resistance around this time occurred regardless of method (Supplementary Figure 14). Recent data suggest that broth dilution (BD-Phoenix) and the gold standard agar dilution have high agreement; 41 thus, rising rates of co-amoxiclav-resistant (as defined by EUCAST breakpoints) EC-BSI/EC-UTI are likely correct.
We also found that primary-care facilities with higher co-amoxiclav prescribing rates in the previous year were more likely to have patients diagnosed with co-amoxiclav resistant EC-UTIs in the subsequent year. Similar associations between trimethoprim use and trimethoprim-resistant urine-associated EC-BSI have been reported in adult women in England, 16 and recently more generally across multiple antibiotic classes for EC-UTI. 17 Over the period with contemporary prescribing data, co-amoxiclav-resistant EC-BSI were too few to investigate associations with antibiotic prescribing within the community. Assessing usage-resistance associations is complicated, since changes in use of one antibiotic are generally accompanied by compensatory prescribing, and may be compounded by multi-drug resistance. Comparisons are ecological, which is a key limitation. Our results may also not be generalizable; for example, although the region we studied is sizeable (~1% of the UK), we did not observe a uniform decrease in cephalosporin-resistant and quinolone-resistant EC-BSIs as seen in BSI caused by Enterobacteriaceae. 15 Such differences likely reflect a complex interplay of selection pressures.
A key limitation is that we were unable to assess associations between individual-patient antibiotic use (not available in the research database) and risk of resistant infections or between specific empiric regimens and outcome; these are important future research priorities. However, there were no clinically important changes in mortality overall, by co-amoxiclav-susceptible/resistant phenotype, or by hospital-exposure. Co-amoxiclav remains our recommended first-line empiric treatment for most severe infections, so the substantial increase in incidence of co-amoxiclav-resistant bacteraemias suggests either that initial inappropriate treatment can be successfully rescued, 42 or that the current definition of co-amoxiclav breakpoints may be suboptimal. 43 Crucially, neither scenario supports a move towards broader empiric antibiotic treatment, consistent with prevailing antimicrobial stewardship messages.
Another inherent limitation is restriction to the routinely collected data available, in particular lack of information on prognostic factors such as illness severity scores, lack of individual antibiotic prescribing data (as above), and having only antibiograms since strain typing was not routinely performed. Representative isolates from 2008-present have been selected for whole genome sequencing and their analysis may increase our understanding of the pathogenesis of EC-BSI. For example, the distinct change in the monthly incidence of community EC-BSI in July 2010 could reflect the introduction and proliferation of a new strain of E. coli to the region. 44
In summary, on-going increases in EC-BSI were driven by community and quasi-community cases, and cannot be attributed only to increased recurrences or an aging population. Absence of changes in mortality and severity do not support ascertainment bias playing a major role, although this cannot be excluded. Whilst urinary foci are clearly important, at present the scope for intervening to prevent UTIs progressing to bacteraemia could be limited. Notably, higher co-amoxiclav use in primary care was associated with higher subsequent rates of co-amoxiclav-resistant EC-UTI, supporting drives to reduce broad-spectrum and inappropriate antibiotic use. However, despite substantial increases in co-amoxiclav-resistant EC-BSI, evidence that patient clinical outcomes are no worse does not support broadening empiric antibiotic prescribing from co-amoxiclav. 11
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for publications from 1999-23 March 2018 , with the terms (“Escherichia coli” OR “E. coli”) AND (“bacteraemia” OR “bloodstream infection”), restricting the search to English language articles, and reviewed titles to identify relevant articles, and reference lists from relevant articles. Escherichia coli (E. coli) is one of the most common causes of bloodstream infection, and the incidence of E. coli bloodstream infection, and particularly antibiotic-resistant infections, is increasing in the UK and Europe. Although a previous study found increases in E. coli bacteraemias in Oxfordshire, this was through 2011 only and did not investigate drivers in detail. The UK government aims to reduce healthcare-associated E. coli bloodstream infection; however, there is only limited evidence to inform appropriate interventions in the current era. Factors that may have contributed to the increasing incidence include the aging population and the increase in antibiotic use and antibiotic-resistant isolates. The percentage of cases that were identified within two days of admission has increased marginally over time. Voluntarily reported data reveals little change in terms of the most likely primary focus over time, with urinary tract infection consistently being the most frequent primary focus for E. coli bacteraemia cases. Incidence generally increases with higher temperatures.
Added value of this study
We investigated potential drivers for these increases in incidence by exploiting available linked electronic health records over 19 years for ~5200 patients with E. coli bloodstream infection and ~140000 with E. coli urinary tract infection, together with community antimicrobial prescribing data for the most recent six years. Our study identified several findings with significant implications for health policy and patient care:
Increases in the incidence of E. coli bloodstream infections were driven mainly by non-hospital-associated cases; standardising for age and sex explained only 10-26%, and standardising additionally for blood cultures taken only 9-28%, of the increase with the smallest percentage explained in non-hospital-associated cases; increases did not appear to be primarily due to patients with evidence of previous urinary tract infections
Co-amoxiclav-resistant bloodstream infections rose significantly faster than co-amoxiclav-susceptible bloodstream infections, with the greatest number of co-amoxiclav-resistant bloodstream infections in 2016 being in patients discharged more than a month previously (i.e. community-associated)
Higher co-amoxiclav use in primary care in the previous year was associated with higher rates of co-amoxiclav-resistant E. coli urinary tract infections in the subsequent year, supporting drives to reduce broad-spectrum and inappropriate antibiotic use in primary care
Despite substantial increases in co-amoxiclav-resistant bloodstream infections there was no evidence that mortality was increasing in these cases; this does not support moving to broader empiric antibiotic prescribing in hospitals (i.e. carbapenems, piperacillin-tazobactam)
Implications of all available advice
This suggests that government strategies to effectively reduce E. coli bloodstream infections should prioritise community settings rather than focus primarily on healthcare-associated settings. The absence of an increased mortality signal suggests that co-amoxiclav resistant E. coli infections are either being successfully treated by dual empiric therapy in severe cases (e.g. with concomitant gentamicin), can be “rescued” once isolate susceptibilities become available, or currently deployed phenotypic susceptibility testing breakpoints do not adequately correlate with clinical outcome. Crucially, none of these explanations support broadening empiric antibiotic prescribing from co-amoxiclav.
Acknowledgements
This work uses data provided by patients and collected by the NHS as part of their care and support. We thank all the people of Oxfordshire who contribute to the Infections in Oxfordshire Research Database.
Research Database Team: R Alstead, C Bunch, DCW Crook, J Davies, J Finney, J Gearing (community), H Jones, L O’Connor, TEA Peto (PI), TP Quan, J Robinson (community), B Shine, AS Walker, D Waller, D Wyllie. Patient and Public Panel: G Blower, C Mancey, P McLoughlin, B Nichols.
NIHR Health Protection Research Unit Steering Committee: J Coia, N French, C Marwick, M Sharland.
Financial support
The research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford in partnership with Public Health England (PHE) [HPRU-2012-10041], and the NIHR Oxford Biomedical Research Centre, and a Medical Research Council UK Clinical Research Training Fellowship to NJF. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or PHE. CCB DWC and TEAP are NIHR Senior Investigators.
Footnotes
Contributions: KDV, NS, DHW, TEAP, and ASW designed the study. TPQ prepared extracts from the IORD database, KDV obtained data from the HSCIC. KDV and ASW analysed the data. KDV, TEAP and ASW prepared the figures. KDV, NS, and ASW prepared the first draft of the manuscript. All authors commented on the data and its interpretation, revised the content critically and approved the final version.
Please contact the corresponding author for questions regarding the analysis, data or code.
Conflicts of interest
Dr. Peto reports grants from Wellcome Trust, grants from Medical Reserach Council, during the conduct of the study. All other authors report no conflicts of interest.
References
- 1.Public Health England. Polymicrobial Bacteraemia and Fungaemia in England, Wales and Northern Ireland 2014. 2015;9 [Google Scholar]
- 2.O’Neill J. Antimicrobial Resistance : Tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. 2014 December;:1–16. [Google Scholar]
- 3.Buetti N, Atkinson A, Marschall J, Kronenberg A. Incidence of bloodstream infections : a nationwide surveillance of acute care hospitals in Switzerland 2008 – 2014. BMJ Open. 2017;7:1–5. doi: 10.1136/bmjopen-2016-013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe: Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2016.
- 5.van der Mee-Marquet NL, Blanc DS, Gbaguidi-Haore H, et al. Marked increase in incidence for bloodstream infections due to Escherichia coli, a side effect of previous antibiotic therapy in the elderly. Front Microbiol. 2015 June 6; doi: 10.3389/fmicb.2015.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerver S, Sinnathamby M, Bou-Antoun S, et al. Annual Epidemiological Commentary: Mandatory MRSA, MSSA and E. Coli Bacteraemia and C. Difficile Infection Data, 2014/15. 2015 [Google Scholar]
- 7.Public Health England. Annual Epidemiological Commentary Mandatory MRSA , MSSA and E. Coli Bacteraemia and C. Difficile Infection Data 2015/16. 2016.
- 8.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JDD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14(11):1041–1047. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 9.Schlackow I, Stoesser N, Walker AS, Crook DW, Peto TEA, Wyllie DH. Increasing incidence of Escherichia coli bacteraemia is driven by an increase in antibiotic-resistant isolates: electronic database study in Oxfordshire 1999-2011. J Antimicrob Chemother. 2012;67:1514–1524. doi: 10.1093/jac/dks082. [DOI] [PubMed] [Google Scholar]
- 10.Abernethy J, Guy R, Sheridan EA, et al. Epidemiology of Escherichia coli bacteraemia in England : results of an enhanced sentinel surveillance programme. J Hosp Infect. 2017;95 doi: 10.1016/j.jhin.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Public Health England. Thirty-Day All-Cause Fatality Subsequent to MRSA , MSSA and E. Coli Bacteraemia and C. Difficile Infection. 2015.
- 12.Livermore DM, Stephens P, Weinberg J, et al. Regional variation in ampicillin and trimethoprim resistance in Escherichia coli in England from 1990 to 1997 , in relation to antibacterial prescribing. J Antimicrob Chemother. 2000;46:411–422. doi: 10.1093/jac/46.3.411. [DOI] [PubMed] [Google Scholar]
- 13.Hay AD, Thomas M, Montgomery A, et al. The relationship between primary care antibiotic prescribing and bacterial resistance in adults in the community : a controlled observational study using individual patient data. J Antimicrob Chemother. 2005 May;:146–153. doi: 10.1093/jac/dki181. [DOI] [PubMed] [Google Scholar]
- 14.Kahlmeter G, Menday P, Cars O. Non-hospital antimicrobial usage and resistance in community-acquired Escherichia coli urinary tract infection. J Antimicrob Chemother. 2003;52(6):1005–1010. doi: 10.1093/jac/dkg488. [DOI] [PubMed] [Google Scholar]
- 15.Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK : links to prescribing change? J Antimicrob Chemother. 2013 June;68:2667–2674. doi: 10.1093/jac/dkt212. [DOI] [PubMed] [Google Scholar]
- 16.Lishman H, Costelloe C, Hopkins S, et al. 26th European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, Netherlands: 2016. Effect of trimethoprim / nitrofurantoin prescribing on the incidence and antibiotic susceptibility patterns of E . coli bacteraemia nationally at the GP practice level ( 2012-2014 ) [Google Scholar]
- 17.Ironmonger D, Edeghere O, Verlander NQ, et al. Effect of general practice characteristics and antibiotic prescribing on Escherichia coli antibiotic non-susceptibility in the West Midlands region of England : a 4 year ecological study. J Antimicrob Chemother. 2018;(73):787–794. doi: 10.1093/jac/dkx465. [DOI] [PubMed] [Google Scholar]
- 18.Ho P-L, Chow K-H, Lai EL, Lau EHY, Cheng VCC. Extended-spectrum-β-lactamase-positive Escherichia coli mainly adds to, rather than replaces, extended-spectrum-β-lactamase-negative E. coli in causing bacteraemia in Hong Kong, 2000–10. J Antimicrob Chemother. 2012;67(3):778–780. doi: 10.1093/jac/dkr502. [DOI] [PubMed] [Google Scholar]
- 19.Llewelyn MJ, Hand K, Hopkins S, Walker AS. Antibiotic policies in acute English NHS trusts: implementation of “Start Smart—Then Focus” and relationship with Clostridium difficile infection rates. J Antimicrob Chemother. 2015;70:1230–1235. doi: 10.1093/jac/dku515. [DOI] [PubMed] [Google Scholar]
- 20.Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2017.
- 21.Finney JM, Walker AS, Peto TEA, Wyllie DH. An efficient record linkage scheme using graphical analysis for identifier error detection. BMC Med Inform Decis Mak. 2011;11:7. doi: 10.1186/1472-6947-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health England. Mandatory enhanced MRSA , MSSA and Escherichia coli bacteraemia , and Clostridium difficile infection surveillance. 2016. Mar,
- 23.Butler C, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007 October; [PMC free article] [PubMed] [Google Scholar]
- 24.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;(16):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 25.Wyllie DH, Walker AS, Peto TEA, Crook DW. Hospital exposure in a UK population , and its association with bacteraemia. J Hosp Infect. 2007;(67):301–307. doi: 10.1016/j.jhin.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 26.NHS Improvement and Public Health England. Guidance on the definition of healthcare associated Gram-negative bloodstream infections. 2017. Jul,
- 27.Cardoso T, Almeida M, Friedman ND, Aragão I, Costa-pereira A. Classification of healthcare-associated infection : a systematic review 10 years after the first proposal. BMC Med. 2014;12(40) doi: 10.1186/1741-7015-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ONS Clinical Commissioning Group mid year population estimates. [Accessed March 17, 2018]. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/clinicalcommissioninggroupmidyearpopulationestimates .
- 29.Schlackow I, Walker SA, Dingle K, et al. Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed Clostridium difficile. PLoS Med. 2012;9(7):e1001279. doi: 10.1371/journal.pmed.1001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995:524–532. doi: 10.2307/2532940. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; http://www.r-project.org/ Published 2017. [Google Scholar]
- 32.Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection : a case - control study. J Antimicrob Chemother. 2007;60(1):92–99. doi: 10.1093/jac/dkm141. [DOI] [PubMed] [Google Scholar]
- 33.Butler C, Francis N, Thomas-Jones E, et al. Variations in presentation, management, and patient outcomes of urinary tract infection: a prospective four-country primary care observational cohort study. Br J Gen Pract. 2017;67(665):e830–e841. doi: 10.3399/bjgp17X693641. http://bjgp.org/content/67/665/e830.abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women : randomised controlled trial. BMJ. 2015;351 doi: 10.1136/bmj.h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashiru-Oredope D, Sharland M, Charani E, Mcnulty C, Cooke J. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart — Then Focus. J Antimicrob Chemother. 2012;67(Suppl 1):i51–i63. doi: 10.1093/jac/dks202. [DOI] [PubMed] [Google Scholar]
- 36.Wyllie DH, Walker AS, Miller R, et al. Decline of meticillin-resistant Staphylococcus aureus in Oxfordshire hospitals is strain-specific and preceded infection-control intensification. BMJ Open. 2011;1:1–10.:e000160. doi: 10.1136/bmjopen-2011-000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duerden B, Fry C, Johnson AP, Wilcox MH. The Control of Methicillin-Resistant Staphylococcus aureus Blood Stream Infections in England. Open Forum Infect Dis. 2015 doi: 10.1093/ofid/ofv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto M, O’Shea AMJ, Livorsi DJ, et al. The Effect of a Nationwide Infection Control Program Expansion on Hospital-Onset Gram-Negative Rod Bacteremia in 130 Veterans Health Administration Medical Centers: An Interrupted Time-Series Analysis. Clin Infect Dis. 2016;63(5):642–650. doi: 10.1093/cid/ciw423. [DOI] [PubMed] [Google Scholar]
- 39.Díez-Aguilar M, Morosini M-I, López-Cerero L, et al. Performance of EUCAST and CLSI approaches for co-amoxiclav susceptibility testing conditions for clinical categorization of a collection of Escherichia coli isolates with characterized resistance phenotypes. J Antimicrob Chemother. 2015 April;70:2306–2310. doi: 10.1093/jac/dkv088. [DOI] [PubMed] [Google Scholar]
- 40.Leverstein-van Hall MA, Waar K, Muilwijk J, et al. Consequences of switching from a fixed 2:1 ratio of amoxicillin/clavulanate (CLSI) to a fixed concentration of clavulanate (EUCAST) for susceptibility testing of escherichia coli. J Antimicrob Chemother. 2013;68(11):2636–2640. doi: 10.1093/jac/dkt218. [DOI] [PubMed] [Google Scholar]
- 41.Davies T, Stoesser N, Abuoun M, et al. 27th European Congress of Clinical Microbiology and Infectious Diseases. Vienna, Austria: 2017. Significant discordance in amoxicillin-clavulanate phenotyping methods and genotypic attribution of phenotypic “resistance” for clinical Escherichia coli isolates. [Google Scholar]
- 42.Fitzpatrick JM, Biswas JS, Edgeworth JD, et al. Gram-negative bacteraemia; a multi-centre prospective evaluation of empiric antibiotic therapy and outcome in English acute hospitals. Clin Microbiol Infect. 2016:244–251. doi: 10.1016/j.cmi.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 43.European Committee on Antimicrobial Susceptability Testing. EUCAST: MIC distributions and ECOFFs. 2007. [Accessed December 16, 2016]. http://www.eucast.org/mic_distributions_and_ecoffs/
- 44.Kallonen T, Brodrick HJ, Harris SR, et al. Systematic longitudinal survey of invasive Escherichia coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res. 2017:1437–1449. doi: 10.1101/gr.216606.116.Freely. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.