Abstract
Background
Chagas disease (CD) is a major neglected vector-borne disease, associated with knowledge gaps and difficulties in clinical drug development.
Methods
Proof-of-concept, double-blinded randomised phase- II clinical trial evaluating efficacy and safety of three oral E1224 regimens, and benznidazole (BZN) versus placebo in adult chronic indeterminate CD. Parasite DNA clearance was assessed by PCR at end of treatment and until 12 months follow-up (sustained response, SR). Trypanolytic anti-α-Gal antibodies (AT-CL-ELISA) and conventional serological methods were also evaluated.
Findings
The trial enrolled 231 patients. Parasite clearance was observed with E1224 during the treatment phase, but no SR was seen with E1224 low-dose (total dose=2000 mg) and short-dose (total dose=2400 mg) regimens, whereas 28·9% of patients had SR with the E1224 high-dose regimen (total dose=4000 mg). BZN (2.5 mg/kg/day orally BID, 60 days) had a rapid and sustained effect on parasite clearance (82·2%, 95%CI=[67·9;92·0]). Placebo group SR was 8·5%(95%CI=[2·4;20·4]). Statistically significant AT-CL-ELISA titer reduction was observed only in BZN-treated patients. Human data on PCR standardization and pharmacokinetic/pharmacodynamics were generated. Both treatments were well tolerated. Reversible, dose-dependent liver enzyme increases were seen with E1224 and BZN.
Interpretation
E1224 is the first new chemical entity developed for CD in decades. E1224 displayed a transient, suppressive effect on parasite clearance, whilst BZN showed early and sustained efficacy until 12 months. Despite PCR limitations, results support increased diagnosis and access to BZN standard regimen, and provide a development roadmap for novel BZN regimens in monotherapy and in combinations with E1224. (Clinical Trials Identifier NCT01489228).
Funding
Various donors through DNDi (see acknowledgments)
Introduction
Chagas disease (CD) is a major endemic vector-borne disease and global public health problem, with an estimated 70 million people at risk in Latin America and approximately six million people infected worldwide. 1 Significant mortality and morbidity is observed in 20-30% of those chronically affected, with development of target organ involvement 10 to 30 years after initial infection. 2
Clinical development in CD is fraught with difficulties relating to the evaluation of therapeutic response and long delays in demonstrating clinical effects. 3 The pharmacokinetic/pharmacodynamics (PK/PD) relationships of treatments for CD are not fully understood, 4 and to date, no markers can adequately predict patients at risk of progression to chronic disease. During the chronic phase, diagnosis depends on serology, or polymerase chain reactions (PCRs), with varying degrees of sensitivity. 2,3,5 Despite the disease being described over a century ago, 6 only two drugs are available for CD treatment, benznidazole (BZN) and nifurtimox (NFX), and there is very limited data from randomized and controlled studies on their use in adults with chronic indeterminate disease. These patients are believed to require prolonged treatment that is often associated with safety concerns. 7,8 There is an urgent need to develop alternative treatments in order to improve disease morbidity. The generation of data that would help to fill existing scientific gaps and inform future drug development is paramount.
Ravuconazole (RAV) is an ergosterol biosynthesis inhibitor with potent in vitro and in vivo activities against CD in animal models. 9--11 Encouraging data raised hopes that E1224, a water-soluble RAV pro-drug, could be a priority candidate for clinical development in CD, and the first new chemical entity developed in over three decades. Overall RAV systemic exposures are substantially higher in humans with E1224 pro-drug administration leading to increased bioavailability and longer plasma terminal half-life. With E1224 loading dose strategy, steady-state is achieved in ≤1 week and allow for once-weekly dosing for maintenance of target plasma concentrations.
We present the results of a proof-of-concept randomized phase II study evaluating three oral E1224 dosing regimens and BZN versus placebo for the treatment of adult chronic indeterminate CD.
Materials and Methods
Study design
The study was conducted in two outpatient units in Bolivia (Cochabamba and Tarija). The trial was a placebo and active-controlled (BZN), randomized, prospective, assessor blind, comparative, dose-finding, and proof-of-concept study of superiority, testing five parallel groups. Double-blinding was limited to the E1224 and placebo arms. Patients were enrolled randomly and equally into each of the five oral treatment arms:
High Dose – eight weeks (HD): E1224 loading dose (400 mg qd D1–3) then 400 mg qw (starting D8) for seven weeks (total dose: 4000 mg).
Low Dose – eight weeks (LD): E1224 loading dose and placebo (200 mg qd D1–3) then 200 mg and placebo qw (starting D8) for seven weeks (total dose: 2000 mg).
Short Dose – four weeks (SD): E1224 loading dose (400 mg qd D1–3) then 400 mg qw (starting D8) three weeks, followed by placebo for seven weeks (total dose: 2400 mg).
Placebo group – eight weeks: four E1224-matched placebo tablets qd Day 1–3 then four placebo tablets qw (starting D8) for seven weeks.
BZN group (Laboratório do Estado de Pernambuco – LAFEPE, tablet 100 mg), 5 mg/kg/day divided in two daily doses, for 60 days.
Patients who did not tolerate treatment were withdrawn and received standard NFX treatment. After unblinding at the end of the study, patients in the placebo arm and those on E1224 with no parasitological clearance were offered BZN treatment, whilst patients allocated to BZN who showed no parasitological clearance were offered NFX treatment.
This study was implemented in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki after the approval by the ethical committees of the participating institutions (Universidad Mayor San Simón, CEADES, Hospital Clínic Barcelona).
Study participants
Subjects eligible for randomisation were >18 to <50 years-old and weighed >40 kg. Women could not be pregnant or breastfeeding, and were required to use 4-month non-hormonal contraception. Inclusion criteria were confirmed diagnosis of T. cruzi infection by two conventional serological tests and serial positive qualitative PCR (at least one of three samples collected over seven days). Included subjects had a normal screening ECG, and no contraindication to study drugs or any azole. They had not received prior BZN or NFX treatment, or systemic treatment with azoles, allopurinol, or any concomitant antimicrobial and immunosuppressant agents. Subjects who had signs and/or symptoms of chronic CD, acute or chronic health conditions, abnormal screening laboratory tests, or history of alcohol abuse or other drug addiction were excluded.
All patients were required to provide written informed consent.
Randomisation and masking
A computer generated randomisation list was prepared by an external provider. The list was stratified by center and used a block size of 10. Treatment packages for the three E1224 regimen and placebo arms were prepared and labeled with numbers corresponding to the randomization list. Placebo tablets were identical to E1224 tablets. Each center received a list of randomization numbers and the corresponding treatment packages. After confirmation that the patient met all entry criteria, the next available randomization number in the corresponding center (in chronological order) was assigned by the study pharmacist, who then delivered the corresponding package for the blinded arms of the study (E1224 or placebo) or prepared the package with an adequate number of tablets for the BZN arm and identified it with the patient’s number. Study subjects, investigators, medical and nursing team remained blinded to treatment allocation. All parasitological, laboratory testing and statistical analyses was done blinded to treatment allocation.
Procedures
The PCR assay method was selected based on the results of the multi-center study for the standardization and laboratory validation of qualitative PCR testing for T. cruzi. 12 A multiplex TaqMan Real-Time quantitative PCR assay, aiming to quantify T. cruzi satellite DNA and an internal amplification control in a single-tube reaction, was further developed and validated. The method limit of detection (LoD) is 0·6979 parasite equivalents (pEq)/mL and limit of quantification (LoQ) is 1·531 pEq/mL. 13 Each PCR experiment included positive and negative controls. External quality control panels for PCR were evaluated during the study period, using blinded seronegative blood samples spiked with serial dilutions of cultured T. cruzi. PCR positivity was defined as a positive result in at least one of the replicates of the three different samples.
For the serological diagnosis, two enzyme linked immunosorbent assays (ELISA) were used, one based on recombinant antigen (Chagatest ELISA recombinante Wiener Lab) and another on a crude antigen (Chagatest ELISA lisado, Wiener). A highly sensitive and specific chemiluminescent ELISA (AT CL-ELISA) was also used, based on the reactivity of sera to mucin glycoproteins purified from infective T. cruzi trypomastigote forms (tGPI-mucins, or F2/3 or AT antigens). 14-16
Blood concentrations of RAV and BZN were measured on the first day of treatment (Day 0, pre-dose), during steady-state (D3–59), at EOT and at the four-month follow-up visit. PK sample analysis was performed using liquid chromatography-electrospray ionization-tandem mass spectrometry. The calibration curve was linear in the concentration range of 50 – 30·000 ng/mL and 50 – 20·000 ng/mL for BZN and RAV, respectively (details methodology and the validation methods included in supplementary information). Population PK parameters included: AUC, Cmax, Cmin, CL, Vd, and t1/2. Age, body mass index, and baseline parasite load were evaluated as covariates.
Safety was evaluated through routine monitoring of adverse events (AEs). Patients were strictly monitored for liver and cardiac safety. If ALT or AST exceeded 3–8 x the upper limit of normal (ULN), treatment was continued if the patient was asymptomatic and did not show bilirubin elevation >2 x ULN. In case of any QTc abnormality, patients were re-dosed only following review of available ECGs. Echocardiography or Holter monitoring was performed on patients with cardiac AEs or clinically significant ECG changes. Patients were withdrawn from the study based on specified liver and cardiac safety criteria. Safety was monitored by an independent Data Safety Monitoring Board and by cardiac safety experts on an ongoing basis.
Outcomes
Following expert consultation, the primary efficacy endpoint was defined as parasitological response at end of treatment (EOT), determined by serial negative qualitative standardized PCR: three negative PCR results, from three samples of 10 ml collected over seven days at EOT. Serial examination was used for increased sensitivity. The secondary efficacy endpoints were sustainability of parasitological clearance (negative qualitative PCR results at D65, and at 4, 6, and 12 months of follow-up), parasite clearance and changes in parasite load (measured by qualitative PCR and quantitative PCR (qPCR) on days 8, 15, 36, EOT, and at 4, 6, and 12 months of follow-up), incidence of conversion to negative response in conventional and non-conventional (lytic anti-α-Gal antibodies measured by AT CL-ELISA) serological response (assessed at EOT, and at 4, 6, and 12 months posttreatment).
Safety endpoints were treatment emergent AE (TEAE), laboratory variables (mean change from baseline for each timepoint D2, D3, D15, D36, and D65) and EKG measurements (ventricular rate (VR), PR interval, RR interval, QRS interval, QT interval, QTcF and QTcB at each timepoint).
Statistical analyses
The study was powered to provide evidence of superior efficacy of each of the E1224 regimens and of BZN relative to placebo. Forty-six patients per treatment group were required for 90% power at a global 5% significance level (two-sided), if the proportion of patients with clear parasitaemia at EOT in the E1224 treatment groups or BZN is 0·60, and the proportion in the placebo group is 0·20, with an estimated drop-out rate of 15%.
The intention-to-treat (ITT) population and the Full analysis set (FAS) comprised all randomized patients by their assigned treatment arms (primary analysis set) and by their actual treatment arms respectively while the per-protocol (PP) population was composed of all ITT patients without any major protocol deviations. A safety population was defined as all patients randomized and having received at least one dose of study therapy. The primary efficacy analysis was performed first on the ITT population and secondarily on FAS and PP populations as sensitivity analyses for the primary and secondary endpoints. The SAF population was used to perform the safety analyses.
Several missing data replacement strategies were used. In case of missing PCR evaluations, the following imputation rule was followed. The outcome was imputed as success if and only if: 1) One unevaluable PCR result and two consecutive negative PCR results at D65; 2) Two unevaluable PCR results and one negative PCR result at D65; 3) Three unevaluable PCR results at D65 and three consecutive negative results at D36. Patients with missing EOT results for any other reason were to be considered as failures for the primary analysis (IMPUT1). A second, more conservative, method of imputation was used on the ITT and FAS populations to assess the sensitivity of the results to the imputation of missing data: all patients with even one missing PCR result at EOT were analysed as failures (IMPUT2).
For demographics and baseline characteristics, descriptive statistics on both the PP and ITT populations were presented. Patient disposition and study discontinuations and their frequencies were tabulated.
The analysis of the primary endpoint used a one-sided Fisher’s exact test of the proportion of ‘cured’ patients on the ITT (primary analysis), FAS and PP (secondary analysis) populations to perform pairwise comparisons between treatment arms and placebo. Hochberg procedure 17 was used to keep the global type I error at the 2.5% level and to address the multiplicity issue. Three sensitivity analyses were performed: (i) ITT with strategy IMPUT1, (ii) FAS and PP with strategy IMPUT2.
Secondary efficacy endpoints were analysed on both the ITT and PP as well as FAS (for most important endpoints) populations by treatment arm. Sustainability of parasitological clearance relative to placebo was analysed at 12 months with a Fisher exact test. Repeated measures model contrasts (dependent variable: parasite at all available timepoints / treatment arm as fixed effect) were used to evaluate the reduction in parasite load over time at EOT and at 4, 6 and 12 months of follow-up.
The time to parasite clearance and the time to first relapse for patients who had cleared parasitaemia at EOT were evaluated by using the Kaplan-Meier survival method with a Logrank test. All tests were performed pairwise and a Hochberg procedure for the 4 comparisons of E1224 and BZN arms to placebo was used. Modeling of the time to first relapse was carried out with a proportional hazard Cox model and a backward stepwise procedure including age, sex, serological markers and biomarkers. Serology markers data were transformed by using geometric mean ratios at each timepoint and a repeated measure linear model was used.
Safety analyses were conducted on the SAF population. Numbers and percentages of patients with at least one reported adverse event (AE) and number of events were evaluated and tabulated by treatment group for: all TEAEs; all TEAEs judged “related” to the treatment; TEAEs leading to drug discontinuation; all TE-SAEs; all TE-SAEs judged “related” to the treatment; and all SAEs; All Deaths. Confidence intervals of proportions (exact two sided 95%) were calculated for each system organ class and for preferred terms with a prevalence of more than 10% in any study arm.
Laboratory safety variables were analysed as mean changes from baseline with each timepoint up to D65 (refer to supplementary material). EKG outcomes (ventricular rate (VR), PR interval, RR interval, QRS interval, QT interval, QTcF and QTcB) were described and analysed as mean changes from the last pre-baseline ECG with each timepoint up to 4M (refer to supplementary material).
A population PK analysis was performed on time-log-concentration data by using a two-compartmental model. Due to sparse data, a model based simulation was used to estimate total AUC and AUC at steady state. A PK/PD analysis dataset was created based on qPCR measurements obtained from patients treated with E1224. Positive but not quantified PCR measurements were imputed by LoQ/2. Negative PCR measurements were imputed by LoD/2.
A semi-mechanistic PK/PD model was developed to adequately describe the observed time course of (quantitative and qualitative) PCR following treatment with E1224. Individual estimated PK parameters were based on a previous population-PK analysis. The model consisted of an indirect response component describing the parasite load based on exposure-dependent parasite proliferation rate and a constant parasite clearance, in combination with a mixture component categorizing patients as sustained responders or relapsers.
Statistical tests were one-sided for analyses on the primary endpoint and two-sided for all other analyses. A p-value below 0·025 (one-sided) or 0·05 (two-sided) was considered significant. All analyses were performed by using the SAS software V9.4 (SAS Institute, Cary, NC, USA).
An interim blinded efficacy analysis of sustained parasitological response on all patients at 6 months was performed to allow administrative decisions of the sponsor by an independent statistician. The principal Investigator and study personnel remained unequivocally blinded until database lock.
Role of funding source
DNDi is a not-for-profit product development organisation, which was the sponsor for this study. DNDi received funding for this study from different sources, which had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
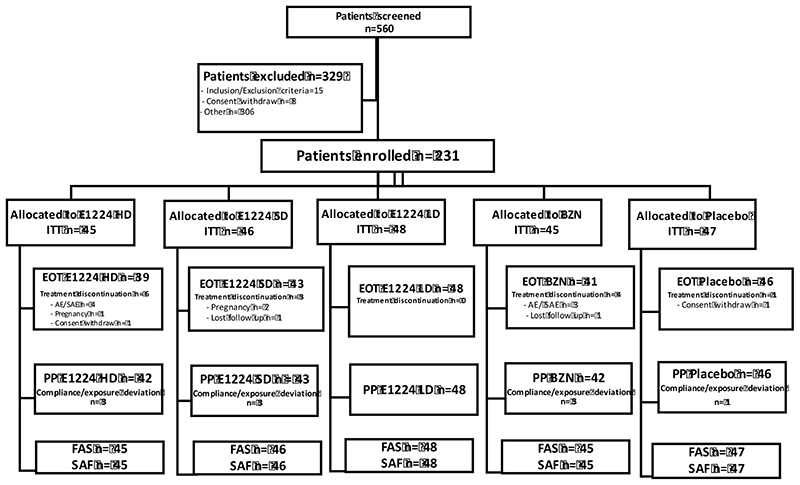
The study was initiated on July 19, 2011 and follow-up was completed on June 13, 2013. Overall, 560 subjects with confirmed CD were screened, among which 231 subjects were eligible for randomization. A total of 45 subjects were allocated to BZN and HD, 48 subjects to LD, 46 subjects in SD arm, and 47 subjects to placebo. During treatment, 14 patients (6%) discontinued treatment due to AEs (n=7), consent withdrawal (n=3), loss to follow-up (n=2), or unspecified reasons (n=2) (Figure 1). Ten significant protocol deviations were documented, resulting in a per-protocol analysis population very close to the intention-to-treat (ITT)/safety population (Figure 1). The only study significant protocol deviation was related to reduced compliance/ exposure, defined as less than 80% of the planned cumulative dose (affecting 1 patient in the placebo arm, 3 in the SD arm, 3 in the HD arm and 3 in the BZN arm).
Figure 1. Study Disposition.
ITT - intention-to-treat analysis set: all randomized patients by their assigned treatment arms (primary analysis set); PP - per-protocol analysis set: a subset of the ITT (and FAS) analysis set composed of all ITT patients without any major protocol deviations (compliance/ exposure deviation defined as less than 80% of the planned cumulative dose); FAS - full analysis as treated set: all randomized patients by their actual treatment arms (patients who did not receive treatment will not be included in this analysis set); SAF - safety (all treated) analysis set: all randomized, patients having received at least one dose of study therapy; EOT - end of treatment: all randomized patients by their actual treatment arms who did not discontinued treatment.
Treatment arms were well balanced, in terms of age, gender, ECG values, serology, hematology, liver function and baseline parasite load (Table 1). Mean age (standard deviation, SD) of enrolled patients was 30·2 (8·8) years, with a 4:1 female to male ratio.
Table 1. Baseline characteristics.
| Description | Placebo (n=47) |
LD (n=48) |
SD (n=46) |
HD (n=45) |
BZN (n=45) |
Total (n=231) |
|---|---|---|---|---|---|---|
| Mean age at screening, years (SD) | 31·0 (9·1) | 31·3 (8·7) | 27·7 (8·3) | 30·1 (8·8) | 30·7 (9·0) | 30·2 (8·8) |
| Gender, % male | 21·3 | 22·9 | 23·9 | 28·9 | 31·1 | 25·5 |
| Conventional ELISA, mean (SD) | 2·4046 (0·4214) | 2·2192 (0·5652) | 2·3248 (0·5569) | 2·2421 (0·5705) | 2·1981 (0·6339) | 2·2783 (0·5537) |
| ELISA, kit recombinant 1 (Wiener), mean (SD) | 2·9409 (0·1397) | 2·8659 (0·3362) | 2·9598 (0·1197) | 2·9049 (0·3000) | 2·8816 (0·3554) | 2·9105 (0·2691) |
| Total white blood cells, 10/mm 3 , mean (SD) | 5538·3 (1352·2) | 5235·4 (847·6) | 5619·6 (1286·1) | 5413·3 (1285·3) | 5571·1 (1139·7) | 5473·6 (1191·7) |
| Neutrophils, 10/mm 3 , mean (SD) | 3134·8 (1003·2) | 2849·2 (699·4) | 3127·4 (901·5) | 3038·8 (898·9) | 3244·8 (1120·9) | 3076·7 (934·3) |
| Lymphocytes, 10/mm 3 , mean (SD) | 1988·6 (634·4) | 2046·6 (487·0) | 2058·5 (660·0) | 2029·2 (597·9) | 1998·9 (464·6) | 2024·5 (569·7) |
| AST, U/L, mean (SD) | 20·5 (4·7) | 20·7 (3·3) | 21·7 (5·0) | 21·8 (4·5) | 21·7 (4·0) | 21·3 (4·3) |
| ALT, U/L, mean (SD) | 21·0 (7·7) | 21·7 (6·9) | 21·5 (7·1) | 24·0 (8·8) | 23·7 (7·9) | 22·4 (7·7) |
| GGT, U/L, mean (SD) | 0·680 (0·155) | 0·646 (0·181) | 0·676 (0·234) | 0·706 (0·198) | 0·689 (0·196) | 0·679 (0·193) |
| Ventricular rate, bpm, mean (SD) | 63·1 (6·9) | 64·1 (6·5) | 64·5 (8·7) | 62·4 (7·4) | 63·6 (9·0) | 63·6 (7·7) |
| QT interval, msec, mean (SD) | 411·9 (27·7) | 414·0 (21·6) | 408·6 (26·1) | 416·4 (25·4) | 415·5 (28·0) | 413·2 (25·8) |
| qPCR, pEq/ml, mean (SD) | 0·9539 (2·3714) | 1·1921 (1·8874) | 0·5853 (0·8722) | 0·7521 (1·1655) | 0·6170 (0·8315) | 0·8251 (1·5640) |
ALT, alanine transaminase; AST, aspartate transaminase; ELISA, enzyme-linked immunosorbent assay; GGT, gamma-glutamyltranspeptidase; qPCR, quantitative polymerase chain reaction; SD, standard deviation
The primary endpoint, parasite DNA clearance at EOT, was significantly different for all active treatment arms than placebo (p<0·001), with the highest clearance observed in the BZN arm (91%) (Table 2). Similar results were obtained in the FAS and PP populations. Sensitivity analyses also provided with similar results.
Table 2. PCR assessment at D65 (EOT) and until 12 months of follow up.
| Placebo (n=47) |
LD (n=48) |
SD (n=46) |
HD (n=45) |
BZN (N=45) |
|
|---|---|---|---|---|---|
| Parasite clearance at D65 (EOT) Yes, n (%) | 12 (25·5, [13·9;4·3]) | 43 (89·6, [77·3;96·5]) | 41 (89·1, [76·4;96·3]) | 34 (75·6, [60·4;87·1]) | 41 (91·1, [78·7;97·5]) |
| p-value for comparison against placebo | 3.10-10 | 4.10-10 | 1·43.10-6 | 5·63.10-11 | |
| Sustainability of parasitological until 12 months of follow up Yes, n (%) | 4 (8·5, [2·4;20·4]) | 4 (8·3, [2·3;20·0]) | 5 (10·9, [3·6;23·6]) | 13 (28·9, [16·4;44·3]) | 37 (82.2, [67·9;92·0]) |
| p-value for comparison against placebo | 0·6547 | 0·6547 | 0·0343 | 1-60·10-13 | |
| Time to first relapse (days) Kaplan Meier estimate of time to 50% reappearance |
84 (55·0;-----) |
111 (60·0;112·0) |
56 (56·0; 60·0) |
287 (111·0;-----) |
--- (305·0;-----) |
| Log-rank test p-value for comparison against placebo | 0·302 | 0·203 | 0·412 | <0·001 | |
| Hazard ratio from Cox Model adjusted on baseline qPCR (HR=1·10, 95%CI [1·03, 1·16]) |
1·17 (0·53;2·56) | 1·68 (0·77;3·67) | 0·60. (0·26;1·37) | 0·06 (0·02;0·21) |
The proportion of patients achieving sustainability of parasite DNA clearance at 12 months in the E1224 SD and LD arms respectively was 10·9% and 8·3%, similar to placebo values (8·5%). SR values for BZN were 82·2% and 28·9% in the E1224 HD arm. A significant difference was measured for the comparison of E1224 HD (p=0·0343) and BZN (p<0·0001) arms vs. placebo, corrected for multiplicity (Table 2).
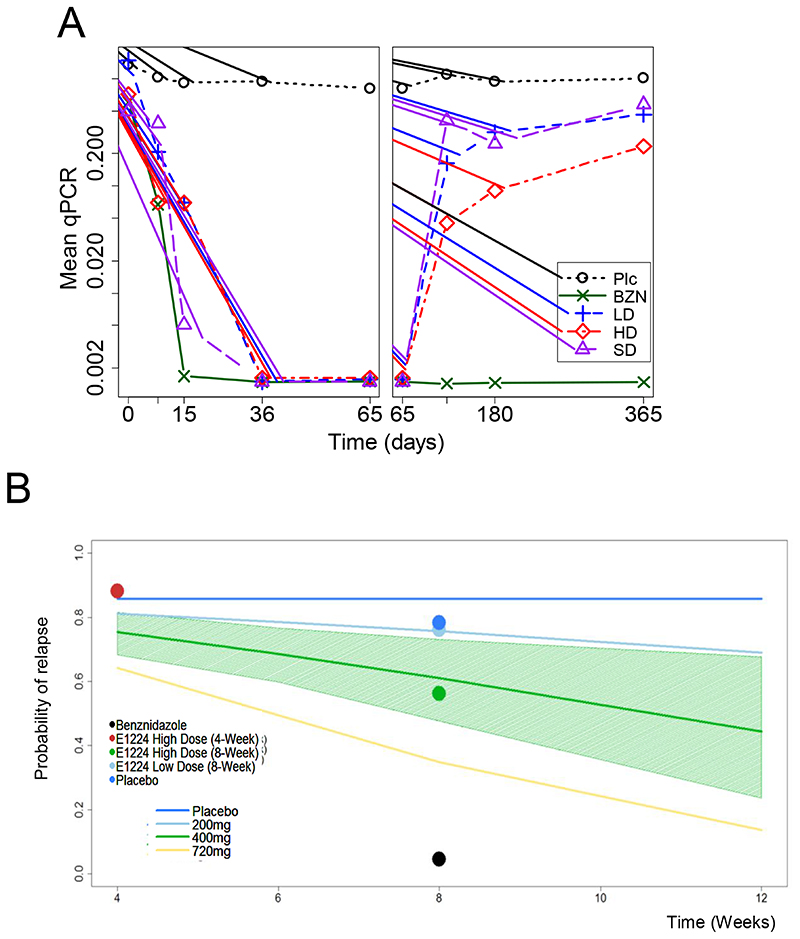
After one week of treatment, mean qPCR repeated measurements showed a significant reduction in parasite load in all treatment arms vs. placebo. All BZN-treated patients cleared parasite after two weeks of treatment. After EOT, the eight patients in the BZN arm that had at least one positive PCR until 12 months (no SR), presented low parasite load. Parasite levels in the LD and SD E1224 arms gradually returned to placebo levels. HD E1224 parasite load remained significantly lower than placebo, with no statistical difference from BZN (Figure 2A).
Figure 2.
(A) Sequential quantitative PCR measurements. The mean of qPCR-Based responses was derived during the treatment phase (left panel) and over time up to 12 months post-treatment (right panel). Plc=Placebo. (B) PKPD model of predicted probability of relapse. The continuous lines in the graph are model predictions of the probability of relapse using E1224 at different treatment regimens. For clarity reasons, the 95% CI (shaded area) for these predictions is only shown for the E1224 High Dose cohort. Circles represent the observed population of patients with relapse.
In a stepwise Cox model, a lower risk of relapse was observed with BZN (HR=0·06 (95%CI 0·02, 0·21)), compared to placebo. An increased hazard of parasitological relapse was independently associated with a significant higher baseline parasite load (HR=1·10, 95% CI: [1·03, 1·16]) (Table 3).
Table 3. Changes from baseline in parasite load until 12 months of follow up in parasite load, conventional serology and AT CL-ELISA.
| Placebo (n=47) |
LD (n=48) |
SD (n=46) |
HD (n=45) |
BZN (N=45) |
|
|---|---|---|---|---|---|
| Changes from baseline in parasite load (GM Ratio (95%CI)) | |||||
| D8 | 1·30(0·78;2·16) | 0·17(0·10;0·28) | 0·09(0·06;0·16) | 0·13(0·08;0·22) | 0·10(0·06;0·17) |
| D15 | 0·81(0·54;1·20) | 0·11(0·07;0·23) | 0·07(0·05;0·11) | 0·09(0·06;0·14) | 0·07(0·05;0·10) |
| D36 | 0·77(0·56;1·06) | 0·06(0·05;0·09) | 0·07(0·05;0·09) | 0·07(0·05;·009) | 0·07(0·05;0·09) |
| EOT (D65) | 0·70(0·49;0·96) | 0·06(0·05;0·09) | 0·07(0·05;0·09) | 0·07(0·05;0·09) | 0·07(0·05;0·09) |
| 4 months | 0·67(0·40;1·12) | 0·12(0·07;0·20) | 0·45(0·27;0·76) | 0·11(0·06;0·18) | 0·07(0·04;0·11) |
| 6 months | 1·08(0·61;1·92) | 0·31(0·18;0·55) | 0·25(0·14;0·46) | 0·19(0·11;0·36) | 0·07(0·04;0·12) |
| 12 months | 0·91(0·50;1·66) | 0·69(0·38;1·23) | 1·05(0·58;1·92) | 0·22(0·12;0·42) | 0·07(0·04;0·12) |
| p-value for comparison against placebo at 12 months* | 0·499 | 0·744 | 0·001 | <0·001 | |
| Changes from baseline in conventional ELISA (mean diff (95%CI)) | |||||
| D36 | -0·05 (-0·17;0·08) | -0·03 (-0·15;0·09) | 0·12 (-0·01;0·24) | -0·06 (-0·19;0·07) | -0·119 (-0·24;0·01) |
| EOT (D65) | -0·07 (-0·19;0·05) | -0·02 (-0·14;0·1041) | 0·08 (-0·05;0·20) | -0·086 (-0·21;0·04) | -0·08 (-0·20;0·05) |
| 4 months | -0·14 (-0·26;-0·02) | -0·031 (-0·15;0·09) | -0·03 (-0·15;0·09) | 0·018 (-0·11;0·15) | -0·05 (-0·18;0·08) |
| 6 months | -0·03 (-0·15;0·08) | -0·042 (-0·15;0·07) | 0·02 (-0·10;0·13) | -0·037 (-0·16;0·08) | -0·09 (-0·21;0·02) |
| 12 months | -0·15 (-0·28;-0·03) | -0·192 (-0·31;-0·07) | -0·05 (-0·18;0·08) | -0·044 (-0·18;0·09) | -0·14 (-0·27;-0·01) |
| p-value for comparison against placebo at 12 months* | 0·670 | 0·254 | 0·233 | 0·868 | |
| Changes from baseline in AT CL-ELISA (mean diff (95%CI)) | |||||
| D36 | 0·04 (-0·02;0·11) | -0·00 (-0·06;0·06) | 0·02 (-0·04;0·09) | -0·01 (-0·07;0·05) | 0·05 (-0·01;0·11) |
| EOT (D65) | 0·01 (-0·06;0·08) | -0·02 (-0·09;0·05) | -0·01 (-0·08;0·06) | -0·04 (-0·11;0·03) | 0·02 (-0·05;0·09) |
| 4 months | -0·01 (-0·07;0·05) | 0·03 (-0·02;0·09) | 0·02 (-0·05;0·08) | -0·03 (-0·09;0·03) | -0·01 (-0·07;0·05) |
| 6 months | -0·02 (-0·09;0·05) | 0·02 (-0·04;0·09) | -0·02 (-0·09;0·05) | -0·05 (-0·13;0·02) | 0·02 (-0·05;0·09) |
| 12 months | -0·02 (-0·08;0·05) | -0·02 (-0·09;0·04) | 0·03 (-0·03;0·09) | 0·04 (-0·02;0·11) | -0·01 (-0·07;0·05) |
| p-value for comparison against placebo at 12 months* | 0·835 | 0·321 | 0·192 | 0·894 | |
| Estimate of geo. mean (95% CI) | 3·01 (2·60, 3·47) | 2·85 (2·47, 3·27) | 2·85 (2·46, 3·29) | 2·55 (2·19, 2·96) | 2·44 (2·11, 2·83) |
| Estimate of geo. mean ratio: treament/placebo (95% CI) | 0·95 (0·77, 1·16) | 0·95 (0·77, 1·16) | 0·85 (0·69, 1·04) | 0·81 (0·66, 0·1) | |
| p-value for ratio to placebo* | 0·593 | 0·596 | 0·1210 | 0·049 | |
EOT, end of treatment.
Estimates from repeated measures linear model with treatment, baseline, sample, treatment by sample and baseline by sample interactions
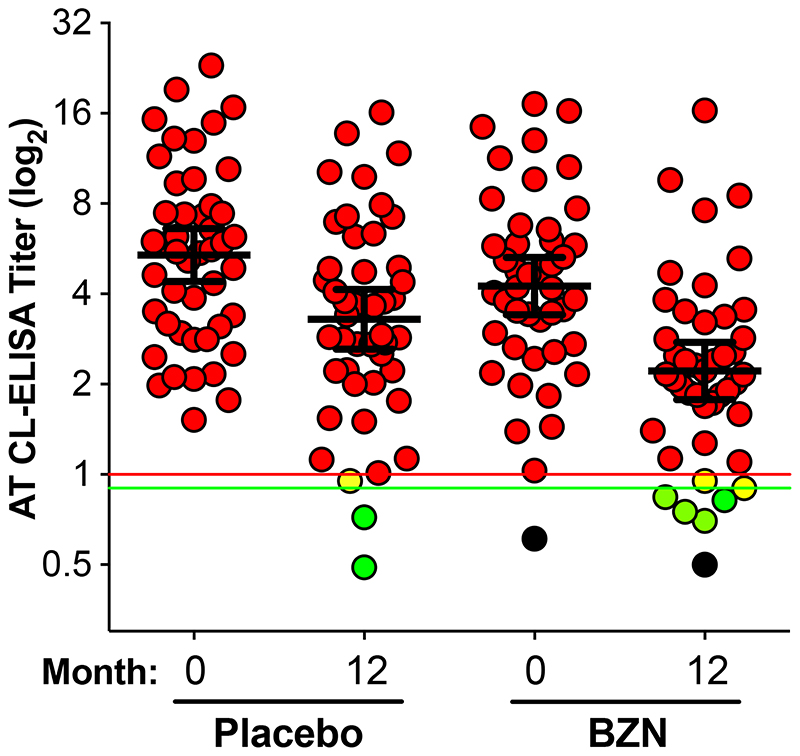
Among adult patients after 12 months of follow-up, the analyses of conventional serology showed no statistically significant differences between active treatment and placebo at any timepoint. However, there was a small, statistically significant reduction in the titers of trypanolytic anti-α-Gal antibodies, as measured by AT CL-ELISA, among BZN-treated patients versus placebo-treated patients at 12 months (geometric mean ratio: treatment/placebo 0·813 (0·662, 0·999), p=0·049); Figure 3). Five patients in the BZN-treated group had a negative AT CL-ELISA result, in contrast to two patients in the placebo-treated group, at the end of the study follow-up. Nine percent (4/44) of treated patients negatively seroconverted for the AT CL-ELISA at the end of the follow-up. Two patients in BZN-treated group and one in the placebo-treated group showed inconclusive results (titer <1.0, ≥0.9).
Figure 3. AT CL-ELISA titers at 0, and 12 months of follow-up in placebo- and BZN-treated patients.
Positive results (red circles): titer ≥1·0; negative results (green circles): titer <0·9; inconclusive results (yellow circles): titer <1·0, ≥0·9. Placebo group: 43 positive (93·5%), 2 negative (4·3%), and 1 inconclusive (2·2%); BZN group: 37 (84.1%), 5 negative (11.4%), and 2 inconclusive (4.5%). Red horizontal line, titer=1·0; green horizontal line, titer=0·9. Black circles: serum samples from the same patient with negative AT CL-ELISA results at months 0 and 12. Only patients with serum samples at 0 and 12 months were considered for this analysis. The geometric mean (long black horizontal line) with 95% C.I. (short black horizontal lines) are indicated for each group. AT CL-ELISA titers were calculated as described. [16]
No deaths occurred during the trial. Overall, 81·0% of subjects developed treatment-emergent AEs (TEAEs) (Table 4). The BZN treatment arm had the highest proportion of TEAEs considered related to treatment (64·4%), compared with 52·2%, 44·4%, and 31·3% in E1224 SD, HD, and LD arms, respectively. Nine subjects experienced 14 TEAEs resulting in treatment suspension or discontinuation, five (11·1%) in the E1224 HD arm and four (8·9%) in the BZN arm (Table 3). Among these, six were ≥grade 3, and included ALT, AST, and GGT increases, and infective cholecystitis.
Table 4. Summary of Adverse Events (AEs).
| Placebo (N=47) |
LD (N=48) |
SD (N=46) |
HD (N=45) |
BZN (N=45) |
|
|---|---|---|---|---|---|
| Any Treatment Emergent Adverse Events (TEAEs) | 38 (8·9%) [95] | 37 (77·1%) [116] | 40 (87..%) [116] | 33 (73·3%) [131] | 39 (86·7%) [165] |
| Any TEAEs judged ‘related’ to treatment | 14 (29·8%) [17] | 15 (31·3%) [28] | 24 (52·2%) [39] | 20 (44·4%) [48] | 29 (64·4%) [89] |
| Any TEAEs resulting in treatment discontinuation | 0 (0·0%) [0] | 0 (0·0%) [0] | 0 (0·0%) [0] | 5 (11·1%) [8] a | 4 (8·9%) [6] b |
| Any Treatment Emergent Serious Adverse Events (SAEs) | 0 (0·0%) [0] | 0 (0·0%) [0] | 1 (2·2%) [1] | 3 (6·7%) [3] | 2 (4·4%) [2] |
| Any Treatment Emergent SAEs judged ‘related’ to treatment | 0 (0·0%) [0] | 0 (0·0%) [0] | 0 (0·0%) [0] | 2 (4·4%) [2] | 0 (0·0%) [0] |
| Any Deaths | 0 (0·0%) [0] | 0 (0·0%) [0] | 0 (0·0%) [0] | 0 (0·0%) [0] | 0 (0·0%) [0] |
Presented: number of patients (percent of patients) [number of events]
TEAEs leading to drug discontinuation: one with infective cholecystitis that showed also increased ALT, AST and GGT). Two patients presented increased ALT and two patients presented increased AST.
TEAEs leading to drug discontinuation: four with hypersensitivity, and one among them with ALT increased
Six patients experienced Serious Adverse Events (SAEs), two of which occurred within 75 days from the start of treatment (n=1 in HD arm, n=1 in BZN arm) (Table 4). Three SAEs occurred in the HD E1224 arm (infective cholecystitis, two spontaneous abortions), two in the BZN arm (bronchitis, blighted ovum), and one in the SD E1224 arm (appendicitis). The SAEs of spontaneous abortion were considered possibly related to study treatment. All other SAEs recovered completely.
Among the most common TEAEs (>10%), there was a higher frequency of headaches, nausea, pruritus, peripheral neuropathy and hypersensitivity with BZN compared with E1224. Patients randomized to HD E1224 had more frequent treatment-related hepatic toxicity and diarrhea. No hepatic safety signal was observed with E1224 at lower doses. ECG outcomes appeared comparable across treatment groups, with no clinically significant increases in QTcF during treatment.
Results showed that the E1224 loading schedule is appropriate to quickly reach steady state and stable trough RAV concentrations. In the range of E1224 doses studied, peak and trough RAV concentrations were proportional to dose. No evidence of accumulation was observed. BZN PK results were compatible with previously reported PK studies. 18
Exploratory evaluations indicated an exposure-dependent effect of E1224 on the dynamics of parasite load (Figure 2A). The PK/PD model confirmed that the predicted probability of relapse decreases with E1224 treatment duration and dose. Model-based simulations indicated that an increase in E1224 HD treatment duration would significantly reduce the probability of relapse from 59% (95% CI, 21%-78%) with eight weeks to 44% (95% CI, 4%-84%) with 12 weeks. Similarly, model-based calculations with the maximum observed average concentrations show that if E1224 dose was increased and treatment duration prolonged to e.g. 12 weeks, the probability of relapse would fall below 20% (Figure 2B).
Discussion
As the first phase II clinical trial performed in Bolivia, this current proof-of-concept study generated key human PK/PD data for BZN, together with the first available data for E1224.
Following a long wait for novel CD drugs, we provide initial evidence that E1224 has manageable toxicity and shows anti-trypanosomal activity during treatment. Disappointingly, but consistent with recently published data on posaconazole, 19,20 SR after 12 months of follow-up was partial/incomplete with eight weeks of treatment. Given this lack of a prolonged effect, E1224 will not be further investigated as monotherapy. However, because of its favorable safety profile, combinations of E1224 with existing drugs, such as BZN or NFX, should be considered, especially given that animal model studies show combination therapy has the potential to improve treatment response and shorten treatment duration. 21,22 Two spontaneous abortions were documented in the study, despite contraceptive measures. Such findings represent a known class effect of azole treatments and indicate the continued need for highly effective contraceptive methods during future clinical trials.
In contrast, BZN was found to have a rapid and sustained effect on parasite DNA clearance with parasite counts having dropped significantly after one week of treatment and with 81.0% of the patients with SR until 12 months. This study provides the first placebo-controlled data on parasite DNA clearance and trypanolytic anti-α-Gal antibodies (AT CL-ELISA) in adult chronic indeterminate CD, with unique evidence on the dynamics of parasite DNA clearance during treatment and its correlation with exposure. Post-treatment follow-up in this study was limited to 12 months. There has been reports of late parasite relapses post-treatment with BZN in patients with advanced Chagas cardiomyopathy, however, data is limited in chronic indeterminate CD. 23 The pattern of treatment-related AEs were similar to those observed in other studies. 8 These results, together with those seen for BZN in recently published studies, 19,20 support increased use of the current treatment, and the evaluation of alternative regimens of BZN, in particular short-course and combination treatments.
Recent expert opinion highlighted that etiological treatment should be offered in adult chronic CD patients. 24, 25 These results should be placed in context of recent placebo-controlled trial data showing no clinical effect of treatment with BZN in patients with different stages of Chagas cardiomyopathy 23 . This study was not sufficiently powered to show smaller impact (< 20%) in cardiac outcome, but it is clear that our current strategies for antiparasitic chemotherapy need to be revisited for patients with chronic Chagas cardiac involvement. Similarly, there is a need to consider early treatment intervention before established cardiac damage, as in the present trial population consisting largely of young adults.
At the same time, double-blinded, placebo-controlled pediatric studies in early chronic T. cruzi infection showed that a 60-day BZN course was safe and effective in producing reduction of titers and negative seroconversion of specific antibodies (including trypanolytic anti-α-Gal antibodies), which had a key impact on treatment policy, based on serology rather than clinical benefit, and justifying recommendation of treatment in seropositive children. 23,26 However, to-date, in the short term, no significant changes in conventional serology have been documented in chronic infected adults. Quite importantly, the present study in asymptomatic patients with indeterminate CD provides placebo-controlled data showing an effect of treatment with BZN on trypanolytic anti-α-Gal antibodies, as measured by a significant decrease in AT CL-ELISA titers at 12 months post-treatment. Nine percent (4/44) of treated patients negatively seroconverted for the AT CL-ELISA at the end of the follow-up, as compared to 4% (2/46) of the placebo-treated group. Higher AT CL-ELISA negative seroconversion (58% and 85%, by per protocol analysis, three and six years post-treatment, respectively) was observed in T. cruzi-infected children treated with BZN. 17,27 Since the follow-up in this current study was only for 12 months, we hypothesize that a higher number of negative seroconversions could have been detected with a longer follow-up period. 27
Finally, due to the lack of early biomarkers of therapeutic efficacy, 28 the need for a long follow-up using conventional serology, and the inability to use the clinical symptoms for this purpose, PCR has emerged as useful tool. 12 This placebo-controlled study found PCR sensitivity to be sufficient as an indicator of therapeutic response, provided that the technique is standardized and multiple samples and serial examination are used for increased sensitivity. Other studies have also shown that PCR is a useful tool for drug development, for revealing therapeutic failure on a short-term basis, and for the follow-up of patients after specific treatment. 5,13,29,30 However, a negative PCR does not exclude the presence of parasite in tissues or circulating in levels below those of detection and there is a need for long term followup data correlating PCR with clinical benefit.
In conclusion, this adequate and well-controlled study provides information on the first new chemical entity to be developed for CD in over three decades. E1224 displayed a transient, suppressive effect, whilst BZN showed early and sustained efficacy by PCR and AT CL-ELISA. These results provide support to scaling up of diagnosis and access to standard regimens of BZN, and provide a roadmap for the development and registration of novel, alternative treatment regimens of BZN in monotherapy and in combinations with E1224 for the treatment of adults with chronic indeterminate CD.
Research in Context.
Evidence before this study
The only two medicines available for the treatment of Chagas disease (CD) - benznidazole (BZN) and nifurtimox - are known to cause some toxicity with limited data on therapeutic response, especially when used in adult chronic CD patients.
Novel antifungal triazole derivatives including ravuconazole pro-drug E1224, have arisen as alternative treatments for CD. Ravuconazole inhibits T. cruzi ergosterol biosynthesis, which is essential for parasite growth and survival, and has pharmacokinetic properties suitable for treatment of disseminated intracellular infection.
We searched MEDLINE and the Cochrane CENTRAL register, for articles published up to December 2015, for randomised controlled trials of trypanocidal drug treatments of CD. This review was cross-referenced to published meta-analyses of randomised controlled trials and non-randomised studies of etiological treatment of CD. Five randomised controlled trials with BZN were identified, three of them placebo-controlled: two placebo-controlled trials in children with acute and early chronic CD, no trials in adult chronic indeterminate CD, and one trial placebo-controlled trial in adult chronic symptomatic CD. In the two placebo-controlled pediatric trials, BZN was superior to placebo in producing negative seroconversion of specific antibodies in children. The only published placebo-controlled trial in adults with advanced chronic cardiac CD concluded that BZN treatment did not impact the clinical progression of Chagas cardiomyopathy, despite significant reduction of circulating parasite load. Serological testing was not available in that study. To-date there has been no previous placebo-controlled randomised trials of BZN in adult patients with chronic indeterminate CD, thus with no target organ involvement.
Added value of this study
E1224 is the first new chemical entity to be evaluated for CD in decades in a doubleblind, placebo-controlled trial with BZN as a comparator. In addition, this study is to the best of our knowledge, the first randomized controlled trial to evaluate a BZN regimen in rigorously assessed adult patients with chronic CD with no evidence of cardiac involvement, using (triplicate) PCR as the primary outcome. Other biological markers of therapeutic response were also assessed, including conventional ELISA, trypanolytic anti-α-Gal antibodies (AT CL-ELISA), and conventional serological methods (CSM).
The size, placebo-controlled design, and primary endpoints enabled demonstration at high significance (p<0·0001) that BZN is highly efficacious, with substantial early and sustained trypanocidal effect, corroborated by significant impact on negative seroconversion for trypanolytic anti-α-Gal antibodies. E1224 displayed a transient, suppressive effect on parasite DNA clearance.
Despite some known limitations of PCR in Chagas disease, the study found sensitivity to be sufficient as an early indicator of therapeutic response, provided that the technique is standardized and multiple samples and serial examination are used for increased sensitivity. In addition, the study corroborates the safety and pharmacokinetic-pharmacodynamic results of BZN and provides the first results of E1224 in humans with CD.
Implications of all the available evidence
This study offers support to scaling up of diagnosis and access to standard regimens of BZN, and provides a roadmap for the development and registration of novel, alternative treatment regimens of BZN in monotherapy and in combinations with E1224 for the treatment of adults with chronic indeterminate CD.
Acknowledgements
We wish to express our sincere and profound thanks to the patients who took part in this study and to the nurses and laboratory staff who contributed to its successful and safe execution.
We thank our Data Safety Monitoring Board who reviewed the study data and provided very valuable and timely responses and advice: Drs. Sergio Sosa Estani (Chairperson), Anis Rassi Jr., Dominique Larrey, Jean-Louis Paillasseur and Pascal Voiriot.
We extend our thanks to Dr Vanessa Gray-Schopfer, OmniScience SA, who provided medical writing services on behalf of DNDi, and to Dr Susan Wells (DNDi) for editing the manuscript. The authors are fully responsible for the contents and editorial decisions for this manuscript.
This clinical trial was funded by through DNDi by the following donors: Strategic Translation Award from the Wellcome Trust (grant number 095422); Médecins Sans Frontières (Doctors without Borders), International; Ministry of Foreign Affairs, Spain; Department for International Development (DFID), UK; Dutch Ministry of Foreign Affairs (DGIS), The Netherlands; Rockefeller Foundation, USA; and anonymous donors. The Platform for a Comprehensive Care of Patients with Chagas disease in Bolivia is a collaborative project between CEADES of Health and Environment; Universidad Mayor de San Simon in Cochabamba, Bolivia; Juan Misael University Saracho, Tarija, Bolivia; and ISGlobal (Barcelona Institute for Global Health, Spain), and supported by the National Chagas Control Program in Bolivia. The Platform is funded by the Spanish Agency for Cooperation and Development (AECID) (grant number 10-CO1-039). ISGlobal Research group receives funds from the Agència de Gestiód’ Ajuts Universitarisi de Recerca (AGAUR) [grant number 2014SGR026], DNDi, CEADES and ISGlobal are members of the Ibero-American NHEPACHA network (New tools for patients with Chagas disease). ICA is supported by NIH/NIMHD grant 2G12MD007592.
Contributor Information
E1224 Study Group:
Glaucia Santina, Bethania Blum, Erika Correia, Luis Izquierdo, Silvia Sanz, Joan C. Reverter, Manuel Morales, Wladimiro Jimenez, Roxana Challapa, Yurly Escobar, Daniel Lozano Helmut Magne, Albert Mendoza, Nilce Mendoza, Jimena Ramos, Gimena Rojas, Lizeth Rojas, Jimy Pinto, Dunia Torrico, Alejandro Palacios, Letty Cardozo, Gabriela Cuellar, Violeta Fernández, Isabel Gonzales, Alejandro Palacios, Rudy Vasco, Lineth Garcia, Rudy Parrado, Anabelle de la Barra, Nair Montaño, Sandro Villarroel, Carlos Hoyos, Tomás Duffy, Margarita Bisio, Juan Carlos Ramirez, Fred Duncanson, Michael Everson, Antonia Daniels, Makoto Asada, Facundo Garcia-Bournisen, Richard Tarleton, Michel Vaillant, Eugene Cox, David Wesche, Alexandre F. Marques, and Alexandre F. Marques
References
- 1.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90:33–44. [PubMed] [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Diez M, Favaloro L, Bertolotti A, et al. Usefulness of PCR strategies for early diagnosis of Chagas’ disease reactivation and treatment follow-up in heart transplantation. Am J Transplant. 2007;7:1633–40. doi: 10.1111/j.1600-6143.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- 4.Pinazo M-J, Guerrero L, Posada E, Rodríguez E, Soy D, Gascon J. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease. Antimicrob Agents Chemother. 2013;57:390–5. doi: 10.1128/AAC.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LM. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88:894–900. doi: 10.1007/s00436-002-0679-3. [DOI] [PubMed] [Google Scholar]
- 6.Coura JR. The discovery of Chagas disease (1908-1909): great successes and certain misunderstandings and challenges. Rev Soc Bras Med Trop. 2013;46:389–90. doi: 10.1590/0037-8682-0143-2013. [DOI] [PubMed] [Google Scholar]
- 7.Jackson Y, Alirol E, Getaz L, Wolff H, Combescure C, Chappuis F. Tolerance and Safety of Nifurtimox in Patients with Chronic Chagas Disease. Clin Infect Dis. 2010;51 doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- 8.Pinazo M-J, Munoz J, Posado E, et al. Tolerance of Benznidazole in Treatment of Chagas ‘ Disease in Adults. Antimicrob Agents Chemother. 2010;54:4896–9. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner FS. Sterol 14-demethylase inhibitors for Trypanosoma cruzi infections. Adv Exp Med Biol. 2008;625:61–80. doi: 10.1007/978-0-387-77570-8_6. [DOI] [PubMed] [Google Scholar]
- 10.Diniz LDF, Caldas IS, Guedes PMDM, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54:2979–86. doi: 10.1128/AAC.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbina Ja. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Schijman AG, Bisio M, Orellana L, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy T, Cura CI, Ramirez JC, et al. Analytical performance of a multiplex Real-Time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis. 2013;7:e2000. doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida IC, Covas DT, Soussumi LM, Travassos LR. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion. 1997;37:850–857. doi: 10.1046/j.1537-2995.1997.37897424410.x. [DOI] [PubMed] [Google Scholar]
- 15.De Andrade AL, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–13. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 16.Izquierdo L, Marques AF, Gállego M, et al. Evaluation of a chemiluminescent enzyme-linked immunosorbent assay for the diagnosis of Trypanosoma cruzi infection in a nonendemic setting. Mem Inst Oswaldo Cruz. 2013;108:928–31. doi: 10.1590/0074-0276130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochberg Y, Tamhane A. Multiple comparison procedures. New York: John Wiley and Sons; 1987. [Google Scholar]
- 18.Raaflaub J. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung. 1980;30:2192–4. [PubMed] [Google Scholar]
- 19.Molina I, Gómez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899–908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 20.Morillo CA, Waskin H, Sosa-Estani S, et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. Cruzi Carriers: The STOP-CHAGAS Trial. J Am Coll Cardiol. 2017 Feb 28;69(8):939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Batista DDGJ, Batista MM, de Oliveira GM, et al. Combined Treatment of Heterocyclic Analogues and Benznidazole upon Trypanosoma cruzi In Vivo. PLoS One. 2011;6:e22155. doi: 10.1371/journal.pone.0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diniz LF, Urbina JA, de Andrade IM, et al. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis. 2013 Aug 15;7(8):e2367.26. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morillo CA, Marin-Neto JA, Avezum A, et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med. 2015 Sep 1; doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 24.Viotti R, Alarcón de Noya B, Araujo-Jorge T, et al. Towards a paradigm shift in the treatment of chronic chagas disease. Antimicrob Agents Chemother. 2014;58:635–9. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias JC, Ramos AN, Jr, Gontijo ED, et al. 2nd Brazilian Consensus on Chagas Disease, 2015. Rev Soc Bras Med Trop. 2016 Dec;49(Suppl 1(Suppl 1)):3–60. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 26.Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am J Trop Med Hyg. 1998;59:526–9. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- 27.Andrade AL, Martelli CM, Oliveira RM, et al. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am J Trop Med Hyg. 2004;71(5):594–7. [PubMed] [Google Scholar]
- 28.Pinazo M-J, Thomas MC, Bua J, et al. Biological markers for evaluating therapeutic efficacy in Chagas disease, a systematic review. Expert Rev Anti Infect Ther. 2014;12:479–96. doi: 10.1586/14787210.2014.899150. [DOI] [PubMed] [Google Scholar]
- 29.Galvão LMC, Chiari E, Macedo AM, Luquetti AO, Silva SA, Andrade ALS. PCR Assay for Monitoring Trypanosoma cruzi Parasitemia in Childhood after Specific Chemotherapy PCR Assay for Monitoring Trypanosoma cruzi Parasitemia in Childhood after Specific Chemotherapy. J Clin Microbiol. 2003;41:5066. doi: 10.1128/JCM.41.11.5066-5070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murcia L, Carrilero B, Munoz MJ, Iborra MA, Segovia M. Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas’ disease: a prospective study in a non-disease-endemic country. J Antimicrob Chemother. 2010;65:1759–64. doi: 10.1093/jac/dkq201. [DOI] [PubMed] [Google Scholar]