Abstract
Objective
Haptoglobin is a haemoglobin-scavenging protein that binds and neutralises free haemoglobin and modulates inflammation and endothelial progenitor cell function. A HP gene copy number variation (CNV) generates HP1 and HP2 allele, the single nucleotide polymorphism rs2000999 influences their levels. HP1 allele is hypothesized to improve outcome after intracerebral haemorrhage (ICH). We investigated the associations of the HP CNV genotype and rs2000999 with haematoma volume, perihaematomal oedema (PHO) volume, and functional outcome as well as mortality after ICH.
Methods
We included patients with neuroimaging-proven ICH, available DNA, and six-month follow-up in an observational cohort study (CROMIS-2). We classified patients into three groups according to the HP CNV: 1-1, 2-1 or 2-2 and also dichotomized HP into HP1-containing genotypes (HP1-1 and HP2-1) and HP2-2 to evaluate the HP1 allele. We measured ICH and PHO volume on CT; PHO was measured by oedema extension distance. Functional outcome was assessed by modified Rankin score (unfavourable outcome defined as mRS 3-6).
Results
We included 731 patients (mean age 73.4, 43.5% female). Distribution of HP CNV genotype was: HP1-1 n=132 (18.1%); HP2-1 n=342 (46.8%); and HP2-2 n=257 (35.2%). In the multivariable model mortality comparisons between HP groups, HP2-2 as reference, were as follows: OR HP1-1 0.73, 95%CI 0.34-1.56 (p-value=0.41) and OR HP2-1 0.5, 95%CI 0.28-0.89 (p-value=0.02) (overall p-value=0.06). We found no evidence of association of HP CNV or rs200999 with functional outcome, ICH volume or PHO volume.
Conclusion
The HP2-1 genotype might be associated with lower 6-month mortality after ICH; this finding merits further study.
Keywords: Intracerebral haemorrhage, Haptoglobin, intracerebral haemorrhage volume, oedema extension distance, perihaematomal oedema volume, functional outcome, death, ALSPAC
Introduction
Spontaneous (non-traumatic) intracerebral haemorrhage (ICH) is the most devastating form of stroke with a mortality of about 40% at one month, and 65% at one year 1–3 . Patients who survive frequently remain severely disabled 4 . Moreover, incidence of ICH is increasing in the elderly population 5–7 , in part due to increasing use of oral anti-coagulation 5–7 .
Spontaneous ICH results from bleeding into the brain parenchyma arising from the rupture of an arterial vessel, most often (>80%) a small arteriole affected by cerebral small vessel diseases (SVD). The commonest sporadic SVD that cause ICH are deep perforator arteriopathy (also termed hypertensive arteriopathy or arteriolosclerosis) and cerebral amyloid angiopathy (CAA). A minority of ICH (less than 20%) is caused by structural or macrovascular bleeding sources such as tumours, arteriovenous malformations, cavernomas or fistulas. Deep perforator arteriopathy is associated with hypertension and is a frequent cause of deep ICH; CAA is caused by amyloid beta deposition in cortical and leptomeningeal blood vessels and is a key cause of lobar ICH.
Haptoglobin is an acute-phase protein which neutralizes free haemoglobin by binding it, and in doing so targets haemoglobin to the CD163 receptor for clearance 8–15 . Haptoglobin prevents the toxic and inflammatory effects of haemoglobin by shielding its iron-containing pocket, and preventing its breakdown into haem and iron, which consequently cause cytotoxicity and brain oedema 8–15 . The HP gene has a copy number variant (CNV), which leads to two co-dominant alleles: HP1 and HP2. Three different HP CNV genotypes exist: HP1-1, HP2-1 and HP2-2, and their respective protein products differ in molecular size and haemoglobin-binding capacity 15–17 . A previous study demonstrated some evidence that patients with the HP2 allele have a larger haematoma volume, though the underlying mechanisms remain unknown 18 . An increase in haematoma volume may be accompanied by more perihaematomal oedema (PHO) 18 19 . ICH and PHO volume have been demonstrated to influence functional outcome 18 19 . A previous study reported worse functional outcome for patients with HP2 allele (HP2-1 or 2-2) compared to HP1-1 patients as well as some evidence for increased mortality for each HP2 allele 18 . The HP CNV might be associated with functional outcome after ICH through differences in haemoglobin clearance and protection from the cytotoxic and inflammatory effects of haemoglobin breakdown products. However most previous studies investigating haptoglobin in ICH are based on investigations in rodents.
The single nucleotide polymorphism (SNP) rs2000999 accounts for up to 50% of variation in circulating haptoglobin levels in the blood independently of the HP CNV 20 . The combined use of the HP CNV and rs2000999 has been suggested as an important genetic tool to discriminate between two potential mechanisms underlying differences between HP1 and HP2 alleles: haptoglobin expression level and functional differences in haptoglobin protein products 21 .
We performed a comprehensible multivariable study investigating the influence of the HP CNV and rs2000999 SNP on functional outcome and mortality after ICH. We also aimed to assess the influence of the HP CNV and the rs2000999 SNP on ICH volume and OED.
Methods
Data collection
We considered patients, of predominantly Caucasian descent, with spontaneous ICH and available blood samples recruited into the Clinical Relevance of Microbleeds in Stroke ICH study 22 . We defined spontaneous ICH as a non-traumatic haemorrhage into the brain parenchyma, presumed due to cerebral SVD after the exclusion of patients with an underlying structural or macrovascular cause.
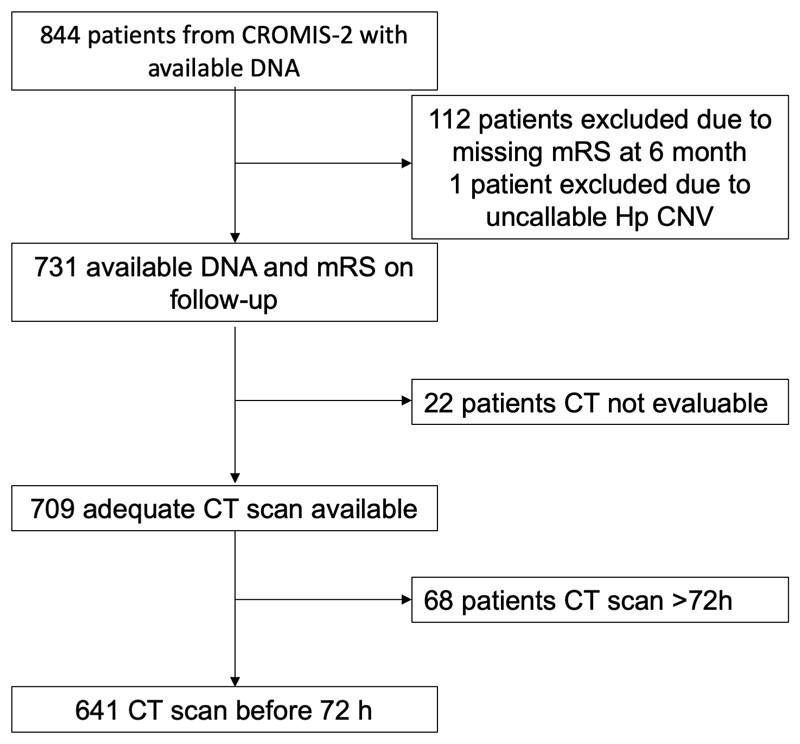
We collected detailed information on demographics, risk factors, medication, clinical presentation, and radiological data. A diagnosis of hypertension, hypercholesterolaemia and diabetes mellitus was present if reported by the patient, stated on medical records or if either drug treatment or any other form of advice (including lifestyle changes) was given. Smoking was defined as current and previous use. All patients had acute brain imaging with CT. Written informed consent was obtained from all participants, or a relative or representative. We excluded patients <18 years, patients without available or adequate CT scan. Patients with a CT scan after 72 hours from symptom onset were excluded from the primary ICH and PHO volume analysis. 18 23 24 . We classified ICH location into lobar, deep (basal ganglia, thalamus), cerebellar and brainstem according to a validated rating scale 25 . Our outcomes were death and functional outcome at 6 months (measured by the modified Rankin Scale [mRS] dichotomized into favorable [mRS 0-2] or unfavorable [mRS 3-6] categories).
Haptoglobin genotyping
To determine the HP CNV we optimised a high-throughput qPCR genotyping assay as described previously 26 . The assay amplified a region in the 5` terminal of the HP gene’s first exon as an internal control (HP5`), and the breakpoint of the HP duplication (HP2). The HP2/HP5` ratio (theoretically either 0, 1, or 2) was used to determine the genotype as HP1-1, HP2-1 or HP2-2 respectively. Samples were run in triplicates; triplicates with a HP2/HP5` ratio coefficient of variation >10% were re-assayed. A second method of HP genotyping by PCR 27 was performed on samples with HP2/HP5’ ratio values between 0.46-077, in order to confirm the HP CNV genotype. Rs2000999 was genotyped using Kompetitive Allele Specific PCR (KASP) assay technology 28 (LGC Genomics Limited, Hertfordshire, UK), call rate was 97.3%.
Measurement of ICH and PHO volume
We measured ICH and PHO volume as previously described via a semi-automated, threshold-based approach 29 . PHO was measured by the oedema extension distance (OED) using a previously described formula 19 ; the rationale behind using OED is that PHO extends a consistent mean linear distance from the border of the ICH, independently of its volume.
Statistical analysis
We present categorical variables using frequency and percentages, continuous variables using mean ± standard deviation (SD). We transformed ICH and PHO volume with cube root transformation to satisfy statistical normal distribution assumptions. We conducted a post hoc sensitivity analysis comparing patients with ICH volume and OED before and after 72 hours. We assessed the distribution of the HP CNV and rs2000999 SNP in the CROMIS-2 cohort compared to ALSPAC (Avon Longitudinal Study of Parents and Children) cohort of healthy individuals, which we used as controls. ALSPAC is a general population cohort study 30 31 ; HP genetic data and rs2000999 SNP data was available from 927 and 748 participants. The ALSPAC study website (http://www.bristol.ac.uk/alspac/researchers/our-data/) contains details of all the data available through a fully searchable data dictionary and variable search tool. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. To evaluate the HP1 allele, we also assessed the HP CNV as a dichotomized variable (HP1-1 and HP2-1 versus HP2-2) according to our pre-specified analysis plan.
We first performed univariable analyses for each of the four outcomes separately with demographic, clinical and radiological variables of interest. We subsequently fitted multivariable logistic regression models with significant variables from the univariable analysis in addition to pre-specified variables. For the analysis of ICH and OED volume we adjusted the models with the pre-specified variables: time from event to imaging, location of ICH, systolic blood pressure (SBP), HP CNV and rs200999 SNP. For functional outcome and mortality analysis, we fitted the multivariable model with the pre-specified variables: age, sex, hypertension, oral anticoagulation (OAC), HP CNV and rs200999 SNP. Additionally, we fitted the multivariable models with variables that were statistically significant at the 20% level in the univariable analysis.
We investigated whether there were interactions between different variables. However, no interaction reached our pre-specified significant threshold for interactions of p<0.001 (chosen to guard against overfitting) and were therefore not included in the models 32 .
Statistical analysis was performed using STATA 15 (StataCorp. 2011. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP).
Ethical approval
The CROMIS-2 study was approved by the local Ethics Committee (reference: 10/H0716/64).
Results
For the primary analysis of functional outcome at 6 months we included 732 patients. One DNA sample was uncallable for the HP CNV and 20 for the rs2000999 SNP. For the secondary analyses of ICH volume and PHO we included 709 patients with an available CT scan (Figure 1). OED mas measured at a mean of 10 hours from ICH onset. Patients who were genotyped (n=844) were not different to those without DNA (n=250) with regard to baseline characteristics and risk factor profile (data not shown). The rs2000999 genotype frequency in CROMIS-2 was as expected when compared to ALSPAC (Supplementary Table 1). However, compared to ALSPAC, CROMIS-2 patients less often had the HP2-2 CNV. We found no systematic difference in demographics, comorbidities and ICH characteristics between those with and without available outcome variable (data not shown).
Figure 1. Patient selection flow diagram.
Mortality
Of 731 patients with available follow-up and genotype data, 112 died within 6 months (15.3%) and 318 (43.5%) were female.
The distribution of the HP CNV was 132 HP1-1 (18.1%), 342 HP2-1 (46.8%) and 257 HP2-2 (35.2%). Distribution of the SNP allele was: 27 A:A (3.8%), 234 A:G (32.9%) and 451 G:G (63.3%), 20 samples were not callable (2.7%).
Patients who died were older, more frequently female, more frequently on OAC, had a lower GCS on admission (GCS <8), a higher ICH and PHO volume, and intraventricular extension (IV). Results of the univariable analysis are shown in supplementary Table 2.
The mortality according to HP CNV was as follows: HP1-1 18.2%; HP2-1 12.6%; HP2-2 17.5%. In the multivariable model (n=608) mortality comparisons between the HP groups, with HP2-2 as a reference group, were as follows: OR HP1-1 0.73, 95% CI 0.34-1.56 (p-value=0.41) and OR HP2-1 0.5, 95% CI 0.28-0.89 (p-value=0.02) (overall p-value=0.06, Table 1).
Table 1. Factors associated with 6 month mortality after ICH in an adjusted multivariable logistic regression model.
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (years) | 1.11 | 1.07-1.14 | <0.001 |
| Female Sex | 1.14 | 0.68-1.92 | 0.63 |
| Hypertension | 1.01 | 0.57-1.76 | 0.99 |
| Diabetes mellitus | 1.31 | 0.65-2.65 | 0.46 |
| Oral anticoagulation | 1.25 | 0.74-2.11 | 0.4 |
| GCS on admission (binary) | |||
| - GCS 3-8 | 4.23 | 1.35-13.28 | 0.01 |
| - GCS 9-15 (reference) | |||
| ICH location | |||
| - Cerebellar (reference) | |||
| - Brainstem | Empty | 0.38 | |
| - Deep | 0.98 | 0.33-2.93 | |
| - Lobar | 0.64 | 0.2-2 | |
| Cr ICH volume (mL) | 2.03 | 1.48-2.8 | <0.001 |
| OED (cm) | 2.82 | 1.01-7.92 | 0.05 |
| IV extension | 1.56 | 0.89-2.72 | 0.12 |
| - HP CNV | 0.06 | ||
| - HP1-1 | 0.73 | 0.34-1.56 | |
| - HP2-1 | 0.5 | 0.28-0.89 | |
| HP2-2 (reference) | |||
| Rs2000999 | 0.74 | ||
| - A:A (reference) | |||
| - A:G | 0.6 | 0.15-2.36 | |
| - G:G | 0.58 | 0.15-2.28 | |
cm = centimeter; CNV = copy number variation; Cr = cube root; CT = computed tomography; GCS = Glasgow Coma Scale; HP = Haptoglobin; ICH = intracerebral haemorrhage; IV = intraventricular; ml = milliliter; OAC: oral anticoagulation; SBP: systolic blood pressure
When dichotomizing HP into HP1-1/2-1 versus HP2-2 there was evidence for association of decreased mortality with the HP1 allele compared to HP2-2 (OR 0.55, 95%CI 0.31-0.95, p=0.03, supplementary Table 3). As expected, there was also evidence for an increase in mortality with increasing age (OR 1.11, 95%CI 1.07-1.14, p<0.001), decreased GCS on admission <9 (OR 4.37, 95%CI 1.39-13.73, p=0.01), and ICH volume (OR 1.99, 95%CI 1.45-2.74, p<0.001).
We further investigated the association between mortality and HP CNV across tertiles of all the covariates included in the multivariable model as a post hoc analysis. Mortality differed between the HP groups for older patients (>80 years) with lower (<12.2mL) ICH volume: in this subgroup, mortality was 26% for HP1-1, 14% for HP2-1 and 42% for HP2-2. Patients died at a median of 3.8 months after ICH. There was no difference (early vs. late death) in the time of death after ICH across HP CNV or rs2000999 groups, in the overall cohort or the subgroup of >80 years and <12.2mL ICH volume (regression data not shown, supplementary Figure 1). The mortality rate was similar across the HP groups for the remaining patients: 15% for HP1-1, 12% for HP2-1 and 12% for HP2-2. The association between mortality and HP CNV was confirmed across tertiles of all the other covariates. Finally, we investigated covariates not included in the multivariable model, to see whether they differed across HP genotypes, but found no bias to explain the association between mortality and HP CNV (data not shown).
Functional outcome
Of 731 patients, 444 (60.7%) suffered an unfavourable outcome (mRS 3-6). Dichotomized unfavourable mRS according to HP CNV was as follows: HP1-1 64.4%; HP2-1 59.7%; HP2-2 60.3%.
Patients with an unfavourable outcome were older, more frequently female, on OAC, more frequently had hypertension, hypercholesterolaemia, presented with a lower GCS (GCS of 3-8), had a higher ICH and PHO volume and IV extension. See supplementary Table 2 for univariable analysis.
In the multivariable model (n=623) age (OR 1.04, 1.02-1.06 95%CI; p<0.001), female sex (OR 2.31; 1.58-3.37; 95%CI; p<0.001) and the cube root of the ICH volume (OR 1.5; 1.22-1.85 95%CI; p<0.001) were significantly associated with functional outcome (Table 2). Neither HP CNV nor rs2000999 SNP were associated with functional outcome.
Table 2. Factors associated with unfavourable outcome after ICH in an adjusted multivariable regression model.
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (years) | 1.04 | 1.02-1.06 | <0.001 |
| Female Sex | 2.31 | 1.58-3.37 | <0.001 |
| Hypertension | 1.37 | 0.92-2.04 | 0.12 |
| Diabetes mellitus | 1.18 | 0.71-1.97 | 0.52 |
| Oral anticoagulation | 1.16 | 0.77-1.73 | 0.49 |
| Antiplatelets | 1.08 | 0.7-1.69 | 0.72 |
| Hypercholesterolaemia | 1.17 | 0.78-1.75 | 0.44 |
| GCS on admission (binary) | |||
| - GCS 3-8 | 3.56 | 0.76-16.5 | 0.11 |
| - GCS 9-15 (reference) | |||
| Cr ICH volume (mL) | 1.5 | 1.22-1.85 | <0.001 |
| IV extension | 1.38 | 0.9-2.12 | 0.14 |
| Surgical evacuation | 1.84 | 0.45-7.5 | 0.39 |
| HP CNV | 0.78 | ||
| - HP1-1 | 1.17 | 0.67-2.03 | |
| - HP2-1 | 0.97 | 0.65-1.45 | |
| - HP2-2 (reference) | |||
| Rs2000999 | 0.66 | ||
| - A:A (reference) | |||
| - A:G | 1.19 | 0.43-3.3 | |
| - G:G | 1.39 | 0.5-3.84 |
CNV = copy number variant; Cr = cube root; CT = computed tomography; GCS = Glasgow Coma Scale; HP = Haptoglobin; ICH = intracerebral haemorrhage; IV = intraventricular; ml = millilitre; OAC: oral anticoagulation; SBP: systolic blood pressure
Intracerebral haemorrhage volume and oedema extension distance
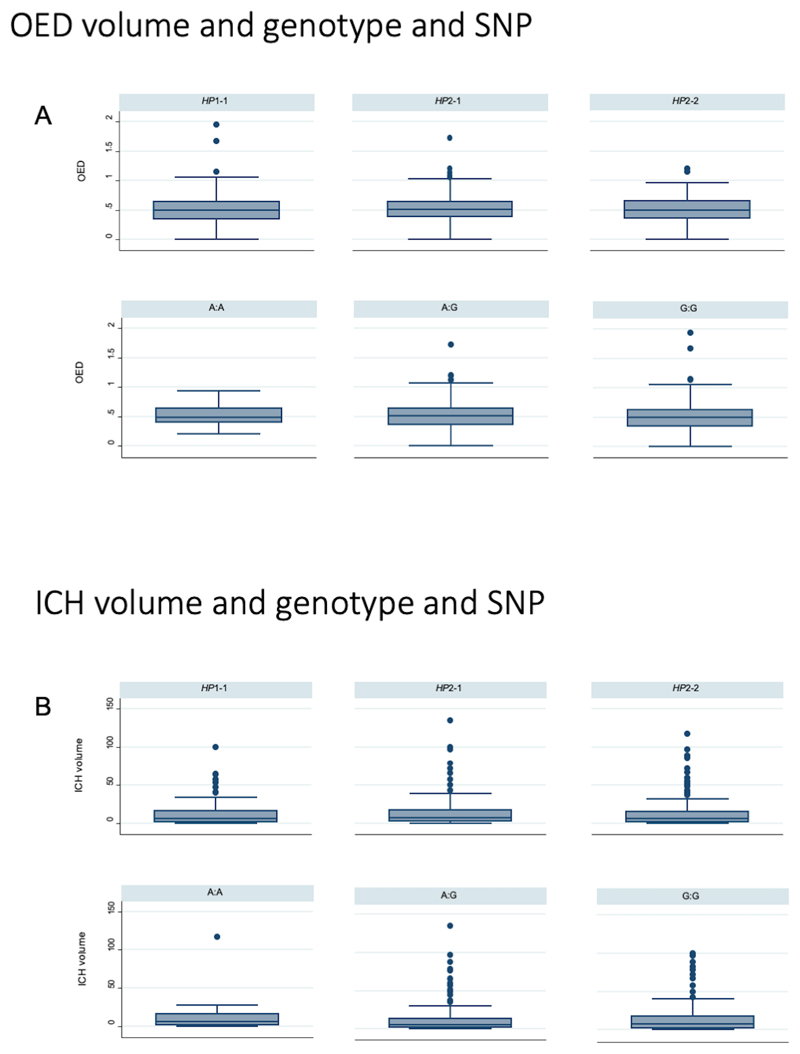
Of the 731 patients included in the functional analysis, 709 had a CT scan available, and of these 68 were >72 hours after symptom onset (Figure 1). Of the remaining 641 individuals, 453 (70.7%) had a scan <24h, 172 (26.8%) between 24-48h and 16 (2.5%) between 48-72h. See Figure 2 for the association of the HP CNV and SNP with OED and ICH volume.
Figure 2.
A) Differences in OED in Haptoglobin genotype and SNP, B) Differences in ICH volume in Haptoglobin genotype and SNP
Mean ICH volume was 13.8 mL (± 18.82 SD), mean PHO volume 19.54 mL (± 20.56 SD) and mean OED 0.51 cm (±0.23 SD). Variables significantly associated with ICH volume in the univariable analysis are listed in the supplementary Table 3.
In the fitted multivariable model (n=604) ICH location (overall p<0.001) and intraventricular extension (coefficient 0.53; 0.37-0.68; p<0.001) were associated with greater ICH volume (Table 3). Neither HP CNV nor the SNP rs2000999 were associated with ICH volume.
Table 3. Factors associated with the cube root ICH volume in an adjusted multivariable regression model.
| Coefficient | 95% CI | P value | |
|---|---|---|---|
| Age (years) | -0.005 | -0.01-0.001 | 0.09 |
| Time Event to CT | 0.35 | ||
| - Day 1 (reference) | |||
| - Day 2 | 0.04 | -0.23-0.31 | |
| - Day 3 | -0.29 | -0.7-0.11 | |
| ICH location | <0.001 | ||
| - Cerebellar (reference) | |||
| - Brainstem | -0.73 | -1.22-0.23 | |
| - Deep | -0.13 | -0.44-0.18 | |
| - Lobar | 0.79 | 0.47-1.1 | |
| SBP (mmHg) | 0.001 | -0.002-0.002 | 0.88 |
| Platelet level (x109/liter) | 0.001 | -0.0004-0.001 | 0.31 |
| Hypercholesterolaemia | 0.09 | -0.05-0.22 | 0.2 |
| IV extension | 0.53 | 0.37-0.68 | <0.001 |
| Neurosurgery | 0.36 | -0.06-0.78 | 0.1 |
| HP CNV | 0.66 | ||
| - HP1-1 | -0.09 | -0.25-0.52 | |
| - HP2-1 | -0.02 | -0.17-0.13 | |
| - HP2-2 (reference) | |||
| Rs2000999 | 0.68 | ||
| - A:A (reference) | |||
| - A:G | 0.14 | -0.25-0.52 | |
| - G:G | 0.16 | -0.22-0.54 | |
CNV = copy number variation; CT = computed tomography; HP = Haptoglobin; ICH = intracerebral haemorrhage; IV= intraventricular; mmHg = millimetre mercury; SBP= systolic blood pressure
After dichotomizing the HP CNV into HP1-1/2-1 versus HP2-2 we did not observe any evidence of an association in univariable or multivariable analyses (p = 0.39 [supplementary Table 4] and p = 0.6 respectively [data not shown]). Similar results were observed when dichotomizing HP CNV into HP1-1 versus HP2-1/2-2 [supplementary Table 4].
Oedema Extension Distance
Variables significantly associated with OED in the univariable analysis are listed in supplementary Table 4. For comparison of HP CNV and SNP for ICH volume and OED see Figure 2.
In the multivariable linear regression model (n=623), ICH location (with lobar and deep ICH locations featuring a longer OED and with a brainstem location featuring a shorter OED, compared to the reference group of cerebellar location, overall p<0.001) and antihypertensive medication (coefficient -0.09; 95%CI -0.16-(-0.02); p=0.01) were significantly associated with OED (Table 4). Neither the univariable nor multivariable analysis showed evidence of association of HP CNV or rs2000999 SNP with OED.
Table 4. Factors associated with size of oedema extension distance in an adjusted multivariable regression model.
| Coefficient | 95% CI | P value | |
|---|---|---|---|
| Female Sex | 0.01 | -0.02-0.05 | 0.44 |
| Time Event to CT | 0.18 | ||
| - Day 1 (reference) | |||
| - Day 2 | 0.07 | -0.008-0.14 | |
| - Day 3 | 0.04 | -0.07-0.15 | |
| ICH location | <0.001 | ||
| - Cerebellar (reference) | |||
| - Brainstem | -0.08 | -0.21-0.06 | |
| - Deep | 0.16 | 0.07-0.24 | |
| - Lobar | 0.24 | 0.15-0.33 | |
| SBP (mmHg) OAC | 0.0002 | -0.0003-0.001 | 0.49 |
| OAC | 0.05 | -0.02-0.12 | 0.17 |
| Antihypertensive medication | -0.09 | -0.16-(-0.02) | 0.01 |
| Platelet level (x109/liter) | 0.0002 | -0.00005-0.0004 | 0.11 |
| IV extension | -0.03 | -0.07-0.008 | 0.11 |
| HP CNV | 0.5 | ||
| - HP1-1 | 0.03 | -0.02-0.09 | |
| - HP2-1 | 0.01 | -0.03-0.05 | |
| HP2-2 (reference) | |||
| Rs2000999 | 0.93 | ||
| - A:A (reference) | |||
| - A:G | 0.01 | -0.09-0.11 | |
| - G:G | 0.003 | -0.1-0.1 |
CNV = copy number variation; CT = computed tomography; HP = Haptoglobin; ICH = intracerebral haemorrhage; mmHg = millimetre mercury; OAC: oral anticoagulation; SBP: systolic blood pressure
Similar to the ICH volume model, dichotomizing HP did not yield any evidence of association in univariable and multivariable models (data not shown).
Discussion
In this large prospective, multicentre cohort study, HP was not associated with functional outcome as assessed by the mRS. The HP CNV distribution was comparable to that reported in a previous study, apart from a slightly higher proportion of HP1-1 patients and lower proportion of HP2-2 18 . Despite the larger sample size, we could not replicate this previous study’s finding of an association of the HP2 allele with functional outcome 18 .
However, we found evidence that mortality was lower in HP2-1 patients compared to HP2-2 homozygotes; our post hoc analyses suggest that this observation is mostly driven by older patients with lower ICH volumes. No association with mortality was found for the rs2000999 SNP (which is associated with haptoglobin expression level) 21 . This suggests that any link between the HP CNV and mortality is mediated by factors other than haptoglobin expression.
While the HP CNV’s association with mortality could have been confounded by bias in a variable excluded from the model, we did not find any evidence for this. Such a factor could still remain unidentified, but a more likely explanation is that patients who died did not contribute to functional outcome analysis. We found evidence of HP2-2 missingness (of subjects of a particular genotype, in this case HP2-2), when comparing CROMIS-2 with ALSPAC cohorts, which might suggest that the HP2-2 genotype confers a mortality risk.
We confirmed previous results showing evidence towards increased mortality with HP2-2 18 , but did not observe a unidirectional dose response of HP alleles in a direction of increasing or decreasing mortality across HP genotypes (mortality: HP1-1 18.2%; HP2-1 12.6%; HP2-2 17.5%). The lower mortality in HP2-1 individuals could be a chance finding. A possible but unlikely explanation is heterozygote advantage or heterosis 33 . At a molecular level, the HP1 allele might protect against the deleterious effect of the HP2 allele only when the two alleles are present together in HP2-1 individuals. Both HP1 and HP2 alleles scavenge haemoglobin, with HP2 being superior 34 35 , and this confers a beneficial effect. However, HP2 has additional off-target effects which are deleterious, mostly pro-inflammatory 36 . In HP2-2 individuals, the better haemoglobin scavenging potential of HP2 versus HP1 is offset by its proinflammatory effects, so that mortality is similar in HP1-1 and HP2-2 individuals. In HP2-1 individuals, the HP1 allele may be negating the deleterious effect of HP2, so that a greater benefit is observed in HP2-1 individuals than is expected by simple co-dominance of the two alleles.
We did not confirm previous findings of worse functional outcome in patients with HP2 allele, which could be due to the significantly smaller cohort size and statistical power of the previous study, with potential for a chance finding 18 .
PHO develops over a continuous period of time in three main stages. It peaks after two weeks, however its evolution is most rapid in the first 2-3 days 37 . PHO is thought to be mediated by a process of toxicity and inflammation 19 37 . We hypothesized that by modulating neurotoxicity and inflammatory processes haptoglobin might have influenced PHO and functional outcome. 38 However, we did not find any association of HP genetic variants (CNV or the rs2000999 SNP) with OED. Similarly, HP genetic variants were not associated with ICH volume, which, like haemtoma expansion, is more likely to be driven by other factors including hydrostatic pressure at the bleeding point 18 .
Despite having a large cohort available, we could not replicate the previous study’s reported finding of an association of the HP2 allele with larger ICH volumes and IV extension 18 . Since ICH volume and OED was assessed on CT scans performed within 72 hours of symptom onset, we cannot exclude an association of HP with ICH volume or OED after this timepoint, although our exploratory analysis of scans beyond 72 hours (n=68) and found no difference in ICH volume and OED across HP genotypes (for both CNV and rs2000999 SNP) (data not shown). We found that long-term antihypertensive medication prior to ICH event is independently associated with decreased OED, even after correcting for SBP. It is possible that patients on antihypertensive medication could have reduced sympathetic activity and inflammatory response when ICH occurs 39 , a hypothesis that merits further study. As we did not collect follow-up scans, we cannot comment on a potential influence of SBP on haematoma growth.
Our study has strengths. Our prospective, multi-centre study is the largest on HP and ICH to date, and should be generalizable to Caucasian populations. We collected detailed baseline clinical and brain imaging data and undertook multivariable regression analysis adjusting and correcting for important predictors of all four outcomes, and took exceptional care to control for covariates.
However, our study also has limitations. Since we obtained informed or proxy consent, our study is biased towards ICH survivors with less severe ICH than would be included in an unselected incident ICH population. However, it is likely that any protective effect of HP is most relevant in ICH patients who survive the acute period. Additionally, CT scans at multiple timepoints were not available and therefore we could not assess the influence of HP CNV and rs200999 SNP on ICH, PHO or OED expansion over time. We also did not have data on the time interval between the ICH and CT scan. However, in a post hoc sensitivity analysis ICH volume before and after 72 hours was very similar although OED was larger in patients with first imaging after 72 hours. As PHO increases beyond 72 hours further studies are needed to assess an influence of the HP CNV and rs2000999 SNP on oedema expansion. Although we excluded patients without blood samples available for genetic analysis, there were no systematic differences in demographics, comorbidities and ICH characteristics between those with and without genetic data available. Finally, it would have been interesting to study plasma and cerebrospinal fluid haptoglobin levels in relation to HP genetic variants, but unfortunately these were not available.
Conclusion
We investigated the association of HP genetic variation (the HP CNV and the rs2000999 SNP) in a large cohort of 731 ICH patients. We found evidence in support of a lower mortality with the HP2-1 genotype, but not functional outcome, ICH volume or OED. While HP genotype may not matter for functional outcome, upregulating or supplementing haptoglobin may still be of benefit, as demonstrated in animal studies 40 , so understanding how different haptoglobin types associate with outcome is important. A future meta-analysis may be appropriate to confirm our observations, and longer follow-up may be needed in case there is an association with longer term outcome.
Supplementary Material
Acknowledgements
We are extremely grateful to all patients, hospital staff and researcher who took part in this study. We also want to thank the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Sources of funding
DJW and DW received funding from the Stroke Foundation/British Heart Foundation. This work was undertaken at UCLH/UCL which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centers funding scheme. MM and IG received funding from the Medical Research Council (MR/L01453X/1). NK received funding from Cancer Research UK program grant C18281/A19169. The UK Medical Research Council (MRC) and Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and ICH will serve as guarantor of the contents of this paper. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf).
Footnotes
Conflict of Interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Bamford J, Sandercock P, Dennis M, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project--1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53(1):16–22. doi: 10.1136/jnnp.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2014;85(6):660–7. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 3.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. The Lancet Neurology. 2010;9(2):167–76. doi: 10.1016/S1474-4422(09)70340-0. [published Online First: 2010/01/09] [DOI] [PubMed] [Google Scholar]
- 4.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke; a journal of cerebral circulation. 1997;28(3):491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 5.Bejot Y, Cordonnier C, Durier J, et al. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain. 2013;136(Pt 2):658–64. doi: 10.1093/brain/aws349. [published Online First: 2013/02/05] [DOI] [PubMed] [Google Scholar]
- 6.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68(2):116–21. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 7.Lovelock CE, Molyneux AJ, Rothwell PM, et al. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6(6):487–93. doi: 10.1016/S1474-4422(07)70107-2. [published Online First: 2007/05/19] [DOI] [PubMed] [Google Scholar]
- 8.Huang FP, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. Journal of neurosurgery. 2002;96(2):287–93. doi: 10.3171/jns.2002.96.2.0287. [published Online First: 2002/02/13] [DOI] [PubMed] [Google Scholar]
- 9.Thiex R, Tsirka SE. Brain edema after intracerebral hemorrhage: mechanisms, treatment options, management strategies, and operative indications. Neurosurg Focus. 2007;22(5):E6. doi: 10.3171/foc.2007.22.5.7. [published Online First: 2007/07/07] [DOI] [PubMed] [Google Scholar]
- 10.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. Journal of neurosurgery. 1998;89(6):991–6. doi: 10.3171/jns.1998.89.6.0991. [published Online First: 1998/12/02] [DOI] [PubMed] [Google Scholar]
- 11.Andersen CB, Torvund-Jensen M, Nielsen MJ, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489(7416):456–9. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Jia Y, Siburt CJ, et al. Haptoglobin alters oxygenation and oxidation of hemoglobin and decreases propagation of peroxide-induced oxidative reactions. Free radical biology & medicine. 2012;53(6):1317–26. doi: 10.1016/j.freeradbiomed.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Cooper CE, Schaer DJ, Buehler PW, et al. Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine beta145. Antioxidants & redox signaling. 2013;18(17):2264–73. doi: 10.1089/ars.2012.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaer CA, Vallelian F, Imhof A, et al. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. Journal of leukocyte biology. 2007;82(1):106–10. doi: 10.1189/jlb.0706453. [DOI] [PubMed] [Google Scholar]
- 15.Bulters D, Gaastra B, Zolnourian A, et al. Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nature reviews Neurology. 2018 doi: 10.1038/s41582-018-0020-0. [published Online First: 2018/06/22] [DOI] [PubMed] [Google Scholar]
- 16.Asleh R, Marsh S, Shilkrut M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circulation research. 2003;92(11):1193–200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 17.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clinical chemistry. 1996;42(10):1589–600. [PubMed] [Google Scholar]
- 18.Murthy SB, Levy AP, Duckworth J, et al. Presence of haptoglobin-2 allele is associated with worse functional outcomes after spontaneous intracerebral hemorrhage. World Neurosurg. 2015;83(4):583–7. doi: 10.1016/j.wneu.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Parry-Jones AR, Wang X, Sato S, et al. Edema Extension Distance: Outcome Measure for Phase II Clinical Trials Targeting Edema After Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2015;46(6):e137–40. doi: 10.1161/STROKEAHA.115.008818. [published Online First: 2015/05/07] [DOI] [PubMed] [Google Scholar]
- 20.Froguel P, Ndiaye NC, Bonnefond A, et al. A genome-wide association study identifies rs2000999 as a strong genetic determinant of circulating haptoglobin levels. PloS one. 2012;7(3):e32327. doi: 10.1371/journal.pone.0032327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazmi N, Koda Y, Ndiaye NC, et al. Genetic determinants of circulating haptoglobin concentration. Clinica chimica acta; international journal of clinical chemistry. 2019;494:138–42. doi: 10.1016/j.cca.2019.03.1617. [published Online First: 2019/03/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charidimou A, Wilson D, Shakeshaft C, et al. The Clinical Relevance of Microbleeds in Stroke study (CROMIS-2) : rationale, design, and methods. International journal of stroke: official journal of the International Stroke Society. 2015;10(Suppl A100):155–61. doi: 10.1111/ijs.12569. [DOI] [PubMed] [Google Scholar]
- 23.Murthy SB, Urday S, Beslow LA, et al. Rate of perihaematomal oedema expansion is associated with poor clinical outcomes in intracerebral haemorrhage. Journal of neurology, neurosurgery, and psychiatry. 2016;87(11):1169–73. doi: 10.1136/jnnp-2016-313653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nature reviews Neurology. 2015;11(2):111–22. doi: 10.1038/nrneurol.2014.264. [DOI] [PubMed] [Google Scholar]
- 25.Charidimou A, Schmitt A, Wilson D, et al. The Cerebral Haemorrhage Anatomical RaTing inStrument (CHARTS): Development and assessment of reliability. J Neurol Sci. 2017;372:178–83. doi: 10.1016/j.jns.2016.11.021. [published Online First: 2016/12/27] [DOI] [PubMed] [Google Scholar]
- 26.Soejima M, Koda Y. TaqMan-based real-time PCR for genotyping common polymorphisms of haptoglobin (HP1 and HP2) Clinical chemistry. 2008;54(11):1908–13. doi: 10.1373/clinchem.2008.113126. [DOI] [PubMed] [Google Scholar]
- 27.Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clinical chemistry. 2002;48(9):1377–82. [PubMed] [Google Scholar]
- 28.Semagn K, Babu R, Hearne S, Olsen M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): overview of the technology and its application in crop improvement. Molecular Breeding. 2014;33:1–14. doi: 10.1007/s11032-013-9917-x. [DOI] [Google Scholar]
- 29.Volbers B, Staykov D, Wagner I, et al. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. European journal of neurology. 2011;18(11):1323–8. doi: 10.1111/j.1468-1331.2011.03395.x. [published Online First: 2011/04/05] [DOI] [PubMed] [Google Scholar]
- 30.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the 'children of the 90s'--the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42(1):111–27. doi: 10.1093/ije/dys064. [published Online First: 2012/04/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International journal of epidemiology. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [published Online First: 2012/04/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauerbrei Ra. Multivariable Model Building. 2008.
- 33.Hedrick PW. What is the evidence for heterozygote advantage selection? Trends Ecol Evol. 2012;27(12):698–704. doi: 10.1016/j.tree.2012.08.012. [published Online First: 2012/09/15] [DOI] [PubMed] [Google Scholar]
- 34.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [published Online First: 2001/02/24] [DOI] [PubMed] [Google Scholar]
- 35.Lipiski M, Deuel JW, Baek JH, et al. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxidants & redox signaling. 2013;19(14):1619–33. doi: 10.1089/ars.2012.5089. [published Online First: 2013/02/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landis RC, Philippidis P, Domin J, et al. Haptoglobin Genotype-Dependent Anti-Inflammatory Signaling in CD163(+) Macrophages. Int J Inflam. 2013;2013:980327. doi: 10.1155/2013/980327. [published Online First: 2013/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke; a journal of cerebral circulation. 2011;42(1):73–80. doi: 10.1161/STROKEAHA.110.590646. [published Online First: 2010/12/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu TY, Sharma G, Strbian D, et al. Natural History of Perihematomal Edema and Impact on Outcome After Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2017;48(4):873–79. doi: 10.1161/STROKEAHA.116.014416. [published Online First: 2017/03/10] [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Luna D, Muchada M, Pineiro S, et al. Potential blood pressure thresholds and outcome in acute intracerebral hemorrhage. European neurology. 2014;72(3–4):203–8. doi: 10.1159/000362269. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Song S, Sun G, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(50):15819–27. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.