Abstract
Goals/background
Animal studies have highlighted how the microbiota acts in a sex-specific manner with sex hormones demonstrating an association with the composition and diversity of the microbiota. This systematic review aimed to gather the available scientific evidence to explore the association between sex hormones and gut microbiota composition and diversity, in humans.
Study
Four bibliographic databases were searched in July 2020 using terms related to “microbiota”, “microflora”, “sex hormones”, “testosterone”, and “estrogen”. Human studies that investigated the correlation between sex hormones and the microbiota composition or diversity using next generation sequencing (NGS) were included.
Results
10,468 records were screened with thirteen studies included in this review. In healthy women, higher estrogen levels were found to be associated with a higher abundance of Bacteroidetes, a lower abundance of Firmicutes, the Ruminococcaceae family and increased diversity. In healthy men, raised testosterone levels positively correlated with Ruminococcus, Acinetobacter, and an increased microbial diversity. Escherichia and Shigella spp. were correlated with raised testosterone in healthy women whereas Ruminococcus spp. was negatively associated with elevated testosterone levels. Women with altered testosterone/oestrogen profiles (such as in PCOS), had a differing gut microbiota compared to healthy women.
Conclusions
The findings gathered highlight an association between sex hormones and the gut microbiota composition/diversity and may contribute to the sex-based variations observed in disease pathogenesis. Factors such as age and medical conditions are implicated in the associations observed and should be accounted for in future studies. As the understanding of the complex symbiotic relationship between humans and their gut microbiota increases, microbiota modulation could be an attractive option for the prevention and treatment of gastrointestinal disorders.
Keywords: Estrogens, gastrointestinal microbiome, gonadal steroid hormones, microbiota, testosterone
Introduction
Growing data supports a critically important and complex relationship between gut microbiota and its host, with dysbiosis implicated in driving various pathophysiological processes. Therefore, maintaining an optimal microbial environment may be a crucial factor for both disease prevention and treatment, in the future.(1, 2) Various host-microbiota interactions impact immune, neuronal and endocrine mediated pathways. Gut microbiota composition is influenced by both host specific and non-specific factors.(3) However, the specific molecular interactions from the level of the microbe to the cellular processes, are still under investigation.
It has been demonstrated that male and female mice have a different microbiota composition.(4) One study has demonstrated that faecal microbiota transfer from male to female mice increased testosterone levels in female mice.(5) Another study observed a substantial effect of gonadectomy and hormone replacement on the composition of microbial species (6). Significant differences in microbial diversity between males and females are first observed following puberty which can be reversed following male castration (6). These data collectively demonstrate the effect of male and female hormones on the microbial environments. Indeed, it appears that the hormones may drive these changes as germ-free (GF) mice given female human faecal suspension show microbial species clustering based on the animals’ sex.(7) In addition, multiple studies on mice have found a direct association between sex hormones and the microbiota diversity and composition.(8-10) In particular, mice models have shown a correlation between the microbiota and hyperandrogenaemia in mice with polycystic ovary syndrome (PCOS).(11-13)t
Estrogen-related disorders include osteoporosis, neurodegenerative disorders and cancers of organs such as breast, ovaries, colon and endometrium of the uterus, whereas testosterone-related disorders include PCOS and infertility. Although non-exhaustive, these conditions emphasise the importance of understanding the manner through which microbiota are influenced by sex hormones and the potential role this may have in disease processes.(14-16) In order to identify if the interactions found in animal studies are also observed in humans, this systematic review was performed to evaluate the current state of research on estrogen and testosterone and microbial composition and diversity in humans.
Methods
This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.(17)
Eligibility criteria
The studies eligible to be included in this systematic review were published analytical observational human studies including cross-sectional, cohort, case-control and nested case-control studies.
Studies exploring the association between testosterone and/or estrogen on the composition and/or diversity (alpha and/or beta) of the human gut microbiota in men and/or women were eligible for inclusion. Only studies which utilised next generation sequencing (NGS), including shotgun sequencing or 16S rRNA amplicon sequencing, to analyse the stool samples were included. Studies that used culture-based techniques were excluded. There was no restriction with regards to the techniques used to measure the sex hormone levels as various techniques including immunoassays and mass spectrometry-based assays have been deemed acceptable.(18) Studies with children (<18 years old), animal models and pregnant women were excluded. In addition, studies investigating the skin, oral, gastric or nasopharyngeal microbiota were excluded.
Only studies published between January 2004 and May 2020 were included. January 2004 was chosen as the start date for the search because it was when the first NGS sequencing platform was marketed.(19) Due to the novelty of the topic, in order to identify as many relevant references as possible no language restriction was applied as long as an abstract in English was provided.
Search strategy and study selection
The literature search was carried out independently with the last search completed on 15th July 2020. As recommended by Bramer et al.(20), to ensure the majority of the relevant references were identified, the following bibliographic databases were searched: EMBASE (OVID), MEDLINE (PubMed), Web of Science, and Google Scholar (first 300 references, as ranked by relevance, were analysed). The search included terms related to “microbiota”, “microflora”, “sex hormones”, “testosterone”, “estrogen”, and “human” in both free text form and database-specific subject heading (e.g. MeSH, EMTREE) whilst using the set Boolean operators “OR”,”AND”. The individual search strategies for EMBASE, MEDLINE, Web of Science, and Google Scholar can be found in Supplementary Figure 1. Furthermore, references of eligible studies and relevant reviews were manually searched to identify any further relevant studies.
Abstracts submitted between 2013 and 2019 to two different conferences proceedings, namely the “United European Gastroenterology (UEG) Week” and the “Annual Meeting of the British Society of Gastroenterology (BSG)”, were manually analysed for inclusion. The abstracts submitted to the 2015 BSG Annual General Meeting were not available thus they could not be screened.
All records identified were combined on an EndNote Web Software (Clarivate Analytics) file and duplicates were removed prior to the screening phase. The titles and abstracts of the studies yielded by the search were then screened and full text of selected studies were subsequently assessed for eligibility.
Data extraction and synthesis
Data extracted from included studies was added to a Microsoft Excel Spreadsheet. Where possible, the following data was extracted from the eligible studies: publication details, study design, demographics of study participants, smoking, diet, pre- and probiotic use, inclusion and exclusion criteria, sex hormone(s) investigated, technique used to measure sex hormone levels, methods utilised to analyse the gut microbiota, alpha and beta diversity indices applied, main study outcome investigated and any further relevant comments. If required, the senior authors of the studies were contacted to gather any missing data.
A narrative synthesis was carried out for all the eligible studies; a meta-analysis was not performed due to the limited number of eligible studies and the heterogeneity of the study designs. For the data interpretation, p values of ≤0.05 were considered significant, and all data is represented as means ± standard deviation (SD).
Results
Literature search
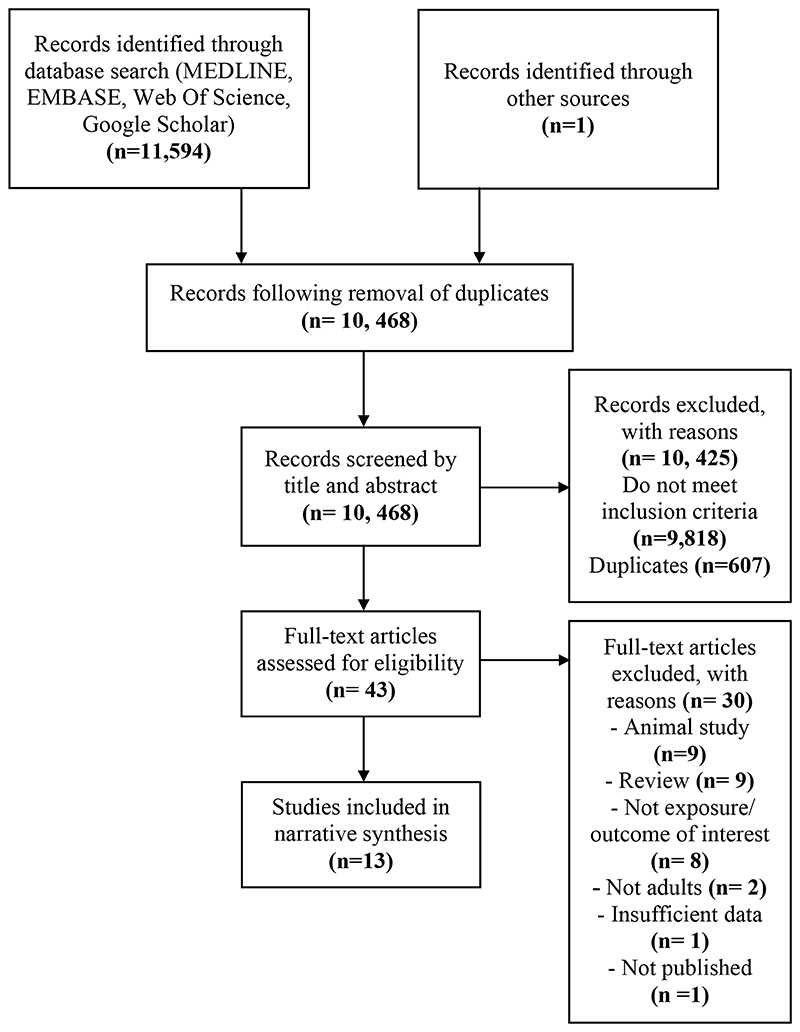
The literature search utilising MEDLINE (n=4,332), EMBASE (n= 5,755), Web of Science (n= 1,207) and Google Scholar (n= 300) yielded a total of 11,594 records. The identified records were transferred to EndNote where duplicates were automatically removed. The title and abstracts of 10,468 records were screened for inclusion; 9,818 records were excluded as they did not meet inclusion criteria while a further 607 duplicates were manually removed. The full texts of 43 articles were read to assess for eligibility with 13 records meeting the inclusion criteria (Figure 1). One relevant conference abstract submitted to the 2018 BSG Annual General Meeting was identified but not enough required information could be gathered to assess eligibility.
Figure 1. Assessment phases (identification, screening and eligibility) of study selection.
Following the removal of duplicates, the search yielded a total of 10, 468 records, of which 43 full-text articles were assessed and 13 subsequently included in the narrative synthesis.(17)
Study characteristics
The 13 included studies were published between 2012-2020. Participants were recruited from the USA (n=5), China (n=5), Poland (n=1), Republic of Korea (n=1) and Spain (n=1). Twelve of the included studies were of a cross-sectional design and one study was a case-control.
All studies combined, yielded a total of 812 participants with 741 women (91%) and 71 men (9%). The mean number of participants for each study was 68 (range 27-163) with a mean age of 41 years (SD 16.2). Two studies utilised the same cohort of participants and thus only the cohort from one of the two studies was included for these calculations (see Table 1).(21, 22)
Table 1. Details and characteristics of included studies.
Details and characteristics of the thirteen studies included. The cross-sectional (n=12) and case-control (n=1) studies were published between 2012-2020. When combining all included studies, there were a total of 812 participants (91% women, 9% men); the mean number of participants per study was 68 (range 27-163) with a. mean age of 41 years (SD 16.2).
PCOS: polycystic ovary syndrome; BMD: bone mineral density; PCOM: polycystic ovarian morphology.
| Study | Year | Design | n* | Population characteristics (age, yrs) |
|---|---|---|---|---|
| Chu et al.(33) | 2020 | Cross-sectional | 28 | Reproductive-aged women with PCOS (Mean 28.14; SD 3.72) (n=14) Reproductive-aged women without PCOS (controls) (Mean 29.43; SD 3.31) (n=14) |
| Flores et al. (23) | 2012 | Cross-sectional | 51 | Healthy men (Mean 38.8; SD 12.27) (n=25) Healthy women (Mean 40.2; SD 12.4) (n=26; premenopausal n=19; postmenopausal n=7) |
| Fuhrman et al. (24) | 2014 | Cross-sectional | 60 | Healthy postmenopausal women (Mean 60.3; SD 3.2) (n=60) |
| Goedert et al. (21) | 2015 | Casecontrol | 96 | Postmenopausal breast cancer patients (Mean 62.17; SD 6.5) (n=48) Postmenopausal normal mammography patients (Mean 61.85; SD 3.6) (n=48) |
| Goedert et al. (22) | 2018 | Cross-sectional | 96 | Postmenopausal breast cancer patients (Mean 62.17; SD 6.5) (n=48) Postmenopausal normal mammography patients (Mean 61.85; SD 3.6) (n=48) |
| He et al.(26) | 2020 | Cross-sectional | 106 | Postmenopausal women with osteopenia (Mean 57.42; SD 5.06)(n=33) Postmenopausal women with osteoporosis (mean 59.69; SD 5.51) (n=42) Postmenopausal women with normal BMD (mean 57.35; SD 3.98) (n=31) |
| Insenser et al. (30) | 2018 | Cross-sectional | 46 | Women with PCOS (Mean 26.7; SD 6.4) (n=15) Non-hyperandrogenic control women (Mean 27.3; SD 5.3) (n=16) Healthy control men (Mean 23.4; SD 3.3) (n=15) |
| Liang et al. (29) | 2020 | Cross-sectional | 27 | Obese women with PCOS (Mean 27.1; SD 3.5)(n=8) Normal-weight PCOS women (Mean 25.7; SD 3.5)(n=10) Healthy normal-weight women (Mean 27.9; SD 3.6) (n=9) |
| Liu et al.(27) | 2017 | Cross-sectional | 48 | Premenopausal women with PCOS (Mean 32.5; SD 5.7) (n=33) Premenopausal women without PCOS (Mean 27.9; SD 5.7) (controls) (n=15) |
| Shin et al. (25) | 2019 | Cross-sectional | 57 | Men (Mean 37.45; SD 1.91) (n=31) Women (Mean 46.15; SD 2.26) (n=26) |
| Torres et al. (31) | 2018 | Cross-sectional | 163 | Healthy premenopausal women (Mean 29.4; SD 4.9) (n=48) Premenopausal women with PCOM (Mean 29.8; SD 5.3) (n=42) Premenopausal women with PCOS (Mean 27.4; SD 4.9) (n=73) |
| Zengul et al. (32) | 2020 | Cross-sectional | 29 | Postmenopausal women with breast cancer (Mean 62.3; SD 8.5) (n=29) |
| Zhou et al. (28) | 2020 | Cross-sectional | 101 | Women with PCOS (Mean 23.6; SD 2.96)(n=60) Healthy women (control group) (Mean 26.5; SD 3.99) (n=41) |
n= Total number of participants.
Multiple factors affecting microbiota were recorded as follows; participant diet was recorded or standardized in 58% of studies (7/13), participants who smoked or took pre- or probiotics were either recorded or excluded in 62% (8/13) of studies. Participants who had recently or were taking antibiotics at the time of the study were excluded in 92% (12/13) of the studies.
Six studies investigated the effects of estrogen, four studies investigated testosterone, and three studies investigated the effects of both sex hormones. Different techniques were adopted to measure the levels of sex hormones with five studies using liquid chromatography and mass spectrometry, seven others employing chemiluminescence or immune assays and one not stating the technique used).
As per the inclusion criteria, all studies utilised NGS. Nine studies used Illumina sequencing of the 16S rRNA gene, two studies used 454 pyrosequencing, one study used sequencing of 16S rRNA using Ion S5 XL and one used shotgun metagenomic sequencing. The hypervariable regions used for analysis included V1-V2 (23-25), V3-V4 (26-29), V4 (22, 30-32) and no specified region (21) whilst one study used deep shotgun metagenome sequencing.(33)
Alpha (α) diversity is a measure of the diversity within a subject while beta (β) diversity compares the diversity between subjects.(34) Alpha and beta diversity were measured in 10 and 8 of the studies respectively. For the studies that investigated alpha diversity, the Shannon index was used in all of the studies; other indices used included the Chao1 index (n=8), richness (number of observed species) (n=8), Simpson’s index (n=5), phylogenetic diversity (PD) (n=4), abundance-based Coverage Estimator (ACE) (n=2) and Pielou’s evenness (n=2). Beta diversity was measured using UniFrac distance analysis (weighted and unweighted) (n=6), Bray-Curtis analyses (n=3), Jaccard index (n=1), Sorensen index (n=1) and principal co-ordinates statistical analysis (PCoA) method with R language (n=1).
Estrogens and the microbiota composition
Three studies performed in healthy men and women found an association between estrogen levels and specific microbial taxa.(23-25, 28) One study performed in men and postmenopausal women found a correlation between total estrogens and non-ovarian estrogen levels, and genera in the Clostridia class including the Ruminococcaceae family (Ruminococcaceae Oscillibacter, Ruminococcaceae Subdoligranulum, Ruminococcaceae genus) and non-Clostridiales.(23) Beta analysis did not find an association between estrogen and a particular class or cluster in the microbiome.(23) Another study performed on healthy women also found an association between the ratio of all estrogen metabolites to parent estrogen and the Clostridiales order and in particular the Ruminococcaceae family.(24) In addition, the total estrogen metabolites (EM) were found to be positively correlated with the Ruminococcus genus (24). Conversely, an inverse relationship was found with the Bacteroides genus.(24) Higher estradiol levels in healthy women were found to correlate with an increased abundance of Bacteroidetes, a lower abundance of Firmicutes, and a decreasing Firmicutes:Bacteroidetes ratio.(25) At the genus level, an inverse correlation was found between estradiol levels and the Slackia and Butyricimonas genera.(25) The study by Zhou et al,(28) found a positive correlation between estradiol (E2) levels and the Subdoligranulum, Collinsella, Sutterella and Agathobacter genera in obese women with PCOS; a negative correlation was observed with the Escherichia-Shigella and Ruminococcus_gnavus_group genera. In non-obese women with PCOS concordance was found between estradiol and the genera Parasutterella and Dialister (28). Two studies did not find an association between estrogens and the microbiota composition (see Tables 2-3).(26, 32) In both healthy and disease states, estrogen (and subsequent metabolites) influence microbial composition by altering abundance of various species.
Table 2. Association between estrogen and the bacterial composition and diversity.
Summary of findings from the included studies (n=6) that investigated the effect of estrogen on the bacterial composition and/or diversity. Three of these studies investigated the effect of estrogen on both composition and diversity, whilst the remaining studies explored its effect on either composition (n=1) or diversity (n=2) alone.
N/A: not applicable; BMD: bone mineral density.
| Study | Estrogen | |
|---|---|---|
| Bacterial Composition | Bacterial Diversity | |
| Flores et al.(23) | ↑ Clostridia taxa including Ruminococcaceae Oscillibacter, Ruminococcaceae Subdoligranulum Ruminococcaceae genus NA and non-Clostridiales in men and post-menopausal women | ↑ Alpha diversity in men and post-menopausal women ↔ Alpha diversity in pre-menopausal women ↔Beta diversity between groups |
| Fuhrman et al.(24) | ↑ Clostridiales order and in particular the Ruminococcaceae family and the Ruminococcus genus in healthy women ↓ Bacteroides genus in healthy women |
↑ Alpha diversity in healthy post-menopausal women |
| Goedert et al.(21) | N/A | ↑ Alpha diversity in healthy post-menopausal women ↔ Alpha diversity in women with breast cancer |
| Goedert et al.(22) | N/A | ↑ Alpha diversity for the IgA-negative microbiota in women (control group) |
| He et al.(26) | ↔ In a cohort of women with normal BMD, osteopenia and osteoporosis | ↔ Alpha diversity in a cohort of women with normal BMD, osteopenia and osteoporosis |
| Zengul et al.(32) | ↔ Any bacterial species in post-menopausal women with breast cancer | N/A |
↑ denotes a positive association between the sex hormone and the outcome of interest.
↓ denotes a negative/inverse association between the sex hormone and the outcome of interest.
↔ denotes a lack of association found between the sex hormone and the outcome of interest
Table 3. Association between estrogen and testosterone and the bacterial composition and diversity.
Summary of findings from the three studies that investigated the association between both estrogen and testosterone and the bacterial composition and/or diversity. Two of these studies investigate the effects of testosterone and estrogen on both the bacterial composition and diversity, whilst one study only investigated their effects on bacterial composition.
N/A: not applicable; PCOS: polycystic ovary syndrome.
| Study | Estrogen | Testosterone | ||
|---|---|---|---|---|
| Bacterial Composition | Bacterial Diversity | Bacterial Composition | Bacterial Diversity | |
| Insenser et al.(30) | ↓ Paraprevotella genus (healthy men, healthy women and women with PCOS were considered as a whole) | ↓ Alpha diversity (healthy men, healthy women and women with PCOS were considered as a whole) | ↑ Raoultella and Paraprevotella genera (healthy men, healthy women and women with PCOS were considered as a whole) | ↑ Alpha diversity (healthy men, healthy women and women with PCOS were considered as a whole) |
| Shin et al.(25) | ↓ Slackia and Butyricimonas genera (All associations seen in healthy women) |
↑ Alpha diversity in healthy women | ↑ Acinetobacter, Dorea, Ruminococcus and Megammonas genera in healthy men | ↑ Alpha diversity in healthy men |
| Zhou et al.(28) | ↑ Subdoligranulum, Collinsella, Sutterella and Agathobacter genera (obese women with PCOS) ↓ Escherichia-Shigella and Ruminococcus_gna vus_group genera (obese women with PCOS) ↑ Parasutt`erella and Dialister genera (non-obese women with PCOS) |
N/A | ↑ Ruminococcus_2 and Collinsella genera (obese women with PCOS) ↑ Prevotella_2 and Megasphaera (non-obese women with PCOS) |
N/A |
↑ denotes a positive association between the sex hormone and the outcome of interest.
↓ denotes a negative/inverse association between the sex hormone and the outcome of interest.
↔ denotes a lack of association found between the sex hormone and the outcome of interest
Estrogens and microbiota diversity
In multiple studies, association were found between estrogen and gut microbiota diversity.(21-25, 30) The study by Insenser et al.(30) found that there was an inverse association between the total estradiol levels and alpha diversity (Chao1 and Shannon indexes) in healthy men, non-hyperandrogenic women, and women with PCOS. In healthy men, Flores et al.(23) found a strong association between the levels of total estrogens (particularly non-ovarian estrogens) and the gut microbiota alpha diversity (Richness, Chao1 and Shannon indexes). The same association was found in post-menopausal women, however, the Chao1 index did not reach statistical significance.(23) In pre-menopausal women there was no association observed between estrogen and diversity.(23) Fuhrman et al.(24) found a correlation between the ratio of estrogen metabolites (EM) to parent estrogen and alpha diversity (PD index) in healthy women, however the role of the EM to parent estrogen ratio in disease remains uncertain. In addition, the study by Goedert et al.(21) did not find an association between EM:parent estrogen and diversity in healthy women or women with breast cancer. However, the study reported a statistically significant association between levels of total estrogens and PD index in control women, which was not observed in women with breast cancer. The study by Shin et al.(25) also found an association between increasing estradiol levels and alpha diversity (Shannon and Simpson’s indexes) in healthy women. In addition, a statistically significant correlation was found between estrogen levels and PD index for the sorted IgA-negative microbiota in healthy women which was not observed in women with breast cancer.(22) He et al.(26) did not find a statistically significant association between estradiol and alpha diversity (richness and Shannon indexes) in a cohort of healthy women and women affected by either osteopenia or osteoporosis (see Tables 2-3). Taken together, there is strong evidence linking estrogen levels and alpha diversity in post-menopausal/ IgA-negative microbiota in healthy women. This is contrasted with there being no statistical difference between estrogens and alpha diversity in pre-menopausal women and beta diversity across all included groups. The variations in microbial diversity highlight the fact that despite efforts to control for confounding factors, microbial diversity is multifactorial.
Testosterone and the microbiota composition
Two of the included studies investigated the effect of testosterone on microbiota composition in men.(25, 30) Insenser et al.(30), reported a correlation between total testosterone, free testosterone and free testosterone:free estrogen ratio with the abundance of the Raoultella genus. In addition, when analysing the genera associated with sex and sex hormones, they found that Paraprevotella had the strongest positive and inverse association with testosterone and estrogen respectively.(30) Shin et al.(25) found that there was a correlation between levels of testosterone in men and abundance of the Acinetobacter, Dorea, Ruminococcus and Megammonas genera, with Ruminococcus being the most sensitive to testosterone levels. Four studies investigated the effects of testosterone on the microbiota composition in healthy women and women with PCOS.(27-29, 33) One study found that four of the metagenomic species (MGS) that were relevant to PCOS, belonged to the Enterobacteriaceae family, were associated with testosterone levels. Despite limited taxonomic annotation information, the MGS related to the women without PCOS exerted a negative correlation with the levels of testosterone.(33) The study by Liu et al.(48) found a positive association between testosterone levels and co-abundance groups (CAG) including Escherichia/Shigella, Streptococcus, Bacteroides, Blautia, Parabacteroides Clostridium XlVa, Alistipes, Weissella Granulicatella, Rothia, Streptococcus and Peptostreptococcus genera in women with and without PCOS. Furthermore, an inverse correlation was found between testosterone levels and CAGs which contained operational taxonomic units (OTU) belonging to the genera Alistipes, Akkermansia, Coprococcus, Collinsella and Ruminococcus.(27) The genera Ruminococcus_2 and Collinsella correlated positively with testosterone levels in obese women with PCOS while in non-obese women with PCOS testosterone levels were associated with the Prevotella_2 and Megasphaera genera.(28) The study by Liang et al.(29), which explored the association between testosterone and the microbiota composition of healthy women, non-obese women with PCOS and obese women with PCOS considered as a whole, found a positive association between testosterone and the Prevotella_9 genus. A negative association was found between testosterone and Halli_group, Subdoligranulum, Fusicatenibacter, Lachospiraceae_ND3007-group, Ruminococcaceae_UCG-003, Ruminiclostridum_5 and Tyzzerella_3 (see Tables 3-4).(29) Despite few studies examining the link between testosterone and microbial composition, there is strong evidence highlighting the positive correlation between testosterone and bacterial species such as Ruminococcus across the population studies for both women and men.
Table 4. Association between testosterone and the bacterial composition and diversity.
Summary of findings gathered in the four studies that investigated the association between testosterone and bacterial composition and/or diversity. Three of these investigated its effects on composition while one explored the effects on diversity.
N/A: not applicable; PCOS: polycystic ovary syndrome; PCOM: polycystic ovarian morphology.
| Study | Testosterone | |
|---|---|---|
| Bacterial Composition | Bacterial Diversity | |
| Chu et al.(33) | ↑ Four of the species related to PCOS (Enterobacteriaceae family) ↓ Species found to be related to healthy women |
N/A |
| Liang et al.(29) | ↑ Prevotella-9 genus ↓ Several species including Subdoligranulum, Halli-group, Fusicatenibacter, Lachospiraceae, Ruminococcaceae, Ruminiclostridium, Tyzzerella (Healthy women, normal-weight women with PCOS and obese women with PCOS considered as a whole) |
N/A |
| Liu et al.(27) | ↑ Multiple genera including Escherichia/Shigella,
Streptococcus, Clostridium XlVa, and Rothia ↓ Multiple genera including Akkermansia, Collinsella and Ruminococcus |
N/A |
| Torres et al.(31) | N/A | ↓ Alpha diversity ↔ Beta diversity between groups (All observed in a cohort of healthy women and women with PCOM and PCOS) |
↑ denotes a positive association between the sex hormone and the outcome of interest.
↓ denotes a negative/inverse association between the sex hormone and the outcome of interest.
↔ denotes a lack of association found between the sex hormone and the outcome of interest
Testosterone and microbiota diversity
In the study by Insenser et al.(30) when all participants were considered as a whole there was a significant correlation between the total testosterone levels and alpha diversity (Chao1 and Shannon indexes) – this association was also observed with the free testosterone to free estradiol ratio. A statistically significant association was found between testosterone levels in healthy men and the microbiota diversity.(25) When combining healthy women and women with polycystic ovarian morphology (PCOM) or PCOS, the study by Torres et al.(31) found a negative correlation between testosterone levels and diversity (richness and Faith PD indexes). The study also found that beta diversity between healthy women and women with PCOS was not due to the effect of different testosterone levels (see Tables 3-4). Akin to the correlation between testosterone and microbial composition, there is strong evidence suggesting testosterone affects alpha diversity, however, much like the studies comparing estrogen and microbial diversity, there is limited mechanistic.
Discussion
The results gathered in this systematic review highlight the interaction and association between estrogen and testosterone levels, and microbial composition and diversity. Increased studies focussing on the effects of estrogen on the gut microbiota, are beginning to solidify the relationship between estrogen and microbial composition. This is especially true for alpha diversity and such results are translatable across both healthy and pathological states. Despite the fact that there are fewer studies investigating the effects testosterone on gut microbiota, there is promising evidence suggesting that testosterone also plays an important role in microbial diversity and composition. However, there is a distinct paucity of data exploring and describing the molecular mechanisms that mediate the effect of estrogen and testosterone on microbial populations. Furthermore, exogenous factors such as age, sex and medical conditions also require further studies. These studies may reveal pathways and traits critical for maintaining a healthy microbial profile.
Clinical implications
It is known that sex is a risk factor for the prevalence and severity of certain pathologies with sex hormones possibly driving these differences.(35) Autoimmune conditions, such as autoimmune hepatitis, have a greater prevalence in females whilst autoinflammatory conditions including primary sclerosing cholangitis (PSC) affect men at a higher proportion.(36-38) Within the gastrointestinal tract, conditions including irritable bowel syndrome (IBS) are more common in women.(39)
Alterations in sex hormones levels may lead to microbial dysbiosis which has been previously implicated in IBD and its symptom severity.(40) For instance, paediatric IBD is more common in males, this is not the case for adult-onset IBD where there is a female predominance thus suggesting that pubertal fluctuations in sex hormones levels may contribute to IBD, and this may be mediated through microbiota alterations.(41)
In IBS, there is a female predominance with a ratio of ~2.5:1 and variations in sex hormone levels may induce microbiota dysbiosis, as IBS patients have different microbial composition compared to healthy controls.(42, 43) Differences in IBS symptom severity between men and women is also reported, with women having increased constipation compared to men, likely due to higher levels of progesterone and oestradiol.(44, 45) Female sex hormones may also modulate visceral hypersensitivity mechanisms through inflammatory mediators such as leukotrienes/prostaglandins interacting with peripheral nociceptor terminals (peripheral sensitization) and subsequent increased excitability of spinal neurones (central sensitization).(46) Low fermentable oligosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diets, a mainstay of IBS management, and small intestinal bacterial overgrowth (SIBO), a complication of IBS, are both related to alterations in the microbiota. Further studies should be carried out to understand the effect of sex hormones in these interactions.(47-50)
The role of sex hormones has been highlighted in the pathophysiology of multiple conditions including osteoporosis, PCOS and breast cancer.(51, 52) Studies in this review have identified that even in diseased states, such as PCOS, sex hormones interact with the gut microbiome. Taking this further, animal models have shown that there could be a bi-directional element of this relationship whereby gut microbiota influence sex hormone levels (and vice-versa) which in turn could influence the disease phenotypes.(53) Gut Microbiota is imperative in the metabolization of steroid hormones such as estrogen and testosterone (through enzyme induction). Through sex hormone metabolism, the systemic effects of sex hormones will vary thus phenotypic presentations of conditions such as PCOS and subfertility may differ accordingly. However, there are limited studies showing this link and more studies are required to further understand this.(54)
The results in this review highlight the importance of alpha diversity and how an increased alpha diversity is beneficial in gut homeostasis. It is known that a healthy gut microbiome consists primarily of Bacteroidetes spp. and Firmicutes spp. with a lower Firmicutes/Bacteroidetes ratio associated with better gut homeostasis and subsequently better health, namely a lower BMI.(55) Shin et al.(25) has demonstrated that higher levels of oestradiol decreases this ratio and be used therapeutically, in tandem with current weight loss techniques, to reduce the incidence of obesity.(55) Taking this idea further, the idea of a bidirectional relationship between sex hormones and enteric bacteria is especially true regarding the estrobolome. Butyric acid is not only produced from dietary fibres but also a metabolite of enteric bacteria and has been shown to play an integral role in the maintenance of the epithelial barrier.(56) In addition, butyrate has been shown to modulate progesterone synthesis and hence explain why elevated levels of progesterone may aid butyrate in regulation of the epithelial barrier through upregulation of tight junctions.(57, 58)
Future studies aimed at gaining a better understanding of this interaction may be translatable to clinical practice. Personalised treatments, including fecal microbiota transplantation (FMT) and probiotics, could become an important treatment paradigm for treating the conditions discussed, and on-going studies in this area will provide data on mechanisms and clinical efficacy.
Molecular mechanisms through which sex hormones may be associated with the microbiota
a. Estrogen
Despite limited evidence to explain the associations shown, several theories have been postulated to demonstrate the association between estrogens and the gut microbiota. A proposed mechanism is the presence of an “estrobolome”. The estrobolome is defined as the group of bacterial genes which are able to metabolize estrogens through their products.(59) The bacterial species which are particularly important in the metabolism of estrogens are the ones capable of producing the β-glucuronidases and β-glucuronides enzymes.(60) These species, for example Escherichia and Collinsella, are believed to impact the enterohepatic circulation of estrogens. Conjugated estrogens released through bile are subsequently de-conjugated by the estrobolome and re-absorbed.(61-63)
Numerous studies support the proposed mechanism which underpins these associations. For example, administration of oral antibiotics, which is known to alter the microbiota composition, results in increased conjugated estrogen excretion and lower urinary estrogens.(64-66) Furthermore, in vitro and in vivo animal model studies further highlight the potential role of the estrobolome in estrogen metabolism.(67-69) Associations between Ruminococcaceae family and activity of β-glucuronidase and β-glucosidase may explain the positive correlation observed between estrogen levels and Ruminococcaceae in two of the studies included in this systematic review.(23, 24, 70) However, it is worth noting that other bacterial species which were found to be associated with levels of estrogen have not been previously shown to have the genes that encode ß-glucuronidase.(60) Flores et al.(44) demonstrated that there was a relatively weak correlation between faecal β-glucuronidase activity and levels of urinary estrogens. This postulates how there could be more than one mechanism to explain the relationship between estrogen and gut microbiota. Diet and probiotics have been shown to alter the β-glucuronidase activity, highlighting the importance of factoring dietary nutrients as a study variable within and across studies investigating the association between sex hormones and the microbiota.(71, 72)
b. Testosterone
There is limited understanding of the underlying mechanisms driving the association between testosterone and microbiota composition and diversity. However, gut bacterial β-glucuronidases facilitate the deconjugation of glucuronidated testosterone and dihydrotestosterone (DHT) into their hydrolysed form which subsequently enter the enterohepatic circulation.(73, 74) Furthermore, the bacterial strain Clostridium scindens is able to convert glucosteroids secreted in bile into androgens in vitro which may have an effect on local and systematic testosterone levels in vivo (75, 76). The negative correlation between testosterone and microbiota diversity in women with PCOS was also observed in a PCOS mouse model while the positive association between testosterone and Ruminococcus in healthy men was also observed in neonatally-androgenized rats.(12, 25, 31)
Reliability of results
The differences in the associations observed in different studies may be partially explainable by the differences in study characteristics which could impact the reliability of the results.
While majority of studies include homogenous population groups, there was inter-study heterogeneity in population. Differences in study designs may also reflect in differences to reported results. For example, exclusion criteria varied amongst studies and confounding variables such as diet, smoking, symbiotic and antibiotics usage and may have influenced the associations observed. Measurement of sex hormone levels was via serum or urine and, quantification assays differed with inter- and intra-assay (CV) variability. Whilst all studies used NGS, one study used shotgun metagenomic sequencing while all the others used 16S rRNA quantification which may have affected the comparability of the results. Different analysis methods were used including DNA extraction techniques, primers and hypervariable regions, bioinformatic pipelines, matching databases and clustering cut-offs which may have influenced the outcomes observed.(77-80)
Limitations
There are several limitations in this systematic review. The lenient study inclusion criteria made data analysis and synthesis more challenging due to the heterogeneity of the studies. Several of the included studies were performed on healthy men and women whilst others were performed in participants with conditions such as PCOS and breast cancer thus rendering it impossible to combine and compare all the studies in a single analysis. However, having more focused inclusion criteria would have resulted in an inadequate number of included studies. Furthermore, only limited data was collected from several studies as the primary aims of the studies were not to directly investigate the association between sex hormones and the microbiota; some studies analysed all participants as a whole rather than based on the participant’s characteristics (e.g., age, sex, medical conditions) therefore rendering the analysis more challenging. The JBI critical appraisal tools, whilst being excellent and widely used, can be rather subjective as they do not allow to quantitatively rate the individual criteria but instead only have the options of “yes”, “no” and “unknown”. An additional limitation is that all studies that met eligibility were included for analysis regardless of the risk of bias assessment, thus, potentially affecting the reliability of the results. In addition, since a meta-analysis was not performed, the narrative synthesis may have been prone to bias.
Overall the investigation of the microbiota using faeces or rectal swabs has numerous limitations; for instance, these methods may only provide a representation of specific parts of the digestive tract and as observed the composition of the microbiota differs between the proximal and distal parts of the intestine and between the luminal and mucosal layers.(81)
Conclusion
Future studies should aim to assess the interaction between sex hormones and the gut microbiota by dividing individuals based on age, sex and medical conditions as these appear to influence the association. Furthermore, as seen by the low percentage of the total participants that were male (9%), additional studies in men are required to further explore the differences in the interaction between sex hormones and the microbiota in each sex. Research should also be directed at gaining an in-depth understanding of the complex mechanisms underlying the interaction between sex hormones and the microbiota. Sex hormones play a role in multiple conditions, thus, identifying and understanding the underlying association between the microbiota and sex hormones may be pivotal in the prevention and treatment of these conditions by targeting the microbiota.
Supplementary Material
Grant Support
M.P. is supported by Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Gut Microbes and Health BB/R012490/1 and its constituent project (BBS/E/F/000PR10355).
Footnotes
Conflict of Interest: All authors declare no conflict of interest.
References
- 1.Schmidt TSB, Raes J, Bork P. The Human Gut Microbiome: From Association to Modulation. Cell. 2018;172(6):1198–215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Kho ZY, Lal SK. The human gut microbiome - A potential controller of wellness and disease. Frontiers in Microbiology. 2018 AUG;9 doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7 doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis DJ, Hecht PM, Jasarevic E, Beversdorf DQ, Will MJ, Fritsche K, et al. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain, Behavior, and Immunity. 2017;59:38–48. doi: 10.1016/j.bbi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 6.Org E, Mehrabian M, Parks BW, Shipkova P, Liu XQ, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–22. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernbom N, Nørrung B, Saadbye P, Mølbak L, Vogensen FK, Licht TR. Comparison of methods and animal models commonly used for investigation of fecal microbiota: effects of time, host and gender. J Microbiol Methods. 2006;66(1):87–95. doi: 10.1016/j.mimet.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Acharya KD, Gao X, Bless EP, Chen J, Tetel MJ. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Scientific Reports. 2019;9(1):1–13. doi: 10.1038/s41598-019-56723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedek G, Zhang J, Nguyen H, Kent G, Seifert HA, Davin S, et al. Estrogen protection against EAE modulates the microbiota and mucosal-associated regulatory cells. Journal of Neuroimmunology. 2017;310:51–9. doi: 10.1016/j.jneuroim.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen KLA, Zhao YC, Hieronymi K, Smith BP, Madak-Erdogan Z. Bazedoxifene and conjugated estrogen combination maintains metabolic homeostasis and benefits liver health. PLoS One. 2017;12(12):e0189911. doi: 10.1371/journal.pone.0189911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, et al. Association between polycystic ovary syndrome and gut microbiota. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley ST, Skarra DV, Rivera AJ, Thackray VG. The Gut Microbiome Is Altered in a Letrozole-Induced Mouse Model of Polycystic Ovary Syndrome. PLoS One. 2016;11(1):e0146509. doi: 10.1371/journal.pone.0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thackray VG. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol Metab. 2019;30(1):54–65. doi: 10.1016/j.tem.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandher RK, Aning J. Diagnosing and managing androgen deficiency in men. Practitioner. 2017;261(1803):19–22. [PubMed] [Google Scholar]
- 15.Carmina E. The spectrum of androgen excess disorders. Fertil Steril. 2006;85(6):1582–5. doi: 10.1016/j.fertnstert.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 16.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009 [PMC free article] [PubMed] [Google Scholar]
- 18.Demers LM, Hankinson SE, Haymond S, Key T, Rosner W, Santen RJ, et al. Measuring Estrogen Exposure and Metabolism: Workshop Recommendations on Clinical Issues. J Clin Endocrinol Metab. 2015;100:2165–70. doi: 10.1210/jc.2015-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barba M, Czosnek H, Hadidi A. Historical Perspective, Development and Applications of Next-Generation Sequencing in Plant Virology. Viruses. 2014;6:106–36. doi: 10.3390/v6010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goedert JJ, Jones G, Hua X, Xu X, Yu GQ, Flores R, et al. Investigation of the Association Between the Fecal Microbiota and Breast Cancer in Postmenopausal Women: a Population-Based Case-Control Pilot Study. Jnci-Journal of the National Cancer Institute. 2015;107(8) doi: 10.1093/jnci/djv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goedert JJ, Hua X, Bielecka A, Okayasu I, Milne GL, Jones GS, et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. British Journal of Cancer. 2018;118(4):471–9. doi: 10.1038/bjc.2017.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores R, Shi JX, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. Journal of Translational Medicine. 2012;10 doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632–40. doi: 10.1210/jc.2014-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Research in microbiology. 2019;170(4–5):192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 26.He JQ, Xu SB, Zhang BZ, Xiao CX, Chen ZR, Si FY, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging-Us. 2020;12(9):8583–604. doi: 10.18632/aging.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Zhang CH, Shi Y, Zhang F, Li LX, Wang XJ, et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Ni ZX, Cheng W, Yu J, Sun S, Zhai DX, et al. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocrine Connections. 2020;9(1):63–73. doi: 10.1530/EC-19-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Y, Ming Q, Liang J, Zhang Y, Zhang H, Shen T. Gut microbiota dysbiosis in polycystic ovary syndrome: Association with obesity — A preliminary report. Canadian Journal of Physiology and Pharmacology. 2020;98(11):803–9. doi: 10.1139/cjpp-2019-0413. [DOI] [PubMed] [Google Scholar]
- 30.Insenser M, Murri M, del Campo R, Martinez-Garcia MA, Fernandez-Duran E, Escobar-Morreale HF. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. Journal of Clinical Endocrinology & Metabolism. 2018;103(7):2552–62. doi: 10.1210/jc.2017-02799. [DOI] [PubMed] [Google Scholar]
- 31.Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–11. doi: 10.1210/jc.2017-02153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zengul AG, Demark-Wahnefried W, Barnes S, Morrow CD, Bertrand B, Berryhill TF, et al. Associations between Dietary Fiber, the Fecal Microbiota and Estrogen Metabolism in Postmenopausal Women with Breast Cancer. Nutrition and cancer. 2020:1–10. doi: 10.1080/01635581.2020.1784444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertility and Sterility. 2020;113(6):1286–98.:e4. doi: 10.1016/j.fertnstert.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, et al. Conducting a Microbiome Study. Cell. 2014;158(2):250–62. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurvich C, Hoy K, Thomas N, Kulkarni J. Sex Differences and the Influence of Sex Hormones on Cognition through Adulthood and the Aging Process. Brain Sci. 2018;8(9) doi: 10.3390/brainsci8090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol (N Y) 2013;9(10):633–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Jansson-Knodell CL, King KS, Larson JJ, Van Dyke CT, Murray JA, Rubio-Tapia A. Gender-Based Differences in a Population-Based Cohort with Celiac Disease: More Alike than Unalike. Dig Dis Sci. 2018;63(1):184–92. doi: 10.1007/s10620-017-4835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ucar-Comlekoglu D, Fox A, Sen HN. Gender Differences in Behçet’s Disease Associated Uveitis. J Ophthalmol. 2014;2014:820710. doi: 10.1155/2014/820710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 40.Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019;8(3) doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greuter T, Manser C, Pittet V, Vavricka SR, Biedermann L on behalf of Swiss IBDnet aowgotSSoG. Gender Differences in Inflammatory Bowel Disease. Digestion. 2020;101(Suppl 1):98–104. doi: 10.1159/000504701. [DOI] [PubMed] [Google Scholar]
- 42.Kim YS, Kim N. Sex-gender differences in irritable bowel syndrome. Journal of Neurogastroenterology and Motility. 2018;24(4):544–58. doi: 10.5056/jnm18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7 doi: 10.12688/f1000research.14592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. 2009;54(7):1542–9. doi: 10.1007/s10620-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choghakhori R, Abbasnezhad A, Amani R, Alipour M. Sex-Related Differences in Clinical Symptoms, Quality of Life, and Biochemical Factors in Irritable Bowel Syndrome. Dig Dis Sci. 2017;62(6):1550–60. doi: 10.1007/s10620-017-4554-6. [DOI] [PubMed] [Google Scholar]
- 46.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56(12):1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. 2017 doi: 10.1136/gutjnl-2017-313750. [DOI] [PubMed] [Google Scholar]
- 48.Sloan TJ, Jalanka J, Major GAD, Krishnasamy S, Pritchard S, Abdelrazig S, et al. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS One. 2018;13(7):e0201410. doi: 10.1371/journal.pone.0201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018;53(7):807–18. doi: 10.1007/s00535-018-1476-9. [DOI] [PubMed] [Google Scholar]
- 50.Lichten E. Are the estrogenic hormonal effects of environmental toxins affecting small intestinal bacterial and microfilaria overgrowth? Med Hypotheses. 2017;109:90–4. doi: 10.1016/j.mehy.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Zborowski JV, Cauley JA, Talbott EO, Guzick DS, Winters SJ. Clinical Review 116: Bone mineral density, androgens, and the polycystic ovary: the complex and controversial issue of androgenic influence in female bone. J Clin Endocrinol Metab. 2000;85(10):3496–506. doi: 10.1210/jcem.85.10.6902. [DOI] [PubMed] [Google Scholar]
- 52.Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5(5):239–47. doi: 10.1186/bcr628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cross TWL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Molecular Metabolism. 2018;15:70–81. doi: 10.1016/j.molmet.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussain T, Murtaza G, Kalhoro DH, Kalhoro MS, Metwally E, Chughtai MI, et al. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim Nutr. 2021;7(1):1–10. doi: 10.1016/j.aninu.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 56.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu N, Li M, Lei H, Jiang X, Tu W, Lu Y, et al. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. J Steroid Biochem Mol Biol. 2017;172:89–97. doi: 10.1016/j.jsbmb.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 58.van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM. A Direct Effect of Sex Hormones on Epithelial Barrier Function in Inflammatory Bowel Disease Models. Cells. 2019;8(3) doi: 10.3390/cells8030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plottel CS, Blaser MJ. Microbiome and Malignancy. Cell Host & Microbe. 2011;10(4):324–35. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwa M, Plottel CS, Blaser MJ, Adams S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heimer GM, Englund DE. Enterohepatic recirculation of oestriol studied in cholecystectomized and non-cholecystectomized menopausal women. Ups J Med Sci. 1984;89(2):107–15. doi: 10.3109/03009738409178470. [DOI] [PubMed] [Google Scholar]
- 62.Mcbain AJ, Macfarlane GT. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. 1998 doi: 10.1099/00222615-47-5-407. [DOI] [PubMed] [Google Scholar]
- 63.Adlercreutz H, Martin F, Pulkkinen M, Dencker H, Rimér U, Sjöberg NO, et al. Intestinal metabolism of estrogens. J Clin Endocrinol Metab. 1976;43(3):497–505. doi: 10.1210/jcem-43-3-497. [DOI] [PubMed] [Google Scholar]
- 64.Martin F, Peltonen J, Laatikainen T, Pulkkinen M, Adlercreutz H. Excretion of progesterone metabolites and estriol in faeces from pregnant women during ampicillin administration. J Steroid Biochem. 1975;6(9):1339–46. doi: 10.1016/0022-4731(75)90363-5. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471–89. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Adlercreutz H, Pulkkinen MO, Hämäläinen EK, Korpela JT. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984;20(1):217–29. doi: 10.1016/0022-4731(84)90208-5. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu K, Muranaka Y, Fujimura R, Ishida H, Tazume S, Shimamura T. Normalization of reproductive function in germfree mice following bacterial contamination. Exp Anim. 1998;47(3):151–8. doi: 10.1538/expanim.47.151. [DOI] [PubMed] [Google Scholar]
- 68.Järvenpää P, K T, Fotsis T, Adlercreutz H. In vitro metabolism of estrogens by isolated intestinal micro-organisms and by human faecal microflora. Journal of Steroid Biochemistry. 1980;13(3):345–9. doi: 10.1016/0022-4731(80)90014-x. [DOI] [PubMed] [Google Scholar]
- 69.Lombardi P, G Barry, Boutin Eugenie, Gorbach Sherwood L. Metabolism of androgens and estrogens by human fecal microorganisms. Journal of Steroid Biochemistry. 1980;9(8):795–801. doi: 10.1016/0022-4731(78)90203-0. [DOI] [PubMed] [Google Scholar]
- 70.Flores R, Shi J, Gail MH, Gajer P, Ravel J, Goedert JJ. Association of Fecal Microbial Diversity and Taxonomy with Selected Enzymatic Functions. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldin BR, Swenson L, Dwyer J, Sexton M, Gorbach SL. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst. 1980;64(2):255–61. doi: 10.1093/jnci/64.2.255. [DOI] [PubMed] [Google Scholar]
- 72.McIntosh FM, Maison N, Holtrop G, Young P, Stevens VJ, Ince J, et al. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ Microbiol. 2012;14(8):1876–87. doi: 10.1111/j.1462-2920.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 73.Collden H, Landin A, Wallenius V, Elebring E, Fandriks L, Nilsson ME, et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. American Journal of Physiology-Endocrinology and Metabolism. 2019;317(6):E1182–E92. doi: 10.1152/ajpendo.00338.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li CY, Basit A, Gupta A, Gáborik Z, Kis E, Prasad B. Major glucuronide metabolites of testosterone are primarily transported by MRP2 and MRP3 in human liver, intestine and kidney. J Steroid Biochem Mol Biol. 2019;191:105350. doi: 10.1016/j.jsbmb.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, et al. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013;54(9):2437–49. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soory M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J Periodontal Res. 1995;30(2):124–31. doi: 10.1111/j.1600-0765.1995.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 77.Greathouse K, Sinha R, Vogtmann E. DNA extraction for human microbiome studies: the issue of standardization. Genome Biology. 2019;20(1):1–4. doi: 10.1186/s13059-019-1843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rintala A, Pietilä S, Munukka E, Eerola E, Pursiheimo JP, Laiho A, et al. Gut Microbiota Analysis Results Are Highly Dependent on the 16S rRNA Gene Target Region, Whereas the Impact of DNA Extraction Is Minor. J Biomol Tech. 2017;28:19–30. doi: 10.7171/jbt.17-2801-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jovel J, Patterson J, Wang W, Hotte N, O’Keefe S, Mitchel T, et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prodan A, Tremaroli V, Brolin H, Zwinderman AH, Nieuwdorp M, Levin E. Comparing bioinformatic pipelines for microbial 16S rRNA amplicon sequencing. PLoS One. 2020;15(1):e0227434. doi: 10.1371/journal.pone.0227434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: A fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11(8):1131–40. doi: 10.3748/wjg.v11.i8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.