Abstract
Photosynthesis is readily impaired by high light levels. Photosynthetic organisms have therefore evolved various mechanisms to cope with the problem. Here, we have dramatically enhanced the light tolerance of the cyanobacterium Synechocystis by adaptive laboratory evolution (ALE). By combining repeated mutagenesis and exposure to increasing light intensities, we generated strains that grow under extremely high light (HL) intensities. HL tolerance was associated with more than 100 mutations in proteins involved in various cellular functions, including gene expression, photosynthesis and metabolism. Co-evolved mutations were grouped into five haplotypes, and putative epistatic interactions were identified. Two representative mutations, introduced into wild-type cells, each confer enhanced HL tolerance, but they affect photosynthesis and respiration in different ways. Mutations identified by ALE that allow photosynthetic microorganisms to cope with altered light conditions could be employed in assisted evolution approaches and strengthen the robustness of photosynthesis in crop plants.
In photosynthetic organisms, excess light can damage cells by promoting the accumulation of reactive oxygen species (ROS), which damage photosystems I and II (PSI/II) and ultimately lead to metabolic breakdown1–5. Repair of PSII involves the replacement of photodamaged D1 proteins6, but rates of damage outstrip repair capacity at high light (HL) intensities, resulting in photoinhibition4. Photodamage to PSI is irreversible5 and the recovery of PSI is thought to involve the replacement of the damaged complex. To minimize photoinhibition, photosynthetic organisms have evolved a variety of mechanisms, including quenching processes that dissipate excess light as heat [e.g. non-photochemical quenching (NPQ)], cyclic electron flow (CEF), O2 photoreduction, photorespiration, protein quality control, and decreasing the PSI/PSII ratio3, 5, 7–13. In addition, non-enzymatic antioxidants (such as ascorbate and glutathione) and specific enzymes (like superoxide dismutase, ascorbate peroxidase, glutathione peroxidase, and catalase) act as scavengers of ROS1, 2.
Increased tolerance to high or fluctuating light conditions could enhance plant productivity and is therefore of great practical interest. Thus, parallel overexpression of three proteins involved in NPQ accelerates photoprotection in tobacco and Arabidopsis thaliana, but has positive effects on growth only in tobacco14, 15. Conversely, overexpression of ROS-scavenging systems can be counterproductive, because hydrogen peroxide also serves as a retrograde signal during acclimation to HL16. Fast forward genetics has been applied to enhance HL acclimation in the green alga Chlamydomomas reinhardtii, and identified a homolog of plant COP1 as a factor involved in NPQ17.
Here, we employed adaptive laboratory evolution (ALE) to select for stable HL tolerance in the cyanobacterium Synechocystis sp. PCC6803 (hereafter: Synechocystis), which is an established platform for genetic engineering and ALE experiments18, 19 and is typically cultivated at low light intensities (20-50 μmol photons m-2 s-1) and a wide temperature range (from 23 °C to 33 °C)20, 21. We have generated and characterized strains that can grow at light intensities exceeding maximal solar irradiance under terrestrial conditions. The effects of two representative protein mutations identified in the adapted strains on HL tolerance were confirmed and functionally characterised in the wild-type background.
Results
Generation and physiological characterisation of HL-tolerant strains
To generate HL-tolerant strains, the mutagenesis scheme previously used to generate thermotolerant Synechocystis strains22, 23 was modified. A batch culture of our Synechocystis laboratory strain (designated “lab type” or “LT”) was mutagenized by exposure to UV light (U) and the resulting strain was subsequently re-mutagenized up to 4 times with methyl methanesulfonate (MMS or M) or U in different permutations (Fig. 1). After each round of mutagenesis, cells were grown under increasing light intensities (from 700 μmol photons m-2 s-1 after the first mutagenesis to up to 2300 μmol photons m-2 s-1 after the final mutagenesis) for 5 to 25 cultivation cycles, each generally lasting for ~15 days (Fig. 1; for further details, see Methods). The light level for initial selection (700 μmol photons m-2 s-1) was chosen so as to induce overt HL stress while allowing for productive growth (Fig. 2a). This experimental design resulted in several lineages of adapted strains, designated as U, UM, UMU, UMM, UMUM, UMMM, and UMUMM. In addition, UMU* and UMU*M strains were generated by subjecting UM cultures to additional selective cycles before the next mutagenesis. LT cells cultivated at 100 μmol photons m-2 s-1 over the entire period served as controls (“LT”) (Fig. 1). A second control population (LT´) was obtained by transferring a LT batch from 100 to 1400 μmol photons m-2 s-1 after 14 months and growing the culture for ~6 weeks instead of the usual 15 days. After 14 cultivation cycles at 1400 μmol photons m-2 s-1 the strain was propagated under increasing light intensities of up to 2200 μmol photons m-2 s-1 (Fig. 1).
Fig. 1. ALE scheme.
Batch cultures (large ovals) were mutagenized by various permutations of UV radiation and MMS treatment, and are designated according to the mutagens employed (U, UV; M, MMS). The numbers of selective cycles are indicated as dots on the left, and the light intensities utilised after each round of mutagenesis are indicated in μmol photons m-2 s-1. For whole-genome sequencing (indicated by double helices), DNA was analysed either from monoclonal cultures (small ovals) or from final and intermediate batch cultures. A light intensity of 700 μmol photons m-2 s-1 was chosen for initial selection as it induced visible signs of HL stress while allowing for productive growth (see Fig. 2a).
Fig. 2. Characterisation of HL-tolerant batch cultures.
a, Image of original LT cells grown under low (100 μmol photons m-2 s-1), intermediate (700 μmol photons m-2 s-1) and lethally high (1100 μmol photons m-2 s-1) light intensities for 7 days at 23 °C under continuous illumination and atmospheric aeration. A light intensity of 700 μmol photons m-2 s-1 induced visible high light stress while still allowing for productive growth. b and c, Images of batch cultures after 12d cultivation under high light (2000 μmol photons m-2 s-1) (b), followed by 12d low light (50 μmol photons m-2 s-1) (c). LT grown at 100 μmol photons m-2 s-1 (LT100) served as control in panel b. d and e, dry mass accumulation of samples from panel b and c. Asterisks indicate significant differences (d, p = 1.82E-02; 4.01E-02; 4.35E-02; 1.71E-10; 9.98E-11; 3.71E-06, and e, p = 2.22E-01; 7.39E-01; 1.06E-04; 1.06E-05; 2.54E-02; 3.26E-05 in corresponding order) from LT controls according to post-hoc Bonferroni-Holm comparisons for n=3 replicates after significant among-group differences were detected by one-factorial ANOVA (two-sided). Error bars indicated SDs from respective average values. f, Images of the batch cultures as in panels b and c, but grown for 12 d at 3000 μmol photons m-2 s-1. As control the LT strain grown at 100 μmol photons m-2 s-1 (LT100) is shown.
Batch cultures of HL-tolerant and control cells were characterised for their performance under high (2000 μmol photons m-2 s-1, HL) and low (50 μmol photons m-2 s-1, LL) light conditions (Fig. 2b-e). LT is inviable under 2000 μmol photons m-2 s-1 (Fig. 2a); therefore, LT grown at 100 μmol photons m-2 s-1 (LT100) served as the control for the 2000 μmol photons m-2 s-1 experiment. Characterisation of the HL-adapted strains revealed that biomass accumulation in some HL-adapted cultures at HL was similar to that of LT100 plants (Fig. 2d). Under LL, biomass accumulation in all HL-adapted cultures was lower than in LT; however, in some strains (UMMM and UM) it approached the LT range (Fig. 2d). Intriguingly, the HL-adapted strains could also cope with light intensities exceeding maximal solar irradiance under terrestrial conditions (3000 μmol photons m-2 s-1) (Fig. 2f). Thus, HL-adapted Synechocystis cultures can grow at extremely high light intensities, with a moderate trade-off under low light.
Mutations in HL-tolerant monoclonal strains and batch cultures
Within the same lineages of batch cultures, phenotypic variation with respect to growth rate and coloration was observed between different selective cycles (Fig. 3a). When monoclonal strains were isolated from batch cultures, variation in coloration and basic chlorophyll fluorescence (Fo) was still noted (Fig. 3b,c). Therefore, we performed whole-genome analysis of adapted strains at the individual clone level, and selected for clones that displayed different levels of Fo to ensure representation of most of the variation within a batch (Fig. 3c-e). To identify HL-tolerance-associated mutations by genome sequencing, four monoclonal strains were selected from each final HL-adapted batch culture (Fig. 3d), as well as appropriate control strains (LT and LT´), giving a total of 28 samples (see Methods). In addition, the starting LT strain at t=0 (LTt=0) was sequenced to serve as the reference genome. LTt=0 exhibited several changes relative to the original Kazusa Synechocystis sequence24. These comprised 13 deletions, 15 insertions, 2 multinucleotide substitutions and 43 SNPs (12 transversions, 31 transitions). In addition, metagenomes of final and intermediary batch cultures, representing branching points of the experimental pedigree (see Fig. 1), were sequenced to recover additional mutant alleles that failed to survive or were not sampled in the course of single-clone isolation.
Fig. 3. Heterogeneity between and within adapted batch cultures.
a, The same batch culture displays varying colouration and density over the course of different selection rounds. b, Individual clones of batch cultures grown at 50 μmol photons m-2 s-1 for 7 days post inoculation (dpi) were isolated by the plate streaking technique and differ in colour. c, Twenty-four individual clones isolated as in panel b display variations in photosynthesis as determined by quantifying basic chlorophyll fluorescence (Fo) and indicated by the colour code. d, Four of the 24 individual clones (from each batch culture) grown at 100 μmol photons m-2 s-1 for 5 dpi were selected and subjected to Fo analysis. e, Pre-selection distributions of Fo in the original n=24 isolated clones (panel c) and post-selection distributions of n=16 samples of four biological replicates of each of the four selected ones (panel d). The four selected clones recapitulate the pre-selection Fo distribution. For boxplot description see Methods.
In total, 612 different mutations were recovered (see Methods), in varying allele frequencies, from all sequenced (meta-)genomes (see Supplementary Data 1). Of these alleles, 74 resided in intergenic regions (comprising 10 small deletions, 15 small insertions, 49 single nucleotide polymorphisms i.e. SNPs). Another 538 mutant alleles were localized to 223 different genes/open reading frames (ORFs) (33 small deletions, 6 small insertions, 499 SNPs), with the SNPs comprising 133 synonymous and 366 non-synonymous mutations (9 premature stop codons, 357 amino-acid exchanges). In addition, large deletions were detected in ycf45 and slr1189. In all, 196 mutant alleles were exclusively detected in HL-adapted mutant material (i.e. allele frequency = 0.0 % in all control LT samples), and 108 of these were non-synonymous SNPs in coding regions.
In the 28 monoclonal strains sequenced, a total of 167 mutant alleles with frequencies ≥80% were detected relative to LTt=0. The majority (151 mutations) localized to protein-coding regions (Supplementary Data 1), comprising 29 synonymous and 114 non-synonymous mutations, as well as eight insertions/deletions, seven of which cause frameshifts. In total, 124 different ORFs were affected. A single non-synonymous mutation (Sll0788D129E) and a single intergenic 8-bp deletion between sll5104 and slr5105 were found at high frequencies (>80%) in all HL-adapted and all LT control clones. They thus represent background mutations relative to the LTt=0 reference and possibly reflect adaptations to our specific growth conditions. Of the remaining 113 non-synonymous mutations, only seven occurred at a frequency ≥80% in LT control clones (with five mutations occurring once, one mutation thrice, and one mutant allele occurring in all four LT control clones). The incomplete segregation of several mutations might indicate that the presence of both WT and mutant variants might allow fine tuning of the respective processes.
Of particular interest were genes with multiple independent non-synonymous mutations (Table 1). This set includes four genes involved in transcription (rpoC1, sigA, sigC, and kaiC-like protein 1), one each in translation (fusB) and photosynthesis/respiration (ndhF), five genes with putative functions in metabolism (slr2124, gltA, ppc, pykF/pyk2, and spoT), one gene involved in chemotaxis (mcpA), four genes with unknown function (slr0753, slr1546, slr6022/slr6081 and ssr5117) and five transposable elements. Intriguingly, only one of our high-frequency, multiply mutated genes, pykF/pyk2, was previously found to be mutated in high-temperature-tolerance ALE experiments23, while none has been reported to play a crucial role in HL acclimation based on transcript profiling or physiological analyses10.
Table 1. Overview of multiple non-synonymous mutations in HL-adapted strains.
Independent non-synonymous mutations. a, UM; b, UMMM; c, UMU*M; d, UMUM; e, UMUMM; f, LT; g, LT´. “x”, clone(s) with high mutant allele frequencies ≥ 80%; “°”, clone(s) with low/medium mutant allele frequencies < 80%; “+”, additional mutant alleles recovered from sequenced batch-culture meta-genomes with frequency ≥ 30%. Additional metagenome-derived alleles are shaded in grey. The two mutations selected for the recapitulation of HL tolerance in LT cells (#1 and #2) are indicated in bold letters.
| Gene | Mutation | Origin | ||||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | ||
| T r a n s c r i p t i o n | ||||||||
| rpoC1 (slr1265): RNA polymerase γ-subunit | K226E | x | ||||||
| F345Y | x | x | ◦ | ◦ | ||||
| K504I | x | x | ||||||
| D551V | x | ◦ | ||||||
| sigA/rpoD1 (slr0653): principal RNA polymerase sigma factor | R96L | ◦ | x | |||||
| K206R | ◦ | x | x | |||||
| Q316R | + | + | ||||||
| M383K | x | |||||||
| K421M | x | |||||||
| sigC (sll0184): group2 RNA polymerase sigma factor | A274V | ◦ | ◦ | ◦ | ◦ | x | ◦ | ◦ |
| E298V | x | |||||||
| kaiC-like protein 1 (sll1595): circadian clock protein homolog | F337S | x | ||||||
| K395E | ◦ | x | ||||||
| T r a n s l a t i o n | ||||||||
| fusB (sll1098): elongation factor G2 | R29Q | x | x | x | ||||
| D138N | + | |||||||
| D189G | x | ◦ | ||||||
| V284A | x | x | ◦ | x | ||||
| K304N | x | ◦ | ||||||
| Y328N | x | ◦ | ||||||
| R461C (#2) | x | x | ◦ | |||||
| P h o t o s y n t h e s i s / R e s p i r a t i o n | ||||||||
| ndhF1 (slr0844): NDH complex subunit 5 | F124L (#1) | x | ◦ | ◦ | ||||
| ndhF3 (sll0026): NDH complex subunit 5 | A283D | x | ||||||
| A347G | x | |||||||
| M e t a b o l i s m | ||||||||
| slr2124: 3-oxoacyl-[acyl-carrier protein] reductase | L57P | x | ||||||
| L116H | ◦ | x | ||||||
| I126T | + | |||||||
| F230L | x | |||||||
| gltA (sll0401): citrate synthase | K329M | x | ||||||
| V387A | x | ◦ | ||||||
| ppc (sll0920): phosphoenol-pyruvate carboxylase | T252M | ◦ | x | |||||
| R1031C | x | ◦ | ◦ | ◦ | ||||
| pykF (sll1275): pyruvate kinase 2 | A260V | ◦ | x | |||||
| P406T | ◦ | x | ||||||
| spoT (slr1325): tentative guanosine-3',5'-bis(diphosphate) 3'-pyrophosphohydrolase | N202D | + | ||||||
| Q416R | x | |||||||
| C h e m o t a x i s | ||||||||
| mcpA (slr1044): methyl-accepting chemotaxis protein | L559I | x | ◦ | |||||
| A609V | ◦ | ◦ | ◦ | ◦ | ◦ | x | ||
| U n k n o w n f u n c t i o n | ||||||||
| slr0753: uncharacterized transporter/p-protein | A116T | ◦ | ◦ | ◦ | x | |||
| T118I | ◦ | x | ||||||
| T162S | x | ◦ | ||||||
| P338S | ◦ | x | ||||||
| T395I | x | |||||||
| slr1546: transmembrane protein, unknown function | +a (407/639 nt) | x | ||||||
| Δ1 bp (536/639 nt) | x | x | x | x | ||||
| slr6022/slr6081: transmembrane protein, unknown function | Q152R | ◦ | x | ◦ | ◦ | ◦ | ◦ | |
| G179A | ◦ | x | ◦ | ◦ | ◦ | ◦ | ◦ | |
| T r a n s p o s o n s / T r a n s p o s a s e g e n e s | ||||||||
| sll8042 | M1 "- "/L2 "- "/R3 "- " | x | ||||||
| slr1682 | E102* | x | ||||||
| slr1902 | V28G | x | ||||||
| slr1903 | R43W | x | ||||||
| slr2062 | T126I | x | ||||||
Adaptive haplotypes and epistatic series
To recognize adaptive patterns in our HL-tolerant clones we investigated the incidence of co-occurrence of high-frequency mutant alleles in multiple strains (i.e. haplotypes) and inferred the chronological order of mutation events by phylogenetic reconstruction (see Methods). In a maximum-likelihood (ML) phylogeny, all adapted monoclonal strains were found to be phylogenetically clearly distinct from LT and contained numerous potentially adaptive mutations (Fig. 4a,b). The UMUM-UMUMM clade that split off first from the UM ancestral lineage in the experiment was clearly separated from all other adapted strains. In this respect, the tree broadly recapitulates the physiological uniqueness of the UMUM and UMUMM batches, which display lower dry mass values than all other strains under HL (see Fig. 2d).
Fig. 4. Haplotypes and mutational epistasis.
a, Adaptive haplotypes in monoclonal strains. Five distinct haplotypes (I-V) are indicated as coloured blocks, with one mutation (RpoC1F345Y) being shared between haplotypes I and IV. Only alleles recovered from two or more clones are considered. Allele frequencies are indicated by colour coding: >0.8, black/coloured; <0.8, grey. Vertical lines at the top indicate genes/loci with single mutations; horizontal lines indicate genes with multiple mutations (named); haplotype-defining mutations are provided, and their spatial arrangements are indexed in Supplementary Data 1. NdhF1F124L (#1) and EF-G2R461C (#2) are highlighted by red arrowheads. Subclades corresponding to haplotypes are indicated in the same colours in the cladogram; asterisks indicate >70 % support over 100 bootstrapping replicates of the ML cladogram (left), which also applies to the phylogram in panel b. Culture drops of corresponding clones (100 μmol photons m-2 s-1) are shown on the right. Epistatic mutations shown in panel b are indicated by corresponding symbols at the bottom. b, Epistatic relationships for selected mutations are indicated in an ML phylogram (drawn to scale). Mutations (designated as &, +, a, b, α and β) occurring independently at least twice in the same order/coupling qualified as epistatic-series candidates.
We found 49 genes/ORFs and 8 intergenic loci to harbour 74 mutations that were shared between at least two monoclonal strains (Supplementary Data 1) with high mutant allele frequency (>80%) in at least one of these clones. Thirty of these mutations with high frequency constituted distinct groups designated as HL-adaptive haplotypes I-V, including 3/9/8/3/9 unique mutations each (Fig. 4a, Supplementary Table 1), with the exception of RpoC1F345Y, which was common to haplotypes I and IV (see Fig. 4a blue/red split). In general, monoclonal isolates sharing a haplotype displayed very similar culture-drop phenotypes (Fig. 4a), with the exception of haplotype III, which yielded two distinct phenotypes (yellow in UM/UMU*M clones, green in LT´ clones). Since none of their mutations was exclusive to one group (i.e. absent in the other), either functionally similar second-site mutations or shared epigenetic modifications25, 26 might account for the phenotypic divergence of UM/UMU*M and LT´ clones. Moreover, analysis of metagenomes from batch cultures clearly indicates that the HL-adapted LT´ strain is not a contaminant derived from other evolved strains (Supplementary Table 2), which implies that effective ALE in Synechocystis does not require deliberate induction of mutations.
The chronologies of mutation events were inferred by assigning shared mutations to internal branches of the ML phylogeny (Fig. 4b), allowing us to identify cases of apparent epistasis. One instance of putative intragenic mutational epistasis was observed for the rpoD1 gene, in which the K206R mutation was followed by an M383K exchange in two independent cases (Fig. 4b). Two cases of assumed intergenic mutational epistasis, Ssr1480F461→slr1546Δnt536 and Ssr1480F46I→EF-G2R29Q, occurred independently at least twice. Moreover, slr1546Δnt536 and EF-G2R29Q apparently co-evolved, since intermediate batch-culture metagenomes of UM, UMMM, UMU*M, and LT´ revealed that both mutations were absent from the common ancestral UM metagenome, but appeared in all respective batches after their separation. In addition, RpoD1K206R and EF-G2Y328N also co-evolved twice independently, apparently in reverse order, in UMUMM4 and UMMM3.
Recapitulating HL tolerance in LT cells
In order to test whether the mutations identified actually confer HL tolerance, we introduced two of them into LT cells (see Methods and Extended Data Fig. 1) and tested their capacity to mediate HL tolerance. The two mutations chosen were F124L in Slr0844 (NAD(P)H-quinone oxidoreductase subunit F) – hereafter NdhF1F124L – and R461C in Sll1098 (elongation factor EF-G2) – hereafter EF-G2R461C. The resultant strains were then grown at different light intensities: 50 μmol (to detect possible trade-offs under low light), 700 μmol (recapitulating the initial selective regime of our experiment, see Fig. 1), 1200 μmol and 2000 μmol photons m-2 s-1 (Fig. 5a-d, Extended Data Fig. 2). Interestingly, growth of some strains slowed between 48 h and 72 h after inoculation, particularly at 700 μmol photons m-2 s-1 (Extended Data Fig. 2a,b), such that the doubling times calculated for the exponential phase do not always correlate well with final dry mass values. Compared to LT, both mutant strains displayed an enhancement in growth, final dry mass and carotenoid content but similar carotenoid profiles under 700 μmol photons m-2 s-1 at 7 days after inoculation (Fig. 5a-e). At 1200 μmol photons m-2 s-1, only EF-G2R461C cells, but not LT and NdhF1F124L strains, continued to grow and accumulate dry mass and pigments as at 700 μmol photons m-2 s-1 (Fig. 5a-d; Extended Data Fig. 2). In contrast to the LT strain, the NdhF1F124L strain was able to retain its pigments at 1200 μmol photons m-2 s-1, despite its lower growth rate after 72 h (Fig. 5d, Extended Data Fig. 2). Neither of the strains survived at 2000 μmol photons m-2 s-1, indicating that the extreme HL tolerance observed in the batch cultures (see Fig. 2) might require multiple mutations. At 50 μmol photons m-2 s-1, in both mutant strains, growth rate and the final dry mass, but not pigment contents, were reduced in comparison to LT cells (Fig. 5b-d, Extended Data Fig. 2), indicating a trade-off for HL tolerance, as seen in the batch cultures (see Fig. 2e).
Fig. 5. HL tolerance of NdhF1F124L, EF-G2R461C, NdhF1F124L EF-G2R461C cells and control strains.
a, Images of cells grown under the indicated light levels and photographed at 7 days post inoculation (dpi). LT, our laboratory-adapted Synechocystis PCC6803 strain; WT, the original motile Synechocystis PCC6803 strain from the Pasteur Collection; #1, NdhF1F124L; #2, EF-G2R461C, #12, NdhF1F124L EF-G2R461C. b-e, Alterations in final optical density (at λ=730 nm, OD730nm) (b), dry mass accumulation (c), overall pigment composition (d) and carotenoid composition (e) in liquid cultures as shown in panel a at 7 dpi. OD730nm and dry mass data were obtained from cultures after re-adjustment to original culture volumes of 90 ml. Data points for dry mass correspond to pooled dry mass of the six replicates, whereas cellular chlorophyll a (Chl) and carotenoid (Car) content was determined from total methanolic pigment extracts and represent data for cell counts equalling OD730nm 0.75 cells ml-1. Carotenoid profiling was based on LC-MS-MS analyses of methanolic pigment extracts of OD730nm 5.0 cells, and UV-Vis absorbance spectra, retention times and molecular masses were used for pigment identification. Relative abundances are presented relative to non-chlorophyll absorbance at 450 nm. Misc, miscellaneous; lyc, lycopene; β-car, β-carotene; ech, echinenone; myx 1, myxoxanthophyll peak 1; zea, zeaxanthin; myx 2, myxoxanthophyll peak 2. f, Phycocyanin to chlorophyll (PC/Chl) ratio, as determined by absorbance spectroscopy (see also Extended Data Fig. 3). Boxplots for PC/Chl ratio for 1200 μmol photons m-2 s-1 cultures are indicated in grey, because spectra are not well resolved due to low OD and high relative carotenoid content (see Ext. Fig. 3) and thus need to be assessed with caution.
Data are derived from six replicates (two biological replicates of three independent clones per genotype). Crosses in boxplots indicate average values, letters signify statistically significant differences with p ≤ 0.05 according to post-hoc Bonferroni-Holm simultaneous comparison of all measurements after significant among-group differences were detected by one-factorial ANOVA (two-sided). Lowercase and uppercase letters in d refer to carotenoid and chlorophyll levels, respectively.
Absorption spectra and 77K fluorescence emission spectra were measured (see Methods) to determine the phycocyanin content relative to chlorophyll (PC/Chl) and the relative amounts of the two photosystems (PSI/PSII) (Extended Data Figs. 3 and 4). At low light (50 μmol photons m-2 s-1), the PC/Chl (Fig. 5f) and PSI/PSII (Fig. 6a) ratios of EF-G2R461C cells are lower than in LT, whereas at 700 μmol photons m-2 s-1 both mutant strains have lower PC/Chl and PSI/PSII values relative to the LT strain. The PSI/PSII ratio of LT and NdhF1F124L but not of EF-G2R461C is markedly increased at 700 μmol photons m-2 s-1 compared to 50 μmol photons m-2 s-1 (Fig. 6a). Accordingly, analysis of the accumulation of thylakoid marker proteins (PsaC, D1, NdhK and CurT) corroborates that the PSI/PSII ratio of the mutant strains is lower than in LT at 700 μmol photons m-2 s-1 (Fig. 6b). However, at 50 μmol photons m-2 s-1 the ratio of PsaC/D1 signal intensities is markedly higher in the mutant strains than in LT (Fig. 6b). This is in contrast to the PSI/PSII ratios obtained by the 77K fluorescence measurements (Fig. 6a) and might be explained by an overproportionally decreased stability of the D1 protein in the mutant strains compared to LT. Moreover, at 1200 μmol photons m-2 s-1, all four thylakoid marker proteins accumulated to much higher levels in EF-G2R461C than in LT and NdhF1F124L cultures (Fig. 6b). This indicates that the EF-G2R461C mutation can preserve the integrity of thylakoid protein complexes under HL conditions.
Fig. 6. PSI/PSII ratio and thylakoid marker protein accumulation in NdhF1F124L, EF-G2R461C, NdhF1F124L EF-G2R461C cells and control strains.
a, PSI to PSII ratio was determined by 77K fluorescence spectroscopy (see also Extended Data Fig. 4) for the same strains as in Fig. 5. b, Marker protein accumulation. Representative constituents of PSII (D1), the NDH complex (NdhK), and PSI (PsaC), as well as CurT as a thylakoid membrane marker protein, were detected in whole-cell extracts corresponding to OD730nm 0.3 cells. Coomassie Brilliant Blue (CBB) staining of the PVDF membrane is provided as loading control. Relative average signal intensities were determined by ImageJ (see Methods) and are also indicated (LT=100).
Data are derived from six replicates (two biological replicates of three independent clones per genotype), except for PSI/PSII molar ratios, which represent results of n=4 independent measurements since two spectra yielded non-evaluable negative fluorescence emission for the PSII F695 signal. Crosses in boxplot indicate average values, letters signify statistically significant differences with p ≤ 0.05 according to post-hoc Bonferroni-Holm simultaneous comparison of all measurements after significant among-group differences were detected by one-factorial ANOVA (two-sided). Boxplots for PSI/PSII ratios for 1200 μmol photons m-2 s-1 cultures are indicated in grey, because spectra are not well resolved due to low OD and high relative carotenoid content (see Ext. Fig. 4) and thus need to be assessed with caution.
Thylakoid electron flow in NdhF1F124L and EF-G2R461C cells
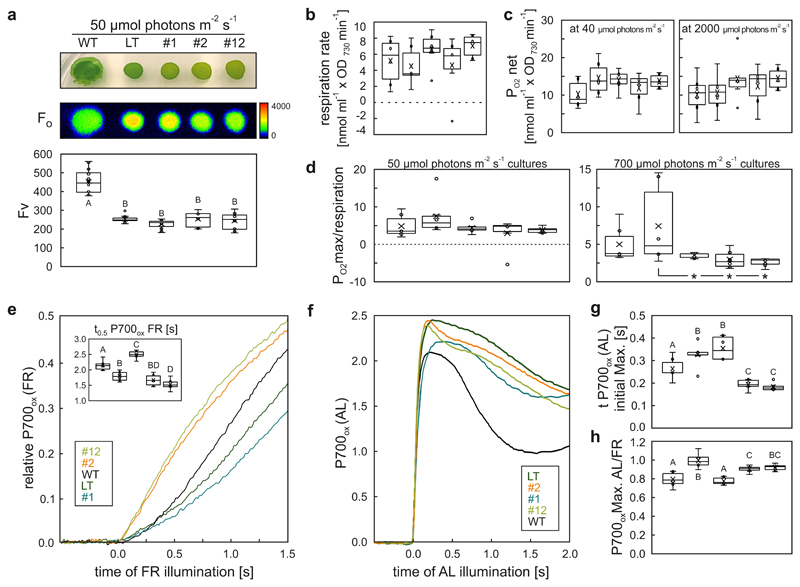
Photosynthesis and respiration were characterised in detail in the mutant strains (Fig. 7). Basic fluorescence (Fo) of chlorophyll a in cultures grown at 50 μmol photons m-2 s-1 was found to be lower in EF-G2R461C cells than in LT and NdhF1F124L, whereas the variable fluorescence (Fv) was not significantly altered in the two mutants compared to LT (Fig. 7a). Respiration was slightly but not significantly increased in NdhF1F124L compared to LT and EF-G2R461C (Fig. 7b). Net O2 production per cell in cultures grown at 50 μmol photons m-2 s-1 did not differ significantly between LT and mutant strains grown under 40 or 2000 μmol photons m-2 s-1. However, at 2000 μmol photons m-2 s-1 the average value of net O2 production relative to the values obtained at 40 μmol photons m-2 s-1 decreased in LT, but not in the mutant strains (Fig. 7c). At 50 and 700 μmol photons m-2 s-1, the ratio of maximum O2 production to respiration was lower in the mutant strains than in LT, but only at 700 μmol photons m-2 s-1 was the difference statistically significant (Fig. 7d).
Fig. 7. Rates of photosynthesis and respiration for NdhF1F124L, EF-G2R461C, and NdhF1F124L EF-G2R461C cells and control strains.
a, Culture drop phenotypes, basal (Fo) and variable (Fv) chlorophyll a fluorescence of the same strains as in Figs. 5 and 6, grown on BG11 plates at 50 μmol photons m-2 s-1 for 3 days post inoculation (dpi) at a starting cell equivalent of OD730nm = 0.02 per drop. Representative images from four technical replicates of three independent clones each are shown. b, Cellular respiration rates of cultures grown for 7 days at 50 μmol photons m-2 s-1 and 23 °C. Data are normalized to OD730nm of cultures. c, Cellular net oxygen production rates (PO2net) of LL (50 μmol photons m-2 s-1)-acclimated cultures after exposure to 40 or 2000 μmol photons m-2 s-1 for 3 min in the course of light-curve measurements. Data are normalized to OD730nm of cultures. d, Relative oxygen production, determined as maximum ratios of oxygen production rate to respiration rate (PO2max/respiration) of cultures grown for 7 d at 50 or 700 μmol photons m-2 s-1 irradiation. Asterisks indicate statistically significant differences (p = 2,52E-02; 2,33E-02; 2,16E-02 in corresponding order) according to post-hoc Bonferroni-Holm simultaneous pairwise comparisons to the LT reference after significant among-group differences were detected by one-factorial ANOVA (two-sided). e-g, P700 oxidation kinetics of cultures grown for 7 d at 50 μmol photons m-2 s-1 induced by far-red light (FR)(e) or actinic light (AL)(f). Data represent early P700 oxidation traces as 820nm-870nm ΔI/I x 103. Prior to measurement of P700 oxidation kinetics, cells were harvested, washed in BG11, adjusted to OD730nm=5.0, and dark-incubated for a minimum of 24 h. The inset in (e) shows half-times of P700 oxidation (t0.5 P700ox FR), whereas the time required to reach the first maximum of AL-induced P700 oxidation is shown in panel g. h, Limitation of electron acceptors from PSI, measured as the ratio of maximum P700 oxidation induced by AL to that induced by FR (P700ox Max. AL/FR). Values less than 1 indicate increasing limitation of electron acceptors28. A ratio of 1 indicates no limitation.
Replicate numbers and statistical analysis were as in Figs. 5 and 6; traces in e and f represent averages over all replicates.
As a measure for CEF activity the kinetics of P700 oxidation induced by far-red (FR) light was determined as described27 (Fig 7e). In this assay, slow oxidation rates are associated with long half-time values of P700 oxidation (t0.5P700ox; see inlet of Fig. 7e) and indicate high CEF activity27. The results indicate that CEF activity is increased in NdhF1F124L cells and decreased in EF-G2R461C relative to LT. We next measured the kinetics of P700 oxidation under actinic light (89 μmol photons m-2 s-1) (Fig. 7f) and determined the time taken to reach the initial maximum of P700 oxidation (t P700ox AL; Fig. 7g) and the ratio of the maximum P700 oxidation induced by white actinic light to that under FR light (P700ox max AL/FR, Fig. 7h). This ratio serves as a measure for the limitation of electron acceptors from PSI and its value is decreased in plants impaired in CEF28. In fact, EF-G2R461C cells oxidize PSI faster (Fig. 7g), most likely because of their reduced CEF activity. Moreover, EF-G2R461C and more particularly NdhF1F124L have lower P700ox max AL/FR values than LT. This indicates that at 89 μmol photons m-2 s-1 the acceptors from PSI are more limited in the mutants than in the LT and might contribute to the trade-off associated with HL tolerance at low light intensities.
Taken together, the physiological mechanisms underlying HL tolerance in the NdhF1F124L and EF-G2R461C strains appear to be different. In accordance with the known function of the NDH complex in CEF and respiration29–31, NdhF1F124L enhances both CEF activity (thereby amplifying this typical HL protective mechanism32, 33) and respiration. Possibly, altered abundance or activity of NdhF1 in the NdhF1F124L strain could not only affect the total abundance of the different variants of NDH complexes29, as indicated by the elevated NdhK levels at 700 and 1200 μmol photons m-2 s-1 (Fig. 6b), but also shift the ratio of CEF to CO2 uptake activities in favour of CEF. Conversely, EF-G2R461C displays reduced CEF, together with a marked increase in stability of the photosystems at 1200 μmol photons m-2 s-1. This is compatible with the pronounced effects of the EF-G redox state on D1 protein biosynthesis previously reported34, and with the observation that redox-insensitive mutants display enhanced D1 turnover and reduced PSII photo-inhibition35, suggesting a possible link between EF-G2R461C and steady-state levels of PSII under HL. Both mutant strains have a lower maximum O2 production-to-respiration ratio at high light intensities, indicating that the mutants might be capable of mitigating oxidative stress more effectively than LT.
Effects of combining NdhF1F124L and EF-G2R461C
Since both mutations have also been found in strain UMMM2 (Fig. 4a, Supplementary Data 1), it is possible that they might act synergistically, or at least have no negative synthetic effect on HL tolerance. To study this, a strain carrying both mutations was generated and analysed. This NdhF1F124L EF-G2R461C strain behaved like EF-G2R461C cells with respect to growth curve, dry mass accumulation, pigment content and photosystem accumulation and stoichiometry, particularly at 1200 μmol photons m-2 s-1 (Figs. 5 and 6), as well as Fo (Fig. 7a), and accelerated P700 oxidation under actinic light (Fig. 7g). With respect to respiration rate and net O2 production per cell, the double mutant behaves more like NdhF1F124L cells (Fig. 7b,c). The EF-G2R461C-induced boost in D1 and NdhK protein levels (at 1200 μmol photons m-2 s-1) (Fig. 5g) and the drop in CEF (Fig. 7e) are both enhanced in the double mutant, indicating a slight additive effect of the two mutations on these phenotypes. Moreover, the ratio of maximum O2 production to respiration at 700 μmol photons m-2 s-1 was lower than that in either single mutant (Fig. 7d).
Thus, the overall effect of EF-G2R461C on HL tolerance persists in the double mutants, with some enhancement in relation to lowered CEF and the stability of photosynthetic proteins at HL. With respect to respiration-related features, the NdhF1F124L mutation shapes the double-mutant phenotype. Consequently, although the two single mutations display diametrically opposite effects with respect to CEF and the accumulation of some marker proteins, their combined impact is still compatible with HL tolerance.
HL tolerance in the original motile Synechocystis PCC6803 strain
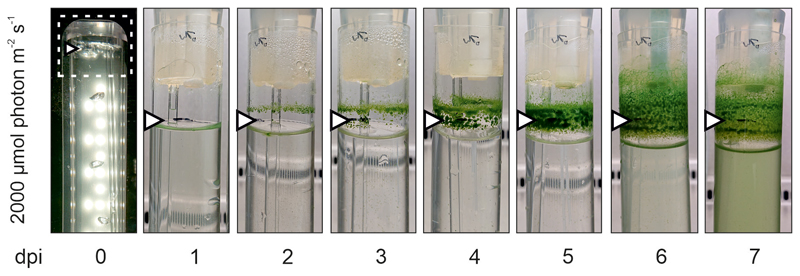
Because our starting strain for ALE has been propagated and adapted for decades under laboratory conditions36–38, it is conceivable that its HL tolerance differs from the original isolate39 deposited in the Pasteur Collection (hereafter “WT”). Therefore, we also included WT in our analysis and found that it could survive exposure to 2000 μmol photons m-2 s-1 (Extended Data Fig. 2c). Closer inspection of these cultures showed that WT‘s motility39, 40 allowed the cells to congregate in shaded regions outside of the liquid medium and avoid dispersion into it prior to 6 days post inoculation, indicating that cells need an extended period of acclimation before they can cope with such high light intensities (Extended Data Fig. 5). In fact, if this strain had been used as the basis for our HL-ALE experiments instead of the laboratory-adapted LT strain that we actually employed, it is very probable that its motility would have interfered with effective metabolic adaptation. At 1200 μmol photons m-2 s-1, however, NdhF1F124L and NdhF1F124L EF-G2R461C cells grew at significantly higher rates than WT (Fig. 5a-c), and the carotenoid amount and profile of WT was more similar to the three mutants than to the LT strain (Fig. 5d,e). Additionally, at 1200 μmol photons m-2 s-1, the WT strain more closely resembled the two strains containing EF-G2R461C than either NdhF1F124L or LT cells with respect to photosystem accumulation and stoichiometry (Fig. 5f,g). At 50 μmol photons m-2 s-1, WT clearly grew at a slower rate than all other strains investigated (Fig. 5a-c), indicating that the natural HL tolerance of WT is accompanied by a strong trade-off under low light conditions.
Hence, our single and double mutants phenocopy in some respects the natural HL-tolerant WT strain. At 1200 μmol photons m-2 s-1 they even grow faster than WT.
Discussion
In principle, genetic factors mediating HL tolerance in cyanobacteria can be identified either by comparing the genomes of HL-tolerant cyanobacteria (like Leptolyngbya ohadii, Nostoc reinholdii and Microcoleus vaginatus)41, 42 with those of their closest non-tolerant relatives. A similar approach was used to determine the genetic basis of the rapid growth of the Synechococcus elongatus strain UTEX 2973 by comparing its genome to the one of its close relative Synechococcus 7942 with much slower growth and then testing Synechococcus 2973 SNPs for their potential to enhance growth of the Synechococcus 7942 strain43. We describe here a different approach by selecting for de-novo HL tolerance in an initially non-tolerant strain by ALE. Our starting material was a laboratory strain adapted to low-light cultivation over many years, which had lost its motility in the process36–38, could not actively evade HL and had to evolve metabolic adaptations during ALE. We began with one culture that was subsequently split into independently evolving subcultures, often giving rise to a set of independent mutations in the same genes. As proof of concept, two representative mutations were tested for their impact on HL tolerance. The results confirmed that one amino-acid exchange in a single protein can suffice to confer a clear increase in HL tolerance. The two mutations have clearly different and in part opposite phenotypical effects, confirming that HL tolerance can be achieved by quite different mechanisms (see Figs. 5 and 6). During ALE, they also occurred together - in the strain UMMM2 (see Fig. 4a). When combined in the LT background they display some additive effects, but the double mutant does not grow faster than the single mutants at 1200 μmol photons m-2 s-1.
The original Synechocystis PCC6803 strain (WT) has a higher HL tolerance than our starting strain for the ALE experiment (see Figs. 5 and 6) since it can actively avoid HL while acquiring the variation needed for acclimation (see Extended Data Fig. 5), and it displays a strong trade-off at low light (see Fig. 5). Nevertheless, in our ALE experiment we were able to restore some of the characteristics of WT, in particular with respect to photosystem stability and stoichiometry at 1200 μmol photons m-2 s-1, but without restoring motility. Therefore, our laboratory strain that could not evade HL through motility might represent the better starting material to identify metabolic adaptations to HL than using a motile strain. In fact, the NdhF1F124L and EF-G2R461C strains grew faster than WT at 50 and 700 μmol photons m-2 s-1 (and EF-G2R461C also at 120 μmol photons m-2 s-1), but it is conceivable that these two mutations might be less effective in complex or natural environments for which WT is supposedly well adapted. Moreover, given that we studied growth of strains until 7 days after inoculation, progressing acclimation of the WT strain and its effect on growth might become apparent only at later growth phases.
Our results demonstrate that microbial photosynthesis can be made more resilient to HL. The mutations identified might also confer tolerance to other challenging conditions that induce oxidative stress. Therefore, photosynthetic organisms adapted by ALE to cope with rapid environmental changes could be employed in efforts to address the consequences of global warming44 – either as independent organisms or as partners in ecologically important symbioses – to enhance the fitness of symbiotic communities, as suggested for the algal endosymbionts in corals45, 46. In addition, attempts to enhance plant photosynthesis have so far relied on expressing heterologous genes or overexpressing endogenous photosynthetic genes47, changing the quantity of a given protein (set). In contrast to this, our approach makes it possible to identify protein variants that might also enhance the robustness of plant photosynthesis, assuming that the corresponding mutated protein or its function is sufficiently conserved.
Methods
Synechocystis strains and culture conditions
Experiments were conducted on glucose-tolerant (GT) wild-type Synechocystis sp. PCC 6803 cells provided by Himadri Pakrasi (Washington University, St. Louis, USA) or on the original isolate from the Pasteur Collection of Cyanobacterial Cultures, obtained from Roman Sobotka (Centrum Algatech, Trebon, Czech Republic). Cultures were routinely grown under continuous illumination with 30 μmol photons m-2 s-1 white fluorescent light (OSRAM HE28W/830 Lumilux warm white Hg fluorescent lamps) at 23 °C if not stated otherwise. Liquid cultures were inoculated to reach an OD=0.05 and grown in BG11 photoautotrophic medium48, which was supplemented with 5 mM glucose (BG11G) for pre-transformation cultures, employing Multi-Cultivator MC 1000-OD devices equipped with an AC-700 cooling unit and a warm-white LED panel (Photon System Instruments, Drasov, Czech Republic). For growth on solid media, BG11 was supplemented with 0.75 % (w/v) bacteriological agar.
Random mutagenesis and adaptive evolution of Synechocystis to high light levels
The experiment was performed as depicted in Fig. 1. Random mutagenesis was performed as described22. Cells were mutagenized chemically by exposure to 1% (v/v) MMS for 60 s, or physically by exposure to UV-C radiation (λ = 254 nm; 35 ml OD730nm = 1.0 cells in 11.5 cm2 petri dish; 233 s at 6610 x 100 μJ cm-2) in a Stratalinker® UV Crosslinker 1800 (Stratagene, La Jolla, CA, USA), resulting in an exposure of the culture surface to 50 J m-2. To avoid blue-light induced DNA repair, UV mutagenized cells were covered with orange long-pass filters for 3 days post mutagenesis. Cultures selected/grown in Multi-Cultivators (see above) were subjected to selection under constant illumination and aeration with atmospheric air. After each round of mutagenesis, cells were grown under increasing light intensities (from 700 μmol photons m-2 s-1 after mutagenesis of the LT strain to 2300 μmol photons m-2 s-1 after the mutagenesis of the UMUM culture, see Fig. 1) for 5 to 25 cultivation cycles, each generally lasting for ~15 days. Cultivation cycles were initiated at OD730nm=0.1 and ended when the batch culture reached OD730nm=2.0; consequently, cycle duration was variable and depended on the growth rate of the batch culture. Afterwards, batch cultures were diluted to OD730nm=0.1 to initiate a new cultivation cycle. Aliquots (10 ml) of mature cultures were cryo-preserved at -80 °C in BG11G + 8 % DMSO (v/v).
Isolation of high-light-adapted Synechocystis clones for genome re-sequencing
Single clones were isolated from each batch culture following the final round of selection by streaking on solid BG11 medium. Clones for whole genome re-sequencing were selected based on their chlorophyll a-fluorescence phenotypes as determined by FluorCam 800MF measurement (Photon Systems Instruments, Drásov, Czech Republic), and grown in liquid culture.
Synechocystis genomic DNA extraction and sequencing
Genomic DNA for whole-genome re-sequencing was extracted from 5 ml of late exponential phase cultures grown photoautotrophically in BG11 at 30 °C and 50 μmol photons m-2 s-1 as described earlier49. DNA integrity was confirmed by agarose gel electrophoresis and subsequent ethidium bromide staining. Illumina HiSeq genome re-sequencing (2x150-bp paired-end reads) was perfomed by NovoGene (Beijing, China). The starting LT strain at t=0 (LTt=0) was sequenced and assembled to serve as the reference genome. Moreover, four monoclonal strains each, obtained from UM, UMMM, UMU*M, UMUM and UMUMM batch cultures, and from the controls LT and LT´ (28 clones in total), were sequenced. Finally, 13 intermediary batch cultures, as well as the 7 final batch cultures used for single-clone isolation (see Fig. 1), were sequenced in order to recover as many extant and “extinct” candidate alleles as possible. Genome resequencing of the LTt=0 reference, the 28 single clones, and batch culture metagenomes yielded average coverages of 1452/1323/927/316/7435/5207-fold, 1007/1452/1323/927/316/7435/5207-fold, and 1099/2940/2845/1606/2781/2068/1342-fold, for chromosome/pSYSM/pSYSA/pSYSG/pCA/pCB, respectively (see Supplementary Data 2). The median insert lengths for the said groups were 312, 310, and 314 bp, respectively. On average, 99.3±1.4 % of sequencing reads mapped to the de novo assembly of the LTt=0 reference genome sequence, and the read mapping error rate was 1 %. De novo assembly was conducted in Spades50, contigs were arranged according to the Kazusa reference genome with Bandage51, genes were annotated with Geneious52, and new genes were predicted with Prokka53. All mutations identified in the experiment were tracked with respect to the new LTt=0 assembly.
Sequence data quality control and filtering
Raw sequencing reads were subjected to two consecutive rounds of adaptor removal using bbduk from BBTools (v.38.67)54. Failure rates were minimal, with remaining adaptor sequences accounting for between 0.16 and 0.34% of the total input base pairs. Next, reads were quality trimmed and filtered (also with bbduk), removing any leading or trailing bases with a PHRED score of 10, and keeping only those reads with an average PHRED score (after trimming) of at least 12. Simultaneously, common sequencing artifacts and laboratory contaminants (e.g. Phi X 174 bacteriophage genome, human genome, and common bacteria) were screened for and removed. The quality of the resulting sequences was very high, since more than 99.97% of reads and more than 99.86% of base pairs were retained after filtering across all samples.
Genome assembly of the LTt=100 strain
To improve the reliability of our variant analysis, we attempted a de-novo assembly, as initial read mapping revealed large insertions and deletions with respect to the Kazusa reference genome (GenBank: GCA_000009725.1), mainly corresponding to transposable elements. Besides detecting the presence of the main chromosome (BA000022.2) and the four canonical plasmids (AP004311.1, AP004312.1, AP004310.1, and AP006585.1), a preliminary assembly of the unmapped reads revealed the presence of two additional small plasmids, which were virtually identical to pCA2.4_M (CP003270.1) and pCB2.4_M (CP003271.1) from GenBank’s assembly GCA_000340785.1.
Cleaned reads from LTt=0 were binned by genome component (references: BA000022.2, AP004311.1, AP004312.1, AP004310.1, AP006585.1, CP003270.1, and CP003271.1) with bbsplit from BBTools (v.38.67)54 and then, for each sequence bin, overlapping paired-end reads were merged using bbmerge from BBTools v.38.6754, 55. Afterwards, merged and non-merged read pairs for each sequence bin were de novo assembled with Spades (v.3.13.0)50 using the k-mer progression 29,53,77,101,125,149,173,197,221,245 and skipping the sequence correction step. The final assembly graph for each genome component was ordered and circularized according to the references using Bandage (v.0.81)51. Finally, the circular sequences were rotated to match the starting position and direction of the published genome and concatenated in a single FASTA file using Geneious R11 (v.11.1.5)52.
Three rounds of sequence polishing were then performed with Pilon (v.1.23)56, the first round resulted in 48 single-base editions, while consecutive rounds yielded no further modifications, achieving a final read recruitment of 99.749% without noticeable breaks in coverage.
An annotation track for the polished assembly was obtained with Prokka (v.1.14.5)53, which was then compared to the annotation for the Kazusa reference genome GCA_000009725.1. Kazusa’s annotation was preferred when it was identical to Prokka’s, while novel genes or discordant annotations were transferred from Prokka’s track. Annotations were transferred using Geneious R11 (v.11.1.5)52.
Variant analysis
Prior to variant calling, reads from all samples were mapped to our newly assembled genome using bbmap from BBTools (v.38.67)54 in paired-end mode, placing ambiguously mapped pairs randomly, and with the flag “local” enabled (bbmap is a global aligner, the “local” flag just enables soft-masking, which is disabled by default). The resulting SAM files were sorted, converted to BAM and then indexed with samtools (v.1.9)57. Average coverage depth ranged from ~673X to ~1742X across samples (Supplementary Data 2, sheet 1). In order to normalize conditions for variant analysis, we capped the coverage depth of the BAM files at ~800X, since this was the lowest value among the clonal samples which are the focus of the paper. Coverage capping was performed with sortsamrefname.jar and biostar154220.jar (available at: http://lindenb.github.io/jvarkit/).
To detect and track mutations in our samples we used the pipeline breseq (v.0.35.0)58, 59, which can take SAM alignment files as input as long as the read names within the files are all unique. To prepare the input for breseq, we converted the capped BAM files to the SAM format, and used a custom script to rename all the reads within the file to match the requirements of the pipeline. breseq was run in two modes: the consensus mode considers a mutation to be fixed when its frequency is ≥0.80 and considers a site to be polymorphic when the frequency of the variant is between 0.20 and 0.80. In polymorphism mode a mutation is considered fixed at frequencies ≥0.95 and polymorphic if it occurs at a frequency of between 0.05 and 0.95. The polymorphism mode is more sensitive to rare mutations, but increases the risk of an excess of false positives. Therefore, we first ran breseq in consensus mode and compiled a list of fixed mutations across all samples (Supplementary Data 2, sheet 2). Thereafter, only these mutations were tracked in polymorphism mode to ensure that they could be detected in any sample even if their frequencies lie below the detection limits of the consensus mode. The unfiltered mutation list from the polymorphism mode run is included in Supplementary Data 2 (sheet 3), and its intersection with the fixed mutations found in consensus mode is included in the data sheet “cons_poly_intersection” (Supplementary Data 2, sheet 4).
To further filter the comprehensive list of mutations “cons_poly_intersection”, we subselected only those mutations that were present in at least two samples from different lineages/treatments or genes that contained at least two mutations; this filter corresponds to table “cons_poly_intersection_min2” (Supplementary Data 2, sheet 5). We also compared the results from breseq (v.0.35.0)58, 59 with those from another popular variant-calling software, freebayes (v.1.3.1-19-g54bf409)60. To match the detection levels used for breseq, freebayes was run in haploid mode (--ploidy 1) with a minimum mutation frequency of 0.8 (--min-alternate-fraction 0.8) directly on the capped BAM files. The raw comparison of both methods is shown in the data sheet “freebayesF80_vs_breseq” (Supplementary Data 2, sheet 6), and after normalizing the representation of the variants for both programs (“fb80_breseq_filtered”, Supplementary Data 2, sheet 7) the only differences are two long deletions at S29_CHROM: 443531 (-69bp deletion in ycf45) and S29_CHROM: 3528077 (-76bp deletion in slr1189). This is not surprising, given that breseq, unlike freebayes, does not attempt to detect this type of mutation. The sheet “fbF80_breseq_filtered_phylo” (see Supplementary Data 2, sheet 8) fully represents the nucleotide variants and recodes indels in order to enable these data to be used to construct the character matrix employed for phylogenetic analysis (“fbF80_breseq_phylip”, Supplementary Data 2, sheet 9).
To compose our final table of mutations we transposed sheet “cons_poly_intersection_min2” into sheet “breseq_fbF80_min2_transposed” (Supplementary Data 2, sheet 10) and added the deletions detected by freebayes. Then we shaded the mutations according to their frequencies (“candidate_mutations_samples”, Supplementary Data 2, sheet 11) and prepared the final table of mutations “candidate_mutations_genes” (Supplementary Data 2, sheet 12), subsets of which were subsumed into five distinct haplotypes (Fig. 4, Supplementary Data 1, sheet 13). All mutations meeting our haplotype candidate criteria (see main text) are summarized in Supplementary Data 2, (sheet 13).
Finally, we compiled a table of Gene Ontologies (sheet “uniprot_gene-ontology”, Supplementary Data 1, sheet 14) for those genes with detected mutations by querying the website https://www.uniprot.org/uniprot/.
Phylogenetic analysis
The phylogenetic matrix (see Supplementary Data 2) had 72 parsimony-informative sites and was used for maximum-likelihood estimation in IQ-TREE v.1.6.561 under the nucleotide substitution model GTR+G and with support derived from 100 bootstrap replicates. The resulting topology is shown in Fig. 4.
Reconstruction of NdhF1F124L and EF-G2R461C in the Synechocystis LT background
Candidate point mutations were introduced into LT cells by homologous recombination using a marker-less gene-replacement system62. Transformation vectors were cloned using Gibson Assembly63 in DH5α E. coli cells. Point mutations were introduced by Q5® site-directed mutagenesis (SDM; NEB, Ipswich, MA, USA). Candidate ORFs and fragments thereof were amplified from LT genomic DNA, while the KanR/sacB double selection cassette (DSC) and the pICH69822 vector backbone (E. Weber; Icon Genetics GmbH, Halle, Germany) were amplified from pDSlux62 using Q5® high-fidelity polymerase (NEB). Correct assembly of vectors was verified by restriction analysis, while functionality of negative selection markers was assayed on LB supplemented with 5 % (w/v) sucrose. Correctly assembled plasmid DNA was used as template for Q5® SDM PCR, re-circularised according to the manufacturer’s instructions, and transformed into DH5α E. coli cells. Successful point mutagenesis and lesion-free auto-ligation were confirmed by sequencing, yielding the plasmids pDS_ndhF1-F124L (pHL1), pDS_fusB-R461C (pHL2). To minimize potential toxicity in E. coli (the cloning host) and off-target effects in Synechocystis, we used the entire coding sequence without any component of the promoter of the respective gene of interest as HR1. This also allowed us to ensure that the gene could not to be knocked out by CDS disruption at any point in the transformation/segregation process, while reducing gene expression in E. coli to a minimum. Transformants were selected on increasing concentrations of kanamycin (10, 25, 50 and 100 μg/ml) for segregation, and the double-selection cassette was removed by negative selection on 5 % sucrose. Full segregation of transgenes was confirmed by Illumina sequencing of PCR-amplified target loci, with 8 separate PCR products per clone being pooled prior to sequence analysis to avoid allelic dropout.
The NdhF1F124L EF-G2R461C double mutant was generated by transforming fully segregated NdhF1F124L and EF-G2R461C mutants with pHL2 and pHL1, respectively (clone 1: pHL1 → pHL2; clones 2-3: pHL2 → pHL1), and segregating/double-selecting the resultant clones as described previously.
Protein extraction, detection and quantification
Whole-cell proteins were prepared by boiling cells (OD730nm=4) in 2x SDS loading buffer (100 mM Tris/HCl pH 6.8, 24 % (v/v) glycerol, 5 % (w/v) SDS, 0.02 % (w/v) bromophenol blue, 100 mM DTT). Cell debris was pelleted by centrifugation, and 7.5 μl of supernatant was fractionated by SDS PAGE on 4 % /10 % polyacrylamide Tris-Tricine gels64. Proteins were blotted onto PVDF membrane (Millipore Immobilon-P Transfer Membrane, 0.45μm) overnight by capillary transfer and marker proteins were immuno-detected as described27. Primary antibodies were purchased (D1 and PsaC, Agrisera Vännäs, Sweden), provided by Roman Sobotka (NdhK), or raised in our lab (CurT)65, and used in 1:10000 dilutions.
Immunoblot ECL signals were quantified using ImageJ66 and normalized to the intensity of the corresponding LT signal on the respective PVDF membrane.
Pigment extraction and quantification
Hydrophobic pigments were extracted from Synechocystis cells at OD730nm=0.75, and quantified as described earlier67. The corresponding cell density had been empirically determined to yield extracts with maximum absorbance ≤ 0.5, which were suitable for approximate pigment quantification.
Pigment extracts for LC-MS-MS analysis were prepared from OD730nm=5.0 cells by extraction for 16 hours at 4 °C under constant agitation in 1 ml of pure methanol. Extracted pigments were dried in a vacuum desiccator and re-dissolved in 100 μl pure methanol. The analyses were performed on a Dionex Ultimate 3000 UHPLC including a DAD array detector (Thermo Fisher Scientific, Waltham, USA). Furthermore, a timsTOF-O-TOF (Bruker Daltonik, Bremen, Germany) was used to verify the mass spectrum, fragments, and isotopic pattern. Samples (10 μL) were injected and separated at a flow rate of 500 μL min-1 on a C30 reversed-phase column (Acclaim C30, 3 μm, 2.1 x 150 mm, Thermo Fisher Scientific) at 15°C. The solvents used were (A) acetonitrile and (B) a mixture of methanol and ethyl acetate (50/50 v/v), both containing 0.1% formic acid. The gradient started with 14.5% B followed by a ramp to 34.5% B within 15 min. The latter was maintained for 10 min, before returning to 14.5% B with additional 5 min of re-equilibration. Lutein was identified using a commercial standard (Extrasynthese, Genay Cedex, France), by retention time, specific m/z values, MSMS fragmentation and the true isotopic pattern, as well as by the full absorption spectrum of the DAD. For absolute quantification, the standard was spiked into Synechocystis LT sample extracts to create a calibration curve within the natural background. Data were acquired by otofControl 4.0 in positive MS mode from the 50-1300 m/z mass range, and evaluated using DataAnalysis 5.0 and MetaboScape 4.0. All software tools were provided by Bruker Daltonik (Bremen, Germany).
Determination of PSII quantum yield parameters
A FluorCam 800MF (Photon Systems Instruments, Drásov, Czech Republic) was used in quenching-analysis mode to determine approximate PSII basal (Fo) and variable (Fv) fluorescence of culture samples dropped onto BG11 agar plates at an initial OD730nm of 2.0 after cultivation for three days. Measuring durations were set to 5 s for Fo, 800 ms Fm pulse duration, 10 s dark pause after Fm measurement (saturating pulse ~1200 μmol photons m-2 s-1), 60 s actinic light exposure at ~40 μmol photons m-2 s-1, 20 s relaxation interval, and 9 s first pulse after actinic light trigger, giving a total of 6 pulses (5 pulses during Kautsky induction; 1 pulse during relaxation). The FluorCam actinic light LED panel 1 was employed, emitting orange-red light (emission peak at 618 nm); the saturating pulse (Super) was provided as a cool-white LED flash of about 1200 μmol photons m-2 s-1.
P700 Dual-PAM measurements
P700 redox kinetics in Synechocystis cells were prepared and performed as described27. The measurement routine involved 3 s dark > 60 s far-red (FR) or actinic light (AL) > 30 s dark at measuring light intensity 4, FR light intensity 3 (38 μmol photons m-2 s-1) or AL intensity 7 (89 μmol photons m-2 s-1), acquisition rate 200 s-1, and high gain (5) and damping (1 ms). Cells for P700 measurements were cultivated for 7 days at 50 μmol photons m-2 s-1 and 23 °C in a multi-cultivator.
Low-temperature fluorescence spectrometry
Low-temperature fluorescence spectra for chlorophyll a and phycobiliproteins were obtained using a HORIBA Fluoromax Plus FL-1013 (HORIBA Jobin Yvon GmbH, Bensheim, Germany). Synechocystis cultures were transferred into suitable glass capillaries (Hilgenberg GmbH, Malsfeld, Germany) straight out of the cultivation device seven days past inoculation, and frozen in liquid nitrogen immediately. Samples were stored light-protected at -80 °C for a maximum of 12 weeks prior to spectrometric analysis and measured as a single batch subsequently. Measurements were performed at 77 K in a Dewar filled with liquid nitrogen, with signal integration time of 0.2 s per nm and a detection bandwidth of 2 nm. Fluorescent signals were detected trough a 500 nm long-pass filter upon excitation with 600 and 435 nm to induce phycobiliprotein68 and chlorophyll a69 fluorescence, respectively. Approximate PSI/PSII molar ratios were calculated as ratio of emission at 720 nm (PSI) and 695 nm (PSII)69.
Determination of phycocyanin:chlorophyll molar ratios
Molar ratios of the peripheral antenna pigment phycocyanin to core antenna pigment chlorophyll a were estimated as described earlier70 using Synechocystis cultures seven days past inoculation.
Oxygen evolution measurements
Light-dependent oxygen evolution in non-disturbed Synechocystis cultures was measured using an Oxytherm+ P Clark-type oxygen electrode (Hansatech Instruments Ltd, Norfolk, UK). Aliquots (1.5 ml) of Synechocystis cultures were transferred from the Multi-Cultivators straight into the Oxytherm+ P measuring cuvette and subjected to a standardized photon flux density protocol. The protocol comprised of thirteen three-minutes treatment steps of illumination with approximately 0 > 40 > 80 > 120 > 160 > 200 > 400 > 600 > 800 > 1200 > 1500 > 1800 > 2000 μmol photons m-2 s-1 provided by a build-in LED panel under controlled temperature of 23 °C (mimicking prior cultivation conditions) and constant stirring at 100 rpm with a magnetic stirrer. Average oxygen evolution rates were calculated for seconds 61-180 of each illumination step and normalized to OD730nm of the respective cultures.
Statistics and boxplot description
Statistical analyses were conducted using one-way ANOVA and post-hoc Bonferroni-Holm-corrected two-sided t-tests as implemented in https://astatsa.com/ (attribution: Navendu Vasavada). Sample sizes were n=6 unless stated otherwise, representing six biological replicates of two separate cultivation cycles of three independent clones (i.e. originating from separate transformation events) for each single and double mutation. WT and LT cultures were raised from isolated clones originating from single batch cultures.
Boxplots represent distributions of data points with the horizontal middle line indicating inclusive medians, crosses indicating average values, boxes indicating second and third quartiles, whiskers indicating first and fourth quartiles, and points outside the whisker ranges representing outliers beyond 1.5-fold interquartile range.
Accession Numbers
The GenBank reference sequences used for read-binning of Synechocystis LTt=0 and subsequent de-novo genome assembly were: BA000022 (Kazusa, chromosome), AP004311 (Kazusa, pSYSA), AP004310 (Kazusa, pSYSM), AP004312 (Kazusa, pSYSG), AP006585 (Kazusa, pSYSX), CP003270 (Freiburg, pCA) and CP003271 (Freiburg, pCB). The LTt=0 reference genome has been deposited at GenBank (ID CP073017–CP073023).
Extended Data
Extended Data Fig. 1. Synechocystis genomic allele-swapping strategy.
Candidate point mutations were introduced into the Synechocystis LT genome employing a modified version of our established single-vector-based, marker-less gene replacement strategy62. The complete ORF of interest (5' CDS + 3' CDS) and a second copy of the 3' CDS were cloned into a non-replicative plasmid on either side of the double-selection cassette (DSC)62. SNPs were introduced by Q5 site-directed mutagenesis. The endogenous genomic ORF was replaced with the mutated ORF by homologous recombination upon positive selection on kanamycin (KanR; nptI), and the original gene structure was re-established by removal of the DSC by intra-chromosomal homologous recombination upon negative selection on sucrose (SucrS; sacB).
Extended Data Fig. 2. Growth curves for mutant and control strains at 50, 700 and 1200 μmol photons m-2 s-1 and HL tolerance at 2000 μmol photons m-2 s-1.
a, Growth of Synechocystis strains over the course of 7 days post inoculation was measured as apparent optical density at λ=720 nm (OD720nm). LT, our laboratory-adapted Synechocystis PCC6803 strain; WT, the original motile Synechocystis PCC6803 strain obtained from the Pasteur Collection; #1, NdhF1F124L; #2, EF-G2R461C; #12, NdhF1F124L EF-G2R461C. Note that cultures were inoculated to reach an OD730nm=0.05, and that cultivation of strains in Multi-Cultivator OD-1000 devices and direct measurement with the in-built system leads to underestimation of the actual OD720nm if actual values exceed ~0.5.
b, Duplication times during the exponential phase of growth curves shown in (a). Duplication time data was inferred from growth curve slopes over eight hours starting at OD=0.1 (i.e. after the first full duplication of culture OD).
c, HL tolerance at 2000 μmol photons m-2 s-1 of NdhF1F124L, EF-G2R461C, NdhF1F124L EF-G2R461C cells, LT and WT strains, analysed as in Fig. 5.
Data are derived from six replicates (two biological replicates of three independent clones per genotype). Error bars indicated SDs from respective average values. Crosses in boxplots indicate average values, letters signify statistically significant differences with p ≤ 0.05 according to post-hoc Bonferroni-Holm simultaneous comparison of all measurements after significant among-group differences were detected by one-factorial ANOVA (two-sided).
Extended Data Fig. 3. Cell culture absorbance spectra.
Absorbance spectra shown are averages of six biological replicates of Synechocystis cultures 7 days post inoculation. Data have been baseline-corrected, such that absorbance at 750 nm equals 0, and were used to estimate phycocyanin-to-chlorophyll a (PC/Chl) molar ratios70.
Extended Data Fig. 4. Low temperature (77K) fluorescence spectra.
a, 77K fluorescence emission spectra upon excitation at 435 nm.
b, 77 K fluorescence emission spectra upon excitation at 600 nm. With the exception of the data for LT at 700 μmol photons m-2 s-1 (for which n=4), all spectra shown are averages of six biological replicates of Synechocystis cultures measured 7 days post inoculation. Data has been baseline corrected for emission at 600 nm (a) and 620 nm (b), and normalized to input culture OD730nm, respectively. Spectra were corrected for dark-signal (dark-offset ON) and the blank value (BG11 medium prior to inoculation) was subtracted.
Extended Data Fig. 5. HL tolerance and motility of the original Synechocystis sp. PCC6803 strain.
Time course of the growth of the original (motile) Synechocystis sp PCC6803 strain at 2000 μmol photons m-2 s-1 and 23 °C over 7 days, after inoculation at OD730nm=0.05. The white arrowheads indicate the initial level of the liquid culture; the dashed outline indicates an area of reduced light exposure owing to the absence of LEDs in the cultivator design. Note that the motile cells migrate out of the liquid medium and only begin to return at 6 days post inoculation (dpi). This observation indicates that this strain requires an extended period of acclimation before it can cope with such high light intensities.
Supplementary Material
Acknowledgments
We thank Paul Hardy for critical reading of the manuscript and the German Science Foundation (DFG, grants TR175 Z1, GRK2062 and EXC2089/1 [e-conversion, funding ID: 390776260)] to D.L.) and the European Research Council (ERC Synergy Grant “PhotoRedesign”) for financial support.
Footnotes
Author contributions D.L., M.D. and A.G. designed adaptive evolution experiments and, together with M.T., performed them. E.M.O. and H.S. designed the sequencing strategy and analysed next-generation sequencing data. M.L. performed carotenoid profiling. D.L. was responsible for conceptualization and management of the entire study and wrote the paper with contributions from all authors.
Competing interests: The authors declare no competing interests.
Additional information
Extended data is available for this paper at https://doi.org/10.1038/s41477-021-00904-2.
Data and materials availability
All data supporting the findings of this study are available within the paper and its Supplementary Information files. Biological material is available upon reasonable request.
References
- 1.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 3.Chaux F, Peltier G, Johnson X. A security network in PSI photoprotection: regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front Plant Sci. 2015;6:875. doi: 10.3389/fpls.2015.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Sonoike K. Photoinhibition of photosystem I. Physiol Plant. 2011;142:56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 6.Jarvi S, Suorsa M, Aro EM. Photosystem II repair in plant chloroplasts--Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim Biophys Acta. 2015;1847:900–909. doi: 10.1016/j.bbabio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 7.de Bianchi S, Ballottari M, Dall'osto L, Bassi R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans. 2010;38:651–660. doi: 10.1042/BST0380651. [DOI] [PubMed] [Google Scholar]
- 8.Kirilovsky D, Kerfeld CA. Cyanobacterial photoprotection by the orange carotenoid protein. Nat Plants. 2016;2:16180. doi: 10.1038/nplants.2016.180. [DOI] [PubMed] [Google Scholar]
- 9.Komenda J, Sobotka R. Cyanobacterial high-light-inducible proteins--Protectors of chlorophyll-protein synthesis and assembly. Biochim Biophys Acta. 2016;1857:288–295. doi: 10.1016/j.bbabio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu M, Hihara Y. Acclimation to high-light conditions in cyanobacteria: from gene expression to physiological responses. J Plant Res. 2012;125:11–39. doi: 10.1007/s10265-011-0454-6. [DOI] [PubMed] [Google Scholar]
- 11.Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16:307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Ruban AV. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonoike K, Hihara Y, Ikeuchi M. Physiological significance of the regulation of photosystem stoichiometry upon high light acclimation of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001;42:379–384. doi: 10.1093/pcp/pce046. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Molina A, Leister D. Accelerated relaxation of photoprotection impairs biomass accumulation in Arabidopsis. Nat Plants. 2020;6:9–12. doi: 10.1038/s41477-019-0572-z. [DOI] [PubMed] [Google Scholar]
- 15.Kromdijk J, et al. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science. 2016;354:857–861. doi: 10.1126/science.aai8878. [DOI] [PubMed] [Google Scholar]
- 16.Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun. 2017;8:49. doi: 10.1038/s41467-017-00074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schierenbeck L, Ries D, Rogge K, Grewe S, Weisshaar B, Kruse O. Fast forward genetics to identify mutations causing a high light tolerant phenotype in Chlamydomonas reinhardtii by whole-genome-sequencing. BMC Genomics. 2015;16:57. doi: 10.1186/s12864-015-1232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen PE, Leister D. Cyanobacteria as an experimental platform for modifying bacterial and plant photosynthesis. Front Bioeng Biotechnol. 2014;2:7. doi: 10.3389/fbioe.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leister D. Experimental evolution in photoautotrophic microorganisms as a means of enhancing chloroplast functions. Essays Biochem. 2018;62:77–84. doi: 10.1042/EBC20170010. [DOI] [PubMed] [Google Scholar]
- 20.Heidorn T, et al. Synthetic biology in cyanobacteria: Engineering and analyzing novel functions. Methods Enzymol. 2011;497:539–579. doi: 10.1016/B978-0-12-385075-1.00024-X. [DOI] [PubMed] [Google Scholar]
- 21.Lopo M, et al. Experimental and modeling analysis of Synechocystis sp. PCC 6803 growth. J Mol Microbiol Biotechnol. 2012;22:71–82. doi: 10.1159/000336850. [DOI] [PubMed] [Google Scholar]
- 22.Tillich UM, Lehmann S, Schulze K, Duhring U, Frohme M. The optimal mutagen dosage to induce point-mutations in Synechocystis sp. PCC6803 and its application to promote temperature tolerance. PLoS One. 2012;7:e49467. doi: 10.1371/journal.pone.0049467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillich UM, Wolter N, Franke P, Duhring U, Frohme M. Screening and genetic characterization of thermo-tolerant Synechocystis sp. PCC6803 strains created by adaptive evolution. BMC Biotechnol. 2014;14:66. doi: 10.1186/1472-6750-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko T, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 25.Hagemann M, et al. Identification of the DNA methyltransferases establishing the methylome of the cyanobacterium Synechocystis sp. PCC 6803. DNA Res. 2018;25:343–352. doi: 10.1093/dnares/dsy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu L, et al. Transgenerational epigenetic inheritance under environmental stress by genome-wide DNA methylation profiling in cyanobacterium. Front Microbiol. 2018;9:1479. doi: 10.3389/fmicb.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dann M, Leister D. Evidence that cyanobacterial Sll1217 functions analogously to PGRL1 in enhancing PGR5-dependent cyclic electron flow. Nat Commun. 2019;10:5299. doi: 10.1038/s41467-019-13223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110:361–371. doi: 10.1016/s0092-8674(02)00867-x. [DOI] [PubMed] [Google Scholar]
- 29.Battchikova N, Eisenhut M, Aro EM. Cyanobacterial NDH-1 complexes: novel insights and remaining puzzles. Biochim Biophys Acta. 2011;1807:935–944. doi: 10.1016/j.bbabio.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa T, Mi H. Cyanobacterial NADPH dehydrogenase complexes. Photosynth Res. 2007;93:69–77. doi: 10.1007/s11120-006-9128-y. [DOI] [PubMed] [Google Scholar]
- 31.Peltier G, Aro EM, Shikanai T. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu Rev Plant Biol. 2016;67:55–80. doi: 10.1146/annurev-arplant-043014-114752. [DOI] [PubMed] [Google Scholar]
- 32.Gao F, et al. The NDH-1L-PSI supercomplex is important for efficient cyclic electron transport in cyanobacteria. Plant Physiol. 2016;172:1451–1464. doi: 10.1104/pp.16.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeremenko N, et al. Open reading frame ssr2016 is required for antimycin A-sensitive photosystem I-driven cyclic electron flow in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2005;46:1433–1436. doi: 10.1093/pcp/pci147. [DOI] [PubMed] [Google Scholar]
- 34.Kojima K, et al. Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol Microbiol. 2007;65:936–947. doi: 10.1111/j.1365-2958.2007.05836.x. [DOI] [PubMed] [Google Scholar]
- 35.Ejima K, Kawaharada T, Inoue S, Kojima K, Nishiyama Y. A change in the sensitivity of elongation factor G to oxidation protects photosystem II from photoinhibition in Synechocystis sp. PCC 6803. FEBS Lett. 2012;586:778–783. doi: 10.1016/j.febslet.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Tajima N, et al. Genomic structure of the cyanobacterium Synechocystis sp. PCC 6803 strain GT-S. DNA Res. 2011;18:393–399. doi: 10.1093/dnares/dsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautmann D, Voss B, Wilde A, Al-Babili S, Hess WR. Microevolution in cyanobacteria: re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012;19:435–448. doi: 10.1093/dnares/dss024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zavrel T, Ocenasova P, Cerveny J. Phenotypic characterization of Synechocystis sp. PCC 6803 substrains reveals differences in sensitivity to abiotic stress. PLoS One. 2017;12:e0189130. doi: 10.1371/journal.pone.0189130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshihara S, Ikeuchi M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem Photobiol Sci. 2004;3:512–518. doi: 10.1039/b402320j. [DOI] [PubMed] [Google Scholar]
- 41.Ohad I, Raanan H, Keren N, Tchernov D, Kaplan A. Light-induced changes within photosystem II protects Microcoleus sp. in biological desert sand crusts against excess light. PLoS One. 2010;5:e11000. doi: 10.1371/journal.pone.0011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raanan H, et al. Towards clarifying what distinguishes cyanobacteria able to resurrect after desiccation from those that cannot: The photosynthetic aspect. Biochim Biophys Acta. 2016;1857:715–722. doi: 10.1016/j.bbabio.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Ungerer J, Wendt KE, Hendry JI, Maranas CD, Pakrasi HB. Comparative genomics reveals the molecular determinants of rapid growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Proc Natl Acad Sci U S A. 2018;115:E11761–E11770. doi: 10.1073/pnas.1814912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaitan-Espitia JD, Hobday AJ. Evolutionary principles and genetic considerations for guiding conservation interventions under climate change. Glob Chang Biol. 2020 doi: 10.1111/gcb.15359. [DOI] [PubMed] [Google Scholar]
- 45.Harvey BJ, Nash KL, Blanchard JL, Edwards DP. Ecosystem-based management of coral reefs under climate change. Ecol Evol. 2018;8:6354–6368. doi: 10.1002/ece3.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Oppen MJ, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proc Natl Acad Sci U S A. 2015;112:2307–2313. doi: 10.1073/pnas.1422301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leister D. Genetic engineering, synthetic biology and the light reactions of photosynthesis. Plant Physiol. 2019;179:778–793. doi: 10.1104/pp.18.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. [Google Scholar]
- 49.Tillett D, Neilan BA. Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. J Phycol. 2000;36:251–258. [Google Scholar]
- 50.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31:3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 54.Bushnell B. BBTools: a suite of bioinformatic tools used for DNA and RNA sequence data analysis. 2016. Available at http://jgi.doe.gov/data-and-tools/bbtools.
- 55.Bushnell B, Rood J, Singer E. BBMerge – Accurate paired shotgun read merging via overlap. PLoS ONE. 2017;12:e0185056–0185015. doi: 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker BJ, et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963–112914. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barrick JE, et al. Identifying structural variation in haploid microbial genomes from short-read resequencing data using breseq. BMC Genomics. 2014;15:1039–1017. doi: 10.1186/1471-2164-15-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deatherage DE, Barrick JE. Engineering and Analyzing Multicellular Systems. Springer; New York: 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. (ed^(eds) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv preprint. 2012:arXiv: 12073907 [q-bioGN] [Google Scholar]
- 61.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viola S, Rühle T, Leister D. A single vector-based strategy for marker-less gene replacement in Synechocystis sp. PCC 6803. Microb Cell Fact. 2014;13:4. doi: 10.1186/1475-2859-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, III, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 64.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 65.Heinz S, et al. Thylakoid membrane architecture in synechocystis depends on CurT, a homolog of the granal CURVATURE THYLAKOID1 proteins. Plant Cell. 2016;28:2238–2260. doi: 10.1105/tpc.16.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zavrel T, Sinetova MA, Cervený J. Measurement of chlorophyll a and carotenoids concentration in cyanobacteria. Bio-protocol. 2015;5:e1467 [Google Scholar]
- 68.Watanabe M, et al. Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria. Proc Natl Acad Sci U S A. 2014;111:2512–2517. doi: 10.1073/pnas.1320599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murakami A. Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PS I/PS II stoichiometries. Photosynth Res. 1997;53:141–148. [Google Scholar]
- 70.Rakhimberdieva MG, Boichenko VA, Karapetyan NV, Stadnichuk IN. Interaction of phycobilisomes with photosystem II dimers and photosystem I monomers and trimers in the cyanobacterium Spirulina platensis. Biochemistry. 2001;40:15780–15788. doi: 10.1021/bi010009t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information files. Biological material is available upon reasonable request.