Abstract
Inspired by physically adaptive, agile, reconfigurable and multifunctional soft-bodied animals and human muscles, soft actuators have been developed for a variety of applications, including soft grippers, artificial muscles, wearables, haptic devices and medical devices. However, the complex performance of biological systems cannot yet be fully replicated in synthetic designs. In this Review, we discuss new materials and structural designs for the engineering of soft actuators with physical intelligence and advanced properties, such as adaptability, multimodal locomotion, self-healing and multi-responsiveness. We examine how performance can be improved and multifunctionality implemented by using programmable soft materials, and highlight important real-world applications of soft actuators. Finally, we discuss the challenges and opportunities for next-generation soft actuators, including physical intelligence, adaptability, manufacturing scalability and reproducibility, extended lifetime and end-of-life strategies.
Soft materials and actuators enable applications that are not possible using traditional rigid robots and actuators. Owing to their flexibility and compliance, soft actuators can adapt to complex and dynamic environments, which makes them exceptionally advantageous for physical interaction with fragile objects or living organisms. However, despite great advances in the field of soft robotics, both replicating the performance of biological actuators and the implementation of soft robots in industrial applications remain a challenge owing to the high-level complexity of biological actuators. Biological muscle and animal limbs have long served as inspiration for the design of soft actuators featuring a range of complex actuations (such as reversible contraction, expansion and rotation) and motions (such as bending and twisting), as well as specific strain, force, energy and power performance metrics1–3 (BOX 1). However, biological muscles have evolved to operate robustly in dynamic environments with high performance (efficiency, speed and power) and complex actions (including sensing, tunable stiffness or repair mechanisms), all of which are challenging to replicate in synthetic systems.
Box 1. Soft actuator performance metrics.
Actuator performance metrics are related to actuation strain (changes in length or geometry upon actuation), actuation force (force generated during actuation), energy (output work generated by the actuator) and power (output work normalized by actuation timescale). The relevant performance metrics for skeletal muscle1 are included for context.
Strain
Actuation strain, ε(%) = (Lactuated – Lnon-actuated)/Lnon-actuated (skeletal muscle: 20-40%)
Actuation strain rate (% s−1) = actuation strain/actuation time (skeletal muscle: 10–50% s−1)
Hysteresis = change in strain over actuation cycles
Locomotion speed = body length/time (for mobile and untethered actuation) Actuator density (kg m−3) = mass/initial volume (external components are typically neglected) (skeletal muscle: 1,037 kg m−3)
Force
Force output (N)
Actuation torque (N m)
Actuation stress (Pa) = force output per area (skeletal muscle: 0.1–0.35 MPa)
Torsional stroke (deg m−1) = rotations per displaced length
Torsional speed (rpm) = rotations per unit of time (minute)
Energy
Work (J) = mlift g Δy (mlift: mass of the lifted object; g: gravitational acceleration, Δy: vertical displacement of the lifted object)
Specific work (J kg−1)=work per mass (skeletal muscle: 8–39 kJ kg−1)
Work density (J m−3)=work per volume (skeletal muscle: 8–40 kJ m−3)
Efficiency, n (%)=work per energy input (skeletal muscle: 35–40%)
Cycle life = maximum actuation cycle before mechanical failure (skeletal muscle >109)
Power
Frequency (Hz) = actuation cycle per second (skeletal muscle: 1–10 Hz)
Power (W) = work done per actuation time
Specific power (W kg−1) = power per mass (skeletal muscle: 48–274 W kg−1)
Power density (W m−3) = power per volume (skeletal muscle: 50–248 W m−3)
Individual performance metrics can be particularly useful for specific tasks and are thus usually designed for specific applications. However, combining the performance and physical intelligence of biological systems (such as self-healing and self-adaptive behaviours) would greatly advance the integration of soft actuator and robot technology in multiple fields (for example, health care4, agriculture5,6, industry7,8 and mobility9).
In this Review, we discuss materials and soft actuation methods that can enable advanced physical intelligence10, function and performance in soft actuator technology. We first examine promising soft actuation methods, including tethered, untethered and biohybrid actuation, and then discuss actuation strategies based on programmable soft materials that improve the performance and multifunctionality of soft actuators. We highlight state-of-the-art demonstrations of soft actuators towards real-world applications, including soft grippers, artificial muscles, sensor-integrated soft robots, haptic displays and biomedical applications. Lastly, we discuss challenges and opportunities for next-generation soft actuators, including integrating physical intelligence, encoding adaptability, manufacturing scalability and reproducibility, and improving the durability of soft robots.
Promising soft actuation methods
Material selection, structural design and fabrication techniques are key factors in designing soft actuators that can convert multiple energy inputs into mechanical energy outputs using tethered, untethered or biohybrid approaches.
Tethered synthetic soft actuation
Tethered soft actuators are usually actuated through a change of fluidic pressure in hollow channels of the soft actuator body, by electrically driven shape change or through passive deformation produced by motion transmitters (for example, cables and tendons) attached to external motors.
Fluidic soft actuators
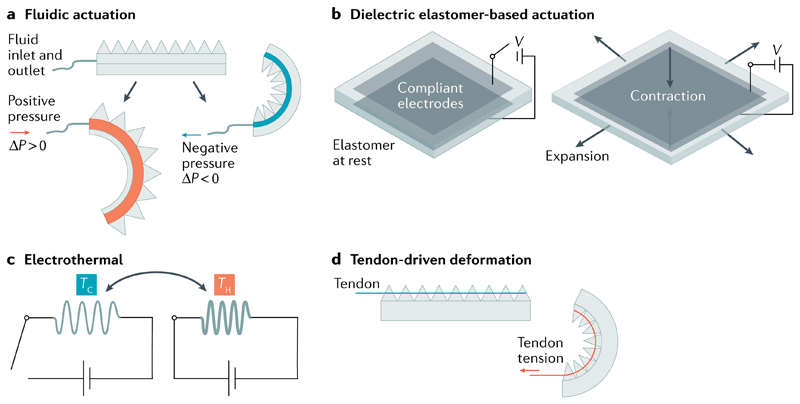
Fluidic soft actuators, which are the most prevalent soft actuator type, work by controlling the fluid pressure inside the hollow channels of their soft body (FIG. 1a). On top of basic gripping and locomotion capabilities, advanced functions are being explored; for example, a single pneumatic input can control the actuation sequence of multiple actuators by exploiting the nonlinearity of soft body deformations and passive flow restrictions. The physical intelligence of this actuation sequence allows a robot with 8 degrees of freedom (8-DOF) to walk without any active control or fluidic valves11. Accessing passive flow resistance (viscous resistance) can be used to program not only the sequence but also the time and degrees of actuation of soft robots12. Additionally, dynamic locomotion gaits, such as jumping, can be realized by harnessing snap-through instabilities of pneumatically inflated shells13. Fluidic actuators are highly compliant, easy to fabricate, and can provide large deformations, but they require bulky pumps, valves and tubes. Moreover, their actuation is typically slow, inefficient and not very precise14,15.
Fig. 1. Working principles of tethered soft actuators.
a | Soft robotic systems can be actuated through changes in the fluid or air pressure ΔP. b | Dielectric elastomers are actuated by an electric field. c | Electrothermal actuation of a single twisted fibre. d | Forces can be transmitted by tendons. TC, cooling temperature; TH, heating temperature.
Electrically driven soft actuators
Electrically driven actuation is the second-most common tethered soft actuation method. Electrically driven soft actuators exploit the Maxwell stress to induce a mechanical shape change in dielectric soft materials. For example, dielectric elastomer actuators (DEAs) have opposing flexible electrodes on both sides of an elastomer sheet, which can contract along the thickness (direction of the electric field) and expand in the area orthogonal to the thickness direction (FIG. 1b). Shape-morphing DEAs can be designed with embedded internal electrodes between layers of elastomer sheets16, and interdigitated DEAs can be 3D-printed to enable multi-axial actuation17. Nevertheless, to deliver large strains, DEAs often require a rigid frame and a pre-stretch step18. DEAs without rigid frames have lower output force, smaller strain and lower energy density, unless they feature multilayer strain-stiffening elastomers19. As an alternative to isotropic elastomers, DEAs can benefit from the anisotropic elasticity of liquid crystal elastomers to achieve high anisotropic strains, because the molecular alignment of liquid crystal elastomers can be programmed; for example, monolithic dielectric liquid crystal elastomers can achieve a strain rate of >120% per second20. DEAs offer high electrical control and electromechanical actuation performance; however, they can suffer from dielectric breakdown at elastomer defects, hindering their large-scale fabrication.
Hydraulically amplified self-healing electrostatic actuators
Hydraulically amplified self-healing electrostatic (HASEL) actuators incorporate the advantages of DEAs and fluid-driven soft actuators7,21,22. Using both electrostatic and hydraulic forces, HASEL actuators can actuate in different modes (such as expansion, contraction and rotation), self-sense their deformation, and electrically self-heal after a dielectric breakdown. They are classified as electroelastic and thermoplastic actuators, and can be scaled up to achieve impressive force outputs22. Electrohydraulic HASEL actuators offer all-round muscle performance with actuation stresses of 0.3 MPa, contraction strains of 24% and energy densities of 70 J kg−1 at resonance. The only limitation remains their operation lifetime, which is less than that of natural muscles by a factor of about 1,000 (REFs7,22). Similar to DEAs, the main challenge for safely operating HASEL actuators in close proximity to the human body is the requirement of very high voltage (of the order of kilovolts). Safety could be ensured by carefully limiting the maximum electrical current flow at the expense of actuation speed22.
Electrohydrodynamic actuators
Electrohydrodynamic actuators use high electric fields to induce motion in dielectric fluid enclosed in elastomeric channels. Stretchable soft pumps based on charge-injection electrohydrodynamic mechanisms enable bi-directional pumping23 and have low power consumption (about 100 mW), low mass, and performance metrics (specific pressure of 10 bar kg−1 and specific flow rate of about 103 ml min−1 kg−1) that are comparable with those of commercial bulky pumps, making them ideal portable tools for soft robots and fluid-driven wearable devices.
Electrothermal actuators
Electrothermal actuators are tethered systems that use electrical current passing through an electrically resistive material to generate Joule heating for expansion or contraction (FIG. 1c). Thermal actuators can be based on shape memory alloys24, shape memory polymers25 or twisted fibres and yarns26–29, which have high specific power (up to about 100 times more power than human muscles30). However, thermo-responsive twisted fibres usually suffer from low actuation efficiency and low frequency owing to slow heating and cooling cycles.
Passive deformation
Although not an actuation mechanism, soft machines can also be designed on the basis of passive mechanisms, that is, deformation caused by tension transmitted through tendons from external motors (FIG. 1d). Here, complex deformations and locomotion can be achieved by using multiple motors connected by tendons to different parts of a soft body. Such tendon-based mechanisms are similar to biological tendons and are thus especially suitable for wearable (assisting and rehabilitating) devices. For example, tendon-driven soft wearable gloves can generate high enough forces, while maintaining a small form factor, to enable rehabilitation of patients with spinal cord injuries31. Similarly, soft machines with discontinuous or architected bodies, which cannot use fluidic pressures, are suitable for tendon-driven actuation32. Other applications include soft, multi-fingered grippers33, continuum robotic arms34, soft pumps35 and efficiency enhancement of fluidic robots36. Using compliant tendons along external rigid motors, tendon-driven soft robots can provide fast and accurate deformations with high output forces, while maintaining an overall compliant structure. These systems, however, are not suitable for scaling down in size, because they require motors, and they cannot be easily scaled up in degrees of freedom, because two motors are required for each degree of freedom to achieve an agonist-antagonist actuation system.
The requirement of motors, pumps or cords for tethered actuation methods constrains device miniaturization and mobility, as well as applications in certain environments. Research is in progress to overcome these miniaturization and mobility constraints, while maintaining the overall softness and performance of the system. Tethered actuation can further be transformed into untethered actuation for specific target applications by integrating external actuation instrumentation (such as fluidic pumps and valves, and high-voltage electronics and batteries), into the actuated robot or device, particularly for centimetre-scale, palm-size or larger soft robots in fluidic37,38 and electrical soft actuation39.
Untethered synthetic soft actuation
Tethered actuators, including those triggered by onboard electronics40 or soft pumps41, have promising features, such as large force output and agile deformation; however, for certain applications, soft actuators powered by untethered stimuli are preferred. Untethered soft actuators can be roughly categorized according to their stimuli: magnetic, light, acoustic and chemical.
Magnetic stimuli
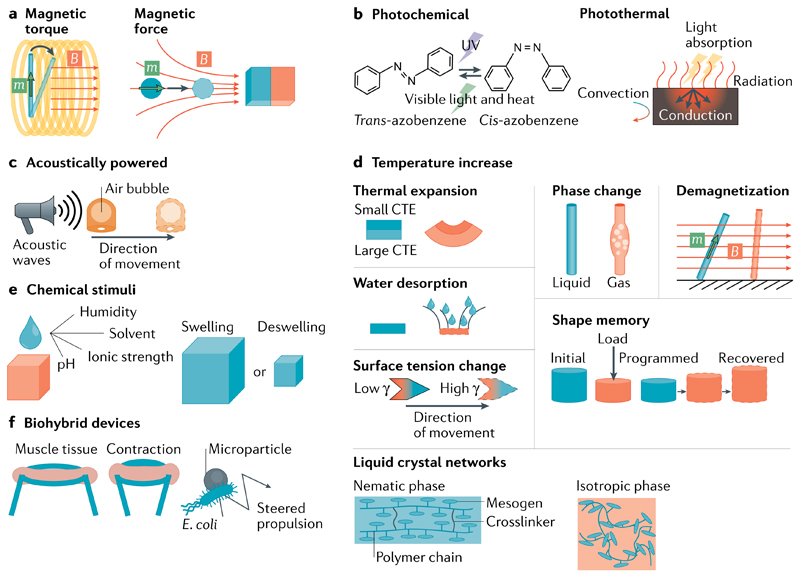
External magnetic field-controlled soft actuators are being intensively investigated, because they can operate in a fixed-volume workspace (for example, a human body) and they can be applied via minimally invasive surgery and targeted drug delivery. The amplitude, gradient and direction of the magnetic field can be modulated with high temporal resolution, enabling deep tissue penetration. Magnetic actuators are actuated by the torques or forces generated by the interaction between controlled external magnetic fields or gradients and the magnetic properties of the actuator (FIG. 2a). With sophisticated control of external magnetic fields and actuator magnetization profiles, composite devices can achieve multimodal locomotion on various terrains42,43, bio-inspired movement and functions44,45 and complex time-varying deformations46,47. By combining magnetic properties with other material attributes and structural designs, more advanced functions can be achieved48–50, such as shape memory, environmental self-adaptation, mechanical tunability and logic circuits, among others. Magnetic actuators can further be encoded with programmed diverse local magnetization profiles51–53 to achieve desired multiple complex deformations under different homogeneous magnetic fields; in addition, the arrangement of the magnetic domains can be reconfigured by heating ferromagnetic materials to their Curie temperatures54 or by re-aligning embedded magnetic particles55. High-frequency magnetic fields can also excite magnetic particles and remotely generate heat to activate soft actuators without any physical contact56.
Fig. 2. Working principles of untethered and biohybrid soft actuators.
a-e | Actuation mechanisms triggered by magnetic fields (panel a), light (panel b), acoustic waves (panel c), temperature increase (panel d, red colour indicates increased temperature) and other environmental stimuli (panel e). f | Biohybrid devices can be designed on the basis of skeletal or cardiac muscle tissues and living microorganisms, such as Escherichia coli. CTE, coefficient of thermal expansion.
Light stimuli
Light is also commonly used as a wireless stimulus for soft actuators. In contrast to magnetic fields, light can be modulated with both high temporal and high spatial resolution using established equipment57, such as optical choppers, lenses and photomasks. Depending on the chemistry and optical absorptive properties of the light-active components in the actuator, the light that stimulates the soft actuator can either be wavelength-selective (provided by an ultraviolet source, a single-colour light-emitting diode (LED) or a laser) or it can be wide-spectrum (provided by white light or solar radiation). The working principle of synthetic light-responsive actuators is usually based on photochemical reactions and/or the photothermal effect (FIG. 2b). In light-responsive liquid crystal networks, the ordering change is often introduced by photoisomerization of azobenzene derivatives58–60 or by photonic–thermal energy conversion59,61,62. In other materials, light can also trigger macroscopic deformation and motion via (including but not limited to) a shape memory effect63,64, an inequivalent thermal strain65,66, water desorption67,68, a change of hydrophobicity69,70, a change of surface tension71, a phase transition72–74 or a change of magnetic properties75,76.
Acoustic stimuli
Acoustic waves are a viable energy source for mechanical soft actuation, owing to their deep penetration into biological tissues and fluidic media. To efficiently convert acoustic energy into motion, mechanical resonance is required, which can be provided, for example, by integrating oscillatory elements, such as gas bubbles77,78 (FIG. 2c) or sharp, flexible structures79,80, into the robot body to produce propulsion. Bubble-based actuators can exert a large thrust force in fluidic medium under a low-amplitude acoustic field; however, the long-term stability of the trapped bubble cannot be guaranteed. By contrast, flexible oscillating structures do not have bubble stability problems, but usually require a higher acoustic energy input for actuation owing to the over-damped displacement response of soft materials. By designing oscillators with well separated resonance frequencies, each oscillating actuator could be selectively addressed by ultrasound waves. Regardless of the type of oscillatory element, an acoustically powered actuator should be in the focal plane of ultrasound waves to enable efficient resonant energy transfer.
Temperature stimuli
In addition to electric field-, magnetic field- and light-induced heating, environmental temperature changes can be used in air and liquids to heat and actuate thermo-responsive soft materials (FIG. 2d). Before heating, several strategies can be applied to accomplish target functions, achieve better output performance, or perform heterogeneous deformation, including optimization of the structural design81, anisotropic alignment of the material82, patterning of material properties83, assembling of parts with different thermal responses84,85 and mechanical pre-treatment of the actuators28,29.
Chemical stimuli
Environmental stimuli, such as humidity, ionic strength and chemical substances (liquid or vapour), can also actuate stimuli-responsive soft materials and, thus, serve as energy inputs for actuation (FIG. 2e). Hygroscopic actuators exploit the volumetric swelling behaviour of anisotropically or heterogeneously designed water-absorbing materials to achieve locomotion86 and programmed shape morphing87,88. Actuators can also respond to changes in ionic strength85, pH89, change of solvent90,91, the presence of solvent vapours27,92, the products (such as gas and heat) of chemical reactions93,94, the surface tension gradient95,96 and selective DNA sequences97.
Such untethered actuators are entirely soft or have only very few rigid parts. Furthermore, they can be easily scaled down in size and are wirelessly controlled, which renders them highly mobile in complex and hard-to-reach environments, such as inside the human body. Untethered actuators can further use ubiquitous energy sources and respond to environmental changes, such as light illumination, temperature change and humidity gradients. However, their work density and force output are usually smaller than those of their tethered counterparts. Also, deformation and motion are dynamically coupled to their relative position from their energy source; for example, in a steady magnetic field, a magnetic actuator experiences different torques or directions of force depending on its orientation and location; and a light-triggered actuator has a different effective illumination area after it deforms because of the self-shadowing effect and incidental angle change. These dynamic changes make deformation more difficult to model and predict and thus, an inverse design strategy is more difficult. Despite these challenges, proper design will enable minimally modulated or even static stimuli inputs to trigger dynamic motions, such as self-sustained oscillation74 and continuous rotation75.
Biohybrid soft actuators
Biological cells and microorganisms have evolved to convert chemical energy into mechanical work with high efficiency and control. Owing to material and fabrication technology limitations, the synthesis of soft robots with sophisticated control and actuation pathways at the length scales of cells and microorganisms remains challenging. Alternatively, biohybrid devices98,99 can be designed in which biological actuating and sensing functions are combined with synthetic structures. For example, cardiomyocytes, smooth muscle cells or skeletal muscle cells contract either spontaneously with a chemical fuel (such as adenosine triphosphate, ATP) or through electrical stimulation (FIG. 2f). These cells can be optogenetically modified to remotely and wirelessly respond to light100,101. Muscle cells can further be co-cultured with neural cells to form neuromuscular units, whose contraction can also be triggered by light102. Such modified muscle cells can be seeded on different substrates or in matrices (including hydrogels, elastomers and extracellular matrices), and their contractile movement can be exploited to perform different functions103, such as in artificial muscles104, pumping105, sensing106 and locomotion100,107,108. Living organisms that can propel themselves (for example, Escherichia coli, sperm cells, jellyfish and insects) can also be combined with control components (FIG. 2f), allowing the design of devices that can be steered to follow desired trajectories109–112 or stimulated on demand to improve motion efficiency113. In addition, biological cells can be used as carriers for abiotic functional dopants, such as micro- or nanoparticles and drugs114,115, to perform biomedical functions.
Integrating biological units into soft actuators enables the design of small-scale devices that combine actuation, sensing and controlling functions without going through an overly complicated miniaturization and integration process. As a trade-off, these biohybrid devices are normally not as robust and durable as abiotic devices, and so the maintenance of the biological activity of cells, tissues and microorganisms requires specific environmental conditions (for example, temperature, proper nutrients, pH and hydration), which limits their potential applications.
Real-world applications
With advances in materials design and fabrication technologies, soft actuators and devices or robots have grown more sophisticated and are now applied in various areas. The mechanical properties of soft actuators are more similar to those of biological tissues than those of their rigid counterparts, making them suitable for replicating the functions of biological appendages, organs and actuators, such as hands, intestines and muscles. The softness of these actuators also allows integration into wearable devices to achieve sensing capabilities, conformability and user comfort. Soft actuators can further interact with humans to provide haptic feedback, not just for the sensing of rigid textures and vibrations, but also to feel continuous surface contours and the softness of textures. In biomedical applications, in particular, for in vivo devices, patient safety is a key priority. Soft actuators have the advantage of being minimally invasive, while enabling contact and interactions with delicate tissues. Here, we discuss real-world applications of soft actuators, ranging from object manipulation and human-machine interactions to biomedical devices (TABLE 1).
Table 1. Application-specific desired abilities and properties (challenges) for soft actuators.
| Applications | Challenges |
|---|---|
| Soft grippers | Precise movement, gripping accuracy, versatility, manipulation across different size scales, adaptability to environmental conditions (dry, wet, vacuum) |
| Artificial muscles | Performance metrics close to those of biological muscles: actuation stress, actuation strain, strain rate, energy density, actuation frequency, life cycle, versatility |
| Sensor-integrated soft robots and wearables | Proprioception and exteroception: sensing shape, position and state; sensing external stimuli (light, heat, sound and electromagnetic fields); stretchable conductive wires and electrodes |
| Haptic displays | Sensing surface properties (texture, friction, softness, tackiness and viscoelasticity); sensing bulk properties (elasticity, elongation and hardness) |
| Biomedical devices | Rehabilitation and surgical devices: safe contact, adaptability, conformability |
Soft grippers
Traditional rigid robotic grippers are superior to human hands in terms of strength and durability, but are out-performed by the compliance, dexterity and conformability of human hands. To prevent damage of fragile objects upon contact or during manipulation, rigid grippers require precise feedback control of their forces and locations. By contrast, the conformability of the soft tissue of human hands enables the gripping of delicate objects, such as a tomato or an egg, without crushing them. Mimicking the soft tissue of human hands, soft constituent materials provide intrinsic compliance and continuum deformation for soft robotic grippers, enabling the high number of degrees of freedom necessary for under-actuated and conformal grasping of arbitrarily-shaped 3D objects.
Soft robotic grippers can perform grasping by actuation, controlled stiffness or controlled adhesion116. The most common strategy is actuation; here, the fingers of the gripper bend around the object to grip it. Fluidic actuation (using positive or negative fluid pressure), which is the most common actuation-based gripping method, can provide high output forces with simple control; for example, an origami-inspired lightweight vacuum-driven soft gripper can lift up to 1,000 times its body weight14. Fluidic actuation is a versatile actuation method, compatible with grippers across size scales (gripping objects with dimensions from around 100 μm (REFS117,118) to around 1 m (REF.119)), and is currently the only commercially available gripping technology (Soft Robotics Inc.). Electroactive polymer-based grippers can provide large forces as well as a quick response (as fast as about 12 ms; REF.21). Shape-memory polymers cannot produce stresses as large as those of fluidic or electroactive actuators, but can be wirelessly actuated by thermal stimulation. Similarly, soft grippers fabricated with other stimuli-responsive materials can be triggered by pH120, humidity121, solvent122, light123 and magnetic fields124. Most of these untethered stimuli induce an indirect, volumetric change-based deformation. Alternatively, magnetic forces and torques can be directly applied to actuators125. Untethered small soft grippers have mainly been applied in biomedical applications thus far, such as targeted drug delivery126 and active biopsy127. Micro-grippers that can operate at approximately human body temperature and can spontaneously grip onto a tissue have also been applied in vivo for sustained drug release128 or to excise cells from tissue129.
Materials or structures of variable stiffness can be applied to enable gripping by controlled stiffness. This method has the advantage that low forces are applied on the object during gripping (soft state), and high forces (stiff state) allow holding of the object, ensuring a conformal grasp. Various materials can be used to modulate the stiffness of the gripper, such as shape memory polymers and low-melting-point alloys130. Shape memory polymers can achieve a substantial stiffness change, enabling the development of grippers that can grasp objects around 925 times heavier than their body weight123. Shape memory polymers, however, have long actuation times (on the order of minutes), whereas granular jamming enables fast gripping (about 0.1 s) at the expense of lower holding forces131.
Grippers with controlled adhesion can control the surface force between itself and the gripped object. Using electro-adhesion, lightweight, fast and versatile soft grippers have been developed that are capable of gripping a wide variety of objects, from a water-filled balloon to a flat sheet of paper5. Such grippers have a very short response time and can be used on both smooth and rough surfaces; however, they require very high electric fields (on the order of kilovolts) to generate electrostatic attractive forces high enough for gripping. By contrast, grippers covered with gecko-inspired, fibre-based dry adhesives do not require power to operate and can achieve high loading capacities (about 280 times their body weight132) and versatile gripping of objects of different shapes133 (FIG. 3a). Micro-crater (sucker cup)-based dry adhesives retain their adhesive strength even in humid conditions and underwater134, but they cannot match microfibres in terms of adhesion strength.
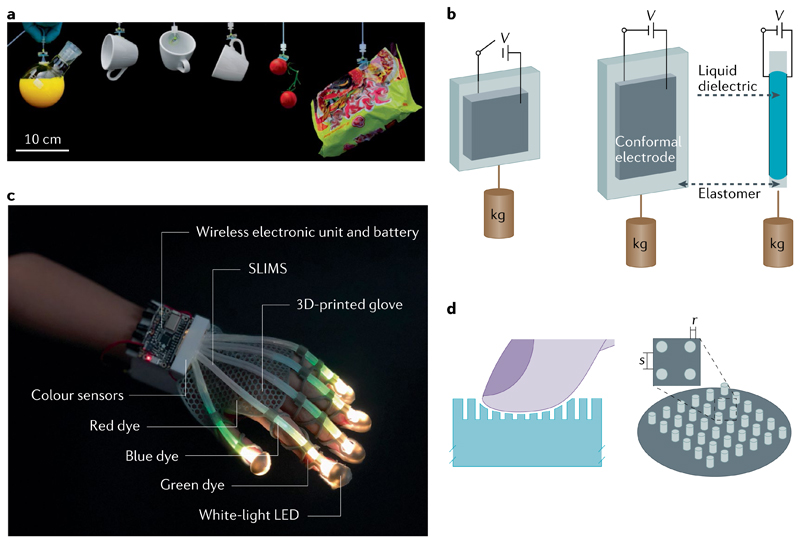
Fig. 3. State-of-the-art soft actuators with potential industrial applications.
a | Dry microfibrillar adhesive-covered soft gripper, shown gripping various objects, that combines 3D surface conformability and high adhesion strength by controllable equal load sharing at the interface133. b | Schematic of a linear hydraulically amplified self-healing electrostatic (HASEL) actuator. c | A high-resolution soft sensor with optical output, fabricated with elastomeric light guides, can be used to differentiate and quantify the location, amplitude and type of mechanical deformation, as demonstrated with a wireless, soft wearable glove144. d | The softness of an object can be perceived by finger touch by optimizing the geometric and material properties of micropatterned pillars163. SLIMS, stretchable lightguide for multimodal sensing; LED, light-emitting diode. Panel a reprinted with permission from REF.133, National Academy of Sciences. Panel c reprinted with permission from REF.144, AAAS. Panel d reprinted with permission from REF.163, AAAS.
Particularly in industrial settings, soft grippers need to provide fast yet precise movements to enable high productivity. The precision and accuracy of soft grippers can be improved by incorporating soft sensors to create a feedback loop. However, although progress has been made, the robustness and sensitivity of rigid electronic sensors have not yet been achieved with soft sensors. In addition, most current soft grippers lack versatility; the same gripper is unable to manipulate objects across different size scales and under various conditions (such as ambient, dry, wet or vacuum). This capability would pave the way for commercialization and wide adoption of soft grippers.
Artificial muscles
Replicating the performance of biological muscles — actuation stresses of up to 0.35 MPa (REF.135), strains of 40% (REF.1) and energy densities of 40 J kg−1 (REF.136) — including their versatility, self-sensing and self-healing capabilities, remains challenging in an artificial soft construct. A range of artificial muscles have been designed, including DEAs19,137, fluid-driven actuators14,56 and stimuli-responsive polymers27–30, based on a variety of working mechanisms2,138. Despite substantial achievements, artificial muscles can only reach or surpass natural muscles in some respects; for example, thermo-responsive polymer fibres have a high specific power, but their actuation efficiency and frequency are low1,27. Fluid-based actuators, which require external pumps and tubes to operate, can morph into complex 3D shapes, but lack speed and efficiency14,15. DEAs have excellent electrical actuation control, but require rigid frames or multilayer stacked designs to achieve high force output and large strains19. HASEL actuators offer all-round muscle performance (FIG. 3b), but their operation cycle falls behind that of natural muscles by three orders of magnitude7,22. The impressive overall muscle-like performance of HASEL actuators stems from the combined benefits of DEAs and fluid-driven actuators, which make such electrohydraulic soft actuators great candidates for scaling up to practical applications.
Sensor-integrated soft robots and wearable devices
To be comparable with biological systems in terms of autonomy, soft robots need to incorporate sensory components and achieve closed-loop control. The sensory feedback of soft robots can be based on proprioception, that is, being able to sense their own shape, position and state, or on exteroception, that is, being able to sense different types of external stimuli. Depending on the application, sensing stimuli, such as light, heat, sound and electromagnetic fields, can be exploited; however, physical and mechanical cues remain the most important signals with which soft robots can navigate their surroundings.
Proprioception is especially difficult to achieve in soft robots owing to their high number of degrees of freedom. Proprioceptive capabilities are usually based on sensing the curvature or strain at discrete points along the soft body, using different types of stretchable strain sensors (such as resistive139, capacitive140 and piezoresistive141). Alternatively, optoelectronic sensory foams can be applied and combined with machine learning algorithms to predict types and degrees of deformation (such as bending and twisting) of a soft actuator142. Similarly, an optical sensory system can be designed using stretchable optical fibres and neural networks to sense volumetric structural deformation during compression143. Such optical fibre-based proprioceptive systems have been used in a soft robotic glove, which can distinguish between all types of finger joint movements in real time144 (FIG. 3c). Liquid metal-based stretchable sensors have also been used to reconstruct the deformation (from actuation as well as from contact with other objects) of soft actuators in real time145.
Most soft actuators capable of proprioception also have exteroceptive capabilities; that is, the same sensors that measure internal pressures can also be used to sense external mechanical stimuli. Additional sensors can be added to achieve exteroceptive contact sensing, for example, by embedding deep and fine contact sensors along with curvature sensors into the soft actuator by 3D printing146. Photonic strain sensors can further be used as curvature, elongation and force sensors to achieve active haptic sensing of shape, texture, softness and even object recognition147. Other non-mechanical external stimuli can be sensed using appropriate soft sensing methodologies; for example, a hybrid bio-LED–gripper composed of engineered bacteria can sense chemicals and convert the chemical signals into optical feedback to make autonomous decisions during a pick-and-place operation148.
User-friendly interactions can improve patient compliance towards wearable assisting and rehabilitation devices. Soft actuators are particularly suitable for wearable devices, allowing close and safe contact with the human body149. Incorporating sensing capabilities and closed feedback loops is crucial for the design of next-generation smart wearable assisting and rehabilitation devices. For example, Exo-Glove Poly, which is a tendon-driven robot made entirely of polymers, helps people with spinal cord injuries with daily grasping tasks31. Through a machine learning model and a first-person-view camera, the robot can detect the user’s intention of object grasping and release and actuate tendons to perform grasping150.
Most soft sensors with stretchable conductive materials provide some form of electronic output, such as change of resistance and capacitance. Most polymers used to fabricate soft robots can stretch up to ten times their original length, which cannot be matched by stretchable conductive wires and electrodes151. Moreover, the use of wires inherently excludes untethered applications. Although wireless communication can be applied for untethered sensors, it requires rigid electronics, decreasing the overall softness of the robot. Modelling the response of such soft sensors to external forces, motions and stimuli is also challenging, because mechanical coupling between the soft robot and the sensor, and, consequently, the sensor response, changes with the actuation state, such as inflation or stretching of the actuator152.
Haptic displays
The field of haptics aims to deliver an artificial sense of touch for humans, mainly through tactile (sense of touch) and kinesthetic (sense of motion and force) feedback. A variety of materials and technologies have been explored for haptic displays153, including soft actuators for tactile displays and remote surgeries. Soft materials offer mechanical compliance, which can be tuned to match human skin, and are easy to produce in different forms153,154. The bulk mechanical properties of soft materials can be manipulated by different transduction mechanisms, including pneumatic155, hydraulic156, electrostatic157,158, electromagnetic159, thermal160 and phase transition161 effects, to enable soft tactile displays. However, to capture a full spectrum of object feeling, the surface properties, including texture, friction, softness and viscoelasticity, must be considered, which can be achieved by controlling the molecular structure of materials162. For example, by independently controlling indentation depth and contact area, the softness of an object can be perceived by finger touch163 (FIG. 3d). Moreover, multi-material 3D printing allows high-resolution voxel-by-voxel control of softness properties at soft actuator interfaces164,165. In addition to mechanical stimulation, electrical stimulation can be used to stimulate mechanosensory neurons by integrating conductive soft materials166,167. Future soft tactile devices should integrate the desired bulk and surface properties of real objects and aim to achieve haptic feedback comparable to what RGB displays and speakers have achieved for vision and hearing.
Biomedical robots and devices
Biomedical tools and drug delivery devices must operate safely in vivo. Thus, soft surgical robots should be flexible enough to navigate through tortuous in vivo environments, yet stiff enough to counter fluidic flow and overcome obstacles; they should also possess efficient force transmission and high dexterity to enable them to perform surgical tasks after arriving at the targeted locations4. For example, soft actuated catheters can be guided and navigated through complex structures to reach target locations that are difficult to access with traditional endoscopes or minimally invasive surgical tools. Various functions and designs have been explored for soft catheters to make them easier to deploy and navigate, and to solve specific physiological problems.
Magnetic navigation is contactless with a high number of degrees of freedom, allowing magnetic catheters to be steered in small and confined 3D spaces168,169. Flow-driven navigation enables a micro-catheter (with the smallest part having a diameter of 25 μm) equipped with a magnetic head to automatically advance in blood vessels and to be magnetically steered at the bifurcation position170 (FIG. 4a). This method is particularly promising for navigating in branching and tortuous small vessels, reducing the risk of iatrogenic damage and the need for human intervention. A flexible sensor array can be integrated with a balloon actuator, which can then be deployed using a catheter; after actuation, the stretchable sensors conform to the cardiac surface, which may allow the design of an entirely soft and conformal electrophysiological device for sensing and therapeutic purposes171,172 (FIG. 4b). Capsule endoscopes, which can be magnetically oriented and anchored to a tumour, can even perform under-tissue biopsy using a fine-needle biopsy tool173 (FIG. 4c).
Fig. 4. Soft actuators in biomedical applications.
a | A functionalized catheter can be wirelessly navigated in blood vessels. b | Balloon catheters integrated with conformal electronics can provide real-time sensing during surgeries. c | Small biopsy devices can locomote in hard-to-access body sites and take biopsy samples. The biopsy device is capsulated in ice for smoother oral ingestion. d | Assisting soft actuators can have safe contact with soft tissues and provide therapeutic functions. e | Targeted drug delivery can be realized using biohybrid soft actuators. f | Artificial soft actuators can avoid the detection by macrophages. anti-TER-119, Ter-119 antibody; DOX, doxorubicin; E. coli MG1655, Escherichia coli strain MG1655; PEG, polyethylene glycol; RBC, red blood cell; SPIONs, superparamagnetic iron oxide nanoparticles; ZW, zwitterionic polymer. Panel a adapted from REF.170, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Panel b adapted from REF.219, Springer Nature Limited. Panel c adapted with permission from REF.173, copyright 2020, Mary Ann Liebert, Inc. Panel d adapted with permission from REF.175, AAAS. Panel e adapted with permission from REF.114, AAAS. Panel f reprinted from REF.176, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Biomedical assistive devices can greatly benefit from the soft nature of soft actuators, which allow user-friendly and safe interactions with the human body ex vivo and in vivo by providing robotic conformability and adaptivity. For example, a ventricular device can assist the recovery of heart function after heart failure. The bracing soft pneumatic actuators apply a rhythmic compressive force to the ventricle-free wall to promote blood ejection volume from the target ventricle (FIG. 4d). This device was tested in vivo in a porcine model, showing potential for future clinical studies174.
Soft untethered milli- and microrobots can also serve as drug carriers. These robots can be navigated to targeted locations and release the loaded drug locally on demand, which is especially desired for the treatment of tumours to increase local drug concentration and avoid side effects in healthy cells and tissues. A biohybrid soft microswimmer based on red blood cells and Escherichia coli can swim with bacterial flexible flagella and can be loaded with magnetic nanoparticles and drugs; it can be steered under magnetic fields and used as a biocompatible drug carrier114 (FIG. 4e). On-demand drug release by milli- and microrobots is commonly triggered by non-invasive stimuli, such as magnetic fields175, light176, enzymes177, ultrasound178 and pH89. Soft microrobots can also be 3D-printed with a zwitterionic photoresist, so that they can be propelled magnetically in a physiological environment without being detected by macrophages as a foreign threat (FIG. 4f). A drug loaded into these microrobots can then be released on demand under light radiation176. Magnetically inert objects can further be made magnetic by applying a magnetic spray, for example, for the design of a drug-loaded capsule that can locomote in a stomach-mimicking environment; application of an oscillating magnetic field then causes disintegration of the capsule shell and release of the drugs179.
A key challenge for surgical and assistive devices is to enable soft contact with human tissue, while maintaining their shape adaptability and conformability in complex environments. They should also be compatible with imaging tools to allow in vivo visualization. In addition, precise control of 3D locomotion, efficient movement in a complex biofluidic environment (fluidic flows and non-Newtonian, viscous biofluids), biocompatibility, on-demand drug release and biodegradability are being intensively investigated for micro- and millirobots (TABLE 1).
Outlook
Although a variety of promising soft actuator technologies have been developed, large-scale deployment and wide acceptance of soft actuators and robots in commercial settings remain limited. To address this inadequacy, the soft robotics community has tapped into the fields of materials science, computer science, biology and mechanical engineering to seek solutions for real-world problems and challenges.
Encoding physical intelligence in the material design
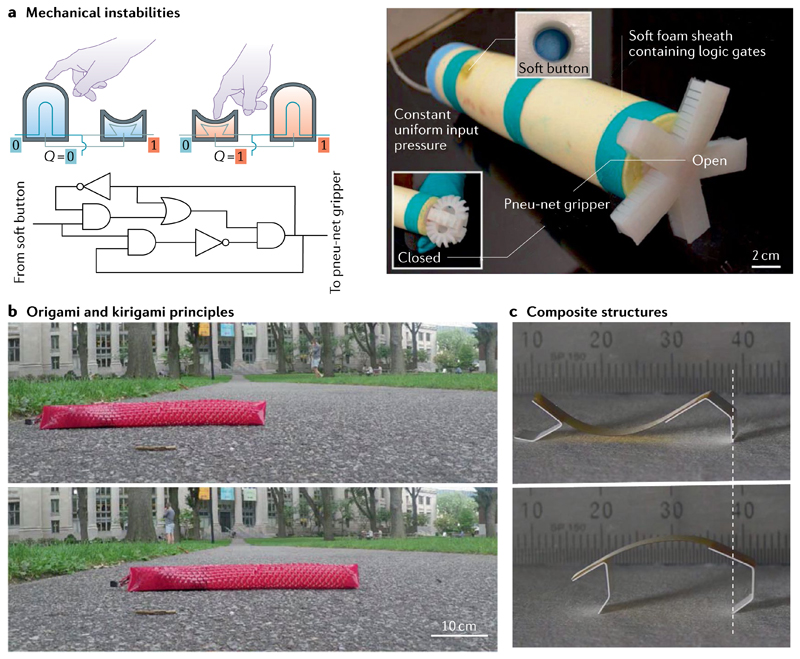
As common mechanical components — such as rigid gears, pin joints and silicon-based electronics — cannot be used in soft actuators, intelligent material designs are being explored to encode physical intelligence10 in soft robots and to implement features that cannot be achieved by bulk constituent materials180. For example, the bistability of spherical membranes can be exploited to create entirely soft valves, which can be used as switches181 to enable binary logic computations182, and to create a soft oscillator183. Similarly, bifurcation-based buckling allows the design of soft actuators that can respond to environmental stimuli in a programmed way based on their encoded logic91. Snap-through instabilities (FIG. 5a) can impart fast motion to soft robots for gripping184 and locomotion185. The distribution of body stiffness can further be programmed in soft swimmers to enable undulatory swimming in the intermediate-flow regime186.
Fig. 5. Encoding physical intelligence in soft robot bodies.
a | Mechanical instabilities can be used to create digital logic gates for binary mechanical computation and human interaction182. b | Introducing cuts (kirigami) allows the buckling-induced transformation of flat sheets into 3D textured surfaces, the directional frictional properties of which can enable efficient crawling gaits187. c | Strategically developing unequal strains, through a difference of materials86 in adjacent layers of a composite structure, can achieve programmable deformations and shape morphing. Panel a adapted with permission from REF.182, National Academy of Sciences. Panel b adapted with permission from REF.187, AAAS. Panel c adapted with permission from REF.86, AAAS.
In addition to mechanical instabilities, kirigami and origami principles can be applied to encode intelligence in soft robot bodies (FIG. 5b). Rationally designed kirigami cuts can introduce frictional asymmetry to cylindrical sheets, allowing a soft robot to crawl187. Kirigami-cut sheets can also be used to develop shape-morphing inflatable structures capable of mimicking a desired target shape188. Moreover, vacuum-based actuators with large linear contractions of origami skeletons can operate as artificial muscles14 and grippers189, with high power densities.
Bilayer structures with different composites can further be used to encode motion and shape in soft structures (FIG. 5c). For example, a bilayer structure made of hygroscopic polyethylene oxide film experiences directional expansion in humid environments and can be used as a low-weight, crawling soft robot86. Pre-stretching one layer in a bilayer system creates actuators with spontaneous curvatures that are capable of zero-power holding (exerting forces in non-actuated states) with programmable shapes and actuation sequences184,190. The property of liquid crystal elastomers to show large deformations under thermal stimulation could be explored in the design of bilayers with different liquid crystal elastomer director alignments to enable untethered shape morphing and propulsion84.
Even without specialized design tools, such as mechanical instability or origami, soft robots demonstrate physical intelligence simply by virtue of their soft bodies. Soft robots have a high number of degrees of freedom, and, although not all of them may be addressed, they can still be deterministically actuated with fairly simple control algorithms. For example, a soft gripper can conformally grip a rigid object owing to the compliance of its body without the requirement to precisely control each contact point. This capability stems from the physical intelligence of soft robots, which greatly simplifies their external control, because the desired functions are encoded in their body materials, structures and mechanisms191–193.
Multimodality, multifunctionality and reconfigurability
Biological actuators show great adaptivity; hands can grab objects of different sizes and shapes; octopi can squeeze and pass through tight tunnels; plants can react to environmental stimuli to protect themselves and promote proliferation. It is desirable for robots to be able to adapt to different tasks and environments either passively or actively with human intervention and feedback control. Soft actuators resemble their biological counterparts in many mechanical aspects, but deformation of their continuum body cannot be as easily controlled as in rigid robots, whose movements are highly discretized with motors and gears.
Moreover, adaptivity is crucial for the safe operation of soft actuators, in particular, for human–machine interfaces, haptics and biomedical devices. In complex and hard-to-predict physiological environments, soft actuators should perform their designated tasks without posing any danger. Thus, mobile soft robots should be able to locomote with multimodalities (such as walking, rolling, jumping and swimming) to reach their target locations in an unfamiliar, changing and multi-terrain environment and to overcome obstacles. Switching modalities usually involves a change of external controls or reconfiguration of shapes; for example, a legged robot performs a walking motion, whereas a helical shape is better for rolling and swimming. Reconfiguration can be triggered by intentionally changing the external stimuli (such as magnetic fields42, light61, electricity194 and air pressure195) and/or passively through environmental changes (such as humidity gradient86, temperature196 and the presence of certain chemicals197). Soft robots that can reconfigure their shapes and respond to stimuli can perform different tasks and become multifunctional. Multifunctionality without sacrificing the performance of individual functions allows the same soft robot to be reapplied in a different task without having to be replaced.
Adaptability can be achieved by designing the physical or chemical properties of materials so that they can change in response to external stimuli. Alternatively, materials can be assembled into different structures, which, in turn, react differently to different stimuli. In addition, origami and kirigami, deployable geometries, heterogeneous stiffness and anisotropic material alignment can be applied as structural strategies. Modular designs can further be employed by assembling individual building blocks into groups with changing geometries or functions based on different needs, such as magnetic swarm robots. Moreover, tethered or untethered feedback loops can be implemented so that a robot can adapt and change its controlling signals; for example, integrated sensors can provide electrical or optical signals, which can change the control signals148, or light-activated robots can have self-shading effects and oscillate74. To coordinate these design parameters, simulations can be performed; in particular, machine-learning and neural networks can help to address the inverse design problem for application-driven problems.
Reproducibility and manufacturing scalability
Repeatability, reproducibility and scalability of manufacturing and operation processes are key for the industrial commercialization of soft actuators. Repeatability measures the consistency of fabrication and operation conducted by the same operator under the same conditions, whereas reproducibility describes how precisely others can replicate the same manufacturing and operation process. Manufacturing repeatability and reproducibility involve material and structural consistency. The properties of many soft materials are affected by even slight environmental changes; for example, the volume and stiffness of hydrogels may be sensitive to temperature and humidity; the mechanical properties of elastomers can vary owing to variations in the temperature under which they are crosslinked; and the magnetization profiles in magnetic actuators can differ depending on particle alignment and doping fraction. However, using the same materials and fabrication protocols, the manufacture of actuators should be consistent and reproducible, resulting in consistent performance. Usually, automated processes with well controlled conditions and repeatable workflows, such as 3D printing, laser cutting, lithography and roll-to-roll processing, provide better material and structural consistency than do manual assembly.
Manufacturing scalability is important for industrial commercialization. Most soft actuators have been demonstrated in the laboratory with only limited large-scale production thus far. Scalability is challenging, because materials processed in large batches can have different properties to materials processed at smaller scales. For example, the fabrication of biohybrid devices, synthetic biomaterials and complex microfabricated devices is highly dependent on human intervention and experience, which limits reproducibility and scalability. Scalability can be achieved by integrating established fabrication techniques for large-scale soft materials with actuating mechanisms using hierarchical designs. For example, soft actuators can be combined with textile technology198 to build wearable devices; here, yarn materials can be varied, the microscale structures of individual yarns can be engineered, various woven or knitting methods can be applied, and functional patches can be patterned. However, a challenge in the fabrication of soft devices with hierarchical designs is the lack of compatibility of fabrication techniques with materials at different length scales; for example, soft micropatterns may be destroyed during roll-to-roll processes, and stimuli-responsive yarns made of soft materials may be too fragile for knitting machines. Although many techniques have been established for soft material fabrication, more efficient and more reliable fabrication tools and methods will be required to improve reproducibility and allow large-scale fabrication for the commercialization of soft actuators and robots.
Lifetime, self-healing and degradation
The generation of electronic waste is rapidly growing and has become a global environmental challenge, with a predicted 52 million tons of electronic waste in the year 2021 (REF.199). Despite current efforts in legislation, collection and recycling, 80% of electronic waste is disposed of by incineration or in landfills, releasing toxic materials (such as volatile organic chemicals and heavy metals) into air, water and soil200. Soft actuation and robotics present an opportunity to mitigate electronic waste generation by avoiding traditional actuator technology. However, widespread application of soft actuators will require consideration of their life cycle as well as sustainable design.
The typical end-of-life of an actuator operating in dynamic environments is caused by injuries that reduce its structural integrity, change its properties or terminate its function. Soft actuators are especially vulnerable to injuries, because soft materials and components are sensitive to mechanical impact and puncture. Self-healing materials can recover their properties after damage, providing a promising way of repairing the function of actuators after catastrophic damage and extending their operational lifetime. Self-healing materials can be divided into extrinsic and intrinsic materials. Extrinsic self-healing materials consist of external healing agents embedded in a material matrix, typically by microencapsulation or vascularization201. Upon damage, the capsules are ruptured, releasing the healing agent to the damaged areas, thereby triggering a healing reaction. For example, soft actuators can be designed with extrinsic self-healing by encapsulating liquid metal droplets in a soft elastomeric matrix202. However, extrinsic healing provides only a limited number of healing cycles, needs access to a reservoir of healing agents, which may be toxic and can leak, and fabrication is challenging. Intrinsic self-healing materials, by contrast, do not require external healing agents, but rely on dynamic covalent bonds and supramolecular chemistry203. For example, self-healing pneumatic soft grippers and muscles use hydrogen-bonded networks204, dissociative Diels-Alder bonds205 or disulfide bonds206, which can recover their function after damage. Similarly, self-healing electronic skins can be designed based on metal-ion coordination207 and ionic interactions208. These intrinsic self-healing properties are inherent to polymer networks, providing an indefinite number of healing cycles (reversibility). However, intrinsic healing relies on polymer chain mobility and reaction rates, and, therefore, healing is limited by kinetics and may require an external activation trigger, such as temperature or light.
Despite progress in the engineering of self-healing materials, their application in soft robotics remains limited, with many challenges ahead209. To design the most suitable self-healing strategy and materials for a soft robot, its end application, performance, function, environment and types of possible damage need to be considered. Healing from mechanical204, electrical7 or thermal damage210, and recovery of mechanical204, conductive211 or thermal properties212 of the robot may require different (and often opposed) strategies. The volume (micro versus bulk) and severity (mild versus catastrophic) of the damage may also determine possible approaches. Furthermore, fast-healing kinetics204 will be required to recover the robot's function quickly and minimize offline repair time. Overcoming these challenges will extend the lifetime of soft actuators and robots and expand their use in real-world applications in demanding environments.
When actuators are damaged beyond repair or at the end of their programmed lifetime, their end-of-life handling needs to be considered to avoid waste generation and environmental impact; in particular, sustainable approaches should be pursued, such as recycling and degradation213. Degradation of transient actuators can bring added value beyond responsible waste management; for example, actuators for medical soft robots can be made to be degradable to avoid the need for recovery and retrieval operations. In addition, programmed and controlled degradation can add functionalities to robots, such as structural changes and/or the release of drugs upon degradation95,214,215. Materials can be degraded by various mechanisms, including hydrolysis216, composting217, enzymatic cleavage218,219 or reversible crosslinking204, enabling the integration of end-of-life management and transient functionalities into soft robotics. However, in addition to degradation mechanisms, the environment surrounding the robots (that is, degradation stimuli or reactive species), degradation kinetics (minutes, weeks), and application and performance in different phases (original, transient and terminal functions) need to be considered.
Despite recent advances, soft actuators cannot yet completely replicate biological behaviours and performances. However, the development of new robotic materials will push the limits of current soft robots and help them to achieve life-like performance. With the objective of bringing robots closer to living things, soft robotics is evolving into a multidisciplinary research field, which is reflected in the very active transdisciplinary soft robotics research community. We hope that the challenges and breakthroughs discussed in this Review will serve as an inspiration for future research and provide a roadmap of materials-based solutions to current and emerging technological roadblocks in the field.
Acknowledgements
This work is funded by the Max Planck Society, the European Research Council (ERC) Advanced Grant SoMMoR project (grant number 834531) and the German Research Foundation (DFG) Soft Material Robotic Systems (SPP 2100) Program (grant number 2197/3-1). M.L., A.P. and A.P.-F. received the Humboldt Postdoctoral Research Fellowship and thank the Alexander von Humboldt Foundation for their financial support.
Footnotes
Author contributions
M.L., A.P., A.A. and A.P.-F. contributed equally to this work. M.S. initiated the Review, and all the authors developed its outline. All authors contributed to the writing and editing of the Review.
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Materials thanks David Gracias, Arianna Menciassi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Madden JDW, et al. Artificial muscle technology: principles and naval prospects. IEEE J Ocean Eng. 2004;29:706–728. [Google Scholar]
- 2.Mirvakili SM, Hunter IW. Artificial muscles: mechanisms, applications, and challenges. Adv Mater. 2018;30:1704407. doi: 10.1002/adma.201704407. [DOI] [PubMed] [Google Scholar]
- 3.Rich SI, Wood RJ, Majidi C. Untethered soft robotics. Nat Electron. 2018;1:102–112. [Google Scholar]
- 4.Cianchetti M, Laschi C, Menciassi A, Dario P. Biomedical applications of soft robotics. Nat Rev Mater. 2018;3:143–153. [Google Scholar]
- 5.Shintake J, Rosset S, Schubert B, Floreano D, Shea H. Versatile soft grippers with intrinsic electroadhesion based on multifunctional polymer actuators. Adv Mater. 2016;28:231–238. doi: 10.1002/adma.201504264. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi A, Mondini A, Mazzolai B. Toward self-growing soft robots inspired by plant roots and based on additive manufacturing technologies. Soft Robot. 2017;4:211–223. doi: 10.1089/soro.2016.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acome E, et al. Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science. 2018;359:61–65. doi: 10.1126/science.aao6139. [DOI] [PubMed] [Google Scholar]
- 8.Ilievski F, Mazzeo AD, Shepherd RF, Chen X, Whitesides GM. Soft robotics for chemists. Angew Chem Int Ed. 2011;50:1890–1895. doi: 10.1002/anie.201006464. [DOI] [PubMed] [Google Scholar]
- 9.Awad LN, et al. A soft robotic exosuit improves walking in patients after stroke. Sci Transl Med. 2017;9:eaai9084. doi: 10.1126/scitranslmed.aai9084. [DOI] [PubMed] [Google Scholar]
- 10.Sitti M. Physical intelligence as a new paradigm. Extrem Mech Lett. 2021;46:101340. [PMC free article] [PubMed] [Google Scholar]
- 11.Gorissen B, et al. Hardware sequencing of inflatable nonlinear actuators for autonomous soft robots. Adv Mater. 2019;31:1804598. doi: 10.1002/adma.201804598. [DOI] [PubMed] [Google Scholar]
- 12.Vasios N, Gross AJ, Soifer S, Overvelde JTB, Bertoldi K. Harnessing viscous flow to simplify the actuation of fluidic soft robots. Soft Robot. 2019;7:1–9. doi: 10.1089/soro.2018.0149. [DOI] [PubMed] [Google Scholar]
- 13.Gorissen B, Melancon D, Vasios N, Torbati M, Bertoldi K. Inflatable soft jumper inspired by shell snapping. Sci Robot. 2020;5:eabb1967. doi: 10.1126/scirobotics.abb1967. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Vogt DM, Rus D, Wood RJ. Fluid-driven origami-inspired artificial muscles. Proc Natl Acad Sci USA. 2017;114:201713450. doi: 10.1073/pnas.1713450114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siefert E, Reyssat E, Bico J, Roman B. Bio-inspired pneumatic shape-morphing elastomers. Nat Mater. 2019;18:24–28. doi: 10.1038/s41563-018-0219-x. [DOI] [PubMed] [Google Scholar]
- 16.Hajiesmaili E, Clarke DR. Reconfigurable shape-morphing dielectric elastomers using spatially varying electric fields. Nat Commun. 2019;10:183. doi: 10.1038/s41467-018-08094-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chortos A, Hajiesmaili E, Morales J, Clarke DR, Lewis JA. 3D printing of interdigitated dielectric elastomer actuators. Adv Funct Mater. 2020;30:1907375 [Google Scholar]
- 18.Pelrine R, Kornbluh R, Pei Q, Joseph J. High-speed electrically actuated elastomers with strain greater than 100% Science. 2000;287:836–839. doi: 10.1126/science.287.5454.836. [DOI] [PubMed] [Google Scholar]
- 19.Duduta M, Hajiesmaili E, Zhao H, Wood RJ, Clarke DR. Realizing the potential of dielectric elastomer artificial muscles. Proc Natl Acad Sci USA. 2019;116:2476–2481. doi: 10.1073/pnas.1815053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson ZS, et al. Monolithic shape-programmable dielectric liquid crystal elastomer actuators. Sci Adv. 2019;5:eaay0855. doi: 10.1126/sciadv.aay0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellaris N, Venkata VG, Smith GM, Mitchell SK, Keplinger C. Peano-HASEL actuators: muscle-mimetic, electrohydraulic transducers that linearly contract on activation. Sci Robot. 2018;3:eaar3276. doi: 10.1126/scirobotics.aar3276. [DOI] [PubMed] [Google Scholar]
- 22.Rothemund P, Kellaris N, Mitchell SK, Acome E, Keplinger C. HASEL artificial muscles for a new generation of lifelike robots — recent progress and future opportunities. Adv Mater. 2020;33:2003375. doi: 10.1002/adma.202003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacucciolo V, et al. Stretchable pumps for soft machines. Nature. 2019;572:516–519. doi: 10.1038/s41586-019-1479-6. [DOI] [PubMed] [Google Scholar]
- 24.Seok S, et al. Meshworm: a peristaltic soft robot with antagonistic nickel titanium coil actuators. IEEE/ASME Trans Mechatron. 2013;18:1485–1497. [Google Scholar]
- 25.Aksoy B, Shea H. Reconfigurable and latchable shape-morphing dielectric elastomers based on local stiffness modulation. Adv Funct Mater. 2020;30:2001597 [Google Scholar]
- 26.Lima MD, et al. Electrically, chemically, and photonically powered torsional and tensile actuation of hybrid carbon nanotube yarn muscles. Science. 2012;338:928–932. doi: 10.1126/science.1226762. [DOI] [PubMed] [Google Scholar]
- 27.Mu J, et al. Sheath-run artificial muscles. Science. 2019;365:150–155. doi: 10.1126/science.aaw2403. [DOI] [PubMed] [Google Scholar]
- 28.Kanik M, et al. Strain-programmable fiber-based artificial muscle. Science. 2019;365:145–150. doi: 10.1126/science.aaw2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, et al. Shape memory nanocomposite fibers for untethered high-energy microengines. Science. 2019;365:155–158. doi: 10.1126/science.aaw3722. [DOI] [PubMed] [Google Scholar]
- 30.Haines CS, et al. Artificial muscles from fishing line and sewing thread. Science. 2014;343:868–872. doi: 10.1126/science.1246906. [DOI] [PubMed] [Google Scholar]
- 31.Kang BB, Choi H, Lee H, Cho K-J. Exo-Glove Poly II: a polymer-based soft wearable robot for the hand with a tendon-driven actuation system. Soft Robot. 2018;6:214–227. doi: 10.1089/soro.2018.0006. [DOI] [PubMed] [Google Scholar]
- 32.Goswami D, Liu S, Pal A, Silva LG, Martinez RV. 3D-architected soft machines withtopologically encoded motion. Adv Funct Mater. 2019;29:1808713 [Google Scholar]
- 33.Schlagenhauf C, et al. Control of tendon-driven soft foam robot hands; 2018 IEEE-RAS 18th Intl Conf on Humanoid Robots (Humanoids); 2018. pp. 1–7. [Google Scholar]
- 34.Mishra AK, Del Dottore E, Sadeghi A, Mondini A, Mazzolai B. SIMBA: tendon-driven modular continuum arm with soft reconfigurable gripper. Front Robot AI. 2017;4:4. [Google Scholar]
- 35.Kim Y, Cha Y. Soft pneumatic gripper with tendon-driven soft origami pump. Front Bioeng Biotechnol. 2020;8:461. doi: 10.3389/fbioe.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren T, et al. A novel tendon-driven soft actuator with self-pumping property. Soft Robot. 2020;7:130–139. doi: 10.1089/soro.2019.0008. [DOI] [PubMed] [Google Scholar]
- 37.Wehner M, et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature. 2016;536:451–455. doi: 10.1038/nature19100. [DOI] [PubMed] [Google Scholar]
- 38.Aubin CA, et al. Electrolytic vascular systems for energy-dense robots. Nature. 2019;571:51–57. doi: 10.1038/s41586-019-1313-1. [DOI] [PubMed] [Google Scholar]
- 39.Li G, et al. Self-powered soft robot in the Mariana Trench. Nature. 2021;591:66–71. doi: 10.1038/s41586-020-03153-z. [DOI] [PubMed] [Google Scholar]
- 40.Ji X, et al. An autonomous untethered fast soft robotic insect driven by low-voltage dielectric elastomer actuators. Sci Robot. 2019;4:eaaz6451. doi: 10.1126/scirobotics.aaz6451. [DOI] [PubMed] [Google Scholar]
- 41.He Q, Cai S. Soft pumps for soft robots. Sci Robot. 2021;6:eabg6640. doi: 10.1126/scirobotics.abg6640. [DOI] [PubMed] [Google Scholar]
- 42.Hu W, Lum GZ, Mastrangeli M, Sitti M. Small-scale soft-bodied robot with multimodal locomotion. Nature. 2018;554:81–85. doi: 10.1038/nature25443. [DOI] [PubMed] [Google Scholar]
- 43.Lu H, et al. A bioinspired multilegged soft millirobot that functions in both dry and wet conditions. Nat Commun. 2018;9:3944. doi: 10.1038/s41467-018-06491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Z, Hu W, Dong X, Sitti M. Multi-functional soft-bodied jellyfish-like swimming. Nat Commun. 2019;10:2703. doi: 10.1038/s41467-019-10549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong X, et al. Bioinspired cilia arrays with programmable nonreciprocal motion and metachronal coordination. Sci Adv. 2020;6:eabc9323. doi: 10.1126/sciadv.abc9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lum GZ, et al. Shape-programmable magnetic soft matter. Proc Natl Acad Sci USA. 2016;113:E6007–E6015. doi: 10.1073/pnas.1608193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu H, et al. Magnetic cilia carpets with programmable metachronal waves. Nat Commun. 2020;11:2637. doi: 10.1038/s41467-020-16458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H-W, et al. Adaptive locomotion of artificial microswimmers. Sci Adv. 2019;5:eaau1532. doi: 10.1126/sciadv.aau1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H, et al. 3D-printed programmable tensegrity for soft robotics. Sci Robot. 2020;5:eaay9024. doi: 10.1126/scirobotics.aay9024. [DOI] [PubMed] [Google Scholar]
- 50.Cao L, et al. Ferromagnetic liquid metal putty-like material with transformed shape and reconfigurable polarity. Adv Mater. 2020;32:2000827. doi: 10.1002/adma.202000827. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, Yuk H, Zhao R, Chester SA, Zhao X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature. 2018;558:274. doi: 10.1038/s41586-018-0185-0. [DOI] [PubMed] [Google Scholar]
- 52.Cui J, et al. Nanomagnetic encoding of shapemorphing micromachines. Nature. 2019;575:164–168. doi: 10.1038/s41586-019-1713-2. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, et al. Voxelated three- dimensional miniature magnetic soft machines via multimaterial heterogeneous assembly. Sci Robot. 2021;6:eabf0112. doi: 10.1126/scirobotics.abf0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alapan Y, Karacakol AC, Guzelhan SN, Isik I, Sitti M. Reprogrammable shape morphing of magnetic soft machines. Sci Adv. 2020;6:eabc6414. doi: 10.1126/sciadv.abc6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng H, et al. Laser reprogramming magnetic anisotropy in soft composites for reconfigurable 3D shaping. Nat Commun. 2020;11:6325. doi: 10.1038/s41467-020-20229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirvakili SM, Sim D, Hunter IW, Langer R. Actuation of untethered pneumatic artificial muscles and soft robots using magnetically induced liquidto-gas phase transitions. Sci Robot. 2020;5:eaaz4239. doi: 10.1126/scirobotics.aaz4239. [DOI] [PubMed] [Google Scholar]
- 57.Sitti M, Wiersma DS. Pros and cons: magnetic versus optical microrobots. Adv Mater. 2020;32:1906766. doi: 10.1002/adma.201906766. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, et al. Humidity- and photo- induced mechanical actuation of cross- linked liquid crystal polymers. Adv Mater. 2017;29:1604792. doi: 10.1002/adma.201604792. [DOI] [PubMed] [Google Scholar]
- 59.Lu X, et al. Liquid-crystalline dynamic networks doped with gold nanorods showing enhanced photocontrol of actuation. Adv Mater. 2018;30:1706597. doi: 10.1002/adma.201706597. [DOI] [PubMed] [Google Scholar]
- 60.Lancia F, Ryabchun A, Nguindjel A-D, Kwangmettatam S, Katsonis N. Mechanical adaptability of artificial muscles from nanoscale molecular action. Nat Commun. 2019;10:4819. doi: 10.1038/s41467-019-12786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahsavan H, et al. Bioinspired underwater locomotion of light-driven liquid crystal gels. Proc Natl Acad Sci USA. 2020;117:5125–5133. doi: 10.1073/pnas.1917952117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuenstler AS, Kim H, Hayward RC. Liquid crystal elastomer waveguide actuators. Adv Mater. 2019;31:e1901216. doi: 10.1002/adma.201901216. [DOI] [PubMed] [Google Scholar]
- 63.Yang H, et al. 3D printed photoresponsive devices based on shape memory composites. Adv Mater. 2017;29:1701627. doi: 10.1002/adma.201701627. [DOI] [PubMed] [Google Scholar]
- 64.Liu JA-C, Gillen JH, Mishra SR, Evans BA, Tracy JB. Photothermally and magnetically controlled reconfiguration of polymer composites for soft robotics. Sci Adv. 2019;5:eaaw2897. doi: 10.1126/sciadv.aaw2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S, et al. Asymmetric elastoplasticity of stacked graphene assembly actualizes programmable untethered soft robotics. Nat Commun. 2020;11:4359. doi: 10.1038/s41467-020-18214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, et al. Light-activated shape morphing and light-tracking materials using biopolymer-based programmable photonic nanostructures. Nat Commun. 2021;12:1651. doi: 10.1038/s41467-021-21764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai G, Ciou J-H, Liu Y, Jiang Y, Lee PS. Leaf-inspired multiresponsive MXene-based actuator for programmable smart devices. Sci Adv. 2019;5:eaaw7956. doi: 10.1126/sciadv.aaw7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, et al. Photothermal bimorph actuators with in-built cooler for light mills, frequency switches, and soft robots. Adv Funct Mater. 2019;340:1808995 [Google Scholar]
- 69.Li C, et al. Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Sci Robot. 2020;5:eabb9822. doi: 10.1126/scirobotics.abb9822. [DOI] [PubMed] [Google Scholar]
- 70.Li C, et al. Supramolecular-covalent hybrid polymers for light-activated mechanical actuation. Nat Mater. 2020;19:900–909. doi: 10.1038/s41563-020-0707-7. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, et al. Direct laser writing of superhydrophobic PDMS elastomers for controllable manipulation via Marangoni effect. Adv Funct Mater. 2017;27:1702946 [Google Scholar]
- 72.Li M, Wang X, Dong B, Sitti M. In-air fast response and high speed jumping and rolling of a light-driven hydrogel actuator. Nat Commun. 2020;11:3988. doi: 10.1038/s41467-020-17775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu QL, et al. Light-steered locomotion of muscle-like hydrogel by self-coordinated shape change and friction modulation. Nat Commun. 2020;11:5166. doi: 10.1038/s41467-020-18801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, et al. Soft phototactic swimmer based on self-sustained hydrogel oscillator. Sci Robot. 2019;4:eaax7112. doi: 10.1126/scirobotics.aax7112. [DOI] [PubMed] [Google Scholar]
- 75.Li M, et al. Flexible magnetic composites for light-controlled actuation and interfaces. Proc Natl Acad Sci USA. 2018;115:8119–8124. doi: 10.1073/pnas.1805832115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M, Kim T, Guidetti G, Wang Y, Omenetto FG. Optomechanically actuated microcilia for locally reconfigurable surfaces. Adv Mater. 2020;32:2004147. doi: 10.1002/adma.202004147. [DOI] [PubMed] [Google Scholar]
- 77.Aghakhani A, Yasa O, Wrede P, Sitti M. Acoustically powered surface-slipping mobile microrobots. Proc Natl Acad Sci USA. 2020;117:3469–3477. doi: 10.1073/pnas.1920099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren L, et al. 3D steerable, acoustically powered microswimmers for single-particle manipulation. Sci Adv. 2019;5:eaax3084. doi: 10.1126/sciadv.aax3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmed D, et al. Artificial swimmers propelled by acoustically activated flagella. Nano Lett. 2016;16:4968–4974. doi: 10.1021/acs.nanolett.6b01601. [DOI] [PubMed] [Google Scholar]
- 80.Kaynak M, Dirix P, Sakar MS. Addressable acoustic actuation of 3D printed soft robotic microsystems. Adv Sci. 2020;7:2001120. doi: 10.1002/advs.202001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T, Bilal OR, Shea K, Daraio C. Harnessing bistability for directional propulsion of soft, untethered robots. Proc Natl Acad Sci USA. 2018;115:5698–5702. doi: 10.1073/pnas.1800386115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotikian A, Truby RL, Boley JW, White TJ, Lewis JA. 3D Printing of liquid crystal elastomeric actuators with spatially programed nematic order. Adv Mater. 2018;30:1706164. doi: 10.1002/adma.201706164. [DOI] [PubMed] [Google Scholar]
- 83.Jin B, et al. Programming a crystalline shape memory polymer network with thermo-and photo-reversible bonds toward a single-component soft robot. Sci Adv. 2018;4:eaao3865. doi: 10.1126/sciadv.aao3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotikian A, et al. Untethered soft robotic matter with passive control of shape morphing and propulsion. Sci Robot. 2019;4:eaax7044. doi: 10.1126/scirobotics.aax7044. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, et al. Stimuli-responsive composite biopolymer actuators with selective spatial deformation behavior. Proc Natl Acad Sci USA. 2020;117:14602–14608. doi: 10.1073/pnas.2002996117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin B, et al. Hygrobot: a self-locomotive ratcheted actuator powered by environmental humidity. Sci Robot. 2018;3:eaar2629. doi: 10.1126/scirobotics.aar2629. [DOI] [PubMed] [Google Scholar]
- 87.Sydney Gladman A, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mater. 2016;15:413–418. doi: 10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- 88.Cao J, et al. Arbitrarily 3D configurable hygroscopic robots with a covalent-noncovalent interpenetrating network and self-healing ability. Adv Mater. 2019;31:e1900042. doi: 10.1002/adma.201900042. [DOI] [PubMed] [Google Scholar]
- 89.Li H, Go G, Ko SY, Park J-O, Park S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater Struct. 2016;25:027001 [Google Scholar]
- 90.Qin H, Zhang T, Li N, Cong H-P, Yu S-H. Anisotropic and self-healing hydrogels with multi-responsive actuating capability. Nat Commun. 2019;10:2202. doi: 10.1038/s41467-019-10243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang Y, Korpas LM, Raney JR. Bifurcation-based embodied logic and autonomous actuation. Nat Commun. 2019;10:1–10. doi: 10.1038/s41467-018-08055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mu J, et al. Molecular-channel driven actuator with considerations for multiple configurations and color switching. Nat Commun. 2018;9:590. doi: 10.1038/s41467-018-03032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartlett NW, et al. A 3D-printed, functionally graded soft robot powered by combustion. Science. 2015;349:161–165. doi: 10.1126/science.aab0129. [DOI] [PubMed] [Google Scholar]
- 94.Yang X, Chang L, Perez-Arancibia NO. An 88-milligram insect-scale autonomous crawling robot driven by a catalytic artificial muscle. Sci Robot. 2020;5:eaba0015. doi: 10.1126/scirobotics.aba0015. [DOI] [PubMed] [Google Scholar]
- 95.Pena-Francesch A, Giltinan J, Sitti M. Multifunctional and biodegradable self-propelled protein motors. Nat Commun. 2019;10:3188. doi: 10.1038/s41467-019-11141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong L, Ambrosi A, Nasir MZM, Guan J, Pumera M. Self-propelled 3D-printed "aircraft carrier" of light-powered smart micromachines for large-volume nitroaromatic explosives removal. Adv Funct Mater. 2019;29:1903872 [Google Scholar]
- 97.Cangialosi A, et al. DNA sequence-directed shape change of photopatterned hydrogels via high-degree swelling. Science. 2017;357:1126–1130. doi: 10.1126/science.aan3925. [DOI] [PubMed] [Google Scholar]
- 98.Ricotti L, et al. Biohybrid actuators for robotics: a review of devices actuated by living cells. Sci Robot. 2017;2:eaaq0495. doi: 10.1126/scirobotics.aaq0495. [DOI] [PubMed] [Google Scholar]
- 99.Alapan Y, et al. Microrobotics and microorganisms. Annu Rev Control Robot Auton Syst. 2019;2:205–230. [Google Scholar]
- 100.Park S-J, et al. Phototactic guidance of a tissue- engineered soft-robotic ray. Science. 2016;353:158–162. doi: 10.1126/science.aaf4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raman R, et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc Natl Acad Sci USA. 2016;113:3497–3502. doi: 10.1073/pnas.1516139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aydin O, et al. Neuromuscular actuation of biohybrid motile bots. Proc Natl Acad Sci USA. 2019;116:19841–19847. doi: 10.1073/pnas.1907051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raman R, Cvetkovic C, Bashir R. A modular approach to the design, fabrication, and characterization of muscle-powered biological machines. Nat Protoc. 2017;12:519–533. doi: 10.1038/nprot.2016.185. [DOI] [PubMed] [Google Scholar]
- 104.Morimoto Y, Onoe H, Takeuchi S. Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci Robot. 2018;3:eaat4440. doi: 10.1126/scirobotics.aat4440. [DOI] [PubMed] [Google Scholar]
- 105.Li Z, et al. Biohybrid valveless pump-bot powered by engineered skeletal muscle. Proc Natl Acad Sci USA. 2019;116:1543–1548. doi: 10.1073/pnas.1817682116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu F, Shang L, Chen Z, Yu Y, Zhao Y. Bioinspired living structural color hydrogels. Sci Robot. 2018;3:eaar8580. doi: 10.1126/scirobotics.aar8580. [DOI] [PubMed] [Google Scholar]
- 107.Xu B, et al. A remotely controlled transformable soft robot based on engineered cardiac tissue construct. Small. 2019;15:e1900006. doi: 10.1002/smll.201900006. [DOI] [PubMed] [Google Scholar]
- 108.Guix M, et al. Biohybrid soft robots with self-stimulating skeletons. Sci Robot. 2021;6:eabe7577. doi: 10.1126/scirobotics.abe7577. [DOI] [PubMed] [Google Scholar]
- 109.Park B-W, Zhuang J, Yasa O, Sitti M. Multifunctional bacteria-driven microswimmers for targeted active drug delivery. ACS Nano. 2017;11:8910–8923. doi: 10.1021/acsnano.7b03207. [DOI] [PubMed] [Google Scholar]
- 110.Singh AV, Hosseinidoust Z, Park B-W, Yasa O, Sitti M. Microemulsion-based soft bacteria-driven microswimmers for active cargo delivery. ACS Nano. 2017;11:9759–9769. doi: 10.1021/acsnano.7b02082. [DOI] [PubMed] [Google Scholar]
- 111.Cao F, Zhang C, Choo HY, Sato H. Insect–computer hybrid legged robot with user-adjustable speed, step length and walking gait. J R Soc Interface. 2016;13:20160060. doi: 10.1098/rsif.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magdanz V, et al. IRONSperm: sperm-templated soft magnetic microrobots. Sci Adv. 2020;6:eaba5855. doi: 10.1126/sciadv.aba5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu NW, Dabiri JO. Low-power microelectronics embedded in live jellyfish enhance propulsion. Sci Adv. 2020;6:eaaz3194. doi: 10.1126/sciadv.aaz3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alapan Y, et al. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Robot. 2018;3:eaar4423. doi: 10.1126/scirobotics.aar4423. [DOI] [PubMed] [Google Scholar]
- 115.Wu M, et al. Photosensitizer-bacteria biohybrids promote photodynamic cancer cell ablation and intracellular protein delivery. Chem Mater. 2019;31:7212–7220. [Google Scholar]
- 116.Shintake J, Cacucciolo V, Floreano D, Shea H. Soft robotic grippers. Adv Mater. 2018;30:1707035. doi: 10.1002/adma.201707035. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y-F, et al. Miniature pneumatic actuators for soft robots by high-resolution multimaterial 3D printing. Adv Mater Techno. 2019;4:1900427 [Google Scholar]
- 118.Paek J, Cho I, Kim J. Microrobotic tentacles with spiral bending capability based on shape-engineered elastomeric microtubes. Sci Rep. 2015;5:10768. doi: 10.1038/srep10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yap HK, Ng HY, Yeow C-H. High-force soft printable pneumatics for soft robotic applications. Soft Robot. 2016;3:144–158. [Google Scholar]
- 120.Li X, Cai X, Gao Y, Serpe MJ. Reversible bidirectional bending of hydrogel-based bilayer actuators. J Mater Chem B. 2017;5:2804–2812. doi: 10.1039/c7tb00426e. [DOI] [PubMed] [Google Scholar]
- 121.Taccola S, et al. Toward a new generation of electrically controllable hygromorphic soft actuators. Adv Mater. 2015;27:1668–1675. doi: 10.1002/adma.201404772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leeladhar, Singh JP. Photomechanical and chemomechanical actuation behavior of graphene–poly(dimethylsiloxane)/gold bilayer tube for multimode soft grippers and volatile organic compounds detection applications. ACS Appl Mater Interfaces. 2018;10:33956–33965. doi: 10.1021/acsami.8b11440. [DOI] [PubMed] [Google Scholar]
- 123.Hubbard AM, Mailen RW, Zikry MA, Dickey MD Genzer J. Controllable curvature from planar polymer sheets in response to light. Soft Matter. 2017;13:2299–2308. doi: 10.1039/c7sm00088j. [DOI] [PubMed] [Google Scholar]
- 124.Diller E, Sitti M. Three-dimensional programmable assembly by untethered magnetic robotic micro-grippers. Adv Funct Mater. 2014;24:4397–4404. [Google Scholar]
- 125.Abbott JJ, Diller E, Petruska AJ. Magnetic methods in robotics. Annu Rev Control. 2020;3:57–90. [Google Scholar]
- 126.Fusco S, et al. An integrated microrobotic platform for on-demand, targeted therapeutic interventions. Adv Mater. 2014;26:952–957. doi: 10.1002/adma.201304098. [DOI] [PubMed] [Google Scholar]
- 127.Jin Q, Yang Y, Jackson JA, Yoon C Gracias DH. Untethered single cell grippers for active biopsy. Nano Lett. 2020;20:5383–5390. doi: 10.1021/acs.nanolett.0c01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malachowski K, et al. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew Chem Int Ed. 2014;53:8045–8049. doi: 10.1002/anie.201311047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Breger JC, et al. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl Mater.Interfaces. 2015;7:3398–3405. doi: 10.1021/am508621s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shintake J, Schubert B, Rosset S, Shea H, Floreano D. Variable stiffness actuator for soft robotics using dielectric elastomer and low-melting-point alloy; 2015IEEE/RSJ Int Conf on Intelligent Robots and Systems (IROS); 2015. pp. 1097–1102. [Google Scholar]
- 131.Amend J, Cheng N, Fakhouri S, Culley B. Soft robotics commercialization: jamming grippers from research to product. Soft Robot. 2016;3:213–222. doi: 10.1089/soro.2016.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hawkes EW, Christensen DL, Kyungwon Han A, Jiang H, Cutkosky MR. Grasping without squeezing: shear adhesion gripper with fibrillar thin film; 2015 IEEE Int Conf on Robotics and Automation (ICRA); 2015. pp. 2305–2312. [Google Scholar]
- 133.Song S, Drotlef D-M, Majidi C, Sitti M. Controllable load sharing for soft adhesive interfaces on three-dimensional surfaces. Proc Natl Acad Sci USA. 2017;114:E4344–E4353. doi: 10.1073/pnas.1620344114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang L, Ha K-H, Rodin GJ, Liechti KM, Lu N. Mechanics of crater-enabled soft dry adhesives: a review. Front Mech Eng. 2020;6:98. [Google Scholar]
- 135.Josephson RK. Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol. 1993;55:527–546. doi: 10.1146/annurev.ph.55.030193.002523. [DOI] [PubMed] [Google Scholar]
- 136.Mirfakhrai T, Madden JDW, Baughman RH. Polymer artificial muscles. Mater Today. 2007;10:30–38. [Google Scholar]
- 137.Christianson C, Goldberg NN, Deheyn DD, Cai S, Tolley MT. Translucent soft robots driven by frameless fluid electrode dielectric elastomer actuators. Sci Robot. 2018;3:eaat1893. doi: 10.1126/scirobotics.aat1893. [DOI] [PubMed] [Google Scholar]
- 138.Hines L, Petersen K, Lum GZ, Sitti M. Soft actuators for small-scale robotics. Adv Mater. 2017;29:1603483. doi: 10.1002/adma.201603483. [DOI] [PubMed] [Google Scholar]
- 139.Yirmibesoglu OD, Menguc Y. Hybrid soft sensor with embedded IMUs to measure motion; 2016 IEEE Int Conf on Automation Science and Engineering (CASE); 2016. pp. 798–804. [Google Scholar]
- 140.Farrow N, McIntire L, Correll N. Functionalized textiles for interactive soft robotics; 2017 IEEE Int Conf on Robotics and Automation (ICRA); 2017. pp. 5525–5531. [Google Scholar]
- 141.Koivikko A, Raei ES, Mosallaei M, Mantysalo M, Sariola V. Screen-printed curvature sensors for soft robots. IEEE Sens J. 2018;18:223–230. [Google Scholar]
- 142.Meerbeek IMV, Sa CMD, Shepherd RF. Soft optoelectronic sensory foams with proprioception. Sci Robot. 2018;3:eaau2489. doi: 10.1126/scirobotics.aau2489. [DOI] [PubMed] [Google Scholar]
- 143.Thuruthel TG, Abidi SH, Cianchetti M, Laschi C, Falotico E. A bistable soft gripper with mechanically embedded sensing and actuation for fast closed-loop grasping. Preprint at https://arxiv.org/abs/1902.04896 .
- 144.Bai H, et al. Stretchable distributed fiber-optic sensors. Science. 2020;370:848–852. doi: 10.1126/science.aba5504. [DOI] [PubMed] [Google Scholar]
- 145.Tapia J, Knoop E, Mutny M, Otaduy MA, Bacher M. Makesense: automated sensor design for proprioceptive soft robots. Soft Robot. 2020;7:332–345. doi: 10.1089/soro.2018.0162. [DOI] [PubMed] [Google Scholar]
- 146.Truby RL, et al. Soft somatosensitive actuators via embedded 3D printing. Adv Mater. 2018;30:1706383. doi: 10.1002/adma.201706383. [DOI] [PubMed] [Google Scholar]
- 147.Zhao H, O'Brien K, Li S, Shepherd RF. Optoelectronically innervated soft prosthetic hand via stretchable optical waveguides. Sci Robot. 2016;1:eaai7529. doi: 10.1126/scirobotics.aai7529. [DOI] [PubMed] [Google Scholar]
- 148.Justus KB, et al. A biosensing soft robot: Autonomous parsing of chemical signals through integrated organic and inorganic interfaces. Sci Robot. 2019;4:eaax0765. doi: 10.1126/scirobotics.aax0765. [DOI] [PubMed] [Google Scholar]
- 149.Chu C-Y, Patterson RM. Soft robotic devices for hand rehabilitation and assistance: a narrative review. J Neuroeng Rehabil. 2018;15:9. doi: 10.1186/s12984-018-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim D, et al. Eyes are faster than hands: a soft wearable robot learns user intention from the egocentric view. Sci Robot. 2019;4:eaav2949. doi: 10.1126/scirobotics.aav2949. [DOI] [PubMed] [Google Scholar]
- 151.Dang W, Vinciguerra V, Lorenzelli L, Dahiya R. Printable stretchable interconnects. Flex Print Electron. 2017;2:013003 [Google Scholar]
- 152.Wang H, Totaro M, Beccai L. Toward perceptive soft robots: progress and challenges. Adv Sci. 2018;5:1800541. doi: 10.1002/advs.201800541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Biswas S, Visell Y. Emerging material technologies for haptics. Adv Mater Techno. 2019;4:1900042 [Google Scholar]
- 154.Miriyev A, Stack K, Lipson H. Soft material for soft actuators. Nat Commun. 2017;8:596. doi: 10.1038/s41467-017-00685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kanjanapas S, Nunez CM, Williams SR, Okamura AM, Luo M. Design and analysis of pneumatic 2-DoF soft haptic devices for shear display. IEEE Robot Autom Lett. 2019;4:1365–1371. [Google Scholar]
- 156.Thai MT, Hoang TT, Phan PT, Lovell NH, Nho Do T. Soft microtubule muscle-driven 3-axis skin-stretch haptic devices. IEEE Access. 2020;8:157878–157891. [Google Scholar]
- 157.Leroy E, Hinchet R, Shea H. Multimode hydraulically amplified electrostatic actuators for wearable haptics. Adv Mater. 2020;32:2002564. doi: 10.1002/adma.202002564. [DOI] [PubMed] [Google Scholar]
- 158.Phung H, Nguyen CT, Jung H, Nguyen TD, Choi HR. Bidirectional tactile display driven by electrostatic dielectric elastomer actuator. Smart Mater Struct. 2020;29:035007 [Google Scholar]
- 159.Kim J, et al. Braille display for portable device using flip-latch structured electromagnetic actuator. IEEE Trans Haptics. 2020;13:59–65. doi: 10.1109/TOH.2019.2963858. [DOI] [PubMed] [Google Scholar]
- 160.Kim S-W, et al. Thermal display glove for interacting with virtual reality. Sci Rep. 2020;10:11403. doi: 10.1038/s41598-020-68362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Torras N, et al. Tactile device based on opto-mechanical actuation of liquid crystal elastomers. Sens Actuators A. 2014;208:104–112. [Google Scholar]
- 162.Lipomi DJ, Dhong C, Carpenter CW, Root NB, Ramachandran VS. Organic haptics: intersection of materials chemistry and tactile perception. Adv Funct Mater. 2020;30:1906850. doi: 10.1002/adfm.201906850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dhong C, et al. Role of indentation depth and contact area on human perception of softness for haptic interfaces. Sci Adv. 2019;5:eaaw8845. doi: 10.1126/sciadv.aaw8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skylar-Scott MA, Mueller J, Visser CW, Lewis JA. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature. 2019;575:330–335. doi: 10.1038/s41586-019-1736-8. [DOI] [PubMed] [Google Scholar]
- 165.Zhai Y, et al. Printing multi-material organic haptic actuators. Adv Mater. 2021;33:2002541. doi: 10.1002/adma.202002541. [DOI] [PubMed] [Google Scholar]
- 166.Kayser LV, Lipomi DJ. Stretchable conductive polymers and composites based on PEDOT and PEDOT:PSS. Adv Mater. 2019;31:1806133. doi: 10.1002/adma.201806133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carpenter CW, et al. Electropneumotactile stimulation: multimodal haptic actuators enabled by a stretchable conductive polymer on inflatable pockets. Adv Mater Technol. 2020;5:1901119. doi: 10.1002/admt.201901119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jeon S, et al. A magnetically controlled soft microrobot steering a guidewire in a three-dimensional phantom vascular network. Soft Robot. 2018;6:54–68. doi: 10.1089/soro.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim Y, Parada GA, Liu S, Zhao X. Ferromagnetic soft continuum robots. Sci Robot. 2019;4:eaax7329. doi: 10.1126/scirobotics.aax7329. [DOI] [PubMed] [Google Scholar]
- 170.Pancaldi L, et al. Flow driven robotic navigation of microengineered endovascular probes. Nat Commun. 2020;11:6356. doi: 10.1038/s41467-020-20195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kashyap V, et al. Multilayer fabrication of durable catheter-deployable soft robotic sensor arrays for efficient left atrial mapping. Sci Adv. 2020;6:eabc6800. doi: 10.1126/sciadv.abc6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Han M, et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat Biomed Eng. 2020;4:997–1009. doi: 10.1038/s41551-020-00604-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Son D, Gilbert H, Sitti M. Magnetically actuated soft capsule endoscope for fine-needle biopsy. Soft Robot. 2019;7:10–21. doi: 10.1089/soro.2018.0171. [DOI] [PubMed] [Google Scholar]
- 174.Payne CJ, et al. Soft robotic ventricular assist device with septal bracing for therapy of heart failure. Sci Robot. 2017;2:eaan6736. doi: 10.1126/scirobotics.aan6736. [DOI] [PubMed] [Google Scholar]
- 175.Wang C, Park J. Magnetic micropump embedded in contact lens for on-demand drug delivery. Micro Nano Syst Lett. 2020;8:1. [Google Scholar]
- 176.Cabanach P, et al. Zwitterionic 3D-printed non-immunogenic stealth microrobots. Adv Mater. 2020;32:2003013. doi: 10.1002/adma.202003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ceylan H, et al. 3D-printed biodegradable microswimmer for theranostic cargo delivery and release. ACS Nano. 2019;13:3353–3362. doi: 10.1021/acsnano.8b09233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park J, Kim J, Pané S, Nelson BJ, Choi H. Acoustically mediated controlled drug release and targeted therapy with degradable 3D porous magnetic microrobots. Adv Healthc Mater. 2020;5:2001096. doi: 10.1002/adhm.202001096. [DOI] [PubMed] [Google Scholar]
- 179.Yang X, et al. An agglutinate magnetic spray transforms inanimate objects into millirobots for biomedical applications. Sci Robot. 2020;5:eabc8191. doi: 10.1126/scirobotics.abc8191. [DOI] [PubMed] [Google Scholar]
- 180.Pal A, Restrepo V, Goswami D, Martinez RV. Exploiting mechanical instabilities in soft robotics: control, sensing, and actuation. Adv Mater. 2021;33:2006939. doi: 10.1002/adma.202006939. [DOI] [PubMed] [Google Scholar]
- 181.Rothemund P, et al. A soft, bistable valve for autonomous control of soft actuators. Sci Robot. 2018;3:eaar7986. doi: 10.1126/scirobotics.aar7986. [DOI] [PubMed] [Google Scholar]
- 182.Preston DJ, et al. Digital logic for soft devices. Proc Natl Acad Sci USA. 2019;116:7750–7759. doi: 10.1073/pnas.1820672116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Preston DJ, et al. A soft ring oscillator. Sci Robot. 2019;4:eaaw5496. doi: 10.1126/scirobotics.aaw5496. [DOI] [PubMed] [Google Scholar]
- 184.Pal A, Goswami D, Martinez RV. Elastic energy storage enables rapid and programmable actuation in soft machines. Adv Funct Mater. 2020;30:1906603 [Google Scholar]
- 185.Tang Y, et al. Leveraging elastic instabilities for amplified performance: spine-inspired high-speed and high-force soft robots. Sci Adv. 2020;6:eaaz6912. doi: 10.1126/sciadv.aaz6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang T, Ren Z, Hu W, Li M, Sitti M. Effect of body stiffness distribution on larval fish-like efficient undulatory swimming. Sci Adv. 2021;7:eabf7364. doi: 10.1126/sciadv.abf7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rafsanjani A, Zhang Y, Liu B, Rubinstein SM, Bertoldi K. Kirigami skins make a simple soft actuator 208. crawl. Sci Robot. 2018;3:eaar7555. doi: 10.1126/scirobotics.aar7555. [DOI] [PubMed] [Google Scholar]
- 188.Jin L, Forte AE, Deng B, Rafsanjani A, Bertoldi K. Kirigami-inspired inflatables with programmable shapes. Adv Mater. 2020;32:2001863. doi: 10.1002/adma.202001863. [DOI] [PubMed] [Google Scholar]
- 189.Li S, et al. A vacuum-driven origami “magic-ball” soft gripper; 2019 Int Conf on Robotics and Automation (ICRA); 2019. pp. 7401–7408. [Google Scholar]
- 190.Cafferty BJ, et al. Fabricating 3D Structures by combining 2D printing and relaxation of strain. Adv Mater Technol. 2018;4:1800299 [Google Scholar]
- 191.Coyle S, Majidi C, LeDuc P, Hsia KJ. Bio-inspired soft robotics: material selection, actuation, and design. Extrem Mech Lett. 2018;22:51–59. [Google Scholar]
- 192.Laschi C, et al. Soft robot arm inspired by the octopus. Adv Robot. 2012;26:709–727. [Google Scholar]
- 193.Laschi C, Cianchetti M. Soft robotics: new perspectives for robot bodyware and control. Front Bioeng Biotechnol. 2014;2:1–5. doi: 10.3389/fbioe.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gu G, Zou J, Zhao R, Zhao X, Zhu X. Soft wall-climbing robots. Sci Robot. 2018;3:eaat2874. doi: 10.1126/scirobotics.aat2874. [DOI] [PubMed] [Google Scholar]
- 195.Hawkes EW, Blumenschein LH, Greer JD, Okamura AM. A soft robot that navigates its environment through growth. Sci Robot. 2017;2:eaan3028. doi: 10.1126/scirobotics.aan3028. [DOI] [PubMed] [Google Scholar]
- 196.Zhang J, et al. Liquid crystal elastomer-based magnetic composite films for reconfigurable shape- morphing soft miniature machines. Adv Mater. 2021;33:2006191. doi: 10.1002/adma.202006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang L, Naumov P, Du X, Hu Z, Wang J. Vapomechanically responsive motion of microchannel- programmed actuators. Adv Mater. 2017;29:1702231. doi: 10.1002/adma.201702231. [DOI] [PubMed] [Google Scholar]
- 198.Sanchez V, Walsh CJ, Wood RJ. Textile technology for soft robotic and autonomous garments. Adv Funct Mater. 2021;31:2008278 [Google Scholar]
- 199.Balde CP, et al. The Global e-Waste Monitor 2017: Quantities, Flows, and Resources. United Nations Univ; 2017. [Google Scholar]
- 200.Wang Z, Zhang B, Guan D. Take responsibility for electronic-waste disposal. Nature. 2016;536:23–25. doi: 10.1038/536023a. [DOI] [PubMed] [Google Scholar]
- 201.Blaiszik BJ, et al. Self-healing polymers and composites. Annu Rev Mater Res. 2010;40:179–211. [Google Scholar]
- 202.Markvicka EJ, Bartlett MD, Huang X, Majidi C. An autonomously electrically self-healing liquid metal-elastomer composite for robust soft- matter robotics and electronics. Nat Mater. 2018;17:618–624. doi: 10.1038/s41563-018-0084-7. [DOI] [PubMed] [Google Scholar]
- 203.Wang S, Urban MW. Self-healing polymers. Nat Rev Mater. 2020;5:562–583. [Google Scholar]
- 204.Pena-Francesch A, Jung H, Demirel MC, Sitti M. Biosynthetic self-healing materials for soft machines. Nat Mater. 2020;19:1230–1235. doi: 10.1038/s41563-020-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Terryn S, Brancart J, Lefeber D, Assche GV, Vanderborght B. Self-healing soft pneumatic robots. Sci Robot. 2017;2:eaan4268. doi: 10.1126/scirobotics.aan4268. [DOI] [PubMed] [Google Scholar]
- 206.Yu K, Xin A, Du H, Li Y, Wang Q. Additive manufacturing of self-healing elastomers. npg Asia Matter. 2019;11:7. [Google Scholar]
- 207.Tee BC-K, Wang C, Allen R, Bao Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat Nanotech. 2012;7:825–832. doi: 10.1038/nnano.2012.192. [DOI] [PubMed] [Google Scholar]
- 208.Tan YJ, et al. A transparent, self-healing and high-κ dielectric for low-field-emission stretchable optoelectronics. Nat Mater. 2020;19:182–188. doi: 10.1038/s41563-019-0548-4. [DOI] [PubMed] [Google Scholar]
- 209.Tan YJ, Susanto GJ, Anwar Ali HP, Tee BCK. Progress and roadmap for intelligent self-healing materials in autonomous robotics. Adv Mater. 2020;33:2002800. doi: 10.1002/adma.202002800. [DOI] [PubMed] [Google Scholar]
- 210.Yang H, et al. Graphene oxide-enabled synthesis of metal oxide origamis for soft robotics. ACS Nano. 2019;13:5410–5420. doi: 10.1021/acsnano.9b00144. [DOI] [PubMed] [Google Scholar]
- 211.Pena-Francesch A, et al. Programmable proton conduction in stretchable and self-healing proteins. Chem Mater. 2018;30:898–905. [Google Scholar]
- 212.Tomko JA, et al. Tunable thermal transport and reversible thermal conductivity switching in topologically networked bio-inspired materials. Nat Nanotech. 2018;13:59–964. doi: 10.1038/s41565-018-0227-7. [DOI] [PubMed] [Google Scholar]
- 213.Hartmann F, Baumgartner M, Kaltenbrunner M. Becoming sustainable, the new frontier in soft robotics. Adv Mater. 2020;33:2004413. doi: 10.1002/adma.202004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Raman R, et al. Light-degradable hydrogels as dynamic triggers for gastrointestinal applications. Sci Adv. 2020;6:eaay0065. doi: 10.1126/sciadv.aay0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bellinger AM, et al. Oral, ultra–long-lasting drug delivery: application toward malaria elimination goals. Sci Transl Med. 2016;8:365ra157. doi: 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Feig VR, Tran H, Bao Z. Biodegradable polymeric materials in degradable electronic devices. ACS Cent Sci. 2018;4:337–348. doi: 10.1021/acscentsci.7b00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Walker S, et al. Using an environmentally benign and degradable elastomer in soft robotics. Int J Intell Robot Appl. 2017;1:124–142. [Google Scholar]
- 218.Baumgartner M, et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat Mater. 2020;19:1102–1109. doi: 10.1038/s41563-020-0699-3. [DOI] [PubMed] [Google Scholar]
- 219.Goudu SR, et al. Biodegradable untethered magnetic hydrogel milli-grippers. Adv Funct Mater. 2020;30:2004975 [Google Scholar]