Abstract
Neurons and glial cells are endowed with membranes that express a rich repertoire of ion channels, transporters, and receptors. The constant flux of ions across the neuronal and glial membranes results in voltage fluctuations that can be recorded from the extracellular matrix. The high frequency components of this voltage signal contain information about the spiking activity, reflecting the output from the neurons surrounding the recording location. The low frequency components of the signal, referred to as the local field potential (LFP), have been traditionally thought to provide information about the synaptic inputs that impinge on the large dendritic trees of various neurons. In this review, we discuss recent computational and experimental studies pointing to a critical role of several active dendritic mechanisms that can influence the genesis and the location-dependent spectro-temporal dynamics of LFPs, spanning different brain regions. We strongly emphasize the need to account for the several fast and slow dendritic events and associated active mechanisms — including gradients in their expression profiles, inter- and intra-cellular spatio-temporal interactions spanning neurons and glia, heterogeneities and degeneracy across scales, neuromodulatory influences, and activitydependent plasticity — towards gaining important insights about the origins of LFP under different behavioral states in health and disease. We provide simple but essential guidelines on how to model LFPs taking into account these dendritic mechanisms, with detailed methodology on how to account for various heterogeneities and electro-physiological properties of neurons and synapses while studying LFPs.
Keywords: ion channels, degeneracy, heterogeneity, computational models, oscillations, neural plasticity
Introduction From Alien Gadgets to Intrinsic Mechanisms
In the quest to dissect the functions of the intricate evolutionary marvel that the human brain is, researchers and engineers have been devising fascinating tools. In vivo neuroscience research on the role of neural activity in mediating behavior has benefitted tremendously from the works of pioneers like Richard Caton — who first performed electroencephalography and recorded electrical activity (electroencephalogram or EEG) from exposed brains of cats, rabbits and monkeys using mirror galvanometers (Caton, 1875, 1877), Adolf Beck — who observed spontaneous activity and rhythmic fluctuations that ceased after sensory stimulation (Coenen et al., 2014), Vladimir Vladimirovich Pravdich-Neminsky — who first reported evoked potentials in dogs (Pravdich-Neminsky, 1912), and Hans Berger — who improved the sensitivity of the apparatus and reported oscillations in EEG signals at frequencies around 10–25 Hz (Berger, 1929; Gloor, 1969b, a; Collura, 1993). We have come a long way from the days of EEG recordings from meditating monks (Benson et al., 1990) to the present where neurosurgeons can implant minimally invasive micro-electrocorticogram (ECoG) grids in patients to record local field potentials (LFPs) from areas as delicate as the Wernicke’s (Kellis et al., 2010). We can now record even single cell spikes from the cortical surface using flexible and organic transistors, and from deep brain areas using high-density silicon probes (Buzsaki et al., 2015; Khodagholy et al., 2016; Tybrandt et al., 2018). Thanks to such advancements in closed-loop translational neuro-electronics and biooptics (Khodagholy et al., 2013; Buzsaki et al., 2015; Chang, 2015; Krook-Magnuson et al., 2015; Rivnay et al., 2017; Cea et al., 2020; Jastrzebska-Perfect et al., 2020), what seemed like “alien gadgets” and yesterday’s science fiction are today’s reality and there has been a burgeoning effort to understand the genesis and function of extracellular spikes and LFPs, in both healthy brains and neurological disorders.
Such an effort has progressed in parallel alongside tremendous strides taken in the field of cellular physiology of neurons and glia, both of which are now recognized to contribute to LFPs (Buzsaki et al., 2012) (Fig. 1A). Classically, LFPs were believed to reflect the synaptic inputs that were received by passive dendritic processes, with the soma as the central processing unit of a neuron (Fig. 1B). While synaptic inputs do play a crucial role in shaping LFPs, it is now well established that dendrites are certainly not mere passive recipients of inputs but express a rich repertoire of active conductances (Magee, 2000; Johnston and Narayanan, 2008; Sjostrom et al., 2008; Spruston, 2008; Narayanan and Johnston, 2012; Nusser, 2012; Major et al., 2013; Stuart and Spruston, 2015) that are equally involved in the processing of inputs and in the genesis and regulation of LFPs (Reimann et al., 2013; Sinha and Narayanan, 2015; Ness et al., 2016, 2018) (Fig. 1C).
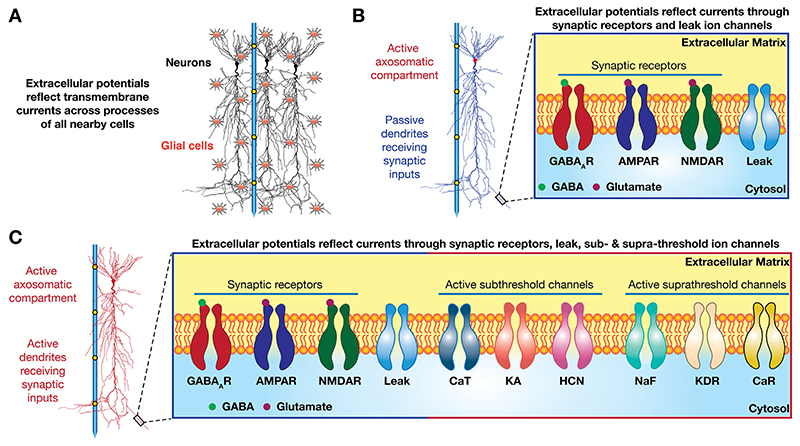
Fig. 1. Sources of LFP.
(A) A schematic of LFP recording from a population of principal neurons and astrocytes using a single electrode with multiple contact points that serve as recording sites. (B) A single recording electrode with multiple sites and a single pyramidal neuron passive dendrites and voltage-gated ion channels only in the axo-somatic compartments. A small dendritic segment is expanded to highlight various synaptic receptors and passive leak channels that contribute to the transmembrane currents. These currents are in addition to the capacitive current consequent to the two ion-conducting media (the cytosol and the cerebrospinal fluid) separated by a dielectric lipid bilayer. (C) Same as B but with active dendrites. An expanded view of a small dendritic segment highlights the diversity of sub- and supra-threshold ion channels/conductances that also contribute to the transmembrane currents. The morphological reconstructions are modified from neuron n123 in the open-source neuronal morphology database Neuromorpho.org (Ascoli et al., 2007).
In this review, we discuss some recent developments in this space at the circuit, cellular, and molecular scales to highlight the complexity involved in the emergence of this gestalt through the presence of active dendrites. We emphasize the need to account for active dendritic properties, gradients in the constitutive components of active dendritic structures, the dynamic nature of these components involving neuromodulation and plasticity, and ubiquitous heterogeneities that span all biological systems in analyzing LFPs. We elucidate the impact of active dendritic structures on LFPs through illustrative examples, both under physiological and pathological conditions. We provide an overview of the different methods currently being employed to address this complexity at various scales and put forward some future directions for making synergistic advances using computational and experimental approaches.
Forms of Local Field Potentials
The constant flux of ions across the neuronal and glial cell membranes through a diverse set of ion channels, pumps, and receptors gives rise to tiny currents and voltage fluctuations which can be recorded from the extracellular matrix. This extracellularly measured voltage signal from the brain has been used to study distinct features of its ongoing activity. The high frequency content of this signal contains information about the spiking activity, reflecting the output from the neurons surrounding the recording location. The low frequency content (<∼300–500 Hz) of the voltage signal, referred to as the LFP, has been traditionally thought to provide information about the synaptic inputs that impinge on the neurons in the form of oscillations and non-rhythmic sensory-evoked events over which the spikes ride (Buzsaki et al., 2012). In this section, we high-light some of the most prominent forms of LFPs observed in the brain.
Unitary LFPs
Unitary LFPs (uLFPs) are the field potentials generated from either a single axon or from the spiking activity of a single unit or presynaptic neuron, typically with a large number of its axon collaterals impinging on a small postsynaptic region. The amplitude, duration, and polarity of uLFPs can vary significantly depending on the type of presynaptic neuron, the location of its axons on the postsynatic target and anatomical connectivity through postsynaptic receptors (Swadlow et al., 2002; Bereshpolova et al., 2006; Stoelzel et al., 2008; Glickfeld et al., 2009; Bazelot et al., 2010). Computational modeling has been utilized to study the possible mechanisms underlying the uLFP signatures generated by fast-spiking inhibitory vs regular-spiking excitatory presynaptic single neurons (Hagen et al., 2017; Telenczuk et al., 2020b). These studies employed anatomically-constrained virtual slices/neuronal columns comprising morphologically-realistic post-synaptic neurons with experimentally determined synapse localization (Hagen et al., 2017), and trimmed axonal arborization of unitary presynaptic neuron to match the realistic size of an in vitro slice (Telenczuk et al., 2020b). They have provided an explanation for how a disynaptic excitatory uLFP can sometimes look like an inhibitory uLFP (Bazelot et al., 2010), highlighting the latter’s dominance when the recording electrode is located inside a predominantly inhibitory population (Telenczuk et al., 2020b). These studies also explain why it can often be difficult to separate excitatory and inhibitory uLFPs in interconnected circuits and how spike-triggered averaged LFP signatures can provide a localized measure of monosynaptic activation (Hagen et al., 2017; Telenczuk et al., 2020b). Thus, it must be emphasized that there is a critical role of axonal morphology, somato-dendritic synaptic distribution profile, anatomical connectivity, timing of input arrival, and electrode location in determining the amplitude, duration, and polarity of uLFPs in particular, and LFPs in general (Sinha and Narayanan, 2020).
LFP oscillations
One of the most prominent set of analyses of LFPs pertains to that of its various rhythms, oscillations in different frequency bands, that have been observed in almost all regions of the brain and are associated with distinct behaviors. Brain rhythms are considered to be one of the key mechanisms for inter-regional communication, stimulus processing, and memory formation (Eckhorn et al., 1988; Nicolelis and Shuler, 2001; Buzsaki and Draguhn, 2004; Buzsaki, 2006; Kay et al., 2009), and are altered in pathological conditions (Sun et al., 2011; Gandal et al., 2012; Yordanova et al., 2013; Mably and Colgin, 2018; Yin et al., 2021). It has been argued that these neural oscillations offer distinct ways of predicting ‘what’ is going to happen ‘when’ in the sensory environment (Kay et al., 2009; Arnal and Giraud, 2012). In what follows, we briefly touch upon some of the most prominent and widely studied LFP oscillations.
Slow Waves: Theta and Alpha Oscillations
Theta oscillations are large amplitude oscillations observed in the ∼4–10 Hz frequency band of the LFPs in several limbic regions of the brain including the hippocampus and entorhinal cortex during awake active exploration, spatial navigation, and rapid eye movement (REM) sleep (Buzsaki et al., 1983; Leung, 1984; Buzsaki, 2002; Buzsaki and Draguhn, 2004; Colgin, 2013, 2016). While they have been well documented in rodent models, thanks to the development of virtual reality and depth stereoelectrode recording tools, theta oscillations have now also been recorded and studied in human and non-human primates (Ekstrom et al., 2005; Watrous et al., 2011; Jutras et al., 2013; Watrous et al., 2013; Bohbot et al., 2017; Goyal et al., 2020) and have been reported to code spatial distance in the absence of sensory cues (Vass et al., 2016). A recent study in humans has observations 2 distinct classes of theta oscillations (Goyal et al., 2020): low-frequency theta oscillations (∼3 Hz) that are prevalent in the anterior hippocampus with their frequency being invariant to movement speed, and high-frequency theta oscillations (∼8 Hz) that are prevalent in the posterior hippocampus with their precise frequency being correlated with the speed of movement. Thus, these two distinct oscillations may reflect nonspatial cognitive and spatial processes, respectively. A similar functional distinction of theta oscillations with reference to spatial vs. nonspatial information has also been observed in rodents along the septo-temporal axis and dorso-ventral hippocampus (Royer et al., 2010; Patel et al., 2012). Theta rhythms have been linked with path integration, spatial navigation, spatial/episodic memory formation, and memory consolidation through temporal sequences of cell assemblies of place and grid cells with cells that are a part of one assembly showing a common preference for a specific phase of these oscillations (O’Keefe and Dostrovsky, 1971; O’Keefe and Conway, 1978; O’Keefe and Nadel, 1978; O’Keefe and Recce, 1993; O’Keefe et al., 1998; O’Keefe and Burgess, 1999; Buzsaki, 2002; Harris et al., 2003; Buzsaki, 2005; Harris, 2005; Hasselmo, 2005; O’Keefe and Burgess, 2005; Huxter et al., 2008; Buzsaki and Moser, 2013; Hasselmo and Stern, 2014; Lisman et al., 2017). Loss or disruption of these rhythms has been reported to result in deficits in spatial memory and its consolidation during REM sleep (Winson, 1978; Boyce et al., 2016).
A lot of heterogeneity has been observed in theta oscillation rhythmogenesis. One form of theta oscillations originates due to the interactions between the interneurons and excitatory neurons in the medial septum – diagonal band of Broca and supramammillary nucleus (Macadar et al., 1970; Roig et al., 1970; Buzsaki, 2002) with the lateral septum playing a regulatory role (Chee et al., 2015). These oscillations travel and propagate along the septotemporal axis of the hippocampus (Lubenov and Siapas, 2009; Patel et al., 2012) and septal lesions disrupt theta oscillations in the hippocampus and entorhinal cortex (Green and Arduini, 1954; Mitchell et al., 1982). However, there is evidence that theta oscillations could also be generated locally within the hippocampal CA1 via afferents from CA3 and the entorhinal cortex, or even independent of these inputs (in vitro) (Buzsaki, 2002; Goutagny et al., 2009; Colgin, 2013, 2016). In the CA1, dendritic Ca2+ spikes have been shown to be associated with the generation of theta oscillations (Kamondi et al., 1998b). Apart from the heterogeneity in theta rhythmogenesis mechanisms, there is also heterogeneity in the modulation of spike timings through theta oscillations. For instance, pyramidal neurons and heterogeneous groups of interneurons found within the CA1 show distinct theta-modulation and phase preference (Csicsvari et al., 1999; Klausberger and Somogyi, 2008; Somogyi et al., 2014). A recent study (Navas-Olive et al., 2020) has cleverly combined computational and experimental approaches to identity the cell-type-specific intrinsic and synaptic mechanisms, as well as cell morphology, in determining theta-phase preference.
The alpha-band of oscillations (8–14 Hz) overlaps with the theta-band and is observed in the striatum, visual, somatosensory, and pre-motor/motor areas in health and disease (Haegens et al., 2011; Oswal et al., 2013; Halgren et al., 2019; Singh and Papa, 2020). It has been shown that alpha oscillations emerge in humans on closing of the eyes, and hence were thought to be associated with “cortical idling” (Berger, 1929; Pfurtscheller et al.,1996). Alpha waves thus offered new areas for the study of (un)consciousness in physiology and pathology such as locked-in syndrome (Palva and Palva, 2007; Rosburg, 2019). It was found that a progressive drug-induced loss of consciousness is tightly linked to the emergence of a hypersynchronous activity in the alpha band that was widely distributed in the frontal cortex (Supp et al., 2011). Based on the results from the same study involving stimulus-related responses to median nerve stimulation, it was suggested that blocking of intracortical communication by hypersynchronous ongoing activity could be a key mechanism for the loss of consciousness.
In contrast, LFP and spiking-activity recordings in the sensorimotor cortex show that the pulsed inhibition from these oscillations exerts a strong influence on both spike timing and firing rate. In addition, a reduction in alpha power is correlated with better discrimination performance, indicating that alpha oscillations could actively suppress irrelevant or interfering stimuli processing (Haegens et al., 2011). Also, these oscillations increase with increase in workload during retention in a short-term memory task (Jensen et al., 2002). This suggests that the alpha generating system is directly or indirectly linked to the circuits responsible for working memory. Furthermore, while the thalamocortical circuits have typically been associated with the generation of alpha oscillations in the past, studies employing LFP and spiking-activity recordings have identified intracortical alpha current generators and their potential for alpha pacemaking in the primary visual (V1), auditory (A1), somatosensory (S1), and sensorimotor cortices (Bollimunta et al., 2008; Haegens et al., 2011; Haegens et al., 2015). Thus, apart from the role of alpha oscillations in fundamental functions of attention such as irrelevant/interfering stimulus suppression, selection of salient stimuli, and in predicting forthcoming visual stimulus (van Dijk et al., 2008; Romei et al., 2010; Haegens et al., 2011; Jensen et al., 2012; Klimesch, 2012), the presence of alpha-generators across cortical depth in the neocortex suggests the involvement of these rhythms in feedforward as well as feedback processes, and is consistent with the view that alpha rhythms may be involved in parsing sensory input streams in a way that facilitates communication across cortical areas (Bollimunta et al., 2008; Haegens et al., 2015).
Fast Waves: Beta and Gamma Oscillations
Beta oscillations (∼13–30 Hz) in the motor cortex have been typically believed to be involved in maintaining the “status quo” or a sensorimotor state of akinesis (Engel and Fries, 2010; Khanna and Carmena, 2017). However, observations from studies involving working-memory tasks with cues and delays have led to the suggestion that beta oscillations in the prefrontal cortex (PFC) and motor cortex could potentially have 2 functions: (1) protection from interference from irrelevant stimuli during delay periods and (2) “clear out” or a stopping process to indicate the end of the cue presentation/task as beta power increases during delays and the end of a trial when working memory information needs to be erased (Swann et al., 2009; Lundqvist et al., 2011; Lundqvist et al., 2016; Lundqvist et al., 2018; Schmidt et al., 2019). Gamma oscillations (∼30–150 Hz) together with beta oscillations have also been implicated to have a role in working memory (Lundqvist et al., 2011; Lundqvist et al., 2016; Lundqvist et al., 2018). A subset of the beta band, the beta2 frequency (20–30 Hz) oscillations have been observed in the somatosensory and motor cortices during motor preparation and have been shown to depend on gap junctional coupling, with the oscillation period being determined by the M-type potassium current (Roopun et al., 2006). Computational analysis has helped reveal the neural mechanisms involved in transient neocortical beta oscillations in several species (Sherman et al., 2016). Within the visual cortical areas, it has been suggested that feedforward and feedback signaling use distinct frequency channels involving the slow (theta and alpha) and the fast (beta and gamma) oscillations through task-dependent dynamic (de)synchronization, and they potentially subserve differential communication requirements (van Kerkoerle et al., 2014; Bastos et al., 2015).
In the olfactory bulb (OB), odors evoke beta-gamma oscillations in the LFP (Kay et al., 2009; Martin and Ravel, 2014; Kay, 2015). These oscillations are generated through intrinsic membrane properties and the dendrodendritic interactions between glutamatergic mitral cells and local inhibitory GABAergic granule cells (GCs) (Debarbieux et al., 2003; Kay et al., 2009; Rojas-Libano et al., 2014; Kay, 2015). Activation of dendrodendritic synapses and oscillatory synchronization of cell assemblies is involved in odor memory and odor discrimination (Stopfer et al., 1997; Nusser et al., 2001; Kay et al., 2009; Lepousez and Lledo, 2013). Through biophysical modeling, it was shown that beta oscillations are produced in the OB primarily through voltage-dependent calcium channel-mediated gamma-aminobutyric acid (GABA) release, independent of N-methyl D-aspartate (NMDA) receptors, and a switch between gamma and beta oscillations can be triggered by an increase in the excitability state of a subpopulation of GCs (Osinski and Kay, 2016). These model predictions were then experimentally verified, and it was shown that even though beta oscillations rely on the same synapse as gamma oscillations, unlike gamma, they could indeed persist in the absence of NMDA receptor activation (Osinski et al., 2018). Together, (Osinski and Kay, 2016) and (Osinski et al., 2018) constitute a good example for how computational and experimental analyses could provide complementary insights about LFP genesis.
Gamma oscillations were initially debated to be not distinguishable from broadband noise (Burns et al., 2011) and are contaminated by spikes (Schomburg et al., 2012; Anastassiou et al., 2015; Ray and Maunsell, 2015), but have emerged to be involved in modulating attention, memory, temporal synchronization of inputs, and interregional communication between brain regions via cross-frequency coupling with theta-oscillations (Bragin et al., 1995; Buzsaki, 1996; Jensen et al., 2007; Jokisch and Jensen, 2007; Benchenane et al., 2011; Schomburg et al., 2014; Buzsaki and Schomburg, 2015b, a; Colgin, 2015b; Sridharan and Knudsen, 2015; Fernandez-Ruiz et al., 2021). In the visual cortex, gamma oscillations are known to be stimulus-evoked and are dependent on stimulus attributes including hue and contrast of the stimulus (Ray and Maunsell, 2011a; Shirhatti and Ray, 2018). By performing simultaneous LFP and single unit recordings from all the layers of primary visual cortex (V1), a recent study showed that the coherence of gamma oscillations and spike-LFP coupling could identify six physiological layers and further sublayers within the V1 thus bridging the mesoscopic LFPs and single unit interactions with the laminar structure of V1 (Senzai et al., 2019). There is ample computational and experimental evidence for the involvement of interneurons, especially the fast-spiking parvalbumin-expressing interneurons, in gamma rhythmogenesis (Traub et al., 1996; Wang and Buzsaki, 1996; Fricker and Miles, 2001; Bartos et al., 2007; Buzsaki and Wang, 2012). Furthermore, slow (∼30– 50 Hz) and fast (∼100–150 Hz) gamma oscillations are differentially implicated in routing flow of information, spatial temporal sequencing, novel-object place pairings, and have been hypothesized to be involved in sending segregated neuronal messages allowing a target “reader” area to disambiguate convergent inputs (Colgin et al., 2009; Colgin and Moser, 2010; Colgin, 2012; Bieri et al., 2014; Colgin, 2015a; Zheng et al., 2015; Zheng et al., 2016b; Zheng et al., 2016a; Fernandez-Ruiz et al., 2017; Trimper et al., 2017; Fernandez-Ruiz et al., 2021).
Disorganized entrainment of local circuits, with sparser dendritic arborization and lower spine density as a cellular substrate for developmental miswiring, is associated with changes in beta-gamma oscillations and neurological and psychiatric disorders (Chini et al., 2020). Increased beta-band oscillatory activity in the basal ganglia network due to resonance of intrinsic oscillations with cortical beta oscillations, with a role for the pallido-striatal feedback loop in amplifying beta oscillations, is associated with Parkinsonian motor symptoms (Koelman and Lowery, 2019; Yin et al., 2021). Deep brain stimulation has been useful in reducing these beta oscillations and improving motor function in patients with Parkin-son’s disease (Kuhn et al., 2006; Kuhn et al., 2008). Gamma oscillations are aberrant/disrupted in disorders like schizophrenia (Sun et al., 2011; Gandal et al., 2012), Alzheimer’s disease and Fragile X syndrome (Mably and Colgin, 2018). Non-invasive transcranial electrical stimulation techniques involving alternating current stimulation may turn out to be useful for resolving such disorders involving rhythmopathies (Beliaeva et al., 2021; Frohlich and Riddle, 2021; Huang et al., 2021; Riddle et al., 2021; Riddle and Frohlich, 2021).
Sharp Wave Ripple Complexes, Delta Waves and Spindles
During quiet wakefulness, slow wave sleep (NREM sleep) and consummatory behaviors, a complex pattern is observed in the LFPs in the CA1, CA2, and CA3 regions of the hippocampus. During these states, afferents on the dendrites of pyramidal neurons lead to their strong depolarization (Kamondi et al., 1998b; Buzsaki, 2015). This leads to the generation of a large sink (i.e., a large negative deflection in the LFP) in the stratum radiatum known as a sharp wave. While sharp waves are slow, they are accompanied by fast oscillations (∼100–200 Hz) in the stratum pyramidale known as ripples. This sharp wave ripple (SPW-R) complex lasts for ∼50–150 ms and is highly irregular (repeating with an average frequency of ∼0.5–3 Hz) (Buzsaki, 1986, 2015). Neuronal spiking and sub-threshold activity during SPW-Rs are controlled by a competition between excitation and inhibition in a sublayer-specific manner (English et al., 2014; Valero et al., 2015; Valero et al., 2017; Valero and de la Prida, 2018). During SPW-Rs, spatial memory associated theta sequences, and emotional/social memory correlates have been observed to be reactivated in a time-compressed manner (Nadasdy et al., 1999; Louie and Wilson, 2001; Cei et al., 2014; Girardeau et al., 2017; Oliva et al., 2020). There is evidence that these ripple-associated replays could be a mechanism for memory consolidation (Girardeau et al., 2009; Carr et al., 2011; Girardeau and Zugaro, 2011; Buzsaki, 2015; Fernandez-Ruiz et al., 2019; Cox et al., 2020; Oliva et al., 2020).
During NREM/slow wave sleep, cortical UP-DOWN states of alternating depolarization and hyperpolarization are observed that are associated with the generation of slow delta waves (∼1–4 Hz) and faster spindles (∼11– 16 Hz) (Steriade and Timofeev, 2003; Fuentealba et al., 2004). Sleep spindles are generated by the interaction between the GABAergic neurons of the thalamic reticular nucleus and the thalamocortical nuclei, and are coupled to the cortical delta waves (Steriade, 1993c, b, a; Steriade et al., 1993c; Steriade et al., 1993b, a; Fernandez and Luthi, 2020). There is emerging evidence for a hippocampal-neocortical dialogue during NREM sleep through SPW-Rs, delta waves, and spindles, and for their role in learning, memory consolidation and interregional transfer of information (Buzsaki, 1996; Buzsaki and Peyrache, 2013; Buzsaki, 2015; Maingret et al., 2016; Seibt et al., 2016; Antony et al., 2019; Kim et al., 2019; Todorova and Zugaro, 2019; Karimi Abadchi et al., 2020; Peyrache and Seibt, 2020; Dickey et al., 2021).
Epileptiform Activity: Interictal Spikes and Fast Ripples
Epilepsy is a complex set of syndromes with common behavioral correlates like recurrent seizures and convulsions associated with hypersynchronous activity in the epileptic tissue (Wyler et al., 1982; Babb et al., 1987; Cohen et al., 2002; Crunelli and Leresche, 2002; Scharfman, 2007). Different waveforms observed in the EEG in the ictal (during a seizure), interictal (between seizures) and postictal (after a seizure) activity enable clinicians to diagnose the focus and timing of seizure-stages in epileptic patients (Fisher et al., 2014). In the hippocampus and temporal cortex of epileptic humans and rodents, interictal spikes lasting 30–500 ms followed by transient high-frequency oscillations called fast ripples (250–500 Hz) are observed in the epileptogenic regions and are associated with a multitude of mechanisms depending on the form of epilepsy and the affected brain region (Dichter and Spencer, 1969a, b; Traub and Wong, 1983; Cohen et al., 2002; Bragin et al., 2004; Bernard, 2005; Urrestarazu et al., 2006; Ibarz et al., 2010; Kohling and Staley, 2011; Jefferys et al., 2012; Avoli, 2014; Valero et al., 2017; Levesque and Avoli, 2019; Levesque et al., 2019). Some of these prominent known mechanisms and associated plasticity involve a disruption of the excitation/inhibition balance, synchronous bursting of pyramidal neurons and interneurons, changes in dendritic GABAergic signaling, and dendritic channelopathies (Chen et al., 2001; Sanabria et al., 2001; Cohen et al., 2002; Su et al., 2002; Mulley et al., 2003; Whittington and Traub, 2003; Bernard et al., 2004; Shah et al., 2004; Cossart et al., 2005; Jung et al., 2007; Yaari et al., 2007; Ibarz et al., 2010; Valero et al., 2017). Such combined studies have enabled the development of several anti-epileptic drugs (Rogawski and Loscher, 2004). Computational simulations have also been used as tools to aid experiments in tackling the complex problem of epileptogenesis (Lytton et al., 2005; Traub et al., 2005; Lytton, 2008).
Active Dendritic Conductances, Dendritic Morphology, and Lfps
With the emergence of new techniques for circuit manipulations and large scale LFP recordings, the focus of neuroscience research has shifted towards teasing apart how brain circuits work together to give rise to behavior. Introduction of ‘alien’ ion channels into neurons, such as channelrhodopsin (Boyden et al., 2005), to control the ionic transmembrane currents towards manipulating large populations of neurons synchronously has become commonplace. At the molecular level, this powerful tool is driven by an ensemble current emergent from tiny single-channel ionic currents (single channel conductance is merely ∼ 1 pS (Lin et al., 2009)), and can introduce massive perturbations to even large nervous systems manifesting as large changes in spikes and LFPs (Kim et al., 2017; Oliva et al., 2018; Fernandez-Ruiz et al., 2019; Jun and Cardin, 2020; McKenzie et al., 2021). The power of such ion channels in altering network physiology begs attention to the study of endogenous ion channels that express on neuronal and glial (Verkhratsky and Steinhauser, 2000) membranes in shaping LFPs, apart from their established roles in altering neural activity and behavior.
As the predominant recipients of afferent synaptic information, dendrites are uniquely placed to process afferent information and critically regulate spike generation in a neuron. Although dendrites were historically considered to be passive structures that were merely housing afferent synapses, it is now abundantly clear that dendritic structures express several ion channels, earning them the moniker active dendrites (Johnston et al., 1996; Poirazi and Mel, 2001; Johnston et al., 2003; Johnston and Narayanan, 2008; Major et al., 2013; Stuart and Spruston, 2015). The presence of these active components has endowed dendrites with specialized processing and propagation capabilities that manifest through strong spatio-temporal interactions across the dendritic arbor. If dendrites were not merely housing synapses and carry transmembrane proteins other than synaptic receptors, shouldn’t field potentials, composite signals that reflect transmembrane currents, be shaped by the presence of these active conductances? Here, we explore this question with reference to the different conductances that express in active dendritic structures.
Suprathreshold dendritic conductances and LFPs
Action potentials or spikes are commonly used as a readout of neuronal responses to stimuli and of information transfer. To understand the role of neurons in generating behavioral outputs and to dissect their function in a neuronal circuit, nowadays, extracellular spike recordings using high-density electrodes and silicon probes are routinely performed from freely moving and head-restrained laboratory animals under awake, asleep, and anesthetized conditions. Most extracellular spikes are of a very short duration (typically < 1–2 ms, >500 Hz) and therefore are filtered out from the LFP (typically < 350 Hz). Yet, a computational study has highlighted that spike-related currents can impact the LFP even below 50 Hz (Reimann et al., 2013). Further computational and experimental studies have shown that a component of the spikes “bleeds through” or ‘contaminates’ the LFP in the lower frequency harmonics of the population spike frequency, especially in the epsilon band (90–150 Hz) and thus a stimulus-triggered spike signature can also be obtained in the LFP (Ray et al., 2008; Reichinnek et al., 2010; Ray and Maunsell, 2011b; Belluscio et al., 2012; Scheffer-Teixeira et al., 2013; Schomburg et al., 2014; Anastassiou and Koch, 2015; Taxidis et al., 2015; Kuokkanen et al., 2018). This is especially useful when multiple electrodes are not available to sort the spikes into individual units and if the focus of the study is to perform a population-level analysis of neuronal activity. However, such population-level analysis can lead to inaccurate conclusions in case the spikes are from a heterogeneous network of neurons, so one must be careful while making assumptions about the homogeneity of the underlying network of neurons (Valero et al., 2015; Valero and de la Prida, 2018). Further, the average of stimulus-triggered LFPs (STA-LFPs), has been used to show that units across several regions can become transiently synchronized specifically during LFP oscillations, even if their spikes are uncorrelated during non-oscillatory periods (Murthy and Fetz, 1996). Using intracellular recordings from the cortex of awake rats, STA-LFP has also been shown to represent the synchrony between the mean synaptic activity of the population and the membrane potential of the single neuron (Okun et al., 2010). But the STA-LFP must be used with caution as highlighted by a detailed analysis of the shape of the STA-LFP from the monkey visual cortex (Ray and Maunsell, 2011b). This study showed that network rhythms can influence the relationship between STA-LFP and functional connectivity, giving a false impression of a traveling LFP wave.
Extracellular spikes reflect the intracellular action potentials that are initiated above a certain membrane voltage threshold. They are a result of a complex interplay between inputs from synaptic receptors, intracellular calcium signaling, and various voltage-gated ion channels. Most in vivo studies focus on spikes recorded from the peri-somatic regions that express several suprathreshold-active spiking conductances such as voltage-gated Na+, K+, and Ca2+ channels. But it is now well established that neuronal dendrites are also endowed with a plethora of suprathreshold active conductances that can both initiate spikes and lead to their active (back)propagation in the dendrites (Stuart and Sakmann, 1994; Johnston et al., 1996; Hoffman et al., 1997; Golding and Spruston, 1998; Schiller et al., 2000; Schiller and Schiller, 2001; Gasparini et al., 2004; Polsky et al., 2004; Losonczy and Magee, 2006; Nevian et al., 2007; Johnston and Narayanan, 2008; Losonczy et al., 2008; Larkum et al., 2009; Hay et al., 2011; Narayanan and Johnston, 2012; Major et al., 2013; Smith et al., 2013; Shai et al., 2015; Gidon et al., 2020). Until recently, the study of intracellular dendritic spikes in vivo from awake-behaving animals was not possible owing to the challenges associated with performing these recordings from thin dendrites while the animal moves around. (Smith et al., 2013) were able to achieve this difficult feat of performing patch clamp recording from the thin dendrites of the visual cortex pyramidal neurons in vivo. They showed that visual stimulation triggered regenerative local dendritic spikes (that were distinct from back-propagating action potentials) that enhanced the orientation selectivity.
Further, in 2017 Mayank Mehta’s group was serendipitously able to record membrane potential and spikes from cortical dendrites using tetrodes in awake behaving rats (Moore et al., 2017). They found that dendritic tips being thinner than the gaps between the tetrode bundles got caught in these gaps and were ensheathed by glial cells, enabling the recording of intracellular (quasi-in cell) dendritic voltage. Through this break-through, they demonstrated striking differences between dendritic and somatic spikes. For instance, the mean rate was found to be higher for dendritic spikes than for somatic spikes, indicating that not all dendritic spikes reach the cell body to yield axo-somatic action potentials (Golding and Spruston, 1998; Losonczy and Magee, 2006; Larkum et al., 2009; Lovett-Barron et al., 2012; Major et al., 2013; Palmer et al., 2014). Furthermore, far more spikes were generated in the dendrites than at the soma during slow wave sleep, providing further evidence for an observation that was made decades ago in anesthetized rats in the hippocampus as well using intracellular sharp electrodes (Kamondi et al., 1998a). Back then, it was discovered that large amplitude fast dendritic spikes coincided with CA1 sharp waves in the LFP and preceded spontaneously occurring dendritic Ca2+ spikes. While the cortical dendritic spikes recorded by (Moore et al., 2017) matched the features of a dendritic Na+ spike, in the same year another study found that slow dendritic calcium spikes were also clearly detectable from the cortical surface of rodents in vivo and in the LFP in response to sensory stimulation (Suzuki and Larkum, 2017). One of the first demonstrations that established the link between slow dendritic calcium spikes and ECoG recordings was by (Helmchen et al., 1999). In this study, (Helmchen et al., 1999) showed that, during whisker stimulation, complex spikes recorded intracellularly from distal dendrites and sharp waves in the ECoG were accompanied by large dendritic calcium transients. The highly active dendritic structure and the role of active dendritic channels in mediating spike initiation and propagation point to the need for accounting for dendritic supra-threshold ion channels and their currents in assessing LFPs.
Recently, another study discovered a new class of calcium-mediated dendritic action potentials (dCaAPs) in slices taken from surgically resected brain tissue of epilepsy patients (Gidon et al., 2020). The authors argue that dCaAPs are different from dendritic sodium spikes, calcium spikes, plateau potentials and NMDA spikes previously reported in rodents (Schiller et al., 1997; Helmchen et al., 1999; Larkum et al., 1999; Oakley et al., 2001; Larkum and Zhu, 2002; Waters et al., 2003; Milojkovic et al., 2005; Zhou et al., 2006a; Zhou et al., 2006b; Nevian et al., 2007; Major et al., 2008; Larkum et al., 2009; De Zeeuw et al., 2011; Moore et al., 2017). These dCaAPs were also observed in brain slices from tumor patients. Through computational analyses, the authors show that these dCaAPs enable dendrites to perform XOR operation, while sodium and NMDA spikes enable AND/OR logical operations at the soma and at tufts and basal dendrites respectively. This opens up interesting avenues for future exploration of the role of these various forms of suprathreshold events and suprathreshold conductances in dendritic computation and LFP signatures, both experimentally and computationally. As dendritic calcium spikes, plateau potentials, and NMDA spikes are relatively slow events, and are critically reliant on active dendritic components, their contributions to the low-frequency LFPs needs further analyses. This is especially important given the role of dendritic slow calcium spikes and plateau potentials in sensory-motor feedback, learning, perception, and plasticity (Takahashi and Magee, 2009; Lavzin et al., 2012; Xu et al., 2012; Larkum, 2013; Bittner et al., 2015; Manita et al., 2015; Takahashi et al., 2016; Bittner et al., 2017; Ranganathan et al., 2018; Aru et al., 2020; Doron et al., 2020; Magee and Grienberger, 2020; Roome and Kuhn, 2020; Takahashi et al., 2020; Bonnan et al., 2021). Thus, signatures of these slow dendritic events in the LFP could provide useful handles to analyses and interpretation of several physiological and behavioral out-comes, and constitutes an important future area for exploration.
Finally, in many scenarios the contribution of active dendritic conductances to LFPs is driven by afferent synaptic inputs and by the nonlinear somato-dendritic processing of these inputs. However, there are several neurons across different brain regions, including the cerebellum, suprachiasmatic nucleus, vestibular nuclei, and the dopaminergic midbrain structures, that are capable of spontaneously firing action potentials or bursts (Hausser et al., 2004; Welsh et al., 2010; Constantin et al., 2013; Hastings et al., 2018; Harvey et al., 2020; Otomo et al., 2020). Given that the ionic basis for such spontaneous activity is well understood in several of these neurons, LFPs in brain regions endowed with spontaneously firing neurons could manifest signatures that are independent of synaptic inputs, instead driven by ionic currents associated with spontaneous action potentials. Importantly, some of these neurons, including the cerebellar Purkinje cells (Hausser et al., 2004; De Zeeuw et al., 2011; Kitamura and Hausser, 2011; Roome and Kuhn, 2018, 2020), are endowed with elaborate dendritic trees and manifest slow dendritic spikes that are associated with spontaneous burst firing. In these neurons, the active dendritic contributions to relatively low-frequency components of the LFP could again be independent of synaptic inputs and could instead be driven by dendritic ionic currents. Thus, an important lacuna in the field pertains to the impact of active dendrites in spontaneously firing and bursting neurons on LFPs in brain regions endowed with such neurons.
Subthreshold dendritic conductances and LFPs
While spikes inarguably provide immense information about neurophysiology, not all activity in neurons is suprathreshold. The underlying subthreshold membrane potential dynamics can vastly change neuronal output and information transfer. What mechanisms exist for a neuron to contribute to, process, and respond to such subthreshold voltage dynamics? The answer lies in the myriad of subthreshold active conductances expressed in the somato-dendritic compartments. In this section, we explore the roles of a few of these mechanisms, with illustrative examples involving different subthreshold ion channels, and argue for a strong impact of subthreshold dendritic conductances on LFPs.
A-type Potassium Channels, Astrocytes, and Calcium Waves
Voltage-gated transient A-type potassium channels, also referred to as “dendritic shock absorbers” (Yuste, 1997) tend to have almost 5-fold higher expression density in the dendrites than in the soma, and despite being subthreshold-active can in fact prevent suprathreshold dendritic spike initiation and propagation, thereby restraining large rapid dendritic depolarization (Hoffman et al., 1997; Migliore et al., 1999). Through morphologically realistic conductance based computational modelling it was shown that these dendritic A-type potassium channels can interact with the endoplasmic reticulum (ER) through inositol triphosphate receptors (InsP3R) and voltage-gated calcium channels, and can regulate the latency and temporal spread of calcium waves in dendrites (Ashhad and Narayanan, 2013). Large scale calcium waves have also been observed travelling through the astrocytic syncytium in vivo (Kuga et al., 2011; Sasaki et al., 2011; Ross, 2012; Ross and Manita, 2012). These astrocyte-mediated calcium waves have a role in synchonizing cortical activity as suggested by the inhibition of spontaneous cortical UP states upon chelation of calcium in cortical astrocytes (Poskanzer and Yuste, 2011). Astrocytic “glissandi” (the regenerative propagation of waves from cell to cell) are correlated with reduced infra-slow rhythms (<0.1–0.5 Hz) in the LFP (Hughes et al., 2011; Kuga et al., 2011).There is a strong phase-locking of interictal events and K-complexes to these infra-slow rhythms during sleep (Vanhatalo et al., 2004; Kang et al., 2005). This together with the fact that astrocytes can regulate the extracellular K+ concentration and modulate inhibitory synaptic activity highlights a significant role of astrocytic function and dysfunction in epilepsy and epileptiform activity (Kang et al., 1998; Vanhatalo et al., 2004; Kang et al., 2005; Coulter and Steinhauser, 2015; Nikolic et al., 2020).
Furthermore, astrocytes themselves express several voltage-gated ion channels and receptors, and can release calcium and neurotransmitter molecules (Verkhratsky and Steinhauser, 2000; Halassa and Haydon, 2010; Araque et al., 2014; Bazargani and Attwell, 2016; Ashhad and Narayanan, 2019). Through this gliotransmission, it has also been observed that astrocytes can induce large, long-lasting and slow excitatory potentials (SEPs) or dendritic plateau potentials in CA1 pyramidal neurons (Ashhad and Narayanan, 2016). The kinetics of these SEPs vary based on the location of stimulated astrocytes (close to proximal vs. distal apical dendritic compartments) and distinct NMDA receptor currents. Pharmacological analyses coupled with morphologically realistic conductance-based computational models have demonstrated that dendritically expressed hyperpolarization-activated cyclic-nucleotide–gated (HCN) and transient A-type potassium channels play critical roles in regulating the amplitude, kinetics, and compartmentalization of such SEPs (Ashhad and Narayanan, 2016, 2019). These studies highlight that while astroglia do not exhibit suprathreshold activity such as spikes, they can definitely regulate both LFPs and sub-/supra-threshold activity through interactions with dendritic subthreshold-active conductances and receptors under physiological and pathological conditions.
T-type Calcium Channels and NREM Sleep Spindles
T-type calcium channels and cation non-specific HCN channels are two other prominent subthreshold-active conductances that are highly expressed in proximal and distal dendrites of hippocampal and thalamo-cortical neurons, and can modulate intrinsic oscillations (McCormick and Pape, 1990; Leresche et al., 1991; Soltesz et al., 1991; Destexhe et al., 1993; Magee and Johnston, 1995; Destexhe et al., 1996; Johnston et al., 1996; Luthi and McCormick, 1998b, a; Magee, 1998, 1999; Hutcheon and Yarom, 2000; Santoro et al., 2000; Perez-Reyes, 2003; Crunelli et al., 2006; Narayanan and Johnston, 2012; Simms and Zamponi, 2014). In the thalamus, during the transition from wakefulness to sleep there is a reduction in the depolarizing tone exerted by afferents onto thalamocortical (TC) neurons and the nucleus reticularis thalami (nRt) (Huguenard and McCormick, 1992; McCormick, 1992a, b; McCormick and Huguenard, 1992; McCormick and von Krosigk, 1992; Steriade and Timofeev, 2003). This is associated with a prolonged hyperpolarization of the majority of the recipient neurons into membrane potentials where activation and inactivation of T channels is possible (Roy et al., 1984; Steriade and Timofeev, 2003; Fuentealba et al., 2004). Once de-inactivated by hyperpolarization, T-type calcium channels mediate low-threshold Ca2+ spikes, which in turn trigger a burst of action potentials during different stages of NREM sleep (Deschenes et al., 1982; Llinas and Jahnsen, 1982; Deschenes et al., 1984; Jahnsen and Llinas, 1984a, c, b; Steriade and Deschenes, 1984; Domich et al., 1986; Llinas and Steriade, 2006). Owing to this oscillatory property and burst synchronization of the thalamocortical circuit by T-type calcium channels, they are regarded as the major pacemakers responsible for rhythmogenesis of thalamocortical sleep spindles during NREM sleep (McCormick and Bal, 1997; Cueni et al., 2008; Astori et al., 2011; Pellegrini et al., 2016).
Further, it has been shown that in the GABAergic nRt neurons, the triad of dendritic small conductance type K+ (SK2) channels, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps, and T-type calcium channels regulates oscillatory dynamics related to sleep. Specifically, the oscillatory bursting is initiated via selective activation of dendritic SK2 channels while the dampening of the oscillation is mediated through Ca2+ uptake by SERCA pumps and cumulative T-type calcium channel inactivation (Cueni et al., 2008). The relevance of SK2 channels was highlighted through studies on SK2-/- mice that lack cellular oscillations, show a large (>3 fold) reduction in low-frequency rhythms during NREM sleep, and have disrupted sleep (Cueni et al., 2008). Deletion of CaV3.2 and CaV3.3 T-type Ca2+ channels has also been shown to suppress sleep spindle rhythmogenesis in mice (Pellegrini et al., 2016) and the absence of CaV3.3 channels prevents oscillatory bursting in the theta-frequency range in nRt cells (Astori et al., 2011). However, sleep spindles are not altered in CaV3.1−/− mice during natural NREM sleep (Lee et al., 2013). This highlights the role of specific ion-channel sub-types/isoforms in mediating biological rhythms and perhaps that in knockouts such as these, a compensatory mechanism is employed by the neurons to sustain oscillatory response dynamics. It is possible that this compensatory mechanism could be mediated through upregulation of HCN channels, which have also been shown to regulate sleep spindles (McCormick et al., 2015). In fact, based on observations in humans, a model for coordination between cortical UP-DOWN states and thalamic spindles during NREM sleep has been proposed which involves both T-type Ca2+ channels and HCN channels (Mak-McCully et al., 2017).
HCN Channels and Theta Oscillations
Unlike most ion channels, HCN channels have a unique property, as the name suggests, of activating upon hyperpolarization. In pyramidal neurons, they are active at the resting membrane potential, have slow activation and deactivation kinetics and their expression density increases from soma to dendrites, being highest in the distal apical dendrites (Magee, 1998; Williams and Stuart, 2000; Lorincz et al., 2002; Migliore and Shepherd, 2002; Kole et al., 2006; Narayanan and Johnston, 2007; He et al., 2014). They regulate neuronal excitability by reducing the temporal summation of EPSPs at the soma (Magee, 1998, 1999, 2000; Williams and Stuart, 2000; Narayanan and Johnston, 2007). As a confluence of these properties, they oppose changes to membrane potential, reduce the excitability of dendrites by reducing their input resistance, confer an increasing gradient of dendritic theta-frequency resonance, contribute an inductive component to the input impedance, and mediate the location- and activity-dependence of the intrinsic phase response (Hutcheon and Yarom, 2000; Ulrich, 2002; Narayanan and Johnston, 2008, 2012). Their somatodendritic expression acts as a gradient of inductance which helps resolve the locationdependent temporal differences in dendritic inputs by synchronizing the theta- and gamma- frequency inputs to a common synchronization frequency (transfer resonance frequency) at the soma (Vaidya and Johnston, 2013). This synchronization frequency happens to be in the theta-frequency range, and is invariant to input location. HCN channels can also affect spike initiation dynamics and have the capability of reducing the coincidence detection window to temporal ranges within the low and high gamma frequency ranges at lower and higher conductance values, respectively (Das and Narayanan, 2014, 2015, 2017; Das et al., 2017).
When an oscillating current in the theta-frequency range is injected in neurons expressing HCN channels, it is observed that the intracellular voltage response leads in phase with respect to the current (Ulrich, 2002; Narayanan and Johnston, 2008) (Fig. 2A), a property of an inductor, and that this phase lead manifests in the theta-frequency range. A morphologically realistic conductance-based experimentally constrained computational model employing a forward modeling scheme for LFPs showed that this phase lead mediated by HCN channels is in fact reflected even in the theta-frequency LFP in the hippocampus (Sinha and Narayanan, 2015). Given that the expression density of HCN channel increases with distance from the soma towards the apical dendrites, it was shown that the phase lead also increases along the somato-apical axis (Fig. 2B). The ability of HCN channels to introduce an inductive phase lead in intracellular voltage responses to theta-modulated synaptic currents played a significant role in altering LFP and spike phases.
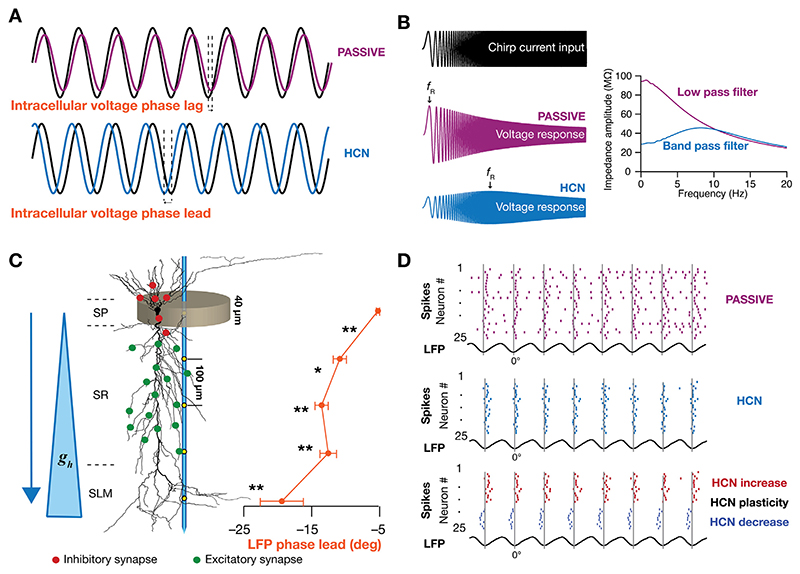
Fig. 2. HCN channels regulate intracellular and extracellular voltage responses to theta frequency inputs.
(A) Voltage response of a passive neuronal compartment (top) and of a neuronal compartment expressing HCN channels (bottom) to a theta-frequency sinusoidal current injection (black traces) for 1 s. Notice that the passive voltage response shows a phase lag with respect to the input current and a reduction in amplitude while the voltage response in the presence of HCN channels shows a corresponding phase lead (Narayanan and Johnston, 2008). (B) Left top: A chirp stimulus, constant amplitude sinusoidal current linearly increasing in frequency from 0 to 20 Hz in 20 s. Left middle: Passive voltage response to the chirp stimulus showing low-pass filtering. Left bottom: Voltage response in the presence of HCN channels showing a band-pass response with the highest amplitude at its resonance frequency (fR). Right: Illustrative impedance amplitude profiles show a low-pass response for the passive compartment and a band-pass response in the presence of HCN channels, with fR in the theta-band. Such low-pass and band-pass responses are also reflected in the LFP power spectral density in the absence and presence of HCN channels, respectively (see (Ness et al., 2016, 2018)) (C) Left: A morphologically realistic CA1 pyramidal neuron model representing a population of neurons in a cylindrical neuropil, with inhibitory synapses targeting the perisomatic regions and excitatory synapses targeting the dendrites in the SR. An experimentally constrained HCN channel gradient was introduced in the model accounting for an increase in HCN conductance (gh) with distance from the soma. A single electrode with seven recording sites, located at the center of the cylindrical neuropil spanned all strata of the CA1. Right: Lead in the LFP phase introduced by the presence of HCN channels increases with HCN conductance along the somato-apical axis ((Sinha and Narayanan, 2015); *: p < 0.05; **: p < 0.005; Wilcoxon signed rank test). (D) Top: Raster plots for 25 passive model neurons with low spike-phase coherence with respect to theta frequency LFP. Middle: Same as top but with a gradient of HCN channels introduced in the model shows enhancement of spike-phase coherence and spike theta-phase preference. Bottom: Same as middle but illustrating the impact of bidirectional HCN plasticity on reconfiguration of cell assemblies. SP: stratum pyramidale, SR: stratum radiatum, SLM: stratum lacunosum moleculare.
CA1 and CA3 pyramidal neurons tend to show phase preference with the maximum firing probability at the trough of a theta cycle (Csicsvari et al., 1999; Klausberger and Somogyi, 2008). (Sinha and Narayanan, 2015) showed that HCN channels can regulate this spike theta-phase preference of individual neurons and at a population level HCN channels enable formation of cell assemblies by enhancing spike thetaphase coherence (Fig. 2D). It was also demonstrated that a single neuron could shift its spike-phase preference through HCN channel plasticity. To our knowledge, this was the first direct evidence of a subthreshold conductance influencing the ongoing LFP and also the spike-phase response of the neurons in the hippocampus. The study further demonstrated a critical role of the phase of inhibitory input arrival with reference to excitatory inputs in altering both the stratum pyramidale LFP and associated spike phases, but not spike phase coherence. Thus, there exists a critical role of HCN channels and synaptic receptors in phase-coding schemas and in the formation and dynamic reconfiguration of neuronal cell assemblies. Following this modeling study, two other modeling studies demonstrated the strong influence of subthreshold HCN channels, this time, on cortical LFPs (Ness et al., 2016, 2018). They showed that a gradient of HCN channels can induce theta-frequency resonance in the LFP signal (Fig. 2C).
Together, these studies present a clear case for the incorporation of subthreshold-activated ion channels, their distribution across the cell membrane, and their plasticity into the computation of LFPs (Sinha and Narayanan, 2015; Ness et al., 2016, 2018). They also strongly highlight how biophysically and morphologically realistic experimentally-constrained computational models can advance our mechanistic understanding of neuronal function in generating neural codes, LFPs, and behavior.
Morphology of active dendrites and LFPs
Dendritic morphology critically regulates neuronal physiology. Although it was traditionally assumed that dendritic arborization merely provides larger surface area to accommodate more synapses, it is clear that the arborization allows for far more functional specializations than acting as a simple funnel for information through a large number of synaptic inputs (Poirazi and Mel, 2001; Poirazi et al., 2003; London and Hausser, 2005; Johnston and Narayanan, 2008; Narayanan and Johnston, 2012; Poirazi and Papoutsi, 2020). The presence of complex dendritic arborization in conjunction with active-dendritic conductances mediates segregation and compartmentalization of afferent inputs, thereby allowing for location dependence in input processing and filtering, dendritic spike initiation, coincidence detection, and even specialized dendro-dendritic communication in certain synapses (Rall and Shepherd, 1968; Golding and Spruston, 1998; Schiller et al., 2000; Schiller and Schiller, 2001; Gasparini et al., 2004; Polsky et al., 2004; Losonczy and Magee, 2006; Narayanan and Johnston, 2007; Nevian et al., 2007; Losonczy et al., 2008; Narayanan and Johnston, 2008; Larkum et al., 2009; Gidon et al., 2020). Dendritic morphology plays a critical role in regulating neural excitability, firing patterns, coincidence detection, and the expression of functional gradients, even in the presence of gradients in ion channel expression (Mainen and Sejnowski, 1996; Vetter et al., 2001; Krichmar et al., 2002; van Ooyen et al., 2002; Cannon et al., 2010; Narayanan and Chattarji, 2010; Ferrante et al., 2013; Dhupia et al., 2015; Ostojic et al., 2015; Das et al., 2017). Changes in any of these functional properties significantly alter the transmembrane currents through ion channels and receptors on the somato-dendritic arbor, thus altering the recorded LFPs at different electrode locations. Thus, the structural organization of the dendritic tree and the arrangement of dendritic arbors of different neurons in the subregion (e.g., open field organization in hippocampus and cerebellum vs. random organization in several other brain regions) should be accounted for as critical regulators of the strength and polarity of field potentials (Johnston and Wu, 1995; Linden et al., 2011; Buzsaki et al., 2012; Einevoll et al., 2013). The arborization of axons and their localization on the dendritic arbor also play essential roles in regulating the spread and shape of unitary LFPs (Glickfeld et al., 2009; Bazelot et al., 2010; Telenczuk et al., 2020b). Databases of neural morphologies (Ascoli et al., 2007; Gouwens et al., 2019) and algorithms for dendritic remodeling (Narayanan et al., 2005; Koene et al., 2009; Cuntz et al., 2010; Narayanan and Chattarji, 2010; Bozelos et al., 2015; Dhupia et al., 2015; Beining et al., 2017), coupled with biophysically realistic computational modeling, could be employed to systematically assess the impact of active dendritic morphology on LFPs.
Gradients, Heterogeneities and Degeneracy
Gradients and heterogeneities in ion-channel expression and intrinsic properties
Neurons manifest gradients in the spatial expression profiles of active ion-channel conductances. There are systematic lines of evidence for the existence of such gradients within individual neurons (Migliore and Shepherd, 2002; Johnston and Narayanan, 2008; Nusser, 2009; Narayanan and Johnston, 2012), as well as across neurons spanning specific anatomical axes (Giocomo et al., 2007; Igarashi et al., 2014; Lee et al., 2014; Malik et al., 2016; Maroso et al., 2016; Malik and Johnston, 2017; Sun et al., 2017; Valero and de la Prida, 2018; Cembrowski and Spruston, 2019; Navas-Olive et al., 2020; Pastoll et al., 2020). Gradients in ionchannel expression profile within a single neuron, along its somatodendritic axis, yield functional maps of neuronal properties. From the standpoint of LFPs, these gradients imply location-dependent contributions of individual ion channels to extracellular field potentials. For instance, hippocampal CA1 pyramidal neurons manifest a gradient in HCN ion channels, with higher expression at distal dendritic locations (Magee, 1998; Lorincz et al., 2002). A primary implication for the expression of this gradient is an increase in the amount of HCN-channel mediated transmembrane currents in distal dendritic locations. However, as HCN channels reduce the excitability of neurons (Gasparini and DiFrancesco, 1997; Magee, 1998; Narayanan and Johnston, 2007), the HCN-channel gradient also implies that the voltage response to a given synaptic current is lower in distal locations. This reduced voltage deflection, in turn, alters the driving forces for the different ion channels and receptors expressed at that location, thereby altering the transmembrane currents through these distinct ion channels and receptors. In addition, the reduced voltage responses also alter the capacitive current in a distance dependent manner as the rate of change in voltage responses is altered by the slow kinetics of HCN channels. The impact of the expression of the HCN channel gradient, therefore, is the cumulative and synergistic impact of changes to all currents, and depends on the patterns of synaptic activation, the ultra-structural morphology, and the relative expression profiles of individual receptors and channels (Sinha and Narayanan, 2015; Ness et al., 2016, 2018; Navas-Olive et al., 2020).
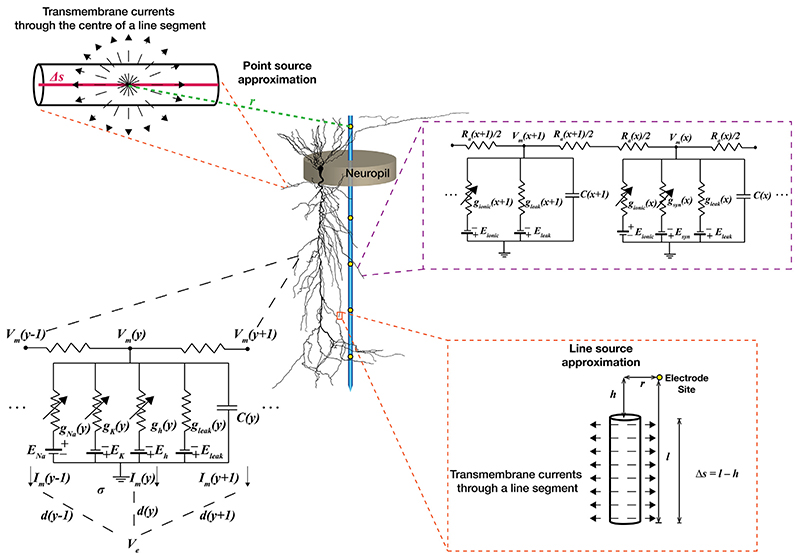
If a computational approach is employed to assess the impact of intraneuronal ion-channel gradients on LFP generation, it is essential to account for neuronal morphology and specific ion-channel gradients. Importantly, these ion-channel gradients should be quantitatively matched with signature somato-dendritic functional maps of that specific neuron (Narayanan and Johnston, 2012; Rathour and Narayanan, 2014; Dhupia et al., 2015). For instance, in assessing the impact of HCN-channel gradients on theta-frequency LFPs, (Sinha and Narayanan, 2015) matched functional maps on local resonance, transfer resonance, and input resistance across the somatodendritic axis before employing the model for computing LFP. (Sinha and Narayanan, 2015) found that the impact of HCN channel gradient on theta-frequency LFP was location-dependent, with changes in LFP phase. Without setting ion-channel gradients and conductances to match electrophysiologically determined functional maps and other physiological properties, the outcomes of such analyses will result in conclusions that overestimate or underestimate the role of a specific ion channel (and its gradients) on the LFP. As LFP is an outcome of distance-dependent summation of different ionic currents emanating from different locations, it is essential that the gradients in all ion channel properties are carefully matched with respective experimental outcomes and the specific patterns of synaptic activity (Linden et al., 2011; Leski et al., 2013) are accounted for in assessing the impact of active dendritic gradients on LFP.
Apart from intraneuronal gradients in ion channel properties, there are also systematic gradients in ion channel expression and intrinsic properties across neurons of the same subtype in different parts of the same brain region. For instance, there are gradients in CA1 pyramidal neuron ion-channel expression and intrinsic properties along the dorso-ventral, proximodistal and deep-superficial axes of the hippocampus (Igarashi et al., 2014; Lee et al., 2014; Malik et al., 2016; Maroso et al., 2016; Malik and Johnston, 2017; Sun et al., 2017; Valero and de la Prida, 2018; Cembrowski and Spruston, 2019; Navas-Olive et al., 2020). An important lacuna in the field pertains to the evaluation of the impact of such inter-neuronal gradients in somato-dendritic properties on LFPs for different patterns of synaptic activation. A systematic electrophysiological and computational evaluation spanning each of these distinct anatomical axes, including the somatodendritic axis of neurons in each subregion, is essential for understanding the impact of intra-neuronal and interneuronal gradients in active dendritic properties on the location-dependence of LFP emergence.
Finally, even within a given subregion, neurons of the same subtype are not identical in terms of their ion channel distributions or their intrinsic properties. Instead, there is considerable cell-to-cell heterogeneity in ion channels and cellular properties even within the same cell types of the same brain region (Rathour and Narayanan, 2014; Malik et al., 2016; Rathour and Narayanan, 2019; Mishra and Narayanan, 2020; Pastoll et al., 2020). In addition, there is animal-to-animal heterogeneity in how gradients manifest along a specified anatomical axis (Pastoll et al., 2020). It is essential that these heterogeneities are not ignored by assuming networks to be composed of homogenous neuronal populations, but are explicitly characterized from specific brain regions and are accounted for in experimental analyses and computational simulations. Without such explicit incorporation, the differential ionic contributions from different neurons, despite them receiving identical input patterns of activity (Mishra and Narayanan, 2021) will be neglected.
Degeneracy in the emergence of characteristic neuronal properties and LFPs
It is now well established that neuronal systems such as single neurons and networks of neurons can perform their characteristic functions despite heterogeneities in underlying system parameters and hence can be quite robust to perturbations (Prinz et al., 2004; Marder, 2011; Marder and Taylor, 2011; Rathour and Narayanan, 2012, 2014; Drion et al., 2015; Mishra and Narayanan, 2019; Rathour and Narayanan, 2019; Goaillard and Marder, 2021). This is an example of degeneracy in the biological context where degeneracy is described as the ability of structurally disparate elements to perform the same function (Tononi et al., 1999; Edelman and Gally, 2001; Stelling et al., 2004; Rathour and Narayanan, 2019). While neurons of the same kind (e.g., pyramidal neurons) manifest characteristic functional outcomes, these neurons are typically morphologically heterogeneous with intricate dendritic arbors. Within these delicate arbors, lies another layer of heterogeneity: the diverse expression profiles of intrinsic properties such as ion channels and receptors. Depending on their location, two dendritic branches even of similar length can express completely different sets of ion channels or their expression profiles in terms of their density could be different. There could even be different subunits (main or auxiliary) of the same ion channel expressed at different locations. On top of that, depending on their location in the morphological tree, dendritic branches can receive completely distinct synaptic inputs, leading to a differential expression of and response from synaptic and extra-synaptic receptors. Furthermore, if the study involves looking at the mechanisms underlying changes in LFPs due to incoming synaptic inputs, there exists the additional involvement of not only activity-dependent synaptic plasticity but also potentially concurrent intrinsic and structural plasticity. Thus, it is highly important that any computational study that is trying to model LFPs for gaining mechanistic insights takes care of introducing the appropriate level of abstraction and heterogeneity to incorporate the underlying variability in the system. This will help avoid incorrectly attributing a single cause for an extracellular phenomenon when there could be multiple underlying causes in a complex neural system generating the LFP.
Finally, whereas these analyses point to the expression of parametric degeneracy in the emergence of single-neuron and network functions, there are strong lines of evidence for the manifestation of degeneracy at the level of LFP rhythmogenesis, whereby LFP rhythms can be generated through disparate mechanisms (Rathour and Narayanan, 2019). It is critical that these disparate routes towards generating signature LFP patterns and rhythms are identified, employing experimental and computational techniques, to exercise extreme caution in making one-to-one relationships between underlying parameters and specific characteristics of LFP recordings (Rathour and Narayanan, 2019).
Accounting for gradients, heterogeneities and degeneracy in models of LFP
Multi-Parametric, Multi-Objective Stochastic Search Algorithms
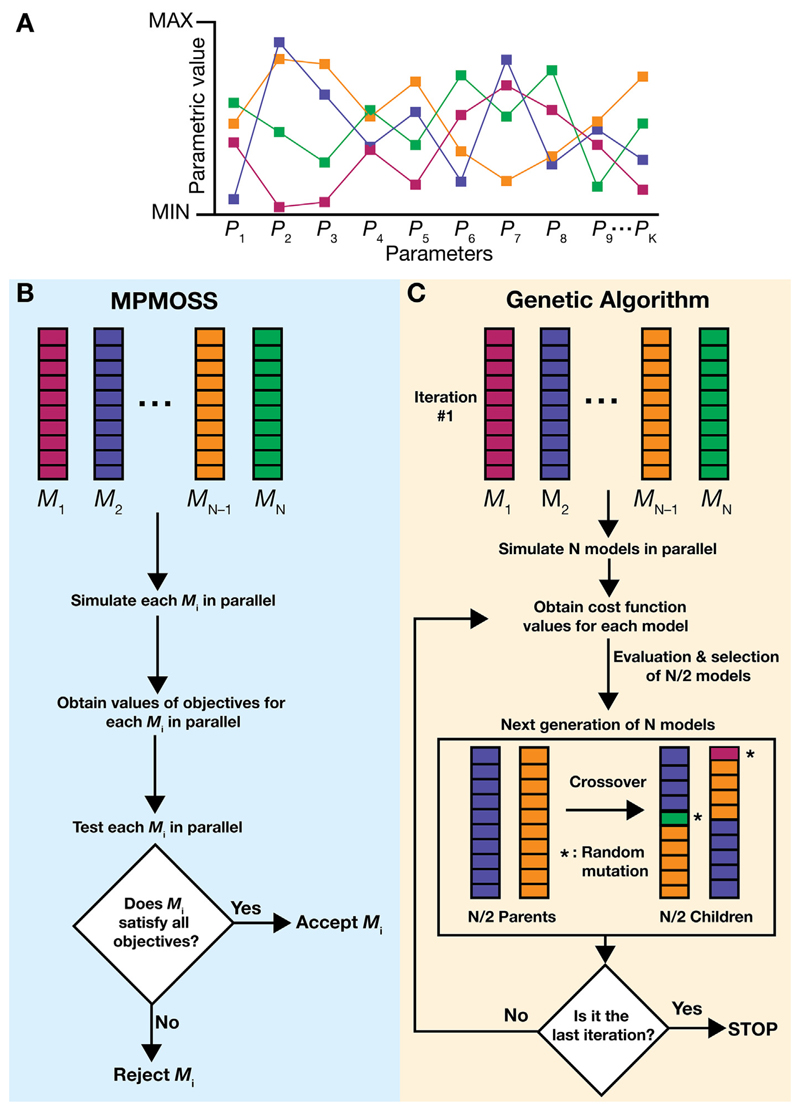
A method that is commonly employed to generate biophysically and physiologically realistic neuron models that account for heterogeneities and degeneracy is the multi-parametric, multi-objective, stochastic search (MPMOSS) (Foster et al., 1993; Taylor et al., 2009; Marder and Taylor, 2011; Rathour and Narayanan, 2012, 2014; Basak and Narayanan, 2018; Mittal and Narayanan, 2018; Mishra and Narayanan, 2019; Jain and Narayanan, 2020; Seenivasan and Narayanan, 2020). The generic algorithm for a specific morphology for a given subtype of neuron in a given subregion involves the following steps (Fig. 3):
Multiple Parameters (MP): Identify the crucial parameters of the neuron. Ideally, these parameters span all passive and active properties of the neuron under consideration, because physiology emerges as a consequence of intricate interactions among all these properties. However, this could also be a subset of parameters, depending on the specific question in hand. The crucial parameters span intrinsic passive (e.g., axial resistance, the leak channel conductance and the specific membrane capacitance, and their location dependent gradients), and active (e.g., conductance, gating properties, (de)activation/inactivation kinetics, reversal potential and location-dependent gradients) properties.
Define the ranges of these parameters from physiological observations for the specific neuronal subtype. Do not employ average or other summary statistics. Consider the range of individual parameters to encompass the entire span of experimental measurements from individual cells, thereby accounting for cell-to-cell variability.
Multiple Objectives (MO): Define the set of objectives that this neuronal model must fulfill based on experimental measurements. For our example, these could be constraints on the resting membrane potential, the input resistance, and the firing rate response to a specific stimulus for this neuronal type. Define lower and upper bounds for each of these measurements from experiments on the specific neuronal subtype. Note that we are not employing summary statistics here as well, but are accounting for the heterogeneities in characteristic neural measurements in that specific region.
- Stochastic Search (SS):
- For each parameter defined in step 1, randomly pick one value within its specified range defined in step 2. This set of parametric values will then be used to simulate one model neuron.
- Perform step 4a multiple times to generate unique combinations of parametric values and thus generate a large number of model neurons.
- Search for valid models: Perform the search by testing each of these models for their validity by assessing whether they satisfy the multiple objectives in Step 3 or not. Declare models that have all their measurements within the bounds defined in Step 3 to be valid models.
Fig. 3. Accounting for heterogeneity and degeneracy in computational models.
(A) Parameter values (Pi) are picked randomly from their respective experimental ranges. A set of parameters constitutes a model/individual, Mi. (B) MPMOSS algorithm. (C) Genetic algorithm (GA). Note that while MPMOSS is a highly parallelized algorithm, GA is by design iterative and spans several generations. There are several variants of the generic GA depicted here, with slight modifications to each step suited for different purposes.
Genetic Algorithms
In a comparative survey of automated parameter-search methods for compartmental neural models, genetic algorithms (GA) outperformed other methods for both simple and complex multicompartmental models (Vanier and Bower, 1999). GA are iterative optimization algorithms inspired from mechanisms of Darwinian evolution. In these, each morphologically realistic multicompartmental model neuron is considered as an individual from a genetically diverse population. Various intrinsic, synaptic, and morphological parameters define an individual (Fig. 3). In the first iteration, a random population is usually generated.
To search for optimal parameters (solutions) for a population of such individuals, first target functions are defined based on experimental data. These could be distance-dependent/independent intrinsic properties such as input resistance, resting membrane potential, resonance frequency, and axial resistance and/or synaptic properties such as excitatory/inhibitory postsynaptic potentials. The values of these target functions are computed for each individual. Then errors (square of difference) are computed between the experimentally and computationally obtained target function values for each individual. These errors help define the fitness of the individuals, towards discerning whether they lie within the experimentally observed variability or not. All individuals are sorted based on their cost function (such as the sum of all the errors of a given individual).
From this list, pairs of individuals are randomly chosen and the individuals with the lower cost function (i.e., they are closer to the experimental observations) are selected for breeding the next generation (iteration). Pairs of these selected individuals act as parents and undergo a crossover (exchange of parameters) to produce pairs of children with genetic diversity. These children may also undergo random mutations (probabilistic changes in the current values of parameters by a small amount that diminishes with each iteration).
This process of evaluation, selection, breeding/ crossover, mutation and new solution generation is continued until either a set bound on the number of iterations has been reached or if a certain criterion for population fitness (such as optimal matches between experimental results and model performance) has been achieved. Several variants of this algorithm have been successfully used in generating a diverse set of model neurons that match experimental observations, and in some cases have been released as open-source software as well (Keren et al., 2005; Druckmann et al., 2007; Menon et al., 2009; Bahl et al., 2012; Friedrich et al., 2014; Van Geit et al., 2016; Neymotin et al., 2017; Gouwens et al., 2018; Navas-Olive et al., 2020).
Analyzing and Utilizing the Valid Model Population
As all models (obtained either through MPMOSS or GA) that are valid manifest signature physiological properties, with biophysically matched parametric ranges, this model population can then be employed to assess parametric dependencies in the single-neuron population (Foster et al., 1993; Taylor et al., 2009; Marder and Taylor, 2011; Rathour and Narayanan, 2012, 2014; Basak and Narayanan, 2018; Mittal and Narayanan, 2018; Mishra and Narayanan, 2019; Jain and Narayanan, 2020; Navas-Olive et al., 2020; Seenivasan and Narayanan, 2020) or to construct heterogeneous network models that are representative of the specific brain region (Prinz et al., 2004; Mishra and Narayanan, 2019, 2021). The same algorithm must be repeated for different morphologies when accounting for heterogeneities in morphologies in the same neuronal subtype (Basak and Narayanan, 2020; Navas-Olive et al., 2020). NeuroMorpho is a great database of neuronal morphologies (Ascoli et al., 2007) and the Allen Cell Types Database (Gouwens et al., 2019) is a very useful database which provides not only neuronal morphologies, with characterization of electro-physiological properties and gene expression in individual neurons, but also a variety of models at different levels of resolution.
The MPMOSS and GA algorithms could be effectively employed for imposing signature intraneuronal functional maps on distinct neuronal morphologies and signature neuronal responses to different patterns of afferent inputs (Rathour and Narayanan, 2014; Basak and Narayanan, 2018, 2020). In addition, these algorithms could be employed to study inter-neuronal gradients (e.g., dorso-ventral), by altering the parametric and objective/target distributions employed in the algorithm to experimentally match specific subregions. Importantly, independent MPMOSS or GA simulations could be performed to generate distinct populations of models for each of the different neuronal subtypes in a given brain region (e.g., principal neurons and interneurons), to explicitly account for characteristic ion-channel properties and intrinsic measurements of each different subtype (Rathour and Narayanan, 2012, 2014; Mittal and Narayanan, 2018; Mishra and Narayanan, 2019; Jain and Narayanan, 2020). Finally, as these algorithms yield models with disparate parametric combinations for matching signature functional outcomes (constituting the manifestation of degeneracy) in any given subregion, they also provide the substrate for accounting for cell-to-cell heterogeneity in network models (Prinz et al., 2004; Mishra and Narayanan, 2019; Navas-Olive et al., 2020; Mishra and Narayanan, 2021). Thus, employing these algorithms for generating a population of models for different subregions constitutes an ideal way to construct heterogeneous networks that account for intra- and inter-neuronal gradients and heterogeneities in ion-channel and intrinsic properties of neuronal subtypes. Such physiologically matched heterogeneous networks should then be employed to assess the impact of active dendritic contributions to LFPs under different synaptic activation patterns.
Within this framework, the impact of individual ion channel subtypes on LFPs could be efficiently analyzed employing the virtual knockout framework (Rathour and Narayanan, 2014; Anirudhan and Narayanan, 2015; Sinha and Narayanan, 2015; Mukunda and Narayanan, 2017; Basak and Narayanan, 2018, 2020; Jain and Narayanan, 2020; Mishra and Narayanan, 2021). In implementing this, the conductance of individual ion channel subtypes in each of the several heterogeneous neurons (of a selected subtype) in the network is set to zero. The LFPs are computed for the two networks, one where the specific ion channel subtype is intact and another where the respective conductance values are set to zero for all neurons of the same subtype, with identical synaptic inputs. Comparison of LFPs in the two networks now provides an estimate of the specific contributions of an ion channel subtype on LFPs (e.g., (Sinha and Narayanan, 2015) for HCN-channel contribution to theta-frequency LFPs). This process could then be repeated for each of the different ion channel subtypes in the different neuronal subtypes to assess their contributions to LFPs at different locations. Together, the MPMOSS and GA algorithms are powerful tools for assessing active dendritic contributions to LFP, while accounting for all physiological constraints including heterogeneities, gradients and degeneracy.
Neuromodulation, Plasticity and Neurological Disorders
The contributions of active dendritic conductances to LFPs are not static, but change in response to neuromodulation, short- and long-term plasticity and pathological insults.
Neuromodulatory impact on active dendrites and LFP
Neuromodulation offers an ideal substrate for reconfiguring functional connectivity in neural circuits towards achieving behavioral context-dependent processing of sensory stimuli (Getting, 1989; Marder and Thirumalai, 2002; Lee and Dan, 2012; Marder, 2012; Bargmann and Marder, 2013; Marder et al., 2014; McCormick et al., 2020). Neuromodulators typically act through receptors expressed on the cellular surface. They can alter intrinsic properties and synaptic efficacy either directly or through activation of downstream signaling cascades. Within an active dendritic framework, such neuromodulatory action translates to changes in the transmembrane currents through dendritic ion channels and receptors (Hoffman and Johnston, 1999; Heckman et al., 2003; Rosenkranz and Johnston, 2006, 2007; Dembrow et al., 2010; Dembrow and Johnston, 2014; Santello and Nevian, 2015; Labarrera et al., 2018; Payeur et al., 2019; Williams and Fletcher, 2019; Yaeger et al., 2019), thereby pointing to a direct neuro-modulatory impact on recorded LFPs. Thus, it is critical that experimental analyses and computational frame-works on LFPs account for neuromodulatory reconfiguration of neural circuits and state-dependent changes to constitutive components of all cell types. Future experimental and computational studies should systematically map the location-dependent impact of different neuromodulatory inputs, impinging on disparate neural circuits with distinct spatiotemporal patterns, on the emergence of LFPs.
Short- and long-term plasticity of intrinsic and synaptic properties
The brain is a highly plastic organ. The continual changes in brain structure and function span all of its components, with a wide variety of time scales over which such changes occur. Whereas changes involving short-term synaptic plasticity (Zucker and Regehr, 2002; Regehr, 2012) and conformational dynamics of molecules, including ion channels and receptors (Johnston and Wu, 1995; Dingledine et al., 1999; Hille, 2001), are on the faster time scale, there are other forms of plasticity that evolve over slower time scales (Kim and Linden, 2007; Gjorgjieva et al., 2016; Turrigiano, 2017; Zenke et al., 2017; Ma et al., 2019). These changes imply that the transmembrane currents through active dendritic receptors and channels would be subjected to long- and short-term changes. For instance, for the same burst of presynaptic activation, a facilitating synapse would manifest progressively increasing postsynaptic responses whereas a depressing synapse would yield progressively reduced responses. Thus, it is important to not treat synapses as linear time invariant structures (Tsodyks and Markram, 1997). Importantly, there are gradients in short-term plasticity profiles across the somato-dendritic axis (Jensen et al., 2021), implying that the profile of transmembrane currents and dendritic voltage responses are critically reliant on the specific short-term profile at individual dendritic locations. Similarly, there are established gradients in ion channel kinetics that regulate different aspects of dendritic physiology. For instance, in CA1 pyramidal neurons, the recovery rate of somatic sodium channels from inactivation is faster for somatic sodium channels compared to their dendritic counterparts. These kinetic differences translate to differences in the amplitudes of dendritic backpropagating action potentials within a train of action potentials (Colbert et al., 1997). These observations imply that the transmembrane currents through the several voltage-gated ion channels along the dendritic arbor respond differentially in response to the same backpropagating action potentials, depending on their dendritic location. Thus, short-term plasticity of synaptic and intrinsic properties, along with somato-dendritic gradients in these plasticity profiles should be systematically accounted for in analyzing the LFP profiles and their dependencies on active dendritic structures.
Long-term forms of plasticity that are involved in learning, memory and stability also span all components of active dendritic structures (Frick and Johnston, 2005; Magee and Johnston, 2005; Kim and Linden, 2007; Johnston and Narayanan, 2008; Narayanan and Johnston, 2012). In addition, synaptic and dendritic intrinsic properties could be altered in the short- and long-term by transmitter and messenger molecules released by the dendrites as well as adjacent glia (Regehr et al., 2009; Araque et al., 2014; Ashhad and Narayanan, 2016; Maroso et al., 2016; Ashhad and Narayanan, 2019). It should be noted that although these forms of plasticity might specifically target one specific ion channel or receptor, the consequent changes in transmembrane currents would span a larger swath of molecules owing to structural or functional interactions. For instance, an increase in the density of a synaptic receptor molecule would alter the postsynaptic voltage deflection, which in turn would change the voltage-dependent opening and driving forces of all ion channels and receptors that are affected by this voltage deflection. Thus, in assessing the impact of behaviorally induced changes on LFPs, changes in all transmembrane currents introduced by these disparate forms of plasticity should be systematically accounted for in a location-dependent manner for the different cells in the circuit. In assessing the impact of bidirectional long-term plasticity in HCN channels (Fan et al., 2005; Brager and Johnston, 2007; Narayanan and Johnston, 2007) on theta-frequency LFPs in hippocampal CA1, (Sinha and Narayanan, 2015) first quantitatively matched cellular plasticity in physiological measurements across the dendritic arbor. Specifically, (Sinha and Narayanan, 2015) matched global changes in input resistance and resonance frequency obtained from electrophysiological recordings (Fan et al., 2005; Brager and Johnston, 2007; Narayanan and Johnston, 2007) for bidirectional HCN-channel plasticity. Employing these models that underwent biophysically realistic plasticity, (Sinha and Narayanan, 2015) showed that graded changes in either HCN conductance or its half-maximal activation voltage resulted in graded changes in phases of theta-frequency LFP and associated spike (Fig. 2D).
Neurological disorders, active dendrites, and LFP
Pathological conditions alter active dendritic properties. Ion channel dysfunction, referred to as channelopathy, is associated with several neurological disorders. Careful experimentation assessing ion channels involved in dendritic information processing has revealed dramatic pathological changes in their composition and densities (Kullmann, 2002; Bernard et al., 2004; Kullmann and Waxman, 2010; Remy et al., 2010; Terzic and Perez-Terzic, 2010; Emoto, 2011; Poolos and Johnston, 2012; Lerche et al., 2013; Brager and Johnston, 2014; Zhang et al., 2014; Johnston et al., 2016; Cook et al., 2021). These changes have been shown to be cell-type dependent, with the same ion channel subtype undergoing changes in opposite directions in different cell types with reference to the same pathological condition (Lee et al., 2011; Brager et al., 2012; Lee and Jan, 2012; Routh et al., 2013; Brager and Johnston, 2014; Zhang et al., 2014; Kalmbach et al., 2015; Routh et al., 2017; Siegel et al., 2017; Brandalise et al., 2020). In addition to channelopathies, neurological disorders alter several aspects of brain structure and function, including connectivity patterns, dendritic morphology and glial physiology (Schauwecker and McNeill, 1996; Maragakis and Rothstein, 2006; McEwen, 2007; Sofroniew and Vinters, 2010; Chattarji et al., 2015; Coulter and Steinhauser, 2015; Khakh and Sofroniew, 2015; Phatnani and Maniatis, 2015; Patel et al., 2019). As active dendritic function, and consequently the transmembrane currents across the different ion channels and receptors are critically regulated by each of these distinct parameters, it is essential that assessment of the impact of active dendrites on LFPs account for synergistic interactions across all these pathological changes. Importantly, such analyses should account for the cell-type and location-dependent changes observed in ion channels, receptors, and connectivity from other cell types.
Computational Methods for LFP Modeling
The mechanisms underlying the origins of LFPs are complex and remain open to exploration (Buzsaki et al., 2012; Einevoll et al., 2013). They involve specific spatiotemporal structures of synaptic inputs, which could be either from long-range afferents or from local synapses. These inputs lead to intra-/inter-cellular spatiotemporal responses from and interactions among intrinsic properties of neuronal dendrites and soma. These in turn generate transmembrane currents that ultimately shape the LFP. Furthermore, these synaptic inputs and intrinsic properties vary across different brain regions depending on varying cell morphologies and topographical arrangements (Buzsaki et al., 2012; Einevoll et al., 2013). In addition to such interregional differences, there are significant heterogeneities in neural properties in any given brain region, which can also undergo changes through neuromodulation, plasticity, or pathological conditions. The fundamental question on the specific contributions of the plethora of active dendritic conductances to LFPs, and how they change in the face of all the gradients, heterogeneities, and dynamics (highlighted in the previous sections) continues to remain largely unexplored. In the face of such complexity, biophysically rooted theoretical and computational modeling tools have become invaluable for uncovering the mechanisms underlying the generation of various forms of LFP – fast vs. slow, rhythmic vs. irregular, unitary vs. population, synchronous vs. asynchronous across multiple locations, highly localized vs. travelling, etc. These tools are also useful for explaining new findings, and in delineating the relative contributions of different cellular and network elements to field potentials (Rall, 1962; Einevoll et al., 2013; Pesaran et al., 2018; Einevoll et al., 2019; Poirazi and Papoutsi, 2020). To highlight these tools, in the following subsections we review the 3 most commonly used approximation methods that are used in computational studies for modeling LFPs. We also point to various free and open-source resources that are available for those researchers who are interested in dabbling with LFP modeling.
Point source approximation
In this approximation method, the extracellular potential generated from a neuronal compartment is approximated to originate from a point source (Holt, 1998; Holt and Koch, 1999; Gold et al., 2006). The transmembrane currents are assumed to originate from a point in the center of a neuronal compartment (Fig. 4). Transmembrane currents are recorded from such point sources for each compartment across the three-dimensional space. For n point sources (indexed by i) at a distance r from the recording location with macroscopic conductivity σ (i.e. the whole neuropil is modeled as a homogeneous conductor), the estimated field potential (ΦFP) is calculated from the transmembrane current (I) as:
Fig. 4. Approaches to account for active dendrites in LFP computation.
Center: A single electrode with multiple recording sites in the center of a cylindrical neuropil of a population of morphologically realistic pyramidal neurons. Note, while a single morphology is depicted here for simplicity, a heterogeneous set of morphologies should be employed to account for the underlying structural heterogeneity. Top Left: Point source approximation for modeling LFP. Bottom Right: Line source approximation for modeling LFP. Top Right: A representation of conductance-based mutli-compartmental model depicting how to account for location-dependent expression of passive leak, active (voltage-gated) ionic, and synaptic conductances. Bottom Left: A representation of a single compartment within the morphology and the transmembrane currents generated through it due to the presence of active and passive conductances. These transmembrane currents are used for computing LFPs. Symbols used: r: radial distance; σ: macroscopic extracellular conductivity; l,h: longitudinal distance from the beginning and end of the line respectively; Δs: length of line segment; Vm: intracellular membrane potential; Im: transmembrane current; x/y: location variables; Ra: axial resistance; g: conductance (subscript represents the type of conductance); E: reversal potential (subscript represents the type of conductance); C: capacitance; d: distance; Ve: extracellular potential.
Line source approximation
Line source approximation (LSA) is one of the most-commonly used methods for forward modeling of LFPs. In this approach, the extracellular potential generated from a neuronal compartment is approximated to originate from a continuous distribution of the transmembrane currents generated from a line that passes through the central axis of the compartment (Fig. 4). Except for distances less than 1 μm away from the recording location (Rosenfalck, 1969; Trayanova et al., 1990; Gold et al., 2006), LSA implementations are known to closely approximate the extracellular signal, and have been employed and validated for hippocampal pyramidal neurons (Gold et al., 2006). For n line sources (indexed by i) of length Δs, the field potential (ΦFP) is computed as a linear sum of the individual field potentials due to each line source as:
where h is the longitudinal distance from the end of the line, l = Δs + h is the distance from the beginning of the line.
There are some important considerations that need to be accounted for while modeling and analyzing LFPs (Buzsaki et al., 2012; Einevoll et al., 2013; Pesaran et al., 2018). First, several models assume homogeneous and isotropic extracellular conductivity, and therefore consider a constant value for σ (typically 0.3–0.4 S/m) in the equations for ΦFP above. However, there are lines of evidence for inhomogeneities in extracellular conductivity (Lopez-Aguado et al., 2001; Goto et al., 2010), which can be accounted for in modeling and understanding LFPs (Gold et al., 2006; Pettersen et al., 2006; Einevoll et al., 2007). Second, the use of σ for the conversion from currents to field potentials implicitly assumes that the neuropil is bereft of frequency-dependent reactances. There have been several studies assessing frequency-dependence of the extracellular space, both experimentally and computationally, with debates about the manifestion and the origins of frequency dependence (Bedard et al., 2004; Logothetis et al., 2007; Bedard et al., 2010; Gomes et al., 2016; Barbour, 2017; Bedard and Destexhe, 2017; Miceli et al., 2017; Pesaran et al., 2018). The broad consensus seems to be that the extracellular medium is largely resistive for the physiological range of signal frequencies (Pesaran et al., 2018). Finally, frequency-dependent features of LFPs should account for intrinsic dendritic membrane filtering and for correlation structures in synaptic inputs (Pettersen and Einevoll, 2008; Linden et al., 2011; Leski et al., 2013). Importantly, membrane filtering should not be assumed to be low-pass in nature (accounting only for the passive time constant), but should also account for location-dependent band-pass filters in neurons that manifest gradients in resonating conductances such as HCN channels (Narayanan and Johnston, 2007; Hu et al., 2009; Kalmbach et al., 2013; Sinha and Narayanan, 2015; Ness et al., 2016, 2018).
Computing a proxy for LFP from point neuronal models
Several studies have used a linear summation of spiking dynamics of a neuronal network as a proxy for its LFP (Hagen et al., 2016; Telenczuk et al., 2020a; Martinez-Canada et al., 2021). A study showed that a specific linear combination of the synaptic currents from leaky integrate-and-fire (LIF) model neurons also provides a reasonable proxy for the LFP (Mazzoni et al., 2015). The spikes from LIF model neurons have also been used to stimulate bio-physically realistic neuron models in a hybrid scheme of LFP generation (Hagen et al., 2016; Skaar et al., 2020). It has also been suggested that the spikes of individual point neurons arranged in space can be convolved with their uLFPs and the linear summation of these uLFPs could be used to provide an imprecise but quick estimation of LFPs from an interconnected network of point neurons (Hagen et al., 2016; Telenczuk et al., 2020b). However, when it comes to understanding the mechanisms of LFP generation, such point neuron or single compartmental models may not fully account for the intricate spatiotemporal dynamics of synaptic inputs on the morphological details of dendrites. Having said that, there are neurons that are structurally and electrotonically compact, with the soma being the main site for synaptic inputs with very few dendritic arbors, such as those in the suprachiasmatic nucleus that is crucial for the maintenance of a 24 h circadian rhythm (Welsh et al., 2010; Harvey et al., 2020). For such neurons, the spiking activity of a network of point neurons could potentially be used as good estimate of the LFP as long as the point neurons are biophysically realistic and account for the diverse set of ion channels and receptors that these neurons express (Welsh et al., 2010; Harvey et al., 2020).
Resources for modeling LFP
While the computational complexity for simulating LFPs is quite high, there are resources available to quickly get initiated. Extracellular Action Potential (EAP) simulations (Holt and Koch, 1999; Gold et al., 2006; Gold et al., 2007) written for NEURON® (Hines and Carnevale, 1997; Carnevale and Hines, 2006) and MATLAB® are available on ModelDB (Hines et al., 2004). While the original code was written to simulate EAPs, it can be easily adapted to model other extracellular phenomena. There are also several relatively new open-source libraries/software packages available to specifically model neuronal networks and LFPs: LFPsim (Parasuram et al., 2016), LFPy (Linden et al., 2014; Hagen et al., 2018), BioNet (Gratiy et al., 2018), NetPyNE (Dura-Bernal et al., 2019), Human Neocortical Neurosolver (HNN) (Neymotin et al., 2020) and Brain Modeling ToolKit (BMTK) (Dai et al., 2020).
Coda and Future Directions
While spikes inarguably provide immense information about neurophysiology, not all events in the brain are above the spiking threshold of a neuron, especially in the dendrites and astrocytes. In addition, based on the ongoing activity and adaptation, the threshold itself can shift. Therefore, due credit must be given to dendritic subthreshold activity because many neuronal conductances, including ion channels, transporters, and receptors, are active in this subthreshold regime. They contribute to membrane potential dynamics and extracellular field potentials in both health and disease. Further, the brain is highly plastic, with heterogeneity and degeneracy emerging to be common phenomena across scales. Thus, the notion of assigning one-to-one functional mappings between mechanisms and their outcomes should be dealt with extreme caution and wherever applicable be replaced with that of many-to-one or even many-to-many mappings through rigorous computational and experimental analyses. Finally, computational studies should adopt a level of abstraction that is appropriate to answer the question at hand. If a model is being used to obtain a quick proxy for the LFP, point neuron network models with linear approximations may be used. But if the goal is to obtain mechanistic insights about the origins of LFPs, the model should incorporate both linear and non-linear biophysical details, not just at the soma but also in the dendrites, and across all cell types.
Future directions for computational models: inverse meets forward to provide mechanistic insights
The first requirement for future computational models is to recognize the complexity underlying the emergence of LFPs, and not oversimplify LFP computations to be merely reflective of synaptic inputs. It is critical that insights about the complex computational capabilities of active dendritic structures, gained through electrophysiological experiments and computational models over the past few decades (Johnston et al., 1996; Segev and London, 2000; Migliore and Shepherd, 2002; Johnston et al., 2003; Johnston and Narayanan, 2008; Major et al., 2013; Grienberger et al., 2015; Stuart and Spruston, 2015; Payeur et al., 2019; Poirazi and Papoutsi, 2020), find their rightful place in defining network physiology and LFPs. Specifically, it is essential that theoretical frameworks and computational models incorporate the complexity of neurons endowed with active dendritic structures, explicitly accounting for the different sub- and supra-threshold conductances, their inter- and intra-neuronal gradients, different forms of modulation and plasticity, the ubiquitous heterogeneities spanning all neural components, the spatio-temporal interactions between different active dendritic components, and how they together drive network computations and LFPs. Given the expanding roles of glial cells in brain physiology (Araque et al., 2014; Ashhad and Narayanan, 2019; Santello et al., 2019; Kol and Goshen, 2020; Nagai et al., 2021), it is equally important to account for glial contributions to network computation and LFPs. To gain a holistic understanding of the origins and roles of LFPs, we recommend the amalgamation of inverse and forward models.
Inverse model
Using some preliminary knowledge about which groups of neurons are present in the circuit under investigation and their anatomical connectivity recurrent neural networks (RNNs), initially using random connectivity matrices, can be trained to match the experimentally obtained LFP data recorded from model animals and humans. Using the predictions from these RNNs mechanistic insights about the anatomical and functional connectivity between the neuronal circuit elements and their dynamics under different behavioral paradigms could be obtained (Rajan et al., 2016; Barak, 2017; Vu et al., 2018; Skaar et al., 2020). Further, where anatomical data is not available forward models can employ the connectivity insights obtained from these RNNs. We envision that such RNNs and other machine learning algorithms will prove to be useful for understanding the role and cellular/network origins of LFPs recorded from human healthy subjects and patients under different behavioral conditions, especially since molecular mechanistic insights may be difficult to obtain in these cases.
Forward model
There are several large-scale models of brain tissue and neuronal databases already available that can prove to be great resources for modeling LFP (Hines et al., 2004; Ascoli et al., 2007; Markram et al., 2015; Bezaire et al., 2016; Arkhipov et al., 2018; Gouwens et al., 2019; Billeh et al., 2020). Using morphologically and biophysically realistic models based on experimentally known intrinsic properties of the neurons and glial cells and their connectivity patterns, transmembrane currents could be computed under different input conditions to generate LFPs and gain insights about the collective roles of intrinsic/synaptic/morphological properties and spatio-temporal dynamics of inputs. Further virtually knocking-out individual intrinsic and synaptic mechanisms, introducing intrinsic/synaptic plasticity, synaptic scaling, and dendritic remodeling could help delineate their specific roles in the generation of LFPs under physiological and pathological conditions. From these insights, testable hypotheses can be obtained and experimentally verified using the extensive set of tools available for neuroscience research (Rivnay et al., 2017; Mendrela et al., 2018; Kim et al., 2020; Moreaux et al., 2020; Nectow and Nestler, 2020; Pal and Tian, 2020; Sabatini and Tian, 2020).
Finally, we strongly urge experimental and computational neuroscientists to collaborate extensively. Through such collaborations, elegant models may be obtained to understand the function of active dendrites and LFPs, and eventually their role in mediating behavior.
Abbreviations
- BMTK
brain modeling toolkit
- dCaAP
dendritic calcium-mediated action potential
- EAP
extracellular action potential
- ECoG
electrocorticogram
- EEG
electroencephalogram
- EPSP
excitatory post-synaptic potential
- ER
endoplasmic reticulum
- GA
genetic algorithm
- GABA
gamma-aminobutyric acid
- GC
granule cell
- HCN channels
hyperpolarization-activated cyclic nucleotide-gated cation non-specific channels
- HNN
human neocortical neurosolver
- InsP3R
inositol triphosphate receptors
- LFP
local field potential
- LSA
line source approximation
- MPMOSS
multi-parametric multi-objective stochastic search
- NMDA
N-methyl D-aspartate
- NREM
non rapid eye movement
- nRt
nucleus reticularis thalami
- PFC
prefrontal cortex
- REM
rapid eye movement
- RNN
recurrent neural network
- SEP
slow excitatory potential
- SERCA
sarco/endoplasmic reticulum Ca2+- ATPase
- SPW-R
sharp wave ripple complex
- STA-LFP
spike-triggered averaged local field potential
- TC
thalamocortical
- uLFP
unitary local field potential.
Acknowledgements
The authors thank members of the cellular neurophysiology laboratory for helpful comments on a draft version of this manuscript. The authors acknowledge funding support from the Wellcome Trust-DBT India Alliance (Senior fellowship to RN; IA/ S/16/2/502727), the Human Frontier Science Program (HFSP) Organization (RN), the Department of Biotechnology through the DBT-IISc partnership program (RN), the Revati & Satya Nadham Atluri Chair at IISc (RN), Microsoft Research India PhD Fellowship Award (MS), and the Ministry of Human Resource Development (MS and RN).
References
- Anastassiou CA, Koch C. Ephaptic coupling to endogenous electric field activity: why bother? Curr Opin Neurobiol. 2015;31:95–103. doi: 10.1016/j.conb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Anastassiou CA, Perin R, Buzsaki G, Markram H, Koch C. Celltype- and activity-dependent extracellular correlates of intracellular spiking. J Neurophysiol. 2015;114:608–623. doi: 10.1152/jn.00628.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anirudhan A, Narayanan R. Analogous synaptic plasticity profiles emerge from disparate channel combinations. J Neurosci. 2015;35:4691–4705. doi: 10.1523/JNEUROSCI.4223-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Schonauer M, Staresina BP, Cairney SA. Sleep spindles and memory reprocessing. Trends Neurosci. 2019;42:1–3. doi: 10.1016/j.tins.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipov A, et al. Visual physiology of the layer 4 cortical circuit in silico. PLoS Comput Biol. 2018;14:e1006535. doi: 10.1371/journal.pcbi.1006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Giraud AL. Cortical oscillations and sensory predictions. Trends Cogn Sci. 2012;16:390–398. doi: 10.1016/j.tics.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Aru J, Suzuki M, Larkum ME. Cellular mechanisms of conscious processing. Trends Cogn Sci. 2020;24:814–825. doi: 10.1016/j.tics.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci. 2007;27:9247–9251. doi: 10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhad S, Narayanan R. Quantitative interactions between the A-type K+ current and inositol trisphosphate receptors regulate intraneuronal Ca2+ waves and synaptic plasticity. J Physiol. 2013;591:1645–1669. doi: 10.1113/jphysiol.2012.245688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhad S, Narayanan R. Active dendrites regulate the impact of gliotransmission on rat hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2016;113:E3280–3289. doi: 10.1073/pnas.1522180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhad S, Narayanan R. Stores, channels, glue, and trees: active glial and active dendritic physiology. Mol Neurobiol. 2019;56:2278–2299. doi: 10.1007/s12035-018-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Luthi A. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M. Mechanisms of epileptiform synchronization in cortical neuronal networks. Curr Med Chem. 2014;21:653–662. doi: 10.2174/0929867320666131119151136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG)and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–482. doi: 10.1016/0013-4694(87)90093-9. [DOI] [PubMed] [Google Scholar]
- Bahl A, Stemmler MB, Herz AV, Roth A. Automated optimization of a reduced layer 5 pyramidal cell model based on experimental data. J Neurosci Methods. 2012;210:22–34. doi: 10.1016/j.jneumeth.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Barak O. Recurrent neural networks as versatile tools of neuroscience research. Curr Opin Neurobiol. 2017;46:1–6. doi: 10.1016/j.conb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Barbour B. Analysis of claims that the brain extracellular impedance is high and non-resistive. Biophys J. 2017;113:1636–1638. doi: 10.1016/j.bpj.2017.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Basak R, Narayanan R. Spatially dispersed synapses yield sharply-tuned place cell responses through dendritic spike initiation. J Physiol. 2018;596:4173–4205. doi: 10.1113/JP275310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak R, Narayanan R. Robust emergence of sharply tuned place-cell responses in hippocampal neurons with structural and biophysical heterogeneities. Brain Struct Funct. 2020;225:567–590. doi: 10.1007/s00429-019-02018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Bazelot M, Dinocourt C, Cohen I, Miles R. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J Physiol. 2010;588:2077–2090. doi: 10.1113/jphysiol.2009.185918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard C, Destexhe A. Is the extracellular impedance high and non-resistive in cerebral cortex? Biophys J. 2017;113:1639–1642. doi: 10.1016/j.bpj.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard C, Kroger H, Destexhe A. Modeling extracellular field potentials and the frequency-filtering properties of extracellular space. Biophys J. 2004;86:1829–1842. doi: 10.1016/S0006-3495(04)74250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard C, Rodrigues S, Roy N, Contreras D, Destexhe A. Evidence for frequency-dependent extracellular impedance from the transfer function between extracellular and intracellular potentials: intracellular-LFP transfer function. J Comput Neurosci. 2010;29:389–403. doi: 10.1007/s10827-010-0250-7. [DOI] [PubMed] [Google Scholar]
- Beining M, Mongiat LA, Schwarzacher SW, Cuntz H, Jedlicka P. T2N as a new tool for robust electrophysiological modeling demonstratedformatureandadult-borndentategranulecells. eLife. 2017;6 doi: 10.7554/eLife.26517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliaeva V, Savvateev I, Zerbi V, Polania R. Toward integrative approaches to study the causal role of neural oscillations via transcranial electrical stimulation. Nat Commun. 2021;12:2243. doi: 10.1038/s41467-021-22468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Benson H, Malhotra MS, Goldman RF, Jacobs GD, Hopkins PJ. Three case reports of the metabolic and electroencephalographic changes during advanced Buddhist meditation techniques. Behav Med. 1990;16:90–95. doi: 10.1080/08964289.1990.9934596. [DOI] [PubMed] [Google Scholar]
- Bereshpolova Y, Stoelzel CR, Gusev AG, Bezdudnaya T, Swadlow HA. The impact of a corticotectal impulse on the awake superior colliculus. J Neurosci. 2006;26:2250–2259. doi: 10.1523/JNEUROSCI.4402-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Uber das elektrenkephalogramm des menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Bernard C. Dogma and dreams: experimental lessons for epilepsy mechanism chasers. Cell Mol Life Sci. 2005;62:1177–1181. doi: 10.1007/s00018-005-5009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bezaire MJ, Raikov I, Burk K, Vyas D, Soltesz I. Interneuronal mechanisms of hippocampal theta oscillations in a full-scale model of the rodent CA1 circuit. eLife. 2016;5 doi: 10.7554/eLife.18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri KW, Bobbitt KN, Colgin LL. Slow and fast gamma rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron. 2014;82:670–681. doi: 10.1016/j.neuron.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeh YN, Cai B, Gratiy SL, Dai K, Iyer R, Gouwens NW, Abbasi-Asl R, Jia X, Siegle JH, Olsen SR, Koch C, et al. Systematic integration of structural and functional data into multi-scale models of mouse primary visual cortex. Neuron. 2020;106:388–403.:e318. doi: 10.1016/j.neuron.2020.01.040. [DOI] [PubMed] [Google Scholar]
- Bittner KC, Milstein AD, Grienberger C, Romani S, Magee JC. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science. 2017;357:1033–1036. doi: 10.1126/science.aan3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18:1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Copara MS, Gotman J, Ekstrom AD. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat Commun. 2017;8:14415. doi: 10.1038/ncomms14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnan A, Rowan MMJ, Baker CA, Bolton MM, Christie JM. Autonomous Purkinje cell activation instructs bidirectional motor learning through evoked dendritic calcium signaling. Nat Commun. 2021;12:2153. doi: 10.1038/s41467-021-22405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352:812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bozelos P, Stefanou SS, Bouloukakis G, Melachrinos C, Poirazi P. REMOD: A tool for analyzing and remodeling the dendritic architecture of neural cells. Front Neuroanat. 2015;9:156. doi: 10.3389/fnana.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Channelopathies and dendritic dysfunction in fragile X syndrome. Brain Res Bull. 2014;103C:11–17. doi: 10.1016/j.brainresbull.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell reports. 2012;1:225–233. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandalise F, Kalmbach BE, Mehta P, Thornton O, Johnston D, Zemelman BV, Brager DH. Fragile X mental retardation protein bidirectionally controls dendritic Ih in a cell type-specific manner between mouse hippocampus and prefrontal cortex. J Neurosci. 2020;40:5327–5340. doi: 10.1523/JNEUROSCI.1670-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Xing D, Shapley RM. Is gamma-band activity in the local field potential of V1 cortex a “clock” or filtered noise? J Neurosci. 2011;31:9658–9664. doi: 10.1523/JNEUROSCI.0660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cerebral cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Buzsaki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Peyrache A. A BOLD statement about the hippocampal-neocortical dialogue. Trends Cogn Sci. 2013;17:57–59. doi: 10.1016/j.tics.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci. 2015a;18:484–489. doi: 10.1038/nn.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci. 2015b doi: 10.1038/nn.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Stark E, Berenyi A, Khodagholy D, Kipke DR, Yoon E, Wise KD. Tools for probing local circuits: high-density silicon probes combined with optogenetics. Neuron. 2015;86:92–105. doi: 10.1016/j.neuron.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RC, O’Donnell C, Nolan MF. Stochastic ion channel gating in dendritic neurons: morphology dependence and probabilistic synaptic activation of dendritic spikes. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale TN, Hines LM. The NEURON book. Cambridge University Press; United Kingdom: 2006. [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton R. The electric currents of the brain. BMJ. 1875;2 [Google Scholar]
- Caton R. Interim report on investigation of the electric currents of the brain. Br Med J. 1877;1(Suppl):62. [Google Scholar]
- Cea C, Spyropoulos GD, Jastrzebska-Perfect P, Ferrero JJ, Gelinas JN, Khodagholy D. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat Mater. 2020;19:679–686. doi: 10.1038/s41563-020-0638-3. [DOI] [PubMed] [Google Scholar]
- Cei A, Girardeau G, Drieu C, Kanbi KE, Zugaro M. Reversed theta sequences of hippocampal cell assemblies during backward travel. Nat Neurosci. 2014;17:719–724. doi: 10.1038/nn.3698. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Spruston N. Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat Rev Neurosci. 2019;20:193–204. doi: 10.1038/s41583-019-0125-5. [DOI] [PubMed] [Google Scholar]
- Chang EF. Towards large-scale, human-based, mesoscopic neurotechnologies. Neuron. 2015;86:68–78. doi: 10.1016/j.neuron.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18:1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Chee SS, Menard JL, Dringenberg HC. The lateral septum as a regulator of hippocampal theta oscillations and defensive behavior in rats. J Neurophysiol. 2015;113:1831–1841. doi: 10.1152/jn.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini M, Popplau JA, Lindemann C, Carol-Perdiguer L, Hnida M, Oberlander V, Xu X, Ahlbeck J, Bitzenhofer SH, Mulert C, Hanganu-Opatz IL. Resolving and rescuing developmental miswiring in a mouse model of cognitive impairment. Neuron. 2020;105:60–74.:e67. doi: 10.1016/j.neuron.2019.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen A, Fine E, Zayachkivska O. Adolf Beck: a forgotten pioneer in electroencephalography. J History Neurosci. 2014;23:276–286. doi: 10.1080/0964704X.2013.867600. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Slow gamma takes the reins in replay. Neuron. 2012;75:549–550. doi: 10.1016/j.neuron.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Do slow and fast gamma rhythms correspond to distinct functional states in the hippocampal network? Brain Res. 2015a;1621:309–315. doi: 10.1016/j.brainres.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Theta-gamma coupling in the entorhinal-hippocampal system. Curr Opin Neurobiol. 2015b;31:45–50. doi: 10.1016/j.conb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17:239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology. 2010;25:319–329. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Collura TF. History and evolution of electroencephalographic instruments and techniques. J Clin Neurophysiol. 1993;10:476–504. doi: 10.1097/00004691-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013;33:9394–9401. doi: 10.1523/JNEUROSCI.0533-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AA, Fields E, Watt AJ. Losing the beat: contribution of purkinje cell firing dysfunction to disease, and its reversal. Neuroscience. 2021;462:247–261. doi: 10.1016/j.neuroscience.2020.06.008. [DOI] [PubMed] [Google Scholar]
- Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Steinhauser C. Role of astrocytes in epilepsy. Cold Spring Harbor Perspect Med. 2015;5:a022434. doi: 10.1101/cshperspect.a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Ruber T, Staresina BP, Fell J. Sharp wave-ripples in human amygdala and their coordination with hippocampus during NREM sleep. Cereb Cortex Commun. 2020;1:tgaa051. doi: 10.1093/texcom/tgaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–190. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Luthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- Cuntz H, Forstner F, Borst A, Hausser M. One rule to grow them all: a general theory of neuronal branching and its practical application. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Gratiy SL, Billeh YN, Xu R, Cai B, Cain N, Rimehaug AE, Stasik AJ, Einevoll GT, Mihalas S, Koch C, Arkhipov A. Brain Modeling ToolKit: An open source software suite for multiscale modeling of brain circuits. PLoS Comput Biol. 2020;16:e1008386. doi: 10.1371/journal.pcbi.1008386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Narayanan R. Active dendrites regulate spectral selectivity in location-dependent spike initiation dynamics of hippocampal model neurons. J Neurosci. 2014;34:1195–1211. doi: 10.1523/JNEUROSCI.3203-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Narayanan R. Active dendrites mediate stratified gamma-range coincidence detection in hippocampal model neurons. J Physiol. 2015;593:3549–3576. doi: 10.1113/JP270688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Narayanan R. Theta-frequency selectivity in the somatic spike triggered average of rat hippocampal pyramidal neurons is dependent on HCN channels. J Neurophysiol. 2017;118:2251–2266. doi: 10.1152/jn.00356.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Rathour RK, Narayanan R. Strings on a violin: location dependence of frequency tuning in active dendrites. Front Cell Neurosci. 2017;11:72. doi: 10.3389/fncel.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12:327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Audinat E, Charpak S. Action potential propagation in dendrites of rat mitral cells in vivo. J Neurosci. 2003;23:5553–5560. doi: 10.1523/JNEUROSCI.23-13-05553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow N, Johnston D. Subcircuit-specific neuromodulation in the prefrontal cortex. Front Neural Circuits. 2014;8:54. doi: 10.3389/fncir.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Roy JP, Steriade M. Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res. 1982;239:289–293. doi: 10.1016/0006-8993(82)90854-x. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Paradis M, Roy JP, Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984;51:1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Babloyantz A, Sejnowski TJ. Ionic mechanisms for intrinsic slow oscillations in thalamic relay neurons. Biophys J. 1993;65:1538–1552. doi: 10.1016/S0006-3495(93)81190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J Neurophysiol. 1996;76:2049–2070. doi: 10.1152/jn.1996.76.3.2049. [DOI] [PubMed] [Google Scholar]
- Dhupia N, Rathour RK, Narayanan R. Dendritic atrophy constricts functional maps in resonance and impedance properties of hippocampal model neurons. Front Cell Neurosci. 2015;8:456. doi: 10.3389/fncel.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus II. Mechanisms underlying origin and restriction. J Neurophysiol. 1969a;32:663–687. doi: 10.1152/jn.1969.32.5.663. [DOI] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I. Characteristics and topographical features. J Neurophysiol. 1969b;32:649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- Dickey CW, Sargsyan A, Madsen JR, Eskandar EN, Cash SS, Halgren E. Travelling spindles create necessary conditions for spike-timing-dependent plasticity in humans. Nat Commun. 2021;12:1027. doi: 10.1038/s41467-021-21298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol. 1986;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron G, Shin JN, Takahashi N, Druke M, Bocklisch C, Skenderi S, de Mont L, Toumazou M, Ledderose J, Brecht M, Naud R, et al. Perirhinal input to neocortical layer 1 controls learning. Science. 2020;370 doi: 10.1126/science.aaz3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drion G, O’Leary T, Marder E. Ion channel degeneracy enables robust and tunable neuronal firing rates. Proc Natl Acad Sci U S A. 2015;112:E5361–5370. doi: 10.1073/pnas.1516400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S, Banitt Y, Gidon A, Schurmann F, Markram H, Segev I. A novel multiple objective optimization framework for constraining conductance-based neuron models by experimental data. Front Neurosci. 2007;1:7–18. doi: 10.3389/neuro.01.1.1.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura-Bernal S, Suter BA, Gleeson P, Cantarelli M, Quintana A, Rodriguez F, Kedziora DJ, Chadderdon GL, Kerr CC, Neymotin SA, McDougal RA, et al. NetPyNE, a tool for data-driven multiscale modeling of brain circuits. eLife. 2019;8 doi: 10.7554/eLife.44494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci. 2013;14:770–785. doi: 10.1038/nrn3599. [DOI] [PubMed] [Google Scholar]
- Einevoll GT, Pettersen KH, Devor A, Ulbert I, Halgren E, Dale AM. Laminar population analysis: estimating firing rates and evoked synaptic activity from multielectrode recordings in rat barrel cortex. J Neurophysiol. 2007;97:2174–2190. doi: 10.1152/jn.00845.2006. [DOI] [PubMed] [Google Scholar]
- Einevoll GT, Destexhe A, Diesmann M, Grun S, Jirsa V, de Kamps M, Migliore M, Ness TV, Plesser HE, Schurmann F. The scientific case for brain simulations. Neuron. 2019;102:735–744. doi: 10.1016/j.neuron.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Emoto K. Dendrite remodeling in development and disease. Dev Growth Differ. 2011;53:277–286. doi: 10.1111/j.1440-169X.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsaki G. Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J Neurosci. 2014;34:16509–16517. doi: 10.1523/JNEUROSCI.2600-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Fernandez LMJ, Luthi A. Sleep Spindles: Mechanisms and Functions. Physiol Rev. 2020;100:805–868. doi: 10.1152/physrev.00042.2018. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berenyi A, Buzsaki G. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron. 2017;93:1213–1226.:e1215. doi: 10.1016/j.neuron.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz A, Oliva A, Fermino de Oliveira E, Rocha-Almeida F, Tingley D, Buzsaki G. Long-duration hippocampal sharp wave ripples improve memory. Science. 2019;364:1082–1086. doi: 10.1126/science.aax0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G, Buzsaki G. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science. 2021;372 doi: 10.1126/science.abf3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA. Functional impact of dendritic branch-point morphology. J Neurosci. 2013;33:2156–2165. doi: 10.1523/JNEUROSCI.3495-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Scharfman HE, deCurtis M. How can we identifyictal and interictal abnormal activity? Adv Exp Med Biol. 2014;813:3–23. doi: 10.1007/978-94-017-8914-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WR, Ungar LH, Schwaber JS. Significance of conductances in Hodgkin-Huxley models. J Neurophysiol. 1993;70:2502–2518. doi: 10.1152/jn.1993.70.6.2502. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Fricker D, Miles R. Interneurons, spike timing, and perception. Neuron. 2001;32:771–774. doi: 10.1016/s0896-6273(01)00528-1. [DOI] [PubMed] [Google Scholar]
- Friedrich P, Vella M, Gulyas AI, Freund TF, Kali S. A flexible, interactive software tool for fitting the parameters of neuronal models. Front Neuroinf. 2014;8:63. doi: 10.3389/fninf.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, Riddle J. Conducting double-blind placebo-controlled clinical trials of transcranial alternating current stimulation (tACS) Transl Psychiatry. 2021;11:284. doi: 10.1038/s41398-021-01391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Timofeev I, Steriade M. Prolonged hyperpolarizing potentials precede spindle oscillations in the thalamic reticular nucleus. Proc Natl Acad Sci U S A. 2004;101:9816–9821. doi: 10.1073/pnas.0402761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S, DiFrancesco D. Action of the hyperpolarization-activated current (Ih) blocker ZD 7288 in hippocampal CA1 neurons. Pflugers Arch. 1997;435:99–106. doi: 10.1007/s004240050488. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Migliore M, Magee JC. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J Neurosci. 2004;24:11046–11056. doi: 10.1523/JNEUROSCI.2520-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Gidon A, Zolnik TA, Fidzinski P, Bolduan F, Papoutsi A, Poirazi P, Holtkamp M, Vida I, Larkum ME. Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science. 2020;367:83–87. doi: 10.1126/science.aax6239. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science. 2007;315:1719–1722. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;21:452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Inema I, Buzsaki G. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat Neurosci. 2017;20:1634–1642. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gjorgjieva J, Drion G, Marder E. Computational implications of biophysical diversity and multiple timescales in neurons and synapses for circuit performance. Curr Opin Neurobiol. 2016;37:44–52. doi: 10.1016/j.conb.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. Hans Berger and the discovery of the electroencephalogram. Electroencephalogr Clin Neurophysiol Suppl. 1969a;28:21–36. [PubMed] [Google Scholar]
- Gloor P. The work of Hans Berger. Electroencephalogr Clin Neurophysiol. 1969b;27:649. doi: 10.1016/0013-4694(69)91207-3. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Marder E. Ion channel degeneracy, variability, and covariation in neuron and circuit resilience. Annu Rev Neurosci. 2021;44:335–357. doi: 10.1146/annurev-neuro-092920-121538. [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C. Using extracellular action potential recordings to constrain compartmental models. J Comput Neurosci. 2007;23:39–58. doi: 10.1007/s10827-006-0018-2. [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: a modeling study. J Neurophysiol. 2006;95:3113–3128. doi: 10.1152/jn.00979.2005. [DOI] [PubMed] [Google Scholar]
- Golding NL, Spruston N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron. 1998;21:1189–1200. doi: 10.1016/s0896-6273(00)80635-2. [DOI] [PubMed] [Google Scholar]
- Gomes JM, Bedard C, Valtcheva S, Nelson M, Khokhlova V, Pouget P, Venance L, Bal T, Destexhe A. Intracellular impedance measurements reveal non-ohmic properties of the extracellular medium around neurons. Biophys J. 2016;110:234–246. doi: 10.1016/j.bpj.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Hatanaka R, Ogawa T, Sumiyoshi A, Riera J, Kawashima R. An evaluation of the conductivity profile in the somatosensory barrel cortex of Wistar rats. J Neurophysiol. 2010;104:3388–3412. doi: 10.1152/jn.00122.2010. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Jackson J, Williams S. Self-generated theta oscillations in the hippocampus. Nat Neurosci. 2009;12:1491–1493. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- Gouwens NW, Berg J, Feng D, Sorensen SA, Zeng H, Hawrylycz MJ, Koch C, Arkhipov A. Systematicgenerationofbiophysicallydetailedmodelsfordiversecorticalneurontypes. Nat Commun. 2018;9:710. doi: 10.1038/s41467-017-02718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwens NW, et al. Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat Neurosci. 2019;22:1182–1195. doi: 10.1038/s41593-019-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Miller J, Qasim SE, Watrous AJ, Zhang H, Stein JM, Inman CS, Gross RE, Willie JT, Lega B, Lin JJ, et al. Functionally distinct high and low theta oscillations in the human hippocampus. Nat Commun. 2020;11:2469. doi: 10.1038/s41467-020-15670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratiy SL, Billeh YN, Dai K, Mitelut C, Feng D, Gouwens NW, Cain N, Koch C, Anastassiou CA, Arkhipov A. BioNet: A Python interface to NEURON for modeling large-scale networks. PLoS ONE. 2018;13:e0201630. doi: 10.1371/journal.pone.0201630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Chen X, Konnerth A. Dendritic function in vivo. Trends Neurosci. 2015;38:45–54. doi: 10.1016/j.tins.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O. alpha-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci U S A. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Barczak A, Musacchia G, Lipton ML, Mehta AD, Lakatos P, Schroeder CE. Laminar profile and physiology of the alpha rhythm in primary visual, auditory, and somatosensory regions of neocortex. J Neurosci. 2015;35:14341–14352. doi: 10.1523/JNEUROSCI.0600-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E, Naess S, Ness TV, Einevoll GT. Multimodal modeling of neural network activity: computing LFP, ECoG, EEG, and MEG signals with LFPy 2.0. Front Neuroinf. 2018;12:92. doi: 10.3389/fninf.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E, Fossum JC, Pettersen KH, Alonso JM, Swadlow HA, Einevoll GT. Focal local field potential signature of the single-axon monosynaptic thalamocortical connection. J Neurosci. 2017;37:5123–5143. doi: 10.1523/JNEUROSCI.2715-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E, Dahmen D, Stavrinou ML, Linden H, Tetzlaff T, van Albada SJ, Grun S, Diesmann M, Einevoll GT. Hybrid scheme for modeling local field potentials from point-neuron networks. Cereb Cortex. 2016;26:4461–4496. doi: 10.1093/cercor/bhw237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren M, Ulbert I, Bastuji H, Fabo D, Eross L, Rey M, Devinsky O, Doyle WK, Mak-McCully R, Halgren E, Wittner L, et al. The generation and propagation of the human alpha rhythm. Proc Natl Acad Sci U S A. 2019;116:23772–23782. doi: 10.1073/pnas.1913092116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Harvey JRM, Plante AE, Meredith AL. Ion channels controlling circadian rhythms in suprachiasmatic nucleus excitability. Physiol Rev. 2020;100:1415–1454. doi: 10.1152/physrev.00027.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?−Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Theta rhythm and the encoding and retrieval of space and time. NeuroImage. 2014;85(Pt 2):656–666. doi: 10.1016/j.neuroimage.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19:453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- Hausser M, Raman IM, Otis T, Smith SL, Nelson A, du Lac S, Loewenstein Y, Mahon S, Pennartz C, Cohen I, Yarom Y. The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J Neurosci. 2004;24:9215–9219. doi: 10.1523/JNEUROSCI.3375-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E, Hill S, Schurmann F, Markram H, Segev I. Models of neocortical layer 5b pyramidal cells capturing a wide range of dendritic and perisomatic active properties. PLoS Comput Biol. 2011;7:e1002107. doi: 10.1371/journal.pcbi.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014;112:1–23. doi: 10.1016/j.pneurobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci. 1999;2:989–996. doi: 10.1038/14788. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sinauer Associates, Inc. 2001 [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hines ML, Morse T, Migliore M, Carnevale NT, Shepherd GM. ModelDB: A database to support computational neuroscience. J Comput Neurosci. 2004;17:7–11. doi: 10.1023/B:JCNS.0000023869.22017.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol. 1999;81:408–411. doi: 10.1152/jn.1999.81.1.408. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holt GR. A critical reexamination of some assumptions and implications of cable theory in neurobiology. 1998. [Google Scholar]
- Holt GR, Koch C. Electrical interactions via the extracellular potential near cell bodies. J Comput Neurosci. 1999;6:169–184. doi: 10.1023/a:1008832702585. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Graham LJ, Storm JF. Complementary theta resonance filtering by two spatially segregated mechanisms in CA1 hippocampal pyramidal neurons. J Neurosci. 2009;29:14472–14483. doi: 10.1523/JNEUROSCI.0187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WA, Stitt IM, Negahbani E, Passey DJ, Ahn S, Davey M, Dannhauer M, Doan TT, Hoover AC, Peterchev AV, Radtke-Schuller S, et al. Transcranial alternating current stimulation entrains alpha oscillations by preferential phase synchronization of fast-spiking cortical neurons to stimulation waveform. Nat Commun. 2021;12:3151. doi: 10.1038/s41467-021-23021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Lorincz ML, Parri HR, Crunelli V. Infraslow (<0.1 Hz) oscillations in thalamic relay nuclei basic mechanisms and significance to health and disease states. Prog Brain Res. 2011;193:145–162. doi: 10.1016/B978-0-444-53839-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–222. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- Huxter JR, Senior TJ, Allen K, Csicsvari J. Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat Neurosci. 2008;11:587–594. doi: 10.1038/nn.2106. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Ito HT, Moser EI, Moser MB. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 2014;588:2470–2476. doi: 10.1016/j.febslet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol. 1984a;122:73–82. [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984b;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984c;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Narayanan R. Degeneracy in the emergence of spike-triggered average of hippocampal pyramidal neurons. Sci Rep. 2020;10:374. doi: 10.1038/s41598-019-57243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska-Perfect P, Spyropoulos GD, Cea C, Zhao Z, Rauhala OJ, Viswanathan A, Sheth SA, Gelinas JN, Khodagholy D. Mixed-conducting particulate composites for soft electronics. Sci Adv. 2020;6:eaaz6767. doi: 10.1126/sciadv.aaz6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M, VanRullen R. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn Sci. 2012;16:200–206. doi: 10.1016/j.tics.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen TP, Kopach O, Reynolds JP, Savtchenko LP, Rusakov DA. Release probability increases towards distal dendrites boosting high-frequency signal transfer in the rodent hippocampus. eLife. 2021;10 doi: 10.7554/eLife.62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM. Foundations of cellular neurophysiology. The MIT Press; Cambridge, Massachusetts: 1995. [Google Scholar]
- Johnston D, Narayanan R. Active dendrites: colorful wings of the mysterious butterflies. Trends Neurosci. 2008;31:309–316. doi: 10.1016/j.tins.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Johnston D, Frick A, Poolos N. In: Dendrites. 3 Edition. Stuart G, Spruston N, Hausser M, editors. Oxford University Press; New York, NY: 2016. Dendrites and disease. [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B BiolSci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun NY, Cardin JA. Activation of distinct channelrhodopsin variants engages different patterns of network activity. eNeuro. 2020;7 doi: 10.1523/ENEURO.0222-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc Natl Acad Sci U S A. 2013;110:13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Johnston D, Brager DH. Cell-type specific channelopathies in the prefrontal cortex of the fmr1-/y mouse model of Fragile X Syndrome(1,2,3) eNeuro. 2015;2 doi: 10.1523/ENEURO.0114-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Chitwood RA, Dembrow NC, Johnston D. Dendritic generation of mGluR-mediated slow afterdepolarizationin layer 5 neurons of prefrontal cortex. J Neurosci. 2013;33:13518–13532. doi: 10.1523/JNEUROSCI.2018-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Buzsaki G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci. 1998a;18:3919–3928. doi: 10.1523/JNEUROSCI.18-10-03919.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998b;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- Karimi Abadchi J, Nazari-Ahangarkolaee M, Gattas S, Bermudez-Contreras E, Luczak A, McNaughton BL, Mohajerani MH. Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. eLife. 2020;9 doi: 10.7554/eLife.51972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Olfactory system oscillations across phyla. Curr Opin Neurobiol. 2015;31:141–147. doi: 10.1016/j.conb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis S, Miller K, Thomson K, Brown R, House P, Greger B. Decoding spoken words using local field potentials recorded from the cortical surface. J Neural Eng. 2010;7:056007. doi: 10.1088/1741-2560/7/5/056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren N, Peled N, Korngreen A. Constraining compartmental models using multiple voltage recordings and genetic algorithms. J Neurophysiol. 2005;94:3730–3742. doi: 10.1152/jn.00408.2005. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Carmena JM. Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. eLife. 2017;6 doi: 10.7554/eLife.24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Zhao Z, Yeh M, Long M, Greenlee JD, Doyle W, Devinsky O, Buzsaki G. Organic electronics for high-resolution electrocorticography of the human brain. Sci Adv. 2016;2:e1601027. doi: 10.1126/sciadv.1601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholy D, Rivnay J, Sessolo M, Gurfinkel M, Leleux P, Jimison LH, Stavrinidou E, Herve T, Sanaur S, Owens RM, Malliaras GG. High transconductance organic electrochemical transistors. Nat Commun. 2013;4:2133. doi: 10.1038/ncomms3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18:222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gulati T, Ganguly K. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell. 2019;179:514–526.:e513. doi: 10.1016/j.cell.2019.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Voroslakos M, Seymour JP, Wise KD, Buzsaki G, Yoon E. Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes. Nat Commun. 2020;11:2063. doi: 10.1038/s41467-020-15769-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Hausser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J Neurosci. 2011;31:10847–10858. doi: 10.1523/JNEUROSCI.2525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelman LA, Lowery MM. Beta-band resonance and intrinsic oscillations in a biophysically detailed model of the subthalamic nucleus-globus pallidus network. Front Comput Neurosci. 2019;13:77. doi: 10.3389/fncom.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene RA, Tijms B, van Hees P, Postma F, de Ridder A, Ramakers GJ, van Pelt J, van Ooyen A. NETMORPH: a framework for the stochastic generation of large scale neuronal networks with realistic neuron morphologies. Neuroinformatics. 2009;7:195–210. doi: 10.1007/s12021-009-9052-3. [DOI] [PubMed] [Google Scholar]
- Kohling R, Staley K. Network mechanisms for fast ripple activity in epileptic tissue. Epilepsy Res. 2011;97:318–323. doi: 10.1016/j.eplepsyres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Goshen I. The memory orchestra: the role of astrocytes and oligodendrocytes in parallel to neurons. Curr Opin Neurobiol. 2020;67:131–137. doi: 10.1016/j.conb.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichmar JL, Nasuto SJ, Scorcioni R, Washington SD, Ascoli GA. Effects of dendritic morphology on CA3 pyramidal cell electrophysiology: a simulation study. Brain Res. 2002;941:11–28. doi: 10.1016/s0006-8993(02)02488-5. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Gelinas JN, Soltesz I, Buzsaki G. Neuroelectronics and biooptics: closed-loop technologies in neurological disorders. JAMA Neurol. 2015;72:823–829. doi: 10.1001/jamaneurol.2015.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. The neuronal channelopathies. Brain. 2002;125:1177–1195. doi: 10.1093/brain/awf130. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Waxman SG. Neurological channelopathies: new insights into disease mechanisms and ion channel function. J Physiol. 2010;588:1823–1827. doi: 10.1113/jphysiol.2010.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen PT, Ashida G, Kraemer A, McColgan T, Funabiki K, Wagner H, Koppl C, Carr CE, Kempter R. Contribution of action potentials to the extracellular field potential in the nucleus laminaris of barn owl. J Neurophysiol. 2018;119:1422–1436. doi: 10.1152/jn.00175.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarrera C, Deitcher Y, Dudai A, Weiner B, Kaduri Amichai A, Zylbermann N, London M. Adrenergic modulation regulates the dendritic excitability of layer 5 pyramidal neurons in vivo. Cell Rep. 2018;23:1034–1044. doi: 10.1016/j.celrep.2018.03.103. [DOI] [PubMed] [Google Scholar]
- Larkum M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36:141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2002;22:6991–7005. doi: 10.1523/JNEUROSCI.22-16-06991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012;490:397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- Lee HY, Jan LY. Fragile X syndrome: mechanistic insights and therapeutic avenues regarding the role of potassium channels. Curr Opin Neurobiol. 2012;22:887–894. doi: 10.1016/j.conb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Song K, Lee K, Hong J, Lee H, Chae S, Cheong E, Shin HS. Sleep spindles are generated in the absence of T-type calcium channel-mediated low-threshold burst firing of thalamocortical neurons. Proc Natl Acad Sci U S A. 2013;110:20266–20271. doi: 10.1073/pnas.1320572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez G, Lledo PM. Odor discrimination requires proper olfactory fast oscillations in awake mice. Neuron. 2013;80:1010–1024. doi: 10.1016/j.neuron.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Lerche H, Shah M, Beck H, Noebels J, Johnston D, Vincent A. Ion channels in genetic and acquired forms of epilepsy. J Physiol. 2013;591:753–764. doi: 10.1113/jphysiol.2012.240606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leski S, Linden H, Tetzlaff T, Pettersen KH, Einevoll GT. Frequency dependence of signal power and spatial reach of the local field potential. PLoS Comput Biol. 2013;9:e1003137. doi: 10.1371/journal.pcbi.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS. Theta rhythm during REM sleep and waking: correlations between power, phase and frequency. Electroencephalogr Clin Neurophysiol. 1984;58:553–564. doi: 10.1016/0013-4694(84)90045-2. [DOI] [PubMed] [Google Scholar]
- Levesque M, Avoli M. High-frequency oscillations and focal seizures in epileptic rodents. Neurobiol Dis. 2019;124:396–407. doi: 10.1016/j.nbd.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Levesque M, Ragsdale D, Avoli M. Evolving mechanistic concepts of epileptiform synchronization and their relevance in curing focal epileptic disorders. Curr Neuropharmacol. 2019;17:830–842. doi: 10.2174/1570159X17666181127124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, Hagen E, Leski S, Norheim ES, Pettersen KH, Einevoll GT. LFPy: a tool for biophysical simulation of extracellular potentials generated by detailed model neurons. Front Neuroinf. 2014;7:41. doi: 10.3389/fninf.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, Tetzlaff T, Potjans TC, Pettersen KH, Grun S, Diesmann M, Einevoll GT. Modeling the spatial reach of the LFP. Neuron. 2011;72:859–872. doi: 10.1016/j.neuron.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017;20:1434–1447. doi: 10.1038/nn.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron. 2007;55:809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Lopez-Aguado L, Ibarz JM, Herreras O. Activity-dependent changes of tissue resistivity in the CA1 region in vivo are layer specific: modulation of evoked potentials. Neuroscience. 2001;108:249–262. doi: 10.1016/s0306-4522(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. 2012;15(423–430):S421–423. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Lansner A. Theta and gamma power increases and alpha/beta power decreases with memory load in an attractor network model. J Cogn Neurosci. 2011;23:3008–3020. doi: 10.1162/jocn_a_00029. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Warden MR, Brincat SL, Miller EK. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat Commun. 2018;9:394. doi: 10.1038/s41467-017-02791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. Gamma and beta bursts underlie working memory. Neuron. 2016;90:152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998a;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron. 1998b;20:553–563. doi: 10.1016/s0896-6273(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Lytton WW. Computer modelling of epilepsy. Nat Rev Neurosci. 2008;9:626–637. doi: 10.1038/nrn2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton WW, Orman R, Stewart M. Computer simulation of epilepsy: implications for seizure spread and behavioral dysfunction. Epilepsy Behav. 2005;7:336–344. doi: 10.1016/j.yebeh.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Turrigiano GG, Wessel R, Hengen KB. Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron. 2019;104:655–664.:e654. doi: 10.1016/j.neuron.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol. 2018;52:182–187. doi: 10.1016/j.conb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadar O, Roig JA, Monti JM, Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav. 1970;5:1443–1449. doi: 10.1016/0031-9384(70)90134-4. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:848. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr Opin Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Magee JC, Grienberger C. Synaptic plasticity forms and functions. Annu Rev Neurosci. 2020;43:95–117. doi: 10.1146/annurev-neuro-090919-022842. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19:959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- Major G, Larkum ME, Schiller J. Active properties of neocortical pyramidal neuron dendrites. Annu Rev Neurosci. 2013;36:1–24. doi: 10.1146/annurev-neuro-062111-150343. [DOI] [PubMed] [Google Scholar]
- Major G, Polsky A, Denk W, Schiller J, Tank DW. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J Neurophysiol. 2008;99:2584–2601. doi: 10.1152/jn.00011.2008. [DOI] [PubMed] [Google Scholar]
- Mak-McCully RA, Rolland M, Sargsyan A, Gonzalez C, Magnin M, Chauvel P, Rey M, Bastuji H, Halgren E. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat Commun. 2017;8:15499. doi: 10.1038/ncomms15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Johnston D. Dendritic GIRK channels gate the integration window, plateau potentials, and induction of synaptic plasticity in dorsal but not ventral CA1 neurons. J Neurosci. 2017;37:3940–3955. doi: 10.1523/JNEUROSCI.2784-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Dougherty KA, Parikh K, Byrne C, Johnston D. Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis. Hippocampus. 2016;26:341–361. doi: 10.1002/hipo.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manita S, Suzuki T, Homma C, Matsumoto T, Odagawa M, Yamada K, Ota K, Matsubara C, Inutsuka A, Sato M, Ohkura M, et al. A top-down cortical circuit for accurate sensory perception. Neuron. 2015;86:1304–1316. doi: 10.1016/j.neuron.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Marder E. Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci U S A. 2011;108(Suppl3):15542–15548. doi: 10.1073/pnas.1010674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Marder E, Taylor AL. Multiple models to capture the variability in biological neurons and networks. Nat Neurosci. 2011;14:133–138. doi: 10.1038/nn.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- Markram H, et al. Reconstruction and simulation of neocortical microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Ravel N. Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks? Front Behav Neurosci. 2014;8:218. doi: 10.3389/fnbeh.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Canada P, Ness TV, Einevoll GT, Fellin T, Panzeri S. Computation of the electroencephalogram (EEG) from networkmodels of point neurons. PLoS Comput Biol. 2021;17:e1008893. doi: 10.1371/journal.pcbi.1008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Linden H, Cuntz H, Lansner A, Panzeri S, Einevoll GT. Computing the local field potential (LFP) from integrate-and-fire network models. PLoS Comput Biol. 2015;11:e1004584. doi: 10.1371/journal.pcbi.1004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol. 1992a;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neuro transmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992b;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Curr Opin Neurobiol. 2015;31:133–140. doi: 10.1016/j.conb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Nestvogel DB, He BJ. Neuromodulation of brain state and behavior. Annu Rev Neurosci. 2020;43:391–415. doi: 10.1146/annurev-neuro-100219-105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Huszar R, English DF, Kim K, Christensen F, Yoon E, Buzsaki G. Preexisting hippocampal network dynamics constrain optogenetically induced place fields. Neuron. 2021;109:1040–1054.:e1047. doi: 10.1016/j.neuron.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrela AE, Kim K, English D, McKenzie S, Seymour JP, Buzsaki G, Yoon E. A high-resolution opto-electrophysiology system with a miniature integrated headstage. IEEE Trans Biomed Circuits Syst. 2018 doi: 10.1109/TBCAS.2018.2852267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Spruston N, Kath WL. A state-mutating genetic algorithm to design ion-channel models. Proc Natl Acad Sci U S A. 2009;106:16829–16834. doi: 10.1073/pnas.0903766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli S, Ness TV, Einevoll GT, Schubert D. Impedance spectrum in cortical tissue: implications for propagation of LFP signals on the microscopic level. eNeuro. 2017;4 doi: 10.1523/ENEURO.0291-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Shepherd GM. Emerging rules for the distributions of active dendritic conductances. Nat Rev Neurosci. 2002;3:362–370. doi: 10.1038/nrn810. [DOI] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci. 1999;7:5–15. doi: 10.1023/a:1008906225285. [DOI] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3940–3951. doi: 10.1523/JNEUROSCI.5314-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Narayanan R. Disparate forms of heterogeneitie sand interactions among them drive channel decorrelation in the dentate gyrus: degeneracy and dominance. Hippocampus. 2019;29:378–403. doi: 10.1002/hipo.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Narayanan R. Heterogeneities in intrinsic excitability and frequency-dependent response properties of granule cells across the blades of the rat dentate gyrus. J Neurophysiol. 2020;123:755–772. doi: 10.1152/jn.00443.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Narayanan R. Ion-channel regulation of response decorrelation in a heterogeneous multi-scale model of the dentate gyrus. Curr Res Neurobiol. 2021;2 doi: 10.1016/j.crneur.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci. 1982;2:292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Narayanan R. Degeneracy in the robust expression of spectral selectivity, subthreshold oscillations and intrinsic excitability of entorhinal stellate cells. J Neurophysiol. 2018;120:576–600. doi: 10.1152/jn.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JJ, Ravassard PM, Ho D, Acharya L, Kees AL, Vuong C, Mehta MR. Dynamics of cortical dendritic membrane potential and spikes in freely behaving rats. Science. 2017;355 doi: 10.1126/science.aaj1497. [DOI] [PubMed] [Google Scholar]
- Moreaux LC, Yatsenko D, Sacher WD, Choi J, Lee C, Kubat NJ, Cotton RJ, Boyden ES, Lin MZ, Tian L, Tolias AS, et al. Integrated neurophotonics:toward dense volumetric interrogation of brain circuit activity-atdepth and in real time. Neuron. 2020;108:66–92. doi: 10.1016/j.neuron.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukunda CL, Narayanan R. Degeneracy in the regulation of short-term plasticity and synaptic filtering by presynaptic mechanisms. J Physiol. 2017;595:2611–2637. doi: 10.1113/JP273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Petrou S, Berkovic SF. Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol. 2003;16:171–176. doi: 10.1097/01.wco.0000063767.15877.c7. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol. 1996;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Yu X, Papouin T, Cheong E, Freeman MR, Monk KR, Hastings MH, Haydon PG, Rowitch D, Shaham S, Khakh BS, et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron. 2021;109:576–596. doi: 10.1016/j.neuron.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rathippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. The h channel mediates location dependence and plasticity of intrinsic phase response in rathippocampal neurons. J Neurosci. 2008;28:5846–5860. doi: 10.1523/JNEUROSCI.0835-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Chattarji S. Computational analysis of the impact of chronic stress on intrinsic and synaptic excitability in the hippocampus. J Neurophysiol. 2010;103:3070–3083. doi: 10.1152/jn.00913.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Functional maps within a single neuron. J Neurophysiol. 2012;108:2343–2351. doi: 10.1152/jn.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Narayan A, Chattarji S. A probabilistic framework for region-specific remodeling of dendrites in three dimensional neuronal reconstructions. Neural Comput. 2005;17:75–96. doi: 10.1162/0899766052530811. [DOI] [PubMed] [Google Scholar]
- Navas-Olive A, Valero M, Jurado-Parras T, de Salas-Quiroga A, Averkin RG, Gambino G, Cid E, de la Prida LM. Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations. Nat Commun. 2020;11:2217. doi: 10.1038/s41467-020-15840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nectow AR, Nestler EJ. Viral tools for neuroscience. Nat Rev Neurosci. 2020;21:669–681. doi: 10.1038/s41583-020-00382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TV, Remme MWH, Einevoll GT. Active subthreshold dendritic conductances shape the local field potential. J Physiol-London. 2016;594:3809–3825. doi: 10.1113/JP272022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TV, Remme MWH, Einevoll GT. h-Type membrane current shapes the local field potential from populations of pyramidal neurons. J Neurosci. 2018;38:6011–6024. doi: 10.1523/JNEUROSCI.3278-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- Neymotin SA, Suter BA, Dura-Bernal S, Shepherd GM, Migliore M, Lytton WW. Optimizing computer models of corticospinal neurons to replicate in vitro dynamics. J Neurophysiol. 2017;117:148–162. doi: 10.1152/jn.00570.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neymotin SA, Daniels DS, Caldwell B, McDougal RA, Carnevale NT, Jas M, Moore CI, Hines ML, Hamalainen M, Jones SR. Human Neocortical Neurosolver (HNN), a new software tool for interpreting the cellular and network origin of human MEG/EEG data. eLife. 2020;9 doi: 10.7554/eLife.51214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Shuler M. Thalamocortical and corticocortical interactions in the somatosensory system. Prog Brain Res. 2001;130:90–110. [PubMed] [Google Scholar]
- Nikolic L, Nobili P, Shen W, Audinat E. Role of astrocyte purinergic signaling in epilepsy. Glia. 2020;68:1677–1691. doi: 10.1002/glia.23747. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Differential subcellular distribution of ion channels and the diversity of neuronal function. Curr Opin Neurobiol. 2012;22:366–371. doi: 10.1016/j.conb.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86:2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus: as a cognitive map. Oxford University Press; 1978. [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Theta activity, virtual navigation and the human hippocampus. Trends Cogn Sci. 1999;3:403–406. doi: 10.1016/s1364-6613(99)01396-0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus. 2005;15:853–866. doi: 10.1002/hipo.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353:1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Schwindt PC, Crill WE. Dendritic calcium spikes in layer 5 pyramidal neurons amplify and limit transmission of ligandgated dendritic current to soma. J Neurophysiol. 2001;86:514–527. doi: 10.1152/jn.2001.86.1.514. [DOI] [PubMed] [Google Scholar]
- Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci. 2010;30:4440–4448. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernandez-Ruiz A, Fermino de Oliveira E, Buzsaki G. Origin of gamma frequency power during hippocampal sharpwave ripples. Cell Rep. 2018;25:1693–1700.:e1694. doi: 10.1016/j.celrep.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernandez-Ruiz A, Leroy F, Siegelbaum SA. Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature. 2020;587:264–269. doi: 10.1038/s41586-020-2758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinski BL, Kay LM. Granule cell excitability regulates gamma and beta oscillations in a model of the olfactory bulb dendrodendritic microcircuit. J Neurophysiol. 2016;116:522–539. doi: 10.1152/jn.00988.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinski BL, Kim A, Xiao W, Mehta NM, Kay LM. Pharmacological manipulation of the olfactory bulb modulates beta oscillations: testing model predictions. J Neurophysiol. 2018;120:1090–1106. doi: 10.1152/jn.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic S, Szapiro G, Schwartz E, Barbour B, Brunel N, Hakim V. Neuronal morphology generates high-frequency firing resonance. J Neurosci. 2015;35:7056–7068. doi: 10.1523/JNEUROSCI.3924-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V. Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr Opin Neurol. 2013;26:662–670. doi: 10.1097/WCO.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Otomo K, Perkins J, Kulkarni A, Stojanovic S, Roeper J, Paladini CA. In vivo patch-clamp recordings reveal distinct subthreshold signatures and threshold dynamics of midbrain dopamine neurons. Nat Commun. 2020;11:6286. doi: 10.1038/s41467-020-20041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Tian L. Imaging voltage and brain chemistry with genetically encoded sensors and modulators. Curr Opin ChemBiol. 2020;57:166–176. doi: 10.1016/j.cbpa.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME. NMDA spikes enhance action potential generation during sensory input. Nat Neurosci. 2014;17:383–390. doi: 10.1038/nn.3646. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Parasuram H, Nair B, D’Angelo E, Hines M, Naldi G, Diwakar S. Computational modeling of single neuron extra cellular electric potentials and network local field potentials using LFPsim. Front Comput Neurosci. 2016;10:65. doi: 10.3389/fncom.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H, Garden DL, Papastathopoulos I, Sürmeli G, Nolan MF. Inter and intra-animal variation in the integrative properties of stellate cells in the medial entorhinal cortex. eLife. 2020;9 doi: 10.7554/eLife.52258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, Tewari BP, Chaunsali L, Sontheimer H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci. 2019;20:282–297. doi: 10.1038/s41583-019-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Fujisawa S, Berenyi A, Royer S, Buzsaki G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron. 2012;75:410–417. doi: 10.1016/j.neuron.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payeur A, Beique JC, Naud R. Classes of dendritic information processing. Curr Opin Neurobiol. 2019;58:78–85. doi: 10.1016/j.conb.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Lecci S, Luthi A, Astori S. Suppression of sleep spindle rhythmogenesis in mice with deletion of CaV3.2 andCaV3.3 T-type Ca(2+) channels. Sleep. 2016;39:875–885. doi: 10.5665/sleep.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Vinck M, Einevoll GT, Sirota A, Fries P, Siegel M, Truccolo W, Schroeder CE, Srinivasan R. Investigating large-scale brain dynamics using field potential recordings:analysis and interpretation. Nat Neurosci. 2018;21:903–919. doi: 10.1038/s41593-018-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen KH, Einevoll GT. Amplitude variability and extra cellular low-pass filtering of neuronal spikes. Biophys J. 2008;94:784–802. doi: 10.1529/biophysj.107.111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen KH, Devor A, Ulbert I, Dale AM, Einevoll GT. Current-source density estimation based on inversion of electrostatic forward solution: effects of finite extent of neuronalactivity and conductivity discontinuities. J Neurosci Methods. 2006;154:116–133. doi: 10.1016/j.jneumeth.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Seibt J. A mechanism for learning with sleep spindles. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190230. doi: 10.1098/rstb.2019.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band-an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Papoutsi A. Illuminating dendritic function with computational models. Nat Rev Neurosci. 2020;21:303–321. doi: 10.1038/s41583-020-0301-7. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Brannon T, Mel BW. Pyramidal neuron as two-layer neural network. Neuron. 2003;37:989–999. doi: 10.1016/s0896-6273(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel BW, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nat Neurosci. 2004;7:621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Dendritic ion channelopathy in acquired epilepsy. Epilepsia. 2012;53(Suppl 9):32–40. doi: 10.1111/epi.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci U S A. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdich-Neminsky V. Ein Versuch der Registrierung der elektrischen Gehirnerscheinungen. Zbl Physiol. 1912;27:951–960. [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Rajan K, Harvey CD, Tank DW. Recurrent network models of sequence generation and memory. Neuron. 2016;90:128–142. doi: 10.1016/j.neuron.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Electrophysiology of a dendritic neuron model. Biophys J. 1962;2:145–167. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Ranganathan GN, Apostolides PF, Harnett MT, Xu NL, Druckmann S, Magee JC. Active dendritic integration and mixed neocortical network representations during an adaptive sensing behavior. Nat Neurosci. 2018;21:1583–1590. doi: 10.1038/s41593-018-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathour RK, Narayanan R. Inactivating ion channels augment robustness of subthreshold intrinsic response dynamics to parametric variability in hippocampal model neurons. J Physiol. 2012;590:5629–5652. doi: 10.1113/jphysiol.2012.239418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathour RK, Narayanan R. Homeostasis of functional maps inactive dendrites emerges in the absence of individual channel ostasis. Proc Natl Acad Sci U S A. 2014;111:E1787–1796. doi: 10.1073/pnas.1316599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathour RK, Narayanan R. Degeneracy in hippocampal physiology and plasticity. Hippocampus. 2019;29:980–1022. doi: 10.1002/hipo.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm andhigh-gamma activity in macaque visual cortex. PLoS Biol. 2011a;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Network rhythms influence the relationship between spike-triggered local field potential and functional connectivity. J Neurosci. 2011b;31:12674–12682. doi: 10.1523/JNEUROSCI.1856-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Do gamma oscillations play a role incerebral cortex? Trends Cogn Sci. 2015;19:78–85. doi: 10.1016/j.tics.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci. 2008;28:7334–7343. doi: 10.1523/JNEUROSCI.1588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG. Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol. 2012;4:a005702. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichinnek S, Kunsting T, Draguhn A, Both M. Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J Neurosci. 2010;30:15441–15449. doi: 10.1523/JNEUROSCI.2535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann MW, Anastassiou CA, Perin R, Hill SL, Markram H, Koch C. A biophysically detailed model of neocortical local fieldpotentials predicts the critical role of active membrane currents. Neuron. 2013;79:375–390. doi: 10.1016/j.neuron.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Beck H, Yaari Y. Plasticity of voltage-gated ion channels in pyramidal cell dendrites. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Riddle J, Frohlich F. Targeting neural oscillations with transcranial alternating current stimulation. Brain Res. 2021:1765147491. doi: 10.1016/j.brainres.2021.147491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J, Alexander ML, Schiller CE, Rubinow DR, Frohlich F. Reduction in left frontal alpha oscillations by transcranial alternating current stimulation in major depressive disorder is context-dependent in a randomized clinical trial. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 doi: 10.1016/j.bpsc.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay J, Wang H, Fenno L, Deisseroth K, Malliaras GG. Next generation probes, particles, and proteins for neural interfacing. Sci Adv. 2017;3:e1601649. doi: 10.1126/sciadv.1601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Roig JA, Budelli R, Macadar O, Monti JM. Hippocampal theta rhythm in relation with unit discharges in septum and hippocampus. Electroencephalogr Clin Neurophysiol. 1970;28:520. [PubMed] [Google Scholar]
- Rojas-Libano D, Frederick DE, Egana JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci. 2014;8:214. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpharhythms over occipito-parietal areas in visual input regulation:correlation or causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roome CJ, Kuhn B. Simultaneous dendritic voltage and calcium imaging and somatic recording from Purkinje neurons inawake mice. Nat Commun. 2018;9:3388. doi: 10.1038/s41467-018-05900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roome CJ, Kuhn B. Dendritic coincidence detection in Purkinje neurons of awake mice. eLife. 2020;9 doi: 10.7554/eLife.59619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Middleton SJ, Cunningham MO, LeBeau FE, Bibbig A, Whittington MA, Traub RD. A beta2-frequency (20-30 Hz)oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci U S A. 2006;103:15646–15650. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T. Alpha oscillations and consciousness in completely locked-in state. Clin Neurophysiol. 2019;130:1652–1654. doi: 10.1016/j.clinph.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Rosenfalck P. Intra- and extracellular potential fields of active nerve and muscle fibres.A physico-mathematical analysis of different models. Acta Physiol Scand Suppl. 1969;321:1–168. [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer Ventorhinal cortex. J Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex. J Neurosci. 2007;27:7054–7069. doi: 10.1523/JNEUROSCI.1744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012;13:157–168. doi: 10.1038/nrn3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WN, Manita S. Imaging calcium waves and sparks incentral neurons. Cold Spring Harbor protocols. 2012;2012:1087–1091. doi: 10.1101/pdb.prot071480. [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Brager DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci. 2013;33:19442–19450. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh BN, Rathour RK, Baumgardner ME, Kalmbach BE, Johnston D, Brager DH. Increased transient Na(+) conductance and action potential output in layer 2/3 prefrontal cortex neurons of the fmr1(-/y) mouse. J Physiol. 2017;595:4431–4448. doi: 10.1113/JP274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JP, Clercq M, Steriade M, Deschenes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol. 1984;51:1220–1235. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsaki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Tian L. Imaging neurotransmitter and neuromodulator dynamics in vivo with genetically encoded indicators. Neuron. 2020;108:17–32. doi: 10.1016/j.neuron.2020.09.036. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Nevian T. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron. 2015;86:233–246. doi: 10.1016/j.neuron.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kuga N, Namiki S, Matsuki N, Ikegaya Y. Locally synchronized astrocytes. Cerebral cortex. 2011;21:1889–1900. doi: 10.1093/cercor/bhq256. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, McNeill TH. Dendritic remodeling of dentate granule cells following a combined entorhinal cortex/fimbria fornix lesion. Exp Neurol. 1996;141:145–153. doi: 10.1006/exnr.1996.0148. [DOI] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Leao RN, Ribeiro S, Tort AB. On high-frequency field oscillations (> 100 Hz) and the spectral leakage of spiking activity. J Neurosci. 2013;33:1535–1539. doi: 10.1523/JNEUROSCI.4217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Schiller Y. NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr Opin Neurobiol. 2001;11:343–348. doi: 10.1016/s0959-4388(00)00217-8. [DOI] [PubMed] [Google Scholar]
- Schiller J, Schiller Y, Stuart G, Sakmann B. Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J Physiol. 1997;505(Pt 3):605–616. doi: 10.1111/j.1469-7793.1997.605ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes inbasal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Herrojo Ruiz M, Kilavik BE, Lundqvist M, Starr PA, Aron AR. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J Neurosci. 2019;39:8231–8238. doi: 10.1523/JNEUROSCI.1163-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg EW, Anastassiou CA, Buzsaki G, Koch C. The spiking component of oscillatory extra cellular potentials in the rat hippocampus. J Neurosci. 2012;32:11798–11811. doi: 10.1523/JNEUROSCI.0656-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg EW, Fernandez-Ruiz A, Mizuseki K, Berenyi A, Anastassiou CA, Koch C, Buzsaki G. Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron. 2014;84:470–485. doi: 10.1016/j.neuron.2014.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seenivasan P, Narayanan R. Efficient phase coding in hippocampal place cells. Phys Rev Res. 2020;2:033393. doi: 10.1103/PhysRevResearch.2.033393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev I, London M. Untangling dendrites with quantitative models. Science. 2000;290:744–750. doi: 10.1126/science.290.5492.744. [DOI] [PubMed] [Google Scholar]
- Seibt J, Timofeev I, Carrier J, Peyrache A. Role of spindle oscillations across lifespan in health and disease. Neural Plast. 2016;2016:8103439. doi: 10.1155/2016/8103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzai Y, Fernandez-Ruiz A, Buzsaki G. Layer-specific physiological features and interlaminar interactions in the primary visual cortex of the mouse. Neuron. 2019;101:500–513.:e505. doi: 10.1016/j.neuron.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai AS, Anastassiou CA, Larkum ME, Koch C. Physiology of layer 5 pyramidal neurons in mouse primary visual cortex:coincidence detection through bursting. PLoS Comput Biol. 2015;11:e1004090. doi: 10.1371/journal.pcbi.1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MA, Lee S, Law R, Haegens S, Thorn CA, Hamalainen MS, Moore CI, Jones SR. Neural mechanisms of transient neocortical beta rhythms: converging evidence from humans,computational modeling, monkeys, and mice. Proc Natl Acad SciU S A. 2016;113:E4885–4894. doi: 10.1073/pnas.1604135113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirhatti V, Ray S. Long-wavelength (reddish) hues induce unusually large gamma oscillations in the primate primary visual cortex. Proc Natl Acad Sci U S A. 2018;115:4489–4494. doi: 10.1073/pnas.1717334115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Chitwood RA, Ding JM, Payne C, Taylor W, Gray R, Zemelman BV, Johnston D. Prefrontal cortex dysfunction infragile X mice depends on the continued absence of fragile Xmental retardation protein in the adult brain. J Neurosci. 2017;37:7305–7317. doi: 10.1523/JNEUROSCI.0571-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Singh A, Papa SM. Striatal Oscillations in Parkinsonian NonHuman Primates. Neuroscience. 2020;449:116–122. doi: 10.1016/j.neuroscience.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Narayanan R. HCN channels enhance spike phase coherence and regulate the phase of spikes and LFPs in the theta-frequency range. Proc Natl Acad Sci U S A. 2015;112:E2207–2216. doi: 10.1073/pnas.1419017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Narayanan R. Unitary sources say: It is inhibition! J Physiol. 2020;598:3815–3816. doi: 10.1113/JP280391. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Skaar JW, Stasik AJ, Hagen E, Ness TV, Einevoll GT. Estimation of neural network model parameters from local field potentials (LFPs) PLoS Comput Biol. 2020;16:e1007725. doi: 10.1371/journal.pcbi.1007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Smith IT, Branco T, Hausser M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature. 2013;503:115–120. doi: 10.1038/nature12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Katona L, Klausberger T, Lasztoczi B, Viney TJ. Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change ofexcitability in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120518. doi: 10.1098/rstb.2012.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Knudsen EI. Gamma oscillations in the midbrain spatial attention network: linking circuits to function. Curr Opin Neurobiol. 2015;31:189–198. doi: 10.1016/j.conb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle 3rd FJ, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Steriade M. Sleep oscillations in corticothalamic neuronal networks and their development into self-sustained paroxysmal activity. Rom J Neurol Psychiatry. 1993a;31:151–161. [PubMed] [Google Scholar]
- Steriade M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Curr Opin Neurobiol. 1993b;3:619–625. doi: 10.1016/0959-4388(93)90064-6. [DOI] [PubMed] [Google Scholar]
- Steriade M. Modulation of information processing in thalamocortical systems: chairman’s introductory remarks. Prog Brain Res. 1993c;98:341–343. doi: 10.1016/s0079-6123(08)62417-6. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984;320:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow(< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993c;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelzel CR, Bereshpolova Y, Gusev AG, Swadlow HA. The impact of an LGNd impulse on the awake visual cortex: synaptic dynamics and the sustained/transient distinction. J Neurosci. 2008;28:5018–5028. doi: 10.1523/JNEUROSCI.4726-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Spruston N. Dendritic integration: 60 years of progress. Nat Neurosci. 2015;18:1713–1721. doi: 10.1038/nn.4157. [DOI] [PubMed] [Google Scholar]
- Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SA. Proximodistal heterogeneity of hippocampal CA3 pyramidal neuron intrinsic properties, connectivity, and reactivation during memory recall. Neuron. 2017;95:656–672.:e653. doi: 10.1016/j.neuron.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, Daskalakis ZJ. gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Supp GG, Siegel M, Hipp JF, Engel AK. Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Curr Biol: CB. 2011;21:1988–1993. doi: 10.1016/j.cub.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Larkum ME. Dendritic calcium spikes are clearly detectable at the cortical surface. Nat Commun. 2017;8:276. doi: 10.1038/s41467-017-00282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci. 2002;22:7766–7773. doi: 10.1523/JNEUROSCI.22-17-07766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Oertner TG, Hegemann P, Larkum ME. Active cortical dendrites modulate perception. Science. 2016;354:1587–1590. doi: 10.1126/science.aah6066. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Ebner C, Sigl-Glockner J, Moberg S, Nierwetberg S, Larkum ME. Active dendritic currents gate descending cortical outputs in perception. Nat Neurosci. 2020;23:1277–1285. doi: 10.1038/s41593-020-0677-8. [DOI] [PubMed] [Google Scholar]
- Taxidis J, Anastassiou CA, Diba K, Koch C. Local field potentials encode place cell ensemble activation during hippocampal sharp wave ripples. Neuron. 2015;87:590–604. doi: 10.1016/j.neuron.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29:5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenczuk B, Telenczuk M, Destexhe A. A kernel-based method to calculate local field potentials from networks of spiking neurons. J Neurosci Methods. 2020a;344:108871. doi: 10.1016/j.jneumeth.2020.108871. [DOI] [PubMed] [Google Scholar]
- Telenczuk M, Telenczuk B, Destexhe A. Modeling unitary fields and the single-neuron contribution to local field potentials in the hippocampus. J Physiol. 2020b;598:3957–3972. doi: 10.1113/JP279452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A, Perez-Terzic C. Channelopathies: decoding disease pathogenesis. Science Transl Med. 2010;2:42.:ps37. doi: 10.1126/scitranslmed.3001433. [DOI] [PubMed] [Google Scholar]
- Todorova R, Zugaro M. Isolated cortical computations during delta waves support memory consolidation. Science. 2019;366:377–381. doi: 10.1126/science.aay0616. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci U S A. 1999;96:3257–3262. doi: 10.1073/pnas.96.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Synaptic mechanisms underlying interictal spike initiation in a hippocampal network. Neurology. 1983;33:257–266. doi: 10.1212/wnl.33.3.257. [DOI] [PubMed] [Google Scholar]
- Traub RD, Contreras D, Whittington MA. Combined experimental/simulation studies of cellular and network mechanisms of epileptogenesis in vitro and in vivo. J Clin Neurophysiol. 2005;22:330–342. [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro andin vivo. J Physiol. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova N, Henriquez CS, Plonsey R. Extra cellular potentials and currents of a single active fiber in a restricted volume conductor. Ann Biomed Eng. 1990;18:219–238. doi: 10.1007/BF02368439. [DOI] [PubMed] [Google Scholar]
- Trimper JB, Trettel SG, Hwaun E, Colgin LL Neuroscience Meeting Planner. Fast gamma rhythms predominate over slow gamma rhythms in superficial layers of medial entorhinal cortex; Society for Neuroscience Annual meeting 2017; Washington, DC. 2017. Program no. 083.23, 2017, http://www.abstractsonline.com/pp8/#!/4376/presentation/5357. [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci U S A. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The dialectic of Hebb and homeostasis. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybrandt K, Khodagholy D, Dielacher B, Stauffer F, Renz AF, Buzsaki G, Voros J. High-density stretchable electro degrids for chronic neural recording. Adv Mater. 2018;30:e1706520. doi: 10.1002/adma.201706520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D. Dendritic resonance in rat neocortical pyramidal cells. J Neurophysiol. 2002;87:2753–2759. doi: 10.1152/jn.2002.87.6.2753. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, Gotman J. High-frequency intracerebral EEG activity (100-500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Vaidya SP, Johnston D. Temporal synchrony and gamma-to-theta power conversion in the dendrites of CA1 pyramidal neurons. Nat Neurosci. 2013;16:1812–1820. doi: 10.1038/nn.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M, de la Prida LM. The hippocampus in depth: asublayer-specific perspective of entorhinal-hippocampal function. Curr Opin Neurobiol. 2018;52:107–114. doi: 10.1016/j.conb.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M, Averkin RG, Fernandez-Lamo I, Aguilar J, Lopez-Pigozzi D, Brotons-Mas JR, Cid E, Tamas G, Menendez de la Prida L. Mechanisms for selective single-cell reactivation duringoffline sharp-wave ripples and their distortion by fast ripples. Neuron. 2017;94:1234–1247.:e1237. doi: 10.1016/j.neuron.2017.05.032. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geit W, Gevaert M, Chindemi G, Rossert C, Courcol JD, Muller EB, Schurmann F, Segev I, Markram H. BluePyOpt: Leveraging open source software and cloud infrastructure to optimise model parameters in neuroscience. Front Neuroinf. 2016;10:17. doi: 10.3389/fninf.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci U S A. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A, Duijnhouwer J, Remme MW, van Pelt J. The effect of dendritic topology on firing patterns in model neurons. Network. 2002;13:311–325. doi: 10.1088/0954-898x/13/3/304. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A. 2004;101:5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier MC, Bower JM. A comparative survey of automated parameter-search methods for compartmental neural models. J Comput Neurosci. 1999;7:149–171. doi: 10.1023/a:1008972005316. [DOI] [PubMed] [Google Scholar]
- Vass LK, Copara MS, Seyal M, Shahlaie K, Farias ST, Shen PY, Ekstrom AD. Oscillations go the distance: low-frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron. 2016;89:1180–1186. doi: 10.1016/j.neuron.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Vetter P, Roth A, Hausser M. Propagation of action potentialsin dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Vu MT, Adali T, Ba D, Buzsaki G, Carlson D, Heller K, Liston C, Rudin C, Sohal VS, Widge AS, Mayberg HS, et al. A shared vision for machine learning in neuroscience. J Neurosci. 2018;38:1601–1607. doi: 10.1523/JNEUROSCI.0508-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibitionin a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Larkum M, Sakmann B, Helmchen F. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2003;23:8558–8567. doi: 10.1523/JNEUROSCI.23-24-08558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Fried I, Ekstrom AD. Behavioral correlates of human hippocampal delta and theta oscillations during navigation. J Neurophysiol. 2011;105:1747–1755. doi: 10.1152/jn.00921.2010. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23:656–661. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series:inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- Williams SR, Fletcher LN. A dendritic substrate for the cholinergic control of neocortical output neurons. Neuron. 2019;101:486–499.:e484. doi: 10.1016/j.neuron.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol. 1982;11:301–308. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- Xu NL, Harnett MT, Williams SR, Huber D, O’Connor DH, Svoboda K, Magee JC. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- Yaari Y, Yue C, Su H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J Physiol. 2007;580:435–450. doi: 10.1113/jphysiol.2007.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger CE, Ringach DL, Trachtenberg JT. Neuromodulatory control of localized dendritic spiking in critical period cortex. Nature. 2019;567:100–104. doi: 10.1038/s41586-019-0963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Zhu G, Zhao B, Bai Y, Jiang Y, Neumann WJ, Kuhn AA, Zhang J. Local field potentials in Parkinson’s disease: a frequency-based review. Neurobiol Dis. 2021;155:105372. doi: 10.1016/j.nbd.2021.105372. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Rothenberger A. Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder. Suppl Clin Neurophysiol. 2013;62:289–301. doi: 10.1016/b978-0-7020-5307-8.00018-1. [DOI] [PubMed] [Google Scholar]
- Yuste R. Potassium channels. Dendritic shock absorbers. Nature. 1997;387(851):853. doi: 10.1038/43063. [DOI] [PubMed] [Google Scholar]
- Zenke F, Gerstner W, Ganguli S. The temporal paradox of Hebbian learning and homeostatic plasticity. Curr Opin Neurobiol. 2017;43:166–176. doi: 10.1016/j.conb.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, Frick A, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Trettel SG, Colgin LL. The relationship between gamma frequency and running speed differs for slow and fast gamma rhythms in freely behaving rats. Hippocampus. 2015;25:924–938. doi: 10.1002/hipo.22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Hwaun E, Colgin LL. Fast gamma rhythms in the hippocampus promote encoding of novel object place pairings. eNeuro. 2016a;3 doi: 10.1523/ENEURO.0001-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Hsiao YT, Colgin LL. Spatial sequence coding differs during slow and fast gamma rhythms in the hippocampus. Neuron. 2016b;89:398–408. doi: 10.1016/j.neuron.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Xiong W, Masurkar AV, Chen WR, Shepherd GM. Dendritic calcium plateau potentials modulate input-output properties of juxtaglomerular cells in the rat olfactory bulb. J Neurophysiol. 2006a;96:2354–2363. doi: 10.1152/jn.00003.2006. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xiong W, Zeng S, Xia A, Shepherd GM, Greer CA, Chen WR. Dendritic excitability and calcium signalling in the mitral cell distal glomerular tuft. Eur J Neurosci. 2006b;24:1623–1632. doi: 10.1111/j.1460-9568.2006.05076.x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]