Conspectus
The story of the non-duplex DNA form known as the G-quadruplex (G4) has traversed a winding path. From initial skepticism followed by debate to a surge in interest, the G4 story intertwines many threads. Starting with computational predictions of a gene regulatory role, which now include epigenetic functions, our group was involved in many of these advances along with many other laboratories. Following a brief background, set in the latter half of the last century when the concept of the G4 as a structure took ground, here we account the developments. This is through a lens that though focused on our groups’ research presents work from many other groups that played significant roles. Together these provide a broad perspective to the G4 story. Initially we were intrigued on seeing potential G4 (pG4)-forming sequences, then known to be found primarily at the telomeres and immunoglobin switch regions, occurring throughout the genome and being particularly prevalent in promoters of bacteria. We further observed that pG4s were not only prevalent but also conserved through evolution in promoters of human, chimpanzee, mouse and rat genomes. This was between 2005 and 2007. Encouraged by these partly and partly in response to the view held by many that genome-wide presence of G4s were genomic “accidents”, the focus shifted to seeking experimental evidence.
In the next year, 2008, two independent findings showed promise. First, on treating human cancer cells with G4-binding ligands, we observed widespread change in gene expression. Second, our search for the missing G4-specific transcription factor, without which, importantly, G4s in promoters posed only half the story, yielded results. We determined how NM23-H2 (also known as NME2 or NDPK-B) interacts with G4s and how interaction of NM23-H2 with a G4 in the promoter of the oncogene c-myc was important for regulation of c-myc transcription. NM23-H2, and subsequently many other similar factors discovered by multiple groups, is possibly giving shape to what might be the “G4-transcriptome”. Later, a close look at NM23-H2-G4 interaction in regulation of the human reverse transcriptase gene (hTERT) revealed the role of G4s in local epigenetic modifications. Meanwhile work from others showed how G4s impact histone modifications following replication. Together these show the intrinsic role of DNA sequence, through formation of DNA structure, in epigenetics.
More recent work, however, was waiting to reveal aspects that tend to bring forth a completely new understanding of G4s. We observed that the telomere-repeat-binding-factor-2 (TRF2), known canonically to be telomere-associated, binds extensively outside telomeres throughout the genome. Moreover, a large fraction of the non-telomeric TRF2 sites comprise G4s. Second, the extent of non-telomeric TRF2 binding at promoters was dependent on telomere length. Thereby TRF2-induced epigenetic gene regulation was telomere-dependent. Together these implicate underlying connections that show signs of addressing an intriguing unanswered question that takes us back to the beginning: Why are G4s prevalent in two distinct regions, the telomeres and gene promoters?
▪. Introduction
Guanine-rich oligonucleotides readily self-assemble to form gels in water. Between 1962 and 1975 chemical studies revealed that this was due to Hoogsteen hydrogen bonding of guanine bases into planar sheet-like arrangements called guanine tetrads (G-tetrads; Figure 1).5 Interest in G-tetrads, however, surged from an altogether different discovery: Elizabeth Blackburn and Joseph Gall showed that DNA at the chromosome termini (telomeres) in the ciliated protozoan Tetrahymena thermophila comprised tandemly repeated G-rich sequences.6 This was 1978.
Figure 1. (A) G-quadruplex formation by stacking of G-tetrads stabilized by Hoogsteen base pairing showing stem and loop configurations. (B) Sequence patterns with varying loop length that adopt the G4 fold readily. GGG trimers are connected through sequences of varying lengths comprising all four bases. Algorithms for G-quadruplex mining are typically based on this formalism. G-quadruplexes with interruption within the GGG trimers (bulge formation) and loop lengths of more than 15 bases have also been reported. (C) Basic topology of intra- and intermolecular G-quadruplexes; many variations of this based on the orientation and directionality of the loop sequence have been reported; the three most common intramolecular forms are shown.
A decade later Sen and Gilbert showed that short singlestranded G-rich repeat sequences form G-tetrads in solution and noted the presence G-repeats outside telomeres in immunoglobin switch regions.7 In the following years leading into the late 1990s, a series of structural studies using telomeric DNA from various organisms including human further established formation of the four-stranded secondary structure called the G-quadruplex (or G4, Figure 1).7,8 Thereafter early work revealed that G4s might be anticancer drug targets as ligand-mediated stabilization of telomeric G4s inhibited telomerase activity.9,10 In support of these, using the macronucleus (with ~108 telomeres) from the ciliate Stylonychia lemnae and G4-specfic antibodies Schaffitzel et al. in 2001 showed in vivo evidence of telomeric G4s.11
Findings in the next few years, however, would introduce a new turn to the developing story. First, in 2002 Hurley and coworkers reported a non-telomeric G-rich sequence.12 This was in the promoter of the oncogene c-myc formed G4 in solution and affected c-myc transcription in cancer cells. Soon sequences with the potential to form G4s (pG4s) were reported throughout the human genome.12–14 In 2006, we made a then intriguing observation. Genomic distribution had a distinct pattern: pG4s were enriched within gene promoters.1 Further, we noted with interest that pG4s were conserved evolutionarily across human, chimpanzee, mouse, and rat promoters genomewide and among hundreds of microbes.1,15,16 Based on these observations, we proposed G4s as global gene regulatory motifs, much like transcription factor binding sites but through a DNA secondary structure instead of the duplex form. A poster on this at the First G-quadruplex Meeting in 200717 was noticed and reported by Chemical and Engineering News in a cover article titled “Ascent of Quadruplexes”.18 This article captured the prevalent excitement in the field and in some sense dawned the theme of G4 chemical biology as we see it today.
Here, we account the progress in G4 research primarily from the vantage of our groups’ work. Over the years, this spanned from computational predictions to the first G4-dependent transcription factor and epigenetic regulation, including recent observations on how G4s might connect telomeres to the rest of the genome.
▪. Enriched and Evolutionarily Conserved Presence of Pg4S in Promoters Suggests Gene Regulatory Function
Using an algorithm that identified DNA sequences of the pattern comprising G4s (Figure 1B describes the formalism of the algorithm used for G4 detection), we scanned newly available microbial genome sequences. In every genome that we studied, a multitude of pG4s spread across the genome. For confirmation, we randomly selected several predicted sequences and experimentally confirmed that they adopted the G4 structure in solution.1 A database, Quadbase, was made in 2008 with pG4s from hundreds of organisms including web-based tools to find new ones and compare pG4s across organisms and updated in 2016.16,19
Several G4 mining algorithms developed by others are available. Although the basic formalism describing G4s remains similar intricate nuances have been introduced at times. Recent ones, like the G4 Hunter and PQS-finder, included attributes like presence of nearby C-nucleotides, the possibility of bulges within G4s (interruptions with the GGG trimer or stem of the G4, Figure 1), and mismatches.20,21 Other G4 mining algorithms include the Quadparser and GRS Mapper.14,22
With the newly made observations, we asked questions about functional relevance. Were pG4s randomly distributed in the genome? This revealed a notable theme: pG4s were particularly enriched within gene promoters relative to other regions of the genome. We related to this as a theme because pG4 enrichment within promoters was evident not only in prokaryotes but also in human, chimpanzee, mouse and rat (vide infra).1,15 In the first report substantiating this, we analyzed >61 000 open reading frames (ORF) across 18 bacteria.1
We next investigated whether pG4s were conserved. This was with the following reasoning. Enrichment of pG4s in promoters of orthologous genes (from different organisms) might suggest a role in gene regulation, as is commonly observed for regulatory elements. Analysis of all reported ORFs across 18 bacteria showed clear signs of conservation of pG4s in promoters of organisms that were otherwise phylogenetically distant.1
Conserved pG4s in Human, Chimpanzee, Mouse, and Rat Promoters
Like bacteria, the presence of a large number of pG4s in not only human but also chimpanzee, mouse, and rat genomes was unmistakable.15,23 Of particular interest was the nature of the genomic distribution of pG4s. When plotted chromosome-wise to focus on all the promoters in a chromosome a remarkable feature emerged. The “hot spots” of pG4s were typically near transcription start sites (TSSs) across all chromosomes in human, chimpanzee, mouse, and rat genomes (Figure 2A),15 whereas G-rich sequences with substitutions that would exclude or destabilize G4 structure formation showed almost no enrichment near TSSs. This importantly indicated that the enrichment of pG4 motifs near TSSs was unlikely due to G-rich sequences per se.15
Figure 2. (A) Potential G4 (pG4) sequences are predominant near transcription start sites (TSSs) of human, chimpanzee, mouse, and rat genomewide. Each row represents a chromosome; TSSs of all genes in a chromosome are centered in each row. (B) Human promoter pG4s conserved within “orthologous” mouse and rat promoters appear in clusters when plotted relative to distance from the TSS; each row represents one promoter; heat map indicates presence of pG4 with respect to distance from TSS in 773 human promoters (plotted in a 100 bp window; lower panel). Frequency of pG4 motifs near TSS shown in upper panel. Adapted with permission from ref 15. Copyright 2008 American Chemical Society.
The occurrence of pG4s was conserved in more than 700 promoters of orthologous genes in human, mouse, and rat genomes. On aligning the conserved pG4s with respect to their distance from the respective TSS, we noted a nonrandom arrangement. The positions of pG4s from TSSs followed systematic patterns, like positions of commonly occurring transcription factor binding sites (Figure 2B).15 Further, we asked if promoters with pG4s were coexpressed.
The 700 human gene promoters harboring pG4s conserved with mouse and rat showed significantly correlated expression in 75 out of 79 human tissues tested.15 This implicated G4s in global gene regulation.
Meanwhile, and in the following years, others made similar observations (reviewed in ref 24). pG4s were reported to be enriched in human and yeast promoters and present in viral genomes.25–27 Together these observations argued for G4-mediated gene regulation in different species.
pG4s Co-occur with Selected Transcription Factor Binding Sites
To understand if pG4s were associated with binding of any specific transcription factor, we looked at 220 transcription factor binding sites (TFBSs).23 These sites represented 187 unique transcription factors (TFs) in >75000 gene promoters of human, chimpanzee, mouse, and rat. The occurrence of nine TFs, including well-known ones like SP1, AP-2, VDR, and MAZ was significant within 100 bases of pG4s.23 Furthermore, the pG4-TFBS combination was conserved in at least 850 “orthologously” related promoters in human, chimpanzee, mouse, and rat genomes. Genes with the pG4-TFBS combinations were expressed in a co-regulated way in human tissues suggesting their significance in gene regulation. We also noted with interest that seven of the nine TFs were zinc-finger binding proteins, showing perhaps a novel characteristic of G4s.23 A similar analysis of pG4-TFBS combinations in E. coli had shown these to be enriched in promoters of genes that are activated during the exponential growth phase of the bacteria.1
G4s Might Confer Selective Advantage in Bacteria
From parallel studies, we made a similar observation in bacteria. Analysis of a large number of bacterial species suggested that groups of bacteria with similar characteristics (clades) showed preference for pG4s in specific gene promoters. This was particularly so for genes that conferred organism-specific selective advantage.28 For example, enrichment of pG4 within promoters of 21 genes crucial for resistance to radiation was noted in three radio-resistant bacteria including Deinococcus radiodurans,28 whereas the promoters of the orthologous genes in E. coli did not show similar enrichment in pG4s. To experimentally test this, D. radiodurans was treated with the intracellular G4-binding ligand NMM (N-methyl mesoporphyrin). This attenuated radio-resistance in D. radiodurans by ~60%. Importantly, genes of the RecF recombination repair pathway harboring promoter pG4s were down-regulated on NMM treatment. This was not the case on treatment with MIX (mesoporphyrin IX dihydrochloride) the non-methylated analogue of NMM with significantly lower affinity for G4s.28 Together, these observations not only supported G4-mediated gene regulation but also implied the importance of this regulation in conferring selective advantage to organisms. It must be mentioned however that any effect due to inhibition of heme biosynthesis by NMM,29 independent of G4 binding, cannot be ruled out.
More Evidence Argues for G4s as Gene Regulatory Motifs
If G4s were involved in gene regulation, stabilizing G4s inside cells using G4-binding ligands might impact gene expression. With this reasoning, we designed the following experiments. Human cancer HeLa S3 and A549 cells were treated with the well-known intracellular G4-binding ligand TmPyP4, and gene expression changes were analyzed.23 Out of 1161 differentially expressed genes, promoters of 711 genes had at least one pG4 within 1 kb of the TSS. Cells treated with TmPyP2, a close analogue of TmPyP4 with no G4 binding affinity, were used to rule out the likelihood of gene expression changes from non-G4-binding effects of the molecule.30
We further reasoned that if G4s were relevant single nucleotide polymorphisms (SNPs) that destabilize formation of the structure might be disallowed. Nucleotides within the pG4 were found to be resistant to polymorphism.31 This was relative to the SNPs in immediately adjacent regions or those within the pG4 sequence that are unlikely to affect the structure (i.e., SNPs within the loop of the pG4 (see Figure 1 for description of the loop).
Using the HapMap data, we noted significant association between SNPs within the promoter pG4s (denoted as Quad-SNP) and differential expression of the corresponding gene in individuals from four different populations. Interestingly, promoter Quad-SNPs when present in a heterozygous fashion (only one allele with SNP) correlated with relatively compensated expression of the gene. Together these observations further implicated promoter G4s in gene expression at a global level.31
A study in 2016 reported that RNA G4s were mostly unfolded in eukaryotic cells and RNA G4-forming sequences were depleted in bacterial transcriptomes.32 Another recent ex vivo G4-sequencing study noted depletion of G4s in bacteria and yeast.33 However, on addition of a G4 stabilizing ligand most of the predicted G4s could be detected. These data suggest prevalence of G4s with low stability in bacteria and yeast. The study also observed enrichment of G4s in promoters of mouse, human, and Trypanosoma genomes.33
Genomic pG4 Distribution Suggests Wider Role in Chromatin Organization and Epigenetics
Analysis of experimentally determined nucleosome occupancy showed relatively high pG4 frequency in nucleosome-free regions.34 For further analysis, two sets of gene promoters, with or without pG4s, were made. Interestingly, genes with pG4-containing and relatively nucleosome-free promoters were associated with signaling events. The nucleosome-enriched promoters without pG4s, on the other hand, were of genes in development related functions. Work from multiple groups in the following years have reported experimental or computational analyses substantiating the role of G4s in chromatin packaging through modulation of nucleosomes (reviewed recently in ref 35).
pG4 distribution was negatively associated with CpG methylation.36 We mapped pG4s within methylated and unmethylated regions of embryonic stem cells or differentiated fibroblasts (obtained from the Human Epigenome Project (HEP)). Enriched presence of pG4s was observed in regions with low average methylation. Conversely, there was reduced occurrence of pG4s in highly methylated regions in both cell types. On closer analysis of individual CpG dinucleotides, it was clear that CpGs present within pG4 motifs had significantly lower average methylation. To confirm, we experimentally determined methylation of >600 000 CpGs from 18 individuals. Significantly lower methylation was found in CpGs within pG4s.36 These observations suggested a role for G4s in CpG methylation through potential involvement of DNA methyltransferases.
Subsequent studies by others were consistent with these findings. DNA methyltransferases like DNMT3A, DNMT3B, and DNMT1 were found to have high affinity for G4s in vitro.37 It was further observed that the interaction of DNMT1 with G4s inhibited DNMT1 enzymatic function resulting in hypomethylation of CpG islands.38
CpG methylation within G4s was postulated as an important factor for G4 stability affecting prognosis of multiple neurodegenerative disorders like amyotrophic lateral sclerosis (ALS) and fronto-temporal dementia (FTD).39 Together these reveal underlying links between formation and presence of G4s and the epigenetic state of DNA in its immediate vicinity. The epigenetic mechanisms in the studies described here implicate the DNA secondary structure of the G4 in roles that impact chromatin through alteration in histone modification or cytosine methylation. Importantly, these studies suggest an intrinsic interdependence of DNA secondary structure (or the non-B DNA form) and the epigenetic state of chromatin.40
▪. The Missing G4-Interacting Transcription Factor
Evidence has implicated G4s in gene regulation. However, without a transcription regulatory protein(s) that specifically associated with G4s inside cells, this was unlikely. We focused on this question. Two papers published about ten years apart were instrumental in this search.
In 2002, the Hurley group showed that a pG4 present within the nuclease hypersensitive region III (NHE III) in the promoter of the oncogene c-myc could be targeted using the G4-stabilizing ligand TMPyP4. This repressed c-myc expression.12 In 1993, Postel et al. had reported that a purine-binding factor, later identified as NM23-H2 (or NME2), associated with the c-myc NHE to transcriptionally regulate c-myc.41 Keeping the two findings in mind, we asked whether the transcription factor NM23-H2 interacted with the c-myc promoter G4.
Results from in vitro experiments showed that recombinant purified NM23-H2 interacted with high affinity with the c-myc promoter G4 in solution.2 For mechanistic understanding, we used two complementary approaches. First, promoter reporter assays were designed with the c-myc promoter upstream of the luciferase gene. The constructs were designed either with the intact G4 or such that the G4 was destabilized by specific nucleotide substitutions in the reporter plasmid. Promoter activity was found to be clearly enhanced on overexpressing NM23-H2 in human cancer HeLa S3 and A549 cells, whereas this was not so for the reporter where the G4 was destabilized. The NM23-H2-mediated activation of c-myc in addition to interaction with G4 might involve other cofactors that act as transcriptional enhancers; however further work will be necessary to understand this fully.
Second, in the presence of intracellular G4-stabilizing ligands, NM23-H2-mediated activation of c-myc transcription was significantly reduced. In late 2008 with these data, we demonstrated that the transcription activity of NM23-H2 was dependent on the presence of intact G4 in the promoter of c-myc (Figure 3).2 Soon after, this was confirmed by an independent study.42
Figure 3. Interaction of NM23-H2 with the parallel type of c-myc promoter G4 transcriptionally activates c-myc expression. Disruption of the G4 results in loss of NM23-H2 binding and reduced transcription of c-myc.
Interaction of proteins with telomeric G4s, for instance, Mre11 in yeast, had been observed.43 However, direct involvement of the G4 in engaging a transcription factor for regulatory function was not reported earlier. These findings therefore led not only to work that focused on the NM23-H2-G4 interaction as a therapeutic target for repression of the oncogene c-myc but also to identification of several other G4-binding regulatory factors (reviewed in refs 40 and 44).
In 2009, using fluorescence and CD experiments, Xodo and co-workers reported that the nuclear protein hnRNPA1/UP1 facilitates unfolding of a G4 in the promoter of the protooncogene KRAS; based on this they inferred a role for G4 in transcription of KRAS.45
In addition to NM23-H2, c-myc was reported to be regulated by G4-binding proteins nucleolin, CNBP, and PARP1 (reviewed in ref 44). Also, many genes including RAS, VEGF, BCL-2, PCGF3, KIT, HIF1A, MYB, PDGFA, PDGFRβ, and RB1 were reported to have promoter G4s; regulatory factors like MAZ and hnRNPA1 were found to interact with promoter G4s for regulation of HRAS and KRAS, respectively; and several helicases were found to have G4-specific functions.44,46,47 Notably, Bugaut et al. and others studied G4s within 5’-UTR RNA and showed a role for RNA G4s in translation regulation.48,49
▪. G4S Show Involvement of DNA Structure in Locally Induced Epigenetic Modifications
Encouraged by the direct role of the NM23-H2-G4 interaction in regulation of c-myc, we investigated if NM23-H2 interacted with G4s at other promoters. Chromatin immunoprecipitation followed by sequencing (ChIP-seq) revealed that a large number of NM23-H2 peaks comprised pG4s.50 Notably, a significant number of NM23-H2 ChIP-seq reads mapped to the promoter of the reverse transcriptase component of the human telomerase gene hTERT, where a relatively high number of G4s had been reported.51 Therefore, we asked whether and, if so, how NM23-H2 might regulate hTERT.
NM23-H2 binding at the hTERT promoter was G4-dependent.52 In case of hTERT, however, unlike c-myc, NM23-H2 was a transcriptional repressor. Using direct and sequential ChIP experiments, we found that recruitment of the REl-silencing-transcription-factor (REST)-co-REST-lysine-specific-histone-demethylase-1 (LSD1) epigenetic repressor complex at the hTERT promoter was NM23-H2-dependent. As expected, there was a marked increase in histone activation marks H3K4me2, H3K4me1, and H3K9ac upon NM23-H2 silencing resulting in enhanced hTERT expression and increase in telomerase activity.52
Together, these showed how G4 within the hTERT promoter was important for local epigenetic modifications (Figure 4).
Figure 4. NM23-H2-G4 interaction recruits the REST-co-REST— LSD1 repressor complex for transcriptional repression of genes.
More support for the role of G4s in epigenetic modulation comes from multiple other studies. In DT40 avian cells devoid of the G4 helicase REV1, the original epigenetically repressed state of the beta-globin locus was lost in daughter cells following replication.53 And artificial introduction of a synthetic G4 resulted in activation of otherwise silent lysozyme C locus in the REV1-deficient cells.53 With use of derivatives of the G4 ligand pyridine 2,6-dicarboximide (PDC), it was further shown that the G4 at the BU-1 locus induced transcriptional reprogramming through H3K4me3 loss and cytosine methylation in DT40 cells.54 Furthermore, deletion of Pif1 helicase, a G4 unwinding factor in Saccharomyces cerevisiae, increased replication related errors and genomic instability.55
In addition to histone modifications, the formation of 8-oxo-7,8-dihydroguanine (OG) due to oxidation of the guanine residue within G-rich pG4 sequences was shown to impact stability of G4s within promoters of KRAS, VEGF, c-Myc, and NTHL1 and recently the DNA repair gene NEIL3.56,57 It was further shown that formation of the OG introduced an abasic (AP) site following base excision repair by 8-oxoguanine DNA glycosylase; melting of the duplex due to the AP promoted G4 formation that ultimately resulted in activation of VEGF and NTHL1.58
Telomere-Binding Factor TRF2 Binds G4s throughout the Genome
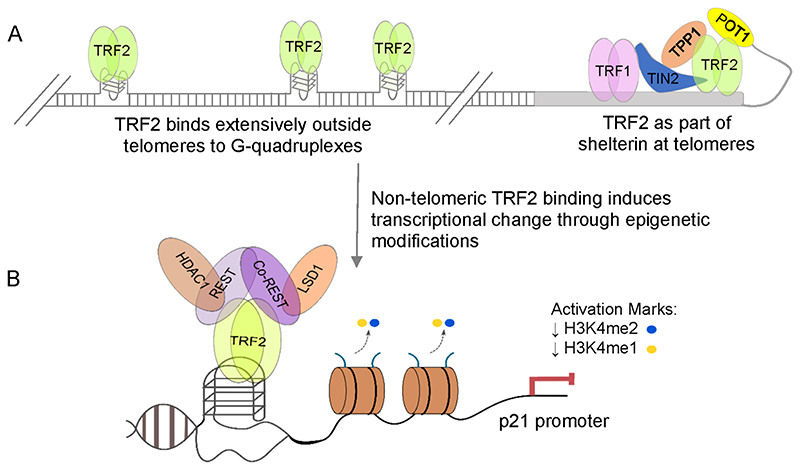
While studying association of NM23-H2 with G4s at telomeres, we observed that NM23-H2 interacts with the telomere-repeat-binding-factor-2 (TRF2).59 Moreover, a truncated form of TRF2 was found to associate with the telomeric G4 in solution. These observations made us test interaction of full-length TRF2 with promoter G4s.60 Purified recombinant TRF2 had high affinity for promoter G4s,3 whereas corresponding single stranded DNA that did not fold into G4s interacted with TRF2 with significantly less affinity.
To test non-telomeric TRF2 binding, we performed ChIP-seq in HT1080 human fibrosarcoma cells. This revealed more than 20 000 TRF2 peaks spread throughout the genome (Figure 5A). Likely reasons for the substantially larger number of sites found by us compared to previous studies have been described in detail earlier.3 Briefly, a difference in sample processing, depth of sequencing (number of reads generated), data analysis, or cell lines used might have contributed to this.
Figure 5. (A) Extensive non-telomeric TRF2 association genome-wide with G-quadruplexes. (B) TRF2-G4 interaction induces recruitment of epigenetic modifier factors for transcription regulation.
Importantly, a large proportion of the TRF2 peaks comprised pG4s within gene promoters. We randomly selected several of these TRF2-pG4 binding sites and checked whether they adopt the G4 structure in solution. All the sequences so tested formed either parallel or mixed type G4s. Also, as expected the folded G4 forms interacted with purified TRF2 with higher affinity relative to corresponding DNA sequences that did not form the G4 structure.3 Together these data suggested extensive TRF2-G4 association throughout the genome.
Non-telomeric TRF2 Interaction with Promoter G4s Induces Gene Regulation through Epigenetic Modifications
Using TRF2 ChIP-seq, we examined the role of TRF2-G4 interaction in gene regulation.3 TRF2-dependent expression was evident in almost all the genes we tested in two different human cell lines. ANXA2 (annexin A2), CHRM2 (cholinergic receptor muscarinic 2), SAMD14 (sterile alpha motif domain-containing protein 14), SMAD7 (mothers against decapentaplegic 7), THRA (thyroid hormone receptor alpha), OBSL1 (obscurin-like protein 1), KCNH2 (potassium voltage-gated channel subfamily H member 2), and INHA (inhibin, alpha) were repressed whereas OPN4 (melanopsin) was activated by TRF2.3 This was supported by TRF2-dependent epigenetic modifications at the respective promoters. Either loss of activation marks like H3K4me3 and H3K4me1 or deposition of the repressor mark H3K27me3 was observed in most cases3 Further, in the presence of the well-known intracellular G4-binding ligand 360A (2,6-N,N’-methyl-quinolinio-3-yl-pyridine dicarboxamide) TRF2 occupancy was significantly excluded.3 Together, these showed TRF2-mediated epigenetic regulation to be G4-dependent.
On similar lines, we found that p21 (also known as CDKN1A), a cyclin dependent kinase inhibitor, was negatively regulated by TRF2 in a G4-dependent manner. This was through recruitment of the REST-co-REST-LSD1 (also known as KDM1A) repressor complex (Figure 5B).61
Of particular note from more recent work is the role of TRF2-G4 interaction in regulation of hTERT (Sharma et al., 2020; unpublished version in bioRxiv).62 TRF2 occupancy at the hTERT promoter up to -600 bp upstream of the TSS was dependent on G4s. TRF2 silencing induced transcriptional activation of hTERT. This was due to TRF2-dependent recruitment of the polycomb repressor PRC2 complex at the hTERT promoter. PRC2-induced trimethylation of H3K27, a well-known repressor histone mark, resulted in repression of hTERT.62 Together, these data substantiate the emerging role of TRF2 in gene regulation, in addition to its canonical function in telomere protection.
Cancer-Specific Highly Recurrent hTERT Promoter Mutations Disrupt TRF2-G4 Interaction Resulting in hTERT Reactivation
Two highly recurrent somatic G → A mutations at the -124 and -146 positions from the translation start site were reported to be associated with several cancers including glioblastoma (>80%), melanoma (71%), and hepatocellular and urothelial carcinomas.63 It was further observed that the mutations disrupted hTERT promoter G4s and overexpressed hTERT.64 We found that both mutations excluded TRF2 binding at the hTERT promoter. Resultant loss of the PRC2 complex induced reactivation of hTERT.62 Importantly, in the presence of G4-stabilizing ligands TRF2 binding was regained in patient-derived glioblastoma cells. As a result, the PRC2 repressor complex and H3K27Me3 marks were re-instated leading to resuppression of hTERT. This induced growth arrest in otherwise highly proliferating glioblastoma cells. These results suggest that the role of TRF2-G4 interaction in hTERT regulation might be of therapeutic importance in multiple cancers directly associated with hTERT promoter mutations.63
TRF2-Mediated Gene Regulation Is Telomere-Sensitive: The Telomere-Sequestration-Partition Model
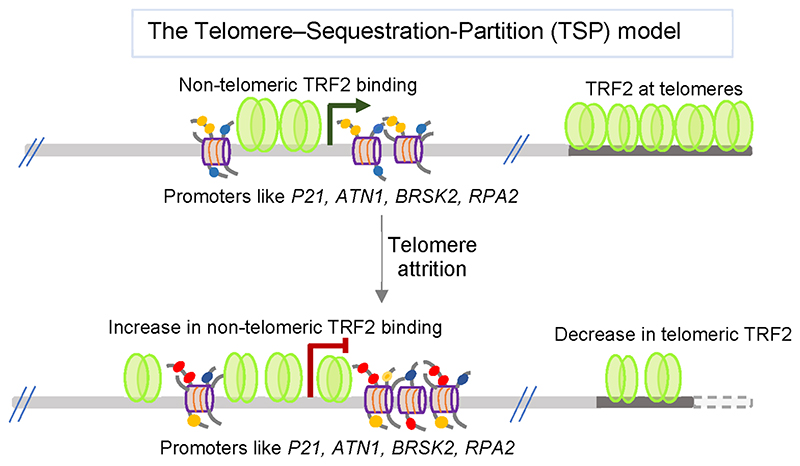
TRF2 is known to associate with telomeres primarily. On observing extensive TRF2 occupancy genome-wide, we asked if telomeres (particularly telomere length) influence non-telomeric TRF2 binding. This revealed notable dependency. This was tested in two distinct cell types, cancer as well as normal, with long or short telomeres (in an otherwise isogenic background). Cells with long telomeres typically had lower TRF2 occupancy at the non-telomeric genomic sites, and conversely, when telomeres were relatively short, non-telomeric TRF2 binding increased significantly.4
Seventeen out of 23 promoters tested showed telomeresensitive TRF2 binding. These promoters were spread across the genome and as far as 62 Mb from the nearest telomere.4 Further, altered promoter TRF2 occupancy in cells with long and short telomeres resulted in epigenetic modifications of the promoters. This was TRF2-dependent and as expected resulted in altered expression of the target gene in almost all cases. Together these observations show how in long telomeres due to the increased number of binding sites TRF2 was majorly bound or sequestered at the telomeres. When telomeres shorten, more TRF2 is available for genomic binding, as would be expected from a partitioning-like phenomenon.4 We denoted this as the telomere-sequestration-partition (TSP) model that is able to influence telomere-dependent gene expression genome-wide (Figure 6). TSP, therefore, is distinct from telomere-dependent gene expression described to influence genes up to 10 Mb away from telomeres in a model called telomere-positioning-effect over long distance (TPE-OLD).65
Figure 6. Non-telomeric TRF2 binding is telomere-sensitive. Sequestered telomeric TRF2 decreases as telomeres shorten resulting in increased non-telomeric TRF2 binding. This leads to TRF2-induced epigenetic regulation in a telomere-dependent way.
▪. Future Perspectives
The quest for understanding the G4 fold continues to reveal new topologies that add to the number and type of G4s.66–68 Several emerging trends offer new opportunities for further investigations. Foremost among these is the likely impact of the G4 on how we perceive DNA structure, that is, the duplex versus the non-duplex form, and their contribution to function.24 Implications of this emanate from a body of literature on the role of G4s including regulation, replication, on-off switching of genes through guanine oxidation, chromatin packaging, and epigenetic modifications.35,40,47,58 Second is the continuing interest in G4s for potential therapeutic interventions including G4-binding molecules that target epigenetic features.27,35,69–73 In addition to these, we draw attention to two emerging facets that might impact our understanding of G4s in distinct ways.
G-Quadruplexes Particularly Enriched at Telomeres and Gene Regulatory Regions: Why?
Outside telomeres, G4s are prevalent within gene regulatory regions. Although this has been known for several years now an intriguing question remains: Is the distinct genomic presence of G4s by design or accident? While the jury is still out on this, recent work might provide early cues. First, results show extensive genome-wide association of TRF2, in addition to TRF2 at telomeres.3 Second, a large fraction of the non-telomeric TRF2 sites comprise G4s.3 Considered with the TSP model described above, these argue for a role of G4s in signaling between telomeres and the non-telomeric genome. In addition to TRF2, telomere-associated factors like telomerase and TERRA (RNA transcribed from telomeres) that interact with G4s and non-telomeric sites may contribute in this signaling.74
Telomeres have long been understood to impact cellular and organismal physiology. However, intriguingly, at a molecular level the influence of telomeres has largely been understood to be limited to the subtelomeric regions.65 With further work, it is possible that G4s help bridge this gap.
TRF2 and Other Factors as Detector of In Vivo G4s in Live Cells
G4-specific antibodies have been instrumental in advancing our understanding of in vivo G4s.75 However, some issues remain. The G4-specific antibody BG4 failed to detect G4s at the hTERT promoter reported to comprise multiple G4s.51,62 On silencing TRF2, however, BG4 occupancy at the hTERT promoter was regained suggesting TRF2-bound hTERT promoter G4s escape detection by BG4.62 Likelihood of this, that is, BG4 excluding protein-bound in vivo G4s was noted by the authors.75 Since many if not all in vivo G4s might be protein-bound, antibodybased detection of in vivo G4s appears nontrivial. Second, the possibility that relatively high-affinity antibodies induce G4s that are otherwise not present in vivo could be a confounding factor. Therefore, TRF2, in addition to other factors that associate with G4s in vivo, might emerge as a viable option for detecting naturally occurring intracellular G4s within live cells.
Key References.
Rawal, P.; Kummarasetti, V. B. R.; Ravindran, J.; Kumar, N.; Halder, K.; Sharma, R.; Mukerji, M.; Das, S. K.; Chowdhury, S. Genome-Wide Prediction of G4 DNA as Regulatory Motifs: Role in Escherichia Coli Global Regulation. Genome Res. 2006, 16, 644-655.1 Whole genome analysis showed that potential G-quadruplexes were prevalent and conserved within promoters of bacteria. Specific transciption factor binding sites in E. coli co-occurred with promoter G-quadruplexes. Based on these data, G-quadruplexes were predicted as global gene regulatory motifs.
Thakur, R. K.; Kumar, P.; Halder, K.; Verma, A.; Kar, A.; Parent, J. L.; Basundra, R.; Kumar, A.; Chowdhury, S. Metastases Suppressor NM23-H2 Interaction with G-Quadruplex DNA within c-MYC Promoter Nuclease Hypersensitive Element Induces c-MYC Expression. Nucleic Acids Res. 2009, 37, 172-183.2 NM23-H2 was identified as a transcription factor that directly interacted with G-quadruplex. The association of NM23-H2 with promoter G-quadruplex inside cancer cells controlled transcription of the oncogene c-myc.
Mukherjee, A. K.; Sharma, S.; Bagri, S.; Kutum, R.; Kumar, P.; Hussain, A.; Singh, P.; Saha, D.; Kar, A.; Dash, D.; Chowdhury, S. Telomere Repeat-Binding Factor 2 Binds Extensively to Extra-Telomeric G-Quadruplexes and Regulates the Epigenetic Status of Several Gene Promoters. J. Biol. Chem. 2019, 294, 17709-17722.3 This showed that TRF2, known for years to bind to telomeres only, associated with thousands of non-telomeric sites genome-wide. A large fraction of these binding sites comprised promoter G-quadruplexes where TRF2-G-quadruplex interaction induced epigenetic gene regulation.
Mukherjee, A. K.; Sharma, S.; Sengupta, S.; Saha, D.; Kumar, P.; Hussain, T.; Srivastava, V.; Roy, S. D.; Shay, J. W.; Chowdhury, S. Telomere Length-Dependent Transcription and Epigenetic Modifications in Promoters Remote from Telomere Ends. PLoS Genet. 2018, 14, e1007782.4 In this paper, TRF2 binding and TRF2-induced regulation of genes remote from telomeres was reported to be sensitive to telomere length. Together with genome-wide TRF2-G-quadruplex association,3 this implicates G-quad-ruplexes in telomere-dependent gene regulation throughout the genome.
Acknowledgments
We acknowledge research fellowships to A.S. and S.S.R. (CSIR). S.C. is supported by research grants from DBT/Wellcome Trust India Alliance Fellowship (Grant IA/S/18/2/504021), Council of Scientific and Industrial Research (CSIR), Dept. of Biotechnology and DST (SERB), India. We thank all members of the Chowdhury laboratory for insightful discussions and suggestions.
Biographies
Biographies
Antara Sengupta received her Master’s Degree in Biotechnology from St. Xavier’s College, Kolkata. In 2016, she joined Shantanu Chowdhury’s group and contributed to the study on non-telomeric functions of TRF2. She is pursuing her doctoral (Ph.D.) studies in functional genomics focusing on hTERT regulation and the potential role of telomeric and non-telomeric G-quadruplexes at the CSIR-Institute of Genomics and Integrative Biology in New Delhi. She works with multiple cellular model systems and utilizes CRISPR technology as one of the genomic tools.
Shuvra Shekhar Roy completed his B. Tech and M. Tech in Biotechnology from KIIT University, Bhubaneswar. He is pursuing his doctoral (Ph.D.) studies in the Shantanu Chowdhury group at the CSIR-Institute of Genomics and Integrative Biology, New Delhi. His thesis work includes understanding the role of G-quadruplexes in modulating chromatin architecture and thus its genomic consequences.
Shantanu Chowdhury, following his Ph.D. in Chemistry from the Indian Institute of Chemical Technology in 1998, trained at the University of Nebraska, Lincoln, and the Max Planck Institute for Terrestrial Microbiology in postdoctoral fellowships. In 2002, he moved to the CSIR-Institute of Genomics and Integrative Biology in New Delhi as a faculty member. Research interests of his group include molecular and cell biology, genomics, and computational approaches in (1) function of non-duplex DNA structures called G-quadruplexes, (2) telomere and telomerase biology, and (3) clinical genomics to understand immune response in triple negative breast cancers.
Footnotes
▪ Author Information
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.0c00431
Notes
The authors declare no competing financial interest.
Contributor Information
Antara Sengupta, Integrative and Functional Biology Unity CSIR-Institute ofGenomics and Integrative Biology, New Delhi 110025, India; Academy ofScientific and Innovative Research (AcSIR), Ghaziabad 201002, India.
Shuvra Shekhar Roy, Integrative and Functional Biology Unit, CSIR-Institute ofGenomics and Integrative Biology, New Delhi 110025, India; Academy ofScientific and Innovative Research (AcSIR), Ghaziabad 201002, India.
Shantanu Chowdhury, Integrative and Functional Biology Unit and GNR Knowledge Centre for Genome and Informatics, CSIR-Institute of Genomics and Integrative Biology, New Delhi 110025, India; Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India.
References
- (1).Rawal P, Kummarasetti VBR, Ravindran J, Kumar N, Halder K, Sharma R, Mukerji M, Das SK, Chowdhury S. Genome-Wide Prediction of G4 DNA as Regulatory Motifs: Role in Escherichia Coli Global Regulation. Genome Res. 2006;16:644–655. doi: 10.1101/gr.4508806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Thakur RK, Kumar P, Halder K, Verma A, Kar A, Parent JL, Basundra R, Kumar A, Chowdhury S. Metastases Suppressor NM23-H2 Interaction with G-Quadruplex DNA within c-MYC Promoter Nuclease Hypersensitive Element Induces c-MYC Expression. Nucleic Acids Res. 2009;37:172–183. doi: 10.1093/nar/gkn919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Mukherjee AK, Sharma S, Bagri S, Kutum R, Kumar P, Hussain A, Singh P, Saha D, Kar A, Dash D, Chowdhury S. Telomere Repeat-Binding Factor 2 Binds Extensively to Extra-Telomeric G-Quadruplexes and Regulates the Epigenetic Status of Several Gene Promoters. J Biol Chem. 2019;294:17709–17722. doi: 10.1074/jbc.RA119.008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mukherjee AK, Sharma S, Sengupta S, Saha D, Kumar P, Hussain T, Srivastava V, Roy SD, Shay JW, Chowdhury S. Telomere Length-Dependent Transcription and Epigenetic Modifications in Promoters Remote from Telomere Ends. PLoS Genet. 2018;14:e1007782. doi: 10.1371/journal.pgen.1007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zimmerman SB, Cohen GH, Davies DR. X-Ray Fiber Diffraction and Model-Building Study of Polyguanylic Acid and Polyinosinic Acid. J Mol Biol. 1975;92:181–192. doi: 10.1016/0022-2836(75)90222-3. [DOI] [PubMed] [Google Scholar]
- (6).Blackburn EH, Gall JG. A Tandemly Repeated Sequence at the Termini of the Extrachromosomal Ribosomal RNA Genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- (7).Sen D, Gilbert W. Formation of Parallel Four-Stranded Complexes by Guanine-Rich Motifs in DNA and Its Implications for Meiosis. Nature. 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- (8).Balagurumoorthy P, Brahmachari SK. Structure and Stability of Human Telomeric Sequence. J Biol Chem. 1994;269:21858–21869. [PubMed] [Google Scholar]
- (9).Mergny JL, Helene C. G-Quadruplex DNA: A Target for Drug Design. Nat Med. 1998;4:1366–1367. doi: 10.1038/3949. [DOI] [PubMed] [Google Scholar]
- (10).Gomez D, Mergny JL, Riou JF. Detection of Telomerase Inhibitors Based on G-Quadruplex Ligands by a Modified Telomeric Repeat Amplification Protocol Assay. Cancer Res. 2002;62:3365–3368. [PubMed] [Google Scholar]
- (11).Schaffitzel C, Berger I, Postberg J, Hanes J, Lipps HJ, Plückthun A. In Vitro Generated Antibodies Specific for Telomeric Guanine-Quadruplex {DNA} React with {Stylonychia} Lemnae Macronuclei. Proc Natl Acad Sci U S A. 2001;98:8572–8577. doi: 10.1073/pnas.141229498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct Evidence for a G-Quadruplex in a Promoter Region and Its Targeting with a Small Molecule to Repress c-MYC Transcription. Proc Natl Acad Sci U S A. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Todd AK. Highly Prevalent Putative Quadruplex Sequence Motifs in Human DNA. Nucleic Acids Res. 2005;33:2901–2907. doi: 10.1093/nar/gki553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Huppert JL. Prevalence of Quadruplexes in the Human Genome. Nucleic Acids Res. 2005;33:2908–2916. doi: 10.1093/nar/gki609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Verma A, Halder K, Halder R, Yadav VK, Rawal P, Thakur RK, Mohd F, Sharma A, Chowdhury S. Genome-Wide Computational and Expression Analyses Reveal G-Quadruplex DNA Motifs as Conserved Cis-Regulatory Elements in Human and Related Species. J Med Chem. 2008;51:5641–5649. doi: 10.1021/jm800448a. [DOI] [PubMed] [Google Scholar]
- (16).Yadav VK, Abraham JK, Mani P, Kulshrestha R, Chowdhury S. Quad Base: Genome-Wide Database of G4 QuadBase: genome-wide database of G4 DNA Occurrence and Conservation in Human, Chimpanzee, Mouse and Rat Promoters and 146 Microbes. Nucleic Acids Res. 2007;36:D381–385. doi: 10.1093/nar/gkm781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bates P, Mergny J-L, Yang D. Quartets in G-Major. The First International Meeting on Quadruplex DNA. EMBO Rep. 2007;8:1003–1010. doi: 10.1038/sj.embor.7401073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Borman S. Ascent of Quadruplexes. Chem Eng News. 2007;85(22):12–17. [Google Scholar]
- (19).Dhapola P, Chowdhury S. Quad Base2: Web Server for Multiplexed Guanine Quadruplex Mining and Visualization. Nucleic Acids Res. 2016;44:W277–283. doi: 10.1093/nar/gkw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bedrat A, Lacroix L, Mergny JL. Re-Evaluation of G-Quadruplex Propensity with G4Hunter. Nucleic Acids Res. 2016;44:1746–1759. doi: 10.1093/nar/gkw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hon J, Martínek T, Zendulka J, Lexa M. Pqsfinder: An Exhaustive and Imperfection-Tolerant Search Tool for Potential Quadruplex-Forming Sequences in R. Bioinformatics. 2017;33:3373–3379. doi: 10.1093/bioinformatics/btx413. [DOI] [PubMed] [Google Scholar]
- (22).D’Antonio L, Bagga P. Computational Methods for Predicting Intramolecular G-Quadruplexes in Nucleotide Sequences; Proceedings. 2004 IEEE Computational Systems Bioinformatics Conference; 2004. p. 561. [Google Scholar]
- (23).Kumar P, Yadav VK, Baral A, Kumar P, Saha D, Chowdhury S. Zinc-Finger Transcription Factors Are Associated with Guanine Quadruplex Motifs in Human, Chimpanzee, Mouse and Rat Promoters Genome-Wide. Nucleic Acids Res. 2011;39:8005–8016. doi: 10.1093/nar/gkr536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lipps HJ, Rhodes D. G-Quadruplex Structures: In Vivo Evidence and Function. Trends Cell Biol. 2009;19:414–422. doi: 10.1016/j.tcb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- (25).Huppert JL, Balasubramanian S. G-Quadruplexes in Promoters throughout the Human Genome. Nucleic Acids Res. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Johnson JE, Smith JS, Kozak ML, Johnson FB. In Vivo Veritas: Using Yeast to Probe the Biological Functions of G-Quadruplexes. Biochimie. 2008;90:1250–1263. doi: 10.1016/j.biochi.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ruggiero E, Richter SN. G-Quadruplexes and G-Quadruplex Ligands: Targets and Tools in Antiviral Therapy. Nucleic Acids Res. 2018;46:3270–3283. doi: 10.1093/nar/gky187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Beaume N, Pathak R, Yadav VK, Kota S, Misra HS, Gautam HK, Chowdhury S. Genome-Wide Study Predicts Promoter-G4 DNA Motifs Regulate Selective Functions in Bacteria: Radioresistance of D. Radiodurans Involves G4 DNA-Mediated Regulation. Nucleic Acids Res. 2013;41:76–89. doi: 10.1093/nar/gks1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).De Matteis F, Gibbs AH, Smith AG. Inhibition of Protohaem Ferro-Lyase by N-Substituted Porphyrins. Structural Requirements for the Inhibitory Effect. Biochem J. 1980;189:645–648. doi: 10.1042/bj1890645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Verma A, Yadav VK, Basundra R, Kumar A, Chowdhury S. Evidence of Genome-Wide G4 DNA-Mediated Gene Expression in Human Cancer Cells. Nucleic Acids Res. 2009;37:4194–204. doi: 10.1093/nar/gkn1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Baral A, Kumar P, Halder R, Mani P, Yadav VK, Singh A, Das SK, Chowdhury S. Quadruplex-Single Nucleotide Polymorphisms (Quad-SNP) Influence Gene Expression Difference among Individuals. Nucleic Acids Res. 2012;40:3800–3811. doi: 10.1093/nar/gkr1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Guo JU, Bartel DP. RNA G-Quadruplexes Are Globally Unfolded in Eukaryotic Cells and Depleted in Bacteria. Science. 2016;353:aaf5371. doi: 10.1126/science.aaf5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Marsico G, Chambers VS, Sahakyan AB, McCauley P, Boutell JM, Antonio MD, Balasubramanian S. Whole Genome Experimental Maps of DNA G-Quadruplexes in Multiple Species. Nucleic Acids Res. 2019;47:3862. doi: 10.1093/nar/gkz179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Halder K, Halder R, Chowdhury S. Genome-Wide Analysis Predicts DNA Structural Motifs as Nucleosome Exclusion Signals. Mol BioSyst. 2009;5:1703–1712. doi: 10.1039/b905132e. [DOI] [PubMed] [Google Scholar]
- (35).Varshney D, Spiegel J, Zyner K, Tannahill D, Balasubramanian S. The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat Rev Mol Cell Biol. 2020;21:459. doi: 10.1038/s41580-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Halder R, Halder K, Sharma P, Garg G, Sengupta S, Chowdhury S. Guanine Quadruplex DNA Structure Restricts Methylation of CpG Dinucleotides Genome-Wide. Mol BioSyst. 2010;6:2439–2447. doi: 10.1039/c0mb00009d. [DOI] [PubMed] [Google Scholar]
- (37).Cree SL, Fredericks R, Miller A, Pearce FG, Filichev V, Fee C, Kennedy MA. DNA G-Quadruplexes Show Strong Interaction with DNA Methyltransferases in Vitro. FEBS Lett. 2016;590:2870–2883. doi: 10.1002/1873-3468.12331. [DOI] [PubMed] [Google Scholar]
- (38).Mao S-Q, Ghanbarian AT, Spiegel J, Martínez Cuesta S, Beraldi D, Di Antonio M, Marsico G, Hansel-Hertsch R, Tannahill D, Balasubramanian S. DNA G-Quadruplex Structures Mold the DNA Methylome. Nat Struct Mol Biol. 2018;25:951–957. doi: 10.1038/s41594-018-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zamiri B, Mirceta M, Bomsztyk K, Macgregor RB, Pearson CE. Quadruplex Formation by Both G-Rich and C-Rich DNA Strands of the C9orf72 (GGGGCC)8·(GGCCCC)8 Repeat: Effect of CpG Methylation. Nucleic Acids Res. 2015;43:10055–10064. doi: 10.1093/nar/gkv1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mukherjee AK, Sharma S, Chowdhury S. Non-Duplex G-Quadruplex Structures Emerge as Mediators of Epigenetic Modifications. Trends Genet. 2019;35:129. doi: 10.1016/j.tig.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Postel E, Berberich S, Flint S, Ferrone C. Human C-Myc Transcription Factor PuF Identified as Nm23-H2 Nucleoside Diphosphate Kinase, a Candidate Suppressor of Tumor Metastasis. Science. 1993;261:478–480. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- (42).Dexheimer TS, Carey SS, Zuohe S, Gokhale VM, Hu X, Murata LB, Maes EM, Weichsel A, Sun D, Meuillet EJ, Montfort WR, et al. NM23-H2 May Play an Indirect Role in Transcriptional Activation of c-Myc Gene Expression but Does Not Cleave the Nuclease Hypersensitive Element III 1. Mol Cancer Ther. 2009;8:1363–1377. doi: 10.1158/1535-7163.MCT-08-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Ghosal G, Muniyappa K. Saccharomyces Cerevisiae Mre11 Is a High-Affinity G4 DNA-Binding Protein and a G-Rich DNA-Specific Endonuclease: Implications for Replication of Telomeric DNA. Nucleic Acids Res. 2005;33:4692–4703. doi: 10.1093/nar/gki777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Balasubramanian S, Hurley LH, Neidle S. Targeting G-Quadruplexes in Gene Promoters: A Novel Anticancer Strategy? Nat Rev Drug Discovery. 2011;10:261–75. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Paramasivam M, Membrino A, Cogoi S, Fukuda H, Nakagama H, Xodo LE. Protein HnRNP A1 and Its Derivative Up1 Unfold Quadruplex DNA in the Human KRAS Promoter: Implications for Transcription. Nucleic Acids Res. 2009;37:2841–2853. doi: 10.1093/nar/gkp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bochman ML, Paeschke K, Zakian VA. DNA Secondary Structures: Stability and Function of G-Quadruplex Structures. Nat Rev Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mendoza O, Bourdoncle A, Boulé JB, Brosh RM, Mergny JL. G-Quadruplexes and Helicases. Nucleic Acids Res. 2016;44:1989–2006. doi: 10.1093/nar/gkw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Bugaut A, Balasubramanian S. 5’-UTR RNA G-Quadruplexes: Translation Regulation and Targeting. Nucleic Acids Res. 2012;40:4727–4741. doi: 10.1093/nar/gks068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Agarwala P, Pandey S, Ekka MK, Chakraborty D, Maiti S. Combinatorial Role of Two G-Quadruplexes in 5’ UTR of Transforming Growth Factor B2 (TGFβ2) Biochim Biophys Acta, Gen Subj. 2019;1863:129416. doi: 10.1016/j.bbagen.2019.129416. [DOI] [PubMed] [Google Scholar]
- (50).Yadav VK, Thakur RK, Eckloff B, Baral A, Singh A, Halder R, Kumar A, Alam MP, Kundu TK, Pandita R, Pandita TK, et al. Promoter-Proximal Transcription Factor Binding Is Transcriptionally Active When Coupled with Nucleosome Repositioning in Immediate Vicinity. Nucleic Acids Res. 2014;42:9602–9611. doi: 10.1093/nar/gku596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Palumbo SML, Ebbinghaus SW, Hurley LH. Formation of a Unique End-to-End Stacked Pair of G-Quadruplexes in the HTERT Core Promoter with Implications for Inhibition of Telomerase by G-Quadruplex-Interactive Ligands. J Am Chem Soc. 2009;131:10878–10891. doi: 10.1021/ja902281d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Saha D, Singh A, Hussain T, Srivastava V, Sengupta S, Kar A, Dhapola P, Dhople V, Ummanni R, Chowdhury S. J Biol Chem. 2017;292:15205–15215. doi: 10.1074/jbc.M117.792077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Schiavone D, Guilbaud G, Murat P, Papadopoulou C, Sarkies P, Prioleau M, Balasubramanian S, Sale JE. Determinants of G Quadruplex-induced Epigenetic Instability in REV 1-deficient Cells. EMBO J. 2014;33:2507–2520. doi: 10.15252/embj.201488398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Guilbaud G, Murat P, Recolin B, Campbell BC, Maiter A, Sale JE, Balasubramanian S. Local Epigenetic Reprograming Induced by G-Quadruplex Ligands. Nat Chem. 2017;9:1110–1117. doi: 10.1038/nchem.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Paeschke K, Bochman ML, Garcia PD, Cejka P, Friedman KL, Kowalczykowski SC, Zakian VA. Pif1 Family Helicases Suppress Genome Instability at G-Quadruplex Motifs. Nature. 2013;497:458–462. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Cogoi S, Ferino A, Miglietta G, Pedersen EB, Xodo LE. The Regulatory G4Motif of the Kirsten Ras (KRAS) Gene Is Sensitive to Guanine Oxidation: Implications on Transcription. Nucleic Acids Res. 2018;46:661–676. doi: 10.1093/nar/gkx1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Fleming AM, Zhu J, Howpay Manage SA, Burrows CJ. Human NEIL3 Gene Expression Regulated by Epigenetic-Like Oxidative DNA Modification. J Am Chem Soc. 2019;141:11036–11049. doi: 10.1021/jacs.9b01847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Fleming AM, Ding Y, Burrows CJ. Oxidative DNA Damage Is Epigenetic by Regulating Gene Transcription via Base Excision Repair. Proc Natl Acad Sci U S A. 2017;114:2604–2609. doi: 10.1073/pnas.1619809114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Kar A, Saha D, Purohit G, Singh A, Kumar P, Yadav VK, Kumar P, Thakur RK, Chowdhury S. Metastases Suppressor NME2 Associates with Telomere Ends and Telomerase and Reduces Telomerase Activity within Cells. Nucleic Acids Res. 2012;40:2554–2565. doi: 10.1093/nar/gkr1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Pedroso IM, Hayward W, Fletcher TM. The Effect of the TRF2 N-Terminal and TRFH Regions on Telomeric G-Quadruplex Structures. Nucleic Acids Res. 2009;37:1541–1554. doi: 10.1093/nar/gkn1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Hussain T, Saha D, Purohit G, Kar A, Mukherjee AK, Sharma S, Sengupta S, Dhapola P, Maji B, Vedagopuram S, Horikoshi NT, et al. Transcription Regulation of CDKN1A (P21/CIP1/WAF1) by TRF2 Is Epigenetically Controlled through the REST Repressor Complex. Sci Rep. 2017;7:11541. doi: 10.1038/s41598-017-11177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Sharma S, Mukherjee AK, Roy SS, Bagri S, Lier S, Verma M, Sengupta A, Kumar M, Nesse G, Pandey DP, Chowdhury S. Human Telomerase Expression Is under Direct Transcriptional Control of the Telomere-Binding-Factor TRF2. bioRxiv. 2020 doi: 10.1101/2020.01.15.907626. [DOI] [Google Scholar]
- (63).Heidenreich B, Kumar R. {TERT} Promoter Mutations in Telomere Biology. Mutat Res, Rev Mutat Res. 2017;771:15–31. doi: 10.1016/j.mrrev.2016.11.002. [DOI] [PubMed] [Google Scholar]
- (64).Kang HJ, Cui Y, Yin H, Scheid A, Hendricks WPD, Schmidt J, Sekulic A, Kong D, Trent JM, Gokhale V, Mao H, et al. A Pharmacological Chaperone Molecule Induces Cancer Cell Death by Restoring Tertiary DNA Structures in Mutant HTERT Promoters. J Am Chem Soc. 2016;138:13673–13692. doi: 10.1021/jacs.6b07598. [DOI] [PubMed] [Google Scholar]
- (65).Shay JW, Wright WE. Telomeres and Telomerase: Three Decades of Progress. Nat Rev Genet. 2019;20:299. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- (66).Pavc D, Wang B, Spindler L, Drevenšek-Olenik I, Plavec J, Šket P. GC Ends Control Topology of DNA G-Quadruplexes and Their Cation-Dependent Assembly. Nucleic Acids Res. 2020;48:2749–2761. doi: 10.1093/nar/gkaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Maity A, Winnerdy FR, Chang WD, Chen G, Phan AT. Intra-Locked G-Quadruplex Structures Formed by Irregular DNA G-Rich Motifs. Nucleic Acids Res. 2020;48:3315. doi: 10.1093/nar/gkaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Butovskaya E, Heddi B, Bakalar B, Richter SN, Phan AT. Major G-Quadruplex Form of HIV-1 LTR Reveals a (3 + 1) Folding Topology Containing a Stem-Loop. J Am Chem Soc. 2018;140:13654–13662. doi: 10.1021/jacs.8b05332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Sengupta A, Ganguly A, Chowdhury S. Promise of G-Quadruplex Structure Binding Ligands as Epigenetic Modifiers with Anti-Cancer Effects. Molecules. 2019;24:582. doi: 10.3390/molecules24030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Mishra SK, Shankar U, Jain N, Sikri K, Tyagi JS, Sharma TK, Mergny JL, Kumar A. Characterization of G-Quadruplex Motifs in EspB, EspK, and Cyp51 Genes of Mycobacterium Tuberculosis as Potential Drug Targets. Mol Ther–Nucleic Acids. 2019;16:698–706. doi: 10.1016/j.omtn.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Sengupta P, Chattopadhyay S, Chatterjee S. G-Quadruplex Surveillance in BCL-2 Gene: A Promising Therapeutic Intervention in Cancer Treatment. Drug Discovery Today. 2017;22:1165–1186. doi: 10.1016/j.drudis.2017.05.001. [DOI] [PubMed] [Google Scholar]
- (72).Xu H, Di Antonio M, McKinney S, Mathew V, Ho B, O’Neil NJ, Santos ND, Silvester J, Wei V, Garcia J, Kabeer F, et al. CX-5461 Is a DNA G-Quadruplex Stabilizer with Selective Lethality in BRCA1/2 Deficient Tumours. Nat Commun. 2017;8:14432. doi: 10.1038/ncomms14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Kaulage MH, Maji B, Pasadi S, Ali A, Bhattacharya S, Muniyappa K. Targeting G-Quadruplex DNA Structures in the Telomere and Oncogene Promoter Regions by Benzimidazolecarbazole Ligands. Eur J Med Chem. 2018;148:178–194. doi: 10.1016/j.ejmech.2018.01.091. [DOI] [PubMed] [Google Scholar]
- (74).Li Y, Tergaonkar V. Noncanonical Functions of Telomerase: Implications in Telomerase-Targeted Cancer Therapies. Cancer Res. 2014;74(74):1639–1644. doi: 10.1158/0008-5472.CAN-13-3568. [DOI] [PubMed] [Google Scholar]
- (75).Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative Visualization of DNA G-Quadruplex Structures in Human Cells. Nat Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]