Abstract
Background
Obesity compromises metabolic health and female fertility, yet not all obese women are similar in metabolic status. The extent to which fecundability is influenced by the metabolic health status of women who are overweight or obese before conception is unknown.
Objective
This study aimed to (1) determine the metabolic health status and (2) examine the association between metabolic health status and fecundability of overweight and obese women trying to conceive in the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO) cohort study.
Study Design
We conducted a prospective preconception cohort study of Asian women (Chinese, Malay and Indian) aged 18 to 45 years trying to conceive who were enrolled from 2015 to 2017 in KK Women’s and Children’s Hospital, Singapore (n=834). We defined women to have metabolically unhealthy status if they had: (1) three or more modified Joint Interim Statement metabolic syndrome (MetS) criteria; or (2) homeostasis model assessmentinsulin resistance (HOMA-IR) index ≥2.5. Body mass index was categorized as normal (18.5-22.9 kg/m2), overweight (23-27.4 kg/m2) or obese (≥27.5 kg/m2) based on the cut-off points for Asian populations. Fecundability was measured by time to pregnancy in menstrual cycles within a year of enrolment. Discrete-time proportional hazards models were used to estimate fecundability odds ratios (FRs), with adjustment for confounders and accounting for left truncation and right censoring.
Results
Of 232 overweight women, 28 (12.1%) and 25 (10.8%) were metabolically unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively. Of 175 obese women, 54 (30.9%) and 93 (53.1%) were metabolically unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively. Compared with metabolically healthy normal weight women, lower fecundability was observed in metabolically unhealthy overweight women based on MetS criteria [FR 0.38 (95% confidence interval 0.15, 0.92)] and HOMA-IR [0.68 (0.33, 1.39)], with MetS criteria showing a stronger association. Metabolically unhealthy obese women showed lower fecundability than the healthy normal weight reference group, by both MetS (0.35; 0.17, 0.72) and HOMA-IR criteria (0.43; 0.26, 0.71). Reduced fecundability was not observed in overweight or obese women who showed healthy metabolic profiles by either definition.
Conclusion
Overweight or obesity was not synonymous with having metabolic syndrome or insulin resistance. In our preconception cohort, metabolically unhealthy overweight and obese women showed reduced fecundability, unlike their counterparts who were metabolically healthy. These findings suggest that metabolic health status, rather than simply being overweight and obese per se, plays an important role in fecundability.
Keywords: conception, fertility, insulin resistance, lipids, metabolic syndrome, overweight, obesity, pregnancy planning, time to pregnancy
Introduction
A new population forecast for the end of the century predicts that most countries around the world will face a marked reduction in total fertility rates (TFRs), resulting in unsustainability of their current population levels.1 This issue is pertinent to Singapore given a TFR of 1.1 reported in 2020.2 Chronic diseases or metabolic disorders which are increasing in prevalence worldwide, including diabetes or dysglycemia, hypertension and dyslipidemia, have each been associated with impaired fertility or longer time to pregnancy (TTP).3–6 Instead of considering each individual component separately, utilizing a cluster of metabolic risk markers in metabolic syndrome (MetS) has been suggested as a better way of identifying women at increased risk of delayed conception.7 However, the association between MetS and TTP among women trying to conceive has not been well studied.
Although obesity increases a predominant risk factor for metabolic dysfunction and chronic diseases, not everyone who is obese experiences metabolic complications.8 This subset of individuals has been considered metabolically healthy,9 although other studies have questioned this concept.10 Given that overweight and obese women have a longer TTP,11–14 this raises the question of whether variations in metabolic health among overweight and obese women play a role in influencing their experience of delayed conception. Thus far, the extent to which fecundability is influenced by the metabolic health status of women who are overweight or obese while trying to conceive remains unknown. This has important implications for health policies and clinical care in the era of precision medicine, where susceptible individuals can be prioritized for intervention and provided with individualized treatment. To date, there is no universally accepted definition of metabolic health. Most studies define metabolic health using MetS components including waist circumference (WC), blood levels of triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), fasting glucose (FG) and blood pressure (BP); many others define it using the homeostasis model assessment-insulin resistance (HOMA-IR) score.9,15
Using data from the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO), we (1) determined the metabolic health status of overweight or obese Asian women who were trying to conceive, and (2) examined whether fecundability as measured by TTP was influenced by the metabolic health status of these overweight or obese women.
Methods
Participants
S-PRESTO (ClinicalTrials.gov, NCT03531658) is a prospective preconception cohort study designed to examine the long-term influences of events occurring before and during early pregnancy on mother-offspring metabolic and mental health.18 The participants recruited were Asian women of Chinese, Malay or Indian ethnicity attempting to conceive within the next 12 months and aged between 18–45 years. Those with known Type 1 diabetes or Type 2 diabetes, had been taking anticonvulsant medication, oral steroids or receiving assisted fertility treatment in the past one month were excluded. Women with newly diagnosed Type 2 diabetes at study baseline were included. Recruitment was between February 2015 and October 2017. This study was conducted according to the guidelines laid down in the Declaration of Helsinki. Ethics approval was obtained from the SingHealth Centralized Institute Review Board (reference 2014/692/D). All participants provided written informed consent.
Study procedure
During the baseline visit, a face-to-face interview was conducted by the research staff in the S-PRESTO cohort center at KK Women’s and Children’s hospital (KKH) to collect information on sociodemographic characteristics, obstetric history and lifestyle factors. This was followed by measurements of weight, height, WC, BP and fasting blood collection. At the end of the clinic visit, women were provided with home urinary pregnancy test kits (Biotron Diagnostics, USA) capable of detecting the beta subunit of human chorionic gonadotropin at a detection limit of 25 mIU/ml, and were reminded to perform the pregnancy test if their expected menstrual period was delayed by 3-4 days, or two weeks after unprotected intercourse. Women were asked to contact the research staff if they had a positive pregnancy test, so that an ultrasound scan could be scheduled to confirm a clinical pregnancy. Research staff contacted women after 6, 9 and 12 months of recruitment to track pregnancy status if no update was received. Those who did not conceive within one-year of recruitment (n=433) or were lost to follow-up (n=13) were withdrawn from the study.
Anthropometric measurements and blood collection
Weight was measured using a SECA 803 weighing scale (Hamburg, Germany) to the nearest 0.1 kg; height was measured using a SECA 213 stadiometer (Hamburg, Germany) to the nearest 0.1 cm. WC was measured using a SECA 212 non-stretchable measuring tape (Hamburg, Germany), at the uppermost lateral border of the ilium. Anthropometric training and standardization sessions were conducted every three months, with details of interobserver technical error of measurement and coefficient of variation reported elsewhere.19 BP was measured at the right upper arm using a semi-automatic blood pressure monitor (Microlife BP 3AS1-2). All measurements were taken in duplicate and averaged.
Fasting blood sample was collected at the same visit for measurements of TG, HDL-C, FG and insulin. TG and HDL-C were measured by colorimetric and enzymatic methods, respectively (Beckman AU5800). FG was measured by hexokinase method (Abbott Architect c8000) and insulin by sandwich immunoassay (Beckman DxI 800). HOMA-IR was calculated as FG (mmol/L) x fasting insulin (μU/mL)/22.5.20
Assessment of metabolic health
We used two definitions to separately classify women as having metabolically unhealthy status (MUS): (1) MetS criteria ≥3; (2) HOMA-IR ≥2.5. MetS was determined by the global definition using WC for Asians, with three or more of the following criteria:16,17 (1) WC ≥80 cm; (2) TG ≥1.7 mmol/L; (3) HDL-C <1.3 mmol/L; (4) FG ≥5.6 mmol/L; and (5) BP ≥130/85 mmHg, or on treatment. The criteria used were modified from the International Diabetes Federation (IDF) 2006 definition for MetS, as described by the Joint Interim Statement in 2009.16 A cut-off of 2.5 was selected for HOMA-IR as it represents the 90th percentile level among women with normoglycemia, defined by the World Health Organization (WHO) 2006 criteria.21 Further, the value of 2.5 was consistent with previous studies utilizing HOMA-IR alone to define metabolic health.15 Women with metabolically healthy status (MHS) were defined separately by having (1) ≤2 MetS criteria or (2) HOMA-IR <2.5.
Assessment of pregnancy and TTP
Pregnancy was initially identified from a positive urinary pregnancy test, followed by ultrasound scan confirmation of an intrauterine gestational sac after six weeks of amenorrhea. In the event where an ultrasound scan was not available or inconclusive, diagnosis of pregnancy was made clinically. The TTP was estimated by the number of menstrual cycles required to achieve a pregnancy over one-year of follow-up. This was calculated based on the interval between the dates of last menstrual period (LMP) at recruitment and before conception (for pregnant women) or last follow-up call (for censored women). The interval was then converted to cycles by dividing by the average cycle length, which was obtained from the reported minimum and maximum lengths of usual cycles at baseline. If women were uncertain of their cycle lengths or had irregular cycles (periods that varied by more than five days in the past six months), we used data from a follow-up questionnaire asking again about cycle length and LMP dates to verify or estimate cycle lengths. The TTP was calculated as the total discrete cycles-at-risk of pregnancy: (days of conception attempt at study entry/average cycle length) + [(date of LMP before conception or the most recent follow-up − date of LMP at recruitment)/average cycle length]. For women who became pregnant, one more conception cycle was added.6,14
Assessment of confounders
Maternal age was calculated from the date of birth and grouped as <35 and ≥35 years, and was included in the analysis as a continuous variable. Ethnicity was based on self-reported parental ethnic group and coded as Chinese, Malay, Indian or mixed ethnicity. Education was assessed by the highest attainment of academic level, classified as below, or at/above tertiary levels. Physical activity was evaluated using the short form of the International Physical Activity Questionnaire.22 Women were classified into three groups (inactive, minimally active and active) based on the metabolic equivalent task scores in minutes (MET-minutes).23 Smoking exposure was defined as any active or passive cigarette smoking. Alcohol intake was assessed based on the consumption of any alcoholic beverage in the past three months. BMI was calculated as weight (kg)/ height (m)2, classified as 18.5-22.9 (normal), 23-27.4 (overweight) and ≥27.5 kg/m2 (obese) using thresholds for Asian populations.24 Given that underweight women with BMI <18.5 kg/m2 (n=78) were not the target group in this study and none of them presented with MUS, we therefore excluded them from the present study.
Statistical analysis
We compared characteristics between women with MHS and MUS, as defined by MetS and HOMA-IR criteria using Pearson’s chi-squared tests or Fisher’s exact tests for categorical variables, and independent t-tests or Mann-Whitney tests for continuous variables. To estimate fecundability odds ratios (FRs) and 95% confidence intervals (CIs), accounting for left truncation and right censoring, we used discrete-time proportional hazards models analyzing TTP as a discrete scale based on the number of menstrual cycles.25,26 The FR represents the odds of conception among exposed women compared to unexposed women. A FR <1 indicates reduced fecundability with longer TTP, while a FR >1 indicates increased fecundability with shorter TTP. To account for left truncation, we based risk sets only on observed cycles-at-risk, i.e. cycles of conception attempt while participating in the study. Data were censored when women (i) had not conceived after one year from the recruitment, (ii) initiated fertility treatment, (iii) were lost to follow-up or withdrew from the study, whichever occurred first.
We used a directed acyclic graph to guide the selection of potential confounders to control for, based on a common cause influencing both metabolic health and fecundability.4,6,7,14 The selected potential confounders were age, ethnicity, education, smoking exposure, alcohol intake, physical activity and BMI. We performed additional separate models with and without adjustment for BMI to assess the contribution of metabolic variables on fecundability independent of overall adiposity as indicated by BMI. Multicollinearity was measured by variance inflation factors (VIFs) in the models with adjustment for BMI. VIFs <2 were observed across models, indicating the absence of multicollinearity between variables. We subsequently tested interactions between MHS/MUS and BMI on fecundability, by introducing each of the cross-product term of MHS/MUS (by MetS and HOMA-IR) and BMI into the fully adjusted formal models. To examine whether fecundability differed by MHS/MUS and BMI, we performed discrete-time proportional hazards model on women who had been classified into six mutually exclusive groups, namely (1) normal weight with MHS, (2) normal weight with MUS, (3) overweight with MHS, (4) overweight with MUS, (5) obesity with MHS and (6) obesity with MUS; normal weight with MHS (group 1) served as the reference group. For the main finding, BMI categories were based on the cut-offs for Asian populations, i.e. 18.5-22.9 (normal), 23-27.4 (overweight) and ≥27.5 kg/m2 (obese). For comparison with other non-Asian populations, additional analysis was performed using BMI at 18.5-24.9 (normal), 25-29.9 (overweight) and ≥30 kg/m2 (obesity) based on the conventional WHO classification.27
In view of the possibility that women who had been attempting to conceive for a long period might have a medical cause for infertility, we performed sensitivity analyses excluding women who had been attempting conception for more than 3 months (n=350), 6 months (n=232) and 12 months prior to study entry (n=122). This would help to consider for varying lengths of conception attempts before study entry and exclude potential cases with underlying pathologies for female and male infertility, given that one-year is a typical length of time after which couples seek infertility treatment. In the sensitivity analysis, we also excluded women with self-reported known polycystic ovarian syndrome (PCOS; n=9). Statistical analyses were conducted using the Stata Statistical Software, Release 16 (StataCorp, College Station, TX, USA).
Results
Participant characteristics
Supplemental Figure 1 shows the participant recruitment flowchart. We included a final sample of 834 women in this study, where 365 (43.8%) conceived naturally during the one-year follow-up and 469 (56.2%) were censored (433 did not conceive, 13 initiated fertility treatment, 13 lost to follow-up or self-withdrew). There were 256 (30.7%) and 349 (41.8%) women who achieved a pregnancy within 6 and 12 cycles of follow-up, respectively. The number of pregnancies by cycle over one-year was shown in Supplemental Figure 2. Compared with excluded women (n=198), included women were similar in majority of the demographic and lifestyle characteristics, except they were older and less likely to be overweight (Supplemental Table 1).
Participant characteristics based on metabolic health status are presented in Table 1. Of 834 included women, 85 (10.2%) and 123 (14.7%) were classified as MUS, defined by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively. In total, there were 154 (18.5%) women who met the criteria of MUS by MetS ≥3 criteria and/or HOMA-IR ≥2.5. Women with MUS as defined by MetS ≥3 criteria and/or HOMA-IR ≥2.5 were more likely to be of Malay or Indian ethnicity, to have attained a lower level of education, to have higher BMI, to spend longer time trying to conceive at study entry and to be exposed to cigarette smoke, but were less likely to consume alcohol in the past three months prior to conception, compared to their counterparts with MHS. The median duration of TTP in women with MUS (vs. MHS) by MetS ≥3 criteria and HOMA-IR ≥2.5 was 9 cycles (vs. 4 cycles) and 5 cycles (vs. 4 cycles), respectively.
Table 1. Characteristics of participants according to metabolic health status, defined by metabolic syndrome and HOMA-IR criteria (n=834).
| Characteristics | Total (n=834) | MetS ≥3 criteria | pa | HOMA-IR ≥2.5 | pa | MetS ≥3 criteria and/or HOMA-IR ≥2.5 | pa | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n=749) | Yes (n=85) | No (n=711) | Yes (n=123) | No (n=680) | Yes (n=154) | |||||
| Age, years | 30.91 + 3.80 | 30.86 + 3.79 | 31.36 + 3.83 | 0.242 | 31.09 + 3.76 | 29.85 + 3.84 | 0.001 | 31.00 + 3.74 | 30.50 + 4.02 | 0.140 |
| Ethnicity | 0.054 | <0.001 | <0.001 | |||||||
| Chinese | 589 (70.6) | 538 (71.8) | 51 (60.0) | 534 (75.1) | 55 (44.7) | 512 (75.3) | 77 (50.0) | |||
| Malay | 140 (16.8) | 119 (15.9) | 21 (24.7) | 98 (13.8) | 42 (34.1) | 94 (13.8) | 46 (29.9) | |||
| Indian | 79 (9.5) | 71 (9.5) | 8 (9.4) | 62 (8.7) | 17 (13.8) | 58 (8.5) | 21 (13.6) | |||
| Mix | 26 (3.1) | 21 (2.8) | 5 (5.9) | 17 (2.4) | 9 (7.3) | 16 (2.4) | 10 (6.5) | |||
| Highest education | <0.001 | <0.001 | <0.001 | |||||||
| Below tertiary | 314 (37.6) | 260 (34.7) | 54 (63.5) | 233 (32.8) | 81 (65.9) | 217 (31.9) | 97 (63.0) | |||
| Tertiary and above | 520 (62.4) | 489 (65.3) | 31 (36.5) | 478 (67.2) | 42 (34.1) | 463 (68.1) | 57 (37.0) | |||
| Physical activity | 0.656 | 0.529 | 0.458 | |||||||
| Inactive | 132 (15.8) | 116 (15.5) | 16 (18.8) | 110 (15.5) | 22 (17.9) | 103 (15.1) | 29 (18.8) | |||
| Minimally active | 425 (51.0) | 385 (51.4) | 40 (47.1) | 368 (51.8) | 57 (46.3) | 352 (51.8) | 73 (47.4) | |||
| Active | 277 (33.2) | 248 (33.1) | 29 (34.1) | 233 (32.8) | 44 (35.8) | 225 (33.1) | 52 (33.8) | |||
| Smoking exposure | 0.008 | <0.001 | <0.001 | |||||||
| No | 636 (76.3) | 581 (77.6) | 55 (64.7) | 562 (79.0) | 74 (60.2) | 539 (79.3) | 97 (63.0) | |||
| Yes | 198 (23.7) | 168 (22.4) | 30 (35.3) | 149 (21.0) | 49 (39.8) | 141 (20.7) | 57 (37.0) | |||
| Alcohol intake | 0.353 | 0.026 | 0.015 | |||||||
| No | 267 (32.0) | 236 (31.5) | 31 (36.5) | 217 (30.5) | 50 (40.7) | 205 (30.1) | 62 (40.3) | |||
| Yes | 567 (68.0) | 513 (68.5) | 54 (63.5) | 494 (69.5) | 73 (59.3) | 475 (69.9) | 92 (59.7) | |||
| Body mass index | <0.001 | <0.001 | <0.001 | |||||||
| 18.5-22.9 kg/m2 | 427 (51.2) | 424 (56.6) | 3 (3.5) | 422 (59.4) | 5 (4.1) | 419 (61.6) | 8 (5.2) | |||
| 23-27.4 kg/m2 | 232 (27.8) | 204 (27.2) | 28 (32.9) | 207 (29.1) | 25 (20.3) | 190 (27.9) | 42 (27.3) | |||
| ≥27.5 kg/m2 | 175 (21.0) | 121 (16.2) | 54 (63.5) | 82 (11.5) | 93 (75.6) | 71 (10.4) | 104 (67.5) | |||
| Attempted time to conceive at study entry, cycles | 1 (0-7) | 1 (0-6) | 4 (0-15) | 0.003 | 1 (0-6) | 3 (0-11) | 0.005 | 1 (0-6) | 4 (0-11) | 0.001 |
| Time to pregnancy, cycles | 4 (2-7) | 4 (2-7) | 9 (3-11) | 0.092 | 4 (2-7) | 5 (3-9) | 0.258 | 4 (2-7) | 5 (3-9) | 0.299 |
| Spontaneous conception | <0.001 | <0.001 | <0.001 | |||||||
| No | 469 (56.2) | 398 (53.1) | 71 (83.5) | 374 (52.6) | 95 (77.2) | 348 (51.2) | 121 (78.6) | |||
| Yes | 365 (43.8) | 351 (46.9) | 14 (16.5) | 337 (47.4) | 28 (22.8) | 332 (48.8) | 33 (21.4) | |||
| Waist circumference (cm) | 84.45 + 11.57 | 83.04 + 10.65 | 96.78 + 12.11 | <0.001 | 81.99 + 9.52 | 98.73 + 12.12 | <0.001 | 81.54 + 9.31 | 97.32 + 11.88 | <0.001 |
| Triglyceride (mmol/L) | 0.92 ± 0.51 | 0.81 ± 0.32 | 1.92 ± 0.76 | <0.001 | 0.82 ± 0.37 | 1.52 ± 0.74 | <0.001 | 0.78 ± 0.30 | 1.57 ± 0.72 | <0.001 |
| HDL-C (mmol/L) | 1.41 ± 0.29 | 1.45 ± 0.28 | 1.08 ± 0.14 | <0.001 | 1.46 ± 0.28 | 1.13 ± 0.18 | <0.001 | 1.48 ± 0.27 | 1.13 ± 0.17 | <0.001 |
| Fasting glucose (mmol/L) | 4.82 ± 0.58 | 4.73 ± 0.33 | 5.55 ± 1.33 | <0.001 | 4.72 ± 0.33 | 5.37 ± 1.15 | <0.001 | 4.70 ± 0.32 | 5.30 ± 1.05 | <0.001 |
| Systolic blood pressure (mmHg) | 106 ± 10 | 105 ± 9 | 115 ± 12 | <0.001 | 105 ± 9 | 112 ± 11 | <0.001 | 104 ± 9 | 112 ± 11 | <0.001 |
| Diastolic blood pressure (mmHg) | 69 ± 8 | 68 ± 7 | 78 ± 10 | <0.001 | 68 ± 8 | 75 ± 9 | <0.001 | 67 ± 7 | 75 ± 9 | <0.001 |
| HOMA-IR (mmol/L x μU/mL) | 1.14 (0.78-1.80) | 1.07 (0.76-1.57) | 3.07 (2.14-4.52) | <0.001 | 1.02 (0.74-1.44) | 3.72 (3.07-4.91) | <0.001 | 1.01 (0.73-1.41) | 3.33 (2.61-4.47) | <0.001 |
Values are presented in n (%) for categorical variables, means ± standard deviations or medians (25th – 75th percentiles) for continuous variables. HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; MetS, metabolic syndrome.
Based on Pearson’s chi-squared test or Fisher’s exact test for categorical variables, independent t-test or Mann-Whitney test for continuous variables.
Classification of participants by metabolic health status and BMI categories
Of 175 obese women, 54 (30.9%) and 93 (53.1%) were identified as unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively; while 43 (24.6%) met the criteria as unhealthy by both definitions (Table 1). Obese women were more likely to be identified as unhealthy by HOMA-IR than MetS criteria. Of 232 overweight women, 28 (12.1%) and 25 (10.8%) were identified as unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively, with 11 (4.7%) of them who met the criteria as unhealthy by both definitions (Table 1). A subgroup of 32 overweight and one obese women were free of any metabolic risk component and HOMA-IR was <2.5.
Association of metabolic health and its components with fecundability
As shown in Table 2, women with MUS based on MetS and HOMA-IR criteria showed lower FR of 0.36 (95% CI 0.21, 0.63) and 0.39 (0.25, 0.62), respectively, compared with women with MHS. Based on the individual components of MetS, lower fecundability was observed in women with raised TG (FR 0.42 (0.23, 0.78)), raised FG (0.45 (0.20, 1.03)), raised BP (0.57 (0.28, 1.17)), reduced HDL-C (0.88 (0.68, 1.12)) and high WC (0.94 (0.73, 1.21)); raised TG was associated with the greatest reduction in fecundability. Compared with the absence of any metabolic risk marker, the FR for each additional MetS component was 1.08 (0.83, 1.38) for one component, 0.91 (0.64, 1.29) for two components, 0.35 (0.18, 0.69) for three components and 0.35 (0.11, 1.15) for four or more components.
Table 2. The association of metabolic health status and its components with fecundability (n=834).
| Metabolic health indicators | n | Pregnancies | Cycles | Crude | Model 1a | Model 2b |
|---|---|---|---|---|---|---|
| FR (95% CI) | FR (95% CI) | FR (95% CI) | ||||
| Metabolic health status | ||||||
| MetS criteria ≥3 | 85 | 14 | 190 | 0.34 (0.20, 0.58) | 0.38 (0.22, 0.65) | 0.36 (0.21, 0.63) |
| HOMA-IR ≥2.5 | 123 | 28 | 290 | 0.47 (0.32, 0.69) | 0.48 (0.32, 0.72) | 0.39 (0.25, 0.62) |
| Metabolic components | ||||||
| High waist circumference (≥80 cm) | 510 | 200 | 1668 | 0.79 (0.65, 0.97) | 0.88 (0.71, 1.11) | 0.94 (0.73, 1.21) |
| Raised triglyceride (≥1.7 mmol/L) | 61 | 11 | 161 | 0.37 (0.20, 0.68) | 0.42 (0.23, 0.77) | 0.42 (0.23, 0.78) |
| Reduced HDL-C (<1.3 mmol/L) | 309 | 117 | 1003 | 0.81 (0.65, 1.01) | 0.85 (0.68, 1.08) | 0.88 (0.68, 1.12) |
| Raised fasting glucose (≥5.6 mmol/L) | 38 | 6 | 47 | 0.37 (0.16, 0.82) | 0.44 (0.19, 0.98) | 0.45 (0.20, 1.03) |
| Raised blood pressure (≥130/85 mmHg) | 36 | 8 | 77 | 0.49 (0.24, 0.99) | 0.56 (0.28, 1.13) | 0.57 (0.28, 1.17) |
| Presence of metabolic risk markers | ||||||
| 0 | 252 | 126 | 1106 | Ref. | Ref. | Ref. |
| 1 | 319 | 153 | 1192 | 0.98 (0.77, 1.24) | 1.06 (0.84, 1.35) | 1.08 (0.83, 1.38) |
| 2 | 178 | 72 | 572 | 0.84 (0.63, 1.13) | 0.94 (0.70, 1.28) | 0.91 (0.64, 1.29) |
| 3 | 63 | 11 | 140 | 0.33 (0.18, 0.62) | 0.38 (0.20, 0.70) | 0.35 (0.18, 0.69) |
| ≥4 | 22 | 3 | 50 | 0.29 (0.09, 0.90) | 0.39 (0.12, 1.23) | 0.35 (0.11, 1.15) |
Data were analysed using discrete-time proportional hazards models. CI, confidence interval; FR, fecundability odds ratio; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; MetS, metabolic syndrome.
Model 1: adjusted for age, ethnicity, education, physical activity, smoking exposure and alcohol intake.
Model 2: adjusted for Model 1 + body mass index.
Association between metabolic health status and fecundability by BMI categories
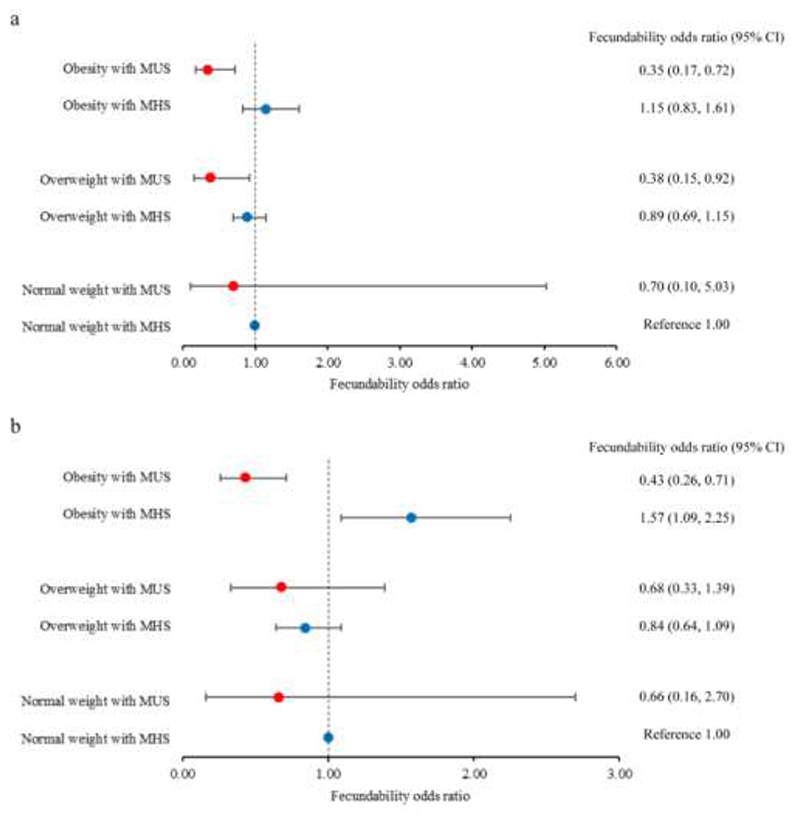
We observed significant interactions between MHS/MUS and BMI on fecundability (MetS or HOMA-IR: P-interaction <0.001). Compared with the healthy normal reference group, obese women with MUS showed lower fecundability, either by using MetS ≥3 criteria (FR 0.35 (0.17, 0.72)) (Figure 1a) or HOMA-IR ≥2.5 (0.43 (0.26, 0.71)) (Figure 1b). In contrast, obese women with MHS based on HOMA-IR <2.5 showed higher fecundability than the healthy normal weight women (1.57 (1.09, 2.25)) (Figure 2b). Similar findings were observed when obesity was defined using the conventional WHO criteria at BMI ≥30 kg/m2 (Supplemental Figure 3).
Figure 1.
Forest plots showing fecundability odds ratios according to metabolic health status of normal weight (18.5-22.9 kg/m2), overweight (23-27.4 kg/m2) and obese women (≥27.5 kg/m2) trying to conceive. Body mass index categories were based on cut-offs for Asian populations. Metabolic health status was defined by (a) metabolic syndrome ≤2 (MHS) vs. ≥3 (MUS) criteria; and (b) HOMA-IR <2.5 (MHS) vs. ≥2.5 (MUS). The dots and capped lines represent point estimates and 95% confidence intervals, respectively, of fecundability odds ratios. The reference group comprised normal weight women with MHS. Data were analyzed using discrete-time proportional hazards models, adjusting for age, ethnicity, education, physical activity, smoking exposure and alcohol intake. HOMA-IR, homeostasis model assessment-insulin resistance; MHS, metabolically healthy status; MUS, metabolically unhealthy status.
Overweight women with MUS showed lower fecundability compared with the healthy normal reference group, based on MetS ≥3 criteria (0.38 (0.15, 0.92)) (Figure 1a) and HOMA-IR ≥2.5 (0.68 (0.33, 1.39)) (Figure 1b); MUS defined by MetS ≥3 criteria was associated with a greater reduction in fecundability than HOMA-IR ≥2.5. For overweight women with MHS, their fecundability was comparable to healthy normal weight women, regardless of the metabolic definition used (MetS ≤2 criteria: 0.89 (0.69, 1.15); HOMA-IR <2.5: 0.84 (0.64, 1.09)) (Figure 1a; Figure 1b). There was a trend towards higher fecundability in this group of women, compared with the healthy normal weight group when the WHO criteria for BMI (25-29.9 kg/m2) was used (MetS ≤2 criteria: 1.49 (1.12, 1.99); HOMA-IR <2.5: 1.35 (1.00, 1.83)) (Supplemental Figure 3).
Normal weight women with MUS showed a trend of lower FR compared with the healthy normal weight group, regardless of how the metabolic health was defined or which BMI cut-offs were used. However, the samples size was restricted to only three women with MUS based on BMI 18.5-22.9 kg/m2 and 14 women with MUS based on BMI 18.5-24.9 kg/m2.
We observed similar findings when sensitivity analyses were performed including only women with an attempted time to conceive of no more than three months (n=484), six months (n=602) or 12 months (n=712) prior to study entry (Supplemental Table 2), or when including women without reported PCOS (n=825) (Supplemental Table 3).
Comment
Principal findings
Of 232 overweight women, 28 (12.1%) and 25 (10.8%) were metabolically unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively. Of 175 obese women, 54 (30.9%) and 93 (53.1%) were metabolically unhealthy by MetS ≥3 criteria and HOMA-IR ≥2.5, respectively. Among the five MetS components, raised TG showed the strongest association with reduced fecundability. MUS was associated with approximately 60% reduction in fecundability. Upon further stratification by BMI status, obese women with MUS consistently showed lower fecundability compared with normal weight women who were metabolically healthy, using either definition of metabolic health. For overweight women with MUS, greater reduction in fecundability was observed in those defined by MetS than HOMA-IR. Both overweight and obese women with MHS were protected from reduced fecundability over a one-year follow-up, suggesting a pivotal role for metabolic health, rather than BMI per se, with fecundability.
Results in the context of what is known
Traditionally, BMI was understood to have a bimodal effect on the relative risk of ovulatory infertility, with the risk of infertility being highest at the two extremes of BMI.28 However, BMI assessment alone is inadequate in determining metabolic health. There are clear biological differences between individuals with MHS and MUS. Obese individuals with MHS demonstrate lower visceral fat, preserved insulin sensitivity and better cardiorespiratory fitness than those with MUS.29 Our data support an impact of metabolic health on fertility. This is evidenced by our findings showing reduced fecundability in overweight and obese women with MUS, but not in those with MHS. The presence of MetS components has been associated with reduced fecundability and increased TTP,3,4,7 which is likely due to the adverse impact on ovarian response and oocyte competence. A detrimental metabolic profile is associated with a chronic inflammatory state, which may compromise embryo development, implantation and endometrial receptivity, with poor reproductive outcomes.12,30 This is accompanied by increased oxidative stress and reactive oxygen species that can reduce oocyte quality and quantity, fertilization and embryo quality.5 Insulin dysregulation has also been shown to disrupt ovarian function,31 contributing to lower fecundability in women with raised HOMA-IR. A milieu of increased ovarian follicular fluid insulin, TG, lactate, and C-reactive protein levels was associated with poorer reproductive outcomes in obese women.31
Further stratification based on BMI reveals a potentially important difference in the utility of these two definitions in defining metabolic health and their association with fecundability. In obese women, HOMA-IR appears as a more sensitive approach than MetS in determining metabolic ill-health that impacts on fecundability. This may be because in an early stage of insulin resistance, FG may not yet be impaired and hence some obese women may be erroneously deemed as metabolically healthy based on MetS criteria. Lifestyle interventions targeting healthy diet such as increasing fruit and vegetable intake and promoting physical activity either moderate- or high-intensity exercise are potential approaches to improving insulin sensitivity and lowering insulin resistance.32,33 This is crucial in counselling obese women who found to be insulin resistant, as reducing their HOMA-IR potentially reverses their subfertility.
Clinical implications
In addition to routine measurement of primary anthropometric indices such as weight and BMI, the present findings suggest that additional profiling of metabolic health status may be a valuable element in preconception care. This represents a paradigm shift towards holistic management, away from the traditional advice of weight loss and optimizing BMI alone. Future research is required to determine whether steps to improve metabolic health of overweight or obese women can indeed improve fecundability. Modifying metabolic risk factors and improving metabolic health, by means of lifestyle modifications aim at reducing BP, normalizing dyslipidemia, reducing FG and/ or weight loss, has far-reaching implications. Even if these women remain in the same BMI category, reversing their metabolic health status from unhealthy to healthy is likely to be beneficial to improving their fecundability in short-term, and reducing their risk of subsequent pregnancy complications and vascular disease development in long-term.35,36 For women who are metabolically healthy, education on keeping a healthy lifestyle to maintain a healthy metabolic profile is required for preventing the transition from healthy to unhealthy status.35
Strengths and limitations
The main strength of this study was the prospective cohort design that began from preconception, with recruitment from community and hospital settings, such that women trying to conceive naturally were assessed before conceiving and followed until pregnancies which were clinically confirmed by ultrasound. This would have improved measurement accuracy and reduced recall bias. In analyzing the metabolic health of participants using both MetS criteria and HOMA-IR, this study provides new insights into the potential of applying different screening criteria for metabolic health by BMI categories during preconception care. Although this study excluded women with known pre-existing diabetes at baseline, the inclusion of those with newly diagnosed Type 2 diabetes suggests that the findings could be generalized to population with diabetes given that these new cases of diabetes were all presented with MUS.
The study has several limitations. The assessment for metabolic health indicators was cross-sectional at baseline, and these measures were not repeated over the preconception follow-up period. Consequently, women may have had a different weight and metabolic profile at the time of conception. Given that overweight women with MHS tended to have increased fecundability compared to the reference group, it is possible that these women were most likely to modify their lifestyle, lose weight and maintain healthy metabolic profile during the follow-up, thereby improving fecundability. HOMA-IR values should be cautiously interpreted; a cut-off of 2.5 was taken as it was the 90th centile level among normoglycemia women in this cohort. Although the value is comparable to previous studies,15 it may not be applicable to other populations as inherent degrees of insulin resistance may differ among populations.37 Applying our findings to individual clinical practice would require adapting it to the unique range of HOMA-IR in the local patient population, given that our study population is of Asian women planning to conceive.
The low conception rate of 44% after one year of natural conception suggests a potential selection bias with recruitment by virtue of our preconception study design and targeting women in the community. The most fertile women would not have had time to consider joining the cohort study before conceiving. Thus, inadvertently, women tending to have a lower fertility than the general population presented for recruitment. Furthermore, we cannot exclude the possibility that some women might have temporarily stopped or delayed their conception attempts mid-study without informing the research team, while others might not have engaged in frequent sexual intercourse despite expressing an intention to conceive. Thus, the number of cycles at-risk might be overestimated, leading to a low overall conception rate. A limitation of our study is that the frequency of sexual intercourse and ovulation timing were not recorded, restricting our ability to verify the cycles at-risk. Further, menstrual cycles were estimated rather than accurately observed; this might introduce error in calculating cycles at-risk and potentially miss early pregnancy losses which could overestimate the fecundability outcome. Hence, the findings should be interpreted cautiously. Nonetheless, the low conception rate observed in this study is consistent with the relatively low TFR in Singapore, and comparable to the conception rate (42% after 12 cycles of conception attempts) among women trying to conceive in China.38
Underweight participants were not included in this study since they were not the target group based on the study aim and none were MUS as defined by either MetS ≥3 or HOMA-IR ≥2.5 criteria. However, the metabolic profile of the underweight women could be examined in future larger cohorts that are specifically designed to address this topic, to provide insights on whether metabolic health screening is required as part of fecundability assessment in this group.
Conclusions
Overweight or obesity was not synonymous with having MetS or insulin resistance. Reduced fecundability was evident in overweight and obese women with MUS, but not in those similarly overweight and obese women with MHS. These findings suggest that metabolic health status, rather than simply being overweight and obese per se, plays an important role in fecundability. In addition to routine measurement of body mass index, assessment of metabolic profile may be a valuable element in preconception care.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
Obesity compromises metabolic health and female fertility, yet not all obese women have a similar metabolic status. The extent to which fecundability is influenced by the metabolic health status of overweight or obese women while trying to conceive is poorly understood.
What are the key findings?
Metabolically unhealthy overweight and obese women showed reduced fecundability, unlike their counterparts who were metabolically healthy.
What does this study add to what is already known?
This study presents new evidence that overweight and obese women who were metabolically healthy did not have reduced fecundability when trying to conceive over one-year. Determining metabolic health status, rather than relying on body mass index per se, enhances our understanding of the relationship of adiposity with fecundability, highlighting the importance of metabolic health screening during preconception care, alongside body weight assessment.
Acknowledgements
We thank the S-PRESTO staff, participants and the study group, including Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Bee Wah Lee, Bernard Chern, Bobby Cheon, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Doris Fok, Elaine Quah, Elizabeth Tham, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Faidon Magkos, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Yu Chen, Heng Hao Tan, Hugo P S van Bever, Izzuddin Bin Mohd Aris, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Kenneth Kwek, Kuan Jin Lee, Lieng Hsi Ling, Ling Wei Chen, Lourdes Mary Daniel, Marielle V. Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Leow, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, Peter D Gluckman, Queenie Ling Jun Li, Sendhil Velan, Seng Bin Ang, Sharon Ng, Shephali Tagore, Shirong Cai, Shu E Soh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue Anne Toh, Teng Hong Tan, Tong Wei Yew, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Yiong Huak Chan, Yung Seng Lee.
Source of Funding
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Program and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. Y.B.C. is supported by a Clinician Scientist Award from the Singapore NMRC (NMRC/CSA/0039/2012). K.M.G. is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042)) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004), the European Union (Erasmus+ Program ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174). S.Y.C. is supported by a Clinician Scientist Award from the Singapore NMRC (NMRC/CSA-INV/0010/2016). J.K.Y.C. is supported by a Clinician Scientist Award from the Singapore NMRC (CSA(SI)/008/2016). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Condensation: Metabolic health status, rather than simply being overweight and obese per se, plays an important role in fecundability.
Conflicts of Interest
K.M.G. and Y.S.C. have received reimbursement to speak at conferences sponsored by companies selling nutritional products. K.M.G., Y.S.C. and S.Y.C. are part of an academic consortium who have received research funding from Abbott, Nutrition, Nestle and Danone. Other authors report no conflict of interest.
Contributor Information
Dr See Ling Loy, Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore; Duke-NUS Medical School, Singapore, Singapore.
Dr Daniel Wei Keong Chan, Department of Pediatrics, KK Women’s and Children’s Hospital, Singapore, Singapore.
Dr Chee Wai Ku, Duke-NUS Medical School, Singapore, Singapore; Department of Obstetrics & Gynecology, KK Women’s and Children’s Hospital, Singapore, Singapore.
Dr Yin Bun Cheung, Program in Health Services & Systems Research and Centre for Quantitative Medicine, Duke-NUS Medical School, Singapore, Singapore; Tampere Centre for Child, Adolescent and Maternal Health Research, Tampere University, Tampere, Finland.
Dr Keith M. Godfrey, Medical Research Council Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom.
Dr Karen Mei Ling Tan, Duke-NUS Medical School, Singapore, Singapore; Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore.
Dr Yap-Seng Chong, Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, National University Health System, Singapore, Singapore.
Dr Lynette Pei-Chi Shek, Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore; Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, National University Health System, Singapore, Singapore; Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore.
Dr Kok Hian Tan, Department of Maternal Fetal Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore.
Dr Shiao-Yng Chan, Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore; Department of Obstetrics & Gynecology, Yong Loo Lin School of Medicine, National University of Singapore, National University Health System, Singapore, Singapore.
Dr Jerry Kok Yen Chan, Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore; Duke-NUS Medical School, Singapore, Singapore.
Dr Fabian Yap, Duke-NUS Medical School, Singapore, Singapore; Department of Pediatrics, KK Women’s and Children’s Hospital, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
References
- 1.Vollset SE, Goren E, Yuan CW, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285–1306. doi: 10.1016/S0140-6736(20)30677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singapore Department of Statistics. Population Trends. 2020. [Accessed Mar 9, 2021]. Available at: https://www.singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/visualising-data/population-trends.
- 3.Schisterman EF, Mumford SL, Browne RW, Barr DB, Chen Z, Louis GM. Lipid concentrations and couple fecundity: the LIFE study. J Clin Endocrinol Metab. 2014;99:2786–94. doi: 10.1210/jc.2013-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugh SJ, Schisterman EF, Browne RW, et al. Preconception maternal lipoprotein levels in relation to fecundability. Hum Reprod. 2017;32:1055–63. doi: 10.1093/humrep/dex052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verit FF, YildizZeyrek F, Zebitay AG, Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med. 2017;44:28–32. doi: 10.5653/cerm.2017.44.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loy SL, Ku CW, Lai AEQ, et al. Plasma glycemic measures and fecundability in a Singapore preconception cohort study. Fertil Steril. 2021;115:138–47. doi: 10.1016/j.fertnstert.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grieger JA, Grzeskowiak LE, Smithers LG, et al. Metabolic syndrome and time to pregnancy: a retrospective study of nulliparous women. BJOG. 2019;126:852–62. doi: 10.1111/1471-0528.15647. [DOI] [PubMed] [Google Scholar]
- 8.Cirulli ET, Guo L, Leon Swisher C, et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019;29:488–500.:e2. doi: 10.1016/j.cmet.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978–89. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Macpherson J, Gray SR, et al. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK Biobank participants. Diabetologia. 2021 doi: 10.1007/s00125-021-05484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28:2856–64. doi: 10.1093/humrep/det333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise LA, Rothman KJ, Mikkelsen EM, Sørensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–64. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnon CJ, Hatch EE, Rothman KJ, et al. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil Steril. 2016;106:451–9. doi: 10.1016/j.fertnstert.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Loy SL, Cheung YB, Soh SE, et al. Female adiposity and time-to-pregnancy: a multiethnic prospective cohort. Hum Reprod. 2018;33:2141–9. doi: 10.1093/humrep/dey300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beh S. Is metabolically healthy obesity a useful concept? Diabet Med. 2019;36:539–45. doi: 10.1111/dme.13869. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KG, Eckel RH, Grundy SM, et al. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 17.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 18.Loo EXL, Soh SE, Loy SL, et al. S-PRESTO Study Group. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO) Eur J Epidemiol. 2021;36:129–42. doi: 10.1007/s10654-020-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu AHY, Aris IM, Ng S, et al. Anthropometric measures and HbA1c to detect dysglycemia in young Asian women planning conception: The S-PRESTO cohort. Sci Rep. 2020;10:9228. doi: 10.1038/s41598-020-66147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. World Hearth Organization; Geneva: 2006. [Google Scholar]
- 22.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire:12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) 2005. [Accessed May 23, 2021]. Available at: www.ipaq.ki.se.
- 24.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 25.Spira A. The use of fecundability in epidemiological surveys. Hum Reprod. 1998;13:1753–6. doi: 10.1093/oxfordjournals.humrep.a019710. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox AJ. Fertility and pregnancy: an epidemiologic perspective. Oxford University Press; United Kingdom: 2010. [Google Scholar]
- 27.Expert panel on the identification, evaluation, and treatment of overweight and obesity in adults. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–67. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 28.Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–90. doi: 10.1097/00001648200203000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Lu Y, Zhu Q, et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol. 2019;221:138.e1–138.e12. doi: 10.1016/j.ajog.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Robker RL, Akison LK, Bennett BD, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94:1533–40. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 32.Fernström M, Fernberg U, Hurtig-Wennlöf A. Insulin resistance (HOMA-IR) and body fat (%) are associated to low intake of fruit and vegetables in Swedish, young adults: the cross-sectional lifestyle, biomarkers and atherosclerosis study. BMC Nutr. 2019;5:15. doi: 10.1186/s40795-019-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan BJ, Schleh MW, Ahn C, et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J Clin Endocrinol Metab. 2020;105:e2941–59. doi: 10.1210/clinem/dgaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30:399–404. doi: 10.1016/j.tcm.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Gao M, Lv J, Yu C, et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020;17:e1003351. doi: 10.1371/journal.pmed.1003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnus MC, Fraser A, Rich-Edwards JW, Magnus P, Lawlor DA, Håberg SE. Time-to-pregnancy and risk of cardiovascular disease among men and women. Eur J Epidemiol. 2021;36:383–91. doi: 10.1007/s10654-021-00718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, Hong X, Zhang H, et al. Pre-pregnancy maternal fasting plasma glucose levels in relation to time to pregnancy among the couples attempting first pregnancy. Human Reprod. 2019;34:1325–33. doi: 10.1093/humrep/dez069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.