Abstract
Calcium ions (Ca2+) act as secondary messengers in a plethora of cellular processes and play crucial role in cellular organelle function and homeostasis. The average resting concentration of Ca2+ is nearly 100 nM and in certain cells it can reach up to 1 μM. The high range of Ca2+ concentration across the plasma membrane and intracellular Ca2+ stores demands a well-coordinated maintenance of free Ca2+ via influx, efflux, buffering and storage. Endoplasmic Reticulum (ER) and Mitochondria depend on Ca2+ for their function and also serve as major players in intracellular Ca2+ homeostasis. The ER-mitochondria interplay helps in orchestrating cellular calcium homeostasis to avoid any detrimental effect resulting from Ca2+ overload or depletion. Since Ca2+ plays a central role in many biological processes it is an essential component of the virus-host interactions. The large gradient across membranes enable the viruses to easily modulate this buffered environment to meet their needs. Viruses exploit Ca2+ signaling to establish productive infection and evade the host immune defense. In this review we will detail the interplay between the viruses and cellular & ER-mitochondrial calcium signaling and the significance of these events on viral life cycle and disease pathogenesis.
Keywords: Virus, Calcium, Mitochondria, Mitochondrial calcium uniporter, Calcium homeostasis, Antiviral signaling
1. Introduction
The ubiquitous role of calcium ions (Ca2+) as a secondary messenger in a plethora of cellular processes is well known. This simple bivalent cation regulates complex signaling mechanisms and acts as a torch-bearer of many extracellular and intracellular signaling pathways (Contreras et al., 2010; Song et al., 2019; Wang et al., 2015). There is a huge variability in Ca2+ concentration across different subcellular compartments; for instance Ca2+ concentration in the intracellular stores like Endoplasmic Reticulum (ER) and Golgi apparatus is around 200–650 μM, whereas in the cytosol the Ca2+ concentration is around 100 nM under resting conditions (Berridge et al., 2000; Raffaello et al., 2016; Zampese and Pizzo, 2012). Ca2+ concentration in the extracellular matrix is also several fold high (1–2 mM) compared to intracellular concentrations. At times the cytosolic Ca2+ concentration sharply increases to 1–3 μM due to certain cellular events like Ca2+ release from intracellular stores, or rapid influx from extracellular milieu. The ER and Golgi help in maintaining cellular Ca2+ homeostasis by acting as cellular Ca2+ stores. Whereas, the mitochondria functions as a Ca2+ buffering organelle and plays a vital role in cellular Ca2+ homeostasis by transiently taking up high levels of Ca2+ directly from cytosol or intracellular stores during conditions involving sharp rise in cellular Ca2+ concentration.
Overall the cellular Ca2+ homeostasis is met by well-coordinated processes of calcium influx, efflux, buffering and storage (Tang et al., 2015). Organelles like ER and mitochondria are an integral part of these processes and are vital to prevent detrimental effects resulting from large Ca2+ gradient flux across the membranes (Wang et al., 2015).
2. Calcium and Mitochondria: The intricate connection
Mitochondria, are highly dynamic double-membrane organelles with pleiotropic functions and serve as a hub of many signaling events (Paupe and Prudent, 2018; Romero-Garcia and Prado-Garcia, 2019). Mitochondria act as transient calcium buffers and can accommodate a broad range of intracellular calcium (50–500 nM) (Romero-Garcia and Prado-Garcia, 2019). While many cellular and mitochondrial functions rely on Ca2+, in turn mitochondria serve a vital role in modulating cellular Ca2+ dynamics, hence both share a symbiotic association. Deregulation or disruption of either of their functions may lead to disruption in cellular homeostasis and lead to cell death (Bravo-Sagua et al., 2017). For instance, a recent study in rat ventricular myocytes suggest that enhanced cytosolic Ca2+ can promote mitochondrial fragmentation or fission. On the other hand, depletion of cytosolic Ca2+ promotes the active movement of mitochondria towards cellular Ca2+ stores like ER and promotes the formation of ER-mitochondrial contacts through mitochondrial associated membranes (MAMs) and uptake of mitochondrial Ca2+. Interestingly, mitochondrial fragmentation will aide/faciitate efficient transport of mitochondria (Bravo-Sagua et al., 2017; Hom et al., 2010). Ca2+ uptake by mitochondria is also affected by the mitochondrial size, morphology and vicinity of mitochondria from the Ca2+ release sites. For instance, the tubular interconnected mitochondrial network has a higher Ca2+ uptake capacity due to a bigger matrix volume (Bravo-Sagua et al., 2017).
Ca2+ plays specific roles in distinct mitochondrial compartments. Ca2+ in the intermembrane space (IMS) regulates the functions of many inner mitochondrial membrane (IMM) resident enzymes. Certain Ca2+ binding EF-hand containing substrate carrier proteins are also regulated by Ca2+. Such substrate carriers can sense an increase in cytosolic Ca2+ level and accordingly pass metabolite and cofactors into the mitochondrial matrix to accelerate several metabolic reactions. In the mitochondrial matrix, Ca2+ is required for ATP production via the TCA cycle and ETC (Denton, 2009). Ca2+ levels in the mitochondrial matrix also regulates cAMP activation through protein kinase A dependent pathway, which in turn regulates ATP production capacity of the mitochondria (Di Benedetto et al., 2013). Although Ca2+ is required for ATP production, excess influx of Ca2+ into mitochondrial matrix to support accelerated ATP production can lead to higher ROS production, which eventually induces loss of mitochondrial membrane potential (MMP). These processes in combination result in the opening of mitochondrial transition pore (mPTP) and release of apoptotic factors. The opening of mPTP can also be triggered by excess ROS, lower H+ ions in the mitochondrial matrix and by direct interaction with polyphosphates (Stansfield, 2014). The crucial interconnections of Ca2+ and mitochondria are shown to be deregulated in many human diseases and are also exploited by invading pathogens for their benefit.
2.1. Mitochondrial calcium transport pathways
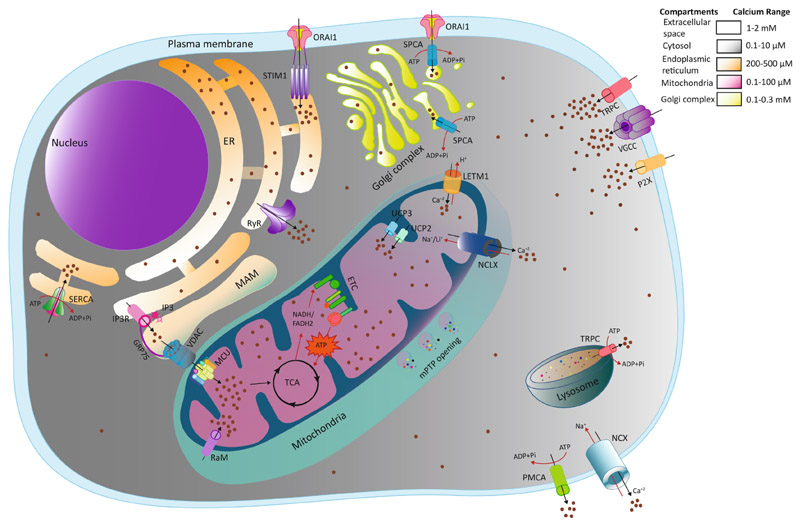
Though a moderate level of mitochondrial Ca2+ is beneficial in terms of cellular energy metabolism, mitochondria cannot resist higher Ca2+ levels for a prolonged period, which may lead to mitochondrial membrane collapse and cell death (Bravo-Sagua et al., 2017). Therefore, the Ca2+ influx and efflux across the mitochondrial membrane are tightly regulated through several ion channels, pumps and Ca2+ binding proteins (Zampese and Pizzo, 2012) (Fig. 1).
Fig. 1. Model depicting cellular calcium trafficking.
Calcium migrates from the extracellular matrix (high Ca2+ concentration) to cytosol (low Ca2+ concentration) across plasma membrane via Voltage-Gated Calcium Channels (VGCCs), Transient Receptor potential Channels (TRPs) and Purinergic receptors (P2Xs). Intracellular stores like ER replenish the depleted Ca2+ level directly through plasma membrane transport via the ORAI1-STIM1 complex, otherwise known as Store-operated Calcium Entry (SOCE). Sarco-Endoplasmic Reticulum Calcium ATPase (SERCA) also maintains the basal ER Ca2+ level by ATP-dependent Ca2+ efflux from the cytoplasm. Likewise, Golgi apparatus restores their Ca2+ store via Secretory Pathway Calcium ATPase (SPCA) from extracellular milieu as well as cytoplasm. Lysosomes use transient receptor potential channels (TRPC) for Ca2+ transport. Ca2+ transport between ER and mitochondria occurs in a highly organized manner through Mitochondrial Associated Membranes (MAMS). IP3 receptor (IP3R) mediated Ca2 + release from ER is sensed and uptaken by mitochondrial Voltage-Dependent Anion Channel (VDAC) and MCU across mitochondrial OMM and IMM respectively. In the mitochondrial matrix, Ca2+ plays a major role in cellular metabolism and oxidative phosphorylation to generate ATP required for innumerable cellular processes. However, some other transporters or modes are known to transport Ca2+ into mitochondria like uncoupling proteins 2 and 3 (UCP2 and UCP3), leucine zipper-EF-hand-containing transmembrane protein1 (LETM1) and rapid uptake mode (RaM). To overcome the increase in mitochondrial calcium concentration, efflux occurs through the Na+/Ca2+/Li + exchanger (NCLX) across the mitochondrial membrane. Unchecked Ca2+ overload in mitochondria leads to loss of mitochondrial membrane potential (MMP), the opening of mitochondrial permeability Transition Pore (mPTP) and release of apoptotic stimuli. Cytosolic Ca2+ returns to its basal level by efflux through Na+/Ca2+exchangers (NCX) and Plasma Membrane Calcium ATPase (PMCA) pump. The range of Ca2+ concentrations (from resting to stimulated) in different cellular compartments are indicated in color-coded gradients. Abbreviations: IP3: Inositol 1, 4, 5 Triphosphate; MCU: mitochondrial Calcium Uniporter; OMM: Outer Mitochondrial Membrane; IMM: Inner Mitochondrial Membrane; ATP: Adenosine Triphosphate.
2.1.1. Mitochondrial Ca2+ uptake
To enter mitochondria, Ca2+ needs to travel through the ion-permeable outer mitochondrial membrane (OMM) followed by the ion impermeable inner mitochondrial membrane (IMM). The permeability of the OMM can be attributed to the voltage-dependent anion channel (VDAC). Earlier studies on IMM have mentioned that it carries out Ca2+ uptake in a rate-limiting manner mediated through electrochemical gradient generated through oxidative phosphorylation (Drago et al., 2011). However, the recent discovery of mitochondrial calcium uniporter (MCU) in 2011 has changed this notion. MCU is the major player involved in Ca2+ transport through IMM in a highly regulated manner (Baughman et al., 2011; De Stefani et al., 2011). Ca2+ uptake through MCU at a very high conductance rate happens through rapid uptake mode (RaM) in specialized cells like heart and liver cells even at sub-optimal cytosolic Ca2+ concentration (50–100 nM) (Gunter and Gunter, 2001; Sparagna et al., 1995). Surprisingly, MCU knockout mice show minimal alteration in mitochondrial bioenergetics despite great reduction in mitochondrial matrix Ca2+ levels. However, uptake of mitochondrial Ca2+ was not completely abolished in these mice suggestive of alternative Ca2+ uptake pathway that may help mitochondria adapt to adverse situations like these. The other channels involved in mitochondrial Ca2+ transport include ryanodine receptor 1 (mRyR1), Letm1 (leucine zipper-EF-hand containing transmembrane protein 1) and uncoupling protein 2 and 3 (UCP2 and 3) (Belosludtsev et al., 2019; Elustondo et al., 2017). mRyr1 is mostly found in metabolically active cells like cardiac cells and neurons owing to their higher conductance rate despite the high energy required by mRyr1 (Holmström et al., 2015). Letm1 is also known to play a role in Ca2+ efflux through H+ exchange and it is also known to act as a K+/H+ exchanger. However, there is a disagreement over its primary role as a Ca2+ or K+ exchanger (Hashimi et al., 2013; Jiang et al., 2009; Li et al., 2019; Nowikovsky et al., 2007). UCP2 and 3 have been recently implicated in mammalian Ca2+ transport as well as in the regulation of MCU in association with other cellular proteins (Trenker et al., 2007).
2.1.2. MCU: Key player in mitochondrial calcium transport
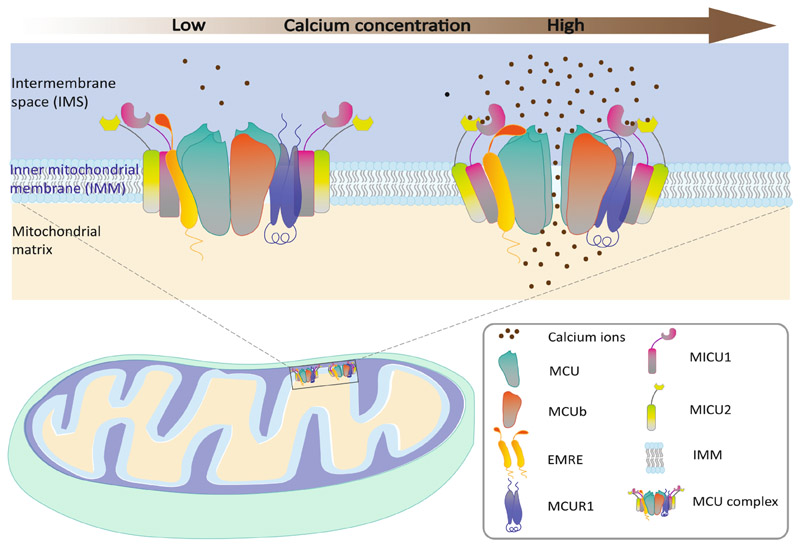
MCU, a product of the ccdc109a (chromosomal location: 10q22.1) gene is a recently discovered transmembrane protein that has gathered attention because of its key role in mitochondrial Ca2+ uptake. The function of MCU is coordinated by many regulatory proteins and the entire complex is termed as the MCU complex (Fig. 2). It consists of two outer subunits named mitochondrial calcium uptake 1 and 2 (MICU1 and MICU2), a ~ 10 kDa single-pass membrane protein EMRE and MCU regulator 1 (MCUR1). While MICU1 and MICU2 act as calcium sensors that enable selective ion transport and control Ca2+ influx (Baughman et al., 2011; Perocchi et al., 2010; Plovanich et al., 2013), EMRE serves as the middle man between components of the MCU complex and its regulators and allows Ca2+ access (Sancak et al., 2013; Tsai et al., 2017). MCUb is a dominant-negative regulator of MCU and shares 50% sequence similarity with MCU (Belosludtsev et al., 2019; Raffaello et al., 2013). Another regulator MCUR1 establishes a connection between MCU and EMRE and helps in MCU complex final assembly (Mallilankaraman et al., 2012; Tomar et al., 2016).
Fig. 2. Schematic diagram of Calcium transport through MCU complex.
Calcium influx mainly occurs via a multiprotein dynamic complex known as MCU in the inner mitochondrial membrane. It is composed of the central pore-forming subunits MCU, the dominant-negative regulator MCUb and the other regulatory subunits MICU1, MICU2, EMRE and MCUR1. At resting conditions (low level of cytosolic Ca2+), MICU1 and MICU2 act as gatekeepers for Ca2+ transport and limit calcium passage limiting Ca2+ passage through MCU (Left Panel). When the cellular Ca2+ signaling is activated leading to an increase in cytosolic Ca2+, MICU1 senses the increase in Ca2+ concentration resulting in the change of conformation and heterodimer formation with MICU2. Then MCUR1 establishes the connection between MCU and EMRE to assemble the MCU complex in an open conformation allowing the Ca2+ entry through the channel (Right panel).
2.1.3. Mitochondrial Ca2+ efflux pathways
Mitochondrial Ca2+efflux is equally important as its uptake to maintain intracellular Ca2+ homeostasis. Ca2+ efflux at the mitochondrial membrane is majorly coordinated by Na+/Ca2+/Li+ exchanger NCLX (Khananshvili, 2013). This process is also coordinated by Letm1, (Shao et al., 2016), the transient opening of mPTP and lipid pores (Briston et al., 2019; Sultan and Sokolove, 2001). Other factors like high levels of ROS (by product of oxidative phosphorylation) can also lead to mPTP opening. Persistent opening of mPTP leads to release of proapoptotic factor from mitochondria and leads to apoptosis. This dysregulation of Ca2+ efflux from mitochondria has been implicated in human pathologies like muscular dystrophy and neurodegenerative disorders (Briston et al., 2019).
2.2. Mitochondria and ER intimacy: The MAM contacts
Cellular Ca2+ signaling occurs by dynamic crosstalk between ER and mitochondria. Ca2+ release from intracellular stores like ER or Ca2+ influx through plasma membrane modulates the mitochondrial redistribution and coupling between ER and mitochondria (Bravo et al., 2011). A Ca2+ rich microenvironment is created near the ER membrane that promotes the association of ER with mitochondria through MAMs. Around 20% of the mitochondrial surface remains in close contact with ER through the MAMs that are enriched with more than 1000 proteins (Gomez-Suaga et al., 2017). The important role of some of them is already discussed elaborately in many previous reviews (Belosludtsev et al., 2019; van Vliet et al., 2014). Transfer of Ca2+ between ER and mitochondria at these contact sites is regulated in a coordinated manner by the ER Ca2+ channels IP3R, mitochondrial outer membrane Ca2+ channel VDAC1 and the cytosolic chaperone Grp75 (Drago et al., 2011; Duchen, 1999; Poston et al., 2013). Loss in the MAMs architecture and its dysfunction has been implicated in neurodegenerative disorders and diabetes.
2.2.1. ER-Mitochondria Ca2+ crosstalk: Role in cellular bioenergetics/metabolism
ER-mitochondrial Ca2+ transport is crucial for cellular metabolism (Rossi et al., 2019) and ER stress regulation. Ca2+ influx and accumulation in mitochondria regulate mitochondrial as well as cellular metabolism through activation of multiple mitochondrial enzymes and ETC components that in turn caters to the increased energy demand of ER-resident chaperones and protein folding enzymes during the management of ER stress (Bravo et al., 2011). RNA-dependent protein kinase (PKR) like ER kinase (PERK) is also a stress sensor in UPR that is abundantly present in MAM that helps propagation of Ca2+ and ROS from ER to mitochondria which in turn contributes to apoptosis (Verfaillie et al., 2012). Perturbance in ER-Mitochondria Ca2+ transfer also reduces the overall efficiency of TCA cycle leading to reduction in NADH and FADH2 levels, which leads to reduced OXPHOS capacity and ATP production. Low levels of cellular ATP can lead to induction of autophagy (Bootman et al., 2018). Hence, it is important to note that ER-mitochondrial Ca2+ signaling plays a major role not only in cellular bioenergetics but other cellular processes as well.
3. Viral infections and calcium signaling: A soft target or savior?
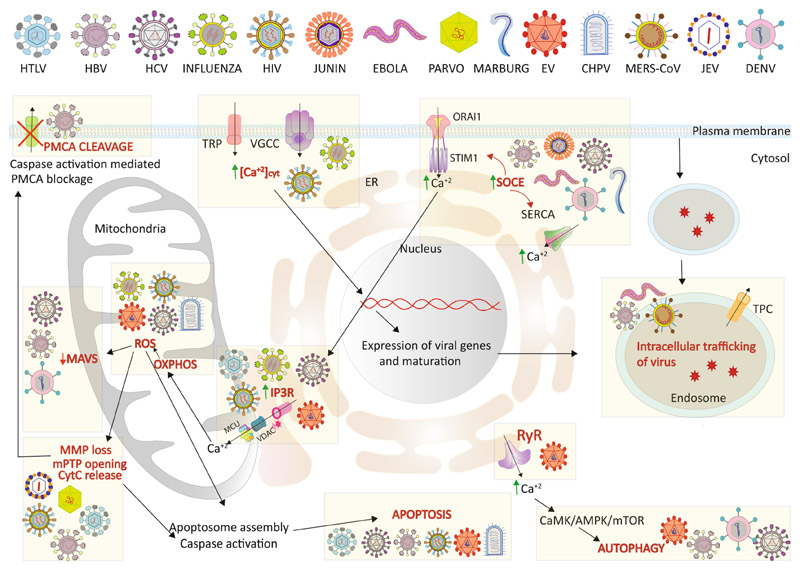
Calcium impacts many cellular processes hence is an important aspect of the virus-host interactions that govern the outcome of viral infections. Viruses can exploit this pivotal arm of cellular signaling to facilitate viral processes such as entry, replication, assembly and egress and to create an intracellular environment conducive to viral dissemination. Many viruses alter the cellular Ca2+ concentration in different compartments of the cell in a spatiotemporal manner, i) to activate Ca2+ dependent enzymes required to meet the high bioenergetic and metabolic demands, ii) to activate transcription factors required to support virus replication and iii) to regulate the innate immune response. The huge Ca2+ concentration gradient maintained across various cellular compartments and high tolerance of the host cell to subtle changes in Ca2+ level allows the viruses to effectively modulate cellular calcium signaling for their benefit without major detrimental effect on the host cells. However, the consequence of these spatiotemporal modulations of calcium concentration and signaling may lead to the onset of disease pathogenesis. In this section, we will address the interaction of the viruses with cellular calcium signaling and attempt to detail the significance of these events on the viral life cycle and disease pathogenesis (Fig. 3).
Fig. 3. Model depicting the influence of viral infections on cellular calcium signaling.
Viruses hijack and alter host cellular Ca2+ signaling and metabolic pathways to accelerate their replication inside host cells. Certain viruses are known to induce intracellular Ca2+ concentrations via Ca2+ uptake through different channels like VGCC, TRP. Some of them also activate SOCE channels (STIM1-ORAI1 mediated or through SERCA pump) to enhance uptake by cellular Ca2+ stores like ER. IP3R mediated ER-mitochondrial Ca2+ transport is also induced in certain viral infections to induce mitochondrial OXPHOS to meet the energy needed for viral replication. Enhanced OXPHOS leads to ROS accumulation that can trigger MMP loss, mPTP opening, Cyt C release and ultimately caspase activation. Caspase activation can block Ca2+ efflux via PMCA cleavage altering intracellular Ca2+ homeostasis. Increased ROS inhibits MAVS signaling and diminishes cellular innate immune response against viruses. High cytosolic Ca2+ can activate PyK2/FAK mediated p53 phosphorylation that in turn enhances specific expression and maturation of viral proteins. A high Ca2+ level also helps in the endosomal trafficking of viral particles. Some viruses induce CaMK/AMPK/mTOR mediated autophagy in response to increased cytosolic Ca2+. Viruses inducing these specific events are indicated in the figure appropriately. Please refer to the text for details. Abbreviations: [Ca2+]cyt: Cytosolic Calcium; SOCE: Store Operated Calcium Entry; VGCC: Voltage-Gated Calcium Channel; OXPHOS: Oxidative Phosphorylation; ROS: Reactive oxygen species; Cyt C: Cytochrome C; MAVS: Mitochondrial Antiviral Signaling protein; PyK2: Protein Tyrosine Kinase2; FAK: Focal Adhesion Kinase; CaMK: Ca2+/Calmodulin-dependent protein kinase; AMPK: 5′ AMP-activated protein kinase; mTOR; HTLV: Human T-lymphotropic virus; HIV: Human Immune deficiency virus; HBV: Hepatitis B virus; HCV: Hepatitis C Virus; EV: Entero Virus; CHPV: Chandipura Virus; MERS-CoV: Middle East Respiratory Syndrome Corona Virus; JEV: Japanese Encephalitis Virus; DENV: Dengue Virus.
3.1. Human immunodeficiency virus (HIV)
HIV infection results in the depletion of functional CD4+ T cells leading to acquired immunodeficiency during infection. HIV infection of the permissive CD4+ T cells results in their death via apoptosis whereas the non-permissive bystander CD4+ T cells undergo death by activation of inflammasomes and pyroptosis (Doitsh and Greene, 2016). HIV Nef protein has been implicated in increase in levels of cytosolic Ca2+. Nef acts as a master switch in the early HIV life cycle and has been associated with enhanced HIV pathogenicity. Distinct mechanisms are involved in Nef-mediated increase in intracellular Ca2+ in different cell lines. In Jurkat T cells, Nef expression promotes extracellular calcium influx while no release of calcium from ER stores is noticed despite Nef interaction with IP3R suggesting that enhanced physical coupling between IP3R and plasma membrane channels promote an increase in cytosolic Ca2+ (Berridge et al., 2000; Manninen and Saksela, 2002). Nef expression in lymphoblastoid CEM cells resulted in the higher filling of non-ER calcium stores (Zegarra-Moran et al., 1999), whereas in pro-myelocytic HL60 differentiated cell lines it has resulted in decreased extracellular calcium influx. Moreover, an increase of non-ER calcium stores occurs via Nef interaction with Src-like protein tyrosine kinase through SH3 domain-mediated protein interaction (Foti et al., 1999). In agreement, agents that enhance cytosolic Ca2+ concentration promote HIV replication whereas agents that block the increase, inhibit HIV replication (Nye and Pinching, 1990; Papp and Byrn, 1995). HIV Nef-mediated release of ER Ca2+ results in activation of the nuclear factor of activated T cells (NFAT), which positively regulates HIV-1 replication and gene expression (Kinoshita et al., 1997). Nef expression is shown to induce transcriptional response imitating T cell receptor activation resulting in upregulation of HIV virulence mediators (Simmons et al., 2001). HIV viral protein R (Vpr), which mainly localizes to nucleus and mitochondria, induces the opening of mPTP by coordinating with Adenine Nucleotide Translocator (ANT) leading to the release of Ca2+ from mitochondria and subsequent apoptosis (Jacotot et al., 2001, 2000). Vpr interacts with and activates the Ca2+ responsive transcriptional coactivators like p300 and CREB-binding protein (CBP) (Kino et al., 2002). It co-operates with Nef in NFAT-dependent T cell activation favoring HIV transcriptional activation (Lahti et al., 2003). The HIV Tat (transactivator of transcription) is also shown to mediate Ca2+ deregulation. HIV Tat localizes to the nucleus and is an important regulator of viral gene expression and replication. HIV-infected cells release Tat into the extracellular milieu which may lead to disruption of Ca2+ signaling in bystander naive cells. HIV-associated dementia may be a consequence of neurotoxicity mediated by extracellular Tat protein. Tat has also been shown to increase cytosolic Ca2+ levels in a dose-dependent manner through the IP3R mediated release of ER Ca2+ pools in primary macrophages (Mayne et al., 2000). HIV gp120 which plays a crucial role in viral entry has also been implicated in dementia (Mattson et al., 2005). In human fetal astrocytes and neurons, HIV gp120 is shown to promote calcium influx through L-type calcium channels, Na+/H+ exchanger and NMDA-type excitatory amino acid receptor resulting in high intracellular Ca2+ levels (Holden et al., 1999) that subsequently promotes caspase-dependent neuronal apoptosis and HIV pathogenesis of dementia (Haughey and Mattson, 2002).
3.2. Hepatitis B virus (HBV)
HBV is a hepatotropic virus that causes chronic hepatitis eventually progressing into end-stage liver disease and hepatocellular carcinoma (HCC) (Shih et al., 2018). During HBV infection, the HBx protein interacts with VDAC and triggers the opening of the mPTP resulting in the release of mitochondrial Ca2+ to the cytoplasm. In parallel, the activation of Ca2+ ATPase transiently facilitates the uptake of Ca2+ by ER which is subsequently released back into the cytosol through the activation of IP3R (Xia et al., 2006). The elevated cytosolic Ca2+ promotes calcium-dependent activation of proline-rich tyrosine kinase 2 (Pyk2) and Focal Adhesion Kinase (FAK), which supports HBV reverse transcription and DNA replication (Bouchard et al., 2006, 2003, 2001). High cytosolic Ca2+ levels also induce NFAT dependent gene expression and are also implicated in the activation of JNK and MAPK signal transduction pathways (Lara-Pezzi et al., 1998; Oh et al., 2003). HBx mediated opening of mPTP also results in the release of cytochrome c from mitochondria which in turn activates caspase-3 that can further enhance cytosolic Ca2+ levels by cleavage of the plasma membrane Ca2+ ATPase (PMCA) required for the efflux of cytosolic Ca2+ into the extracellular milieu (Chami et al., 2003). High levels of cytosolic Ca2+ also facilitates HBV core assembly (Choi et al., 2005). Overall, these studies demonstrate that HBx-induced Ca2+ signaling alterations not only promote HBV replication but also drive the onset of liver disease pathogenesis and progression into hepatocellular carcinoma.
3.3. Hepatitis C virus (HCV)
Like HBV, HCV is also a hepatotropic virus and is among the major etiological agents that cause chronic liver disease and HCC. HCV core and non-structural protein NS5A have been shown to modulate host calcium-signaling (Dionisio et al., 2009). HCV core and NS3/4A protease also localize to the outer mitochondrial membrane. HCV core protein promotes ER Ca2+ depletion by impairing SERCA pump function (Benali-Furet et al., 2005). The expression of HCV core fragment 37–191 is sufficient to upregulate ER oxidoreductin 1, triggers ER Ca2+ efflux and uptake by mitochondria via MCU (Ivanov et al., 2015). HCV may promote mitochondrial ATP production by facilitating mitochondrial Ca2+ uptake eventually leading to ROS production and apoptosis of HCV-infected cells (Li et al., 2007). The HCV viroporin protein p7 tends to form a hexameric channel on the ER membrane and may promote the release of ER Ca2+ to the cytosol. Molecules that block p7 have been effective in inhibiting viral replication (Griffin et al., 2003). HCV NS5A has also been implicated in ER Ca2+ efflux and uptake by mitochondria leading to elevated ROS levels and activation of NF-κB and STAT3, which supports cell growth and protects HCV-infected cells from apoptosis (Gong et al., 2001). A recent study suggests that treatment of primary monocyte-derived macrophages and THP-1 macrophages with HCV core protein induces a rapid increase of intracellular calcium through phospholipase C, which then leads to the activation of the NLRP3 inflammasome and IL-1β production (Negash et al., 2019). These detrimental effects of HCV viral proteins on calcium signaling pave way for the pathogenesis of the chronic liver disease and carcinogenesis associated with HCV infection.
3.4. Human T-lymphotropic Virus type-I (HTLV-1)
HTLV-1 infection is associated with adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) (Rajamani et al., 1995). p13II is an HTLV accessory protein that localizes to the inner mitochondrial membrane (IMM) (Ciminale et al., 1999) and is shown to promote mitochondria depolarization and swelling. p13II affects the mitochondrial Ca2+ retention and sensitizes the p13II expressing T lymphocytes to ceramide or Fas ligand-induced apoptosis (D’Agostino et al., 2005). Further study suggests that p13II reduces mitochondrial Ca2+ uptake by triggering inward K+ current and inner membrane depolarization (Biasiotto et al., 2010). Another protein p12I encoded by the ORF I of HTLV-1 is enriched in the ER and cis-Golgi compartments where it binds to two ER-resident proteins calreticulin and calnexin which play a major role in ER calcium storage (Ding et al., 2001). p12I expression results in increased basal levels of cytoplasmic Ca2+ due to the release of ER Ca2+ by IP3R as well as by activation of Calcium Release-Activated Calcium (CRAC) channels in the plasma membrane. The increase in cytosolic Ca2+ levels promotes NFAT mediated transcriptional upregulation and facilitates proviral DNA integration and optimal infectivity of HTLV by activation of host cells (Ding et al., 2002). p12I interaction with calcineurin and calreticulin results in retention of the calreticulin-MHC-I complexes in the ER or cis-Golgi leading to the inhibition of its association with β2-micro-globulin and subsequent trafficking to the cell surface (Johnson et al., 2000). Furthermore, expression of p12I in Jurkat T cells resulted in a calcium-dependent increase in expression of transcriptional co-activator p300 and p300-dependent transcription resulting in T cell activation and efficient viral infection (Nair et al., 2006).
3.5. Enteroviruses
Enteroviruses are non-enveloped RNA viruses such as poliovirus and coxsackievirus. Enteroviral infections are associated with many human diseases such as aseptic meningitis, hand-foot and mouth disease, paralysis and myocarditis. Enterovirus 2B protein is a viroporin that can enhance membrane permeability by forming membrane lesions on the ER and plasma membrane. The 2B protein has been implicated in disruption of intracellular Ca2+ homeostasis via depletion of ER Ca2+ stores and elevation of cytosolic Ca2+ concentration (Peischard et al., 2019; van Kuppeveld et al., 2005). Infection with poliovirus and coxsackievirus is shown to increase cytosolic Ca2+ concentration partly through its release from ER via IP3R and RyR channels and subsequent uptake by mitochondria through Ca2+ uniporter and VDAC, resulting in high mitochondrial Ca2+ overload, mitochondrial dysfunction and apoptosis (Brisac et al., 2010; Peischard et al., 2019). Similarly, coxsackievirus infection is associated with 2B protein-mediated release of Ca2+ from the ER and Golgi stores with a concomitant increase in extracellular Ca2+ uptake. This leads to a decline in the Ca2+ release capacity of the intracellular stores and inhibition of stimulus-induced mitochondrial uptake of Ca2+ resulting in perturbation of overall intracellular Ca2+ homeostasis (Campanella et al., 2004; van Kuppeveld et al., 1997). The 2B protein-mediated decline in ER and Golgi Ca2+ load is also implicated in disrupting the Golgi secretory trafficking resulting in inhibition of cytokine release, recycling of receptors and antigen presentation, thereby affecting the innate and adaptive immune responses during infection (de Jong et al., 2006). The disruption of intracellular Ca2+ homeostasis also leads to a decline in mitochondrial ATP production capacity and induction of autophagy via activation of the AMPK/MEK/ERK and Ras/Raf/MEK/ERK signaling pathways facilitating enterovirus pathogenesis (Xin et al., 2015). Some groups also report antiapoptotic function of 2B due to disruption of apoptotic signaling between ER and mitochondria as a consequence of reduced Ca2+ concentration in ER (Campanella et al., 2004). With the global disruption of intracellular Ca2+ homeostasis during enterovirus infection, what fine-tunes the switch from proapoptotic to antiapoptotic signaling remains an enigma. Infection of coxsackievirus also perturbs mitochondrial metabolism and infection of mouse cardiomyocytes with coxsackievirus B3 showed decreased activities of ETC complexes I and III causing cardiac insufficiency (Peischard et al., 2019).
3.6. Kaposi’s sarcoma associated herpesvirus (KHSV)
KSHV, also termed Human Herpesvirus 8 (HHV8), infects circulating B cells and endothelial cells and causes Kaposi’s sarcoma, primary effusion lymphoma and a subset of Castleman’s disease. KHSV infection is known to perturb cellular Ca2+ homeostasis and several of its proteins, K15, v-GPCR, K1, K7, VMIP I and II and v-Bcl2 contribute to this. The K1 protein of KHSV is a B cell receptor-like protein that triggers a downstream cascade of tyrosine phosphorylation leading to a prolonged increase in intracellular Ca2+ concentration resulting in NFAT activation, upregulation of inflammatory cytokines and viral dissemination (Lee et al., 2005). The KHSV protein K7 localizes to the mitochondria and maintains mitochondrial membrane potential. It induces cytoplasmic Ca2+ mobilization by promoting ER Ca2+ release and activation of capacitative Ca2+ entry (CCE) via its interaction with cellular Calcium-Modulating Cyclophilin Ligand (CAML). As a result, increased cytosolic calcium levels prevent cellular apoptosis and enable the successful completion of viral replication (Feng et al., 2002). Intracellular calcium mobilization was shown to trigger expression of lytic viral accessory protein PF-8 or the late-lytic viral envelope glycoprotein gpK8 through calcineurin resulting in reactivation of latent KHSV infection (Zoeteweij et al., 2001). KHSV encoded v-Bcl2, which shares structural and functional homology with Bcl-2, promotes viral persistence, latency and development of Kaposi sarcoma by enhancing the viability of infected cells (Boshoff and Chang, 2001).
3.7. Human herpes Simplex viruses (HSV)
Herpes Simplex Virus type-1 and 2 (HSV-1 and HSV-2) are wide-spread across the globe and lead to lifelong infections. HSV1 is usually associated with oral sores and HSV2 infection leads to genital herpes. HSV-1 and HSV-2 infection has been shown to result in a rapid transient increase in cytosolic Ca2+ concentration through release from IP3-sensitive ER stores. This increase in cytosolic Ca2+ levels leads to FAK phosphorylation and reorganization of the actin cytoskeleton facilitating the nuclear trafficking of internalized virus (Chami et al., 2006; Cheshenko et al., 2003). In HSV-1 infected macrophages, an increase in cytosolic Ca2+ levels due to release from mitochondria results in activation of NFkB ultimately leading to the enhancement of proinflammatory responses (Mogensen et al., 2003). Increased cytosolic Ca2+ levels also lead to cell death and virus release thereby helping in the spread of HSV-1 infection (Yura et al., 2003). HSV-2 glycoprotein H has been shown to interact with integrin αvβ3 and promote the release of Ca2+ from intracellular stores which facilitate viral entry and dissemination (Cheshenko et al., 2014).
3.8. Influenza a virus (IAV)
Influenza A virus (IAV) infection of neurons and neutrophils is shown to promote Ca2+ release from ER stores through activation of IP3R pathway resulting in an increase in cytosolic Ca2+ overload and disruption in neuronal and neutrophil physiology (Brask et al., 2001; Hartshorn et al., 1988). The L-type channel, Cav1.2 serves as a receptor for IAV binding and entry and chemical inhibition or knockdown of voltage-gated calcium channels inhibited H1N1 and H3N2 IAV infection in multiple cell lines (Chandrasekaran et al., 2008; Fujioka et al., 2018). Influenza virus protein PB1-F2 has been shown to localize to the mitochondria and triggers mitochondrial depolarization and apoptosis (Chanturiya et al., 2004). Synthetic liposomes harboring the PB1-F2 suggest that it facilitates Ca2+ conductance and functions as a canonical channel for the conductance of cations and anions with low selectivity (Henkel et al., 2010).
3.9. Epstein-Barr virus (EBV)
EBV, also known as human herpesvirus 4 is among the most common human virus and associated with benign and malignant tumors of epithelial and lymphoid cells. EBV entry into the cells is dependent on host cell calcium. Verapamil, an L-type Ca2+ channel blocker is effective in inhibiting EBV entry and EBV-mediated transformation of B cells (Dugas et al., 1988). During latent infection, EBV expresses the latent membrane protein 2A (LMP2A) which aggregates on the plasma membrane and mimics B cell receptor and prevents calcium mobilization upon B cell stimulation by competitively binding to the cellular phospho-tyrosine kinases (PTKs) (Fruehling and Longnecker, 1997; Miller et al., 1994, 1993).
EBV oncoprotein latent membrane protein 1 (LMP1) reactivates EBV in a calcium-dependent manner via Ca2+/calmodulin dependent protein kinase type IV/Gr (CaMKIV/Gr) mediated upregulation of the transcription of transcriptional activators of the lytic phase through their calcium-responsive sites in promoter regions (Chatila et al., 1997; Liu et al., 1997; Mosialos et al., 1994)
3.10. Ebola virus (EBOV)
EBOV is a deadly RNA virus that causes the lethal Ebola hemorrhagic fever. Infection with EBOV affects the endolysosomal calcium Two Pore Channels (TPCs) resulting in increased cytosolic Ca2+ levels through release from endolysosomal stores. This Ca2+ influx into cytosol facilitates intracellular trafficking of virus-containing endosomal vesicles. Disruption of TPC function by genetic knockout or chemical inhibition was effective in inhibiting virus entry (Sakurai et al., 2015). Inhibition of Ca2+ release from endolysosomal stores by small molecule antagonist like Ned19, NAADP and tetrandrine inhibits EBOV infection of macrophages (Sakurai et al., 2015). In addition, EBOV, Marburg virus (MARV) and Junin virus (JUNV) have also been implicated in STIM1/ORAI1-mediated calcium signaling to facilitate their budding and egress from host cells (Han et al., 2015).
3.11. Rotavirus (RV)
Rotavirus is a non-enveloped RNA virus that causes viral gastroenteritis. Many studies implicate the role of Ca2+ in the cytopathology of infected enterocytes. Ca2+ chelation with BAPTA, blocking L-type Ca2+ channel with verapamil and reduction in extracellular Ca2+ concentration are protective against rotavirus-associated cytopathy. NSP4 protein of this virus acts as an enterotoxin that elevates cytosolic Ca2+ concentration due to phospholipase C activation and IP3 production (Dong et al., 1997; Tian et al., 1995). Also, activation of L-type Ca2+ channel has been shown to serve in increasing cytosolic Ca2+ concentration. Studies have shown that the initial increase in cytosolic Ca2+ was due to increased permeability of cell membrane, whereas both extracellular Ca2+ influx and release from ER-Ca2+ stores contribute to the later elevation in cytosolic Ca2+ concentration (Brunet et al., 2000). Moreover, the release of Ca2+ from ER, impairs N-glycosylation of rotavirus proteins VP7 and NSP4 and has been implicated in cell death (Poruchynsky et al., 1991).
3.12. Other viruses
Tulane virus (TV), a rhesus enteric calicivirus disrupts Ca2+ homeostasis and high cytosolic Ca2+ levels is important for its replication. The TV NS1-2 protein has a characteristic viroporin domain (VPD) and functions like a viroporin. It localizes to the ER and causes aberrant Ca2+ signaling when expressed in mammalian cells and truncation of the VP domain abrogated these functions of NSP1-2 (Strtak et al., 2019). Similarly, human cytomegalovirus (HCMV) also increases intracellular Ca2+ levels to favor virus replication by triggering the depletion of ER Ca2+ stores via the viral protein UL37X1 mediated activation of host’s P2Y2 purinergic receptors and PLC-IP3 pathway (Chen et al., 2019a). Further murine cytomegalovirus (MCMV) is shown to significantly alter cytoplasmic Ca2+ level and MMP in mice cochlear neurons (Panel et al., 2019).
Japanese encephalitis virus (JEV) is an arbovirus that is the most common agent of acute viral encephalitis in South-East Asia. JEV infection increases intracellular Ca2+ levels and disrupts mitochondrial membrane potential (MMP). Treatment of infected cells with CW-33, a synthesized derivative of furoquinolines reduced intracellular Ca2+ levels, raised mitochondrial membrane potential, and upregulated anti-apoptotic signaling pathways of AKT/mTOR. Besides, CW-33 activated the expression of IFN-stimulated genes (ISGs). Results demonstrate that the CW-33 exhibits significant potential against JEV (Huang et al., 2016).
Chandipura virus (CHPV), another neurotropic virus associated with acute viral encephalitis and is shown to promote neuronal cell death by disrupting intracellular Ca2+ signaling which leads to mitochondrial dysfunction and oxidative stress leading to neuronal apoptosis through the FasL-FADD pathway during CHPV infection. Minocycline, a broad-spectrum antibiotic with anti-oxidative and anti-inflammatory properties was found to be effective in mitigating the increase in cytoplasmic Ca+2 and ROS levels and CHPV induced cellular apoptosis (Verma et al., 2018)
Dengue virus (DENV) is shown to promote colocalization of STIM1 and ORAI1 leading to their interaction and activation of store operated Ca2+ entry (SOCE). Manipulation of the intracellular and extracellular Ca2+ levels by Ca2+ chelators SOC inhibitor SKF96365 decreased DENV yield suggesting that DENV alters cellular Ca2+ homeostasis to favor viral replication and dissemination (Cheshenko et al., 2007; Dionicio et al., 2018). Many viruses have also been shown to depend on STIM1 and ORAI1 interaction and Ca2+ signaling for the budding and egress of mature virus particles (Chen and Lamb, 2008; Chen et al., 2019b; Freedman and Harty, 2016; Han et al., 2015; Yao et al., 2018). The expression of several TRP channels like TRPV1, TRPA1 and TRPM8 is increased during infection with respiratory viruses such as respiratory syncytial virus (RSV), measles virus (MV) and human rhinovirus (HRV). While the increase in expression of TRPA1 and TRPV1 is mediated by soluble factors released during viral infections, enhanced expression of TRPM8 is dependent on virus replication. These findings provide insights into the possible utilization of these TRP channels by respiratory viruses to create a favorable intracellular Ca2+ environment to favor their replication (Abdullah et al., 2014; Omar et al., 2017).
Parvovirus VP1 protein is also shown to increase cytosolic Ca2+ concentration by mediating the activation of ICRAC channels of the plasma membrane (Nykky et al., 2014).
Porcine Delta Corona Virus (PDCoV), an enteropathogenic virus that causes severe vomiting and diarrhea in infected piglets upregulates intracellular Ca2+ concentrations which cause calpain-mediated lysosomal disruption with subsequent release of cathepsin B and L. Upregulated expression of cathepsin L and B facilitate virus infection and L-type Ca2+ channel blocker diltiazem hydrochloride significantly inhibited PDCoV infection (Zhang et al., 2019b).
Like EBOV, Middle East Respiratory Syndrome Corona Virus (MERS-CoV) also utilizes the NAADP dependent TPC channels for Ca2+ mobilization from the endolysosomal stores to facilitate viral entry and trafficking (Gunaratne et al., 2018).
We have briefly summarized the cytobiological effects on Ca2+ signaling induced by specific viral proteins or the complete viral particle in the following Table 1.
Table 1. Role of viruses/viral proteins on Ca2+ homeostasis.
| Virus | Protein | Cytobiological consequences on Ca2+ signaling | Refs. |
|---|---|---|---|
| HIV-1 | Nef | Activates T cell Receptors via NFAT activation.Enhances viral infectivity. | (Simmons et al., 2001) (Chami et al., 2006) |
| Vpr | Interacts with cellular Ca2+ regulators.Induces mitochondrial Ca2+ leakage through membrane permeabilization. | (Jacotot et al., 2001, 2000) | |
| Tat | Induces Ca2+ dependent apoptosis | (Kruman et al., 1998) | |
| gp120 | Increases [Ca2+]cyt that leads to increased ROS accumulation | (Haughey and Mattson, 2002) | |
| Env | Mediates Ca2+ dependent viral fusion | (Zaitseva et al., 2017) | |
| HTLV-1 | pI2I | Increases Ca2+ outflow from ER via binding to calreticulin and calnexin.Enhances viral infection through Ca2+ dependent activation of transcriptional co-activator p300. | (Ding et al., 2001) (Nair et al., 2006) |
| pI3II | Affects mitochondrial Ca2+ permeability across IMMIncreases the sensitivity of cells to apoptosis. | (D’Agostino et al., 2002)(D’Agostino et al., 2005) | |
| HCV | Core | Decreases [Ca2+]er, induces apoptosis via Bax-mediated mitochondrial membrane depolarization.Stimulates ER Ca2+ efflux to mitochondria through MCU activation. | (Benali-Furet et al., 2005; John et al., 1998)(Ivanov et al., 2015; Li et al., 2007) |
| NS5A | Increases [Ca2+]mt and ROS production and affects transmembrane potential. | (Gong et al., 2001) | |
| Activates Ca2+ -dependent degradation of NFκB inhibitory subunit Iκβα. | (Waris et al., 2003) | ||
| p7 | Allows Ca2+ -flow from ER by forming ion channels in the cellular membrane | (Griffin et al., 2003) | |
| Poliovirus | Viral particle | Accumulates Ca+2 in mitochondria that leads to mitochondrial dysfunction and apoptosis. | (Brisac et al., 2010) |
| Coxsackie virus B3 | 2B | Increases [Ca2+]cyt by rigorous Ca2+ outflow from ER. | (Peischard et al., 2019) |
| 3C | Promotes mitochondrial Ca2+mediated apoptosis through cleavage of procaspase 8. | (Peischard et al., 2019) | |
| 2A | Along with 3C, it destabilizes cellular homeostasis by increased expression of Bax, p53 and decreased expression of Bcl2 leading to cell death | (Peischard et al., 2019) | |
| Viral particle | Disturbs mitochondrial homeostasis. Activates the AMPK/MEK/ERK and Ras/Raf/MEK/ERK signaling pathways to induce autophagy in host cells. | (Xin et al., 2015) | |
| Rabies virus | Viral particle | Hampers Ca2+ homeostasis and GABAergic neurotransmission by loss of host proteins calbindin-D-28 k and elevation of m-calpin respectively. | (Schwaller et al., 2002; Torres-Fernández et al., 2005; Ubol et al., 2005) |
| Influenza virus | Viral particle | Targets Cav1.2 channel for binding and entry into host cells. | (Fujioka et al., 2018) |
| M2 | Regulates ROS dependent Mitochondrial Antiviral Signaling (MAVS) | (Wang et al., 2019) | |
| Ebola virus | Viralparticle | Affects TPCs to control the movement of endosome-containing virus particles.STIM1/ORAI1-mediated Ca2+ signal critical for their budding from host cells | (Han et al., 2015; Kintzer and Stroud, 2016; Sakurai et al., 2015) |
| Rotavirus | NSP4 | Decreases [Ca2+]er and increases [Ca2+]cyt through PLC activation and IP3 production. | (Dong et al., 1997; Tian et al., 1995) |
| Chandipura virus | Viral particle | Affects intracellular Ca2+ signaling mediated by angiotensin II.Causes neuronal apoptosis through FasL-FADD pathway. | (Verma et al., 2018) |
| HBV | HBx | Activates mitochondrial PTP opening to release mitochondrial Ca2+ to cytoplasm and activates Ca2+ ATPase to accumulate Ca2+ in ER that ultimately releases through IP3R, thus, results in increased [Ca2+]cyt. Enhances viral replication through activation of proline-rich tyrosine kinase 2 (Pyk2) and Focal Adhesion Kinase (FAK). | (Xia et al., 2006)(Bouchard et al., 2006) |
| HHV-8 | K1 | Increases prolonged intracellular Ca2+ concentration through a phosphorylation cascade of downstream elements. | (Lee et al., 2005) |
| K7 | Elevates [Ca2+]cyt by interacting with CAML protein of ER and activation of CCE pathway. | (Feng et al., 2002) | |
| HSV | Increased [Ca2+]cyt results in FAK phosphorylation required for actin cytoskeleton reorganization and nuclear trafficking of the internalized virus. Enhances proinflammatory response through activation of NFkB.Targets T-type Ca2+ channels, downregulates VGCC and alters sensory neurons to transmit pain information. | (Chami et al., 2006; Chen et al., 2019b; Cheshenko et al., 2003; Mogensen et al., 2003; Zhang et al., 2019a, 2017) | |
| Epstein-Barr virus | LMP1 | Reactivates EBV in a calcium-dependent manner inducing a Ca2+/calmodulin dependent protein kinase type IV/Gr (CaMKIV/Gr). | (Chatila et al., 1997; Mosialos et al., 1994) |
| LMP2A | Binds cellular phospho-tyrosine kinases (PTKs), prevent calcium mobilization and acts as a negative regulator of calcium-signaling. | (Fruehling and Longnecker, 1997; Miller et al., 1994, 1993) | |
| HPV | E6 | Interacts with E6BP, a Ca2+ binding protein of ER. | (Chen et al., 1995; Weis et al., 1994) |
| E7 | Allows virus-induced oncogenic activity. | (Tugizov et al., 2005) | |
| HCMV | UL37X1 | Activates STIM1/ORAI1 mediated calcium influx. | (Chen et al., 2019b) |
| Dengue virus | Viral particle | Activates SOC channels altering cellular Ca2+ homeostasis via STIM1/ORAI1 interaction. | (Cheshenko et al., 2007; Dionicio et al., 2018) |
4. ER-Mitochondria calcium signaling in cellular stress responses
Ca2+ plays a central role in many cell-signaling pathways and hence the regulation of cellular Ca2+ homeostasis is crucial for cell survival and function. Ca2+ homeostasis is ensured by coordinated efforts of the Ca2+ channels, binding, transport, sensor and buffer proteins, including the cellular organelles that serve as Ca2+ stores to manage/buffer the transient spikes in intracellular Ca2+.
The ER comprises a major part of the cellular reticular network, which is involved in many vital processes in the cell including Ca2+ homeostasis. Disruption in ER function upregulates the ER stress responses such as the Unfolded Protein Response (UPR). The upregulation of UPR results in the immediate shutdown of protein translation, enhanced transcription of genes that encode proteins that enhance ER protein folding capacity and ER-Associated Degradation (ERAD) of misfolded proteins. If the ER stress is beyond the threshold of repair UPR also activates the cell death pathways. The integral membrane proteins of ER, dsDNA activated protein kinase R like ER-resident kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1) orchestrate the transduction of ER stress signal and activation of UPR. This important topic has been covered in substantial detail by many good reviews (Hetz and Glimcher, 2009; Ron and Walter, 2007). In this review, we will focus on the integral role of cellular Ca2+ signaling in the modulation of ER stress responses.
Thapsigargin, an inhibitor of SERCA is a widely used compound to induce ER stress suggesting that disturbance in ER Ca2+ homeostasis directly contributes to ER stress. ER Ca2+ depletion results in rapid accumulation of misfolded proteins in the ER and UPR activation due to disruption in the activity of the ER chaperones and folding proteins such as calreticulin, BiP and protein disulfide isomerase. In addition to the requirement of Ca2+ for their effective function, these proteins also contribute to Ca2+ buffering in ER lumen hence promote ER Ca2+ homeostasis. The Ca2+ dependent immunoglobulin binding protein (BiP) plays a very central role in this process as it is involved in sensing misfolded proteins and activation of the ER stress transducers. Calreticulin, the ER chaperone and Ca2+ buffer is also known to co-ordinate with BiP to maintain ATF6 in an inactive state and during ER stress it dissociates from ATF6 to promote UPR activation (Hong et al., 2004). Upon depletion of ER Ca2+, an ER-resident oxidoreductase PDIA6 has been shown to stimulate IRE1 activity by interacting with its ER luminal domain. PDIA6 expression is tightly regulated by microRNA-322 and an increase in cytosolic Ca2+ concentration through depletion of ER Ca2+ stores and SOCE reduces miR-322 abundance, thereby increasing PD1A6 levels (Groenendyk et al., 2014). Lipid overload during obesity may lead to increased delivery of saturated fatty acids to the ER which has been shown to enhance saturation of the ER phospholipids and direct activation of UPR transducers PERK and IRE1. Similarly, enhanced delivery of unesterified cholesterol to ER leads to inhibition of SERCA ATPase resulting in disruption of ER Ca2+ homeostasis and UPR activation (Li et al., 2004; Volmer et al., 2013).
Disruption in ER Ca2+ homeostasis disrupts mitochondrial functions due to calcium overload thereby affecting mitochondrial bioenergetics and lipid metabolism. The mitochondrial Ca2+ uniporter-dependent uptake of Ca2+ released from ER stores at the contact sites is shown to be facilitated by the mitochondrial fusion protein, Mitofusin 2 (Mfn2) (de Brito and Scorrano, 2008). Mfn2 is enriched in these contact sites and an optimum level of Mfn2 expression is required to facilitate proper interaction between ER and mitochondria and smooth Ca2+ transport (Guo et al., 2007). Under normal physiological conditions, the Bcl2 family anti-apoptotic protein localizes to the ER membrane and preserves ER luminal Ca2+ concentration by regulating IP3R mediated ER Ca2+ release and the Bcl2 family pro-apoptotic proteins have been implicated in reduced ER luminal Ca2+ concentration. Few studies also suggest that these Bcl2 family proteins also interact with SERCA to modulate Ca2+ uptake and release from ER (Lewis et al., 2014). Signaling pathways that are activated in ER stress responses have also been implicated to promote autophagy. Besides, ER Ca2+ release or SOCE can result in high cytosolic Ca2+ concentrations which activate CamKKβ leading to AMPK mediated mTOR inhibition and activation of autophagy (Decuypere et al., 2013; Høyer-Hansen et al., 2007). On the other hand, Ca2+ overloading in mitochondria can promote mitochondrial dysfunction and injury subsequently leading to activation of PINK1-PARKIN mediated mitochondrial-selective autophagy or Mitophagy (Chen and Dorn, 2013). Thus, the dynamics of ER-mitochondrial calcium transport govern overall cellular homeostasis and perturbation in this process has been implicated in many chronic and debilitating human diseases (Krebs et al., 2015).
4.1. Ca2+ signaling in cellular innate immune and antiviral signaling
The crucial role of calcium in immune cell function and activation of resting immune cells is well defined. Here, we will review the significance of ER-mitochondrial calcium signaling in the cellular innate immune response against viral infections. The cellular antiviral response is activated upon pathogen recognition by Pathogen Associated Molecular Patterns (PAMPs) via an array of Pathogen Recognition Receptors (PRRs). The activation of PRRs triggers a cascade of signaling events that promote the production of type-1 interferons which then activate the antiviral signaling in paracrine and autocrine mode to trigger transcription of antiviral genes (Brubaker et al., 2015). Most studied PRRs include Toll-like Receptors (TLRs), Retinoic Acid-Inducible Gene I (RIG1) like Receptors (RLRs), Nucleotide-binding Oligomerization Domain (NOD)-Like Receptors (NLRs) and cyclic GMP-AMP (cGAMP) synthase (cGAS) (Refolo et al., 2020; Streicher and Jouvenet, 2019). In this section, we will discuss the modulation of the receptor-induced antiviral responses via intracellular Ca2+ signaling.
4.1.1. Calcium mediated modulation of TLR signaling
TLRs are one of the important receptors of the innate immune system recognizing PAMPs expressed on the surface of invading microbial pathogens. Post activation, TLRs promote inositol-1, 4, 5-trisphosphate (IP3) and diacylglycerol production giving rise to a cascade of events which lead to the release of Ca2+ from the ER lumen thereby reducing the load of ER Ca2+ stores (Feske et al., 2012; Hogan et al., 2010). The reduced Ca2+ concentration in ER lumen leads to the confirmational change in Stromal Interaction Molecule1 (STIM1) an ER Ca2+ sensor on the ER membrane which results in its interaction with calcium release-activated calcium channel protein 1 (ORAI1) located on the plasma membrane thereby opening Ca2+ permeable ORAI1 ion channels to initiate Ca2+ entry (Feske et al., 2012; Hogan et al., 2010). Once activated the TLRs rapidly activate the extracellular signal-regulated kinase (ERK1/2) pathway by coordinating GTPases Ras and Rap1 activation in immune cells which stimulate the production of inflammatory cytokines and chemokines (Feske et al., 2012; Hogan et al., 2010; Tang et al., 2017, 2015). The entry of Ca2+ effected the activation of ERK1/2 (Limnander and Weiss, 2011) suggesting that Ca2+ may play an essential role in regulating the activation of ERK1/2 triggered by TLRs (Tang et al., 2015). A study on human Mesenchymal Stem Cells (hMSCs) showed TLR3/4 priming results in the induction of cytokines (IFNs and interleukins) that are closely dependent on intracellular Ca2+ signaling (Park et al., 2016). The high concentration of Ca2+ induces dsRNA sensors like TLR3, MDA5 and RIG-I in epidermal keratinocytes. Other TLRs (TLR1 and 2) and IFNα are also induced in response to high Ca2+. It also enhances antiviral activity of epidermal keratinocytes by upregulating Human β-Defensins 2 and 3 (HBD2 & HBD3) in response to HSV1 infection (Yamamura et al., 2018).
4.1.2. Calcium mediated modulation of RLR signaling
Viral infection can induce IFN response and ER stress simultaneously. ER stress can directly contribute to mitochondrial dysfunction by Ca2+ overload and ROS accumulation and perturb mitochondrial antiviral signaling (Csordás and Hajnóczky, 2009; West et al., 2011). The mitochondria serve as a hub for orchestrating the cellular antiviral signaling mediated by RIG-I-like Receptors (RLR). RIG-I-like receptor family belongs to a class of PRRs that responds to RNA virus infections and comprises three helicases namely Retinoic-Inducible Gene-I (RIG-I), Melanoma Differentiation-Associated gene 5 (MDA5) and Laboratory of Genetics and Physiology 2 (LGP2). RIG-I and MDA5 contain a helicase domain and N-terminal Caspase Recruitment Domain (CARD). Through the CARD-CARD interaction, the RLR proteins interact with the mitochondrial antiviral signaling (MAVS) protein on the mitochondrial outer membrane leading to the activation of the IRF3 and NFκB pathways (Jacobs and Coyne, 2013). It has been demonstrated that ER stress further enhances interferon β production upon stimulation with ISGs and the mitochondrial calcium uniporter interacts with MAVS. Knockout of MCU indicated that MCU positively regulates RLR signaling and MAVS-dependent IFNβ production during ER stress conditions (Cheng et al., 2016). The reduced mitochondrial potential has also been implicated in reduced anti-viral signaling due to its negative effect on MAVS signaling (Koshiba et al., 2011). Dissipation of mitochondrial membrane potential did not disrupt the signaling downstream of MAVS suggesting the mitochondrial function and homeostasis likely affect RLR signaling at the MAVS level (Koshiba et al., 2011). The viruses may exploit this aspect by increasing ER Ca2+ depletion and uptake by mitochondria to disrupt mitochondrial membrane potential and negatively affect RLR signaling and host antiviral response.
4.1.3. Calcium mediated modulation of inflammasome activation
NOD-like Receptor Protein 3 (NLRP3) inflammasome is a multimeric protein complex that triggers the release of proinflammatory cytokines IL-1β and IL-18. A wide variety of agents and DAMPs, such as silica and uric acid crystals and PAMPS such as dsDNA activate the NLRP3 inflammasome. The Calcium Sensing Receptor (CASR) activates the NLRP3 inflammasome through phospholipase C, which catalyzes IP3 production and thereby induces the release of Ca2+ from ER stores leading to a decrease in cAMP concentration and activation of NLRP3 inflammasome (Guo et al., 2015; Horng, 2014; Lee et al., 2012). Additionally, Ca2+ release from ER and influx through plasma membrane induces mitochondrial damage, ROS production, MMP loss and mitochondrial DNA release to the cytosol, activating the NLRP3 inflammasome. Inflammasome complex in turn helps in cytokine maturation (majorly proinflammatory cytokines IL1β and IL-18) via proteolytic processing by caspase 1 (Murakami et al., 2012). The above-mentioned studies also unraveled the role of NLRP3 inflammasome activators like ATP and Nigericin in promoting ER Ca2+ release via IP3R and other Ca2+ channels that in turn result in activation of the complex (Lee et al., 2012; Murakami et al., 2012). However, along with the direct involvement of Ca2+ signaling, there are additional regulators of Ca2+ related signaling pathways like TRP channels (TRPV2 and TRPM2) that also play a role in modulating NLRP3 inflammasome activation (Compan et al., 2012; Elliott and Sutterwala, 2015; Zhong et al., 2013). These findings strengthen the fact that ER-mitochondrial Ca2+ signaling is a key modulator of NLRP3 inflammasome-mediated innate immune response.
4.1.4. Calcium mediated modulation of STING activity
STING (Stimulator of IFN Genes) is a member of the ISGs (Interferon Stimulating Genes) family that plays a crucial role in host IFN response against pathogens. Ca2+ chelator (BAPTA-AM) suppresses IRF3 activation and IFN production via inhibition of STING translocation from ER to the cytoplasm (Hirabayashi et al., 2017) suggesting the role of intra-cellular Ca2+ in STING signaling. During viral infections (HSV1 and HCMV) increase in intracellular Ca2+ may activate STING via multiple pathways. High Ca2+ levels are sensed by Calmodulin (CaM) that phosphorylates CaMKII and AMPK. They in turn suppress ULK1 (inhibitor of STING) phosphorylation activating STING. Activated CaMKII and AMPK also phosphorylate Beclin 1 that induces autophagy and STING. Using Sendai virus and HCMV particles, it was demonstrated that virus entry and fusion with endosomal membranes induce cytoplasmic Ca2+ oscillations and disruption of Ca2+ signaling with 2-APB completely blocking the antiviral activity by inhibiting the relocation of STING from ER to the cytoplasm as well as nuclear translocation of IRF3 suggesting that Ca2+ is required for STING activation in the response to HCMV infection (Hare et al., 2015). Enhanced intracellular Ca2+ also triggers loss of mitochondrial membrane potential and opening of mPTP resulting in the leakage of mtDNA into the cytosol. This is sensed by cGAS that gets activated and in turn leads to STING activation (Hare et al., 2015; Holm et al., 2012; Mathavarajah et al., 2019; Sharon-Friling and Shenk, 2014). A study on mouse macrophages indicates that STIM1 (an ER Ca2+ sensor) interacts with STING resulting in its activation to trigger IFN response (Srikanth et al., 2019). In this context, it is important to note that STING also tightly regulates Ca2+ fluxes in association with SERCA2 (Mathavarajah et al., 2019).
4.2. Modulation of innate immune signaling via autophagy induction
Autophagy plays a crucial role in defense against intracellular pathogens and many viral pathogens are also known to exploit autophagy for their benefit. Ca2+ is crucial for autophagic induction however it is unclear if an increase in cytosolic Ca2+ levels or modifications of ER Ca2+ levels contributes to the induction of autophagy (Brady et al., 2007; Gao et al., 2008; Gordon et al., 1993; Høyer-Hansen et al., 2007). During stress conditions like starvation, Ca2+ released from lysosomes activates the Transcription Factor EB (TFEB) by a calcium-dependent phosphatase, Calcineurin. TFEB migrates to the nucleus to activate the expression of autophagic pathway genes (Medina et al., 2015). On the other hand, deregulation of IP3R mediated Ca2+ signaling reduces mitochondrial Ca2+ uptake, amputates OXPHOS and stimulates autophagy due to activation of AMPK. IP3R also regulates autophagy through its interaction with Beclin-1. IP3R antagonist, Xestospongin B induces autophagy by disrupting the molecular complex formed by IP3R and Beclin 1 (Cárdenas et al., 2010; Decuypere et al., 2011; Vicencio et al., 2009).
With all these facts, Ca2+ signaling proves to be a major modulator of host innate immune responses towards many pathogens and hence can be a potent target for developing therapeutic approaches.
5. Mitochondrial Ca2+ signaling as a therapeutic target
Ca2+ being the central player in cellular bioenergetics as well as organelle homeostasis, can be explored as a potential therapeutic target against innumerable human diseases including those manifested upon pathogen infestation. Owing to its role in many host cellular pathways, its modulation will be a better approach in the development of a broad spectrum drug against many human illnesses. The anti-JEV compound CW33, reduces mitochondrial Ca2+ overload and stabilizes membrane potential via activation of Akt/mTOR and Jak/STAT1 pathway in JEV infected BHK21 cells. It further activates the GTPases and induces STAT1 mediated antiviral response against JEV (Huang et al., 2016). During HBV infection, cytosolic Ca2+ dependent Proline-rich tyrosine Kinase (PyK2) is activated by HBV X protein that enhances viral DNA replication and assembly (Bouchard et al., 2001; Choi et al., 2005; Zhang et al., 2016). A specific L-type Ca2+ channel blocker Oxethazaine, generally used as a gastric pain reliever is found to significantly decrease cytosolic Ca2+ concentration and thereby inhibits PyK2 mediated HBV replication and capsid assembly. Anti-influenza compound Amantadine ameliorates HCV proteins induced mitochondrial dysfunction and nitro oxidative stress via prevention of mitochondrial Ca2+ overload, ROS production, induction of mitochondrial hyperpolarization and delaying Ca2+ induced mPTP opening (Piccoli et al., 2009; Quarato et al., 2014). In addition to Amantadine, another antiviral drug Alisporivir also prevents HCV-induced mitochondrial Ca2+ overload, enhances cellular respiration and limits ROS production. Being a Cyclophilin D inhibitor, it also reverts HCV-induced mitochondrial dysfunction via blocking Cyclophilin D interaction with mPTP and desensitizing its opening (Quarato et al., 2012). MicroRNA 222 downregulates Thrombospondin-1 (THBS1) and Cluster of Differentiation 47 (CD47) expression which are known to induce mitochondrial dysfunction by modulating mitochondrial Ca2+ level and MMP upon Transmissible Gastro Enterovirus (TGEV) infection (Zhao et al., 2019). Hepatic ischemia/reperfusion injury includes mitochondrial dysfunction as a result of mPTP opening. A study involving an array of small molecule inhibitors of cyclophilin D suggested these inhibitors prevent mPTP opening in hepatocytes, thus restoring the hepatocytes Ca2+ retention capacity, oxidative phosphorylation and reduced liver damage in diseased cellular as well as animal model (Panel et al., 2019). An anti-inflammatory and anti-oxidative agent Minocycline has Ca2+ chelating property that is proved useful in normalizing CHPV induced cytoplasmic Ca2+ levels thereby limiting ROS production and p38 phosphorylation that otherwise leads to activation of the apoptotic cascade (Verma et al., 2018). Neurotrophins are proved to protect neurons against HIV infection mediated destabilization of Ca2+ homeostasis and oxidative stress. LM11A-31 is a novel non-peptide that mimics a p75 neurotrophin receptor ligand-binding domain of nerve growth factor (NGF). LM11A-31 stabilizes Ca2+ homeostasis and prevents the development of disease conditions in HIV-infected neurons by PI3K-dependent activation of AKT1 signaling (Meeker et al., 2016). Ebola virus entry into host cells is dependent on the host endosomal Two Pore Channel (TPC). Tetrandrine is a small molecule inhibitor of the TPC channel that restricts Ebola virus entry and infection in macrophages as well as in vivo models (Sakurai et al., 2015). A study on mitochondrial Ca2+ stabilization hypothesizes a hybrid trifunctional neuroprotective compound possessing the ability to ameliorate the deregulation of Ca2+ dynamics during certain diseases like Alzheimer’s. It contains three different moieties for regulation of different Ca2+ related pathways; (i) a dihydropyridine moiety to block L type Ca2+channels restricting Ca2+ entry, (ii) a benzothiazepine moiety to block NCLX and slow down Ca2+ efflux from mitochondria, and (iii) a polyphenol moiety to mask excess free radicals. This leads to delayed apoptosis and can act as an effective neuroprotector useful in treating Alzheimer’s by delaying disease progression (Fernández-Morales et al., 2012).
In addition to these approved drug molecules and newly synthesized chemical compounds, certain host factors play a protective role against the deregulation of Ca2+ homeostasis. Forkhead box O3a (FOXO3a) is a transcription factor that up-regulates Bcl2/adenovirus F1B 19KDa protein Interacting Protein 3 (BNIP3) expression in adult cardiomyocytes that results in increased mitochondrial Ca2+ leading to MMP loss, mitochondrial fragmentation and apoptosis. dn-FX3a, an Adeno associated virus encoding dominant-negative FOXO3a is proved to attenuate BNIP3 expression and improves mitochondrial structure and function (Chaanine et al., 2016) in normal as well as stressed cardiomyocytes. Hax1 is another host factor localized in the cytosolic surface of ER and mitochondria (Yap et al., 2011) that regulates Ca2+ signaling and inhibits apoptosis (Li et al., 2009). It is the central regulator of ER Ca2+ storage in interaction with Ca2+ pumps. HIV and CSFV virus proteins interact with Hax1 and disrupt its localization pattern and induce cell death (Jacotot et al., 2000). Hax1 also regulates lymphocyte development, trafficking and survival (Chao et al., 2008). Deregulation of the same is seen in cases of cancer, neurological disorder and psoriasis (Simmen, 2011). Taking into account their importance in cellular Ca2+ homeostasis and associated functions, both FOXO3a and Hax1 can be explored as potential targets for therapeutics development against certain diseases resulting from Ca2+ signaling deregulation.
Certain pharmacological modulators have been found to regulate Ca2+ transport, dynamics, and homeostasis during preliminary research. However, their role as potential therapeutics against human diseases needs further research. Ruthenium Red (RuR/ Ru360) reduces Ca2+ uptake and mitochondrial Ca2+ overload (García-Rivas et al., 2006). Veratridine treatment enhances mitochondrial Ca2+ accumulation, oxidative stress and p38-MAPK linked neural cell death in mouse hippocampal slices. CGP-37157 is a mitochondrial NCLX channel inhibitor that regulates the mitochondrial Ca2+ level, cellular energy metabolism and rescues neurons from veratridine induced death (Nicolau et al., 2010). CGP-37157 also affects Ca2+ transport by affecting mRyR, SERCA pump in striated muscles, inhibiting L type Ca2+ channel in β cells and modulating VGCC in neurons (Neumann et al., 2011). Certain Benzodiazepines and Benzothiazepines like Clonazepam and Diltiazem respectively also inhibit L type Ca2+ channel in the plasma membrane (Kinnally et al., 1993). Another in silico study including 700 FDA-approved drugs, suggests Mitoxanthone to specifically inhibit Ca2+ uptake by MCU without affecting mitochondrial bioenergetics (Arduino and Perocchi, 2018). Dantrolene, an antispasmodic drug inhibits ER Ca2+ channels and limits Ca2+ accumulation (Piccoli et al., 2007). Acyclovir blocks mitochondrial permeability transition pore and thus restricts deregulated leakage across the mitochondrial membrane that leads to apoptosis (Scrima et al., 2018). Though these compounds are effective in preserving mitochondrial Ca2+ homeostasis, their in vivo application is still under question. There are certain limitations like lack of specificity, interference with the function of other organelles and inadequate efficiency that demands further research for its clinical validation and usage.
6. Conclusion and future perspectives
Ca2+ is an indispensable ion for almost every cellular process and is tightly maintained in a state of homeostasis. Perturbances in cellular Ca2+ homeostasis have been implicated in many human pathologies and infectious diseases. Many viruses target cellular Ca2+ homeostasis to proliferate and disseminate successfully. The broad range of intracellular Ca2+ concentrations across membranes enable the viruses to make subtle changes in Ca2+ concentrations for their own benefit without major detrimental effects to the host cells. Targeting cellular Ca2+ signaling is an attractive therapeutic option to curb viral infections and counteract viral disease pathogenesis. It has to be noted that Ca2+ is pivotal for many cellular processes and therapeutics targeting Ca2+ signaling may have detrimental and toxic effects. However, being a major target of many viruses, modulation of cellular Ca2+ signaling may have a broad antiviral effect and facilitate development of panantivirals. Due to its function as a central node of multiple signaling events, understanding the direct effect of Ca2+ perturbations during viral infections has been a challenge and needs further detailed investigations. The transient and rapid spatiotemporal changes in Ca2+ concentration and signaling urge for development of more specific and flexible tools to investigate Ca2+ signaling pathways and unravel the precise mechanisms involved. Many FDA approved drugs targeting specific Ca2+ signaling components are already in use to treat several human pathological conditions. The probable repurposing of such drugs may be an attractive option to rapidly develop cost-effective antiviral therapeutics targeting the Ca2+ signaling.
Acknowledgement
Gulam Hussain Syed is thankful for the funds received from DBT-Wellcome Trust India Alliance (IA/I/15/1/501826), DST SERB (CRG/2020/006238/BHS) and research funds from the Institute of Life Sciences (ILS), Bhubaneswar. Swagatika Panda is thankful to the National Postdoctoral Fellowship (PDF/2019/001189). Mohd. Faraz Alam and Suchismita Behera are thankful for the research fellowship from ILS, Bhubaneswar.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdullah H, Heaney LG, Cosby SL, McGarvey LPA. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax. 2014;69(1):46–54. doi: 10.1136/thoraxjnl-2013-203894. [DOI] [PubMed] [Google Scholar]
- Arduino DM, Perocchi F. Pharmacological modulation of mitochondrial calcium homeostasis. J Physiol. 2018;596(14):2717–2733. doi: 10.1113/JP274959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosludtsev KN, Dubinin MV, Belosludtseva NV, Mironova GD. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochem. 2019;84(6):593–607. doi: 10.1134/S0006297919060026. [DOI] [PubMed] [Google Scholar]
- Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, Paterlini-Bréchot P. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24(31):4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Biasiotto R, Aguiari P, Rizzuto R, Pinton P, D’Agostino DM, Ciminale V. The p13 protein of human T cell leukemia virus type 1 (HTLV-1) modulates mitochondrial membrane potential and calcium uptake. BBA. 2010;1797(6-7):945–951. doi: 10.1016/j.bbabio.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Chehab T, Bultynck G, Parys JB, Rietdorf K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium. 2018;70:32–46. doi: 10.1016/j.ceca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Chang Y. Kaposi’s sarcoma-associated herpesvirus: a new DNA tumor virus. Annu Rev Med. 2001;52(1):453–470. doi: 10.1146/annurev.med.52.1.453. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Puro RJ, Wang L, Schneider RJ. Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication. J Virol. 2003;77(14):7713–7719. doi: 10.1128/JVI.77.14.7713-7719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Wang L, Schneider RJ. Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J Virol. 2006;80(9):4406–4414. doi: 10.1128/JVI.80.9.4406-4414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science (80-) 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- Brask J, Owe-Larsson B, Hill RH, Kristensson K. Changes in calcium currents and GABAergic spontaneous activity in cultured rat hippocampal neurons after a neurotropic influenza A virus infection. Brain Res Bull. 2001;55(3):421–429. doi: 10.1016/S0361-9230(01)00536-6. [DOI] [PubMed] [Google Scholar]
- Bravo-Sagua R, Parra V, López-Crisosto C, Díaz P, Quest AF, Lavandero S. Calcium Transport and Signaling in Mitochondria. Compr Physiol. 2017;7:623–634. doi: 10.1002/cphy.c160013. [DOI] [PubMed] [Google Scholar]
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124:2511. doi: 10.1242/jcs.095455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisac C, Téoulé Francois, Autret A, Pelletier I, Colberè-Garapin F, Brenner C, Lemaire C, Blondel B. Calcium flux between the endoplasmic reticulum and mitochondrion contributes to poliovirus-induced apoptosis. J Virol. 2010;84(23):12226–12235. doi: 10.1128/JVI.00994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briston T, Selwood DL, Szabadkai G, Duchen MR. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol Sci. 2019;40(1):50–70. doi: 10.1016/j.tips.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33(1):257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J-P, Cotte-Laffitte J, Linxe C, Quero A-M, Géniteau-Legendre M, Servin A. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74(5):2323–2332. doi: 10.1128/JVI.74.5.2323-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, de Jong AS, Lanke KWH, Melchers WJG, Willems PHGM, Pinton P, Rizzuto R, van Kuppeveld FJM. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J Biol Chem. 2004;279(18):18440–18450. doi: 10.1074/jbc.M309494200. [DOI] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Muller M, Vais H, Cheung K-H, Yang J, Parker I, Thompson CB, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaanine AH, Kohlbrenner E, Gamb SI, Guenzel AJ, Klaus K, Fayyaz AU, Nair KS, Hajjar RJ, Redfield MM. FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am J Physiol Hear Circ Physiol. 2016;311(6):H1540–H1559. doi: 10.1152/ajpheart.00549.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Ferrari D, Nicotera P, Paterlini-Braéchot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278(34):31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- Chami M, Oulès B, Paterlini-Bréchot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. BBA. 2006;1763(11):1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A, Srinivasan A, Raman R, Viswanathan K, Raguram S, Tumpey TM, Sasisekharan V, Sasisekharan R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol. 2008;26(1):107–113. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- Chanturiya AN, Basañez G, Schubert U, Henklein P, Yewdell JW, Zimmerberg J. PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes. J Virol. 2004;78(12):6304–6312. doi: 10.1128/JVI.78.12.6304-6312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J-R, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452(7183):98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- Chatila T, Ho N, Liu P, Liu S, Mosialos G, Kieff E, Speck SH. The Epstein-Barr virus-induced Ca2+/calmodulin-dependent kinase type IV/Gr promotes a Ca(2 +)-dependent switch from latency to viral replication. J Virol. 1997;71:6560–6567. doi: 10.1128/JVI.71.9.6560-6567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology. 2008;372(2):221–232. doi: 10.1016/j.virol.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Reid C, Band V, Androphy E. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science(80-.) 1995;269(5223):529–531. doi: 10.1126/science:7624774. [DOI] [PubMed] [Google Scholar]
- Chen S, Shenk T, Nogalski MT. P2Y2 purinergic receptor modulates virus yield, calcium homeostasis, and cell motility in human cytomegalovirus-infected cells. Proc Natl Acad.Sci USA. 2019a;116(38):18971–18982. doi: 10.1073/pnas.1907562116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Cao R, Zhong Wu. Host Calcium Channels and Pumps in Viral Infections. Cells. 2019b;9(1):94. doi: 10.3390/cells9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Liao Y, Zhou L, Peng S, Chen H, Yuan Z. Amplified RLR signaling activation through an interferon-stimulated gene-endoplasmic reticulum stress-mitochondrial calcium uniporter protein loop. Sci Rep. 2016;6:20158. doi: 10.1038/srep20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Del Rosario B, Woda C, Marcellino D, Satlin LM, Herold BC. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol. 2003;163:283–293. doi: 10.1083/jcb.200301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Liu W, Satlin LM, Herold BC, Linstedt A. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol Biol Cell. 2007;18(8):3119–3130. doi: 10.1091/mbc.e07-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Trepanier JB, González PA, Eugenin EA, Jacobs WR, Herold BC. Herpes simplex virus type 2 glycoprotein H interacts with integrin αvβ3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J Virol. 2014;88:10026–10038. doi: 10.1128/JVI.00725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Gyoo Park S, Yoo J-h, Jung G. Calcium ions affect the hepatitis B virus core assembly. Virology. 2005;332(1):454–463. doi: 10.1016/j.virol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Ciminale V, Zotti L, D’Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I(HTLV-I) Oncogene. 1999;18(31):4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- Compan V, Baroja-Mazo A, López-Castejón G, Gomez A, Martínez C, Angosto D, Montero María T, Herranz A, Bazán E, Reimers D, Mulero V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37(3):487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. BBA. 2010;1797(6-7):607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. BBA. 2009;1787(11):1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino DM, Ranzato L, Arrigoni G, Cavallari I, Belleudi F, Torrisi MR, Silic-Benussi M, Ferro T, Petronilli V, Marin O, Chieco-Bianchi L, et al. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J Biol Chem. 2002;277(37):34424–34433. doi: 10.1074/jbc.M203023200. [DOI] [PubMed] [Google Scholar]
- D’Agostino DM, Silic-Benussi M, Hiraragi H, Lairmore MD, Ciminale V. The human T-cell leukemia virus type 1 p13II protein: effects on mitochondrial function and cell growth. Cell Death Differ. 2005;12(Suppl 1):905–915. doi: 10.1038/sj.cdd.4401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- de Jong AS, Visch H-J, de Mattia F, van Dommelen MM, Swarts HG, Luyten T, Callewaert G, Melchers WJ, Willems PH, van Kuppeveld FJ. The coxsackievirus 2B protein increases efflux of ions from the endoplasmic reticulum and Golgi, thereby inhibiting protein trafficking through the Golgi. J Biol Chem. 2006;281(20):14144–14150. doi: 10.1074/jbc.M511766200. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere J-P, Bultynck G, Parys JB. A dual role for Ca(2+) in autophagy regulation. Cell Calcium. 2011;50(3):242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Decuypere J-P, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB, Holowka D. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS ONE. 2013;8(4):e61020. doi: 10.1371/journal.pone.0061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. BBA. 2009;1787(11):1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013;17(6):965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Ding W, Albrecht Björn, Kelley RE, Muthusamy N, Kim S-J, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76(20):10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Albrecht Björn, Luo R, Zhang W, Stanley JRL, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin. J Virol. 2001;75(16):7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionicio CL, Peña F, Constantino-Jonapa LA, Vazquez C, Yocupicio-Monroy M, Rosales R, Zambrano JL, Ruiz MC, Del Angel RM, Ludert JE. Dengue virus induced changes in Ca. Virus Res. 2018;245:17–28. doi: 10.1016/j.virusres.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, Majano PL, Benedicto I, Rosado JA, Salido GM, Gonzalez-Gallego J. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50(5):872–882. doi: 10.1016/j.jhep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Doitsh G, Greene WC. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe. 2016;19:280–291. doi: 10.1016/j.chom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zeng C-Q-Y, Ball JM, Estes MK, Morris AP. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci USA. 1997;94(8):3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516(Pt 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas B, Delfraissy JF, Calenda A, Peuchmaur M, Wallon C, Rannou MT, Galanaud P. Activation and infection of B cells by Epstein-Barr virus. Role of calcium mobilization and of protein kinase C translocation. J Immunol. 1988;141:4344–4351. [PubMed] [Google Scholar]
- Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265(1):35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elustondo PA, Nichols M, Robertson GS, Pavlov EV. Mitochondrial Ca. J Bioenerg Biomembr. 2017;49:113–119. doi: 10.1007/s10863-016-9676-6. [DOI] [PubMed] [Google Scholar]
- Feng P, Park J, Lee B-S, Lee S-H, Bram RJ, Jung JU. Kaposi’s sarcoma- associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002;76(22):11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Morales JC, Arranz-Tagarro J-A, Calvo-Gallardo E, Maroto M, Padín J-F, García AG. Stabilizers of neuronal and mitochondrial calcium cycling as a strategy for developing a medicine for Alzheimer’s disease. ACS Chem Neurosci. 2012;3(11):873–883. doi: 10.1021/cn3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Skolnik EY, Prakriy M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 2012;12(7):532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti M, Cartier L, Piguet V, Lew DP, Carpentier J-L, Tron D, Krause K-H. The HIV Nef protein alters Ca2+ signaling in myelomonocytic cells through SH3-mediated protein-protein interactions. J Biol Chem. 1999;274(49):34765–34772. doi: 10.1074/jbc.274.49.34765. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Harty RN. Calcium and filoviruses: a budding relationship. Futur Microbiol. 2016;11(6):713–715. doi: 10.2217/fmb-2016-0057. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235(2):241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- Fujioka Y, Nishide S, Ose T, Suzuki T, Kato I, Fukuhara H, Fujioka M, Horiuchi K, Satoh AO, Nepa P, Kashiwagi S, et al .A Sialylated Voltage-Dependent Ca. Cell Host Microbe. 2018;23:809–818.:e5. doi: 10.1016/j.chom.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Gao W, Ding W-X, Stolz DB, Yin X-M. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4(6):754–761. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rivas G de J, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, Mille CCJ. The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr Biol. 2017;27(3):371–385. doi: 10.1016/j.cub.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PB, Hole I, Fosse M, Røtnes JS, Seglen PO. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem. 1993;268(35):26107–26112. [PubMed] [Google Scholar]
- Griffin SD, Beales LP, Clarke DS, Worsfol O, Evans SD, Jaege J, Harris MP, Rowlands DJ. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Peng Z, Dudek E, Fan X, Mizianty MJ, Dufe E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y, Kim DH, et al. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal. 2014;7:ra54. doi: 10.1126/scisignal.2004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne GS, Yan Y, Li F, Walseth TF, Marchant JS. NAADP-dependent Ca. Cell Calcium. 75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK. Uptake of calcium by mitochondria: transport and possible function. IUBMB Life. 2001;52(3-5):197–204. doi: 10.1080/15216540152846000. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting J-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chen K-H, Gu Y, Liao H, Tang J, Xiao R-P. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res. 2007;101(11):1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- Han Z, Madara JJ, Herbert A, Prugar LI, Ruthel G, Lu J, Liu Y, Liu W, Liu X, Wrobel JE, Reitz AB, et al. Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention. PLoS Pathog. 2015;11(10):e1005220. doi: 10.1371/journal.ppat.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DN, Collins SE, Mukherjee S, Loo Y-M, Gale M, Janssen LJ, Mossman KL, Sandri-Goldin RM. Membrane Perturbation-Associated Ca2+ Signaling and Incoming Genome Sensing Are Required for the Host Response to Low-Level Enveloped Virus Particle Entry. J Virol. 2015;90(6):3018–3027. doi: 10.1128/JVI.02642-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- Hashimi H, McDonald L, Stříbrná E, Lukeś J. Trypanosome letm1 protein is essential for mitochondrial potassium homeostasis. J Biol Chem. 2013;288(37):26914–26925. doi: 10.1074/jbc.M113.495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Henkel M, Mitzner D, Henklein P, Meyer-Almes F-J, Moroni A, DiFrancesco ML, Henkes LM, Kreim M, Kast SM, Schubert U, Thiel G, et al. The proapoptotic influenza A virus protein PB1-F2 forms a nonselective ion channel. PLoS ONE. 2010;5(6):e11112. doi: 10.1371/journal.pone.0011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Kwon S-K, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, Polleux F. ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science. 2017;358(6363):623–630. doi: 10.1126/science:aan6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28(1):491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91(4):1369–1378. doi: 10.1016/S0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, Gonzalez-Dosal R, Rasmussen SB, Christensen MH, Yarovinsky TO, Rixon FJ, et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13(8):737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, Springer D, et al. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol. 2015;85:178–182. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom J, Yu T, Yoon Y, Porter G, Sheu S-S. Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. BBA. 2010;1797(6-7):913–921. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Luo S, Baumeister P, Huang J-M, Gogia RK, Li M, Lee AS. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response. J Biol Chem. 2004;279(12):11354–11363. doi: 10.1074/jbc.M309804200. [DOI] [PubMed] [Google Scholar]
- Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35(6):253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Huang S-H, Lien J-C, Chen C-J, Liu Y-C, Wang C-Y, Ping C-F, Lin Y-F, Huang A-C, Lin C-W. Antiviral Activity of a Novel Compound CW-33 against Japanese Encephalitis Virus through Inhibiting Intracellular Calcium Overload. Int J Mol Sci. 2016;17(9):1386. doi: 10.3390/ijms17091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AV, Smirnova OA, Petrushanko IY, Ivanova ON, Karpenko IL, Alekseeva E, Sominskaya I, Makarov AA, Bartosch B, Kochetkov SN, Isaguliants MG. HCV core protein uses multiple mechanisms to induce oxidative stress in human hepatoma Huh7 cells. Viruses. 2015;7:2745–2770. doi: 10.3390/v7062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol. 2013;425(24):5009–5019. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Métivier D, Lenoir C, Geuskens M, Vieira HL, et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca 2+/H+ antiporter. Science(80-) 2009;326(5949):144–147. doi: 10.1126/science:1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Mulloy JC, Ciminale V, Fullen J, Nicot C, Franchini G. The MHC class I heavy chain is a common target of the small proteins encoded by the 3’ end of HTLV type 1 and HTLV type 2. AIDS Res Hum Retroviruses. 2000;16(16):1777–1781. doi: 10.1089/08892220050193308. [DOI] [PubMed] [Google Scholar]
- Khananshvili D. The SLC8 gene family of sodium-calcium exchangers (NCX)-Structure, function, and regulation in health and disease. Mol Aspects Med. 2013;34(2-3):220–235. doi: 10.1016/j.mam.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A. 1993;90(4):1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human Immunodeficiency Virus Type 1 (HIV-1) Accessory Protein Vpr Induces Transcription of the HIV-1 and Glucocorticoid-Responsive Promoters by Binding Directly to p300/CBP Coactivators. J Virol. 2002;76(19):9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6(3):235–244. doi: 10.1016/S1074-7613(00)80326-X. [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531(7593):258–264. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147.. [DOI] [PubMed] [Google Scholar]
- Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460(1):114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154(2):276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lahti AL, Manninen A, Saksela K. Regulation of T cell activation by HIV-1 accessory proteins: Vpr acts via distinct mechanisms to cooperate with Nef in NFAT-directed gene expression and to promote transactivation by CREB. Virology. 2003;310(1):190–196. doi: 10.1016/S0042-6822(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Lara-Pezzi E, Luis Armesilla A, Majano PL, Miguel Redondo J, López-Cabrera M. The hepatitis B virus X protein activates nuclear factor of activated T cells (NF-AT) by a cyclosporin A-sensitive pathway. EMBO J. 1998;17(23):7066–7077. doi: 10.1093/emboj/17.23.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-S, Lee S-H, Feng P, Chang H, Cho N-H, Jung JU. Characterization of the Kaposi’s sarcoma-associated herpesvirus K1 signalosome. J Virol. 2005;79(19):12173–12184. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Hayashi T, Su T-P, Betenbaugh MJ. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioenergetics and cell survival. J Bioenerg Biomembr. 2014;46(1):1–15. doi: 10.1007/s10863-013-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-B, Feng J, Geng S-M, Zhang P-Y, Yan X-N, Hu G, Zhang C-Q, Shi B-J. Induction of apoptosis by Hax-1 siRNA in melanoma cells. Cell Biol Int. 2009;33(4):548–554. doi: 10.1016/j.cellbi.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Boehning DF, Qian T, Popov VL, Weinman SA. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J. 2007;21(10):2474–2485. doi: 10.1096/fsb2.v21.1010.1096/fj.06-7345. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apopto. J Biol Chem. 2004;279(35):37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- Li Y, Tran Q, Shrestha R, Piao L, Park S, Park J, Park J. LETM1 is required for mitochondrial homeostasis and cellular viability (Review) Mol Med Rep. 2019;19:3367–3375. doi: 10.3892/mmr.2019.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Weiss A. Ca-dependent Ras/Erk signaling mediates negative selection of autoreactive B cells. Small GTPases. 2011;2:282–288. doi: 10.4161/sgtp.2.5.17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu P, Borras A, Chatila T, Speck SH. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 1997;16:143–153. doi: 10.1093/emboj/16.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, Miller R, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14(12):1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen A, Saksela K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J Exp Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavarajah S, Salsman J, Dellaire G. An emerging role for calcium signalling in innate and autoimmunity via the cGAS-STING axis. Cytokine Growth Factor Rev. 2019;50:43–51. doi: 10.1016/j.cytogfr.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mayne M, Holden CP, Nath A, Geiger JD. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol. 2000;164:6538–6542. doi: 10.4049/jimmunol.164.12.6538. [DOI] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17(3):288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Poulton W, Clary G, Schriver M, Longo FM. Novel p75 neurotrophin receptor ligand stabilizes neuronal calcium, preserves mitochondrial movement and protects against HIV associated neuropathogenesis. Exp Neurol. 2016;275(Pt 1):182–198. doi: 10.1016/j.expneurol.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Lee JH, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci U S A. 1994;91(2):772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/JVI.67.6.3087-3094.1993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH, Melchjorsen J, Höllsberg P, Paludan SR. Activation of NF-kappa B in virus-infected macrophages is dependent on mitochondrial oxidative stress and intracellular calcium: downstream involvement of the kinases TGF-beta-activated kinase 1, mitogen-activated kinase/extracellular signal-regulated kin. J Immunol. 2003;170:6224–6233. doi: 10.4049/jimmunol.170.12.6224. [DOI] [PubMed] [Google Scholar]
- Mosialos G, Hanissian SH, Jawahar S, Vara L, Kieff E, Chatila TA. A Ca2 +/calmodulin-dependent protein kinase, CaM kinase-Gr, expressed after transformation of primary human B lymphocytes by Epstein-Barr virus (EBV) is induced by the EBV oncogene LMP1. J Virol. 1994;68:1697–1705. doi: 10.1128/JVI.68.3.1697-1705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109(28):11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AM, Michael B, Datta A, Fernandez S, Lairmore MD. Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I. Virology. 2006;353(2):247–257. doi: 10.1016/j.virol.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash AA, Olson RM, Griffin S, Gale M, Walker CM. Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. PLoS Pathog. 2019;15(2):e1007593. doi: 10.1371/journal.ppat.1007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann JT, Diaz-Sylvester PL, Fleischer S, Copello JA. CGP-37157 inhibits the sarcoplasmic reticulum Ca2+ ATPase and activates ryanodine receptor channels in striated muscle. Mol Pharmacol. 2011;79(1):141–147. doi: 10.1124/mol.110.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau SM, Egea J, López MG, García AG. Mitochondrial Na+/Ca2+ exchanger, a new target for neuroprotection in rat hippocampal slices. Biochem Biophys Res Commun. 2010;400(1):140–144. doi: 10.1016/j.bbrc.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 2007;14(9):1647–1656. doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]
- Nye KE, Pinching AJ. HIV infection of H9 lymphoblastoid cells chronically activates the inositol polyphosphate pathway. AIDS. 1990;4(1):41–46. doi: 10.1097/00002030-199001000-00006. [DOI] [PubMed] [Google Scholar]
- Nykky J, Vuento M, Gilbert L, Gallyas F. Role of mitochondria in parvovirus pathology. PLoS ONE. 2014;9(1):e86124. doi: 10.1371/journal.pone.0086124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JC, Jeong D-L, Kim I-K, Oh S-H. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp Mol Med. 2003;35(4):301–309. doi: 10.1038/emm.2003.41. [DOI] [PubMed] [Google Scholar]
- Omar S, Clarke R, Abdullah H, Brady C, Corry J, Winter H, Touzelet O, Power UF, Lundy F, McGarvey LPA, Cosby SL, et al. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS ONE. 2017;12(2):e0171681. doi: 10.1371/journal.pone.0171681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel M, Ruiz I, Brillet R, Lafdil F, Teixeira-Clerc F, Nguyen CT, Calderaro J, Gelin M, Allemand F, Guichou J-F, Ghaleh B, et al. Small-Molecule Inhibitors of Cyclophilins Block Opening of the Mitochondrial Permeability Transition Pore and Protect Mice From Hepatic Ischemia/Reperfusion Injury. Gastroenterology. 2019;157(5):1368–1382. doi: 10.1053/j.gastro.2019.07.026. [DOI] [PubMed] [Google Scholar]
- Papp B, Byrn RA. Stimulation of HIV expression by intracellular calcium pump inhibition. J Biol Chem. 1995;270(17):10278–10283. doi: 10.1074/jbc.270.17.10278. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim SH, Das A, Yang SN, Jung KH, Kim MK, Berggren PO, Lee Y, Chai JC, Kim HJ, Chai YG. TLR3-/4-Priming Differentially Promotes Ca (2+) Signaling and Cytokine Expression and Ca(2+)-Dependently Augments Cytokine Release in hMSCs. Sci Rep. 2016;6:23103. doi: 10.1038/srep23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupe V, Prudent J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem Biophys Res Commun. 2018;500(1):75–86. doi: 10.1038/srep23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peischard S, Ho HT, Theiss C, Strutz-Seebohm N, Seebohm G. A Kidnapping Story: How Coxsackievirus B3 and Its Host Cell Interact. Cell Physiol Biochem. 2019;53:121–140. doi: 10.33594/000000125. [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467(7313):291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, D’Aprile A, Scrima R, Ripoli M, Boffoli D, Tabilio A, Capitanio N. Role of reactive oxygen species as signal molecules in the pre-commitment phase of adult stem cells. Ital J Biochem. 2007;56:295–301. [PubMed] [Google Scholar]
- Piccoli C, Quarato G, Ripoli M, D’Aprile A, Scrima R, Cela O, Boffoli D, Moradpour D, Capitanio N. HCV infection induces mitochondrial bioenergetic unbalance: causes and effects. BBA. 2009;1787(5):539–546. doi: 10.1016/j.bbabio.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, et al. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PLoS ONE. 2013;8(2):e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poruchynsky MS, Maass DR, Atkinson PH. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J Cell Biol. 1991;114:651–656. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston CN, Krishnan SC, Bazemore-Walker CR. In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM) J Proteomics. 2013;79:219–230. doi: 10.1016/j.jprot.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Quarato G, D’Aprile A, Gavillet B, Vuagniaux G, Moradpour D, Capitanio N, Piccoli C. The cyclophilin inhibitor alisporivir prevents hepatitis C virus- mediated mitochondrial dysfunction. Hepatology. 2012;55(5):1333–1343. doi: 10.1002/hep.25514. [DOI] [PubMed] [Google Scholar]
- Quarato G, Scrima R, Ripoli M, Agriesti F, Moradpour D, Capitanio N, Piccoli C. Protective role of amantadine in mitochondrial dysfunction and oxidative stress mediated by hepatitis C virus protein expression. Biochem Pharmacol. 2014;89(4):545–556. doi: 10.1016/j.bcp.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabò I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum Mitochondri, and Lysosomes. Trends Biochem Sci. 2016;41(12):1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani K, Rugman FP, Savant CS, Vakil SD. HTLV 1 associated myelopathy and adult T cell leukaemia-lymphoma in the same patient: report of a case. J Neurol Neurosurg Psychiatry. 1995;59(5):560–561. doi: 10.1136/jnnp.59.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial Interactome: A Focus on Antiviral Signaling Pathways. Front Cell Dev Biol. 2020;8:8. doi: 10.3389/fcell.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Garcia S, Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review) Int J Oncol. 2019;54:1155–1167. doi: 10.3892/ijo.2019.4696. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rossi A, Pizzo P, Filadi R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim Biophys Acta - Mol Cell Res. 2019;1866(7):1068–1078. doi: 10.1016/j.bbamcr.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347(6225):995–998. doi: 10.1126/science:1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342(6164):1379–1382. doi: 10.1126/science:1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. "New" functions for "old" proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1(4):241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Scrima R, Piccoli C, Moradpour D, Capitanio N. Targeting Endoplasmic Reticulum and/or Mitochondrial Ca. Front Chem. 2018;6:73. doi: 10.3389/fchem.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Fu Z, Ji Y, Guan X, Guo S, Ding Z, Yang X, Cong Y, Shen Y. Leucine zipper-EF-hand containing transmembrane protein 1(LETM1) forms a Ca. Sci Rep. 2016;6:34174. doi: 10.1038/srep34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon-Friling R, Shenk T. Human cytomegalovirus pUL37x1-induced calcium flux activates PKCα, inducing altered cell shape and accumulation of cytoplasmic vesicles. Proc Natl Acad Sci U S A. 2014;111:E1140–E1148. doi: 10.1073/pnas.1402515111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C, Yang C-C, Choijilsuren G, Chang C-H, Liou A-T. Hepatitis B Virus. Trends Microbiol. 2018;26(4):386–387. doi: 10.1016/j.tim.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Simmen T. Hax-1: a regulator of calcium signaling and apoptosis progression with multiple roles in human disease. Expert Opin Ther Targets. 2011;15(6):741–751. doi: 10.1517/14728222.2011.561787. [DOI] [PubMed] [Google Scholar]
- Simmons A, Aluvihare V, McMichael A. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity. 2001;14(6):763–777. doi: 10.1016/S1074-7613(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Song Z, Wang Yu, Zhang F, Yao F, Sun C. Calcium signaling pathways: Key pathways in the regulation of obesity. Int J Mol Sci. 2019;20(11):2768. doi: 10.3390/ijms20112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu S-S, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270(46):27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Woo JS, Wu B, El-Sherbiny YM, Leung J, Chupradit K, Rice L, Seo GJ, Calmettes G, Ramakrishna C, Cantin E, et al. The Ca(2+) sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat Immunol. 2019;20(2):152–162. doi: 10.1038/s41590-018-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield WE, Ranek M, Pendse A, Schisler JC, Wang S, Pulinilkunnil T, Willis MS. Cellular and Molecular Pathobiology of Cardiovascular Disease. Elsevier; 2014. pp. 51–78. [DOI] [Google Scholar]
- Streicher F, Jouvenet N. Stimulation of Innate Immunity by Host and Viral RNAs. Trends Immunol. 2019;40(12):1134–1148. doi: 10.1016/j.it.2019.10.009. [DOI] [PubMed] [Google Scholar]
- Strtak AC, Perry JL, Sharp MN, Chang-Graham AL, Farkas T, Hyser JM. Recovirus NS1-2 Has Viroporin Activity That Induces Aberrant Cellular Calcium Signaling To Facilitate Virus Replication. mSphere. 2019:4. doi: 10.1128/mSphere.00506-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Sokolove PM. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch Biochem Biophys. 2001;386(1):37–51. doi: 10.1006/abbi.2000.2194. [DOI] [PubMed] [Google Scholar]
- Tang S, Chen T, Yang M, Wang L, Yu Z, Xie B, Qian C, Xu S, Li N, Cao X, Wang J. Extracellular calcium elicits feedforward regulation of the Toll-like receptor-triggered innate immune response. Cell Mol Immunol. 2017;14(2):180–191. doi: 10.1038/cmi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Wang X, Shen Q, Yang X, Yu C, Cai C, Cai G, Meng X, Zou F. Mitochondrial Ca2+ uniporter is critical for store-operated Ca2+ entry-dependent breast cancer cell migration. Biochem Biophys Res Commun. 2015;458(1):186–193. doi: 10.1016/j.bbrc.2015.01.092. [DOI] [PubMed] [Google Scholar]
- Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/JVI.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar D, Dong Z, Shanmughapriya S, Koch D, Thomas T, Hoffman N, Timbalia S, Goldman S, Breves S, Corbally D, Nemani N, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016;15(8):1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fernández Orlando, Yepes Gloriae, Gomez Javiere, Pimienta Hernanj. Calbindin distribution in cortical and subcortical brain structures of normal and rabies-infected mice. Int J Neurosci. 2005;115(10):1375–1382. doi: 10.1080/00207450590956396. [DOI] [PubMed] [Google Scholar]
- Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9(4):445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-W, Wu Y, Pao P-C, Phillips CB, Williams C, Miller C, Ranaghan M, Tsai M-F. Proteolytic control of the mitochondrial calcium uniporter complex. Proc Natl Acad Sci U S A. 2017;114(17):4388–4393. doi: 10.1073/pnas.1702938114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/ 14 protein complex. J Virol. 2005;79(2):1099–1112. doi: 10.1128/JVI.79.2.1099-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubol S, Kasisith J, Pitidhammabhorn D, Tepsumethanol V. Screening of pro-apoptotic genes upregulated in an experimental street rabies virus-infected neonatal mouse brain. Microbiol. Immunol. 2005;49(5):423–431. doi: 10.1111/mim.2005.49.issue-510.1111/j.1348-0421.2005.tb03746.x. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppeveld FJM, de Jong AS, Melchers WJG, Willems PHGM. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol. 2005;13(2):41–44. doi: 10.1016/j.tim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- van Vliet AR, Verfaillie T, Agostinis P. New functions of mitochondria associated membranes in cellular signaling. BBA. 2014;1843(10):2253–2262. doi: 10.1016/j.bbamcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere J-P, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19(11):1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Ghosh S, Basu A. Chandipura Virus Induced Neuronal Apoptosis via Calcium Signaling Mediated Oxidative Stress. Front Microbiol. 2018;9:1489. doi: 10.3389/fmicb.2018.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio JM, Ortiz C, Criollo A, Jones AWE, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16(7):1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A. 2013;110(12):4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang X, Shen Y. Molecular mechanism of mitochondrial calcium uptake. Cell Mol Life Sci. 2015;72(8):1489–1498. doi: 10.1007/s00018-014-1810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhu Y, Lin X, Ren C, Zhao J, Wang F, Gao X, Xiao R, Zhao L, Chen H, Jin M, et al. Influenza M2 protein regulates MAVS-mediated signaling pathway through interacting with MAVS and increasing ROS production. Autophagy. 2019;15(7):1163–1181. doi: 10.1080/15548627.2019.1580089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G, Livolsi A, Imbert V, Peyron JF, Siddiqui A. Hepatitis C virus NS5A and subgenomic replicon activate NF-kappaB via tyrosine phosphorylation of IkappaBalpha and its degradation by calpain protease. J Biol Chem. 2003;278:40778–40787. doi: 10.1074/jbc.M303248200. [DOI] [PubMed] [Google Scholar]
- Weis K, Griffiths G, Lamond AI. The endoplasmic reticulum calcium-binding protein of 55 kDa is a novel EF-hand protein retained in the endoplasmic reticulum by a carboxyl-terminal His-Asp-Glu-Leu motif. J Biol Chem. 1994;269(29):19142–19150. [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Shen Y, Xie H, Zheng S. Involvement of endoplasmic reticulum in hepatitis B virus replication. Virus Res. 2006;121(2):116–121. doi: 10.1016/j.virusres.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Xin L, Ma X, Xiao Z, Yao H, Liu Z. Coxsackievirus B3 induces autophagy in HeLa cells via the AMPK/MEK/ERK and Ras/Raf/MEK/ERK signaling pathways. Infect Genet Evol. 2015;36:46–54. doi: 10.1016/j.meegid.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Morizane S, Yamamoto T, Wada J, Iwatsuki K. High calcium enhances the expression of double-stranded RNA sensors and antiviral activity in epidermal keratinocytes. Exp Dermatol. 2018;27(2):129–134. doi: 10.1111/exd.2018.27.issue-210.1111/exd.13456. [DOI] [PubMed] [Google Scholar]
- Yao J-H, Liu Z-J, Yi J-H, Wang J, Liu Y-N. Hepatitis B Virus X Protein Upregulates Intracellular Calcium Signaling by Binding C-terminal of Orail Protein. Curr Med Sci. 2018;38(1):26–34. doi: 10.1007/s11596-018-1843-z. [DOI] [PubMed] [Google Scholar]
- Yap SV, Koontz JM, Kontrogianni-Konstantopoulos A. HAX-1: a family of apoptotic regulators in health and disease. J Cell Physiol. 2011;226(11):2752–2761. doi: 10.1002/jcp.22638. [DOI] [PubMed] [Google Scholar]
- Yura Y, Matsumoto R, Sumi T, Kusaka J. Effect of Ca2+-dependent cell death on the release of herpes simplex virus. Arch Virol. 2003;148(2):221–235. doi: 10.1007/s00705-002-0924-1. [DOI] [PubMed] [Google Scholar]
- Zaitseva E, Zaitsev E, Melikov K, Arakelyan A, Marin M, Villasmil R, Margolis LB, Melikyan GB, Chernomordik LV. Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine. Cell Host Microbe. 2017;22(1):99–110.:e7. doi: 10.1016/j.chom.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampese E, Pizzo P. Intracellular organelles in the saga of Ca2+ homeostasis: different molecules for different purposes? Cell Mol Life Sci. 2012;69(7):1077–1104. doi: 10.1007/s00018-011-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Moran O, Rasola A, Rugolo M, Porcelli AM, Rossi B, Galietta LJ. HIV-1 nef expression inhibits the activity of a Ca2+-dependent K+ channel involved in the control of the resting potential in CEM lymphocytes. J Immunol. 1999;162:5359–5366. [PubMed] [Google Scholar]
- Zhang L, Liu C, Xiao Y, Chen X. Oxethazaine inhibits hepatitis B virus capsid assembly by blocking the cytosolic calcium-signalling pathway. J Gen Virol. 2016;97:1198–1209. doi: 10.1099/jgv.0.000417. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hsia SC, Martin-Caraballo M. Regulation of T-type Ca. J Neurochem. 2019a;151:238–254. doi: 10.1111/jnc.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hsia SC, Martin-Caraballo M. Regulation of T-type Ca. J Neurovirol. 2017;23:657–670. doi: 10.1007/s13365-017-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun R, Geng S, Shan Y, Li X, Fang W, Jung JU. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J Virol. 2019b;93(7) doi: 10.1128/JVI.01784-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Song X, Bai X, Tan Z, Ma X, Guo J, Zhang Z, Du Q, Huang Y, Tong D. microRNA-222 Attenuates Mitochondrial Dysfunction During Transmissible Gastroenteritis Virus Infection. Mol Cell Proteomics. 2019;18(1):51–64. doi: 10.1074/mcp.RA118.000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeteweij JP, Moses AV, Rinderknecht AS, Davis DA, Overwijk WW, Yarchoan R, Orenstein JM, Blauvelt A. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:2374–2380. doi: 10.1182/blood.V97.8.2374. [DOI] [PubMed] [Google Scholar]