Abstract
Evidence is increasing for positive effects of α-diversity on ecosystem functioning. We highlight here the crucial role of β-diversity – a hitherto underexplored facet of biodiversity – for a better process-level understanding of biodiversity change and its consequences for ecosystems. A focus on β-diversity has the potential to improve predictions of natural and anthropogenic influences on diversity and ecosystem functioning. However, linking the causes and consequences of biodiversity change is complex because species assemblages in nature are shaped by many factors simultaneously, including disturbance, environmental heterogeneity, deterministic niche factors, and stochasticity. Because variability and change are ubiquitous in ecosystems, acknowledging these inherent properties of nature is an essential step for further advancing scientific knowledge of biodiversity–ecosystem functioning in theory and practice.
The Importance of β-Diversity for Understanding the Causes and Consequences
There is a growing body of evidence showing that biodiversity is important for generating and stabilizing ecosystem functions, and thus ensures the provisioning of numerous ecosystem services to society [1]. Theoretical, experimental, and observational studies across different types of ecosystems and biomes [2,3] confirm positive effects of local-scale biodiversity on ecosystem functions. The largest body of evidence exists for the linkages between local species richness (α-diversity – the number and abundance of species within local communities of interacting species) of plants and biomass productivity. Studies on species richness–productivity relationships have substantially advanced our understanding of the mechanisms underlying the functional roles of biodiversity [4–7]. Recently, research on the functional role of biodiversity has broadened its view beyond a strong focus on productivity, simultaneously considering the effects of diversity on multiple ecosystem functions [8–15]. A series of studies have demonstrated that high levels of species richness are important for sustaining multiple functions and services, and thus a loss of species can adversely affect the functionality of ecosystems. However, another facet of biodiversity, which – as we posit here – is essential in the context of ecosystem multifunctionality, has been considered relatively scantily in investigations of biodiversity–ecosystem functioning (see Glossary) relationships to date. The multiscale nature of biodiversity, and specifically β-diversity – the variation in the identities and abundances of species among local assemblages – has received much less attention compared to α-diversity. Our aim here is to highlight the crucial role of β-diversity, by synthesizing its mechanistic effect on biodiversity organization (and its responses to natural and anthropogenic drivers), and by describing its association with the provisioning of multiple ecosystem functions.
Glossary.
- β-Diversity
the variation in the identities and abundances of species among local species assemblages. It can be quantified in different ways, including taxonomic, functional, and phylogenetic dissimilarity, either weighted by relative abundances or not. Biotic homogenization is the outcome of a human-induced reduction in β-diversity.
- Biodiversity–ecosystem functioning
the study framework that investigates possible consequences of biodiversity change on ecosystem functions. In experimental studies, species diversity is manipulated to quantify the net effects of biodiversity loss on ecosystem functioning. With the help of advanced statistical methods, non-manipulative studies are also increasingly feasible for the evaluation of the relationships between biodiversity and ecosystem functioning in real-world settings.
- Biotic homogenization
an anthropogenic impact on biodiversity. Because of human-induced decreases in environmental variability (environmental homogenization), species assemblages could increase in similarity in terms of their taxonomic, functional, and phylogenetic composition across locations. The term was originally used to describe the replacement of native by non-native species that can result in a decline in community dissimilarity over spatial and temporal scales.
- Community assembly
considers the mechanisms by which local species assemblages are organized, and describes the final outcome of these organization processes. There is debate about whether the outcomes of community assembly processes result in a single, stable equilibrium, alternative stable states, or an alternative transient state. It is often difficult to define the final timepoint of community assembly processes.
- Deterministic processes
contribute to the processes of community assembly in predictable, non-random ways. Important processes of deterministic assembly include species–environment associations, habitat filtering, competitive hierarchy among species, and interspecific niche partitioning. Note that some other processes such as dispersal limitation and priority effect, which are often considered to be stochastic assembly processes (see below), can also be under the control of deterministic processes.
- Stochastic processes
these contribute to the process of community assembly that follows the mathematical theory of stochasticity and is not necessarily predictable. Important factors behind stochastic assembly include historic contingency (the order of arrival, i.e., the priority effect), ecological drift (demographic or environmental stochasticity), and dispersal limitation. Note that it is often difficult to identify the roles and contributions of these factors to community assembly, especially for observational studies, which is why they are often considered to be seemingly random. However, some deterministic processes can also operate within the frame of these stochastic processes.
Biodiversity–Ecosystem Multifunctionality
There is increasing concern regarding the causes and consequences of human-induced β-diversity change [16,17], including biotic homogenization [18,19]. Homogenization of ecological communities could affect ecosystem functioning as strongly as, or even more strongly than, the effects of local species losses or gains (changes in α-diversity; cf the spatial insurance hypothesis [20]). While potential degradation of ecosystem functions and services has been reported in response to a decline of α-diversity, for instance, similar investigations are still widely lacking for β-diversity (Box 1). A perspective based on β-diversity is especially important in the context of multifunctionality. This is because there is no ubiquitous species assemblage that can simultaneously support all functions at high levels. Consequently, sustaining multiple functions requires different sets of local species assemblages (i.e., β-diversity) in a heterogeneous environment [21]. Given that people depend on multiple, rather than individual, ecosystem services simultaneously for human well-being, the growing theoretical and empirical evidence for a positive contribution of biodiversity to ecosystem multifunctionality is of high practical importance.
Box 1. Possible Effects of Biotic Homogenization on Ecosystem Multifunctionality in a Landscape.
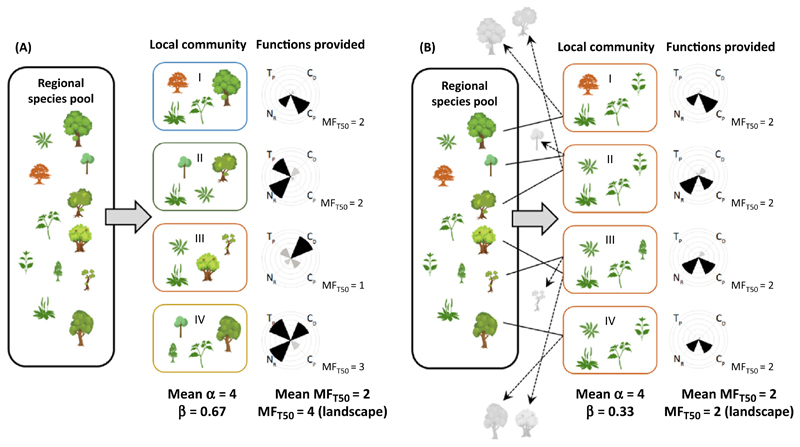
Figure I shows that plant species assemblages in each locality support some focal functions (i.e., biomass production, crop production, nutrient retention, and conservation of faunal diversity). Supporting all ecosystem functions in all single localities is unrealistic [14], and different sets of species in different local communities are expected to support ecosystem functioning in a different manner [10]. Supporting multiple functions in a landscape thus needs a variety of species [13]. Reflecting the spatial variation in species composition, ecosystem functions supported by local species assemblages should also be spatially variable in natural systems.
In this landscape (Figure I), plant species in local communities are drawn from a regional species pool consisting of different types of plants, including trees, grasses, wildflowers, and ferns (A). Suppose this landscape has been affected by agricultural development, and thus only a small subset of plant species (mainly, non-woody plants) remain in local communities. As a result of such anthropogenic filtering and the associated environmental homogenization (e.g., through irrigation, fertilization or landscape simplification), species composition is similar among localities, leading to a decline in β-diversity (biotic homogenization) (B). Note that, in this landscape, land-use intensification does not lead to a decline in the number of species and the functions supported by them; in other words α-diversity and the number of functions above the threshold value of 50% of the maximum performance (MFT50) are maintained before and after biotic homogenization [mean α-diversity = 4 and mean MFT50 = 2 for both (A) and (B)]. However, the decline in β-diversity results in a loss of functionality (i.e., loss of high functionality for timber production and conservation of faunal diversity as a result of the loss of tree diversity; e.g., [13]), leading to a decrease in the total number of ecosystem functions in this landscape [β-diversity of 0.67 and 0.33, and a landscape-level MFT50 of 4 and 2 for (A) and (B), respectively].
α-Diversity has been receiving special attention in the study of biodiversity–ecosystem functioning [11]; however, this example emphasizes that potential threats to ecosystem functionality cannot be fully captured by such an exclusive focus on one spatial level of biodiversity (see also [23,31,46,58]).
In addition, note that landscape structure (e.g., complex vs simple landscape; Figure II) has been a long focus in applied ecology [66]; however, potential consequences for β-diversity are less clear from these studies. An explicit focus on this scale of diversity would add to our mechanistic understanding of diversity effects beyond a phenomenological characterization of heterogeneity.
Figure I. Schematic Analysis of the Relationship between Plant Diversity and Four Ecosystem Functions at Different Scales.
(A) Before and (B) after abiotic and biotic homogenization caused by human activities. Each plant icon represents a different species, and those shown in grey are lost from a meta-community because of anthropogenic filtering (systematically lost species [98]). Such environmental filters select a subset of species with specific traits that can persist in a human-modified landscape [66]. α-Diversity is the mean number of species in local communities, and β-diversity is calculated based on Whittaker’s multiplicative index (β = 1 –mean α/γ). Radial bar charts indicate the percent levels of individual functions (TP, timber production; CP, crop production; NR, nutrient retention; CD, conservation of faunal diversity), which are evaluated based on the threshold approach [9]. Functions greater or equal to the threshold of 50%, and those less than the threshold of 50%, are shown by black and grey bars in the diagram, respectively. MFT50 indicates the number of functions that exceed the threshold of 50% of the observed maximum values for the given function. MFT50 is evaluated both at the local and landscape levels.
Figure II. Aerial Photographs of Different Landscape Structures.
Examples for relatively (A) complex and (B) simple landscape configurations are shown. Photographs are from Google Inc. The spatial extents are same for the two figure panels (roughly 2 km × 2 km).
With increasing dimensionality of the functional context, any species could become fundamentally irreplaceable [10]. This and related notions (i.e., low multifunctional redundancy [9,14,22]) are increasingly recognized in ecology, and underline the imperative to conserve high levels of local diversity. While studies of biodiversity–multifunctionality have substantially contributed to understanding why and how biodiversity is important, remaining uncertainties include the inevitable trade-offs between different functions [12,14]. Sustaining all functions at high levels in a single locality is unrealistic because ecosystems are heterogeneous in nature (with spatial differences in species richness, identity, and composition), and because some functions might be mutually exclusive. Recognizing the heterogeneous distribution of species and functions across space and time calls for a more dynamic appraisal of diversity as a factor that is not static but changes over space and time (e.g., the course of patch dynamics and succession in a forest ecosystem).
The Causes and Consequences of Biodiversity Changes
Focusing on spatial attributes and levels of diversity is not necessarily new in the study of biodiversity–ecosystem functioning [20,23]. Previous work showed that spatial and temporal turnover in species can contribute towards simultaneously supporting different functions [8–10]. New evidence for the effects of β-diversity (Table 1) is becoming available for different groups of organisms from experimental [11], theoretical [24], and observational studies [13–15]. Nevertheless, the observed patterns are not always consistent, likely resulting from different definitions and metrics being used to define β-diversity, as well as a possible dependence between α- and β-diversity (Table 1) (also see [25]). We thus cannot yet deduce a generalized theory on the role of β-diversity in ecosystem functioning. Nonetheless, some important implications have emerged that should be considered in future research. First, it is indeed important to consider the effect of diversity at multiple spatial scales [26]. The role of β-diversity and spatial scale in general in mediating the functional consequences of biodiversity change is linked with the variations in local-scale diversity (different number and identities of species in a local assemblage), resulting in local changes in ecosystem functioning that can scale up to large-scale changes in the provisioning of multiple ecosystem functions [14,15]. Second, it is important to account for the mechanisms driving spatial variation in local diversity so as to understand (and subsequently manage) diversity–ecosystem functioning relationships. In these regards, it is worth focusing on the notion of Mokany et al. [27], who stated that the ‘insurance effects of β-diversity’ (to support ecosystem functioning [11,20]) may only significantly manifest itself under spatiotemporal interactions between communities that are distributed non-randomly across large areas of space. That is, they emphasized the importance of natural processes that organize biodiversity and in so doing support ecosystem function. It is important to note that β-diversity is useful to infer environmental, spatial, and stochastic determinants of community assembly for numerous organism groups [28–30]. Taken together, focusing on this dimension of diversity has profound potential not only for quantifying the large-scale importance of biodiversity to sustain the (multi)functionality of ecosystems but also because it may contribute to a mechanistic understanding of processes underlying the emergence of the observed patterns of spatial variation in species assemblages and functions.
Table 1. Characteristics of Recent Studies that Quantify the Relationship between β-Diversity and Ecosystem Function(s)a.
| Study and region | Focal taxa | Focal function(s) | Approach and dataset | Note | Refs |
|---|---|---|---|---|---|
| Pasari et al. (USA) | Grassland plants | Aboveground productivity, root biomass, soil carbon, nitrogen retention, invasion resistance, insect richness, insect abundance | Simulated artificial landscapes based on experimental data were used. β-Diversity was calculated with Sørensen’s index; it is not fully independent of α-diversity [33]. The averaging and threshold approach [9,42] were used to evaluate diversity–multifunctionality relationships | α-Diversity had strong positive effects on individual functions and multifunctionality, and positive effects of β-diversity emerged only when multiple functions were simultaneously considered. The study suggests that, in addition to conserving important species, maintaining ecosystem multifunctionality will require a landscape mosaic of diverse communities | [11] |
| Silva Pedro et al. (Germany) | Forest trees | Primary productivity | Simulations with a process-based forest landscape and disturbance model were conducted for a temperate forest landscape. β-Diversity was calculated via the multiplicative law (γ = αβ), representing the effective number of distinct communities on the landscape [38]. Productivity was the focal ecosystem function | β-Diversity had a larger effect on productivity than α-diversity, especially at the later stages of succession following disturbance. The study suggests that instead of homogenizing areas affected by natural disturbances, forest management should incorporate diversity created by disturbances into stand development to capitalize on a positive diversity effect on productivity | [24] |
| Mori et al. (Japan) | Soil fungi | Belowground primary production, soil carbon sequestration, plant litter decomposition (three different substrates), amount of plant-available nitrogen, nitrogen retention | Observational data from a real landscape were used. Local- and landscape-level dissimilarities of communities and functions were quantified. Effects of α-diversity on β-diversity were removed, based on the modified Raup–Crick index [96]. The averaging method [97], multiple thresholds [42], and a method based on mixed models [14] were applied to evaluate the diversity–multifunctionality relationships | Unlike the positive effects of α-diversity on multifunctionality at the local scale, effects of β-diversity on multifunctionality were only prominent at the landscape level. The study suggests that making species assemblages depauperate may result in a loss of multifunctionality | [14] |
| van der Plas et al. (six European countries) | Forest trees | Timber quality, timber production, root biomass, litter decomposition, wood decomposition, microbial biomass, soil carbon stock, tree regeneration, drought resistance, insect herbivory resistance, mammal browsing resistance, pathogen resistance, earthworm biomass, bird diversity, bat diversity, understory plant diversity | Simulated artificial landscapes based on observational data were used. β-Diversity was calculated with Lennon’s index; it is not fully independent of α-diversity [34]. The threshold approach [9] was used to evaluate diversity–multifunctionality relationships | The relationships between β-diversity and landscape-scale multifunctionality were always positive. The study suggests that it is important to conserve the landscape-scale biodiversity that is being eroded by biotic homogenization if multifunctionality is to be maintained | [13] |
| Hautier et al. (65 study sites of the Nutrient Network Global Research Cooperative) | Grassland plants | Aboveground live biomass, resource capture aboveground (light interception), resource pools belowground (percentage total soil nitrogen and extractable soil phosphorus and potassium), soil carbon storage, litter decomposition, invasion resistance | Observational data from the Nutrient Network Global Research Cooperative (NutNet). Pretreatment data on community-level functions were used, which means that communities in the real-world ecosystems were focused. β-Diversity was calculated with Sørensen’s dissimilarity index. The averaging and threshold approaches [9,42] were used to evaluate diversity–multifunctionality relationships. The effects of mean α-diversity and of β-diversity on the multifunctionality in each of 65 sites and their interactive effects were compared across these study sites | Grassland ecosystems with both high α-diversity and β-diversity had higher levels of multifunctionality. In addition, the identity of species influencing ecosystem function differed among functions and across local communities, likely explaining why more diverse grasslands maintained greater multifunctionality when more functions and localities were considered | [15] |
The Many Faces of β-Diversity
Different patches of a natural system can be in different developmental stages at any given point in time. Such asynchronous development enhances spatial variation in local biodiversity (i.e., β-diversity). Spatial and temporal processes underlying the origin and organization of biodiversity can thus not be fully separated. In the following we focus in particular on β-diversity in the context of spatial differences in species composition across local communities within a landscape (i.e., areas of 1–1000 km2). Assessing the spatial variability of species composition is a useful measure to understand responses of communities to variable environmental conditions and their consequences for ecological properties [31] (effect-and-response framework of β-diversity).
The Additive Partitioning Methods
β-Diversity can be quantified in many ways [32–35], including factors such as species turnover, nestedness, and richness differences [36,37]. The many definitions of β-diversity may be one reason why this facet of diversity has not been a focus of studies on biodiversity–ecosystem functioning to date. Depending on the choice of metrics, one can gain different results for β-diversity. Consequently, it generally remains unknown which drivers behind the observed pattern of β-diversity are most tightly associated with focal ecosystem processes. In manipulative studies it may be feasible to control and separate the different factors underlying β-diversity. However, because β-diversity is usually related to landscape-scale variation, the applicability of manipulative studies is limited and their replication nearly impossible. In this context, the method of β-diversity partitioning, which has strongly contributed to a more rigorous understanding of community organization [37–41], could be an effective way to further advance a mechanistic understanding of the roles of β-diversity in the support of ecosystem functioning. Similar achievements have been made for the relationships between α-diversity and ecosystem functioning based on methods to partition the net diversity effect into its selection and complementarity components [4]. A possible linkage between the two partitioning methods is illustrated in Figure 1. It is worth focusing here on the initial evidence from experiments of biodiversity–multifunctionality based on species turnover in functional contributions [8,10]. As illustrated by Byrnes et al. [42], their results lie between the two extremes of no species turnover and no species redundancy across functions. In other words, both the number and identity of species are important to simultaneously supporting multiple functions. Quantifying the relative contributions of richness and identity is important in theory and practice [43]. Smith and Knapp [44] showed that dominant species can support ecosystem function when species loss is nonrandom, although the long-term consequences of species loss remain unclear. Lohbeck et al. [45] similarly found a primary control of dominant tree species on supporting multifunctionality in tropical forests, although high levels of species richness were important because of spatial and temporal turnover of species. Furthermore, Mori et al. [14] identified a set of functionally important species of soil fungi that support belowground multi-functionality, although the overall importance of species richness was larger than the contributions of these species. If the number of species is more important than the identity of species, management needs to shift its focus from a small set of focal species towards species richness to ensure the provisions of ecosystem services. If species with a disproportionate effect on ecosystem functioning can be identified, however, such information could be useful for management to select and prioritize species of interest and concern. In these regards, β-diversity partitioning may be an intriguing approach with high theoretical and practical utility.
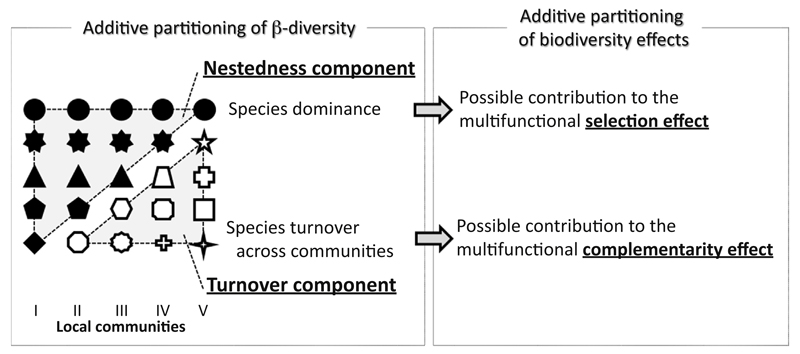
Figure 1. Schematic Representation of Potential Linkages between the Additive Partitioning Methods of β-Diversity and Biodiversity Effects [4,37].
Separating the different effects of diversity is not easy, especially for communities in the real world [6], because of large variations in species composition and their functionalities under variable environmental conditions [21]. This figure represents a possible approach to cope with this issue. Each black and white icon represents a different species. Icons in the same column make up the individual local communities (I–V). Across local communities, there are dominant species that contribute to the nestedness component of β-diversity. Such species could play a crucial role in supporting multiple ecosystem functions; they could impose a diversity effect that may be (if not fully) equivalent to the selection effect by virtue of their competitive dominance. The other issue illustrated here is that species turnover occurs across communities in a landscape [15], most frequently as a result of environmental variation. Because different species perform differently under different environmental conditions, they could complement each other in utilizing available resources, and thus enhance the niche space occupation across locations. Therefore, the complementarity effect of diversity for multiple functions could be linked to the turnover component of β-diversity at larger spatial scales. Note that, in reality, dominant species (or functionally important species) can also change across communities [8]; therefore, it is likely that species turnover does not always contribute to the multifunctional species complementarity and could instead be associated with species selection, especially at smaller spatial scales. In addition, no formal approach exists to partition the diversity effects for ecosystem multifunctionality [99]; this diagram considers a multifunctional context and thus may differ from new partitioning approaches developed for a single functional context (cf [100]).
Linkages among α-, β-, and γ-Diversity
Another complexity is that β-diversity can be affected by diversity at other scales, including α- and γ-diversity (total number of species in a region) [46–48]. Karp et al. [46] demonstrated the scale-dependency of β-diversity responses to land-use intensification because of a sampling effect. They showed that land-use intensification filtered bird species and thus reduced local species richness of bird communities. At small spatial scales, drawing small samples from the meta-community with low α-diversity could increase the likelihood that species composition differs between locations (indicating high β-diversity in a highly intensified landscape). Once this sampling effect was removed, β-diversity substantially decreased in highly intensified landscapes. They further found that community homogenization at large scales as a result of trait filtering was followed by a decline in functional diversity (a similar example for plants is illustrated in Box 1). Given the importance of avian functional diversity in supporting ecosystem services [49,50] such as pest control [51] and seed dispersal [52], the impacts of such a diversity loss could be enormous. This example highlights the difficulties as well as the potential of focusing on β-diversity to infer anthropogenic influences on biodiversity and ecosystem functioning.
Heterogeneity, Determinism, and Stochasticity in Natural Ecosystems
Research on biodiversity and ecosystem functions is now moving towards evaluating the potential importance of these relationships in real-world ecosystems, advancing beyond an initial focus on experimental and manipulative results in model systems such as common gardens [6,14,53–55]. Compared to experimental systems, natural systems have a high level of spatial variation in biotic and abiotic characteristics. Considering environmental heterogeneity and interactions between species as well as stochastic factors affecting species assembly, β-diversity could play a central role in understanding how these naturally diverse and fluctuating communities are organized, and how such processes influence the functioning of ecosystems (Box 2).
Box 2. Community Assembly and Biodiversity–Ecosystem Functioning.
Disentangling the mechanisms underlying biodiversity organization (community assembly) and understanding the functional contributions of ecological communities (biodiversity–ecosystem functioning) are both central issues of community ecology (Figure I). However, these topics have thus far been discussed largely in parallel rather than together (if not always; e.g., [23]). We illustrate here how these closely associated issues of community ecology can be unified by accounting for different scales of biodiversity.
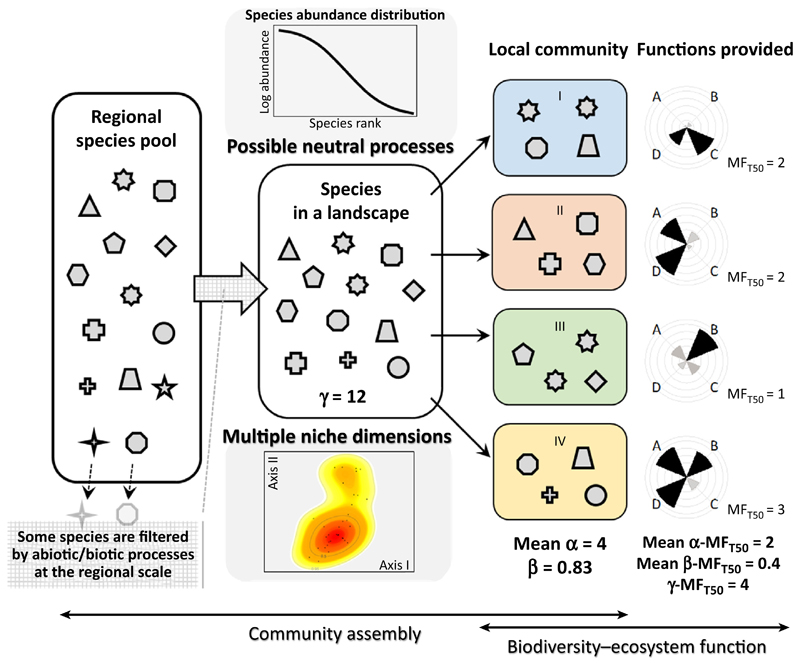
Figure I highlights important processes of community assembly operating at different spatial scales. In this system there are many species in a regional species pool. When a focus is given to a specific landscape, some species may not be observed but the majority of species can still be found. As generally observed in natural ecosystems [101], the number of individuals is not equally distributed across different species in the meta-community of a landscape; species abundance distribution is characterized by the dominance of a few species and the rarity of numerous others. Possible reasons for why these rare species can coexist include neutral processes [102] and other stochastic processes [103], as well as disturbances and the priority effect. In addition, because of a high level of species diversity, the meta-community is characterized by different suites of species traits, likely suggesting the existence of multiple niche dimensions. This is another important reason for how different species can coexist and thus local species diversity can be maintained [103]. In Figure I, for example, six traits of soil invertebrate assemblages in a natural forest [31] are summarized in a 2D spectrum based on kernel density estimation [104]. As a result of a variety of different assembly mechanisms including stochastic and deterministic processes, β-diversity arises and communities are differentiated among localities, contributing to the maintenance of multiple ecosystem functions in this landscape (the relationship between β-diversity and landscape-level multifunctionality is outlined in Box 1). Overall, β-diversity can be an important mediator between community assemblage and functioning, reflecting processes of community organization and determining the provisioning of multiple ecosystem functions.
Figure I. Schematic Illustration of Key Processes of Community Assembly and Biodiversity–Ecosystem Functioning Operating at Different Spatial Scales.
Each icon represents different species, and those shown in grey outline are lost from the meta-community of a landscape. The number of species in each local community, and in the landscape containing these communities, constitute α- and γ-diversity, respectively. β-Diversity is calculated based on Sørensen index [33]. To illustrate meta-community structure, the species abundance distribution [101] and kernel density estimation of the trait spectrum [104] are shown. For the latter, contour lines indicate 0.5 and 0.95 quantiles of the occurrence probability of traits. Spatial variation in local environmental conditions is shown by different colors (blue, red, green, and yellow) for the background of local communities [I–IV]. Evaluation of individual functions (A–D) is based on the threshold approach [9]. Levels of individual functions are shown with radial bar diagrams (as shown in Figure I in Box 1). Unlike in Figure I in Box 1, multifunctionality is assessed here at α-, β-, and γ-scales [13]; p-MFT50 values are calculated based on Bray–Curtis distance, and the mean value is shown.
Biotic Homogenization
In this regard, one important issue to be considered is biotic homogenization [18] (Box 1), a phenomenon describing the decline in β-diversity that is observed for many terrestrial [31,46,56–59] and, to a lesser extent, marine assemblages [60]. It occurs because of the loss of endemic species and/or the gain of cosmopolitan species [18]. The term is now widely used to describe the homogenizing process in communities regarding their taxonomic, functional, or phylogenetic diversity that is caused by anthropogenic influences such as land-use intensification [31,46,56–59] and climate change [60]. Environmental homogenization is often responsible for the observed patterns of biotic homogenization through trait filtering and the resulting dominance by specific combinations of species with a narrow set of selected traits [31,46,57]. Accordingly, functional homogenization often occurs simultaneously with biotic homogenization, and can be even more significant for functional characteristics than taxonomic homogenization [58]. In nature, community assembly processes are influenced by both deterministic processes (e.g., niche partitioning and species sorting based on competitive hierarchy) and stochastic processes (e.g., ecological drift, priority effect, and other forms of historic contingencies), and the relative importance of these processes changes in space and time [28,29]. Biotic homogenization often results from the elimination of one or several of these community assembly processes (Box 3). Recent evidence has shown that biotic homogenization does not necessarily correspond to a loss of local species richness [31,60]. This underlines the fact that a sole focus on α-diversity is not sufficient to capture the consequences of human alterations to ecosystems for their functioning. Detecting biotic homogenization via β-diversity is important because a loss of functional traits from regional species pools may be hidden behind the observed patterns of biotic homogenization. This loss could severely threaten functional diversity [31,46], an important facet of diversity when considering ecosystem functions [22,61].
Box 3. Anthropogenic Impacts on the Processes of Community Assembly and Their Potential Consequences on Biodiversity–Ecosystem Functioning.
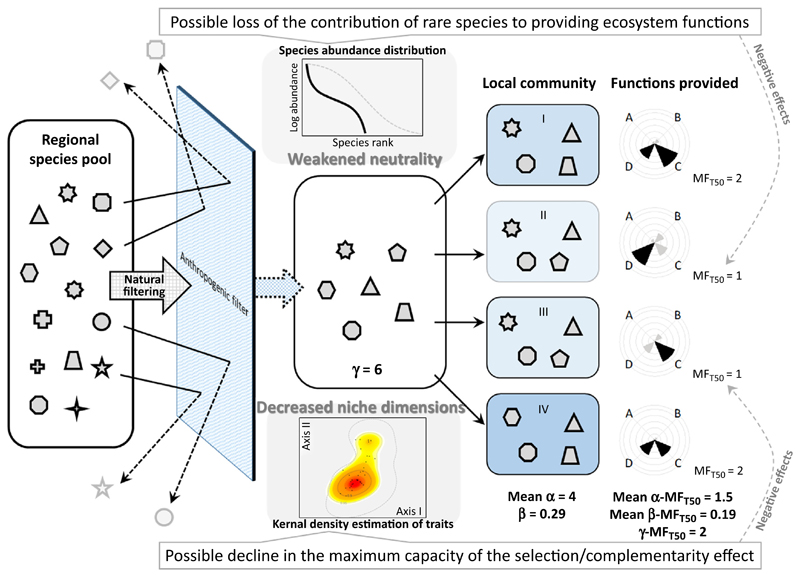
Figure I illustrates possible alterations of key processes underpinning community assembly and biodiversity–functioning relationships. In this example of biotic homogenization [β-diversity declines from 0.83 (Figure I in Box 1) to 0.29 (Figure I of this box), despite no change in α-diversity], some species are filtered by strong anthropogenic influences (e.g., land-use intensification) [31,46,57,59]. Note that this anthropogenic filter is exemplified here at the landscape level; however, such filtering can occur at any spatial scale. As a result, species that occur in the landscape are a small subset of those originally found in the region. Strong anthropogenic filtering deterministically selects species and thus may weaken important processes, most likely including neutrality and historic contingency. Species abundance distribution could change relative to that observed before human influences were intensified. In this example, the species abundance distribution is characterized by a log-normal distribution that tends to lack rare species (for instance, compared to those showing a log-series distribution [101]). Species lost tend to be rare and may be an endemic or a large-sized species, a frequently observed pattern of biotic homogenization [18]. Furthermore, strong filtering only allows species with specific traits (e.g., cosmopolitan species with high environmental tolerances) to exist in the landscape [57], affecting functional characteristics more than taxonomic characteristics of local communities [58]. Such selection could potentially affect the trait spectrum in many ways. Possibilities include a reduction of trait hypervolume in terms of size (volume) and complexity (dimension). In Figure I a trait spectrum was constructed using the dataset of Figure I in Box 2, but instead of using community data from a natural forest, communities homogenized by forest conversion were used to estimate the kernel density of the trait distribution; consequently, traits are more aggregated within a 2D plain (compared to those in Figure I Box 2). Such alternations of trait spectra may have large consequences for ecosystem functions.
Two important mechanisms underlying positive diversity effects on functions are the selection and complementarity effects [4]. If the aggregate of niche occupation (in terms of the absolute unit, not based on the relative unit) is reduced because of the reduction of trait space, ecosystem properties that emerge as a community-level aggregate of resource use could become weak; that is, even if a niche is effectively partitioned among species (niche complementarity), niche space itself is small enough to adversely affect overall functionality. This may also be the case for the selection effect; the maximum amount of resources that can be utilized by the dominant species could be limited, or important species that drive the selection effect have been already lost. Such weakening in local community characteristics (illustrated as ‘negative effects’ on MFT50 in Figure I) may provide an explanation for the important notion that the number of species is not necessarily a strong predictor of ecosystem function (e.g., [105]) (for instance, in the present explanation, α-diversity is kept constant at 4 in both Figure I of Box 2 and Figure I in this box). In this example, ecosystem multifunctionality at both the local and landscape scales is threatened (loss of the local and spatial insurance effect of diversity). These and other changes triggered by human influences could be assessed based on simultaneously focusing on different scales of biodiversity and ecosystem functioning relationships.
In Figure I a reduction in γ-diversity leads to a decline in β-diversity (i.e., sampling effect [48]). However, loss of β-diversity can occur in a variety of ways. A threat to β-diversity can even be masked by a simultaneous increase in α-diversity [46]. Our explanation is aimed at explicitly describing the importance of different scales of biodiversity with a particular focus on β-diversity, which has been considered in the context of understanding the processes of community organization [28,29,106] but has so far been widely disregarded with regard to its possible linkages with ecosystem functions [11]. Many tools and approaches, including simulation [24], theoretical [20], and empirical studies [25,55], are now available to carefully assess these interactive processes and the large-scale influences of environmental changes on small-scale outcomes of ecosystem functions and services.
Figure I. Schematic Illustration of Key Processes of Community Assembly and Biodiversity–Ecosystem Functioning, as Altered by Anthropogenic Influences (indicated as the blue-shaded filter).
Environmental conditions (shown by the light-blue background) and species composition (icons) in local communities (I–IV) are similar in functional traits to some extent (because of human-induced environmental homogenization). Explanations of the icons, inset displays, diversity indices, and multifunctionality indices are given in Figure I in Box 2. For species abundance distribution, a possible change is illustrated [there is a shift from the grey dotted curve (shown in Figure I in Box 2) to the black solid curve]. For kernel density estimation of traits, the same dataset [31] as Figure I in Box 2 is used but, instead of those in a natural forest, those homogenized because of forest conversion are shown here (0.95 quantiles of occurrence probability in Figure I in Box 2 is shown with a grey dotted line).
Changes in β-diversity [19,62] could thus be a potent indicator of threats on ecosystem functions and services. In a northern oak savanna, MacDougall et al. [63] demonstrated how human activities can homogenize environmental conditions and thus the diversity of ecosystems, leading to the hidden risk of abrupt and potentially irreversible change in the system after disturbance, despite the fact that it previously appeared to be stable. There is a growing body of literature on using spatial patterns as early-warning indicators of ecological regime shifts and critical transitions [64], but β-diversity has not yet been mainstreamed into these efforts. Scholars have only now started to shed light on possible linkages between heterogeneous dynamics of ecological communities and ecosystem functions, a theme that deserves further attention [21]. This is particularly the case when studying biodiversity–ecosystem multifunctionality in natural or close-to-natural ecosystems, which are characterized by heterogeneity and both deterministic and stochastic processes. A focus on β-diversity may yield insights into possible alternations of assembly processes (e.g., a shift from neutrality to niche-based assembly) and alert to possible consequences for ecosystem functions (e.g., loss of spatial insurance) (Box 3).
Landscape Complexity and Multifunctionality
The provisioning of multiple ecosystem services depends on the composition and configuration of landscapes [65,66]. Consequently, if different land-cover types deliver services to varying degrees, landscape diversification is an intuitive means to foster ecosystem multifunctionality [67]. There is thus an increasing demand for landscape diversification, as well as for identifying configurations that support multifunctionality [68,69]. The landscape scale is the scale that is often most relevant for informing policy related to land management. Landscape perspectives in restoration of biodiversity-based ecosystem services are increasingly gaining attention [70,71]. There has been a substantial effort to identify how landscape complexity is linked to biodiversity and ecosystem services [66,72]. Although the importance of β-diversity has not necessarily been a focus in these efforts, there are several implications for ecosystem functioning following a decline in β-diversity in human-modified landscapes. For instance, there is a substantial shift in species composition towards the dominance of less-specialized taxa across localities because of land-use intensification and homogenization, subsequently threatening functional diversity and ecosystem functions [31,46,50,57]. These studies have – implicitly – tested a landscape-moderated insurance hypothesis, which expects landscape complexity and heterogeneity to foster biodiversity, and consequently to support stability of ecosystem processes and provide insurance against changing environments [66]. However, a serious knowledge gap still exists regarding the contributions of β-diversity in supporting multifunctional landscapes.
In principle, β-diversity can be calculated at any spatial scale [34]. In the context of landscape diversification, however, a coarse scale of β-diversity is important (e.g., the diversity between stands and patches in a forest landscape). This is because providing multiple ecosystem services often requires patches in different successional stages or different types of land cover, acknowledging that broad and diverse areas of land are able to provide different services. Tylianakis et al. [73] showed for bee and wasp communities that, although α-diversity was higher in intensively used agroecosystems, β-diversity was higher in less intensively used agroecosystems owing to greater habitat heterogeneity and associated community dissimilarity contributing to γ-diversity. Lamy et al. [65] recently showed that both landscape configuration and composition influence the provisioning of ecosystem services, and that different bundles of services are associated with specific configurations and compositions on the landscape. Combining such evidence for landscape effects on biodiversity and ecosystem services has profound potential not only in the context of co-benefits (e.g., reducing emissions from deforestation and forest degradation, REDD+) but also for the functional roles of β-diversity in supporting ecosystem services. Importantly, Winfree et al. [74] recently showed the importance of bee β-diversity on pollination services at landscape-scale. A related issue is the scale-dependency of ecosystem services and their possible mismatches across scales [72]. Uncertainties exist for how accumulating local-scale evidence of positive biodiversity effects on ecosystem functioning can be scaled up to large scales at which policy can be informed [1]. In this regard, focusing on the variation of biodiversity in space and time is relevant for exploring potential schemes to secure ecosystem service provisioning in heterogeneous landscapes.
Heterogeneous Dynamics of Communities and Ecosystem Functions
History and Disturbance
Another issue is the effect of history and disturbance on biodiversity and ecosystem functioning because these factors are inherent, fundamental, and ubiquitous in nature. Disturbance often leaves long-term imprints on ecological properties such as species composition and functioning [75–77]. Disturbance legacies contribute to ecological integrity by virtue of carrying over important characteristics into the post-disturbance state, facilitating succession and the regeneration of biota [78]. Unlike anthropogenic disturbances, which often cause abiotic and biotic homogenization and a loss of β-diversity, natural disturbances often increase β-diversity, partly because they are often patchy, complex in shape, and variable in severity. Consequently, while human disturbances often lead to detrimental effects on ecosystem functioning, natural disturbances create niches for many taxa including rare and specialized species and prevent competitive exclusion, ultimately fostering the maintenance of biological diversity, as well as biodiversity-dependent ecosystem functioning.
While deterministic processes including niche theory have helped experimental and theoretical studies to explore the underlying mechanisms of biodiversity–ecosystem functioning [79,80], stochastic processes such as disturbance, historic contingency, and ecological drift have received considerably less attention. The latter processes could, however, be the reasons why many communities are diverse in terms of composition [81] and functioning [82,83]. At this juncture, a further focus on disturbances is of paramount importance. As a consequence of the spatial and temporal variability in disturbance regimes, species composition can diverge strongly between localities. Although the classical conception of disturbance assumed that ecosystem development was reset completely, more recent insights assert that ecosystems can indeed be diverse from the very beginning of their development [84]. Disturbance history therefore matters for the subsequent stages of ecological development, and disturbances of different severity and frequency can lead to complex structural and compositional patterns [85]. Recent advances in community ecology highlight that even subtle differences in community characteristics, such as the order of arrival of species after a high-severity disturbance (priority effect), can have long-lasting effects on species composition and ecosystem functions [82,83]. Because species assemblages are inherently prone to a variety of environmental perturbations, it is necessary to focus on the causes of biodiversity under given environmental conditions (i.e., assembly processes), in addition to its consequences for ecological properties (i.e., ecosystem functioning). It is thus worthwhile to further focus on β-diversity as the link between processes of community assembly and diversity–functioning relationships (also see Box 2).
Climate change could alter disturbance regimes in many regions [86]. A likely consequence of such changes in disturbance regimes is that the structure and functioning of ecological systems will also change, and could even lead to novel ecosystems under a changing climate [87]. Changes in disturbance regimes remain uncertain, and the short-term, direct influences on the provision of ecosystem services are complex and sometimes detrimental [77]. At the same time, disturbances could contribute to heterogeneity and diversification of future ecosystems at large scales. Recent works have shown that disturbances can act as an important mediator for ecosystems to autonomously adapt to changing environmental conditions [24,88]. More specifically, these studies found that disturbances contribute to the enhancement and rapid recovery of biodiversity at different scales [24,88], which subsequently supports ecosystem functioning [24]. Disturbances can also provide opportunities for species to respond to climate change [89], for example if disturbances help them to spread into new areas at the leading edge of their current range [90]. Summing up, unlike anthropogenic drivers such as nitrogen deposition, which directly enhances ecosystem function (e.g., productivity) in the short term but can indirectly deteriorate it in the long term (through the decline of biodiversity [91]), natural disturbances support ecosystem functioning through their positive influences on local and regional biodiversity.
Non-Equilibrium and Alternative States
Ecological communities are always dynamic and vary in space and time. They often develop along relatively predictable successional trajectories, but can also abruptly change to alternative states. There is a large body of theory and terminology for describing this dynamic nature of communities, including alternative stable states [92], alternative transient states [81], dynamic equilibrium [88], and non-equilibrium dynamics [75,76]. An in-depth analysis of these concepts is beyond the scope of this contribution, but we suggest that spatial variation at the landscape scale is a key element in understanding this dynamic behavior of ecosystems. Considering the increasing importance of variability in ecological systems, particularly in the context of global change [93], research should give further attention to spatial heterogeneity and temporal variabilities in terms of the structure, composition (including taxonomic, functional, and phylogenetic characteristics), and functioning of ecosystems.
Concluding Remarks
The objective of this commentary is to call for an expansion of our perspectives on the roles of biodiversity in supporting humanity. This call is motivated by the observation that, hitherto, studies on biodiversity–ecosystem functioning have not yet sufficiently considered the dynamic nature of ecosystems, or the spatiotemporal diversity arising from it. This β-diversity has large potential to be a cornerstone of biodiversity research, improving our understanding of the causes (through the processes of community assembly) and consequences (for ecosystem functioning) of biodiversity change (Box 2). Such an improved understanding of biodiversity–ecosystem functioning relationships is essential to ensure the sustained supply of multiple societal benefits of ecosystems [1] (see Outstanding Questions).
Outstanding Questions.
How are spatial and temporal variations in the identities and abundances of species among local assemblages (β-diversity) important to supporting ecosystem functioning? How can such importance be different and amplified when multiple ecosystem functions are simultaneously focused?
Howare the key processes of driving the spatial and temporal variations in local diversity (β-diversity) linked with the mechanisms underpinning diversity– ecosystem functioning relationships? How can such processes of local community assembly change in responses to natural and human influences? What are the consequences of such alterna-tions of local processes (e.g., ashiftfrom neutrality to niche-based community assembly) for ecosystem functions?
How can local changes in the causes of biodiversity (community assembly) and its functional consequences (ecosystem functioning) scale up to large-scale changes in the provisioning of multiple ecosystem services?
Linking the causes and consequences of biodiversity changes is not easy [6,94] because ecological communities are dynamic and complex [95]. This is especially true under mounting anthropogenic impacts. In addition to the further need for fundamental ecological research, we note that the perspectives highlighted in this commentary are currently not sufficiently incorporated into practical frameworks of biodiversity conservation and management. We thus encourage advancing knowledge in this area to allow ongoing assessment bodies, such as the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), to better assess the status of biodiversity and ecosystem services. Because variability and change are all ubiquitous in socioecological systems, acknowledging such inherent properties of nature is an essential step in making scientific knowledge practically applicable.
Highlights.
A rich body of evidence shows how biodiversity can help to sustain pools and fluxes of matter and energy in ecosystems. Understanding such diversity effects on ecosystem functioning is crucial to predicting the potential consequences of biodiversity loss.
Although α-diversity has received great attention in the literature, there is a serious knowledge gap for the roles and functions of β-diversity.
β-Diversity provides insights into the mechanisms driving biodiversity changes and their consequences for multiple ecosystem functions. Focusing on β-diversity is especially important in ecological communities that are subject to large environmental fluctuations and disturbances.
Considering the increasing importance of variability in ecological systems, particularly in the context of global change, insights gained from studying β-diversity are of importance for both theoretical and applied ecology.
Acknowledgments
A.S.M. was supported by the Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (15KK0022), and the Environment Research and Technology Development Fund of the Japanese Ministry of the Environment (S-14). R.S. acknowledges support from the Austrian Science Fund FWF through START grant Y895-B25. F.I. acknowledges support from the US National Science Foundation Long-Term Ecological Research (LTER) program (DEB 1234162) and the LTER Network Communications Office (DEB-1545288). We acknowledge thoughtful comments by anonymous reviewers.
References
- 1.Isbell F, et al. Linking the influence and dependence of people on biodiversity across scales. Nature. 2017;546:65–72. doi: 10.1038/nature22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 3.Duffy JE, et al. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature. 2017;549:261–264. doi: 10.1038/nature23886. [DOI] [PubMed] [Google Scholar]
- 4.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 5.Tilman D, et al. Biodiversity and ecosystem functioning. Ann Rev Ecol Evol Syst. 2014;45:471–493. [Google Scholar]
- 6.Mori AS. Environmental controls on the causes and functional consequences of tree species diversity. J Ecol. 2018;106:113–125. [Google Scholar]
- 7.Cadotte MW. Functional traits explain ecosystem function through opposing mechanisms. Ecol Lett. 2017;20:989–996. doi: 10.1111/ele.12796. [DOI] [PubMed] [Google Scholar]
- 8.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448:188–190. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 9.Zavaleta ES, et al. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc Natl Acad Sci U S A. 2010;107:1443–1446. doi: 10.1073/pnas.0906829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 11.Pasari JR, et al. Several scales of biodiversity affect ecosystem multifunctionality. Proc Natl Acad Sci U S A. 2013;110:10219–10222. doi: 10.1073/pnas.1220333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamfeldt L, et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat Commun. 2013;4:1340. doi: 10.1038/ncomms2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Plas F, et al. Biotic homogenization can decrease landscape-scale forest multifunctionality. Proc Natl Acad Sci U S A. 2016;113:3557–3562. doi: 10.1073/pnas.1517903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori AS, et al. Low multifunctional redundancy of soil fungal diversity at multiple scales. Ecol Lett. 2016;19:249–259. doi: 10.1111/ele.12560. [DOI] [PubMed] [Google Scholar]
- 15.Hautier Y, et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat Ecol Evol. 2018;2:50–56. doi: 10.1038/s41559-017-0395-0. [DOI] [PubMed] [Google Scholar]
- 16.Socolar JB, et al. How should beta-diversity inform biodiversity conservation? Trends Ecol Evol. 2016;31:67–80. doi: 10.1016/j.tree.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Loiseau N, et al. Multi-component β-diversity approach reveals conservation dilemma between species and functions of coral reef fishes. J Biogeogr. 2017;44:537–547. [Google Scholar]
- 18.Olden JD. Biotic homogenization: a new research agenda for conservation biogeography. J Biogeogr. 2006;33:2027–2039. [Google Scholar]
- 19.Dornelas M, et al. Assemblage time series reveal biodiversity change but not systematic loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- 20.Loreau M, et al. Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci U S A. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillebrand H, Matthiessen B. Biodiversity in a complex world: consolidation and progress in functional biodiversity research. Ecol Lett. 2009;12:1405–1419. doi: 10.1111/j.1461-0248.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 22.Mori AS, et al. Response diversity determines there silience of ecosystems to environmental change. Biol Rev. 2013;88:349–364. doi: 10.1111/brv.12004. [DOI] [PubMed] [Google Scholar]
- 23.France KE, Duffy JE. Diversity and dispersal interactively affect predictability of ecosystem function. Nature. 2006;441:1139–1143. doi: 10.1038/nature04729. [DOI] [PubMed] [Google Scholar]
- 24.Silva Pedro M, et al. A disturbance-induced increase in tree species diversity facilitates forest productivity. Landsc Ecol. 2016;31:989–1004. [Google Scholar]
- 25.Barnes AD, et al. Species richness and biomass explain spatial turnover in ecosystem functioning across tropical and temperate ecosystems. Philos Trans R Soc B. 2016;371:20150279. doi: 10.1098/rstb.2015.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bracken MES, et al. Spatial scale mediates the effects of biodiversity on marine primary producers. Ecology. 2017;98:14341443. doi: 10.1002/ecy.1812. [DOI] [PubMed] [Google Scholar]
- 27.Mokany K, et al. beta diversity contributes to ecosystem processes more than by simply summing the parts. Proc Natl Acad Sci U S A. 2013;110:E4057. doi: 10.1073/pnas.1313429110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chase JM. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 2010;328:1388–1391. doi: 10.1126/science.1187820. [DOI] [PubMed] [Google Scholar]
- 29.Mori AS, et al. Community assembly processes shape an altitudinal gradient of forest biodiversity. Global Ecol Biogeogr. 2013;22:878–888. [Google Scholar]
- 30.Matsuoka S, et al. Disentangling the relative importance of host tree community, abiotic environment and spatial factors on ectomycorrhizal fungal assemblages along an elevation gradient. FEMS Microbiol Ecol. 2016;92:fiw044. doi: 10.1093/femsec/fiw044. [DOI] [PubMed] [Google Scholar]
- 31.Mori AS, et al. Biotic homogenization and differentiation of soil faunal communities in the production forest landscape: taxonomic and functional perspectives. Oecologia. 2015;177:533544. doi: 10.1007/s00442-014-3111-7. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MJ, et al. Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol Lett. 2010;14:19–28. doi: 10.1111/j.1461-0248.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 33.Koleff P, et al. Measuring beta diversity for presence–absence data. J Anim Ecol. 2003;72:367–382. [Google Scholar]
- 34.Lennon JJ, et al. The geographical structure of British bird distributions: diversity, spatial turnover and scale. J Anim Ecol. 2001;70:966–979. [Google Scholar]
- 35.Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. [Google Scholar]
- 36.Legendre P. Interpreting the replacement and richness difference components of beta diversity. Global Ecol Biogeogr. 2014;23:1324–1334. [Google Scholar]
- 37.Baselga A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr. 2010;19:134–143. [Google Scholar]
- 38.Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- 39.Baselga A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecol Biogeogr. 2012;21:1223–1232. [Google Scholar]
- 40.Carvalho JC, et al. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Global Ecol Biogeogr. 2012;21:760–771. [Google Scholar]
- 41.Leprieur F, Oikonomou A. The need for richnessindependent measures of turnover when delineating biogeographical regions. J Biogeogr. 2014;41:414–417. [Google Scholar]
- 42.Byrnes JEK, et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Method Ecol Evol. 2014;5:111–124. [Google Scholar]
- 43.Hector A, et al. BUGS in the analysis of biodiversity experiments: species richness and composition are of similar importance for grassland productivity. PLoS One. 2011;6:e17434. doi: 10.1371/journal.pone.0017434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MN, Knapp AK. Dominant species maintain ecosystem function with non-random species loss. Ecol Lett. 2003;6:509–517. [Google Scholar]
- 45.Lohbeck M, et al. The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology. 2016;97:2772–2779. doi: 10.1002/ecy.1499. [DOI] [PubMed] [Google Scholar]
- 46.Karp DS, et al. Intensive agriculture erodes beta-diversity at large scales. Ecol Lett. 2012;15:930–970. doi: 10.1111/j.1461-0248.2012.01815.x. [DOI] [PubMed] [Google Scholar]
- 47.Mori AS, et al. Null model approaches to evaluating the relative role of different assembly processes in shaping ecological communities. Oecologia. 2015;178:261–273. doi: 10.1007/s00442-014-3170-9. [DOI] [PubMed] [Google Scholar]
- 48.Kraft NJB, et al. Disentangling the drivers of beta diversity along latitudinal and elevational gradients. Science. 2011;333:1755–1758. doi: 10.1126/science.1208584. [DOI] [PubMed] [Google Scholar]
- 49.Tscharntke T, et al. Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology. 2008;89:944–951. doi: 10.1890/07-0455.1. [DOI] [PubMed] [Google Scholar]
- 50.Sekercioglu CH. Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J Ornithol. 2012;153:153–161. [Google Scholar]
- 51.Martínez-Salinas A, et al. Bird functional diversity supports pest control services in a Costa Rican coffee farm. Agric Ecosyst Environ. 2016;235:277–288. [Google Scholar]
- 52.Lavabre JE, et al. How does the functional diversity of frugivorous birds shape the spatial pattern of seed dispersal? A case study in a relict plant species. Philos Trans R Soc B. 2016;371:20150280. doi: 10.1098/rstb.2015.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujii S, et al. Disentangling relationships between plant diversity and decomposition processes under forest restoration. J Appl Ecol. 2017;54:80–90. [Google Scholar]
- 54.Mori AS, et al. Biodiversity and ecosystem services in forest ecosystems: a research agenda for applied forest ecology. J Appl Ecol. 2017;54:12–27. [Google Scholar]
- 55.Soliveres S, et al. Biodiversity at multiple trophic levels isneeded for ecosystem multifunctionality. Nature. 2016;536:456–459. doi: 10.1038/nature19092. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues JL, et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc Natl Acad Sci U S A. 2013;110:988–993. doi: 10.1073/pnas.1220608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gamez-Virues S, et al. Landscape simplification filters species traits and drives biotic homogenization. Nat Commun. 2015;6:8568. doi: 10.1038/ncomms9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori AS, et al. Concordance and discordance between taxonomic and functional homogenization: responses of soil mite assemblages to forest conversion. Oecologia. 2015;179:527–535. doi: 10.1007/s00442-015-3342-2. [DOI] [PubMed] [Google Scholar]
- 59.Gossner MM, et al. Land-use intensification causes multitrophic homogenization of grassland communities. Nature. 2016;540:266–269. doi: 10.1038/nature20575. [DOI] [PubMed] [Google Scholar]
- 60.Magurran AE, et al. Rapid biotic homogenization ofmarine fish assemblages. Nat Commun. 2015;6:8405. doi: 10.1038/ncomms9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cadotte MW, et al. Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol. 2011;48:1079–1087. [Google Scholar]
- 62.Newbold T, et al. Global patterns of terrestrial assemblage turnover within and among land uses. Ecography. 2016:1151–1163. [Google Scholar]
- 63.MacDougall AS, et al. Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse. Nature. 2013;494:86–89. doi: 10.1038/nature11869. [DOI] [PubMed] [Google Scholar]
- 64.Scheffer M, et al. Anticipating critical transitions. Science. 2012;338:344–348. doi: 10.1126/science.1225244. [DOI] [PubMed] [Google Scholar]
- 65.Lamy T, et al. Landscape structure affects the provisionof multiple ecosystem services. Environ Res Lett. 2016;11:124017 [Google Scholar]
- 66.Tscharntke T, et al. Landscape moderation of biodiversity patterns and processes – eight hypotheses. Biol Rev Camb Philos Soc. 2012;87:661–685. doi: 10.1111/j.1469-185X.2011.00216.x. [DOI] [PubMed] [Google Scholar]
- 67.Knoke T, et al. Compositional diversity of rehabilitated tropical lands supports multiple ecosystem services and buffers uncertainties. Nat Commun. 2016;7:11877. doi: 10.1038/ncomms11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Triviño M, et al. Optimizing management to enhance multifunctionality in a boreal forest landscape. J Appl Ecol. 2017;54:61–70. [Google Scholar]
- 69.Law EA, et al. Mixed policies give more options in multifunctional tropical forest landscapes. J Appl Ecol. 2017;54:51–60. [Google Scholar]
- 70.Montoya D, et al. Emerging perspectives in the restoration of biodiversity-based ecosystem services. Trends Ecol Evol. 2012;27:666–672. doi: 10.1016/j.tree.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Rey Benayas JM, et al. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science. 2009;325:1121–1124. doi: 10.1126/science.1172460. [DOI] [PubMed] [Google Scholar]
- 72.Bullock JM, et al. Restoration of ecosystem services and biodiversity: conflicts and opportunities. Trends Ecol Evol. 2011;26:541–549. doi: 10.1016/j.tree.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Tylianakis JM, et al. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology. 2005;86:3296–3302. [Google Scholar]
- 74.Winfree R, et al. Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science. 2018;359:791–793. doi: 10.1126/science.aao2117. [DOI] [PubMed] [Google Scholar]
- 75.Spugel DG. Disturbance, equilibrium, and environmental variability: what is 'natural' vegetation in a changing environment? Biol Conserv. 1991;51:1–18. [Google Scholar]
- 76.Mori AS. Ecosystem management based on natural disturbances: hierarchical context and non-equilibrium paradigm. J Appl Ecol. 2011;48:280–292. [Google Scholar]
- 77.Thom D, Seidl R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev. 2016;91:760–781. doi: 10.1111/brv.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidl R, et al. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol Appl. 2014;24:2063–2077. doi: 10.1890/14-0255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turnbull LA, et al. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol Lett. 2013;16:116–127. doi: 10.1111/ele.12056. [DOI] [PubMed] [Google Scholar]
- 80.Turnbull LA, et al. Understanding the value of plant diversity for ecosystem functioning through niche theory. Proc R Soc Lond B. 2016;283:20160536. doi: 10.1098/rspb.2016.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukami T, Nakajima M. Community assembly: alternative stable states or alternative transient states? Ecol Lett. 2011;14:973–984. doi: 10.1111/j.1461-0248.2011.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukami T, et al. Assembly history dictates ecosystem functioning: evidence from wood decomposer communities. Ecol Lett. 2010;13:675–684. doi: 10.1111/j.1461-0248.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- 83.Dickie IA, et al. Do assembly history effects attenuate from species to ecosystem properties? A field test with wood-inhabiting fungi. Ecol Lett. 2012;15:133–141. doi: 10.1111/j.1461-0248.2011.01722.x. [DOI] [PubMed] [Google Scholar]
- 84.Donato DC, et al. Multiple successional pathways and precocity in forest development: can some forests be born complex? J Veg Sci. 2012;23:576–584. [Google Scholar]
- 85.Tepley AJ, et al. Fire-mediated pathways of stand development in Douglas-fir/western hemlock forests of the Pacific Northwest, USA. Ecology. 2013;94:1729–1743. doi: 10.1890/12-1506.1. [DOI] [PubMed] [Google Scholar]
- 86.Seidl R. Forest disturbances under climate change. Nat Clim Change. 2017;7:395–402. doi: 10.1038/nclimate3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Millar CI, et al. Climate change and forests of the future: managing in the face of uncertainty. Ecol Appl. 2007;17:2145–2151. doi: 10.1890/06-1715.1. [DOI] [PubMed] [Google Scholar]
- 88.Thom D, et al. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Global Change Biol. 2017;23:269–282. doi: 10.1111/gcb.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thom D, et al. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J Appl Ecol. 2017;54:28–38. doi: 10.1111/1365-2664.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serra-Diaz JM, et al. Disturbance and climate micro-refugia mediate tree range shifts during climate change. Landsc Ecol. 2015;30:1039–1053. [Google Scholar]
- 91.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci U S A. 2013;110:11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beisner BE, et al. Alternative stable states in ecology. Front Ecol Environ. 2003;1:376–382. [Google Scholar]
- 93.De Laender F, et al. Reintroducing environmental change drivers in biodiversity-ecosystem functioning research. Trends Ecol Evol. 2016;31:905–915. doi: 10.1016/j.tree.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mori AS, et al. Biodiversity-ecosystem function relationships change through primary succession. Oikos. 2017;126:1637–1649. [Google Scholar]
- 95.Chapin FS, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 96.Chase JM, et al. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere. 2011;2:art24 [Google Scholar]
- 97.Maestre FT, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leach MK, Givnish TJ. Ecological determinants of species loss in remnant prairies. Science. 1996;273:1555–1558. [Google Scholar]
- 99.van der Plas F, et al. Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in European forests. Nat Commun. 2016;7:11109. doi: 10.1038/ncomms11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Isbell F, et al. Quantifying effects of biodiversity on ecosystem functioning across times and places. Ecol Lett. 2018;21:763–778. doi: 10.1111/ele.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGill BJ, et al. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol Lett. 2007;10:995–1015. doi: 10.1111/j.1461-0248.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 102.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press; 2001. [DOI] [PubMed] [Google Scholar]
- 103.Chase JM, Myers JA. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B. 2011;366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diaz S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- 105.Gagic V, et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc R Soc Lond B. 2015;282:20142620. doi: 10.1098/rspb.2014.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Myers JA, et al. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol Lett. 2013;16:151–157. doi: 10.1111/ele.12021. [DOI] [PubMed] [Google Scholar]